Rhizosphere biology and crop productivity—a review
M. Watt A B , J. A. Kirkegaard A and J. B. Passioura AA CSIRO Plant Industry, PO Box 1600, Canberra, ACT 2601, Australia.
B Corresponding author. Email: michelle.watt@csiro.au
Australian Journal of Soil Research 44(4) 299-317 https://doi.org/10.1071/SR05142
Submitted: 15 September 2005 Accepted: 18 April 2006 Published: 27 June 2006
Abstract
There is great potential to use the wide genotypic and agronomically induced diversity of root systems and their exuded chemicals to influence rhizosphere biology to benefit crop production. Progress in the areas of pathogens and symbionts in this regard is clear. Further progress, especially related to interactions with non-pathogenic organisms, will rely on an appreciation of the properties of rhizospheres in the field: the spatial and temporal boundaries of these rhizospheres, and the effects of structural, chemical, and physical soil heterogeneity in which the roots and associated microorganisms exist and function. We consider the rhizosphere environment within Australian cropping systems in relation to the likely success of biological interventions, and provide 3 case studies that highlight the need to characterise the rhizosphere and the microbial interactions therein to capture agronomic benefits. New techniques are available that allow direct visualisation and quantification of rhizosphere processes in field conditions. These will no doubt help develop better genetic and agronomic approaches. Future success, as with those in the past, will rely on integrating interventions related to rhizosphere biology with other management constraints of specific farming systems.
Additional keywords: roots, exudates, soil, microorganisms, agronomy, genetics.
Introduction
With the notable exceptions of symbionts and pathogens, the study of soil biology in agriculture has historically dealt with the effect of agricultural practices on free-living organisms in the soil. There have been many studies of how agronomic practice and the variety of the seasons have affected the populations or activities of particular classes of soil organisms per se in the bulk soil. Data from such studies are difficult to convert into integrated information that can be used to improve the productivity of crops and the viability of cropping systems. What is important agriculturally is how the interactions between management and soil biology affect the performance of crops (Fig. 1). Roots are thus an integral component of the soil biology.
|
The study of soil biology considering roots as integral offers us insights into how we might improve agronomic practices and cultivars, i.e. how we might facilitate the innovative management of Australian farms that has enabled rises in productivity to keep ahead of the steadily falling terms of trade over the last few decades. There is, as yet, no substantial edifice of theory that can connect improved practice with the extraordinarily complex interactions between roots and organisms in the soil. Nevertheless, the idea of the rhizosphere, now 100 years old, has laid the foundation for such an edifice.
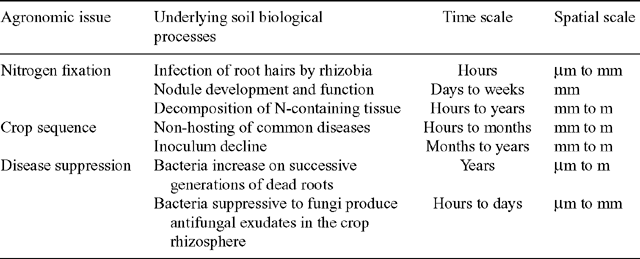
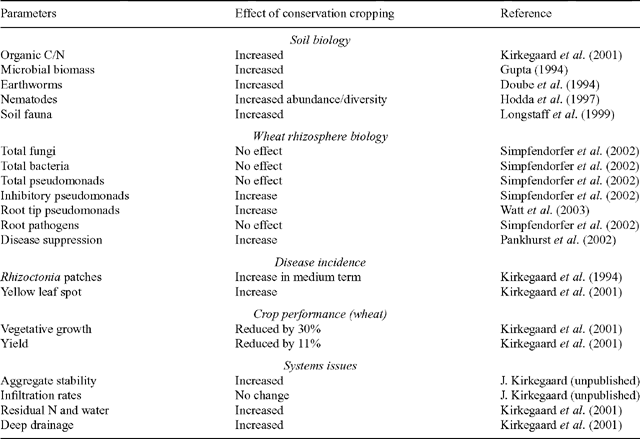
There is strong evidence that changes in agronomic practice have improved the productivity and sustainability of Australian farming systems by influencing (amongst other things) the soil biology. Processes that have been successfully harnessed include symbiotic nitrogen fixation, crop sequences to control disease and inhibitory organisms, and longer-term suppression of disease (Table 1). These operate around roots (either living, or as dead remnants) and in rhizospheres over wide spatial and temporal scales.
|
Evidence from fumigation experiments and from puzzling agronomic responses in field trials suggests that we can capitalise further on interactions between crop management and soil biology (Kirkegaard 1995; Bever 2003). An example of agronomic responses that implicate influential changes in soil biology are those associated with conservation farming practices. A particular puzzle has been that such practices invariably improve many attributes of soil that are associated with high fertility; structural stability, infiltration rates, faunal and microbiological activity, soil organic matter, are all typically increased. Yet farmers’ evaluations of the crop performance in conservation farming, both in Australia and worldwide, have been highly variable (Kirkegaard 1995; Lyon et al. 2004). What is clear is that the apparently major improvements in soil properties do not always translate reliably into better crop yields. The range of possible contributory factors includes: increased pests and diseases; toxic chemicals arising from retained stubble; greater residual effects of herbicides; growth-inhibitory bacteria in the rhizosphere; inhibited root growth in the harder unploughed seed bed; inhibitory signals passing from roots to leaves when the roots are experiencing less than ideal soil conditions; and concentration of nutrients in the surface soil. Unravelling the underlying causes of crop response to changed agronomic practice such as this is difficult, but processes occurring within the rhizosphere are central.
This review aims to take stock of these various issues and the implications they might have for designing and interpreting agronomic experiments that aim to capture benefits from soil biology. It is organised into: (1) a section on the ecology of the rhizosphere, and especially of perennial rhizospheres — the niches that successive generations of roots occupy in unploughed soil, how their properties differ from those of bulk soil, and how diverse the properties of the roots are when growing in field soil; (2) three case studies of major agronomic effects that are becoming explicable in terms of interactions between roots and soil organisms; and (3) future directions including an analysis of diagnostic tools, and prospects for novel ways of harnessing biological activity in the soil to improve the performance of crops.
Ecology of the rhizosphere
The rhizosphere in laboratory and field
Most information about important processes in the rhizosphere comes from studies in controlled environments where roots are grown in simple uniform media, and organisms of interest are applied. Attempts to model rhizosphere processes have been instructive, but are too simplistic in their depictions of the rhizosphere to be agriculturally useful. They do highlight that compounds diffusing from roots stimulate bacterial growth (Newman and Watson 1977), that the water solubility of exudates and their pattern of exudation along a root affect how organisms grow (Darrah 1991), that wetter soil allows bacteria from the seed to migrate further along a root (Scott et al. 1995), and that bacterial death rates and predation can account for numbers of rhizosphere bacteria (Zelenev et al. 2000).
In the field, rhizospheres are much more complex than in the laboratory (McCully 1999). Field plants have more root types compared with those in the laboratory and a very broad range of chemicals is exuded from them and their associated organisms. The rhizospheres of field-grown roots experience large variation in environmental variables: in temperature, both diurnally and seasonally; in soil water, especially in the topsoil, which can range from air-dry to saturated in periods of hours to weeks; in structure, which offers niches at a range of scales, from continuous macropores that may enable rapid root extension in otherwise hard soil, to microcavities within soil aggregates that may protect resident bacteria from predation by larger organisms; in nutritional status, that may include concentrated pockets of nutrients, both organic and inorganic; in inocula, as in remnant roots that harbour large populations of microorganims; and in oxygen status, which may range from well-aerated to hypoxic within even a single aggregate. The inhabitants of the rhizosphere include microorganisms, macrofauna and insects. Bacteria generally are the most abundant (approx. 1010 cells/g of soil, or 106 cells/mm3 rhizosphere biofilm), followed by fungi, protozoa, nematodes and insects (see Watt et al. 2006b; Doube and Brown 1998 for a review of rhizosphere macrofauna and insects). The diversity of each group is still being discovered (see New methodologies section below), and the abundance and diversity of organisms in the rhizosphere depend on the cropping environment, the plant species, the types of roots, their ages, and the chemicals exuded from them.
Wide spatial and temporal boundaries of the rhizosphere
The rhizosphere of a single root occupies a volume that extends from the root to an ill-defined position in the soil that depends on the diffusion of exudates and the stage of development and biochemistry of the roots (Hinsinger et al. 2005). Huisman (1982) used the distance within which fungi respond to root exudates to estimate rhizosphere width, and found it to be approximately 1.0 mm for most, but 5 and 12 mm for Rhizoctonia and Gaemannomyces gramminis, respectively. Bacteria tightly bound to the root, observed in solution under a microscope, sit within 0.03 mm of the root surface (Watt et al. 2006a). The characteristic time for exudates to diffuse and interact with a soil organism is the quotient of the distance squared and the diffusivity (see Watt et al. 2006b). Exudates exchange between roots and organisms much more quickly close to the root than further away, creating heterogeneity across the rhizosphere at a point in time. The rhizosphere can extend from the interior of the root along the length of hyphae of any fungi associated with that root. Within older roots, spaces left behind after cells have decomposed can harbour organisms (McCully 2001).
A rhizosphere is born when a root tip arrives in a volume of soil and ends when that piece of root decays (Jones et al. 2004). Over time, a root develops hairs and branch roots, and, in dicotyledon roots, secondary thickening. Depending on whether the crop is annual or perennial, the rhizosphere can persist for years. Once the root dies, it continues to harbour a succession of organisms that have important nutritional, disease, or other effects on new crop roots, as seen in rotations with legumes or canola (see below, Case study 3).
Shoots sense many rhizospheres of different ages from the various members of the root system. Cereal root systems consist of seminal roots, branch roots, and nodal roots. Dicotyledonous root systems have a tap root with successive orders of branch roots, and roots extending from the hypocotyl with their own branch roots. We know little about the infection dynamics of diseases, arbuscular mycorrhizal fungi (AMF), and other organisms, on these different types of roots. Sivasithamparam and Parker (1979) conducted one of the few studies of rhizosphere microorganisms on different root types. They found that seminal roots of wheat had more bacteria and actinomycetes than nodal roots both closely and loosely associated with the roots, whereas fungi were similar for both root types only in the closely associated fraction.
Bacterial populations change with root age. Populations on root tips differed from those at the root base of the seminal roots of wheat seedlings (Liljeroth et al. 1991). Sheathed and bare roots of field-grown maize had similar total numbers of bacteria, but the older bare roots were dominated by actinomycetes (Gochnauer et al. 1989). Specific compounds in exudates can be a major reason for differences in colonisation of different root types. McCully and Canny (1985) found that young and old regions of maize roots exude similar amounts of total carbon; however, the composition is different from each, and Liljeroth et al. (1991) found that bacteria from tips rather than those from the base, preferred citrate.
Generally, root systems occupy progressively deeper parts of the profile with time, so that the youngest regions of the axes are deepest. The various types of roots, and their extension rates and orientation, determine the extent of occupation of soil with depth, and the differences in soil structure, temperature, nutrients, and water at various depths influence how soil organisms interact dynamically with roots to affect crop growth. Designing temporal and spatial sampling strategies for agronomic experiments on, for example, biological inoculants, crop sequences, or the development of disease-suppressive soils, is difficult but crucially important.
Exudates and chemical signals
For nearly 200 years, scientists have known that roots exude chemicals that stimulate or suppress the activity of organisms in soil (Schroth and Hildebrand 1964). These organisms include microorganisms, seeds of root parasites, and other roots (Bertin et al. 2003; Bais et al. 2004). The central role of root exudates is particularly well recognised for fungi, some of which only break dormancy and germinate when exposed to such chemicals (see Akiyama et al. 2005, who showed that strigolactone induces branching of mycorrhizal fungi in culture).
