Sensitivity of organic matter mineralisation to water availability: role of solute diffusivity and the ‘Birch effect’
Weiwen Qiu A * , Denis Curtin
A * , Denis Curtin  A , Wei Hu
A , Wei Hu  A and Mike Beare
A and Mike Beare  A
A
A The New Zealand Institute for Plant and Food Research Limited, Private Bag 4704, Christchurch 8140, New Zealand.
Soil Research 61(1) 9-19 https://doi.org/10.1071/SR22013
Submitted: 17 January 2022 Accepted: 19 June 2022 Published: 11 July 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Several functions are used to describe the effects of soil water content on organic matter mineralisation. A meta-analysis of published studies identified relative water content (RWC; available water relative to the soil’s available water holding capacity) as the best water descriptor for N mineralisation.
Aims: To evaluate RWC as a predictor of C and N mineralisation in New Zealand soils; and to investigate how solute diffusivity and the ‘Birch effect’ may help to explain this relationship.
Methods: Three agricultural soils (0–15 cm), differing in water holding capacity were incubated (8-week; 20°C) under a range of RWCs to measure carbon (respiration) and net N mineralisation. After 4 weeks, a subset of samples from each treatment were re-wetted to field capacity for a further 4-weeks to quantify the respiration response to re-wetting.
Key results: For all three soils, there was a linear relationship between respiration and RWC where the C respired at the wilting point (RWC = 0) was ∼25–30% of that at field capacity (RWC = 1.0). Results from a solute diffusivity model suggested that a decrease in microbial substrate supply, owing to restricted diffusion of dissolved organic compounds, contributed to moisture-induced decline in respiration. A respiration flush was not observed when RWC was >0 at re-wetting. Nitrogen mineralisation was non-linearly related to RWC, with small decreases in RWC below 1.0 (optimum) having a greater effect on N, than C, mineralisation.
Conclusions: RWC may be a reliable ‘water modifier’ to describe the influence of soil moisture on respiration. Further work is recommended to verify the RWC vs net N mineralisation relationship observed in this study.
Keywords: Birch effect, first-order model, incubation, net N mineralisation, relative water content, soil respiration, SOM mineralisation-moisture relationship, substrate diffusion.
Introduction
Turnover of soil organic matter (SOM) is strongly influenced by environmental parameters, particularly soil temperature and water availability (Raich and Schlesinger 1992; Lützow et al. 2006). While the effects of rising temperatures on soil C stocks and soil heterotrophic respiration (HR) have attracted considerable attention, much less work has been carried out to understand soil water–respiration responses (Falloon et al. 2011; Moyano et al. 2013). Mechanisms determining the response of respiration to soil moisture remain poorly quantified (Moyano et al. 2013).
Knowledge of the moisture–SOM mineralisation response is needed to predict how soil C stocks may change in response to moisture changes associated with climate change and irrigation (Falloon et al. 2011; Mudge et al. 2017), and to predict the release of plant-available mineral N (mineralisation) under field conditions where soil moisture is temporally dynamic (Paul et al. 2003). It is well accepted that SOM mineralisation is greatest at about field capacity and decreases as the soil dries (Orchard and Cook 1983). However, the reported sensitivity of mineralisation to changes in soil moisture shows considerable variability. Falloon et al. (2011) showed that use of moisture response functions from five different soil C models (RothC, TRIFFID, SOILN, Bethy, Sim-Cycle) may result in either large losses or small gains in modelled future global C stocks.
The factors determining the moisture sensitivity of SOM mineralisation are not completely understood. The critical water content for microbial desiccation stress varies between taxa, with fungi being more tolerant of dry conditions than bacteria (Harris 1981; Manzoni et al. 2012). However, at water contents between desiccation stress (matric potential ∼−1500 kPa) and field capacity (−10 kPa), the main impacts of soil water content on microbial respiration may be indirect (owing to the influence of water content on diffusion of organic substrates), rather than a direct physiological response of microorganisms to water stress (Linn and Doran 1984; Davidson et al. 2000; Schjønning et al. 2003).
Based on a meta-analysis of 12 published laboratory incubation studies, Paul et al. (2003) evaluated ways of expressing soil water status with respect to its influence on N mineralisation. From the wide range of soil water descriptors evaluated (including gravimetric and volumetric water content; water-filled pore space; soil water potential), relative water content (RWC) was selected as providing a robust relationship with net N mineralisation. One objective of our study was to assess whether RWC (i.e. available water as a proportion of available water holding capacity) could be successfully used to describe the relationships of soil water content with C respiration and N mineralisation in New Zealand soils.
