Microbial community composition and activity in paired irrigated and non-irrigated pastures in New Zealand
Suzanne M. Lambie A * , Paul L. Mudge A and Bryan A. Stevenson A
A * , Paul L. Mudge A and Bryan A. Stevenson A
A Manaaki Whenua – Landcare Research, Private Bag 3127, Hamilton 3240, New Zealand.
Soil Research 60(4) 337-348 https://doi.org/10.1071/SR21149
Submitted: 4 June 2021 Accepted: 1 October 2021 Published: 17 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Microorganisms are key for carbon (C) and nitrogen (N) cycling in soils supporting agricultural production.
Aims: We investigated the impacts of irrigation on microbial community structure and activity in New Zealand on 28 paired non-irrigated and irrigated grazed pasture sites where C and N had decreased under irrigation.
Methods: Microbial community structure and microbial biomass (phospholipid fatty acids) and activity (basal respiration, substrate-induced respiration (SIR), aerobically mineralisable N (AerMN)) were assessed.
Key results: Microbial biomass did not differ between irrigated and non-irrigated soils, but irrigated soils had increased gram-negative bacteria (P < 0.05), lower gram-positive:gram-negative ratio (P < 0.001) and lower fungal:bacterial ratio (P < 0.001) compared to non-irrigated soils. SIR and AerMN were greater in irrigated compared to non-irrigated soils. There were no differences in basal respiration between irrigation treatments. Greater prevalence of gram-negative bacteria (r-strategist) as well as decreases in actinomycetes and fungal to bacterial ratio, and increased SIR and AerMN suggest more rapid cycling of C and nutrients in irrigated systems where C had been lost.
Conclusions: We found clear evidence that irrigation alters microbial community structure and activity in New Zealand pasture systems.
Implications: Irrigation driven alteration of microbial populations may contribute to losses of soil SOM and soils’ ability to deliver ecosystem services.
Keywords: aerobically mineralisable nitrogen, carbon cycling, gram-negative bacteria, fungal:bacterial ratio, irrigation, microbial community composition, nitrogen, substrate induced respiration.
Introduction
Humans have used irrigation to improve food production for over 4000 years (Thenkabail et al. 2006). In response to a rapidly growing human population, the amount of irrigated land has greatly increased in recent history, such that by 2012, about 275 million ha were irrigated globally (FAO 2016). In New Zealand, the amount of land utilising irrigation has increased by 57% between 2002/03 and 2011/12 and the economic value of irrigation to dairy and sheep production (2011/12) was estimated to be NZD2.17 billion (Corong et al. 2014). Despite increasing use of irrigation around the world, there is relatively limited information on the long-term effects of irrigation on microbially mediated soil carbon and nitrogen cycling.
Microbial community structure and function are directly affected by water availability (Harris 1981; Paul et al. 2003; Moinet et al. 2016; Siebielec et al. 2020). Appropriately applied irrigation reduces water stress for microbes (Harris 1981; Williams and Rice 2007; Siebielec et al. 2020), changes microbial activity and biomass, and alters community structure. For example, Ma et al. (2016) reported decreased bacterial biomass, increased fungal biomass, and therefore an increased fungal:bacterial (F:B) ratio in response to irrigation in arid soils from Inner Mongolia. Changes in microbial parameters generally increased after 2 years of irrigation compared to a single year (Ma et al. 2016). After 7 years of irrigation in a temperate tall grass prairie ecosystem (Kansas, USA), Williams and Rice (2007) reported increased fungal biomass, decreased bacterial stress, and an additional increase in catabolic diversity. Further, Kenngott et al. 2021 measured increased carbon and nitrogen stocks and gram-negative bacteria but a decrease in fungal:bacterial ratio in irrigated temperate grasslands in Germany.
A number of studies in New Zealand have shown lower soil C and N stocks in irrigated compared to non-irrigated soils (Kelliher et al. 2012; Schipper et al. 2012; Mudge et al. 2017), which has been linked to decreased labile C (e.g. Schipper et al. 2019) that may be a feedback loop associated with changes in microbial community structure (Moreno et al. 2021). The objective of our work was to assess microbial community composition and activity in paired irrigated and non-irrigated pasture soils, where C and N stocks were lower under irrigation.
Materials and methods
Experimental method
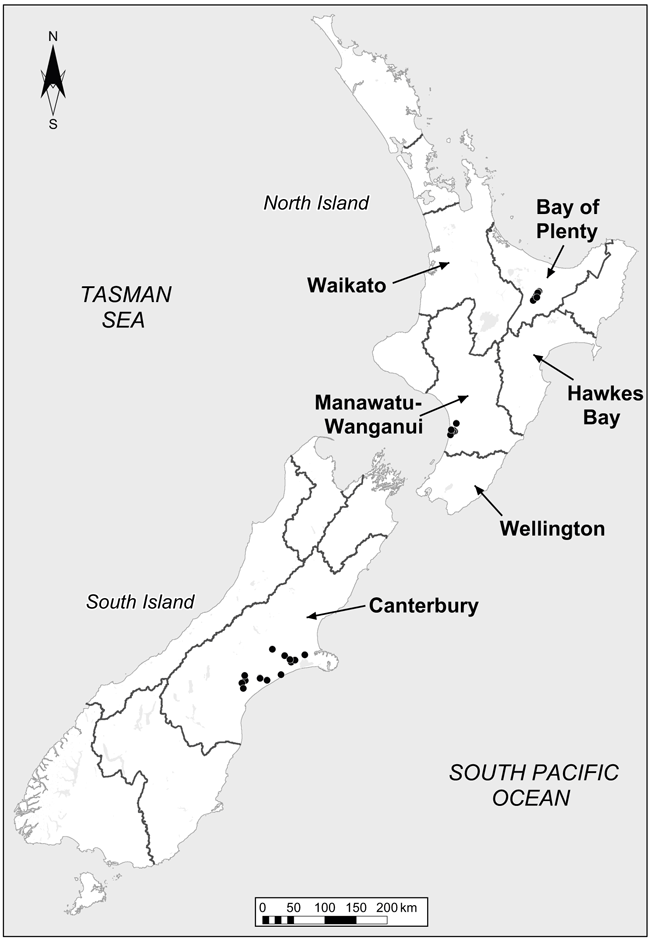
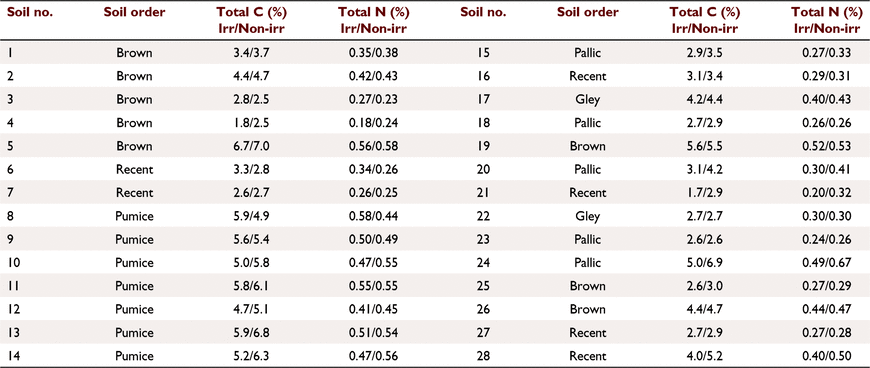
A subset of the sites sampled in Mudge et al. 2017 (where mean total carbon and nitrogen stocks were significantly lower under irrigation) were used in this study. The six sites sampled in the Otago region by Mudge et al. 2017 were omitted giving a total of 28 out of the original 34 sites sampled. The omitted Otago sites were predominantly flood irrigated vs sprinkler irrigation for the other sites. Each of the 28 sites sampled contained irrigated and non-irrigated paired treatments and were sampled to a 0–10 cm depth. The irrigated and non-irrigated pairs within each site were within 100 m of each other and were on the same soil, landform and usually the same farm with the same farm management on both treatments (Mudge et al. 2017). Sites were under intensively grazed pastures, 14 in the North Island (Bay of Plenty and Manawatū regions) and 14 in the South Island (Canterbury region) of New Zealand (Supplementary Table S1; Fig. 1). Pumice soils were collected from Bay of Plenty (n = 7), Recent (n = 2) and Brown (n = 5) soils from Manawatū, and Pallic (n = 5), Recent (n = 4), Gley (n = 2) and Brown (n = 3) soils from Canterbury region (Table 1). Soil carbon and nitrogen contents for the irrigated and non-irrigated pairs within each site are shown in Table 1. On average, the irrigated treatment had 8 ± 3% lower C content and 6 ± 3% lower N content compared to the paired non-irrigated treatment. This is comparable to Mudge et al. (2017) where C decreased by 8% in the top 30 cm of the profile.
