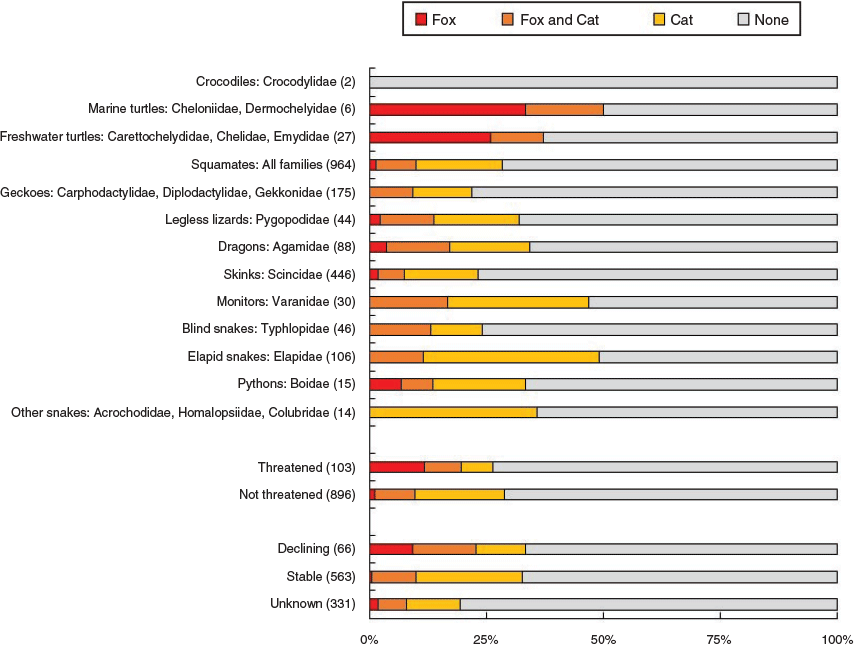Reptiles as food: predation of Australian reptiles by introduced red foxes compounds and complements predation by cats
Alyson M. Stobo-Wilson A M , Brett P. Murphy A , Sarah M. Legge B , David G. Chapple C , Heather M. Crawford D , Stuart J. Dawson
A M , Brett P. Murphy A , Sarah M. Legge B , David G. Chapple C , Heather M. Crawford D , Stuart J. Dawson  D , Chris R. Dickman E , Tim S. Doherty
D , Chris R. Dickman E , Tim S. Doherty  F , Patricia A. Fleming
F , Patricia A. Fleming  D , Matthew Gentle G , Thomas M. Newsome H , Russell Palmer I , Matthew W. Rees J , Euan G. Ritchie K , James Speed G , John-Michael Stuart D , Eilysh Thompson K , Jeff Turpin L and John C. Z. Woinarski
D , Matthew Gentle G , Thomas M. Newsome H , Russell Palmer I , Matthew W. Rees J , Euan G. Ritchie K , James Speed G , John-Michael Stuart D , Eilysh Thompson K , Jeff Turpin L and John C. Z. Woinarski  A
A
A NESP Threatened Species Recovery Hub, Charles Darwin University, Darwin, NT 0909, Australia.
B NESP Threatened Species Recovery Hub, Centre for Biodiversity and Conservation Research, University of Queensland, St Lucia, Qld 4072, Australia.
C School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
D Centre for Terrestrial Ecosystem Science and Sustainability, Harry Butler Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
E NESP Threatened Species Recovery Hub, Desert Ecology Research Group, School of Life and Environmental Sciences, Heydon-Laurence Building A08, University of Sydney, Camperdown, NSW 2006, Australia.
F School of Life and Environmental Sciences, Heydon-Laurence Building A08, University of Sydney, NSW 2006, Australia.
G Pest Animal Research Centre, Invasive Plants and Animals, Biosecurity Queensland, 203 Tor Street, Toowoomba, Qld 4350, Australia.
H Global Ecology Lab, School of Life and Environmental Sciences, University of Sydney, Sydney, NSW 2006, Australia.
I Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia.
J Quantitative and Applied Ecology Group, School of Biosciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
K Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
L School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
M Corresponding author. Email: alyson.stobowilson@gmail.com
Wildlife Research 48(5) 470-480 https://doi.org/10.1071/WR20194
Submitted: 17 November 2020 Accepted: 9 March 2021 Published: 14 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Invasive species are a major cause of biodiversity loss across much of the world, and a key threat to Australia’s diverse reptile fauna. There has been no previous comprehensive analysis of the potential impact of the introduced European red fox, Vulpes vulpes, on Australian reptiles.
Aims: We seek to provide an inventory of all Australian reptile species known to be consumed by the fox, and identify characteristics of squamate species associated with such predation. We also compare these tallies and characteristics with reptile species known to be consumed by the domestic cat, Felis catus, to examine whether predation by these two introduced species is compounded (i.e. affecting much the same set of species) or complementary (affecting different groups of species).
Methods: We collated records of Australian reptiles consumed by foxes in Australia, with most records deriving from fox dietary studies (tallying >35 000 samples). We modelled presence or absence of fox predation records against a set of biological and other traits, and population trends, for squamate species.
Key results: In total, 108 reptile species (~11% of Australia’s terrestrial reptile fauna) have been recorded as consumed by foxes, fewer than that reported for cats (263 species). Eighty-six species have been reported to be eaten by both predators. More Australian turtle species have been reported as consumed by foxes than by cats, including many that suffer high levels of predation on egg clutches. Twenty threatened reptile species have been reported as consumed by foxes, and 15 by cats. Squamate species consumed by foxes are more likely to be undergoing population decline than those not known to be consumed by foxes. The likelihood of predation by foxes increased with squamate species’ adult body mass, in contrast to the relationship for predation by cats, which peaked at ~217 g. Foxes, but not cats, were also less likely to consume venomous snakes.
Conclusions: The two introduced, and now widespread, predators have both compounding and complementary impacts on the Australian reptile fauna.
Implications: Enhanced and integrated management of the two introduced predators is likely to provide substantial conservation benefits to much of the Australian reptile fauna.
Key words: invasive species, lizard, snake, threatened species, turtle.
Introduction
The Australian reptile fauna is rich and highly distinctive, comprising 1050 native species, of which ~95% are endemic (Chapple et al. 2019). An increasing proportion of this fauna is recognised as threatened (Chapple et al. 2019; Tingley et al. 2019), with the first known extinction occurring in the past decade (Andrew et al. 2018). For terrestrial squamates (lizards and snakes), which comprise >95% of the reptile fauna, the threat affecting the most species (138) is ‘invasive and other problematic species and disease’ (Tingley et al. 2019). The two carnivorous mammals introduced to Australia, the domestic cat, Felis catus (hereafter cat), and the European red fox, Vulpes vulpes (hereafter fox), are the most pervasive of these invasive species. A recent review estimated that 650 million reptiles are killed annually by cats in Australia, and reported that cats are known to consume 258 Australian reptile species (Woinarski et al. 2018). Predation by the fox may also be significant, but there has been no previous assessment of its magnitude.
