Bringing back the endangered bridled nail-tailed wallaby at Taunton National Park (Scientific) through effective predator control
John Augusteyn A G , Michael A. McCarthy B , Alan Robley C , Anthony Pople D , Barry Nolan E , Graham Hemson A , Rhonda Melzer
A G , Michael A. McCarthy B , Alan Robley C , Anthony Pople D , Barry Nolan E , Graham Hemson A , Rhonda Melzer  A , Samuel Richards A and Andrew Dinwoodie F
A , Samuel Richards A and Andrew Dinwoodie F
A Queensland Parks and Wildlife Service, PO Box 3130, Red Hill, Qld 4701, Australia.
B School of BioSciences, The University of Melbourne, Vic. 3010, Australia.
C Arthur Rylah Institute for Environmental Research, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
D Biosecurity Queensland, Ecosciences Precinct, GPO Box 267, Brisbane, Qld 4001, Australia.
E Queensland Parks and Wildlife Service, PO Box 5332, Airlie Beach, Qld 4802, Australia.
F PO Box 56, Central Queensland University, Rockhampton, Qld 4701, Australia.
G Corresponding author. Email: John.Augusteyn@des.qld.gov.au
Wildlife Research 49(4) 382-398 https://doi.org/10.1071/WR21067
Submitted: 21 April 2021 Accepted: 19 October 2021 Published: 31 January 2022
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Context: Feral cats (Felis catus), wild dogs/dingoes (Canis familiaris) and foxes (Vulpes vulpes) are predators of the endangered bridled nail-tailed wallaby (BNTW; Onychogalea frenata). Predator-proof fencing is advocated as a solution to ensure their conservation in the wild.
Aims: The aims of this study were to determine whether predator control translated into a reduction in their activity, find evidence of cats preying on BNTWs and understand factors that influence changes in the BNTW population size living in an unfenced reserve, particularly focusing on the influence of cat and dog control and rainfall.
Methods: An activity index, calculated using spoor on sand pads and images on remote cameras, was undertaken to monitor predator activity. The stomach contents of cats caught were examined to determine how commonly BNTWs feature as a prey item. The size of the BNTW population and annual survival of individuals was assessed through annual capture–mark–recapture (CMR) surveys and vehicle spotlight counts. Rainfall was measured at the study site and using data from the Bureau of Meteorology.
Key results: The core BNTW population estimated by CMR data increased by 214% over 4 years (2013–2017), to 400 individuals in 2017, whereas spotlight data indicated that the population had increased by 262% over 8 years (2012–2020), to 1265 individuals in 2020. The percentage of small (≤3.5 kg) BNTWs caught increased substantially over the study period. There was no detectable difference in cat or dog activity following control and no correlation was found among predator activity, rainfall and BNTW survival. The remains of BNTWs were found in 20% of cats removed from the core BNTW area.
Conclusions: The study confirmed that cats frequently ate BNTWs, and a combination of control methods is required to manage the threat, but there was no statistical support for a relationship between predator activity and BNTW survival.
Implications: The study found that native species conservation in fox-free environments is possible without the need for predator-proof fences and the ongoing maintenance costs.
Keywords: predation, capture–mark–recapture, endangered species, conservation, feral cat, dingo, fox, Onychogalea, pest control.
Introduction
Feral cat (cat) predation is recognised as one of the most important threats to small to medium-sized mammals in Australia (Woinarski et al. 2014; Murphy et al. 2019; Woolley et al. 2019). Evidence from dietary studies (Stokeld et al. 2018; Doherty et al. 2019) and other species-specific studies (Oakwood 2000; Fisher et al. 2001; Augusteyn et al. 2021) indicates that wild dogs (dogs; includes dingoes) limit the recovery of some threatened species. Conservation agencies and several non-government groups have installed predator-proof fences to conserve threatened species but the cost of installing these fences is high, and the ongoing maintenance costs are not insignificant (Hayward and Kerley 2009; Roshier et al. 2020). These maintenance costs may even be higher than the ongoing cost of reducing predators in non-fenced areas. While trapping cats can reduce their abundance in the short term (Bengsen et al. 2011a), the long-term suppression of cats and dogs using trapping, or a combination of trapping, baiting and shooting, has not been demonstrated on mainland Australia in unfenced areas. Effective predator control is considered more likely when a suite of options is used in combination at an appropriate temporal and spatial scale (DEWHA 2008).
The BNTW is listed as endangered under the Queensland Nature Conservation Act (1992) and the Commonwealth Environment Protection and Biodiversity Conservation Act (1999) and vulnerable under the International Union for the Conservation of Nature Red List. BNTWs were thought to be extinct in 1937, until they were rediscovered in 1973 on Taunton Station (what is now Taunton National Park (Scientific); hereafter Taunton or the Park) near Dingo, in central Queensland (Gordon and Lawrie 1980). Initial surveys estimated the Taunton area population to be up to 600 (Tierney 1985). Spotlight surveys in the early 1990s estimated the population for the Park to be 1430 (Evans 1992). In 1993, the whole park estimate was just under a maximum of 2267 (on the basis of an extrapolation of density estimates), declining to <100 by 1998 (Lundie-Jenkins et al. 1998).
BNTWs previously ranged from the Murray River in Victoria to Charters Towers in northern Queensland and were considered common, at least over part of this range (Fig. 1). The young and juvenile individuals of this medium-sized wallaby (female and male BNTWs weigh up to 6 and 8 kg respectively; Evans and Gordon 2008) fall within the so-called ‘critical weight range’ (35−5500 g) of species that have declined in Australia as a result of predation (Burbidge and McKenzie 1989; Murphy and Davies 2014). Fisher et al. (2000, 2001) found that cat predation was the main cause of juvenile and subadult BNTW (up to 3.5 kg) mortality during drought periods, when ground cover was lacking. Cat predation accounted for ~31% (determined from 13 collared juvenile wallabies) of juvenile wallabies (<2 kg and 4–6-month-old from conception) and dogs killed 17% of adults (>6 months old) fitted with collars (55 wallabies were collared; Fisher et al. 2001). BNTW hair has been found in ~10% of dog scats at Taunton (Evans 1992; Bennison 2008). Until this study, no BNTW remains had been found in cat stomachs. In addition to direct mortality through predation, predators also spread zoonotic diseases such as toxoplasmosis (Toxoplasma gondii) and hydatids (Echinococcus granulosus; AWHN 2009; Fancourt and Jackson 2014; Ross et al. 2020). Foxes have not been detected on Taunton but are present on neighbouring or near-neighbouring properties (less than 5 km from the core BNTW area on the Park; C. Pearce, pers. comm., Warrigal Station; C. Burns, pers. comm., Charlevue Station). They are also killed on the Capricorn Highway and Fitzroy Developmental Road that run adjacent to the Park (G. Porter, pers. comm., J. Augusteyn, pers. obs.).

|
Effective predator control is considered essential to the BNTW’s recovery (Lundie-Jenkins and Lowry 2005). However, the amount and the scale of predator control on Taunton both before and after becoming a National Park has varied (Cameron 2005). Cats were not targeted for control on Taunton until 2013, other than through occasional spotlight shooting, and no cats were detected taking a bait during bait-uptake monitoring on Taunton in 2005 and 2006 (Bennison 2007). Sodium fluoroacetate (1080) was not registered for use on cats in Queensland until 2008 when the Queensland Curiosity Cat ® bait (125 g 1080 meat bait) was approved (limited to 5000 ha across the whole of Queensland). However, a recent study found that the prescribed method for deploying meat baits for cats on Taunton rendered them ineffective (Fancourt et al. 2021), even though three radio-collared cats took a bait on separate baiting events (B. Fancourt, pers. comm.). Prior to 2008, all baits deployed had to weigh more than 250 g, which is likely to have made them less effective on cats. Since 2011, 125 g meat baits have been used on Taunton for dog control, thereby making cat by-catch more probable. Between 2013 and 2016, a total of 19 dogs and 119 cats were either trapped or shot. Further details on the success of the different trap configurations can be found in Augusteyn and Nolan (2021).
Queensland Parks and Wildlife Service (QPWS) currently spends A$40 000 per year directly on pest animal management at Taunton (Nolan and Wykes 2019), which includes staff labour, travel, vehicle lease and fuel, and material costs. However, there is often debate regarding the costs and benefits of installing predator-proof fencing at Taunton as a long-term solution to conserve BNTWs. The aims of this study were to determine whether the effort to remove cats and dogs has reduced their activity and improved the viability of the BNTW population and, in doing so, determine whether it is possible to conserve BNTWs at Taunton without fencing. The results of a cat stomach analysis are also reported.