Mineralisation pulses often occur when dry soils are wetted by rainfall or irrigation, a phenomenon commonly known as the ‘Birch effect’ (Birch 1958). In locations where alternating wet–dry conditions are common, these pulses may account for a significant portion of the total mineralisation. Substrates fuelling these re-wetting pulses may include osmo-regulatory compounds, synthesised by the microbial community to protect against osmotic stress in dry soil, and SOM solubilised as a result of soil drying/re-wetting (Slessarev and Schimel 2020). The intensity and duration of dry conditions determine the magnitude of the response to re-wetting (Barnard et al. 2020). However, the moisture threshold at which a mineralisation pulse is triggered has not been well defined, even though such information is important to assess the potential significance of the Birch effect in a given environment. A second objective was to identify the critical soil water content below which there is a mineralisation pulse upon re-wetting.
Materials and methods
Soil types, physical and chemical properties
Three soil types that are common in the Canterbury region of New Zealand were selected for this study: (1) Temuka clay loam (Mollic Endoaquept, United States Department of Agriculture (USDA) Soil Classification System); (2) Templeton silt loam (Udic Haplustept); and (3) Waimakariri silt loam (Typic Ustorthents). All three soils were under grass (predominantly perennial ryegrass); long-term (>25 years) at the Temuka and Waimakariri sites and short-term (∼5 years) at the Templeton site. The soils differed in texture, organic matter content, and water holding capacity (Table 1). These soils, which are derived from sedimentary parent materials, were sampled in winter when they had been at, or close to, field capacity for some weeks. At each site, several sub-samples (0–15 cm) were taken using a spade and combined. In the laboratory, the soils were sieved through a 4-mm mesh in field-moist condition. Each soil was split in two parts: one part was air-dried, and the other part was maintained field-moist in a cold room at ∼4°C prior to the incubation studies.

|
Physical and chemical properties were determined using the air-dried soils. Texture was assessed by the sieving-sedimentation method (Gee and Or 2002). Water content at field capacity was determined using a tension table at −10 kPa and wilting point of the soils was measured at −1500 kPa using pressure plates. Total carbon (TC) and nitrogen (TN) were determined using Dumas combustion (LECO TruMac, Leco Corporation, St. Joseph, MI, USA). Hot water extractable C (HWEC), a measure of labile C, was extracted at 80°C as described by Ghani et al. (2003). Soil pH was determined at a soil:water ratio of 1:2 using a glass electrode. The ammonium acetate method was used to measure cation exchange capacity (CEC) (Thomas 1982).
The soils, which were sampled at sites with little or no input of fertiliser N for several years, were low in mineral N (Table 1). All three soils were acidic (pH 5.3–5.9). The Waimakariri and Temuka soils had higher SOM content (43 and 37 g C kg−1, respectively) than the Templeton soil (23 g C kg−1). HWEC followed the same order as total C in the three soils (range 1222–2362 mg kg−1). The Temuka soil had higher clay content and CEC than the other soils (Table 1).
Experimental treatments
There were seven (Temuka and Templeton soils) or eight (Waimakariri soil) moisture treatments, with RWC ranging from 1.0 (field capacity) to −0.04 (below wilting point). The RWC was computed as soil available water content relative to the available water capacity, defined in terms of an upper (i.e. −10 kPa; field capacity) and lower (−1500 kPa; wilting point) limit:
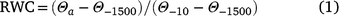
where Θa is actual soil water content (w/w, %), and Θ−10 and Θ−1500 are field capacity (w/w, %) and wilting point (w/w, %), respectively. On average, the RWC increment between successive moisture treatments was 0.15.
Soil preparation
Field-moist soil (the three soils were at field capacity when sampled) was spread thinly on plastic trays on a laboratory bench (20°C) and carefully dried to achieve different target water contents (soils were turned frequently to ensure that they were drying uniformly and avoiding excessive drying at the edges of the soil pile). Once soil had reached the first RWC target (0.84–0.87; Table 2), enough soil was removed for the incubations and other measurements. The drying process then continued with soil samples sequentially removed for the progressively decreasing RWC target values (Table 2). The moisture-adjusted soils were placed in sealed plastic bags and stored in a cold room prior to incubation. For each soil, the entire moisture adjustment procedure was completed within 24 h.

|
Mineralisation of N and C in aerobic incubation
The moisture-adjusted soils were incubated at 20°C for 8 weeks to measure C and N mineralisation (three replicates of each moisture treatment). To measure C mineralisation, samples of the moisture-adjusted soils (equivalent to 25-g of oven-dry soil) were weighed into 50-mL vials (covered by Parafilm with punctured holes), which were placed in 1-L air-tight jars (fitted with rubber septa to facilitate headspace gas sampling). The jars were incubated at 20°C for 8 weeks. A sample of headspace air (∼20 mL) was periodically collected (total of 11 samplings over the 8-week incubation period) with a Hamilton syringe for CO2 measurement using an infra-red gas analyser (LI-COR, Lincoln, Nebraska, USA). After CO2 measurement, each jar was flushed with fresh air to bring CO2 concentration back to the ambient level. De-ionised water was added to the soils to maintain constant water content during the incubation.
