Applications of chemical bird repellents for crop and resource protection: a review and synthesis
Shelagh T. DeLiberto A and Scott J. Werner A *
A *
A
Abstract
Non-lethal repellents are needed to protect newly planted and ripening crops, to prevent valuable resources from being damaged by some wild birds worldwide. We systematically searched all scientific publications, patents and product registrations to develop a current review and synthesis regarding chemical bird repellents for wildlife researchers, ecologists, managers and conservationists. We then developed a database regarding the testing procedures and repellency results associated with the published and unpublished literature. For this comprehensive database, we developed an ‘index of success’, or relative efficacy level (e.g. effective in most experiments), associated with each tested bird repellent. We found 345 papers published in 1948–2022, including 2994 tests of 1478 repellent chemicals. From 224 publications regarding seed repellents, chemicals that were effective in most experiments and tested in three or more experiments include fungicides (cycloheximide, thiuram), insecticides (carbamates, imidacloprid), starlicide (3-chloro-p-toluidine hydrochloride), human pharmaceuticals (aminopyridine, quinine sulfate), petroleum distillate (paranapthalene), alkaloids (caffeine, quinine sulfate), monoterpenes (d-pulegone) and naturally occurring or synthetic polyphenolic compounds (anthraquinone). Among 114 publications regarding repellents used for foliar/fruit applications, chemicals that were effective in most experiments include activated charcoal, anthraquinone and carbamate. Among other bird repellents that were reportedly effective in most experiments, chemicals used for water applications and tested in three or more experiments include benzaldehyde, ortho-aminoacetophenone and sodium chloride; chemicals used as bait repellents include anthraquinone, methyl anthranilate and 2-carbamoyloxyethyl(trimethyl)azanium chloride; and the single chemical regarded as an area repellent was methyl anthranilate. There are currently 17 registered bird repellent products in the USA for five active ingredients, including anthraquinone, capsaicin, methiocarb, methyl anthranilate and polybutene. Future research and development of chemical bird repellents should include biopesticides (i.e. pesticides derived from natural materials) and pesticides that are already registered for human food use. The future discovery of repellent active ingredients and repellent products can be facilitated by an understanding of the scientific literature, patents and product registrations regarding bird repellent applications summarised in this review.
Keywords: agricultural pests, bird repellent, crop protection, management strategies, pest management, repellent chemical, resource protection, wildlife management.
Introduction
The incidence of agricultural and domestic pests, including weeds, pathogens and vertebrate pests, affects crop productivity, as well as human health and safety worldwide. Across the globe, pests affect an average of 35% of potential crop yield loss prior to harvest (Popp et al. 2013). Strategies for protecting crops from vertebrate and invertebrate pests date back to the beginning of agricultural systems (ca 11 500 years ago; DeLiberto and Werner 2016). Through crop protection measures, including pesticides, producers can alleviate crop losses due to pests (Oerke 2006). Pesticides, broadly defined as chemicals and other products used to kill, repel or control pests, include non-lethal animal repellents (Schierow and Esworthy 2012).
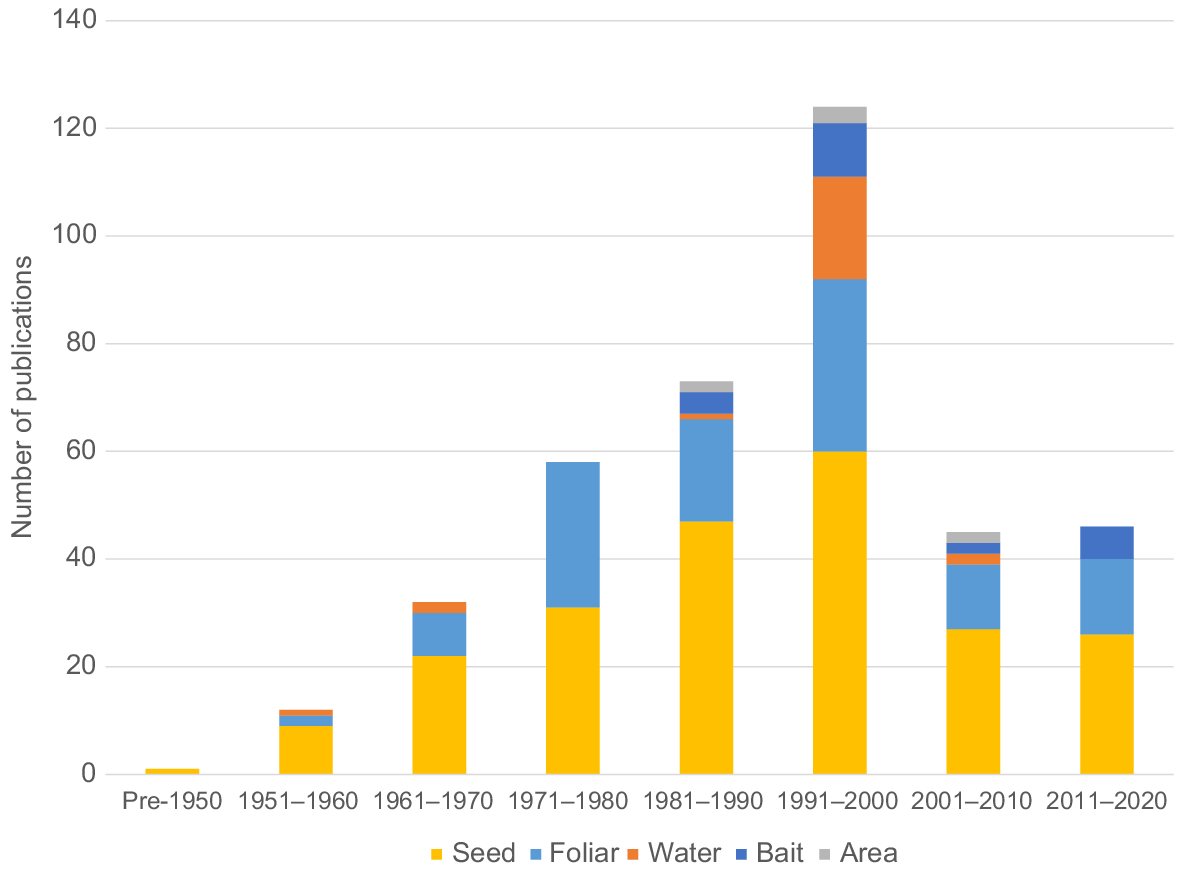
Beginning in the late 1990s, bird repellent registrations decreased by 41% compared with previous decades (N = 32–33 in 1978–1988 and N = 18 in 1998–2008; Table 1) (Clark 1998). However, the number of bird repellent patents published between 2008 (11 patents, 40 active ingredients) and 2021 (25 patents, 73 active ingredients) has increased by 100% after remaining stable for decades (Table 1). Patents allow manufacturers to protect their intellectual property and thereby enable subsequent commercial development, availability and use of their invention (e.g. chemical bird repellents). When patents expire or otherwise become invalid, products related to the patents become available from multiple sources and the prices drop, ultimately cutting revenues to the patent holder (Neumeyer et al. 1969; Pelaez et al. 2013). After the research and development phase (i.e. 5–10 years) and the additional time needed to register a chemical (approximately 2 years), only 7–10 years for recovering costs and garnering profit remain (St. Aubin 1977).
| Products/patents | 1978 | 1988 | 1998 | 2008 | 2021 | |
|---|---|---|---|---|---|---|
| Product labels | 32 | 33 | 18 | 18 | 23 | |
| Active ingredients | 10 | 10 | 5 | 6 | 6 | |
| Patents | 15 | 9 | 13 | 11 | 25 | |
| Active ingredients | 67 | 8 | 91 | 40 | 73 |
Chemical repellents have generally been classified as primary and secondary repellents. Birds reflexively withdraw from primary repellents because they irritate the peripheral chemical senses (Sayre and Clark 2001). Secondary repellents cause conditioned aversion responses, or target-oriented avoidance (Bullard et al. 1983). The ‘unpleasant experience’ of secondary repellents promotes learned or conditioned avoidance of foods paired with these repellents. Relative to the behavioural response of European starlings in the negative control group (gavaged only with propylene glycol), starlings similarly avoided food treated with methyl anthranilate (primary repellent) or methiocarb (secondary repellent) after either repellent was delivered enterically via gavage (Sayre and Clark 2001). Primary repellents may therefore be converted to secondary repellents via gastrointestinal delivery, thus potentially increasing the cost-effectiveness of the repellent application (Sayre and Clark 2001).
In addition to primary and secondary repellent classifications, we categorised repellent chemicals based upon repellent application type (i.e. seed repellents, foliar/fruit repellents, water repellents, bait repellents, area repellents). Seed repellents are often used to manage bird damages to newly planted crops, including seeds or pre-emergent seedlings. Pre-plant seed treatments are often necessary to protect seeds and pre-emergent seedlings from avian depredation without negatively affecting the germination of treated seeds (DeLiberto and Werner 2016). Many repellent experiments are conducted with seeds to protect newly planted crops from wild birds. Often, seed repellent tests are conducted as a first step in evaluating chemicals as bird repellents; see procedures described by Schafer and Brunton (1971) and Starr et al. (1964). Repellents that showed promise for bird repellency may have been tested further with additional species or field tests. Seed repellent testing can also identify repellents that may be effective for foliar use. Once identified for further testing, bioscientists and resources managers may conduct foliar repellent testing in captive settings followed by controlled field studies (e.g. enclosures within agricultural fields) or field studies in areas of known bird damage.
Foliar/fruit repellents are often used to manage bird damages to fruit or nut crops, as well as maturing crops and turf. For this review, we classified foliar/fruit repellents as those applied to crops post-planting. Some examples of commodities protected with foliar applications include fruit and tree nuts, turf, corn and other grains sprayed to prevent damage as crops ripen (pre-harvest; e.g. soybean damage by geese).
Water repellents are used to discourage birds from depredating fish hatcheries, utilising temporary pools of water at airports, and from using settling and tailing ponds containing oil or toxic chemicals (Belant et al. 1995). In addition, laboratory screening tests with repellents in solution have also been considered water-related repellents (Duncan 1963; Clark 1995). Bait repellents are used to protect non-target birds from pesticide-treated baits. Near 100% repellency is needed to prevent non-target bird mortality and to prevent egg depredation in threatened and endangered birds (Day et al. 2003). In contrast to other repellent application types, area repellents provide spatial repellency, or repellency to a given area (not feeding repellency) associated with a valued resource.
Considering the changing environment for tolerances or maximum residue levels in global trade and the growing moral concern surrounding animal testing (Goodman et al. 2015), there is a need for a comprehensive review of bird repellents tested through time and their efficacy with different species and crop types. A compendium to identify chemicals that have been tested and their published efficacy is needed for future use. Our objective was to review and synthesise all tests published in English, current US patents and current US registrations of chemical bird repellents for both bioscientists and resource managers.
Methods
We used Google Scholar and approximately 20 single search terms (e.g. repellent, avian, bird, chemical, damage) and Boolean combinations (e.g. ‘repellent’ AND bird, OR goose, OR blackbird) to search all scientific literature published through January 2022. We also utilised manual searches to identify relevant studies from reference lists, conferences, internet sites and popular articles. We searched all bird repellents tests published in English, current US patents and current US registrations of chemical bird repellents for the purpose of comprehensively reviewing chemical bird repellents used for crop and resource protection.
