Mitigation of nitrous oxide emissions with nitrification inhibitors in temperate vegetable cropping in southern Australia
D. A. Riches A D , S. W. Mattner B , R. Davies C and I. J. Porter A DA Department of Animal, Plant and Soil Sciences, La Trobe University, Bundoora, Vic. 3086, Australia.
B Victorian Strawberry Industry Certification Authority, 1015 Myers Road, Toolangi, Vic. 3777, Australia.
C BASF Australia Ltd, 28 Freshwater Place, Southbank, Vic. 3006, Australia.
D Corresponding authors. Email: d.riches@latrobe.edu.au; i.porter@latrobe.edu.au
Soil Research 54(5) 533-543 https://doi.org/10.1071/SR15320
Submitted: 30 October 2015 Accepted: 17 March 2016 Published: 18 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Intensive vegetable production in southern Australia is characterised by high inputs of nitrogen (N) fertiliser, water, and occasionally animal manures, which creates the potential for high nitrous oxide (N2O) emissions. Three field experiments were conducted to investigate the effects of the nitrification inhibitors 3, 4-dimethylpyrazole phosphate (DMPP), 3-methyl pyrazole plus 1H-1,2,4 triazole (3MP+TZ), and dicyandiamide (DCD) on N2O emissions and yields in broccoli (Brassica oleracea), lettuce (Lactuca sativa) and cauliflower (Brassica oleracea) crops in southern Australia. The inhibitor treatments on fertilisers and poultry manure were compared with standard commercial practice for vegetable crops in this region, and N2O emissions were measured using manual chambers through to harvest. Daily fluxes ranged from 0.81 g N2O-N ha–1 day–1 for untreated soil to 11.65 g N2O-N ha–1 day–1 for manure treated soil. Extrapolation of these results translate to annual emissions of 0.30 kg N2O-N ha–1 year–1 to 4.24 kg N2O-N ha–1 year–1, respectively. Cumulative soil N2O fluxes from the manure treatments were ~4-fold greater than the standard inorganic fertiliser program for a given crop. Nitrous oxide direct emission factors were in the range 0.02–0.16% for inorganic fertilisers and from 0.19% to 0.43% for poultry manure. The greatest decrease in N2O emissions occurred when DMPP or a combination of 3MP+TZ were added to poultry manure (62% and 66% decrease, respectively). Decreases in N2O emissions from nitrification inhibitors were smaller and less consistent when used with inorganic fertilisers, but DMPP decreased emissions in two out of three trials, with a maximum decrease of 32% observed in the broccoli trial. DCD proved ineffective for mitigating N2O emissions in all trials.
Additional keywords: dicyandiamide, dimethylpyrazole phosphate, fertiliser, manure, nitrogen.
Introduction
Intensive horticultural production is highly dependent on nitrogen (N) fertilisers and animal manures to maintain crop yields in the face of declining natural soil fertility. In high value horticulture, N inputs are often supplied in excess of the crop requirements to maximise yields. Addition of fertiliser N to crops creates potential environmental problems through both nitrate leaching and increased production of nitrous oxide (N2O), a potent greenhouse gas (GHG) (IPCC 2007) that also results in stratospheric ozone depletion (Crutzen 1981; Ravishankara et al. 2009). Estimates of the contribution of agriculture to global anthropogenic N2O emissions range from 58% to 84% (Mosier et al. 2004; Smith et al. 2007; Smith et al. 2008). Nitrous oxide production is favoured by high levels of available carbon (C), N and soil moisture (Weier et al. 1993; Dalal et al. 2003); conditions that often occur in vegetable production. This is especially the case when animal manures and N fertilisers are used in combination in the cropping system.
Improved N management in agriculture is essential to minimise detrimental environmental impacts while maximising yields and profitability. This can be achieved by increasing nutrient use efficiency through: (1) matching nutrient amount and timing of application to the crop requirements; and (2) using fertiliser products with lower potential for loss through leaching or gaseous emissions. Nitrification inhibitors (NIs) slow the conversion of ammonium to nitrate in the soil, thereby decreasing the potential for N loss through leaching and N2O production from nitrification and denitrification processes in soil (Chen et al. 2008). While a global meta-analysis has shown that NIs decrease N2O emissions by an average of 38% (Akiyama et al. 2010), to date there have been no studies on the high clay content, red sodosols in southern Australia. The few studies in other southern Australian vegetable production regions have been confined to sandy soils during winter cropping (Porter et al. 2014; Lam et al. 2015). As temperature and soil texture are known to influence the efficacy of NIs (Bronson et al. 1989), the findings from these studies cannot necessarily be extrapolated to the sodosols found in the Werribee region of Victoria. The Werribee South market garden area comprises in excess of 3000 ha and, combined with the immediate surrounding production areas, produce 10% of Victoria’s vegetables and 90%, 44% and 38% of the state’s cauliflower, broccoli and lettuce crops, respectively (ABS 2015). Currently there are several NI products available commercially, including DMPP (as ENTEC®, Incitec Pivot, Australia), 3MP+TZ (Piadin®, SKW Piesteritz, Germany) and DCD+TZ (Alzon®, SKW Piesteritz, Germany), but their efficacy for mitigating N2O emissions from soils in vegetable production systems in southern Australia is largely unknown. This study is the first report on the effect of DMPP and other NIs applied with manures and fertiliser on cumulative N2O emissions, yields and soil mineral N contents in the red sodosols of the Werribee South vegetable production region. This information will improve knowledge of GHG emissions from vegetable production and assess the potential for N2O mitigation using NIs.
Materials and methods
Study site
Three experiments were conducted on a commercial vegetable farm in Werribee South, Victoria, located ~35 km south-west of Melbourne, Victoria, Australia (37°56’S, 144°41’E, elevation 17 m above sea level). The climate is temperate with a mean annual rainfall of 538 mm. The mean daily minimum and maximum temperatures are 13.8°C and 25.7°C in summer and 5.0°C and 13.7°C in winter. The soil is classified as a Red Sodosol (Isbell 2002) with a texture (0–10 cm) consisting of 36.5% sand, 29.4% silt, 34.1% clay and a pH of 8.4. A description of a typical soil profile in the region can be obtained at http://vro.agriculture.vic.gov.au/dpi/vro/portregn.nsf/pages/werribee_soil_pit_wp1 (accessed 25 September 2015).
