Identifying important environmental variables in the niche partitioning of two keystone ecosystem engineers (Bettongia gaimardi and Potorous tridactylus) in Tasmania
Isaac Standaloft A * and Jamie B. Kirkpatrick A
A * and Jamie B. Kirkpatrick A
A School of Geography, Planning, and Spatial Sciences, University of Tasmania, Sandy Bay, Tas. 7005, Australia.
Wildlife Research 50(7) 507-516 https://doi.org/10.1071/WR21110
Submitted: 10 August 2021 Accepted: 2 June 2022 Published: 14 July 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The eastern bettong (Bettongia gaimardi) and the long-nosed potoroo (Potorous tridactylus) are mycophagous marsupials regarded as both keystone species and ecosystem engineers. Despite Tasmania being a refuge for these declining species, their niche partitioning is poorly understood.
Aims: Our aim was to identify factors that distinguish the distributions of B. gaimardi and P. tridactylus, and to develop a better explanation of their individual niches.
Methods: The Department of Primary Industries, Parks, Water and Environment conducted mammal surveys between 1975 and 2019. We used GIS to analyse these data, and geospatial information to identify relationships between B. gaimardi and P. tridactylus presence/absence and environmental variables. We then developed a model describing the distributions of these species in Tasmania.
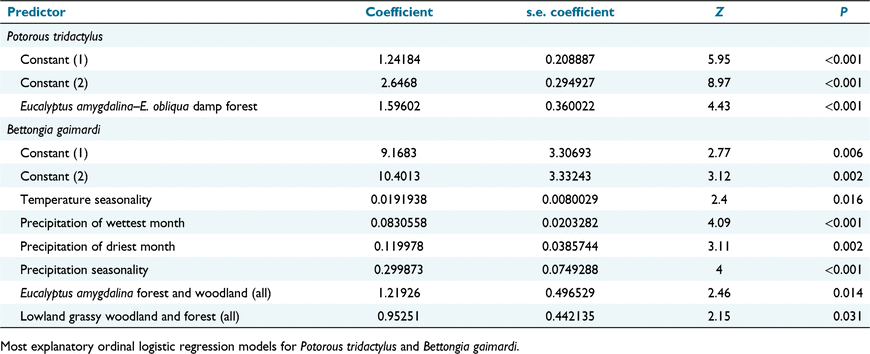
Key results: Temperature seasonality (s.d. × 100), precipitation of wettest month (mm), precipitation of the driest month (mm), precipitation seasonality (coefficient of variation), the presence of vegetation dominated by Eucalyptus amygdalina and the presence of lowland grassy woodland/forest were the components in the best model for B. gaimardi. Our model broadly predicts that the distribution of B. gaimardi is restricted to the more fertile eastern half of Tasmania. P. tridactylus was associated with very few variables, with the presence of E. amygdalina–Eucalyptus obliqua damp forest being the only component in a very weak model. Transects with P. tridactylus and not B. gaimardi were more associated with rainforest and wet forest communities and areas of higher annual and wettest-month precipitation than were those with B. gaimardi and not P. tridactylus.
Conclusions: The importance of infertile sites to B. gaimardi may have been overstated in the literature, with moderate to high fertility being more characteristic of its range. B. gaimardi is adapted to persist in environments of low truffle (food) density, typical of the eastern half of Tasmania, through its ability to adopt a larger home range than for P. tridactylus, which requires dense ground vegetation.
Implications: Sites of high fertility in fragmented landscapes should be considered to be potential habitat for B. gaimardi. This challenges previous assumptions that infertile sites are the primary habitat of the species, with fertile sites offering poorer-quality habitat.
Keywords: biogeography, conservation, ecological niche, ecology, geographical range, habitat preference, modelling, spatial ecology, threatened species, wildlife management.
Introduction
Since 1788, Australia has been subject to some of the most rapid extinctions of small mammals in modern history (Woinarski et al. 2015). Many of the destructive processes responsible for decline in these species continue (Wintle et al. 2019). The protection of remaining populations of a species cannot be achieved without a detailed understanding of their niche (Wiens et al. 2010). Without understanding the specific needs of a threatened species, we risk insufficient coverage of their habitats in protected areas (Possingham et al. 2006).
The eastern bettong (Bettongia gaimardi, Desmarest 1822) and the long-nosed potoroo (Potorous tridactylus, Kerr 1792) are nocturnal marsupials, historically restricted to temperate eastern Australia and Tasmania (Rose and Rose 1998; Trent 2015; Frankham et al. 2016). Although two subspecies are recognised for B. gaimardi, only the Tasmanian subspecies remains extant (Rose and Rose 1998). Three subspecies are recognised for P. tridactylus, two on mainland Australia and one in Tasmania, the latter of which occurs in two genetically distinct populations (Frankham et al. 2016). B. gaimardi and P. tridactylus average 1.6–1.8 kg and 0.6–1.64 kg respectively (Rose and Rose 1998; Johnston 2010, p. 95). Sexual dimorphism is pronounced only in P. tridactylus, with males larger than females (Norton et al. 2010a). Whereas P. tridactylus has an average home range of 2–19.4 ha, B. gaimardi has an unusually large home range for its size, at 35–149 ha. In both species, males often occupy a larger home range, incorporating those of multiple females (Kitchener 1973; Rose and Rose 1998; Norton et al. 2010a; Gardiner et al. 2019b). Reproduction in both species occurs year-round (Bennett 1987; Rose and Rose 1998).