Each repellent experiment offered repellent-treated food/feed or water treated with at least one concentration, but sometimes a range of repellent concentrations. Most publications described one or more repellent experiment(s). We categorised each experiment according to the repellent application type (i.e. seed repellents, foliar/fruit repellents, water repellents, bait repellents, area repellents) and to the active principle of the main ingredient used (e.g. anthraquinone, methiocarb), identified by the Chemical Abstract Service (CAS) number, a short string of text that refers to a particular chemical substance (e.g. CAS 84-65-1, anthraquinone). We also recorded publication year, testing location (country, state), captive vs field evaluation, chemical concentration evaluated, bird species tested and food type used among all published tests, current patents and current registrations of chemical bird repellents. We summarised the food type and bird species used for each repellent test within each application considered. Each food type was categorised according to the US Department of Agriculture Economic Research Service Crop categories. These categories include corn (Zea mays) and other feed grains (e.g. sorghum (Sorghum bicolor), barley (Hordeum vulgare) and oats (Avena sativa)), fruit and tree nuts, rice (Oryza sativa), soybeans (Glycine max) and oil crops (e.g. sunflower (Helianthus spp.)), sugar and sweeteners, vegetables and pulses and wheat (Triticum aestivum). We categorised each bird species by bird family.
In addition to a systematic search of scientific literature, we conducted a patent search using single keywords (e.g. bird, avian, repellent) and PatSnap, a global patent platform. This search identified all patents, current and expired, that described a repellent used specifically for birds. We categorised each patent according to the application and identified the principal main ingredient used by CAS number. Many patents contained multiple active ingredients or unspecified combinations of active ingredients, making classification difficult.
Finally, we searched bird repellent products registered as of 2021 using the National Pesticide Information Retrieval System (NPIRS). The US EPA maintains this database, and US states and territories voluntarily provide their state registration data. This search identified all active federal registrations (e.g. registered repellent products). We categorised these by active ingredient and identified the registered uses (seed, foliar/fruit, water, bait, or area) and the bird species identified.
We then created an ‘index of success’, or relative efficacy level, for each repellent application type (i.e. seed repellents, foliar/fruit repellents, water repellents, bait repellents, area repellents). For the index of success, we defined a repellent ‘experiment’ as the testing of a single repellent. We based the index of success on three factors: (1) percentage repellency (i.e. [consumption of repellent-treated food ÷ consumption of untreated food] × 100); (2) reported R50 repellency index, or minimum concentration of a chemical repellent that causes birds (e.g. 3–5 of 5 tested birds) to consume ≤50% of repellent-treated food offered during no-choice experiment (Schafer and Brunton 1971; Bruggers et al. 1984); and (3) qualitative reports in the absence of calculated repellency (e.g. ‘damage was less,’ ‘harvest was greater in treated plots’). The index of success was classified into four relative efficacy levels: (1) effective in most experiments (≥75% repellency and/or R50 ≤ 0.1); (2) effective in some experiments (50–74% calculated repellency and/or R50 > 0.1 ≤ 0.2); (3) less effective in most experiments (25–49% calculated repellency and/or R50 > 0.2 ≤ 1.0); and (4) not effective in most experiments (<25% calculated repellency and/or R50 > 1.0).
Results
Systematic review
Our literature search identified 345 papers published in English in 1948–2022, including 2994 repellent experiments of 1478 repellent chemicals (Table 2). Repellency experiments consisted of 81% seed repellent, 9% foliar/fruit repellent, 8% water repellent, 1% bait repellent and 1% area repellent (Fig. 1). We discuss the specifics of each of these repellent categories separately.
| Publication variables | Seed | Foliar/fruit | Water | Bait | Area | Total | |
|---|---|---|---|---|---|---|---|
| # publications | 224 | 114 | 26 | 22 | 7 | 345 | |
| # experiments | 2428 | 256 | 235 | 60 | 15 | 2994 | |
| # countries | 23 | 20 | 3 | 4 | 2 | 36 | |
| Most frequent country (# publications) | USA (171) | USA (82) | USA (20) | USA (34) | USA (13) | USA (2564) | |
| # species | 60 | 32 | 12 | 21 | 4 | 93 | |
| Most frequent species (# experiments) | Agelaius phoeniceus (1538) | Agelaius phoeniceus (38) | Sturnus vulgaris (195) | Corvus ossifragus (9) | Sturnus vulgaris (6) | Agelaius phoeniceus (1585) | |
| # families | 24 | 17 | 10 | 12 | 3 | 31 | |
| Most frequent family (# experiments) | Icteridae (1759) | Icteridae (49) | Sturnidae (195) | Corvidae (20) | Sturnidae (6) | Icteridae (1759) | |
| Number of chemicals tested | 1384 | 42 | 138 | 11 | 6 | 1478 | |
| Most frequent substance (# experiments) | 9,10 Anthraquinone (184) | 3,5-dimethyl-4-methylthio phenol methylcarbamate (104) | Methyl anthranilate (23) | 9,10 anthraquinone (19) | Methyl anthranilate (9) | 3,5-dimethyl-4-methylthio phenol methylcarbamate (258) | |
| # crop types tested | 30 | 30 | na | na | na | 45 | |
| Most frequent crop type (# experiments) | Rice (532) | Grass (17) | na | na | na | Rice (368) | |
| # commodities tested | 11 | 9 | na | na | na | 12 | |
| Most frequent commodity (# experiments) | Rice (532) | Fruit and tree nuts (40) | na | na | na | Rice (368) | |
| % field studies | 10% | 64% | 2% | 30% | 73% | 14% |
Seed repellents
Seed repellent tests were discussed in 224 of the published papers. Of these, 76% were conducted in the USA, 5% in the United Kingdom (UK), 3% in Spain, 2% in each of India and Pakistan, 1% in each of Africa, Canada, Israel, South America and New Zealand. Seed-based repellents were mainly tested on corn and other feed grains (28%), rice (22%) and soybean and oil crops (11%). The predominant bird family tested was Icteridae (44%), consisting of red-winged blackbirds (Agelaius phoeniceus), grackles and brown-headed cowbirds (Molothrus ater). Bird families tested in 4–10% of published papers include Sturnidae (e.g. starlings), Passeridae (e.g. house sparrow (Passer domesticus)), Phasianidae (e.g. pheasants), Columbidae (e.g. pigeons) and Corvidae (e.g. crows).
Index of success – seed repellents
There were 2428 seed repellent experiments conducted with 1384 repellents. Of these, 1274 repellents were tested only one or two times, and 68% (N = 864) were classified as not effective in most experiments, 22% (N = 278) as less effective in most experiments, 7% (N = 84) as effective in some experiments and only 4% (N = 48) effective in most experiments (Supplementary Table S1, available in Supplementary Material). Based on only one or two tests, these results should be given less weight, because ineffectiveness with one or two species or at a single concentration is not comparable to repellents tested across many concentrations or with multiple species. The remaining 104 repellents were tested in 1044 experiments. Of the 100 repellents tested more than two times, 18% (N = 19) were classified as not effective in most experiments, 44% (N = 46) were classified as less effective in most experiments, 25% (N = 26) as effective in some experiments and 13% (N = 13) as effective in most experiments (Table 3).
| CAS number | Chemical name | Number of experiments | Index of success | |
|---|---|---|---|---|
| 84-65-1 | 9,10-anthraquinone | 184 | a | |
| 2032-65-7 | 3,5-dimethyl-4-methylthio phenol methylcarbamate (methiocarb) | 144 | a | |
| 137-26-8 | Tetramethyl thiuram disulfide | 59 | - | |
| 504-24-5/ 1124-33-0 | 4-aminopyridine/4-nitropyridine-N-oxide | 43 | a | |
| 138261-41-3 | Imidacloprid | 21 | a | |
| 89-82-7 | d-pulegone | 19 | a | |
| 58-08-2 | Caffeine | 12 | a | |
| 120-12-7 | Paranapthalene | 7 | a | |
| 66-81-9 | Cyclohexamide | 6 | a | |
| 137-30-4 | Zinc dimethyldithiocarbamate | 5 | a | |
| 35 A | Dinol sulfite | 4 | a | |
| 33240-95-8 | 3-chloro-p-toluidine hydrochloride | 4 | a | |
| 6119-70-6 | Quinine sulfate | 4 | a | |
| 134-20-3 | Methyl anthranilate | 64 | b | |
| 2686-99-9/ 12407-86-2 | Trimethacarb | 24 | b | |
| 621-79-4 | Cinnamamide | 17 | b | |
| 59398-71-3 | Dolomitic hydrated lime | 11 | b | |
| 1401-55-4 | Tannic acid/wattle tannin | 9 | b | |
| 2921-88-2 | Chlorpyrifos | 9 | b | |
| 64365-11-3/ 7440-44-0/ 8021-99-6 | Activated charcoal or animal charcoal | 9 | b | |
| 65-30-5 | Nicotine sulfate | 8 | b | |
| 7447-41-8 | Lithium chloride | 8 | b | |
| 37918-25-5 | 2-methyl-α,α-diphenyl-1-pyrrolidine butyramide | 7 | b | |
| 82-05-3 | Benzanthrone | 7 | b | |
| 10380-28-6 | Copper-8-quinilinolate | 6 | b | |
| 1302-78-9 | Bentonite | 5 | b | |
| 130-89-2 | Quinine hydrochloride | 5 | b | |
| 20427-59-2 | Copper hydroxide | 4 | b | |
| 66332-96-5 | N-[3-(propan-2-yloxy)phenyl]-2-(trifluoromethyl)benzamide | 4 | b | |
| 9 A | Zinc dimethyl dithiocarbamate cyclohexamine | 3 | b | |
| 18 A | Blue dye | 3 | b | |
| 330-64-3 | 3,5-diisopropylphenyl N-methylcarbamate | 3 | b | |
| 3696-28-4 | Omadine disulfide | 3 | b | |
| 541-35-5 | n-Butyramide | 3 | b | |
| 6012-92-6 | 3-(p-chlorophenyl)-5-methylrhodanine | 3 | b | |
| 84-11-7 | Phenanthraquinone | 3 | b | |
| 85-52-9 | 2-benzoylbenzoic acid | 3 | b | |
| 87-25-2 | Ethyl anthranilate | 3 | b | |
| 90-44-8 | 9,10-dihydro-9-oxoanthracene | 3 | b | |