Many rhizosphere chemicals are common constituents of root cells that have leaked from living roots or lysing, decomposing cells. Others are controlled metabolically by transport processes in the roots. Root exudates include protons and hydroxyl ions, water-soluble sugars such as sucrose and carboxylic anions, water-insoluble polysaccharides that become mucilage that protects roots and organisms, nitrogen-containing compounds such as amino acids (Merbach et al. 1999), and a very large range of secondary metabolites and signals. The net carbon (since, importantly, roots can take up rhizosphere carbon) released from roots ranges from 5 to 10% of net carbon fixed by the plant (reviewed in Farrar et al. 2003). These exudates support microbial activity in the rhizosphere. Modelling suggests that the growth of the microbial biomass in the rhizosphere, supported by such carbon efflux, can, in itself, inhibit plant growth (~20% growth depression, Darrah 1998).
Rhizosphere organisms also contribute to the rhizosphere chemistry, releasing mineral nutrients from dead cells that can be taken up by roots, antibiotics and antifungal agents, phytotoxins (Gerhardson et al. 1985), and mucilages. One of the best characterised compounds of microbial origin is the antifungal toxin 2,4-diacetylphloroglucinol (DAPG) produced by Pseudomonas spp. that suppress pathogen growth (Keel et al. 1992). We focus here on mucilages, signals, and volatiles from roots.
Roots are covered by mucilage from roots and microorganisms (Greaves and Darbyshire 1972), which binds soil tightly when dry (Watt et al. 1993, 1994). Root mucilage is primarily produced by the root cap (border) cells, is left behind as the root grows forward, and contains complex polysaccharides with charged carboxyl groups, neutral sugars, proteins, and phenolics, depending on species (Miki et al. 1980). Root cap cells and their mucilage can selectively stimulate or inhibit rhizosphere bacteria (Gochnauer et al. 1990), and stimulate hyphal branching of the AMF, Gigaspora gigantea (Nagahashi and Douds 2004). The specific properties of mucilages are worthy of further research, in particular as sources of genetic variation between and within plant species.
Signals regulate numbers and activities of organisms, and root and shoot growth. The best characterised is the nod-factor-flavonoid exchange between legume root hairs and rhizobia that initiates nodule development (Brencic and Winans 2005). Specific signals regulating infection and invasion of roots by AMF or root disease organisms have not been identified. Acyl-homoserine lactone (AHLs) molecules produced by Gram-negative bacteria, regulate expression of genes within a group or ‘quorum’ of bacteria (Sharma et al. 2003). AHLs can be rapidly degraded in specific rhizosphere soils (Wang and Leadbetter 2005). Further, roots produce compounds that mimic the AHLs and therefore confuse communication between rhizosphere bacteria (Teplitski et al. 2000). Given this complexity the importance of AHLs to crop production, particularly in pores where successive roots are colonised (see below), remains to be demonstrated.
Signals of microbial origin can influence shoot processes, including AHLs that can stimulate transpiration (Joseph and Phillips 2003) and lumichrome and other rhizobia molecules that stimulate leaf expansion (Matiru and Dakora 2005). Kirkegaard et al. (1999b) found that wheat leaf extension was inhibited by Rhizoctonia in the absence of water or nutrient stress in these leaves, and suggested that the plant responded to signals from the roots induced by low levels of interaction with the fungus. Rhizosphere bacteria such as Pseudomonas spp. also cause slower leaf growth in the absence of invading or reducing growth of roots (M. Watt, unpublished data). Given the enormous variety of compounds in the rhizosphere, the best strategy may be simply to screen for genotypes that do not respond to negative signals from the rhizosphere by maintaining leaf growth in the presence of deleterious organisms.
Root exudates that move in the gaseous phase of soils include isothiocyanates (ITC) released from members of the Brassica genus including canola (Rumberger and Marschner 2003), and hydrogen gas released from the nodules as a by-product of nitrogen fixation in certain legumes (Dong et al. 2003). ITCs may modify the bacteria around canola roots (see Case study 3). Hydrogen injected into soil stimulates plant growth, notably that of wheat (Dong et al. 2003), but not if the soil is sterilised, suggesting that hydrogen promotes growth via soil organisms that stimulate plant growth. As with canola roots and ITCs, hydrogen effects depend on distances between nodules and new roots, and the stage of succession of organisms around nodules. These dynamics remain to be unravelled in the field to identify new agronomic practices to take advantage of legume hydrogen.
Manipulating exudates for agronomic advantage
Exudate regulation of rhizosphere organisms in the field is still largely unknown. Gaps in knowledge include: the fate of exudates in soil, sites of synthesis and transport in and out of the root, sensing and receptors within the plant, and the specificity of different compounds for root and organism responses. Research, by necessity, is still done in simple systems devoid of the physical, chemical, and biological complexities of field soil, which influence diffusion distances and longevity of exudates. Recently, a Pseudomonas syringae leaf pathogen was found to block the synthesis of an antibacterial compound released from the roots of Arabidopsis exposed to other strains of Pseudomonas syringae (Bais et al. 2005). Charcoal rendered this antibacterial compound inactive as, presumably, would natural soil. Exudates, however, remain a critical target for manipulating soil biology for agronomic benefit because different genotypes with different roots can be used to modify the soil chemistry (O’Connell et al. 1996; Rengel and Marschner 2005). Developing screens to study and select for plants with exudate-mediated microorganism interactions, in conditions relevant to farming systems, remains a challenge.
Characteristics of the rhizosphere in Australian cropping soils
Soil structure and successive generations of roots in soil cracks and macropores
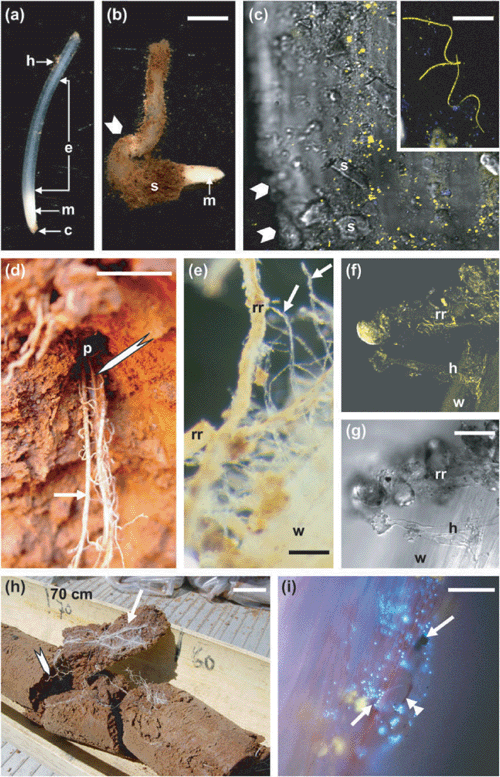
Most Australian cropping soils are difficult for roots to penetrate. Untilled soils are hard and force roots to grow much slower than in cultivated soil (Watt et al. 2005). Hard pans of soil can form below the ploughed layer, impeding roots and movement of soil organisms. Most subsoils are very dense, exceeding 1.6 g/cm3 bulk density. When roots encounter hard soil, extension is restricted and apices have much shorter elongation zones and are distorted (Fig. 2a, b). Roots in hard soil are more heavily infected by Fusarium in bean and Rhizoctonia in wheat (Burke et al. 1980; Gill et al. 2004). Bacteria accumulate on the apices of wheat roots (cv. Janz) in untilled soil (Fig. 2c; Watt et al. 2003). Case study 1 below outlines how interactions between soil hardness and inhibitory bacteria in the rhizosphere can explain lowered productivity of wheat in conservation farming systems. Increases in organisms around roots in hard soil seem due to an interaction between slower growth rate and more exudates (Watt et al. 2006b). For example, roots in hard soil release more root cap cells and mucilage (Iijima et al. 2000) that are carbon-rich substrates for bacteria.
|
Perhaps more important than uniform strength in soil is the presence of cracks and large pores (Cresswell and Kirkegaard 1995), within which roots are often constrained to grow (Fig. 2d), leading to variation in plant growth (Stirzaker et al. 1996). Often, previous roots have occupied these spaces, which become niches that successive generations occupy. The surrounding soil has more microbial biomass that consumes more substrates than that in the bulk soil (Pierret et al. 1999). These niches may be in the unploughed surface soil in conservation cropping systems, and in the undisturbed soil below the plough layer.
Direct contact between new roots and dead remnants from previous crops or weeds is substantial (Fig. 2e). At least half of new roots were in direct contact with dead roots of previous crops in direct-drilled soils in south-eastern NSW (Watt et al. 2005). These dead roots harboured as many bacteria as young, living wheat roots; however, many more were filamentous, such as actinomycetes (inset, Fig. 2c). Root-to-root contact alters the bacterial population on the young roots (Fig. 2f, g), favouring filamentous bacteria, with fewer Pseudomonas (Watt et al. 2006a).
Organisms in cracks and pores may affect crops through nutrients, disease, symbiotic interactions, or other unknown effects on plant growth. Van Noordwijk et al. (1993) found more nitrogen mineralisation in cracks in soil with organic matter and new roots. McNeill et al. (1999) and Khan et al. (2001) found greater nitrogen in dead roots than in dead shoots of crops, and that this root nitrogen can be taken up by a subsequent wheat crop. Such pores can be richer in phosphorus (Pierret et al. 1999). Macrofauna and insects, and organisms transported by water that flows through spaces larger than 0.03 mm in diameter, would move quickly through pores. Subsoil roots as deep as 1.5 m are heavily colonised by bacteria and fungi (Fig. 2h, i), possibly carried from above through pores. Rhizosphere dynamics in pores may affect the worth of ‘primer’ plants that make holes in subsoils that can be used by subsequent crops (see final section).
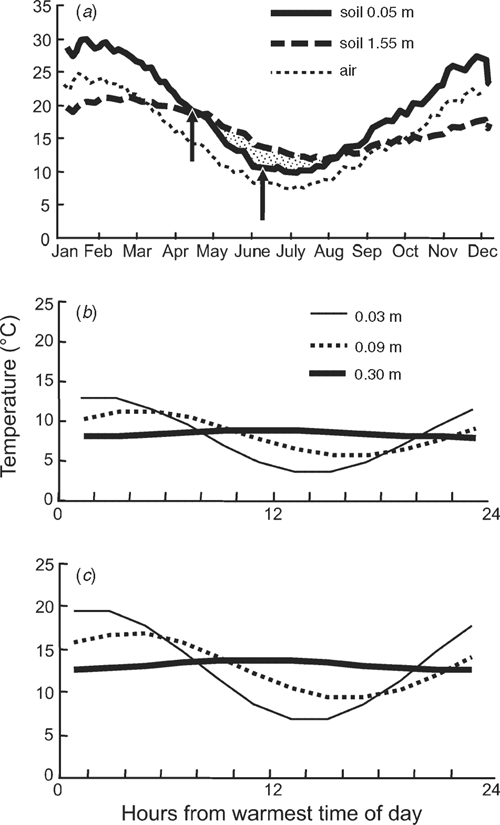
Soil temperature
Roots and organisms encounter different temperatures depending on the depth of soil, time of year, and cropping region (Fig. 3). Most wheat in Australia sown in autumn has roots within the top 0.2 m where soil is on average 8°C in south-eastern New South Wales, and 13°C in south-eastern Queensland. During a season in south-eastern NSW, surface soil temperature varies by 20°C (Fig. 3a). Crops sown in April encounter soil 10°C warmer than if sown in June. The subsoil oscillates 10°C over the season, and in winter, is warmer than the surface soil. Temperature affects the rates of growth and metabolism of roots and organisms, which affect exudation, although the temperature optima for these root and organism processes can be different. Wheat root extension is more inhibited by Pseudomonas at 15°C than at 20°C (Elliott and Lynch 1985), and strawberry roots grown at cool temperatures (5 and 10°C) produce exudates that strongly stimulate germination and hyphal growth of Rhizoctonia, whereas roots grown at 20 and 30°C produced no such exudates (Husain and McKeen 1963).
|
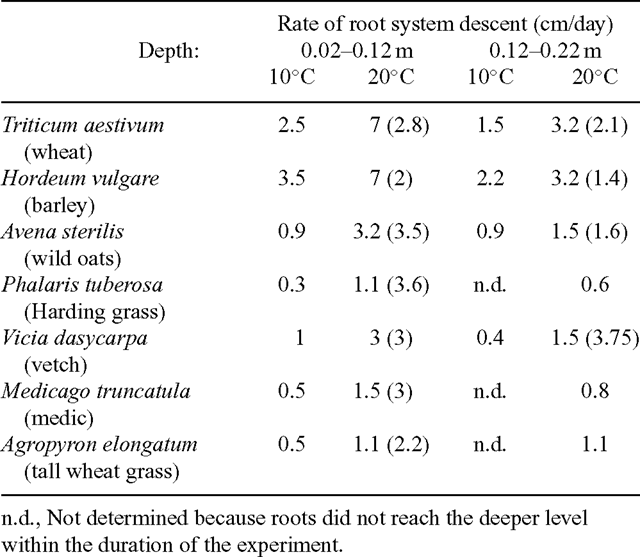
Few studies have quantified the effect of temperature on individual root extension rates, and these few generally focus on young seminal axes. The extension rate of wheat seminal roots is 3.5 times slower at 7°C than at 15°C (S. Refshauge and M. Watt, unpublished), and that of maize is 2.8 times slower at 16°C than at 29°C (Pahlavian and Silk 1988). Cohen and Tadmor (1969; summarised here in Table 2) measured rates of descent of root systems, finding, as above, that young seedling root growth is reduced 3-fold when the temperature is reduced by half. The large variation across species was greater at cooler temperature (10-fold) than at warm (6-fold), and in surface soil compared with deeper (5-fold variation at both 10 and 20°C in deeper soil). Rates of descent in field environments also depend on other factors such as soil density, availability of continuous cracks and macropores, toxic elements, soil water content, and pathogenic organisms. Thus, rates in Table 2 may well overestimate rates in most field environments.
|
Microorganism activity also depends strongly on temperature. Of particular relevance to the rhizosphere is differential effects of temperature on growth rates of different types of soil organisms, and their relative ability to colonise root surfaces at different temperatures. Pietikäinen et al. (2005) found that both fungi and bacteria in soil grew quickest between 25 and 30°C, but that fungi were more inhibited above 30°C than bacteria, and that bacteria were more inhibited by cooler temperatures, regardless of soil type. Fungi to bacteria ratios may increase at cool temperatures, and decrease at warm in the rhizosphere. Leach (1947) showed that temperature differentially affected hyphal elongation rates, with Pythium ultimum growing 3.5 times faster than Rhizoctonia solani at 12°C, but only 1.7 times faster at 20°C. Agronomic effects of organisms can be influenced by soil temperature, as suggested by the inability of AMF to enhance P uptake in wheat during the cool autumn conditions in southern Australia, as discussed in more detail in Case study 2.