Bettongia gaimardi and P. tridactylus share a largely mycophagous diet. Although non-fungal elements such as seeds, insects, foliage and tree sap may be included in the diet, this material is taken less frequently, or to supplement the diet in times of scarcity (Taylor 1992; Johnson 1994d; Tory et al. 1997; Rose and Rose 1998). Most of the diet is made up by the fruiting bodies (hereafter truffles) of ectomycorrhizal fungi, especially those associated with eucalypts, such as the Mesophelliaceae (Claridge et al. 1992, 1993; Johnson 1994c; Tory et al. 1997; Andren et al. 2018). This dietary preference is supported by a well-developed olfactory sense, used to locate truffles, and forelimbs adapted to digging (Donaldson and Stoddart 1994; Rose and Rose 1998; Vernes and Jarman 2014). In addition, the enlarged foregut of B. gaimardi may be specifically adapted to optimise digestion of truffles, which are otherwise nutritionally deficient (Rose and Rose 1998). This may explain the positive effect on adult body condition and growth rate in pouch young when truffle consumption is increased (Johnson 1994d).
The habitat of B. gaimardi and P. tridactylus is very likely to be limited by truffle availability (Taylor 1992; Tory et al. 1997; Rose and Rose 1998; Claridge 2002). As the presence and abundance of ectomycorrhizal fungi are influenced by precipitation and soil fertility, B. gaimardi and P. tridactylus habitat is also predicted by these factors (Slankis 1974; Bougher 1995). However, whereas B. gaimardi is common over a wide temperature range and in areas of lower rainfall (Taylor 1993a; Proft et al. 2021), P. tridactylus is associated with higher rainfall and milder temperatures (Claridge et al. 1993, 2019; Claridge and Barry 2000; Robley et al. 2014). This means that B. gaimardi is recorded largely from dry sclerophyll habitats in Tasmania (Rose and Rose 1998). An open understorey and high tree canopy cover are associated with foraging by B. gaimardi and areas of dense vegetation are important for denning (Gardiner et al. 2019a). P. tridactylus is associated with a broader range of habitats than is B. gaimardi, such as heathy woodland and rainforest (Taylor et al. 1985; Andren et al. 2013). The dependency of this species on dense ground cover and high tree canopy cover is well documented (Norton et al. 2010b; Andren et al. 2018; Claridge et al. 2019). Furthermore, there is evidence that communities with greater structural heterogeneity support larger populations of P. tridactylus (Norton et al. 2010b; Andren et al. 2018).
Bettongia gaimardi and P. tridactylus are both keystone species and ecosystem engineers. The symbiotic relationship between plants and ectomycorrhizal fungi is important for the health of forest communities (Claridge 2002). Through depositing viable spores in their faeces, B. gaimardi and P. tridactylus are important dispersal agents for ectomycorrhizal fungi (Claridge et al. 1992; Johnson 1994c). Mesophelliaceae appears particularly dependent on spore dispersal by mammals, which may explain why this is the most common family in both the diet of B. gairmardi and in southern dry sclerophyll forests (Johnson 1994c). Additionally, passage through the gut of a mycophagous mammal may be necessary for spore germination in some Mesophelliaceae species (Claridge et al. 1992). Some of these ectomycorrhizal fungi significantly increase truffle production after a fire event. This increase in production is followed by immediate population growth (through emigration), and truffle consumption, in B. gaimardi and P. tridactylus (Claridge et al. 1992; Johnson 1995, 1997). An increase in spore production and dispersal ensures that fungi will be spread widely, ready to rapidly inoculate regenerating plants. Finally, B. gaimardi and P. tridactylus are true ecosystem engineers in their ability to improve soil quality through turn-over, aeration, moderation of soil-surface temperature, and by providing a germination niche for native seedlings (Davies et al. 2019; Munro et al. 2019; Ross et al. 2019, 2020).
Bettongia gaimardi is extinct on mainland Australia and the Tasmanian population has reduced genetic diversity (Rose and Rose 1998; Proft et al. 2021). Although still extant, the decline of P. tridactylus on mainland Australia is well documented (i.e. Andren et al. 2013, 2018) and populations have become genetically isolated since European settlement (Frankham et al. 2016). Both species fall within the 450–5000 g weight range on which fox (Vulpes vulpes, Linnaeus 1758) predation is suggested to be substantial (Dickman 1996; Rose and Rose 1998; Robley et al. 2014; Norton et al. 2015). Other contributors to decline include predation by the feral cat (Felis catus, Linneaus 1758) and dog (Canis lupus, Linneaus 1758), habitat loss and fragmentation, inappropriate fire regime and drought (Dickman 1996; Johnson 1997; Rose and Rose 1998; Norton et al. 2010b, 2015; Andren et al. 2013, 2018; Gardiner et al. 2019b; Proft et al. 2021). The mainland subspecies, P. tridactylus tridactylus, is listed as Vulnerable under the Environmental Protection and Biodiversity Conservation Act 1999, whereas B. gaimardi is listed as Vulnerable on the Mammal Action Plan and Near Threatened on the IUCN Red List (Woinarski et al. 2015).
While Tasmania is considered a refuge for B. gaimardi and P. tridactylus, precisely how their respective niches differ there is unclear. In the present study, we use a long-standing mammal data set and open-source geospatial information to improve understanding of the possible causes of distributions of these species. We aim to (1) identify a set of macro-scale vegetation, geology and climate variables for each species that best describe their distributions, and (2) use this information to better explain their individual niches.
Materials and methods
Study area
The present study was conducted on the main island of Tasmania. Tasmania has a temperate maritime climate. Average annual rainfall exceeds 1000 mm in the west, whereas rainfall in the eastern half of the island is lower and less reliable. Mean monthly daily maximum temperatures ranges from 18°C to 23°C in summer and from 9°C to 14°C in winter. Siliceous rocks dominate in the west. Basalt and limestone are common in the north-west. Dolerite dominates in the eastern and the central highlands with sedimentary rocks, recent sediments, Quaternary deposits, sandstone, and siltstone in places. Consequently, soil fertility is low in the west and low to high in the north-west, centre and east. A strong west to east vegetation gradient is present with moorland, wet eucalypt forest and rainforest in the west, dry forests and grassy woodland in the east and alpine and subalpine vegetation in the central highlands (Kitchener and Harris 2013).