After 4 weeks, a subset of samples from each treatment was re-wetted to field capacity to identify the water content threshold below which a respiration flush occurs upon re-wetting. These soils were kept at field capacity for a further 4-week period to quantify the respiration response to re-wetting (headspace CO2 was measured seven times during the 4-week incubation).
For the N mineralisation study, moisture-adjusted soil samples (equivalent to 25 g of oven-dry soil) were weighed into plastic vials (50 mL) and incubated at 20°C. All vials were covered with Parafilm (holes were punctured in the film to facilitate aeration) to minimise moisture loss; water was added at weekly intervals to offset any water lost by evaporation. Mineral N (NO3−-N and NH4+-N) was extracted using 2 M KCl after 2, 4 and 8 weeks of incubation (enough samples were incubated to allow sacrificial sampling at each time point) and determined using an automated colorimeter (QuickChem 8000 FIA+, Lachat Instruments, Loveland, CO, USA) (Keeney and Nelson 1983). Net N mineralisation was calculated by subtracting mineral N at the establishment of the incubation from the amount determined at each sampling point.
Data analysis
Analysis of variance (ANOVA) and the least significant differences (l.s.d.) at the 5% level were performed using the R package ‘agricolae’ (ver. 4.0.5).
Results and discussion
Effect of water content on C mineralisation in 8-week incubation
In all three soils, the amount of C respired during the 8-week incubation increased systematically as water content increased (Fig. 1). The cumulative amount of C mineralised in 8 weeks at field capacity (RWC 1.0) was greatest (P < 0.05) for Waimakariri soil (1158 mg kg−1), followed by the Temuka soil (958 mg kg−1), with the Templeton soil having the lowest value of 466 mg kg−1. These respiration values are directly in line with the amounts of total and hot water-extractable C in the soils (Table 1).
|
|
To compare the C mineralisation vs RWC relationship across the three soils, we normalised mineralisation values measured at all RWCs to mineralisation at field capacity (RWC = 1.0), which our results confirmed to produce the highest mineralisation rates over the range of volumetric water contents measured (Fig. 1). For all three soils, there was a linear (R2 = 0.98) relationship between relative C mineralisation (8-week) and RWC (Fig. 2). The relationship was similar across soils, but especially so for the Temuka and Waimakariri soils. For those two soils, C mineralisation at wilting point (i.e. RWC = 0) was 25–26% of that at field capacity. Mineralisation appeared to be slightly less sensitive to water content in the Templeton soil, where mineralisation at wilting point was 32% of that at field capacity.
For shorter incubation periods, respiration also exhibited a linear relationship with RWC (results for the first week of incubation shown in Fig. 2). However, respiration in the Temuka and Waimakariri soils appeared slower to adjust to lowered soil moisture than in the Templeton soil. Thus, in the first week of incubation, the estimated amount of respiration at wilting point, as a proportion of that at field capacity, was 40–47% for these soils (vs 25–26% for the full 8-week incubation) (Fig. 2). This observation may suggest that there was a lag period (or equilibration period) during which the microbial population did not experience the full impact of the lowered moisture status. Drying will concentrate dissolved microbial substrate (dissolved organic matter, DOM) and this could temporarily buffer the effect of drying in decreasing solute diffusion. This lag period was relatively brief and respiration data for the second week of incubation had a similar relationship with RWC as data for the full 8-week incubation (data not shown).
Pool size and respiration kinetics
The C mineralisation data were fitted to a single-pool, first-order model of the form:

where Cmin is cumulative C mineralised in time t, C0 is potentially mineralisable C, and k is the mineralisation rate constant. Values of C0 and k were estimated by least square iteration using SigmaPlot 14.0.
