Linking nest microhabitat selection to nest survival within declining pheasant populations in the Central Valley of California
Ian A. Dwight A , Jessica H. Vogt A , Peter S. Coates A D , Joseph P. Fleskes A , Daniel P. Connelly B and Scott C. Gardner C
A D , Joseph P. Fleskes A , Daniel P. Connelly B and Scott C. Gardner C
A US Geological Survey, Western Ecological Research Center, Dixon Field Station, 800 Business Park Drive, Suite D, Dixon, CA 95620, USA.
B Pheasants Forever, 1783 Buerkle Circle, Saint Paul, MN 55110, USA.
C California Department of Fish and Wildlife, 1416 9th Street, 12th Floor, Sacramento, CA 95819, USA.
D Corresponding author. Email: pcoates@usgs.gov
Wildlife Research 47(5) 391-403 https://doi.org/10.1071/WR18199
Submitted: 21 December 2018 Accepted: 24 February 2020 Published: 15 July 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Context: The ring-necked pheasant (Phasianus colchicus) has experienced considerable population declines in recent decades, especially in agricultural environments of the Central Valley of California. Although large-scale changes in land cover have been reported as an important driver of population dynamics, the effects of microhabitat conditions on specific demographic rates (e.g. nesting) are largely unknown.
Aims: Our goal was to identify the key microhabitat factors that contribute to wild pheasant fitness by linking individual-level selection of each microhabitat characteristic to the survival of their nests within the California Central Valley.
Methods: We radio- or GPS-marked 190 female ring-necked pheasants within five study areas and measured nest-site characteristics and nest fates during 2013–2017. Specifically, we modeled microhabitat selection using vegetation covariates measured at nest sites and random sites and then modeled nest survival as a function of selecting each microhabitat characteristic.
Key results: Female pheasants tended to select nest sites with greater proportions of herbaceous cover and avoided areas with greater proportions of bare-ground. Specifically, perennial grass cover was the most explanatory factor with regard to nest survival, but selection for increasing visual obstruction alone was not shown to have a significant effect on survival. Further, we found strong evidence that pheasants selecting sites with greater perennial grass height were more likely to have successful nests.
Conclusions: Although pheasants will select many types of vegetation available as cover, our models provided evidence that perennial grasses are more beneficial than other cover types to pheasants selecting nesting sites.
Implications: Focusing management actions on promoting perennial grass cover and increased heights at the microsite level, in lieu of other vegetative modifications, may provide improved quality of habitat for nesting pheasants and, perhaps, result in increased productivity. This is especially important if cover is limited during specific times of the nesting period. Understanding how microhabitat selection influences fitness can help land managers develop strategies to increase the sustainability of hunted populations of this popular game-bird species.
Additional keywords: California, Central Valley, habitat selection, nest survival, perennial grass, Phasianus colchicus, ring-necked pheasant, vegetation composition.
Introduction
The reproductive success of wild bird populations is inextricably linked to a multitude of environmental factors and individual behaviors, including habitat selection. Nest-site selection and related behaviors are likely to be influenced by both large-scale factors at the landscape level, as well as conditions at the site level (Orians and Wittenberger 1991; Clark et al. 1999). Furthermore, habitat selected by an individual may not necessarily be optimal habitat for maximising reproductive success (Kristan 2003). Habitat-selection behaviors appear to be the result of an individual’s response to available environmental cues, rather than the assessment of habitat quality and how it might increase reproductive success (Kristan 2003). Decisions to settle and, subsequently, breed in a given area may be contingent on current habitat conditions, which could make predictions of future habitat quality difficult. This is especially important if the decision pertains to a specific breeding site (Orians and Wittenberger 1991). Certain landscapes may provide features that appear suitable but actually act as ‘ecological traps’ (Coates et al. 2017a), especially in agricultural systems (Best 1986). Identifying links between reproductive success and microhabitat selection of avian populations is critical to understanding mechanisms affecting population trends (Clark et al. 1999). This knowledge is especially important for farmland bird species because the landscapes they inhabit have been altered such that quality nesting habitat for these species is either highly fragmented or is no longer available (Benton et al. 2003; Coates et al. 2017b).
The ring-necked pheasant (Phasianus colchicus; hereafter, pheasant) was introduced to the western United States from eastern Asia in the late 1800s and was successfully established in California during the early 1900s (Lever 1987). However, in the past 30 years, populations of pheasants in agricultural and upland areas of California have sharply declined (Coates et al. 2017b). Multiple aspects of agricultural land-use changes, including habitat fragmentation as the result of hedgerow removal (Chamberlain et al. 2000; Benton et al. 2003), shifts in crop type and in timing of crop harvest (Glemnitz et al. 2015), increased predation associated with habitat alteration (Evans 2004; Seymour et al. 2004), and increased use of pesticides (Dahlgren 1988; Mineau and Whiteside 2006) are likely to have contributed to these declines. Previous management efforts to slow the decline of pheasant populations included predator removal (Chesness et al. 1968) and agricultural management techniques meant to increase the biodiversity and productivity of grassland communities (Warner and Etter 1989; Atkinson et al. 2005), but all were met with varying levels of success. Therefore, it is prudent to research alternative management approaches and their application toward pheasant populations in California. It follows that pheasants are charismatic, non-migratory and sensitive to changes in the environment (Coates et al. 2017b), and relative annual abundance can be tracked using standardised survey methodology (Rice 2003), which lend well to pheasants acting as a potential indicator of the overall health of agricultural landscapes in supporting avian populations (Nielson et al. 2008). Hence, populations of pheasants, particularly in the Central Valley of California, may shed light on the overall function of farmland habitats for other avian species that are often logistically challenging to monitor.
Studies examining habitat selection can be important tools for pheasant population recovery because they identify habitat deficiencies that may cause low reproductive success, which could provide land managers with specific management goals aimed at improving nest survival. Previous literature has focussed on pheasant nest-site selection (Wood and Brotherson 1981; Matthews et al. 2012) and nest survival (Patterson and Best 1996), but few studies have sought to link selection to the probability of reproductive success (Haensly et al. 1987). Therefore, we quantitatively linked microhabitat characteristics of pheasant nest-site selection to nest survival in the Central Valley of California. Specifically, our goal was to identify microhabitat components associated with nest-site selection in female pheasants and evaluate the extent to which selected habitat influences nest survival of pheasants in northern California. Female pheasants are known to select nest sites concealed by vegetation on the ground (Dumke and Pils 1979; Clark et al. 1999). Hence, vegetation that maintains structure throughout the breeding season, such as perennial grasses and forbs, may provide optimal nesting cover. We predicted that individual pheasants that selected nesting sites with relatively greater grass cover (e.g. annual, perennial) than that of the available habitat and sought to maximise visual obstruction around the nest were more likely to produce young than those that chose sites with less grass and visual obstruction. Our findings can inform effective habitat-management practices within agricultural landscapes by identifying unique ecological factors that are selected by this ground-nesting bird and by identifying the extent to which those factors contribute to nest survival.
Materials and methods
Study area
The study area comprised five study sites spanning across the Sacramento Valley and Sacramento–San Joaquin River Delta regions of California, USA (Fig. 1). Four study sites, Gray Lodge Wildlife Area (GLWA), Little Dry Creek unit of Upper Butte Basin Wildlife Area (UBBWA), Parker and Twin Lakes units of Yolo Bypass Wildlife Area (YBWA), and Roosevelt Ranch, were located in the Sacramento Valley region. These sites were 3723 ha, 1522 ha, 1742 ha and 1570 ha in size respectively. Mandeville Island Duck Club, a man-made island of ~2200 ha, was located in the Sacramento–San Joaquin River Delta region. Mandeville Island and Roosevelt Ranch were private hunting clubs that primarily managed pheasant and waterfowl; GLWA, UBBWA, YBWA, were public wildlife refuges managed by the Calfornia Department of Fish and Wildlife for multiple game and migratory bird species.
