Irruptive dynamics of the brush-tailed bettong (Bettongia penicillata) when reintroduced to a fenced sanctuary with feral cats present
Jeff Short A B *
A B *
A Wildlife Research and Management Pty Ltd, PO Box 1360, Kalamunda, WA 6926, Australia.
B Environmental and Conservation Sciences, Murdoch University, South Street, Murdoch, WA 6150, Australia.
Wildlife Research 50(2) 85-95 https://doi.org/10.1071/WR22063
Submitted: 17 June 2021 Accepted: 4 October 2022 Published: 25 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The fluctuating fortunes of the brush-tailed bettong have seen this species classified as ‘Rare or Likely to Become Extinct’ in the 1970s, delisted and hailed as a conservation success in the 1990s, and re-listed as ‘Critically Endangered’ in 2008. Key actions to recover this species, broad-scale fox control and reintroduction to fox-free habitat, have had variable success.
Aims: To monitor the reintroduction of bettong to fox-free habitat of Wadderin Sanctuary in the eastern wheatbelt of Western Australia.
Methods: Growth of the population was monitored over a 12-year period by regular trapping throughout the sanctuary.
Key results: The population increased monotonically to peak at 305 individuals (0.71 ha−1) at 7.5 years following release, before subsequent decline. Population growth was accompanied by a significant decline in mean condition and a reduction in the proportion of females with pouch young or lactating, presumably owing to resources per head gradually declining. The proportion of large pouch young relative to total young carried by females declined as woylie numbers increased, suggesting that, increasingly, pouch young did not go to full term. Declines in population number, condition and reproduction were exacerbated by dry seasons. Bettongs established successfully, despite a succession of single feral cats within the 427-ha sanctuary. The removal of the last cat, 8 years following the establishment of bettongs, had no obvious impact on bettong numbers, as, at this point, their population was stabilising because of declining body condition and reduced reproductive output.
Conclusions: The observed pattern of population growth of bettongs was consistent with an herbivore irruption consequent of a release of a species to new habitat. Decline following peak numbers appeared as a result of density-dependent resource limitation (declining resources per head with increased abundance) interacting with years of low rainfall.
Implications: The dynamics of irruption and decline of herbivores are relevant to management of reintroduced populations to fenced predator-free sites and, potentially, to unfenced populations following release from predation.
Keywords: drought, eruption, feral cat, irruption, overpopulation, reintroduction, translocation, woylie.
Introduction
The European red fox (Vulpes vulpes) and the feral cat (Felis catus) were introduced to the Australian continent following European settlement and have had a devastating impact on the native fauna (Short and Smith 1994; Kinnear et al. 2002; Woinarski et al. 2015). A key conservation response has been to create sanctuaries fenced to exclude these predators for the reintroduction of predator-vulnerable species (Legge et al. 2017). This paper documents the reintroduction and establishment of a critically endangered macropodid to one such sanctuary where predator exclusion was not entirely effective.
The brush-tailed bettong (Bettongia penicillata) is a small (1.2–1.5 kg) macropodid within the family Potoroidae that formerly had a wide distribution through semi-arid and arid Australia (de Tores and Start 2008). It is one of a group of species collectively known as rat-kangaroos that suffered major declines in the late 19th and early 20th century, coinciding with the colonisation of their habitat by the European fox (Vulpes vulpes; Short 1998). The eastern subspecies (B. penicillata penicillata) is now presumed extinct, and the western subspecies (B. penicillata ogilbyi) has survived only as three relict populations in the south-west of Western Australia (Dryandra Woodland, Tutanning Nature Reserve, and Perup Forest; Christensen 1995).
The western subspecies (hereafter referred to as ‘woylie’ or ‘bettong’) was listed in 1973 as ‘Rare or Likely to Become Extinct’ under Western Australia’s Wildlife Conservation Act(Groom 2010). In the late 1970s and early 1980s, populations were barely detectable, with trap success and spotlight sightings close to or at zero (Kinnear et al. 2002). Broad-scale fox control to an area of >30 000 km2 of conservation land in south-western Western Australia (Woinarski et al. 2014) resulted in a resurgence in populations through the 1980s and 1990s (Christensen 1995; Kinnear et al. 2002), and led to it being delisted in 1996. However, another major decline (∼90% from 1999, Wayne et al. 2013) led to its being re-listed. It is currently listed under the Commonwealth Environmental Protection and Biodiversity Conservation Act as ‘Endangered’ and has an IUCN status of ‘Critically Endangered’.
Translocation has been widely used in an attempt to improve the conservation status of the species, with Morris et al. (2015) reporting 56 translocations within Western Australia, and with at least a further 16 attempts in South Australia and New South Wales (Finlayson et al. 2010). Translocations of this species began in the late 1970s with transfers within Perup in south-western Western Australia and to South Australian islands (Delroy et al. 1986; Short et al. 1992), and, subsequently, to numerous sites in Western Australia, South Australia and New South Wales. However, many translocations have shown to be unsuccessful (Robinson et al. 1996; Mawson 2004; Priddel and Wheeler 2004; de Tores and Start 2008; Woinarski et al. 2014) or are of indeterminate status. Successful translocations appear to be associated with the complete exclusion of foxes and feral cats (Felis catus; de Tores and Start 2008), although Wayne et al. (2013) listed persistent reintroduced populations at numerous unfenced sites in Western Australia where foxes are effectively controlled, but feral cats are not.
Groom (2010) reported a significant decline in all free-ranging populations of woylie, both natural and reintroduced. Despite translocations and widespread baiting to control foxes, the woylie population was perceived to have declined by >90% in the 10 years to 2012 (Wayne et al. 2013). Marlow et al. (2015) showed that feral cat populations increase in the absence of foxes and are likely to have a detrimental impact on woylie populations.
In 2010, woylies were translocated to Wadderin Sanctuary, 280 km east of Perth in the Western Australian wheatbelt. This is a site fenced to exclude exotic predators, but has an imperfect record of predator exclusion. The aim of this study was to monitor the establishment of woylies and to detail attributes of their biology and ecology.