|
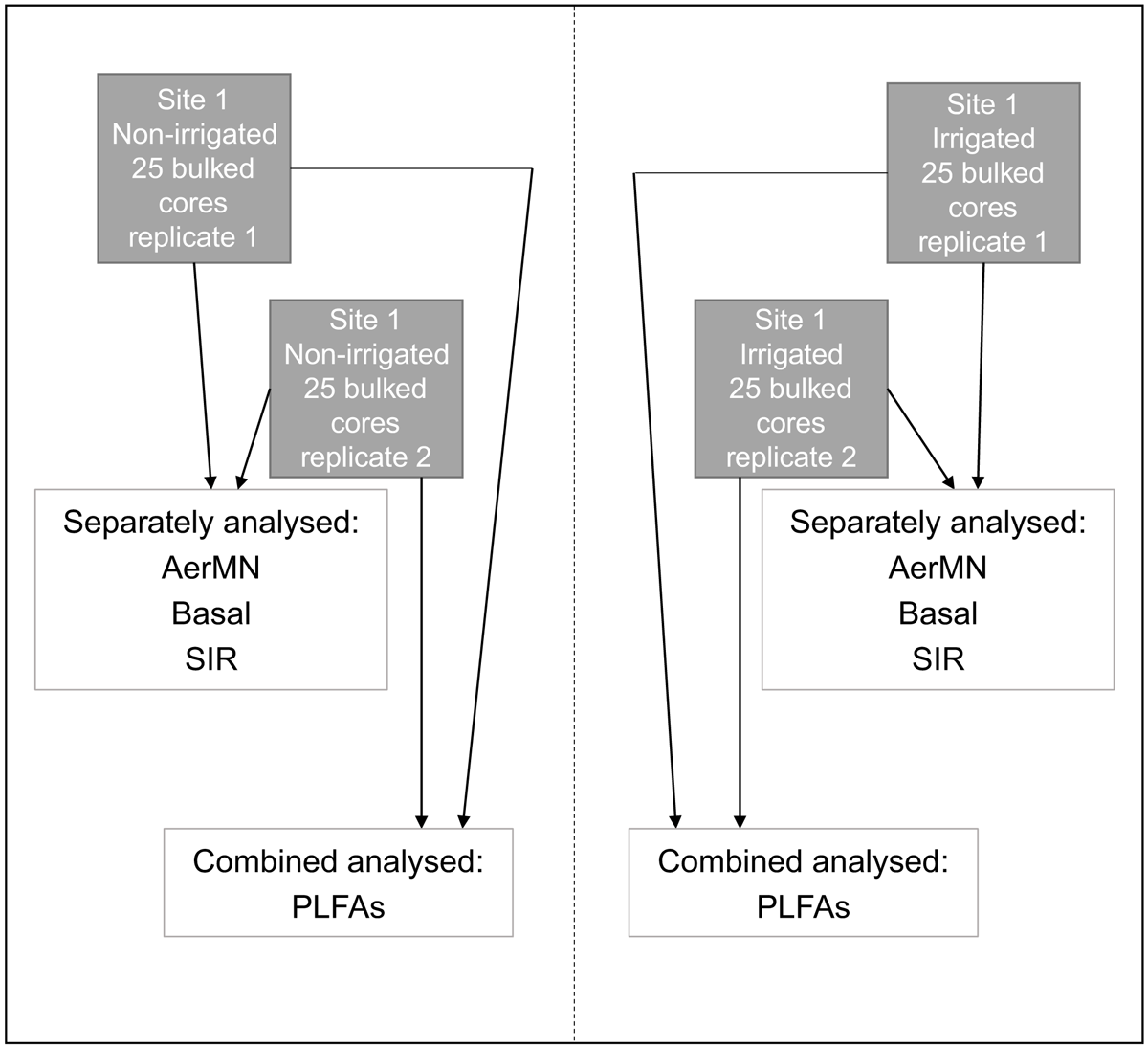
Irrigation was predominantly via centre pivots and mean annual precipitation across the sites ranged between 590 and 1550 mm (Mudge et al. 2017). The length of time under irrigation varied between the paired sites and ranged between 3 and 33 years (Supplementary Table S1). At each paired site, two 10 m × 10 m sampling grids were randomly established in each treatment and 25 cores (25 mm diameter by 100 mm deep) were collected and bulked within each grid, for a total of two replicates at each treatment at each site (Fig. 2).
To avoid seasonal effects on microbial parameters (e.g. Williams and Rice 2007), soils were sampled at same time of the year, but in two sequential years. Soils in the North Island were collected in November 2014 and soils in the South Island were collected in November 2015. It is important to note that in both years, sampling for the paired sites occurred in spring (a relatively high precipitation period) prior to the start of irrigation. Our objective was to assess the long-term effect of irrigation, not the shorter-term effect of recent irrigation during the dry season.
Soil analysis
Four soil samples taken at each site (two non-irrigated and two irrigated) were sieved separately to 2 mm at field moisture and a sub-sample taken from each and air dried at 35°C. The moisture content ranged between 6 and 58% (Supplementary Table S2).
Functional measurements included basal respiration and net N mineralisation as measures of C and N cycling. Substrate-induced respiration (SIR) was also measured as an indication of potential change in microbial community composition affecting the metabolic response to carbon substrate addition. The remaining soil was adjusted to 60% of water holding capacity and pre-incubated for 7 days at 25°C in the dark. Basal respiration was then determined over a subsequent 7-day incubation in the dark at 25°C by measurement of the CO2 concentration in the headspace at the end of the incubation (West and Sparling 1986). SIR was determined over a 4-hour incubation (25°C) following the addition of glucose to the ratio of 0.15 g to 0.1 kg of oven-dry equivalent soil (West and Sparling 1986). Aerobically mineralisable N (AerMN) was determined following a 56-day incubation (25°C) at 60% water holding capacity, after which the soils were extracted with 2 M potassium chloride and quantified for ammonium and nitrate (Parfitt et al. 2005). AerMN was the difference in ammonium and nitrate concentrations pre- and post-incubation. Basal respiration, SIR and AerMN were analysed on each of the field replicates (non-irrigated = 2, irrigated = 2) for a total of four samples at each of the 28 paired sites (Fig. 2).
For a general assessment of microbial community composition and indication of biomass of specific groups (e.g. total microbial, bacterial, fungal, gram-positive, gram-negative and actinomycete biomasses), we used phospholipid fatty acid (PLFA) analysis. PLFA is suitable for assessment of microbial community composition and representative of a range of ecosystem functions (Orwin et al. 2018; Lin et al. 2020). For PLFA analysis, field duplicates at each paired sampling site within each treatment (irrigated vs non-irrigated) were combined (Fig. 2), freeze-dried and frozen at −80°C. PFLAs were quantified following the method of Bligh and Dyer (1959), as modified by White et al. (1979) and Bardgett et al. (1996). Briefly, lipids were extracted from 1.5 g of fresh soil, fractionated and methylated, and the resulting fatty acid methyl esters (FAMEs) analysed using an Agilent 7890A GC with Agilent 5975C VL MSD (Agilent, Santa Clara, CA, USA). The resulting peaks were identified using retention times relative to two added internal standards (C13 and C19) and a bacterial methyl ester standard mixture (Supelco Bacterial Acid Methyl Esters CP Mix 47080-U; Sigma-Aldrich Corporation, St Louis, MO, USA). Peak size was quantified using the FAME 19:0 internal standard, and the abundance of each of the individual fatty acids extracted expressed as relative μg g−1 of dry soil using standard nomenclature and converted to μmol based on the molecular weight of the individual FAMEs. Microbial biomass was expressed as the sum of all FAME peaks.
