Improved house mouse control in the field with a higher dose zinc phosphide bait
Wendy A. Ruscoe A * , Peter R. Brown
A * , Peter R. Brown  A , Lyn A. Hinds
A , Lyn A. Hinds  A , Steve Henry A , Nikki Van de Weyer
A , Steve Henry A , Nikki Van de Weyer  A B , Freya Robinson A , Kevin Oh A B and Richard P. Duncan
A B , Freya Robinson A , Kevin Oh A B and Richard P. Duncan  A C
A C
A CSIRO Health and Biosecurity, GPO Box 1700, Canberra, ACT 2601, Australia.
B Applied BioSciences, Macquarie University, Sydney, NSW 2109, Australia.
C Centre for Conservation Ecology and Genomics, Institute for Applied Ecology, University of Canberra, Bruce, ACT 2617, Australia.
Wildlife Research 50(5) 335-343 https://doi.org/10.1071/WR22009
Submitted: 21 January 2022 Accepted: 26 May 2022 Published: 18 July 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Recent studies have shown that the sensitivity of wild house mice to zinc phosphide (ZnP) in Australia is significantly lower than previously assumed, which may account for the reported variability in efficacy of ZnP baits used for broadacre control of house mice in grain-growing regions. Under laboratory conditions ZnP-coated grains with a new higher dose (50 g ZnP/kg grain) were readily consumed but the efficacy of using grains with this higher dose under natural field conditions has not been tested.
Aims: To test whether the newly derived ZnP50 (50 g ZnP/kg grain) was more effective under field conditions than the currently registered ZnP25 (25 g ZnP/kg grain) in reducing populations of house mice during a mouse population irruption.
Methods: We used a before–after-control–impact (BACI) design to assess changes in mouse population size under different baiting treatments in a replicated field trial. We assessed changes in mouse abundance in recently sown paddocks with either ZnP50 (n = 3) or ZnP25 (n = 3) compared with unbaited control sites (n = 3).
Key results: Baiting with ZnP50 led to a median reduction in mouse numbers of >85%. Our modelling showed that under similar circumstances, using the ZnP50 formulation should deliver >80% reduction in population size most (>90%) of the time. In contrast, the current registered bait (ZnP25) achieved approximately 70% reduction in population size, but with more variable results. We would be confident of getting an 80% reduction in population size only 20% of the time by using the currently registered ZnP25 bait under similar field conditions.
Conclusions: Consistent with laboratory studies, this study demonstrated the higher probability of achieving a consistently high kill rate under field conditions with the new ZnP50 bait compared with the currently registered formulation (ZnP25).
Implications: By using the new ZnP50 bait, farmers are far more likely to get good kill rates, thereby reducing the need for repeated baiting (which is costly and generally ineffective at protecting newly sown crops). Using the new bait should result in lower control costs for farmers and fewer toxic grains being spread to control mice.
Keywords: Bayesian capture–mark–recapture models, broadacre cereal farms, efficacy, Mus musculus, pest control, population modelling, rodent, toxin, ZnP.
Introduction
Wild house mice (Mus musculus) in Australia undergo periodic plagues over vast areas of agricultural land, with densities exceeding 800 mice/ha, resulting in serious damage to agricultural crops (Singleton et al. 2005). Records of mouse plagues in New South Wales (Saunders and Giles 1977), Victoria and South Australia (Mutze 1989), coupled with reports from Queensland (Caughley et al. 1994; Pople et al. 2013), and to a lesser extent Western Australia (Chapman 1981), indicate that, on average, a plague occurs in at least one state in Australia every 4 years, most commonly in the cereal-growing regions (Singleton et al. 2005). The 1993, mouse plague that affected South Australia and Victoria was estimated to have caused A$64.5 million (in 1993) worth of damage to cereal crops (Caughley et al. 1994), with off-farm costs (reflecting mouse damage to infrastructure, produce, and the cost of cleaning up) conservatively estimated to be A$1 million (Caughley et al. 1994). The 2011 mouse plague reportedly caused over A$200 million in crop damage alone (S. Humphrys, National Mouse Management Working Group, in Hinds et al. (2014)), and the 2021 plague was of similar magnitude, extending into Western Australia as of January 2022 (G. Martin, Crop Protection Manager, GRDC, pers. comm.).
Farmers attempt to control mouse populations in their fields by applying the acute rodenticide zinc phosphide. Registered rodenticide baits for in-field application in Australia contain 25 g of zinc phosphide (Zn3P2, hereafter referred to as ZnP) per kilogram wheat bait as a surface coating (25 g ZnP/kg grain, hereafter referred to as ZnP25; 2.5% w/w). At this dose rate, a single grain was assumed to be lethal to a 20 g mouse (Staples et al. 2003). The current label application rate is 1 kg bait/ha, which equates to approximately 2–3 grains/m2 or about 25 000 toxin-coated grains/ha. This rate should be sufficient for effective population control, assuming single grains are lethal.