Specifically, this study considered the following hypotheses and predictions:
-
Woylie populations are regulated by food resources, when predation is low or absent.
(1) Population size will follow an irruptive pattern; i.e. increase from low numbers, then reach a plateau and decline; (2) body condition and reproductive parameters will decline over time as woylie numbers increase and resources per head decrease; and; (3) the population will decrease in dry years and this will be exacerbated if at high numbers.
-
Woylie populations can establish and persist in the presence of feral cats under some conditions.
(1) Woylies can persist in the presence of feral cats if these predators are maintained at a fixed low number; and (2) the absence of cat predation will result in an increase in woylie numbers.
Materials and methods
Study site
Wadderin (31°59.2′S, 118°24.8′E) is a vegetation remnant isolated within a landscape almost entirely cleared for cereal cropping. It is located approximately 8 km north of the town of Narembeen, Western Australia. Wadderin is vested with the Water Corporation (Water Reserve 20 022), but in 2004 a lease was granted to the Shire of Narembeen to create a wildlife sanctuary that excluded foxes and feral cats. In 2007, 427 ha of the remnant were fenced by volunteers from the Narembeen community to exclude exotic predators. The perimeter fence is 9 km in length and is a wire netting fence 1.8 m high, supported by galvanised steel posts, with an outward-facing overhang and a 50 cm apron at the ground level on both the inside and outside of the fence. The fence, as originally built, was not cat-proof. It was substantially upgraded in 2015 and 2016 by widening the overhang to 60 cm, supporting the overhang with fence droppers placed horizontally to ensure it was not drooping at its outer margin to maintain fence height, adding an electrified wire at mid-height, and ensuring the height of the fence was everywhere ≥1.8 m tall.
Wadderin has a series of granite outcrops surrounded by a mix of woodland and shrubland vegetation. Sixty-seven hectares of the sanctuary are granite outcrop (16%), with woodland communities making up 297 ha (69%), dominated by extensive areas of jam (Acacia acuminata), York gum (Eucalyptus loxophleba), salmon gum (E. salmonophloia), and rock she-oak (Allocasuarina heugeliana). A further 63 ha are shrubland (Melaleuca spp. and Allocasuarina campestris) or mallee Eucalyptus spp. (a combined 15%). Poison bush Gastrolobium parviflorum and G. spinosum occur sparsely in shrubland habitat, but not in other habitats.
Wadderin has no history of grazing by domestic stock, having been managed as a water catchment since the 1920s. The sanctuary is long-unburnt (>40 years). Narembeen has an annual average rainfall of 332 mm, with the bulk falling in winter (www.bom.gov.au).
Western grey kangaroos (Macropus fuliginosus) increased to high densities in 2010, following fencing, and at that time had a major visible impact on native vegetation. They were subsequently reduced to and maintained at a low level by culling. Rabbits were maintained at low levels by poisoning using both 1080 and phostoxin. Echidnas (Tachyglossus aculeatus) are common and regularly burrow under the fence and, on occasions, create potential pathways into the sanctuary for foxes and feral cats, despite weekly fence inspections by community volunteers.
Foxes and feral cats were removed from the sanctuary prior to translocations of native fauna by baiting with ‘1080’ dried meat baits to control foxes and trapping with cage traps to remove feral cats. Despite initial removal, Wadderin has been subject to periodic incursions by feral cats and, less frequently, by foxes. A network of fixed remote camera-traps was used to monitor for the presence of predators. The key control action to deal with incursions by foxes was the use of 1080 meat baits tethered to star pickets at a height of about 0.6 m above the ground (to limit take by woylies). Since reintroduction of native mammals, feral cats have been controlled variously by use of cat-specific 1080 baits, by trapping with small treadle-operated cage traps baited with a bait-type potentially attractive to cats, or by use of large treadle-operated cage traps raised on stilts or on horizontal branches at >1.5 m to reduce non-target capture of woylies.
Reintroductions
Woylies were sourced from captive colonies managed by wildlife carers in Perth, established from animals from Dryandra Woodland, and Perup and Tutanning Nature Reserves. They were measured, microchipped, and transported to Wadderin in individual bags within pet packs and released in the evening following their capture. Some supplementary feed was provided over the first summer (October 2010–February 2011), because of exceptionally dry conditions. Further woylies were added in 2012, also sourced from wildlife carers.
Fauna monitoring
One or two major surveys of the woylie population were conducted each year from 2010 to 2021 (n = 16 surveys, mean of 351 trap-nights per survey). These were supplemented by typically two or three minor surveys each year to provide detail on seasonal condition and reproduction (n = 25 surveys; mean of 113 trap-nights). Small treadle-operated wire-mesh cage traps (Sheffield Wire Products, 220 × 220 × 560 mm) were used to assess the presence and abundance of woylies and reintroduced quenda (Isoodon obesulus). Cages, each partially covered with a hessian bag, were placed at 100 m intervals around the track network and baited with universal bait (peanut butter, rolled oats and sardines). Trap locations were fixed and marked by 180 numbered pickets. The distance from any point in the sanctuary to a trap line was <400 m, suggesting multiple trap locations within the range of all woylies (home range size of ∼65 ha, Yeatman and Wayne 2015). Captured woylies were pit-tagged, sexed and measured (weight and pes) and, if female, the presence and size of pouch young or presence of a lactating nipple was noted.
The presence of feral cats (and/or foxes) was assessed by using fixed cameras (Bushnell, Reconyx and Swift). Five cameras were used from January 2011, increasing to six cameras in January 2012 and eight from November 2013.
Analysis
The population of woylies was estimated by both the ‘minimum number alive’ (MNA) method and by the Jolly–Seber mark–recapture method (Krebs 1999). MNA used all available capture data; mark–recapture estimates used major surveys only and were calculated using the Jolly–Seber full model (Krebs 2002).
Annual recruitment was estimated from the number of new individuals first caught in any particular year, whereas the annual mortality was assumed to be the sum of all individuals last caught in any particular year (and uncaught in any subsequent survey). Mortality for 2021 (the last year of assessment) was calculated from animals uncaught in the major survey of February 2022. Earlier major surveys have caught 87% of the MNA population established by additional captures at subsequent surveys.
