Soil biodiversity and biogeochemical function in managed ecosystems
X. D. Chen A F G , K. E. Dunfield B , T. D. Fraser C , S. A. Wakelin D , A. E. Richardson E and L. M. Condron
E and L. M. Condron  F H
F H
A Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, 10016, China.
B School of Environmental Sciences, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G 2W11, Canada.
C Charlottetown Research and Development Centre, Agriculture and Agri-Food Canada, 440 University Avenue, Charlottetown, Prince Edward Island C1A 4N6, Canada.
D Scion, PO Box 29237, Riccarton, Christchurch 8440, New Zealand.
E CSIRO Agriculture and Food, PO Box 1700, Canberra, ACT 2601, Australia.
F Agriculture and Life Sciences, PO Box 85084, Lincoln University, Christchurch 7647, New Zealand.
G Institute of Environment, Resource, Soil and Fertilizer, Zhejiang Academy of Agricultural Sciences, Hangzhou, 310021, China.
H Corresponding author. Email: leo.condron@lincoln.ac.nz
Soil Research 58(1) 1-20 https://doi.org/10.1071/SR19067
Submitted: 21 March 2019 Accepted: 27 September 2019 Published: 31 October 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
A complex combination of environmental, biological, chemical, and physical properties and processes determine soil biodiversity and its relationship to biogeochemical functions and ecosystem services. Vegetation, land-use, and land management, in turn, influence diversity and function in the soil ecosystem. The objective of this review was to assess how different land-use systems (crop production, animal production, and planted forest) affect soil biodiversity, and how consequent changes in soil biodiversity influence energy (carbon) and nutrient dynamics. Deficiencies in understanding relationships between soil biodiversity and biogeochemical function in managed ecosystems are highlighted, along with the need to investigate how diversity influences specific processes across different functional groups and trophic levels. The continued development and application of molecular techniques and data informatics with descriptive approaches will contribute to advancing our understanding of soil biodiversity and function in managed agricultural and forest ecosystems.
Additional keywords: animal production, crop production, planted forest.
Introduction
Soils have a key role in food security by producing 95% of the global food supply (FAO 2015). As the global population rises, there is mounting pressure on land resources to provide food and fibre, either through more extensive (expanding the land-base) or intensive agriculture, horticulture, and forestry. However, productive land per person is predicted to decline to one-fourth of the level of the 1960s unless new practices are adopted (FAO 2015). In a time when we are seeking to optimise productivity in managed ecosystems in order to meet the demands of a global population for food and fibre, we are also experiencing climate variability and increasing public awareness of the environmental and socio-cultural impacts of land-use change and intensification at local, catchment, regional, to global scales. In order to understand and manage opportunities and trade-offs between managed and natural ecosystems, we need to fully appreciate the drivers of biogeochemical cycles that support soil ecosystem services, such as nutrient cycling, greenhouse gas emissions, regulation of hydraulic processes, soil stability, security of production, and sustaining biodiversity.
The aesthetic and functional benefits of biodiversity derive from the presence of many different plant and animal species in an environment. Soil biodiversity similarly encompasses a vast variety and numbers of organisms that spend all or most of their life cycle in the soil. Biodiversity ‘hotspots’ such as tropical rainforests support an abundance of plant and animal diversity, which includes up to 80 000 plant, 50 000 insect, 1500 bird, and 2000 mammal and amphibian described species (Myers et al. 2000). The corresponding level of biodiversity present in the underlying soil environment is much greater, yet is clearly less understood. Described species of soil bacteria and fungi exceed 15 000 and 97 000, respectively, compared with 20 000–25 000 species of nematodes, 21 000 species of protists (protozoa, protophyta, and moulds), and 40 000 species of mites (Orgiazzi et al. 2016). However, the ‘true’ identity of much of the soil biota remains unknown, as those identified to date are estimated to represent only 1.5–6.5% of soil bacteria and fungi diversity, compared with 0.2–2.5%, 0.03–0.3%, and 55% for nematodes, protists, and mites, respectively (Orgiazzi et al. 2016). In addition to the high level of biodiversity found in soil, the numbers of individual species present and their combined mass is large. A single gram of soil typically contains up to 1 billion bacteria and 10 m of fungal hyphae, while the total topsoil biomass can be up to 1.5 kg per square metre or 15 tonnes per hectare (http://globalsoilweek.org/soilatlas-2015).
Given the extent of soil biodiversity, it is important to understand how this biodiversity relates to key functions that determine the ecosystem productivity such as organic matter and nutrient dynamics (Fitter et al. 2005; Bahram et al. 2018; Crowther et al. 2019), which in turn influence soil quality and health (Brussaard et al. 2004; Ferris and Tuomisto 2015). It has been proposed that the collective actions of soil biota drive aboveground biodiversity and productivity, which then determines overall ecosystem stability (Yang et al. 2018). In manipulated systems, high soil biodiversity has been shown to increase plant productivity by enhancing nutrient uptake and reducing nutrient loss (Wagg et al. 2014; Bender and Van der Heijden 2015). However, during long-term ecosystem development no relationship was observed between soil biodiversity and plant diversity, which were mainly driven by changes in plant cover and soil pH (Delgado-Baquerizo et al. 2019).
The biodiversity and function of biota in soil are primarily influenced through a combination of environmental (temperature and rainfall), biological (plant species, growth-turnover, root exudates, and herbivory) and physico-chemical (soil structure, nutrient availability, soil pH, and mineralogy) properties and processes (Bahram et al. 2018; Delgado-Baquerizo et al. 2019). Changes in the overall expression of these variables and ecosystem disturbance shape the habitat of biodiversity present and the biophysical space and conditions in which soil functions can be supported. All ecosystems are subject to spatial and temporal disturbance but the nature, complexity, and severity of disturbance will differ in managed ecosystems (agriculture, horticulture, and tree plantations) compared with native forest and unmanaged grassland. Increasing demands on land for agricultural purposes has resulted in continued large-scale conversion of natural ecosystems to managed ecosystems (MEA 2005; Jackson et al. 2009). Anthropogenic activities, intensification, and land-use change can significantly influence soil biodiversity with consequent impacts on functional processes such as nutrient bioavailability and cycling (Mäder et al. 2002; de Vries et al. 2013; Orgiazzi et al. 2016).
In this review we specifically focus on a range of different managed ecosystems: crop production, animal production, and planted forest systems. The main objective was to assess how various land-use and management systems and practices influence soil biodiversity, and how consequent changes in soil biodiversity may affect soil carbon (C) flows and nutrient dynamics, and the provision of other key ecosystem services in the respective systems.
Crop production systems
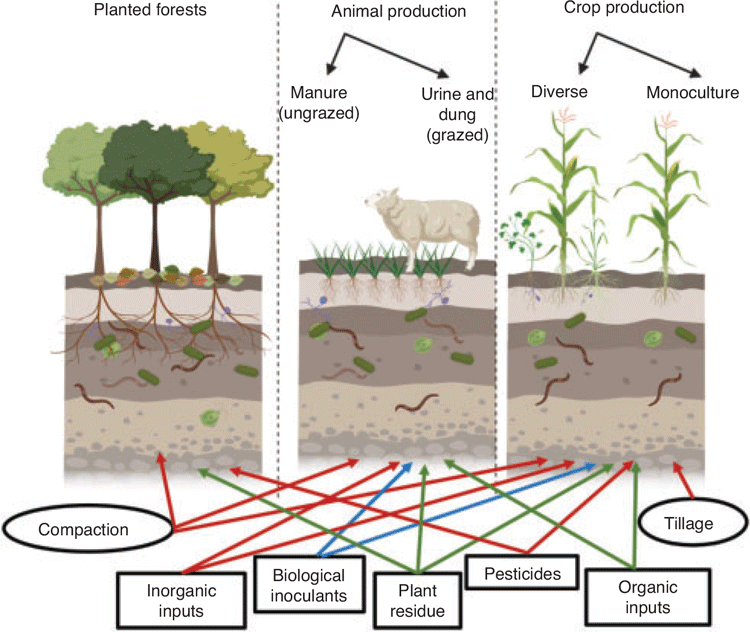
Soil organisms play a vital role in many important ecosystem functions that influence soil and crop productivity and system sustainability. Microbial communities are the main drivers of biogeochemical cycles, therefore changes in their abundance, activity, and community structure may affect nutrient flows in soil (Schmidt et al. 2011; Markussen et al. 2018). This includes direct processes such as crop decomposition, nutrient mineralisation and mobilisation, denitrification, and nitrogen (N) fixation, in addition to cascades of impacts (C and nutrient flow) throughout the soil food web (Fig. 1).
|
Various studies have indicated that the taxonomic diversity of soil microbial communities is intrinsically linked to the quality of crop residue returned to soil (Pascault et al. 2013). Both land-use change and intensification have similarly been shown to have impacts on belowground organisms and associated functions (Postma-Blaauw et al. 2010; Tsiafouli et al. 2015). Under intensive land-use, Tsiafouli et al. (2015) reported simplified soil food web diversity, with a shift towards smaller organisms and potential implications for function, where the effects of agricultural intensification were more severe for larger soil biota (lower taxonomic richness) than smaller organisms (Postma-Blaauw et al. 2010).
Agro-ecosystem resilience refers to a cropping system’s ability to maintain yields when challenged by environmental stress (Gaudin et al. 2015). It has been suggested that complex agroecosystems that closely mimic diverse natural ecosystems are more resilient and environmentally sustainable (Lin 2011). Systems with low biodiversity are often highly productive, but have increased vulnerability to system perturbation, including effects of climate change and extreme weather events (Gaudin et al. 2015). Soil ecosystem resilience is the capacity to maintain soil function and its resistance and recovery from disturbance and, as such, plays a key role in agro-ecosystem stability (Peterson et al. 2018). Resilience in soil ecosystems is commonly linked to the microbial biodiversity present, particularly within and across key functional groups (Grandy et al. 2012). Poor land-management practices that result in declines in soil biodiversity in turn are widely suggested to impede delivery of soil ecosystem services, such as C sequestration, climate and gas regulation, and nutrient cycling (de Vries et al. 2012).
Changes in biogeochemical pathways, soil structure, and microbial ecology resulting from agricultural management practices may subsequently impact plant growth, soil health, and ecosystem sustainability. Agricultural soils provide a unique system because they can be managed to facilitate the conservation of soil biodiversity and the functions and services they provide. Some specific examples of the impacts of common agriculture practices (e.g. tillage and crop rotations) on soil biota are discussed below, along with how sustainable farming practices aim to promote diverse and active soil biological communities in order to enhance soil fertility and maintain soil C while sustaining crop yields (Hartman et al. 2018; Knapp and van der Heijden 2018). Although other practices may impact soil biology and functions (e.g. chemical fumigation, plastic mulching, and biological amendments), the practices of tillage, nutrient amendment, and crop rotations are commonly the foundation of global agroecosystems – with the majority of studies focusing on these practices.
Tillage
Tillage involves the mechanical disruption of soil for preparation of the seed bed, soil aeration, and weed control (Lal et al. 2007). However, continual disturbance of agricultural soil has been shown to be a major contributor to soil degradation by impacting soil structure (Young and Ritz 2000), increasing erosion, diminishing soil organic matter (SOM) and nutrients, and changing water holding capacity (Lal 1993; Lal et al. 2007). The effects of tillage on soil biota have been reported extensively, with several reviews outlining the effects on soil ecology (Kladivko 2001; Roger-Estrade et al. 2010), functional diversity of soil biota (van Capelle et al. 2012), microbial habitat space and function (Young and Ritz 2000), and the abundance and biomass of earthworms (Briones and Schmidt 2017).
The effects of tillage on soil biota are both direct (death, injury, and exposure to predation) and indirect (disruption of habitat) (Kladivko 2001; Roger-Estrade et al. 2010), with different responses reported for different groups. A majority of studies demonstrate higher soil microbial biomass under no-till versus conventional tillage systems (Wardle 1995; D’Hose et al. 2018). Soils under reduced or no-till systems also exhibit altered microbial communities in comparison to conventional tillage (Helgason et al. 2009; Kuntz et al. 2013; Smith et al. 2016). These shifts in microbial diversity can change the functional capacity of the communities (Mangalassery et al. 2015; Nivelle et al. 2016). For example, Smith et al. (2010) showed that tillage changed microbial community structure and diversity of nitrifier and denitrifier populations. The results indicated that under no-till management practices there was decreased seasonal nitrous oxide (N2O) emissions from soil. The diversity of the arbuscular mycorrhizal community was similarly altered according to level of soil disturbance (Mirás-Avalos et al. 2011). A higher number of functional gene sequences related to N-cycling processes was observed in a maize (Zea mays)–soybean (Glycine max) rotation under no-till compared with conventional tillage, which was attributed to changes in the nutrient resources between the systems (Smith et al. 2016). Due to lack of soil disturbance, long-term no-till management also results in stratification of organic C and N (Edwards and Cresser 1992; Zibilske et al. 2002; Gál et al. 2007), which can shift microbial community composition. In a maize-based rotation, after 30 years under no-till versus conventional till (mouldboard plough) management, significant effects of depth on bacterial ammonia-oxidiser abundance and gene expression were observed, whereby no-till supported higher abundances of total archaea and ammonia-oxidising archaea than the conventional till system across the growing season (Munroe et al. 2016).
In general, practices that contribute to greater topsoil organic matter have a positive effect on soil biodiversity. Conservation tillage is defined by having more than 30% of the crop residue remaining on the soil surface (Triplett and Dick 2008). This results in increased potential for C and nutrients to be returned to the upper horizon only, with subsequent influence on the detritus food web (Hendrix et al. 1986). In conjunction with this, the abundances of mites, collembola, ground beetles, spiders, and earthworms all showed significant increase. The nematode communities were also influenced by tillage treatment, with an increase in fungivores and herbivores but a decrease in bacterivores (Hendrix et al. 1986). Other studies also found positive effects of no-till practices on earthworm populations (Jordan et al. 1997; Briones and Schmidt 2017) whereas, in contrast, combined effects of chemical herbicides and pesticides used in reduced till systems may negate some of the observed positive effects (Bai et al. 2018). Positive effects of reduced tillage on bacterial, fungal, nematode, collembolan, and earthworm communities have however been widely reported (Fu et al. 2000; Sánchez-Moreno et al. 2006; Zheng et al. 2008; van Capelle et al. 2012; Kuntz et al. 2013; D’Hose et al. 2018). By contrast, enchytraeid worms have shown to have higher numbers under conventional tillage (House and Parmelee 1985; Hendrix et al. 1986; Wardle 1995). Meta-analysis by Spurgeon et al. (2013) showed a consistent trend of increased fungal and earthworm abundance and community complexity in response to conversion to a lower intensity system, such as in the case of implementing reduced tillage management. The combination of non-inversion tillage and organic amendments resulted in increases in abundance of earthworms, abundance of bacterivorous nematodes, bacterial biomass, and microbial biomass C when assessed over 60 European multi-year field trials (D’Hose et al. 2018).
Despite the potential negative effects of tillage on SOM and biological communities, it continues to be an important practice in many agricultural systems for seed bed preparation, weed control, and to suppress soil-borne diseases (Hobbs et al. 2008). Response of soil biota to tillage may be confounded by other management decisions including crop rotation. In a 12-year study of intensive cropping in Mexico, nematode numbers increased in maize under no-tillage but there was no effect of tillage in the wheat phase (Govaerts et al. 2007). When no-till was combined with continuous cropping, Sánchez-Moreno et al. (2006) reported an increase in fungal and plant-parasitic nematodes. When practiced alone, no-till resulted in a decrease in bacterial, fungal, and plant feeders, while predatory nematodes were more affected by the crop rotation than tillage. Crop rotation, tillage, and residue management can result in changes to the resource base (especially C flow into soil), thereby initiating important multi-trophic effects where not all nematode functional groups or species react in the same way (Yeates et al. 1999). Collembola density and species richness were 3–4 times higher in systems implementing residue retention compared with conventional cultivation after four years of rotation with five different agricultural treatments in France (Coulibaly et al. 2017). Optimising tillage management should consider the entire soil system including soil C management; soil ecology and ecological models should reciprocally include soil management parameters (Roger-Estrade et al. 2010).
Crop rotation
Crop rotation is extensively used to suppress disease caused by pests and pathogens and to maintain soil fertility, especially where legumes are integrative to the rotation (Liebman and Davis 2000). Rotations that are more diverse have generally been found to increase total yield and yield stability across a wide range of soil types and climatic conditions (Raimbault and Vyn 1991; Légère et al. 2011; Munkholm et al. 2013; Gaudin et al. 2015). Soils under crop rotation tend to contain higher organic matter, have increased structural stability, and show increased quantities of microbial biomass and activities (Munkholm et al. 2013), along with enhanced soil enzyme activities (Dick 1992). Belowground communities are tightly linked to aboveground communities through trophic interactions and plant–soil feedbacks (Fig. 1). In agroecosystems where single plant species are commonly grown over large spatial areas, the crop rotation sequence and length can have an important influence on soil biodiversity.