Crop management and treatments
At each site, treatments were selected to allow comparison with the standard fertiliser and manure application practices routinely used by commercial growers in the region. The aim of trials 1 and 2 was to determine the effect of NIs on cumulative N2O emissions, and the respective yields, of broccoli (Brassica oleracea) and lettuce (Lactuca sativa). Trial 2 also investigated the impact of NIs on N2O emissions from different base fertilisers with various urea, ammonium and nitrate contents. Trial 3 investigated the effects of NIs on cauliflower (Brassica oleracea) yields under decreased N application rates. Soil mineral N measurements were also taken in trial 3 to determine the effect NIs had on nitrification.
Trial 1: Broccoli 2011
After bed forming, composted poultry manure (3.5% total N, Fresh Weight (FW)) was applied at 5 t ha–1 (FW) to the full width of the bed on 1 March 2011. No inorganic fertiliser was applied to the manure treatments to enable direct comparison between the manure treatment without a NI (Manure) and with a NI (Manure+NI). The Manure+NI treatment had 4 L ha–1 of 3MP+TZ solution applied as a broadacre spray over the manure in a water volume equivalent to 3390 L ha–1. The manure was then incorporated using a rototiller. Container-grown broccoli seedlings (cultivar ‘Viper’) were planted on 2 March 2011 in beds spaced 1.62 m apart at a density of 30 000 transplants ha–1 (two transplant rows per bed; between plant spacing of 41 cm). The standard commercial treatment consisted of Nitrophoska Special® (NP) (Incitec Pivot, Australia) containing 6.5% NH4+-N and 5.5% NO3–-N applied as a base dressing at planting with a side dressing of calcium nitrate (CN) two weeks after planting (NP+CN) (Table 1). In the fertiliser treatments, NIs were compared at the same N dose (87 kg N ha–1) as the commercial treatment (NP+CN), with the N applied in a single application at planting. These treatments consisted of: (1) no NI (NP 87); (2) DMPP (DMPP NP 87) as ENTEC Nitrophoska Special® (Incitec Pivot, Australia); and (3) DCD+TZ (Alzon 87) containing a 50 : 50 mixture of Alzon® 46 (SKW Piesteritz, Germany) and a low N content NPK fertiliser. Fertilisers were applied to the transplant row by hand immediately after transplants were sown on 2 March 2011. The control treatment received no fertiliser until 20 April 2011 when a broadcast application of fertiliser was applied to the whole field. Gas samples for N2O measurement were collected until 11 March 2011 for treatment NP 87, 5 April 2011 for the NP+CN and Alzon 87 treatments, and 11 May 2011 (harvest) for Control, Manure, Manure+NI, and DMPP NP 87 treatments.
All fertiliser treatments were applied to plots 9.1 m in length and replicated four times in a randomised complete block design. The site was irrigated by overhead sprinklers according to commercial practice (typically 10–20 mm every few days as required). All plots received a broadcast application of 39 kg ha–1 of N as ammonium sulfate nitrate (N-Sure®, Incitec Pivot, Australia; 28.5 kg N as NH4+-N, 10.5 kg N as NO3–-N) on 20 April 2011. Broccoli heads at commercial maturity were harvested from the centre 5 m section of each plot on 4 May 2011, 7 May 2011 and 12 May 2011, and the total yield from each plot was calculated.
Trial 2: Lettuce 2011–2012
Container-grown iceberg lettuce seedlings (cultivar ‘Avatar’) were planted on 6 December 2011 in beds spaced 1.62 m apart at a density of 40 000 plants ha–1 (four transplant rows per bed). Fertilisers were applied into the planting furrow at sowing. Fertiliser treatments were applied to plots 10 m in length replicated three times in a randomised complete block design. Fertiliser treatments and N application rates are shown in Table 1 and consisted of: (1) no base fertiliser (Control); (2) Nitrophoska Special at 720 kg ha–1 (NP); (3) ENTEC Nitrophoska Special at 720 kg ha–1 (DMPP NP); (4) Nitrophoska Special + 2% DCD (dicyandiamide) at 720 kg ha–1 (DCD NP); (5) urea blend NPK base fertiliser (30 : 70 blend of urea and NPK base) at 403 kg ha–1 (Urea); (6) urea with DMPP (30 : 70 blend of Urea with ENTEC® (Incitec Pivot, Australia) and NPK base fertiliser) at 403 kg ha–1 (DMPP Urea); and (7) urea with DCD+TZ (30 : 70 blend of Alzon 46 and NPK base fertiliser) at 403 kg ha–1 (Alzon 46). All treatments, including the control (no base fertiliser), received a side dressing of 12 kg ha–1 N as Hydrocomplex® (Yara, Australia) on 21 December 2011, followed by fertigation on 14 January 2012 and 20 January 2012 with 15 kg ha–1 N as CN and 7.5 kg ha–1 N as Diamond White® (Campbells, Australia), respectively (Table 1). The site was irrigated by overhead sprinklers according to the standard commercial practice (typically 10–20 mm every few days as required). The crop was harvested on 24 January 2012 and yield was assessed on 120 lettuces from the middle of each plot.
Trial 3: Cauliflower 2013–2014
Container-grown cauliflower (cultivar ‘Pamplona’) seedlings were planted on 20 November 2013 in beds spaced 1.67 m apart at a density of 24 000 plants ha–1 (two transplant rows per bed, plant spacing 50 cm). Fertilisers were applied by hand in two bands over the top of transplants to plots 8 m in length, replicated four times in a randomised block factorial design on 21 November 2013. The factorial consisted of three rates of NP fertiliser at 50%, 75% and 100% of the standard commercial rate and three inhibitor treatments: (1) no NI; (2) DMPP (ENTEC Nitrophoska Special); and (3) 0.054% 3MP + 0.108% TZ (w/w) (Table 1). Fertilisers were applied in bands by hand over the transplant rows. Side dressings were made with CN + boron (Campbells, Australia) on 18 December 2013 and 9 January 2014 at 100, 75 and 50% of the commercial fertiliser rate (Table 1). Two additional treatments were included to determine the N2O emissions from Manure and Manure+NI without any inorganic fertiliser. Composted poultry manure (3.0% total N, FW) was applied at the rate of 5 t ha–1 (FW) in a band (30 cm wide) between the cauliflower transplant rows on 21 November 2013 and incorporated into the surface soil layer (0–10 cm). Four replicate plots were treated with manure in a split-plot design with half of each manure plot (randomly allocated) treated with DMPP solution at a rate equivalent to 1.90 kg DMPP ha–1 in 6000 L ha–1 of water. An additional control treatment received no fertiliser or manure. Cauliflower heads were harvested at commercial maturity on 28 January 2014, 31 January 2014, 3 February 2014, and 6 February 2014.