Gilligan (1980) made direct measurements of hyphae on wheat roots grown in sand, using microscopy. The wheat pathogenic fungus, Gaemannomyces graminis, extended 2.8 times faster along roots at 19°C than at 10°C. Most fascinating was that at 19°C the fungus grew preferentially towards the root base, but that this directional growth was absent at the cooler temperature. The hyphae thus extended 3 times faster towards the root tip at 10°C. The author suggests that this directional growth is related to assimilate supply to the fungus, although the exact mechanisms by which this occurred remains unclear, particularly since root elongation rate (e.g. position of tip) was not tracked simultaneously. Gilligan’s study shows how dynamics of growth between organisms and roots can be learned from direct, microscopic quantification, and that environmental factors relevant to the field, such as temperature, differentially affect the rates of the various processes. New techniques combining in situ tracking of organisms on roots, and microscopic image analysis (discussed below), offer opportunities to extend such work to a number of different organisms relevant to crop production.
Soil water and organism mobility
Soil water provides a film of water on surfaces within which organisms may propel themselves (e.g. with flagella), and can provide flows of water that can carry organisms substantial distances through large pores, if they do not stick strongly to soil surfaces (Camper et al. 1993). Boelens et al. (1994) used a motile and non-motile strain of a growth-promoting Pseudomonas fluorescens inoculant to show that motility did not influence the ability to colonise roots. Neither seed inoculation nor organism motility reliably helped distribute the inoculant. The flow of water plus extensive mixing of the inoculant through the tilled layer were much more effective in distributing the inoculant across the root system.
Moisture affects the diffusion of water-soluble chemicals in soil, and thence how quickly organisms receive a signal from a root. In wet soils, the rhizosphere is wide for organisms that respond to water-soluble compounds, and in dry soils the rhizosphere for such organisms is much closer to the root (Watt et al. 2006b). Very dry soil may cause organisms, including roots, to desiccate and die. Irrigated and flooded agricultural systems provide a special case where organisms, e.g. pathogens such as Fusarium oxysporum in cotton, can be spread large distances in soil, and re-wetting events will cause organisms to redistribute particularly within the surface soil.
Chemistry
Soil chemistry can influence the rhizosphere organisms directly, or indirectly by modifying root growth and exudates (see Rengel and Marschner 2005; Nelson and Mele 2006, this issue). Australian soils are generally low in available phosphorus and added phosphorus is quickly bound or ‘fixed’ to soil surfaces, or is bound within organic matter. Soils can be too acidic or alkaline, or have high salt concentrations or toxic levels of elements such as Al, Mn, and B. Many herbicides applied to weeds either drip from leaves into surface soil, or are incorporated into surface soil. Some, such as sulfonylurea, can increase damage to roots by Rhizontonia (Smiley and Wilkins 1992), possibly by inhibiting root extension (Wheal et al. 1998).
The surface soil can be readily ameliorated, with lime or gypsum to alleviate acidity or sodicity, or with fertilisers as required. Subsoils are much less readily affected by management, and toxic elements or physicochemical hostilities, such as severe sodicity, can severely limit root depth and thence crop production. The behaviour of subsoil roots and their rhizospheres, particularly perennial rhizospheres in biopores that are repeatedly colonised by successive generations of roots and that contain many remnant roots, may hold the key to improving how root systems of current crops can make better use of subsoils.
Agronomic effects linked to rhizosphere processes
In this section we present three case studies in which significant agronomic effects can be linked to rhizosphere processes. In each, understanding the interactions of roots and organisms in field-relevant rhizospheres (as discussed in the previous section) was necessary to understand and benefit from the different agronomic interventions under investigation. Other examples of such agronomic effects related to rhizosphere processes include optimising specific rhizobia to legume combinations (Brockwell et al. 1995), and management to encourage proliferation of specific disease-suppressive organisms in the soil (see Barnett et al. 2006, this issue).
Case study 1: productivity of direct-drilled wheat in SE Australia
Conservation cropping systems involving direct-drilling of crops into uncultivated seedbeds have been developing for over 30 years in Australia, and systems have been tuned to specific regions (Steed et al. 1993). The level of adoption of direct-drilling has varied significantly within different regions of Australia, from an estimated 98% of crops direct-drilled in the sand-plains of Western Australia to only 13% in central NSW (Connell and Hooper 2002). In southern Australia, particularly in higher rainfall areas, adoption has been slow and the benefits to crop yields flowing from seemingly improved soil conditions harder to demonstrate (Kirkegaard 1995). There are several contributing reasons: stubble loads are generally higher, erosion risks are lower, regular pasture phases maintain soil organic matter, the incidence of soil- and stubble-borne disease is high, and, in many winter-dominant rainfall areas, the conservation of water is less critical for crop yield.
From the outset, a consistent problem with direct-drilled wheat in southern Australia was the reduced early vigour of crops compared with those sown into cultivated soil (Kirkegaard et al. 1995; Simpfendorfer et al. 2002). In a survey of glasshouse and field studies worldwide, Lekberg and Koide (2005) also found evidence for constrained growth (14% shoot biomass reduction) in direct-drilled crops compared with those in disturbed soil. Causes for this constrained growth include changes in temperature and water content of the surface soil, reduced nutrient availability and/or uptake, increased soil strength and reduced root growth, increased incidence of foliar and root disease, and increases in inhibitory microorganisms and phytotoxins. The surprising results of Chan et al. (1987) and later Kirkegaard et al. (1995), showing that soil fumigation could overcome the early growth reductions, pointed to the role of soil biological constraints.
In a subsequent investigation at 39 farm sites over 3 years in southern NSW, Simpfendorfer et al. (2001, 2002) demonstrated that the problem was widespread (62% of sites), was not related to any of the major soil-borne cereal disease organisms, or to general changes in soil biology, but was strongly related to the inhibitory activity of Pseudomonas isolated from the rhizosphere of wheat seedlings at each site. The most likely mechanism for this effect was recently elucidated by Watt et al. (2003, 2005) who studied the architecture, distribution, morphology, and associated soil biology of intact field-grown roots of direct-drilled wheat at a long-term tillage experiment at Harden in southern NSW, where reduced early growth had persisted for many years. The studies showed a higher proportion of contorted, slow-growing root tips constrained by the harder direct-drilled soil, and an associated build-up of Pseudomonas on the slow-growing root tips. The Pseudomonas preferentially built up in the zone around the root tip, whereas the general rhizosphere bacterial population did not.
Thus an interaction between the intact field structure and a specific component of the soil biology was generating a pattern of rhizosphere colonisation that was associated with inhibited wheat growth in direct-drilled soil. This finding could explain why management strategies such as early sowing into warmer soils and cultivation below the seed, both of which increase seedling root growth rates, reduced the effect of direct-drilling on early growth. It also provided opportunities to explore other management and/or genetic options to increase the rate of root growth to avoid the problem (Watt et al. 2005). This work provides a good example of the importance of examining the intact soil/root system in the field when trying to unravel puzzling plant growth responses, as well as the importance of interactions between the soil biology, the soil structure, and the patterns of root growth, in determining those responses.
During the course of this work, the long-term field site at Harden attracted other research on the effect of conservation cropping on soil biology, mostly concerned with effects on the populations of particular classes of organisms. The widely promoted improvements in soil biology expected under direct-drill systems were also evident at the Harden site where increases in soil organic matter, microbial biomass, populations of earthworms, nematode and faunal diversity, as well as disease suppression were all evident on direct-drill/stubble-retained treatments compared with late-burn/single-tine-cultivation treatment (Table 3). In spite of these general ‘improvements’, the growth and yield of wheat throughout the 15-year period have been lower on the direct-drill/stubble-retained treatment compared with the most commonly used management system in the region, comprising a late-burn/single cultivation prior to sowing (Table 3). In addition, more residual subsoil water and mineral N remained in the soil following harvest of direct-drill/ stubble-retained crops, representing an increased risk of deep drainage and N leaching under the conservation cropping system. Thus, in these high-rainfall, mixed farming systems in south-eastern Australia, the promotion of direct-drill/stubble-retained systems on the basis of ‘improvements’ in aspects of the soil biology, and promoted as soil ‘health’ or ‘quality’ (see also Letey et al. 2003), may overlook the associated production constraints and sustainability issues such as acidification and salinisation.
|
Case study 2: the role of arbuscular mycorrhizal fungi (AMF) in wheat production
AMF are obligate symbionts that colonise the roots of most crop plants, taking up nutrients such as P and Zn in return for assimilates from the host. They have also been credited with improving soil structure through their external hyphal structures and the production of the polysaccharide glomalin (Wright and Anderson 2000), increasing water availability, and suppressing disease (Graham 2001). As a major component of the below-ground ecosystem, their potential importance in crop production has been studied intensively. However, their effects on productivity of agricultural systems have been difficult to assess and contradictory (Ryan and Graham 2002; Lekberg and Koide 2005).
The clearest example of AMF benefits to crop growth in the field in Australia is on Vertisols in the northern wheatbelt, where crops grown after 12–18 months of bare fallow grew poorly owing to P and Zn deficiencies associated with low levels of AMF, a condition known as ‘long-fallow disorder’ (Thompson 1987). Such a problem can be managed in the field either by avoiding sequences of fallow or non-host followed by AMF-dependent crops, or by ensuring that P and Zn nutrition of the following crop is adequate. The latter is sometimes problematic on these soils, which are prone to extended periods of surface drying, making P and Zn fertilisers unavailable. Observations that less dependent crops such as wheat could also be affected when grown after non-host crops such as canola raised concerns regarding the general effect of canola on AMF and wheat productivity, not only in the northern wheatbelt (Thompson et al. 2001), but also elsewhere in Australia, because canola and another non-host, lupin, were the most widely grown broadleaf break crops rotated with wheat in the southern and western wheatbelt.
A comprehensive study of the effect of AMF on wheat productivity in south-eastern Australia on both alkaline Vertosols similar to those in the northern wheatbelt and on acidic Kandosols was conducted by Ryan and colleagues (Ryan et al. 2002; Ryan and Angus 2003). They manipulated levels of AMF in commercial fields and in previously uncropped soils by using combinations of different pre-crops that varied in host status, P fertiliser application, and cultivation, to generate AMF root colonisation, varying from 5 to 70%, in subsequent wheat and pea crops. They also monitored a range of other important agronomic parameters including soil water, mineral N, and soil-borne root pathogens to enable clear interpretation of the results under commercial field conditions. They showed that high AMF colonisation in wheat and field pea did not increase nutrient uptake, biomass, or yield in autumn-sown crops in spite of a strong P limitation on crop growth and yield. The authors concluded that high colonisation by AMF is unimportant for the productivity of wheat or field pea grown on these soils, which occupy large areas of cropland in temperate south-eastern Australia. In some experiments, higher AMF colonisation led to greater uptake of Zn and P after anthesis and higher grain concentrations, suggesting a great activity of AMF late in the season. The authors hypothesised that for these autumn-sown crops, cool soil prior to spring reduced nutrient uptake by AMF, and that AMF was likely to be parasitic then. This hypothesis is supported by the lower levels of water-soluble carbohydrates and reduced growth of seedlings as AMF colonisation of the roots increased (Ryan et al. 2005). AMF commonly require up to 20% of total fixed host photosynthate to support their colonisation of roots, and parasitism in the absence of nutritional benefits has been documented for other crops (Graham 2000). Rather than reducing nutrient uptake and productivity, lower levels of AMF colonisation may partly explain the superior growth of wheat following non-host crops such as canola in south-eastern Australia, due to reduced drain on C from the seedling roots. On highly calcareous P-fixing soils elsewhere in southern Australia (such as upper Eyre Peninsula), recent studies showed that fumigation could significantly reduce wheat growth in the absence of applied P, suggesting a role for AMF and other P-solubilising microorganisms in those areas, although this problem is readily addressed in wheat when commercial rates of P are applied (D. K. Roget, CSIRO Adelaide, pers. comm.). As for the northern Australian case, the growth of more highly AMF-dependent pasture species such as lucerne or medic could not be restored with fertiliser application following fumigation, and for successful establishment of these species, AMF management is likely to be more critical.
