Determining the impacts of conservation fencing on woma pythons (Aspidites ramsayi)
Joshua Magro A * , Reece Pedler
A * , Reece Pedler  A , John Read
A , John Read  A B and Rebecca West
A B and Rebecca West  A
A
A
B
Abstract
Fenced conservation reserves are an effective management tool for the conservation of many threatened species. However, conservation fencing is known to inadvertently affect non-target species, ranging from barrier effects to direct mortality. There is a paucity of information on the negative impacts of fencing on reptiles.
Using the woma python, a species of conservation significance, this research aimed to improve our knowledge of how reptiles interact with fences.
The spatial ecology of womas was explored in relation to fencing at the Wild Deserts project partnership site, a rabbit-, cat- and fox-proof fenced area of Sturt National Park in arid Australia. A 6-year dataset of opportunistic observations of womas at the study site were analysed for demographic, spatial and temporal patterns in woma fence interactions. Nine adult pythons were radiotracked over a year to assess space use in relation to fencing.
Twenty-two per cent of all opportunistic woma observations at the site were mortalities associated with entanglements. All 20 entanglement deaths were in 30-mm netting despite 50-mm netting comprising lower segments of 21% of the fence network. Fencing encounters were greatest in dune habitats and during summer and autumn. Fence crossings were infrequent among telemetered pythons and most encounters did not result in entanglement, with four of the nine individuals recorded to have crossed the fence successfully, despite one mortality.
Thirty-millimetre netting, particularly in areas of netting overlap, represents an entanglement risk to womas.
This research is applicable to the management of conservation fences and can be extended to other large snake and reptile species. The impacts of small-aperture netting on large snakes and other non-target species should be considered in the planning phases for conservation fencing and mitigation strategies should be sought in the planning phases where possible. Large-aperture netting is preferable to 30-mm netting for pythons, where exclusion of rabbits is not necessary. However, larger netting apertures may disproportionately affect other non-target species such as bearded dragons.
Keywords: entanglement, fence, home range, mortality, radiotelemetry, reptile, safe haven, snake.
Introduction
Conservation practitioners regularly employ fences as a tool to protect biodiversity from threatening processes (Hayward and Kerley 2009). Although globally widespread, conservation fencing is particularly popular in Australia (Dickman 2012; Legge et al. 2018), New Zealand (Burns et al. 2012) and southern Africa (Hayward and Kerley 2009). In Oceania, conservation fencing is routinely used to exclude invasive mammalian predators for the in situ protection or reintroduction of threatened predator-vulnerable species, with currently at least 19 predator free-fenced safe havens in Australia and 28 in New Zealand, covering a combined area of 350 km2 and 84 km2 respectively (Burns et al. 2012; Legge et al. 2018). Conversely, in southern Africa, conservation fencing is primarily used to alleviate human–wildlife conflict and restrict human persecution of threatened species, often by containing megaherbivores and large carnivores in protected areas away from human settlements and agriculture (Kassilly 2002; Lindsey et al. 2012).
Until there is effective landscape management of threatening processes, conservation fencing will continue to be a valuable solution in the conservation of many threatened species (Ringma et al. 2018; Tulloch et al. 2023). However, conservation fencing does produce inadvertent negative impacts on some non-target species, ranging from barrier effects to direct mortality (Jakes et al. 2018). Fencing in general poses a physical barrier to migration, dispersal movements and genetic exchange for many species. Inappropriate fence alignment is known to restrict access to water resources, which may result in dehydration and subsequent mortality (Mbaiwa and Mbaiwa 2006). Mortalities also often result from collisions (Baines and Andrew 2003), entanglements, or by electrocution when contacting electric fences (Lee et al. 2021). Further, fences can change population dynamics and induce behavioural changes such as those in predator–prey relationships in which predators may preferentially hunt along fence lines (McGregor et al. 2020).
Although there is some information on the negative impact of conservation fencing on birds and mammals, the impacts of fencing on reptiles remains lacking. Reptile mortalities associated with fencing are frequently observed in snakes, turtles and Tiliqua skinks in Australia (Long and Robley 2004; Ferronato et al. 2014) and tortoises in South Africa (Holt et al. 2021; Lee et al. 2021). It is thought that large wide-ranging and migratory species are at a greater risk to fencing impacts because their more extensive movements increase the frequency of fencing encounters (Ferronato et al. 2014; Maida et al. 2020; Smith et al. 2020). Testudine mortalities are often linked to exposure and dehydration of dispersing individuals as well as electrocution, whereas snakes and large lizards more often die via entanglements (Long and Robley 2004; Beck 2009; Pietersen 2022). Unlike birds that typically interact with fences by accident, the spatial distribution of reptile fence encounters may be less random, given that squamates use chemoreception to gain information about their social environment and often follow specified pheromone trails during certain activities such as mate searching (Bryant et al. 2011). Gaining an understanding of both spatial and temporal correlates with reptile mortality attributed to fencing as well as behavioural responses to fencing will better inform our understanding of the magnitude of fencing impacts on reptiles as well as in the development of species-specific mitigation strategies (Woltz et al. 2008; Macpherson et al. 2021).
Snakes are often reported as the taxon that is most often entangled in netting and other mesh products (Long and Robley 2004; Ebert et al. 2019). Entanglements have been reported for numerous species, including the woma python (Aspidites ramsayi), a data-deficient species of conservation significance. Although these arid-zone reptiles have been candidates for reintroduction into fenced conservation reserves themselves (Read et al. 2011), recent mortality entanglements demonstrated in this present study have raised concerns that conservation fences may be detrimental to this and other large snake species. Herein, the spatiotemporal ecology of womas is explored in relation to the fencing of a safe haven site in arid Australia to guide management and mitigation strategies, which may be applicable not only to this species, but also to other snakes and large reptiles. Specifically, this study aimed to (1) use existing datasets to establish whether there were any broad-scale spatiotemporal or population demographic patterns in opportunistic woma observations including entanglements and (2) describe woma space use to better understand the nature, frequency and outcome of woma fencing encounters.
Materials and methods
Study area
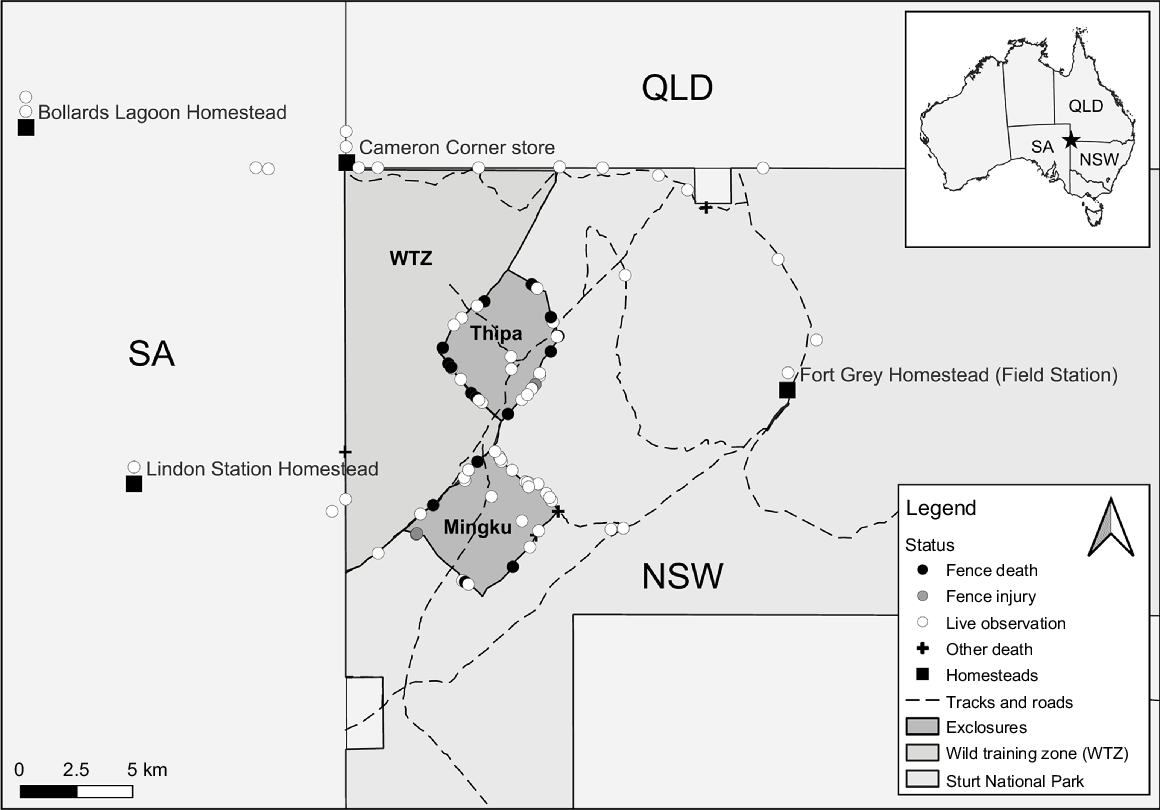
This study was conducted at the Wild Deserts project partnership site in Sturt National Park, a fenced safe haven located in the Strzelecki Desert of far north-western New South Wales (NSW), Australia. The site includes two 20-km2 exclosures designed to exclude alien rabbits (Oryctolagus cuniculus), cats (Felis catus) and foxes (Vulpes vulpes) for the reintroduction of seven locally extinct mammal species. There is also a 104-km2 ‘wild training zone’ to assist in enabling populations of these native mammals to co-exist with low numbers of alien predators (Fig. 1) (Pedler et al. 2018).