Materials and methods
Study area
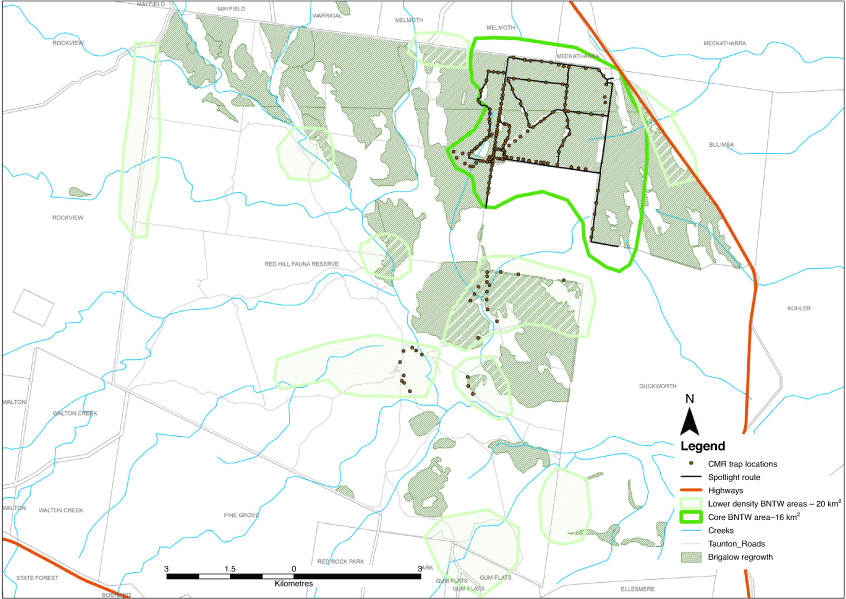
Taunton is situated in the Brigalow Belt Bioregion, and is located near the town of Dingo, central Queensland. The reserve covers an area of 11 676 ha. Today, a majority of the BNTWs live in the north-eastern corner of the Park, an area otherwise known as the core BNTW area (~16 km2; Fig. 2). Brigalow regrowth (Acacia harpophylla) and A. harpophylla–Eucalyptus cambageana woodland to open forest on fine-grained sedimentary rocks (Regional Ecosystem (RE) 11.9.1; Queensland Herbarium 2018) dominate this area. To improve food availability for BNTWs, weeds are actively controlled (Melzer 2015) and there is supplementary feeding in drought.
Rainfall data
The average rainfall for the area is 665 mm, which mostly falls between December and March (BOM 2019). Rainfall data were obtained from a weather station on the Park and using data recorded at the nearby Dingo Post Office and obtained from the Bureau of Meteorology (BOM 2019). The long-term average monthly rainfall data were obtained from the Bureau of Meteorology (BOM 2019). We calculated monthly deviations from the long-term average rainfall for that month to identify months of above- and below-average rainfall.
Predator activity
Thirty-eight sand pads were used to monitor cat and dog activity (Allen et al. 1996; Engeman and Allen 2000) between 2006 and 2016. The sand pads consisted of a rectangle of sand spread across the width of a road (~2–3 m wide) and were installed at 1-km intervals along tracks within the eastern section of Taunton, including the core BNTW area. All sand pads were raked to remove tracks before the start of the monitoring session and daily for at least three consecutive nights in February/March and again in May/June/July most years. No attractant was used. The sand pads were checked in the morning and an estimate of the number of animals that crossed each pad and left tracks was recorded. Rain and other factors sometimes limited the monitoring period to two nights, but sessions that were less than three nights were excluded from the analysis.
To assess changes in cat and dog activity over time, 38 Reconyx Hyperfire (HC600) camera traps were set within 100 m of existing sand pads between September 2012 and August 2015. No attractant was used. Each camera was mounted no higher than 60 cm above the ground on a star picket or tree situated on the edge of a road. Cameras were oriented so that they faced south at an angle of ~20°, to the direction of the track to avoid sunlight being directed into the lens in the morning and afternoon and to ensure that the 42° field of view of the camera covered the entire road. Cameras were set to ‘high’ trigger sensitivity and to record three still images, with a 1-s delay between each photo.
The number of cats or dogs observed per camera was used as an activity index and assumed to be proportional to their population sizes (Bengsen et al. 2011b; Meek et al. 2015). The cameras remained in place for 4–6 months and individual cameras operated for between 17 and 73 days within these deployments. A fault in the cameras compromised the sample size and we analysed data only from periods when at least 26 cameras were operating for at least 30 days, so as to ensure that these data were representative samples of the activity. There were insufficient pre-cat control data collected in comparable periods (months or seasons) to investigate a difference in the cat activity before (2012) and following (2013–2016) cat control.
To investigate whether there were any trends in cat and dog activity, daily counts of the estimated number of identifiable individual cats and dogs on sand pads or photographed using remote cameras were analysed with Bayesian generalised (non)-linear mixed models, using two possible distributions (negative binomial and zero-inflated negative binomial). These distributions were chosen to account for the relatively high proportion of zeroes in the data. We incorporated the approach developed by Engeman et al. (1998) to partition the variance component of the fixed effects, with ‘night’ and ‘sand plot’ being included as fixed effects. Models were run in the R (version 3.2.4) statistical software package ‘BRMS’ (Buerkner 2016).
Cat abundance
The pelt patterns of individual tabby cats caught on camera, set across half of the Park within the core BNTW area, were used to count cats in autumn and winter 2015. The number of cats was calculated by doubling the number of tabby cats because 50% of the cats caught in traps were black and the other 50% were tabby. Individual black cats are indistinguishable from the images recorded. This count is likely to represent half of the total cat population on Park owing to the coverage of the cameras.
Cat stomach content analysis
The stomach contents of cats caught were examined between 2013 and 2016 and, where possible, animals were identified to species level. Hair and other body parts that were not easily recognised in the field were sent either to the Queensland Museum or to Scats About (http://www.scatsabout.com.au) for identification.
BNTW population size
The BNTW population size for the core area was estimated using two methods, including capture–mark–recapture (CMR) and line-transect surveys. The four-day CMR surveys were undertaken between 2007 and 2017. The surveys were conducted biannually for the first 3 years (2007–2010) and annually thereafter. In total, 220 cage traps (38 cm × 38 cm × 76 cm) were set, mostly within the core BNTW area. Lucerne was placed in traps wired open for up to 3 weeks before each CMR survey, so as to habituate BNTWs to enter traps. Traps were placed along roads (usually not closer than 5 m to the road edge), with most being placed well away from the road edge and near or within shrub clumps. To prevent the similar-sized, black-striped wallaby (Notamacropus dorsalis) from entering the trap and interfering with the counts, an excluder, that consisted of weld mesh with a 100 mm × 400 mm slot cut into the bottom to allow BNTWs to enter was placed in front of each trap. Traps were checked and cleared two to three times each night throughout the survey. Captured animals were held in hessian bags for measuring and were marked with a microchip. All animals were released at the point of capture.
The MARK program was used to calculate BNTW population size (White and Burnham 1997). The data were treated as robust, where it was assumed that mortality of individuals can occur between trapping sessions (open), but not from night-to-night within trapping sessions (closed). Model selection was based on Akaike’s information criteria (AIC) and evidence of model convergence. Models with unrealistically narrow confidence intervals (<1 animal) were rejected (Anderson et al. 1994; Buckland et al. 1997). Pooled nightly data, rather than each individual trap-session data (two or three trap sessions per night), were analysed because of the short time between capture sessions and the need to maintain the MARK program assumption of statistical independence, i.e. the capture of an individual in a trap does not affect the capture of another animal in that session.
In addition to the modelled population estimates, we estimated the number of BNTWs ‘known to be alive’ (KTBA) by retrospectively adding animals to previous surveys that were caught in subsequent surveys and were alive at the time of the previous survey/s. The following criteria were used to add animals to estimate the KTBA in a survey (we will refer to the survey of interest as Survey A):
-
Animals not caught in Survey A, but captured in a preceding and subsequent survey.
-
Animals not caught in Survey A, but captured in a subsequent survey and whose weight indicated that they would have been alive during Survey A.
The age estimates used in (2) above were based on weight (weight age) and were very conservative. Some animals could have been older, but we used the lower-weight age to ensure that we did not overestimate the number KTBA. Males weighing >5 kg and females weighing >3.5 kg were considered to be at least 12 months old. Males weighing >3 kg but <5 kg and females weighing >3 kg but <3.5 kg were considered to be at least 6 months old.
The spotlight line-transect surveys were conducted along the same tracks (50–104 km divided into 10–14 transects) through the core BNTW area over four nights per survey, each year between 2012 and 2020 (except 2013, 2017 and 2019). Two observers each used 100 W spotlights and rangefinders to measure the perpendicular distance from the vehicle to the animal clusters. The surveys were conducted from the back of a vehicle travelling at 5–20 km h−1.
