The pH of Australian soils: field results from a national survey
Patrice de Caritat A B , Michelle Cooper A and John Wilford AA Geoscience Australia, GPO Box 378, Canberra, ACT 2601, Australia.
B Corresponding author. Email: patrice.decaritat@ga.gov.au
Soil Research 49(2) 173-182 https://doi.org/10.1071/SR10121
Submitted: 10 June 2010 Accepted: 1 September 2010 Published: 10 March 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
The pH is one of the fundamental soil properties governing nutrient availability, metal mobility, elemental toxicity, microbial activity, and plant growth. The field pH of topsoil (0–0.10 m depth) and subsoil (~0.60–0.80 m depth) was measured on floodplain soils collected near the outlet of 1186 catchments covering >6 Mkm2 (6 × 1012 m2) or ~80% of Australia. Field pH duplicate data, obtained at 124 randomly selected sites, indicate a precision of 0.5 pH unit (or 7%), and mapped pH patterns are consistent and meaningful. The median topsoil pH is 6.5, while the subsoil pH has a median of 7 but is strongly bimodal (6–6.5 and 8–8.5). In most cases (64%) the topsoil and subsoil pH values are similar; among the sites exhibiting a pH contrast, those with more acidic topsoils are more common (28%) than those with more alkaline topsoils (7%). The distribution of soil pH at the national scale indicates the strong controls exerted by precipitation and ensuing leaching (e.g. low pH along the coastal fringe, high pH in the dry centre), aridity (e.g. high pH where calcrete is common in the regolith), vegetation (e.g. low pH reflecting abundant soil organic matter), and subsurface lithology (e.g. high pH over limestone bedrock). The new data, together with existing soil pH datasets, can support regional-scale decision-making relating to agricultural, environmental, infrastructural, and mineral exploration decisions.
Additional keywords: acidity, alkalinity, baseline, regolith, soil quality.
Introduction
The pH is a fundamental soil property that can impact on soil quality (acid soil, alkaline soil) and use (nutrient availability). In Australia, ~50% of agricultural land (~50 Mha or 5 × 1011 m2) has surface pH values ≤5.5 (below optimum for extremely acid-sensitive agricultural plants and below the optimal level to prevent subsoil acidification); 12–24 Mha (1.2–2.4 × 1011 m2) of agricultural land is extremely to highly acidic, with pH values ≤4.8 (below optimum for acid-sensitive agricultural plants); and 23 Mha (2.3 × 1011 m2) of agricultural land has subsoils with pH ≤5.5 (Australian Agriculture Assessment 2001). In the absence of remedial lime applications (to neutralise acidity), it was estimated that 29–60 Mha (2.9–6 × 1011 m2) will reach the limiting soil pH value of 4.8 within 10 years, and a further 14–39 Mha (1.4–3.9 × 1011 m2) will reach pH 5.5, where growth of sensitive plant species is impaired (Australian Agriculture Assessment 2001).
There are several state-wide or more detailed studies of soil pH in Australia (e.g. Helyar et al. 1990; Powell et al. 1991; Crawford et al. 1994; Beeton et al. 2006), some of which have been compiled into the Australian Soil Resource Information System (www.asris.csiro.au/index_ie.html) national dataset. Those datasets can give detailed information on the spatial distribution of soil pH within catchments and from a variety of landforms and substrates. There are fewer studies, however, that have focused from the outset on collecting nation-wide soil pH data from a single type of soil or landform. The advantage of this type of study is that it can help highlight soil pH controls other than substrate or landform, since all sampled soils have developed on the same substrate or landform. This affords a focus on large-scale pH patterns and processes that control or influence them. The drawback, conversely, is that such datasets lack the spatial resolution to represent the variability of pH inside catchments, for instance from soils developed on other parent materials or landforms.
We report results from a nation-wide survey, during which the pH of surface and subsurface soils from a single type of landform, namely alluvial landform, was measured in the field. The new results represent a low-density but geographically widespread data layer that can have multi-disciplinary applications and establish a baseline of background data against which future changes at the national scale will be able to be compared.
Methods
Background methodology
The National Geochemical Survey of Australia (NGSA) project was initiated in late 2006 as part of Geoscience Australia’s Onshore Energy Security Program (Johnson 2006). The Program provides pre-competitive data and knowledge to support exploration for energy resources in Australia. As a spin-off, the NGSA will also establish a baseline geochemical database and atlas, which will find applications in various disciplines (de Caritat et al. 2008). The NGSA is carried out collaboratively between the Federal geoscience agency, Geoscience Australia, and each of the State and the Northern Territory geoscience agencies.