The impacts of foxes may be most significant for threatened reptile species (Webb et al. 2014). Fox predation has been listed as a threat affecting 12 of the 61 Australian reptile species listed nationally (as at 2008) as threatened (Department of the Environment Water Heritage and the Arts 2008). However, in many cases, this attribution of impact was conjectural or circumstantial, rather than definitive. Several lines of evidence have indicated that foxes have had, and continue to have, a significant detrimental impact on at least some components of the Australian reptile fauna. The most substantial evidence relates to very high rates of predation (often locally >95%) by foxes on egg clutches of many Australian freshwater turtles (Thompson 1983; Kennett et al. 2009; Fielder et al. 2014; Dawson et al. 2016; Robley et al. 2016) and of marine turtles at some breeding sites (Limpus and Reimer 1994; Chaloupka and Limpus 2001). This predation pressure is sufficient to cause a long-term population decline of at least some turtle species (Spencer 2002; Spencer and Thompson 2005; Spencer et al. 2016; Spencer et al. 2017). Indeed, experimental studies and management interventions have demonstrated that fox control or exclusion has led to substantial increases in turtle reproductive success (Chaloupka and Limpus 2001; Spencer and Thompson 2003; Spencer et al. 2006).
There have been no comparable studies of the impacts of fox predation on the population viability of any Australian squamate species. However, several studies have reported substantial (up to 5-fold) increases in the abundance of large monitor species (Varanus gouldii, V. varius) in responses to fox baiting (leading to reduced fox density; Olsson et al. 2005; Hu et al. 2019; Stobo-Wilson et al. 2020), and exclusion of foxes and cats (Read and Scoleri 2015). Although foxes commonly consume the eggs of monitors (Cogger 1959), the substantial increase in the abundance of monitors in response to a decrease in fox abundance due to fox control suggests that the impacts of foxes on the Australian reptile fauna may be due not only to predation but also competition (Sutherland et al. 2011; Glen 2014; Hu et al. 2019).
There are now substantial areas in south-western and south-eastern Australia in which broad-scale management of foxes is being implemented (Reddiex et al. 2006; Saunders et al. 2010). However, most fox-inhabited areas have no effective management, and foxes persist even in managed areas (albeit at lower densities), indicating that impacts of fox predation on Australian reptiles may be pervasive and ongoing, and a more comprehensive assessment of such impact is warranted. Here, we provide such an assessment, through collation of records of predation by foxes on Australian reptiles, using an approach similar to that described by Woinarski et al. (2018) for records of predation by cats on reptiles. We compare tallies of species consumed by foxes and by cats, and examine whether squamate species consumed by foxes vary in morphological or other traits from species consumed by cats. In part, this analysis aims to determine whether the predation pressure on Australian reptiles by these two introduced mammals is complementary (i.e. they eat different species) or compounding (predation by both species falls largely on the same set of species).
Methods
Foxes and cats in Australia
As context for these comparisons, we note some similarities and contrasts in the ecology of these two introduced predators. Both species are now widespread in Australia; however, whereas cats occur pervasively across all habitats on the Australian mainland and many islands (including Tasmania), foxes are largely absent from monsoonal northern Australia, absent from Tasmania and occur on far fewer islands. Both species are widely recognised as highly opportunistic and generalist in their diet, capable of switching dietary intake with variations in the abundance of prey species (Sutherland et al. 2011; Doherty et al. 2015). Cats are obligate carnivores whereas foxes may consume significant amounts of plant material (notably fruits; Woinarski et al. 2019; Fleming et al. 2021). Foxes (male weight 4.7–8.3 kg) are typically larger than cats (3.4–7.3 kg; Van Dyck and Strahan 2008). Cats are more adept climbers, but foxes are also known to climb trees to hunt prey (Mella et al. 2018). Foxes are more likely than cats to dig up prey, including the buried eggs of some reptile species and reptiles sheltering in burrows (Spencer and Thompson 2005; Nielsen and Bull 2016). For both predators, reptiles comprise a significant component of the diet in Australia (Doherty et al. 2015; Fleming et al. 2021). Cats also have a metabolism that favours consumption of multiple small meals during foraging periods (Woinarski et al. 2019).
Reptile species eaten by foxes
Australian reptile taxonomy is fluid. We followed the checklist used in Chapple et al. (2019) for squamates, except that we excluded a set of ~30 reptile species described since 2017 (see appendix 4.1 in Chapple et al. 2019), and we excluded all 31 Australian sea-snake species on the grounds that fox predation on these marine species is improbable. These exclusions left 956 Australian native terrestrial squamate species. In addition to these, we also included eight introduced squamate species, two crocodile species, and six marine turtle species listed in Cogger (2014). For freshwater turtles, we follow the checklist of the IUCN Turtle Taxonomy Working Group (Rhodin et al. 2017). With 27 species of freshwater turtles (including one introduced species), the tally of terrestrial reptiles in Australia considered here is 999 species (see Table S1 for list of species, available as Supplementary material to this paper). Given the recent rate of discovery and taxonomic revision in the Australian reptile fauna (Tingley et al. 2016), records of some of the reptile species named in earlier fox dietary studies may now not be readily attributable to currently accepted species. Where necessary, we used the taxonomic clarification and mapping of Chapple et al. (2019) to re-allocate the species names used in earlier fox dietary studies.
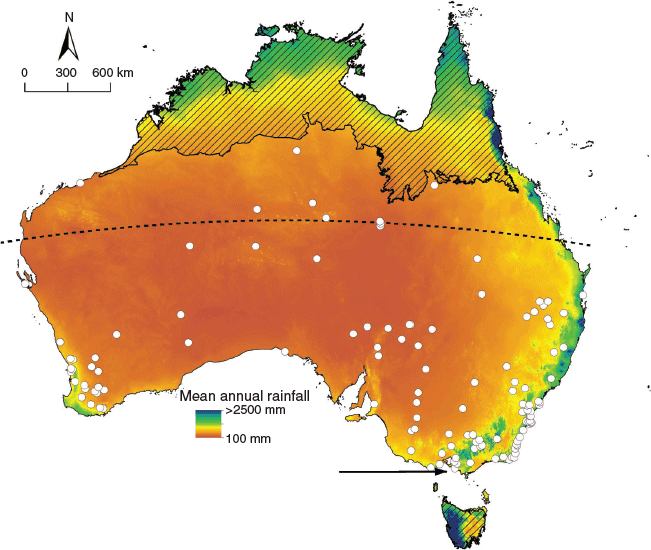
Our listing of Australian reptile species consumed by foxes derived largely from 73 fox diet studies, many with multiple study sites (collectively including 35 488 stomach and scat samples) spread across the fox’s extensive Australian distribution (Fig. 1). We also included information from autecological studies and reviews of reptile species and their sources of mortality (e.g. Spencer and Thompson 2005; Heard et al. 2006), and more general reviews of fox impacts (Saunders et al. 1995; Saunders and McLeod 2007; Department of the Environment Water Heritage and the Arts 2008; Saunders et al. 2010). However, we did not include such information if it was based on inference or presumption of predation rather than definitive records of fox predation. A listing of all sources from which records were extracted is given in Table S2. From this collation, we tallied the number of reptile species, and the number of threatened reptile species (those recognised as threatened under Australian legislation or globally by the International Union for the Conservation of Nature (IUCN), as at September 2020) known to be consumed by foxes. Australian legislation allows for the listing of subspecies as threatened; in such cases, we treated the species as threatened given that our records of fox predation of reptiles typically did not discriminate to subspecies level, and we assume that if a fox can consume one subspecies of a species, it is capable of consuming any other subspecies of that species.
|
We compared these tallies with the equivalent numbers for reptile species reported to be eaten by cats (Woinarski et al. 2018). The main basis of the collation of records of predation by cats on reptiles was from 60 cat dietary studies, many with multiple study sites (including 10 744 cat stomachs or scats), a substantial tally but, nonetheless, a smaller sample size than for our fox collation. Note that tallies for cat-eaten reptiles vary in minor detail from those presented in the earlier study (Woinarski et al. 2018) as we have added a few more records of cat-eaten reptiles and there have been some subsequent changes in taxonomy and the conservation status of Australian reptiles.