The rhizosphere is the plant–soil interface, where the diversity and activity of the microbial community are strongly influenced by the plant through root exudates and specific chemical signalling (Raaijmakers et al. 2009; Philippot et al. 2013; Lareen et al. 2016). Up to 40% of total plant C is typically allocated belowground, with some 5–20% of the photosynthetic C being reported to be returned directly to soil through either root (rhizo) deposition or by release of root exudates (Jones et al. 2009). As a result, the rhizosphere supports a marked proliferation and enrichment of specific microorganisms (Jones et al. 2009). Beneficial microorganisms associated with plant roots can play an important role in nutrient uptake and plant growth (Gaiero et al. 2013), disease suppression (Peralta et al. 2018), reducing damage from pests (Elhady et al. 2018), and resilience during periods of stress (Gaudin et al. 2015). The advent of molecular techniques has facilitated a more comprehensive investigation of biodiversity and function at the root surface. Recent focus has been directed on factors that mediate the composition of the rhizosphere microbiome, realising that changing the biodiversity of the community associated with the roots of plants may have beneficial effects on plant growth and health (Berg and Smalla 2009; Berendsen et al. 2012; Quiza et al. 2015; Bender et al. 2016; Dessaux et al. 2016; Wallenstein 2017).
Although intensively managed systems are often reported to have less diversity than natural systems (Tsiafouli et al. 2015), the direct link to specific functions and plant growth can be difficult to isolate. The concept of ‘microbiome engineering’ attempts to use knowledge gained from biodiversity studies and potential for promoting beneficial communities in the rhizosphere to increase yields by promoting plant and soil health. Efforts to consolidate global datasets on soil communities combined with soil chemical data have highlighted the importance of soil organisms and their influence on biogeochemistry at a global scale (Crowther et al. 2019), where trends have been more difficult to confirm at local scales. Understanding these processes are imperative in predicting and adapting to future scenarios under climate change.
The inclusion of legumes in rotation such as red clover (Trifolium pratense) contribute N to the soil system and to the N requirements of the succeeding crop (Gaudin et al. 2013), and also support diverse organisms and functions. Legumes form symbiotic associations with various genera of rhizobia bacteria, enabling them to fix atmospheric N, thereby reducing N fertiliser requirements when incorporated in a crop rotation. There is strong evidence that pea (Pisum sativum) hosts a unique microbial consortia in general in addition to rhizobia alone (Turner et al. 2013). Although the rhizospheres of pea, oat (Avena sativa), and wheat all showed high abundances of nematodes and bacterivorous protozoa, the pea rhizosphere contained a higher proportion of fungi to bacteria (Turner et al. 2013). The influence of legumes on soil biology may be dependent on the specific plant or cultivar; however, increased frequency of legumes (pea, lentil (Lens culinaris), and chickpea (Cicer arietinum)) in a wheat-based cropping system has also been shown to shift bacterial diversity and function in the rhizosphere of the subsequent wheat crop, where the response was partially attributed to biological N-fixation in the legume phase of the rotation (Hamel et al. 2018). Including legumes in crop rotation influences other N-cycling functional genes. In a 30-year field study, a diversified maize-based rotation that included wheat and red clover in the rotation had higher bacterial ammonia-oxidiser abundance compared with a monoculture (Munroe et al. 2016). Distinct N-cycling bacterial communities were also associated with an alfalfa (Medicago sativa)-based hay crop versus annual crops, and this was associated with lower N2O emissions in the perennial system (Thompson et al. 2018). In addition to being important rotational crops, legumes can also be included in agricultural systems as cover crops.
A strategy to mimic natural ecosystems, by integrating cover crops or intercrops into annual cropping systems has been shown to ‘protect’ soil because it is never left fallow, and to diversify annual crop rotations (Scherr and McNeely 2008). Cover crop mixtures have the potential to contribute to sustainable agroecosystems by supporting aboveground and belowground diversity, building SOM, retaining nutrients, stabilising soil between crops, as well as influencing soil biota (Fageria et al. 2005; Snapp et al. 2005; Fernandez et al. 2016). Cover crop systems supply a variety of biomass inputs (crop residues and root exudates) providing a greater range of C substrates to support microbial diversity and growth. In a 15-year rotation study by Schmidt et al. (2018), cover crops were shown to increase the abundance of bacteria and shifted bacterial community composition to organisms with more diverse metabolic capacities and moderate rates of growth (Schmidt et al. 2018). A meta-analysis from 37 different sites showed significant increases in soil organic C when cover crops were included in the rotation over the control crop only system (Poeplau and Don 2015). Incorporation of mixed-species cover crops showed changes in abundance of several nematode taxa, supporting the notion that nematode communities can provide sensitive indicators of soil food web dynamics (DuPont et al. 2009). Higher abundances of facultative plant parasitic, bacterial feeding, and predatory nematodes have also been reported with inclusion of a legume cover crop in a maize rotation system, in addition to higher densities of termites, earthworms, millipedes, and centipedes (Blanchart et al. 2006).
Crop diversification may not translate into a more diverse soil ecosystem. For example, Peralta et al. (2018) reported a decrease in bacterial diversity in soils under a more diverse crop rotation when comparing from one to five species and a bare fallow treatment. However, disease suppression potential was highest in the rotation with the highest diversity and diminished in soils with no plants (Peralta et al. 2018). This highlights potential uncoupling between diversity and function, although the study did not include information on fungal communities or other groups of soil biota that may have benefited from a diversified rotation.
Nutrient inputs
Nutrient inputs to agriculture include mineral fertilisers, organic amendments (e.g. cover crops, animal manure, sludge, and various other waste products) and crop residues. Direct impacts of mineral fertiliser are spatially limited, but may strongly affect soil microbial biomass and microbial community composition and function, and abiotic properties such as pH (Ryan et al. 2009). The addition of mineral fertilisers to either crop or pasture systems can have significant effects on the structure of microbial communities. In the tillage experiment by Bissett et al. (2013) there was strong interaction between seasonal application of mineral N and tillage practice that was not manifest across all sampling times, indicating a strong responsiveness of the community and interaction with environment and management. Bacterial and fungal community responses to pasture management were investigated by Wakelin et al. (2009), who showed large effects in response to lime application and time of sampling, which also had significant and positive effects on key N-cycling genes including nifH and amoA gene abundance. Structure of microbial communities also responded to long-term fertilisation with phosphorus (P), whereby strong seasonal effects were similarly evident, with fungal communities in particular being most responsive to levels of soil P fertility in terms of both community structure and richness. A 28-year field experiment under continuous maize, comparing no nutrient inputs to annual inputs of mineral N, animal manure, or a combination, showed that mineral N without P inputs decreased organic P cycling and shifted microbial communities containing key P-cycling gene phoD (Chen et al. 2019). Organic amendments generally have greater impact on soil microbial biomass, activity, and diversity, and can have disease suppression effects (Hallmann et al. 1999; Bailey and Lazarovits 2003; Fu et al. 2017), depending on the biological properties of the added material (Bonanomi et al. 2010). Type and rate of organic amendment can also impact N-cycling communities, whereby bacterial and archaeal ammonia-oxidising communities and abundance differed in soils that received straw versus peat amendments (Wessén et al. 2010). Animal manures similarly have significant impacts on community structure and function associated with N cycling, which is discussed below in relation to animal production systems.
Agronomic management systems designed to increase organic matter, including C-rich fertilisers and organic amendments, may diversify nematode populations and other soil biota, thus improving the resilience of arable cropping systems. For example, the application of organic fertiliser resulted in fundamentally different protist community structure and function compared with mineral fertiliser, with an increase in bacterivorous and omnivorous protists, and a decrease in plant pathogens (Xiong et al. 2018). Soil microbial biomass increased in treatments amended with organic fertiliser for 27 years (cattle manure, biodynamic), where no negative effects of N fertiliser application were observed on collembola density and an increase in species richness occurred in response to mineral fertiliser application (Coulibaly et al. 2017). A meta-analysis by Liu et al. (2016) examined crop systems with different fertiliser regimes (unfertilised, inorganic N only, inorganic N, P, potassium (K), organic fertiliser, organic and inorganic N, P, and K), and where organic fertilisers were categorised as animal manure, cover crop, straw compost, straw, sludge (sewage or sugarcane), and waste products (food, paper, or bio-solids). In this analysis, high inorganic N resulted in a more simplified nematode community structure with higher abundance and richness in organic compared with inorganic fertiliser treatments. Bacterivores, fungivores, and omnivores showed the greatest response to C inputs, and N-rich animal manures appeared to control plant-parasitic nematodes (Liu et al. 2016).
Management practices in organic farming systems aim to tighten nutrient cycles, in which plant residues or manure from livestock are applied to land, along with greater use of perennial and leguminous plants. In addition, neither synthetic fertilisers nor agro-chemicals are applied (Lori et al. 2017). Bacteria and fungi play a key role in nutrient cycling in these systems through decomposition of organic matter, and transformation of important soil nutrients like N and P (Knapp and van der Heijden 2018). Kallenbach et al. (2015) used in situ 13C isotopic tracing to demonstrate increased microbial growth rates and higher microbial C use-efficiency in an organic verses a conventional system, which resulted in higher retention of C inputs and increased abundance of microbial-derived SOM (Kallenbach et al. 2015). A meta-analysis of 56 studies revealed that organic farming had a positive effect on total abundance and activity of soil microbial communities on a global scale, for example through intensified N mineralisation capacity, as indicated by greater dehydrogenase, protease, and urease activities in organic systems (Lori et al. 2017).
The Glenlea long-term crop rotation experiment (Winnipeg, Manitoba) is Canada’s longest running trial comparing organic and conventional systems. Higher alkaline phosphatase enzyme activity, higher abundance of bacteria containing the alkaline phosphatase (phoD) gene, and a shift in the composition of the active phoD-containing bacterial communities occurred in response to lower concentrations of labile P in the organically managed soils (Fraser et al. 2015a, 2015b). Similarly, the Swiss DOK experiment (established in 1978) has compared the long-term effects of organic and conventional management on ecosystem properties (Raupp et al. 2006). The organic system increased richness, decreased evenness, and shifted the structure of soil microbiota compared with conventionally managed soils using mineral fertilisation. This effect was largely attributed to the use and quality of the organic amendments (Hartmann et al. 2015). Microbial co-occurrence networks of bacterial and fungal rRNA gene sequences indicated the presence of unique microbial hubs in the organic versus the conventional systems (van der Heijden and Hartmann 2016).
Animal production systems
Animal production systems occupy and impact a significant proportion of agricultural land globally. Animals can be permanently or seasonally grazed on pasture, or housed indoors for various times at different levels of intensity. In either system, most of the organic matter and nutrients consumed by livestock are excreted in urine and dung and most often returned to soils, albeit disproportionally. Grazed animals excrete organic matter and nutrients directly onto the soil surface in randomly distributed discrete urine and dung deposits (Haynes and Williams 1993). However, excreta from housed animals is collected (often stored) and returned to soil as liquid slurries, bedding materials (straw and sawdust), or as manures or composts (farmyard manure and composted manure) (Sims and Maguire 2005; He et al. 2016). These represent direct and indirect pathways of organic matter and nutrient addition to soil, which influence the magnitude and trajectory of the impacts on soil biodiversity and function.
Urine and dung returned through grazing
Grazing animals excrete 75–95% of the N they consume in feed as urine and dung, resulting in patches of soil with extremely high N concentrations (Haynes and Williams 1993). The large amount of excreta N in relatively small patches usually exceeds the immediate plant requirements, so excreta patches are considered ‘hotspots’ for N transformation and loss. The transfer of N within and out of the soils requires interactions between various microorganisms that carry out nitrification (conversion of ammonia or ammonium to nitrate) and denitrification (reduction of nitrate to gaseous forms of N). Application of sheep and cattle urine has been shown to alter the microbial pathways for N2O emissions, whereby urine significantly promoted N2O production by denitrification and nitrifier denitrification in both field and incubation experiments (Mahmood and Prosser 2006; Di et al. 2009; Pan et al. 2018). Various studies have shown that urine application increased the abundance of ammonia-oxidising bacteria, amoA, and nirK genes, and changed community structure which coincided with production of N2O. In contrast, there was no consistent impact on community structure and abundance of N-cycling genes in ammonia-oxidising archaea, amoA and nirK genes, and other denitrification functional genes (nirS, nosZ, and rpoB) (Mahmood and Prosser 2006; Di et al. 2009, 2010; Orwin et al. 2009; O’Callaghan et al. 2010; Morales et al. 2015; Pan et al. 2018; Yao et al. 2018). Most studies have found that urine application had little impact on numbers or estimated biomass of bacteria and fungi based on different methods (Williams et al. 2000; Nunan et al. 2006; Singh et al. 2009). Moreover, community composition and traits of key species or groups and their relative abundance and complementarities have been shown to be influenced by urine patches in soil, rather than an overall change in species richness. By comparison, using high-throughput sequencing, Morales et al. (2015) recently found that urine application decreased bacteria richness, while the community structure was consistently stable.
Studies that have investigated the impact of dung on soil microorganisms and soil microflora are more limited. Dung is rich in organic C and N, and the deposition of dung on the soil surface provides a continuous supply of nutrients for microbial growth and metabolism (Cardenas et al. 2016). Most studies associated with dung patches have focused on greenhouse gas emission (e.g. N2O), while changes in microorganism community structure in relation to nutrient cycling have received less attention. In a typical steppe grassland, denitrification was responsible for most of the N2O emissions in dung-treated soils, with significant increase in the bacterial amoA gene abundance, but there was no difference in the archaeal amoA gene abundance (Pan et al. 2018). The impacts of such shifts in functional genes or community composition as a significant driver of soil processes and function in relation to N hotspots in dung and urine remain to be more fully investigated.
Soil fauna have also been found to have a consistent positive effect on litter decomposition including interaction with urine and dung. Species richness and community structure of soil fauna has in particular focused on interaction with nematodes and earthworms in grazed pasture ecosystems (Bardgett et al. 1998; Mikola et al. 2009; Villenave et al. 2011; Hu et al. 2015). For example, the abundance of fungivorous nematodes and Aporrectodea earthworms showed significant increases in grazed pasture, but decreased the abundance of detritivorous enchytraeids and Lumbricus earthworms (Mikola et al. 2009). Other studies have also found positive effects of urine or dung on nematode community structure and showed positive correlations with microbial C and N (Bardgett et al. 1998; Wang et al. 2006; Hu et al. 2015). However, the study of urine and dung patch impacts on other soil fauna diversity and community has received less attention largely because of difficulties in collection of soil samples from patches and methodological challenges concerning soil fauna distribution (André et al. 2002). Nonetheless diversity of other fauna such as springtails, nematodes, earthworms, and arthropods have been assessed (Waite et al. 2003; Hogg and Hebert 2004; Griffiths et al. 2006; Read et al. 2006; Bienert et al. 2012; Porco et al. 2013; Oliverio et al. 2018). However, the relationship between soil fauna diversity and functional interactions in grazed ecosystems remains poorly understood.
Animal manures
Animal excreta in the form of slurry, farmyard manure, and composted manure is widely used to add organic C and nutrients (e.g. N and P) to soil in both organic-based and conventional farming systems (Francioli et al. 2016). Various studies have shown that organic fertilisers stimulated bacterial and fungal biomass in soil, increased the abundance of organisms, and induced marked change in bacterial and fungal community structures (Birkhofer et al. 2008; Hartmann et al. 2015; Wang et al. 2015; Francioli et al. 2016; van der Bom et al. 2018), whereas other studies with cattle slurry have demonstrated little difference (De Goede et al. 2003; de Vries et al. 2006). This indicates that the impacts of manure-based inputs depend on multiple factors including form, addition rate, soil type, and environmental conditions (Bünemann et al. 2006). For example, farmyard manure increased bacterial diversity, and stimulated specific microbial groups known to prefer nutrient-rich environments (e.g. Firmicutes and Proteobacteria), which are also involved in the degradation of complex organic compounds (Francioli et al. 2016).
Microbial-based functions in soil have been shown to respond to organic fertiliser application. For example, repeated inputs of excreta and consequent increases in SOM have resulted in major change to N-cycling rates with enhanced nitrification and denitrification (Wang et al. 2014, 2015; Liu et al. 2018). In terms of nitrification, bacteria had a more significant role in ammonia oxidation following long-term slurry or composted manure application with greater ammonia or ammonium concentrations and potentially higher nitrification rates (Wang et al. 2014, 2015). In contrast, Zhou et al. (2015) observed that ammonia-oxidising archaea in a permanent grassland soil was increased by 44 years of cattle slurry amendment. This was also observed in response to long-term manure application to rice paddy soils (Liu et al. 2018), where both ammonia-oxidising archaea and nitrite-oxidising community structures were more sensitive to long-term manure application. This suggests that ammonia-oxidising archaea are better adapted to growth at low pH and low substrate availability.