These studies highlight the need for careful studies in the field to quantify the importance of AMF for different crops within specific farming systems.
Case study 3: Brassica break crops and biofumigation
Substantial productivity improvements in Australian wheat crops in the last decade were underpinned by controlling root diseases using broadleaf break crops such as canola (Brassica napus) and lupin (Lupinus angustifolia) grown in sequence with cereals (Angus 2001). Kirkegaard et al. (2004) reviewed yield responses of wheat to preceding break crops and the mechanisms responsible. They concluded that the average yield improvement of 20% in wheat was remarkably consistent across broad regions and time scales and that much is known about the mechanisms responsible such as disease control, improved nutrition, and water supply. However, there remained inexplicable ‘rotation’ effects apparently associated with poorly understood or inadequately defined factors, particularly soil biology and soil structure.
An interesting case study in this regard is the effect of canola on wheat crops in south-eastern Australia, where wheat grew better following Brassica break crops than when following other broadleaf break crops in the early 1990s. Angus et al. (1991) and Kirkegaard et al. (1994) explored possible causes, and could not attribute the effect to the non-hosting of root disease because all break crops were non-hosts, or to nitrogen nutrition. One possibility was that Brassica crops were improving the soil structure, both in the surface layers as a result of their extensive fine roots (Chan and Heenan 1996) and in the subsoil as a result of biopores created by their deep taproots, which were used by subsequent wheat crops to penetrate the soil. However, Cresswell and Kirkegaard (1995) subsequently found no evidence that canola could improve subsoil structure, and although Schönhammer and Fischbeck (1987) had previously found some evidence for improved soil structure following canola, such effects would be transient under the conventional cultivation regimes used in Australian canola production at the time.
Another hypothesis was that allelochemicals unique to brassicas, principally isothiocyanates (ITCs), may actively suppress disease organisms in a process termed ‘biofumigation’ (Kirkegaard et al. 1993; Angus et al. 1994). In this process, the ITCs were thought to be released during canola root growth or decomposition, reducing the levels of disease inoculum to infect subsequent wheat crops. Subsequent laboratory and pot studies demonstrated that cereal pathogens such as take-all (Gaeumannomyces graminmis, Ggt) were highly sensitive to the ITCs released by canola roots, whether in a pure form applied in Petri dish agar (Sarwar et al. 1998; Smith and Kirkegaard 2002), or when canola root tissues were added to soil at rates likely to be present in the field (Smith et al. 1999). A subsequent series of field experiments showed that Ggt inoculum fell to lower levels under canola crops than under linseed crops during the period from flowering to maturity (Kirkegaard et al. 2000). This coincided with a fall in concentration of the ITC-precursor glucosinolates (GSLs) in the canola taproots.
These results supported in part the original ‘biofumigation’ hypothesis: GSLs contained predominately in the canola tap roots were released and hydrolysed when roots decomposed late in the season and reduced the levels of Ggt inoculum compared with non-Brassica break crops. However the differences in Ggt inoculum measured at the time of canola harvest did not always persist during the subsequent 5-month summer fallow prior to the following wheat crop, as Ggt inoculum declines whenever there is no host present and soil moisture facilitates decomposition. As a result, the effects of the Ggt suppression during the canola year on the disease development and yield in subsequent wheat crops were limited. This was confirmed by Smith et al. (2004) who failed to detect any evidence that brassicas influenced the levels of Ggt or other rhizosphere organisms on the roots of subsequent wheat crops differently from other break crops, raising further doubt about ‘biofumigation’ as it was originally conceived. Thus, despite reports of significant ITC-induced changes in the rhizosphere bacteria of canola (Rumberger and Marschner 2003), it appeared that such effects did not necessarily persist to influence the levels of disease in a subsequent season.
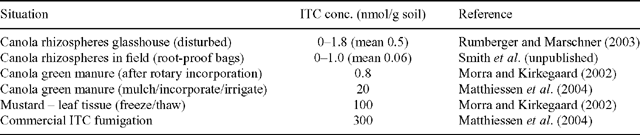
Recent studies of ITC concentrations in the soil around canola roots and the conditions necessary for their release, together with broader consideration of the overall effects of canola on the soil biology in crop rotations, indicate that the original biofumigation hypothesis was simplistic. GSLs and the myrosinase enzyme necessary for the hydrolysis to form ITCs are physically separated in intact tissues, so that significant tissue disruption is required for significant ITC release. Rumberger and Marschner (2003) measured mean ITC concentrations of 0.5 nmol/g and maximum concentrations of 1.8 nmol/g in the rhizosphere of canola grown in glasshouse rhizotrons, whereas only traces were found in the bulk soil (Table 4). ITC added to soil was degraded by microorganisms within 96 h. The concentrations of ITC measured periodically in soil, within root-proof pouches buried adjacent to canola plants growing in the field, also did not exceed 1 nmol/g, and were, more often than not, undetectable (B. Smith and J. A. Kirkegaard, unpublished). Morra and Kirkegaard (2002) showed that less than 1% of the potential ITC was released in the field (0.8 nmol/g soil) following rotary cultivation of flowering canola crops into the soil, whereas full tissue maceration and irrigation could increase this to 20 nmol/g soil (Matthiessen et al. 2004). Freezing leaf tissue released around 30% of available ITCs into soil upon thawing (100 nmol/g soil) which is approaching the levels of ITCs detected following commercial fumigation (300 nmol/g soil) reported by Matthiessen et al. (2004) (Table 4). These results show that the levels of ITC released in soil from canola roots in broad-acre production are likely to be too low for biofumigation, except perhaps for the most sensitive of soil organisms such as Ggt.
|
Although biofumigation to directly influence pathogenic fungi such as Ggt seems unlikely due to the low ITC concentrations in the rhizosphere of dryland canola, these or other compounds specific to Brassica rhizospheres can influence the rhizosphere biology (Rumberger and Marschner 2003), and in some cases this can significantly influence following cereal crops. For example, Kirkegaard et al. (2004) showed that Brassica break-crops led to higher levels of the Trichoderma spp. isolated from the crowns of following wheat crops than after chickpea or cereal crops. Trichoderma are known antagonists of cereal disease such as crown rot (Fusarium pseudograminiearum) and have been shown to be highly tolerant of ITCs in vitro (Smith and Kirkegaard 2002). Further evidence that Brassica break crops could significantly affect soil biology was that different amounts of mineral nitrogen accumulated in the summer fallow following brassicas than following legumes (Kirkegaard et al. 1999a), an observation that could not be explained by the amount, nitrogen content, or carbon : nitrogen ratio of the crop residues. What caused this effect is uncertain, but populations of organisms associated with nitrogen cycling such as free-living nitrogen-fixing bacteria, Azospirillum spp., and ammonium-oxidising bacteria were generally lower following canola, whereas total bacterial populations did not differ. Further studies (Ryan et al. 2006, this issue) have shown that the effects that accelerate mineralisation are transitory under laboratory conditions, but in the field the are strongly influenced by the growth of subsequent wheat crops.
It is now clear that the rotational benefits of Brassica break crops can derive from many effects on soil biology in addition to the reduction in hosting of cereal pathogens. Although the specific effects of ITCs on rhizosphere biology cannot be ruled out, most of these effects appear to be general changes in rhizosphere organisms, rather than the direct killing of disease inoculum by ITCs.
Future directions
Research
Field research
Research in the field is essential to link rhizosphere biology and crop productivity. The role of the agronomist as integrator is crucial. Quantifying rhizosphere biology in the field may involve direct harvesting and analysis of crop roots and their rhizospheres, or the use of fumigants and other chemicals toxic to specific organisms. With the former, careful consideration is needed as to when and where rhizosphere organisms can be expected on different root types in the profile, based on a good estimation of the dynamics among soil structure, temperature, moisture, and chemistry (Watt et al. 2006b). With fumigants, caution is needed regarding confounding interactions with nutrients released from killed cells. Such treatments are gross disturbances of the soil organisms at best, rather than complete sterilisation, but nevertheless remain our best method for assessing pervasive roles for soil organisms in cropping systems.
New methodologies
Molecular methodologies have dominated the study of rhizosphere organisms in the past 20 years (reviewed in Prosser 2002). Nucleic acids can be extracted from soil or isolated organisms, sequenced, and positioned on phylogenic trees to identify previously uncultured organisms and their diversity (Marschner et al. 2001; Johnson et al. 2003). Uncultured organisms that divide in response to substrate can be identified (Borneman 1999). Molecular methods are combined with more traditional techniques such as BIOLOG to assess which substrates are used by isolated organisms, and Janssen et al. (2002) have used novel culturing methods with such studies to culture up to 19% of microscopically counted cells from soil (many times more than previously possible) to characterise the physiology of the isolates.
Oligonucleotide probes can be designed to quantify (e.g. with Real Time PCR) rDNA or rRNA in extracted samples to, for example, predict root disease on farmers’ crops. Combined with knowledge of summer rain and breakdown of leaf substrates that host the disease, these have been particularly successful in predicting the dynamics of Take-all disease of wheat in South Australia (D. K. Roget, unpublished). Such analyses will continue to be valuable to combine with yield mapping and knowledge of soil type and other paddock-scale attributes to help manage spatial variability of yield.
Oligonucleotide probes are conjugated to fluorochromes to bind to rRNA of organisms on roots for direct visualisation (fluorescence in situ hybridisation, FISH) (Amann et al. 1995). FISH is powerful for showing where bacteria are on roots in relation to other features identified in the same microscopic field of view, particularly when combined with the 3-dimensional capabilities of laser confocal microscopy. However, the field of view is small compared with even a single root (<1% of the rhizosphere of a 1-cm piece of root), and FISH cannot be used to detect gross treatment differences at the paddock scale. Probe number is limited by interference from soil particles that emit in the same range as fluorochromes (see Bouvier and Del Giorgio 2003 for comprehensive review of limitations of FISH), and the organisms observed are those left behind after sample preparation. FISH was recently used to quantify rhizosphere bacteria on wheat roots grown in the field (Watt et al. 2006a). Pseudomonas constituted 10% of the total labelled bacteria, and was present in numbers 10–100 times less than evident in controlled environment studies reported in the literature. However, root caps were heavily colonised by bacteria, and contact points with remnant roots had more filamentous bacteria than other regions.