Spotlight data were analysed as conventional distance sampling in Distance 7.3 (Thomas et al. 2010) and by using AIC to select among the default recommended detection models. Data were truncated at 60 m. An area estimated to be occupied by BNTW (16 km2) was used to convert the density estimates to population size. Spotlight population estimates were not compared with CMR estimates of population size because of the lack of years when both types of data were collected (n = 4).
BNTW survival
Size-specific BNTW survival estimates were used to determine the effectiveness of management actions and the response of BNTWs to rainfall and indices of cat and dog abundance. BNTWs weighing 3.5 kg or less, at the time of capture, were analysed as ‘small’ and were conservatively considered to be within the prey range of cats. Animals above 3.5 kg were analysed as ‘large’.
Modelling of survival probabilities of BNTWs for each period between trapping sessions, and probabilities of trapping individuals conditional on their being alive is described in further detail in Appendix S1 (available as Supplementary material to this paper). The analysis allowed for the trapping probability to vary from session to session, and to potentially vary between males and females (for large individuals), and between large and small individuals. Models that had separate random effects for juvenile females and males (i.e. the fitted values were free to vary randomly from year to year) were fitted to determine the degree to which the cat and dog indices and rainfall explained the variation in the annual survival rate. The fitted values that included these separate random effects can be considered as ‘observed’ survival probabilities. These ‘observed’ survival probabilities were compared with the fitted values (without random effects) of the model.
Various modifications of the mark–recapture model were analysed. First, different measures of explanatory variables were trialled. Different measures of rainfall were used (actual rainfall, residual rainfall, and a power mean of rainfall with an exponent of 0.1 rather than a simple arithmetic mean to place more weight on low-rainfall periods). Power means were calculated by raising each number to a power A (in this case A = 0.1), taking the arithmetic mean of these values, and then raising that result to the power 1/A. The explanatory variables (cats, dogs, rainfall) were also converted to binary values in some versions of the analysis to distinguish between high (>1 standard deviation above the mean) and lower values. In these latter cases, the values were classified as high in few time periods, which meant that the model parameters were commonly non-identifiable; without sufficient replication of the high index class, it was not possible to estimate the effects of cats separately from that of dogs. Second, in cases where the data were sufficient to estimate the parameters, the results were qualitatively the same as when the variables were treated as continuous variables. Thus, these additional models are not discussed further. The final model allowed the survival rate to vary among juveniles and sexes, but the trapping probability was the same for all individuals. A model that allowed for differences between ages and sexes in the trapping probability produced qualitatively similar results; so, this paper focuses on the simpler models.
Results
Cat and dog activity
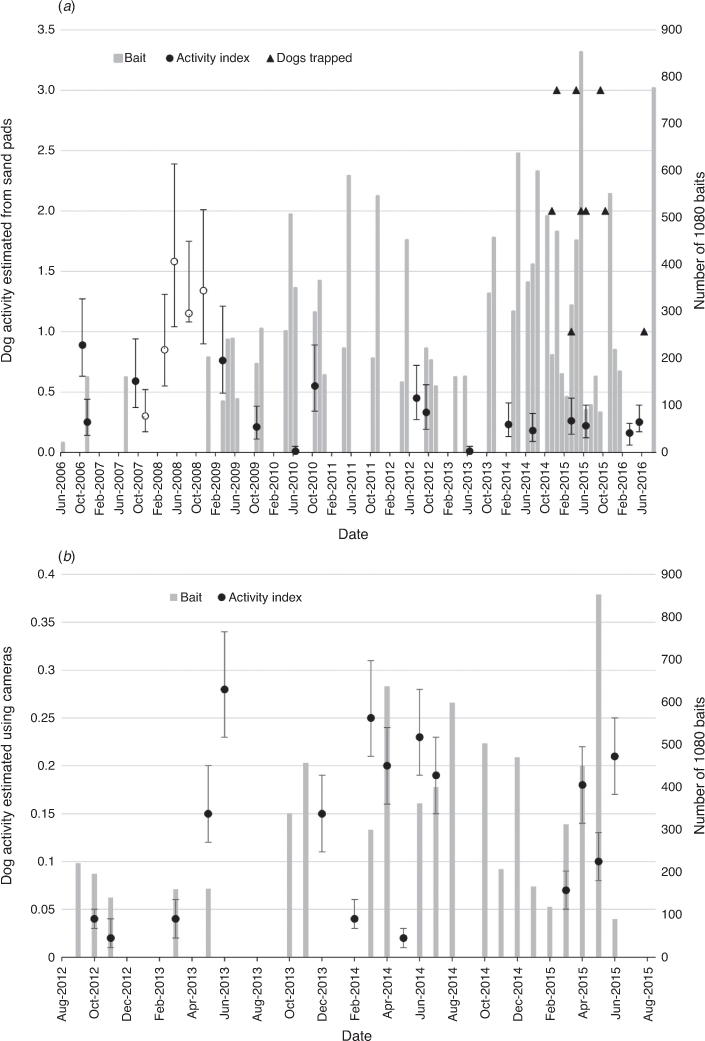
Sand plot activity monitoring occurred six times between June 2013 and June 2016 following the commencement of cat control and three times before cat control. Sand plot cat activity was significantly higher in 2012, but it declined before control commenced. The only significant decline in sand plot cat activity recorded following control was in June 2015. This reading roughly coincided with 12 cats being removed in March/April 2015. This represents 24% of the 50 cats estimated (from cat pelt work, see below) to be on the Park at the time and the activity index was the lowest recorded for the entire project.
Monthly dog activity obtained using cameras was variable. Despite several large short-term declines, dog activity was generally higher in 2015 than in 2012 (Fig. 3). Sand plot dog activity declined significantly when pulse baiting started in November 2008 and then remained relatively stable for the rest of the study. No correlation was found between dog activity estimated using camera data and sand pad data.
Cat activity, determined using remote cameras, was not significantly different between before and following control (on the basis of 95% CIs), but there were some small significant changes immediately after control work (Fig. 4). Cat control commenced in May–June 2013 and trapping occurred on 19 occasions over the following 25 months to July 2015. Sixteen cats were removed between August 2013 and October 2013 and there was a significant increase in cat activity between June and December 2013. A further 36 cats were removed between March and December 2014; however, cat activity remained unchanged during this period. Another 23 cats were removed between March and June 2015 and cat activity decreased significantly between April to June 2015. No camera data were captured after June 2015.
Cat abundance
In the winter of 2015, half of the Park supported ~25 cats. Traps set across the Park resulted in the capture of 33 cats during 2015, which is ~66% of the population at the time (if the estimated number of cats (25) is extrapolated across the entire park (50)). The actual percentage of cats removed is likely to be less than this because cats from surrounding areas migrate into the Park to fill the territorial vacuums created following control.
Cat stomach contents
The stomach contents of 118 cats were removed for analysis. Seventeen of these had empty stomachs, possibly because they had been in a trap all night, and so were omitted from the analysis. The remains of BNTWs were found in ~10% of the 101 cat stomachs that contained food (Fig. 5). This increased to 20% if the cats found outside of the core BNTW area were excluded from the analysis.

|
BNTW population size
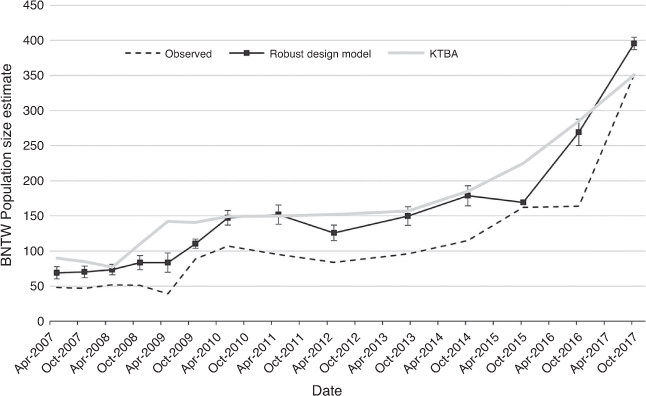
The BNTW population size in the core area was estimated to have increased by 214% over 4 years (2013–2017; Fig. 6), whereas the estimates calculated using line-transect data found that the population had increased by 262% over 8 years (2012–2020; Fig. 7). The 95% confidence intervals for the CMR data strongly suggest that this increase was real. Although this was not the case for the spotlight data, which had very large confidence intervals, the matching trends from CMR and spotlighting add further confidence. The CMR data showed that there had been five significant (on the basis of 95% CIs) increases since 2007, including November 2009, June 2010, and October 2014, 2016 and 2017. The CMR data also suggested that the population dipped slightly in May 2012 and October 2015. However, the KTBA data suggested that there may have been problems with the trapping in those years because the difference between the population estimate and the KTBA increased in those years. The line-transect sample size was sufficient (n > 77) in most surveys to model year-specific detection functions. Although, in 2015, a global detection function (i.e. all years combined) was used because the sample size was too small (n = 26). The density estimates ranged from 11.0 BNTWs per km2 in 2015 to 79.0 BNTWs per km2 in 2020 and a mean of 37.2 BNTWs per km2.