ZnP is a fast-acting, acute (single dose) toxic agent used for vertebrate pest control in multiple countries, including Australia, India, China, various countries in Southeast Asia, parts of Europe and the USA, with its use as a rodenticide beginning as early as 1911 in Italy (Tickes 1985). It is used around the world because of its good safety record, low cost, and reasonably high efficacy against a range of target rodent species (Sugihara et al. 1995). Although several effective anticoagulant rodenticides are available for the control of rodents in industrial and domestic situations, they are not suitable for the control of broadscale rodent infestations in crops primarily because of the potential for bioaccumulation in the animal food chain (see Lohr and Davis (2018) for review). When ingested, ZnP reacts immediately with stomach acids to release highly toxic phosphine gas that is quickly absorbed into the bloodstream to affect the lungs, liver, kidneys, heart and central nervous system (Guale et al. 1994; Erickson and Urban 2004). Death usually results from heart and kidney failure within 24 h but can occur up to 112 h after ingestion (Henry et al. 2022; Hinds et al. 2022). One benefit of using acute poisons is that secondary poisoning risks to predators and scavengers from ZnP exposure are low, especially when compared with other rodenticides (anticoagulants; Erickson and Urban 2004). As ZnP and phosphine do not bioaccumulate in the tissue of target animals, secondary poison risk is related to only the consumption of undigested bait in the gastro-intestinal tract of target animals (Tkadlec and Rychnovský 1990; Sterner and Mauldin 1995). Zinc phosphide is a dull grey–black colour that is unattractive to birds. It also causes an emetic (vomiting) response in most animals (but rodents cannot vomit), reducing non-target fatalities (McLeod and Saunders 2013).
ZnP is the only rodenticide registered for in-crop use in Australia (APVMA 2000). Its application has been a key management tool for grain-growers for many years (Mutze 1993; Brown et al. 1997, 2002; Mutze and Sinclair 2004) and it is easy to apply by aerial or ground methods. In Australia, ZnP can reduce mouse populations by up to 95% (Brown et al. 2002; Mutze and Sinclair 2004), but its efficacy can be variable (Caughley et al. 1998b; Brown et al. 2002). For example, mouse numbers in the southern Australian cereal growing area were high in 2016 (Ruscoe et al. 2022) but multiple anecdotal reports from farmers claimed that baiting at the prescribed rate had variable success in controlling mouse numbers (S. Henry and P. R. Brown, pers. comm.). Previously, landholders in the Eyre Peninsular reported that ZnP baiting failure and variability in kill success has been attributed to the presence of alternative food sources, such as mature crops containing seed heads, post-harvest spilled grain, or bait aversion (Mutze 2017). However, a recent study investigating how alternative food affected bait-take by mice found that mice ingesting bait at doses far higher than the published lethal dose were surviving and becoming averse to toxic grains (Henry et al. 2022). This led to a re-examination of the median lethal dose (LD50) using oral gavage trials on both Australian wild-caught and a laboratory strain of mice. These laboratory trials found that the LD50 was 72–79 mg ZnP/kg body weight (BW; Hinds et al. 2022), a value significantly higher than the 32.7 mg ZnP/kg BW previously reported by Li and Marsh (1988), which was the value on which the currently registered bait is formulated. Given the results of Hinds et al. (2022), a new higher-strength bait was developed on the basis of a lethal dose (LD90: 110 mg ZnP/kg BW), which equates to an application rate of 50 g ZnP/kg wheat bait (hereafter referred to as ZnP50), whereby each wheat grain is coated with ~2 mg ZnP (LD90 dose for 15 g mouse). Laboratory feeding trials showed that mice readily consumed this new ZnP50 bait and mortality was high (94%, n = 18) in the absence of alternative food (Hinds et al. 2022).
The purpose of this study was to evaluate the performance of the new ZnP50 bait compared with currently registered ZnP25 baits in cropping fields as farmers were baiting their paddocks during a mouse outbreak. We used a randomised experiment to determine whether the new bait could deliver better population reductions of house mice than does the currently registered bait (ZnP25). We were able to test the new bait after the regulatory authority, Australian Pesticide and Veterinary Medicines Authority (AVPMA), approved an emergency permit for the use of ZnP50 during the 2021 mouse plague in eastern Australia (APVMA permit PER90799, May 2021). We used a before–after-control–impact (BACI) design to compare changes in the density of mouse populations subject to the following three treatments: Treatment 1, baiting using ZnP25; Treatment 2, baiting using ZnP50; and untreated controls.
Materials and methods
Study design
We selected nine sites (paddocks) in newly sown wheat or canola crops (generally cultivars of Triticum aestivum, Brassica napus, L. or Brassica rapa L.) around Parkes, central New South Wales, Australia (33.1373°S, 148.1747°E) in May 2021. On all sites, farmers had rolled and burnt stubble in efforts to reduce mouse numbers in preparation for sowing. Our trial commenced 4 weeks after crop sowing, meaning that the sites had very little ground cover. At the time of our trial, eastern Australia was experiencing high to ‘plague’ numbers of house mice in cropping landscapes and nearby towns. Although many farmers were undertaking mouse control using ZnP baits to protect newly sown crops from mouse damage, we selected sites where no in-crop ZnP baiting had occurred in the current year or for at least 10 years prior (the last time there was a widespread mouse outbreak in this region of eastern Australia).
Experimental sites
Each experimental site was approximately 16 ha in size, with sites clustered in two ‘blocks’ located about 20 km apart, with three sites in Block 1 and six sites in Block 2. Sites in each block were within 2 km of each other. Two of the sites were slightly smaller than the others (~11 ha) and were designated control sites (one in each block). The remaining sites were randomly allocated to experimental treatments such that the three sites at Block 1 comprised 1 × Control, 1 × ZnP25, and 1 × ZnP50 treatments, and the six sites at Block 2 comprised 2 × Control, 2 × ZnP25, and 2 × ZnP50 treatments. To census mouse populations, we located trapping grids as centrally as possible within each site. Each trapping grid covered 0.64 ha, to establish a sufficient buffer area around each grid where mice had been controlled to minimise immigration into the trapping grid following baiting. Previous trapping studies of house mice in Australia show substantial home-range overlap and estimated median home-range sizes of 0.119–0.199 ha during the non-breeding season (Krebs et al. 1995; Chambers et al. 2000).