Map of the Wild Deserts project partnership site, highlighting the distribution of opportunistic woma python records since 2018. Woma observation(s) recorded from homesteads are visually displayed by stacking the observation status symbols vertically above the homestead symbol.
Rainfall is low and highly variable (Pook et al. 2014), with a long-term annual average of 176.7 mm (at Fort Grey, 1899–2023; Bureau of Meteorology 2024). The landscape consists of longitudinal sand dunes typically oriented north–east to south–west, separated by interdunal clay swales. Shrublands dominated by mulga (Acacia aneura), needlewood (Hakea leucoptera) and hopbush (Dodonaea viscosa) occupy the sand dunes and chenopods and grasses dominate the swales (Keith 2004).
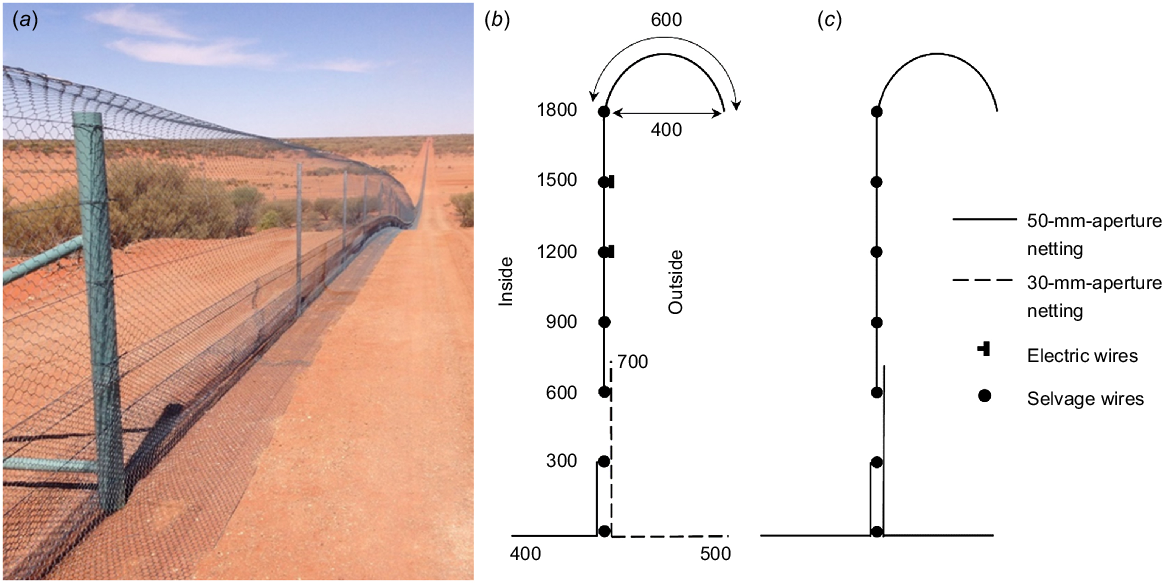
The Wild Deserts fencing network was completed in November 2018 and is approximately 50 km in length, including the 9.8-km-long discontinuous ‘wild training zone’ fence and 37.7 km of exclosure fencing. This does not include the dog fence to which the wild training zone fence adjoins to. The rabbit-/cat-/fox-proof exclosure fence design consists of a 30-mm aperture external foot apron to 700 mm in height to exclude juvenile rabbits (Moseby and Read 2006) and a 50-mm-aperture internal foot apron to 350-mm height (Fig. 2). Netting between 600 and 1800 mm in height is of 50-mm aperture to exclude cats and foxes. The wild training-zone fence has the same specifications as does the exclosure fence, except that the external foot apron is of 50-mm aperture, because it does not aim to exclude rabbits.
Study species
Woma pythons (Aspidites ramsayi) are large (up to 2.5-m snout–vent length, SVL), secretive pythons of primarily sandy habitats across much of arid and semi-arid Australia (Shine and Slip 1990; Pedler 2011; Cogger 2014). Little information on woma ecology and biology is available, given their mostly fossorial and nocturnal habits and occupancy of remote areas. Most detailed ecological and behavioural information have come from the only two published radiotelemetry studies on this species, including one of a wild population in the Brigalow Belt area of eastern Queensland (Bruton 2014) and the other of a captive-bred population reintroduced into the arid rangelands of South Australia (Read et al. 2011). Womas spend extended periods of time underground, where their low metabolic rate enables them to endure long periods of fasting in burrows, which are thermally buffered and important shelter sites in dryland environments (Bruton et al. 2014). Small mammals and reptiles are consumed in similar proportions, with birds also supplementing woma diets (Shine and Slip 1990).
Womas are likely to have a patchy distribution across their range, with significant declines being reported in some regions (Sadlier 1994), notably in the Western Australian Wheatbelt (Pearson 1993). This species was most recently assessed nationally as Least Concern by the IUCN, de-listed from their Endangered status in 1996 on the basis of the assumption that although the Wheatbelt population is in decline, other populations are not suspected to be declining to the same extent across their expansive range (Bruton et al. 2017). However, womas are listed as Priority One (for ‘south-western subspecies’) in Western Australia, Vulnerable in New South Wales, Near Threatened in both Queensland and Northern Territory and Rare in South Australia (Bruton et al. 2017). Land-clearing for agriculture, grazing, and cropping are major threats, particularly in the Wheatbelt and Brigalow Belt (Covacevich 1996). Predation by cats and foxes is also suspected to be a threat through direct predation of juvenile womas as well as an indirect threat by reducing the abundance of woma prey species.
Womas naturally occur within the study site in Sturt National Park and are important predators that are likely to assist in regulating populations of reintroduced species within the exclosures (Read et al. 2011). During a comprehensive environmental-impact assessment and approval process, the planned rabbit-, cat-, and fox-proof fences were not expected to significantly affect this species (West and Pedler 2017), given that there were only two confirmed records of womas in Sturt National Park prior to the construction of the fence network (Atlas of Living Australia 2023).
Spatial, temporal and demographic patterns in opportunistic woma records
Field activities associated with the implementation of the Wild Deserts project between January 2018 and February 2024 have led to opportunistic field records of woma pythons (n = 93), including from neighbouring properties outside Sturt National Park (Fig. 1). Opportunistic records were compiled in a dedicated database, with date, location, morphometrics (SVL, total length, mass) and status (alive, fence-injured, fence death, other death) being included. Locational data were collected using a Garmin GPS (Garmin International, USA) with 3–5-m precision. Twenty-five pythons did not have GPS location coordinates recorded but a location description, which was assigned approximate coordinates. Womas captured post-2020 were also microchipped to aid in capture–recapture analysis.
The spatial distribution of woma records around the fence was analysed by first plotting all woma records on a map of the Wild Deserts project area by using mapping software QGIS (ver. 3.36.0; QGIS 2024), followed by excluding all records greater than 100 m from a Wild Deserts fence. Given that there were no records from the wild training zone fence, only the exclosure fencing was analysed. This returned 60 total woma observations. The number of woma records from fence segments that perpendicularly intersect multiple dunes (the north–east and south–west segments of both exclosures) was compared to that in segments that ran mostly parallel to dunes (the north–west and south–east segments of both exclosures) (range = 20,186–17,441 m) (Fig. 1). A chi-squared test was used to determine whether womas were randomly distributed around the exclosures, with the null hypothesis being that python abundance would be equal between the two fence-segment groups. Spatial correlates in woma observations were analysed by classing all woma locations within 100 m of a Wild Deserts fence into two habitat categories, namely, dune and swale. By using QGIS, an equivalent number of random points to woma observations along the fence line were generated and scored for habitat type. Given the distinct contrast in vegetation between the two habitat categories, this was done visually by overlaying high-resolution aerial imagery. The habitat type of each python record was compared with these randomly located points and habitat preference was assessed using a chi-squared test to determine whether the observed distribution of python-location between habitat type differed from that expected (random locations).