Pheasant habitat within the study area was typical of managed wetland–riparian, upland, and open grassland habitat surrounded by irrigated agriculture that included rice (Oryza sativa), orchards, alfalfa (Medicago sativa), barley (Hordeum vulgare), winter wheat (Triticum spp.) and a variety of row crops, such as tomato (Solanum lycopersicum), sunflower (Helianthus spp.), safflower (Carthamus tinctorus), and corn (Zea mays). Orchards in close proximity to the study area consisted primarily of almonds (Prunus dulcis), pistachios (Pistacia vera), walnuts (Juglans regia), olives (Olea europaea L.), and peaches (Prunus persica). Agricultural practices were similar among study sites, but crops harvested varied among sites and were rotated annually. Common herbaceous cover types in the wetland–riparian communities included tule (Schoenoplectus acutus var. occidentalis), cattail (Typha latifolia), smart weed (Persicaria punctata), watergrass (Echinochloa crus-galli), swamp timothy (Crypsis schoenoides) and sprangletop (Leptochloa fascicularis). Major cover types within upland communities consisted of white sweet clover (Melilotus albus), common sunflower (Helianthus annuus), gumweed (Grindelia camporum), cocklebur (Xanthium strumarium), curly dock (Rumex crispus) and pepperweed (Lepidium latifolium). Grassland communities contained perennial grasses such as creeping wild rye (Elymus triticoides), California fescue (Festuca californica), common timothy (Phleum pratense), as well as, annual grasses such as Italian rye grass (Lolium multiflorum) and wild oat (Avena fatua).
Capture and monitoring
We captured female pheasants in the winter and early spring during November to April. To avoid disturbing nesting females, we concluded our trapping efforts when pheasants began to nest in April. We modified spotlighting techniques (Labisky 1959) developed for greater sage-grouse (Centrocercus urophasianus) trapping (Giesen et al. 1982; Wakkinen et al. 1992) to capture adult pheasant at night by using an all-terrain vehicle (ATV). Pheasants were captured at nocturnal roosting locations by using spotlights and nets attached to 3-m extension handles. An ATV was used to locate roosting birds, and females were preferentially targeted for capture when flushed. Captured females were outfitted with battery-powered necklace-style very high frequency (VHF) transmitters (Advanced Telemetry Systems, Isanti, Minnesota, USA) equipped with a mortality sensor. The weights of transmitters with collaring materials (~23 g) were well under the recommended values on the basis of pheasant body mass to minimise post-release researcher-induced stress and mortality (Schroeder et al. 1999). Birds were processed within 30 min of capture and released at their capture location to minimise stress and disorientation. Pheasant capture and radio-marking procedures were permitted under California Department of Fish and Wildlife (SC-12940) and approved by the U.S. Geological Survey Western Ecological Research Center Animal Care and Use Committee.
We conducted on-the-ground monitoring of pheasant reproduction following release of marked birds during 2014 through 2017. We used a three-element Yagi antenna and portable receiver (Advanced Telemetry Systems) to track radio-marked females. We minimised relocation error by circling each bird at a radius of 10–20 m and then walked within 10 m of the bird’s location. We then approximated the distance and recorded the azimuth from the observer’s location (recorded using GPS) to estimate the location coordinates (universal transverse mercator, UTM) of the marked pheasant. On the approach to obtaining the pheasant’s location, we sought to prevent the bird from flushing or running.
When females were found in the same location on two consecutive observations, we collected locations from multiple azimuths relative to the bird, so as to ensure location accuracy and increase the observer’s efficiency in locating the nest following nest fate. Each nest was then monitored ≥2 times per week until its fate was determined. Nests were verified visually after the nest failed or the eggs hatched to minimise nest abandonment. A nest was considered successful if more than one chick hatched, ascertained by visual assessment of eggshell remains or observing more than one chick in the nest bowl. If the entire clutch failed to hatch, nests were considered unsuccessful and classified as depredated or abandoned. We further classified depredations as suspected mammalian or avian, by using diagnostic indicators observed at the nest (Rearden 1951). Specifically, nests with crushed eggs, eggshells found outside of the nest edge, clean eggshells with no yolk residue, and highly disturbed vegetation surrounding the nest site were suspected to be depredated by a mammalian predator. On the basis of previous literature, mammalian nest predators will often disturb the nest bowl and the surrounding vegetation, as well as leave coarsely crushed eggshells in and around the nest bowl (Rearden 1951). Nests with mostly undisturbed nest bowls and surrounding vegetation, and containing whole eggshells with a small hole in one end or missing eggs, were classified as depredated by an avian predator (Rearden 1951). As a caveat, these data were not used in subsequent analyses of nest survival, but were included for descriptive purposes because using diagnostic indicators alone to assess the cause of failure for individual nests can be subject to observation error.
Habitat measurements
During 2015–2017, following nest success or failure at the nest bowl, we measured horizontal cover with a Robel pole (Robel et al. 1970), which is a 1.5-m tall pole marked with numbered bands that correspond to a height in decimetres from the ground. A 1-m tall viewing pole is placed 2 m from the Robel pole and is used to read the numbered bands. The measurement was taken by recording the lowest band visible before the pole is completely covered by vegetation. This measurement was taken from three directions relative to the pole and then averaged to attain a single value. The first direction was chosen at random and the remaining two directions were sequentially oriented 120° from the previous direction. Because this method does not provide a value for vertical cover, we used a coverboard (Jones 1968), which consists of 25 equal squares (3.175 cm × 3.175 cm) marked on a board. The coverboard was placed flat with squares facing upward within the nest bowl. An observer counted the number of squares with >50% visibility from 2 m above, and the proportion of visible squares provided a measure of vertical visual obstruction. During 2014 only, we also used the coverboard method to measure horizontal cover by placing the board upright in the nest bowl and measuring at 0 and 45 degree angles in three random directions. However, the Robel pole replaced the coverboard as the measure of horizontal cover after 2014, because the Robel pole provided a measure of visual obstruction that did not crush the underlying vegetation. Hence, the coverboard was used as a measure of vertical cover during 2014–2017, and as a measure of horizontal cover in 2014. We also measured vegetation composition cover at seven subplots (20 × 50 cm) along three transects located ≤25 m of each nest using the Daubenmire method (Daubenmire 1959). Orientation of the first transect was randomly assigned and the remaining two transects were sequentially oriented at 120° intervals to the previous transect. Measurements were recorded at the nest bowl (0 m), and at 10- and 25-m distances from the nest along all three transect lines. These distances correspond to an overall spatial scale of 0, 0.03, and 0.2 ha respectively. Last, we measured the height of vegetation within 0.5 m of all subplots for each cover type (e.g. grass, forb and shrub). Residual cover was defined as dead vegetation from the previous year that was still rooted and had not yet become loose litter. Bare ground was defined as being devoid of vegetation, rocks or other substrate. All vegetation cover and height variables used for analyses are listed in Table 1.