Organic fertiliser application has been shown to influence the overall abundance and diversity patterns of a range of other N-cycling functional groups, including denitrifiers. For example, Pereg et al. (2018) reported that sheep manure application promoted denitrification in grapevine soils, which was accompanied with higher abundance of denitrifiers (based on nirK, nirS, and nosZ gene quantification), resulting in a potential reduction in N2O emissions. Higher abundance of denitrifiers in response to organic fertiliser application has been reported in other studies (Hai et al. 2009; Hallin et al. 2009; Clark et al. 2012). However, changes in community composition and measured abundance of genes only reflects the potential for enhanced function and without associated rate measurements does not necessarily translate to actual turnover of nutrients in soils. With the exception of phoD, few studies have investigated the effects of animal excreta on functional groups and genes involved in P cycling (Fraser et al. 2015b; Chen et al. 2017).
Shifts in soil fauna species due to animal excreta application to soil and resulting effects on decomposition and nutrient cycling are becoming widely recognised (Koch et al. 2013). Murchie et al. (2015) found that earthworm genera respond to cattle slurry in a successional pattern, with L. rubellus feeding on fresh slurry and Allolobophora chlorotica benefiting subsequently, resulting in enhanced decomposition. Several studies have shown that inputs of manure, slurry, and compost significantly affect earthworm biomass, numbers, and density (Whalen et al. 1998; Leroy et al. 2007, 2008; Birkhofer et al. 2008; van Eekeren et al. 2009; Koblenz et al. 2015; Guo et al. 2016; Zavattaro et al. 2017). Cattle slurry and farmyard manure application similarly shifted the structure of nematode populations with increased numbers of bacterivorous nematodes, but decreased numbers of plant-parasitic nematodes. The relative abundance of different species of collembola, however, was more negatively affected by application of cattle slurry. Similar increased biomass or numbers in response to manure applications have been observed for protozoa (Griffiths et al. 1998), nematodes (Opperman et al. 1993; Griffiths et al. 1998; De Goede et al. 2003; Birkhofer et al. 2008; Leroy et al. 2009; van Eekeren et al. 2009), and various arthropods (Pfiffner and Niggli 1996). Overall, the responses of soil fauna and community interactions in soil are complex and may be related to differences in excreta types, soil properties, and management systems. More studies focusing on interactions among diversity of soil fauna and nutrient cycling are needed to elucidate how inputs of animal excreta affect soil function.
Planted forest systems
Forested ecosystems cover 4 billion ha (Keenan et al. 2015), which is ~30% of the total ice-free land area, and are thus key regulators of global biogeochemical processes. Approximately 60% of terrestrial C is held in forests, with nearly half of that in forest ecosystem soils (Pan et al. 2011). In addition to nutrient cycling, forests support a broad range of other ecosystem services such as watershed protection, arresting soil erosion, and maintaining global biodiversity. Broad diversity in forest ecosystems include boreal, temperate, and tropical systems. Within each of these there is a continuum from primary through to modified natural, semi-natural, and plantations grown for productive or protective uses, through to ‘trees outside of forests’, such as those in urban environments (Evans 2009; FAO 2012). Planted (production) forests represent ~7% of the total forest area, which is increasing with greater demand for ecosystem services they provide and the associated opportunity for sequestration of C (Payn et al. 2015). A further important but underappreciated ecosystem service provided by plantation forestry is protection of native (sensu primary) forests from direct harvest of timber. Plantation forests therefore play a further role in supporting global biodiversity (Buongiorno and Zhu 2014). The majority of planted forests globally are represented by Pinus and Eucalyptus spp. (68% cover), with the remainder comprising acacia (6%), teak (5%), and various other soft- and hard-woods (Indufor 2012). Given this, much of the available literature on the biology and functioning of forest systems soils comes from planted Pinus and Eucalyptus forests.
Nutrient and energy flow
Unlike intensively managed agricultural systems, nutrient cycling within forest systems is relatively closed (Mahendrappa et al. 1986; Attiwill and Adams 1993). While the ‘closed and nested’ nature of forest nutrient cycling is generally beneficial to long-term sustainability of production, provision of exogenous nutrients are required where soil nutrient resources are poor, or where multiple forest rotations and extraction of system resources (wood and other materials) occur resulting in nutrient depletion (Akselsson et al. 2007; Zabowski et al. 2007; Smaill and Garrett 2016). However, these fertiliser inputs are generally highly targeted and limited in their use.
Central to recycling of nutrients within forest ecosystems is the return and recycling of plant-derived biomass through microbial and faunal decomposition (Gosz et al. 1973; Harmon et al. 1986). The rates and dynamics of decomposition are important as they drive energy flow within the ecosystem and regulate the release of nutrients (Odum et al. 1962; Harmon et al. 1986). As the demand for nutrients by trees varies greatly over time, the supply of energy (originally from plant photosynthate) within the ecosystem supports microbial and faunal function. This is both expressed belowground with continuous inputs from root exudation, root turnover, and mycorrhizal C allocations, and aboveground with large but episodic inputs from leaf-fall and biomass turnover, which includes pruning, thinning, and debris derived from harvesting operations. The SOM pool, and the living microbial biomass as the active component of this, comprise the dynamic interface that integrates organic inputs with nutrient inputs from belowground through the weathering of soil minerals. Collectively, this active pool can hold over 90% of the soils’ total N and sulfur (S), and 50% of the P across terrestrial biomes (Condron et al. 2010), and similarly within forest ecosystems provides a dynamic supply of nutrients.
The microbial decomposition of woody debris and leaf material in various forest systems is well described. Indeed, characterising the cascade of microorganisms from a phylogenetic and functional perspective (e.g. lignolytic and cellulolytic) has been the subject of previous studies. Major fungi involved in lignocellulosic conversion have been identified within the Ascomycetes, Deuteromycetes, and Basidiomycetes (Krishna 2007), with many thousands of individual species recognised (Horwath 2007; Tedersoo et al. 2014). In addition to microorganisms, numerous studies have also demonstrated importance of microfauna and macrofauna in enhancing litter decomposition (Frouz et al. 2015). Within forests, 20–100% of the litter fall can be initially processed through interaction and decomposition activity of the fauna within the litter layer (Frouz et al. 2015). As the leaf and litter material is initially decomposed, key nutrients are lost with a change in the bioenergetic content of the remaining C. Decomposition of altered C, such as macrofauna faeces (frass), or faunal necromass and detritus is lower than that of the original litter (Filser et al. 2016). These interactions demonstrate the importance of energy cascades (energy content of the organic matter) and nutrient depletion (C : nutrient stoichiometry) in affecting decomposition kinetics. Sequential transformations of the original plant-derived C also influences the longer-term recalcitrance or persistence of the SOM generated (Fox et al. 2006), often by reducing energy density (Williams and Plante 2018). That is, the translation of the plant C through microbial and faunal activity into necromass and other forms is a key driver for generation and persistence of C in soils (Schmidt et al. 2011; Clemmensen et al. 2013; Lehmann and Kleber 2015). Structure of microbial and faunal communities in forest ecosystems thus have a strong influence on the long-term dynamics and retention of SOM in forest soils (Schmidt et al. 2011). Indeed the role of soil biota is generally overlooked in SOM modelling studies, effectively reducing their power to predict gross primary productivity or global soil C (Luo et al. 2015; Filser et al. 2016). The inclusion of faunal and microbial elements into new C-models is seen as essential to better predict the net balance and dynamics of SOM stocks (Fox et al. 2006; Filser et al. 2016; Grandy et al. 2016). This is particularly important as environments change from relatively quasi semi-state conditions into increasingly ‘changeable’ and disrupted ecosystems. For forest systems, this applies at a lesser scale than in agricultural systems.
For planted forest systems the contributions of pollen and root exudates to nutrient cycling and microbial processes are often overlooked. Pine pollen, for example, is deposited in forests over a relatively short period (Lee et al. 1996) and although its contribution as compared with leaf or litter fall may be small, pollen still returns significant amounts of N, P, S, and cations into the forest at specific times (Cho et al. 2003). Such small but highly episodic deposition may have a trigger or stimulatory effect on fungi and litter decomposition, thus impacting wider ecosystem nutrient dynamics (Stark 1972). Similarly, exudation of low molecular weight organic compounds (LMWOCs) into soils by tree species (compared with annual agricultural plants) is poorly understood in terms of absolute quantity, proportion of total plant photosynthate that is delivered belowground, and the role that the C has on microbial dynamics and ecosystem processes. As in other ecosystems, difficulty in addressing these questions extends from the high turnover of these molecules in the rhizosphere, with half-lives of 1–5 h in soil typically being reported. Thus, while point-in-time quantifications of LMWOCs demonstrate significant concentrations of these in soils (as a percentage of total dissolved C), their gross efflux over time is often underestimated (Jones 1998; Jones et al. 2003). It is evident that tree roots such as Pinus radiata exude a diverse range of LMOWCs into soil that affect the structure and function of the root microbiome (Shi et al. 2012). As such these exudates may have a disproportionally large role on nutrient cycling and plant–microbial interactions.
Nitrogen
In planted forest ecosystems, N availability is a key driver of net primary productivity (Magnani et al. 2007; Johnson and Turner 2014). In addition, N has an important role in SOM cycling and decomposer activity, thereby having further influence on the availability of other nutrients (Hobbie 2008). The general limitation of plant-available N extends from the relatively small pool of available N in the soil, slow turnover of N within SOM and high plant demand, particularly while the canopy is established (Johnson and Turner 2014).
Nitrogen inputs from fertiliser application, or deposition from the atmosphere, enrich the N status of managed ecosystems globally, including planted forests (Johnson and Turner 2014). Particularly in the northern hemisphere, external inputs can in some cases result in N-saturation, moving N cycling from a closed or internal system to a more open state (Magnani et al. 2007) with elevated levels of soil ammonia or ammonium, nitrate, or nitrite (Galloway et al. 2003). These forms of N can result in losses of N through volatilisation (e.g. ammonia), denitrification resulting in formation of nitrous oxides (nitric oxide and N2O) and N2, or leaching of nitrate-N into groundwater or rivers (Galloway et al. 2003, 2004). Within these reactions a diverse range of biogeochemical transformations occur with process rates mediated by availability of key substrates (forms of N), supply of energy (labile C), microsite redox conditions, and soil pH, temperature, and moisture (Bateman and Baggs 2005; Baggs et al. 2010; Cuhel et al. 2010; Harrison-Kirk et al. 2013; Cui et al. 2016). Associated with this is a vast array of different consortia of microbial taxa and soil fauna (Schloss and Handelsman 2006) that are dependent on the soil habitat (Wakelin et al. 2008), the aboveground community composition (Marschner et al. 2001; Garbeva et al. 2004), and the soil compartment (e.g. horizon and depth) (Pereira et al. 2017). As many of the N transformation processes, particularly those associated with N reduction or denitrification, are undertaken by a range of taxa, the microbiology linked with these ecosystem functions changes over space and time (Nelson et al. 2016; Albright et al. 2018). In this instance, the use of environmental RNA based characterisation (i.e. N-cycling functional-gene expression via environmental meta-transcriptomics) provides a more useful approach in establishing the links between soil biology and N-cycling function than the more widespread use of DNA-based analyses that are directed at potential function only (Albright et al. 2018).
Given the high dependency on internal nutrient cycling in forest ecosystems, nitrification plays an important role in N dynamics through the coupling of SOM decomposition with supply of mineral N to plants and other organisms (Ivarson and Sowden 1959; Galloway et al. 2003). As previously discussed, the oxidation of ammonia or ammonium to nitrate by ammonia-oxidising bacteria and archaea is a rate-limiting step in nitrification. These domain kingdoms differ markedly in ecophysiology, with the latter tending to be more abundant in low N systems. Regardless, both groups can be qualitatively and quantitatively assessed through detection of their respective ammonia monooxygenase genes (amoA) (Rotthauwe et al. 1997; Stephen et al. 1999; Tourna et al. 2008). Unlike diazotrophic function (nifH), nitrification is restricted to a narrow phylogenetic range of taxa, and their activities are closely connected to ammonia oxidation (chemolithoautotrophy). Accordingly, the assessment of these genes provides a relatively robust link to actual or potential process rates occurring in the environment (Wakelin et al. 2014), and remains a highly useful tool for assessment of this important soil function (Kowalchuk and Stephen 2001).
In the absence of direct N inputs, non-symbiotic (free-living) N-fixing microorganisms (diazotrophs) may play an important role in supply of N inputs and therefore ecosystem productivity (Binkley et al. 2000). However, compared with the quantifiable inputs of N that occur through biological N-fixation in legume species (e.g. Acacia and legume crops used in agriculture), the extent of N fixation that can be accounted for by diazotrophic activity is poorly understood (in both forest and agricultural systems). It is most often masked by inputs of the more readily identifiable sources of N (e.g. fertiliser, bird and animal waste, and rainfall), or as otherwise estimated values derived from the δ-N in whole-system N budgets (Johnson and Turner 2014). Binkley et al. (2000), for example, found little evidence to support significant inputs of N from diazotrophic activity across a range of forest systems. However, difficulty in determining this empirically is not surprising with rates as low as 1–3 kg N ha–1 year–1 being typically reported (Cleveland et al. 1999; Son 2001). Other work, however, has suggested more significant inputs of diazotrophic N across different forest systems, with the magnitude varying strongly on a site-by-site basis (Jurgensen et al. 1990). Nonetheless, while input rates may be small they could still offset a significant portion of N lost during harvesting, particularly when inputs are aggregated over periods of long rotation (Burgoyne and Deluca 2009). Certainly, there remains a research gap to fully define the importance and therefore potential of free-living N-fixing microorganisms as a contributor to the fertility and productivity of managed ecosystems.
The ability to fix atmospheric N into plant available (mineral N) forms is widely distributed through the bacterial and archaeal kingdoms (Raymond et al. 2004). In forest soils (pine, spruce, and other tree species), functional genes associated with N-fixation ability (nifH genes) have been widely detected across at least 11 phyla with strong associations within the α- and γ-Proteobacteria, Actinobacteria, and Acidobacteria (Rösch and Bothe 2009). A similar predominance of α- and γ-Proteobacteria type nifH genes has been found in hardwood forest (maple and oak) soils, albeit with vast difference in the presence of total diversity of N-fixing taxa (Izquierdo and Nüsslein 2015). Under Eucalyptus spp. plantations, Bradyrhizobium (a genus within the α-Proteobacteria) and Burkholderia (β-Proteobacteria) were the most dominant genera, although nifH genotypes were associated with at least 25 further genera (da Silva et al. 2014). Given that nifH is broadly distributed across the bacterial kingdom, it is possible that the distribution in abundance and diversity of this function is related to general shifts in the total bacteria population across different habitats. Indeed, there appears to be a large disparity between the active and ‘potentially’ active diazotrophic community (da Silva et al. 2016). Consequently, such nifH gene abundance may not relate to diazotrophic activity, but rather provide a view of total potential activity (or capacity factor) which is unlikely to be realised. Nonetheless, the actual value of diazotrophs in providing N in forest ecosystems as compared with that provided by leguminous species warrants further investigation.
Mineral weathering
Importantly for forestry, which by its very nature is managed over long-term (decadal) periods, the weathering of rocks and soil minerals play an important part in the overall ecosystem nutrient budgets, especially in subsoils (Morford et al. 2016). These processes are interconnected and integrated through biogeochemical interactions of microorganisms that release N, P, and other nutrients from soil minerals (Jongmans et al. 1997). Plant roots, particularly those of long-lived tree species, penetrate deep into soil (Canadell et al. 1996) and so have a major influence on the weathering of the regolith through physical interactions and release of exudates that include protons and organic anions (Pierret et al. 2016). Soil microbial processes related to the weathering of rock (geogenic N inputs) may thus provide a significant input of plant-available N (and P) into forest ecosystems (Morford et al. 2016; Houlton et al. 2018). While igneous rock has relatively low N content, sedimentary materials typically hold ~500 mg N kg–1 rock material (Johnson and Goldblatt 2015). Indeed, discrepancies in global N fluxes can be resolved by considering traditional and geochemical (rock and sediment) fluxes together (Houlton et al. 2018). However, the extent of microbiological processes in mineral weathering and N-release remain unquantified.
Mycorrhizal fungi as well as various groups of saprotrophic fungi (e.g. Penicillium spp.) and mineral-solubilising bacteria (e.g. Pseudomonas spp.) have been shown to be actively involved in soil weathering processes and mobilisation of nutrients (especially P) from soil minerals (Blum et al. 2002; Glowa et al. 2003; Watmough and Dillon 2003). However, the extent of this function and its quantification within whole soil ecosystems, and particularly the species and mechanisms involved in subsoils and deeper remains relatively unexplored (Pierret et al. 2016). Despite this, these processes are of critical importance to long-term functioning of forest ecosystems; for example, as shown in temperate forests on acidic soils (Zabowski et al. 2007; Uroz et al. 2009). Given that the microbiology and functioning of the topsoil is poorly described relative to its importance (Wakelin 2018), then describing and quantifying the many biogeochemical processes and associated microbiology in the subsoil represents a new frontier.