Absolute and relative bacterial biomass were calculated from PLFAs associated with gram-positive bacteria (i-15:0, a-15:0, i-16:0, i-17:0 and a-17:0), gram-negative bacteria (cy-17:0, cy-19:0, 16:1 ω7c and 18:1ω7c) (Zelles 1999; Waldrop and Firestone 2004), and the general bacterial marker 15:0 (Bardgett et al. 1996; Orwin et al. 2016). Relative biomass (i.e. percentage of community composition) for each biomarker was determined by dividing the absolute biomass by the total PFLA biomass. The gram-positive:gram-negative ratio was calculated by dividing the sum of all gram-positive PLFAs by the sum of all gram-negative PLFAs. The fungal PLFA marker (18:2ω6,9c) was used to calculate fungal biomass and the F:B ratio. Also, actinomycetes were calculated as the sum of PLFA markers (10Me16:0, 10Me17:0 and 10Me18:0), and a bacterial stress indicator was calculated as (cy17:0 + cy19:0)/(16:1ω7c + 18:1ω7c) (Kaur et al. 2005).
Data analysis
For those analyses where both of the field replicates within a treatment unit were analysed separately (e.g. AerMN, Basal respiration, SIR), the data was averaged before statistical analysis. PLFA groups and microbial activity for each paired site are presented in Supplementary Table S3. Statistical analysis of soil parameters between the irrigated and non-irrigated pairs within each site was undertaken using a paired, two-tailed, t-test (n = 28). For those analyses found to have a non-normal distribution, the Wilcoxin-Matched-Pairs test was used to assess differences between treatments non-parametrically. ANOVA with blocking by site and post hoc analysis (Student–Newman–Keuls) was used to determine the effect of soil order. Two-tailed t-tests were used to determine differences between soils in the North and South Islands for each of the parameters measured. All analyses were undertaken in Genstat 14 (VSN International, Hemel Hempstead, UK).
Regression analyses were conducted on the differences between irrigated and non-irrigated pairs (Δ) within each site (irrigated minus non-irrigated data). Regressions were used to assess if microbial parameters were affected by the length of time under irrigation, and if changes in microbial community structure were related to changes in microbial function. Microbial total abundance and functional data were log transformed before analysis to meet assumptions of normality and equality of variance. Box and whisker plots were used to show the absolute values and distribution of the overall irrigated and non-irrigated data. Outliers showed no consistent pattern between soils within treatments or between parameters and no data was excluded from the analyses.
Multivariate statistics on the full multivariate pattern of relative PLFA abundances were run using the Primer software Package (Primer v6 and PERMANOVA, 2012, Primer-E Ltd, Plymouth, UK). Data were square root transformed and the Bray-Curtis metric was used. The PLFA abundance data was visually examined using non-linear multi-dimensional scaling (NMD). The irrigation and location (i.e. region or island) effects on the multivariate pattern of PLFAs was analysed by permutational multivariate analysis of variance (PERMANOVA). The variance explained by the main effects was determined using the DISTLM programme in Primer.
Statistical analyses were significant if P < 0.05. Data are presented on a soil dry weight basis (105°C) unless otherwise stated.
Results
Microbial composition and abundance
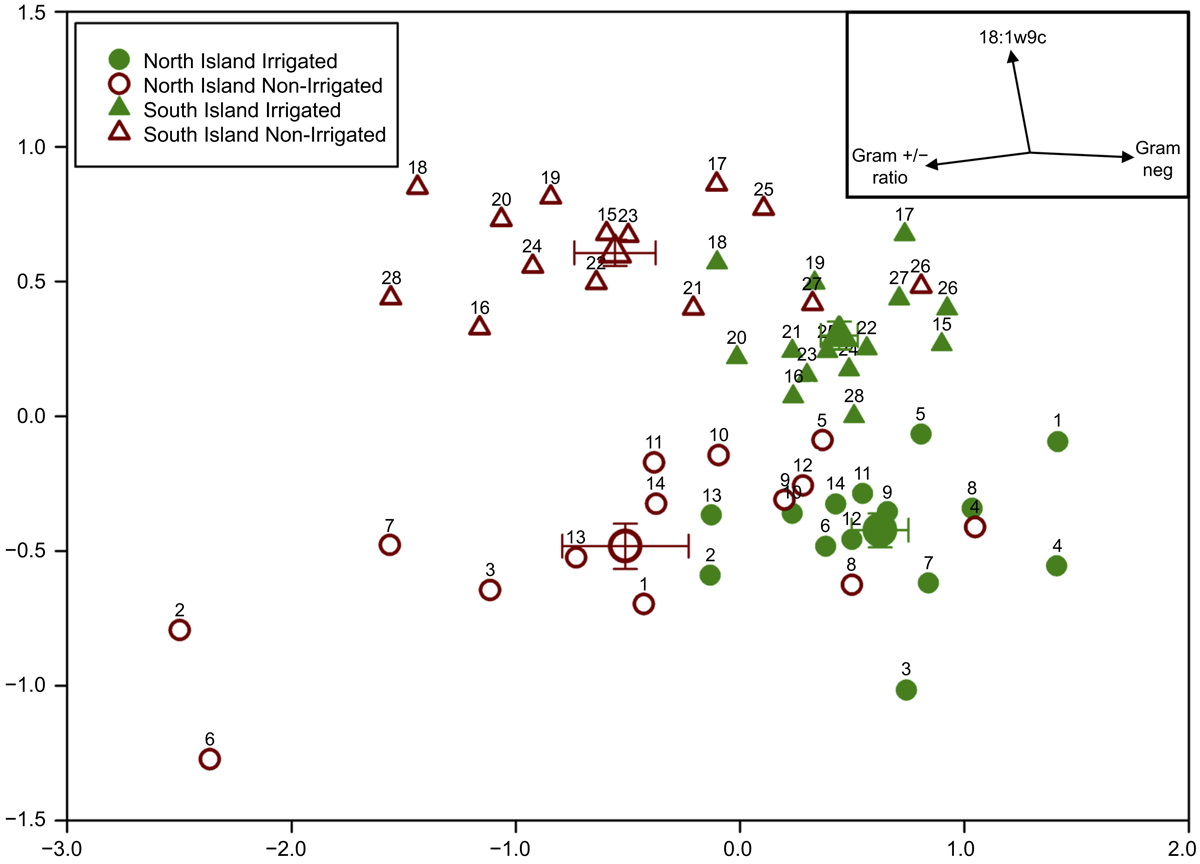
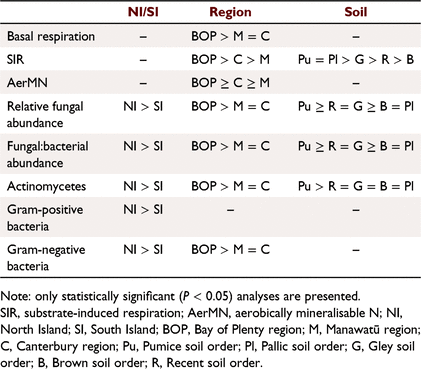
The multi-dimensional scaling plot (Fig. 3) showed a clear shift along the x axis in the irrigated vs non-irrigated treatment within a paired site. The analysis also clearly separated the soils by North and South Island sites (along the y axis) as well as irrigation presence/absence. Soil number assignments are presented in Table 1 and Supplementary Table S1. Correlation analysis of the individual PLFAs and PLFA groups indicated that the gram-positive:gram-negative ratio and gram-negative abundance were highly correlated (>0.9) to the first NMD (i.e. x) axis and the alternate fungal biomarker 18:1ω9c highly correlated to the second NMD (i.e. y) axis.
|
When considering the multivariate pattern of the relative abundances of all the individual PLFA markers, permutational analysis of variance indicated that irrigation was highly significant (P < 0.001) and explained approximately 20% of the total PLFA variation in multivariate space. While sites in both North Island regions were significantly different from the South Island sites (P < 0.001), they were not different from each other (P = 0.19). Thus for simplicity, sites are grouped by North or South Island (Fig. 3). Location of sampling (i.e. North or South Island) explained 16% of the total variation (inclusion of region did not significantly increase the variance explained). Region * Δ was not significant (P = 0.86), indicating that the irrigation effect was consistent across regions. It is interesting to note, however, that when considered on a North Island vs South Island basis, the multivariate distance between irrigated and non-irrigated treatment centroids on each of the Islands was relatively similar (3.6 vs 3.3 units), whereas the difference between the North Island vs South Island dry treatments was greater than that of the irrigated treatments (3.5 vs 2.5 units).
