Predator-free short-hydroperiod wetlands enhance metamorph output in a threatened amphibian: insights into frog breeding behaviour evolution and conservation management
Chad T. Beranek A B C , Samantha Sanders A , John Clulow A B and Michael Mahony A
A B C , Samantha Sanders A , John Clulow A B and Michael Mahony A
A School of Environmental and life Sciences, Biology Building, University of Newcastle, University Drive, Callaghan, NSW 2308, Australia.
B FAUNA Research Alliance, PO Box 5092, Kahibah, NSW 2290, Australia.
C Corresponding author. Email: chad.beranek@uon.edu.au
Wildlife Research 49(4) 360-371 https://doi.org/10.1071/WR21049
Submitted: 7 March 2021 Accepted: 12 October 2021 Published: 30 October 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Context: Knowledge on the drivers of breeding behaviour is vital to understand amphibian ecology and conservation. Proposed drivers of amphibian reproductive behaviour include selection of optimum water quality, and avoidance of tadpole predators and competition. These hypotheses are underpinned by the logic that amphibians will choose breeding habitat that will result in enhanced metamorph output.
Aims: We aimed to infer key drivers that influence metamorph output in the threatened green and golden bell frog (Litoria aurea). We hypothesised that (1) metamorph output would be higher in recently refilled wetlands than in wetlands with a longer hydroperiod, (2) metamorph output would be negatively correlated with tadpole predator abundance, and (3) waterbodies with long hydroperiods would have higher abundances of aquatic predators and lower abundances of L. aurea tadpoles.
Methods: We tested these hypotheses by monitoring breeding, tadpole and predator abundances in a wild population of L. aurea. We coupled this with metamorph counts that were adjusted to represent per capita numbers via genetic means. We also ruled out the influence of detection probability in explaining the results with a manipulative experiment.
Key results: We found support for all three hypotheses and hence provide evidence that the adaptive behaviour of L. aurea to preference recently refilled wetlands is governed by the abundance of tadpole predators. We found metamorph counts per clutch to be 8.2-fold greater in short-hydroperiod wetlands (26 ± 15–44 95% CI) than in long-hydroperiod wetlands (3 ± 2–5 95% CI). Four predator taxa were associated with low metamorph output and two of these occurred in higher abundances in longer-hydroperiod wetlands.
Conclusions and implications: These results have provided evidence that the behavioural adaptation of L. aurea to select recently refilled wetlands has evolved in response to tadpole predation pressure. We recommend practitioners to conduct tadpole releases in newly refilled wetlands to enhance survival to metamorphosis in future reintroductions.
Keywords: amphibian ecology, wetland hydrology, predation, freshwater macroinvertebrates, tadpoles, reintroduction.
Introduction
Knowledge of the drivers of amphibian breeding behaviour is vital to understand amphibian ecology and conservation (Wells 2010). There are many features of aquatic breeding habitat that are important to amphibians worldwide, including hydro-period, presence of wetland vegetation, shading, temperature, salinity, landscape position and landscape connectivity (Lemckert and Mahony 2010; Hamer and Parris 2011; Hamer 2016; Hamer et al. 2021).
The hydro-period of a wetland has obvious links to amphibian breeding habitat selection (Van Buskirk 2005). Tadpoles from species that primarily breed in permanent wetlands have adaptive features to avoid predation from aquatic predators (such as unpalatability), but these features are lacking in tadpoles from ephemeral breeding species (Kats et al. 1988). It is thought that this is due to avoidance of tadpole predation because such situations contain fewer aquatic predators (Pintar and Resetarits Jr 2017).
There is compelling evidence that predator presence is a major driver of frog breeding habitat occupancy (Resetarits Jr and Wilbur 1989; Hopey and Petranka 1994; Petranka et al. 2007; Pollard et al. 2017). Experimental mesocosm and field studies have identified that many species of amphibians will avoid breeding in water bodies with fish and tadpole predators (Resetarits Jr and Wilbur 1989; Hopey and Petranka 1994; Binckley and Resetarits Jr 2002). In addition, recent attention has been drawn to the role of freshwater macroinvertebrate predators driving breeding habitat selection (Pintar and Resetarits Jr 2020) and larval anuran mortality (Valdez 2018; Gould et al. 2019). A common adaptive response of adult amphibians to avoid offspring predation is through predator recognition and avoidance (Blaustein 1999).
There are other factors beyond predation that explain drivers of amphibian breeding habitat selection (Resetarits Jr and Wilbur 1989). Including selection for enhanced tadpole food resources (Odendaal et al. 1982), selection for certain water-quality attributes (Wilder and Welch 2014) and avoidance of intraspecific and interspecific tadpole competition (Stein and Blaustein 2015). It is likely that responses to these factors are species specific and multivariate. The adaptive evolutionary response that underpins all these behaviours is that anurans will choose breeding habitat that will result in enhanced breeding success and offspring survival, which results in increased metamorph output.
Understanding the drivers of breeding site-selection behaviours of anurans provides insights into conservation management. This is important because many anuran species are in decline worldwide from numerous threats (Scheele et al. 2019). Understanding the factors that influence breeding behaviours enables insight into conservation management strategies that maximise breeding quantity, tadpole survival and metamorph output. Constructed wetland designs can be optimised to promote breeding of targeted amphibians (Beranek et al. 2020b). Reintroduction programs can target tadpole releases into wetlands that have spatial and temporal attributes that enable high survival rates of tadpoles (Mendelson III and Altig 2016).
The green and golden bell frog (Litoria aurea) is a threatened species that has declined in south-eastern Australia over the past ~45 years (White and Pyke 1996). Its distribution extended as far west as Bathurst, and covered the eastern coast from northern New South Wales (NSW) to far-eastern Gippsland in Victoria (White and Pyke 1996). Only 31 populations remain within NSW (White and Pyke 2008). Many of the remaining populations are at risk of stochastic extinction, yet some appear stable (Goldingay et al. 2017). To halt declines, conservation management has emphasised breeding habitat creation and restoration (O’Meara and Darcovich 2015; Beranek et al. 2020b) and reintroductions (Pyke et al. 2008; White and Pyke 2008), despite a lack of knowledge of the ecological factors that drive breeding habitat selection and metamorph output.
There is abundant knowledge regarding the habitat correlations of adult L. aurea (Hamer and Mahony 2010; Valdez et al. 2015, 2016, 2017; Fardell et al. 2018; Hamer 2018), but a paucity of information on the drivers of breeding habitat selection and how this relates to selection of habitats that enhance offspring survival. Larger numbers of metamorphs have been observed in ephemeral wetlands than in permanent wetlands, and this was attributed to higher predation rates in the latter by native fish (Goldingay and Newell 2005). Similarly, L. aurea has been observed to be generally absent from wetlands that contain the invasive fish Gambusia holbrooki (Pyke et al. 2002). However, L. aurea can persist in wetlands containing G. holbrooki with high emergent vegetation density (see van de Mortel and Goldingay 1998; Hamer et al. 2002b). In a study that undertook opportunistic experimental manipulation, increased L. aurea calling occurred in wetlands that were drained and refilled, where G. holbrooki was eliminated (Pollard et al. 2017). It is unknown whether this response was due to the removal of G. holbrooki or the recharging of the wetland. The study of Pollard et al. (2017), coupled with analysis of movement in L. aurea by Hamer et al. (2008), showed that L. aurea has an innate behaviour to move to and select impermanent wetlands for breeding that have recently refilled after being dry. We postulate that there must be selection fitness benefits to explain this behaviour, but this has not been investigated.