Mycorrhizal associations
While mycorrhizal associations are of questionable value to net primary production in highly intensive agricultural ecosystems (Guppy and Mclaughlin 2009), they are of major importance in both natural and managed forest ecosystems. In areas lacking suitable mycorrhizal symbionts, pine and other plantation trees neither effectively establish nor grow without inoculation (Reinhart and Callaway 2006; Nuñez et al. 2009) or provision of appropriately treated nursery stock (Smaill and Walbert 2013; Chen et al. 2014). The main mycorrhizal associations with planted forest trees are generally ectomycorrhizal (Pampolina et al. 2002; Smith and Read 2010). However, some important plantation species, such as Eucalyptus, Salix, and Populus spp. form both arbuscular and ectomycorrhizal associations (Lodge 1989; Adams et al. 2006). This ability to dual-associate may provide benefits for establishment of trees on new sites or on poor resource quality soils (Adjoud-Sadadou and Halli-Hargas 2017).
Trees invest considerable energy to supporting mycorrhizal symbiosis. For example, as much as 30% of all photosynthetically fixed C is supplied to mycorrhizal fungi (Hobbie 2006); such that in forest soils, mycorrhizal mycelia can comprise up to one-third of the total microbial biomass (Högberg and Högberg 2002). The extent of the mycorrhizal biomass in forest soils, and the interaction between mycorrhizae and host-tree supply of phytosynthate means that these fungi have a key role in ecosystem-level C budgets (Hasselquist et al. 2012), mediating C persistence in soil (Gadgil and Gadgil 1971; Rygiewicz and Andersen 1994; Chapela et al. 2001), and the wider nutrient economy in forests (Phillips et al. 2013). Thus, small changes at the plant–microbiome level, can have major influence on total ecosystem functioning and the global cycling of C. There is need therefore that mycorrhizae should be more explicitly modelled in global C-models (Meyer et al. 2012).
The primary benefits of mycorrhizal association are generally attributed to provision of P for plant growth (Smith and Read 2010). The large surface area of mycorrhizal mycelia in soil confers an ability to explore and acquire P, water, and other nutrients that far exceeds the capacity of the host-tree root system alone (Smith and Read 2010). In addition to the acquisition of inorganic and organic P from soil, mycorrhizal symbioses have increasingly been identified as important to the wider nutrition of the host plant, including amino acids, sugars, N, S, K, and various other macro- and micro-elements such as zinc (Casieri et al. 2013). Given this, the beneficial role of mycorrhizal symbioses is increasingly recognised as being of wider importance to nutrient cycling in forest ecosystems than the supply of inorganic P either with or without fertilisation. Furthermore, in addition to nutrient provision, mycorrhizal fungi support other important ecosystem services associated with improved soil structure and aggregation, water infiltration and purification (Simard and Austin 2010), along with enhancing host-tree resistance to abiotic stress, pests, and diseases (Branzanti et al. 1999; Reivant Munters 2014).
While over 90% of the P requirement of trees can be met through mycorrhizal symbioses, variation in the physiology of the fungi–soil and fungi–plant interfaces can alter the uptake of P from soils, resulting in variation in the efficacy of mycorrhizal associations (Plassard and Dell 2010). These interactions are likely to similarly extend to other symbiosis outcomes (e.g. protection of the host from disease) and also other ecosystem services provided by mycorrhizae, including adaptation to climate change (Rillig et al. 2001; Simard and Austin 2010). As such, there is significant interest and potential to manage or direct outcomes of mycorrhizal associations that extend well beyond lifting the productive capacity of tree growth alone.
In forest ecosystems mycorrhizal fungal mycelia that link the roots of plants in a network are ubiquitous (Van der Heijden and Horton 2009). Thus, as well as providing a pathway for nutrient flow, they also comprise a network for signalling and communication within the forest ecosystem (Brownlee et al. 1983; Simard and Durall 2004). A future and potentially important role of mycorrhizal fungi may be to provide forest ecosystems with greater resilience to environmental stress, particularly as a result of climatic change (Simard and Austin 2010; Gorzelak et al. 2015; Simard 2018). However, there is need to develop molecular-based tools to more effectively characterise mycorrhizae field populations coupled with better understanding of the ecology of the symbiosis in forest soils and the services they provide in both plantation and natural forest ecosystems.
Conclusions
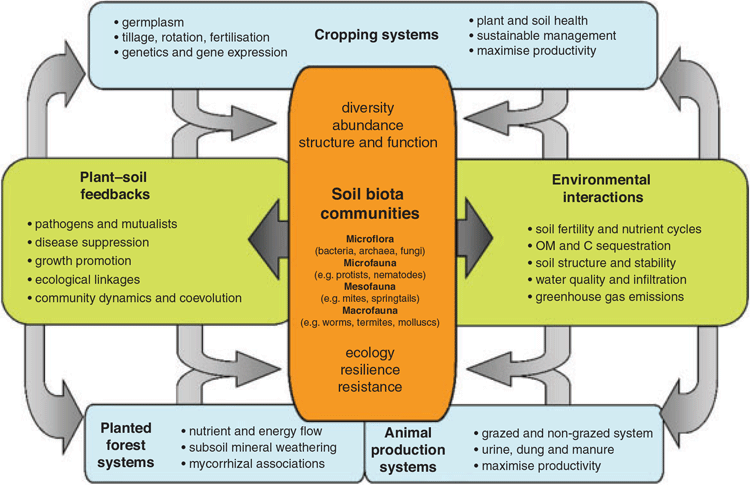
The findings of this review highlight our relatively poor understanding of plant–soil-biota interactions and relationships between soil biodiversity and biogeochemical function across a range of managed ecosystems (Fig. 2). Most reported research has focused on describing and quantifying the impact of land-use management practices on the taxonomic diversity of bacteria and fungi, but few studies have investigated how diversity influences specific functions across different groups and trophic levels. There is a need to improve understanding across trophic levels and to further investigate interactions between community members. In particular, it is essential that descriptive approaches are linked with analysis of both functional genes and functional groups, and more importantly, to associate this with measured actual and potential process rates that operate under realistic environmental conditions. Nutrient dynamics and microbe–plant interactions are most often driven by small continual or episodic resource inputs (organic C and nutrients), seasonal factors, and a wide range of edaphic environmental factors, whereby the functional consequences of these interactions require more detailed investigation. The capacity to predict and model the effects of system perturbation and anthropogenic interventions on structure and function of microbial communities in soil environments will be important. When linked with system process outcomes, the continued development and application of metagenomic and metatranscriptomic techniques and approaches in environmental metabolomics have the potential to further our understanding of the impacts of land-use, land management, and climate change on multi-trophic diversity, interactions, and biogeochemical functions in soil ecosystems (Bouchez et al. 2016; Wakelin et al. 2016; Bahram et al. 2018; Crowther et al. 2019)
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
SA Wakelin was funded by the Growing Confidence in Forestry Futures program (Contract number CO4X1306), which was co-funded by the Forest Growers Levy Trust and the New Zealand Ministry of Business Innovation and Employment. KE Dunfield was funded by the Canada Research Chairs program and Food From Thought, a Canada First Research Excellence Fund Grant. XD Chen acknowledges the support from the UCAS Joint PhD Training Program (China).
References
Adams F, Reddell P, Webb MJ, Shipton WA (2006) Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical north-eastern Australia. Australian Journal of Botany 54, 271–281.| Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Adjoud-Sadadou D, Halli-Hargas R (2017) Dual mycorrhizal symbiosis: an asset for eucalypts out of Australia? Canadian Journal of Forest Research 47, 500–505.
| Dual mycorrhizal symbiosis: an asset for eucalypts out of Australia?Crossref | GoogleScholarGoogle Scholar |
Akselsson C, Westling O, Sverdrup H, Gundersen P (2007) Nutrient and carbon budgets in forest soils as decision support in sustainable forest management. Forest Ecology and Management 238, 167–174.
| Nutrient and carbon budgets in forest soils as decision support in sustainable forest management.Crossref | GoogleScholarGoogle Scholar |
Albright M, Johansen R, Lopez D, Gallegosgraves V, Steven B, Kuske CR, Dunbar J (2018) Short-term transcriptional response of microbial communities to N-fertilization in a pine forest soil. Applied and Environmental Microbiology 84, e00598–18
| Short-term transcriptional response of microbial communities to N-fertilization in a pine forest soil.Crossref | GoogleScholarGoogle Scholar | 29802185PubMed |
André HM, Ducarme X, Lebrun P (2002) Soil biodiversity: myth, reality or conning? Oikos 96, 3–24.
| Soil biodiversity: myth, reality or conning?Crossref | GoogleScholarGoogle Scholar |
Attiwill PM, Adams MA (1993) Tansley review No. 50. Nutrient cycling in forests. New Phytologist 124, 561–582.
| Tansley review No. 50. Nutrient cycling in forests.Crossref | GoogleScholarGoogle Scholar |
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biology and Fertility of Soils 46, 793–805.
| Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil.Crossref | GoogleScholarGoogle Scholar |
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J, Medema MH, Maltz MR, Mundra S, Olsson PA, Pent M, Põlme S, Sunagawa S, Ryberg M, Tedersoo L, Bork P (2018) Structure and function of the global topsoil microbiome. Nature 560, 233–237.
| Structure and function of the global topsoil microbiome.Crossref | GoogleScholarGoogle Scholar | 30069051PubMed |
Bai Z, Caspari T, Gonzalez MR, Batjes NH, Mäder P, Bünemann EK, de Goede R, Brussaard L, Xu M, Ferreira CSS, Reintam E, Fan H, Mihelič R, Glavan M, Tóth Z (2018) Effects of agricultural management practices on soil quality: a review of long-term experiments for Europe and China. Agriculture, Ecosystems and Environment 265, 1–7.
| Effects of agricultural management practices on soil quality: a review of long-term experiments for Europe and China.Crossref | GoogleScholarGoogle Scholar |
Bailey K, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil and Tillage Research 72, 169–180.
| Suppressing soil-borne diseases with residue management and organic amendments.Crossref | GoogleScholarGoogle Scholar |
Bardgett RD, Keiller S, Cook R, Gilburn AS (1998) Dynamic interactions between soil animals and microorganisms in upland grassland soils amended with sheep dung: a microcosm experiment. Soil Biology and Biochemistry 30, 531–539.
| Dynamic interactions between soil animals and microorganisms in upland grassland soils amended with sheep dung: a microcosm experiment.Crossref | GoogleScholarGoogle Scholar |
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biology and Fertility of Soils 41, 379–388.
| Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space.Crossref | GoogleScholarGoogle Scholar |
Bender S, Van der Heijden M (2015) Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. Journal of Applied Ecology 52, 228–239.
| Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses.Crossref | GoogleScholarGoogle Scholar |
Bender SF, Wagg C, van der Heijden MGA (2016) An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends in Ecology and Evolution 31, 440–452.
| An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability.Crossref | GoogleScholarGoogle Scholar | 26993667PubMed |
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends in Plant Science 17, 478–486.
| The rhizosphere microbiome and plant health.Crossref | GoogleScholarGoogle Scholar | 22564542PubMed |
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiology Ecology 68, 1–13.
| Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 19243436PubMed |
Bienert F, de Danieli S, Miquel C, Coissac E, Poillot C, Brun JJ, Taberlet P (2012) Tracking earthworm communities from soil DNA. Molecular Ecology 21, 2017–2030.
| Tracking earthworm communities from soil DNA.Crossref | GoogleScholarGoogle Scholar | 22250728PubMed |
Binkley D, Son Y, Valentine DW (2000) Do forests receive occult inputs of nitrogen? Ecosystems 3, 321–331.
| Do forests receive occult inputs of nitrogen?Crossref | GoogleScholarGoogle Scholar |
Birkhofer K, Bezemer TM, Bloem J, Bonkowski M, Christensen S, Dubois D, Ekelund F, Fliessbach A, Gunst L, Hedlund K, Mader P, Mikola J, Robin C, Setala H, Tatin-Froux F, Van der Putten WH, Scheu S (2008) Long-term organic farming fosters below and aboveground biota: implications for soil quality, biological control and productivity. Soil Biology and Biochemistry 40, 2297–2308.
| Long-term organic farming fosters below and aboveground biota: implications for soil quality, biological control and productivity.Crossref | GoogleScholarGoogle Scholar |
Bissett A, Brown MV, Siciliano SD, Thrall PH (2013) Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecology Letters 16, 128–139.
| Microbial community responses to anthropogenically induced environmental change: towards a systems approach.Crossref | GoogleScholarGoogle Scholar | 23679012PubMed |
Blanchart E, Villenave C, Viallatoux A, Barthès B, Girardin C, Azontonde A, Feller C (2006) Long-term effect of a legume cover crop (Mucuna pruriens var. utilis) on the communities of soil macrofauna and nematofauna, under maize cultivation, in Southern Benin. European Journal of Soil Biology 42, S136–S144.
| Long-term effect of a legume cover crop (Mucuna pruriens var. utilis) on the communities of soil macrofauna and nematofauna, under maize cultivation, in Southern Benin.Crossref | GoogleScholarGoogle Scholar |
Blum JD, Andrea K, Nezat CA, Driscoll CT, Johnson CE, Siccama TG, Christopher E, Fahey TJ, Likens GE (2002) Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 417, 729–731.
| Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems.Crossref | GoogleScholarGoogle Scholar | 12066181PubMed |
Bonanomi G, Antignani V, Capodilupo M, Scala F (2010) Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biology and Biochemistry 42, 136–144.
| Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases.Crossref | GoogleScholarGoogle Scholar |
Bouchez T, Blieux A, Dequiedt S, Domaizon I, Dufresne A, Ferreira S, Godon JJ, Hellal J, Joulian C, Quaiser A, Martin-Laurent F, Mauffret A, Monier JM, Peyret P, Schmitt-Koplin P, Sibourg O, D’oiron E, Bispo A, Deportes I, Ranjard L (2016) Molecular microbiology methods for environmental diagnosis. Environmental Chemistry Letters 14,
| Molecular microbiology methods for environmental diagnosis.Crossref | GoogleScholarGoogle Scholar |
Branzanti MB, Rocca E, Pisi A (1999) Effect of ectomycorrhizal fungi on chestnut ink disease. Mycorrhiza 9, 103–109.
| Effect of ectomycorrhizal fungi on chestnut ink disease.Crossref | GoogleScholarGoogle Scholar |
Briones MJI, Schmidt O (2017) Conventional tillage decreases the abundance and biomass of earthworms and alters their community structure in a global meta-analysis. Global Change Biology 23, 4396–4419.
| Conventional tillage decreases the abundance and biomass of earthworms and alters their community structure in a global meta-analysis.Crossref | GoogleScholarGoogle Scholar | 28464547PubMed |
Brownlee C, Duddridge JA, Malibari A, Read DJ (1983) The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport. Plant and Soil 71, 433–443.
| The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport.Crossref | GoogleScholarGoogle Scholar |
Brussaard L, Kuyper WT, Didden WAM, de Goede RGM, Bloem J (2004) Biological soil quality from biomass to biodiversity - importance and resilience to management stress and disturbance. In ‘Managing soil quality - challenges in modern agriculture’. (Eds P Schjønning, S Elmholt, B Christensen) pp. 139–161. (CAB International: Wallingford, UK)
Bünemann EK, Schwenke GD, Van Zwieten L (2006) Impact of agricultural inputs on soil organisms—a review. Australian Journal of Soil Research 44, 379–406.
| Impact of agricultural inputs on soil organisms—a review.Crossref | GoogleScholarGoogle Scholar |
Buongiorno J, Zhu S (2014) Assessing the impact of planted forests on the global forest economy. New Zealand Journal of Forestry Science 44, S2
| Assessing the impact of planted forests on the global forest economy.Crossref | GoogleScholarGoogle Scholar |
Burgoyne TA, Deluca TH (2009) Short-term effects of forest restoration management on non-symbiotic nitrogen-fixation in western Montana. Forest Ecology and Management 258, 1369–1375.
| Short-term effects of forest restoration management on non-symbiotic nitrogen-fixation in western Montana.Crossref | GoogleScholarGoogle Scholar |
Canadell J, Jackson RB, Ehleringer JB, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108, 583–595.
| Maximum rooting depth of vegetation types at the global scale.Crossref | GoogleScholarGoogle Scholar | 28307789PubMed |
Cardenas L, Misselbrook T, Hodgson C, Donovan N, Gilhespy S, Smith K, Dhanoa M, Chadwick D (2016) Effect of the application of cattle urine with or without the nitrification inhibitor DCD, and dung on greenhouse gas emissions from a UK grassland soil. Agriculture, Ecosystems and Environment 235, 229–241.