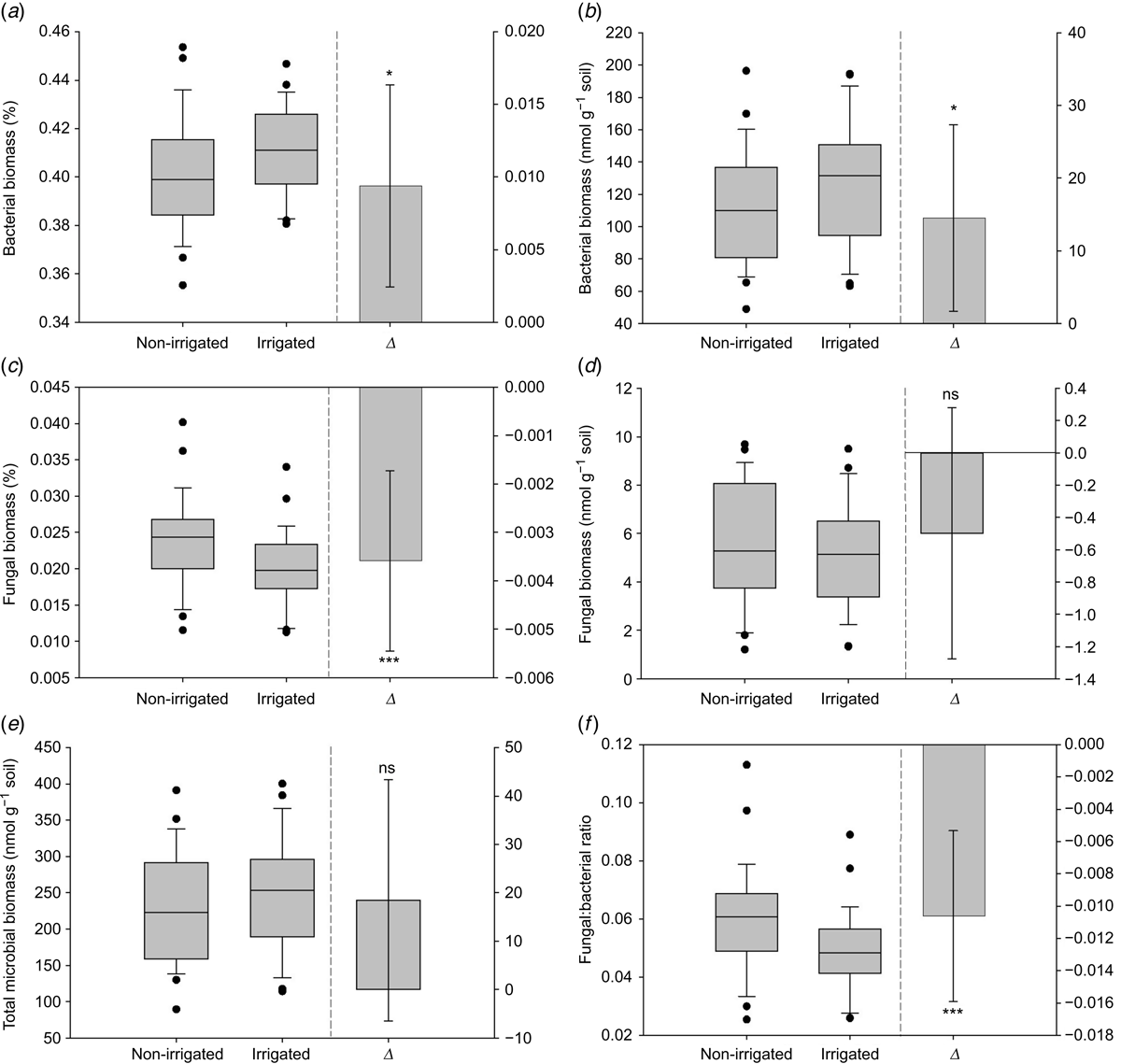
Mean bacterial biomass Δ was significantly (P < 0.05) greater in the irrigated treatment and the median relative bacterial biomass was lower in the irrigated compared to the non-irrigated treatment (Fig. 4a). Absolute bacterial biomass Δ was significantly (P < 0.05) greater in the irrigated treatment and the median was higher in the irrigated compared to the non-irrigated treatment (Fig. 4b). Relative fungal biomass Δ was significantly (P < 0.001) lower in the irrigated treatment and the median was lower in the irrigated compared to non-irrigated treatment (Fig. 4c). Absolute fungal biomass Δ was not significant and the median was similar between treatments (Fig. 4d). Total biomass Δ was not significant and the median was higher in the irrigated compared to non-irrigated treatment (Fig. 4e). F:B ratio Δ was significantly (P < 0.001) lower in the irrigated treatment and the median was lower in the irrigated compared to the non-irrigated treatment (Fig. 4f).