Aims and hypotheses
The present study aimed to investigate per capita L. aurea metamorph output in wetlands with different hydroperiods. The investigation was undertaken in a created wetland mosaic and provides evidence on the factors that contribute to differences in survival rates to metamorphosis. Specifically, we aimed to (1) determine whether there is a higher per clutch metamorph output in recently refilled wetlands (referred to as short-hydroperiod wetlands) than in wetlands that have not recently refilled (referred to as long-hydroperiod wetlands), (2) determine what explanatory variables best predict the number of L. aurea metamorphs, and (3) determine whether there are differences in the abundance of potential tadpole predators and competitors in recently refilled wetlands compared with long-hydroperiod wetlands.
Given that bell frogs have a well documented behaviour to seek out newly refilled short-hydroperiod wetlands to breed in (Hamer et al. 2008), we hypothesise for Aim 1 that such situations will lead to a greater metamorph output per clutch than in long-hydroperiod wetlands. Furthermore, given that the density of aquatic predators in wetlands is likely to increase with longer hydroperiods, we hypothesised for Aim 2 that differences in metamorph output are due to a lower abundance of tadpole predators in short-hydroperiod than in long-hydroperiod wetlands, and not due to other explanations, such as water quality and competition. For Aim 3, we hypothesised that significant predators identified explaining the metamorph counts will be more abundant in not recently refilled wetlands than in recently refilled wetlands, and the opposite would be true for L. aurea tadpoles. We also describe observations of tadpole predation and temporal trends in wetland refilling to further support cause and effect relationships in the interpretation of the results.
Materials and methods
Study site
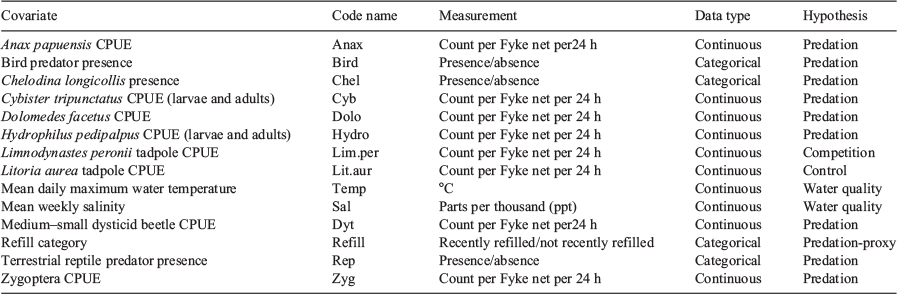
The study site was located on Kooragang Island (32°50–54′S, 151°42–47′E; see Fig. 1), occurring within the mouth of the Hunter River in NSW, Australia. The island is ~30 km2 and comprises numerous wetlands including man-made and natural waterbodies (Klop-Toker et al. 2018; Beranek et al. 2020b). This area contains one of the largest extant populations of L. aurea (Hamer et al. 2002a; Hamer and Mahony 2010; Mahony et al. 2013). The study site contained 11 wetlands that were created in 2015 and 2016 to enhance local L. aurea populations. These wetlands were made to passively reduce disease and exclude fish (Beranek et al. (2020b). Four of these wetlands have a permanent hydroperiod, whereas the rest have ephemeral hydroperiods of varying duration. Two of the four permanent wetlands had salinity levels that were consistently outside the tadpole survival threshold of L. aurea (Kearney et al. 2012), and these were not included in any further analysis. There was an additional ephemeral natural wetland that was located between two created wetlands and was included in the present study. See Beranek et al. (2020b) for a comprehensive description of the study site.

|
Fauna surveys
Visual encounter surveys (VES) for metamorphs were conducted almost weekly during the breeding season of L. aurea (September–April) for four breeding seasons, from 2016 to 2020. VES consisted of two to eight observers actively searching the entire surface area of each wetland, including aquatic vegetation, for metamorphs. The microhabitat that each metamorph occupied was recorded and this included emergent wetland vegetation (Bolboschoenus caldwelli, B. fluviatalis, Schoenoplectus vallidus, Typha sp.), grasses (including the species Lachnagrostis filiformis, Cynodon dactylon, Paspalum dilitatum, P. distichum), or open water and algae mat.
Fyke netting of wetlands was used to quantify the abundance of aquatic fauna, including tadpoles, potential predators and competitors. Fyke nets are the most effective survey method for detecting the tadpoles of L. raniformis, the sister species of L. aurea, and are commonly used in fish surveys (Wassens et al. 2017). The Fyke nets used in the study were 0.7 m in height and 5.7 m in length from wing to wing, with a mesh size of 4 mm. Fyke-netting was undertaken monthly in each of the nine wetlands from September to March each year. This was considered a sufficient sampling regime to have confidence that most breeding events would be detected because L. aurea has a tadpole development period of ~1.5–3 months (Beranek et al. 2020b). Fyke nets were placed with the mouth open towards emergent or submerged vegetation to optimise capture of aquatic fauna. There was approximately one Fyke net set per 1 ML volume of wetland water, and were activated for a period of 14–18 h from late afternoon to the morning. During VES and Fyke-netting, opportunistic observations of potential egg and tadpole predators were also made.
For the purpose of analysis, we defined a ‘breeding event’ for L. aurea as an instance of breeding that occurred in a particular wetland, as evidenced by either an observation of an egg clutch, tadpoles or metamorphs. Differential size classes of tadpoles were also to quantify breeding events.
To ensure that we could uncouple metamorph and tadpole counts from the number of egg clutches laid in a wetland, several lines of evidence were used. (1) We measured snout–vent length (SVL) during each instance of tadpole netting to identify whether there were distinct size cohorts, and we considered there were more than one breeding event if a standard deviation of ±2 mm was observed. (2) In wetlands where there was prolonged calling, recruitment patterns of metamorphs were investigated to identify peaks and troughs. Because metamorph output in anurans is staggered, we considered each peak to represent the maximum metamorph count of a particular clutch. (3) In wetlands where there were large explosive breeding congregations, we conducted genetic relatedness analysis to estimate the number of clutches that were laid in each event, and used the mean of this for breeding events we did not obtain genetic material from.
Bird surveys were conducted weekly during the frog-breeding season to determine the potential impacts of predatory waterbirds on metamorph output. Bird surveys were completed by doing an ~20 min survey around and within the perimeter of the bunding of each wetland in the afternoon from ~4 h before sunset to sunset, by using binoculars. Incidental encounters of birds were also made during nocturnal frog surveys and while conducting other activities.
Similarly, reptile surveys were conducted weekly during the frog-breeding season to determine the potential impacts of terrestrial reptile predators on metamorph output. Reptiles were surveyed using artificial herpetofauna refuges consisting of 1 × 1 m corrugated iron sheets, placed haphazardly ~5 m from the edge of the wetland on the bunding wall. Incidental encounters of terrestrial reptile predators were also made during nocturnal frog surveys, bird surveys, Fyke-netting and other activities.
Physiochemical sampling
Temperature and salinity were recorded from wetlands and used as covariates to model against metamorph counts. At each of the nine wetlands, repeated measures were taken at the same location that was ~5 m from the wetland edge. Salinity was recorded by inserting a hand-held water probe (YSI Professional Plus with a multi-probe attachment) ~5 cm below the water surface. Salinity was measured in parts per thousand (ppt). This was recorded weekly throughout the L. aurea breeding season for each wetland. The mean salinity value of each breeding event was used as a covariate modelled against metamorph numbers, which was estimated between the known or estimated date of conception and the date of the first metamorph observed for each breeding event.
Water temperature was recorded 5 cm below the wetland water surface by placing iButton thermochrons (Model: DS1921G) into floating canisters in each wetland. Thermochrons were wrapped in parafilm before deployment to prevent water damage. They were set to record temperature at hourly intervals. They were targeted for deployment at the beginning of each breeding season and collected when wetlands dried or within 3 months of deployment for data collection. They were redeployed later in the season before heavy summer rain was predicted. This was undertaken to ensure that water temperature data were captured from the start of breeding until metamorphosis.