The data set
The data for B. gaimardi and P. tridactylus was sourced from the ‘spotlight survey’ data set of the Department of Primary Industries, Parks, Water and Environment (DPIPWE). Surveys began in 1975 and, currently, 173 transects are surveyed annually on the main island of Tasmania (DPIPWE 2010). Transects are placed in strategic locations, which sought to inform wildlife management actions. Wildlife management for the control of herbivore impacts on primary industries is the present stated purpose of the survey. All transects occur along permanent sealed, gravel or forestry service roads, but avoid main roads and highways. The survey undersamples in the south-western third of the state, where there are fewer roads and therefore limited access to establish transect routes. This region of the state has a concentration of low-fertility soils and high rainfall; therefore, survey data are biased towards fertile dry areas of the state. Nevertheless, the data set covers most Tasmanian terrestrial environments.
Surveys are conducted between the third week in November and the end of December 40 min after sunset, avoiding conditions of high winds, rain, or fog. A driver, whose head is approximately 1.6 m from the ground, makes observations and operates a roof-mounted 100 W sealed beam spotlight, from a vehicle travelling 20 km/h. A passenger records all non-domestic mammal and nocturnal bird observations made by the driver, as well as weather condition, moon phase and traffic volume (DPIPWE 2010). Transects are approximately 10 km long. Mammal observations are attributed to a given transect only, rather than to a specific point location along a transect. Data from this long-term survey were provided in the form of a Microsoft Access database, which contained B. gaimardi and P. tridactylus presence records as well as coordinates of the approximate central point for each transect. A file with line features representing all transects was provided by DPIPWE.
This survey style was developed targeting the detection and enumeration of mid-sized Tasmanian native and introduced mammals such as kangaroo, wallaby and deer; however, all mammal observations are recorded and B. gaimardi and P. tridactylus sightings do consistently occur. Owing to their small size and the sometimes-dense nature of roadside vegetation, this method is not particularly efficacious for sightings and identification of these two species, especially P. tridactylus, of which the females can be mistaken for Isoodon obesulus and the males for Thylogale billardierii (Michael Driessen and Robbie Gaffney, pers. comm., 14 June 2020).
Vegetation, geology and climate data sets
We used the open source TASVEG 4.0 geospatial vegetation data set released in 2020 by DPIPWE (2020). This digital state-wide vegetation map comprises 157 mapping units (vegetation communities). It is interpreted from aerial photographs (Michaels 2006). TASVEG 4.0 data are provided as an Esri Shapefile. Detailed descriptions of vegetation communities can be found in the TASVEG companion manual (Kitchener and Harris 2013).
Geology was determined using the open-source Geology of Tasmania 1:250 000 scale geospatial data set provided by Mineral Resources Tasmania (https://www.mrt.tas.gov.au/products/digital_data). This state-wide data package is supplied as an ESRI Shapefile and is compiled from several sources. We used the ‘description’ field for our analysis, which provides a text description of the geological mapping units (Department of Energy Infrastructure and Resources 2014).
The Worldclim open-source database (Fick and Hijmans 2017) was used to determine climate values. Eight measurements of temperature and rainfall were used, following previous work on B. gaimardi climate range conducted by Proft et al. (2021). These were mean annual temperature, temperature seasonality (s.d. × 100), maximum temperature of the warmest month, minimum temperature of the coolest month, annual precipitation, precipitation seasonality, precipitation of the wettest month and precipitation of the driest month. The 2.5 min spatial resolution version of this data set was used, because this was determined to be an acceptable tradeoff between precision and file size.
Sampling approach
Spotlight survey data for B. gaimardi and P. tridactylus were taken from the Microsoft Access database and transferred to Microsoft Excel for manipulation. The number of records for each species was extracted from these data.
Data collection for environmental variables was achieved using Arc GIS Pro by Esri. The file containing all transect line features was loaded as well as vegetation, geology and climate geospatial data files. A polygon feature was created around each transect using the ‘buffer’ tool in Arc GIS Pro at 1 km for B. gaimardi and 0.5 km for P. tridactylus. These distances best represent the radii of the home ranges of these two species (Kitchener 1973; Rose and Rose 1998; Gardiner et al. 2019b). These polygons were then used to extract presence or absence of all vegetation and geological classes from the TASVEG 4.0 and Geology of Tasmania 1:250 000 spatial data sets by using the ‘summarise within’ tool in Arc GIS Pro. Presence or absence for each environmental variable within transect polygons was recorded in Excel for all transects, for both B. gaimardi and P. tridactylus.
The central point provided for each transect in the DPIPWE spotlight survey data set were used as the location at which climate variables were extracted. The ‘Zonal Statistics as Table’ tool was used to sample data from each of the eight Worldclim geospatial data sets for each transect. These data were stored in Excel and were used for both B. gaimardi and P. tridactylus analyses, because factoring in home range size was not necessary for climate data, given that the scale of grid cells within the Worldclim data set was larger than the two home-range sizes.