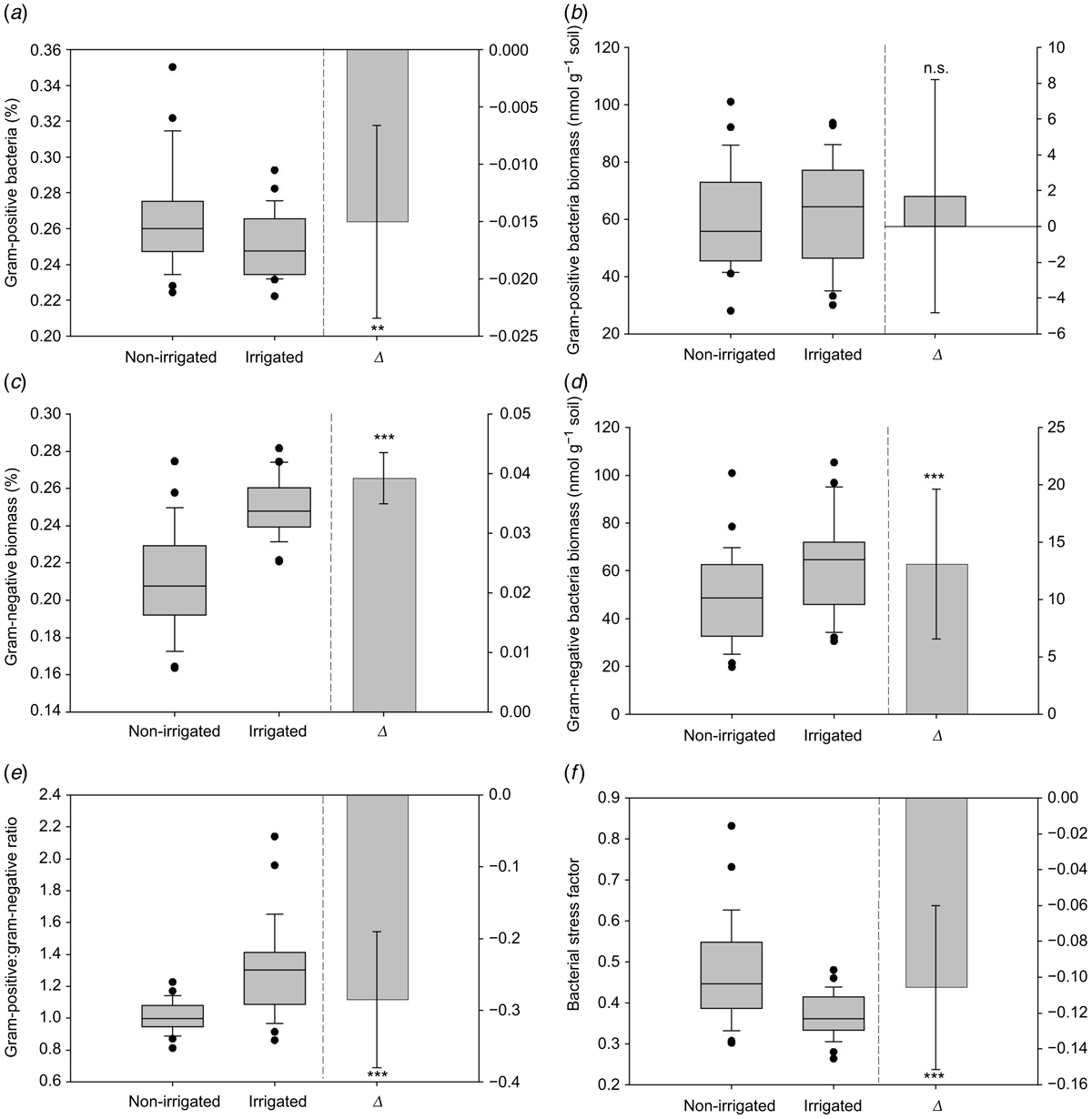
Relative abundance of gram-positive bacteria Δ was significantly (P < 0.001) lower in the irrigated treatment and the median was lower in the irrigated compared to the non-irrigated treatment (Fig. 5a). Absolute gram-positive Δ was not significant and the median biomass was higher in the irrigated compared to non-irrigated treatment (Fig. 5b). Relative and absolute abundance of gram-negative bacteria Δ was significantly (P < 0.001) greater in the irrigated treatment and the median of both was higher in the irrigated compared to non-irrigated treatment (Fig. 5c, d). Gram-positive:gram-negative ratio Δ was significantly (P < 0.001) greater in the irrigated treatment and the median was higher in the irrigated treatment (Fig. 5e). Bacterial stress factor Δ was significantly (P < 0.001) lower in the irrigated treatment and the median was lower in the irrigated compared to non-irrigated treatment (Fig. 5f). Although the bacterial stress factor had non-normal distribution, non-parametric testing supported the findings of the parametric testing.
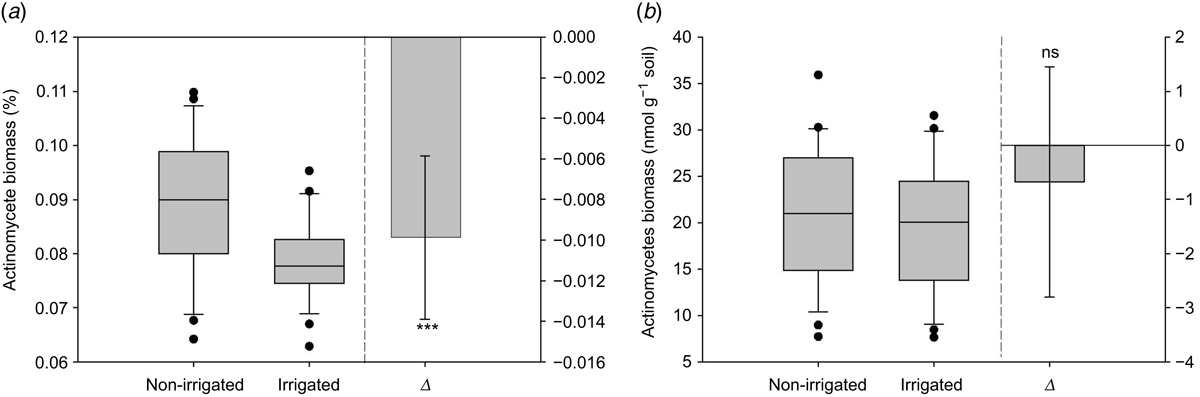
Relative abundance of actinomycetes Δ was significantly (P < 0.001) lower in the irrigated treatment and the median was lower in the irrigated treatment compared to non-irrigated (Fig. 6a). Absolute actinomycetes Δ was not significant and the median was similar between the irrigated and non-irrigated treatments (Fig. 6b).
The relative abundance of fungi, the F:B ratio and the absolute amounts of actinomycete, gram-positive, gram-negative biomass were significantly greater in the North Island compared to South Island soils (Table 2).
|
Microbial activity
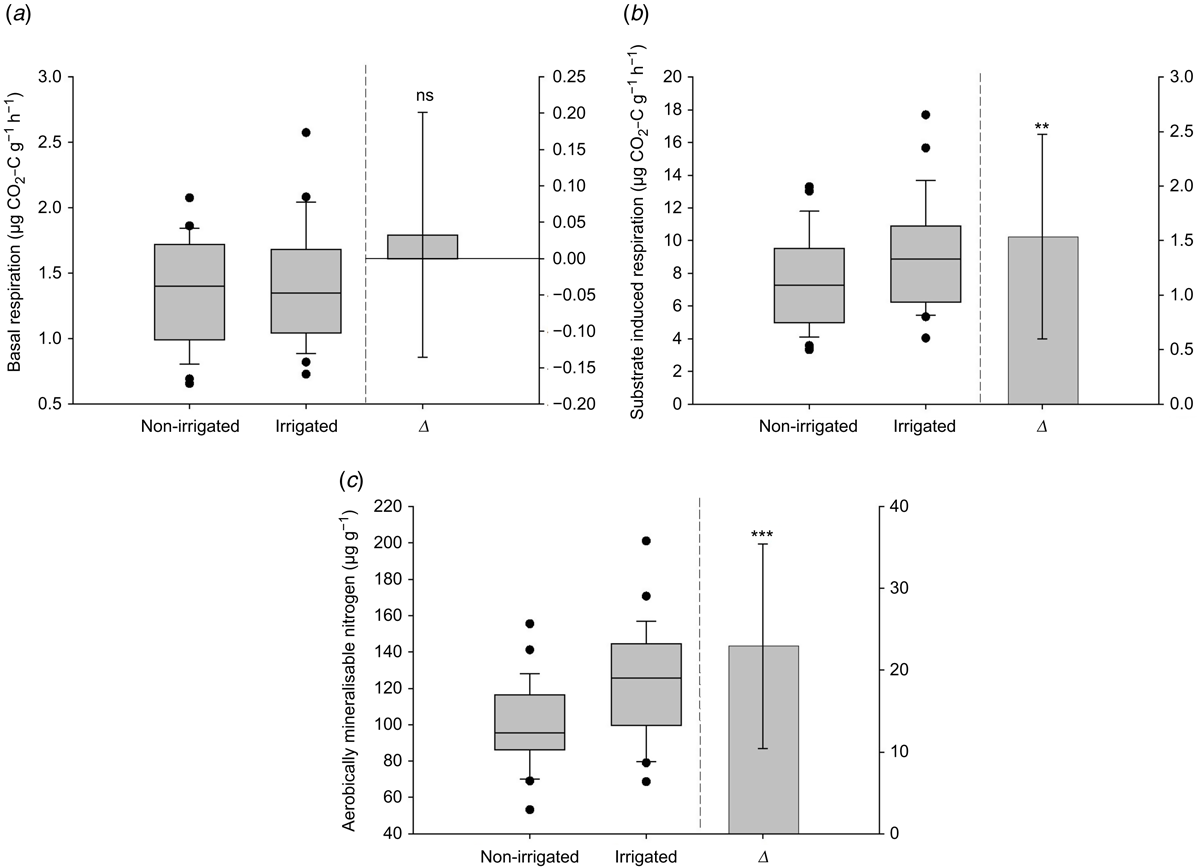
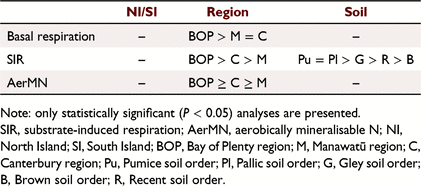
Basal respiration Δ was not significant and the median was similar between the treatments (Fig. 7a). SIR Δ was significantly (P < 0.001) greater in the irrigated treatment and the median was higher in the irrigated compared to non-irrigated treatment (Fig. 7b). AerMN Δ was significantly (P < 0.001) greater in the irrigated treatment and the median was higher in irrigated compared to non-irrigated treatment (Fig. 7c). There were also no differences in SIR, basal respiration and AerMN between the North Island and South Island soils, but pumice soils from the Bay of Plenty (North Island) had greater rates for these parameters (Table 3).