‘Catchment outlet sediments’, which are similar to floodplain sediments in most cases, were the target sampling material for the NGSA. Floodplain sediments have been widely used in geochemical surveys (the main aim of the present sampling exercise) because they are representative of the average regolith composition in a catchment (Ottesen et al. 1989). Although this holds true for most geochemical compositional parameters of the sediments, inherited from the original lithologies from which they are derived through weathering and transport, a property such as soil pH is not expected to be influenced by provenance to such an extent. Pedogenic processes at the site of soil development and over time will control the soil’s pH to a much greater extent. Therefore, we do not imply that the field pH measured here is representative of the whole catchment; rather, it is representative of the landform unit from which it was sampled (as demonstrated by duplicate analyses, see Results). The generalised description of ‘catchment outlet sediments’ (compared to floodplain or overbank sediments) used here accounts for the locally important aeolian influence on transported regolith in some areas of Australia. Catchments were sampled as close as possible to their lowest point (usually their outlet), targeting alluvial landforms (AL00 in RTMAP, see Pain et al. 1991); the most commonly sampled alluvial landform units in this project were floodplain (AL11), alluvial plain (AL10), and alluvial terrace (AL20). Small coastal catchments and small islands were not included in the survey.
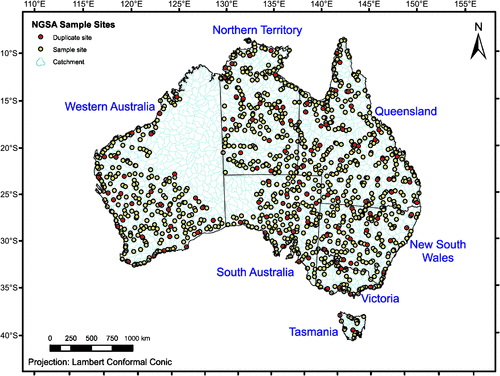
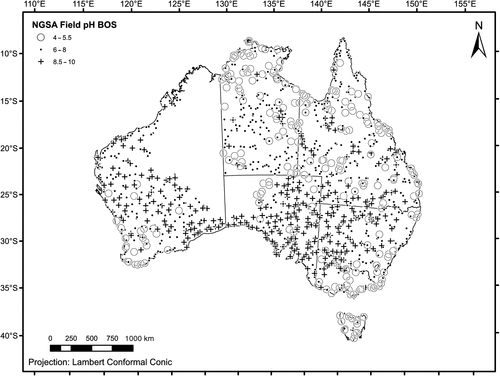
For the NGSA, catchment outlet sediments were sampled from two depths at 1315 sites representing 1186 large catchments (124 of which were sampled in duplicate for quality control; five large ones were sampled also in the middle of the catchment) covering much of the Australian continent (Fig. 1). The area represented by the sampled catchments exceeds 6 Mkm2 (6 × 1012 m2) or ~80% of Australia, a far greater coverage than afforded, for instance, by the ASRIS compilation (see below). The unsampled part of Australia is mostly subject to access restrictions and/or remote, and could not be accessed in time. First a ‘top outlet sediment’ (TOS) sample was taken between 0 and 0.10 m below the surface (on occasions where a dense root layer was encountered, the 0.10-m thickness of the TOS sample was measured from just below that layer to avoid comparing mineral-rich materials with organic-rich materials in the geochemical analysis of the samples). Then, a deeper ‘bottom outlet sediment’ (BOS) sample was taken from a depth of 0.60–0.80 m, on average. Both types of samples are composites that were homogenised either from a shallow soil pit (TOS) or from a minimum of three hand-augered holes (BOS). Herein, the TOS sample will be synonymous with ‘surface soil’ or ‘topsoil’, and the BOS sample with ‘subsurface soil’ or ‘subsoil’.
|
At each sampling location, a full site description was recorded (landform, landuse, soil properties, etc.), digital photos were taken, and GPS coordinates saved. Apart from field pH, dry and moist Munsell soil colours were also recorded for both TOS and BOS samples (Cooper et al. 2010). Finally, TOS and BOS samples were collected for geochemical analyses (see www.ga.gov.au/ngsa). The average sampling density for the area sampled is ~1 sample/5500 km2 (5.5 × 109 m2). Sample collection and preparation were carried out as per the Field Manual (Lech et al. 2007) and Sample Preparation Manual (de Caritat et al. 2009) developed for the NGSA project. The preliminary soil pH map of Australia constitutes the first data release of the NGSA project (de Caritat et al. 2010), and the field pH data, the subject of the present contribution, can be downloaded from Geoscience Australia’s website (www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=70105).
pH measurement
Field pH was determined in the field by the saturated paste method using the widely available Inoculo Laboratories soil pH test kits developed by CSIRO (www.inoculo.com.au). This method was selected because it is cheap and easy; it is also recommended by the Australian Soil and Land Survey Field Handbook (McDonald et al. 1990). The field pH method entails: (i) saturating a small amount of soil (about half a teaspoon or ~2 mL) with a few drops of universal indicator; (ii) mixing soil and indicator to form a paste; (iii) dusting the saturated paste with white barium sulfate powder; (iv) waiting ~60 s for the indicator to react with the soil; (v) matching the colour of the powder to a chart with increments of half a pH unit.