| 85-91-6 | Dimethyl anthranilate | 29 | c | |
| 126-14-7 | Sucrose octaacetate | 10 | c | |
| 8006-64-2 | Turpentine | 10 | c | |
| 58-89-9 | Lindane | 9 | c | |
| 1332-40-7 | Copper oxychloride | 8 | c | |
| 81-64-1 | 1,4-dihyrdroxyanthraquinone | 8 | c | |
| 104-54-1 | Cinnamic alcohol | 6 | c | |
| 1074-36-8 | Mercaptobenzoic acid | 6 | c | |
| 14371-10-9/ 104-55-2 | Cinnamaldehyde | 6 | c | |
| 57-06-7 | Allyl isothiocyanate | 6 | c | |
| 7429-90-5 | Aluminum powder | 6 | c | |
| 8000-78-0 | Garlic oil | 6 | c | |
| 814-91-5 | Copper oxalate | 6 | c | |
| 82-22-4 | 1,1′ dianthrimide | 6 | c | |
| 94-62-2 | Piperine | 6 | c | |
| 117-80-6 | 2,3-dichloro-1,4-napthoquinone | 5 | c | |
| 1328-53-6 | Monastral Green Pigment | 5 | c | |
| 5234-68-4 | 5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide | 5 | c | |
| 10 A | di-brom benzanthrone | 4 | c | |
| 1135-24-6 | 3-methoxy, 4-hydroxycinnamic acid | 4 | c | |
| 116-06-3 | Aldicarb | 4 | c | |
| 131341-86-1 | 4-(2,2-difluoro-1,3-benzdioxol-4-yl)-1h-pyrrole-3-carbonitrile | 4 | c | |
| 133-06-2 | 3a, 4,7,7a-tetrahydrophthalimide | 4 | c | |
| 140-10-3 | Cinnamic acid and trans-cinnamic acid | 4 | c | |
| 16909-11-8 | 3,5 dimethoxy cinnamic acid | 4 | c | |
| 57520-17-9 | Guazitine triacetate | 4 | c | |
| 91465-08-6 | Lambda-cyhalothrin | 4 | c | |
| 2439-10-3 | 1-dodecylguanidine acetate | 3 | c | |
| 6099-04-3 | 3-methoxy cinnamic acid | 3 | c | |
| 109-08-0 | 2-methoxy-3-methylpyrazine | 3 | c | |
| 119446-68-3 | 1-[[2-[2-chloro-4-(4-chlorophenoxy)phenyl]-4]methyl-1,3-dioxolan-2-yl]methyl]-1,2,4-triazole | 3 | c | |
| 12427-38-2 | Manganese ethylene-1,2,-bisdithiocarbamate | 3 | c | |
| 1305-62-0 | Calcium hydroxide | 3 | c | |
| 133-18-6 | Phenyl ethyl anthranilate | 3 | c | |
| 1461-22-9 | Tributyl tin chloride | 3 | c | |
| 150-84-5 | Citronellyl acetate | 3 | c | |
| 35554-44-0 | 1-[2-(allyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole | 3 | c | |
| 551-93-9 | Ortho-aminoacetophenone | 3 | c | |
| 72-20-8 | 1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8-dimethano-napthalene | 3 | c | |
| 7393-66-0 | S-(10-Phenoxyarsinyl)phenoxythiolacetic acid | 3 | c | |
| 7704-34-9 | Sulfur | 3 | c | |
| 82-45-1 | 1-amino-9,10-anthracenedione | 3 | c | |
| 830-09-1 | 4-methoxycinnamic acid | 3 | c | |
| 85-01-8 | Phenanthrene | 3 | c | |
| 94-59-7 | 5-(2-propenyl)-1,3-benzodioxole | 3 | c | |
| 99-92-3 | Para-aminoacetophenone | 3 | c | |
| 471-34-1 | Calcium carbonate | 7 | d | |
| 120068-37-3 | Fipronil | 5 | d | |
| 8002-65-1 | Neem oil | 5 | d | |
| 102-25-0 | 1,3,5-triethylbenzene | 4 | d | |
| 331-39-5 | 3,4 dihydroxycinnamic acid | 4 | d | |
| 57-50-1 | Sucrose | 4 | d | |
| 90-50-6 | 3,4,5-Trimethoxycinnamic acid | 4 | d | |
| 1132-21-4 | 3,5 dimethoxybenzoic acid | 3 | d | |
| 118-75-2 | Tetra chloro-para-benzoquinone | 3 | d | |
| 131-09-9 | 2-chloroanthraquinone | 3 | d | |
| 13463-67-7 | Titanium dioxide | 3 | d | |
| 13851-11-1 | 1,3,3-Trimethylbicyclo[2.2.1]heptan-2-yl acetate | 3 | d | |
| 14808-60-7 | White quartz sand | 3 | d | |
| 327-97-9 | Chlorogenic acid | 3 | d | |
| 3734-33-6 | Denatonium benzoate | 3 | d | |
| 530-59-6 | 3,5 dimethoxy, 4 hydroxycinnamic acid | 3 | d | |
| 60-57-1 | 1R,4S,4aS,5R,6R,7S,8S,8aR)-1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4,5,8-dimethanonapthalene | 3 | d | |
| 65-85-0 | Benzoic acid | 3 | d | |
| 7784-25-0 | Aluminum ammonium sulfate | 3 | d |
The index of success for seed repellent chemicals tested in one or two experiments (N = 1274) is summarised in Table S1. The CAS number is a short string of text that refers to a particular chemical substance (e.g. CAS 84-65-1 for 9,10-anthraquinone). For each repellent chemical, ‘index of success’ includes: effective in most experiments (a); effective in some experiments (b); less effective in most experiments (c); and not effective in most experiments (d).
From 224 publications regarding seed repellents, chemicals that were effective in most experiments and tested in more than three experiments include aminopyridine, anthraquinone, caffeine, carbamates, cycloheximide, d-pulegone, dinol sulfite, imidacloprid, paranapthalene, quinine sulfate, thiuram and 3-chloro-p-toluidine hydrochloride (Table 3). These seed-repellent chemicals include fungicides (cycloheximide, thiram), insecticides (carbamates, imidacloprid), starlicide (3-chloro-p-toluidine hydrochloride), human pharmaceuticals (aminopyridine, quinine sulfate), petroleum distillate (paranapthalene), alkaloids (caffeine, quinine sulfate), monoterpenes (d-pulegone), and naturally occurring or synthetic polyphenolic compounds (anthraquinone).
There were 48 seed repellent chemicals that were effective in most experiments and tested in two or fewer experiments. These included chemicals such as alpha-aminoacetophenone, linayl anthranilate, monocrotophos, pennyroyal oil and strychnine (Table S1). Some of the 864 chemicals that were not effective in most experiments and tested in two or fewer experiments included ammonia, formic acid, furan, green dye, lead oxide, limonene, red dye and trifloxystrobin.
Foliar/fruit repellents
Tests of foliar/fruit repellents were discussed in 114 of the published papers. Of these, 68% were conducted in the USA, 3% in each of Canada, the UK, India, Israel and Uruguay, and 2% in each of Kenya, Mali, Senegal, Somalia, Sudan, Tanzania, Australia and the Philippines. Foliar/fruit repellents were mainly tested on fruit and tree nuts (28%), corn and other feed grains (19%), soybean and oil crops (15%), turf (12%) and rice (9%). The predominant bird family tested was Icteridae (22%), consisting of mixed flocks of red-winged blackbirds, grackles and brown-headed cowbirds. Birds in the Anatidae family were also tested frequently (18%). Bird families tested in 4–7% of published papers include Sturnidae (e.g. starlings), Passeridae (e.g. house sparrow), Turdidae (e.g. robins), Columbidae (e.g. pigeons) and Ploceidae (e.g. weavers).
Index of success – foliar/fruit repellents
There were 256 foliar/fruit repellent experiments conducted, with 42 repellents. Of the 42 repellents tested, 29% (N = 12) were classified as not effective in most experiments, 38% (N = 16) were classified as less effective in most experiments, 24% (N = 10) as effective in some experiments and 7% (N = 3) as effective in most experiments (Table 4).
| CAS number | Chemical name | Number of experiments | Index of success | |
|---|---|---|---|---|
| 2032-65-7 | 3,5-dimethyl-4-methylthio phenol methylcarbamate (methiocarb) | 104 | a | |
| 84-65-1 | 9,10-anthraquinone | 42 | a | |
| 64365-11-3/ 7440-44-0 | Activated charcoal | 2 | a | |
| 137-26-8 | Tetramethyl thiuram disulfide (Thiram) | 10 | b | |
| 504-24-5 | 4-aminopyridine | 7 | b | |
| 7784-25-0 | Aluminum ammonium sulfate (Curb) | 4 | b | |
| 1305-62-0 | Calcium hydroxide | 3 | b | |
| 58-08-2 | Caffeine | 2 | b | |
| 89-82-7 | (R)-5-Methyl-2-(1-methylethylidene) cyclohexanone (d-pulegone) | 1 | b | |
| 137-30-4 | Zinc dimethyldithiocarbamate (Ziram) | 2 | b | |
| 55285-14-8 | 2,3-dihydro methylcarbamate | 1 | b | |
| 2631-40-5 | 2-isopropyl methylcarbamate | 1 | b | |
| 8006-90-4 | Peppermint oil | 1 | b | |
| 134-20-3 | Methyl anthranilate | 35 | c | |
| 2686-99-9 | 3,4,5-trimethylphenyl-methylcarbamate (Trimethacarb) | 5 | c | |
| 85-91-6 | Dimethyl anthranilate | 3 | c | |
| 59398-71-3 | Hydrated lime | 3 | c | |
| 63-25-2 | 1-naphthyl methylcarbamate (Sevin) | 2 | c | |
| 1309-48-4 | Magnesium oxide | 2 | c | |
| 12136-45-7 | Potassium oxide | 2 | c | |
| 551-93-9 | Ortho-aminoacetophenone | 1 | c | |
| 621-79-4 | Cinnamamide | 1 | c | |
| 9 A | Zinc dimethyl dithiocarbamate cyclohexamine | 1 | c | |
| 1401-55-4 | Gallotannin | 1 | c | |
| 1328-53-6 | Monastral green pigment | 1 | c | |
| 2634-33-5 | 1,2-benzisothiazol-3-one | 1 | c | |
| 16 A | SiO2 (70%) and Al2O3 (13.5%) | 1 | c | |
| 32 A | N,N,-diethyl-tert-octyl sulfinamide | 1 | c | |
| 33 A | N,N,-di-n-butyl-tert-octyl sulfinamide | 1 | c | |
| 31 A | Proprietary micronutrient formulation (percent w/w 4.0 S, 1.5 Mg, 0.75 Mn, 3.5 Fe, 0.75 Zn, 0.006 Cu, 0.16 B and 0.003 Mo) | 3 | d | |
| 3734-33-6 | Denatonium benzoate | 1 | d | |
| 18 A | Blue food dye | 1 | d | |
| 471-34-1 | Calcium carbonate | 1 | d | |
| 999-81-5; 24307-147-6 | Chloride (10.7%), chlormequat | 1 | d | |
| 29883-15-6 | D-amygdalin hydrate | 1 | d | |
| 8000-78-0 | Garlic oil | 1 | d | |
| 2921-88-2 | O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Chlorpyrifos; Lorsban) | 1 | d | |
| 60207-90-1 | Propiconazole | 1 | d | |
| 51609-52-0 | Putrescent egg solids | 1 | d | |
| 120068-37-3 | Fipronil | 1 | d | |
| 7758-87-4 | Calcium phosphate | 1 | d | |
| 56-72-4 B | O-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl) O,O-diethyl thiophosphate | 1 | – |
The CAS number is a short string of text that refers to a particular chemical substance (e.g. CAS 2032-65-7 for methiocarb). For each repellent chemical, ‘index of success’ includes: effective in most experiments (a); effective in some experiments (b); less effective in most experiments (c); and not effective in most experiments (d).
From 114 publications regarding foliar/fruit repellents, chemicals that were effective in most experiments included activated charcoal, anthraquinone and methiocarb (Table 4). These foliar repellent chemicals include an insecticide (methiocarb) and a naturally occurring or synthetic polyphenolic compound (anthraquinone). Some of the 12 chemicals that were not effective in most experiments included blue food dye, denatonium benzoate, calcium carbonate, fipronil, garlic oil and propiconazole (Table 4).