Model development
To prevent plant phenology from confounding differences between successful and failed nests, we implemented a date-adjustment recommendation proposed by Gibson Blomberg and Sedinger (2016). Measurements taken at the time of nest success or failure did not necessarily represent plant growth throughout the season and, therefore, may not be an accurate representation of vegetation height and density at the time of nest-site selection (Hausleitner et al. 2005). We fit linear mixed-effect models and adjusted height and cover measurements for vegetation types that warranted adjustment using the estimated slope coefficient. Model parameters for this and subsequent nest habitat-selection analyses were estimated in program R (R Core Team 2014) using package ‘lme4’ (Bates et al. 2015). We specified ordinal date as the only fixed effect and vegetation measurements at used locations as the response. We also fit a random intercept of study site for all models to account for differences among study sites. The slope coefficients of each model provided an estimated growth rate for each microhabitat variable measured. We assessed support for growth-rate changes for each microhabitat variable, by evaluating 95% confidence intervals of the slope coefficient, and we did not consider the growth rate to be different from zero if the interval overlapped zero. Vegetation measurements were adjusted according to the estimated slope coefficient for variables with evidenced growth rates. Measurements were adjusted to a mean peak nesting date (greatest frequency of females incubating) specific to each year for variables that required date-adjustment to account for phenology. Variables with phenological adjustements are reported in Appendix S1, available as Supplementary material to this paper.
To examine nest-site microhabitat selection, defined as habitat use disproportionate to availability (Manly et al. 2002), we compared vegetation characteristics at nest sites to available habitat within the study area. To characterise available locations, we generated independent random points in a GIS and conducted the same microhabitat measurements at those locations. The number of independent random locations surveyed was approximately equivalent to the number of nests surveyed at each study site during each year of the study. The boundary of the study area in which available sites were surveyed was established by combining the property borders of study sites and adjacent private lands we were given permission to access, with a minimum convex polygon being derived from all telemetry locations at each study site. Most marked females at our study sites stayed within study-site boundaries throughout the field season, and the majority of marked females moved no more than 1 km from their capture location during the study period.
We used a Design II approach (Manly et al. 2002) to evaluate habitat selection; so, habitat use was identified at the individual-pheasant level but availability was assessed at the population level. Hence, measured resource units were classified as used or available. To contrast used and available resource units, we developed generalised linear mixed models (GLMM) and specified a binomial distribution (Zuur et al. 2009). All selection models included random intercept terms for year and study site. Additionally, data were standardised such that variables had a mean of 0 and standard deviation of 1. Coefficents were back-transformed when interpreting and visualising the data. Models were based on a priori hypotheses that pheasant nest site-selection behaviors are influenced by available cover. We employed a multi-step exploratory approach using Akaike’s information criterion with a second-order bias correction (AICc) to evaluate model support (Burnham and Anderson 2002) in all model-selection processes, except for the preliminary variable-reduction step.
The first step in the model-development process was to compare the effects of horizontal cover, vertical cover, and Robel pole height relative to selection across specific years in restricted datasets (Appendix S2). As mentioned above, we adopted the Robel pole method in 2015, in lieu of using the coverboard as measure of horizontal cover. Thus, we restricted the dataset and compared horizontal and vertical cover relative to an intercept-only model for 2014 data, and compared Robel pole height and vertical cover relative to an intercept-only model for 2015–2017 data. To account for variation among years and field sites, we also fit field site as a random intercept in all models, and year as a random intercept in models that comprised multiple years of data.
We then conducted a preliminary variable-reduction step using all candidate predictors meausured across the entire study period (Table 1). This process used a ‘for loop’ in R, in which GLMM models with random intercept terms for site and year were run using the lme4 package in R version 3.4.3 (R Core Team 2014). We computed a correlation matrix for all predictors and ran 10 000 iterations of the loop. We then used it to randomly generate a new matrix of uncorrelated predictors (R < 0.65). This was undertaken by, first, randomly selecting any predictors from the candidate set of predictors and then randomly adding additional predictors under the condition that correlation was <0.65. The loop randomly selected between two and five model predictors to include in a model fit for each iteration. This process was undertaken to minimise omission of important variables, to reduce the influence of potential model mis-specification, and to avoid over-parameterisation from including too many predictors. We stored the results for the β coefficient estimate for each predictor included in the model as well as the AIC value of that model. We then calculated variable importance for each predictor by computing the AIC of a new model without that predictor and taking the difference (dAIC) between AIC values of the model with and without the predictor. A positive value indicated improvement in model fit when the predictor was included (Laforge et al. 2015). At the conclusion of the loop, we averaged across all dAIC scores for all possible predictors, and ranked correlated predictors by their average dAIC across all models that included them (Appendix S3). Variables with a positive dAIC value were carried forward to subsequent analyses of selection and survival.
We examined the data for nonlinearity by evaluating single-variable models for each measured microhabitat characteristic carried forward from the variable reduction step. Each variable was examined at each of the microsite spatial scales (0, 0.03 and 0.20 ha). A microsite spatial scale of 0 ha corresponded to measurements taken at the nest bowl. Univariate models, each representing a single spatial scale for each variable were evaluated, with and without a quadratic term included, and the most explanatory models were extracted (Appendix S4). Models were considered supported by the data if AICc values were at least 2 units less than that of a ‘null’ model (i.e. intercept-only model with random effects). We again looked for correlation among significant variables and dropped the variable with the least support when two variables were highly correlated (R ≥ 0.65), namely variables describing the same habitat component at different scales. Last, we investigated more complex relationships among variables by developing higher-order models, which included the top-performing parameters as well as interactions among fixed effects. Models supported by the data were those with AICc values at least 2 units less than that of a ‘base’ model (e.g. random effects and main fixed effects without interactions). These global models are listed and described in Appendices S5 and S6.
We estimated cumulative nest-survival probability over a 37-day exposure period consisting of ~14 days of laying (Labisky and Jackson 1966) and 23 days of incubation (Dumke and Pils 1979), by using a maximum-likelihood estimation approach. Both the laying and incubation stages were used to derive cumulative nest-survival probabilities to avoid positive bias associated with only including the incubation stage in nest-survival estimates (Blomberg et al. 2015). We developed an encounter history of individual nests on the basis of the date each nest was found, last checked, and the fate determined.
We evaluated the same variables carried forward from the variable reduction step (Appendix S3) by using R statistical software with the ‘RMark’ package (R Version 3.43, Laake and Rexstad 2007) that implements MARK (White and Burnham 1999), so as to estimate the effects of environmental factors on pheasant nest-survival probability. We were interested in linking selection behavior with respect to nest-site vegetation characertistics to nest-survival probability. In this analysis, we first investigated variation in survival among years and study sites, to determine whether effects of site or year should be included as fixed effects because RMark does not allow for specification of random effects. Second, we investigated groups of models using the same model-selection process as in the first analysis. Last, we compared models to identify variables that were supported by the data. To evaluate model uncertainty, we calculated model probabilities (wmodel i) for the most parsimonious model compared with other models within the group (Anderson 2008). Changes in AICc and AICc weights were used to evaluate individual model strength relative to an intercept-only model (Anderson 2008). Individual models were considered supported by the data if AICc values were at least 2 units less than those for an intercept-only model. We removed three nests from the survival analysis because they were abandoned following camera installation and we knew abandonment to be researcher induced (that is, the pheasant did not return to nest following incidental flush). We did not attempt to install cameras again after the first year, to minimise abandonment. However, habitat-survey data for these nests were included in the habitat-selection analysis to avoid losing informative data.
We used the same modeling approach as outlined in Lockyer et al. (2015) to evaluate nest survival relative to nest-site selection parameters using R statistical software with the RMark package (R version 3.43; Laake and Rexstad 2007). First, we calculated logarithmic (log) ratios of selection by dividing the used point measurement by the mean of available habitat at the population level and log-transforming the result. This transformation normalised selection coefficients to be used in the nest-survival analysis in relation to what was available at that study site. By incorporating the log selection ratios as covariates in a nest-survival analysis, we were able to estimate whether pheasants selected for a particular habitat characteristic that translated into significant changes in nest survival probability. Models were considered supported by the data in explaining survival if AICc values were at least 2 units less than that for the intercept-only model.