N2O measurements
Static chambers made from PVC pipe 15 cm diameter and 16 cm in height, with one end threaded to enable attachment of a cap, were used to measure N2O emissions. The chambers were driven into the ground to a depth of 8 cm, leaving a headspace height of 8 cm. In trials 1 and 3, one chamber was used in each experimental plot. In trial 2, two chambers were used in each plot. Chambers were inserted into the plant rows to cover the fertiliser bands in all trials and in similar positions in control plots. This chamber placement allowed direct comparison of the effect of NIs on N2O emissions without any dilution effect of the untreated soils. Cumulative N2O emissions were calculated for both the chamber area and the entire field by partitioning the field into treated and untreated areas and assuming that the non-treated soil in fertilised plots had the same N2O flux as the unfertilised control treatment. Chamber caps consisted of a threaded PVC lid with a rubber O-ring to form an airtight seal. A 13 mm rubber septum was fitted into the cap. At each sampling, gas samples were collected from chambers at fixed intervals after capping, by inserting a double-ended needle into the cap septum and also into a 12 mL evacuated sample tube (Exetainer, Labco UK). Chamber air temperature during the closure (capped) time was recorded with a data logger (Tinytag, Gemini loggers UK) inside one chamber on each sampling occasion.
Gas sampling occurred 1, 2, 3, 5, 7, 10, and 13 days after fertiliser applications, and weekly thereafter. For all trials sampling coincided with the scheduled commercial irrigations, which often occurred daily during the plant establishment phase. In trial 1, the majority of gas samples were collected between 1100 and 1600 hours following irrigation. Sampling usually commenced within 1 to 2 h of irrigation when soil moisture was elevated. In this trial, soil temperatures were relatively constant due to mild air temperatures (autumn cropping) and the cooling effects of irrigation. For trial 2, the majority of gas samples were collected between 1200 and 1430 hours. For trials 1 and 2, gas samples were collected immediately before (0 min) capping chambers and 60 min after closure. Time course measurements were taken at 15 min intervals over 1.25 h on selected sampling occasions. N2O flux generally showed a linear trend over the measurement period. For trial 3, gas samples were collected 0, 20 and 40 min after capping using a syringe and needle before transfer to an evacuated tube. Samples were collected between 1100 and 1300 hours, a timing most likely to represent the average daily N2O flux (Scheer et al. 2014). For trial 3, soil volumetric water content (VWC) was measured at each gas sampling with a HydroSense soil water monitoring system (Model CS620, 12 cm probes, Campbell Scientific, Australia). Water-filled pore space (WFPS) was calculated using the soil bulk density and assuming a particle density of 2.65 g cm–3 (Werner et al. 2006). Rainfall, irrigation and soil WFPS data for trial 3 (cauliflower) is shown in Fig. 1. While soil moisture data was not available for trials 1 and 2, they used the same irrigation regime as trial 3, so similar average moisture conditions were expected.

|
Gas samples were analysed for N2O concentrations by gas chromatography in the laboratory using the method of Rowlings et al. (2012). Nitrous oxide fluxes were determined from the linear increase in concentration over the closure period, correcting for air temperature and atmospheric pressure. It was assumed that the daily flux changed linearly between sampling dates, and cumulative N2O fluxes were estimated by integration using the trapezoidal rule. Cumulative N2O fluxes were calculated separately for both the chamber area and the whole plot. Whole plot fluxes from planting until harvest were estimated by assuming the untreated area had the same emission rate as the unfertilised control using Eqn 1.
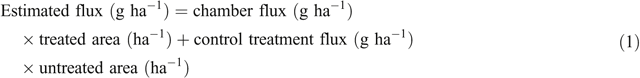
This allowed a comparison of NI effect within the chamber and an estimation of N2O emissions per hectare. In trial 1, when treatments were sampled late in the season and N2O fluxes had dropped to near background levels before sampling ended, the flux rate of the last three sampling points were assumed to be constant through to harvest to calculate cumulative emissions for the entire cropping period. Direct N2O emission factors for fertiliser and manure treatments (proportion of applied N emitted as N2O) were calculated by subtracting the N2O emission from the control treatment as the background emission (Rashti et al. 2015).
Soil N measurements
In trial 3, soil was collected from each plot by taking three cores of 3 cm diameter to a depth of 10 cm from three evenly spaced points (one next to each plant row and another from the plot centre) on a diagonal transect across the bed. Samples were air-dried at 40°C for 48 h before analysis for mineral N (NO3– and NH4+). The dried soil samples were extracted with 2M KCl and the NO3–-N and NH4+-N concentrations were determined colourimetrically in filtered extracts (Rayment and Lyons 2011).
Statistical analysis
Statistical analysis was performed using Genstat (version 16, VSI International). Analysis of variance (ANOVA) was used to test for significance of treatment effects for yield, N2O emissions and soil mineral N data. Where data were not normally distributed, data were transformed before analysis.
Results
Yield
Trial 1: Broccoli
Broccoli yields for treatments that received fertiliser or manure (8.2, 8.2, 8.6, 8.7, 8.8 and 9.5 t ha–1 for treatments DMPP NP 87, Alzon 87, Manure, Manure+NI, NP 87 and NP+CN 87, respectively) were not significantly different, but all were significantly higher than the control treatment (6.9 t ha–1).
Trial 2: Lettuce
Lettuce yields were significantly higher in all fertiliser treatments (22.6, 24.0, 24.9, 25.2, 26.8 and 28.4 t ha–1 for treatments DMPP NP, Urea, Alzon, DMPP urea, DCD NP and NP, respectively) than the control treatment (17.8 t ha–1). The addition of DMPP to the Nitrophoska (nitrate/ammonium N) base fertiliser (DMPP NP) resulted in a significantly lower yield than Nitrophoska without NI (NP) or with DCD (DCD NP). There were no significant differences in yield between the urea base fertiliser treatments (Urea, Alzon and DMPP urea).
Trial 3: Cauliflower
Cauliflower yield was 55% higher in plots treated with all rates of fertilisers (mean 42.4 t ha–1) compared with the untreated control (27.4 t ha–1). There was no significant effect of either fertiliser rate or NI on yield. There was no significant difference (P = 0.64) in cauliflower yields between the Manure treatment and the Manure+NI treatment (35.6 and 33.8 t ha–1, respectively) and the untreated control (27.4 t ha–1).