There were instances where water temperature data were missed owing to late iButton deployment. To predict the water temperature at these times, data were imputed by using an analysis of covariance (ANCOVA) to generate a predictive formula for each wetland between the variables daily maximum air temperature (sourced from the Bureau of Meteorology, Williamstown RAAF weather station, number = 061078) and the daily maximum water temperature (°C). This was conducted on JMP version 14. The mean maximum daily water temperature was used as a covariate modelled against metamorph output and was estimated by calculating the mean daily maximum water temperature between the known or estimated date of conception to the date of the first metamorphs observed for each breeding event.
Calculation of the metamorph detectability offset
A manipulative experiment was conducted to calculate detection probability of metamorphs in each wetland, so as to rule out the possibility that differences in vegetation structure and composition could influence observation in the VES sampling. This was completed because each wetland was different in terms of physical structure, size and water-plant composition, and hence the detection rates of metamorphs may differ among wetlands. To conduct this, silicon frog models (floating fishing lures, Hengjia, China), marked with a unique identification code, were used.
The experimental procedure followed that of Valdez et al. (2017) and we present a summarised version. The models were allocated to wetlands (1A, 2A, 2C, 3A, 4A, 4B, 4C and 14B) where metamorphs were regularly observed. Placement of models was stratified as follows. The dominant microhabitat types of each wetland were determined, and models were allocated by an independent observer, proportional to the coverages of primary microhabitats. A random number generator (30–55) was used to determine the exact placement of models in a wetland as a further step to remove bias from the experiment. Models were placed in positions that represented the natural microhabitat usage of L. aurea.
Once the models were in position, two independent surveyors conducted nocturnal visual encounter surveys (VES) with the objective of recording models as would occur in a VES survey for metamorphosing frogs. The nocturnal VES were conducted in late July through to late September, from after sunset between 1800 hours and 2030 hours. When models were found, they were collected, and their unique numbers were recorded. An independent observer relocated models that were found and not found in each wetland the following day, so that three repeat surveys were conducted per wetland.
Differences in seven different habitat types were tested, consisting of floating algae mat (filamentous algae), and stands of the following emergent reed plants that occurred in most of the wetlands: Bolboschoenus caldwelli, B. fluviatalis, grass, Phagmites australis, Schoenoplectus vallidus, Typha sp. The detection probability for metamorphs occurring in open water was assumed to be 0.76, as determined in (Valdez et al. 2017). A general linear model with a binomial distribution was used to determine whether there was a significant difference in detection probability among habitat types, where each model frog in a wetland was allocated to a Bernoulli success (1 = model frog detected, 0 = model frog not detected). The differences in detection probability were analysed among all habitat types with the emmeans package in R statistics, where an α level of P = 0.05 was used. See Supplementary material Appendix S1 for statistical methods and results of this analysis.
There was a significant difference between grass and all other habitat types. Hence, detection probability values for three groups of habitat type were considered, namely, open water (d = 0.77 ± 0.12 s.e.), grass (d = 0.22 ± 0.06 s.e.) and all other habitat types lumped into one detection probability group (d = 0.47 ± 0.07 s.e.). To calculate the overall detection probability of metamorphs in a survey, a product sum was applied to the estimated detection probabilities of each habitat type that were weighted by the proportion of metamorphs observed in each habitat type during the survey, as per the equation below:
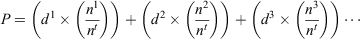
where P = the overall detection probability of metamorphs in the survey, n1 = the number of metamorphs observed using a certain habitat type in one survey, nt = the total number of metamorphs observed in the survey across all habitat types and d = the detection probability of the specific habitat type.
Determining the number of mating pairs per tadpole/metamorph cohort
It is likely that metamorph output from a wetland would be influenced by the number of breeding pairs that have contributed to a breeding event in a wetland. Because we were interested in the metamorph output per clutch, we accounted for this by using genetic analysis on tadpole samples from cohorts to identify full-sibling clusters, so as to quantify the number of independent clutches. This resolved one of the challenges of assessing the number of clutches in a wetland that occur in explosive breeding events, because all egg masses are deposited at the same time and therefore differences in size and developmental stages of tadpoles cannot be used to identify different cohorts. Tadpole siblings were identified by analysing 1988 single-nucleotide polymorphisms (SNP) derived from DArTseq (Wenzl et al. 2008) that were obtained through filtering a total SNP dataset of 41 151 polymorphic loci. Loci were filtered on the basis of a 100% call rate, 100% reproducibility, minimum minor allele frequency (MAF) of 5%, and removal of secondaries and linkage disequilibrium was filtered at threshold of <90% by using the R package SNPRelate (Zheng et al. 2012). All previous filtering steps were performed in the R package, DArTR (Gruber et al. 2018).
COLONY (version 2) was used to assign the relationships among tadpoles within each cohort as either full-siblings, half-siblings or unrelated. (Beranek et al. 2021a). From this analysis, the number of sib-ship clusters was used to determine how many mating pairs contributed to the tadpole cohort within a wetland. The metamorph count data were divided by this number to produce the number of metamorphs per clutch. Given that there was a limited number of genotypes that could be analysed emphasis was given to determining the number of mating pairs in tadpole cohorts produced in short-hydroperiod wetlands, as opposed to long-hydroperiod wetlands. This was deemed as a valid approach because we had a means to determine when multiple mating pairs contributed to tadpole cohorts in permanent wetlands (differences in size-classes), and we had an a priori expectation that we would observe larger numbers of L. aurea metamorphs emerging from short-hydroperiod wetlands because this has been observed before (Goldingay and Newell 2005). This meant that weight was given to the null hypothesis of Aim 1.
Statistical analysis: comparing differences in metamorph numbers between hydroperiod categories (Aim 1)
To test the difference in metamorph counts between hydroperiod categories, a negative binomial model was used, because preliminary data investigations identified over-dispersion within the data. Negative binomial models provide an elegant solution to dealing with over-dispersion by stretching a Poisson distribution with an additional parameter (theta) to fit the degree of over-dispersion present (Ver Hoef and Boveng 2007). The metamorph counts were standardised to represent the mean number of metamorphs per mating pair of each breeding event. This was achieved by dividing the metamorph count by the number of full-sibling tadpole groups quantified with molecular pedigree analysis. The adjusted metamorph counts were also tested with and without a detection probability offset to determine whether this variable influenced the result where an increase of 3 scores in Akaike information criterion (AIC) was considered a significant improvement of the model.
Statistical analysis: factors explaining variations in metamorph numbers (Aim 2)
To test the influence of the environmental explanatory variables, the same procedure as above was followed. However, there were two instances where 100% mortality of tadpoles was observed and these were removed from this analysis because, for this aim, we were interested in knowing which factors contributed to metamorph survival in wetlands where they were able to successfully metamorphose. This resulted in a sample size of n = 35. Each variable was inserted into models where the strength of the variable in explaining metamorph counts were assessed on the basis of AIC values and effect sizes (see Table 1 for summary of covariates and Appendix S2 for a summary of species included in each predator covariate). Effect sizes were derived from odds ratios.
|
Statistical analysis: comparison of catch per unit effort (CPUE) in aquatic fauna between wetland refill categories (Aim 3)
The abundances of aquatic fauna were tested for differences between wetland refill treatments following the same statistical procedure as for the previous analyses. However, the data for this analysis focussed on catch per unit effort of aquatic fauna in Fyke nets modelled against refill category (recently refilled, n = 23; not recently refilled, n = 81). This was applied to Lim. peronii tadpoles, Lit. aurea tadpoles, Anax papuensis larvae, Cybister tripunctatus (adults and larvae), Notonectidae, Dytiscidae and Zygoptera. Another difference was that wetland name was used as a random effect. The means and standard errors are plotted for each comparison and an ANOVA using the function glmmTMB:::Anova.glmmTMB from the glmmTMB package was used to determine a statistically significant difference with an α level of P = 0.05.