To test for patterns in temporal distribution, all opportunistic records were summed by month and a chi-squared test was used to determine whether woma encounters were equally distributed among months. To determine whether the temporal distribution of woma encounters corresponded to the predictor variable monthly rainfall, a generalised linear model was used with the number of monthly woma observations as the dependent variable and monthly sampling effort and rainfall as continuous fixed predictor variables. A negative binomial distribution was used to account for overdispersion. Sampling effort was included as a covariate to account for the unstandardised collection of woma observations and was measured as the cumulative number of days on site for each visitor (includes monitoring staff, volunteers, etc.) to Wild Deserts per month. Given that sampling-effort data were available only for periods of which visitor data were collected (latter half of 2020, first half of 2021 and all of 2022–2024), the entire dataset was truncated.
The total body length of entangled pythons was compared with that of pythons that were not entangled by using Welch’s t-test because of unequal sample sizes. Total body length was used because more values were recorded than for SVL. To determine whether the fixed predictor variable habitat (dune or swale) contributed to woma mortalities, a logistic regression was used on the observations recorded within 100 m of a Wild Deserts fence, with death status as the binary dependent variable.
Interaction with conservation fences
Nine adult pythons (two females, seven males) were hand-captured during opportunistic and targeted nocturnal searches of the 50-km Wild Deserts conservation fence network up to 10 days prior to radiotransmitter implantation. Each python was temporarily housed in a 65 × 41 × 28 cm ventilated plastic tub located within a dark, climate-controlled room. Tubs were filled with 50 mm of sand substrate and contained a 254 × 230-mm electric heat mat (MICROclimate, UK) and water tray. Morphometrics were collected including mass, snout–vent and total length, and sex (determined via cloacal probing or manual eversion of hemipenes) (Table 1). From 14 to 16 March 2023, a veterinarian surgically implanted VHF S1-2T Holohil temperature-sensitive radiotransmitters (Holohil, Canada) in the coelomic cavities of pythons following the general procedures of (Reinert and Cundall 1982). Either a 9-g (12-month lifespan, 30 × 11-mm, 250-mm whip) or a 13-g (24-month lifespan, 50 × 11-mm, 250-mm whip) unit was fitted, depending on the size of the python, with transmitters ranging between 0.34% and 1.48% of body mass. Pythons were then released at their point of capture after a minimum of 12 h post-surgery for recovery. Data collection on telemetered pythons began the day following their release.
| Snake ID | Sex | SVL (m) | Tail length (m) | Total length (m) | Mass (kg) | |
|---|---|---|---|---|---|---|
| Cranky Pants | M | 1.460 | 0.135 | 1.595 | 1.450 | |
| Figure 8 | M | 1.145 | 0.110 | 1.255 | 0.722 | |
| Galileo | M | 1.260 | 0.110 | 1.370 | 0.840 | |
| Nick of Time | M | 1.250 | 0.115 | 1.365 | 0.874 | |
| Nyctophilus | F | 1.210 | 0.115 | 1.225 | 0.804 | |
| Ozimops | M | 2.165 | 0.165 | 2.330 | 3.875 | |
| T-Bone | F | 1.240 | 0.115 | 1.355 | 0.900 | |
| Tough Nut | M | 1.390 | 0.125 | 1.515 | 1.150 | |
| Wriggly One | M | 1.850 | 0.130 | 2.090 | 2.600 | |
| Mean s.e. | 1.441 0.115 | 0.124 0.006 | 1.567 0.129 | 1.468 0.358 |
Radiotracking occurred over a 12-month period from 16 March 2023 to 01 March 2024. The sampling schedule was irregular (due to availability of field personnel) and involved two main tracking regimes. Pythons were intensively radiotracked during late March, April and September 2023 approximately every 24-h with a maximum of two fixes per 24 h period. Tracking was less frequent during other times with a minimum of one fix per month. Late March, April and September were selected for fine-scale sampling as they generally experience a similar temperature profile and are predicted to be within the woma active season on either ends of the over-wintering period.
Telemetered pythons were located using a three-piece Yagi antenna and receiver (Lotek, Canada). Woma home ranges were estimated using weighted autocorrelated kernel-density estimation (wAKDE) (Fleming et al. 2018). This method was selected, given the temporally autocorrelated nature of the tracking data and irregular sampling violates the independently and identically distributed data assumptions of traditional home-range estimators, such as minimum convex polygon (MCP) and kernel-density estimation (KDE) (Crane et al. 2021; Silva et al. 2022). However, extended periods of inactivity exhibited by the womas resulted in a very low number of relocations across all individuals (range = 4–18). Further, the majority of the variograms (which visualises the autocorrelation structure of the tracking data by plotting the average square displacement over different time lags) displayed for each python did not demonstrate home-range stability and all individuals had very low effective sample sizes (mean ± s.e. = 7.51 ± 1.65, range = 2.8–18) (Fig. S1). Therefore, most home-range estimates did not meet the AKDE range-residency assumption. Despite this, given space usage was intended to be visualised in relation to fencing over a short timeframe and not infer true woma home range, tentative wAKDE estimates were still provided for individuals with ≥10 relocations. The phrase ‘short-term home range’ is therefore a more accurate descriptor of space use than home range in this study, given that the short study period almost certainly underestimated or misrepresented true woma home-range size (Goldingay 2015). Tracking data also determined the proportion of short-term home-range area intersected by the fence, the frequency of fencing encounters, and each python’s daily movement distances, which were calculated as the straight-line distance moved between consecutive fixes divided by the number of days between fixes (Natusch et al. 2022).
Observations of pythons were classed as either direct aboveground observations or indirect underground shelter-site observations. Snake body temperatures were calculated using a calibrated transmitter pulse rate, for which a stopwatch was used to record the time (ms) over 10 transmitter-pulse intervals. On every occasion a snake was located, records were taken of the location coordinates, date, time, telemetry-pulse interval and habitat. A linear mixed-effects model was used to test the influence of the fixed predictor variable month (late March/April and September) on the response variable snake body temperature. Snake ID was included as a random effect for both models to account for repeated observations of the same individual.
To preclude any potential bias in search effort between certain habitats in the opportunistic records, the habitat preferences for telemetered snakes were also determined. A generalised linear mixed-effects model was used to test whether the number of telemetered woma relocations (dependent variable) differed between the levels of the fixed predictor variable habitat (dune or swale), with the null hypothesis being that the number of python relocations would be equal between habitats. Given that the response variable was count data, a negative binomial distribution was used. Snake ID was included as a random effect.
Eight active telemetered woma over-winter burrow systems (four inside the exclosures, four outside) were selected for camera trap surveys to determine whether location (inside or outside exclosure), influenced the activity levels of prey species at woma burrow entrances. Camera traps have demonstrated to be useful in detecting species that co-exist in burrows with fossorial ectotherms (Ridley and Schlesinger 2023). One Swift Enduro camera (Outdoor Cameras Australia, Australia) was deployed at each burrow system and mounted to a star picket 0.5 m above the ground, angled downward at approximately 60° and at a distance of at least 1 m from the largest burrow entrance (if there were multiple burrows) (Fig. S2). The setup produced a 1-m2 field of view of the burrow entrance or of the track leading into a burrow concealed by vegetation. Within a species, an individual was considered unique if at least 10 min had passed since the last observation of that species. Surveys occurred between 11 September 2023 and 24 September 2023. Images were collated using Reconyx MapView™ Professional (ver. 3.7.2.2; Reconyx Inc., USA). Potential differences in prey activity (measured as mean observations per day) at woma burrow entrances were compared between inside and outside of an exclosure by using a Student’s t-test. Species recorded during surveys were assessed whether they were potential woma prey items, following comparisons with diet analysis by Shine and Slip (1990). All species were included except for womas themselves.
In the event that a telemetered python was observed interacting with the Wild Deserts fence, the distance and length of time taken to travel along the fence and number of cross attempts were recorded. Observations of behaviours during the initial fence interactions were formulated into an ethogram for the scoring of behaviours in subsequent small-scale pen trial experiments involving non-telemetered pythons (J. Magro, R. Pedler, J. Read, R. West, unpubl. data).