Water repellents
Water repellents were discussed in 26 of the published papers. Of these, 80% were conducted in the USA, 15% in the UK and 0.5% in Australia. Water repellents were tested in captivity (using drinkers) and in the field on ponds or other bodies of standing water. The predominant bird family tested was Sturnidae (45%), followed by Laridae (e.g. gulls) and Anatidae (e.g. mallards).
Index of success – water repellents
There were 235 water repellent experiments conducted, with 138 repellents. Of the 138 repellents tested, 35% (N = 48) were classified as not effective in most experiments, 26% (N = 36) were classified as less effective in most experiments, 28% (N = 38) as effective in some experiments, and 11% (N = 16) as effective in most experiments (Table 5).
| CAS number | Chemical name | Number of experiments | Index of success | |
|---|---|---|---|---|
| 551-93-9 | Ortho-aminoacetophenone | 6 | a | |
| 100-52-7 | Benzaldehyde | 3 | a | |
| 7440-23-5 | Sodium chloride | 3 | a | |
| 135-02-4 | o-anisaldehyde | 2 | a | |
| 85-91-6 | Dimethyl anthranilate | 2 | a | |
| 89-82-7 | (R)-5-Methyl-2-(1-methylethylidene) cyclohexanone | 2 | a | |
| 100-6-1 | p-methoxyacetophenone | 1 | a | |
| 108-44-1 | m-Toluidine | 1 | a | |
| 119-65-3 | 2-azanaphthalene | 1 | a | |
| 143-33-9 | Sodium cyanide | 1 | a | |
| 14371-10-9 | Cinnamaldehyde | 1 | a | |
| 271-58-9 | 2,1-benzisoxazole | 1 | a | |
| 88-15-3 | 2-acetylthiophene | 1 | a | |
| 95-53-4 | o-toluidine | 1 | a | |
| 7447-40-7 | Potassium chloride | 1 | a | |
| 10043-52-4 | Calcium chloride | 1 | a | |
| 134-20-3 | Methyl anthranilate | 23 | b | |
| 4079-52-1 | 2-methoxyacetophenone | 3 | b | |
| 5763-61-1 | Veratryl amine | 3 | b | |
| 90-16-4 | 4-ketobenztriazine | 3 | b | |
| 130-89-2 | Quinine hydrochloride | 3 | b | |
| 7647-01-0 | Hydrochloric acid | 3 | b | |
| 606-45-1 | Methyl-2-methoyxbenzoate | 2 | b | |
| 87-25-2 | Ethyl anthranilate | 2 | b | |
| 104-54-1 | Cinnamic alcohol | 2 | b | |
| 118-93-4 | 2-hydroxyacetophenone | 2 | b | |
| 126-14-7 | Sucrose octaacetate | 2 | b | |
| 140-11-4 | Benzyl acetate | 2 | b | |
| 4101-30-8 | 2-amino-4,5-dimethoxyacetophenone | 2 | b | |
| 529-20-4 | o-tolualdehyde | 2 | b | |
| 60-12-8 | Phenethanol | 2 | b | |
| 7149-26-0 | Linalyl anthranilate | 2 | b | |
| 88-68-6 | Anthranilamide | 2 | b | |
| 120-72-9 | 2,3-benzopyrrole | 1 | b | |
| 7149-10-2 | N-acetyl vanillyl amine | 1 | b | |
| 100-06-1 | 4-methoxyacetophenone | 1 | b | |
| 101-41-7 | Methylphenyl acetate | 1 | b | |
| 106-49-0 | p-Toluidine | 1 | b | |
| 109-97-7 | Pyrrole | 1 | b | |
| 110-86-1 | Pyridine | 1 | b | |
| 121-69-7 | N,N-dimethyl aniline | 1 | b | |
| 137-26-8 | Tetramethyl thiuram disulfide | 1 | b | |
| 1401-55-4 | Tannic acid | 1 | b | |
| 150-84-5 | Citronyll acetate | 1 | b | |
| 24295-03-2 | 2-acetylthiazole | 1 | b | |
| 288-47-1 | Thiazole | 1 | b | |
| 3576-63-4 | Veratryl acetamide | 1 | b | |
| 36556-6-6 | 5,6,7,8-tetrahydroquinoline | 1 | b | |
| 5344-90-1 | 2-amino benzyl alcohol | 1 | b | |
| 621-82-9 | Ethylcinnamyl acetate | 1 | b | |
| 64-19-7 | Acetic acid | 1 | b | |
| 91-20-3 | Napthalene | 1 | b | |
| 98-86-2 | Acetophenone | 1 | b | |
| Synthesised | N-acetyl veratryl amine | 1 | b | |
| 84-65-1 | 9,10-anthraquinone | 3 | c | |
| 90-02-8 | 2-hydroxybenzaldehyde | 3 | c | |
| 99-92-3 | Para-aminoacetophenone | 3 | c | |
| 2032-65-7 | 3,5-dimethyl-4-methylthio phenol methylcarbamate (methiocarb) | 2 | c | |
| 104-53-0 | Hydrocinnamic aldehyde | 2 | c | |
| 122-78-1 | Phenyl acetylaldehyde | 2 | c | |
| 133-18-6 | Phenyl ethyl anthranilate | 2 | c | |
| 150-13-0 | 4-aminobenzoic acid | 2 | c | |
| 25628-84-6 | Propionyl methyl anthranilate | 2 | c | |
| 586-37-8 | 3-methoxyacetophenone | 2 | c | |
| 7779-77-1 | Isobutyl anthranilate | 2 | c | |
| 93-03-8 | Veratryl alcohol | 2 | c | |
| 97404-53-0 | Xanthoxylum piperitum | 2 | c | |
| 99-03-6 | Meta-aminoacetophenone | 2 | c | |
| 99-05-8 | 3-aminobenzoic acid | 2 | c | |
| 10043-67-1 | Aluminum potassium sulfate | 1 | c | |
| 101651-31-4 | Vanillyl acetamide | 1 | c | |
| 101-97-3 | Ethylphenyl acetate | 1 | c | |
| 102-06-7 | 1,3-Diphenylguanidine | 1 | c | |
| 103-45-7 | Phenethyl acetate | 1 | c | |
| 104-55-2 | Cinnamaldehyde | 1 | c | |
| 105-54-4 | Ethyl butyrate | 1 | c | |
| 110-85-0 | Piperazine | 1 | c | |
| 119-36-8 | Methyl salicylate | 1 | c | |
| 135-19-3 | 2-Naphthol | 1 | c | |
| 1758-62-9 | Pyrazine | 1 | c | |
| 41-68-9 | Benzothiole | 1 | c | |
| 4180-23-8 | Anethole | 1 | c | |
| 50-78-2 | Acetyl salicylic acid | 1 | c | |
| 53751-40-9 | Veratryl acetate | 1 | c | |
| 5392-40-5 | 3,7-dimethyl-2,6-octadienal | 1 | c | |
| 592-88-1 | Allyl sulfide | 1 | c | |
| 621-79-4 | Cinnamamide | 1 | c | |
| 73-22-3 | (S)-2-amino-3-(3-indolyl)propionic acid | 1 | c | |
| 7784-25-0 | Aluminum ammonium sulfate | 1 | c | |
| 8002-65-1 | Neem oil | 1 | c | |
| 57-50-1 | Sucrose | 5 | d | |
| 404-86-4 | Capsaicin (synthetic) | 4 | d | |
| 118-92-3 | Anthranilic acid | 3 | d | |
| 50-99-7 | D-glucose | 3 | d | |
| 100-09-4 | p-anisic acid | 2 | d | |
| 118-48-9 | Isatoic anhyride | 2 | d | |
| 119-61-9 | Diphenyl ketone | 2 | d | |
| 121-71-1 | 3-hydroxyacetophenone | 2 | d | |
| 55-21-0 | Benzamide | 2 | d | |
| 579-75-9 | 2-methoxybenzoic acid | 2 | d | |
| 586-38-9 | m-anisic acid | 2 | d | |
| 616-79-5 | 5-nitro anthranilic acid | 2 | d | |
| 65505-24-0 | Isobutyl methyl anthranilate | 2 | d | |
| 65-85-0 | Benzoic acid | 2 | d | |
| 68480-21-7 | Isobutyl-N,N-dimethyl anthranilate | 2 | d | |
| 69-72-7 | 2-hydroxybenzoic acid | 2 | d | |
| 90147-57-2 | Yucca schidigera root | 2 | d | |
| 34 A | Yucca extract + Xanthoxylum fruit extract | 1 | d | |
| 107-95-9 | B-alanine | 1 | d | |
| 121-98-2 | Methyl-4-methoxybenzoate | 1 | d | |
| 123-77-3 | Azodicarbonamide | 1 | d | |
| 1754-62-7 | Methyl trans-cinnamate | 1 | d | |
| 22839-61-8 | Aspartame | 1 | d | |
| 3196-73-4 | B-alanine, methyl ester | 1 | d | |
| 4602-84-0 | Farnesol | 1 | d | |
| 532-32-1 | Sodium benzoate | 1 | d | |
| 56-40-6 | Aminoacetic acid | 1 | d | |
| 56-41-7 | Aminopropanoic acid | 1 | d | |
| 5653-40-7 | 2-amino-4,5-dimethoxybenzoic acid | 1 | d | |
| 56-84-8 | L-2-Aminobutanedioic acid | 1 | d | |
| 56-85-9 | 2,5-diamino-5-oxopentanoic acid | 1 | d | |
| 56-86-0 | 4-amino-5-hydroxypentanamide | 1 | d | |
| 57683-71-3 | o-carboethyoxybenzene sulfonamide | 1 | d | |
| 59398-71-3 | Ca(OH)2MgO | 1 | d | |
| 60-18-4 | L-tyrosine | 1 | d | |
| 635-46-1 | 1,2,3,4-tetrahydroisoquinoline | 1 | d | |
| 63-68-3 | (S)-2-Amino-4-(methylthio)butyric acid | 1 | d | |
| 63-91-2 | L-phenylalanine | 1 | d | |
| 64-17-5 | Ethyl alcohol | 1 | d | |
| 70-47-3 | L-S-aminosuccinamic acid | 1 | d | |
| 71-00-1 | L-histidine | 1 | d | |
| 74-79-3 | (S)-2-amino-5-guanidinopentanoic acid | 1 | d | |
| 7784-26-1 | Aluminum ammonium sulfate dodecahydrate | 1 | d | |
| 81-07-2 | Sacharin | 1 | d | |
| 82385-42-0 | Sodium sacharin | 1 | d | |
| 93-58-3 | Methyl benzoate | 1 | d | |
| 99-93-4 | p-hydroxyacetophenone | 1 | d | |
| Synthesised | Veratryl nonanoate | 1 | d |
The CAS number is a short string of text that refers to a particular chemical substance (e.g. CAS 551-93-9 for ortho-aminoacetophenone). For each repellent chemical, ‘index of success’ includes: effective in most experiments (a); effective in some experiments (b); less effective in most experiments (c); and not effective in most experiments (d).