| Effect of the application of cattle urine with or without the nitrification inhibitor DCD, and dung on greenhouse gas emissions from a UK grassland soil.Crossref | GoogleScholarGoogle Scholar | 27974862PubMed |
Casieri L, Ait Lahmidi N, Doidy J, Veneault-Fourrey C, Migeon A, Bonneau L, Courty PE, Garcia K, Charbonnier M, Delteil A, Brun A, Zimmermann S, Plassard C, Wipf D (2013) Biotrophic transportome in mutualistic plant-fungal interactions. Mycorrhiza 23, 597–625.
| Biotrophic transportome in mutualistic plant-fungal interactions.Crossref | GoogleScholarGoogle Scholar | 23572325PubMed |
Chapela IH, Osher LJ, Horton TR, Henn MR (2001) Ectomycorrhizal fungi introduced with exotic pine plantations induce soil carbon depletion. Soil Biology and Biochemistry 33, 1733–1740.
| Ectomycorrhizal fungi introduced with exotic pine plantations induce soil carbon depletion.Crossref | GoogleScholarGoogle Scholar |
Chen YL, Liu RJ, Bi YL, Feng G (2014) Use of mycorrhizal fungi for forest plantations and minesite rehabilitation. In ‘Mycorrhizal fungi: use in sustainable agriculture and land restoration’. (Eds Z Solaiman, L Abbott, A Varma) pp. 325–355. (Springer: Berlin, Heidelberg)
Chen X, Jiang N, Chen Z, Tian J, Sun N, Xu M, Chen L (2017) Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Applied Soil Ecology 119, 197–204.
| Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials.Crossref | GoogleScholarGoogle Scholar |
Chen X, Jiang N, Condron LM, Dunfield KE, Chen Z, Wang J, Chen L (2019) Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long-term nitrogen and manure inputs. Geoderma 349, 36–44.
| Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long-term nitrogen and manure inputs.Crossref | GoogleScholarGoogle Scholar |
Cho YJ, Kim IS, Kim PG, Lee EJ (2003) Deposition of airborne pine pollen in a temperate pine forest. Grana 42, 178–182.
| Deposition of airborne pine pollen in a temperate pine forest.Crossref | GoogleScholarGoogle Scholar |
Clark IM, Buchkina N, Jhurreea D, Goulding KWT, Hirsch PR (2012) Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 1235–1244.
| Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment.Crossref | GoogleScholarGoogle Scholar | 22451109PubMed |
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618.
| Roots and associated fungi drive long-term carbon sequestration in boreal forest.Crossref | GoogleScholarGoogle Scholar | 23539604PubMed |
Cleveland CC, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, Fischer JCV, Elseroad A (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochemical Cycles 13, 623–645.
| Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems.Crossref | GoogleScholarGoogle Scholar |
Condron LM, Stark C, O’Callaghan M, Clinton P, Huang Z (2010) The role of microbial communities in the formation and decomposition of soil organic matter. In ‘Soil microbiology and sustainable crop production’. (Eds G Dixon, E Tilston) pp. 81–118. (Springer: Dordrecht)
Coulibaly SFM, Coudrain V, Hedde M, Brunet N, Mary B, Recous S, Chauvat M (2017) Effect of different crop management practices on soil collembola assemblages: a 4-year follow-up. Applied Soil Ecology 119, 354–366.
| Effect of different crop management practices on soil collembola assemblages: a 4-year follow-up.Crossref | GoogleScholarGoogle Scholar |
Crowther TW, van den Hoogen J, Wan J, Mayes MA, Keiser AD, Mo L, Averill C, Maynard DS (2019) The global soil community and its influence on biogeochemistry. Science 365, eaav0550
| The global soil community and its influence on biogeochemistry.Crossref | GoogleScholarGoogle Scholar | 31439761PubMed |
Cuhel J, Simek M, Laughlin RJ, Bru D, Cheneby D, Watson CJ, Philippot L (2010) Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Applied and Environmental Microbiology 76, 1870–1878.
| Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity.Crossref | GoogleScholarGoogle Scholar | 20118356PubMed |
Cui PY, Fan FL, Yin C, Song AL, Huang PR, Tang YJ, Zhu P, Peng C, Li TQ, Wakelin SA, Liang YC (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biology and Biochemistry 93, 131–141.
| Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes.Crossref | GoogleScholarGoogle Scholar |
D’Hose T, Molendijk L, Van Vooren L, van den Berg W, Hoek H, Runia W, van Evert F, ten Berge H, Spiegel H, Sanden T, Grignani C, Ruysschaert G (2018) Responses of soil biota to non-inversion tillage and organic amendments: an analysis on European multiyear field experiments. Pedobiologia 66, 18–28.
| Responses of soil biota to non-inversion tillage and organic amendments: an analysis on European multiyear field experiments.Crossref | GoogleScholarGoogle Scholar |
da Silva MC, Paula TA, Moreira BC, Carolino M, Cruz C, Bazzolli DM, Silva CC, Kasuya MC (2014) Nitrogen-fixing bacteria in Eucalyptus globulus plantations. PLoS One 9, e111313
| Nitrogen-fixing bacteria in Eucalyptus globulus plantations.Crossref | GoogleScholarGoogle Scholar |
da Silva MDS, Mendes IR, Paula TD, Dias RS, de Paula SO, Silva CC, Bazzolli DMS, Kasuya MCM (2016) Expression of the nifH gene in diazotrophic bacteria in Eucalyptus urograndis plantations. Canadian Journal of Forest Research 46, 190–199.
| Expression of the nifH gene in diazotrophic bacteria in Eucalyptus urograndis plantations.Crossref | GoogleScholarGoogle Scholar |
De Goede RGM, Brussaard L, Akkermans ADL (2003) On-farm impact of cattle slurry manure management on biological soil quality. NJAS Wageningen Journal of Life Sciences 51, 103–133.
| On-farm impact of cattle slurry manure management on biological soil quality.Crossref | GoogleScholarGoogle Scholar |
de Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J (2006) Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biology and Biochemistry 38, 2092–2103.
| Fungal/bacterial ratios in grasslands with contrasting nitrogen management.Crossref | GoogleScholarGoogle Scholar |
de Vries FT, Manning P, Tallowin JRB, Mortimer SR, Pilgrim ES, Harrison KA, Hobbs PJ, Quirk H, Shipley B, Cornelissen JHC, Kattge J, Bardgett RD (2012) Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecology Letters 15, 1230–1239.
| Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities.Crossref | GoogleScholarGoogle Scholar | 22882451PubMed |
de Vries FT, Thébault E, Liiri M, Birkhofer K, Tsiafouli MA, Bjørnlund L, Jørgensen HB, Brady MV, Christensen S, de Ruiter PC (2013) Soil food web properties explain ecosystem services across European land use systems. Proceedings of the National Academy of Sciences 110, 14296–14301.
| Soil food web properties explain ecosystem services across European land use systems.Crossref | GoogleScholarGoogle Scholar |
Delgado-Baquerizo M, Bardgett RD, Vitousek PM, Maestre FT, Williams MA, Eldridge DJ, Lambers H, Neuhauser S, Gallardo A, García-Velázquez L, Sala OE, Abades SR, Alfaro FD, Berhe AA, Bowker MA, Currier CM, Cutler NA, Hart SC, Hayes PE, Hseu ZY, Kirchmair M, Peña-Ramírez VM, Pérez CA, Reed SC, Santos F, Siebe C, Sullivan BW, Weber-Grullon L, Fierer N (2019) Changes in belowground biodiversity during ecosystem development. Proceedings of the National Academy of Sciences 116, 6891–6896.
| Changes in belowground biodiversity during ecosystem development.Crossref | GoogleScholarGoogle Scholar |
Dessaux Y, Grandclément C, Faure D (2016) Engineering the rhizosphere. Trends in Plant Science 21, 266–278.
| Engineering the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 26818718PubMed |
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience 2, 621–624.
| Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiology Ecology 72, 386–394.
| Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions.Crossref | GoogleScholarGoogle Scholar | 20370827PubMed |
Dick RP (1992) A review: long-term effects of agricultural systems on soil biochemical and microbial parameters. Agriculture, Ecosystems and Environment 40, 25–36.
| A review: long-term effects of agricultural systems on soil biochemical and microbial parameters.Crossref | GoogleScholarGoogle Scholar |
DuPont ST, Ferris H, Van Horn M (2009) Effects of cover crop quality and quantity on nematode-based soil food webs and nutrient cycling. Applied Soil Ecology 41, 157–167.
| Effects of cover crop quality and quantity on nematode-based soil food webs and nutrient cycling.Crossref | GoogleScholarGoogle Scholar |
Edwards AC, Cresser MS (1992) Advances in soil science. In ‘Advances in soil science’. (Ed. BA Stewart) pp. 57–59. (Springer: New York)
Elhady A, Adss S, Hallmann J, Heuer H (2018) Rhizosphere microbiomes modulated by pre-crops assisted plants in defense against plant-parasitic nematodes. Frontiers in Microbiology 9, 1133
| Rhizosphere microbiomes modulated by pre-crops assisted plants in defense against plant-parasitic nematodes.Crossref | GoogleScholarGoogle Scholar | 29915566PubMed |
Evans J (2009) ‘Planted forests: uses, impacts and sustainability’. (CAB International and Food and Agriculture Organization of the United Nations)
Fageria NK, Baligar VC, Bailey BA (2005) Role of cover crops in improving soil and row crop productivity. Communications in Soil Science and Plant Analysis 36, 2733–2757.
| Role of cover crops in improving soil and row crop productivity.Crossref | GoogleScholarGoogle Scholar |
FAO (2012) ‘FRA 2015: Terms and definitions. FAO Forestry Paper No. 180’ (Food and Agriculture Organization of the United Nations: Rome)
FAO (2015) Available at http://www.fao.org/news/story/en/item/270812/icode/ [verified 6 October 2019].
Fernandez AL, Sheaffer CC, Wyse DL, Staley C, Gould TJ, Sadowsky MJ (2016) Structure of bacterial communities in soil following cover crop and organic fertilizer incorporation. Applied Microbiology and Biotechnology 100, 9331–9341.
| Structure of bacterial communities in soil following cover crop and organic fertilizer incorporation.Crossref | GoogleScholarGoogle Scholar | 27464828PubMed |
Ferris H, Tuomisto H (2015) Unearthing the role of biological diversity in soil health. Soil Biology and Biochemistry 85, 101–109.
| Unearthing the role of biological diversity in soil health.Crossref | GoogleScholarGoogle Scholar |
Filser J, Faber JH, Tiunov AV, Brussaard L, Frouz J, Deyn GD, Uvarov AV, Berg MP, Lavelle P, Loreau M (2016) Soil fauna: key to new carbon models. Soil (Göttingen) 2, 565–582.
| Soil fauna: key to new carbon models.Crossref | GoogleScholarGoogle Scholar |
Fitter AH, Gilligan C, Hollingworth K, Kleczkowski A, Twyman RW, Pitchford J (2005) Biodiversity and ecosystem function in soil. Functional Ecology 19, 369–377.
| Biodiversity and ecosystem function in soil.Crossref | GoogleScholarGoogle Scholar |
Fox O, Vetter S, Ekschmitt K, Wolters V (2006) Soil fauna modifies the recalcitrance-persistence relationship of soil carbon pools. Soil Biology and Biochemistry 38, 1353–1363.
| Soil fauna modifies the recalcitrance-persistence relationship of soil carbon pools.Crossref | GoogleScholarGoogle Scholar |
Francioli D, Schulz E, Lentendu G, Wubet T, Buscot F, Reitz T (2016) Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Frontiers in Microbiology 7, 1446
| Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies.Crossref | GoogleScholarGoogle Scholar | 27683576PubMed |
Fraser TD, Lynch DH, Entz MH, Dunfield KE (2015a) Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 257–258, 115–122.
| Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial.Crossref | GoogleScholarGoogle Scholar |
Fraser TD, Lynch DH, Bent E, Entz MH, Dunfield KE (2015b) Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biology and Biochemistry 88, 137–147.
| Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management.Crossref | GoogleScholarGoogle Scholar |
Frouz J, Roubíčková A, Heděnec P, Tajovský K (2015) Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. European Journal of Soil Biology 68, 18–24.
| Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies.Crossref | GoogleScholarGoogle Scholar |
Fu S, Coleman DC, Hendrix PF, Crossley DA (2000) Responses of trophic groups of soil nematodes to residue application under conventional tillage and no-till regimes. Soil Biology and Biochemistry 32, 1731–1741.
| Responses of trophic groups of soil nematodes to residue application under conventional tillage and no-till regimes.Crossref | GoogleScholarGoogle Scholar |
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R, Shen Q (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biology and Biochemistry 104, 39–48.
| Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease.Crossref | GoogleScholarGoogle Scholar |
Gadgil RL, Gadgil PD (1971) Mycorrhiza and litter decomposition. Nature 233, 133
| Mycorrhiza and litter decomposition.Crossref | GoogleScholarGoogle Scholar | 16063238PubMed |
Gaiero JR, McCall CA, Thompson KA, Day NJ, Best AS, Dunfield KE (2013) Inside the root microbiome: Bacterial root endophytes and plant growth promotion. American Journal of Botany 100, 1738–1750.
| Inside the root microbiome: Bacterial root endophytes and plant growth promotion.Crossref | GoogleScholarGoogle Scholar | 23935113PubMed |
Gál A, Vyn TJ, Michéli E, Kladivko EJ, McFee WW (2007) Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with tilled-zone sampling depths. Soil and Tillage Research 96, 42–51.
| Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with tilled-zone sampling depths.Crossref | GoogleScholarGoogle Scholar |
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. BioScience 53, 341–356.
| The nitrogen cascade.Crossref | GoogleScholarGoogle Scholar |
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226.
| Nitrogen cycles: past, present, and future.Crossref | GoogleScholarGoogle Scholar |
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology 42, 243–270.
| Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness.Crossref | GoogleScholarGoogle Scholar | 15283667PubMed |
Gaudin ACM, Westra S, Loucks CES, Janovicek K, Martin RC, Deen W (2013) Improving resilience of northern field crop systems using inter-seeded red clover: a review. Agronomy (Basel) 3, 148–180.
| Improving resilience of northern field crop systems using inter-seeded red clover: a review.Crossref | GoogleScholarGoogle Scholar |
Gaudin AC, Tolhurst TN, Ker AP, Janovicek K, Tortora C, Martin RC, Deen W (2015) Increasing crop diversity mitigates weather variations and improves yield stability. PLoS One 10, e0113261
| Increasing crop diversity mitigates weather variations and improves yield stability.Crossref | GoogleScholarGoogle Scholar | 25658914PubMed |
Glowa KR, Arocena JM, Massicotte HB (2003) Extraction of potassium and/or magnesium from selected soil minerals by piloderma. Geomicrobiology Journal 20, 99–111.
| Extraction of potassium and/or magnesium from selected soil minerals by piloderma.Crossref | GoogleScholarGoogle Scholar |
Gorzelak MA, Asay AK, Pickles BJ, Simard SW (2015) Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 7, plv050
| Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities.Crossref | GoogleScholarGoogle Scholar | 25979966PubMed |
Gosz JR, Likens GE, Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecological Monographs 43, 173–191.
| Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire.Crossref | GoogleScholarGoogle Scholar |
Govaerts B, Fuentes M, Mezzalama M, Nicol JM, Deckers J, Etchevers JD, Figueroa-Sandoval B, Sayre KD (2007) Infiltration, soil moisture, root rot and nematode populations after 12 years of different tillage, residue and crop rotation managements. Soil and Tillage Research 94, 209–219.
| Infiltration, soil moisture, root rot and nematode populations after 12 years of different tillage, residue and crop rotation managements.Crossref | GoogleScholarGoogle Scholar |
Grandy AS, Fraterrigo JM, Billings SA (2012) Soil ecosystem resilience and recovery. In ‘Soil ecology and ecosystem services’. (Ed. DH Wall) pp. 366–376. (Oxford University Press Oxford: UK)
Grandy AS, Wieder WR, Wickings K, Kyker-Snowman E (2016) Beyond microbes: are fauna the next frontier in soil biogeochemical models? Soil Biology and Biochemistry 102, 40–44.
| Beyond microbes: are fauna the next frontier in soil biogeochemical models?Crossref | GoogleScholarGoogle Scholar |
Griffiths BS, Wheatley RE, Olesen T, Henriksen K, Ekelund F, Ronn R (1998) Dynamics of nematodes and protozoa following the experimental addition of cattle or pig slurry to soil. Soil Biology and Biochemistry 30, 1379–1387.
| Dynamics of nematodes and protozoa following the experimental addition of cattle or pig slurry to soil.Crossref | GoogleScholarGoogle Scholar |
Griffiths BS, Donn S, Neilson R, Daniell TJ (2006) Molecular sequencing and morphological analysis of a nematode community. Applied Soil Ecology 32, 325–337.