|
The limited number of samples (n = 4) where both methods (spotlight and trapping) were used meant that it was not possible to statistically test for differences. Despite this, there appears to be good alignment in the data for the 4 years in common.
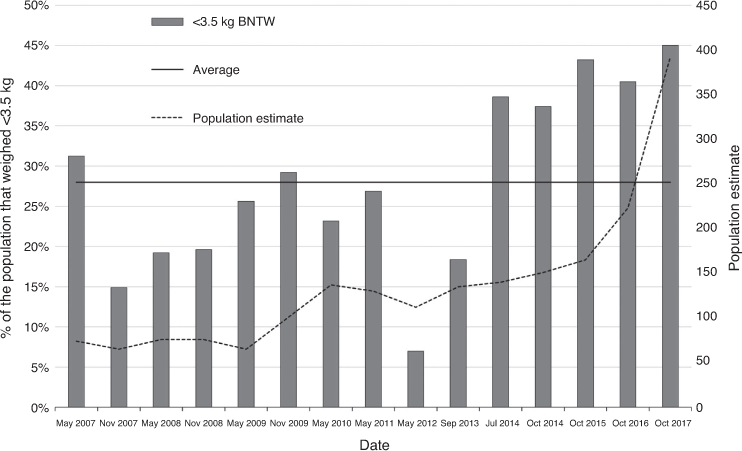
The percentage of BNTWs caught in a CMR survey that weighed ≤3.5 kg and the average percentage for the entire period (2007–2017) are plotted in Fig. 8. The size of the ≤3.5 kg cohort varied between 7% and 31% (22% average) of the population from May 2007 to May 2012 and between 18% and 45% (37% average) from July 2014 to October 2017.
BNTW survival
The fitted annual survival results (r) for juvenile, adult male and adult female were 0.83, 0.92 and 0.97 respectively. The estimated annual survival of small (≤3.5 kg) BNTWs tended to be higher from 2013 onward (Fig. 9). The effects of variables thought to affect adult and juvenile survival are statistically small or uncertain. The graphs of the relationships suggest positive correlations between BNTW and cats and dogs (Figs 10, 11), but negative relationships with rainfall (Fig. 12); however, the β values and CI overlap with zero indicate that there is little predictive power in these relationships. The effects seen in the fitted relationships (Figs 10–12) reflect the parameter estimates of the statistical model. The regression coefficients have relatively wide 95% credible intervals that encompass zero (Table 1). The model included a standard deviation that defined the residual variation in survival that was not explained by the cat and dog indices and rainfall. This standard deviation was estimated to be 0.38 with a 95% credible interval of [0.08, 0.76], which corresponds to a moderate level of unexplained variation in survival; correlations between the fitted values without the residual error and with the residual error ranged from 0.84 to 0.95. Note that this measure of the variation explained by the explanatory variables will tend to be inflated because the number of explanatory variables (3) is quite large relative to the number of time periods between trapping sessions (13), leading to the potential for over-fitting.

|

|

|

|

|
Discussion
Predator activity
Although we found small significant changes in predator activity (on the basis of the 95% CIs) that could be related to management actions, the overall trend was not significantly different between before and after control. Despite killing cats, cat activity increased after some trapping events and decreased after others. Fancourt et al. (2021) found that baiting with fresh-meat baits only minimally reduced cat abundance, leading to a negligible reduction in the number of cat detections, naïve occupancy and relative abundance during their study conducted at Taunton. The increase in cat activity may have been due to rapid immigration (e.g. Lazenby et al. 2015). The cat population for the Park was crudely estimated in 2015 to be 50 on the basis of pelts. In the same year, Fancourt et al. (2021) estimated the abundance of cats at Taunton to be 68.4 (2.28 cats per grid cell, 95% CI 1.26–4.12). Thirty-three cats were removed through trapping and shooting in 2015 and it is most likely that some cats would have taken a bait, which would have reduced the population by ~50% if there was no immigration. Cat activity did decrease significantly following this control, suggesting that the cameras could be used to detect large decreases in the cat population.
Robley et al. (2016) conducted a power analysis of the Taunton activity data and found that cameras had a greater ability to detect smaller levels of change in feral cat activity (>51% with 95% confidence at α = 0.05) than did sand plots (>75%, with 95% confidence at α = 0.05); however, neither method was sensitive enough to determine the effectiveness of cat control at Taunton. They predicted that increasing the number of cameras from 38 to 55 would enable changes of >42% to be detected. Smaller levels of change may be possible to detect with more sand plots. However, the spatial limitations on the number of plots that could be reasonably placed within Taunton and still ensure spatial independence were an issue. For dogs, cameras and sand pads were only able to detect large changes in activity (>75% with 95% confidence at α = 0.05). These results differed from those of Lyra-Jorge et al. (2008) and Ballard et al. (2014) who compared sand pad and camera activity and found that sand pads were able to match and sometimes improve the detection of large and medium-sized mammals. Other studies, such as those of Robley et al. (2010) and Southwell et al. (2019), have found results similar to those of our study and concluded that cameras provided more robust detection than did sand pads.
Several studies have used activity indices as a measure of abundance with mixed success (Allen et al. 1996; Burrows et al. 2003; Hopkins and Kennedy 2004; Robley et al. 2010; Doherty and Algar 2015; Fancourt 2016; Fancourt et al. 2021b). The main concern with using activity indices is that they do not consider variations in detection probability (Hayward and Marlow 2014; Fancourt et al. 2021) and may even be unreliable for monitoring carnivores in general (Royle et al. 2008). The second problem with indices is that unless detection probability and factors that may cause activity to change disproportionately to abundance (e.g. as a result of changes in weather, resource changes, breeding and behavioural (avoidance) shifts) are known and accounted for, then it can lead to an incorrect interpretation of change in abundance (Fleming et al. 1996; Allen et al. 2011; Fancourt 2016; Geyle et al. 2020; Roshier and Carter 2021). At Taunton, radio-tracking studies have shown that cats spend only ~4% of their time on tracks (Fancourt et al. 2021) and dogs avoid roads altogether during winter (Allen and Byrne 2008), making track-based activity indices extremely unreliable at times. The placement of sand pads and cameras and the effect it can have on detection and estimating population change have been discussed in earlier publications (Southwell et al. 2019; Geyle et al. 2020). At Taunton, cameras were placed to improve the detection of dogs, yet this may have under-sampled cat activity. Stokeld et al. (2015) also reported that the number of cameras needed to adequately detect cats is likely to be higher in northern Australia than in other parts of the country.
Predators and BNTWs
The cat stomach content analysis confirmed that cats are a major predator of BNTWs, which is similar to the results obtained from other studies conducted at Taunton (Evans 1992; Horsup and Evans 1993; Fisher et al. 2001). This result suggests that cat control can play a role in BNTW conservation, particularly in areas that do not have predator-proof fences. The percentage of cats culled containing BNTW remains was at least double the percentage of BNTW remains found in dog scats (6%, Evans 1992; 10% in the core BNTW area, Bennison 2008) and this demonstrated that BNTWs are a major prey species of cats. Fisher et al. (2001) found that cats predominantly preyed on juvenile BNTWs. The size and age of a cat is likely to determine the size of the prey captured, with older, bigger cats generally taking larger prey but some smaller cats being able to tackle and consume prey larger than themselves (Fleming et al. 2020). Tigers and other solitary felids are known to prey on species with a body mass similar to theirs (Hayward et al. 2012; Kutt 2012; Fancourt 2015; Murphy et al. 2019). We therefore consider that a 3.5 kg BNTW would be well within the predatory capabilities of most adult cats (weighing up to 5.5 kg) and they most likely can take adult BNTWs as well. We postulate that before 2013, when cat control at Taunton began, cats were likely to be killing more small BNTWs than other age classes. This is because adult BNTWs may be perceived as riskier prey and juvenile naiveté or other behavioural traits of young BNTWs, particularly when they are being crèched (Fisher and Goldizen 2001), predispose them to cat predation.
Gibson et al. (1994) reported a sudden spike in the number of cat-related hare-wallaby deaths and noted that the change was likely to be due to the movement of other, possibly larger, cats into the area or due to seasonal changes causing the resident cats to change their prey. The movement of cats is also most likely to be a response to seasonal changes and its effect on food availability, breeding and juvenile dispersal, location, and cat density (Moseby et al. 2009; Fisher et al. 2015). And in the same way that seasonal change can alter prey choice, so too can it affect cat control success and our ability to monitor cats (Edwards et al. 1997; Short et al. 2002; Moseby et al. 2009).