From 26 publications regarding water repellents, chemicals that were effective in most experiments and tested in three or more experiments include ortho-aminoacetophenone, benzaldehyde and sodium chloride (Table 5). These water repellent chemicals included an aromatic ketone, an aromatic aldehyde and an ionic compound, respectively. Chemicals that were not effective in most experiments and tested in three or more experiments included anthranilic acid, capsaicin (synthetic), D-glucose and sucrose (Table 5).
Bait repellents
Bait repellents were used in bait-safening operations (to protect non-targets) or other resource protection needs, including protecting the eggs of endangered bird species. Repellents used in baiting applications were discussed in 22 of the published papers. Of these, 57% were conducted in the USA and 40% were conducted in New Zealand. The predominant bird family tested was Corvidae (32%), followed by Nestoridae (e.g. New Zealand endemic Kea; 16%), Petroicidae (e.g. Australasian robin species; 10%), Psittaculidae (e.g. new world parrots; 10%) and Odontophoridae (e.g. pheasants; 6%). There were 60 repellent experiments conducted for bait applications, with 11 repellents.
Index of success – bait repellents
Of the 11 repellents tested, 18% (N = 2) were classified as not effective in most experiments, 45% (N = 5) were classified as effective in some experiments and 27% (N = 3) as effective in most experiments. One repellent had no efficacy data provided (Table 6).
| CAS number | Chemical name | Number of experiments | Index of success | |
|---|---|---|---|---|
| 84-65-1 | 9,10 anthraquinone | 19 | a | |
| 51-83-2 | 2-carbamoyloxyethyl(trimethyl)azanium chloride | 7 | a | |
| 134-20-3 | Methyl anthranilate | 4 | a | |
| 2032-65-7 | 3,5-dimethyl-4-methylthio phenol methylcarbamate (methiocarb) | 8 | b | |
| 89-82-7 | (R)-5-Methyl-2-(1-methylethylidene) cyclohexanone | 5 | b | |
| 12407-86-2 | 3,4,5-and 2,3,5-trimethylphenyl methylcarbamate | 4 | b | |
| 84-65-1/89-82-7 | 9,10 anthraquinone + d-pulegone | 1 | b | |
| 16423-68-0 | Erythrosine | 1 | b | |
| 8007-80-5 | Cinnamon oil | 9 | d | |
| 2437-29-8 | Special Green V200A dye | 1 | d | |
| 81-64-1 A | 1,4-dihyrdroxyanthraquinone | 1 | – |
The CAS number is a short string of text that refers to a particular chemical substance (e.g. CAS 84-65-1 for 9,10-anthraquinone). For each repellent chemical, ‘index of success’ includes: effective in most experiments (a); effective in some experiments (b); less effective in most experiments (c); and not effective in most experiments (d).
From 22 publications regarding bait repellents, chemicals that were effective in most experiments include 2-carbamoyloxyethyl(trimethyl)azanium chloride, anthraquinone and methyl anthranilate (Table 6). These bait repellent chemicals included a cholinergic agonist, a naturally occurring or synthetic polyphenolic compound, and a naturally occurring or synthetic irritant, respectively. Chemicals that were not effective in most experiments and tested in three or more experiments included anthranilic acid, capsaicin (synthetic), D-glucose and sucrose (Table 6).
Area repellents
Area repellents were discussed in seven of the published papers. Of these, 86% were conducted in the USA, and 14% were conducted in the UK The predominant bird family tested was Sturnidae (57%) and Icteridae (29%).
Index of success – area repellents
There were 15 repellent experiments conducted with six area repellents. Of the six repellents tested, 50% (N = 2) were classified as not effective in most experiments, 25% (N = 1) were classified as effective in some experiments and 25% (N = 1) as effective in most experiments. Two repellents had no efficacy data provided (Table 7).
| CAS number | Chemical name | Number of experiments | Index of success | |
|---|---|---|---|---|
| 134-20-3 | Methyl anthranilate | 9 | a | |
| 3734-33-6 | Denatonium benzoate | 1 | b | |
| 7704-34-9 | Sulfur | 2 | d | |
| 91-20-3 | Napthalene | 1 | d | |
| 105-54-4 A | Ethyl butyrate | 1 | – | |
| 5989-54-8 A | s-limonene | 1 | – |
The CAS number is a short string of text that refers to a particular chemical substance (e.g. CAS 134-20-3 for methyl anthranilate). For each repellent chemical, ‘index of success’ includes: effective in most experiments (a); effective in some experiments (b); less effective in most experiments (c); and not effective in most experiments (d).
From seven publications regarding area repellents, methyl anthranilate was effective in most experiments, denatonium benzoate was effective in some experiments and sulfur and naphthalene were not effective in most experiments. Ethyl butyrate and s-limonene were tested as area repellents, but no results were reported.
US patents
There have been 181 bird repellent patents worldwide, belonging to 73 simple patent families (i.e. same priority date or combination of priority dates), since 1944. Of these, 26% (N = 19) are currently active, and the remainder have expired due to time limits, non-payment or otherwise withdrawn. Of the 73 simple patent families, 49% (N = 36) were seed repellents, 33% (N = 24) were area repellents, 14% (N = 10) were foliar/fruit repellents, 3% (N = 2) were bait repellents and 1% (N = 1) were water repellents.
The 73 simple patent families identified chemicals and chemical combinations of 213 patented bird repellent chemicals. One-quarter of the 213 chemicals that are patented as bird repellents are represented by six chemicals, including methyl anthranilate (N = 14), anthraquinone (N = 10), dimethyl anthranilate (N = 8), ortho-aminoacetophenone (N = 7), cinnamamide (N = 7) and methyl phenyl acetate (N = 6). Of these 213 bird repellent chemicals, 47% (N = 100) were tested in a published research paper. The species of bird was not often specified in the patent, with most patents referencing ‘birds’ in general. Birds mentioned in these patents included woodpeckers, waterfowl, starlings, magpies, gulls and cockatoos.
US registered repellents
At the time of publication, there were 17 registered bird repellent products for five active ingredients (see Graphical Abstract). This manuscript will not discuss an additional registered product for one active ingredient for a bird toxicant. The 17 registered products have applications for foliar/fruit repellents (13 products; three active ingredients: anthraquinone, methiocarb, methyl anthranilate), area repellents (13 products; one active ingredient: methyl anthranilate), water repellents (five products; two active ingredients: capsaicin, methyl anthranilate), seed repellents (three products; three active ingredients: anthraquinone, methiocarb, methyl anthranilate) and bait repellents (two products; two active ingredients: anthraquinone, methyl anthranilate). Specific groups of birds associated with these registered repellents included blackbird species, gulls, geese, pigeons and sparrows.
Discussion
Seed repellents
Anthraquinone (CAS 84-65-1) is the most tested repellent for seed applications. Repellent seed tests for anthraquinone protection of seeds from birds have been published from the 1940s to the present. With few exceptions, seed testing with a variety of grains (e.g. corn, rice, millet, oat, sunflower) and many species in the families Icteridae, Corvidae and Anatidae, and the order Galliformes (e.g. turkey, pheasant, quail), have displayed excellent repellency (DeLiberto and Werner 2016). However, the anthraquinone concentrations needed to provide repellent efficacy differ significantly among the species tested. Concentration-response testing with horned larks (Eremophila alpestris) offered anthraquinone-treated wheat seeds demonstrated that 0.3% anthraquinone provided 100% feeding repellency. However, lark repellency was not related to actual anthraquinone concentration (Werner et al. 2015). In contrast, ring-necked pheasants (Phasianus colchicus) offered anthraquinone-treated corn exhibited 82% repellency for corn treated with 0.9% anthraquinone (Werner et al. 2009). Another observation from seed testing with anthraquinone is that seed-handling time affects exposure to the repellent. The residue of seed hulls decreases as seed-handling time increases (Avery et al. 1997). For example, red-winged blackbirds exhibited 72% repellency for rough rice treated with 0.25% anthraquinone but 79% repellency for brown rice treated with 0.15% anthraquinone (unpubl. data; United States Department of Agriculture, National Wildlife Research Center). Researchers have attempted to exploit this by adding inert binders (e.g. starches, clays) to planted seeds to increase handling time (Daneke and Decker 1988). Additionally, experiments have indicated that the formulation of the test diet (i.e. contained within the pellet vs topical or surface treatments) affects the efficacy of anthraquinone-based repellents. Anthraquinone (6275 ppm) was an effective repellent for European starlings (Sturnus vulgaris) on pellets, achieving 80% repellency, whereas up to 35 000 ppm of anthraquinone was ineffective when the anthraquinone was not topically applied (Tupper et al. 2014).
Methiocarb (CAS 2032-65-7) ranked second in the number of seed repellent tests conducted. Like anthraquinone, seed testing of methiocarb has occurred with various grain species (corn, rice, sorghum, sunflower) and many species of birds. Several tests with methiocarb-treated seeds were conducted with international bird species in the development of repellents to help reduce bird depredation of grain crops in Africa, India and Southeast Asia (Bruggers 1979; Hamsa et al. 1982; Bruggers et al. 1984; Sultana et al. 1986; Sandhu et al. 1987). Although most published repellent tests showed excellent repellency (Guarino 1972; Bruggers 1979), the registrant voluntarily withdrew registration for all food uses in the USA between 1989 and 1992. Methiocarb registrations have continued in other countries (e.g. Australia), but in 2019, the European Commission proposed non-renewal of all approvals due to the potential toxicity of methiocarb.
Nine additional chemicals tested with seeds reliably showed effective bird repellency, including thiram (CAS 137-26-8), caffeine (CAS 58-08-2) and 4-aminopyridine (CAS 504-24-5). Thiram was identified as a potential bird repellent in the 1950s and has been tested extensively. An early indicator of success for thiram was direct seeding evaluations of pine seeds. Birds exposed to thiram demonstrated repellency when used as a fungicide (Mann et al. 1956; Abbott 1958; Royall and Ferguson 1962). These tests led to more rigorous captive testing with various bird species (Neff and Meanley 1957; Schafer et al. 1977, 1983). Captive tests with thiram were generally effective, although repellency was always higher when thiram was offered in a choice test with an untreated test diet or other chemical repellent treatments (Neff and Meanley 1957; Lopez-Antia et al. 2014). Differences in efficacy between choice and no-choice testing indicate that thiram is unpalatable to birds but may not successfully suppress the intake of treated material under the most challenging conditions (Clark 1995). This is observed in the results of field testing conducted with thiram. Often, treatment levels successful in captive testing were not successful in field testing, although with increased thiram concentrations, repellency was achieved (Mann et al. 1956; Kennedy and Connery 2008).
Evaluation of caffeine (CAS 58-08-2) as a seed repellent identified it as a non-toxic (LD50 316 mg/kg) repellent (R50 0.18–0.43%) in small-scale screening trials with red-winged blackbirds (Schafer et al. 1983). This led to further captive feeding trials to evaluate caffeine at 0.1, 0.15 and 0.25% seed treatment levels. Repellencies of 76% and 72% were observed with male red-winged blackbirds and brown-headed cowbirds, respectively (Avery and Cummings 2003; Avery et al. 2005). Simulated field testing (conducted with captive birds in a flight pen) and field tests in Louisiana demonstrated additional positive results: 92% efficacy at 0.2% seed treatment levels in captivity and 90% repellency at 0.75% seed treatment levels in the field (Avery et al. 2005). Formulation improvements were needed to alleviate solubility and phytotoxicity issues, resulting in the addition of sodium benzoate to caffeine seed treatments. In water, sodium benzoate shows little repellency to European starlings (Clark 1995). Concentration-response testing with the new formulation was highly repellent to red-winged blackbirds and alleviated the decreased germination issues (Werner et al. 2007). However, caffeine was never registered as a bird repellent in the USA. Publication, or public disclosure of, caffeine efficacy data and the optimised repellent formulation precluded the commercial development of caffeine as a chemical bird repellent (pers. comm., S.J. Werner). This is a prime example of how protection (or lack of protection) of intellectual property can influence subsequent commercial development, availability and use of wildlife management methods.