Captures were collated to season in each year (winter, spring, summer, and autumn). Records of woylies recaptured within the same season as a prior capture of that individual were omitted from analyses to ensure independence; hence, any repeat captures of the same individual were typically >90 days apart. Hence, consecutive surveys were considered independent.
Body condition was assessed using the method of Krebs and Singleton (1993). A regression of skeletal size (pes length, mm) and body mass (g) was calculated using all available data. This regression was used to predict body mass from the pes length of each individual. The condition of each individual (condition index, CI) was then assessed as the ratio of observed to predicted body mass. An individual in average condition has a CI = 1, those with a CI > 1 are in better condition (i.e. observed > predicted body mass), and those with a CI < 1 are in poorer condition. A mixed model (Gallucci 2019) was used to assess the effect of the factors gender (male, female), season (winter, spring, summer, autumn) and ‘age’ (a binary variable distinguishing first capture of an individual, typically as a subadult, versus all subsequent captures as an adult) and the covariates years since first reintroduction, woylie population size, rainfall over the previous 3 months and rainfall over the previous 12 months on condition of all individuals >500 g (i.e. excluding dependent young). Individual ID was included as a random effect to account for recaptures of individual woylies over time.
Changes in reproductive activity were assessed over season and over time. Female reproductive activity (a binary variable differentiating female with or without pouch young or in lactation) was analysed using a generalised mixed-model with logit-link function (The Jamovi Project 2021). Potential explanatory variables included years since first reintroduction, ‘age’ of woylie, woylie condition, woylie population size, season, and rainfall over the previous 3 and 12 month periods. Individual ID was included in the model as a random effect. Where two variables were highly correlated, one of the pair was omitted from further analysis. Scaling of covariates (rainfall over the previous 3 months, woylie population size, and woylie condition) was standardised.
The number of females with pouch young or lactating as a percentage of total females caught by season was plotted against year. The proportion of large pouch young (large unfurred, furred and young at foot) as a proportion of total young carried by females at each survey was regressed against woylie population size.
The impact of the feral cat on woylie recruitment was assessed by comparing recruitment across two sequential 3-year periods (one with cat present; one without) in areas close to and distant from the centre of the cat’s activity as determined from sightings on eight fixed cameras. Results were analysed using a chi-squared analysis.
Results
Reintroduction of woylies
Forty-two woylies were reintroduced to Wadderin Sanctuary in 2010 and 2012 (Table 1). Survival of each cohort of released animals was quite variable (Fig. 1), with 50% of woylies surviving to 4 years for the October 2010 release, compared with 17% of woylies released in June 2010.

|
In total, >740 woylies (over and above those translocated) were marked over the 12 years to February 2022. The population grew steadily, to peak at ∼305 individuals in January 2018, then declined to ∼170 in January 2021, before making a slight recovery by February 2022 (Fig. 2). Estimates of population size from MNA and mark–recapture were broadly similar for the first 7 years of monitoring, but with MNA failing to adequately predict peak numbers. Trap success for woylies averaged 72% (range 55.3–81.4%) for all major surveys from 2013. Other species captured included quenda (mean 6.4% trap success) and brushtail possum (Trichosurus vulpecula; 2.7%).
Woylies established despite significant droughts in 2010 (annual rainfall 54% of long-term average), 2015 (annual rainfall 68% of long-term average) and 2019 (annual rainfall 59% of long-term average; Fig. 3). There was no step change in numbers of woylies (Fig. 2) or their condition, recruitment or reproduction (Figs 4, 5, 6) for the dry years of 2014 and 2015, in comparison to the major declines in 2019 and 2020. This suggests the adverse impact of drought is contingent on high numbers of woylies relative to resource availability.
|
|
|
|
|
|
Recruitment and mortality of woylies
Woylie recruitment substantially exceeded mortality in the first 6 years of the reintroduction (2011–2016), but thereafter (2017, 2018, and 2020) mortality exceeded recruitment, resulting in the population reaching a plateau and, subsequently, declining (Fig. 4). Recruitment exceeded mortality in 2021, resulting in a small increase in the number of woylies in this year (Fig. 2).
Condition of woylies
Condition (CI) varied with years since first reintroduction (F1, 3032 = 148.1; P < 0.001), woylie population size (F1, 2946 = 20.0; P < 0.001), rainfall over the previous 12 months (F1, 2498 = 42.1; P < 0.001), gender (F1, 645 = 10.35; P = 0.001), season (F3, 2977 = 26.5; P < 0.001), and ‘age’ (F1, 3038 = 331.2; P < 0.001), with the only higher-level interaction significant being season by ‘age’ (F3, 3030 = 324.1; P < 0.001).
Woylie condition declined with years since first reintroduction (CI of 1.09 at Year 0; 0.93 at Year 12), woylie population size (1.04 at a population size of 50; 0.98 at a population size of 300) and increased with rainfall over the previous 12 months (0.97 at 200 mm; 1.05 at 500 mm). Rainfall over the previous 3 months was not significant and was omitted from the analysis. Mean condition of female woylies was higher than that of males (1.02 vs 0.99), woylies were in better condition in spring and summer (1.03, 1.02) than in winter and autumn (0.99, 0.97), and older woylies (second and subsequent captures vs first capture) were in better condition than were woylies at first capture (1.03 vs 0.93). The significant interaction between season and ‘age’ was as a result of woylies at first capture being at poorest condition in autumn (0.90) and at best in spring (0.97), whereas woylies at subsequent captures were in poorest condition in winter (0.99) and at best in summer (1.05).
Mean seasonal condition (Fig. 5) declined at a significant rate over time (F1,37 = 31.59; P < 0.001). For the population has a whole, mean seasonal condition averaged 10% above predicted in 2011, but declined to 15% below predicted in 2020, with lowest values occurring in Autumn 2017, Autumn 2019, and Autumn and Winter 2020. Oscillations in condition from highs in spring and summer to lows in autumn and winter increased markedly in later years. Field notes from captures at times of lowest values of condition described woylies as ‘very skinny’, ‘exceptionally thin (bones of spine very sharp and defined)’ and ‘all very bony’, suggesting that poor condition was due to increasing food shortages.