Data analysis
Statistical analyses were performed in R (ver. 4.0.4; R Core Team 2021). All tests were considered significant at the α = 0.05 level. Packages dplyr (ver. 1.0.8; https://cran.r-project.org/web/packages/dplyr/; Wickham et al. 2023) and lubridate (ver. 1.8.0; https://cran.r-project.org/web/packages/lubridate/; Grolemund and Wickham 2011) were used for general data manipulation and ggplot2 (ver. 3.3.5; https://cran.r-project.org/web/packages/ggplot2/; Wickham 2016) and cowplot (ver. 1.1.1; https://cran.r-project.org/web/packages/cowplot/; Wilke 2023) for graphics. The lme4 package (ver. 1.1.29; https://cran.r-project.org/web/packages/lme4/; Bates et al. 2015) was used to fit mixed models and the MASS package (ver. 7.3.53; Venables and Ripley 2002) was used to fit the generalised linear model that had a negative binomial distribution. Residuals were diagnosed using the DHARMa package (ver. 0.4.6; https://cran.r-project.org/web/packages/DHARMa/; Hartig 2023). Continuous variables in models containing more than one predictor variable were standardised using the scale function. Shapiro–Wilks tests were used to test for normality prior to running Student’s t-tests. Weighted autocorrelated kernel density estimation and movement trajectories were developed using the ctmm package (ver. 1.1.0; https://cran.r-project.org/web/packages/ctmm/; Calabrese et al. 2016) and the adehabitatLT package (ver. 0.3.25; https://cran.r-project.org/web/packages/adehabitatLT/; Calenge 2006) respectively.
Results
Spatial, temporal and demographic patterns in opportunistic woma records
A significantly higher proportion of the 60 woma records along the fence network were from dune habitats than from swales (χ2 = 10, d.f. = 1, P < 0.05), despite no difference in the frequency of sightings along exclosure segments that intersected dune crests rather than not (χ2 = 1.07, d.f. = 1, P = 0.3). There were notably no records from the 50-mm-aperture wild training-zone fence, which comprised 21% of total fence length. Observations were not equally distributed among months (χ2 = 91.39, d.f. = 11, P < 0.001), with more frequent sightings occurring during late summer/early autumn.
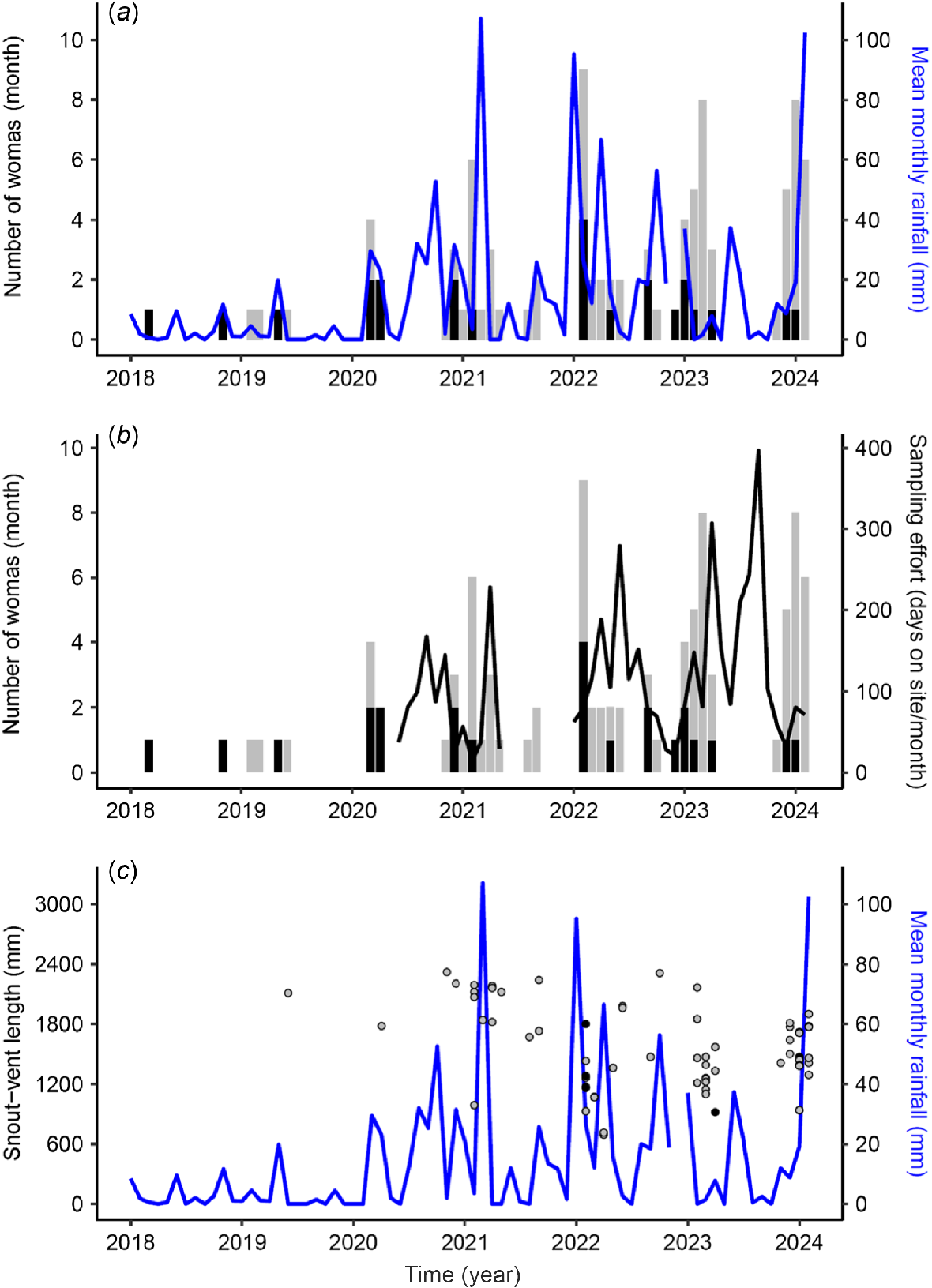
Of the 93 total woma observations across the study area recorded between 20 March 2018 and 18 February 2024, 63 observations constituted 55 unique individuals implanted with microchips. Six snakes were observed more than once, with one of these on four occasions on different sides of the Minkgu exclosure fence, and on one occasion being observed climbing through the 50-mm-aperture netting at 700–900 mm above the ground level. This same individual (subsequently named Wriggly One) was implanted with a radiotransmitter. Woma SVL ranged between 0.694 and 2.320 m and the total length of entangled pythons was comparable to non-entangled pythons (t = 0.59, d.f. = 18.57, P = 0.56) (Table 2). Neither mean monthly rainfall (β = −0.07, s.e. = 0.24, P = 0.78) nor monthly sampling effort (β = −0.31, s.e. = 0.25, P = 0.23) influenced monthly woma observations (Fig. 3). There were 20 recorded fence deaths, and these occurred only during summer and autumn. All entanglements were observed in 30-mm netting, particularly in areas of netting overlap where internal and external foot aprons join (Fig. S3). Entanglement incidence in swale habitats was comparable with that in dune habitats (β = 0.88, s.e. = 0.58, P = 0.13).
| Status | SVL (m) | Tail length (m) | Total length (m) | Mass (kg) | |
|---|---|---|---|---|---|
| Alive non-entangled | 1.581 ± 0.06 (n = 58) | 0.132 ± 0.004 (n = 58) | 1.699 ± 0.06 (n = 64) | 1.93 ± 0.15 (n = 60) | |
| Entangled A | 1.411 ± 0.12 (n = 7) | 0.124 ± 0.005 (n = 6) | 1.635 ± 0.10 (n = 11) | 1.24 ± 0.24 (n = 3) | |
| Total B | 1.562 ± 0.05 (n = 65) | 0.131 ± 0.004 (n = 64) | 1.696 ± 0.06 (n = 76) | 1.90 ± 0.14 (n = 63) |
Monthly woma observation rates in relation to (a) mean monthly rainfall and (b) sampling effort from 1 January 2018 to 18 February 2024, and (c) size distribution in relation to mean monthly rainfall. Grey and black bars in (a) and (b) and points in (c) indicate live and fence-death observations respectively. Monthly-rainfall data were sourced from Bureau of Meteorology (2024) (Fort Grey Recording Station 46006).