Reporter genes are inserted into bacteria or fungi to express ice-nucleating or fluorescing proteins (generally lux or the green fluorescing protein, gfp). These may express continuously (Bloemberg et al. 2000), or in response to a chemical process in those organisms (with an inducible promoter), which may be related to a rhizosphere exudate or signal (Jaeger et al. 1999; Steidle et al. 2001). The transformed bacteria are generally viewed in situ, and sometimes combined with FISH to identify associated organisms. Such ‘biosensors’ can help identify local chemistry in the rhizosphere; however, as with FISH, samples must be very well defined because only a small area of the rhizosphere is viewed at any one time under a microscope. Larger areas can be seen with a CCD camera at the cm scale, such as in the study of carbon efflux from barley root systems (Darwent et al. 2003), or by extracting cells and combining with flow cytometry. Sample preparation and what compounds remain in the rhizosphere (volatiles, water-soluble ones) over what time frame for biosensors to express are also important, as is soil autofluorescence, which will restrict the types of biosensors.
Novel imaging techniques, adapted largely from medical and earth sciences, combined with in situ organism interactions, will give insights into rhizosphere structure and processes across a broad range of organisms. These include cryo-analytical scanning electron microscopy to localise phosphorus concentrations to arbuscular mycorrhizas grown in soil (Ryan et al. 2003) and necrotrophic fungi such as Rhizoctonia within rotting roots (Refshauge et al. 2006), and synchrotron-based methods to image and quantify mineral-organic complexes on and within roots (Hansel et al. 2001). Computed tomography (CT) offers opportunities for non-invasive imaging of root–water–organism interactions (Grose et al. 1996; Johnson et al. 2004). Improvements in resolution and imaging software will allow studies in larger volumes of soil, and distinction between water, organic material, and solids in intact field soil. These exciting technologies need to be combined with existing, long-standing techniques to relate to processes at different scales.
Consistent units for rhizosphere processes
Soil bound to roots after excavation from pots or the field is used as the ‘rhizosphere’ in many studies. However, this depends on root hair length (e.g. 1 mm for barley), root and organism mucilages, and water (drier soil increasing hair length and soil adhesion, Watt et al. 1993, 1994). Further, bacteria such as Cytophaga were more associated with soil tightly bound to barley roots, compared with Pseudomonas which were associated with the loosely bound soil (Olsson and Persson 1999). Using adhered soil will overestimate or underestimate different rhizosphere organisms and processes (Hinsinger et al. 2005). Different studies are thus analysing different fractions of the rhizosphere biology that depend on the spatial and temporal definition of the rhizosphere, adhesion of organisms to roots and soil, and methods of extraction.
The behaviour of rhizosphere organisms is rarely followed through time. There is a lack of consistent units to express dynamic processes. This is a major problem for comparing studies since expression per length, weight, or volume of root or soil gives different numbers (Duineveld and van Veen 1999). This lack of consistency means that we often have little idea if some process in the rhizosphere is happening quickly or slowly compared with something else, which makes it difficult to connect a given rhizosphere process to agronomic practices and yield. Expression of roots and organisms in units of distance and time (rates) helps to reveal how the rhizosphere develops in different cropping conditions, such as direct-drilling or biopores in the subsoil, and how it thus can be managed with agronomy or breeding (Watt et al. 2006b).
Prospects to harness rhizosphere processes in novel ways to improve crop performance
We have emphasised the central role of roots in regulating soil biology. Here we suggest 4 ways that roots could be used to help manage soil biology. They all use roots of different genotypes to improve plant growth. The greatest gains will be through targetting specific traits of different plants to specific farming systems. For example, a wheat genotype developed for vigorous leaf growth was recently found to be less affected by soil organisms in direct-drilled soil compared with a conventional cultivar, Janz (Watt et al. 2005). Thus, vigorous genotypes may present a new opportunity for increasing productivity in conservation farming.
Manipulating roots and exudates of the current crops
Genotypes could be selected with roots and exudates to modify the rhizosphere biology to benefit the current crop (O’Connell et al. 1996; Rengel and Marschner 2005). For example, genotypes may vary in the extent that their rhizospheres overlap because their root axes respond differently to gravity, resulting in different root architectures (Ge et al. 2000). The extent of rhizosphere overlap would create differences in net concentrations of exudates around roots and thus the numbers and diversity of some microorganisms. Differences in root-hair length may change the size of the rhizosphere and extent of close contact between soil organisms and the root, and 2-fold variation in root-hair length and density has been identified in barley (Gahoonia et al. 1997). Neal et al. (1973) and Miller et al. (1990) reported differences in bacterial populations between 2 wheat genotypes that differed in one chromosome. Azcón and Ocampo (1981) found wide variation in wheat cultivars for VA infection, which was not related to nutrition, and possibly related to carbon efflux from roots. More recently, the more vigorous wheat line, V18, was less stimulated by fumigation compared with the conventional cultivar, Janz, suggesting that its roots either did not host organisms detrimental to growth, or that it was less affected by such organisms (Watt et al. 2005). V18 had fewer Pseudomonas on its root tips from the field compared with Janz (unpublished data). This suggests that there is genotypic variation in the amount or type of exudates from the root tips. Gupta et al. (2004) showed 3-fold variation in the number of copiotrophic bacteria (‘fast-growing’) on the roots of wheat cultivars grown after a preceding cereal crop, and postulated that Trident, a wheat cultivar that grows well after previous wheat crops, induces smaller populations of copiotrophic bacteria and larger populations of oligotrophic bacteria in its rhizosphere, due to either the amount or composition of exudates. It appears that larger populations of copiotrophic bacteria (e.g. Pseudomonas) relative to oligotrophic bacteria may be detrimental to the performance of some wheat varieties, and that pre-crop species and genotypes can influence this ratio.
Exploiting remnant roots of cereals
Based on the evidence in the previous section, it follows that genotypes could be selected with roots and exudates that, as remnants, host organisms that benefit the subsequent crop. This ‘rotation’ has been extensively exploited in cereal farming systems using legume and oilseed break crops (Table 1); however, attention could now focus on the variation in the rhizosphere effects of remnant cereal roots. Mazzola et al. (2004) showed that one wheat cultivar out of 6 stimulated the presence of DAPG-producing, disease-suppressive Pseudomonas strains in soil. Sowing these cultivars may speed up natural suppressiveness in paddocks. The chances for success in using crop or remnant roots to improve soil biology will depend on agronomic history and soil type. It may be that soils low in organic matter and remnant roots, such as sands or newly cropped soil, will make for distinct rhizospheres, whereas soil with high organic matter and remnant roots may swamp the developing rhizospheres with pre-existing populations of organisms on the background organic materials (Garbeva et al. 2004).
Using specific genotypes for delivering inoculants
Substantial research has gone into developing strategies for inoculating seeds and soil with organisms that can stimulate crop growth in the laboratory, but few inoculants are successful in the field (Stewart 2001). A better understanding of what parts of root systems inoculants come from could help improve how inoculants perform in the field. An important trait is the ability to keep pace with the growing root tip of a main axis. Simons et al. (1996) selected inoculants from the tips of roots that had been growing the longest, and found that a specific root tip exudate was one factor critical to some isolates keeping pace with the root tip. McCully (2001) proposed that endophytic inoculants are likely to be living in decaying cortices of roots. Such spaces could be exploited as niches to encourage inoculants to proliferate. If an inoculant applied with the seed proliferates in decaying cortical cells, genotypes with more and faster cortical decay at the root base may be more likely to support the inoculant. Residues from previous crops can be used to host inoculants (Bowen 1980) such as beneficial actinomycetes, or suppressive organisms such as those reported in Barnett et al. (2006, this issue).
Using roots to develop good soil structure
Roots change the soil structure by growing between aggregates and reshaping the spaces within soil. They are a powerful management tool. Worldwide, roots of lucerne are used to create ‘biopores’ in deeper soil layers that a subsequent crop can use (Cresswell and Kirkegaard 1995; Davies and Peoples 2003). Root systems can also be used to improve soil structure in the surface soil. Over time, agricultural soils typically harden. Such ‘coalescence’, particularly obvious on irrigated soils (Cockroft and Olsson 2000), can be reversed with ryegrass roots, accompanied by appropriate tillage and by gentle irrigation (B. Cockcroft, pers. comm.). This reversal appears to be driven primarily by how populous ryegrass roots and hairs are, and by accompanying mucilages that bind soil aggregates.
Conclusions
Major leaps in the productivity of agricultural systems rarely arise from interventions related to single factors, but rather from synergistic interactions among many interventions working together. This is most famously demonstrated by the English agricultural revolution in the 1700s in which the synergistic interactions among the individual components of the Norfolk system — use of marl and clay, rotation of crops, the culture of turnips hand-hoed, and the culture of clover and rye — most of which had been promoted individually since ancient times, made it such an effective agent of improvement (Evans 1998). More recent examples of such effective interactions in Australian agriculture include the ‘pasture improvement revolution’, involving adapted legume species, inoculation with effective rhizobia, application of P and in particular molybdenum so vital for the effective activity of rhizobia (Williams and Andrew 1970), and the more recent doubling of average wheat yields in south-eastern Australia underpinned by root-disease control using break crops such as canola, and the consequent responses of semi-dwarf varieties to N fertiliser applications (Angus 2001).
These examples serve to illustrate the need to carefully consider any planned manipulation of soil biology to improve crop production in the context of both the future farming systems in which we are expecting such interventions to be effective, and the actual root and rhizosphere environment in which we expect them to function. The current trend in dryland farming systems towards no-till farming with controlled traffic or precision guidance systems, together with other economic imperatives to increase the scale and efficiency of operations, are likely to continue. Aspects of these systems relevant to rhizosphere biology include the preservation of intact soil structure and the increased longevity of root residues from season to season, as well as a capacity to more precisely deliver seed, fertilizer, and other products in the soil.
Acknowledgments
This review, and much of the research reported, were supported by the Grains Research and Development Corporation (GRDC) of Australia. We are grateful to Margaret McCully for stimulating research on field-grown roots at CSIRO, Plant Industry, and in Australia via Root and Rhizosphere Workshops. We also thank Rosemary White and the CSIRO Plant Industry Microscopy Centre, and Geoff Howe and the Ginnindera Research Station staff for experimental support. We are grateful to Linda Magee for Figs 2d, 3b, and c and the reference section, to Julianne Lilley for the annual temperature analysis from APSIM in Fig. 3a, and to Alan Richardson for valuable suggestions on the manuscript.
Akiyama K,
Matsuzaki K, Hayashi H
(2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Amann RI,
Ludwig W, Schleifer KH
(1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Reviews 59, 143–169.
| PubMed |