Data analysis
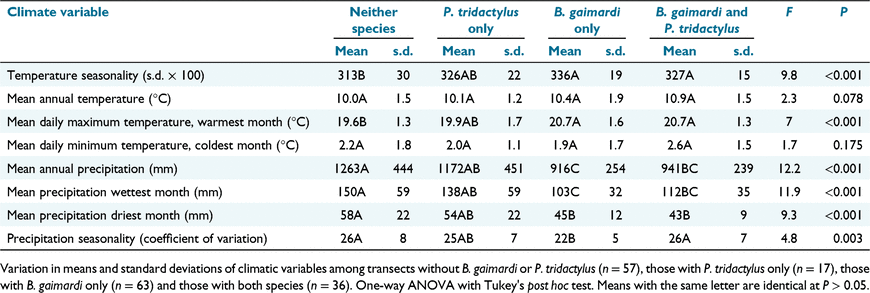
After examination of histograms, the data for B. gaimardi were converted to classes of record number (0, 1–2, >2), as was that for P. tridactylus (0, 1, >1). A variable was created to compare the distributions of the two species. The four classes of this variable were as follows: neither species; only P. tridactylus; only B. gaimardi; both species. The relationship of these four classes to each of the climate variables was determined using one-way ANOVA followed by Tukey’s multiple-range test in R (R Core Team 2020). Chi-squared analyses determined the relationships of these classes with each of the vegetation and geological types from the TASVEG and Geology of Tasmania data sets with sufficient data for the test in R (R Core Team 2020). Given the strong associations detected between B. gaimardi and Eucalyptus amygdalina communities and with lowland grassy woodland and forest communities, these TASVEG communities were consolidated into the following two aggregate groups: E. amygdalina forest and woodland (all) and lowland grassy woodland and forest (all). Where the overall chi-squared value was significant, the transects with only P. tridactylus were compared with those with only B. gaimardi, to gain insights into differences in their distributions.
Model fitting and selection
Ordinal logistic regression in the software package Minitab 16™ using default settings was used to reduce the variables to a best predictive model for each species, by successively removing the least significant component, until all slopes were significant
Results
P. tridactylus
Transects with at least one P. tridactylus record were distributed evenly, with a slight concentration around the Tamar River (Fig. 1). A model of very low explanatory power (37.6% concordant, 53.4% ties) consisted of only E. amygdalina–E. obliqua damp sclerophyll forest (Table 1).
|
|
|
B. gaimardi
The majority of transects with at least one observation of B. gaimardi occurred in the eastern half of the state, with the highest proportion of presence transects occurring in the Central Highlands and on the north-eastern coast (Fig. 2). The ordinal regression model was concordant in its predictions in 77.5% of cases and tied in 0.4%. The most significant components in the model were precipitation in the wettest month and rainfall seasonality, but the model also included rainfall in the driest month, temperature seasonality, E. amygdalina forest and woodland and lowland grassy woodland and forest (Table 1). In climate space (e.g. Fig. 3), there were eight transects with only one observation of the species mixed with the absences, all in high-rainfall areas in which there were no other observations of the species. These may be observation errors. In contrast, with the puzzling exception of the Deddington transects, the absences mixed with the presences in the graphs were from 12 areas where the species had been observed several times on other transects. The thresholds for more than two observations for the climatic variables in the model were <136 mm for precipitation of the wettest month, <34 for precipitation seasonality, >306 for temperature seasonality and <68 for precipitation of the driest month. The two vegetation types were positively associated with B. gaimardi.
|
|
Comparison of the distributions of the two species
The two species were discriminated in their climatic ranges by mean annual precipitation and precipitation in the wettest month, with P. tridactylus occurring in areas of higher rainfall than for B. gaimardi, but with major overlap of ranges in the lower-rainfall areas (Table 2). The standard deviations for climatic variables that are significantly different among the four classes are consistently higher for P. tridactylus by itself than for B. gaimardi by itself or the two species together (Table 2). P. tridactylus occurred at a higher percentage frequency than did B. gaimardi in many rainforest and wet eucalypt communities (Table 3), whereas the reverse pertained for eastern riparian scrub (Table 3).
|

|
Discussion
The association we identified between B. gaimardi presence and lowland dry eucalypt communities either dominated by E. amygdalina and/or with grassy understories is well established in the literature (Rose and Rose 1998; Davies et al. 2019; Gardiner et al. 2019a; Proft et al. 2021). The preference of B. gaimardi for tree-dominated vegetation with open understories (Johnson 1994a, 1997; Davies et al. 2019) is consistent with these associations.
Surprisingly, none of the dry eucalypt communities that we identified to be positively associated with B. gaimardi presence was noted to contain E. tenuiramis, despite B. gaimardi being previously observed in E. tenuiramis forests in Tasmania (Johnson 1994a, 1994b, 1994c, 1994d, 1997; Davies et al. 2019).
Gardiner et al. (2019a, 2019b) demonstrated that B. gaimardi adapts to disturbed landscapes so long as its resource needs are met. Therefore, where B. gaimardi habitat is replaced by disturbance-related communities, it may be that B. gaimardi is able to persist by adjusting its home range accordingly. We speculate that the ability of B. gaimardi to persist in disturbed landscapes may exist on a spectrum with an upper limit on the proportion of disturbed land to natural habitat that the species will tolerate.
Our results supported the consensus that B. gaimardi seldom occurs in wetter habitats (Rose and Rose 1998; Davies et al. 2019), unlike P. tridactylus. We identified a preference for dry and damp eucalypt communities by P. tridactylus. Eastern riparian scrub was preferred by B. gaimardi but not by P. tridactylus. This community usually occurs as linear vegetation in open or cleared areas. P. tridactylus avoids areas with low or no cover (Claridge et al. 1992, 2019; Norton et al. 2010b; Trent 2015; Andren et al. 2018). B. gaimardi may be able to utilise disturbed landscapes, whereas P. tridactylus may not (Gardiner et al. 2019a, 2019b). Our results confirmed that P. tridactylus is able to utilise a broader range of vegetation communities than is B. gaimardi, including vegetation types in areas of high rainfall (Rose and Rose 1998), such as rainforest (Taylor et al. 1985).
Eucalyptus ovata forest and woodland is listed as a threatened community under the Nature Conservation Act 2002. We found the two species to be associated with this community, emphasising the need to conserve the remaining fragments of E. ovata forest and woodland.
An association between B. gaimardi and sand or sandstone geology is recorded in the literature (Taylor 1993a; Johnson 1994a, 1994b, 1994c). The association between B. gaimardi and sand or sandstone geology and associated sandy soils has been explained by higher densities of ectomycorrhizal fungi on infertile sites (Slankis 1974), especially species in the family Mesophelliaceae, which disproportionately contribute to the diet of B. gaimardi (Johnson 1994c). Yet, two-third of the transects with B. gaimardi were at least partially underlain by Jurassic dolerite, with transects containing only the species having the highest concentration of this argillaceous rock. B. gaimardi is thus associated with a wide range of soil fertility.