N2O emissions
Trial 1: Broccoli
There was an initial high N2O flux from soil in the period following the base fertiliser application at planting (Fig. 2a). Comparing N2O flux within the chamber area when fertilisers were applied as a single basal application of 87 kg N ha–1, the cumulative N2O emissions over the first 10 days of the crop for DMPP NP 87 decreased by ~70% compared with NP 87 (Fig. 2b) and was not significantly different to the untreated control. No decrease in cumulative N2O emissions occurred for the Alzon 87 treatment over this 10 day period. The cumulative N2O emissions within the chamber area over the first 35 days of crop growth decreased by 75% in the DMPP NP 87 treatment compared with the standard commercial practice of applying NP at planting with a side dressing of CN (NP+CN 87). The Alzon 87 treatment had higher cumulative N2O emissions than the DMPP NP 87 treatment but was not significantly different to the NP+CN 87 treatment. Calculated emission reductions for NI treatments on a whole plot basis were smaller than those calculated for just the chamber area.
Cumulative N2O emission from the Manure treatment was approximately 5-fold higher than the standard commercial practice treatment (NP+CN 87) (Fig. 2c and 2d), although the N application rate per hectare was approximately double for the Manure treatment. Cumulative N2O emission was ~60% lower in the Manure+NI treatment compared with the Manure treatment on both chamber area and whole plot basis.
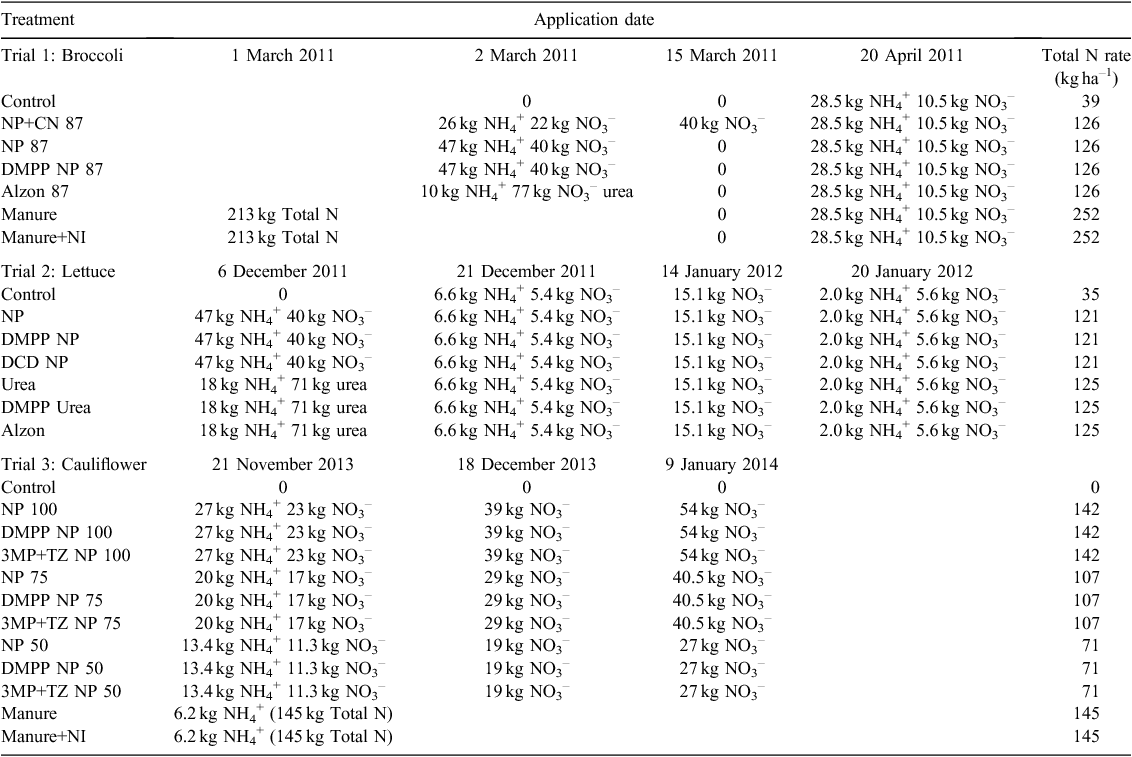
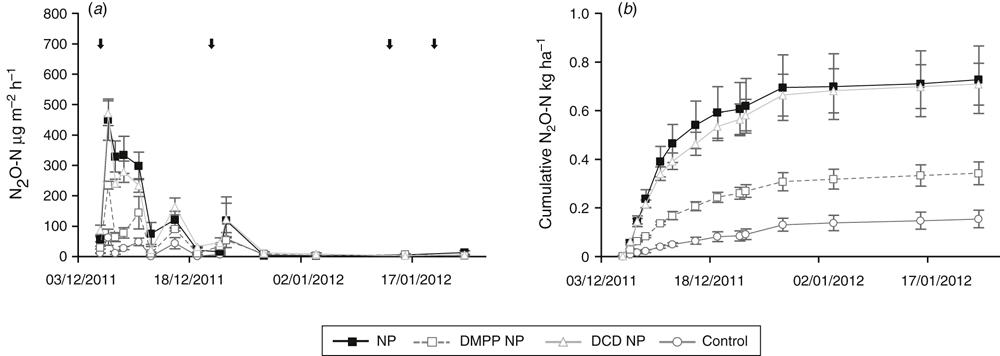
Trial 2: Lettuce
There was a period of high N2O flux from soil in the first 10 days following base fertiliser application at planting (Fig. 3a and Fig. 4a). Cumulative N2O emissions for the chamber area over the growing season decreased by more than 50% when DMPP was added to NP (nitrate/ammonium N) or urea fertiliser compared with fertilisers applied without DMPP (Fig. 3b and Fig. 4b). Emission decreases with DMPP on a per plot basis were ~28% and 36% for NP and urea-based fertilisers respectively. DCD was not effective in decreasing cumulative N2O emissions from NP fertiliser or when it was used in combination with TZ with urea N fertiliser.
Trial 3: Cauliflower
Daily N2O fluxes were lower in inorganic fertiliser plus NI treatments and the untreated control, compared with commercial fertiliser treatments for the period between base fertiliser application and the side dress fertiliser application ~1 month later (Fig. 5a). However, whilst cumulative fluxes with the NI treatments were similar to the control, and 32–45% lower than the commercial fertiliser treatment over the same period, the large variation in emissions from replicates meant they were not significantly different (Fig. 5b). Cumulative N2O flux over the entire cropping period was significantly lower for the control treatment compared with the commercial fertiliser treatment.