Results
Nest-site selection
Over the course of the study, a total of 163 nests (n = 55, 2014; n = 40, 2015; n = 38, 2016; n = 31, 2017) and 162 independent random locations was assessed. One hundred and eight were first nesting attempts (n = 35, 2014; n = 27, 2015; n = 28, 2016; n = 18, 2017), 46 were second attempts (n = 15, 2014; n = 11, 2015; n = 9, 2016; n = 11, 2017) and 10 were third attempts (n = 5, 2014; n = 2, 2015; n = 1, 2016; n = 2, 2017). Nest-initiation rate across all females and all years of the study was 92.9% (s.e. = 6.0). We found the strongest support for vertical cover over horizontal cover and Robel pole height in both restricted datasets (Appendix S2). Furthermore, vertical cover with a quadratic term included in the model was the strongest microhabitat component that distinguished nest sites from random locations across all field sites (w = 0.98; Appendix S4). Therefore, Robel pole height and horizontal cover were not carried forward into nest-survival analyses.
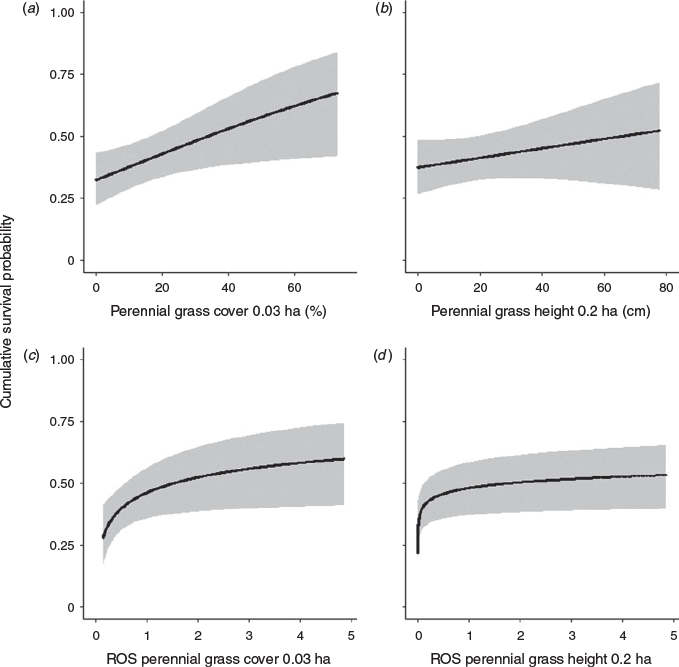
The prelimainary variable reduction step demonstrated greatest model support based on dAIC for the following habitat variables: vertical cover, residual vegetation cover, residual vegetation height, bare ground cover, perennial grass height, and annual grass height (Appendix S3). These variables, including each spatial scale with a positive dAIC value, were carried forward in all subsequent analyses. Investigation of functional relationships for these variables showed improvement when a quadratic term was included for vertical cover, residual vegetation cover and residual vegetation height. Evidence of support for linear or quadratic effects for a single variable are listed in Appendix S4. Correlated variable–spatial scale combinations were then evaluated, such that only variables with the greatest model support at a given spatial scale were brought forward. We found the greatest model support when a quadratic term was included for vertical cover and residual cover at the nest bowl, as well as, residual cover height at the 0.2-ha scale (Appendix S4). Linear terms were well supported for bare ground cover at the nest, perennial grass height at the 0.2-ha scale, and annual grass height at the 0.2-ha scale (Appendix S4; Fig. 2). Therefore, the base global model included vertical cover, residual cover and bare ground cover at the nest, as well as perennial grass height, residual vegetation height and annual grass height at the 0.2-ha scale (Appendix S5). The most supported global model included interactions of vertical cover with annual grass height and residual cover with bare ground cover (Table 2). The two interactions are illustrated in Fig. 3, and demonstrate the effect of vertical cover varying with annual grass height and the effect of residual vegetation cover varying with bare ground cover. Last, we found evidence of selection for perennial grass cover at the 0.03-ha scale, but it was highly correlated with perennial grass height (R > 0.65) and was not included in the final global model.
|
Nest survival
The cumulative nest-survival estimate across all five field sites was 44.2% (95% CI: 34.0–54.0%). Of the 163 nests we monitored during the study, 46% (n = 75) of the nests failed. Of the failed nests, 65% (n = 49) were evidenced to be depredated by either avian (n = 27), mammalian (n = 20) or unknown (n = 2) predators. Other causes for nest failure included abandonment (n = 15), plowing or discing of the nest site by farming equipment (n = 4), flooding of the nest site (n = 4) and unknown causes (n = 3).
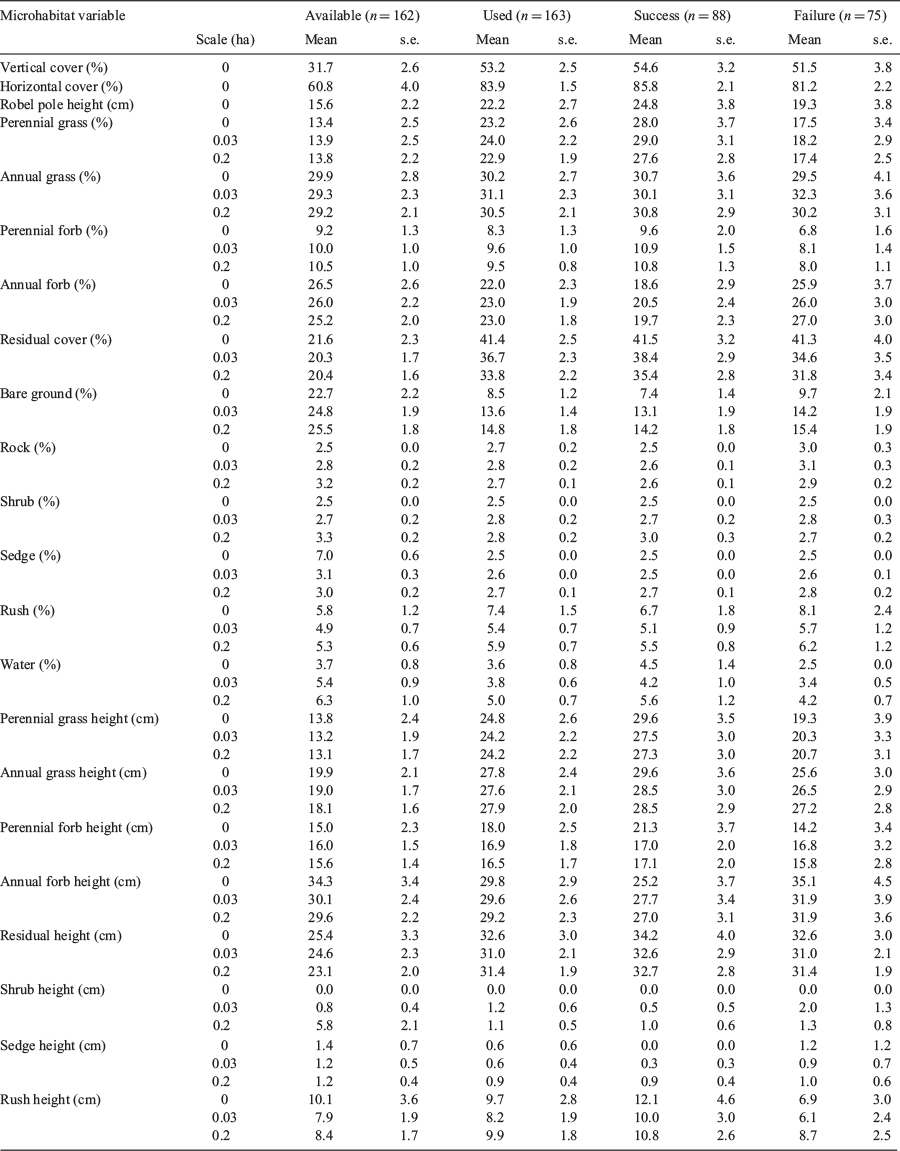
We did not find support for effects of interannual variation or variation across study sites relative to the intercept-only model (Appendix S4). Therefore, we did not include study site or year as an additive fixed effect in the subsequent modeling steps. The most explanatory microhabitat feature predicting nest survival across all study sites was perennial grass cover at the 0.03-ha spatial scale (w = 0.35; Appendix S4). Nest survival increased by 0.504% (95% CI: 0.500–0.507%) for every 1% increase in perennial grass cover within the 0.03-ha radius of the nest (Fig. 4a). Perennial grass cover at the nest bowl was the only other model that was substantially better than was the intercept-only model (Appendix S4), but was removed from the table because it was highly correlated with perennial grass at the 0.03-ha spatial scale. For comparative purposes, we also illustrated the effects of perennial grass height at the 0.2-ha spatial scale (Fig. 4b), largely because this covariate was stronger in explaining selection.