Experimental treatments
There were three treatments, each with three replicates in this experiment, for a total of nine sites. The treatments were
-
Untreated Control: an untreated control where no bait was applied,
-
Treatment ZnP25: commercially available ZnP25 bait (25 g ZnP/kg bait), equating to approximately 1 mg ZnP per grain, applied at 1 kg bait/ha (label rate),
-
Treatment ZnP50: newly derived ZnP50 bait (50 g ZnP/kg bait), equating to approximately 2 mg ZnP per grain, applied at 1 kg bait/ha.
Mouse baits were sourced from a commercial bait manufacturer as toxin-coated sterilised wheat grain in sealed metal drums (Last Supper Supreme Zinc Phosphide (Broadacre) Mouse Bait, Wilhelm Rural Pty Ltd) in either the 25 g ZnP/kg (ZnP25) or 50 g ZnP/kg (ZnP50) active ingredient baits.
Toxic baits were applied to each treatment site using a Sharman Bait Spreader (MD & LA Sharman Pty Ltd) mounted on the back of a four-wheel-drive farm utility vehicle. The same vehicle, driver and bait spreader were used on all sites for consistency, with the spreader emptied of residual bait and refilled between each site. All sites were baited on the same day, with ZnP25 sites baited first, followed by ZnP50 sites. The spreader was calibrated according to manufacturer instructions to deliver 1 kg of toxic grain per hectare as it was driven over the paddocks at a set speed in parallel lines 15 m apart to give complete coverage of the area.
Population monitoring
A pre-treatment survey of mouse populations at all sites was undertaken from 29 May to 3 June 2021 (four nights trapping). Treatments were applied on 5 June 2021 and post-treatment surveys were conducted 3 days later, from 8 to 13 June 2021 (five trapping nights). Trapping was extended to five nights post-treatment because the first two nights were wet (15 and 22 mm rain), which may affect capture probabilities and, hence, population estimates, especially when few animals were expected to be trapped on treated grids. Mouse populations were surveyed using capture–mark–recapture (CMR) techniques, based on live-capture data from traps laid out in a single grid within each of the nine sites. Sixty-four live-capture Longworth box traps (Longworth Scientific, Abingdon, UK) were placed on an 8 × 8 grid at 10-m spacing. Traps were baited with wheat grains and contained bonded polyester fibre (Dacron) for bedding. Traps were checked each morning and re-set each afternoon during a trapping session. All animals were individually marked (Biomark RFID PitTags), and their weight, body length, sex and breeding condition were recorded before being released at the point of capture. This study was approved by the CSIRO Wildlife and Large Animal, Animal Ethics Committee (approval number: 2019-18) and adheres to the 8th Edition of the Australian Code and Use of Animals for Scientific Purposes.
Population-size estimation
We analysed the capture–mark–recapture (CMR) data to estimate the numbers of mice on each trapping grid at each survey, assuming that populations were closed during each trapping survey. We used the method described in Royle et al. (2009), which allowed us to model heterogeneity in detection probabilities implemented in a Bayesian framework via data augmentation (see Supplementary material: data analysis for details). The outcome of the CMR analysis was an estimate of population size on each grid at each survey, expressed as a posterior distribution specifying the probability that the number of individuals took a particular value, having accounted for variation in capture probability across grids, nights, and among individuals. From this, we derived the best estimate of population size on each grid at each survey as the mean of the posterior distribution, along with two measures of uncertainty, namely the variance and 95% credible intervals (CIs) of the posterior distribution. A 95% CI defines the bounds within which we are 95% confident that a parameter value lies. We converted numbers of mice to density by dividing the estimated population size by the grid size (80 × 80 m, which includes a 5 m buffer around the outer traps = 0.64 ha) and converting the values to mice per hectare.
Before–after-control–impact analysis
We used a replicated before–after-control–impact (BACI) design comprising population-size estimates taken before and after implementation of the treatments. To test treatment efficacy, we modelled log-transformed estimates of mouse population size as a function of survey (pre-treatment vs post-treatment), experimental treatment (Control, ZnP25, ZnP50) and their interaction. We incorporated the uncertainty in mouse population-size estimates into the analysis by modelling the (log-transformed) mean number of mice at the jth site during the kth survey (Njk), as drawn from a normal distribution with variance that was a function of within-site-survey variation (the uncertainty in estimated population size,  , which was the variance in log (Njk) derived from the posterior distribution) and among-site-survey variation (unexplained random variation, σ2, estimated in model fitting) as follows (Eqn 1):
, which was the variance in log (Njk) derived from the posterior distribution) and among-site-survey variation (unexplained random variation, σ2, estimated in model fitting) as follows (Eqn 1):

where sPost is a dummy variable having value 1 for sites measured post-survey and 0 otherwise, t25 and t50 are dummy variables having value 1 for the ZnP25 and ZnP50 treatments respectively, and 0 otherwise, iPost_25 and iPost_50 are dummy variables for interaction terms having value 1 for the post-ZnP25 and post-ZnP50 surveys respectively, and 0 otherwise, and b2 is a dummy variable coding for Block 1 (0) and Block 2 (1). The βs are parameters estimated in model fitting. We fitted the above model in a Bayesian framework using Markov-chain Monte Carlo (MCMC) methods as implemented in the JAGS software (Plummer 2003), by using the package jagsUI (Kellner 2015) called from R v. 4.0.1 (R Core Team 2021). The model was run with three chains for 10 000 iterations following a burn-in of 5000 iterations, which was sufficient to achieve convergence as judged by the Gelman–Rubin statistic (Gelman and Rubin 1992).