|
Microbial composition vs activity
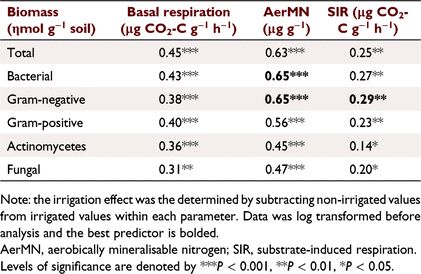
Correlations between microbial community composition Δ and activity Δ were positive indicating the greater the irrigation effect on microbial activity the greater the irrigation effect on community structure (Table 4). The irrigation effect on total bacteria and gram-negative bacteria explained the greatest (and essentially equal) amounts of the variation in the irrigation effect on AerMN (r2 = 0.65); while the irrigation effect on gram-negative bacteria explained the most variance (r2 = 0.29) in the irrigation effect on SIR though total bacterial abundance explained only slightly less total variation (Table 4).
|
Discussion
We assessed the response of microbial community composition and activity to irrigation but our work did not support the hypothesis that irrigation would shift to a microbial community indicative of slower C and N cycling. It was not surprising that we measured differences in microbial community composition between the irrigated and non-irrigated paired sites, as redistribution of microbial groups has been shown in response to changes in water inputs (e.g. Williams and Rice 2007; Araya et al. 2013; Ma et al. 2016). However, we did observe some key patterns in changes in microbial communities.
We identified a lower gram-positive:gram-negative ratio in irrigated soils, which was driven by an increase in gram-negative bacteria under irrigation. Gram-negative bacteria may be more sensitive to changes in soil moisture (Harris 1981), which is consistent with lower abundances in non-irrigated compared to irrigated soils. Gram-negative bacteria also commonly target labile C compounds that require less energy for degradation and can therefore indicate a change in C cycling (Treseder et al. 2011; Whitaker et al. 2014). de Vries and Shade (2013) proposed that gram-negative bacteria represent r-strategists and gram-positive bacteria represent K-strategists, and therefore the gram-positive:gram-negative ratio can indicate changes in the predominance of r- and K-strategists. R-strategists are copiotrophs, which have rapid growth and low resource use efficiency and predominantly degrade fresh organic matter inputs, whereas K-strategists are oligotrophs with low growth rates and high resource use efficiency and degrade SOM (Klappenbach et al. 2000; Fontaine et al. 2003; Fierer et al. 2007), and these two approaches to C cycling can have an impact on SOM storage. R-strategists are also thought to be key in priming events associated with the addition of C and N substrates, and therefore enhanced r-strategist abundance increases the possibility of priming events contributing to decreases in SOM content (Kuzyakov et al. 2000; Chen et al. 2014). Increasing prevalence of gram-negative bacteria may also explain greater SIR in irrigated soils, as r-strategists have adapted to respond more rapidly to fresh organic matter inputs which may be greater under irrigation (Knapp et al. 2001; Kelliher et al. 2012; Kenngott et al. 2021). Although the changes in microbial community only ranged between 1 and 4% of relative abundance, they are indicative of a changing dynamic with respect to SOM cycling.
Contrary to Ma et al. (2016) in arid soils and Williams and Rice (2007) in temperate soils, we measured a decrease in F:B ratio in the irrigated compared to the non-irrigated soils, which was due to a decrease in the relative amount of fungal biomass. Intensively grazed systems are generally associated with bacteria-dominated communities (de Vries et al. 2011, 2012), and the sites utilised by Ma et al. (2016) and Williams and Rice (2007) were more likely to be fungal-dominated communities as they were sheep-grazed grassland and annually burnt or ungrazed native grasslands (de Vries et al. 2011, 2012). Consistent with our results, Kenngott et al. (2021) also measured decreased fungal biomass in temperate, hay production grasslands, but in contrast, they found increased carbon and nitrogen stocks associated with irrigation. The lack of consistency across climatic zones and grazing intensity interactions with irrigation on microbial community structure indicates a complex microbial response complicated by a range of known and unknown factors. However, given the fact that in our study both the gram-positive:gram-negative ratio and F:B ratios suggest a more rapid cycling (r-dominated) system under irrigation (Carmona et al. 2020), and that other studies have found differing results with regard to changes in the fungal community, it may be that different facets of the microbial system oppose each other.
Actinomycetes are thought to be pivotal in the formation of stable SOM, degrading complex C substrates and undertaking N fixation (Bhatti et al. 2017), and were negatively impacted by irrigation in our soils. Actinomycetes are considered to be slower growing than both bacteria and fungi (Bhatti et al. 2017) and therefore support the theory that our soils are shifting from a more K-strategist population to an r-strategist community under irrigation. However, the difference in their abundances between the treatments was minor, so must be treated with some caution but are suggestive of a change occurring in irrigated soils. Actinomycetes are catalysts for the provision of N to plants and soil humus formation (Bhatti et al. 2017), and therefore decreasing actinomycetes abundance may lead to increasing fertiliser demands to maintain fodder production within pasture systems and at least partially mitigating the positive effects of irrigation on plant production.
We found separation in the distribution of microbial communities and driving factors between North Island and South Island soils (Fig. 5), which may be due to several factors. Firstly, there were some significant differences in both soil chemical and biological factors that may be related to pedogenesis factors (Wakelin et al. 2013) such as parent material. The North Island soils (Bay of Plenty and Manawatū combined) had greater amounts of mineral N and microbial PLFA groups than South Island soils (Canterbury). The patterns with respect to soil order were variable and often showed little difference between the soil orders with respect to microbial parameters (Supplementary Table S2). Secondly, though the soils were collected during the same season, they were sampled in consecutive years due to logistical constraints, the North Island sites in 2014, and the South Island sites in 2015. While there are some differences between the soils themselves, we cannot rule out the different times at which the samples were collected was a factor in the separation between North Island and South Island soils. Thirdly, different climatic conditions between the North and South Island sites may influence microbial communities. We could not find strong evidence that either mean annual precipitation or mean annual temperature were correlated to the separation in sites (data not shown), though it is interesting to note the separation between North and South Island sites appear to decrease with irrigation.
Irrigation substantially increased AerMN rates in our soils, consistent with others (Paul et al. 2003; Barakat et al. 2016; Feyissa et al. 2021) who also found that soil moisture and precipitation rates were important determining factors in N mineralisation. This is particularly interesting given that there was no significant decrease in mineral N concentrations under irrigation, suggesting a shift towards more aerobically degradable forms of nitrogen under irrigation. Increasing mineralisation rates can have important implications for plant production and environmental impacts with respect to water and air quality. Pakrou and Dillon (2000) reported that increased mineralisation rates contributed to greater N leaching and gaseous losses despite greater plant N uptake in irrigated dairy pastures.
There was no difference in basal respiration in response to irrigation, which supports the findings of Moinet et al. (2016) and Condron et al. (2014) in New Zealand soils, who also found no dependence on soil water regime for heterotrophic respiration. They concluded that respiration is controlled by accessibility of substrate rather than water content. Soil respiration has been shown to increase with irrigation or along increasing rainfall gradients in semi-arid areas (e.g. Miao et al. 2017; Throop et al. 2020), and these soils indicated a non-linear respiration response to soil moisture whereby drought had a greater effect than irrigation (Miao et al. 2017). This suggests that impacts of irrigation on respiration are likely to be less in soils with higher natural rainfall rates (Paul et al. 2003). Hawkes et al. (2020) found that legacy effects afforded soil communities a high resistance to changes in rainfall which lasted over 4.5 years and was reflected in no differences in soil respiration, but they also found no changes in community structure, which is contrary to our findings. Although basal respiration did not increase under irrigation, SIR was enhanced, inferring a shift either towards more degradable inputs and/or more rapidly responsive organisms in irrigated soils as also indicated by changes in the microbial community in these soils.