Interaction with conservation fences
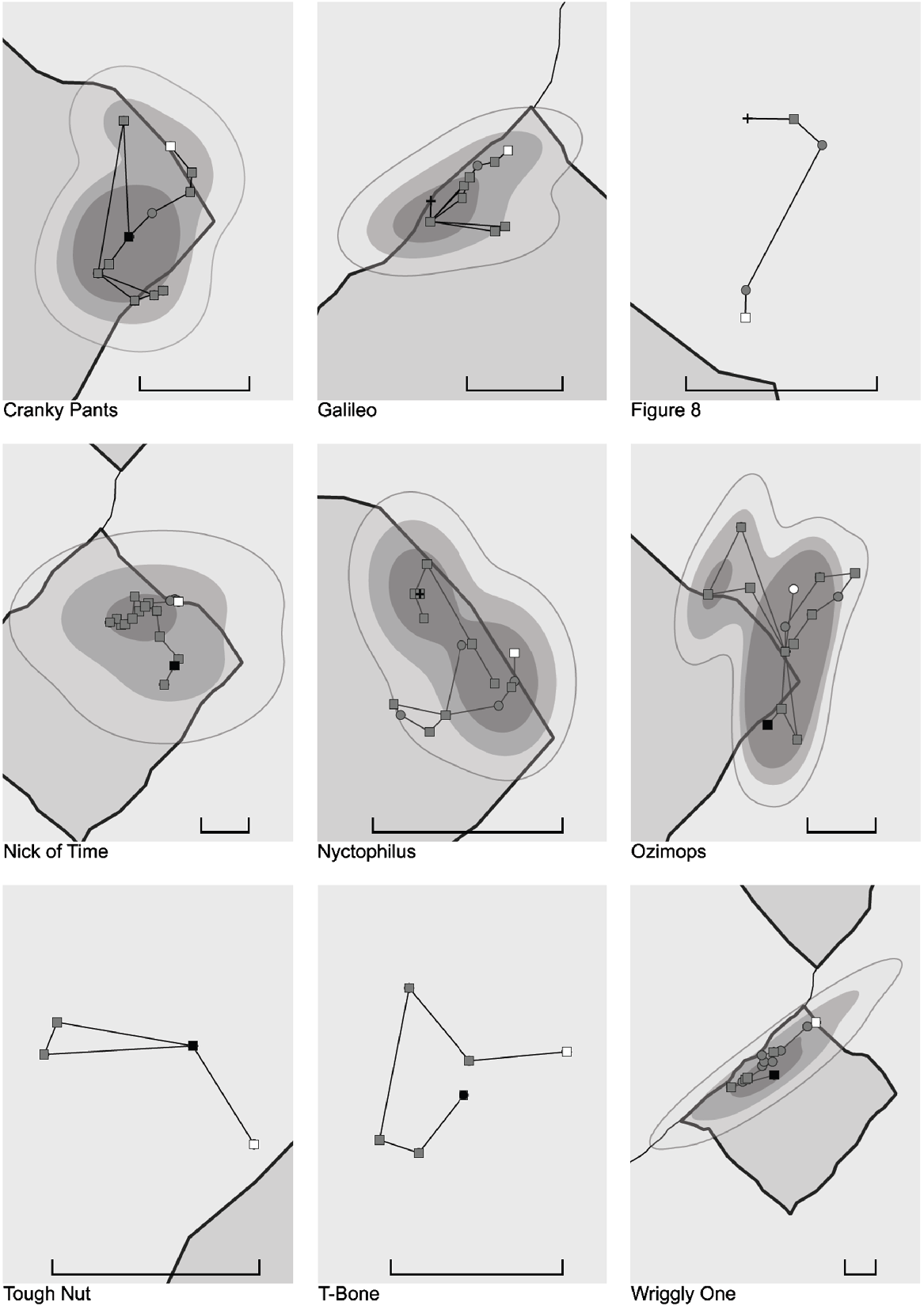
In total, 498 locational fixes were collected from nine pythons over 303 ± 22 days (mean ± s.e.) per individual. The pythons were recorded from a total of 72 shelter sites, with 48 ± 12 days (mean ± s.e.) (max = 254 days) being recorded stationary at a shelter site and a straight-line distance moved between consecutive shelter sites of 613 ± 94 m (max = 1932 m) (Table S1, Fig. S4). Tracking terminated prematurely for four of the nine pythons. One individual was lost after 10 August 2023, possibly owing to transmitter failure; the radiotransmitters of two pythons were found expelled in python faeces and another python was found deceased by entanglement. Home-range analyses were not conducted for three individuals because of ≤10 relocations (Table 3). Mean woma wAKDE short-term home-range size was 4.018 km2 (Table 3, Fig. 4). Four of the nine pythons successfully crossed through the exclosure fence (SVL range = 1.21–1.85 m) (Table 3), with three crossing only once and another crossing three times. However, the first fence-crossing attempt recorded for a fifth individual resulted in an entanglement mortality (SVL = 1.26 m).
| Snake ID | wAKDE lower CI (km2) | wAKDE estimate (km2) | wAKDE upper CI (km2) | Movement model | % inside exclosures | Number of fence crosses | |
|---|---|---|---|---|---|---|---|
| Cranky Pants | 0.840 | 1.980 | 3.60 | OUF | 79 | 3 | |
| Figure 8 | NA | NA | NA | NA | NA | 0 | |
| Galileo | 0.421 | 1.434 | 3.056 | OUF anisotropic | 66 | 0A | |
| Nick of Time | 1.133 | 7.623 | 20.169 | OU anisotropic | 83 | 1 | |
| Nyctophilus | 0.348 | 0.716 | 1.214 | OUF anisotropic | 71 | 1 | |
| Ozimops | 3.213 | 5.424 | 8.207 | OUF anisotropic | 24B | 0 | |
| T-Bone | NA | NA | NA | NA | NA | 0 | |
| Tough Nut | NA | NA | NA | NA | NA | 0 | |
| Wriggly One | 1.827 | 6.931 | 15.374 | OUF anisotropic | 81 | 1 |
Note that short-term home ranges were not all calculated over the same timeframe for each individual (see Table S1).
wAKDE lower and upper CI 95%, lower and upper confidence intervals for the 95% wAKDE estimate; % inside exclosures, percentage of wAKDE estimate contained within a Wild Deserts exclosure; Number of fence crosses, number of times an individual was known to have crossed the Wild Deserts exclosure fencing during study; Model acronyms: OU, Ornstein–Uhlenbeck; OUF, Ornstein–Uhlenbeck Foraging.
Movement trajectories and short-term home-range maps of telemetered pythons in relation to Wild Deserts fencing. Short-term home ranges were constructed only for pythons with ≥10 relocations. White-, grey-, and black-coloured shapes represent first, intermediate and final relocations respectively. Squares, circles and crosses represent shelter sites, aboveground and other fixes respectively. Shaded area represents inside of an exclosure. Scale bar: 1 km. Note that short-term home ranges were not all calculated over the same timeframe for each individual (see Table S1).
Daily movement distance over the study period was 38.55 ± 12.15 m (mean ± s.e.), with reduced movements in late autumn, winter and early spring (Table S2). Despite the slightly lower difference in mean maximum temperature (0.9°C) and higher difference in mean minimum temperature (0.7°C) in April than in September (Fig. S5), which were the 2 months that experienced intensive tracking, telemetered woma body temperatures were considerably lower during September (β = −6.25, s.e. = 0.29, P < 0.001) (Table S2, Fig. S6). Body temperature across the entire study duration was 28.27°C ± 0.14 (mean ± s.e.). Seven of the nine tracked individuals were directly observed above the ground (4% of total fixes) across various times of the day and night and only in March–April (Fig. S6). The greatest number of snakes aboveground on a given tracking event was four after dark during early April, which also coincided with slight precipitation, the highest maximum temperature of the month and a full moon. Pythons were observed actively moving, basking or resting, with one instance of an observed predation event of a python consuming a central bearded dragon (Pogona vitticeps). Reintroduced greater bilbies (Macrotis lagotis) were also recorded as a prey item following inspection of the scat of a 1.26-m-SVL python prior to tracking.