To some extent this empirical method depends on the user’s ability to match colours and on lighting conditions; it is limited to the range of pH 2–10; it also only has a resolution of one half of a pH unit and hence is a relatively ‘crude’ measurement. Therefore, results should be considered a ‘first pass’ only. Preliminary comparisons with (a) pH values determined in the laboratory on a subset of samples using slurries with a soil : water ratio of 1 : 5 (pH1 : 5), and (b) with pH estimates derived from visible near-infrared adsorption spectroscopy also available from a subset of samples, however, suggest general correlation and overall robustness of the field pH data. Importantly, the data presented are based on one site per catchment and cannot, therefore, inform on intra-catchment pH heterogeneity, as discussed above.
Soil pH was measured in duplicate at 124 randomly selected sites, where the duplicate sample was collected generally 10–100 m upstream from the original site and in the same landform unit to ensure comparability.
Results and discussion
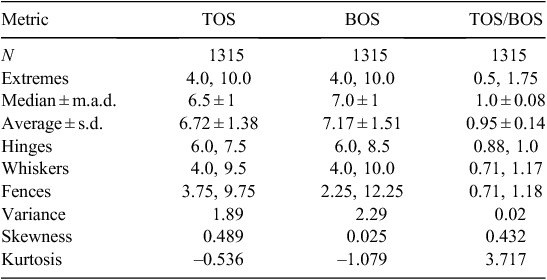
The soil pH measurements recorded during this project are summarised in Table 1. Soil pH values between 4 and 10 were recorded (Table 1), spanning the range from extremely acidic, through neutral, to extremely alkaline (nomenclature after USDA 1998). Ratios of TOS/BOS pH values were calculated to determine the pH contrast between topsoil and subsoil. The raw data are available from: www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=70105.
|
From the duplicate soil pH measurements at 124 randomly selected sites, an overall (sampling and analytical) precision of 0.5 pH units (or 7% in relative terms) was established.
Topsoil pH
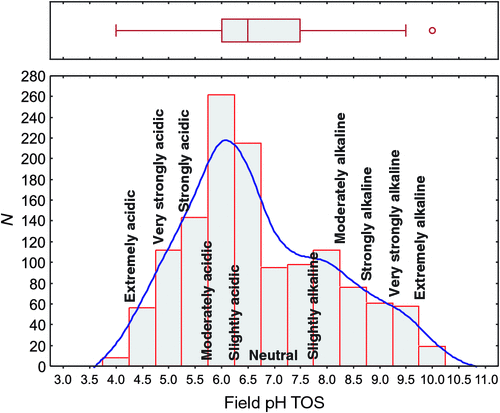
The pH of the topsoil or TOS samples ranged from 4 to 10, with a median value of 6.5 (Table 1). The inner 50% of the data fall within the narrow interval of pH 6–7.5 (25th and 75th percentiles), and there are upper outliers and no lower outliers, following the definition of Tukey (1977). The data are positively skewed (skewness 0.489), and a histogram (Fig. 2) displays a dominant mode at pH 6–6.5 and a distinct shoulder around pH 8.
|
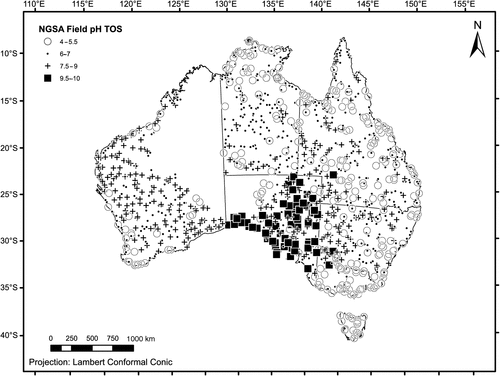
Spatially, the topsoil pH results form a striking pattern (Fig. 3), with the most elevated values (pH 9.5–10) clustering along the coast of South Australia and extending inland into the lower Murray River system (western border region between Victoria and New South Wales). Another cluster of elevated topsoil pH values occurs in north-eastern South Australia. Topsoil pH values between 7.5 and 9 were recorded principally scattered over much of Western Australia and South Australia, but also occur in western New South Wales, Queensland, and southern Northern Territory. The alkaline conditions are largely attributed to subcropping carbonate rocks (Eucla Basin in south-eastern Western Australia and southern South Australia; Eromanga Basin in central Queensland) or secondary soil carbonates or ‘calcretes’ commonly occurring in the interior of South Australia, Yilgarn Craton (the southern inland part of Western Australia, from which most of the NGSA samples from that State derive), and western New South Wales (Chen et al. 2002). Slightly acidic, neutral, and slightly alkaline pH conditions (6–7) typify topsoils from parts of the Northern Territory and Queensland, but are also found scattered in the other jurisdictions, with the exception of Tasmania. Victoria and Tasmania and most coastal sites in New South Wales and Queensland and in south-west Western Australia have lower topsoil pH values (4–5.5), as do quite a few sites in the Northern Territory and Queensland. The more acidic soil conditions are likely caused by the abundance of organic matter (e.g. in Tasmania), heavier precipitation (coastal fringes), as discussed below, or simply the presence of underlying acid rocks in the subsurface. Acid sulfate soils occupy an estimated ~6 Mha (6 × 1010 m2) of the coastal zone around Australia (Hicks et al. 2009).