Last, we investigated ratios of selection to elucidate links between individual pheasant choice of microhabitats to their survival outcome. We found strong model support for perennial grass cover at the 0.03-ha scale (Appendix S4; Fig. 4c) and perennial grass height at the 0.2-ha scale (Appendix S4; Fig. 4d) on the basis of ratios of selection relative to an intercept-only model. We did not find support for other variables, even though vertical cover, bare ground and residual cover were found to be significant relative to the intercept-only model in the nest-selection analysis.
Discussion
Within northern California, an area that has undergone major landscape alterations from changes in agricultural practices over the past three decades (Coates et al. 2017b), we identified a clear link between vegetation characterisics that were selected by nesting pheasants with the probability of their nests surviving through an incubation period. Specifically, female pheasants tended to select nest sites with intermediate amounts of vertical cover and residual cover at the nest bowl, while avoiding nesting in areas with an increasing bare ground cover (Fig. 3). However, nest survival was largely a function of perennial grass cover and height.
Estimated cumulative nest survival was 44.2% (95% CI: 34.0–54.0%), a rate that was similar to other estimates on nesting pheasant throughout the United States. For example, Clark et al. (1999) estimated average nest survival for pheasants in Palo Alto and Kossuth County, Iowa, at 53.8% and 39.8% respectively, and recommended adding blocks of undisturbed Conservation Reserve Program grasslands >15 ha in size, so as to improve nest success. Haensly et al. (1987) investigated rates of predation on pheasant nests in Oregon and estimated overall nest success at 28% in strip habitats and 49% in non-strip habitats. Last, fates of pheasant nests in Illinois were estimated at 13% in harvested hayfields and 35% in unharvested hayfields, and the authors recommended providing small undisturbed plots of nesting cover near forage crops to sustain pheasant populations (Warner and Etter 1989). Although we fully recognise the importance of managing pheasant habitat in relation to larger, landscape-scale factors, our research highlights management actions that can promote management of microhabitat at much smaller spatial scales, such as increasing perennial grass cover and height, within a multi-scale management framework.
Although not always directly measured at the microsite scale, the importance of perennial grass to nesting pheasants has been corroborated elsewhere within the United States (Joselyn et al. 1968; Warner 1994; Clark et al. 1999). One study found that both nest success and overall hen success were higher at sites with a greater proportion of perennial grasses in northern Iowa (Clark et al. 1999). In eastern Illinois, Warner (1994) found that the percentage of hatched pheasant nests was positively correlated with the amount of grassland available to female pheasants during spring. Last, an earlier study in Illinois by Joselyn et al. (1968) investigated the effects of manipulating roadside cover by planting a mixture of perennial grasses with the intent of providing suitable nesting cover for pheasant. Although cumulative nest survival was similar between managed and unmanaged roadsides (27% and 24% respectively), seeded roadsides had a greater density of nests than did any of the other cover types during all 4 years of the study, suggesting there being strong selection for seeded areas. These higher densities resulted in two times more successful nests per hectare (0.32 and 0.16 respectively) than at unmanaged roadsides.
Although perennial grass cover best explained nest survival in the present study, a quadratic effect for percentage vertical cover best explained nest-site selection. We originally predicted a linear relationship between probability of selection and vertical cover, such that pheasant would select the greatest available vertical cover. The importance of suitable amounts of vegetation cover for nesting pheasant has been thoroughly investigated (Dahlgren 1988; Robertson 1996; Smith et al. 1999) and, for decades, dense cover has been thought to provide greater protection from predators, because it provides a physical barrier between the senses of the predator and the nest site (Elton 1939). However, our investigation of higher-order effects indicated that pheasants selected intermediate levels of vertical cover in that the probability of selection was lowest at the upper and lower end of the continuum. Female pheasants appear to seek an intermediate level of cover to balance the benefits of increased concealment from predators with costs associated with barriers that might prevent the female from evading capture by a predator. Benefits associated with increased cover have been corroborated by multiple studies. For example, in Minnesota, predation by both mammalian and avian predators was highest among poorly concealed pheasant nests (Chesness et al. 1968). A nest study using artificial nests in Idaho demonstrated that greater cover was most protective against nest predators such as the black-billed magpie (Pica hudsonia) because it decreased the visibility of eggs within the nest (Jones and Hungerford 1972). Within an agricultural setting in Illinois, pheasant nest destruction was highest in years when both pheasants and predators were concentrated in areas of low cover (Warner 1994). For ground-nesting birds that employ crypsis as the main form of nest defense, such as pheasants, habitat changes such as decreased vegetative density and loss of heterogeneity may increase nest predation rates (Evans 2004).
The ‘ghosts of predators past’ hypothesis (Peckarsky and Penton 1988; Byers 1997) predicts that prey species maintain specific antipredator behaviors because throughout evolutionary history they were subject to a certain level of predation. Although pheasants are likely to have co-evolved with both aerial and terrestrial predators, landscape alterations such as habitat fragmentation may have changed the predator community in such a way that pheasant habitat-selection behaviors may no longer be optimal in the current conditions. If the predator community is primarily mammalian, too much cover or too great height might not be beneficial, because they do not provide adequate escape opportunities. However, high cover and height provide more concealment from avian predators. Thus, choosing a moderate level of cover may make the most sense in mixed-predator environments.
Like many other farmland bird species, pheasants are ground-nesting birds, which exposes them to a vast array of both aerial and terrestrial predators (Draycott et al. 2008). Principal terrestrial nest predators of pheasant include raccoons (Procyon lotor), striped skunks, coyotes (Canis latrans) and red foxes (Vulpes vulpes; Chesness et al. 1968; Frey et al. 2003). Aerial nest predators include American crows (Corvus brachyrhynchos), common ravens (Corvus corax) and black-billed magpies (Chesness et al. 1968; Jones and Hungerford 1972; Kallioniemi et al. 2015). Corvid populations have increased dramatically over the past century, particularly in the western United States (Marzluff et al. 1994), and this is likely to be leading to a lessened advantage when selecting only moderate levels of cover at the nest site. By using a modeling approach based on count-survey data, Coates et al. (2017b) found strong support for corvid abundance negatively affecting pheasant abundance in California, particularly in the northern central region. Over the course of our study, suspected corvid depredations comprised 19% (n = 5) of all avian depredations (n = 27). Increases in raven numbers have been linked to decreased nesting success in other Galliformes as well (Coates and Delehanty 2010). The deleterious effects related to nest predators are likely to be intensified by modern farming practices, which reduce the availability of suitable nest cover (Evans 2004).