| Molecular sequencing and morphological analysis of a nematode community.Crossref | GoogleScholarGoogle Scholar |
Guo L, Wu G, Li Y, Li C, Liu W, Meng J, Liu H, Yu X, Jiang G (2016) Effects of cattle manure compost combined with chemical fertilizer on topsoil organic matter, bulk density and earthworm activity in a wheat–maize rotation system in Eastern China. Soil and Tillage Research 156, 140–147.
| Effects of cattle manure compost combined with chemical fertilizer on topsoil organic matter, bulk density and earthworm activity in a wheat–maize rotation system in Eastern China.Crossref | GoogleScholarGoogle Scholar |
Guppy CN, McLaughlin MJ (2009) Options for increasing the biological cycling of phosphorus in low-input and organic agricultural systems. Crop and Pasture Science 60, 116–123.
| Options for increasing the biological cycling of phosphorus in low-input and organic agricultural systems.Crossref | GoogleScholarGoogle Scholar |
Hai B, Diallo NH, Sall S, Haesler F, Schauss K, Bonzi M, Assigbetse K, Chotte JL, Munch JC, Schloter M (2009) Quantification of key genes steering the microbial nitrogen cycle in the rhizosphere of sorghum cultivars in tropical agroecosystems. Applied and Environmental Microbiology 75, 4993–5000.
| Quantification of key genes steering the microbial nitrogen cycle in the rhizosphere of sorghum cultivars in tropical agroecosystems.Crossref | GoogleScholarGoogle Scholar | 19502431PubMed |
Hallin S, Jones CM, Schloter M, Philippot L (2009) Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. The ISME Journal 3, 597–605.
| Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment.Crossref | GoogleScholarGoogle Scholar | 19148144PubMed |
Hallmann J, Rodríguez-Kábana R, Kloepper JW (1999) Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control. Soil Biology and Biochemistry 31, 551–560.
| Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control.Crossref | GoogleScholarGoogle Scholar |
Hamel C, Gan Y, Sokolski S, Bainard LD (2018) High frequency cropping of pulses modifies soil nitrogen level and the rhizosphere bacterial microbiome in 4-year rotation systems of the semiarid prairie. Applied Soil Ecology 126, 47–56.
| High frequency cropping of pulses modifies soil nitrogen level and the rhizosphere bacterial microbiome in 4-year rotation systems of the semiarid prairie.Crossref | GoogleScholarGoogle Scholar |
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell J, Lienkaemper GW, Cromack K Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell J, Lienkaemper GW, Cromack K (1986) Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15, 133–302.
| Ecology of coarse woody debris in temperate ecosystems.Crossref | GoogleScholarGoogle Scholar |
Harrison-Kirk T, Beare MH, Meenken ED, Condron LM (2013) Soil organic matter and texture affect responses to dry/wet cycles: effects on carbon dioxide and nitrous oxide emissions. Soil Biology and Biochemistry 57, 43–55.
| Soil organic matter and texture affect responses to dry/wet cycles: effects on carbon dioxide and nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar |
Hartman K, van der Heijden MGA, Wittwer RA, Banerjee S, Walser JC, Schlaeppi K (2018) Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 6, 14
| Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming.Crossref | GoogleScholarGoogle Scholar | 29338764PubMed |
Hartmann M, Frey B, Mayer J, Mader P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. The ISME Journal 9, 1177–1194.
| Distinct soil microbial diversity under long-term organic and conventional farming.Crossref | GoogleScholarGoogle Scholar | 25350160PubMed |
Hasselquist NJ, Metcalfe DB, Högberg P (2012) Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Global Change Biology 18, 3596–3605.
| Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest.Crossref | GoogleScholarGoogle Scholar |
Haynes RS, Williams PM (1993) Nutrient cycling and soil fertility in the grazed pasture ecosystem. Advances in Agronomy 49, 119–199.
| Nutrient cycling and soil fertility in the grazed pasture ecosystem.Crossref | GoogleScholarGoogle Scholar |
He ZQ, Pagliari PH, Waldrip HM (2016) Applied and environmental chemistry of animal manure: a review. Pedosphere 26, 779–816.
| Applied and environmental chemistry of animal manure: a review.Crossref | GoogleScholarGoogle Scholar |
Helgason BL, Walley FL, Germida JJ (2009) Fungal and bacterial abundance in long-term no-till and intensive-till soils of the Northern Great Plains. Soil Science Society of America Journal 73, 120–127.
| Fungal and bacterial abundance in long-term no-till and intensive-till soils of the Northern Great Plains.Crossref | GoogleScholarGoogle Scholar |
Hendrix PF, Parmelee RW, Crossley DA, Coleman DC, Odum EP, Groffman PM (1986) Detritus food webs in conventional and no-tillage agroecosystems. Bioscience 36, 374–380.
| Detritus food webs in conventional and no-tillage agroecosystems.Crossref | GoogleScholarGoogle Scholar |
Hobbie EA (2006) Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology 87, 563–569.
| Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies.Crossref | GoogleScholarGoogle Scholar | 16602286PubMed |
Hobbie SE (2008) Nitrogen effects on decomposition: a five‐year experiment in eight temperate sites. Ecology 89, 2633–2644.
| Nitrogen effects on decomposition: a five‐year experiment in eight temperate sites.Crossref | GoogleScholarGoogle Scholar | 18831184PubMed |
Hobbs PR, Sayre K, Gupta R (2008) The role of conservation agriculture in sustainable agriculture. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 543–555.
| The role of conservation agriculture in sustainable agriculture.Crossref | GoogleScholarGoogle Scholar | 17720669PubMed |
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytologist 154, 791–795.
| Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil.Crossref | GoogleScholarGoogle Scholar |
Hogg ID, Hebert PD (2004) Biological identification of springtails (Hexapoda: Collembola) from the canadian arctic, using mitochondrial DNA barcodes. Canadian Journal of Zoology 82, 749–754.
| Biological identification of springtails (Hexapoda: Collembola) from the canadian arctic, using mitochondrial DNA barcodes.Crossref | GoogleScholarGoogle Scholar |
Horwath W (2007) Carbon cycling and formation of soil organic matter. In ‘Soil microbiology, ecology and biochemistry’. pp. 303–339. (Academic Press: Oxford, UK)
Houlton BZ, Morford SL, Dahlgren RA (2018) Convergent evidence for widespread rock nitrogen sources in earth’s surface environment. Science 360, 58–62.
| Convergent evidence for widespread rock nitrogen sources in earth’s surface environment.Crossref | GoogleScholarGoogle Scholar | 29622648PubMed |
House GJ, Parmelee RW (1985) Comparison of soil arthropods and earthworms from conventional and no-tillage agroecosystems. Soil and Tillage Research 5, 351–360.
| Comparison of soil arthropods and earthworms from conventional and no-tillage agroecosystems.Crossref | GoogleScholarGoogle Scholar |
Hu J, Wu JH, Ma MJ, Nielsen UN, Wang J, Du GZ (2015) Nematode communities response to long-term grazing disturbance on Tibetan plateau. European Journal of Soil Biology 69, 24–32.
| Nematode communities response to long-term grazing disturbance on Tibetan plateau.Crossref | GoogleScholarGoogle Scholar |
Indufor (2012) Strategic review on the future of forest plantations. Report to Forest Stewardship Council (FSC)(ID 11914). Indufor: Helsinki, Finland.
Ivarson KC, Sowden FJ (1959) Decomposition of forest litters: I. Production of ammonia and nitrate nitrogen, changes in microbial population, and rate of decomposition. Plant and Soil 11, 237–248.
| Decomposition of forest litters: I. Production of ammonia and nitrate nitrogen, changes in microbial population, and rate of decomposition.Crossref | GoogleScholarGoogle Scholar |
Izquierdo JA, Nüsslein K (2015) Variation in diazotrophic community structure in forest soils reflects land use history. Soil Biology and Biochemistry 80, 1–8.
| Variation in diazotrophic community structure in forest soils reflects land use history.Crossref | GoogleScholarGoogle Scholar |
Jackson L, Rosenstock T, Thomas M, Wright J, Symstad A (2009) Managed ecosystems: Biodiversity and ecosystem functions in landscapes modified by human use. In ‘Biodiversity, ecosystem functioning, and human wellbeing: an ecological and economic perspective’. (Eds S Naeem, DE Bunker, A Hector, M Loreau, C Perrings) pp. 178–194. (Oxford University Press: New York)
Johnson B, Goldblatt C (2015) The nitrogen budget of earth. Earth-Science Reviews 148, 150–173.
| The nitrogen budget of earth.Crossref | GoogleScholarGoogle Scholar |
Johnson DW, Turner J (2014) Nitrogen budgets of forest ecosystems: a review. Forest Ecology and Management 318, 370–379.
| Nitrogen budgets of forest ecosystems: a review.Crossref | GoogleScholarGoogle Scholar |
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant and Soil 205, 25–44.
| Organic acids in the rhizosphere – a critical review.Crossref | GoogleScholarGoogle Scholar |
Jones DL, Dennis PG, Owen AG, Hees PAWV (2003) Organic acid behavior in soils–misconceptions and knowledge gaps. Plant and Soil 248, 31–41.
| Organic acid behavior in soils–misconceptions and knowledge gaps.Crossref | GoogleScholarGoogle Scholar |
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant and Soil 321, 5–33.
| Carbon flow in the rhizosphere: carbon trading at the soil-root interface.Crossref | GoogleScholarGoogle Scholar |
Jongmans A, Van Breemen N, Lundström U, Van Hees P, Finlay R, Srinivasan M, Unestam T, Giesler R, Melkerud P-A, Olsson M (1997) Rock-eating fungi. Nature 389, 682–683.
| Rock-eating fungi.Crossref | GoogleScholarGoogle Scholar |
Jordan D, Stecker JA, Cacnio-Hubbard VN, Li F, Gantzer CJ, Brown JR (1997) Earthworm activity in no-tillage and conventional tillage systems in Missouri soils: A preliminary study. Soil Biology and Biochemistry 29, 489–491.
| Earthworm activity in no-tillage and conventional tillage systems in Missouri soils: A preliminary study.Crossref | GoogleScholarGoogle Scholar |
Jurgensen MF, Tonn JR, Graham RT, Harvey AE, Geier-Hayes K (1990) Nitrogen fixation in forest soils of the Inland Northwest. In ‘Management and productivity of western montane forest soils’. (Eds AE Harvey, LF Neuenschwand) pp. 101–109. (USDA Forest Service: Washington, USA)
Kallenbach CM, Grandy AS, Frey SD, Diefendorf AF (2015) Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biology and Biochemistry 91, 279–290.
| Microbial physiology and necromass regulate agricultural soil carbon accumulation.Crossref | GoogleScholarGoogle Scholar |
Keenan RJ, Reams GA, Achard F, Freitas JVD, Grainger A, Lindquist E (2015) Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. Forest Ecology and Management 352, 9–20.
| Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015.Crossref | GoogleScholarGoogle Scholar |
Kladivko EJ (2001) Tillage systems and soil ecology. Soil and Tillage Research 61, 61–76.
| Tillage systems and soil ecology.Crossref | GoogleScholarGoogle Scholar |
Knapp S, van der Heijden MGA (2018) A global meta-analysis of yield stability in organic and conservation agriculture. Nature Communications 9, 3632
| A global meta-analysis of yield stability in organic and conservation agriculture.Crossref | GoogleScholarGoogle Scholar | 30194344PubMed |
Koblenz B, Tischer S, Rücknagel J, Christen O (2015) Influence of biogas digestate on density, biomass and community composition of earthworms. Industrial Crops and Products 66, 206–209.
| Influence of biogas digestate on density, biomass and community composition of earthworms.Crossref | GoogleScholarGoogle Scholar |
Koch A, McBratney A, Adams M, Field D, Hill R, Crawford J, Minasny B, Lal R, Abbott L, O’Donnell A, Angers D, Baldock J, Barbier E, Binkley D, Parton W, Wall DH, Bird M, Bouma J, Chenu C, Flora CB, Goulding K, Grunwald S, Hempel J, Jastrow J, Lehmann J, Lorenz K, Morgan CL, Rice CW, Whitehead D, Young I, Zimmermann M (2013) Soil security: solving the global soil crisis. Global Policy 4, 434–441.
| Soil security: solving the global soil crisis.Crossref | GoogleScholarGoogle Scholar |
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annual Review of Microbiology 55, 485–529.
| Ammonia-oxidizing bacteria: a model for molecular microbial ecology.Crossref | GoogleScholarGoogle Scholar | 11544365PubMed |
Krishna C (2007) Fungal solid-state fermentation systems for bioconversion of lignocellulosic biomass: process protocol and applications. In ‘Manual of environmental microbiology, third edition’. (Eds C Hurst, R Crawford, J Garland, D Lipson, A Mills, L Stetzenbach) pp. 1107–1111. (American Society for Microbiology Press: Washington DC)
Kuntz M, Berner A, Gattinger A, Scholberg JM, Mäder P, Pfiffner L (2013) Influence of reduced tillage on earthworm and microbial communities under organic arable farming. Pedobiologia 56, 251–260.
| Influence of reduced tillage on earthworm and microbial communities under organic arable farming.Crossref | GoogleScholarGoogle Scholar |
Lal R (1993) Tillage effects on soil degradation, soil resilience, soil quality, and sustainability. Soil and Tillage Research 27, 1–8.
| Tillage effects on soil degradation, soil resilience, soil quality, and sustainability.Crossref | GoogleScholarGoogle Scholar |
Lal R, Reicosky DC, Hanson JD (2007) Evolution of the plow over 10,000 years and the rationale for no-till farming. Soil and Tillage Research 93, 1–12.
| Evolution of the plow over 10,000 years and the rationale for no-till farming.Crossref | GoogleScholarGoogle Scholar |
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Molecular Biology 90, 575–587.
| Plant root-microbe communication in shaping root microbiomes.Crossref | GoogleScholarGoogle Scholar | 26729479PubMed |
Lee EJ, Kenkel NC, Booth T (1996) Pollen deposition in the boreal forest of west central Canada. Canadian Journal of Botany 74, 1265–1272.
| Pollen deposition in the boreal forest of west central Canada.Crossref | GoogleScholarGoogle Scholar |
Légère A, Stevenson FC, Vanasse A (2011) Short communication: a corn test crop confirms beneficial effects of crop rotation in three tillage systems. Canadian Journal of Plant Science 91, 943–946.
| Short communication: a corn test crop confirms beneficial effects of crop rotation in three tillage systems.Crossref | GoogleScholarGoogle Scholar |
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528, 60–68.
| The contentious nature of soil organic matter.Crossref | GoogleScholarGoogle Scholar | 26595271PubMed |
Leroy BL, Van den Bossche A, De Neve S, Reheul D, Moens M (2007) The quality of exogenous organic matter: short-term influence on earthworm abundance. European Journal of Soil Biology 43, S196–S200.
| The quality of exogenous organic matter: short-term influence on earthworm abundance.Crossref | GoogleScholarGoogle Scholar |
Leroy BLM, Schmidt O, Van den Bossche A, Reheul D, Moens M (2008) Earthworm population dynamics as influenced by the quality of exogenous organic matter. Pedobiologia 52, 139–150.
| Earthworm population dynamics as influenced by the quality of exogenous organic matter.Crossref | GoogleScholarGoogle Scholar |
Leroy BL, De Sutter N, Ferris H, Moens M, Reheul D (2009) Short-term nematode population dynamics as influenced by the quality of exogenous organic matter. Nematology 11, 23–38.
| Short-term nematode population dynamics as influenced by the quality of exogenous organic matter.Crossref | GoogleScholarGoogle Scholar |
Liebman M, Davis AS (2000) Integration of soil, crop and weed management in low-external-input farming systems. Weed Research 40, 27–47.
| Integration of soil, crop and weed management in low-external-input farming systems.Crossref | GoogleScholarGoogle Scholar |
Lin BB (2011) Resilience in agriculture through crop diversification: adaptive management for environmental change. BioScience 61, 183–193.
| Resilience in agriculture through crop diversification: adaptive management for environmental change.Crossref | GoogleScholarGoogle Scholar |
Liu T, Chen X, Hu F, Ran W, Shen Q, Li H, Whalen JK (2016) Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agriculture Ecosystems and Environment 232, 199–207.
| Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities.Crossref | GoogleScholarGoogle Scholar |
Liu HY, Li J, Zhao Y, Xie KX, Tang XJ, Wang SX, Li ZP, Liao YL, Xu JM, Di HJ, Li Y (2018) Ammonia oxidizers and nitrite-oxidizing bacteria respond differently to long-term manure application in four paddy soils of south of China. The Science of the Total Environment 633, 641–648.
| Ammonia oxidizers and nitrite-oxidizing bacteria respond differently to long-term manure application in four paddy soils of south of China.Crossref | GoogleScholarGoogle Scholar |
Lodge DJ (1989) The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix. Plant and Soil 117, 243–253.