Reproduction of woylies over time
The percentage of females carrying pouch young or that were lactating declined over time at Wadderin, from highs of 70–100% of females in the early years (2011–2014), declining to values of less than 50% by 2019 (Fig. 6). These declining values were due largely to an increased seasonality of reproduction, with periods where females carried few or no young. For example, less than 15% of females had pouch young or were lactating in the summers of 2017–2018, 2018–2019 and 2019–2020. This period with few or no young extended from summer through autumn to early winter in 2018 and 2020. There appeared to be substantial recovery in reproduction in late 2020 and in 2021.
Rainfall over the previous 3 and 12 months was highly correlated (r = 0.42) as were woylie population size and years since first reintroduction (0.66) and ‘age’ and condition (−0.29), so only one of each pair was utilised in the mixed model. Rainfall over the previous 3 months (β = 0.49, z = 5.92; P < 0.001) and woylie condition (β = 1.15, z = 10.97; P < 0.001) had significant positive effects on probability of females carrying a young or lactating. This probability was least for summer and increased for autumn (β = 0.69, z = 3.82; P < 0.001), spring (β = 1.15, z = 4.78; P < 0.001) and winter (β = 1.59, z = 7.04; P < 0.001). Woylie population size (β = −0.61, z = −6.89; P < 0.001) had a negative effect. The number of large pouch young [large unfurred, furred and young at foot (i.e. lactating nipple)] as a proportion of total young carried by females significantly declined with woylie population size (F1,19 = 27.06; P < 0.001) from ∼25% in 2011, when the population of woylies was at about 50 (0.12 ha−1), to <10% in 2018–2020, when numbers were at 250–300 (0.64 ha−1).
Feral cats and foxes within the sanctuary
At least one feral cat was present within the sanctuary for much of the period of establishment of woylies. A feral cat was detected on 89 occasions on widely spaced cameras over 12 years of monitoring (Fig. 2). Seven feral cats were either removed or disappeared from the sanctuary over the period of monitoring (Table 2). All those captured were males (weight range: 3.75–4.7 kg), and largely serially replaced each other until a major fence upgrade in 2015 and 2016 successfully excluded further incursions. Camera images suggested very little temporal overlap between successive cats. The final record of a cat was in July 2018; this cat was first recorded within the sanctuary in September 2015, and, so, had a comparatively long residency without capture relative to other cats recorded. Most cats were removed by trapping (five of seven) in either ground or raised cage traps. Trapping for cats was greatly impeded by frequent capture of woylies and possums in traps set for cats.
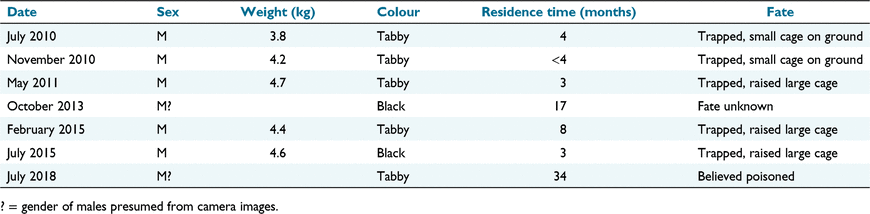
|
Two incursions by foxes, namely, one in July 2013 and another in June 2018, were detected over 12 years of monitoring (Fig. 2). The 2013 incursion lasted 12 days, the 2018 incursion lasting 19 days. The incursion in 2018 was from a fox digging under the fence, commencing its dig outside the line of the netting skirt.
Impact of the feral cat
In all, 33 of 36 sightings (92%) of the last tabby cat at Wadderin (Table 2) were from three cameras within 500 m of each other in open woodland in the north of the sanctuary. The camera with most records (61%) was assumed to be the centre of the cat’s hunting activity. The number of recruits to the woylie population with first capture within or beyond 1.5 km from this point were compared for the ∼3 years that the cat was present and for the subsequent 3 years when no cat was present. There was a significant association between areas and time periods ( = 9.8; P < 0.005). The area within 1.5 km of the centre of the cat’s range (an area of ∼300 ha within the fence) generated 65% of new woylies when the cat was present, compared with 80% for the period when it was absent.
= 9.8; P < 0.005). The area within 1.5 km of the centre of the cat’s range (an area of ∼300 ha within the fence) generated 65% of new woylies when the cat was present, compared with 80% for the period when it was absent.
Discussion
Woylie dynamics
Woylies were reintroduced to Wadderin Sanctuary in mid-2010 and the population has been extant for 12 years. The population increased steadily for the first 7 years, before reaching a plateau at about 0.7 ha−1 (a population size of ∼300), and subsequently declining. Peak density was attained approximately 7.5 years following release and density declined by 45% 3 years thereafter. This increase in the number of woylies at Wadderin to peak density was accompanied by a decline in body condition and reproductive output. The proportion of large pouch young relative to total young carried by females declined with time, reflecting poorer body condition.
This pattern of rise in numbers followed by a decline and accompanied by a steady decline in body condition and reproduction as numbers increased suggests an herbivore (consumer) irruption (Caughley 1976; Duncan et al. 2020). This is when a newly established consumer and its food resource interact to generate substantial fluctuations in both over time. Typically, a small founding population is released among abundant resources and as the consumers increase in number, both total food resources and food resources per head decline. After a series of fluctuations of decreasing oscillation over time, consumer density and vegetation density stabilise at substantially lower levels than the initial peaks (Caughley 1976).
Duncan et al. (2020) demonstrated that the dynamics of interaction between a consumer and its food supply shift from a monotonic increase to an asymptote (logistic growth) to increasingly stronger fluctuations around an equilibrium density as the rate of increase of the food supply declines relative to that of the herbivore. This indicates that a rise in numbers followed by a substantial fall could be due to the dynamic interaction of an herbivore with its food supply alone, without the need to invoke other agents of mortality such as predation or inter-specific competition for resources. In all, 5 of 25 time series of woylie numbers examined by Duncan et al. (2020) showed an irruptive pattern that could be interpreted as the dynamic interaction between a consumer and its food supply. Although this explanation emphasises woylie abundance as a key driver, it is likely that, at Wadderin, the conjunction of high woylie population size, a sequence of dry years and competition from other herbivores and omnivores all contributed to the subsequent woylie decline.