Shifts in agricultural management techniques and intensification of farming practices have led to widespread habitat fragmentation, which alters the suitability of habitats, especially those in close proximity to agricultural land (Benton et al. 2003). All of our study sites were associated with agricultural lands, such that sites were adjacent to crop fields, and crop fields were often leased to farmers or managed within sites. Hence, much of the pheasant habitat at each site was broken up into smaller units separated by canals that allow for efficient irrigation delivery and quick drawdown of flood-irrigated units such as seasonal wetlands. Habitat elements related to loss of heterogeneity, such as reduced patch size and proximity to edges have been associated with an increase in nest-predation rates (Andrén 1992; Seymour et al. 2004). For example, Seymour et al. (2004) investigated the effects of reserve size on nest predation by red foxes and concluded that the probability of a nest being encountered by ground predators was dependent on patch area. In Sweden, Andrén (1992) found that the density of corvids increased with habitat fragmentation intermixed with agricultural land and observed an edge-related increase in nest-predation rates.
Behaviors of the individual about where to nest are not only driven by habitat characteristics (Smith et al. 2007), but also by the distribution of predators (Fahrig 2007), the nature of the nest (Collias and Collias 1984), food availability (Evans 2004) and previous breeding experience (Sedgwick 2004). Although individuals cannot directly observe habitat quality, they respond to cues that carry information about habitat suitability (Kristan 2003). Whereas organisms normally use these cues to determine where to settle, rapid landscape changes resulting from agricultural intensification can decouple known environmental indicators from the perceived suitability of a given habitat element, resulting in an ‘ecological trap’ (Fahrig 2007). For example, at least eight nests (4.9% of all nests) from marked pheasants in our study were flooded by summer irrigations, or were destroyed by farming equipment. In these cases, female pheasants selected nesting sites in agricultural fields or seasonal wetlands, likely on the basis of suitable cover availability, but active management of these habiatats did not allow for the completion of nest incubation. Generally, prey species make the decision to settle in habitat types that grant security from predation (Lauridsen and Lodge 1996), but mismatched cues resulting from landscape alterations can make habitat types that were once suitable for protection from predators unfit, contributing to increased predation rates (Fahrig 2007). If animals are able to choose habitat accurately, selection should have a positive impact on population growth, but ecological-trap situations intensify the detrimental effects of poor-quality habitat when observable cues are no longer correlated with their expected fitness (Kristan 2003).
In conclusion, we found clear predictable relationships between microhabitat vegetation components and nest-site selection and nest survival for pheasants in the Central Valley of California. Although pheasants generally selected intermediate levels of vertical cover, those pheasants that chose greater perennial grass cover and heights showed greater fitness advantage. An understanding of such mechanisms in choice and the consequence for nesting pheasants helps inform factors that are likely to be important to land managers interested in sustaining pheasant populations in human-modified environments. Similar analytical methods could be extended to other farmland birds (Nielson et al. 2008), which could also help direct future management strategies in similarly changing agricultural landscapes. Such indicator species are important because agricultural intensification continues to be a leading driver in substantial declines of farmland biodiversity (Chamberlain et al. 2000; Benton et al. 2003) and, without guided management, may imperil the productivity of these systems. Therefore, understanding mechanistic links between changing agricultural practices and declines in farmland biodiversity can lead to better-informed management actions and aid in the development of an adaptive framework in the face of changing landscapes.
Management implications
On the basis of our results, we emphasise the importance of perennial grass cover and height to nesting pheasants. To improve reproductive success for pheasants, our models indicated that ≥35% total perennial grass cover (corresponding to ≥50% nest survival) is required in pheasant nesting habitat (e.g. primarily upland habitats). Additionally, our models indicated that land-use activities that reduce grass height (annual and perennial), especially during the spring breeding season, can have adverse impacts to pheasant reproduction. However, it remains important to recognise that while land management focussed on microhabitat could prove beneficial to reproduction in pheasant populations, actions conducted at a single spatial scale can be limiting, given that ecological factors operate across multiple scales (Holland et al. 2004). Therefore, where perennial grasses are present, increasing field size may prove beneficial to nesting pheasant (Haensly et al. 1987; Clark et al. 1999) within the context of a multi-scale management framework. Additionally, land cover that sometimes surrounds pheasant management areas at even larger spatial scales could either facilitate or constrain local level management efforts (Jorgensen et al. 2014). This indicates that further research to identify all ecological factors influencing pheasant nest success and consequent pheasant abundance, at both the micro- and macrohabitat scales, could improve management efficacy.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was part of a cooperative effort with U.S. Geological Survey, Pheasants Forever, and the California Department of Fish and Wildlife. We thank M. Meshriy, B. Burkholder, L. Souza, J. Stoddard, A. Atkinson and D. Van Baren with California Department of Fish and Wildlife for their expertise and financial and logistical support. We also thank Mandeville Island and Roosevelt Ranch for providing financial support and access to private lands. Last, we are grateful to J. Beckstrand and S. Freitas of the U.S. Department of Fish and Wildlife for logistical support and advice during field operations. Any use of trade, product, website, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
Anderson, D. R. (2008). ‘Model Based Inferences in the Life Sciences.’ (Springer Science: New York, NY, USA.)Andrén, H. (1992). Corvid density and nest predation in relation to forest fragmentation: a landscape perspective. Ecology 73, 794–804.
| Corvid density and nest predation in relation to forest fragmentation: a landscape perspective.Crossref | GoogleScholarGoogle Scholar |
Atkinson, P. W., Fuller, R. J., Vickery, J. A., Conway, G. J., Tallowin, J. R. B., Smith, R. E. N., Haysom, K. A., Ings, T. C., Asteraki, E. J., and Brown, V. K. (2005). Influence of agricultural management, sward structure, and food resources on grassland field use by birds in lowland England. Journal of Applied Ecology 42, 932–942.
| Influence of agricultural management, sward structure, and food resources on grassland field use by birds in lowland England.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Maechler, M., Bolker, B., and Steve Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Benton, T. G., Vickery, J. A., and Wilson, J. D. (2003). Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecology & Evolution 18, 182–188.
| Farmland biodiversity: is habitat heterogeneity the key?Crossref | GoogleScholarGoogle Scholar |
Best, L. (1986). Conservation tillage: ecological traps for nesting birds? Wildlife Society Bulletin (1973–2006) 14, 308–317.
Blomberg, E. J., Gibson, D., and Sedinger, J. S. (2015). Biases in nest survival associated with choice of exposure period: a case study in North American upland game birds. The Condor 117, 577–588.
| Biases in nest survival associated with choice of exposure period: a case study in North American upland game birds.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference: a Practical Information-theoretic Approach.’ 2nd edn. (Springer: New York, NY, USA.)
Byers, J. (1997). ‘American Pronghorn. Social Adaptations and the Ghosts of Predators Past.’ (University of Chicago Press: Chicago, IL, USA.)