| The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix.Crossref | GoogleScholarGoogle Scholar |
Lori M, Symnaczik S, Mäder P, De Deyn G, Gattinger A (2017) Organic farming enhances soil microbial abundance and activity—a meta-analysis and meta-regression. PLoS One 12, e0180442
| Organic farming enhances soil microbial abundance and activity—a meta-analysis and meta-regression.Crossref | GoogleScholarGoogle Scholar | 28700609PubMed |
Luo Y, Keenan TF, Smith M (2015) Predictability of the terrestrial carbon cycle. Global Change Biology 21, 1737–1751.
| Predictability of the terrestrial carbon cycle.Crossref | GoogleScholarGoogle Scholar | 25327167PubMed |
Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296, 1694–1697.
| Soil fertility and biodiversity in organic farming.Crossref | GoogleScholarGoogle Scholar | 12040197PubMed |
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S, Grelle A, Hari P, Jarvis PG, Kolari P, Kowalski AS, Lankreijer H, Law BE, Lindroth A, Loustau D, Manca G, Moncrieff JB, Rayment M, Tedeschi V, Valentini R, Grace J (2007) The human footprint in the carbon cycle of temperate and boreal forests. Nature 447, 849–851.
| The human footprint in the carbon cycle of temperate and boreal forests.Crossref | GoogleScholarGoogle Scholar |
Mahendrappa M, Foster N, Weetman G, Krause H (1986) Nutrient cycling and availability in forest soils. Canadian Journal of Soil Science 66, 547–572.
| Nutrient cycling and availability in forest soils.Crossref | GoogleScholarGoogle Scholar |
Mahmood S, Prosser JI (2006) The influence of synthetic sheep urine on ammonia oxidizing bacterial communities in grassland soil. FEMS Microbiology Ecology 56, 444–454.
| The influence of synthetic sheep urine on ammonia oxidizing bacterial communities in grassland soil.Crossref | GoogleScholarGoogle Scholar | 16689876PubMed |
Mangalassery S, Mooney SJ, Sparkes DL, Fraser WT, Sjögersten S (2015) Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. European Journal of Soil Biology 68, 9–17.
| Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils.Crossref | GoogleScholarGoogle Scholar |
Markussen T, Happel EM, Teikari JE, Huchaiah V, Alneberg J, Andersson AF, Sivonen K, Riemann L, Middelboe M, Kisand V (2018) Coupling biogeochemical process rates and metagenomic blueprints of coastal bacterial assemblages in the context of environmental change. Environmental Microbiology 20, 3083–3099.
| Coupling biogeochemical process rates and metagenomic blueprints of coastal bacterial assemblages in the context of environmental change.Crossref | GoogleScholarGoogle Scholar | 30084235PubMed |
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biology and Biochemistry 33, 1437–1445.
| Soil and plant specific effects on bacterial community composition in the rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Meyer A, Grote R, Butterbach-Bahl K (2012) Integrating mycorrhiza in a complex model system: effects on ecosystem C and N fluxes. European Journal of Forest Research 131, 1809–1831.
| Integrating mycorrhiza in a complex model system: effects on ecosystem C and N fluxes.Crossref | GoogleScholarGoogle Scholar |
Mikola J, Setälä H, Virkajärvi P, Saarijärvi K, Ilmarinen K, Voigt W, Vestberg M (2009) Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecological Monographs 79, 221–244.
| Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture.Crossref | GoogleScholarGoogle Scholar |
Millennium Ecosystem Assessment(MEA) (2005) ‘Ecosystems and human well-being’. (Island Press: Washington, DC)
Mirás-Avalos JM, Antunes PM, Koch A, Khosla K, Klironomos JN, Dunfield KE (2011) The influence of tillage on the structure of rhizosphere and root-associated arbuscular mycorrhizal fungal communities. Pedobiologia 54, 235–241.
| The influence of tillage on the structure of rhizosphere and root-associated arbuscular mycorrhizal fungal communities.Crossref | GoogleScholarGoogle Scholar |
Morales SE, Jha N, Saggar S (2015) Impact of urine and the application of the nitrification inhibitor DCD on microbial communities in dairy-grazed pasture soils. Soil Biology and Biochemistry 88, 344–353.
| Impact of urine and the application of the nitrification inhibitor DCD on microbial communities in dairy-grazed pasture soils.Crossref | GoogleScholarGoogle Scholar |
Morford SL, Houlton BZ, Dahlgren RA (2016) Direct quantification of long-term rock nitrogen inputs to temperate forest ecosystems. Ecology 97, 54–64.
| Direct quantification of long-term rock nitrogen inputs to temperate forest ecosystems.Crossref | GoogleScholarGoogle Scholar | 27008775PubMed |
Munkholm LJ, Heck RJ, Deen B (2013) Long-term rotation and tillage effects on soil structure and crop yield. Soil and Tillage Research 127, 85–91.
| Long-term rotation and tillage effects on soil structure and crop yield.Crossref | GoogleScholarGoogle Scholar |
Munroe JW, McCormick I, Deen W, Dunfield KE (2016) Effects of 30 years of crop rotation and tillage on bacterial and archaeal ammonia oxidizers. Journal of Environmental Quality 45, 940–948.
| Effects of 30 years of crop rotation and tillage on bacterial and archaeal ammonia oxidizers.Crossref | GoogleScholarGoogle Scholar | 27136161PubMed |
Murchie AK, Blackshaw RP, Gordon AW, Christie P (2015) Responses of earthworm species to long-term applications of slurry. Applied Soil Ecology 96, 60–67.
| Responses of earthworm species to long-term applications of slurry.Crossref | GoogleScholarGoogle Scholar |
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
Nelson MB, Martiny AC, Martiny JBH (2016) Global biogeography of microbial nitrogen-cycling traits in soil. Proceedings of the National Academy of Sciences 113, 8033–8040.
| Global biogeography of microbial nitrogen-cycling traits in soil.Crossref | GoogleScholarGoogle Scholar |
Nivelle E, Verzeaux J, Habbib H, Kuzyakov Y, Decocq G, Roger D, Lacoux J, Duclercq J, Spicher F, Nava-Saucedo JE, Catterou M, Dubois F, Tetu T (2016) Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Applied Soil Ecology 108, 147–155.
| Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization.Crossref | GoogleScholarGoogle Scholar |
Nunan N, Singh B, Reid E, Ord B, Papert A, Squires J, Prosser JI, Wheatley RE, McNicol J, Millard P (2006) Sheep urine induced changes in soil microbial community structure. FEMS Microbiology Ecology 56, 310–320.
| Sheep urine induced changes in soil microbial community structure.Crossref | GoogleScholarGoogle Scholar | 16629760PubMed |
Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90, 2352–2359.
| Lack of belowground mutualisms hinders Pinaceae invasions.Crossref | GoogleScholarGoogle Scholar | 19769113PubMed |
O’Callaghan M, Gerard EM, Carter PE, Lardner R, Sarathchandra U, Burch G, Ghani A, Bell N (2010) Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biology and Biochemistry 42, 1425–1436.
| Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine.Crossref | GoogleScholarGoogle Scholar |
Odum EP, Connell CE, Davenport LB (1962) Population energy flow of three primary consumer components of old‐field ecosystems. Ecology 43, 88–96.
| Population energy flow of three primary consumer components of old‐field ecosystems.Crossref | GoogleScholarGoogle Scholar |
Oliverio AM, Gan HJ, Wickings K, Fierer N (2018) A DNA metabarcoding approach to characterize soil arthropod communities. Soil Biology and Biochemistry 125, 37–43.
| A DNA metabarcoding approach to characterize soil arthropod communities.Crossref | GoogleScholarGoogle Scholar |
Opperman MH, Wood M, Harris PJ, Cherrett CP (1993) Nematode and nitrate dynamics in soils treated with cattle slurry. Soil Biology and Biochemistry 25, 19–24.
| Nematode and nitrate dynamics in soils treated with cattle slurry.Crossref | GoogleScholarGoogle Scholar |
Orgiazzi A, Bardgett RD, Barrios E, Behan-Pelletier V, Briones MJI, Chotte JL, De Deyn GB, Eggleton P, Fierer N, Fraser T, Hedlund K, Jeffery S, Johnson NC, Jones A, Kandeler E, Kaneko N, Lavelle P, Lemanceau P, Miko L, Montanarella L, Moreira FMS, Ramirez KS, Scheu S, Singh BK, Six J, van der Putten WH, Wall DH (2016) ‘Global soil biodiversity atlas.’ (European Commission: Luxembourg)
Orwin KH, Bertram JE, Clough TJ, Condron LM, Sherlock RR, O’Callaghan M (2009) Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes. Applied Soil Ecology 42, 271–278.
| Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes.Crossref | GoogleScholarGoogle Scholar |
Pampolina NM, Dell B, Malajczuk N (2002) Dynamics of ectomycorrhizal fungi in an Eucalyptus globulus plantation: effect of phosphorus fertilization. Forest Ecology and Management 158, 291–304.
| Dynamics of ectomycorrhizal fungi in an Eucalyptus globulus plantation: effect of phosphorus fertilization.Crossref | GoogleScholarGoogle Scholar |
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG (2011) A large and persistent carbon sink in the world’s forests. Science 333, 988–993.
| A large and persistent carbon sink in the world’s forests.Crossref | GoogleScholarGoogle Scholar | 21764754PubMed |
Pan H, Ying S, Liu H, Zeng L, Zhang Q, Liu Y, Xu J, Li Y, Di H (2018) Microbial pathways for nitrous oxide emissions from sheep urine and dung in a typical steppe grassland. Biology and Fertility of Soils 54, 717–730.
| Microbial pathways for nitrous oxide emissions from sheep urine and dung in a typical steppe grassland.Crossref | GoogleScholarGoogle Scholar |
Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S, Mathieu O, Leveque J, Mougel C, Henault C, Lemanceau P, Pean M, Boiry S, Fontaine S, Maron PA (2013) Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 16, 810–822.
| Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect.Crossref | GoogleScholarGoogle Scholar |
Payn T, Carnus JM, Freer-Smith P, Kimberley M, Kollert W, Liu S, Orazio C, Rodriguez L, Silva LN, Wingfield MJ (2015) Changes in planted forests and future global implications. Forest Ecology and Management 352, 57–67.
| Changes in planted forests and future global implications.Crossref | GoogleScholarGoogle Scholar |
Peralta AL, Sun YM, McDaniel MD, Lennon JT (2018) Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 9, e02235
| Crop rotational diversity increases disease suppressive capacity of soil microbiomes.Crossref | GoogleScholarGoogle Scholar |
Pereg L, Morugán-Coronado A, McMillan M, García-Orenes F (2018) Restoration of nitrogen cycling community in grapevine soil by a decade of organic fertilization. Soil and Tillage Research 179, 11–19.
| Restoration of nitrogen cycling community in grapevine soil by a decade of organic fertilization.Crossref | GoogleScholarGoogle Scholar |
Pereira A, Pam A, Bini D, Durrer A, Robin A, Bouillet JP, Andreote FD, Ejbn C (2017) Shifts in the bacterial community composition along deep soil profiles in monospecific and mixed stands of Eucalyptus grandis and Acacia mangium. PLoS One 12, e0180371
| Shifts in the bacterial community composition along deep soil profiles in monospecific and mixed stands of Eucalyptus grandis and Acacia mangium.Crossref | GoogleScholarGoogle Scholar | 29176902PubMed |
Peterson CA, Eviner VT, Gaudin ACM (2018) Ways forward for resilience research in agroecosystems. Agricultural Systems 162, 19–27.
| Ways forward for resilience research in agroecosystems.Crossref | GoogleScholarGoogle Scholar |
Pfiffner L, Niggli U (1996) Effects of bio-dynamic, organic and conventional farming on ground beetles (col. Carabidae) and other epigaeic arthropods in winter wheat. Biological Agriculture and Horticulture 12, 353–364.
| Effects of bio-dynamic, organic and conventional farming on ground beetles (col. Carabidae) and other epigaeic arthropods in winter wheat.Crossref | GoogleScholarGoogle Scholar |
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WM (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nature Reviews. Microbiology 11, 789–799.
| Going back to the roots: the microbial ecology of the rhizosphere.Crossref | GoogleScholarGoogle Scholar | 24056930PubMed |
Phillips RP, Edward B, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist 199, 41–51.
| The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests.Crossref | GoogleScholarGoogle Scholar | 23713553PubMed |
Pierret A, Maeght JL, Clément C, Montoroi JP, Hartmann C, Gonkhamdee S (2016) Understanding deep roots and their functions in ecosystems: an advocacy for more unconventional research. Annals of Botany 118, 621–635.
| Understanding deep roots and their functions in ecosystems: an advocacy for more unconventional research.Crossref | GoogleScholarGoogle Scholar |
Plassard C, Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree Physiology 30, 1129–1139.
| Phosphorus nutrition of mycorrhizal trees.Crossref | GoogleScholarGoogle Scholar | 20631011PubMed |
Poeplau C, Don A (2015) Carbon sequestration in agricultural soils via cultivation of cover crops – a meta-analysis. Agriculture, Ecosystems and Environment 200, 33–41.
| Carbon sequestration in agricultural soils via cultivation of cover crops – a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Porco D, Decaëns T, Deharveng L, James SW, Skarżyński D, Erséus C, Butt KR, Richard B, Hebert PD (2013) Biological invasions in soil: DNA barcoding as a monitoring tool in a multiple taxa survey targeting European earthworms and springtails in North America. Biological Invasions 15, 899–910.
| Biological invasions in soil: DNA barcoding as a monitoring tool in a multiple taxa survey targeting European earthworms and springtails in North America.Crossref | GoogleScholarGoogle Scholar |
Postma-Blaauw MB, de Goede RG, Bloem J, Faber JH, Brussaard L (2010) Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 91, 460–473.
| Soil biota community structure and abundance under agricultural intensification and extensification.Crossref | GoogleScholarGoogle Scholar | 20392011PubMed |
Quiza L, St-Arnaud M, Yergeau E (2015) Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Frontiers in Plant Science 6, 507
| Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering.Crossref | GoogleScholarGoogle Scholar | 26236319PubMed |
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant and Soil 321, 341–361.
| The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms.Crossref | GoogleScholarGoogle Scholar |
Raimbault BA, Vyn TJ (1991) Crop rotation and tillage effects on corn growth and soil structural stability. Agronomy Journal 83, 979–985.
| Crop rotation and tillage effects on corn growth and soil structural stability.Crossref | GoogleScholarGoogle Scholar |
Raupp J, Pekrun C, Oltmanns M, Köpke U (2006) ‘Long-term field experiments in organic farming’. (Verlag Dr. H. J. Köster: Berlin)
Raymond J, Siefert JL, Staples CR, Blankenship RE (2004) The natural history of nitrogen fixation. Molecular Biology and Evolution 21, 541–554.
| The natural history of nitrogen fixation.Crossref | GoogleScholarGoogle Scholar | 14694078PubMed |
Read DS, Sheppard SK, Bruford MW, Glen DM, Symondson WOC (2006) Molecular detection of predation by soil micro-arthropods on nematodes. Molecular Ecology 15, 1963–1972.
| Molecular detection of predation by soil micro-arthropods on nematodes.Crossref | GoogleScholarGoogle Scholar | 16689911PubMed |
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytologist 170, 445–457.
| Soil biota and invasive plants.Crossref | GoogleScholarGoogle Scholar | 16626467PubMed |
Reivant Munters A (2014) The foliar bacterial endophyte community in native Pinus radiata: a role for protection against fungal disease? http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-234871
Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS (2001) Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant and Soil 233, 167–177.
| Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils.Crossref | GoogleScholarGoogle Scholar |
Roger-Estrade J, Anger C, Bertrand M, Richard G (2010) Tillage and soil ecology: partners for sustainable agriculture. Soil and Tillage Research 111, 33–40.
| Tillage and soil ecology: partners for sustainable agriculture.Crossref | GoogleScholarGoogle Scholar |
Rösch C, Bothe H (2009) Diversity of total, nitrogen-fixing and denitrifying bacteria in an acid forest soil. European Journal of Soil Science 60, 883–894.
| Diversity of total, nitrogen-fixing and denitrifying bacteria in an acid forest soil.Crossref | GoogleScholarGoogle Scholar |
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Applied and Environmental Microbiology 63, 4704–4712.