The impact of drought on woylie numbers, condition and reproduction appeared contingent on high densities of woylie. The decline in woylie population began in 2018 and continued to 2020. Hence, it coincided with, but preceded the dry years of 2019 and 2020. There was no obvious impact of a dry 2014 and 2015 on the numbers of woylies or their body condition, recruitment or reproduction.
Drought was considered to have a major impact on bettong numbers elsewhere (Short et al. 1998), as with other macropods (Robertson 1986). Even though woylies have a distinctive dietary niche (e.g. Zosky et al. 2018), drought is likely to have induced some competition for food resources with other species. However, possums, a potential food competitor (Davis 2005), were at low numbers, as were bandicoots, relative to woylies. European rabbits and kangaroos were maintained at low numbers by management and were less likely to overlap in diet.
Future monitoring at Wadderin may provide some insight as to what weight to put on these various explanations (irruption vs drought and/or competition). Woylies are unlikely to return to their former peak density under the former, having eroded the capital base of their food supply (Caughley 1976), but may do so with a return to average or above-average rainfall years if their decline was due to drought or competition. Consistent with both explanations, woylies showed a small increase in population size through 2021 following a return to above-average annual rainfall, with new recruits exceeding presumed deaths.
The population at peak density at Wadderin (0.71 ha−1) can be compared to estimates from a fenced site (1.45 ha−1: Karakamia Sanctuary; Eikelboom 2010) and unfenced natural populations subject to predation [1.6 ha−1 (Upper Warren), 1.14 ha−1 (Dryandra Woodland), and 0.18 ha−1 (Tutanning Nature Reserve; Wayne et al. 2013)]. Observed peak densities appear broadly correlated with rainfall, with sites being located on a substantial rainfall gradient ranging from ∼1000 mm per annum at Upper Warren to 332 mm per annum at Wadderin.
Yeatman (2010) reported demographic changes very similar to those at Wadderin for a reintroduced population of woylies at Karakamia Sanctuary, namely, declining trends in condition and reproduction over time since reintroduction. Unfortunately, no clear information was available on changing abundance at this site (Yeatman 2010). However, the population was perceived to be overabundant. In total, 535 woylies were removed from the Karakamia site over the 7 years from 2000 to 2006, in an effort to reduce intra-specific and inter-specific competition (Australian Wildlife Conservancy 2006–2010; Richards et al. 2009).
Most female woylies at Wadderin carried young or were lactating in the early years of the reintroduction, but this declined greatly (often to zero) as numbers increased and body condition decreased over time. In these latter years, there was typically a brief spike to 60–80% of females with young in late winter or spring, followed by extended periods when few or no females carried young. This suggests that females at this time were producing, at most, only a single young each year.
Woylies are widely reported to breed continuously throughout the year (Sampson 1971; de Tores and Start 2008; Thompson et al. 2015). Christensen (1995) reported that females give birth to their first young at the age of 170–180 days, and approximately every 100 days thereafter for the rest of their 4–6 years. However, this assumes ad libitum resources, which is often not the case in the wild. For example, Priddel and Wheeler (2004) reported a low incidence of breeding on Saint Peter Island, SA (only 25% of females captured had pouch young). Similarly, woylies translocated to Baird Bay Island and Island A, Venus Bay, both in South Australia, typically had low numbers of females with pouch young (Nelson et al. 1992).
Condition of woylies at Wadderin was typically lowest in late autumn and winter and relates to the seasonal pattern of winter-dominant rainfall. This is at a time of year when fungi makes up a greater proportion of the diet of woylies in south-western Western Australia (Zosky et al. 2018). The poor condition of woylies in late autumn and winter leads to a period of apparent vulnerability. Armstrong (2008) reported a major die-off (>90%) of woylies in winter 2005 at Venus Bay, SA, owing to the exhaustion of food resources from overpopulation coinciding with a series of six frosts in a week. Presumably, poor body condition, resulting from food shortages, increased vulnerability to cold stress. Similarly, de Tores (2020) found mortality of translocated woylies to be highest in winter.
Woylies at Wadderin increased to the point of perceived overpopulation (i.e. available food resources unable to support the current population) before declining, a pattern observed elsewhere for bettong populations translocated to islands and sanctuaries (Williams and Donaldson 2008; Linley et al. 2017; Moseby et al. 2018). Overpopulation at Wadderin was assessed from declining parameters of body condition and reproduction as well as a prominent observed fence effect in vegetation in the latter years of the study. The latter is consistent with observations of vegetation change elsewhere following the reintroduction of native herbivores (lower plant species richness and a higher percentage of bare ground: Kemp et al. 2021); although such an impact in this study cannot be solely attributed to woylies.
Bettongs are opportunistic omnivores with a diverse diet, that includes fungi, roots, tubers, bulbs, dicots, monocots, fruits, seeds, bark, insects, arachnids and carrion (Zosky et al. 2018, and references therein). Little is known about the rate of renewal of a bettongs’ food resources following depletion, or how that renewal is influenced by drought. Johnson (1994) suggested that fruit-bodies of ectomycorrhizal fungi may be substantially depleted by foraging by bettongs. Similarly Zosky (2011) found that a site with a high density population of woylies had a significantly lower number and dry weight of fungal sporocarps than did other sites with lower densities.
This suggests the need for a direct assessment of food resources of woylie populations over time and a concurrent monitoring of the demographic parameters of the populations. Key questions include the following: (1) to what extent do bettongs deplete the capital stock of their food plants when they peak at high densities; (2) how do prolonged periods of low rainfall affect available food resources; (3) what is the rate of replenishment of food sources, particularly their fungal staple; (4) to what extent does poor condition and poor reproductive output resulting from food shortages or drought affect the resilience of the bettong population to predation; and (5) to what extent can predators play a role, by reducing the realised rate of increase of bettongs, in stabilising the irruptive relationship between bettongs and their food supply?