In this analysis, most attention focuses on parameters β4 and β5, which estimate the post-treatment difference in mouse population size between the control and the ZnP25 and ZnP50 treatments respectively, having accounted for pre- and post-treatment changes at the control site (β1), any overall difference in population size between the control and ZnP treatments (β2 and β3), and any overall difference in population size between blocks (β6). Negative parameter estimates for β4 and β5 would indicate that application of the ZnP baiting treatments resulted in lower post-treatment mouse populations than in the control sites.
Because we modeled Njk on the log scale, transforming the parameters to  or
or  estimates the ratio of control (C) to treatment (T) population sizes post-treatment, e.g. C/T. We can then estimate the proportional reduction in population size at treatment sites relative to control sites as (C − T) / T, which, for the ZnP25 treatment, is given by
estimates the ratio of control (C) to treatment (T) population sizes post-treatment, e.g. C/T. We can then estimate the proportional reduction in population size at treatment sites relative to control sites as (C − T) / T, which, for the ZnP25 treatment, is given by  – 1. We used this proportional reduction in population size for each treatment relative to control sites as a measure of treatment efficacy. Figures were produced in R, using the package ggplot2 (Wickham 2016).
– 1. We used this proportional reduction in population size for each treatment relative to control sites as a measure of treatment efficacy. Figures were produced in R, using the package ggplot2 (Wickham 2016).
Bait toxicity testing
A sample of 10 toxic grains was taken from each drum used for each treatment site as the bait spreader was being loaded. These ZnP-coated grains were individually analysed to confirm the expected coating rate of approximately 1 and 2 mg ZnP per grain for the ZnP25 and ZnP50 baits respectively. Analysis was undertaken by ACS Laboratories (Australia, 37 Stubbs Street, Kensington, Vic. 3031, Australia), a commercial, independent analytical-service provider accredited by the National Association of Testing Authorities (NATA), Australia (NATA Accreditation No. 16973). Grains coated with ZnP were individually analysed by microwave digestion followed by inductively coupled plasma optical emission spectroscopy (ICP–OES). Each grain was weighed directly into a teflon digestion tube to which 5 mL of concentrated nitric acid and 1 mL of 30% hydrogen peroxide were added. Each digestion tube was then microwaved for 65 min by using a temperature gradient from 0 to 200°C in a Milestone Connect Ethos Lean Compact Microwave Digestor. Once cooled, the contents of the digestion tube were diluted to 50 mL with deionised water, followed by a further 10-fold dilution with deionised water for ICP analysis using an Agilent 5110 ICP–OES (Dual View). The operating conditions for the instrument were as follows: 1.20 kW RF power, plasma 15.0 L/min, auxiliary 1.50 L/min, AVS7 sample injection system, injection pump rate 15.7 mL/min, detection intensity, Zn 206.2 nm/c.s.
Ethics approval
This study was approved by the CSIRO Wildlife and Large Animal, Animal Ethics Committee (approval number: 2019-18) and adheres to the 8th Edition of the Australian Code and Use of Animals for Scientific Purposes. This article does not contain any studies with human participants performed by any of the authors.
Results
Population-size estimation
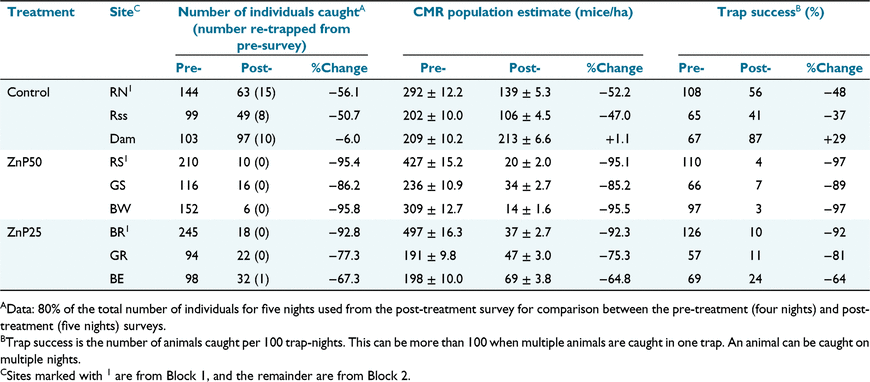
We trapped 1651 individuals over the 2-week study (1261 in the pre-treatment survey and 390 in the post-treatment survey). Mouse body weight averaged 12.8 g over all grids and surveys, indicating a predominantly young adult population. Very few younger animals (<10 g) were trapped, and no pregnant/lactating females or scrotal males were observed. Estimates of mouse population size from the capture–mark–recapture analyses from each survey on each grid are shown in Table 1. Pre-treatment mouse numbers were estimated at 200 and 500 mice per hectare.
Population size was uniformly higher on Block 1 sites and these sites also experienced greater population reductions pre- to post-treatment, including on the control site (Table 1, Fig. 1). Fig. 1 shows CMR estimates of population density (per ha) for each site in each treatment by survey. On average, the number of mice present on the control sites decreased between the two surveys. However, larger population reductions pre- to post-treatment were seen on the treated sites, particularly the ZnP50 sites (Table 1) in number of individuals caught, CMR population estimates and trap success. The number of individuals caught, and trap success, is presented (Table 1) for comparison with previous Australian baiting studies.