Angus JF
(2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277–288.
| Crossref | GoogleScholarGoogle Scholar |

Angus JF,
Gardner PA,
Kirkegaard JA, Desmarchelier JM
(1994) Biofumigation: Isothiocyanates released from Brassica roots inhibit the growth of the take-all fungus. Plant and Soil 162, 107–112.
| Crossref | GoogleScholarGoogle Scholar |

Angus JF,
van Herwaarden AF, Howe GN
(1991) Productivity and break crop effect of winter-growing oilseeds. Australian Journal of Experimental Agriculture 31, 669–677.
| Crossref | GoogleScholarGoogle Scholar |

Azcón R, Ocampo JA
(1981) Factors affecting the vesicular-arbuscular infection and mycorrhizal dependency of thirteen wheat cultivars. New Phytologist 87, 677–685.
| Crossref |

Bais HP,
Park S,
Weir TL,
Callaway RM, Vivanco JM
(2004) How plants communicate using the underground information superhighway. Trends in Plant Science 9, 26–32.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bais HP,
Prithiviraj B,
Jha AK,
Ausubel FM, Vivanco JM
(2005) Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434, 217–221.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Barnett SJ,
Roget DK, Ryder MH
(2006) Suppression of Rhizoctonia solani AG-8 induced disease on wheat by the interaction between Pantoea, Exiguobacterium, and Microbacteria. Australian Journal of Soil Resaerch 44, 331–342.