BNTW survival
Despite the lack of a significant change in cat activity following culling, the BNTW population increased since the control efforts increased, suggesting that predator control was effective and activity indices may not be the best measure. The initial increase in the BNTW population was modest, but after 2015, despite 2015 being a drought year, the increase accelerated. The mean population estimates from this study were consistently higher that those obtained in the mid-1990s, which were most likely drought affected (Fisher et al. 2000). The combination of providing animals with drought-relief food and predator control in the present study is likely to have bolstered survival, making the impact of these events difficult to detect.
Historical BNTW density estimates derived from spotlight data suggest that it ranged from 0.822 in March 1998 to 19.55 in March 1993. However, Lundie-Jenkins et al. (1998) highlighted problems with the accuracy of the data and the large confidence intervals because of the low sample sizes. They extrapolated the density estimates across the entire park (rather than just for the core BNTW area) to give a population estimate of between 95 and 2267, which is the largest population estimate ever recorded on the Park. We suggest that these estimates are likely to be an overestimate of the population at the time, which means that the current population size is probably greater than the population size in the late 1980s/early 1990s and possibly the highest on record. In the current study, we used two independent methods, spotlight and CMR data, to measure the trends in the population. The similarity in the data trends between these two methods gives us confidence that the estimates from the current study are reasonably accurate. The population estimates derived from the spotlight data were slightly larger but also had much larger confidence intervals. The small disparity in the abundance estimate between the two methods may highlight some of the problems associated with trapping and having sufficient traps available in the higher-density areas to adequately sample the population. Although both techniques surveyed much of the same area, traps were also set in some of the lower-density areas outside the core area, which may have affected the result. We also found a disparity between the modelled population estimate and the KTBA, which effectively represents a minimum count. The KTBA data showed that a larger portion of the population was missed in April 2021 and October 2015 than other years and this contributed to a dip in the modelled population estimate. This difference highlights potential problems with the trapping method and the statistical approach. Both 2012 and 2015 were dry years and animals may have dispersed across the core area and not just along roads where we trapped and spotlighted. Fisher et al. (2000) reported that the CMR data were negatively biased because the wallaby’s behaviour did not conform to the assumptions of the CMR models. They reported problems with unequal capture probabilities and recommended that the line-transect method be used for assessing trends in the BNTW population at Taunton, despite the line-transect data containing large confidence intervals. Large confidence intervals reduce the detection of trends and, where possible, methods should try to improve precision. The precision of distance-sampling estimates can be improved through the use of multiple-covariate distance sampling (Marques et al. 2007); however, this approach did not improve data precision in the current study. Stratifying the transects according to sightability may also improve precision; however, this approach did not work for Lundie-Jenkins et al. (1998) at Taunton, who also reported problems with precision.
Despite the increase in BNTWs, neither cat or dog activity nor rainfall were found to have a significant effect on BNTW survival. Line-transect data collected in the 1990s showed that survival correlated with rainfall (Lundie-Jenkins et al. 1998), and Fisher et al. (2001) reported that rainfall, food, predator activity, year, sex and habitat did not affect adult survival, whereas drought and predation were negatively correlated with juvenile survival. They estimated a slightly lower annual survival rate of 0.80 (0.75–0.84, 95% CI)) than the 0.83–0.97 recorded in the present study. The slightly higher survival rate obtained in the present study could reflect the improved survival rates that have contributed to the population increase since cat control commenced.
The small positive effect of cats on juvenile wallaby survival observed in the present study lacks a clear possible mechanism, whereas a negative effect of cats on adult survival is plausible and possible (i.e. a negative effect could be accommodated within the uncertainty around the regression line; black dotted lines in Fig. 10). However, a negative effect of cats on adults but little effect on juveniles seems unlikely; so, one could conclude that there is little relationship between the cat index and wallaby survival. The similar lack of any relationship between wallaby survival and dog activity might be because they had been baited for many years and the introduction of dog trapping did not decrease the dog population to the same extent as the commencement of cat control decreased the cat abundance. Alternatively, the measure for dogs was too insensitive. The analysis provided some evidence that survival of BNTWs increases with dog activity, which is consistent with meso-predator effects (whereby dogs help limit the impacts of other potential predators such as cats; Estes 1996; Wang and Fisher 2012). This estimated effect is consistent for adult and juvenile BNTWs, although the effect for juveniles is uncertain. However, the results are also consistent with survival of wallabies varying with an unmeasured factor that also correlates with dog activity. For example, conditions that might change wallaby survival might also change dog activity. Using camera data, Fancourt et al. (2019) found that cats and dogs exhibited a marked overlap in both temporal and spatial activity, indicating co-existence at Taunton. Further, the evidence that survival of juvenile wallabies increases with the dog index is only somewhat suggestive within the 95% credible interval encompassing zero.
Limitations of the study
If one were to interpret the credible intervals in a null hypothesis significance-testing framework, none of the effects would be significant (with the null hypothesis being of no effect, all the credible intervals encompass zero; Nakagawa and Cuthill 2007; Cumming et al. 2012). Given that (1) none of the putative effects are significant (all the credible intervals encompass zero), (2) the results are correlative rather than experimental, and (3) the index of dog activity correlates with impacts of dogs only partially, the implications of these results for management are merely suggestive. Even though the dataset is large, with 14 trapping sessions over 10 years, the capacity to examine temporal variation in survival (and the factors that might influence it) is still limited. Indeed, the analysis in the present paper is analogous to fitting a regression model with three explanatory variables (cat index, dog index, and rainfall) to 13 data points; such a low ratio of data points to explanatory variables will limit statistical power and can lead to over-fitting. Over-fitting of statistical models will tend to mean that credible intervals are unrealistically precise. Further, given that the true values for survival of each of the 13 data points is uncertain (the data are estimated with error because the number of captured animals is finite), and that the explanatory variables will at best measure the driving factors indirectly, it is perhaps not surprising that the estimated effects are uncertain. The effects are also slightly more uncertain for small individuals because of the low number of individuals caught. Given the uncertainty in the results, the limitations of the correlative analysis, and the fact that further years of data collection will reduce the uncertainty quite slowly, studies that target possible mechanisms more directly seem warranted. Further, more direct measures of cat and dog abundance might help clarify any possible relationships with wallaby survival.
The lack of a suitable nil-treatment or control site with which to compare the current study also limits our ability to assess the effectiveness of predator control. As a result, it is difficult to identify the importance of other drivers of population dynamics because we could not compare what would have happened if we had not provided supplementary food or killed predators. The lack of a correlation with rainfall for years when food was not provided suggests that bottom-up effects did not influence the population and therefore top-down effects, for years when rainfall is average to above average, are more likely to have influenced the population. Had our measure of cat and dog activity been more sensitive, then perhaps it may have been possible to find a relationship between the predators and BNTW survival.
Management implications
For most species, abundance measures are the preferred method for assessing population trends (Caughley and Gunn 1996). However, for threatened species, understanding population dynamics using data on age-specific survival and fecundity may be a more appropriate (Taylor and Gerrodette 1993; Caughley and Gunn 1996; Fisher et al. 2000). Since 2013, the percentage of small BNTWs has increased markedly, particularly in comparison to the percentage from surveys conducted before the intensive cat and dog control began. Juvenile and young adult survivorship has also tended to be above average after targeted cat control and dog trapping commenced. Although we believe that controlling predators was the main factor responsible for the observed BNTW population increase, other factors influenced their population dynamics, particularly food supply, and this is affected by rainfall, weed control and supplementary feeding in drought.
The findings from this study and others conducted in unfenced reserves (Comer et al. 2020) have demonstrated that predator-proof fences may not be necessary in areas without foxes, and this is the case even though it is sometimes not possible to reduce predators across the broader landscape (neighbours). Predator-proof fencing is a rapidly expanding conservation action aimed at stopping the influx of introduced predators (Dickman 2012; Hayward and Somers 2012; Legge et al. 2018; Berry et al. 2019). However, poorly designed and planned fences, particularly those that contain species well outside their known range or climate change-affected range, could potentially do more harm than good and some of the concerns with predator-exclusion fences have been discussed elsewhere (Hayward and Kerley 2009; Hayward et al. 2014; Legge et al. 2018; Berry et al. 2019; Roshier et al. 2020; Kemp et al. 2021).
Our results suggest that the timing and frequency of baiting and trapping and the scale over which these activities were applied was sufficient to enable the BNTW population to increase. Kearney et al. (2020) emphasised the importance of removing all threats, but our findings argue that this is not necessary to conserve a species. The failure of the statistical analysis to detect large negative influences of the key threats to BNTWs seems irrelevant, given the non-trivial improvement in juvenile survivorship and population size following management activities. Nevertheless, it remains an important challenge to not only improve the efficiencies of control, but also increase our knowledge and ability to track the populations of both the BNTWs and their predators in unfenced reserves. We suggest that future studies should investigate other statistically robust ways to monitor cats and dogs, particularly on smaller reserves such as Taunton. These methods should consider the animal’s use of the habitat, spacing intervals, encounter rates in general and ensure that the detection device does not bias the animal’s behaviour.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The work was funded by the Federal Government’s Clean Energy and Biodiversity Fund.