The single-pool model provided a good fit to the experimental data for all soils and RWC treatments (R2 > 0.98) (Fig. 1). We hypothesised that the pool of mineralisable C (C0) would be independent of the water treatment, whereas the rate constant (k) would increase as water content increases, with a maximum at about field capacity. However, in all three soils, C0 increased as RWC increased, while the rate constant tended to decrease as RWC increased (Fig. 3). The largest value of C0 was always observed in the field capacity treatment and the lowest value in the driest treatment; C0 in the field capacity treatment was about three times greater than that of the driest treatment. In keeping with total C and HWEC, C0 values of Temuka and Waimakariri soils (RWC 1.0 treatment) were ∼2.5 times that of the Templeton soil (Fig. 3a). Although, in all soils, the rate constant tended to decrease as RWC increased (Fig. 3b), the relationship with RWC was not as close as that between RWC and C0.
|
|
Caution is required in interpreting these model results, given the relatively short duration of the incubation (8 weeks). Nevertheless, there are reports in the literature that mineralisable C may increase with increase in soil moisture (Zak et al. 1999; Curtin et al. 2012). Whereas some studies have shown that microbial biomass is positively related to water-filled pore volume when substrate supply is held constant (Rakhsh et al. 2020), there is also evidence that the mineralisation of native soil organic matter is not necessarily regulated by the size or composition of the soil microbial biomass (Kemmitt et al. 2008). Zak et al. (1999) hypothesised that the effect of moisture on the pool size could be a reflection of the positive influence of moisture on the flux of organic substrates to microbial cells via diffusion (discussed further below). The Mineralisable C pool (C0) at field capacity, estimated from the first-order model, represented less than 5% of total C and was less than the amount of HWEC in the soils. This is consistent with the results of McNally et al. (2018) who reported that the mineralisable C pool (C0) predicted from short-term static incubation data underestimates the amount of C that can be lost from soils following conversion of long-term pastures to continuous cropping.
Respiration–moisture relationship
Our results suggest that there was a consistent and robust relationship between respiration (a measure of microbial activity) and soil moisture, expressed as relative water content, that allows for differences in soil water holding capacity that are associated with differences in the texture and SOM content of the three soils investigated in this study. Respiration at wilting point was substantial (∼one-quarter to one-third of that at field capacity), confirming that at least some soil microorganisms are more tolerant of moisture stress than are plants. However, even relatively small reductions in the moisture content of wet soil (a decrease in RWC from 1.0 to 0.84–0.87) resulted in a significant decrease in respiration. These apparently contradictory observations can probably be explained in terms of the influence of substrate supply on the respiration rate. Dissolved organic matter (DOM), considered to be the immediate substrate for soil microorganisms (Marschner and Kalbitz 2003; Kemmitt et al. 2008), is transported to microbial cells by diffusion. The observed relationship between respiration and RWC may reflect the fact that solute diffusivity is a function of moisture content, provided that the moisture content is above the threshold at which diffusion ceases (the point at which water films on soil particles becomes discontinuous). Solute diffusivity in the three experimental soils was calculated using the model developed by Moldrup et al. (2007) and Schjønning et al. (2003):
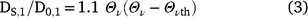
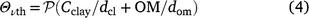
where DS,l represents the diffusion coefficient of a solute (DOM in this study) in soil, and D0,l is the diffusion coefficient of that solute in free water (i.e. DS,l/D0,l is relative solute diffusivity, Eqn 3). Θv is volumetric water content, and Θvth is the threshold water content at which solute diffusion ceases. Θvth was calculated from Eqn 4. In Eqn 4, 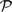 , Cclay, and OM represent the soil bulk density, clay and the organic matter content, respectively, while dcl and dom are the particle densities of clay and organic matter assumed equal to 2.7 and 1.0 g cm−3 (Moldrup et al. 2007). Fig. 4a shows that solute diffusivity (m2 s−1) declined in a non-linear manner as RWC decreased. The soils had very low diffusivity rates (i.e. 0.003, 0.0001 and 0.006 m2 s−1 for Temuka, Templeton and Waimakariri soils, respectively) at the wilting point. At field capacity, the solute diffusivity rate in the Templeton soil was about half that in the other two soils. When solute diffusivity was plotted as a proportion of that at field capacity, the relationship with RWC was similar for the three soils (Fig. 4b). These results support the suggestions that reduced substrate supply, owing to slowed diffusion, may be an important cause of the decline in respiration in response to declining soil moisture. However, the response of solute diffusivity to RWC showed some differences from that observed between respiration and RWC; i.e. the solute diffusion relationship was curvilinear (polynomial relationship), rather than the linear relationship observed for respiration vs RWC. Also, solute diffusivity appeared to be more sensitive to low water content than was respiration (diffusivity rate at wilting point was estimated to be 2–6% of that at field capacity vs ∼25–30% for respiration).