|
Topsoil pH was compared with the Prescott Index (PI) (Prescott 1950; McKenzie and Ryan 1999) of soil leaching at the collection site. The 250-m grid-size PI data layer used here was obtained from CSIRO where PI is defined as:
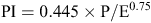
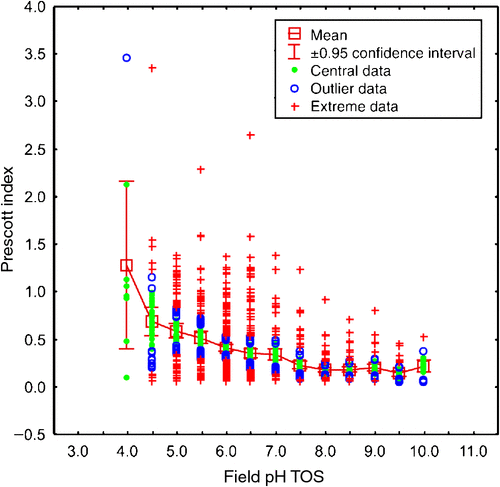
where P is average monthly precipitation and E is average monthly evaporation from a free water surface. Higher PI values indicate greater rainfall, lesser evaporation, and overall greater leaching of soils. A weak but significant decrease of the PI with increasing field pH was found (Fig. 4), especially for the surface soils:
|
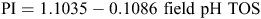
which has a correlation coefficient r = –0.4385 (r2 = 0.1923), which, for N = 1315, is significant (P << 0.01). The mean PI per half pH-unit increment drops sharply from 1.28 for the pH 4 class (which has eight samples with widely variable PI values) to 0.69 (for pH 4.5), then more regularly to 0.18 (for pH 8), then remains between 0.22 and 0.14 up to pH 10.
Figure 4 further indicates that extremely to slightly acidic topsoils (pH 4–6.5) can be found in a range of climatic conditions (PI 0.06–3.5), but most of the moderately to extremely alkaline topsoils (pH 8–10) are found in the most arid (least leached) parts of Australia (PI < 0.5).
Subsoil pH
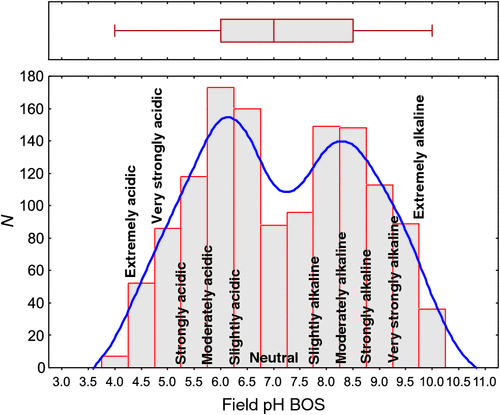
The pH of the subsoil or BOS samples ranges from 4 to 10, with a median of 7 (Table 1), slightly higher than the median TOS pH. The inner 50% of the data fall within the interval of pH 6–8.5, and there are no outliers according to the definition of Tukey (1977). The data have a much lower skewness of 0.025 but are not normally distributed as shown by the histogram (Fig. 5), which displays two, almost equally important, modes at 6–6.5 and at 8–8.5.
|
The spatial distribution of subsoil pH (Fig. 6) has many similarities with the topsoil pH map, but without the existence of outliers. Much of the continent south of 25°S, except Victoria and Tasmania, is dominated by strongly to extremely alkaline subsoil (pH 8.5–10), with interspersed, slightly acidic, neutral, and slightly alkaline values (pH 6–8) in eastern New South Wales and in the Yilgarn of the south of Western Australia. The more alkaline subsoils (pH 8.5–10) reflect climate and secondary/pedogenic carbonates (arid interior of Western Australia, South Australia, and western New South Wales) and/or primary carbonates in the underlying bedrock (Eucla Basin along the south coast of South Australia and the east of Western Australia; Eromanga Basin in central Queensland). Northern Australia and much of Victoria and Tasmania have mostly circum-neutral (pH 6–8) or more acidic (pH 4–5.5) subsoil pH values. Again, these lower pH values are attributed to organic matter in tropical or temperate vegetation zones, more abundant rainfall, or simply lithology.