Acknowledgements
We thank the numerous QPWS and Biosecurity Queensland staff and volunteers, who assisted with trapping predators and BNTWs, and particularly Kerrie Bennison, John Wyland, Peter Mowatt, Bronwyn Fancourt, James Speed, Matthew Gentle, Janelle Lowry, Rosie Kerr and Bluey Harris. Jakub Stoklosa, Joanne Potts, Paul Moloney, Bob Augusteyn and Andrew Weeks are thanked for their assistance with data analysis. The Fitzroy Basin Association is thanked for administering this project and for their support with off-park management activities. Graeme Armstrong and Cassandra Tracey are thanked for their contribution to the project submission and their ongoing general support. The reviewers are thanked for their comments on earlier drafts. The predator monitoring and the monitoring of the BNTW population was conducted under Animal Ethics permits DERM/2010/2/04, SA 2013/06/430 and SA 2016/05/55.
References
Allen, L., and Byrne, D. (2008). Are we focusing wild dog control in the wrong time of the year and going about it the wrong way? In ‘Proceedings of the 14th Australasian Vertebrate pest Conference’, June 2008, Darwin, NT, Australia. (Eds G. Saunders and C. Lane.) p. 95. (The Vertebrate Pests Committee and the Invasive Animals Cooperative Research Centre: Canberra, ACT, Australia.)Allen, L., Engeman, R., and Krupa, H. (1996). Evaluation of three relative abundance indices for assessing dingo populations. Wildlife Research 23, 197–205.
| Evaluation of three relative abundance indices for assessing dingo populations.Crossref | GoogleScholarGoogle Scholar |
Allen, B. L., Engeman, R. M., and Allen, L. R. (2011). Wild dogma: an examination of recent ‘evidence’ for dingo regulation of invasive mesopredator release in Australia. Current Zoology 57, 568–583.
| Wild dogma: an examination of recent ‘evidence’ for dingo regulation of invasive mesopredator release in Australia.Crossref | GoogleScholarGoogle Scholar |
Anderson, D. R., Burnham, K. P., and White, G. C. (1994). AIC model selection in over dispersed capture–recapture data. Ecology 75, 1780–1793.
| AIC model selection in over dispersed capture–recapture data.Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J. D., and Nolan, B. J. (2021). Evaluating methods for controlling feral cats that minimise non-target impacts at Taunton National Park (Scientific). Ecological Management & Restoration , .
| Evaluating methods for controlling feral cats that minimise non-target impacts at Taunton National Park (Scientific).Crossref | GoogleScholarGoogle Scholar |
Augusteyn, J., Rich, M., Story, G., and Nolan, B. (2021). Canids potentially threaten bilbies at Astrebla Downs National Park. Australian Mammalogy 43, 300–310.
| Canids potentially threaten bilbies at Astrebla Downs National Park.Crossref | GoogleScholarGoogle Scholar |
Australian Government Bureau of Meteorology (BOM) (2019). Rainfall data. Available at http://www.bom.gov.au.
Australian Wildlife Health Network (AWHN) (2009). Hydatid disease (Echinococcus granulosus) in Australian wildlife: fact sheet. AWHN.
Ballard, G., Meek, P. D., Doak, S., Fleming, P. J., and Sparkes, J. (2014). Camera traps, sand plots and known events: what do camera traps miss. In ‘Camera Trapping: Wildlife Management and Research’. (Eds P. D. Meek and P. J. S. Fleming.) pp. 189–202. (CSIRO Publishing: Melbourne, Vic., Australia.)
Bengsen, A. J., Leung, L. K., Lapidge, S. J., and Gordon, I. J. (2011a). Using a general index approach to analyse camera‐trap abundance indices. The Journal of Wildlife Management 75, 1222–1227.
| Using a general index approach to analyse camera‐trap abundance indices.Crossref | GoogleScholarGoogle Scholar |
Bengsen, A., Butler, J., and Masters, P. (2011b). Estimating and indexing feral cat population abundances using camera traps. Wildlife Research 38, 732–739.
| Estimating and indexing feral cat population abundances using camera traps.Crossref | GoogleScholarGoogle Scholar |
Bennison, K. (2007). Summary of monitoring of canine activity, baiting, predation and bridled nail-tailed population size, on Taunton National Park (Scientific) between 1980 and 2007. Pest Arrest report, Technical Support Unit, Central Region, Queensland Parks and Wildlife Service, Australia. Unpublished report.
Bennison, K. (2008). Conservation of Taunton National Park (Scientific) and the bridled nail-tailed wallaby population through pest management. Taunton Pest Arrest Report. Unpublished report.
Berry, L. E., L’Hotellier, F. A., Carter, A., Kemp, L., Kavanagh, R. P., and Roshier, D. A. (2019). Patterns of habitat use by three threatened mammals 10 years after reintroduction into a fenced reserve free of introduced predators. Biological Conservation 230, 1–9.
| Patterns of habitat use by three threatened mammals 10 years after reintroduction into a fenced reserve free of introduced predators.Crossref | GoogleScholarGoogle Scholar |
Buckland, S. T., Burnham, K. P., and Augustin, N. H. (1997). Model selection: an integral part of inference. Biometrics 53, 603–618.
| Model selection: an integral part of inference.Crossref | GoogleScholarGoogle Scholar |
Buerkner, P.-C. (2016). brms: Bayesian Regression Models using Stan. R package version 0.8.0. Available at https://CRAN.R-project.org/package=brms [verified 12 April 2016].
Burbidge, A. A., and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Burrows, N. D., Algar, D., Robinson, A. D., Sinagra, J., Ward, B., and Liddelow, G. (2003). Controlling introduced predators in the Gibson Desert of Western Australia. Journal of Arid Environments 55, 691–713.
Cameron, D. (2005). Report on the bridled nail-tailed wallaby and resource management history of Taunton National Park (Scientific) Central Queensland. March 2005. Queensland Parks and Wildlife Service, Environmental Protection Agency. Unpublished report.
Caughley, G., and Gunn, A. (1996). Conservation biology in theory and practice. No. 333.9516 C3. Blackwell Science, Cambridge, MA, USA.
Comer, S., Clausen, L., Cowen, S., Pinder, J., Thomas, A., Burbidge, A. H., Tiller, C., Algar, D., and Speldewinde, P. (2020). Integrating feral cat (Felis catus) control into landscape-scale introduced predator management to improve conservation prospects for threatened fauna: a case study from the south coast of Western Australia. Wildlife Research 47, 762–778.
| Integrating feral cat (Felis catus) control into landscape-scale introduced predator management to improve conservation prospects for threatened fauna: a case study from the south coast of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cumming, G., Fidler, F., Kalinowski, P., and Lai, J. (2012). The statistical recommendations of the American Psychological Association Publication Manual: effect sizes, confidence intervals, and meta‐analysis. Australian Journal of Psychology 64, 138–146.
| The statistical recommendations of the American Psychological Association Publication Manual: effect sizes, confidence intervals, and meta‐analysis.Crossref | GoogleScholarGoogle Scholar |
Department of the Environment, Water, Heritage and the Arts (DEWHA) (2008). ‘Threat Abatement Plan for Predation by Feral Cats.’ (DEWHA: Canberra, ACT, Australia.)
Dickman, C. R. (2012). Fences or ferals? Benefits and costs of conservation fencing in Australia. In ‘Fencing for Conservation’. (Eds M. J. Somers, and M. Hayward.) pp. 43–63. (Springer: New York, NY, USA.)
Doherty, T. S., and Algar,, D. (2015). Response of feral cats to a track‐based baiting programme using Eradicat® baits. Ecological Management & Restoration 16, 124–130.
Doherty, T. S., Davis, N. E., Dickman, C. R., Forsyth, D. M., Letnic, M., Nimmo, D. G., Palmer, R., Ritchie, E. G., Benshemesh, J., Edwards, G., and Lawrence, J. (2019). Continental patterns in the diet of a top predator: Australia’s dingo. Mammal Review 49, 31–44.
| Continental patterns in the diet of a top predator: Australia’s dingo.Crossref | GoogleScholarGoogle Scholar |
Edwards, G. P., Piddington, K. C., and Paltridge, R. M. (1997). Field evaluation of olfactory lures for feral cats (Felis catus L.) in central Australia. Wildlife Research 24, 173–183.