Telemetered womas were more frequently located in dune habitats (β = 1.29, s.e. = 0.37, P < 0.001), with 86% of shelter sites and 68% of aboveground fixes on dunes. Animal burrows were the only shelter sites used, with bilby or bilby-modified rabbit burrow systems being most frequently used inside the exclosures and those outside tending to be rabbit warrens, but reptile burrows were also frequented. The camera-trap surveys identified all over-winter shelter sites inside the exclosures as active bilby burrows and that prey activity was greater at woma burrow entrances inside the exclosures (t = 4.29, d.f. = 3.26, P < 0.05) (Fig. S7). Only one woma was recorded in the camera-trap surveys and was observed basking over 3 days between 09:40 hours and 12:10 hours. Other noteworthy behavioural observations acquired during tracking included locating a small male and a large male outside the exclosure in the same shelter site as a small female 26 h (moved 310 m) and 35 h (moved 926 m) respectively, following the post-surgery release of the small female. All three individuals remained in the same shelter-site for at least 6 days. The small male was then located in a new shelter site within the exclosure at most 6 days after being detected in the communal burrow, followed by the female 6 days later.
Two of the telemetered snakes were observed interacting with the Wild Deserts exclosure fence. One small male made 0.36 crossing attempts per minute and moved 16 m along the fence over 22 min, but was unsuccessful, returning to its most recently used shelter site outside the exclosure. This same python was then detected to have successfully crossed the fence at most 4 days later. Of the eight crossing attempts, five involved the python raising up to the 50-mm netting, at least 700 mm above the ground. A second python (small female) made 0.1 crossing attempts per minute in a 30-min period along 20 m of fence line and successfully crossed the fence into the exclosure after approximately 80 min.
Discussion
This is the first study to investigate the impact of conservation fencing on snake entanglement. Despite entanglements, rabbit-proof conservation fences were not a completely impermeable barrier to woma pythons and fencing encounters were more frequent during warmer months and in dune habitats. The results broadly highlight that certain aspects of fence design can cause impacts to non-target species and that modification of these could reduce such impacts.
The observed woma short-term home ranges overlapped with the fences despite infrequent fence crossings. Low fence-crossing rates may be attributed to the largely sedentary behaviour of womas over the study duration. This is supported by the literature, with Bruton (2013) reporting that womas spend 74% of their time underground and exhibit prolonged overwintering periods over an average of 134 days. In recently released captive-bred snakes that were naïve to their release site, Read et al. (2011) reported an initial mean sedentary period of 28 days, followed by a move, on average, every 5.2 days from late September 2007 to late January 2008, suggesting movements are more frequent during warmer months. Reported minimum mean movement distances between consecutive shelter-sites are short at 109 m (Read et al. 2011) and an average distance of 393 m per move within 55 h (Bruton 2014), which are slightly shorter to movements observed in this study. Both active and ambush foraging strategies are known from this species, possibly explaining why movements are comparatively shorter to those of obligate active foragers such as the sympatric mulga snake (Pseudechis australis) (Read et al. 2011). Therefore, although the low number of fence crossings recorded may be an artefact of short study duration and/or the irregular tracking regime, crossings may be naturally low, given womas move infrequently and rather short distances. As to why woma short-term home ranges overlapped with fencing may be due to a prey availability differential. The greater prey activity recorded at woma burrow entrances within the exclosures may suggest that areas within fences provide a selective advantage for individuals able to cross and, therefore, also potentially encourage woma fence interactions and contribute to entanglement incidence. This may well be the case, given womas were recorded consuming bilbies at this location. The fences also exhibit a barrier on other prey items, including bearded dragons, which may subsequently concentrate womas in certain areas.
This study demonstrated that womas have strong seasonal preferences in activity, which is common in other ectotherms (Shine and Lambeck 1985; Shine 1991; Brown and Shine 2002). As was expected, womas were more active over the warmer seasons. However, womas were more active and had higher body temperatures in late March/April than in September, despite the similarity in air temperatures between the 2 months. This may suggest that they actively regulate their body temperature rather than relying wholly on changes in ambient temperature, or that ground temperature (which may have been warmer in April than in September) may have a greater influence on activity than has air temperature alone. During overwinter periods following active seasons when womas are not likely to be feeding or mating (Bruton 2013), the lowering of body temperatures is likely to assist in reducing their already low metabolic rate and associated energy expenditure (Slip and Shine 1988; Bedford and Christian 1998; Pearson et al. 2003). Further, snake activity is known to increase with rainfall and high humidity, possibly as a mechanism to prevent water loss (Henderson and Hoevers 1977; Daltry et al. 1998). Annual variability in the number of opportunistic woma records at the study site may be partially due to rainfall. Despite neither sampling effort nor monthly rainfall explaining a significant proportion of the variation in the number of woma observations per month, the truncated timeframe over which the data were analysed may have limited the ability to achieve a significant result. Similarly, more encounters following periods of rainfall have been observed in another arid-zone python species over a monthly timescale (McDonald et al. 2011). This all suggests potential high-risk times for fencing entanglements to be during the warmer seasons and possibly following rainfall events or periods of high relative humidity.
This study demonstrated that womas prefer sand-dune habitat over clay swales, which corresponds with Read et al. (2011) who found 98% of all woma shelter sites on dunes. The greater number of woma observations, particularly woma shelter sites in dunes may reflect a potentially greater availability of underground burrow systems and associated prey items in this habitat (Moseby and O’Donnell 2003; Moseby et al. 2005), as pre-excavated animal burrows have been found to be the most influential shelter variable influencing woma occurrence (Bruton et al. 2014). Further, woma movement trajectories often followed the north–east to south–west orientation of the longitudinal dunes in the site. Although there was no difference between entanglement incidence in swale habitat and that in dune habitat, searches for entanglements should target dunes (i.e. north–east and south–west segments of both exclosures), given fencing encounters were more frequent in this habitat.
Despite one instance of an entanglement mortality in a telemetered python, four of the nine telemetered individuals successfully crossed the fence without resulting in an entanglement, suggesting entanglement incidence is potentially low. Further, 14 live woma observations including the capture location of six of the nine telemetered pythons were also located along a 4-km section of the Mingku exclosure fence from which no entanglements had been recorded over the 5 years since the fence was installed, implying that many womas frequent near fencing but seldom become entangled.
Entanglement incidence may be related to extended exposure of pythons attempting fence crossings to high diurnal temperatures, which can result in rapid overheating and subsequent mortality (Ferronato et al. 2014; Eye et al. 2018). The lack of entanglement records for large pythons (SVL > 1.5 m) potentially stems from the fact that there may be proportionally fewer larger pythons than small-medium sized pythons at this site. Larger individuals may also have a head or neck diameter too broad to attempt crossing through the 30-mm or even the 50-mm netting and/or the ability to use their length to reach to the 50-mm netting at 700 mm aboveground to cross between sides. Although mortalities were frequently observed in small–medium-sized pythons, factors other than body length may be at play, given entangled womas were similar in SVL to telemetered womas able to cross unhindered. Body circumference is a factor not considered in this study yet is a useful measure in determining the susceptibility of certain individuals to entanglement, as smaller fencing apertures will prevent snakes of a certain body circumference from passing through.
Ebert et al. (2019) demonstrated that circumference was the only morphometric measure they tested (that included SVL) to demonstrate a positive relationship with snake entanglements in plastic erosion-control blanket netting. However, measuring body circumference of entangled snakes in this study was problematic, because many were found desiccated, which, if measured, would provide an underestimate of true body circumference. Some womas found entangled also had large food items in their gut cavities (e.g. bearded dragons), which bulged and dramatically increased their girth and almost certainly contributed to their entanglement incidence in some instances. Pizzatto et al. 2007 found woma mid-body circumference expressed as a proportion of SVL to be, on average, 0.09 ± 0.01 s.d. (range = 0.07–0.12) from 26 museum specimens. Using these data, inferred woma mid-body circumference of the womas that became entangled and those known to cross would be witihin a range of 82.8–162 mm and of 108.9–166.5 mm respectively. It is important to note that if girth does contribute to mortality, body circumference of entangled womas would be greater than this inferred estimate, given that circumference was calculated on the basis of SVL.
Pheromone trails appear to be important in the social behaviour of this species, influencing movement trajectories. Direct observations of a small telemetered female woma on the fence, including the many direction changes in movement, indicated that this individual was attempting to cross the fence in a specific area, suggesting possible pheromone-trailing behaviour and that it is not limited to males. After sharing a shelter site, then crossing the fence in the same area that a small male had crossed six nights before, the female followed the small male into his new shelter site. This and another communal burrowing event, which are the first such instances reported for this species, and the spatial clustering of opportunistic records, support the use of pheromone trails. Considering this, insights into the manipulation of reptile pheromone trails to control invasive reptile species such as brown tree snakes (Boiga irregularis) in Guam (Mason and Greene 2001) suggests its potential applicability to the context of fence-impact mitigation. Reptiles including womas could be actively lured to and through fences at specified locations, using the pheromone trail of a conspecific or a potential prey item. Future studies should explore how the manipulation of snake pheromones sourced from sloughed skins or live animals affects their behaviour and movement in relation to fencing for the development of mitigation strategies.