Woylies and cat predation
Woylies established as a new population at Wadderin despite at least one feral cat being present for much of the time. The cat density for much of the establishment phase of the woylie population was 0.24 km−2 (a single cat assumed to be resident in 427 ha); comparable to the calculated average across Australia of 0.27 km−2 (Legge et al. 2017). Predator:prey ratios increased from ∼1:100 woylies in 2012 to 1:300 in 2018, with new recruits exceeding losses in the years 2011–2016. All cats detected were either known, or believed to, be males, and it is assumed that intraspecific aggression prevented more than a single male from establishing within the fenced area. Short and Turner (2005) reported that feral cats attempting to reinvade a reserve at Shark Bay were predominantly male (2.5 male:1 female).
There was no direct evidence of predation by feral cats on woylies at Wadderin, such as woylie carcases attributable to feral cats. However, there was significantly less recruitment of woylies in the area within 1.5 km of the centre of cat activity than in the area beyond. This area (∼300 ha) compares to an average home range (248 ha) occupied by feral cats in an agricultural landscape in central-western New South Wales (Molsher et al. 2005). The feral cat at Wadderin appeared to be having a localised impact on the woylie population. Contrary to prediction, the removal of the cat did not result in an increase in woylie numbers. Rather numbers stabilised and then declined. This reinforced the conclusion that woylies at Wadderin were regulated by their food supply.
Evidence for the impact of feral cats on woylie populations, and other bettong species, elsewhere are ambiguous (Abbott et al. 2014). Studies suggest that woylies are susceptible to cat predation when foxes are controlled (Priddel and Wheeler 2004; Marlow et al. 2015). However, Wayne et al. (2013) listed 10 populations of woylies in south-western Western Australia that were extant in 2010, some 5–28 years after establishment by translocation. These populations were in areas subject to intensive and regular fox control (Orell 2004), so have presumably persisted in the presence of expanding feral cat populations following meso-predator release.
The impact of feral cats on any particular prey population is likely to be influenced by a variety of factors, including top–down factors (density of cats, their functional and numerical responses, the availability of and preference for other prey species at any point in time, individual experience) and bottom–up factors (the prey’s realised rate of increase and changes in density of habitat and availability of shelter sites). Prey populations may be particularly vulnerable to predation when other factors increase mortality and reduce fecundity. For example, cat predation often interacts with drought, when productivity of native mammals often declines markedly (Short 2016).
Managing feral cat density to some threshold value below which bettongs persist is not an easy task; the threshold is likely to change over time with drought and over-population and the technologies for cat control are far from precise. If managers are able to restrict a feral cat population within a fenced reserve to male-only (or one sex sterilised), this precludes a numerical response by the cat population and can potentially provide a level of predation that may permit persistence while enhancing the bettongs’ anti-predator response (Tay et al. 2021).
Conclusions
The woylie was successfully translocated to Wadderin Sanctuary and has been extant there for 12 years. Woylies established despite the presence of feral cats within the sanctuary, with cats being present at a density equivalent to their estimated mean density across Australia. Woylies showed an irruptive pattern of growth, growing strongly, to reach plateau at 0.7 ha−1, before subsequently declining. Changing body condition and reproductive parameters suggested that the species had overshot its food supply and this was exacerbated by drought. Hence, woylies at Wadderin appear to be regulated by their food supply. This seems to be a common pattern of growth in newly reintroduced and recovering populations of bettong not subject to high levels of predation and suggests the need for management intervention (either harvest or predation from a fixed density population of feral cats) during the upswing in numbers to prevent over-abundance with detrimental impact on food resources and the wellbeing of the reintroduced population.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research was funded by Wildlife Research and Management with support from the Western Australian State Natural Resource Management Program and from the Australian Government’s National Landcare Program (21st Anniversary Landcare Grant).
Acknowledgements
I thank Andrew Hide for assisting with the translocation of woylies and with initial monitoring; the Narembeen community, particularly Brian Cusack, for assistance throughout, numerous volunteers for assistance with monitoring, staff at Department of Parks and Wildlife for facilitating translocations, and A.R.E. Sinclair and three anonymous referees for providing comments on an earlier draft of this manuscript.
References
Abbott IJ, Peacock D, Short J (2014) The new guard: the arrival and impacts of cats and foxes. In ‘Carnivores of Australia’. (Eds AS Glen, CR Dickman) pp. 69–104. (CSIRO Publishing: Melbourne, Vic., Australia)Armstrong D (2008) 4.7 Venus Bay Peninsula (SA) woylie summary. In ‘Progress report of the woylie conservation research project: diagnosis of recent woylie (Bettongia penicillata ogilbyi) decline in southwestern Australia’. pp. 199–202. (Department of Environment and Conservation: Perth, WA, Australia)
Australian Wildlife Conservancy (2006–2010) Karakamia Sanctuary annual reports. Australian Wildlife Conservancy Pty Ltd, Perth, WA, Australia.
Caughley G (1976) Wildlife management and the dynamics of ungulate populations. In ‘Applied biology. Vol. 1’. (Ed. TH Coaker) pp. 183–246. (Academic Press: London, UK)
Christensen P (1995) Brush-tailed bettong Bettongia penicillata Gray, 1837. In ‘The mammals of Australia’. (Ed. R Strahan) pp. 292–293. (Australian Museum/Reed Books: Sydney, NSW, Australia)
Davis JE (2005) Mycophagy in the brushtail possum Trichosurus vulpecula leading to dietary overlap with the woylie Bettongia penicillata in Dryandra Woodland, Western Australia. BSc(Hons) thesis, Murdoch University, Perth, WA, Australia.