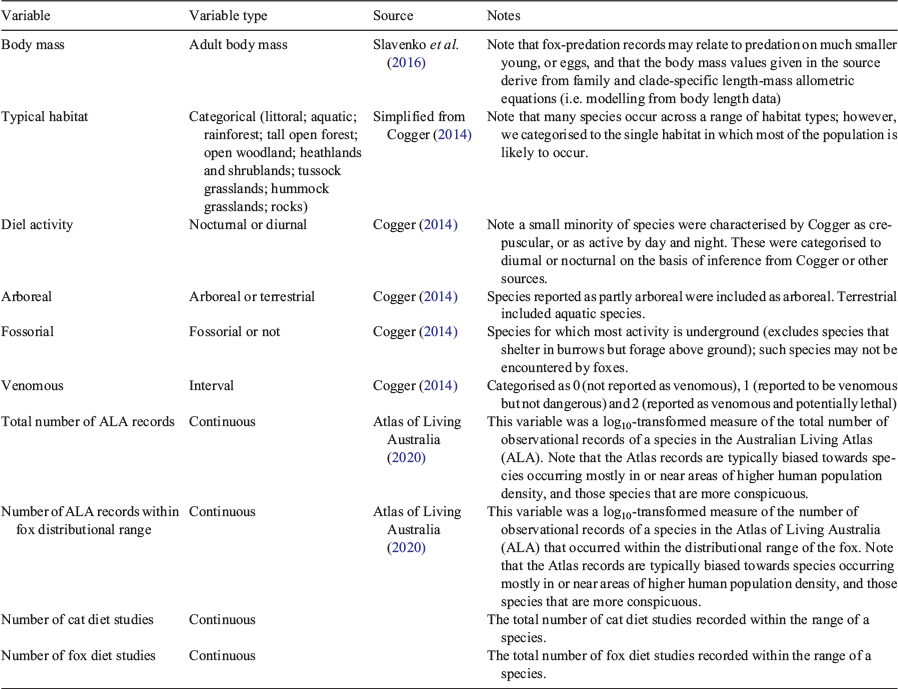
We developed a set of ecological traits for squamate species (Table 1), and contrasted these traits among squamate species known to be consumed by foxes and those known to be consumed by cats, against those not recorded as eaten. We excluded the few non-squamate reptiles (i.e. turtles, crocodiles) from the trait analyses, because their inclusion would have swamped variation among squamate species. Additionally, we categorised all squamate species by whether or not they occurred within the distributional range of the fox (~80% of Australia’s land area). Almost all Australian reptile species occur within the distributional range of the cat. As a surrogate for the distributional extent and abundance of each squamate species within the range of the cat and fox, we tallied for each squamate species (1) the total number of records in the Atlas of Living Australia (ALA; the main distributional database for Australian biodiversity), and (2) only those records that occur within the distributional range of the fox. For the latter, we tallied the number of ALA records in all bioregions (Thackway and Cresswell 1995) for which there were valid fox records. We also tallied the number of fox and cat dietary studies that have occurred within the range of each squamate species, as a measure of research effort, to account for potential sampling biases.
|
We include records of predation on reptile eggs within our collation, where this is attributable to the species. We acknowledge that some of the records of reptiles in fox (or cat) diet may have been as a result of the fox (or cat) scavenging on carrion; however, in most cases, such distinction was not made in the sources that we collated. In general, foxes take more carrion than do cats (Fleming et al. 2020, 2021). However, most carrion consumption is of larger mammals (Catling 1988), although we note that the sole record we collated for fox consumption of the large-sized Varanus spenceri was explicitly noted to be from the fox scavenging on an already dead individual (Mifsud and Woolley 2012). We use ‘consumption’ or ‘eaten’ preferentially in the present paper; however, in most cases, this can be assumed to indicate predation.
Analysis
We tallied the number of squamate species recorded within the following four predation classes: those known to be eaten by foxes but not cats (FX); those known to be eaten by both foxes and cats (FC); those known to be eaten by cats but not foxes (XC); and those not known to be eaten by either predator (XX). We used a likelihood ratio test to assess whether there was a statistically significant difference in the frequency distribution of species among these groups. Additionally, before trait-based modelling, we used an analysis of variance (ANOVA) to assess whether there was a significant difference in the average number of ALA records for each squamate species, and the average number of dietary studies within each species’ range, among these four predation classes.
To assess the extent to which introduced predators may be associated with population trends for Australian squamates, we calculated the proportion of squamate species in the four predation classes (FX, FC, XC and XX) that were evaluated as ‘decreasing’, ‘stable’ or ‘unknown’ – noting that no squamate species is considered to have a population that is increasing, with these trend categorisations sourced from Chapple et al. (2019). We used χ2 tests to compare the proportional number of species with declining relative to stable population trends (omitting species with ‘unknown’ trends) for species known and not known to be consumed by foxes, and for species known and not known to be consumed by cats. Given that there are no comparable recent assessments of population trends for most Australian non-squamate reptile species, this assessment of association of predation with population trends relates to squamates only.
All analyses were conducted in program R (R Core Team 2017). Prior to modelling, we followed the protocol for data exploration provided by Zuur et al. (2010). Continuous explanatory variables were centred and standardised by deducting the mean and dividing by twice the standard deviation (Gelman 2008).
We used generalised linear models (GLMs), with binomial error family, to identify squamate species’ traits that were associated with the relative likelihood of being found in the diet of foxes and cats. We modelled whether or not a species was recorded as consumed by foxes (yes/no) or cats (yes/no) for Australian squamate species against all possible combinations of the following six species’ traits as predictor variables: body mass, diel activity (i.e. nocturnal cf. diurnal), typical habitat, whether the species was predominantly arboreal, or predominantly fossorial (burrowing), and whether the species was venomous (non-venomous; mildly venomous; highly venomous; Table 1). We used adult body mass, but recognise that, for larger species, foxes may selectively kill or consume the much smaller young (or eggs). Furthermore, we note that other squamate traits (such as furtiveness, colouration, aggressiveness or escape speed) may also influence the likelihood of predation by cats or foxes, but such traits are not straightforward to categorise. Note also that our classification of species as venomous is based on information relating to risks to humans, and no studies have compared the impacts of venom of different Australian snake species on cats or foxes; however, we assume that the rankings are broadly comparable.