Before–after-control–impact analysis
The BACI analysis used to evaluate treatment efficacy (Eqn 1) estimates the reductions in populations sizes as a function of survey, experimental treatment and their interaction, and incorporates uncertainty in mouse population-size estimates. The posterior distribution of the proportional reduction in population size for treatment relative to control sites (treatment efficacy) estimated from the BACI analysis is shown in Fig. 2. Using the ZnP25 bait, the median expected reduction in population size was approximately 70%, with a 50% probability of observing a population reduction of between 58% and 78% (Fig. 2). In contrast, the median expected population reduction associated with using the ZnP50 bait was approximately 90%, with a 50% probability of a population reduction between 84% and 92%.
Assuming at least an 80% reduction in population size was the desired outcome of a baiting program, the new ZnP50 bait had a 95% probability of achieving that outcome or better, whereas the currently registered ZnP25 bait had a 20% probability of achieving that outcome or better (Fig. 3).
Bait testing
Independent analysis ZnP-coated grains confirmed the expected average toxin coating rate per grain. On average, individual grains of the ZnP25 batch (25 g ZnP/kg grain) were coated with 0.97 ± 0.06 mg ZnP per grain (mean ± s.e.; range: 0.48–1.66 mg; n = 30), those from the ZnP50 batch (50 g ZnP/kg grain) were coated with 1.83 ± 0.13 mg ZnP per grain (mean ± s.e.; range: 0.81–4.0 mg; n = 30).
Discussion
This study showed that the new ZnP50 bait was highly effective in reducing mouse population sizes. The proportional reduction in mouse population size pre- to post-treatment relative to control sites (treatment efficacy) for the ZnP50 bait had a median value of >85%. Accounting for variation among experimental sites and uncertainties in capture–mark–recapture population estimates, we estimate that the ZnP50 bait delivered a reduction in mouse population size of >80%, with >90% certainty. In contrast, the outcome of using the currently registered bait (ZnP25) was far less certain. We estimated a median reduction in population size of <75%, and that there was only a 20% chance that this bait would deliver >80% reduction in population size.
Similarly, variable kill rates using the currently registered ZnP25 have been reported; Brown et al. (2002) surveyed mouse populations associated with aerial baiting of cereal crop paddocks and reported population density reductions ranging between 56% and 94% in trial sites in north-western Victoria, compared with a 20% reduction in populations on the unbaited control sites. Studies by Mutze and Sinclair (2004) found that ZnP could reduce mouse populations by >90% when used either 5 days before sowing or 6 weeks after sowing cereal crops. In contrast, when baiting occurred only 2 days after sowing, mouse numbers were reduced by only 30% and obvious mouse diggings were abundant along the seed rows. Caughley et al. (1998b) reported mortality rates ranging from 95% down to slight population increases associated with ZnP baiting control operations in Queensland cereal/sorghum/soybean crops at various stages of the crop cycle. The least successful kills occurred when stubble was baited immediately following harvest when presumably spilled grain was abundant on the ground (Caughley et al. 1998a, 1998b). In a separate study in wheat paddocks, we have measured spilled grain on the ground in the months following wheat harvest at up to150 kg/ha (n = 4 paddocks; W. A. Ruscoe, P. R. Brown and S. Henry, unpubl. data). In a laboratory trial, wild house mice ate more toxic wheat grain and mortality rate was significantly higher (85%) in the presence of a less-favoured alternative grain (lentils), than were mortalities of 47% and 53% in the presence of a more-favoured cereal grain (wheat and barley respectively; Henry et al. 2022). These studies all suggest that alternative food availability could be reducing the bait consumption by mice, leading to reduced efficacy if a single toxic grain is not lethal.
The paddocks in which we conducted our trials were recently emerged canola and wheat crops in early autumn. We trapped very few younger animals, and no pregnant/lactating females or scrotal males were observed, indicating that the summer breeding probably finished in March (early autumn) 2021. Estimates of mouse population size from the CMR analyses showed pre-treatment mouse numbers between 300 and 500 mice per hectare, which is high, but not as high as estimated during other plagues. Prior to sowing, in desperation, farmers had burned the (previous year) crop-stubble to reduce food and cover available to mice. In this respect, the paddock-scape had virtually all background food removed and resembled the paddocks of 30 years ago before conservation agriculture became prominent. As such, there was not much else on the ground, and the mice should have readily found toxic grains. We calculated the percentage population reduction on each site by using trap success (for comparison with previous studies); ZnP25 showed similarly variable reductions (64–92%) in population size as in previous studies (Brown et al. 2002; Mutze and Sinclair 2004), whereas the ZnP50 showed 89–97% reductions. It is concerning that our ZnP25-treated sites showed large variability in population reductions as varying levels of background food would not have been a contributing factor in this, as has been suggested previously. The effect of higher levels of background food availability, associated with the more recent changes to conservation agricultural practises (that include leaving standing stubble and chaff that provides cover habitat for mice and ploughing, which buries residual grain) on toxic grain uptake, requires in-field evaluation.