The work of Williams and Rice (2007) suggests that the time under irrigation is an important factor in the response of microbial communities to irrigation. The length of time under irrigation in our soils ranged between 3 and 33 years when sampled, and we tested this theory but found no evidence to support increasing effects of irrigation over time. Ma et al. (2016) found that changes in microbial parameters increased after 2 years of irrigation in arid soils, and it is possible that changes in community structure or function may be more strongly affected immediately after irrigation begins and may not have been captured within the time range we tested. Paul et al. (2003) suggested that the responsiveness of microbial communities to irrigation lessens with increasing annual rainfall rates. However, we found no significant correlations between annual rainfall and changes in microbial parameters under irrigation.
Conclusions
In a series of paired irrigated and non-irrigated pasture soils in New Zealand, we found that long-term irrigation shifted the microbial community to one indicative of faster C and N cycling with decreases in both the gram positive:gram negative ratio and F:B ratio. This has important implications for intensive grazing in New Zealand. Enhanced degradation of new and existing SOM pools may lead to decreased C and N stocks, which is supported by the conclusions of Mudge et al. (2017) who found that New Zealand soils have a propensity to lose SOM under irrigation. If a shift to a faster C cycling system under irrigation facilitates decreasing SOM, then soil ecosystem services, such as provision of nutrients to plants and C storage, will be moderated and may ultimately led to decreased agricultural production.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by the Sustainable Land Use Research Initiative, Manaaki Whenua – Landcare Research, and the Ministry for Business, Innovation and Employment (MBIE Endeavour funded project C09X1613).
Acknowledgements
The authors thank Scott Fraser and Trevor Knight for sampling North and South Island soils respectively, and the Environmental Chemistry Laboratory, David Hunter, and Alexandra McGill for undertaking sample analysis. Further, we thank Loius Schipper and the anonymous reviewers for their contributions to this manuscript.
References
Araya JN, Gowing DJ, Dise N (2013) Does soil nitrogen availability mediate the response of grassland composition to water regime? Journal of Vegetation Science 24, 506–517.| Does soil nitrogen availability mediate the response of grassland composition to water regime?Crossref | GoogleScholarGoogle Scholar |
Barakat M, Cheviron B, Angulo-Jaramillo R (2016) Influence of the irrigation technique and strategies on the nitrogen cycle and budget: a review. Agricultural Water Management 178, 225–238.
| Influence of the irrigation technique and strategies on the nitrogen cycle and budget: a review.Crossref | GoogleScholarGoogle Scholar |
Bardgett RD, Hobbs PJ, Frostegård Å (1996) Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biology and Fertility of Soils 22, 261–264.
| Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland.Crossref | GoogleScholarGoogle Scholar |
Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microbial Pathogenesis 111, 458–467.
| Actinomycetes benefaction role in soil and plant health.Crossref | GoogleScholarGoogle Scholar | 28923606PubMed |
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917.
| A rapid method of total lipid extraction and purification.Crossref | GoogleScholarGoogle Scholar | 13671378PubMed |
Carmona CR, Clough TJ, McNally SR, Beare MH, Tregurtha CS, Hunt JE (2020) Seasonal irrigation affects the partitioning of new photosynthate carbon in soil. Soil Biology and Biochemistry 143, 107751
| Seasonal irrigation affects the partitioning of new photosynthate carbon in soil.Crossref | GoogleScholarGoogle Scholar |
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determines the priming effect: microbial N mining and stoichometric decomposition theories. Global Change Biology 20, 2350–2367.
| Soil C and N availability determines the priming effect: microbial N mining and stoichometric decomposition theories.Crossref | GoogleScholarGoogle Scholar |
Condron LM, Hopkins DW, Gregorich EG, Black A, Wakelin SA (2014) Long-term irrigation effects on soil organic matter under temperate grazed pasture. European Journal of Soil Science 65, 741–750.
| Long-term irrigation effects on soil organic matter under temperate grazed pasture.Crossref | GoogleScholarGoogle Scholar |
Corong E, Hensen M, Journeaux P (2014) Value of irrigation in New Zealand: an economy-wide assessment. NZ Institute of Economic Research Ltd and AgFirst Consultants Ltd, Wellington, NZ.
de Vries FT, Liiri ME, Bjørnlund L, Bowker MA, Christensen S, Setälä HM, Bardgett RD (2012) Land use alters the resistance and resilience of soil food webs to drought. Nature Climate Change 2, 276–280.
| Land use alters the resistance and resilience of soil food webs to drought.Crossref | GoogleScholarGoogle Scholar |
de Vries FT, Shade A (2013) Controls on soil microbial community stability under climate change. Frontiers in Microbiology 4,
| Controls on soil microbial community stability under climate change.Crossref | GoogleScholarGoogle Scholar | 24032030PubMed |
de Vries FT, van Groenigen JW, Hoffland E, Bloem J (2011) Nitrogen losses from two grassland soils with different fungal biomass. Soil Biology and Biochemistry 43, 997–1005.
| Nitrogen losses from two grassland soils with different fungal biomass.Crossref | GoogleScholarGoogle Scholar |
FAO (2016) Aquastat website. Available at http://www.fao.org/nr/water/aquastat/didyouknow/index3.stm [Accessed 3 February 2017]
Feyissa A, Yang F, Wu J, Chen Q, Zhang D, Cheng X (2021) Soil nitrogen dynamics at a regional scale along a precipitation gradient in secondary grassland of China. Science of The Total Environment 781, 146736
| Soil nitrogen dynamics at a regional scale along a precipitation gradient in secondary grassland of China.Crossref | GoogleScholarGoogle Scholar |
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364.
| Toward an ecological classification of soil bacteria.Crossref | GoogleScholarGoogle Scholar | 17601128PubMed |
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biology and Biochemistry 35, 837–843.
| The priming effect of organic matter: a question of microbial competition?Crossref | GoogleScholarGoogle Scholar |
Harris R (1981) Effect of water potential on microbial growth and activity in soils. In ‘Water potential relations on soil microbiology’. (Eds JF Parr, WR Gardner, LF Elliott) pp. 23–96. (Soil Science Society of America: Madison, WI)
Hawkes CV, Shinada M, Kivlin SN (2020) Historical climate legacies on soil respiration persist despite extreme changes in rainfall. Soil Biology and Biochemistry 143, 107752
| Historical climate legacies on soil respiration persist despite extreme changes in rainfall.Crossref | GoogleScholarGoogle Scholar |
Kaur A, Chaudhary A, Kaur A, Choudhary R, Kaushik R (2005) Phospholipid fatty acid – a bioindicator of environment monitoring and assessment in soil ecosystems. Current Science 89, 1103–1112.