To account for potential sampling bias, we included the number of ALA records for each squamate species (either the total number of records or the number within only the distributional range of the fox) and the number of cat or fox dietary studies within each species’ distributional range as ‘offset’ terms, which were stipulated a priori for inclusion in all candidate models. We log10-transformed reptile body mass and the number of ALA records, and allowed the effect of body mass to be non-linear by introducing a quadratic term, stipulating its inclusion in a model only with the linear term (i.e. body mass2 + body mass). Because our collation identified no records of fox predation on squamates that occur primarily in rainforest and littoral habitats, and no records of cat predation on squamates that occur primarily in littoral habitat (when considering only those species within the distributional range of the fox), we did not have records for all of the classes of the habitat trait in these analyses, and, therefore, excluded rainforest and littoral squamates from the fox-eaten analyses, and littoral squamates from the cat-eaten analyses (when considering only those species within the distributional range of the fox). This reduced the habitat trait to seven classes for the fox-eaten analysis (aquatic, rocks, hummock grasslands, tussock grasslands, heathlands and shrublands, open woodlands and tall open forest) and eight classes for the corresponding cat-eaten analysis (i.e. also including rainforests).
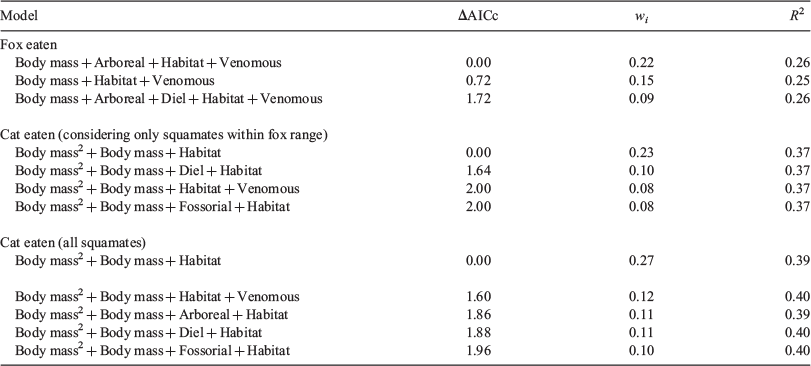
We developed a set of candidate models to explain whether squamates were fox-eaten and cat-eaten, including all combinations of the six explanatory (trait) variables, without interactions (i.e. 64 models). To take account of model-selection uncertainty, we used a model-averaging approach, incorporating the predictions of multiple candidate models weighted according to the second-order form of the Akaike information criterion, corrected for small sample size (AICC; Burnham and Anderson 2002). In this way, we examined several competing models simultaneously to identify the top set of models (95% confidence model set; R package MuMIn, Barton 2018). We identified highly influential variables by calculating relative variable importance, defined as the sum of Akaike weights, for all models containing a given predictor variable. Variables with a relative variable importance of ≥0.73 (equivalent to an AICC difference of 2, which is a common ‘rule-of-thumb’ used to indicate a significant effect; Richards 2005) were retained in the best model, which was used to identify the most influential traits and visualise variable effects.
Results
Tallies of consumed reptiles
The majority of fox dietary studies we collated analysed the contents of fox scats (~92 study site combinations, with 30 487 samples), rather than fox stomachs (~48 study site combinations, with 5001 samples). However, far more reptile species were identified from the fox stomach samples (81 species) than from fox scats (18 species).
With information from dietary studies and other sources, we collated records of 108 reptile species (all native) being consumed by foxes in Australia. These represented 10.8% of the terrestrial reptile fauna (999 species) and 16.0% of the reptile fauna that occurs within the current distribution of foxes in Australia (677 species; Table S1). The tally for cats was greater; 263 reptile species were recorded as consumed by cats, with 237 of these occurring within the distribution of the fox. Eighty-six reptile (including 82 squamate) species were reported as eaten by both foxes and cats, 22 reptile (13 squamate) species by foxes but not cats, 178 reptile (all squamate) species by cats but not foxes, and 714 reptile (692 squamate) species by neither predator (Fig. 2). In contrast to the lower overall tally of reptile species consumed by foxes than by cats, more turtle species were consumed by foxes than by cats.
The frequency distribution of species tallies across the four predation classes was highly non-random (χ23,995 = 1096, P < 0.01). Given the proportional tallies of species consumed by foxes and consumed by cats, reptile species were more likely to be consumed by both predators or neither. In part, this may reflect a sampling bias in that the reptile species known to be consumed by both predators are relatively widespread or abundant, whereas reptile species not known to be consumed by either predator are relatively restricted or rare. Reptile species that were not reported as consumed by either cats or foxes had significantly fewer ALA records (mean 111 ALA records; ANOVA: F3,995 = 58.8, P < 0.01), and fewer predator diet studies within their distributional range (mean 11 studies; ANOVA: F3, 995 = 224.1, P < 0.01) than did reptiles that were only consumed by cats (mean 484 ALA records, 36 studies), only consumed by foxes (mean 377 ALA records, 29 studies) and consumed by both predators (mean 1 040 ALA records, 65 studies). This influence of sampling effort and species abundance or distributional extent validates the inclusion of the number of ALA records and number of studies for each squamate species as offset terms in the GLMs.
Squamate species consumed by foxes were significantly more likely to have decreasing (rather than stable) population trends than were those not recorded as consumed by foxes (χ21,620 = 8.1, P < 0.01). There was no comparable association of population trends for species recorded (versus not recorded) as consumed by cats (χ21,620 = 1.3, P > 0.1).
Of Australia’s 103 threatened reptile species (excluding sea-snakes), 20 have been recorded as being consumed by foxes (including eight marine and freshwater turtles), whereas cat predation has been reported for 15 (Fig. 2). Twelve threatened species were recorded as being consumed by foxes but not cats; these include six turtle and six squamate species (Table S1). In contrast, the seven Australian threatened reptile species known to be consumed by cats but not foxes were all squamates.