Results
In total, 37 breeding events were observed over the four breeding seasons within the nine studied wetlands. Of these, 14 occurred in short-hydroperiod wetlands that had recently refilled (| < | 25 days), as explosive breeding events (mean days since drying = 6 days ± 2.2 s.e., range: 0–25). The other 23 occurred in long-hydroperiod wetlands that had remained charged for over | > | 170 days (mean days since drying = 627 days ± 86 s.e., range: 170–1473). Of the 37 breeding events observed, five were confirmed by egg clutch observations, 31 were confirmed by the presence of tadpoles and one was confirmed by the presence of metamorphs. Of the breeding events confirmed by the presence of tadpoles, five were confirmed by differential size-classes.
Genetics showed a further 31 mating pairs from 13 tadpole cohorts (originally assigned as one breeding event each). Hence, it was estimated that a minimum of 28 mating pairs bred in wetlands that remained charged, and 40 bred in wetlands that had been newly refilled.
Timing of recruitment in differing hydroperiod categories
There were distinct temporal patterns in the occurrence of breeding events in each wetland hydroperiod category. Metamorph output from short-hydroperiod wetlands occurred primarily in March and April, that is, towards the latter quarter of the breeding season, with one instance occurring in early September (Table 2). Overwintering tadpoles were observed in August 2018, which resulted in metamorph recruitment in September 2018 (n = 2 metamorphs). Metamorph output from long-hydroperiod wetlands occurred only in the months of December, January and February. These patterns were evident in almost every breeding season. The one exception being that there was no metamorph output in March and April of the 2018/19 season, because there was no rain in the preceding January or February to produce wetland replenishment, and wetlands that were dry during this period remained dry until the breeding season ended. There were two instances of 100% mortality of tadpoles owing to wetland drying, both occurring in recently refilled ephemeral wetlands that had been recharged during spring, but there were no follow-up rains to maintain the wetlands. In these instances, breeding was estimated to have occurred on 10 October 2018 in 4C and 19 September 2019 in 2B. Subsequent drying occurred about a month later (8 November 2018) in 4C and about a month and a half later (1 November 2019) in 2B.

|
Comparing differences in metamorph output between wetland hydroperiod categories (Aim 1)
There was a higher mean metamorph output from breeding events that occurred in short-hydroperiod wetlands than from those that occurred in long-hydroperiod wetlands (Fig. 2). This was true whether detection probability was included as an offset (Deviance[1,35] = 38.80, P = 0.000) or not (Deviance[1,35] = 36.02, P = 0.000). The model containing an offset had a smaller difference between the two categories. With the offset not included, the mean metamorph output in the short-hydroperiod category was ~8.2 times greater than was the alternative (short = 26 ± 15–44 95% CI, long = 3 ± 2–5 95% CI). With the offset included, the mean metamorph output in the short-hydroperiod category was ~9.2 times greater (short = 29 ± 17–50 95% CI, long = 3 ± 2–5 95% CI). The AIC was smaller in the model that did not contain the offset (227.8 > 226.5).

|
Factors explaining variations in metamorph output (Aim 2)
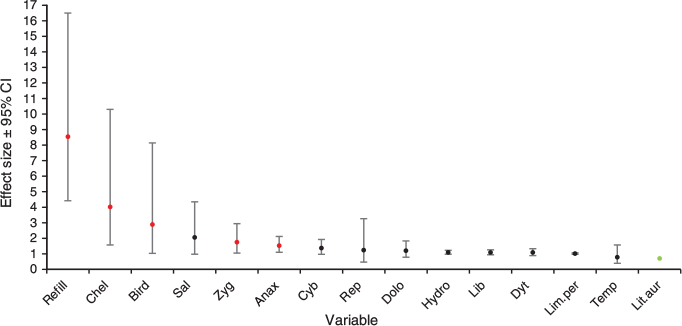
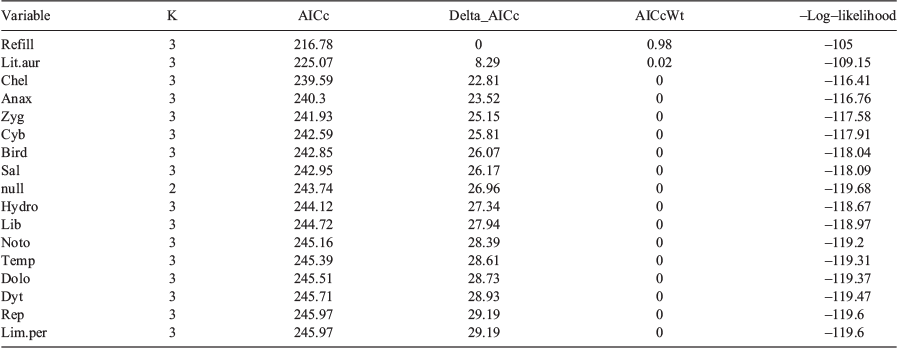
Wetland hydroperiod category was the strongest predictor for explaining observed metamorph counts (Table 3). This variable had the greatest AIC weight (0.98) and improved the model by 8.3 AIC values, compared with the next-best model. The second-most parsimonious model contained Lit.aur as a covariate, which had an AIC weight of 0.02 and improved the model by 14.52 AIC values, compared with the model containing Chel. Including Chel, there were three models that contained a predator covariate (Anax and Zyg) in the top five models.
|
Effect sizes showed that five variables had significant negative relationships with metamorph counts, and one variable had a significant positive relationship (Fig. 3). Four of the five variables with significant negative relationships were predator variables, including Chelodina longicollis presence, predatory waterbird presence, Zygopteran larvae abundance and Anax papuensis abundance, ordered from the strongest to the weakest. Refill category had the strongest negative relationship with mean metamorph count among all variables. Litoria aurea tadpole abundance was the only variable that had a positive relationship with mean metamorph counts. The predator variable Cybister tripunctatus abundance and the water quality variable average salinity had negative relationships with metamorph counts; however, these were not statistically significant because their confidence intervals marginally overlapped zero. All other variables had no obvious relationship with the metamorph counts because their effect sizes were near one, with confidence intervals in most instances widely overlapping this threshold.
Comparison of CPUE in aquatic fauna between wetland hydroperiod categories (Aim 3)
Confirmed predators and several other inferred predators were mostly found in higher abundances in wetlands that had not recently dried and refilled (Fig. 4; see Appendix S3 for observations of predator–prey interactions among aquatic fauna). In contrast, the tadpoles of L. aurea were observed to be in higher abundances in short-hydroperiod wetlands (χ2[1] = 22.97, P = 0.000). The tadpoles of Lim. peronii showed no obvious difference between hydroperiod categories (χ2[1] = 0.28, P = 0.595). Long-hydroperiod wetlands had significantly higher CPUE of Cybister tripunctatus (χ2[1] = 8.26, P = 0.004), Noto (χ2[1] = 7.00, P = 0.008), Anax papuensis (χ2[1] = 13.93, P = 0.000) and Zygopteran larvae (χ2[1] = 7.41, P = 0.007). No difference was observed between the hydroperiod categories in the freshwater-macroinvertebrate taxa Dolomedes facetus (χ2[1] = 0.08, P = 0.776), Hydro (χ2[1] = 0.29, P = 0.592) and small dytiscid beetles (χ2[1] = 0.12, P = 0.730).

|
Discussion
These findings have implications for understanding amphibian ecology, evolution and conservation biology of Litoria aurea, which are likely to be applicable to its threatened sister species, Litoria raniformis, and potentially other amphibians globally. We found support for all hypotheses, namely (1) that short hydroperiod wetlands produced higher numbers of L. aurea metamorphs, (2) that tadpole predators featured as determining factors of metamorph output, and (3) that tadpole predators of L. aurea were more abundant in short-hydroperiod wetlands than in long-hydroperiod wetlands, whereas the opposite was true for L. aurea tadpoles. Hence, we focus our discussion on tadpole predators in regard to understanding of amphibian ecology, innate behavioural evolution in L. aurea and formulating conservation strategies.