|
Comparison of topsoil and subsoil pH
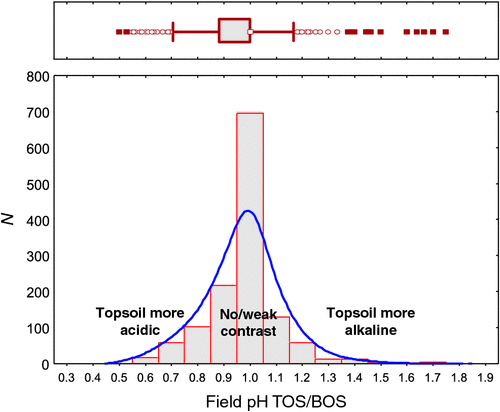
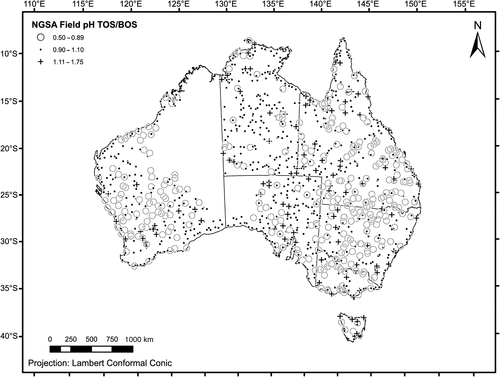
The ratio of topsoil to subsoil pH ranged from 0.5 to 1.75, with a median of 1.0 (Table 1). Of 1315 samples, 469 (36%) have the same pH in the topsoil as in the subsoil. The inner 50% of the TOS/BOS data fall between a pH ratio of 0.88 and 1.0, and there are 75 lower and 60 upper outliers following the definition of Tukey (1977). The data have a skewness of 0.432, and the histogram (Fig. 7) displays a strongly dominant mode at a ratio value of 1.0.
|
Of 1315 samples, 847 (64%) have either no pH contrast (topsoil/subsoil pH ratio = 1) or a weak pH contrast between the surface and depth (defined here as topsoil/subsoil pH ratio 0.9–1.1), and there were many more samples with a more acidic topsoil (373 or 28% with topsoil/subsoil pH ratio < 0.9) than with a more alkaline topsoil (95 or 7% with topsoil/subsoil pH ratio > 1.1).
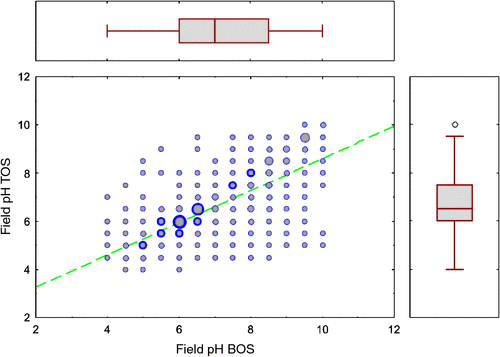
The topsoil and subsoil field pH values recorded during this survey correlate strongly, according to the linear regression (Fig. 8):
|
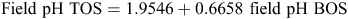
which has a correlation coefficient r = 0.7357 (r2 = 0.5413, N = 1315, P < 0.01).
The spatial distribution of the ratio TOS/BOS field pH (Fig. 9) can be useful to identify areas prone to more acidic topsoils (ratio < 0.9) or more alkaline topsoils (ratio > 1.1) relative to the subsoil. Although the resulting map is quite noisy, more acidic topsoils appear to be common over much of the Yilgarn Craton, and in New South Wales and southern Queensland. The areas with alkaline soil pH (at either depth level), as discussed above, seem mostly to have a ratio around unity, indicating a strong pH-buffering role played by subcropping carbonates or the calcretes. Sites with more alkaline topsoils than subsoils do not appear to form consistent patterns and are mostly isolated sites or form small clusters.