Squamate traits associated with fox and cat predation
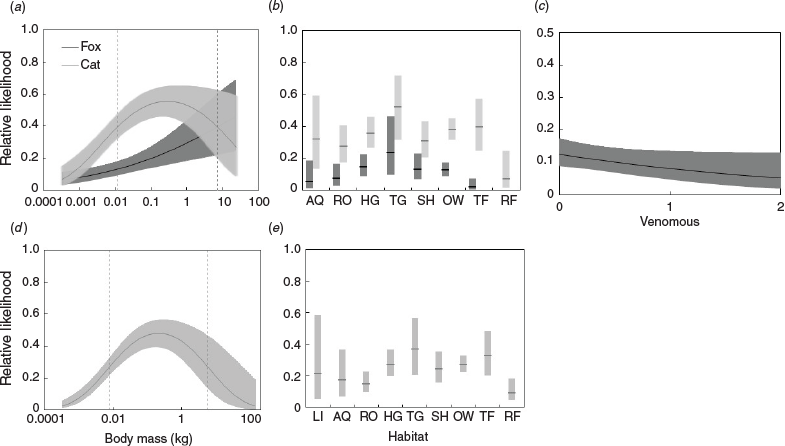
Model averaging showed that larger, non-venomous squamates that occur predominantly in tussock grasslands were more likely to be eaten by foxes, than were all other squamates (Table 2, Fig. 3). In contrast, cats were more likely to consume medium-sized squamates (peaking at adult bodyweight of ~217 g, about the size of a slatey-grey snake, Stegonotus cucullatus) across the cat’s entire range. Cats also showed a significant habitat effect, with squamates that predominantly occur in rainforest habitat less likely to be consumed by cats than those from other habitats. Neither cat nor fox predation showed any significant association with the other reptile traits we considered.
|
When considering only the influence of body mass, the 25% of squamate species most likely to be eaten by foxes were species with an adult body mass of ≥1.4 kg. In contrast, the 25% of squamate species most likely to be eaten by cats were those with an adult body mass of 0.07–1.16 kg. Only the seven squamate species with an adult body mass of >7.2 kg were more likely to be fox-eaten than cat-eaten, all other species (in the range of the fox) were more likely to be cat-eaten (651 species). Notwithstanding a tendency for foxes to eat larger squamates than cats do, both predators were recorded eating squamate species across almost the entire size range, from the smallest species (<2 g) to (at least eggs or subadults of) some of the largest monitors and pythons (~10 kg).
Discussion
Our study has documented consumption by the introduced red fox on 108 Australian native reptile species. We have shown that there is a marked overlap in the reptile species consumed by foxes and those consumed by the other main introduced predator in Australia, the feral cat, such that much of the predation impact is likely to be compounding across the two predator species. However, our results suggest that impacts are also complementary, with the most notable difference between the two predators being the propensity for foxes (far more so than for cats) to consume the eggs (and nestlings) of Australian freshwater and marine turtles, with well documented impacts on the population viability of many of these species (Thompson 1983; Fielder et al. 2014; Dawson et al. 2016), with comparable impacts arising from fox predation being widely reported elsewhere in the world (e.g. Kurz et al. 2012).
For the far more speciose Australian squamate fauna, fewer species are known to be consumed by foxes (95 species) than by cats (259 species), in part because the distributional range of cats overlaps with more Australian squamate species than does that of foxes, and in part because far more Australian squamate species are of the size that we show to be most likely consumed by cats. This result is also consistent with previous studies that have found significantly lower incidence of reptiles in fox diet than in cat diet in Australia (Doherty et al. 2015; Woinarski et al. 2018; Fleming et al. 2021).
Our tallies, for both predators, are likely to be very incomplete, as is evident by a highly significant sampling effect; squamate species with more restricted distributions within which there were few predator dietary studies were less likely to have been recorded as consumed by these predators. We also demonstrated that the most widely used dietary sampling method, inspection of the prey composition in scats, is far less likely to provide evidence of predation that is attributable to individual reptile species than is the case for inspection of prey in predator stomachs, presumably because there is far less diagnostic material left in scats. Many Australian reptile species are challenging to identify definitively to species from live individuals in the hand; it is far harder to do so from semi-digested remnants in a predator stomach, and even more so from a few scales or claws in a scat. Although foxes also consume many reptile eggs, identification to species of eggshell remnants is almost impossible (Dawson et al. 2016); so, we have undoubtedly under-estimated the squamate species consumed through egg predation by foxes. Recent advances in identification of remains in predator dietary samples, such as through DNA meta-barcoding (Dawson et al. 2016; de Sousa et al. 2019), may help provide more resolution of reptile species in fox and cat diets, and may be especially worth considering in assessment of the causes and the extent of mortality in threatened reptiles.
To some extent, foxes and cats show some partitioning in squamate consumption, with foxes consuming larger squamate species and cats consuming medium-sized squamate species. We recognise that these body size relationships need to be interpreted with caution because our metric for squamate size is adult mass, and both predators may selectively prey on individuals that are not at full body size. This may be especially so for larger squamate species, some of which are larger than cats or foxes. Furthermore, some of the consumption by foxes (and cats) of larger-bodied reptiles may derive from carrion, with foxes in particular more likely to scavenge. Nonetheless, the evidence from several previous studies has demonstrated that the abundance of large monitor species (V. gouldii and V. varius) increases in areas where fox numbers are reduced (Olsson et al. 2005; Hu et al. 2019; Stobo-Wilson et al. 2020), providing at least indirect support for the conclusion that foxes prey preferentially on larger squamate species (although we note also that competition for food may also influence the responses of varanids to foxes).
We also note a potential caveat that to some extent the apparent preference by foxes (and cats) against small reptile species may be a sampling artefact, with very small reptile prey more likely to be overlooked (and/or harder to identify) in inspection of predator dietary samples. Nonetheless, the apparent stronger preference by foxes than by cats for larger squamate species is consistent with previous studies that have reported that foxes are more likely to eat larger animals, than are cats, although this has previously been recorded mostly for mammal prey (Glen et al. 2011; Murphy et al. 2019). Dietary samples may also have some biases associated with reptile physical characteristics, with soft-bodied geckoes being less likely to leave identifiable traces in scats than for harder-scaled reptiles (such as monitors and agamids); such differential digestion may explain the low proportion of geckoes recorded as consumed by foxes and cats (Fig. 2).
We found that venomous squamates were less likely to be consumed by foxes, whereas there was no such association for cats. Previous studies have shown that cats can catch and kill many snakes (Shine and Koenig 2001), and are adept at killing even large venomous snakes (McGregor et al. 2015; Fleming et al. 2020). Of the other reptile traits that we considered, the relative likelihood of a species being consumed by foxes showed some variation across habitat, with none of the many Australian reptile species occurring primarily in rainforest or the few species associated with littoral habitats known to be consumed by foxes. Rainforest squamates were also less likely to be eaten by cats. To some extent, this relationship reflects fox distribution and habitat use, with Australia’s rainforest squamates occurring mostly beyond the range of the fox, and foxes rarely using that habitat anyway (Rowland et al. 2020). In contrast, a relatively high proportion of reptile species occurring in tussock grasslands were consumed by foxes, where foxes occur abundantly and may be able to hunt effectively for reptiles in these more open habitats (Molsher et al. 2000). The likelihood of squamates to be consumed by foxes showed no association with the diel activity of squamate species, possibly because foxes (and also cats) are not exclusively nocturnal (Phillips and Catling 1991), and foxes may also be able to detect and dig up diurnal squamates in their nocturnal shelter sites. Likewise, the likelihood of fox predation did not vary significantly between arboreal and terrestrial squamates, probably because most arboreal squamates (with the notable exception of some rainforest species) also spend some time on the ground.
We have collated many records of consumption by foxes, and by cats, on Australian reptiles, but we recognise that such a compilation does not demonstrate the magnitude of that predation pressure or impacts on the population viability of those reptile species. For cats, a previous study complemented the inventory of reptile species consumed, with an estimate of the numbers of individual reptiles killed per year (Woinarski et al. 2018). A comparable analysis, for the number of Australian reptiles killed by foxes annually, has not yet been undertaken.