, Cclay, and OM represent the soil bulk density, clay and the organic matter content, respectively, while dcl and dom are the particle densities of clay and organic matter assumed equal to 2.7 and 1.0 g cm−3 (Moldrup et al. 2007). Fig. 4a shows that solute diffusivity (m2 s−1) declined in a non-linear manner as RWC decreased. The soils had very low diffusivity rates (i.e. 0.003, 0.0001 and 0.006 m2 s−1 for Temuka, Templeton and Waimakariri soils, respectively) at the wilting point. At field capacity, the solute diffusivity rate in the Templeton soil was about half that in the other two soils. When solute diffusivity was plotted as a proportion of that at field capacity, the relationship with RWC was similar for the three soils (Fig. 4b). These results support the suggestions that reduced substrate supply, owing to slowed diffusion, may be an important cause of the decline in respiration in response to declining soil moisture. However, the response of solute diffusivity to RWC showed some differences from that observed between respiration and RWC; i.e. the solute diffusion relationship was curvilinear (polynomial relationship), rather than the linear relationship observed for respiration vs RWC. Also, solute diffusivity appeared to be more sensitive to low water content than was respiration (diffusivity rate at wilting point was estimated to be 2–6% of that at field capacity vs ∼25–30% for respiration).
Higher water contents would result in a larger inter-connected water-filled pore network, enabling microorganisms or enzymes to easily utilise diffusing organic substrates (Franzluebbers 1999; Arnold et al. 2015). Conversely, substrate diffusivity and nutrient availability to soil microbes in dry treatments will be retarded at low water content because the channels connecting capillary water are disrupted as the soil dries (Moldrup et al. 2001; Manzoni et al. 2012). However, the results in Fig. 4c show that significant mineralisation occurred in dry soils (∼wilting point) when solute diffusion had effectively ceased. At this point, only DOM that is co-located with microorganisms (and therefore can be degraded locally without significant diffusion) will be mineralised.
In soil, capillary force forms a thin meniscal water film surrounding individual microorganisms, and the curvature radius of the meniscal film has an inverse relation with water potential (Ilstedt et al. 2000). When a larger meniscus covers soil microorganisms, more substrate (DOM) is available for microbial decomposers to utilise. Change in the size of water filled-pores in response to changes in RWC may be another important factor affecting the respiration–moisture relationship. The field capacity treatment (−10 kPa, 30-μm water-filled pore size diameter) in this study maintained a ratio of air-filled and water-filled pores that provided an adequate oxygen and solute supply for microbes to metabolise organic matter. Thus, the greatest microbial respiration rates were observed at field capacity. In the intermediate or low RWC treatments, water was mainly contained in medium and small pores (ranging from <0.2 μm to <30 μm). These pores may be uninhabitable by large microorganisms (mostly, bacteria and enzymes will be active) (Van der Linden et al. 1989; Sleutel et al. 2012). As such, the large C decomposers may suffer water/substrate stress and enter a dormant stage (or die if they succumb to the stress).
C mineralisation after soil re-wetting
After 4 weeks, a set of soils that had been incubated at the range of RWCs described above (Table 2) was re-wetted to field capacity and respiration measured for a further 4-week period. The intent was to address the question of how dry soil must be to induce the ‘Birch effect’ (pulse of respiration after re-wetting dry soil). There was no evidence of a flush of C mineralisation when these soils were re-wetted to field capacity, even when soil water content was close to wilting point when re-wetting occurred (Fig. 5). The amounts of C mineralised post re-wetting (data for the first week and the 2-week post-wetting incubation periods in Fig. 5a, b) were similar (P > 0.05) regardless of the antecedent water content (i.e. water content prior to wetting to field capacity).
|
|
These results would suggest that a flush of mineralisation may not occur if soil water content at the time of re-wetting is above the wilting point. However, this seems to be at variance with the results of Harrison-Kirk et al. (2013) who observed a respiration flush after re-wetting moderately dry soils (i.e. soils that had been maintained at 120% of wilting point water content prior to re-wetting).
We confirmed that a flush of respiration occurred when air-dried samples of our three soils (gravimetric water content 2–3%) were wetted to field capacity. In the day after re-wetting, the soils respired 2.9–4.6 times as much CO2–C as the control soils (soils that had been maintained at field capacity, not dried) (Fig. 6a). However, the flush was short-lived. In the second and third days post-re-wetting, the wetted soil respired 2.6–3.3 times as much CO2–C as the control soils (Fig. 6b), and the respiration rates in re-wetted soils were 1.6–2.2 times those of control soils in the 3–7 day period (Fig. 6c).