Ryan PR, Dessaux Y, Thomashow LS, Weller DM (2009) Rhizosphere engineering and management for sustainable agriculture. Plant and Soil 321, 363–383.
| Rhizosphere engineering and management for sustainable agriculture.Crossref | GoogleScholarGoogle Scholar |
Rygiewicz PT, Andersen CP (1994) Mycorrhizae alter quality and quantity of carbon allocated below ground. Nature 369, 58–60.
| Mycorrhizae alter quality and quantity of carbon allocated below ground.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Moreno S, Minoshima H, Ferris H, Jackson L (2006) Linking soil properties and nematode community composition: effects of soil management on soil food webs. Nematology 8, 703–715.
| Linking soil properties and nematode community composition: effects of soil management on soil food webs.Crossref | GoogleScholarGoogle Scholar |
Scherr SJ, McNeely JA (2008) Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 477–494.
| Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes.Crossref | GoogleScholarGoogle Scholar | 17652072PubMed |
Schloss PD, Handelsman J (2006) Toward a census of bacteria in soil. PLoS Computational Biology 2, e92
| Toward a census of bacteria in soil.Crossref | GoogleScholarGoogle Scholar | 16848637PubMed |
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56.
| Persistence of soil organic matter as an ecosystem property.Crossref | GoogleScholarGoogle Scholar |
Schmidt R, Gravuer K, Bossange AV, Mitchell J, Scow K (2018) Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS One 13, e0192953
| Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil.Crossref | GoogleScholarGoogle Scholar | 30036389PubMed |
Shi S, O’Callaghan M, Jones EE, Richardson AE, Walter C, Stewart A, Condron LM (2012) Investigation of organic anions in tree root exudates and rhizosphere microbial communities using in situ and destructive sampling techniques. Plant and Soil 359, 149–163.
| Investigation of organic anions in tree root exudates and rhizosphere microbial communities using in situ and destructive sampling techniques.Crossref | GoogleScholarGoogle Scholar |
Simard SW (2018) Mycorrhizal networks facilitate tree communication, learning, and memory. In ‘Memory and learning in plants’. (Eds F Baluska, M Gagliano, G Witzany) pp. 191–213. (Springer: Berlin, Heidelberg)
Simard SW, Austin M (2010) The role of mycorrhizas in forest soil stability with climate change. In ‘Climate change and variability’. (Ed. SW Simard) pp. 275–302. (IntechOpen: Online)
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Canadian Journal of Botany 82, 1140–1165.
| Mycorrhizal networks: a review of their extent, function, and importance.Crossref | GoogleScholarGoogle Scholar |
Sims JT, Maguire RO (2005) Manure management. In ‘Encyclopedia of soils in the environment’. (Ed. D Hillel) pp. 402–410. (Elsevier: New York)
Singh BK, Nunan N, Millard P (2009) Response of fungal, bacterial and ureolytic communities to synthetic sheep urine deposition in a grassland soil. FEMS Microbiology Ecology 70, 109–117.
| Response of fungal, bacterial and ureolytic communities to synthetic sheep urine deposition in a grassland soil.Crossref | GoogleScholarGoogle Scholar | 19622069PubMed |
Smaill SJ, Garrett LG (2016) Multi-rotation impacts of increased organic matter removal in planted forests. Journal of Soil Science and Plant Nutrition 16, 287–293.
| Multi-rotation impacts of increased organic matter removal in planted forests.Crossref | GoogleScholarGoogle Scholar |
Smaill SJ, Walbert K (2013) Fertilizer and fungicide use increases the abundance of less beneficial ectomycorrhizal species in a seedling nursery. Applied Soil Ecology 65, 60–64.
| Fertilizer and fungicide use increases the abundance of less beneficial ectomycorrhizal species in a seedling nursery.Crossref | GoogleScholarGoogle Scholar |
Smith SE, Read DJ (2010) ‘Mycorrhizal symbiosis.’ (Academic Press: Cambridge, UK)
Smith J, Wagner-Riddle C, Dunfield K (2010) Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Applied Soil Ecology 44, 138–146.
| Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring.Crossref | GoogleScholarGoogle Scholar |
Smith CR, Blair PL, Boyd C, Cody B, Hazel A, Hedrick A, Kathuria H, Khurana P, Kramer B, Muterspaw K, Peck C, Sells E, Skinner J, Tegeler C, Wolfe Z (2016) Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem. Ecology and Evolution 6, 8075–8084.
| Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem.Crossref | GoogleScholarGoogle Scholar | 27878079PubMed |
Snapp SS, Swinton SM, Labarta R, Mutch D, Black JR, Leep R, Nyiraneza J, O’Neil K (2005) Evaluating cover crops for benefits, costs and performance within cropping system niches. Agronomy Journal 97, 322–332.
| Evaluating cover crops for benefits, costs and performance within cropping system niches.Crossref | GoogleScholarGoogle Scholar |
Son Y (2001) Non-symbiotic nitrogen fixation in forest ecosystems. Ecological Research 16, 183–196.
| Non-symbiotic nitrogen fixation in forest ecosystems.Crossref | GoogleScholarGoogle Scholar |
Spurgeon DJ, Keith AM, Schmidt O, Lammertsma DR, Faber JH (2013) Land-use and land-management change: relationships with earthworm and fungi communities and soil structural properties. BMC Ecology 13, 46–59.
| Land-use and land-management change: relationships with earthworm and fungi communities and soil structural properties.Crossref | GoogleScholarGoogle Scholar | 24289220PubMed |
Stark N (1972) Nutrient cycling pathways and litter fungi. Bioscience 22, 355–360.
| Nutrient cycling pathways and litter fungi.Crossref | GoogleScholarGoogle Scholar |
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT, Flemming CA, White DC (1999) Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Applied and Environmental Microbiology 65, 95–101.
Tedersoo L, Bahram M, Polme S, Koljalg U, Yorou NS, Wijesundera R, Villarreal Ruiz L, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Poldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Partel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson KH, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo LD, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S (2014) Fungal biogeography. Global diversity and geography of soil fungi. Science 346, 1256688
| Fungal biogeography. Global diversity and geography of soil fungi.Crossref | GoogleScholarGoogle Scholar | 25430773PubMed |
Thompson KA, Deen B, Dunfield KE (2018) Impacts of surface-applied residues on N-cycling soil microbial communities in miscanthus and switchgrass cropping systems. Applied Soil Ecology 130, 79–83.
| Impacts of surface-applied residues on N-cycling soil microbial communities in miscanthus and switchgrass cropping systems.Crossref | GoogleScholarGoogle Scholar |
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia‐oxidizing archaea and bacteria in soil microcosms. Environmental Microbiology 10, 1357–1364.
| Growth, activity and temperature responses of ammonia‐oxidizing archaea and bacteria in soil microcosms.Crossref | GoogleScholarGoogle Scholar | 18325029PubMed |
Triplett GB, Dick WA (2008) No-tillage crop production: a revolution in agriculture! Agronomy Journal 100, 153–165.
| No-tillage crop production: a revolution in agriculture!Crossref | GoogleScholarGoogle Scholar |
Tsiafouli MA, Thebault E, Sgardelis SP, de Ruiter PC, van der Putten WH, Birkhofer K, Hemerik L, de Vries FT, Bardgett RD, Brady MV, Bjornlund L, Jorgensen HB, Christensen S, D’ Hertefeldt T, Hotes S, Hol WHG, Frouz J, Liiri M, Mortimer SR, Setala H, Tzanopoulos J, Uteseny K, Pizl V, Stary J, Wolters V, Hedlund K (2015) Intensive agriculture reduces soil biodiversity across Europe. Global Change Biology 21, 973–985.
| Intensive agriculture reduces soil biodiversity across Europe.Crossref | GoogleScholarGoogle Scholar | 25242445PubMed |
Turner TR, Ramakrishnan K, Walshaw J, Heavens D, Alston M, Swarbreck D, Osbourn A, Grant A, Poole PS (2013) Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. The ISME Journal 7, 2248–2258.
| Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants.Crossref | GoogleScholarGoogle Scholar | 23864127PubMed |
Uroz S, Calvaruso C, Turpault MP, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends in Microbiology 17, 378–387.
| Mineral weathering by bacteria: ecology, actors and mechanisms.Crossref | GoogleScholarGoogle Scholar | 19660952PubMed |
van Capelle C, Schrader S, Brunotte J (2012) Tillage-induced changes in the functional diversity of soil biota – a review with a focus on German data. European Journal of Soil Biology 50, 165–181.
| Tillage-induced changes in the functional diversity of soil biota – a review with a focus on German data.Crossref | GoogleScholarGoogle Scholar |
van der Bom F, Nunes I, Raymond NS, Hansen V, Bonnichsen L, Magid J, Nybroe O, Jensen LS (2018) Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biology and Biochemistry 122, 91–103.
| Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field.Crossref | GoogleScholarGoogle Scholar |
van der Heijden MGA, Hartmann M (2016) Networking in the plant microbiome. PLoS Biology 14, e1002378
| Networking in the plant microbiome.Crossref | GoogleScholarGoogle Scholar |
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. Journal of Ecology 97, 1139–1150.
| Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems.Crossref | GoogleScholarGoogle Scholar |
van Eekeren N, de Boer H, Bloem J, Schouten T, Rutgers M, de Goede R, Brussaard L (2009) Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers. Biology and Fertility of Soils 45, 595–608.
| Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers.Crossref | GoogleScholarGoogle Scholar |
Villenave C, Saj S, Attard E, Klumpp K, Le Roux X (2011) Grassland management history affects the response of the nematode community to changes in above-ground grazing regime. Nematology 13, 995–1008.
| Grassland management history affects the response of the nematode community to changes in above-ground grazing regime.Crossref | GoogleScholarGoogle Scholar |
Wagg C, Bender S, Widmer F, Van der Heijden M (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proceedings of the National Academy of Sciences 111, 5266–5270.
| Soil biodiversity and soil community composition determine ecosystem multifunctionality.Crossref | GoogleScholarGoogle Scholar |
Waite IS, O’Donnell AG, Harrison A, Davies JT, Colvan SR, Ekschmitt K, Dogan H, Wolters V, Bongers T, Bongers M, Bakonyi G, Nagy P, Papatheodorou EM, Stamou GP, Boström S (2003) Design and evaluation of nematode 18S rDNA primers for PCR and denaturing gradient gel electrophoresis (DGGE) of soil community DNA. Soil Biology and Biochemistry 35, 1165–1173.
| Design and evaluation of nematode 18S rDNA primers for PCR and denaturing gradient gel electrophoresis (DGGE) of soil community DNA.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA (2018) Managing soil microbiology: realising opportunities for the productive land-based sectors. New Zealand Journal of Agricultural Research 61, 358–376.
| Managing soil microbiology: realising opportunities for the productive land-based sectors.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biology and Biochemistry 40, 803–813.
| Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Gregg AL, Simpson RJ, Li GD, Riley IT, McKay AC (2009) Pasture management clearly affects soil microbial community structure and N-cycling bacteria. Pedobiologia 52, 237–251.
| Pasture management clearly affects soil microbial community structure and N-cycling bacteria.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Williams E, O’Sullivan CA, Cameron KC, Di HJ, Cave V, O’Callaghan M (2014) Predicting the efficacy of the nitrification inhibitor dicyandiamide in pastoral soils. Plant and Soil 381, 35–43.
| Predicting the efficacy of the nitrification inhibitor dicyandiamide in pastoral soils.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Cave VM, Dignam BE, D’Ath C, Tourna M, Condron LM, Zhou J, Van Nostrand JD, O’Callaghan M (2016) Analysis of soil eDNA functional genes: potential to increase profitability and sustainability of pastoral agriculture. New Zealand Journal of Agricultural Research 59, 333–350.
| Analysis of soil eDNA functional genes: potential to increase profitability and sustainability of pastoral agriculture.Crossref | GoogleScholarGoogle Scholar |
Wallenstein MD (2017) Managing and manipulating the rhizosphere microbiome for plant health: a systems approach. Rhizosphere 3, 230–232.
| Managing and manipulating the rhizosphere microbiome for plant health: a systems approach.Crossref | GoogleScholarGoogle Scholar |
Wang KH, McSorley R, Bohlen P, Gathumbi SM (2006) Cattle grazing increases microbial biomass and alters soil nematode communities in subtropical pastures. Soil Biology and Biochemistry 38, 1956–1965.
| Cattle grazing increases microbial biomass and alters soil nematode communities in subtropical pastures.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Zhu G, Song L, Wang S, Yin C (2014) Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. Journal of Basic Microbiology 54, 190–197.
| Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil.Crossref | GoogleScholarGoogle Scholar | 23686819PubMed |
Wang X, Han C, Zhang J, Huang Q, Deng H, Deng Y, Zhong W (2015) Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China. Soil Biology and Biochemistry 84, 28–37.
| Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China.Crossref | GoogleScholarGoogle Scholar |
Wardle DA (1995) Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Advances in Ecological Research 26, 105–185.
| Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices.Crossref | GoogleScholarGoogle Scholar |
Watmough SA, Dillon PJ (2003) Mycorrhizal weathering in base-poor forests. Nature 423, 823–824.
| Mycorrhizal weathering in base-poor forests.Crossref | GoogleScholarGoogle Scholar | 12815420PubMed |
Wessén E, Nyberg K, Jansson JK, Hallin S (2010) Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Applied Soil Ecology 45, 193–200.
| Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management.Crossref | GoogleScholarGoogle Scholar |
Whalen J, Parmelee R, Edwards C (1998) Population dynamics of earthworm communities in corn agroecosystems receiving organic or inorganic fertilizer amendments. Biology and Fertility of Soils 27, 400–407.
| Population dynamics of earthworm communities in corn agroecosystems receiving organic or inorganic fertilizer amendments.Crossref | GoogleScholarGoogle Scholar |
Williams EK, Plante AF (2018) A bioenergetic framework for assessing soil organic matter persistence. Frontiers of Earth Science 6, 143
| A bioenergetic framework for assessing soil organic matter persistence.Crossref | GoogleScholarGoogle Scholar |
Williams BL, Grayston SJ, Reid EJ (2000) Influence of synthetic sheep urine on the microbial biomass, activity and community structure in two pastures in the Scottish uplands. Plant and Soil 225, 175–185.
| Influence of synthetic sheep urine on the microbial biomass, activity and community structure in two pastures in the Scottish uplands.Crossref | GoogleScholarGoogle Scholar |
Xiong W, Jousset A, Guo S, Karlsson I, Zhao Q, Wu H, Kowalchuk GA, Shen Q, Li R, Geisen S (2018) Soil protist communities form a dynamic hub in the soil microbiome. The ISME Journal 12, 634–638.
| Soil protist communities form a dynamic hub in the soil microbiome.Crossref | GoogleScholarGoogle Scholar | 29028001PubMed |
Yang G, Wagg C, Veresoglou SD, Hempel S, Rillig MC (2018) How soil biota drive ecosystem stability. Trends in Plant Science 23, 1057–1067.
| How soil biota drive ecosystem stability.Crossref | GoogleScholarGoogle Scholar | 30287162PubMed |
Yao B, Di HJ, Cameron KC, Podolyan A, Shen JP, He JZ (2018) Understanding the mechanisms for the lower nitrous oxide emissions from fodder beet urine compared with kale urine from dairy cows. Journal of Soils and Sediments 18, 85–93.
| Understanding the mechanisms for the lower nitrous oxide emissions from fodder beet urine compared with kale urine from dairy cows.Crossref | GoogleScholarGoogle Scholar |
Yeates GW, Wardle DA, Watson RN (1999) Responses of soil nematode populations, community structure, diversity and temporal variability to agricultural intensification over a seven-year period. Soil Biology and Biochemistry 31, 1721–1733.
| Responses of soil nematode populations, community structure, diversity and temporal variability to agricultural intensification over a seven-year period.Crossref | GoogleScholarGoogle Scholar |
Young IM, Ritz K (2000) Tillage, habitat space and function of soil microbes. Soil and Tillage Research 53, 201–213.
| Tillage, habitat space and function of soil microbes.Crossref | GoogleScholarGoogle Scholar |
Zabowski D, Skinner M, Payn T (2007) Nutrient release by weathering: implications for sustainable harvesting of Pinus radiata in New Zealand soils. New Zealand Journal of Forestry Science 37, 336–354.
Zavattaro L, Bechini L, Grignani C, van Evert FK, Mallast J, Spiegel H, Sandén T, Pecio A, Giráldez Cervera JV, Guzmán G, Vanderlinden K, D’Hose T, Ruysschaert G, ten Berge HFM (2017) Agronomic effects of bovine manure: a review of long-term European field experiments. European Journal of Agronomy 90, 127–138.
| Agronomic effects of bovine manure: a review of long-term European field experiments.Crossref | GoogleScholarGoogle Scholar |
Zheng Y, Zhang LM, Zheng YM, Di HJ, He JZ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. Journal of Soils and Sediments 8, 406–414.
| Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices.Crossref | GoogleScholarGoogle Scholar |
Zhou X, Fornara D, Wasson EA, Wang D, Ren G, Christie P, Jia Z (2015) Effects of 44 years of chronic nitrogen fertilization on the soil nitrifying community of permanent grassland. Soil Biology and Biochemistry 91, 76–83.
| Effects of 44 years of chronic nitrogen fertilization on the soil nitrifying community of permanent grassland.Crossref | GoogleScholarGoogle Scholar |
Zibilske LM, Bradford JM, Smart JR (2002) Conservation tillage induced changes in organic carbon, total nitrogen and available phosphorus in a semi-arid alkaline subtropical soil. Soil and Tillage Research 66, 153–163.
| Conservation tillage induced changes in organic carbon, total nitrogen and available phosphorus in a semi-arid alkaline subtropical soil.Crossref | GoogleScholarGoogle Scholar |