The present study collated records of consumption by foxes on 20 threatened reptile species. This tally in part reflects the previously documented severe impact of fox predation on turtles; however, our study indicated that impacts may also be significant for some squamate species. Even low rates of predation on such imperilled species may be of concern, because local extirpation of Australian reptile species, attributable to these invasive carnivores, has been demonstrated (Bamford and Calver 2012).
We found that squamate species known to be consumed by foxes are more likely to be suffering population declines than are species not known to be consumed by foxes, providing some inference of population-level impacts of such predation, even on reptiles that may still be common and not yet recognised as threatened. That inference is consistent with evidence from the few studies that have attempted to estimate rates and causes of mortality in Australian squamate species (Shine and Fitzgerald 1996). Separately and collectively, the two mammalian carnivores introduced to, and now widespread in, Australia are likely to be taking a significant toll on, and contributing to the decline of, Australia’s highly distinctive reptile fauna. Effective targeted and broad-scale actions taken to control foxes and cats will provide conservation benefits to this fauna.
Conflicts of interest
Sarah M. Legge and Thomas M. Newsome are Associate Editors. Despite this relationship, they did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. The authors have no further conflicts of interest to declare.
Acknowledgements
This research received support from the Australian Government's National Environmental Science Program through the Threatened Species Recovery Hub. MR received funding from the Conservation Ecology Centre, Holsworth Wildlife Research Endowment and Victorian Environmental Assessment Council. SD receives funding from the Centre for Invasive Species Solutions. We thank Joanna Riley for contributing fox diet data.
References
Andrew, P., Cogger, H., Driscoll, D., Flakus, S., Harlow, P., Maple, D., Misso, M., Pink, C., Retallick, K., Rose, K., Tiernan, B., West, J., and Woinarski, J. C. Z. (2018). Somewhat saved: a captive breeding program for two endemic Christmas Island lizard species, now extinct in the wild. Oryx 52, 171–174.| Somewhat saved: a captive breeding program for two endemic Christmas Island lizard species, now extinct in the wild.Crossref | GoogleScholarGoogle Scholar |
Atlas of Living Australia (2020). Occurrence records. Available at https://spatial.ala.org.au/.
Australian Bureau of Meteorology (2016). Gridded climate data. Available at http://www.bom.gov.au/climate/averages/climatology/gridded-data-info/gridded-climate-data.shtml.
Bamford, M. J., and Calver, M. C. (2012). Cat predation and suburban lizards: a 22 year study at a suburban Australian property. The Open Conservation Biology Journal 6, 25–29.
| Cat predation and suburban lizards: a 22 year study at a suburban Australian property.Crossref | GoogleScholarGoogle Scholar |
Barton, K. (2018). Package “MuMIn” Title Multi-Model Inference. Available at https://cran. r-project.org/web/packages/MuMIn/MuMIn.pdf.
Burnham, K. P., and Anderson, D. R. (2002). ‘Model selection and multimodel inference: a practical information-theoretic approach.’ (Springer: Berlin, Germany.)
Catling, P. C. (1988). Similarities and contrasts in the diets of foxes, Vulpes vulpes, and cats, Felis catus, relative to fluctuating prey populations and drought. Wildlife Research 15, 307–317.
| Similarities and contrasts in the diets of foxes, Vulpes vulpes, and cats, Felis catus, relative to fluctuating prey populations and drought.Crossref | GoogleScholarGoogle Scholar |
Chaloupka, M., and Limpus, C. J. (2001). Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biological Conservation 102, 235–249.
| Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters.Crossref | GoogleScholarGoogle Scholar |
Chapple, D. G., Tingley, R., Mitchell, N. J., Macdonald, S. L., Keogh, J. S., Shea, G. M., Bowles, P., Cox, N. A., and Woinarski, J. C. Z. (2019). ‘The Action Plan for Australian Lizards and Snakes 2017.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Cogger, H. G. (1959). Australian goannas. Australian Museum Magazine 13, 71–75.
Cogger, H. G. (2014). ‘Reptiles and amphibians of Australia.’ 7th edn. (CSIRO Publishing: Melbourne, Vic., Australia.)
Dawson, S. J., Crawford, H. M., Huston, R. M., Adams, P. J., and Fleming, P. A. (2016). How to catch red foxes red handed: identifying predation of freshwater turtles and nests. Wildlife Research 43, 615–622.
| How to catch red foxes red handed: identifying predation of freshwater turtles and nests.Crossref | GoogleScholarGoogle Scholar |
de Sousa, L. L., Silva, S. M., and Xavier, R. (2019). DNA metabarcording in diet studies: unveiling ecological aspects in aquatic and terrestrial ecosystems. Environmental DNA 1, 199–214.
| DNA metabarcording in diet studies: unveiling ecological aspects in aquatic and terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar |
Department of the Environment Water Heritage and the Arts (2008). ‘Threat abatement plan for predation by the European red fox.’ (Department of the Environment, Water, Heritage and the Arts: Canberra, ACT, Australia.)
Doherty, T. S., Davis, R. A., Etten, E. J. B., Algar, D., Collier, N., Dickman, C. R., Edwards, G., Masters, P., Palmer, R., and Robinson, S. (2015). A continental‐scale analysis of feral cat diet in Australia. Journal of Biogeography 42, 964–975.
| A continental‐scale analysis of feral cat diet in Australia.Crossref | GoogleScholarGoogle Scholar |
Fielder, D. P., Limpus, D. J., and Limpus, C. J. (2014). Reproduction and population ecology of the vulnerable western sawshelled turtle, Myuchelys bellii, in the Murray–Darling Basin, Australia. Australian Journal of Zoology 62, 463–476.
| Reproduction and population ecology of the vulnerable western sawshelled turtle, Myuchelys bellii, in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Fleming, P. A., Crawford, H. M., Auckland, C. H., and Calver, M. C. (2020). Body size and bite force of stray and feral cats: are bigger or older cats taking the largest or more difficult-to-handle prey? Animals (Basel) 10, 707.
| Body size and bite force of stray and feral cats: are bigger or older cats taking the largest or more difficult-to-handle prey?Crossref | GoogleScholarGoogle Scholar |
Fleming, PA, Crawford, HM, Stobo-Wilson, AM, Dundas, SJ, Dawson, SJ, Stuart, J-MD, O’Connor, J, Speed, J, Gentle, M, Riley, J, Turpin, JM, Thompson, E, Ritchie, EG, Palmer, R, Newsome, TM, Dickman, CR, Saunders, G, and Woinarski, JCZ (2021). The diet of the introduced red fox Vulpes vulpes in Australia: analysis of temporal and spatial patterns. Mammal Review , .
Gelman, A. (2008). Scaling regression inputs by dividing by two standard deviations. Statistical Methods 27, 2865–2873.
| Scaling regression inputs by dividing by two standard deviations.Crossref | GoogleScholarGoogle Scholar |
Glen, A. S. (2014). Fur, feathers and scales: interactions between mammalian, reptilian and avian predators. In ‘Carnivores of Australia: past, present and future’. (Eds A. S. Glen and C. R. Dickman.) pp. 279–299. (CSIRO Publishing: Melbourne, Vic., Australia.)