The intensity of the respiration flush is likely to be inversely related to soil water content at the time of re-wetting (Fierer and Schimel 2003). A hypothesised/hypothetical relationship between the flush size and RWC at time of re-wetting is in Fig. 7 for soils producing either a large or a small respiration flush (large or small Birch effect). We assume a linear increase in flush size as RWC decreases, with the slope of the relationship between flush size and RWC being the same for both soils. If this model is correct, the RWC required to initiate a respiration flush upon re-wetting would be lower for soils exhibiting a small Birch effect (Fig. 7). This model might explain why our soils, where the Birch effect was apparently relatively small, did not exhibit a respiration flush when RWC at re-wetting was ≥−0.04 (slightly below wilting point; Fig. 6) whereas other workers have reported a respiration pulse after re-wetting relatively moist soil (Harrison-Kirk et al. 2013). The magnitude of the Birch effect has been shown to vary depending on physico-chemical characteristics (e.g. texture, compaction) as well as on the quantity and composition of organic matter in the soil (Beare et al. 2009; Butterly et al. 2010). Work is needed to determine if the relationship depicted in Fig. 7 between drying intensity and the size of the respiration flush after re-wetting is valid for soils exhibiting the Birch effect to different degrees.
|
|
Relationship between net N mineralisation and relative water content
Nitrogen mineralisation, measured over an 8-week incubation period, decreased in the three soils as water content decreased (Fig. 8). Relative mineralisation (N mineralised as a proportion of mineralisation at field capacity) was non-linearly related to RWC; i.e. small decreases in RWC from 1.0 (field capacity) resulted in significant decline in N mineralisation. The relationship of N mineralisation with RWC thus differed from the linear relationship observed between C mineralisation and RWC. The water content where N mineralisation was at its maximum in this study was at field capacity; on average, N mineralisation at wilting point (RWC 0) was estimated to be 33% of that at field capacity.
|
|
In all three soils, N mineralisation was low relative to C mineralisation: the mean ratio of mineralised C to mineralised N (±s.d.) being 18.1 (±1.9), 17.7 (±3.2), and 16.5 (±2.7) in the Temuka, Waimakariri and Templeton soils, respectively. Low N mineralisation may be a reflection of the historically low fertiliser N inputs to the three soils.
Our N mineralisation vs RWC relationship showed some significant differences to that reported by Paul et al. (2003), based on their meta-analysis. They found that optimal RWC for N mineralisation is ≥0.7, with N mineralisation at wilting point being 42% of that at field capacity. Overall, our study indicated that N mineralisation is more sensitive to soil drying than the meta analysis of Paul et al. (2003) would suggest. Use of our mineralisation vs RWC equation (Fig. 8) may result in predictions of net N mineralisation that are somewhat lower than those obtained using the Paul et al. (2003) function. Even so, our data support their conclusion that RWC may be a reliable (and practical) ‘water modifier’ to describe the influence of soil moisture on N mineralisation.
Conclusions
The results indicate that the response of C mineralisation to soil water content can be predicted as a simple function of RWC. Changes in solute diffusivity (substrate accessibility) and size characteristics of the water-filled soil pores are main driving factors underpinning the SOM moisture–mineralisation relationship. Physical characteristics required to estimate RWC (upper and lower limit of available water content) are available for many soils (or approximate values can be estimated). Therefore, RWC provides a practical index for moisture-adjustment of N mineralisation values. However, N mineralisation appeared more sensitive than C mineralisation to declining soil water content. Further work is needed to extend our findings to other soil types and to verify the observation that a flush of mineralisation does not occur if soil water content at the time of re-wetting is above the wilting point.
Data availability
The data that support our findings are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was completed under The New Zealand Institute for Plant and Food Research Limited’s Sustainable Agro-ecosystems (SAE) programme, with funding from the Ministry for Business, Innovation and Employment under the Strategic Science Investment Fund (SSIF), contract number: C11X1702.
Acknowledgements
We are grateful to Sarah Glasson and Rebekah Tregurtha from New Zealand Institute for Plant and Food Research Limited for their technical help.
References
Arnold C, Ghezzehei TA, Berhe AA (2015) Decomposition of distinct organic matter pools is regulated by moisture status in structured wetland soils. Soil Biology and Biochemistry 81, 28–37.| Decomposition of distinct organic matter pools is regulated by moisture status in structured wetland soils.Crossref | GoogleScholarGoogle Scholar |
Barnard RL, Blazewicz SJ, Firestone MK (2020) Rewetting of soil: revisiting the origin of soil CO2 emissions. Soil Biology and Biochemistry 147, 107819
| Rewetting of soil: revisiting the origin of soil CO2 emissions.Crossref | GoogleScholarGoogle Scholar |
Beare MH, Gregorich EG, St-Georges P (2009) Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biology and Biochemistry 41, 611–621.
| Compaction effects on CO2 and N2O production during drying and rewetting of soil.Crossref | GoogleScholarGoogle Scholar |
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant and Soil 10, 9–31.