Understanding the role of aquatic predators in amphibian ecology
We found support for the hypothesis of Aim 1 that there is a greater metamorph output in short-hydroperiod wetlands than in long-hydroperiod wetlands. This result is in contrast to those of a similar study on the Columbia spotted frog Rana luteiventris where wetlands with semi-permanent and permanent hydrology were more productive for metamorph output (McCaffery et al. 2014). This disparity between studies highlights the importance of understanding the unique ecology of each species and has obvious implications for conservation management.
We found that metamorph output in L. aurea was lower in wetlands that contained lower numbers of tadpole predators. This agrees with the hypotheses of Aims 2 and 3, namely that metamorph output is dependent on wetland hydroperiod, where short-hydroperiod wetlands had higher abundances of tadpole predators. We identified a L. aurea tadpole predator that has not yet been recognised (Pyke and White 2001), the larvae of the Australian emperor dragonfly (Anax papuensis; see S3 for predation observation descriptions). Our results indicated that this predator occurs in greater numbers in long-hydroperiod wetlands, and we found the opposite trend for L. aurea tadpole and metamorph abundance. Several other studies have highlighted the role of native freshwater macroinvertebrate predators in decreasing the recruitment of metamorphs in anuran populations (Loman 2002; Cole et al. 2016). However, it is likely that other tadpole predators that we did not investigate may produce similar impacts and this presents an avenue for future research.
The wider variance and the significantly greater mean metamorph output in short-hydroperiod wetlands than in long hydroperiod wetlands provided evidence of a reproductive fitness trade-off. The trade-off is between (1) the risk of total loss of reproductive effort if an ephemeral wetland dries, with the potential gains in tadpole survival as a result of reduced numbers of predators if the wetland retains water, and (2) the heightened risk of tadpole predation in permanent wetlands but the absent risk of tadpole desiccation. Short-hydroperiod wetlands have a higher risk of expedient drying, which can result in 100% mortality of tadpoles (Beranek et al. 2020a). We observed two such events and both occurred in wetlands with the shortest hydroperiods. Further adding to the variance in metamorph output in short-hydroperiod wetlands, we observed dispersal of the turtle C. longicollis into recently refilled wetlands, which is a behaviour commonly observed in this species (Ryan and Burgin 2007). Similarly, we found that dispersive wetland birds were negatively correlated with L. aurea metamorph output. This group of potential predators also possesses behaviours to seek out shallow ephemeral wetlands for foraging (Fasola 1982; Beranek 2020). Although only one cohort of tadpoles co-occurred with the mosquito fish (Gambusia holbrooki), which precluded analysis, it is likely that their presence would contribute to reductions in metamorph output (van de Mortel and Goldingay 1998). This fish species is also dispersive and readily colonises newly refilled wetlands (Chapman and Warburton 2006). It is likely that stochastic colonisation events of dispersive predators’ produce additional variance to metamorph output in short-hydroperiod wetlands.
Although desiccation is less likely to occur in long-hydroperiod wetlands, the risk of predation is increased. We found greater numbers of tadpole predators in long-hydroperiod wetlands, and found some of these predators to be significantly negatively correlated with the metamorph output. Some of these predators were freshwater macroinvertebrates and this gives weight to the importance of this group as a driver of amphibian population dynamics (Valdez 2018).
Understanding the role of aquatic predators in the evolution of breeding habitat selection
Despite the risks associated with desiccation and total reproductive loss (Darwinian fitness of zero) in short-hydroperiod wetlands, to maximise offspring survival and thereby their own genetic fitness, L. aurea has evolved behaviours attuned to seek out such situations (see Hamer et al. 2008). As hypothesised, this can be explained by the substantially higher mean metamorph output in short-hydroperiod wetlands, owing to fewer predators. Short-hydroperiod wetlands have a ~8.2-fold larger mean metamorph output, per clutch, than do clutches conceived in long-hydroperiod wetlands, and a lower chance of containing predators. This gives insight to the evolutionary processes underlying the explosive breeding behaviours during large rainfall events of the bell frog complex and potentially other explosive breeding amphibians that occupy mesic areas where both permanent and ephemeral water bodies are available.
Insight to conservation strategies
Our results suggest that ephemeral wetlands, coupled with permanent wetlands, may be vital for supporting L. aurea populations affected by disease, by providing enhanced recruitment. The higher metamorph output in short-hydroperiod wetlands highlights the importance of this breeding habitat for the restoration ecology of L. aurea in chytrid-affected populations. Other studies should confirm whether similar tadpole recruitment dynamics occur in L. raniformis and other chytrid-affected amphibians, so as to determine whether short-hydroperiod wetlands are also useful to increase recruitment.
These findings also have implications for improving tadpole reintroduction protocols in L. aurea and other amphibians. Because there was a higher metamorph output and inferred higher tadpole survival in short-hydroperiod wetlands than in long-hydroperiod wetlands, such situations should be sought after for the introduction of initial propagules. In past tadpole reintroduction attempts of L. aurea, practitioners have not regarded the hydroperiod of release sites and, in most instances, have released tadpoles into permanent water bodies (Daly et al. 2008; Pyke et al. 2008; White and Pyke 2008; Klop-Toker et al. 2021). The practitioners of these attempts did control for large predators by conducting fish removal. However, our results highlighted that freshwater macroinvertebrates may also contribute to tadpole mortality and, hence, only targeting vertebrate predators may not be sufficient to reduce predation pressure.
A solution for reintroduction protocols to counter high tadpole mortality in L. aurea is to time releases to coincide with late heavy summer rain, and introduce propagules into wetlands that were previously dry. This strategy is guided by the behavioural adaptation of L. aurea to preferentially select and disperse to recently charged wetlands. Our findings showed that this set of behaviours have been selected because of increased survival rates of offspring. Our results indicated that this would maximise the metamorph output by 8.2-fold, and would mostly avoid heavy predation rates of freshwater-macroinvertebrate predators, such as Anax papuensis. This strategy of timing tadpole release to match the natural spatial and temporal patterns in breeding is supported by the reintroduction procedure reviewed by Mendelson III and Altig (2016). Combining this strategy with long-term planning of dealing with issues arising from sex-specific maturation periods (Beranek et al. 2021b) may result in more successful reintroductions of L. aurea and other similar species.
This reintroduction strategy can be backed up with temporary predator-proof barriers around the release site (Rahel 2013), or manual removal of large predators. Predator removal would be best targeted to large and dispersive tadpole eaters such as the eastern-long necked turtle (Chelodina longicollis) and eels (Anguilla sp.; Roe and Georges 2008). Indeed, we found the presence of C. longicollis to be the most powerful predictor among examined predators of low metamorph output in L aurea. This predator nests in the banks of wetlands, where their young hatch during late-summer rainfall events (Roe and Georges 2008). The occurrence and abundance of this native species should be monitored if the project aim is to enhance the threatened frog population. However, there may be unintended consequences of managing native food webs. For example, the turtle may limit the abundance of other smaller predators. In comparison, permanent exclusion of introduced tadpole predators, such as the mosquito fish, is warranted where feasible, to maximise recruitment (Beranek et al. 2021c).
Owing to the low sample sizes of breeding events (n = 37 breeding events, in n = 10 wetlands), we did not have sufficient power to test multivariate models, and hence there is scope to further unravel the importance of factors in determining the recruitment of L. aurea. It seems likely that trophic cascades (Ripple et al. 2016) and interactions among aquatic fauna may influence tadpole predator abundance and, hence, metamorph output. Despite the low sample sizes, alternative hypotheses that could potentially explain the conclusions of this study were explored, such as differences in detectability of metamorphs between wetlands and the potentially greater amount of mating pairs contributing to breeding in explosive events. Finally, it is possible that other factors we did not measure could also be important in determining metamorph output, such as food availability and chytrid loads. More research is needed to further unravel the importance of each driver in determining metamorph output in amphibians.