|
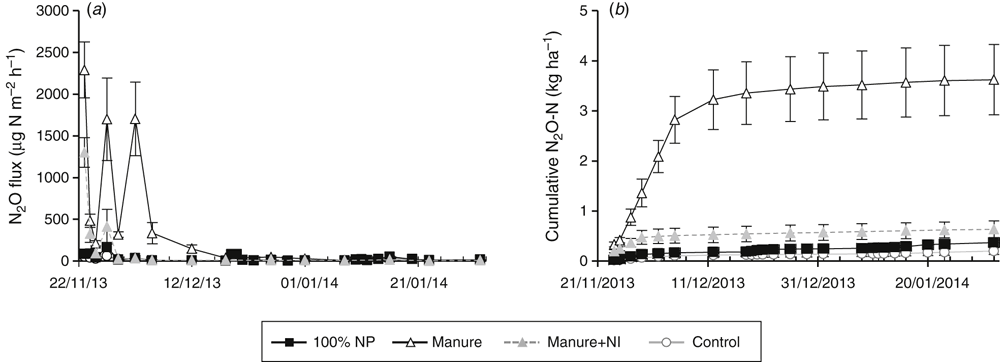
There was a period of very high N2O flux (peaks of >2000 µg m2 h–1) during the first 12 days of the study (Fig. 6a) in the poultry manure treatment. Cumulative N2O flux for the chamber area from the poultry manure treatment was ~10 times higher than the inorganic fertiliser treatment over the entire cropping period (Fig. 6b). The addition of DMPP to manure decreased cumulative N2O flux by more than 80%. The high N2O flux in the manure persisted for approximately one month before dropping back to levels similar to the control treatment (Fig. 6a).
|
Comparison of whole plot daily emissions from the three trials and emission factors
The average daily N2O flux on a whole plot basis from the control treatment was approximately three times higher in trials 2 (lettuce) and 3 (cauliflower) compared with trial 1 (broccoli) (Table 2). In the trials where manure was used (Trials 1 and 3), total N2O flux from manures without NI was ~4-fold higher than the inorganic fertiliser programme. In trials 1 and 2, the DMPP treatments were the only NI treatments that decreased total N2O flux relative to the commercial fertiliser programs. The addition of NI to manure decreased total N2O flux by 62% in trial 1 (3MP+TZ) and by 66% in trial 2 (DMPP). The proportion of fertiliser N or manure N emitted as N2O over the measurement period ranged from 0.02% to 0.16% for inorganic fertilisers and from 0.19% to 0.43% for Manure+NI (Table 2).

|
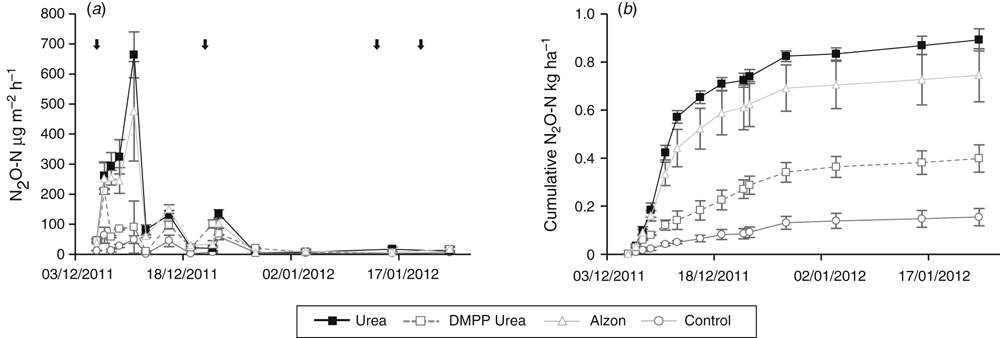
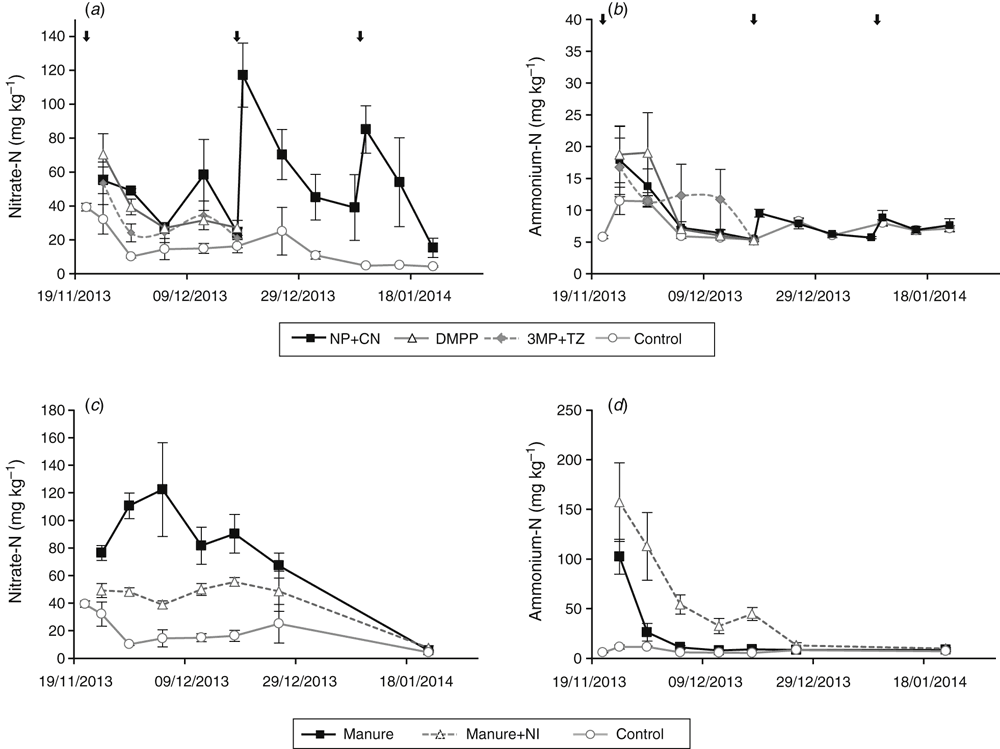
Soil nitrogen
Differences in soil nitrate (NO3–) were small for the inorganic fertiliser treatments following the base fertiliser application, but there was a large increase in soil NO3– observed after the CN top dressing (Fig. 7a). There was little difference in soil ammonium (NH4+) concentrations between fertiliser treatments (Fig. 7b). The Manure+NI treatment had lower soil NO3– and higher soil NH4+ concentrations than the Manure treatment (Fig. 7c, d). Both manure treatments had higher soil NO3– and NH4+ concentrations than the control treatment.