Management implications
The findings from this research are particularly important given the large number of fenced safe havens in Australia and planned construction of further fences in the future to better conserve threatened mammal species currently not protected within safe havens (Ringma et al. 2019). The study has highlighted that fencing impacts on the behavioural and spatial ecology of large non-target reptiles should be considered in the management and development planning stages of conservation fencing, particularly with the use of small netting apertures. Although this study focused on conservation fencing, the findings can be applied to fences and netting in general. Erosion-control netting and road-exclusion fencing used to prevent wildlife road mortalities are both particularly common in North America and Europe; however, both frequently result in snake entanglements or dispersal disruptions (Clark et al. 2010; Ebert et al. 2019; Maida et al. 2020).
Large apertures should be preferentially selected where possible in future conservation fencing projects to reduce fencing impacts on large snakes. Given womas were not encountered along the wild training zone fence and were entangled only in 30-mm aperture netting, it is likely that 50-mm aperture netting is permeable to most womas, but potentially not to the largest individuals with large food items in their gut cavities. However, it is important to note that anecdotal observations suggest entanglements of bearded dragons (Pogona vitticeps) are more likely in 50-mm aperture netting.
Thirty-millimetre rabbit-proof netting possibly presents entanglement and barrier risks to a range of snakes and should be limited only to circumstances where it is necessary to exclude rabbits. Further, the overlapping of netting may also present an entanglement risk, because the aperture for snakes to cross the fence is further reduced in areas of netting overlap. Snake-specific gates or portals may be effective tools for resolving entanglement issues associated with conservation fencing because they can be retrofitted to existing fences. Further investigation into the use of portals in mitigating fence impacts will be an important next step in resolving the growing concerns around large snake entanglements in conservation fencing.
Data availability
Data for this paper are available at the Dryad data repository, https://doi.org/10.5061/dryad.mpg4f4r96.
Declaration of funding
Funding for this study was provided by the Centre for Ecosystem Science, UNSW Sydney, and the New South Wales Government Department of Climate Change, Energy, Environment and Water through the Wild Deserts Feral Predator Free Partnership Project.
Acknowledgements
The authors thank team members who contributed to the development of this study including Richard Kingsford, Katherine Moseby and significant field support in python tracking and capture from Brianna Coulter, David Damschke, Peta and Andrew Murray. The significant contributions from Larry Vogelnest (Taronga Conservation Society) in transmitter implantation are gratefully acknowledged.
References
Atlas of Living Australia (2023) Occurrence download. Atlas of Living Australia. Available at https://doi.org/10.26197/ala.da11cdd7-2eaf-4034-9a2b-82704350bddb
Baines D, Andrew M (2003) Marking of deer fences to reduce frequency of collisions by woodland grouse. Biological Conservation 110, 169-176.
| Crossref | Google Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1-48.
| Crossref | Google Scholar |
Bedford GS, Christian KA (1998) Standard metabolic rate and preferred body temperatures in some Australian pythons. Australian Journal of Zoology 46(4), 317-328.
| Crossref | Google Scholar |
Brown GP, Shine R (2002) Influence of weather conditions on activity of tropical snakes. Austral Ecology 27(6), 596-605.
| Crossref | Google Scholar |
Bruton MJ (2013) Arboreality, excavation, and active foraging: novel observations of radiotracked woma pythons Aspidites ramsayi. Memoirs of the Queensland Museum – Nature 56(2), 313-329.
| Google Scholar |
Bruton MJ, McAlpine CA, Smith AG, Franklin CE (2014) The importance of underground shelter resources for reptiles in dryland landscapes: a woma python case study. Austral Ecology 39(7), 819-829.
| Crossref | Google Scholar |
Bruton M, Wilson S, Shea G, Ellis R, Venz M, Hobson R, Sanderson C (2017) Aspidites ramsayi. The IUCN Red List of Threatened Species 2017, e.T2176A83765377.
| Crossref | Google Scholar |
Bryant GL, Bateman PW, Fleming PA (2011) Tantalising tongues: male carpet pythons use chemoreception to differentiate among females. Australian Journal of Zoology 59(1), 42-48.
| Crossref | Google Scholar |
Bureau of Meteorology (2024) Climate data online. Department of the Environment (Australia). Available at http://www.bom.gov.au/climate/data/index.shtml [accessed 1 June 2024]
Calabrese JM, Fleming CH, Gurarie E (2016) ctmm: an R package for analyzing animal relocation data as a continuous-time stochastic process. Methods in Ecology and Evolution 7(9), 1124-1132.
| Crossref | Google Scholar |
Calenge C (2006) The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197(3–4), 516-519.
| Crossref | Google Scholar |
Clark RW, Brown WS, Stechert R, Zamudio KR (2010) Roads, interrupted dispersal, and genetic diversity in timber rattlesnakes. Conservation Biology 24(4), 1059-1069.
| Crossref | Google Scholar | PubMed |
Covacevich JA (1996) Aspidites ramsayi (Boidae) in the Brigalow Biogeographic Region of Queensland: occurrence, conservation status and possible bilby associations. Memoirs of The Queensland Museum 39, 243-246.
| Google Scholar |
Crane M, Silva I, Marshall BM, Strine CT (2021) Lots of movement, little progress: a review of reptile home range literature. PeerJ 9, e11742.
| Crossref | Google Scholar | PubMed |
Daltry JC, Ross T, Thorpe RS, Wüster W (1998) Evidence that humidity influences snake activity patterns: a field study of the Malayan pit viper Calloselasma rhodostoma. Ecography 21, 25-34.
| Crossref | Google Scholar |
Ebert SE, Jobe KL, Schalk CM, Saenz D, Adams CK, Comer CE (2019) Correlates of snake entanglement in erosion control blankets. Wildlife Society Bulletin 43(2), 231-237.
| Crossref | Google Scholar |
Eye DM, Maida JR, McKibbin OM, Larsen KW, Bishop CA (2018) Snake mortality and cover board effectiveness along exclusion fencing in British Columbia, Canada. The Canadian Field-Naturalist 132(1), 30-35.
| Crossref | Google Scholar |
Ferronato BO, Roe JH, Georges A (2014) Reptile bycatch in a pest-exclusion fence established for wildlife reintroductions. Journal for Nature Conservation 22(6), 577-585.
| Crossref | Google Scholar |
Fleming CH, Sheldon D, Fagan WF, Leimgruber P, Mueller T, Nandintsetseg D, Noonan MJ, Olson KA, Setyawan E, Sianipar A, Calabrese JM (2018) Correcting for missing and irregular data in home-range estimation. Ecological Applications 28(4), 1003-1010.
| Crossref | Google Scholar | PubMed |
Goldingay RL (2015) A review of home-range studies on Australian terrestrial vertebrates: adequacy of studies, testing of hypotheses, and relevance to conservation and international studies. Australian Journal of Zoology 63(2), 136-146.
| Crossref | Google Scholar |
Grolemund G, Wickham H (2011) Dates and times made easy with lubridate. Journal of Statistical Software 40(3), 1-25.
| Crossref | Google Scholar |
Hayward MW, Kerley GIH (2009) Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142(1), 1-13.
| Crossref | Google Scholar |
Henderson RW, Hoevers LG (1977) The seasonal incidence of snakes at a locality in northern Belize. Copeia 1977(2), 349-355.
| Crossref | Google Scholar |
Holt S, Horwitz LK, Wilson B, Codron D (2021) Leopard tortoise Stigmochelys pardalis (Bell, 1928) mortality caused by electrified fences in central South Africa and its impact on tortoise demography. African Journal of Herpetology 70(1), 32-52.
| Crossref | Google Scholar |
Jakes AF, Jones PF, Paige LC, Seidler RG, Huijser MP (2018) A fence runs through it: a call for greater attention to the influence of fences on wildlife and ecosystems. Biological Conservation 227, 310-318.
| Crossref | Google Scholar |
Kassilly FN (2002) The fence as a moderator of the wildlife menace in Kenya. African Journal of Ecology 40(4), 407-409.
| Crossref | Google Scholar |
Lee ATK, Macray MB, Ryan PG, Alexander GJ (2021) Tortoise mortality along fence lines in the Karoo region of South Africa. Journal for Nature Conservation 59, 125945.