|
Comparison with the ASRIS dataset
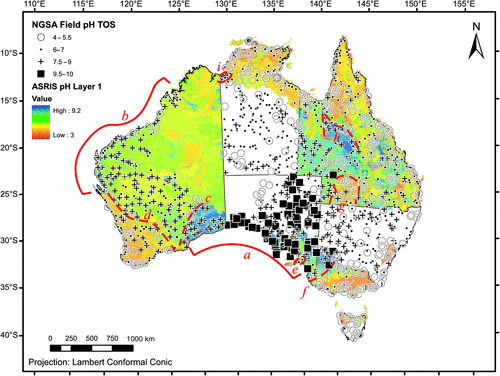
The new field pH data for topsoil is overlain on a raster image of the ASRIS Layer 1 pH dataset (www.asris.csiro.au/index_ie.html) in Fig. 10. The Layer 1 pH dataset obtained from ASRIS is a combination of Level 4 and Level 5 data that produces a ‘best of’ raster (McKenzie et al. 2005). Several estimation methods for pH are represented in the ASRIS dataset, including ‘based on measurements for replicate soil profiles in the land-unit tract’, to ‘based on single measurements in the land-unit tract’, to ‘based on experience with similar soils’ (e.g. same taxa in the Australian Soil Classification of Isbell (2002) but from other regions) (McKenzie et al. 2005). The ASRIS pH data are presented as 1 : 5 soil : CaCl2 solution, whether they were measured directly as such or converted from 1 : 5 soil : water measurements according to Henderson and Bui’s (2002) conversion table (McKenzie et al. 2005). Therefore, NGSA and ASRIS pH values may not be directly equivalent, but relative levels and large-scale patterns can still be compared.
|
Several observations are made from this comparison. Where the two datasets overlap, the coincidence of large pH patterns is surprisingly good both for first-order features and for some more-detailed features. For examples of the former, see the low soil pH along much of the coastline of Australia except the Eucla Basin in South Australia and the south-east of Western Australia (‘a’ in Fig. 10), and between the northern Carnarvon and Canning Basins in the north-west of Western Australia (‘b’). Further, the generally more acidic soils over much of the ‘Top End’ in the Northern Territory, on the Cape York Peninsula in Queensland, and over most of Victoria are reflected in both datasets. Examples for more detailed features include the north-western limit of the Eucla Basin (‘c’); the more acidic soils of the south-west corner of Western Australia (‘d’); the Fleurieu Peninsula being identified as more acidic than the surrounding Adelaide Hills/Murray Basin (‘e’); the south-eastern limit of the Murray Basin where it abuts the Victorian high lands (‘f’); the more acidic soils in the Thargomindah–Quilpie region of south-western Queensland (‘g’); the higher pH values east of Mt Isa in central Queensland compared to areas further north, west, or east (‘h’); and the circum-neutral soils of the Victoria River estuary region near the border of the Northern Territory and Western Australia compared to the surrounding more acidic soils (‘i’). One notable region where the two datasets give clearly divergent results is in central-western Queensland (between ‘g’ and ‘h’) where the NGSA measurements indicate fairly acidic conditions (pH 4–7), compared to alkaline conditions according to ASRIS.
The NGSA samples were collected over a short period of time (2007–09) and the field pH was measured by the same technique all over the country, potentially making it a useful cross-check between the large number of State/Northern Territory datasets collated in ASRIS, which were collected over several decades using different methods (e.g. pH measured in water or CaCl2 solutions). It is hoped that the recently released NGSA soil field pH dataset can be integrated into the ASRIS compilation to assist in providing a better and more widespread knowledge on this fundamental soil property across the country.
Finally, while the ASRIS dataset covers approximately 4.6 Mkm2 (4.6 × 1012 m2) and the NGSA dataset 6 Mkm2 (6 × 1012 m2), the overlap is only ~3.5 Mkm2 (3.5 × 1012 m2), indicating that the new dataset fills a significant gap (~2.5 Mkm2 or 2.5 × 1012 m2) in today’s best available knowledge. This gap is over most of the Northern Territory, South Australia, New South Wales and Tasmania.
Conclusions
Based on a recent continent-wide survey of floodplain sediments covering 6 Mkm2 (6 × 1012 m2), a field pH dataset and maps are now available for soils developed on a single landform unit in Australia. The preliminary maps shown here result from field pH data gathered using the saturated paste method, which has a resolution of one-half of a pH unit. As such, the data and maps presented here are a preliminary dataset. In addition, the field pH data presented refer to a single type of landform unit where parent material is alluvial sediment; this has advantages and drawbacks as discussed.
Soil field pH was collected on surface samples (0–0.10 m) or topsoils, and on deeper samples (~0.60–0.80 m) or subsoils. The pH data and maps show distinct areas of alkaline conditions, which generally either overlie known carbonate lithologies (limestone, marble, etc.) or correspond to the extent of calcretes, where evaporation is large compared to precipitation (low Prescott Index). More-acid conditions are found along much of the coast (high leaching, high Prescott Index) or in areas of luxuriant vegetation (organic acids) or where basic rocks supplying base cations to the soils are absent.