Our results indicated that the distribution of B. gaimardi is strongly influenced by climate. There is a disparity between our work and that of Proft et al. (2021), who found precipitation in the driest quarter, minimum temperature of the coldest month and annual precipitation to be most associated with B. gaimardi occurrence. However, we did find precipitation of the driest month to be comparable to precipitation in the driest quarter reported by Proft et al. (2021). The higher number (254) and more varied sources of B. gaimardi records used by Proft et al. (2021) in constructing their Species Distribution Model (SDM) is likely to be the reason for any discrepancies. Examples from the literature support our modelled B. gaimardi climate range (Taylor 1992; Davies et al. 2019; Ross et al. 2019).
We identified no statistically significant relationships between P. tridactylus presence and any climate variables. This is contrary to work by Trent (2015), who found that habitat suitability was negatively correlated with mean annual temperature and positively correlated with mean annual precipitation and that these were the best predictors of presence, along with two environmental predictors. These environmental predictors, potential habitat within 1 km and undergrowth cover, were positively correlated with P. tridactylus habitat suitability. The difference from our results may reflect a greater range of annual precipitation at sites in Victoria and New South Wales from which P. tridactylus is reported (Claridge et al. 1993; Robley et al. 2014), than at our sites. It may be that P. tridactylus distribution is not easily predicted by climate in Tasmania within the climatic range of the observations we used.
Mycorrhizal fungi are known to achieve maximum infection and sporulation in soils of low fertility and high water availability (Slankis 1974). In Australia, the mycorrhizal fungi most common in both eucalypt communities and mycophagous mammal diets, Mesophelliaceae, show the same pattern of higher biomass in infertile wet environments and lower biomass in fertile dry environments (Taylor 1992; Johnson 1994b, 1994c; Bougher 1995). However, this ectomycorrhizal family is known to tolerate a wide range of conditions. Therefore, in most cases, suboptimal conditions will simply result in low abundance of Mesophelliaceae, rather than complete absence.
Over the past three decades, much of the work undertaken on B. gaimardi in Tasmania has been disproportionately focused on populations in areas with infertile soils, where truffle density, and therefore B. gaimardi density, is high (Johnson 1994a, 1994d, 1995; Rose and Rose 1998; Claridge and Barry 2000). Furthermore, many of these studies have been conducted at the same infertile site near Colebrook Tasmania. Most of these works refer to Taylor (1993a), who came to the conclusion that, although B. gaimardi distribution is extensive across the eastern half of the state, densities are highest on infertile sites. We believe that the emphasis in the literature on B. gaimardi density being higher on infertile sites does not accurately describe the breadth of the niche which B. gaimardi fills in the Tasmanian landscape.
Although B. gaimardi may take advantage of high food availability on infertile sites by increasing its own population density, our results suggest that these sites are not representative of the species’ habitat across the full extent of its distribution. Modelling by Taylor (1993a), Proft et al. (2021), and our own work, has amply demonstrated that B. gaimardi is widespread on fertile soils. The eastern half of Tasmania has higher soil fertility and lower rainfall than does the western half of the state, where B. gaimardi is largely absent, implying that B. gaimardi is broadly associated with areas of low truffle density.
Gardiner et al. (2019a, 2019b) showed that B. gaimardi has the ability to expand its home range in fragmented landscapes, so as to meet its resource requirements. B. gaimardi is also known to have a much larger home range than for many other small mammals of a similar size (Taylor 1993b; Rose and Rose 1998). Therefore, we postulate that the ability of B. gaimardi to adopt a larger home range to meet its resource requirements is indicative of the species niche. That is, B. gaimardi is adapted to persist in habitats of low truffle density where other mycophagous specialists are not, through its ability to travel large distances and its willingness to traverse unsuitable habitats in search of food.
Evidence in support of this hypothesis can be seen in the physiognomy of the species. Heavily muscled hind limbs with long tibiae and fibulae and elongated feet enable rapid movement over large distances (Rose and Rose 1998). These features may be particularly useful in open habitats, typical of the vegetation communities in eastern Tasmania. Conversely, these features may make moving through dense vegetation difficult. Therefore, dense habitats may favour P. tridactylus rather than B. gaimardi, such as at the Peter Murrell Reserves, south of Hobart (Driessen and Jarman 2014; Driessen et al. 2021). Furthermore, the smaller home range of P. tridactylus and unwillingness to go far from dense vegetation, makes it more suited than B. gaimardi to the wet and infertile western half of the state where truffle density is likely to be higher.
Conclusions
We have used a novel GIS approach to analyse a long-standing data set in a new way. B. gaimardi habitat in Tasmania can generally be described as open dry sclerophyll in the eastern half of the state. B. gaimardi is associated with fertile soils across the majority of its distribution. On the basis of these findings we suggest that B. gaimardi is adapted to persist in environments of low truffle density where other mycophagous mammals cannot, through its ability to adopt a larger home range. This is where the niche of B. gaimardi differs from that of P. tridactylus, which appears to favour wet and infertile environments where groundcover and truffle density are likely to be higher, meaning that P. tridactylus can meet its food requirements over a smaller home range. Although the overemphasis in the literature on B. gaimardi populations in infertile areas does highlight the importance of high-quality B. gaimardi habitat, care should be taken by land managers to avoid discounting fertile sites that represent the majority of its distribution. Furthermore, we found that E. ovata forest and woodland constitutes habitat for both species. Because this vegetation community is listed as threatened under the Nature Conservation Act 2002, our work emphasises the importance of conserving remaining fragments.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We thank the Department of Primary Industries, Parks Water and Environment for access to their invaluable spotlight survey data set. In particular, thanks go to Robbie Gaffney and Michael Driessen who happily gave their time throughout the research to provide input and assist in interpretation of the data set. We thank Mark Williams and Vanessa Adams of the School of Geography, Planning and Spatial Sciences, University of Tasmania, for their technical support in implementing Arc GIS Pro. Finally, we thank Chris Johnson of the School of Natural Sciences, University of Tasmania, for input from his wealth of knowledge.