Engeman, R. M., and Allen, L. (2000). Overview of a passive tracking index for monitoring wild canids and associated species. Integrated Pest Management Reviews 5, 197–203.
| Overview of a passive tracking index for monitoring wild canids and associated species.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M., Allen, L., and Zerbe, G. O. (1998). Variance estimate for the Allen activity index. Wildlife Research 25, 643–648.
| Variance estimate for the Allen activity index.Crossref | GoogleScholarGoogle Scholar |
Estes, J. A. (1996). Predators and ecosystem management. Wildlife Society Bulletin 24, 390–396.
Evans, A. M. (1992). The bridled nail-tailed wallaby: ecology and management: final report. A Report to the Australian National Parks and Wildlife Service. Department of Environment and Heritage.
Evans, M., and Gordon, G. (2008). Bridled nail-tailed wallaby. In ‘The Mammals of Australia’. (Eds S. Van Dyck, and R. Strahan.) pp. 355–356. (New Holland Publishers: Sydney, NSW, Australia.)
Fancourt, B. A. (2015). Making a killing: photographic evidence of predation of a Tasmanian pademelon (Thylogale billardierii) by a feral cat (Felis catus). Australian Mammalogy 37, 120–124.
| Making a killing: photographic evidence of predation of a Tasmanian pademelon (Thylogale billardierii) by a feral cat (Felis catus).Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A. (2016). Avoiding the subject: the implications of avoidance behaviour for detecting predators. Behavioral Ecology and Sociobiology 70, 1535–1546.
| Avoiding the subject: the implications of avoidance behaviour for detecting predators.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., and Jackson, R. B. (2014). Regional seroprevalence of Toxoplasma gondii antibodies in feral and stray cats (Felis catus) from Tasmania. Australian Journal of Zoology 62, 272–283.
| Regional seroprevalence of Toxoplasma gondii antibodies in feral and stray cats (Felis catus) from Tasmania.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Cremasco, P., Wilson, C., and Gentle, M. N. (2019). Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not. Journal of Applied Ecology 56, 2584–2595.
| Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Augusteyn, J., Cremasco, P., Nolan, B., Richards, S., Speed, J., Wilson, C., and Gentle, M. (2021). Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case study Journal of Environmental Management 280, 111691.
| Measuring, evaluating and improving the effectiveness of invasive predator control programs: feral cat baiting as a case studyCrossref | GoogleScholarGoogle Scholar | 33272660PubMed |
Fancourt, B. A., Harry, G., Speed, J., and Gentle, M. N. (2021b). Efficacy and safety of Eradicat® feral cat baits in eastern Australia: population impacts of baiting programmes on feral cats and non-target mammals and birds. Journal of Pest Science , .
| Efficacy and safety of Eradicat® feral cat baits in eastern Australia: population impacts of baiting programmes on feral cats and non-target mammals and birds.Crossref | GoogleScholarGoogle Scholar |
Fisher, D. O., and Goldizen, A. W. (2001). Maternal care and infant behaviour of the bridled nail tailed wallaby (Onychogalea fraenata). Journal of Zoology 255, 321–330.
| Maternal care and infant behaviour of the bridled nail tailed wallaby (Onychogalea fraenata).Crossref | GoogleScholarGoogle Scholar |
Fisher, D. O., Hoyle, S. D., and Blomberg, S. P. (2000). Population dynamics and survival of an endangered wallaby: a comparison of four methods. Ecological Applications 10, 901–910.
| Population dynamics and survival of an endangered wallaby: a comparison of four methods.Crossref | GoogleScholarGoogle Scholar |
Fisher, D. O., Blomberg, S. P., and Hoyle, S. D. (2001). Mechanisms of drought-induced population decline in an endangered wallaby. Biological Conservation 102, 107–115.
| Mechanisms of drought-induced population decline in an endangered wallaby.Crossref | GoogleScholarGoogle Scholar |
Fisher, P., Algar, D., Murphy, E., Johnston, M., and Eason, C. (2015). How does cat behaviour influence the development and implementation of monitoring techniques and lethal control methods for feral cats? Applied Animal Behaviour Science 173, 88–96.
| How does cat behaviour influence the development and implementation of monitoring techniques and lethal control methods for feral cats?Crossref | GoogleScholarGoogle Scholar |
Fleming, P. J. S., Thompson, J. A., and Nicol, H. I. (1996). Indices for measuring the efficacy of aerial baiting for wild dog control in north-eastern NSW. Wildlife Research 23, 665–674.
| Indices for measuring the efficacy of aerial baiting for wild dog control in north-eastern NSW.Crossref | GoogleScholarGoogle Scholar |
Fleming, P. A., Crawford, H. M., Auckland, C. H., and Calver, M. C. (2020). Body size and bite force of stray and feral Cats—are bigger or older cats taking the largest or more difficult-to-handle prey? Animals (Basel) 10, 707.
| Body size and bite force of stray and feral Cats—are bigger or older cats taking the largest or more difficult-to-handle prey?Crossref | GoogleScholarGoogle Scholar |
Geyle, H. M., Stevens, M., Duffy, R., Greenwood, L., Nimmo, D. G., Sandow, D., Thomas, B., White, J., and Ritchie, E. G. (2020). Evaluation of camera placement for detection of free‐ranging carnivores; implications for assessing population changes. Ecological Solutions and Evidence 1, p.e12018.
Gibson, D. F., Lundie-Jenkins, G., Langford, D. G., Cole, J. R., and Johnson, K. A. (1994). Predation by feral cats, Felis catus, on the rufous hare-wallaby, Lagorchestes hirsutus, in the Tanami Desert. Australian Mammalogy 17, 103–107.
Gordon, G., and Lawrie, B. C. (1980). The rediscovery of the bridled nail-tailed wallaby, Onychogalea fraenata (Gould) (Marsupialia: Macropodidae) in Queensland. Australian Wildlife Research 7, 339–345.
| The rediscovery of the bridled nail-tailed wallaby, Onychogalea fraenata (Gould) (Marsupialia: Macropodidae) in Queensland.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., and Kerley, G. I. (2009). Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142, 1–13.
| Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes?Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., and Somers, M. J. (2012). An introduction to fencing for conservation. In ‘Fencing for conservation’. (Eds M. J. Somers, and M. W. Hayward.) pp. 1–6. (Springer: New York, NY, USA.)
Hayward, M. W., Jędrzejewski, W., and Jedrzejewska, B. (2012). Prey preferences of the tiger Panthera tigris. Journal of Zoology 286, 221–231.
| Prey preferences of the tiger Panthera tigris.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., and Marlow, N. (2014). Will dingoes really conserve wildlife and can our methods tell? Journal of Applied Ecology 51, 835–838.
Hayward, M. W., Moseby, K., and Read, J. L. (2014). The role of predator exclosures in the conservation of Australian fauna. Carnivores of Australia: Past, Present and Future , 353–371.
Hopkins, H. L., and Kennedy, M. L. (2004). An assessment of indices of relative and absolute abundance for monitoring populations of small mammals. Wildlife Society Bulletin 32, 1289–1296.
| An assessment of indices of relative and absolute abundance for monitoring populations of small mammals.Crossref | GoogleScholarGoogle Scholar |
Horsup, A., and Evans, M. (1993). Predation by feral cats, Felis catus, on an endangered marsupial, the bridled nail-tailed wallaby, Onychogalea frenata. Australian Mammalogy 16, 83–84.
| Predation by feral cats, Felis catus, on an endangered marsupial, the bridled nail-tailed wallaby, Onychogalea frenata.Crossref | GoogleScholarGoogle Scholar |
Kearney, S. G., Adams, V. M., Fuller, R. A., Possingham, H. P., and Watson, J. E. (2020). Estimating the benefit of well-managed protected areas for threatened species conservation. Oryx 54, 276–284.
| Estimating the benefit of well-managed protected areas for threatened species conservation.Crossref | GoogleScholarGoogle Scholar |
Kemp, J. E., Jensen, R., Hall, M. L., Roshier, D. A., and Kanowski, J. (2021). Consequences of the reintroduction of regionally extinct mammals for vegetation composition and structure at two established reintroduction sites in semi‐arid Australia. Austral Ecology 46, 653–669.
| Consequences of the reintroduction of regionally extinct mammals for vegetation composition and structure at two established reintroduction sites in semi‐arid Australia.Crossref | GoogleScholarGoogle Scholar |
Kutt, A. S. (2012). Feral cat (Felis catus) prey size and selectivity in north‐eastern Australia: implications for mammal conservation. Journal of Zoology 287, 292–300.
| Feral cat (Felis catus) prey size and selectivity in north‐eastern Australia: implications for mammal conservation.Crossref | GoogleScholarGoogle Scholar |
Lazenby, B. T., Mooney, N. J., and Dickman, C. R. (2015). Effects of low-level culling of feral cats in open populations: a case study from the forests of southern Tasmania. Wildlife Research 41, 407–420.