The present, freely downloadable dataset and maps show a close correspondence to the ASRIS national soil data compilation, both at the first-order level and to some degree of detail. It is hoped that the NGSA field pH dataset can complement the ASRIS compilation and be seen as valuable because it was collected over a short period of time, contains pH data measured with the same method across the country, is easily available from one website, and fills some large holes (~2.5 Mkm2 or 2.5 × 1012 m2) in the ASRIS coverage with first-pass, low-density data of good quality.
The new soil pH data can, in conjunction with other available datasets (e.g. ASRIS), help inform decisions, for example, about geochemical exploration (e.g. dispersion of metals in the regolith) (Butt et al. 2000), soil nutrient status (e.g. likely abundance of base cations), soil amendment requirements (e.g. fertiliser application) (Chorom and Rengasamy 1997), agricultural practices (e.g. deep furrowing), infrastructure protection (e.g. road deterioration, pipelines), soil acidification (Brennan et al. 2004) and alkalinisation (e.g. background or baseline status for future change, inland and coastal acid sulfate soils), and metal mobility and environmental risks deriving therefrom (Ward et al. 2004) at the large scale.
Acknowledgments
The NGSA project was carried out under the Onshore Energy Security Program funded by the Commonwealth Government of Australia. Collaboration with the geoscience agencies of all Australian States and the Northern Territory took place under a National Geoscience Agreement. We are grateful to the teams who went in the field to collect the samples and record the field data used here. We also thank the land owners for granting access to the field sites. Published with permission of the Chief Executive Officer, Geoscience Australia.
References
Australian Agriculture Assessment (2001) Australian Agriculture Assessment 2001 report, National Land and Water Resources Audit, Land & Water Australia. Available at: www.anra.gov.au/topics/land/pubs/national/agriculture_contents.html (accessed 5 August 2010).Beeton RJS, Buckley KI, Jones GJ, Morgan D, Reichelt RE, Trewin D (2006) Australia State of the Environment 2006. Department of the Environment, Water, Heritage and the Arts. Available at: www.environment.gov.au/soe/2006/index.html (accessed 5 August 2010).
Brennan RF, Bolland MDA, Bowden JW (2004) Potassium deficiency, and molybdenum deficiency and aluminium toxicity due to soil acidification, have become problems for cropping sandy soils in south-western Australia. Australian Journal of Experimental Agriculture 44, 1031–1039.
| Potassium deficiency, and molybdenum deficiency and aluminium toxicity due to soil acidification, have become problems for cropping sandy soils in south-western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVaisrnF&md5=2f78674abf3049609891bca783418d66CAS |
Butt CRM, Lintern MJ, Anand RR (2000) Evolution of regoliths and landscapes in deeply weathered terrain—implications for geochemical exploration. Ore Geology Reviews 16, 167–183.
| Evolution of regoliths and landscapes in deeply weathered terrain—implications for geochemical exploration.Crossref | GoogleScholarGoogle Scholar |
Chen XY, Lintern MJ, Roach IC (2002) Calcrete: Characteristics, distribution and use in mineral exploration. Cooperative Research Centre for Landscape Environments and Mineral Exploration Monograph. Available at: http://crcleme.org.au/Pubs/Monographs/Calcrete%20book.html (accessed 5 August 2010).
Chorom M, Rengasamy P (1997) Carbonate chemistry, pH, and physical properties of an alkaline sodic soil as affected by various amendments. Australian Journal of Soil Research 35, 149–161.
| Carbonate chemistry, pH, and physical properties of an alkaline sodic soil as affected by various amendments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXpt1ehtQ%3D%3D&md5=8eb62d6eeeafa5084e897d76f42c9baaCAS |
Cooper M, de Caritat P, Burton G, Fidler R, Green G, House E, Strickland C, Tang J, Wygralak A (2010) National Geochemical Survey of Australia: Field Data. Geoscience Australia, Record 2010/18. Available at: www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=70478 (accessed 9 August 2010).
Crawford DM, Baker TG, Maheswara J (1994) Soil pH changes under Victorian pastures. Australian Journal of Soil Research 32, 105–115.
| Soil pH changes under Victorian pastures.Crossref | GoogleScholarGoogle Scholar |
de Caritat P, Cooper M, Burton G, Fidler R, Green G, House E, Strickland C, Tang J, Wygralak A (2010) Preliminary soil pH map of Australia. AusGeo News 97, March 2010. Available at: www.ga.gov.au/ausgeonews/ausgeonews201003/soil.jsp (accessed 5 August 2010).
de Caritat P, Cooper M, Lech M, McPherson A, Thun C (2009) National Geochemical Survey of Australia: Sample Preparation Manual. Geoscience Australia, Record 2009–08. Available at: www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=68657 (accessed 5 August 2010).
de Caritat P, Lech ME, McPherson AA (2008) Geochemical mapping ‘down under’: selected results from pilot projects and strategy outline for the National Geochemical Survey of Australia. Geochemistry: Exploration, Environment, Analysis 8, 301–312.