Longevity of ZnP baits may vary depending on the environmental factors where the baits are applied, whether the baits are pellets or whole grain, and time since deployment. ZnP degradation has been shown to increase under higher rainfall and humidity levels (Hilton and Robison 1972; Sterner and Mauldin 1995; Twigg et al. 2001). Twigg et al. (2001) demonstrated a reduction of ZnP on whole-wheat bait under seasonal wet conditions compared with dry conditions, but the available bait was considered lethal to rats for 8–14 days under either condition. Variability in baiting success was also found in Hawaii, where the effectiveness of ZnP on both pelleted bait and oat grain bait (groats) was tested against pest rats (Rattus rattus, R. norvegicus and R. exulans; Sugihara et al. 1995). Post-baiting captures of all rat species did not differ between the ZnP treatments and the untreated controls, and the authors concluded that ZnP was not effective in reducing the pest populations, particularly of R. norvegicus, which may have in part been due to rain during the trial. In contrast, Bell (1972) found that ZnP loss from baits occurred over time but that it was some factor or factors other than temperature and precipitation that were responsible for the deterioration of the bait toxicity. Although exposure to weather hastens the chemical decomposition of the ZnP, particularly in the first week post-deployment, some deterioration, albeit at a slower rate, occurred when bait was stored in the opened original bags at room temperatures.
In our study we had three fine nights following the application of the baiting treatments, followed by two nights of modest rain (15 and 22 mm per night) during the post-treatment trapping. There should have been sufficient time for animals to encounter baits before any degradation occurred if, indeed, it did in the 8 days post-baiting that we surveyed.
Without background food availability and wet weather affecting bait take, we conclude that, as found in the laboratory trials of Henry et al. (2022) and Hinds et al. (2022), the ZnP25-formulated bait containing, on average, 1 mg ZnP was not sufficient to kill mice unless they found and consumed multiple baits before starting to feel sick. Any animal that found and ate only one grain of ZnP25 probably consumed a sublethal dose and became averse to the baits, likely refusing to take more (Henry et al. 2022). Such aversion can last for 20–30 days (Parker and Hannan-Jones 1996) and has been reported in other rodents (Shepherd and Inglis 1993). This highlights the importance of each grain delivering an adequate dose to kill each mouse. The ZnP50 bait grains contained, on average, 2 mg of ZnP each, which for a 15 g mouse should deliver a toxic dosage equivalent to the newly derived LD90 (Hinds et al. 2022).
The average weight of mice in our study was 12.8 g (range: 6.5–24 g). According to Hinds et al. (2022), 13 g mice would need to ingest at least 1.4 mg ZnP to receive a lethal dose (LD90). There is some variability in the amount of toxin on individual grains as a result of the manufacturing process. From the grain samples that we had independently analysed, 50% of ZnP25 grain samples contained ≥1.0 mg ZnP, but only four grains of 30 (13%) had ≥1.4 mg. This means that 87% of ZnP25 grains available to be consumed (at 1 kg/ha) contained a sublethal dose for the average mouse, and, if eaten in isolation, would likely result in bait aversion. In contrast, 77% of ZnP50 grains contained ≥1.4 mg ZnP, reducing the probability of sublethal dose-related aversion. In competition for a mouse’s attention while foraging is spilled grain, (plus weed seeds and invertebrates) in paddocks.
The best evaluation of pest animal control effectiveness is the reduction in damage. However, this can be difficult to assess because counts of seedlings need to be measured several weeks after emergence, by which time the damage is done, and re-baiting is ineffectual. Here, we report the treatment efficacy on the basis of the change in population size of the target pest population. Efficacy should incorporate cost and benefit analysis of the treatment (management and crop loss data) as well as humaneness and hazards to non-target animals. The benefit of using one bait formulation over the other must include cost considerations. The process for making and delivering the alternative ZnP baits used in this study is the same, the only cost difference is in the additional quantity of base ZnP technical powder, which is about A$1/kg of bait (A. Shilling, pers. comm.).
In southern cropping regions of Australia, most damage to crops occurs at sowing when mouse abundance is high at the end of the breeding season. The best option for bait application remains to bait at sowing or shortly before to protect crops at this critical time, and when alternative food is likely to be at a nadir. Given the time constraints involved, it is imperative to bait only once and with a bait that gives the best chance of a successful outcome. This study has demonstrated the far higher certainty of achieving a high kill rate with the new ZnP50 bait than with the currently registered formulation, hopefully negating the need for repeat baiting.
Supplementary material
Supplementary material is available online.
Data availability
Data are held by the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia, and may be made available upon reasonable request to the senior author.
Conflicts of interest
PRB and LAH are Associate Editors for Wildlife Research. Despite this relationship, they did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practise when handling manuscripts submitted by an editor of this journal. The authors have no further conflicts of interest to declare.
Declaration of funding
This study was funded by the Grains Research and Development Corporation (CSP1804-012RTX and CSP1806-015RTX) and supported by CSIRO Health and Biosecurity.
Author contributions
WAR, PRB, LAH and SH designed this research. All authors (except RPD) collected experimental field data. WAR and RPD analysed the data. WAR, RPD and PRB wrote the first-draft manuscript. All authors contributed to the final manuscript.
Acknowledgements
We sincerely thank the farmers for allowing us to undertake this experiment on their properties. We thank Leigh Nelson and Ken Young (GRDC) for ongoing support. We are also grateful for logistical and field support from NSW Department of Primary Industries (Dave Forsyth, Stephen Jackson, Emma Sawyers, Pat Taggart). Peter Caley (Data61, CSIRO) reviewed our R code, analysis, and the draft manuscript. Two anonymous reviewers helped improve the manuscript.