Glen, A. S., Pennay, M., Dickman, C. R., Wintle, B. A., and Firestone, K. B. (2011). Diets of sympatric native and introduced carnivores in the Barrington Tops, eastern Australia. Austral Ecology 36, 290–296.
| Diets of sympatric native and introduced carnivores in the Barrington Tops, eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Heard, G. W., Robertson, P., Black, D., Barrow, G. J., Johnson, P. J., Hurley, V. G., and Allen, G. (2006). Canid predation: a potentially significant threat to relic populations of the Inland Carpet Python Morelia spilota metcalfei (Pythonidae) in Victoria. Victorian Naturalist 123, 68–74.
Hu, Y., Gillespie, G., and Jessop, T. S. (2019). Variable reptile responses to introduced predator control in southern Australia. Wildlife Research 46, 64–75.
| Variable reptile responses to introduced predator control in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Kennett, R, Roe, J, Hodges, K, and Georges, AJ (2009). Chelodina longicollis (Shaw 1794): eastern long-necked turtle, common long-necked turtle, common snake-necked turtle. Chelonian Research Monographs 5, 031.1–031.8.
| Chelodina longicollis (Shaw 1794): eastern long-necked turtle, common long-necked turtle, common snake-necked turtle.Crossref | GoogleScholarGoogle Scholar |
Kurz, D. J., Straley, K. M., and DeGregorio, B. A. (2012). Out-foxing the red fox: how best to protect the nests of the Endangered loggerhead marine turtle Caretta caretta from mammalian predation? Oryx 46, 223–228.
| Out-foxing the red fox: how best to protect the nests of the Endangered loggerhead marine turtle Caretta caretta from mammalian predation?Crossref | GoogleScholarGoogle Scholar |
Limpus, C., and Reimer, D. (1994). The loggerhead turtle, Caretta caretta, in Queensland: a population in decline. In ‘Proceedings of the Australian Marine Turtle Conservation Workshop’. (Ed. R. James.) pp. 39–47. (Australian Nature Conservation Agency: Canberra, ACT, Australia.)
McGregor, H., Legge, S., Jones, M. E., and Johnson, C. N. (2015). Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS One 10, e0133915.
| Feral cats are better killers in open habitats, revealed by animal-borne video.Crossref | GoogleScholarGoogle Scholar | 26288224PubMed |
Mella, V. S. A., McArthur, C., Frend, R., and Crowther, M. S. (2018). Foxes in trees: a threat for Australian arboreal fauna? Australian Mammalogy 40, 103–105.
| Foxes in trees: a threat for Australian arboreal fauna?Crossref | GoogleScholarGoogle Scholar |
Mifsud, G., and Woolley, P. A. (2012). Predation of the Julia Creek dunnart (Sminthopsis douglasi) and other native fauna by cats and foxes on Mitchell grass downs in Queensland. Australian Mammalogy 34, 188–195.
| Predation of the Julia Creek dunnart (Sminthopsis douglasi) and other native fauna by cats and foxes on Mitchell grass downs in Queensland.Crossref | GoogleScholarGoogle Scholar |
Molsher, R. L., Gifford, E. J., and McIlroy, J. C. (2000). Temporal, spatial and individual variation in the diet of red foxes (Vulpes vulpes) in central New South Wales. Wildlife Research 27, 593–601.
| Temporal, spatial and individual variation in the diet of red foxes (Vulpes vulpes) in central New South Wales.Crossref | GoogleScholarGoogle Scholar |
Murphy, B. P., Woolley, L. A., Geyle, H. M., Legge, S. M., Palmer, R., Dickman, C. R., Augusteyn, J., Brown, S. C., Comer, S., Doherty, T. S., Eager, C., Edwards, G., Fordham, D. A., Harley, D., McDonald, P. J., McGregor, H., Moseby, K., Myers, C., Read, J., Riley, J., Stokeld, D., Trewella, G. J., Turpin, J. M., and Woinarski, J. C. Z. (2019). Introduced cats (Felis catus) eating a continental mammal fauna: the number of individuals killed in Australia. Biological Conservation 237, 28–40.
| Introduced cats (Felis catus) eating a continental mammal fauna: the number of individuals killed in Australia.Crossref | GoogleScholarGoogle Scholar |
Nielsen, T. P., and Bull, C. M. (2016). Impact of foxes digging for the pygmy bluetongue lizard (Tiliqua adelaidensis). Transactions of the Royal Society of South Australia 140, 228–233.
| Impact of foxes digging for the pygmy bluetongue lizard (Tiliqua adelaidensis).Crossref | GoogleScholarGoogle Scholar |
Olsson, M., Wapstra, E., Swan, G., Snaith, E., Clarke, R., and Madsen, Y. (2005). Effects of long‐term fox baiting on species composition and abundance in an Australian lizard community. Austral Ecology 30, 899–905.
| Effects of long‐term fox baiting on species composition and abundance in an Australian lizard community.Crossref | GoogleScholarGoogle Scholar |
Phillips, M., and Catling, P. C. (1991). Home range and activity patterns of red foxes in Nadgee Nature Reserve. Wildlife Research 18, 677–686.
| Home range and activity patterns of red foxes in Nadgee Nature Reserve.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2017). ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/.
Read, J. L., and Scoleri, V. (2015). Ecological implications of reptile mesopredator release in arid South Australia. Journal of Herpetology 49, 64–69.
| Ecological implications of reptile mesopredator release in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Reddiex, B., Forsyth, D. M., McDonald-Madden, E., Einoder, L. D., Griffioen, P. A., Chick, R. R., and Robley, A. J. (2006). Control of pest mammals for biodiversity protection in Australia. I. Patterns of control and monitoring. Wildlife Research 33, 691–709.
| Control of pest mammals for biodiversity protection in Australia. I. Patterns of control and monitoring.Crossref | GoogleScholarGoogle Scholar |
Rhodin, A. G. J., Iverson, J. B., Bour, R., Fritz, U., Georges, A., Shaffer, H. B., and van Dijk, P. P. (2017). Turtles of the world. Annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status. Eighth edition. Chelonian Research Monographs 7, .
Richards, S. A. (2005). Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86, 2805–2814.
| Testing ecological theory using the information-theoretic approach: examples and cautionary results.Crossref | GoogleScholarGoogle Scholar |
Robley, A., Howard, K., Lindeman, M., Cameron, E., Jardine, A., and Hiscock, D. (2016). The effectiveness of short‐term fox control in protecting a seasonally vulnerable species, the Eastern Long‐necked Turtle (Chelodina longicollis). Ecological Management & Restoration 17, 63–69.
| The effectiveness of short‐term fox control in protecting a seasonally vulnerable species, the Eastern Long‐necked Turtle (Chelodina longicollis).Crossref | GoogleScholarGoogle Scholar |
Rowland, J., Hoskin, C. J., and Burnett, S. (2020). Distribution and diet of feral cats (Felis catus) in the Wet Tropics of north-east Australia, with a focus on the upland rainforest. Wildlife Research 47, 649–659.
| Distribution and diet of feral cats (Felis catus) in the Wet Tropics of north-east Australia, with a focus on the upland rainforest.Crossref | GoogleScholarGoogle Scholar |
Saunders, G., and McLeod, L. (2007). ‘Improving fox management strategies in Australia.’ (Bureau of Rural Sciences: Canberra, ACT, Australia.)