Conclusions
In conclusion, we have demonstrated that predators may be an important factor guiding the adaptive response of L. aurea to select recently refilled wetlands as breeding habitat. We suggest amphibian conservation practitioners investigate and use the adaptive responses of amphibians to guide reintroduction protocols when undertaking tadpole and metamorph releases. In this instance, releasing L. aurea tadpoles into recently refilled wetlands at the end of summer after heavy rainfall is likely to result in an increased in metamorph output.
Data availability statement
The data are available on Mendeley Data: 10.17632/fdkkpd86wp.1.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was funded by BHP and Earthwatch Australia.
Acknowledgements
We thank Earthwatch for organising teams of students to assist fieldwork (see Appendix S4 for list of students involved in field research). We thank summer scholarship recipients Robbie Fay, Giorginna Xu, John Hembra, Kathleen Bushell, Lachlan McCrae, Max Manion, Kate Schmal and Alexandra Cottle for their assistance in fieldwork. We also thank many others for fieldwork assistance, namely Rennay Baldwin, Hayden Bond, Lachlan Burgess, Lucille Courthaudon, Rachel Ivory, Dean Lenga, Stephen Mahony, Kim McGloury, Jessica McGreggor, Lily Mickaill, Heidi Pritchard, Ben Rochester, Josh Smart, and Bonni Yare. We thank Kim Colyvas for advice regarding statistics and Ray Marten for reviewing the manuscript. Fieldwork was conducted under approval from the University of Newcastle Animal Care and Ethics Committee (ARA 2010-154) and University of Newcastle Work Health and Safety policy. Approvals to operate fieldwork were granted under NSW Scientific Licences SL100529 and SL10090. We thank Ross Goldingay and Skye Wassens who reviewed and improved the paper.
References
Beranek, C. T. (2020). Nocturnal detection of Australian Little Bittern and Australian Painted-snipe–Prospects for nocturnal survey methods for rare wetland birds. The Whistler 14, 48–53.Beranek, C. T., Clulow, J., and Mahony, M. (2020a). A simple design feature to increase hydro‐period in constructed ephemeral wetlands to avoid tadpole desiccation‐induced mortality. Ecological Management & Restoration 21, 250–253.
| A simple design feature to increase hydro‐period in constructed ephemeral wetlands to avoid tadpole desiccation‐induced mortality.Crossref | GoogleScholarGoogle Scholar |
Beranek, C. T., Clulow, J., and Mahony, M. (2020b). Wetland restoration for the threatened green and golden bell frog (Litoria aurea): development of a breeding habitat designed to passively manage chytrid-induced amphibian disease and exotic fish. Natural Areas Journal 40, 362–374.
| Wetland restoration for the threatened green and golden bell frog (Litoria aurea): development of a breeding habitat designed to passively manage chytrid-induced amphibian disease and exotic fish.Crossref | GoogleScholarGoogle Scholar |
Beranek, C. T., Clulow, J., and Mahony, M. (2021a). Genetic evidence for polyandry in the threatened green and golden bell frog. Genetica , .
| Genetic evidence for polyandry in the threatened green and golden bell frog.Crossref | GoogleScholarGoogle Scholar | 34655370PubMed |
Beranek, C.T., Maynard, C., McHenry, C., Clulow, J., and Mahony, M. (2021b). Identifying a limiting factor in the population dynamics of a threatened amphibian: the influence of extended female maturation and operational sex ratio. Austral Ecology , .
| Identifying a limiting factor in the population dynamics of a threatened amphibian: the influence of extended female maturation and operational sex ratio.Crossref | GoogleScholarGoogle Scholar |
Beranek, C. T., Maynard, C., McHenry, C., Clulow, J., and Mahony, M. (2021c). Rapid population increase of the threatened Australian amphibian Litoria aurea in response to wetlands constructed as a refuge from chytrid-induced disease and introduced fish. Journal of Environmental Management 291, 112638.
| Rapid population increase of the threatened Australian amphibian Litoria aurea in response to wetlands constructed as a refuge from chytrid-induced disease and introduced fish.Crossref | GoogleScholarGoogle Scholar | 33962282PubMed |
Binckley, C. A., and Resetarits, W. J. (2002). Reproductive decisions under threat of predation: squirrel treefrog (Hyla squirella) responses to banded sunfish (Enneacanthus obesus). Oecologia 130, 157–161.
| Reproductive decisions under threat of predation: squirrel treefrog (Hyla squirella) responses to banded sunfish (Enneacanthus obesus).Crossref | GoogleScholarGoogle Scholar | 28547021PubMed |
Blaustein, L. (1999). Oviposition site selection in response to risk of predation: evidence from aquatic habitats and consequences for population dynamics and community structure. In ‘Evolutionary theory and processes: modern perspectives’. (Ed. S. P. Wasser.) pp. 441–456. (Springer Publishing: Amsterdam, The Netherlands.)
Chapman, P., and Warburton, K. (2006). Postflood movements and population connectivity in gambusia (Gambusia holbrooki). Ecology Freshwater Fish 15, 357–365.
| Postflood movements and population connectivity in gambusia (Gambusia holbrooki).Crossref | GoogleScholarGoogle Scholar |
Cole, E. M., Hartman, R., and North, M. P. (2016). Hydroperiod and cattle use associated with lower recruitment in an r-selected amphibian with a declining population trend in the Klamath Mountains, California. Journal of Herpetology 50, 37–43.
| Hydroperiod and cattle use associated with lower recruitment in an r-selected amphibian with a declining population trend in the Klamath Mountains, California.Crossref | GoogleScholarGoogle Scholar |
Daly, G., Johnson, P., Malolakis, G., Hyatt, A., and Pietsch, R. (2008). Reintroduction of the Green and Golden Bell Frog Litoria aurea to Pambula on the south coast of New South Wales. Australian Zoologist 34, 261–270.
| Reintroduction of the Green and Golden Bell Frog Litoria aurea to Pambula on the south coast of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Fardell, L., Valdez, J., Klop–Toker, K., Stockwell, M., Clulow, S., Clulow, J., and Mahony, M. (2018). Effects of vegetation density on habitat suitability for the endangered green and golden bell frog, Litoria aurea. Herpetological Conservation and Biology 13, 47–57.
Fasola, M. (1982). Feeding dispersion in the Night Heron Nycticorax nycticorax and Little Egret Egretta garzetta and the information centre hypothesis. The Italian Journal of Zoology 49, 177–186.