References
APVMA (2000) Evaluation of the new active zinc phosphide in the product MouseOff zinc phosphide bait. (National Registration Authority for Agricultural and Veterinary Chemicals: Canberra, ACT, Australia) Available at https://apvma.gov.au/node/14101Bell HB (1972) ‘The hazards of secondary poisoning from zinc phosphide to selected vertebrate species.’ (University of Tennessee)
Brown, PR, Singleton, GR, Kearns, B, and Griffiths, J (1997). Evaluation and cost-effectiveness of strychnine for control of populations of wild house mice Mus domesticus in Victoria. Wildlife Research 24, 159–172.
| Evaluation and cost-effectiveness of strychnine for control of populations of wild house mice Mus domesticus in Victoria.Crossref | GoogleScholarGoogle Scholar |
Brown, PR, Chambers, LK, and Singleton, GR (2002). Pre-sowing control of house mice (Mus domesticus) using zinc phosphide: efficacy and potential non-target effects. Wildlife Research 29, 27–37.
| Pre-sowing control of house mice (Mus domesticus) using zinc phosphide: efficacy and potential non-target effects.Crossref | GoogleScholarGoogle Scholar |
Caughley J, Monamy V, Heiden K (1994) ‘Impact of the 1993 mouse plague.’ (GRDC: Canberra, ACT, Australia)
Caughley J, Bomford M, Parker B, Sinclair R, Griffiths J, Kelly D (1998a) ‘Managing vertebrate pests: rodents.’ (Bureau of Resource Sciences and Grains Research Development Corporation: Canberra, ACT, Australia)
Caughley J, Donkin C, Strong K (1998b) Managing mouse plagues in rural Australia. In ‘Proceedings of the eighteenth vertebrate pest conference’. (Eds RO Baker, AC Crabb) pp. 160–165. (University of California: Davis, CA, USA)
Chambers, LK, Singleton, GR, and Krebs, CJ (2000). Movements and social organization of wild house mice (Mus domesticus) in the wheatlands of northwestern Victoria, Australia. Journal of Mammology 81, 59–69.
| Movements and social organization of wild house mice (Mus domesticus) in the wheatlands of northwestern Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Chapman, A (1981). Habitat preference and reproduction of feral house mice, Mus musculus, during plague and non-plague situations in Western Australia. Wildlife Research 8, 567–579.
| Habitat preference and reproduction of feral house mice, Mus musculus, during plague and non-plague situations in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Erickson WA, Urban DJ (2004) ‘Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach.’ (US Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances: Washington, DC, USA)
Gelman, A, and Rubin, DB (1992). Inference from iterative simulation using multiple sequences. Statistical Science 7, 457–472.
| Inference from iterative simulation using multiple sequences.Crossref | GoogleScholarGoogle Scholar |
Guale, FG, Stair, EL, Johnson, BW, Edwards, WC, and Haliburton, JC (1994). Laboratory diagnosis of zinc phosphide poisoning. Veterinary and Human Toxicology 36, 517–519.
| 7900268PubMed |
Henry, S, Brown, PR, Van de Weyer, N, Robinson, F, and Hinds, LA (2022). Effects of background food on alternative grain uptake and zinc phosphide efficacy in wild house mice. Pest Management Science 78, 1090–1098.
| Effects of background food on alternative grain uptake and zinc phosphide efficacy in wild house mice.Crossref | GoogleScholarGoogle Scholar | 34786822PubMed |
Hilton, HW, and Robison, WH (1972). Fate of zinc phosphide and phosphine in the soil-water environment. Journal of Agricultural and Food Chemistry 20, 1209–1213.
| Fate of zinc phosphide and phosphine in the soil-water environment.Crossref | GoogleScholarGoogle Scholar | 5083526PubMed |
Hinds LA, Henry S, Sharma S, Leung L, Dyer C, Mayer L (2014) Effects or oral uptake of the chemosterilant 4-vinylcyclohexene diepoxide in wild house mice, Mus domesticus. In ‘26th Vertebrate pest control conference’. (Eds RM Timm, JM O’Brien) pp. 380–385. (University of California: Davis, CA, USA)
Hinds, LA, Henry, S, Van de Weyer, N, Robinson, F, Ruscoe, WA, and Brown, PR (2022). Acute oral toxicity of zinc phosphide: an assessment for wild house mice in Australia. Integrative Zoology , .
| Acute oral toxicity of zinc phosphide: an assessment for wild house mice in Australia.Crossref | GoogleScholarGoogle Scholar | 35651323PubMed |
Kellner K (2015) jagsUI: a wrapper around rjags to streamline JAGS analyses. R package version 1. Available at https://cran.r-project.org/web/packages/jagsUI
Krebs, CJ, Kenney, AJ, and Singleton, GR (1995). Movements of feral house mice in agricultural landscapes. Australian Journal of Zoology 43, 293–302.
| Movements of feral house mice in agricultural landscapes.Crossref | GoogleScholarGoogle Scholar |
Li JH, Marsh RE (1988) LD50 determination of zincphosphide toxicity for house mice and albino laboratory mice. In ‘Proceedings of the 13th Vertebrate Pest Conference’, Monteray, CA, USA, 1–3 March 1988. (Eds A Crabb, R Marsh) pp. 91–94. (University of California: Davis, CA, USA)
Lohr, MT, and Davis, RA (2018). Anticoagulant rodenticide use, non-target impacts and regulation: a case study from Australia. Science of the Total Environment 634, 1372–1384.
| Anticoagulant rodenticide use, non-target impacts and regulation: a case study from Australia.Crossref | GoogleScholarGoogle Scholar | 29710637PubMed |
McLeod L, Saunders G (2013) ‘Pestcides used in the management of vertebrate pests in Australia: a review.’ (NSW Department of Primary Industries: Orange, NSW, Australia)
Mutze, GJ (1989). Mouse plagues in South Australian cereal-growing areas. 1. Occurrence and distribution of plagues from 1900 to 1984. Wildlife Research 16, 677–683.