Discussion
Estimated average daily N2O fluxes from inorganic fertiliser over the period from planting to harvest in the current trials were in the range 1.2–6.9 g ha–1 day–1 N2O-N, which are higher than those previously observed in lettuce (1.0–3.0 g ha–1 day–1) (Rashti et al. 2015) and broccoli (0.33–0.97 g ha–1 day–1) (Scheer et al. 2014) trials in sub-tropical Australia. When poultry manure was applied to soil, N2O fluxes were ~4-fold greater than emissions from inorganic fertilisers for the whole plot area (and up to 10 times greater for the chamber area), with approximately double the N application rate for manure (trial 1) or similar N application rates (trial 3). Daily fluxes ranged from 0.81 g N2O-N ha–1 day–1 for untreated soil to 11.65 g N2O-N ha–1 day–1 for manure treated soil. These results for the cropping period translated to annual emissions of 0.30 kg N2O-N ha–1 year–1 to 4.24 kg N2O-N ha–1 year–1 respectively. Other studies have also shown that the addition of animal manures result in large increases in N2O emissions from agricultural soils relative to background levels (Chang et al. 1998; Khalil et al. 2002; Rochette et al. 2008; Dalal et al. 2009; Jäger et al. 2011; Mori and Hojito 2012). Field studies on grassland soils amended with cattle manures have recorded peak emissions of >1.6 mg m–2 h–1 (Mori and Hojito 2012), which is similar to the maximum emissions from manure treatments observed in this study of 2.3 mg m–2 h–1. In general, poultry manure contains greater amounts of readily mineralisable N and C than cattle manures (Chadwick et al. 2000) and thus would be likely to have greater N2O emissions (Velthof et al. 2003).
Commercial vegetable production practices, where manures are used in combination with inorganic fertilisers, would be expected to produce greater N2O emissions than observed in this study where the manure was the sole N addition, due to the increased availability of both mineral N and labile C. Peak emissions of 10 mg m–2 h–1 N2O-N have been observed in a groundnut–maize rotation where poultry manure was used in combination with crop residues and inorganic N (Khalil et al. 2002).
The period of elevated N2O emissions following manure application in this study was ~3 to 4 weeks in duration which agrees with the findings of Rochette et al. (2008) who reported 60–90% of the seasonal N2O emission occurred within the first 40 days after manure application. When manure is applied at commercial rates (5–10 t ha–1), as well as contributing a large amount of N – typically up to 300 kg N ha–1 (mainly as organic N) – the availability of soil organic C is also increased. This favours N2O production by increasing substrate availability for microbial activity (Weier et al. 1993; Dalal et al. 2003), and the resultant increased microbial respiration decreases oxygen availability leading to more anaerobic soil conditions which promotes denitrification (Dalal et al. 2003; Jäger et al. 2011). Large increases in N2O emissions have also been observed following other organic matter (cane trash) additions in sugarcane crops (Weier 1996) or vegetables when crop residue is incorporated post-harvest (Baggs et al. 2000; Scheer et al. 2014).
In this study, NIs decreased N2O emissions in the chamber area by more than 80% when applied with manure, or ~60% on a whole plot basis taking into account both the treated and untreated areas. This is a considerably greater decrease in N2O emissions compared with another study where a 44% decrease in N2O emissions from DMPP applied to poultry manure was observed in a celery crop grown in a sandy soil (Lam et al. 2015). Large decreases in N2O emissions (48–49%) have also been observed from cattle manure slurries applied to grasslands when treated with DMPP (Merino et al. 2005). In this study, the mechanism for the decrease in N2O emissions from manure plus DMPP appeared to be through decreased soil nitrate concentrations, with more soil N retained as ammonium. The decrease in soil nitrate with DMPP usage is also likely to lower the potential for nitrate leaching (Zerulla et al. 2001; Trenkel 2010).
When the NIs, DMPP and 3MP+TZ, were applied to inorganic fertilisers, N2O emission mitigation results were more variable, but in two of the three trials decreases in cumulative N2O emissions of 50% or more occurred in the fertiliser treated area during the cropping period, and decreases up to 36% occurred for the whole plot area. The magnitude of these decreases is similar to those observed in previous vegetable experiments (Pfab et al. 2012) and grain cropping (Weiske et al. 2001; Liu et al. 2013). Decreases in N2O emissions with these NIs occurred irrespective of whether the N source was ammonium or urea. In contrast, when DCD was used either alone with an ammonium and nitrate N fertiliser, or in combination with TZ in a urea based fertiliser, cumulative N2O emissions did not decrease. DCD has been less effective than DMPP and other NIs for reducing N2O emissions in laboratory (Kou et al. 2015; Chaves et al. 2006) and field experiments (Weiske et al. 2001; Zhang et al. 2015), although efficacy was similar for DCD and DMPP in a wheat–maize system (Liu et al. 2013). A laboratory study has shown DMPP was able to inhibit nitrification for at least twice as long as DCD under the same conditions (Chaves et al. 2006). The temperature regime in this laboratory experiment was lower than the soil temperatures in our field experiments. Field experiments have also shown a longer duration of nitrification inhibition for DMPP compared with DCD and this was related to faster decomposition kinetics for DCD (Weiske et al. 2001). The high water solubility, and thus mobility, of DCD may result in leaching through the soil profile which will also limit its effectiveness (Weiske et al. 2001; Vogeler et al. 2007). DMPP has similar mobility in soil to ammonium (Trenkel 2010) and would be less prone to leaching. In heavily irrigated summer grown vegetable crops, such as those in this study, these factors may partially explain the difference in effectiveness between DMPP and DCD. The effectiveness of DCD, and possibly DMPP, may have decreased because of the elevated soil temperatures (max. soil temperature frequently >25°C) during the Australian summer/early autumn conditions under which this study was conducted, as both NIs are reported to be less effective at elevated temperatures (Irigoyen et al. 2003; Di and Cameron 2004).
This preliminary work suggests that certain NIs have the potential to significantly decrease N2O emissions from soils in temperate vegetable production systems, particularly when composted animal manures are used. The use of NIs with organic products, such as manures, is likely to result in greater decreases in N2O emissions compared with inorganic fertiliser by decoupling the supply of easily oxidisable C and the supply of NO3–-N as the substrate for denitrification (Ruser and Schulz 2015). The current commercial availability of DMPP and other NI-treated fertiliser products and liquid NI formulations means that immediate adoption of NIs is possible if the industry can see a benefit. The key benefit of using NIs, as determined in the current study, is environmental through decreased N2O emissions and the potential for lowering nitrate leaching, rather than by increasing yields. Increased yields relative to current farm practices is not a sensible aim as growers are currently using N rates in excess of the crop requirement to maximise yield. However, the results of this study indicate that there is scope for decreasing N application rates in these production systems without yield loss, thereby increasing farm profits and making NIs more likely to be adopted. Results from the cauliflower trial showed growers could potentially lower application rates by 25% to 50% without any decrease in yield. Using NIs with lower fertiliser rates may be expected to decrease N2O emissions considerably, since the relationship between N2O emissions and N input is reported to be exponential rather than linear (Shcherbak et al. 2014).