References
Andren, M, Milledge, D, Scotts, D, and Smith, J (2013). The distribution of long-nosed potoroo Potorous tridactylus tridactylus habitat on the far north coast of New South Wales. Australian Zoologist 36, 494–506.| The distribution of long-nosed potoroo Potorous tridactylus tridactylus habitat on the far north coast of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Andren, M, Milledge, D, Scotts, D, and Smith, J (2018). The decline of the northern long-nosed Potoroo Potorous tridactylus tridactylus on the far north coast of New South Wales. Australian Zoologist 39, 414–423.
| The decline of the northern long-nosed Potoroo Potorous tridactylus tridactylus on the far north coast of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Bennett AF (1987) Conservation of mammals within a fragmented forest environment: the contributions of insular biogeography and autecology. In ‘Nature conservation: the role of remnants of native vegetation’. (Eds DA Saunders, GW Arnold, AA Burbidge, AJ Hopkins) pp. 41–52. (Surrey Beatty: Sydney, NSW, Australia)
Bougher NL (1995) Diversity of ectomycorrhizal fungi associated with eucalypts in Australia. In ‘Ectomycorrhizal research for forestry in Asia’. (Eds M Brundrett, B Dell, N Malajczuk, M Gong) pp. 8–15. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
Claridge, AW (2002). Ecological role of hypogeous ectomycorrhizal fungi in Australian forests and woodlands. Plant and Soil 244, 291–305.
| Ecological role of hypogeous ectomycorrhizal fungi in Australian forests and woodlands.Crossref | GoogleScholarGoogle Scholar |
Claridge, AW, and Barry, SC (2000). Factors influencing the distribution of medium-sized ground-dwelling mammals in southeastern mainland Australia. Austral Ecology 25, 676–688.
| Factors influencing the distribution of medium-sized ground-dwelling mammals in southeastern mainland Australia.Crossref | GoogleScholarGoogle Scholar |
Claridge, AW, Tanton, MT, Seebeck, JH, Cork, SJ, and Cunningham, RB (1992). Establishment of ectomycorrhizae on the roots of two species of Eucalyptus from fungal spores contained in the faeces of the long-nosed potoroo (Potorous tridactylus). Australian Journal of Ecology 17, 207–217.
| Establishment of ectomycorrhizae on the roots of two species of Eucalyptus from fungal spores contained in the faeces of the long-nosed potoroo (Potorous tridactylus).Crossref | GoogleScholarGoogle Scholar |
Claridge, AW, Cunningham, RB, and Tanton, MT (1993). Foraging patterns of the long-nosed potoroo (Potorous tridactylus) for hypogeal fungi in mixed-species and regrowth eucalypt forest stands in southeastern Australia. Forest Ecology and Management 61, 75–90.
| Foraging patterns of the long-nosed potoroo (Potorous tridactylus) for hypogeal fungi in mixed-species and regrowth eucalypt forest stands in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Claridge, AW, Paull, DJ, and Welbourne, DJ (2019). Elucidating patterns in the occurrence of threatened ground-dwelling marsupials using camera-traps. Animals 9, 913.
| Elucidating patterns in the occurrence of threatened ground-dwelling marsupials using camera-traps.Crossref | GoogleScholarGoogle Scholar |
Davies, GTO, Kirkpatrick, JB, Cameron, EZ, Carver, S, and Johnson, CN (2019). Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland. Royal Society Open Science 6, 180621.
| Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland.Crossref | GoogleScholarGoogle Scholar | 30800338PubMed |
Department of Energy, Infrastructure and Resources (2014) ‘Structure of datasets. Mineral resources Tasmania.’ (Department of Energy, Infrastructure and Resources)
DPIPWE (2010) ‘Spotlight survey manual.’ (Department of Primary Industries, Parks, Water and Environment)
DPIPWE (2020) ‘TASVEG 4.0.’ (Tasmanian Vegetation Monitoring and Mapping Program, Natural and Cultural Heritage Division, Department of Primary Industries, Parks, Water and Environment)
Dickman, CR (1996). Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 2, 185–195.
| Impact of exotic generalist predators on the native fauna of Australia.Crossref | GoogleScholarGoogle Scholar |
Donaldson, R, and Stoddart, M (1994). Detection of hypogeous fungi by Tasmanian bettong (Bettongia gaimardi: Marsupialia; Macropodoidea). Journal of Chemical Ecology 20, 1201–1207.
| Detection of hypogeous fungi by Tasmanian bettong (Bettongia gaimardi: Marsupialia; Macropodoidea).Crossref | GoogleScholarGoogle Scholar | 24242315PubMed |
Driessen MM, Jarman PJ (2014) Comparison of camera trapping and live trapping of mammals in Tasmanian coastal woodland and heath-land. In ‘Camera trapping: wildlife management and research’. (Eds PD Meek, PJS Flemming, G Ballard, P Banks, AW Claridge, J Sanderson, D Swann) pp. 253–262. (CSIRO Publishing: Melbourne, Vic., Australia)
Driessen, MM, Jarman, PJ, Visoiu, M, and Dewar, E (2021). Mammal responses to moderate-intensity planned burning in a small, isolated woodland reserve. Wildlife Research 48, 561–576.
| Mammal responses to moderate-intensity planned burning in a small, isolated woodland reserve.Crossref | GoogleScholarGoogle Scholar |
Fick, SE, and Hijmans, RJ (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37, 4302–4315.
| WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas.Crossref | GoogleScholarGoogle Scholar |
Frankham, GJ, Handasyde, KA, and Eldridge, MDB (2016). Evolutionary and contemporary responses to habitat fragmentation detected in a mesic zone marsupial, the long-nosed potoroo (Potorous tridactylus) in south-eastern Australia. Journal of Biogeography 43, 653–665.
| Evolutionary and contemporary responses to habitat fragmentation detected in a mesic zone marsupial, the long-nosed potoroo (Potorous tridactylus) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gardiner, R, Hamer, R, Leos-Barajas, V, Peñaherrera-Palma, C, Jones, ME, and Johnson, C (2019a). State-space modeling reveals habitat perception of a small terrestrial mammal in a fragmented landscape. Ecology and Evolution 9, 9804–9814.
| State-space modeling reveals habitat perception of a small terrestrial mammal in a fragmented landscape.Crossref | GoogleScholarGoogle Scholar | 31534695PubMed |
Gardiner, R, Proft, K, Comte, S, Jones, M, and Johnson, CN (2019b). Home range size scales to habitat amount and increasing fragmentation in a mobile woodland specialist. Ecology and Evolution 9, 14005–14014.
| Home range size scales to habitat amount and increasing fragmentation in a mobile woodland specialist.Crossref | GoogleScholarGoogle Scholar | 31938498PubMed |
Johnson, CN (1994a). Distribution of feeding activity of the Tasmanian bettong (Bettongia gaimardi) in relation to vegetation patterns. Wildlife Research 21, 249–255.
| Distribution of feeding activity of the Tasmanian bettong (Bettongia gaimardi) in relation to vegetation patterns.Crossref | GoogleScholarGoogle Scholar |
Johnson, C (1994b). Fruiting of hypogeous fungi in dry sclerophyll forest in Tasmania, Australia: seasonal variation and annual production. Mycological Research 98, 1173–1182.
| Fruiting of hypogeous fungi in dry sclerophyll forest in Tasmania, Australia: seasonal variation and annual production.Crossref | GoogleScholarGoogle Scholar |
Johnson, CN (1994c). Mycophagy and spore dispersal by a rat-kangaroo: consumption of ectomycorrhizal taxa in relation to their abundance. Functional Ecology 8, 464–468.
| Mycophagy and spore dispersal by a rat-kangaroo: consumption of ectomycorrhizal taxa in relation to their abundance.Crossref | GoogleScholarGoogle Scholar |
Johnson, CN (1994d). Nutritional ecology of a mycophagous marsupial in relation to production of hypogeous fungi. Ecology 75, 2015–2021.
| Nutritional ecology of a mycophagous marsupial in relation to production of hypogeous fungi.Crossref | GoogleScholarGoogle Scholar |
Johnson, CN (1995). Interactions between fire, mycophagous mammals, and dispersal of ectromycorrhizal fungi in Eucalyptus forests. Oecologia 104, 467–475.
| Interactions between fire, mycophagous mammals, and dispersal of ectromycorrhizal fungi in Eucalyptus forests.Crossref | GoogleScholarGoogle Scholar | 28307662PubMed |
Johnson, CN (1997). Fire and habitat management for a mycophagous marsupial, the Tasmanian bettong Bettongia gaimardi. Australian Journal of Ecology 22, 101–105.
| Fire and habitat management for a mycophagous marsupial, the Tasmanian bettong Bettongia gaimardi.Crossref | GoogleScholarGoogle Scholar |
Johnston PG (2010) Long-nosed potoroo. In ‘Field companion to the mammals of Australia’. (Eds S VanDyck, I Gynther, A Baker) pp. 95. (Reed New Holland: Sydney, NSW, Australia)
Kitchener, DJ (1973). Notes on the home range and movement in two small macropods, the Potoroo (Potorous apicalis) and the Quokka (Setonix brachyurus). Mammalia 37, 231–240.
| Notes on the home range and movement in two small macropods, the Potoroo (Potorous apicalis) and the Quokka (Setonix brachyurus).Crossref | GoogleScholarGoogle Scholar |
Kitchener A, Harris S (2013) ‘From forest to fjaeldmark: descriptions of Tasmania’s vegetation.’ 2nd edn. (Department of Primary Industries, Parks, Water and Environment: Tas., Australia)
Michaels K (2006) ‘A manual for assessing vegetation condition in Tasmania. Version 1.’ (Resource Management and Conservation, Department of Primary Industries, Water and Environment: Hobart, Tas., Australia)
Munro, NT, McIntyre, S, Macdonald, B, Cunningham, SA, Gordon, IJ, Cunningham, RB, and Manning, AD (2019). Returning a lost process by reintroducing a locally extinct digging marsupial. PeerJ 7, e6622.
| Returning a lost process by reintroducing a locally extinct digging marsupial.Crossref | GoogleScholarGoogle Scholar | 31179166PubMed |
Norton, MA, Claridge, AW, French, K, and Prentice, A (2010a). Population biology of the long-nosed potoroo (Potorous tridactylus) in the southern highlands of New South Wales. Australian Journal of Zoology 58, 362–368.
| Population biology of the long-nosed potoroo (Potorous tridactylus) in the southern highlands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Norton, MA, French, K, and Claridge, AW (2010b). Habitat associations of the long-nosed potoroo (Potorous tridactylus) at multiple spatial scales. Australian Journal of Zoology 58, 303–316.
| Habitat associations of the long-nosed potoroo (Potorous tridactylus) at multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Norton, MA, Prentice, A, Dingle, J, French, K, and Claridge, AW (2015). Population characteristics and management of the long-nosed potoroo (Potorous tridactylus) in high-quality habitat in the Southern Highlands of New South Wales. Australian Mammalogy 37, 67–74.