Goldingay, R., and Newell, D. (2005). Aspects of the population ecology of the green and golden bell frog Litoria aurea at the northern end of its range. Australian Zoologist 33, 49–59.
| Aspects of the population ecology of the green and golden bell frog Litoria aurea at the northern end of its range.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R. L., Parkyn, J., and Newell, D. A. (2017). No evidence of protracted population decline across 17 years in an unmanaged population of the green and golden bell frog in north-eastern New South Wales. Australian Journal of Zoology 65, 87–96.
| No evidence of protracted population decline across 17 years in an unmanaged population of the green and golden bell frog in north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Gould, J., Valdez, J. W., Clulow, J., and Clulow, S. (2019). Diving beetle offspring oviposited in amphibian spawn prey on the tadpoles upon hatching. Entomological Science 22, 393–397.
| Diving beetle offspring oviposited in amphibian spawn prey on the tadpoles upon hatching.Crossref | GoogleScholarGoogle Scholar |
Gruber, B., Unmack, P. J., Berry, O. F., and Georges, A. (2018). dartr: an r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18, 691–699.
| dartr: an r package to facilitate analysis of SNP data generated from reduced representation genome sequencing.Crossref | GoogleScholarGoogle Scholar | 29266847PubMed |
Hamer, A. J. (2016). Accessible habitat delineated by a highway predicts landscape-scale effects of habitat loss in an amphibian community. Landscape Ecology 31, 2259–2274.
| Accessible habitat delineated by a highway predicts landscape-scale effects of habitat loss in an amphibian community.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J. (2018). Accessible habitat and wetland structure drive occupancy dynamics of a threatened amphibian across a peri-urban landscape. Landscape and Urban Planning 178, 228–237.
| Accessible habitat and wetland structure drive occupancy dynamics of a threatened amphibian across a peri-urban landscape.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., and Mahony, M. J. (2010). Rapid turnover in site occupancy of a pond-breeding frog demonstrates the need for landscape-level management. Wetlands 30, 287–299.
| Rapid turnover in site occupancy of a pond-breeding frog demonstrates the need for landscape-level management.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., and Parris, K. M. (2011). Local and landscape determinants of amphibian communities in urban ponds. Ecological Applications 21, 378–390.
| Local and landscape determinants of amphibian communities in urban ponds.Crossref | GoogleScholarGoogle Scholar | 21563570PubMed |
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2002a). Management of freshwater wetlands for the endangered green and golden bell frog (Litoria aurea): roles of habitat determinants and space. Biological Conservation 106, 413–424.
| Management of freshwater wetlands for the endangered green and golden bell frog (Litoria aurea): roles of habitat determinants and space.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2002b). The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia. Oecologia 132, 445–452.
| The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2008). Movement patterns of adult Green and Golden Bell Frogs Litoria aurea and the implications for conservation management. Journal of Herpetology 42, 397–407.
| Movement patterns of adult Green and Golden Bell Frogs Litoria aurea and the implications for conservation management.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., Schmera, D., and Mahony, M. J. (2021). Multi‐species occupancy modeling provides novel insights into amphibian metacommunity structure and wetland restoration. Ecological Applications 31, e2293.
| 33432692PubMed |
Hopey, M. E., and Petranka, J. W. (1994). Restriction of wood frogs to fish-free habitats: how important is adult choice? Copeia 1994, 1023–1025.
| Restriction of wood frogs to fish-free habitats: how important is adult choice?Crossref | GoogleScholarGoogle Scholar |
Kats, L. B., Petranka, J. W., and Sih, A. (1988). Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 69, 1865–1870.
| Antipredator defenses and the persistence of amphibian larvae with fishes.Crossref | GoogleScholarGoogle Scholar |
Kearney, B. D., Byrne, P. G., and Reina, R. D. (2012). Larval tolerance to salinity in three species of Australian anuran: an indication of saline specialisation in Litoria aurea. PLoS One 7, e43427.
| Larval tolerance to salinity in three species of Australian anuran: an indication of saline specialisation in Litoria aurea.Crossref | GoogleScholarGoogle Scholar | 22916260PubMed |
Klop-Toker, K., Valdez, J., Stockwell, M., Clulow, S., Clulow, J., and Mahony, M. (2018). Community level impacts of invasive mosquitofish may exacerbate the impact to a threatened amphibian. Austral Ecology 43, 213–224.
| Community level impacts of invasive mosquitofish may exacerbate the impact to a threatened amphibian.Crossref | GoogleScholarGoogle Scholar |
Klop-Toker, K., Callen, A., King, J.-P., Beranek, C., Lenga, D., Valdez, J., Clulow, S., Pizzatto, L., Stockwell, M., Clulow, J., and Mahony, M. (2021). Reintroduction of green and golden bell frogs into created habitats on Kooragang Island, Australia. In ‘Global conservation translocation perspectives: 2021. Case studies from around the globe’. (Ed. P. S. Soorae.) pp. 70–75. (IUCN SSC Conservation Translocation Specialist Group, Environment Agency: Abu Dhabi; and Calgary Zoo, Canada: Gland, Switzerland.)
Lemckert, F. L., and Mahony, M. J. (2010). The relationship among multiple-scale habitat variables and pond use by anurans in northern New South Wales, Australia. Herpetological Conservation and Biology 5, 537–547.
Loman, J. (2002). Rana temporaria metamorph production and population dynamics in the field: effects of tadpole density, predation and pond drying. Journal for Nature Conservation 10, 95–107.
| Rana temporaria metamorph production and population dynamics in the field: effects of tadpole density, predation and pond drying.Crossref | GoogleScholarGoogle Scholar |
Mahony, M. J., Hamer, A. J., Pickett, E. J., McKenzie, D. J., Stockwell, M. P., Garnham, J. I., Keely, C. C., Deboo, M. L., O’Meara, J., Pollard, C. J., Clulow, S., Lemckert, F. L., Bower, D. S., and Clulow, J. (2013). Identifying conservation and research priorities in the face of uncertainty: a review of the threatened bell frog complex in eastern Australia. Herpetological Conservation and Biology 8, 519–538.
McCaffery, R. M., Eby, L. A., Maxell, B. A., and Corn, P. S. (2014). Breeding site heterogeneity reduces variability in frog recruitment and population dynamics. Biological Conservation 170, 169–176.
| Breeding site heterogeneity reduces variability in frog recruitment and population dynamics.Crossref | GoogleScholarGoogle Scholar |
Mendelson, J. R., and Altig, R. (2016). Tadpoles, froglets, and conservation: a discussion of basic principles of rearing and release procedures. Amphibian & Reptile Conservation 10, 20–27.
O’Meara, J., and Darcovich, K. (2015). Twelve years on: ecological restoration and rehabilitation at Sydney Olympic Park. Ecological Management & Restoration 16, 14–28.
| Twelve years on: ecological restoration and rehabilitation at Sydney Olympic Park.Crossref | GoogleScholarGoogle Scholar |
Odendaal, F. J., Bull, C. M., and Nias, R. C. (1982). Habitat selection in tadpoles of Ranidella signifera and R. riparia (Anura: Leptodactylidae). Oecologia 52, 411–414.
| Habitat selection in tadpoles of Ranidella signifera and R. riparia (Anura: Leptodactylidae).Crossref | GoogleScholarGoogle Scholar | 28310404PubMed |
Petranka, J. W., Harp, E. M., Holbrook, C. T., and Hamel, J. A. (2007). Long-term persistence of amphibian populations in a restored wetland complex. Biological Conservation 138, 371–380.
| Long-term persistence of amphibian populations in a restored wetland complex.Crossref | GoogleScholarGoogle Scholar |
Pintar, M. R., and Resetarits, W. J. (2017). Out with the old, in with the new: oviposition preference matches larval success in Cope’s gray treefrog, Hyla chrysoscelis. Journal of Herpetology 51, 186–189.
| Out with the old, in with the new: oviposition preference matches larval success in Cope’s gray treefrog, Hyla chrysoscelis.Crossref | GoogleScholarGoogle Scholar |
Pintar, M. R., and Resetarits, W. J. (2020). Aquatic beetles influence colonization of disparate taxa in small lentic systems. Ecology and Evolution 10, 12170–12182.
| Aquatic beetles influence colonization of disparate taxa in small lentic systems.Crossref | GoogleScholarGoogle Scholar | 33209279PubMed |
Pollard, C. J., Stockwell, M. P., Bower, D. S., Garnham, J. I., Pickett, E. J., Darcovich, K., O’Meara, J., Clulow, J., and Mahony, M. J. (2017). Removal of an exotic fish influences amphibian breeding site selection. The Journal of Wildlife Management 81, 720–727.
| Removal of an exotic fish influences amphibian breeding site selection.Crossref | GoogleScholarGoogle Scholar |
Pyke, G. H., and White, A. W. (2001). A review of the biology of the Green and Golden Bell Frog Litoria aurea. Australian Zoologist 31, 563–598.