Legge, S., Woinarski, J. C., Burbidge, A. A., Palmer, R., Ringma, J., Radford, J. Q., Mitchell, N., Bode, M., Wintle, B., Baseler, M., and Bentley, J. (2018). Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildlife Research 45, 627–644.
| Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators.Crossref | GoogleScholarGoogle Scholar |
Lundie-Jenkins, G., and Lowry, J. (2005). Recovery plan for the bridled nail-tailed wallaby (Onychogalea fraenata) 2005–2009. Report to the Department of Environment and Heritage, Canberra. Environmental Protection Agency/Queensland Parks and Wildlife Service, Brisbane, Qld, Australia.
Lundie-Jenkins, G., Hoolihan, D., Porter, G., and Pitt, L. (1998). Surveys of macropod and predator species on Taunton National Park (Scientific), central Queensland 1993 to 1998. Unpublished report. Department of Environment and Heritage, Toowoomba, Qld, Australia.
Lyra-Jorge, M. C., Ciocheti, G., Pivello, V. R., and Meirelles, S. T. (2008). Comparing methods for sampling large-and medium-sized mammals: camera traps and track plots. European Journal of Wildlife Research 54, 739–744.
| Comparing methods for sampling large-and medium-sized mammals: camera traps and track plots.Crossref | GoogleScholarGoogle Scholar |
Marques, T. A., Thomas, L., Fancy, S. G., and Buckland, S. T. (2007). Improving estimates of bird density using multiple-covariate distance sampling. The Auk 124, 1229–1243.
| Improving estimates of bird density using multiple-covariate distance sampling.Crossref | GoogleScholarGoogle Scholar |
Meek, P. D., Ballard, G. A., Vernes, K., and Fleming, P. J. S. (2015). The history of wildlife camera trapping as a survey tool in Australia. Australian Mammalogy 37, 1–12.
| The history of wildlife camera trapping as a survey tool in Australia.Crossref | GoogleScholarGoogle Scholar |
Melzer, R. I. (2015). When is stock grazing an appropriate ‘tool’ for reducing ‘Cenchrus ciliaris’ (buffel grass) on conservation reserves? Proceedings of the Royal Society of Queensland 120, 53–68.
Moseby, K. E., Stott, J., and Crisp, H. (2009). Improving the effectiveness of poison baiting for the feral cat and European fox in northern South Australia: the influence of movement, habitat use and activity. Wildlife Research 36, 1–14.
Murphy, B. P., and Davies, H. F. (2014). There is a critical weight range for Australia’s declining tropical mammals. Global Ecology and Biogeography 23, 1058–1061.
| There is a critical weight range for Australia’s declining tropical mammals.Crossref | GoogleScholarGoogle Scholar |
Murphy, B. P., Woolley, L. A., Geyle, H. M., Legge, S. M., Palmer, R., Dickman, C. R., Augusteyn, J., Brown, S. C., Comer, S., Doherty, T. S., and Eager, C. (2019). Introduced cats (Felis catus) eating a continental fauna: the number of mammals killed in Australia. Biological Conservation 237, 28–40.
| Introduced cats (Felis catus) eating a continental fauna: the number of mammals killed in Australia.Crossref | GoogleScholarGoogle Scholar |
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society 82, 591–605.
| Effect size, confidence interval and statistical significance: a practical guide for biologists.Crossref | GoogleScholarGoogle Scholar | 17944619PubMed |
Nolan, B. J., and Wykes, B. (2019). Review of feral cat trapping program at Taunton National Park Scientific 2019: Department of Environment and Science, Queensland Government, Australia.
Oakwood, M. (2000). Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48, 519–539.
| Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Queensland Herbarium (2018). ‘Regional Ecosystem Description Database (REDD). Version 10.1 (March 2018).’ (DSITI: Brisbane, Qld, Australia.)
Robley, A., Gormley, A., Woodford, L., Lindeman, M., Whitehead, B., Albert, R., Bowd, M., and Smith, A. (2010). Evaluation of camera trap sampling designs used to determine change in occupancy rate and abundance of feral cats. Arthur Rylah Institute for Environmental Research Technical Report No. 201. Department of Sustainability and Environment, Melbourne, Vic., Australia.
Robley, A., Potts, J., and Moloney, P. (2016). Bridled nail-tailed wallaby and predator monitoring project: occupancy and the power to detect change. Arthur Rylah Institute for Environmental Research Unpublished Client Report for the Department of National Parks, Sport and Racing, Queensland. Department of Environment, Land, Water and Planning, Melbourne, Vic., Australia.
Roshier, D. A., and Carter, A. (2021). Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape. Ecosphere 12, e03628.
| Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape.Crossref | GoogleScholarGoogle Scholar |
Roshier, D. A., Hotellier, F. L., Carter, A., Kemp, L., Potts, J., Hayward, M. W., and Legge, S. M. (2020). Long-term benefits and short-term costs: small vertebrate responses to predator exclusion and native mammal reintroductions in south-western New South Wales, Australia. Wildlife Research 47, 570–579.
| Long-term benefits and short-term costs: small vertebrate responses to predator exclusion and native mammal reintroductions in south-western New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Ross, A. K., Lowry, J., Elphinstone, A., and Lawes, J. C. (2020). Toxoplasma gondii seroprevalence in endangered bridled nailtail wallabies and co-occurring species. Australian Mammalogy 42, 167–170.
| Toxoplasma gondii seroprevalence in endangered bridled nailtail wallabies and co-occurring species.Crossref | GoogleScholarGoogle Scholar |
Royle, J. A., Stanley, T. R., and Lukacs, P. M. (2008). Statistical modeling and inference from carnivore survey data. In ‘Noninvasive survey methods for carnivores’. (Eds R. A. Long, P. MacKay, J. Ray, and W. Zielinski.) pp. 293–312. (Island Press: Washington, DC, USA.)
Short, J., Turner, B., and Risbey, D. (2002). Control of feral cats for nature conservation. III. Trapping. Wildlife Research 29, 475–487.
Southwell, D. M., Einoder, L. D., Lahoz‐Monfort, J. J., Fisher, A., Gillespie, G. R., and Wintle, B. A. (2019). Spatially explicit power analysis for detecting occupancy trends for multiple species. Ecological Applications 29, e01950.
| Spatially explicit power analysis for detecting occupancy trends for multiple species.Crossref | GoogleScholarGoogle Scholar | 31187919PubMed |
Stokeld, D., Frank, A. S., Hill, B., Choy, J. L., Mahney, T., Stevens, A., Young, S., Rangers, D., Rangers, W., and Gillespie, G. R. (2015). Multiple cameras required to reliably detect feral cats in northern Australian tropical savanna: an evaluation of sampling design when using camera traps. Wildlife Research 42, 642–649.
| Multiple cameras required to reliably detect feral cats in northern Australian tropical savanna: an evaluation of sampling design when using camera traps.Crossref | GoogleScholarGoogle Scholar |
Stokeld, D., Fisher, A., Gentles, T., Hill, B., Triggs, B., Woinarski, J. C., and Gillespie, G. R. (2018). What do predator diets tell us about mammal declines in Kakadu National Park? Wildlife Research 45, 92–101.
| What do predator diets tell us about mammal declines in Kakadu National Park?Crossref | GoogleScholarGoogle Scholar |
Taylor, B. L., and Gerrodette, T. (1993). The uses of statistical power in conservation biology: the vaquita and northern spotted owl. Conservation Biology 7, 489–500.
| The uses of statistical power in conservation biology: the vaquita and northern spotted owl.Crossref | GoogleScholarGoogle Scholar |
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., Bishop, J. R., Marques, T. A., and Burnham, K. P. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology 47, 5–14.
Tierney, P. J. (1985). Habitat and ecology of the bridled nail-tailed wallaby, Onychogalea fraenata, with implications for management. M.Sc. Thesis, Department of Biology and Environmental Science, Queensland Institute of Technology, Brisbane, Qld, Australia.
Wang, Y., and Fisher, D. O. (2012). Dingoes affect activity of feral cats, but do not exclude them from the habitat of an endangered macropod. Wildlife Research 39, 611–620.
| Dingoes affect activity of feral cats, but do not exclude them from the habitat of an endangered macropod.Crossref | GoogleScholarGoogle Scholar |
White, G. C., and Burnham, K. P. (1997). ‘Program Mark: survival estimation from populations of marked animals.’ (Colorado State University: Fort Collins, CO, USA.)
Woinarski, J. C., Burbidge, A., and Harrison, P. (2014). ‘The action plan for Australian mammals 2012.’ (CSIRO Publishing.)
Woolley, L. A., Geyle, H. M., Murphy, B. P., Legge, S. M., Palmer, R., Dickman, C. R., Augusteyn, J., Comer, S., Doherty, T. S., Eager, C., and Edwards, G. (2019). Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed. Mammal Review 49, 354–368.
| Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed.Crossref | GoogleScholarGoogle Scholar |