| The effect of soil drying on humus decomposition and nitrogen availability.Crossref | GoogleScholarGoogle Scholar |
Butterly CR, Marschner P, McNeill AM, Baldock JA (2010) Rewetting CO2 pulses in Australian agricultural soils and the influence of soil properties. Biology and Fertility of Soils 46, 739–753.
| Rewetting CO2 pulses in Australian agricultural soils and the influence of soil properties.Crossref | GoogleScholarGoogle Scholar |
Curtin D, Beare MH, Hernandez-Ramirez G (2012) Temperature and moisture effects on microbial biomass and soil organic matter mineralization. Soil Science Society of America Journal 76, 2055–2067.
| Temperature and moisture effects on microbial biomass and soil organic matter mineralization.Crossref | GoogleScholarGoogle Scholar |
Davidson EA, Verchot LV, Cattanio JH, Ackerman IL, Carvalho JEM (2000) Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48, 53–69.
| Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia.Crossref | GoogleScholarGoogle Scholar |
Falloon P, Jones CD, Ades M, Paul K (2011) Direct soil moisture controls of future global soil carbon changes: an important source of uncertainty. Global Biogeochemical Cycles 25, GB3010
| Direct soil moisture controls of future global soil carbon changes: an important source of uncertainty.Crossref | GoogleScholarGoogle Scholar |
Fierer N, Schimel JP (2003) A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Science Society of America Journal 67, 798–805.
| A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil.Crossref | GoogleScholarGoogle Scholar |
Franzluebbers AJ (1999) Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biology and Biochemistry 31, 1083–1090.
| Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture.Crossref | GoogleScholarGoogle Scholar |
Gee WG, Or D (2002) Particle size analysis. In ‘Methods of soil analysis’. (Eds JH Dane, GC Topp) Part 2.4. pp. 255–293. (Soil Science Society of America: Madison, USA)
| Crossref |
Ghani A, Dexter M, Perrott KW (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biology and Biochemistry 35, 1231–1243.
| Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation.Crossref | GoogleScholarGoogle Scholar |
Harris RF (1981) Effect of water potential on microbial growth and activity. In ‘Water potential relations in soil microbiology. Vol. 9.’ (Eds JF Parr, WR Gardner, LF Elliott) pp. 23–95. (Soil Science Society of America, Inc)
| Crossref |
Harrison-Kirk T, Beare MH, Meenken ED, Condron LM (2013) Soil organic matter and texture affect responses to dry/wet cycles: effects on carbon dioxide and nitrous oxide emissions. Soil Biology and Biochemistry 57, 43–55.
| Soil organic matter and texture affect responses to dry/wet cycles: effects on carbon dioxide and nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar |
Ilstedt U, Nordgren A, Malmer A (2000) Optimum soil water for soil respiration before and after amendment with glucose in humid tropical acrisols and a boreal mor layer. Soil Biology and Biochemistry 32, 1591–1599.
| Optimum soil water for soil respiration before and after amendment with glucose in humid tropical acrisols and a boreal mor layer.Crossref | GoogleScholarGoogle Scholar |
Keeney DR, Nelson DW (1983) Nitrogen—inorganic forms. In ‘Methods of soil analysis: Part 2. Chemical and microbiological properties’. (Ed. AL Page) pp. 643–698. (American Society of Agronomy, Inc., Soil Science Society of America, Inc)
| Crossref |
Kemmitt SJ, Lanyon CV, Waite IS, Wen Q, Addiscott TM, Bird NRA, O’Donnell AG, Brookes PC (2008) Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass – a new perspective. Soil Biology and Biochemistry 40, 61–73.
| Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass – a new perspective.Crossref | GoogleScholarGoogle Scholar |
Linn DM, Doran JW (1984) Effect of water-filled pore-space on carbon-dioxide and nitrous-oxide production in tilled and nontilled soils. Soil Science Society of America Journal 48, 1267–1272.
| Effect of water-filled pore-space on carbon-dioxide and nitrous-oxide production in tilled and nontilled soils.Crossref | GoogleScholarGoogle Scholar |
Lützow Mv, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review. European Journal of Soil Science 57, 426–445.
| Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review.Crossref | GoogleScholarGoogle Scholar |
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93, 930–938.
| Responses of soil microbial communities to water stress: results from a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 22690643PubMed |
Marschner B, Kalbitz K (2003) Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113, 211–235.
| Controls of bioavailability and biodegradability of dissolved organic matter in soils.Crossref | GoogleScholarGoogle Scholar |
McNally S, Beare M, Curtin D, Tregurtha C, Qiu WW, Kelliher F, Baldock J (2018) Assessing the vulnerability of organic matter to C mineralisation in pasture and cropping soils of New Zealand. Soil Research 56, 481–490.