| Crossref | Google Scholar |
Legge S, Woinarski JCZ, Burbidge AA, Palmer R, Ringma J, Radford JQ, Mitchell N, Bode M, Wintle B, Baseler M, Bentley J, Copley P, Dexter N, Dickman CR, Gillespie GR, Hill B, Johnson CN, Latch P, Letnic M, Manning A, McCreless EE, Menkhorst P, Morris K, Moseby K, Page M, Pannell D, Tuft K (2018) Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildlife Research 45(7), 627-644.
| Crossref | Google Scholar |
Macpherson MR, Litzgus JD, Weatherhead PJ, Lougheed SC (2021) Barriers for big snakes: incorporating animal behaviour and morphology into road mortality mitigation design. Global Ecology and Conservation 26, e01471.
| Crossref | Google Scholar |
Maida JR, Bishop CA, Larsen KW (2020) Migration and disturbance: impact of fencing and development on Western Rattlesnake (Crotalus oreganus) spring movements in British Columbia. Canadian Journal of Zoology 98(1), 1-12.
| Crossref | Google Scholar |
Mason RT, Greene MJ (2001) Invading pest species and the threat to biodiversity: pheromonal control of guam brown tree snakes, Boiga irregularis. In ‘Chemical signals in vertebrates 9’. (Eds A Marchlewska-Koj, JJ Lepri, D Müller-Schwarze) pp. 361–368. (Kluwer Academic/Plenum Publishers: New York, NY, USA)
Mbaiwa JE, Mbaiwa OJ (2006) The effects of veterinary fences on wildlife populations in Okavango Delta, Botswana. International Journal of Wilderness 12(3), 17-41.
| Google Scholar |
McDonald PJ, Luck GW, Wassens S, Pavey CR (2011) Ecology of stimson’s python (Antaresia stimsoni) in the MacDonnell ranges of central Australia. Australian Journal of Zoology 59(2), 95-102.
| Crossref | Google Scholar |
McGregor H, Read J, Johnson CN, Legge S, Hill B, Moseby K (2020) Edge effects created by fenced conservation reserves benefit an invasive mesopredator. Wildlife Research 47(8), 677-685.
| Crossref | Google Scholar |
Moseby KE, O’Donnell E (2003) Reintroduction of the greater bilby, Macrotis lagotis (Reid) (Marsupialia : Thylacomyidae), to northern South Australia: survival, ecology and notes on reintroduction protocols. Wildlife Research 30(1), 15-27.
| Crossref | Google Scholar |
Moseby KE, Read JL (2006) The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biological Conservation 127(4), 429-437.
| Crossref | Google Scholar |
Moseby KE, De Jong S, Munro N, Pieck A (2005) Home range, activity and habitat use of European rabbits (Oryctolagus cuniculus) in arid Australia: implications for control. Wildlife Research 32(4), 305-311.
| Crossref | Google Scholar |
Natusch D, Lyons J, Shine R (2022) Spatial ecology, activity patterns, and habitat use by giant pythons (Simalia amethistina) in tropical Australia. Scientific Reports 12(1), 5274.
| Crossref | Google Scholar |
Pearson D, Shine R, Williams A (2003) Thermal biology of large snakes in cool climates: a radio-telemetric study of carpet pythons (Morelia spilota imbricata) in south-western Australia. Journal of Thermal Biology 28(2), 117-131.
| Crossref | Google Scholar |
Pedler RD (2011) Recent records of the woma python (Aspidites ramsayi) in South Australia, with and evaluation of distribution, habitat and status. Herpetofauna 41(1-2), 31-52.
| Google Scholar |
Pedler RD, West RS, Read JL, Moseby KE, Letnic M, Keith DA, Leggett KD, Ryall SR, Kingsford RT (2018) Conservation challenges and benefits of multispecies reintroductions to a national park – a case study from New South Wales, Australia. Pacific Conservation Biology 24(4), 397-408.
| Crossref | Google Scholar |
Pietersen DW (2022) Body size, defensive behaviour, and season influence mortality probability in wildlife interactions with electrified fences. African Journal of Wildlife Research 52(1), 172-184.
| Crossref | Google Scholar |
Pizzatto L, Almeida-Santos SM, Shine R (2007) Life-history adaptations to arboreality in snakes. Ecology 88(2), 359-366.
| Crossref | Google Scholar | PubMed |
Pook MJ, Risbey JS, Ummenhofer CC, Briggs PR, Cohen TJ (2014) A synoptic climatology of heavy rain events in the Lake Eyre and Lake Frome catchments. Frontiers in Environmental Science 2, 54.
| Crossref | Google Scholar |
QGIS (2024) QGIS Project. Available at http://www.qgis.org/ [accessed 21 September 2024]
Read JL, Johnston GR, Morley TP (2011) Predation by snakes thwarts trial reintroduction of the endangered woma python Aspidites ramsayi. Oryx 45(4), 505-512.
| Crossref | Google Scholar |
Reinert HK, Cundall D (1982) An Improved Surgical Implantation Method for Radiotracking Snakes. Copeia 3, 702–-705.
| Google Scholar |
Ridley JCH, Schlesinger CA (2023) Activity of tjakura (great desert skinks) at burrows in relation to plant cover and predators. Ecology and Evolution 13(8), e10391.
| Crossref | Google Scholar | PubMed |
Ringma J, Legge S, Woinarski J, Radford J, Wintle B, Bode M (2018) Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens. Nature Ecology & Evolution 2, 410-411.
| Crossref | Google Scholar | PubMed |
Ringma J, Legge S, Woinarski JCZ, Radford JQ, Wintle B, Bentley J, Burbidge AA, Copley P, Dexter N, Dickman CR, Gillespie GR, Hill B, Johnson CN, Kanowski J, Letnic M, Manning A, Menkhorst P, Mitchell N, Morris K, Moseby K, Page M, Palmer R, Bode M (2019) Systematic planning can rapidly close the protection gap in Australian mammal havens. Conservation Letters 12(1), e12611.
| Crossref | Google Scholar |
Sadlier RA (1994) Conservation status of the reptiles and amphibians in the Western Division of New South Wales – an overview. In ‘Future of the fauna of Western New South Wales’. (Eds D Lunney, S Hand, P Reed, D Butcher) pp. 161–167. (The Royal Zoological Society of New South Wales: Sydney. NSW, Australia)
Shine R, Lambeck R (1985) A radiotelemetric study of movements, thermoregulation and habitat utilization of Arafura filesnakes (Serpentes: Acrochordidae). Herpetologica 41(3), 351-361.
| Google Scholar |
Shine R, Slip DJ (1990) Biological aspects of the adaptive radiation of Australasian pythons (Serpentes: Boidae). Herpetologica 46(3), 283-290.
| Google Scholar |
Silva I, Fleming CH, Noonan MJ, Alston J, Folta C, Fagan WF, Calabrese JM (2022) Autocorrelation-informed home range estimation: a review and practical guide. Methods in Ecology and Evolution 13(3), 534-544.
| Crossref | Google Scholar |
Slip DJ, Shine R (1988) Thermoregulation of free-ranging diamond pythons, Morelia spilota (Serpentes, Boidae). Copeia 1988(4), 984-995.
| Crossref | Google Scholar |
Smith D, King R, Allen BL (2020) Impacts of exclusion fencing on target and non-target fauna: a global review. Biological Reviews 95(6), 1590-1606.
| Crossref | Google Scholar | PubMed |
Tulloch AIT, Jackson MV, Bayraktarov E, Carey AR, Correa-Gomez DF, Driessen M, Gynther IC, Hardie M, Moseby K, Joseph L, Preece H, Felipe Suarez-Castro A, Stuart S, Woinarski JCZ, Possingham HP (2023) Effects of different management strategies on long-term trends of Australian threatened and near-threatened mammals. Conservation Biology 37(2), e14032.
| Crossref | Google Scholar |
West R, Pedler R (2017) Wild Deserts: a project to reintroduce locally extinct mammals to Sturt National Park in NSW. Review of Environmental Factors. Prepared for the NSW Office of Environment and Heritage. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Parks-reserves-and-protected-areas/Parks-management-other/sturt-national-park-review-environmental-factors-reintroduce-locally-extinct-mammals-170451.pdf
Woltz HW, Gibbs JP, Ducey PK (2008) Road crossing structures for amphibians and reptiles: informing design through behavioral analysis. Biological Conservation 141(11), 2745-2750.
| Crossref | Google Scholar |