Bertin C,
Yang X, Weston LA
(2003) The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil 256, 67–83.
| Crossref | GoogleScholarGoogle Scholar |

Bever JD
(2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytologist 157, 465–473.
| Crossref | GoogleScholarGoogle Scholar |

Bloemberg GV,
Wijfjes AHM,
Lamers GEM,
Stuurman N, Lugtenberg BJJ
(2000) Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: New perspectives for studying microbial communities. Molecular Plant-Microbe Interactions 13, 1170–1176.
| PubMed |

Boelens J,
Vande Woestyne M, Verstraete W
(1994) Ecological importance of motility for the plant growth-promoting rhizopseudomonas strain ANP15. Soil Biology and Biochemistry 26, 269–277.
| Crossref | GoogleScholarGoogle Scholar |

Borneman J
(1999) Culture-independent identification of microorganisms that respond to specified stimuli. Applied and Environmental Microbiology 65, 3398–3400.
| PubMed |

Bouvier T, Del Giorgio PA
(2003) Factors influencing the detection of bacterial cells using fluorescence in situ hybridization (FISH): a quantitative review of published reports. FEMS Microbiology Ecology 44, 3–15.
| Crossref | GoogleScholarGoogle Scholar |

Brencic A, Winans SC
(2005) Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbial and Molecular Biology Reviews 69, 155–194.
| Crossref | GoogleScholarGoogle Scholar |

Brockwell J,
Bottomley PJ, Thies JE
(1995) Manipulation of rhizobia microflora for improving legume productivity and soil fertility: A critical assessment. Plant and Soil 174, 143–180.
| Crossref | GoogleScholarGoogle Scholar |

Burke DW,
Miller DE, Barker AW
(1980) Effects of soil temperature on growth of beans in relation to soil compaction and fusarium root rot. Phytopathology 70, 1047–1049.