In our trials, the N2O direct emission factors calculated for the period from planting until harvest were in the range 0.02–0.16% for inorganic fertilisers and from 0.19% to 0.43% for manure with NI. These values are considerably lower than the default IPCC values of 1% (IPCC 2006) but are similar in those determined by Rashti et al. (2015). Because gas measurements in the current work were not continued post-harvest, this may have resulted in an underestimation of the true total emissions from treatments. Research in sub-tropical Australian vegetable cropping has shown N2O emissions in the fallow period after incorporation of crop debris can be much greater than those produced from planting until harvest (Scheer et al. 2014). As N2O flux rates and cumulative emissions observed during the cropping period in these sub-tropical studies were much lower than those observed during the cropping period in this study, post-harvest emissions from crop residues may comprise a considerably smaller proportion of the total emissions (within crop + post-harvest) in southern Australian when manure is used in the production system. Further studies are required to determine if this relationship is consistent in temperate vegetable systems. Additional studies also need to look at ways to optimise N use efficiency, including the use of lower fertiliser rates to achieve the same maximum yields and continuing evaluation of the environmental and economic benefits.
Acknowledgements
This work was funded by the Australian Department of Agriculture, Fisheries and Forestry and Incitec-Pivot Limited. The authors would like to thank James Carthcart for providing some of the fertiliser/nitrification products used and Harry Velisha for providing the trial sites.
References
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Global Change Biology 16, 1837–1846.| Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Australian Bureau of Statistics (ABS) (2015) ‘Agricultural Commodities Australia 2013–2014 No. 71210.’ (ABS: Canberra, ACT).
Baggs EM, Rees RM, Smith KA, Vinten AJA (2000) Nitrous oxide emission from soils after incorporating crop residues. Soil Use and Management 16, 82–87.
| Nitrous oxide emission from soils after incorporating crop residues.Crossref | GoogleScholarGoogle Scholar |
Bronson KF, Touchton JT, Hauck RD (1989) Decomposition rate of dicyandiamide and nitrification inhibition. Communications in Soil Science and Plant Analysis 20, 2067–2078.
| Decomposition rate of dicyandiamide and nitrification inhibition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhtFOktbY%3D&md5=10048b55d5153af411fbb67f35f994b5CAS |
Chadwick DR, John F, Pain BF, Chambers BJ, Williams J (2000) Plant uptake of nitrogen from the organic nitrogen fraction of animal manures: a laboratory experiment. The Journal of Agricultural Science 134, 159–168.
| Plant uptake of nitrogen from the organic nitrogen fraction of animal manures: a laboratory experiment.Crossref | GoogleScholarGoogle Scholar |
Chang C, Janzen HH, Cho CM (1998) Nitrous oxide emission from long-term manured soils. Soil Science Society of America Journal 62, 677–682.
| Nitrous oxide emission from long-term manured soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXks1eqsb4%3D&md5=d2ac91a567ad4debe3f7063514eaa53eCAS |
Chaves B, Opoku A, De Neve S, Boeckx P, Van Cleemput O, Hofman G (2006) Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biology and Fertility of Soils 43, 62–68.
| Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosFWktro%3D&md5=05304825ebf7401cd345e22a9a9dae69CAS |
Chen D, Suter H, Islam A, Edis R, Freney JR, Walker CN (2008) Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers. Soil Research 46, 289–301.
| Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1OktLw%3D&md5=fec61f7dd15fe4ea2a2bc5ea7113200cCAS |
Crutzen PJ (1981) Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide. In ‘Denitrification, nitrification and atmospheric nitrous oxide’. (Ed. CC Delwiche) pp. 17–44. (Wiley: New York, NY)
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
Dalal R, Gibson I, Menzies N (2009) Nitrous oxide emission from feedlot manure and green waste compost applied to Vertisols. Biology and Fertility of Soils 45, 809–819.
| Nitrous oxide emission from feedlot manure and green waste compost applied to Vertisols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGisrnO&md5=824ad8dd0fdab041ea63d6f3191f95e8CAS |
Di HJ, Cameron KC (2004) Effects of temperature and application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate and microbial biomass in a grazed pasture soil. Soil Research 42, 927–932.
| Effects of temperature and application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate and microbial biomass in a grazed pasture soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOqsLnF&md5=9407b3bc4e533d19ff75495f9a3413ecCAS |
Intergovernmental Panel on Climate Change (IPCC) (2007) Summary for policymakers. In ‘Climatic Change 2007: the physical science basis’. (IPCC: Geneva, Switzerland)
IPCC (2006) ‘IPCC guidelines for national greenhouse gas inventories. Agriculture, forestry and other land use. Vol. 4’. (IGES: Kanagawa, Japan)
Irigoyen I, Muro J, Azpilikueta M, Aparicio-Tejo P, Lamsfus C (2003) Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Soil Research 41, 1177–1183.
| Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1Cgt70%3D&md5=92d1b1fadc60b4d0482344e6dd14bd3cCAS |
Isbell RF (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Jäger N, Stange C, Ludwig B, Flessa H (2011) Emission rates of N2O and CO2 from soils with different organic matter content from three long-term fertilization experiments—a laboratory study. Biology and Fertility of Soils 47, 483–494.
| Emission rates of N2O and CO2 from soils with different organic matter content from three long-term fertilization experiments—a laboratory study.Crossref | GoogleScholarGoogle Scholar |
Khalil MI, Rosenani AB, Van Cleemput O, Fauziah CI, Shamshuddin J (2002) Nitrous oxide emissions from an ultisol of the humid tropics under maize–groundnut rotation. Journal of Environmental Quality 31, 1071–1078.
| Nitrous oxide emissions from an ultisol of the humid tropics under maize–groundnut rotation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlslOmsLw%3D&md5=21d3db0526cefc449894fdafeeeb50c5CAS | 12175023PubMed |
Kou Y, Wei K, Chen G, Wang Z, Xu H (2015) Effects of 3, 4-dimethylpyrazole phosphate and dicyandiamide on nitrous oxide emission in a greenhouse vegetable soil. Plant, Soil and Environment 61, 29–35.