Delroy, LB, Earl, J, Radbone, I, Robinson, AC, and Hewett, M (1986). The breeding and reestablishment of the brush-tailed bettong, Bettongia penicillata, in South-Australia. Australian Wildlife Research 13, 387–396.
| The breeding and reestablishment of the brush-tailed bettong, Bettongia penicillata, in South-Australia.Crossref | GoogleScholarGoogle Scholar |
de Tores P (2020) Native fauna response to large scale fox control in the northern jarrah forest of south-west Western Australia: Operation Foxglove. PhD thesis, University of New South Wales, Sydney, NSW, Australia.
de Tores PJ, Start AN (2008) Woylie Bettongia penicillata Gray, 1837. In ‘The mammals of Australia’. (Eds S Van Dyck, R Strahan) pp. 291–292. (Reed New Holland: Australia)
Duncan, RP, Dexter, N, Wayne, A, and Hone, J (2020). Eruptive dynamics are common in managed mammal populations. Ecology 101, e03175.
| Eruptive dynamics are common in managed mammal populations.Crossref | GoogleScholarGoogle Scholar |
Eikelboom TH (2010) A field comparison of survey methods for estimating the population density of woylies (Bettongia penicillata) at Karakamia Wildlife Sanctuary, Western Australia. Honours thesis, University of Western Australia, Perth, WA, Australia.
Finlayson GR, Finlayson ST, Dickman CR (2010) Returning the rat-kangaroos: translocation attempts in the family Potoroidae (superfamily Macropdoidea) and recommendations for conservation. In ‘Macropods: the biology of kangaroos, wallabies and rat-kangaroos’. (Eds G Coulson, MDB Eldridge) pp. 245–262. (CSIRO Publishing: Melbourne, Vic., Australia)
Gallucci M (2019) GAMLj: general analyses for linear models [jamovi module]. Available at https://gamlj.github.io/
Groom, C (2010). Justification for continued conservation efforts following the delisting of a threatened species: a case study of the woylie, Bettongia penicillata ogilbyi (Marsupialia:Potoroidae). Wildlife Research 37, 183–193.
| Justification for continued conservation efforts following the delisting of a threatened species: a case study of the woylie, Bettongia penicillata ogilbyi (Marsupialia:Potoroidae).Crossref | GoogleScholarGoogle Scholar |
Johnson, CN (1994). Mycophagy and spore dispersal by a rat-kangaroo: consumption of ectomycorrhizal taxa in relation to their abundance. Functional Ecology 8, 464–468.
| Mycophagy and spore dispersal by a rat-kangaroo: consumption of ectomycorrhizal taxa in relation to their abundance.Crossref | GoogleScholarGoogle Scholar |
Kemp, JE, Jensen, R, Hall, ML, Roshier, D, and Kanowski, J (2021). Consequences of the reintroduction of regionally extinct mammals for vegetation composition and structure at two established reintroduction sites in semi-arid Australia. Austral Ecology 46, 653–669.
| Consequences of the reintroduction of regionally extinct mammals for vegetation composition and structure at two established reintroduction sites in semi-arid Australia.Crossref | GoogleScholarGoogle Scholar |
Kinnear, JE, Sumner, NR, and Onus, ML (2002). The red fox in Australia: an exotic predator turned biocontrol agent. Biological Conservation 108, 335–359.
| The red fox in Australia: an exotic predator turned biocontrol agent.Crossref | GoogleScholarGoogle Scholar |
Krebs CJ (1999) ‘Ecological methodology.’ (Addison Wesley Longman: Menlo Park, CA, USA)
Krebs CJ (2002) ‘Programs for ecological methodology.’ 2nd edn. Available at https://www.zoology.ubc.ca/∼krebs/books.html
Krebs, CJ, and Singleton, GR (1993). Indexes of condition for small mammals. Australian Journal of Zoology 41, 317–323.
| Indexes of condition for small mammals.Crossref | GoogleScholarGoogle Scholar |
Legge, S, Murphy, BP, McGregor, H, Woinarski, JCZ, Augusteyn, J, Ballard, G, Baseler, M, Buckmaster, T, Dickman, CR, Doherty, T, Edwards, G, Eyre, T, Fancourt, BA, Ferguson, D, Forsyth, DM, Geary, WL, Gentle, M, Gillespie, G, Greenwood, L, Hohnen, R, Hume, S, Johnson, CN, Maxwell, M, McDonald, PJ, Morris, K, Moseby, K, Newsome, T, Nimmo, D, Paltridge, R, Ramsey, D, Read, J, Rendall, A, Rich, M, Ritchie, E, Rowland, J, Short, J, Stokeld, D, Sutherland, DR, Wayne, AF, Woodford, L, and Zewe, F (2017). Enumerating a continental-scale threat: how many feral cats are in Australia? Biological Conservation 206, 293–303.
| Enumerating a continental-scale threat: how many feral cats are in Australia?Crossref | GoogleScholarGoogle Scholar |
Linley, GD, Moseby, KE, and Paton, DC (2017). Vegetation damage caused by high densities of burrowing bettongs (Bettongia lesueur) at Arid Recovery. Australian Mammalogy 39, 33–41.
| Vegetation damage caused by high densities of burrowing bettongs (Bettongia lesueur) at Arid Recovery.Crossref | GoogleScholarGoogle Scholar |
Marlow, NJ, Thomas, ND, Williams, AAE, Macmahon, B, Lawson, J, Hitchen, Y, Angus, J, and Berry, O (2015). Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control. Australian Journal of Zoology 63, 18–27.
| Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control.Crossref | GoogleScholarGoogle Scholar |
Mawson, PR (2004). Translocations and fauna reconstruction sites: Western Shield review: February 2003. Conservation Science Western Australia 5, 108–121.
Molsher, R, Dickman, C, Newsome, A, and Müller, W (2005). Home ranges of feral cats (Felis catus) in central-western New South Wales. Wildlife Research 32, 587–595.
| Home ranges of feral cats (Felis catus) in central-western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Morris KD, Page M, Kay R, Renwick J, Desmond A, Comer S, Burbidge A, Kuchling G, Sims C (2015) Forty years of fauna translocations in Western Australia: lessons learned. In ‘Advances in reintroduction biology of Australian and New Zealand fauna’. (Eds DP Armstrong, MW Hayward, D Moro, PJ Seddon) pp. 217–235. (CSIRO Publishing: Melbourne, Vic., Australia)
Moseby, KE, Lollback, GW, and Lynch, CE (2018). Too much of a good thing; successful reintroduction leads to overpopulation in a threatened mammal. Biological Conservation 219, 78–88.
| Too much of a good thing; successful reintroduction leads to overpopulation in a threatened mammal.Crossref | GoogleScholarGoogle Scholar |
Nelson LS, Storr RF, Robinson AC (1992) ‘Plan of management for the brush-tailed bettong, Bettongia penicillata Gray, 1837 (Marsupialia, Potoridae) in South Australia.’ (Department of Environment and Planning: Adelaide, SA, Australia)
Orell, P (2004). Fauna monitoring and staff training: Western Shield review: February 2003. Conservation Science Western Australia 5, 51–95.