| Assessing the vulnerability of organic matter to C mineralisation in pasture and cropping soils of New Zealand.Crossref | GoogleScholarGoogle Scholar |
Moldrup P, Olesen T, Komatsu T, Schjønning P, Rolston DE (2001) Tortuosity, diffusivity, and permeability in the soil liquid and gaseous phases. Soil Science Society of America Journal 65, 613–623.
| Tortuosity, diffusivity, and permeability in the soil liquid and gaseous phases.Crossref | GoogleScholarGoogle Scholar |
Moldrup P, Olesen T, Blendstrup H, Komatsu T, de Jonge LW, Rolston DE (2007) Predictive-descriptive models for gas and solute diffusion coefficients in variably saturated porous media coupled to pore-size distribution: IV. Solute diffusivity and the liquid phase impedance factor. Soil Science 172, 741–750.
| Predictive-descriptive models for gas and solute diffusion coefficients in variably saturated porous media coupled to pore-size distribution: IV. Solute diffusivity and the liquid phase impedance factor.Crossref | GoogleScholarGoogle Scholar |
Moyano FE, Manzoni S, Chenu C (2013) Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models. Soil Biology and Biochemistry 59, 72–85.
| Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models.Crossref | GoogleScholarGoogle Scholar |
Mudge PL, Kelliher FM, Knight TL, O’Connell D, Fraser S, Schipper LA (2017) Irrigating grazed pasture decreases soil carbon and nitrogen stocks. Global Change Biology 23, 945–954.
| Irrigating grazed pasture decreases soil carbon and nitrogen stocks.Crossref | GoogleScholarGoogle Scholar | 27483409PubMed |
Orchard VA, Cook FJ (1983) Relationship between soil respiration and soil moisture. Soil Biology and Biochemistry 15, 447–453.
| Relationship between soil respiration and soil moisture.Crossref | GoogleScholarGoogle Scholar |
Paul KI, Polglase PJ, O’Connell AM, Carlyle JC, Smethurst PJ, Khanna PK (2003) Defining the relation between soil water content and net nitrogen mineralization. European Journal of Soil Science 54, 39–47.
| Defining the relation between soil water content and net nitrogen mineralization.Crossref | GoogleScholarGoogle Scholar |
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B: Chemical and Physical Meteorology 44, 81–99.
| The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate.Crossref | GoogleScholarGoogle Scholar |
Rakhsh F, Golchin A, Al Agha AB, Nelson PN (2020) Mineralization of organic carbon and formation of microbial biomass in soil: effects of clay content and composition and the mechanisms involved. Soil Biology and Biochemistry 151, 108036
| Mineralization of organic carbon and formation of microbial biomass in soil: effects of clay content and composition and the mechanisms involved.Crossref | GoogleScholarGoogle Scholar |
Schjønning P, Thomsen IK, Moldrup P, Christensen BT (2003) Linking soil microbial activity to water- and air-phase contents and diffusivities. Soil Science Society of America Journal 67, 156–165.
Slessarev EW, Schimel JP (2020) Partitioning sources of CO2 emission after soil wetting using high-resolution observations and minimal models. Soil Biology and Biochemistry 143, 107753
| Partitioning sources of CO2 emission after soil wetting using high-resolution observations and minimal models.Crossref | GoogleScholarGoogle Scholar |
Sleutel S, Bouckaert L, Buchan D, Van Loo D, Cornelis WM, Sanga HG (2012) Manipulation of the soil pore and microbial community structure in soil mesocosm incubation studies. Soil Biology and Biochemistry 45, 40–48.
| Manipulation of the soil pore and microbial community structure in soil mesocosm incubation studies.Crossref | GoogleScholarGoogle Scholar |
Thomas GW (1982) Exchangeable cations. In ‘Methods of soil analysis’. (Ed. AL Page) pp. 159–165. (America Society of Agronomy: Madison, WI, USA)
| Crossref |
Van der Linden AMA, Jeurissen LJJ, Van veen JA, Schippers B (1989) Turnover of the soil microbial biomass as influenced by soil compaction. In ‘Nitrogen in organic wastes.’ (Eds JAA Hansen, KAJ Henriksen) pp. 25–26. (Academic Press Limited: London)
| Crossref |
Zak DR, Holmes WE, MacDonald NW, Pregitzer KS (1999) Soil temperature, matric potential, and the kinetics of microbial respiration and nitrogen mineralization. Soil Science Society of America Journal 63, 575–584.
| Soil temperature, matric potential, and the kinetics of microbial respiration and nitrogen mineralization.Crossref | GoogleScholarGoogle Scholar |