| Mouse plagues in South Australian cereal-growing areas. 1. Occurrence and distribution of plagues from 1900 to 1984.Crossref | GoogleScholarGoogle Scholar |
Mutze, GJ (1993). Cost-effectiveness of poison bait trails for control of house mice in mallee cereal crops. Wildlife Research 20, 445–455.
| Cost-effectiveness of poison bait trails for control of house mice in mallee cereal crops.Crossref | GoogleScholarGoogle Scholar |
Mutze G (2017) Mice in crops: again, and again, and again. In ‘Stubble management workshop’. (NRM Biosecurity, Primary Industries and Regions SA: Port Lincoln, SA, Australia) Available at https://airep.com.au/wp-content/uploads/2020/08/9-Mice-MUTZE-EP-Stubbles-Extravaganza.pdf
Mutze, G, and Sinclair, R (2004). Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops. Wildlife Research 31, 249–257.
| Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops.Crossref | GoogleScholarGoogle Scholar |
Parker RW, Hannan-Jones M (1996) ‘The potential of zinc phosphide for mouse plague control in Australia.’ (QDoNRa Mines: Brisbane, Qld, Australia)
Plummer M (2003) JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In ‘Proceedings of the 3rd international workshop on distributed statistical computing’, Vienna, Austria, 20–22 March 2003. (Eds K Hornik, F Leisch, A Zeileis) Available at http://www.R-project.org/conferences/DSC-2003/
Pople, A, Scanlan, J, Cremasco, P, and Farrell, J (2013). Population dynamics of house mice in Queensland grain-growing areas. Wildlife Research 40, 661–674.
| Population dynamics of house mice in Queensland grain-growing areas.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org/
Royle, JA, Nichols, JD, Karanth, KU, and Gopalaswamy, AM (2009). A hierarchical model for estimating density in camera-trap studies. Journal of Applied Ecology 46, 118–127.
| A hierarchical model for estimating density in camera-trap studies.Crossref | GoogleScholarGoogle Scholar |
Ruscoe, WA, Brown, PR, Henry, S, van de Weyer, N, Robinson, F, Hinds, LA, and Singleton, GR (2022). Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice. Journal of Pest Science 95, 493–503.
| Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice.Crossref | GoogleScholarGoogle Scholar |
Saunders, GR, and Giles, JR (1977). A relationship between plagues of the house mouse, Mus musculus (Rodentia: Muridae) and prolonged periods of dry weather in south-eastern Australia. Wildlife Research 4, 241–247.
| A relationship between plagues of the house mouse, Mus musculus (Rodentia: Muridae) and prolonged periods of dry weather in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Shepherd, DS, and Inglis, IR (1993). Toxic bait aversions in different rat strains exposed to an acute rodenticide. The Journal of Wildlife Management 57, 640–647.
| Toxic bait aversions in different rat strains exposed to an acute rodenticide.Crossref | GoogleScholarGoogle Scholar |
Singleton, GR, Brown, PR, Pech, RP, Jacob, J, Mutze, GJ, and Krebs, CJ (2005). One hundred years of eruptions of house mice in Australia – a natural biological curio. Biological Journal of the Linnean Society 84, 617–627.
| One hundred years of eruptions of house mice in Australia – a natural biological curio.Crossref | GoogleScholarGoogle Scholar |
Staples L, Smith M, Pontin K, Hunt W (2003) Use of zinc phosphide to overcome rodent infestations. In ‘Proceedings of the Australian postharvest technical conference’, Canberra, ACT, Australia. (Eds EJ Wright, MC Webb, E Highley) pp. 110–115. (CSIRO Stored Grain Research Laboratory)
Sterner, RT, and Mauldin, RE (1995). Regressors of whole-carcass zinc phosphide/phosphine residues in voles: indirect evidence of low hazards to predators/scavengers. Archives of Environmental Contamination and Toxicology 28, 519–523.
| Regressors of whole-carcass zinc phosphide/phosphine residues in voles: indirect evidence of low hazards to predators/scavengers.Crossref | GoogleScholarGoogle Scholar | 7755404PubMed |
Sugihara, RT, Tobin, ME, and Koehler, AE (1995). Zinc phosphide baits and prebaiting for controlling rats in Hawaiian sugarcane. The Journal of Wildlife Management 59, 882–889.
| Zinc phosphide baits and prebaiting for controlling rats in Hawaiian sugarcane.Crossref | GoogleScholarGoogle Scholar |
Tickes, BR (1985). Zinc phosphide in subterranean burrow systems. Bulletin of Environmental Contamination and Toxicology 34, 557–559.
| Zinc phosphide in subterranean burrow systems.Crossref | GoogleScholarGoogle Scholar | 3986394PubMed |
Tkadlec, E, and Rychnovský, B (1990). Residues of Zn3P2 in the common vole (Microtus arvalis) and secondary poisoning hazards to predators. Folia Zoologica 39, 147–156.
Twigg, LE, Martin, GR, Wilson, N, Goddard, D, Watkins, R, and Armstrong, PJ (2001). Longevity of zinc phosphide on wheat bait in tropical Australia. Wildlife Research 28, 261–267.
| Longevity of zinc phosphide on wheat bait in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2016) ‘ggplot2: elegant graphics for data analysis.’ (Springer: New York, NY, USA) Available at https://ggplot2.tidyverse.org