| Geochemical mapping ‘down under’: selected results from pilot projects and strategy outline for the National Geochemical Survey of Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVCks77O&md5=1cefbb36dda276cd22a9cbc6f5c69112CAS |
Helyar KR, Cregan PD, Godyn DL (1990) Soil acidity in New South Wales—Current pH values and estimates of acidification rates. Australian Journal of Soil Research 28, 523–537.
| Soil acidity in New South Wales—Current pH values and estimates of acidification rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlslSqtr8%3D&md5=6621884f6c9bd85e3a4e5e62ae13c7b1CAS |
Henderson BL, Bui EN (2002) An improved calibration curve between soil pH measured in water and CaCl2. Australian Journal of Soil Research 40, 1399–1405.
| An improved calibration curve between soil pH measured in water and CaCl2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlvFeitg%3D%3D&md5=45b47f87f7441c27e434ec3c2604ff20CAS |
Hicks WS, Bowman GM, Fitzpatrick RW (2009) Effect of season and landscape position on the aluminium geochemistry of tropical acid sulfate soil leachate. Australian Journal of Soil Research 47, 137–153.
| Effect of season and landscape position on the aluminium geochemistry of tropical acid sulfate soil leachate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvVajsrk%3D&md5=f5391a7e371b8f592d359f9150b2c996CAS |
Isbell RF (2002) ‘The Australian Soil Classification.’ Rev. edn (CSIRO Publishing: Melbourne)
Johnson J (2006) Onshore Energy Security Program underway. AusGeo News 84, December 2006. Available at: www.ga.gov.au/ausgeonews/ausgeonews200612/onshore.jsp (accessed 5 August 2010).
Lech ME, de Caritat P, McPherson AA (2007) National Geochemical Survey of Australia: Field Manual. Geoscience Australia, Record 2007/08. Available at: www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=65234 (accessed 5 August 2010).
McDonald RC, Isbell RF, Speight JG, Walker J, Hopkins MS (1990) ‘Australian soil and land survey field handbook.’ 2nd edn (Inkata Press: Melbourne)
McKenzie NJ, Ryan PJ (1999) Spatial prediction of soil properties using environmental correlation. Geoderma 89, 67–94.
| Spatial prediction of soil properties using environmental correlation.Crossref | GoogleScholarGoogle Scholar |
McKenzie NJ, Jacquier DW, Maschmedt DJ, Griffin EA, Brough DM (2005) The Australian Soil Resource Information System Technical Specifications. Australian Collaborative Land Evaluation Program. Available at: www.asris.csiro.au/methods.html#Method_Downloads (accessed 5 August 2010).
Ottesen RT, Bogen J, Bølviken B, Volden T (1989) Overbank sediment: a representative sample medium for regional geochemical sampling. Journal of Geochemical Exploration 32, 257–277.
| Overbank sediment: a representative sample medium for regional geochemical sampling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXkvVWmtL4%3D&md5=0e719ac2bba2464775e5583848849e79CAS |
Pain C, Chan R, Craig M, Hazell M, Kamprad J, Wilford J (1991) RMAP: BMR Regolith Database Field Handbook. Bureau of Mineral Resources, Record 1991/29. Available at: www.ga.gov.au/products/servlet/controller?event=GEOCAT_DETAILS&catno=14415 (accessed 5 August 2010).
Powell B, McBratney AB, Macleod DA (1991) The application of fuzzy classification to soil pH profiles in the Lockyer Valley, Queensland, Australia. Catena 18, 409–420.
| The application of fuzzy classification to soil pH profiles in the Lockyer Valley, Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmtFais7w%3D&md5=21ef6b43fe838d2652b1dbfffaf6d97bCAS |
Prescott JA (1950) A climatic index for the leaching factor in soil formation. Journal of Soil Science 1, 9–19.
| A climatic index for the leaching factor in soil formation.Crossref | GoogleScholarGoogle Scholar |
Tukey JW (1977) ‘Exploratory data analysis.’ (Addison Wesley: Reading, MA)
USDA (1998) Soil quality indicators: pH. United States Department of Agriculture (USDA) Natural Resources Conservation Service, Soil Quality Information Sheet. Available at: http://soils.usda.gov/sqi/publications/files/indicate.pdf (accessed 5 August 2010).
Ward NJ, Sullivan LA, Bush RT (2004) Soil pH, oxygen availability, and the rate of sulfide oxidation in acid sulfate soil materials; implications for environmental hazard assessment. Australian Journal of Soil Research 42, 509–514.
| Soil pH, oxygen availability, and the rate of sulfide oxidation in acid sulfate soil materials; implications for environmental hazard assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnslShu7w%3D&md5=264e09f9d494eb5670708cdf85dff71eCAS |