| A review of the biology of the Green and Golden Bell Frog Litoria aurea.Crossref | GoogleScholarGoogle Scholar |
Pyke, G., White, A., Bishop, P., and Waldman, B. (2002). Habitat-use by the green and golden bell frog Litoria aurea in Australia and New Zealand. Australian Zoologist 32, 12–31.
| Habitat-use by the green and golden bell frog Litoria aurea in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
Pyke, G., Rowley, J., Shoulder, J., and White, A. (2008). Attempted introduction of the endangered Green and Golden Bell Frog to Long Reef Golf Course: a step towards recovery? Australian Zoologist 34, 361–372.
| Attempted introduction of the endangered Green and Golden Bell Frog to Long Reef Golf Course: a step towards recovery?Crossref | GoogleScholarGoogle Scholar |
Rahel, F. J. (2013). Intentional fragmentation as a management strategy in aquatic systems. Bioscience 63, 362–372.
| Intentional fragmentation as a management strategy in aquatic systems.Crossref | GoogleScholarGoogle Scholar |
Resetarits, W. J., and Wilbur, H. M. (1989). Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70, 220–228.
| Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors.Crossref | GoogleScholarGoogle Scholar |
Ripple, W. J., Estes, J. A., Schmitz, O. J., Constant, V., Kaylor, M. J., Lenz, A., Motley, J. L., Self, K. E., Taylor, D. S., and Wolf, C. (2016). What is a trophic cascade? Trends in Ecology & Evolution 31, 842–849.
| What is a trophic cascade?Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2008). Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system. Austral Ecology 33, 1045–1056.
| Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system.Crossref | GoogleScholarGoogle Scholar |
Ryan, M., and Burgin, S. (2007). Gone walkabout? Movement of the eastern long-necked turtle Chelodina longicollis from farm dams in northwest peri-urban Sydney (Australia). The Journal of Biological Sciences 8, 119–127.
Scheele, B. C., Pasmans, F., Skerratt, L. F., Berger, L., Martel, A., Beukema, W., Acevedo, A. A., Burrowes, P. A., Carvalho, T., Catenazzi, A., De la Riva, I., Fisher, M. C., Flechas, S. V., Foster, C. N., Frías-Álvarez, P., Garner, T. W. J., Gratwicke, B., Guayasamin, J. M., Hirschfeld, M., Kolby, J. E., Kosch, T. A., La Marca, E., Lindenmayer, D. B., Lips, K. R., Longo, A. V., Maneyro, R., McDonald, C. A., Mendelson, J., Palacios-Rodriguez, P., Parra-Olea, G., Richards-Zawacki, C. L., Rödel, M.-O, Rovito, S. M., Soto-Azat, C., Toledo, L. F., Voyles, J., Weldon, C., Whitfield, S. M., Wilkinson, M., Zamudio, K. R., and Canessa, S. (2019). Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463.
| Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity.Crossref | GoogleScholarGoogle Scholar | 30923224PubMed |
Stein, M., and Blaustein, L. (2015). Larval performance and oviposition habitat selection of the tree frog, Hyla savignyi, in response to conspecific larval density. Israel Journal of Ecology & Evolution 61, 61–66.
| Larval performance and oviposition habitat selection of the tree frog, Hyla savignyi, in response to conspecific larval density.Crossref | GoogleScholarGoogle Scholar |
Valdez, J. W. (2018). Predaceous diving beetles (Coleoptera: Dytiscidae) may affect the success of amphibian conservation efforts. Australian Journal of Zoology 66, 352–355.
| Predaceous diving beetles (Coleoptera: Dytiscidae) may affect the success of amphibian conservation efforts.Crossref | GoogleScholarGoogle Scholar |
Valdez, J. W., Stockwell, M. P., Klop-Toker, K., Clulow, S., Clulow, J., and Mahony, M. J. (2015). Factors driving the distribution of an endangered amphibian toward an industrial landscape in Australia. Biological Conservation 191, 520–528.
| Factors driving the distribution of an endangered amphibian toward an industrial landscape in Australia.Crossref | GoogleScholarGoogle Scholar |
Valdez, J., Klop-Toker, K., Stockwell, M. P., Clulow, S., Clulow, J., and Mahony, M. J. (2016). Microhabitat selection varies by sex and age class in the endangered green and golden bell frog Litoria aurea. Australian Zoologist 38, 223–234.
| Microhabitat selection varies by sex and age class in the endangered green and golden bell frog Litoria aurea.Crossref | GoogleScholarGoogle Scholar |
Valdez, J. W., Klop-Toker, K., Stockwell, M. P., Fardell, L., Clulow, S., Clulow, J., and Mahony, M. J. (2017). Differences in microhabitat selection patterns between a remnant and constructed landscape following management intervention. Wildlife Research 44, 248–258.
| Differences in microhabitat selection patterns between a remnant and constructed landscape following management intervention.Crossref | GoogleScholarGoogle Scholar |
Van Buskirk, J. (2005). Local and landscape influence on amphibian occurrence and abundance. Ecology 86, 1936–1947.
| Local and landscape influence on amphibian occurrence and abundance.Crossref | GoogleScholarGoogle Scholar |
van de Mortel, T., and Goldingay, R. (1998). Population assessment of the endangered Green and Golden Bell Frog Litoria aurea at Port Kembla, New South Wales. Australian Zoologist 30, 398–404.
| Population assessment of the endangered Green and Golden Bell Frog Litoria aurea at Port Kembla, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Ver Hoef, J. M., and Boveng, P. L. (2007). Quasi-poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88, 2766–2772.
| Quasi-poisson vs. negative binomial regression: how should we model overdispersed count data?Crossref | GoogleScholarGoogle Scholar | 18051645PubMed |
Wassens, S., Hall, A., and Spencer, J. (2017). The effect of survey method on the detection probabilities of frogs and tadpoles in large wetland complexes. Marine and Freshwater Research 68, 686–696.
| The effect of survey method on the detection probabilities of frogs and tadpoles in large wetland complexes.Crossref | GoogleScholarGoogle Scholar |
Wells, K. D. (2010). ‘The ecology and behavior of amphibians.’ (University of Chicago Press: Chicago, IL, USA.)
Wenzl, P., Huttner, E., Carling, E. J., Xia, L., Blois, H., Caig, V., Heller-Uszynska, K., Jaccoud, D., Hopper, C., and Kilian, A. G. (2008). Diversity Arrays Technology (DArT): a generic high-density genotyping platform. In ‘7th International Safflower Conference’, 3–6 November 2008, Wagga Wagga, NSW, Australia. Available at http://www.australianoilseeds.com/__data/assets/pdf_file/0006/6837/final_Wenzl_oral_paper.pdf
White, A., and Pyke, G. (1996). Distribution and conservation status of the green and golden bell frog Litoria aurea in New South Wales. Australian Zoologist 30, 177.
White, A., and Pyke, G. (2008). Frogs on the hop: translocations of Green and Golden Bell Frogs Litoria aurea in Greater Sydney. Australian Zoologist 34, 249–260.
| Frogs on the hop: translocations of Green and Golden Bell Frogs Litoria aurea in Greater Sydney.Crossref | GoogleScholarGoogle Scholar |
Wilder, A. E., and Welch, A. M. (2014). Effects of salinity and pesticide on sperm activity and Oviposition site selection in green Treefrogs, Hyla cinerea. Copeia 2014, 659–667.
| Effects of salinity and pesticide on sperm activity and Oviposition site selection in green Treefrogs, Hyla cinerea.Crossref | GoogleScholarGoogle Scholar |
Zheng, X., Levine, D., Shen, J., Gogarten, S. M., Laurie, C., and Weir, B. S. (2012). A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 28, 3326–3328.
| A high-performance computing toolset for relatedness and principal component analysis of SNP data.Crossref | GoogleScholarGoogle Scholar | 23060615PubMed |