Chamberlain, D. E., Fuller, R. J., Bunce, R. G. H., Duckworth, J. C., and Shrubb, M. (2000). Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. Journal of Applied Ecology 37, 771–788.
| Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales.Crossref | GoogleScholarGoogle Scholar |
Chesness, R., Nelson, M., and Longley, W. (1968). The effect of predator removal on pheasant reproductive success. The Journal of Wildlife Management 32, 683–697.
| The effect of predator removal on pheasant reproductive success.Crossref | GoogleScholarGoogle Scholar |
Clark, W., Schmitz, R., and Bogenschutz, T. (1999). Site selection and nest success of ring-necked pheasants as a function of location in Iowa landscapes. The Journal of Wildlife Management 63, 976–989.
| Site selection and nest success of ring-necked pheasants as a function of location in Iowa landscapes.Crossref | GoogleScholarGoogle Scholar |
Coates, P. S., and Delehanty, D. J. (2010). Nest predation of greater sage-grouse in relation to microhabitat factors and predators. The Journal of Wildlife Management 74, 240–248.
| Nest predation of greater sage-grouse in relation to microhabitat factors and predators.Crossref | GoogleScholarGoogle Scholar |
Coates, P. S., Prochazka, B. G., Ricca, M. A., Gustafson, K. B., Ziegler, P., and Casazza, M. L. (2017a). Pinyon and juniper encroachment into sagebrush ecosystems impacts distribution and survival of greatersage-grouse. Rangeland Ecology and Management 70, 25–38.
| Pinyon and juniper encroachment into sagebrush ecosystems impacts distribution and survival of greatersage-grouse.Crossref | GoogleScholarGoogle Scholar |
Coates, P. S., Brussee, B. E., Howe, K. B., Fleskes, J. P., Dwight, I. A., Connelly, D. P., Meshriy, M. G., and Gardner, S. C. (2017b). Long-term and widespread changes in agricultural practices influence ring-necked pheasant abundance in California. Ecology and Evolution 7, 2546–2559.
| Long-term and widespread changes in agricultural practices influence ring-necked pheasant abundance in California.Crossref | GoogleScholarGoogle Scholar | 28428846PubMed |
Collias, N., and Collias, E. (1984). ‘Nest Building and Bird Behavior.’ (Princeton University Press: Princeton, NJ, USA.)
Dahlgren, R. B. (1988). Distribution and abundance of the ring-necked pheasant in North America. In ‘Proceedings of the Midwest Fish and Wildlife Conference’.(Eds D. L. Hallett, W. R. Edwards, and G. V. Burger.) pp. 29–43. (North Central Section of The Wildlife Society: Bloomington, IN, USA.)
Daubenmire, R. (1959). A canopy-coverage method of vegetation analysis. Northwest Science 33, 43–64.
Draycott, R. A. H., Hoodless, A. N., Woodburn, M. I. A., and Sage, R. B. (2008). Nest predation of common pheasants Phasianus colchicus. The Ibis 150, 37–44.
| Nest predation of common pheasants Phasianus colchicus.Crossref | GoogleScholarGoogle Scholar |
Dumke, R., and Pils, C. (1979). Renesting and dynamics of nest site selection by Wisconsin pheasants. The Journal of Wildlife Management 43, 705–716.
| Renesting and dynamics of nest site selection by Wisconsin pheasants.Crossref | GoogleScholarGoogle Scholar |
Elton, C. (1939). On the nature of cover. The Journal of Wildlife Management 3, 332–338.
| On the nature of cover.Crossref | GoogleScholarGoogle Scholar |
Evans, K. L. (2004). The potential for interactions between predation and habitat change to cause population declines of farmland birds. The Ibis 146, 1–13.
| The potential for interactions between predation and habitat change to cause population declines of farmland birds.Crossref | GoogleScholarGoogle Scholar |
Fahrig, L. (2007). Non-optimal animal movement in human-altered landscapes. Functional Ecology 21, 1003–1015.
| Non-optimal animal movement in human-altered landscapes.Crossref | GoogleScholarGoogle Scholar |
Frey, S. N., Majors, S., Conover, M. R., Messmer, T. A., and Mitchell, D. L. (2003). Effect of predator control on ring-necked pheasant populations. Wildlife Society Bulletin 31, 727–735.
Gibson, D., Blomberg, E. J., and Sedinger, J. S. (2016). Evaluating vegetation effects on animal demographics: the role of plant phenology and sampling bias. Ecology and Evolution 6, 3621–3631.
| Evaluating vegetation effects on animal demographics: the role of plant phenology and sampling bias.Crossref | GoogleScholarGoogle Scholar | 27148444PubMed |
Giesen, K. M., Schoenberg, T. J., and Braun, C. E. (1982). Methods for trapping sage grouse in Colorado. Wildlife Society Bulletin 10, 224–231.
Glemnitz, M., Zander, P., and Stachow, U. (2015). Regionalizing land use impacts on farmland birds. Environmental Monitoring and Assessment 187, 336.
| Regionalizing land use impacts on farmland birds.Crossref | GoogleScholarGoogle Scholar | 25957192PubMed |
Haensly, T., Crawford, J., and Meyers, S. (1987). Relationships of habitat structure to nest success of ring-necked pheasants. The Journal of Wildlife Management 51, 421–425.
| Relationships of habitat structure to nest success of ring-necked pheasants.Crossref | GoogleScholarGoogle Scholar |
Hausleitner, D., Reese, K., and Appa, A. (2005). Timing of vegetation sampling at greater sage-grouse nests. Rangeland Ecology and Management 58, 553–556.
| Timing of vegetation sampling at greater sage-grouse nests.Crossref | GoogleScholarGoogle Scholar |
Holland, J. D., Bert, D. G., and Fahrig, L. (2004). Determining the spatial scale of species’ response to habitat. Bioscience 54, 227–233.
| Determining the spatial scale of species’ response to habitat.Crossref | GoogleScholarGoogle Scholar |
Jones, R. E. (1968). A board to measure cover used by prairie grouse. The Journal of Wildlife Management 32, 28–31.
| A board to measure cover used by prairie grouse.Crossref | GoogleScholarGoogle Scholar |
Jones, R. E., and Hungerford, K. E. (1972). Evaluation of nesting cover as protection from magpie predation. The Journal of Wildlife Management 36, 727–732.
| Evaluation of nesting cover as protection from magpie predation.Crossref | GoogleScholarGoogle Scholar |
Jorgensen, C. F., Powell, L. A., Lusk, J. J., Bishop, A. A., and Fontaine, J. J. (2014). Assessing landscape constraints on species abundance: does the neighborhood limit species response to local habitat conservation programs? PLoS One 9, e99339.
| Assessing landscape constraints on species abundance: does the neighborhood limit species response to local habitat conservation programs?Crossref | GoogleScholarGoogle Scholar | 24918779PubMed |
Joselyn, G. B., Warnock, J. E., and Etter, S. L. (1968). Manipulation of roadside cover for nesting pheasants: a preliminary report. The Journal of Wildlife Management 32, 217–233.
| Manipulation of roadside cover for nesting pheasants: a preliminary report.Crossref | GoogleScholarGoogle Scholar |
Kallioniemi, H., Väänänen, V.-M., Nummi, P., and Virtanen, J. (2015). Bird quality, origin and predation level affect survival and reproduction of translocated pheasants Phasianus colchicus. Wildlife Biology 21, 269–276.
| Bird quality, origin and predation level affect survival and reproduction of translocated pheasants Phasianus colchicus.Crossref | GoogleScholarGoogle Scholar |
Kristan, W. B. (2003). The role of habitat selection behavior in population dynamics: source–sink systems and ecological traps. Oikos 103, 457–468.
| The role of habitat selection behavior in population dynamics: source–sink systems and ecological traps.Crossref | GoogleScholarGoogle Scholar |
Laake, J., and Rexstad, E. (2007). Appendix C. RMark: an alternative approach to building linear models. In ‘Program MARK: a Gentle Introduction’. (Eds E. Cooch and G. White.) pp. C1–C115. (Colorado State University: Fort Collins, CO, USA.)