An innovative seed repellent that relies on bait acceptance of seed and is used to protect ripening crops as a roost dispersal or area dispersal (e.g. buildings, feedlots) is 4-aminopyridine (CAS 504-24-5). Initial testing with a related pyridine chemical, 4-nitropyridine-N oxide (CAS 1124-33-0), illustrated the unique response of birds after consuming treated baits. Shortly after consuming baits treated with 4-aminopyridine and related compounds, birds cannot fly and emit distress sounds that alert their flock mates to danger, causing them to disperse (Goodhue and Baumgartner 1965). Thorough testing of 4-aminopyridine in a number of field situations has shown birds are reliably dispersed from fields of ripening corn, sorghum, grapes and peanuts (De Grazio et al. 1971; Mott et al. 1972; Besser 1978; Gadd 1992), as well as from feedlots and structures (Goodhue and Baumgartner 1965). Despite the successes of 4-aminopyridine, it has not proven effective in all situations. Some lessons learned from extensive field testing indicate that bait acceptance and treatment level can affect the efficacy of 4-aminopyridine (e.g. time to distress for affected birds resulting in lack of bird dispersal; Kelly and Dolbeer 1984). Additionally, field testing of baiting at the edge of fields or utilising elevated feeding platforms increased the visibility of reacting birds to the rest of the flock (Besser 1978; Gadd 1992). The timing of field treatment with 4-aminopyridine is critical for efficacy; treating fields after the damage has begun results in decreased repellent efficacy (Woronecki et al. 1979).
The remaining chemicals identified as effective repellents included: imidacloprid (CAS 138261-41-3), paranapthalene or anthracene (CAS 120-12-7), cyclohexamide (66-81-9), ziram (CAS 137-30-4) and d-pulegone (CAS 89-82-7). These compounds demonstrated promising efficacy in captive bird experiments but had minimal or no field testing. Interestingly, anthracene had good results in cage testing with red-winged blackbirds. However, the calculated R50 for anthracene and red-winged blackbirds was greater than 1.0% (Schafer et al. 1983). This illustrates the potential unreliability of R50 data by itself. Similarly, ziram has a calculated R50 of 0.65%, but excellent repellency in captive cage tests (repellency >76%; Frank and Dischner 1970; Cummings et al. 1994). Ziram is registered as a bird repellent in the European Union, UK and Australia as a seed repellent to protect corn from rooks (Corvus fugilegus) and crows (Corvus spp.). None of the other chemicals have been registered as bird repellents, although d-pulegone does appear in patents for bait safening and as an area repellent (US20170367327A1, US20050186237A1). Another 45 compounds tested only one to two times, mainly having R50 values with red-winged blackbirds, had excellent repellency (R50 values <0.1%). However, 30% (N = 14) of these chemicals are phytotoxic to at least one plant species (Schafer and Bowles 2004). Other considerations for chemical repellents that may preclude them from further testing as bird repellents include toxicity to mammals, humans and secondary toxicity to predators.
In total, 26 (25%) of the repellents tested as seed repellents in three or more experiments were categorised as effective in some experiments. These included compounds: methyl anthranilate (CAS 134-20-3); dimethyl anthranilate (CAS 85-91-6); cinnamamide (CAS 621-79-4); trimethacarb (CAS 2686-99-9/12407-86-2); and dolomitic hydrated lime (CAS 59398-71-3). There are many reasons for repellents to fall into this category. The concentration of the chemical exposure, the availability of alternative food and the bird’s level of hunger interact to determine the degree of irritation it will tolerate to continue feeding on treated food (Werner and Avery 2017). In addition, repellency and sensitivity vary widely among species. These characteristics can all be found in the testing of these ‘less effective’ repellents.
Methyl anthranilate has been tested extensively with a range of species. The testing shows some of these differences among species. Red-winged blackbirds are repelled by 2.5% methyl anthranilate treated rice (Avery et al. 1995), but 1.0% methyl anthranilate-treated food was eaten at the same rate or at an increased rate (Avery et al. 1988). European starlings, however, decreased consumption of 0.5% methyl anthranilate treated food for up to 9 days (Mason et al. 1991). These and other results led researchers to conclude that red-winged blackbirds are not as sensitive to methyl anthranilate as European starlings (Avery et al. 1988; Mason et al. 1991). Similar results have been observed in testing with Canada geese (Branta canadensis) and mallards (Anas platyrhynchos;Cummings et al. 1992).
Formulation of the repellent itself can lead to repellent efficacy problems. Published testing of lime as a bird repellent had varying results (Belant et al. 1997; Cummings et al. 1998). It was found that a contributing factor in repellency was the varying particulate sizes and pH of lime from different sources (Clark and Belant 1998). Cinnamamide has been tested with a few species of birds and has repellency ranging from 50 to 70% for most species evaluated (Crocker and Reid 1993; Watkins et al. 1995, 1999). However, cinnamamide has not been registered as a bird repellent, although mentioned in eight patents. The lack of registrations for cinnamamide could be attributed to the volatility of cinnamon oil, with 40% of the applied chemical being lost within 8 weeks of application (Cowan and Crowell 2017).
Another 1338 chemicals have been evaluated as seed repellents with bird species. Ninety percent of these chemicals have been classified as less effective or not effective based on our criteria. Nevertheless, this information is helpful for future testing and development of bird repellents to protect seeds and pre-emergent seedlings. Many of the repellents already described were first evaluated in broad screening evaluations. Having these data in a single source may help eliminate the need for initial testing of many of these repellent compounds in the future (Tables S2a and S2b).
Foliar/fruit repellents
Considerably fewer tests have been published describing the testing of foliar/fruit repellents compared with seed repellents. Seventy-five percent of this review’s foliar/fruit repellents were also tested as seed repellents.
Methiocarb and anthraquinone were the compounds with the most foliar/fruit repellent tests identified (57%, N = 146). Both compounds are considered effective in most experiments in foliar applications. Although considered effective in most experiments, foliar repellents have many challenges compared with seed repellents. Identified problems may include residue levels post-application and at the time of crop harvest and application methods for specific foliar applications. One example of complicated application methods is repellents to protect ripening corn. Methiocarb failed to protect ripening field corn from starling damage in Ontario, Canada. Authors speculated that weather and application timing might have contributed to the ineffectiveness (Joyner et al. 1980). The lack of field efficacy among foliar repellents can also be attributed to insufficient concentrations of the repellent on the protected surface. For example, sunflower repellent applications coincide with growth patterns and floral components of sunflower that limit repellent residues on achenes and, consequently, contact with foraging birds (Kaiser et al. 2021).
Many foliar repellent tests (N = 41) have been conducted to evaluate repellents for protecting fruit crops (e.g. cherry, grape, blueberry). A majority (59%) of these tests were conducted with the repellent methiocarb. The repellent methiocarb marketed as Mesurol® (Mobay Chemical Corporation, Pittsburgh, Pennsylvania) was registered as a bird repellent for cherries in 1978 and blueberries in 1983 (Dolbeer and Ickes 1994). However, despite the effectiveness of methiocarb as a bird repellent, registrations were voluntarily pulled in 1988 (blueberries) and 1989 (cherries) by the registrant to avoid additional costs associated with EPA data requirements (Dolbeer and Ickes 1994). Generally recognised as an effective repellent for fruit applications, methiocarb still presented some challenges. Fruits (i.e. figs) that have a tough outer skin that the birds do not consume had less success with methiocarb treatments. Birds could peck and remove the skins with little contact with the repellent (Crabb 1979). In later testing, methiocarb residue levels were also a problem, with residue levels too high at harvest or too low to effectively protect the crop (Guarino et al. 1974; Avery et al. 1993).
Other repellents evaluated as foliar repellents for fruit include methyl anthranilate, ortho-aminoaceteophenone (CAS 551-93-9) and d-pulegone (CAS 89-82-7). Methyl anthranilate testing for fruit crops generally showed little efficacy as a bird repellent. Several tests conducted with blueberries, cherries and grapes demonstrated no difference in damage among treated or control plots (Curtis et al. 1994; Cummings et al. 1995). Early formulations of methyl anthranilate evaluated in the field also caused discolouration of leaves and, in some cases, fruit (Curtis et al. 1994). Despite alterations to the formulation to reduce these effects, the use of methyl anthranilate as a foliar bird repellent has been limited. Ortho-aminoacetophenone and d-pulegone had intermediate success as bird repellents in captive tests with apple quarters (observed repellency of 39% and 59% for ortho-aminoacetophenone and d-pulegone, respectively) (Wager-Page and Mason 1996a, 1996b). No further testing with either of these compounds for foliar/fruit use was discovered, perhaps because of the strong odours associated with these compounds.
A recent review of anthraquinone applications for pest management (DeLiberto and Werner 2016) provides an in-depth discussion of the various foliar/fruit bird repellent uses and testing. Recent testing in the USA indicated efficacy in protecting soybeans in foliar applications with Canada geese (Werner et al. 2019). According to the NPIRS database, there are three registered products with anthraquinone as the active ingredient. These include a seed treatment for the protection of recently planted rice and corn seed (AV-1011; Arkion® Life Sciences LLC, New Castle, Delaware) and a foliar treatment for the protection of grass and other outdoor spaces from geese (Flight Control, Arkion). Anthraquinone is one of the most patented active ingredients identified in our search. There are nine simple patent families identifying anthraquinone as a bird repellent (Table S3). In addition, four simple patent families discuss a combination of anthraquinone with d-pulegone (bait safening, New Zealand). Five more simple patent families discuss the use of polycyclic quinones in general.
Water repellents
Fourteen of the repellents tested with water were classified as effective in most experiments. Of these, six were only tested in water and two were classified as ineffective due to R50 results of >1.0% (Schafer et al. 1983). Only d-pulegone (CAS 89-92-7) was classified as effective in most experiments in both water and seed applications. Five chemicals that demonstrated promising efficacy in water repellent testing had limited efficacy as seed repellents. These included cinnamaldehyde (CAS 14371-10-9), dimethyl anthranilate (CAS 85-91-6) and ortho-aminoacetophenone (CAS 55-93-9). Ortho-aminoacetophenone testing as a seed repellent demonstrated repellency at all treatment levels but without a dose-dependent concentration response (Clark et al. 1991). Anthraquinone has been shown to have repellent properties in seed and foliar testing with many species and feed types. Still, water repellent tests showed only moderate efficacy, primarily due to its insolubility in water (Duncan 1963; Clark 1995). Belant et al. (1995) testing showed that lower levels of methyl anthranilate were needed to repel birds from water than from food. These results illustrate the importance of testing repellents in the intended application.