Kelliher FM, Condron LM, Cook FJ, Black A (2012) Sixty years of seasonal irrigation affects carbon storage in soils beneath pasture grazed by sheep. Agriculture, Ecosystems & Environment 148, 29–36.
| Sixty years of seasonal irrigation affects carbon storage in soils beneath pasture grazed by sheep.Crossref | GoogleScholarGoogle Scholar |
Kenngott KGJ, Riess K, Muñoz K, Schaumann GE, Buhk C, Diehl D (2021) Flood pulse irrigation of meadows shapes soil chemical and microbial parameters more than mineral fertilization. Soil Systems 5, 24
| Flood pulse irrigation of meadows shapes soil chemical and microbial parameters more than mineral fertilization.Crossref | GoogleScholarGoogle Scholar |
Klappenbach JA, Dunbar JM, Schmidt TM (2000) rRNA operon copy number reflects ecological strategies of bacteria. Applied and Environmental Microbiology 66, 1328–1333.
| rRNA operon copy number reflects ecological strategies of bacteria.Crossref | GoogleScholarGoogle Scholar | 10742207PubMed |
Knapp AK, Briggs JM, Koelliker JK (2001) Frequency and extent of water limitation to primary production in a mesic temperate grassland. Ecosystems 4, 19–28.
| Frequency and extent of water limitation to primary production in a mesic temperate grassland.Crossref | GoogleScholarGoogle Scholar |
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biology and Biochemistry 32, 1485–1498.
| Review of mechanisms and quantification of priming effects.Crossref | GoogleScholarGoogle Scholar |
Lin D, McCulley RL, Nelson JA, Jacobsen KL, Zhang D (2020) Time in pasture rotation alters soil microbial community composition and function and increases carbon sequestration potential in a temperate agroecosystem. Science of the Total Environment 698, 134233
| Time in pasture rotation alters soil microbial community composition and function and increases carbon sequestration potential in a temperate agroecosystem.Crossref | GoogleScholarGoogle Scholar |
Ma H-K, Bai G-Y, Sun Y, Kostenko O, Zhu X, Lin S, Ruan W-B, Zhao N-X, Bezemer TM (2016) Opposing effects of nitrogen and water addition on soil bacterial and fungal communities in the Inner Mongolia steppe: a field experiment. Applied Soil Ecology 108, 128–135.
| Opposing effects of nitrogen and water addition on soil bacterial and fungal communities in the Inner Mongolia steppe: a field experiment.Crossref | GoogleScholarGoogle Scholar |
Miao Y, Han H, Du Y, Zhang Q, Jiang L, Hui D, Wan S (2017) Nonlinear responses of soil respiration to precipitation changes in a semiarid temperate steppe. Scientific Reports 7, 45782
| Nonlinear responses of soil respiration to precipitation changes in a semiarid temperate steppe.Crossref | GoogleScholarGoogle Scholar | 28361982PubMed |
Moinet GYK, Cieraad E, Hunt JE, Fraser A, Turnbull MH, Whitehead D (2016) Soil heterotrophic respiration is insensitive to changes in soil water content but related to microbial access to organic matter. Geoderma 274, 68–78.
| Soil heterotrophic respiration is insensitive to changes in soil water content but related to microbial access to organic matter.Crossref | GoogleScholarGoogle Scholar |
Moreno G, Hernández-Estebar A, Rolo V, Igual JM (2021) The enduring effects of sowing legume-rich mixtures on the soil microbial community and soil carbon in semi-arid wood pastures. Plant and Soil 465, 563–582.
Mudge PL, Kelliher FM, Knight TL, O’Connell D, Fraser S, Schpper LA (2017) Irrigated grazed pasture decreases soil carbon and nitrogen stocks. Global Change Biology 23, 945–954.
| Irrigated grazed pasture decreases soil carbon and nitrogen stocks.Crossref | GoogleScholarGoogle Scholar | 27483409PubMed |
Orwin KH, Dickie IA, Holdaway R, Wood JR (2018) A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biology and Biochemistry 117, 27–35.
| A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions.Crossref | GoogleScholarGoogle Scholar |
Orwin KH, Dickie IA, Wood JR, Bonner KI, Holdaway RJ (2016) Soil microbial community structure explains the resistance of respiration to a dry-rewet cycle, but not soil functioning under static conditions. Functional Ecology 30, 1430–1439.
| Soil microbial community structure explains the resistance of respiration to a dry-rewet cycle, but not soil functioning under static conditions.Crossref | GoogleScholarGoogle Scholar |
Pakrou N, Dillon P (2000) Key processes of the nitrogen cycle in an irrigated and a non-irrigated pasture. Plant and Soil 224, 231–250.
| Key processes of the nitrogen cycle in an irrigated and a non-irrigated pasture.Crossref | GoogleScholarGoogle Scholar |
Parfitt RL, Yeates GW, Ross DJ, Mackay AD, Budding PJ (2005) Relationships between soil biota, nitrogen and phosphorus availability and pasture growth under organic, and conventional management. Applied Soil Ecology 28, 1–13.
| Relationships between soil biota, nitrogen and phosphorus availability and pasture growth under organic, and conventional management.Crossref | GoogleScholarGoogle Scholar |
Paul KI, Polglase PJ, O’Connell AM, Carlyle JC, Smethurst PJ, Kanna PK (2003) Defining the relation between soil water content and net nitrogen mineralization. European Journal of Soil Science 54, 39–48.
| Defining the relation between soil water content and net nitrogen mineralization.Crossref | GoogleScholarGoogle Scholar |
Schipper LA, Dodd MB, Pronger J, Mudge PL, Upsell M, Moss RA (2012) Decadal changes in soil carbon and nitrogen under a range of irrigation and phosphorus fertilizer treatments. Soil Science Society of America Journal 77, 246–256.
| Decadal changes in soil carbon and nitrogen under a range of irrigation and phosphorus fertilizer treatments.Crossref | GoogleScholarGoogle Scholar |
Schipper LA, Petrie OJ, O’Neill TA, Mudge PL, Liáng LL, Robinson JM, Arcus VL (2019) Shifts in temperature response of soil respiration between adjacent irrigated and non-irrigated grazed pastures. Agriculture, Ecosystems & Environment 285, 106620
| Shifts in temperature response of soil respiration between adjacent irrigated and non-irrigated grazed pastures.Crossref | GoogleScholarGoogle Scholar |
Siebielec S, Siebielec G, Klimkowicz-Pawlas A, Gałązka A, Grządziel J, Stuczyński T (2020) Impact of water stress on microbial community and activity in sandy and loamy soils. Agronomy 10, 1429
| Impact of water stress on microbial community and activity in sandy and loamy soils.Crossref | GoogleScholarGoogle Scholar |
Thenkabail PS, Biradar CM, Turral H, Noojipady P, Li YJ, Vinthanage J, Dheerarath V, Velpuri M, Schull M, Cai XL, Dutta R (2006) ‘An irrigated area map of the world (1999) derived from remote sensing’. (International Water Management Institute: Colombo, Sir Lanka)
Throop HL, Seely MK, Marufu VJ Throop HL, Seely MK, Marufu VJ (2020) Multiple scales of spatial heterogeneity control soil respiration responses to precipitation across a dryland rainfall gradient. Plant and Soil 453, 423–443.
| Multiple scales of spatial heterogeneity control soil respiration responses to precipitation across a dryland rainfall gradient.Crossref | GoogleScholarGoogle Scholar |
Treseder KK, Kivlin SN, Hawkes CV (2011) Evolutionary trade-offs among decomposers determine responses to nitrogen enrichment. Ecology Letters 14, 933–938.
| Evolutionary trade-offs among decomposers determine responses to nitrogen enrichment.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, van Koten C, O’Callaghan M, Brown M (2013) Physicochemical properties of 50 New Zealand pasture soils: a starting point for assessing and managing soil microbial resources. New Zealand Journal of Agricultural Research 56, 248–260.
| Physicochemical properties of 50 New Zealand pasture soils: a starting point for assessing and managing soil microbial resources.Crossref | GoogleScholarGoogle Scholar |
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138, 275–284.
| Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities.Crossref | GoogleScholarGoogle Scholar |
West AW, Sparling GP (1986) Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. Journal of Microbiological Methods 5, 177–189.
| Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents.Crossref | GoogleScholarGoogle Scholar |
Whitaker J, Ostle N, Nottingham AT, Ccahuana A, Salinas N, Bardgett RD, Meir P, McNamas NP (2014) Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. Journal of Ecology 102, 1058–1071.
| Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient.Crossref | GoogleScholarGoogle Scholar |
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40, 51–62.
| Determination of the sedimentary microbial biomass by extractable lipid phosphate.Crossref | GoogleScholarGoogle Scholar |
Williams MA, Rice CW (2007) Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community. Applied Soil Ecology 35, 535–545.
| Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community.Crossref | GoogleScholarGoogle Scholar |
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biology and Fertility of Soils 29, 111–129.
| Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review.Crossref | GoogleScholarGoogle Scholar |