Priddel, D, and Wheeler, R (2004). An experimental translocation of brush-tailed bettongs (Bettongia penicillata) to western New South Wales. Wildlife Research 31, 421–432.
| An experimental translocation of brush-tailed bettongs (Bettongia penicillata) to western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Richards, JD, Gardner, T, and Copley, M (2009). Bringing back the animals. Landscope 24, 54–61.
Robertson, G (1986). The mortality of kangaroos in drought. Australian Wildlife Research 13, 349–354.
| The mortality of kangaroos in drought.Crossref | GoogleScholarGoogle Scholar |
Robinson AC, Canty PD, Moody T, Rudduck P (1996) ‘South Australia’s offshore islands.’ (Department of Environment and Natural Resources, South Australia & Australia Heritage Commission: Adelaide, SA, Australia)
Sampson JC (1971) The biology of Bettongia penicillata Gray, 1837. PhD thesis, University of Western Australia, Perth, WA, Australia.
Short, J (1998). The extinction of rat-kangaroos (Marsupialia:Potoroidae) in New South Wales, Australia. Biological Conservation 86, 365–377.
| The extinction of rat-kangaroos (Marsupialia:Potoroidae) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Short, J (2016). Predation by feral cats key to the failure of a long-term reintroduction of the western barred bandicoot (Perameles bougainville). Wildlife Research 43, 38–50.
| Predation by feral cats key to the failure of a long-term reintroduction of the western barred bandicoot (Perameles bougainville).Crossref | GoogleScholarGoogle Scholar |
Short, J, and Smith, A (1994). Mammal decline and recovery in Australia. Journal of Mammalogy 75, 288–297.
| Mammal decline and recovery in Australia.Crossref | GoogleScholarGoogle Scholar |
Short, J, and Turner, B (2005). Control of feral cats for nature conservation. IV. Population dynamics and morphological attributes of feral cats at Shark Bay, Western Australia. Wildlife Research 32, 489–501.
| Control of feral cats for nature conservation. IV. Population dynamics and morphological attributes of feral cats at Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Short, J, Bradshaw, SD, Giles, J, Prince, RIT, and Wilson, GR (1992). Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia: a review. Biological Conservation 62, 189–204.
| Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Short, J, Turner, B, and Majors, C (1998). The fluctuating abundance of endangered mammals on Bernier and Dorre Islands, Western Australia - conservation implications. Australian Mammalogy 20, 53–61.
| The fluctuating abundance of endangered mammals on Bernier and Dorre Islands, Western Australia - conservation implications.Crossref | GoogleScholarGoogle Scholar |
Tay, NE, Fleming, PA, Warburton, NM, and Moseby, KE (2021). Predator exposure enhances the escape behaviour of a small marsupial, the burrowing bettong. Animal Behaviour 175, 45–56.
| Predator exposure enhances the escape behaviour of a small marsupial, the burrowing bettong.Crossref | GoogleScholarGoogle Scholar |
The Jamovi Project (2021) jamovi (version 2.2) [Computer Software]. Available at https://www.jamovi.org
Thompson, CK, Wayne, AF, Godfrey, SS, and Thompson, RCA (2015). Survival, age estimation and sexual maturity of pouch young of the brush-tailed bettong (Bettongia penicillata) in captivity. Australian Mammalogy 37, 29–38.
| Survival, age estimation and sexual maturity of pouch young of the brush-tailed bettong (Bettongia penicillata) in captivity.Crossref | GoogleScholarGoogle Scholar |
Wayne, AF, Maxwell, MA, Ward, CG, Vellios, CV, Ward, BG, Liddelow, GL, Wilson, I, Wayne, JC, and Williams, MR (2013). Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wildlife Research 40, 169–183.
| Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata.Crossref | GoogleScholarGoogle Scholar |
Williams J, Donaldson F (2008) Boodieful Island: an account of the success of a boodie (Bettongia lesueur) translocation to Faure Island, WA. In ‘Proceedings of the 21st Australasian Wildlife Management Society Conference’. (Ed. AS Glen) p. 135. (Australasian Wildlife Management Society)
Woinarski JCZ, Burbidge AA, Harrison PL (2014) ‘The action plan for Australian mammals 2012.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Woinarski, JCZ, Burbidge, AA, and Harrison, RL (2015). Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112, 4531–4540.
| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
Yeatman G (2010) Demographic changes of a woylie (Bettongia penicillata) population: a response to increasing density and climate drying? Honours thesis, University of Western Australia, Perth, WA, Australia.
Yeatman, GJ, and Wayne, AF (2015). Seasonal home range and habitat use of a critically endangered marsupial (Bettongia penicillata ogilbyi) inside and outside a predator-proof sanctuary. Australian Mammalogy 37, 157–163.
| Seasonal home range and habitat use of a critically endangered marsupial (Bettongia penicillata ogilbyi) inside and outside a predator-proof sanctuary.Crossref | GoogleScholarGoogle Scholar |
Zosky KL (2011) Food resources and the decline of woylies Bettongia penicillata ogilbyi in southwestern Australia. PhD thesis, Murdoch University, Perth, WA, Australia.
Zosky, KL, Wayne, AF, Bryant, KA, Calver, MC, and Scarff, FR (2018). Diet of the critically endangered woylie Bettongia penicillata ogilbyi in south-western Australia. Australian Journal of Zoology 65, 302–312.
| Diet of the critically endangered woylie Bettongia penicillata ogilbyi in south-western Australia.Crossref | GoogleScholarGoogle Scholar |