As discussed previously, methyl anthranilate has been tested as both seed and foliar/fruit repellents. Methyl anthranilate is also the repellent most tested as a water repellent. In addition to captive trials, methyl anthranilate has been field tested and/or small-scale captive tested to prevent bird access to a body of water (i.e. pool, pond, puddle; Avery et al. 1992; Dolbeer et al. 1993; Belant et al. 1995) and for the protection of catfish ponds (Dorr et al. 1998). Although methyl anthranilate successfully reduced bill contacts by mallards and ring-billed gulls (Dolbeer et al. 1991), area applications of methyl anthranilate were not effective in limiting catfish predation by herons (Dorr et al. 1998). Current label restrictions limit the use of methyl anthranilate to non-fish-bearing water, such as temporary pools or mine tailing ponds.
Bait repellents
Extensive testing of repellents for pesticide baits has been conducted in New Zealand for the protection of local endangered birds, including Kea (Nestor notabilis) and North Island Robins (Petroica australis longipes), during 1080 bait applications for the control of brushtail possums (Trichosurus vulpecula). Repellents selected for use on pesticide baits must not prevent acceptance by the target species. A repellent acceptable to the target species but repellent to non-target species will need to be selected. For example, repellent testing to protect non-target birds from zinc phosphide rodenticide applications successfully prevented zinc phosphide toxicosis among Canada geese, horned larks and ring-necked pheasants (Werner et al. 2011).
Early repellent testing for pesticide baits included primary repellents such as methyl anthranilate (Mason et al. 1993) and cinnamon oil (Spurr 1993). Methyl anthranilate (1.0% concentration) successfully prevented brown-headed cowbird consumption of treated pesticide granules (Mason et al. 1993). Trials with 0.1% cinnamon oil did not eliminate the consumption of treated 1080 baits by rare captive birds in New Zealand. An initial delay in accepting baits treated with cinnamon oil was observed, but the effect was quickly extinguished (Spurr 1993). Another primary repellent successfully evaluated for deterring ingestion of pesticide baits is d-pulegone, which deterred northern bobwhite (Colinus virginianus) consumption of granular pesticides (Mastrota and Mench 1995). The mode of action of methyl anthranilate, cinnamon oil and d-pulegone requires birds to sample the repellent before avoidance is achieved. Depending on the toxicity of the pesticide to the non-target species, small amounts of sampling may not be lethal and these types of repellents may be appropriate.
Anthraquinone, a chemical tested with success as a seed and foliar/fruit repellent, has also been tested in New Zealand as a repellent for 1080 pesticide baits. The anthraquinone concentrations tested have been between 0.045% and 2.7% and have had varying degrees of success (Day et al. 2003; Orr-Walker et al. 2012; Clapperton et al. 2014; Nichols et al. 2020). Anthraquinone trials have all included the addition of colour (i.e. blue or green dyes) and a taste repellent (e.g. d-pulegone, cinnamon oil). D-pulegone was proven effective in bait trials but may be cost-prohibitive as part of a large-scale eradication effort (Clapperton et al. 2014). In areas where target species are low, efforts have been made to condition aversion to the treated baits with higher levels of anthraquinone with some success (Nichols et al. 2020). However, these anthraquinone levels would also likely repel target species and could not be used in regular bait operations.
Synthesis of systematic review
We found 345 papers regarding chemical bird repellents that were published in 1948–2022, including 2994 tests of 1478 repellent chemicals. Most of these publications were associated with bird-repellent seed treatments (65%, N = 224; e.g. rice) and foliar/fruit repellent applications (33%, N = 114; e.g. grass, fruit and tree nuts). Of the 17 bird repellents that are currently registered in the USA, two registered repellents are naturally occurring or synthetic irritants (capsaicin and methyl anthranilate). Other chemical classes for these registered bird repellents include a naturally occurring or synthetic polyphenolic compound (anthraquinone), an insecticide (methiocarb) and an organic polymer (polybutene).
Conclusions
From 224 publications regarding seed repellents, chemicals that were effective in most experiments and tested in three or more experiments include fungicides (cycloheximide, thiuram), insecticides (carbamates, imidacloprid), starlicide (3-chloro-p-toluidine hydrochloride), human pharmaceuticals (aminopyridine, quinine sulfate), petroleum distillate (paranapthalene), alkaloids (caffeine, quinine sulfate), monoterpenes (d-pulegone) and naturally occurring or synthetic polyphenolic compounds (anthraquinone). Among 114 publications regarding repellents used for foliar/fruit applications, chemicals that were effective in most experiments include activated charcoal, anthraquinone and carbamate. Among other bird repellents that were reportedly effective in most experiments, chemicals used for water applications and tested in three or more experiments include benzaldehyde, ortho-aminoacetophenone and sodium chloride; chemicals used as bait repellents include anthraquinone, methyl anthranilate and 2-carbamoyloxyethyl(trimethyl)azanium chloride; and the single chemical regarded as an area repellent was methyl anthranilate. There are currently 17 registered bird repellent products in the USA for five active ingredients, including anthraquinone, capsaicin, methiocarb, methyl anthranilate and polybutene.
This systematic and comprehensive review illustrates the amazing quantity and quality of wildlife research regarding bird repellents and repellent applications published in 1948–2022. We have shown how these research studies (both laboratory and field efficacy tests) have contributed to registered products (e.g. pre-plant seed treatments, foliar/fruit repellents) and patented bird repellents. The continued protection of intellectual property (i.e. patented inventions) will safeguard the commercial development, availability and use of future wildlife management methods, including chemical bird repellents. Future research and development of chemical bird repellents should include biopesticides (i.e. pesticides derived from natural materials) and pesticides that are already registered for human food use. The future discovery of repellent active ingredients and repellent products can be facilitated by an understanding of the scientific literature, patents and product registrations regarding bird repellent applications summarised in this review.
Data availability
Public access to all Supplementary material is available online on FigShare at: https://doi.org/10.6084/m9.figshare.25152803.v1.
Declaration of funding
This research was supported by the United States Department of Agriculture’s National Wildlife Research Center.
Acknowledgements
We thank J.D. Taylor for his thoughtful review of a previous draft of this manuscript. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official determination or policy of the United States Department of Agriculture or US government.
References
Abbott HG (1958) Application of avian repellents to Eastern white pine seed. The Journal of Wildlife Management 22, 304-306.
| Crossref | Google Scholar |
Avery ML, Cummings JL, Decker DG, Johnson JW, Wise JC, Howard JI (1993) Field and aviary evaluation of low-level application rates of methiocarb for reducing bird damage to blueberries. Crop Protection 12, 95-100.
| Crossref | Google Scholar |
Avery ML, Decker DG, Humphrey JS, Aronov E, Linscombe SD, Way MO (1995) Methyl anthranilate as a rice seed treatment to deter birds. Journal of Wildlife Management 59, 50-56.
| Crossref | Google Scholar |
Avery ML, Fischer DL, Primus TM (1997) Assessing the hazard to granivorous birds feeding on chemically treated seeds. Pesticide Science 49, 362-366.
| Crossref | Google Scholar |
Avery ML, Werner SJ, Cummings JL, Humphrey JS, Milleson MP, Carlson JC, Primus TM, Goodall MJ (2005) Caffeine for reducing bird damage to newly seeded rice. Crop Protection 24, 651-657.
| Crossref | Google Scholar |
Belant JL, Gabrey SW, Dolbeer RA, Seamans TW (1995) Methyl anthranilate formulations repel gulls and mallards from water. Crop Protection 14, 171-175.
| Crossref | Google Scholar |
Belant JL, Ickes SK, Tyson LA, Seamans TW (1997) Comparison of four particulate substances as wildlife feeding repellents. Crop Protection 16, 439-447.
| Crossref | Google Scholar |
Besser JF (1978) Improvements in the use of 4-aminopyridine for protecting agricultural crops from birds. Proceedings of the Vertebrate Pest Conference 8, 51-53.
| Google Scholar |
Bruggers RL (1979) Summary of methiocarb trials against pest birds in Senegal. Proceedings of the Bird Control Seminar 8, 172-184.
| Google Scholar |
Bruggers RL, Sultana P, Brooks JE, Fiedler LA, Rimpel M, Manikowski S, Shivanarayan N, Santhaiah N, Okuno I (1984) Preliminary investigations of the effectiveness of trimethacarb as a bird repellent in developing countries. Proceedings of the Vertebrate Pest Conference 11, 192-203.
| Google Scholar |
Clapperton BK, Morgan DKJ, Day TD, Oates KE, Beath AM, Cox NR, Matthews LR (2014) Efficacy of bird repellents at deterring North Island robins (Petroica australis longipes) and tomtits (P. macrocephala toitoi) from baits. New Zealand Journal of Ecology 38, 116-123.
| Google Scholar |
Clark L (1998) Review of bird repellents. Proceedings of the Vertebrate Pest Conference 18, 330-337.
| Google Scholar |
Clark L, Belant JL (1998) Contribution of particulates and pH on cowbirds’ (Molothrus ater) avoidance of grain treated with agricultural lime. Applied Animal Behaviour Science 57, 133-144.
| Crossref | Google Scholar |
Clark L, Shah PS, Mason JR (1991) Chemical repellency in birds: relationship between chemical structure and avoidance response. Journal of Experimental Zoology 260, 310-322.
| Crossref | Google Scholar | PubMed |
Cowan P, Crowell M (2017) Visual and taste cues for minimising native bird interactions with toxic 1080 baits – a review of current practices. New Zealand Journal of Ecology 41, 178-185.
| Crossref | Google Scholar |
Crabb AC (1979) A report on efficacy of methiocarb as an avian repellent in figs and results of industry-wide bird damage assessments. Proceedings of the Bird Control Seminar 8, 25-30.
| Google Scholar |
Crocker DR, Reid K (1993) Repellency of cinnamic acid derivatives to rooks and chaffinches. Wildlife Society Bulletin 21, 456-460.
| Google Scholar |
Cummings JL, Otis DL, Davis JE, Jr (1992) Dimethyl and methyl anthranilate and methiocarb deter feeding in captive Canada geese and Mallards. The Journal of Wildlife Management 56, 349-355.
| Crossref | Google Scholar |
Cummings JL, Mason JR, Otis DL, Davis JEJ (1994) Evaluation of methiocarb, ziram, and methyl anthranilate as bird repellents applied to dendrobium orchids. Wildlife Society Bulletin 22, 633-638.
| Google Scholar |
Cummings JL, Avery ML, Pochop PA, Davis JE, Jr, Decker DG, Krupa HW, Johnson JW (1995) Evaluation of a methyl anthranilate formulation for reducing bird damage to blueberries. Crop Protection 14, 257-259.
| Crossref | Google Scholar |
Cummings JL, Pochop PA, Yoder CA, Davis JJ (1998) Potential bird repellents to reduce bird damage to lettuce seed and seedlings. Proceedings of the Vertebrate Pest Conference 18, 350-353.
| Google Scholar |
Curtis PD, Merwin IA, Pritts MP, Peterson DV (1994) Chemical repellents and plastic netting for reducing bird damage to sweet cherries, blueberries, and grapes. HortScience 29, 1151-1155.
| Crossref | Google Scholar |
Daneke D, Decker DG (1988) Prolonged seed handling time deters red-winged blackbirds feeding on rice seed. Proceedings of the Vertebrate Pest Conference 13, 287-292.