Lam SK, Suter H, Davies R, Bai M, Sun J, Chen D (2015) Measurement and mitigation of nitrous oxide emissions from a high nitrogen input vegetable system. Scientific Reports 5, 1–4.
| Measurement and mitigation of nitrous oxide emissions from a high nitrogen input vegetable system.Crossref | GoogleScholarGoogle Scholar |
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat–maize cropping system. Biogeosciences 10, 2427–2437.
| Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltlGktLg%3D&md5=7ae6f766dbe0c2a108f66906ecb09266CAS |
Merino P, Menéndez S, Pinto M, González-Murua C, Estavillo JM (2005) 3, 4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use and Management 21, 53–57.
| 3, 4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application.Crossref | GoogleScholarGoogle Scholar |
Mori A, Hojito M (2012) Effect of combined application of manure and fertilizer on N2O fluxes from a grassland soil in Nasu, Japan. Agriculture, Ecosystems & Environment 160, 40–50.
| Effect of combined application of manure and fertilizer on N2O fluxes from a grassland soil in Nasu, Japan.Crossref | GoogleScholarGoogle Scholar |
Mosier A, Wassmann R, Verchot L, King J, Palm C (2004) Methane and nitrogen oxide fluxes in tropical agricultural soils: Sources, sinks and mechanisms. Environment, Development and Sustainability 6, 11–49.
| Methane and nitrogen oxide fluxes in tropical agricultural soils: Sources, sinks and mechanisms.Crossref | GoogleScholarGoogle Scholar |
Pfab H, Palmer I, Buegger F, Fiedler S, Müller T, Ruser R (2012) Influence of a nitrification inhibitor and of placed N-fertilization on N2O fluxes from a vegetable cropped loamy soil. Agriculture, Ecosystems & Environment 150, 91–101.
| Influence of a nitrification inhibitor and of placed N-fertilization on N2O fluxes from a vegetable cropped loamy soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVCksb8%3D&md5=21f8aeda4dd0f41f8a925bb293d83492CAS |
Porter I, Riches D, Deuter P, Boersma M, Scheer C, Firrell M, Keane P, Swarts N, Daynes C, Close D, Rogers G, Hardie M (2014) Mitigation of nitrous oxide emissions in the Australian horticultural industries. In ‘Proceedings of the Soil Science Australia National Soil Conference’, 23–27 November 2014, Melbourne, Australia.
Rashti MR, Wang WJ, Harper SM, Moody PW, Chen CR, Ghadiri H, Reeves SH (2015) Strategies to mitigate greenhouse gas emissions in intensively managed vegetable cropping systems in subtropical Australia. Soil Research 53, 475–484.
| Strategies to mitigate greenhouse gas emissions in intensively managed vegetable cropping systems in subtropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtl2qurnO&md5=6c8b449de2ffbef6e20cd50cf258c3d4CAS |
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125.
| Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtF2hs7jF&md5=d412beb03e751c999a0044beb213d53fCAS | 19713491PubMed |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO Publishing: Melbourne)
Rochette P, Angers DA, Chantigny MH, Gagnon B, Bertrand N (2008) N2O fluxes in soils of contrasting textures fertilized with liquid and solid dairy cattle manures. Canadian Journal of Soil Science 88, 175–187.
| N2O fluxes in soils of contrasting textures fertilized with liquid and solid dairy cattle manures.Crossref | GoogleScholarGoogle Scholar |
Rowlings DW, Grace PR, Kiese R, Weier KL (2012) Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Global Change Biology 18, 726–738.
| Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Ruser R, Schulz R (2015) The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—a review. Journal of Plant Nutrition and Soil Science 178, 171–188.
| The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkvVahsrs%3D&md5=f0ad4ead1942107e1f9d32b8156d6654CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=ee89302220febfea18060a4a6d021cc2CAS |
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proceedings of the National Academy of Sciences of the United States of America 111, 9199–9204.
| Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpsVamurY%3D&md5=8382fab4f0e01c7b581e0fdf91fd058aCAS | 24927583PubMed |
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O (2007) Agriculture. In ‘Climate change 2007: Mitigation. Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change’. (Eds B Metz, OR Davidson, PR Bosch, R Dave, LA Meyer). (Cambridge University Press: Cambridge, UK and New York, USA)
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider W, Towprayoon S, Wattenbach M, Smith J (2008) Greenhouse gas mitigation in agriculture. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 789–813.
| Greenhouse gas mitigation in agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXislGgtb8%3D&md5=0038abc8ae4b0afaa7bd4999687cb8a9CAS | 17827109PubMed |
Trenkel ME (2010) ‘Slow and controlled-release and stabilized fertilizers. An option for enhancing nutrient use efficiency in agriculture.’ (International Fertilizer Industry Association: Paris)
Velthof G, Kuikman P, Oenema O (2003) Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biology and Fertility of Soils 37, 221–230.
Vogeler I, Blard A, Bolan N (2007) Modelling DCD effect on nitrate leaching under controlled conditions. Soil Research 45, 310–317.
| Modelling DCD effect on nitrate leaching under controlled conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsV2gs7g%3D&md5=8097b8b7efba5afd8353a02f68edcf6cCAS |
Weier KL (1996) Trace gas emissions from a trash blanketed sugarcane field in tropical Australia. In ‘Sugarcane: research towards efficient and sustainable production’. (Eds JR Wilson, DM Hogarth, JA Campbell, AL Garside) pp. 271–272. (CSIRO Division of Tropical Crops and Pastures: Brisbane, Qld)
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Science Society of America Journal 57, 66–72.
| Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeqtLk%3D&md5=fd56bf11bee9b78d91071f533d1e8f08CAS |
Weiske A, Benckiser G, Herbert T, Ottow JCG (2001) Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biology and Fertility of Soils 34, 109–117.
| Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqtL4%3D&md5=b2d586c1d498dafda647d5eaea7be43cCAS |
Werner C, Zheng X, Tang J, Xie B, Liu C, Kiese R, Butterbach-Bahl K (2006) N2O, CH4 and CO2 emissions from seasonal tropical rainforests and a rubber plantation in Southwest China. Plant and Soil 289, 335–353.
| N2O, CH4 and CO2 emissions from seasonal tropical rainforests and a rubber plantation in Southwest China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1WnsrjM&md5=58b519d33a4b299a85d0cb578eb9a75aCAS |
Zerulla W, Barth T, Dressel J, Erhardt K, Horchler von Locquenghien K, Pasda G, Rädle M, Wissemeier A (2001) 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils 34, 79–84.
| 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |
Zhang M, Fan CH, Li QL, Li B, Zhu YY, Xiong ZQ (2015) A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agriculture, Ecosystems & Environment 201, 43–50.
| A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1yhsr4%3D&md5=173074d7fa14c174daaa47d7480ff7a4CAS |