Labisky, R. F. (1959). Night-lighting: a technique for capturing birds and mammals. Biological Notes 040, 1–11.
Labisky, R. F., and Jackson, G. L. (1966). Characteristics of egg-laying and eggs of yearling pheasants. The Wilson Bulletin 78, 379–399.
Laforge, M. P., Vander Wal, E., Brook, R. K., Bayne, E. M., and McLoughlin, P. D. (2015). Process-focussed, multi-grain resource selection functions. Ecological Modelling 305, 10–21.
| Process-focussed, multi-grain resource selection functions.Crossref | GoogleScholarGoogle Scholar |
Lauridsen, T., and Lodge, D. (1996). Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnology and Oceanography 41, 794–798.
| Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat.Crossref | GoogleScholarGoogle Scholar |
Lever, C. (1987). ‘Naturalized birds of the world.’ (John Wiley and Sons, Inc.: New York, NY, USA.)
Lockyer, Z. B., Coates, P. S., Casazza, M. L., Espinosa, S., and Delehanty, D. J. (2015). Nest‐site selection and reproductive success of greater sage‐grouse in a fire‐affected habitat of northwestern Nevada. The Journal of Wildlife Management 79, 785–797.
Manly, F. J., McDonald, L. L., Thomas, D. L., McDonald, T. L., and Erickson, W. P. (2002). ‘Resource Selection by Animals: Statistical Design and Analysis for Field Studies.’ (Chapman and Hall: London, UK.)
Marzluff, J. M., Boone, R. B., and Cox, G. W. (1994). Native pest bird species in the West: why have they succeeded where so many have failed? Studies in Avian Biology 15, 202–220.
Matthews, T. W., Taylor, J. S., and Powell, L. A. (2012). Ring-necked pheasant hens select managed Conservation Reserve Program grasslands for nesting and brood-rearing. Wildlife Management 76, 1653–1660.
| Ring-necked pheasant hens select managed Conservation Reserve Program grasslands for nesting and brood-rearing.Crossref | GoogleScholarGoogle Scholar |
Mineau, P., and Whiteside, M. (2006). Lethal risk to birds from insecticide use in the United States: spatial and temporal analysis. Environmental Toxicology and Chemistry 25, 1214–1222.
| Lethal risk to birds from insecticide use in the United States: spatial and temporal analysis.Crossref | GoogleScholarGoogle Scholar | 16704051PubMed |
Nielson, R. M., McDonald, L. L., Sullivan, J. P., Burgess, C., Johnson, D. S., Johnson, D. H., and Howlin, S. (2008). Estimating the response of ring-necked pheasants (Phasianus colchicus) to the conservation reserve program. The Auk 125, 434–444.
| Estimating the response of ring-necked pheasants (Phasianus colchicus) to the conservation reserve program.Crossref | GoogleScholarGoogle Scholar |
Orians, G. H., and Wittenberger, J. F. (1991). Spatial and temporal scales in habitat selection. American Naturalist 137, S29–S49.
| Spatial and temporal scales in habitat selection.Crossref | GoogleScholarGoogle Scholar |
Patterson, M., and Best, L. (1996). Bird abundance and nesting success in Iowa CRP fields: the importance of vegetation structure and composition. American Midland Naturalist 135, 153–167.
| Bird abundance and nesting success in Iowa CRP fields: the importance of vegetation structure and composition.Crossref | GoogleScholarGoogle Scholar |
Peckarsky, B., and Penton, M. (1988). Why do Ephemerella nymphs scorpion posture: a ‘ghost of predation past’? Oikos 53, 185–193.
| Why do Ephemerella nymphs scorpion posture: a ‘ghost of predation past’?Crossref | GoogleScholarGoogle Scholar |
R Core Team (2014). ‘R: a Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.)
Rearden, J. D. (1951). Identification of waterfowl nest predators. The Journal of Wildlife Management 15, 386–395.
| Identification of waterfowl nest predators.Crossref | GoogleScholarGoogle Scholar |
Rice, C. G. (2003). Utility of pheasant call counts and brood counts for monitoring population density and predicting harvest. Western North American Naturalist 63, 178–188.
Robel, R. J., Briggs, J. N., Dayton, A. D., and Hulbert, L. C. (1970). Relationships between visual obstruction measurements and weight of grassland vegetation. Journal of Range Management 23, 295–297.
| Relationships between visual obstruction measurements and weight of grassland vegetation.Crossref | GoogleScholarGoogle Scholar |
Robertson, P. A. (1996). Does nesting cover limit abundance of ring-necked pheasants in North America? Wildlife Society Bulletin 24, 98–106.
Schroeder, M. A., Young, J. R., and Braun, C. E. (1999). Sage grouse (Centrocercus urophasianus). In ‘The Birds of North America’. (Eds A. Poole and F. Gill.) pp. 425. (The Academy of Natural Sciences: Philadelphia, PA, USA.)
Sedgwick, J. (2004). Site fidelity, site territoriality, and natal philopatry in willow flycatchers (Empidonax traillii). The Auk 121, 1103–1121.
| Site fidelity, site territoriality, and natal philopatry in willow flycatchers (Empidonax traillii).Crossref | GoogleScholarGoogle Scholar |
Seymour, A. S., Harris, S., and White, P. C. L. (2004). Potential effects of reserve size on incidental nest predation by red foxes Vulpes vulpes. Ecological Modelling 175, 101–114.
| Potential effects of reserve size on incidental nest predation by red foxes Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Smith, S., Stewart, N., and Gates, J. (1999). Homes ranges, habitat selection, and mortality of ring-necked pheasants (Phasianus colchicus) in north central Maryland. American Midland Naturalist 141, 185–197.
| Homes ranges, habitat selection, and mortality of ring-necked pheasants (Phasianus colchicus) in north central Maryland.Crossref | GoogleScholarGoogle Scholar |
Smith, P. A., Gilchrist, H. G., and Smith, J. N. M. (2007). Effects of nest habitat, food, and parental behavior on shorebird nest success. The Condor 109, 15–31.
| Effects of nest habitat, food, and parental behavior on shorebird nest success.Crossref | GoogleScholarGoogle Scholar |
Wakkinen, W. L., Reese, K. P., Connelly, J. W., and Fischer, R. A. (1992). An improved spotlighting technique for capturing sage-grouse. Wildlife Society Bulletin 20, 425–426.
Warner, R. (1994). Agricultural land use and grassland habitat in Illinois: future shock for Midwestern birds? Conservation Biology 8, 147–156.
| Agricultural land use and grassland habitat in Illinois: future shock for Midwestern birds?Crossref | GoogleScholarGoogle Scholar |
Warner, R., and Etter, S. (1989). Hay cutting and the survival of pheasants: a long-term perspective. The Journal of Wildlife Management 53, 455–461.
| Hay cutting and the survival of pheasants: a long-term perspective.Crossref | GoogleScholarGoogle Scholar |
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S138.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
Wood, A., and Brotherson, J. (1981). Microenvironment and nest site selection by ring-necked pheasants in Utah. The Great Basin Naturalist 41, 457–460.
Zuur, A. F., Leno, E. N., Valker, N. J., Saveliev, A. A., and Smith, G. M. (2009). ‘Mixed Effects Models and Extensions in Ecology with R: Statistics for Biology and Health.’ (Springer Science + Business Media, LLC: New York, NY, USA.)