| Population characteristics and management of the long-nosed potoroo (Potorous tridactylus) in high-quality habitat in the Southern Highlands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Possingham HP, Wilson KA, Andelman SJ, Vynne CH (2006) Protected areas: goals, limitations, and design. In ‘Principles of conservation biology’. (Eds MJ Groom, GK Meefe, CR Carroll) pp. 516–519. (Sinauer Associates, Inc.: Sunderland, MA, USA)
Proft, KM, Bateman, BL, Johnson, CN, Jones, ME, Pauza, M, and Burridge, CP (2021). The effects of weather variability on patterns of genetic diversity in Tasmanian bettongs. Molecular Ecology 30, 1777–1790.
| The effects of weather variability on patterns of genetic diversity in Tasmanian bettongs.Crossref | GoogleScholarGoogle Scholar | 33590590PubMed |
R Core Team (2020) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org/
Robley, A, Gormley, AM, Forsyth, DM, and Triggs, B (2014). Long-term and large-scale control of the introduced red fox increases native mammal occupancy in Australian forests. Biological Conservation 180, 262–269.
| Long-term and large-scale control of the introduced red fox increases native mammal occupancy in Australian forests.Crossref | GoogleScholarGoogle Scholar |
Rose, RW, and Rose, RK (1998). Bettongia gaimardi. Mammalian Species 584, 1–6.
| Bettongia gaimardi.Crossref | GoogleScholarGoogle Scholar |
Ross, CE, Munro, NT, Barton, PS, Evans, MJ, Gillen, J, Macdonald, BCT, McIntyre, S, Cunningham, SA, and Manning, AD (2019). Effects of digging by a native and introduced ecosystem engineer on soil physical and chemical properties in temperate grassy woodland. PeerJ 7, e7506.
| Effects of digging by a native and introduced ecosystem engineer on soil physical and chemical properties in temperate grassy woodland.Crossref | GoogleScholarGoogle Scholar | 31497393PubMed |
Ross, CE, McIntyre, S, Barton, PS, Evans, MJ, Cunningham, SA, and Manning, AD (2020). A reintroduced ecosystem engineer provides a germination niche for native plant species. Biodiversity and Conservation 29, 817–837.
| A reintroduced ecosystem engineer provides a germination niche for native plant species.Crossref | GoogleScholarGoogle Scholar |
Slankis, V (1974). Soil factors influencing formation of mycorrhizae. Annual Review of Phytopathology 12, 437–457.
| Soil factors influencing formation of mycorrhizae.Crossref | GoogleScholarGoogle Scholar |
Taylor, RJ (1992). Seasonal changes in the diet of the Tasmanian bettong (Bettongia gaimardi), a mycophagous marsupial. Journal of Mammalogy 73, 408–414.
| Seasonal changes in the diet of the Tasmanian bettong (Bettongia gaimardi), a mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Taylor, RJ (1993a). Habitat requirements of the Tasmanian bettong (Bettongia gaimardi), a mycophagous marsupial. Wildlife Research 20, 699–710.
| Habitat requirements of the Tasmanian bettong (Bettongia gaimardi), a mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Taylor, RJ (1993b). Home range, nest use and activity of the Tasmanian bettong, Bettongia gaimardi. Wildlife Research 20, 87–95.
| Home range, nest use and activity of the Tasmanian bettong, Bettongia gaimardi.Crossref | GoogleScholarGoogle Scholar |
Taylor, RJ, Bryant, SL, Pemberton, D, and Norton, TW (1985). Mammals of the Upper Henty River region, Western Tasmania. Papers and Proceedings of the Royal Society of Tasmania 119, 7–14.
| Mammals of the Upper Henty River region, Western Tasmania.Crossref | GoogleScholarGoogle Scholar |
Tory, MK, May, TW, Keane, PJ, and Bennett, AF (1997). Mycophagy in small mammals: a comparison of the occurrence and diversity of hypogeal fungi in the diet of the long-nosed potoroo Potorous tridactylus and the bush rat Rattus fuscipes from southwestern Victoria, Australia. Australian Journal of Ecology 22, 460–470.
| Mycophagy in small mammals: a comparison of the occurrence and diversity of hypogeal fungi in the diet of the long-nosed potoroo Potorous tridactylus and the bush rat Rattus fuscipes from southwestern Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Trent SW (2015) Assessment of habitat factors and development of a species distribution model for the long nosed potoroo (Potorous tridactylus tridactylus) in SEQ. Master’s thesis, University of Southern Queensland, Toowoomba, Qld, Australia.
Vernes, K, and Jarman, P (2014). Long-nosed potoroo (Potorous tridactylus) behaviour and handling times when foraging for buried truffles. Australian Mammalogy 36, 128–130.
| Long-nosed potoroo (Potorous tridactylus) behaviour and handling times when foraging for buried truffles.Crossref | GoogleScholarGoogle Scholar |
Wiens, JJ, Ackerly, DD, Allen, AP, Anacker, BL, Buckley, LB, Cornell, HV, Damschen, EI, Davies, TJ, Grytnes, J-A, Harrison, SP, Hawkins, BA, Holt, RD, McCain, CM, and Stephens, PR (2010). Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13, 1310–1324.
| Niche conservatism as an emerging principle in ecology and conservation biology.Crossref | GoogleScholarGoogle Scholar | 20649638PubMed |
Wintle, BA, Cadenhead, NCR, Morgain, RA, Legge, SM, Bekessy, SA, Cantele, M, Possingham, HP, Watson, JEM, Maron, M, Keith, DA, Garnett, ST, Woinarski, JCZ, and Lindenmayer, DB (2019). Spending to save: what will it cost to halt Australia’s extinction crisis? Conservation Letters 12, e12682.
| Spending to save: what will it cost to halt Australia’s extinction crisis?Crossref | GoogleScholarGoogle Scholar |
Woinarski, JCZ, Burbidge, AA, and Harrison, PL (2015). A review of the conservation status of Australian mammals. Therya 6, 155–166.
| A review of the conservation status of Australian mammals.Crossref | GoogleScholarGoogle Scholar |