Saunders, G., Coman, B., Kinnear, J., and Braysher, M. (1995). ‘Managing vertebrate pests: foxes.’ (Australian Government Publishing Service: Canberra, ACT, Australia.)
Saunders, G. R., Gentle, M. N., and Dickman, C. R. (2010). The impacts and management of foxes Vulpes vulpes in Australia. Mammal Review 40, 181–211.
| The impacts and management of foxes Vulpes vulpes in Australia.Crossref | GoogleScholarGoogle Scholar |
Shine, R., and Fitzgerald, M. (1996). Large snakes in a mosaic rural landscape: the ecology of carpet pythons Morelia spilota (Serpentes: Pythonidae) in coastal eastern Australia. Biological Conservation 76, 113–122.
| Large snakes in a mosaic rural landscape: the ecology of carpet pythons Morelia spilota (Serpentes: Pythonidae) in coastal eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Shine, R., and Koenig, J. (2001). Snakes in the garden: an analysis of reptiles ‘rescued’ by community-based wildlife carers. Biological Conservation 102, 271–283.
| Snakes in the garden: an analysis of reptiles ‘rescued’ by community-based wildlife carers.Crossref | GoogleScholarGoogle Scholar |
Slavenko, A., Tallowin, O. J. S., Itescu, Y., Raia, P., and Meiri, S. (2016). Late Quaternary reptile extinctions: size matters, insularity dominates. Global Ecology and Biogeography 25, 1308–1320.
| Late Quaternary reptile extinctions: size matters, insularity dominates.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J. (2002). Experimentally testing nest site selection: fitness trade‐offs and predation risk in turtles. Ecology 83, 2136–2144.
| Experimentally testing nest site selection: fitness trade‐offs and predation risk in turtles.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Thompson, M. B. (2003). The significance of predation in nest site selection of turtles: an experimental consideration of macro‐and microhabitat preferences. Oikos 102, 592–600.
| The significance of predation in nest site selection of turtles: an experimental consideration of macro‐and microhabitat preferences.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Thompson, M. B. (2005). Experimental analysis of the impact of foxes on freshwater turtle populations. Conservation Biology 19, 845–854.
| Experimental analysis of the impact of foxes on freshwater turtle populations.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., Janzen, F. J., and Thompson, M. B. (2006). Counterintuitive density‐dependent growth in a long‐lived vertebrate after removal of nest predators. Ecology 87, 3109–3118.
| Counterintuitive density‐dependent growth in a long‐lived vertebrate after removal of nest predators.Crossref | GoogleScholarGoogle Scholar | 17249235PubMed |
Spencer, R.-J., Van Dyke, J. U., and Thompson, M. B. (2016). The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions. Ecological Applications 26, 1969–1983.
| The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions.Crossref | GoogleScholarGoogle Scholar | 27755718PubMed |
Spencer, R. J., Van Dyke, J. U., and Thompson, M. B. (2017). Critically evaluating best management practices for preventing freshwater turtle extinctions. Conservation Biology 31, 1340–1349.
| Critically evaluating best management practices for preventing freshwater turtle extinctions.Crossref | GoogleScholarGoogle Scholar | 28319283PubMed |
Stobo-Wilson, A. M., Brandle, R., Johnson, C. N., and Jones, M. E. (2020). Management of invasive mesopredators in the Flinders Ranges, South Australia: effectiveness and implications. Wildlife Research 47, 720–730.
| Management of invasive mesopredators in the Flinders Ranges, South Australia: effectiveness and implications.Crossref | GoogleScholarGoogle Scholar |
Sutherland, D. R., Glen, A. S., and Paul, J. (2011). Could controlling mammalian carnivores lead to mesopredator release of carnivorous reptiles? Proceedings of the Royal Society of London. Series B, Biological Sciences 278, 641–648.
| Could controlling mammalian carnivores lead to mesopredator release of carnivorous reptiles?Crossref | GoogleScholarGoogle Scholar |
Thackway, R., and Cresswell, I. D. (1995). ‘An Interim Biogeographic Regionalisation for Australia: a framework for establishing a national system of reserves, Version 4.’ (Australian Nature Conservation Agency: Canberra, ACT, Australia.)
Thompson, M. B. (1983). Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Australian Wildlife Research 10, 363–371.
| Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Tingley, R., Meiri, S., and Chapple, D. G. (2016). Addressing knowledge gaps in reptile conservation. Biological Conservation 204, 1–5.
| Addressing knowledge gaps in reptile conservation.Crossref | GoogleScholarGoogle Scholar |
Tingley, R., Macdonald, S. L., Mitchell, N. J., Woinarski, J. C. Z., Meiri, S., Bowles, P., Cox, N. A., Shea, G. M., Böhm, M., Chanson, J., Tognelli, M. F., Harris, J., Walke, C., Harrison, N., Victor, S., Woods, C., Amey, A. P., Bamford, M., Catt, G., Clemann, N., Couper, P. J., Cogger, H., Cowan, M., Craig, M., Dickman, C. R., Doughty, P., Ellis, R., Fenner, A., Ford, S., Gaikhorst, G., Gillespie, G. R., Greenlees, M. J., Hobson, R., Hoskin, C. J., How, R., Hutchinson, M. N., Lloyd, R., McDonald, P., Melville, J., Michael, D. R., Moritz, C., Oliver, P. M., Peterson, G., Robertson, P., Sanderson, S., Somaweera, R., Teale, R., Valentine, L., Vanderduys, E., Venz, M., Wapstra, E., Wilson, S., and Chapple, D. G. (2019). Geographic and taxonomic patterns of extinction risk in Australian squamates. Biological Conservation 238, 108203.
| Geographic and taxonomic patterns of extinction risk in Australian squamates.Crossref | GoogleScholarGoogle Scholar |
Van Dyck, S., and Strahan, R. (2008). ‘The Mammals of Australia.’ 3rd edn. (Reed New Holland: Sydney, NSW, Australia.)
Webb, J. K., Harlow, P. S., and Pike, D. A. (2014) Australian reptiles and their conservation. In ‘Austral Ark: the state of wildlife in Australia and New Zealand’. (Eds A. Stow, N. Maclean, and G. I. Holwell.) pp. 354–381. (Cambridge University Press: Cambridge.)
Woinarski, J. C. Z., Murphy, B. P., Palmer, R., Legge, S. M., Dickman, C. R., Doherty, T. S., Edwards, G., Nankivell, A., Read, J. L., and Stokeld, D. (2018). How many reptiles are killed by cats in Australia? Wildlife Research 45, 247–266.
| How many reptiles are killed by cats in Australia?Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Legge, S. M., and Dickman, C. R. (2019). ‘Cats in Australia: companion and killer.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Zuur, A. F., Ieno, E. N., and Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14.
| A protocol for data exploration to avoid common statistical problems.Crossref | GoogleScholarGoogle Scholar |