| Google Scholar |
Day TD, Matthews LR, Waas JR (2003) Repellents to deter New Zealand’s North Island robin Petroica australis longipes from pest control baits. Biological Conservation 114, 309-316.
| Crossref | Google Scholar |
De Grazio JW, Besser JF, DeCino TJ, Guarino JL, Starr RI (1971) Use of 4-Aminopyridine to protect ripening corn from blackbirds. The Journal of Wildlife Management 35, 565-569.
| Crossref | Google Scholar |
DeLiberto ST, Werner SJ (2016) Review of anthraquinone applications for pest management and agricultural crop protection. Pest Management Science 72, 1813-1825.
| Crossref | Google Scholar | PubMed |
Dolbeer RA, Ickes SK (1994) Red-winged blackbird feeding preferences and response to wild rice treated with portland cement or plaster. Proceedings of the Vertebrate Pest Conference 16, 279-282.
| Google Scholar |
Dolbeer RA, Clark L, Woronecki PP, Seamans TW (1991) Pen tests of methyl anthranilate as a bird repellent in water. Proceedings of the Eastern Wildlife Damage Control Conference 5, 112-116.
| Google Scholar |
Dolbeer RA, Belant JL, Clark L (1993) Methyl anthranilate formulations to repel birds from water at airports and food at landfills. Proceedings of the Great Plains Wildlife Damage Control Workshop 11, 42-52.
| Google Scholar |
Dorr B, Clark L, Glahn JE, Mezine I (1998) Evaluation of a methyl anthranilate-based bird repellent: toxicity to channel catfish Ictalurus punctatus and effect on great blue heron Ardea herodias feeding behavior. Journal of the World Aquaculture Society 29, 451-462.
| Crossref | Google Scholar |
Duncan CJ (1963) The response of the feral pigeon when offered the active ingredients of commercial repellents in solution. Annals of Applied Biology 51, 127-134.
| Crossref | Google Scholar |
Frank VH, Dischner MvU (1970) The testing of repellents intended to prevent consumption of seed grain by pheasants. Z.Jagdwiss 16, 14-22.
| Google Scholar |
Gadd P (1992) Avitrol use in the protection of wine grapes from the house finch (linnet) in Sonoma County. Proceedings of the Vertebrate Pest Conference 15, 89-92.
| Google Scholar |
Goodhue LD, Baumgartner FM (1965) Applications of new bird control chemicals. The Journal of Wildlife Management 29, 830-837.
| Crossref | Google Scholar |
Goodman J, Chandna A, Roe K (2015) Trends in animal use at US research facilities. Journal of Medical Ethics 41, 567-569.
| Crossref | Google Scholar | PubMed |
Guarino JL (1972) Methiocarb, a chemical bird repellent: a review of its effectiveness on crops. Proceedings of the Vertebrate Pest Conference 5, 108-111.
| Google Scholar |
Guarino JL, Shake WF, Schafer EW, Jr (1974) Reducing bird damage to ripening cherries with methiocarb. The Journal of Wildlife Management 38, 338-342.
| Crossref | Google Scholar |
Hamsa M, Ali B, El Haig I, Bohl W, Besser JF, De Grazio JW, Bruggers RL (1982) Evalutating the repellency of methiocarb. Malimbus 4, 33-41.
| Google Scholar |
Joyner DE, Somers JD, Gilbert FF, Brooks RJ (1980) Use of methiocarb as a blackbird repellent in field corn. The Journal of Wildlife Management 44, 672-676.
| Crossref | Google Scholar |
Kaiser BA, Johnson BL, Ostlie MH, Werner SJ, Klug PE (2021) Inefficiency of anthraquinone-based avian repellents when applied to sunflower: the importance of crop vegetative and floral characteristics in field applications. Pest Management Science 77, 1502-1511.
| Crossref | Google Scholar | PubMed |
Kelly ST, Dolbeer RA (1984) Decline in use of avitrol R to reduce blackbird damage to field corn. Wildlife Society Bulletin 12, 252-255.
| Google Scholar |
Kennedy TF, Connery J (2008) An investigation of seed treatments for the control of crow damage to newly-sown wheat. Irish Journal of Agricultural and Food Research 47, 79-91.
| Google Scholar |
Lopez-Antia A, Ortiz-Santaliestra ME, Mateo R (2014) Experimental approaches to test pesticide-treated seed avoidance by birds under a simulated diversification of food sources. Science of The Total Environment 496, 179-187.
| Crossref | Google Scholar | PubMed |
Mann WFJ, Derr HJ, Meanley B (1956) Bird repellents for direct seeding longleaf pine. Forests and People 6, 16-17 48.
| Google Scholar |
Mason JR, Avery ML, Glahn JF, Otis DL, Matteson RE, Nelms CO (1991) Evaluation of methyl anthranilate and starch-plated dimethyl anthranilate as bird repellent feed additives. The Journal of Wildlife Management 55, 182-187.
| Crossref | Google Scholar |
Mason JR, Clark L, Miller TP (1993) Evaluation of a pelleted bait containing methyl anthranilate as a bird repellent. Pesticide Science 39, 299-304.
| Crossref | Google Scholar |
Mastrota FN, Mench JA (1995) Evaluation of taste repellents with northern bobwhites for deterring ingestion of granular pesticides. Environmental Toxicology and Chemistry 14, 631-638.
| Crossref | Google Scholar |
Mott DF, Besser JF, West RR, De Grazio JW (1972) Bird damage to peanuts and methods for alleviating the problem. Proceedings of the Vertebrate Pest Conference 5, 118-120.
| Google Scholar |
Nichols M, Bell P, Mulgan N, Taylor A (2020) Conditioned aversion in kea to cereal bait: a captive study using anthraquinone. Applied Animal Behaviour Science 230, 105077.
| Crossref | Google Scholar |
Oerke E-C (2006) Crop losses to pests. The Journal of Agricultural Science 144, 31-43.
| Crossref | Google Scholar |
Orr-Walker T, Adams NJ, Roberts LG, Kemp JR, Spurr EB (2012) Effectiveness of the bird repellents anthraquinone and d-pulegone on an endemic New Zealand parrot, the kea (Nestor notabilis). Applied Animal Behaviour Science 137, 80-85.
| Crossref | Google Scholar |
Pelaez V, da Silva LR, Araujo EB (2013) Regulation of pesticides: a comparative analysis. Science and Public Policy 40, 644-656.
| Crossref | Google Scholar |
Popp J, Pető K, Nagy JG (2013) Pesticide productivity and food security. A review. Agronomy for Sustainable Development 33, 243-255.
| Crossref | Google Scholar |
Royall WC, Jr, Ferguson ER (1962) Controlling bird and mammal damage in direct seeding loblolly pine in East Texas. Journal of Forestry 60, 37-39 10.1093/jof/60.1.37.
| Google Scholar |
Sandhu PS, Dhindsa MS, Toor HS (1987) Evaluation of methiocarb and thiram as seed treatments for protecting sprouting maize from birds in Punjab (India). Tropical Pest Management 33, 370-372.
| Google Scholar |
Sayre RW, Clark L (2001) Effect of primary and secondary repellents on European starlings: an initial assessment. The Journal of Wildlife Management 65, 461-469.
| Crossref | Google Scholar |
Schafer EW, Jr, Brunton RB (1971) Chemicals as bird repellents: two promising agents. The Journal of Wildlife Management 35, 569-572.
| Crossref | Google Scholar |
Schafer EW, Jr, Bowles WA, Jr, Hurlbut J (1983) The acute oral toxicity, repellency, and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Archives of Environmental Contamination and Toxicology 12, 355-382.
| Crossref | Google Scholar | PubMed |
Snijders L, Thierij NM, Appleby R, St. Clair CC, Tobajas J (2021) Conditioned taste aversion as a tool for mitigating human-wildlife conflicts. Frontiers in Conservation Science 2, 744704.
| Crossref | Google Scholar |
Spurr EB (1993) Feeding by captive rare birds on baits used in poisoning operations for control of brushtail possums. New Zealand Journal of Ecology 17, 13-18.
| Google Scholar |
Starr RI, Besser JF, Brunton RB (1964) A laboratory method for evaluating chemicals as bird repellents. Agricultural and Food Chemistry 12, 342-344.
| Crossref | Google Scholar |
St. Aubin F (1977) How much do regulations inhibit pesticide development. Pest Control 45, 16-17 20, 62, 64, 66–68.
| Google Scholar |
Sultana P, Brooks JE, Bruggers RL (1986) Repellency and toxicity of bird control chemicals to pest birds in Bangladesh. Tropical Pest Management 32, 246-248.
| Google Scholar |
Tupper SK, Werner SJ, Carlson JC, Pettit SE, Wise JC, Lindell CA, Linz GM (2014) European starling feeding activity on repellent treated crops and pellets. Crop Protection 63, 76-82.
| Crossref | Google Scholar |
Wager-Page SA, Mason JR (1996a) Exposure to volatile d-pulegone alters feeding behavior in European starlings. Journal of Wildlife Management 60, 917-922.
| Google Scholar |
Wager-Page SA, Mason JR (1996b) Ortho-aminoacetophenone, a non-lethal repellent: the effect of volatile cues vs. direct contact on avoidance behavior by rodents and birds. Pesticide Science 46, 55-60.
| Google Scholar |
Watkins RW, Gill EL, Bishop JD (1995) Evaluation of cinnamamide as an avian repellent: determination of a dose-response curve. Pesticide Science 44, 335-340.
| Crossref | Google Scholar |
Watkins RW, Lumley JA, Gill EL, Bishop JD, Langton SD, MacNicoll AD, Price NR, Drew MGB (1999) Quantitative structure-activity relationships (QSAR) of cinnamic acid bird repellents. Journal of Chemical Ecology 25, 2825-2845.
| Crossref | Google Scholar |
Werner SJ, Cummings JL, Tupper SK, Hurley JC, Stahl RS, Primus TM (2007) Caffeine formulation for avian repellency. The Journal of Wildlife Management 71, 1676-1681.
| Crossref | Google Scholar |
Werner SJ, Carlson JC, Tupper SK, Santer MM, Linz GM (2009) Threshold concentrations of an anthraquinone-based repellent for Canada geese, red-winged blackbirds, and ring-necked pheasants. Applied Animal Behaviour Science 121, 190-196.
| Crossref | Google Scholar |
Werner SJ, Linz GM, Carlson JC, Pettit SE, Tupper SK, Santer MM (2011) Anthraquinone-based bird repellent for sunflower crops. Applied Animal Behaviour Science 129, 162-169.
| Crossref | Google Scholar |
Werner SJ, DeLiberto ST, Mangan AM, Pettit SE, Ellis JW, Carlson JC (2015) Anthraquinone-based repellent for horned larks, great-tailed grackles, American crows and the protection of California’s specialty crops. Crop Protection 72, 158-162.
| Crossref | Google Scholar |
Werner SJ, Gottlob M, Dieter CD, Stafford JD (2019) Application strategy for an anthraquinone-based repellent and the protection of soybeans from Canada goose depredation. Human-Wildlife Interactions 13, 308-316.
| Google Scholar |
Woronecki PP, Dolbeer RA, Ingram CR, Stickley AR, Jr (1979) 4-Aminopyridine effectiveness reevaluated for reducing blackbird damage to corn. The Journal of Wildlife Management 43, 184-191.
| Crossref | Google Scholar |