Camper AK,
Hayes JT,
Sturman PJ,
Jones WL, Cunningham AB
(1993) Effects of motility and adsorption rate coefficient on transport of bacteria through saturated porous media. Applied and Environmental Microbiology 59, 3455–3462.
| PubMed |

Chan KY, Heenan DP
(1996) The influence of crop rotation on soil structure and soil physical properties under conventional tillage. Soil and Tillage Research 37, 113–125.
| Crossref | GoogleScholarGoogle Scholar |

Chan KY,
Mead JA, Roberts WP
(1987) Poor early growth and yield of wheat under direct drilling. Australian Journal of Agricultural Research 38, 791–800.

Cockroft B, Olsson KA
(2000) Degradation of soil structure due to coalescence of aggregates in no-till, no-traffic beds in irrigated crops. Australian Journal of Soil Research 38, 61–70.
| Crossref | GoogleScholarGoogle Scholar |

Cohen Y, Tadmor NH
(1969) Effects of temperature on the elongation of seedling roots of some grasses and legumes. Crop Science 9, 189–192.

Cresswell HP, Kirkegaard JA
(1995) Subsoil amelioration by plant roots – the process and the evidence. Australian Journal of Soil Research 33, 221–239.
| Crossref | GoogleScholarGoogle Scholar |

Darrah PR
(1991) Models of the rhizosphere. I. Microbial population dynamics around a root releasing soluble and insoluble carbon. Plant and Soil 133, 187–199.
| Crossref | GoogleScholarGoogle Scholar |

Darwent MJ,
Paterson E,
McDonald AJS, Tomos AD
(2003) Biosensor reporting of root exudation from Hordeum vulgare in relation to shoot nitrate concentration. Journal of Experimental Botany 54, 325–334.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Davies SL, Peoples MB
(2003) Identifying potential approaches to improve the reliability of terminating a lucerne pasture before cropping: a review. Australian Journal of Experimental Agriculture 43, 429–447.
| Crossref | GoogleScholarGoogle Scholar |

Dong Z,
Wu L,
Kettlewell B,
Caldwell CD, Layzell DB
(2003) Hydrogen fertilization of soils – is this a benefit of legumes in rotation? Plant, Cell & Environment 26, 1875–1879.
| Crossref | GoogleScholarGoogle Scholar |

Doube BM,
Buckerfield JC, Kirkegaard JA
(1994) Short-term effects of tillage and stubble management on earthworm populations in cropping systems in southern New South Wales. Australian Journal of Agricultural Research 45, 1587–1600.
| Crossref | GoogleScholarGoogle Scholar |

Duineveld BM, van Veen JA
(1999) The number of bacteria in the rhizosphere during plant development: relating colony-forming units to different reference units. Biology and Fertility of Soils 28, 285–291.
| Crossref | GoogleScholarGoogle Scholar |

Elliott LF, Lynch JM
(1985) Plant growth-inhibitory pseudomonads colonizing winter wheat (Triticum aestivum L.) roots. Plant and Soil 84, 57–65.
| Crossref | GoogleScholarGoogle Scholar |

Farrar J,
Hawes M,
Jones D, Lindow S
(2003) How roots control the flux of carbon to the rhizosphere. Ecology 84, 827–837.

Gahoonia TS,
Care D, Nielsen NE
(1997) Root hairs and phosphorus acquisition in wheat and barley cultivars. Plant and Soil 191, 181–188.
| Crossref | GoogleScholarGoogle Scholar |

Garbeva P,
van Veen JA, van Elsas JD
(2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology 42, 243–270.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ge Z,
Rubio G, Lynch JP
(2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant and Soil 218, 159–171.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gerhardson B,
Alström S, Ramert B
(1985) Plant reactions to inoculation of roots with fungi and bacteria. Phytopathologische Zeitschrift 114, 108–117.

Gill JS,
Hunt S,
Sivasithamparam K, Smettem KRJ
(2004) Root growth altered by compaction of a sandy loam soil affects severity of rhizoctonia root rot of wheat seedlings. Australian Journal of Experimental Agriculture 44, 595–599.
| Crossref | GoogleScholarGoogle Scholar |

Gilligan CA
(1980) Dynamics of root colonization by the take-all fungus, Gaeumannomyces graminis. Soil Biology and Biochemistry 12, 507–512.
| Crossref | GoogleScholarGoogle Scholar |

Gochnauer MB,
McCully ME, Labbe H
(1989) Different populations of bacteria associated with sheathed and bare regions of roots of field-grown maize. Plant and Soil 114, 107–120.
| Crossref | GoogleScholarGoogle Scholar |

Gochnauer MB,
Sealey LJ, Mccully ME
(1990) Do detached root-cap cells influence bacteria associated with maize roots? Plant, Cell & Environment 13, 793–801.
| Crossref |

Graham JH
(2001) What do root pathogens see in mycorrhizas? New Phytologist 149, 357–359.
| Crossref | GoogleScholarGoogle Scholar |

Greaves MP, Darbyshire JF
(1972) The ultrastructure of the mucilaginous layer on plant roots. Soil Biology and Biochemistry 4, 443–449.
| Crossref | GoogleScholarGoogle Scholar |

Grose MJ,
Gilligan CA,
Spencer D, Goddard BVD
(1996) Spatial heterogeneity of soil water around single roots: use of CT-scanning to predict fungal growth in the rhizosphere. New Phytologist 133, 261–272.
| Crossref |

Hansel CM,
Fendorf S,
Sutton S, Newville M
(2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environmental Science & Technology 35, 3863–3868.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hinsinger P,
Gobran GR,
Gregory PJ, Wenzel WW
(2005) Rhizosphere geometry and heterogenity arising from root-mediated physical and chemical processes. New Phytologist 168, 293–303.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Huisman OC
(1982) Interrelations of root growth dynamics to epidemiology of root-invading fungi. Annual Review of Phytopathology 20, 303–327.
| Crossref | GoogleScholarGoogle Scholar |

Husain SS, McKeen WE
(1963) Interactions between strawberry roots and Rhizoctonia fragariae. Phytopathology 53, 541–545.

Iijima M,
Griffiths B, Bengough AG
(2000) Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist 145, 477–482.
| Crossref | GoogleScholarGoogle Scholar |

Jaeger C,
Lindow S,
Miller S,
Clark E, Firestone M
(1999) Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Applied and Environmental Microbiology 65, 2685–2690.
| PubMed |

Janssen PH,
Yates PS,
Grinton BE,
Taylor PM, Sait M
(2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria and Verrucomicrobia. Applied and Environmental Microbiology 68, 2391–2396.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Johnson MJ,
Lee KY, Scow KM
(2003) DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 114, 279–303.
| Crossref | GoogleScholarGoogle Scholar |

Johnson SN,
Read DB, Gregory PJ
(2004) Tracking larval insect movement within soil using high resolution X-ray microtomography. Ecological Entomology 29, 117–122.
| Crossref | GoogleScholarGoogle Scholar |

Jones DL,
Hodge A, Kuzyakov Y
(2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytologist 163, 459–480.
| Crossref | GoogleScholarGoogle Scholar |

Joseph CA, Phillips DA
(2003) Metabolites from soil bacteria affect plant water relations. Plant Physiology and Biochemistry 41, 189–192.
| Crossref | GoogleScholarGoogle Scholar |

Keel C,
Schnider U,
Maurhofer M,
Voisard C,
Laville J,
Burger U,
Wirthner P,
Haas D, Defago G
(1992) Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Molecular Plant-Microbe Interactions 5, 4–13.

Kirkegaard JA
(1995) A review of trends in wheat yield responses to conservation cropping in Australia. Australian Journal of Experimental Agriculture 35, 835–848.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Gardner PA,
Angus JF, Koetz E
(1994) Effect of Brassica break crops on the growth and yield of wheat. Australian Journal of Agricultural Research 45, 529–545.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Howe GN, Mele P
(1999a) Enhanced accumulation of mineral-N following canola. Australian Journal of Experimental Agriculture 39, 587–593.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Munns R,
James RA,
Gardner PA, Angus JF
(1995) Reduced growth and yield of wheat with conservation cropping. 2. Soil biological factors limit growth under direct drilling. Australian Journal of Agricultural Research 46, 75–88.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Munns R,
James RA, Neate SM
(1999b) Does water and phosphorus uptake limit leaf growth of Rhizoctonia-infected wheat seedlings? Plant and Soil 209, 157–166.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Sarwar M,
Wong PTW,
Mead A,
Howe G, Newell M
(2000) Field studies on the biofumigation of take-all by Brassica break crops. Australian Journal of Agricultural Research 51, 445–456.
| Crossref | GoogleScholarGoogle Scholar |

Kirkegaard JA,
Simpfendorfer S,
Holland J,
Bambach R,
Moore KJ, Rebetzke GJ
(2004) Effect of previous crops on crown rot and yield of durum and bread wheat in northern NSW. Australian Journal of Agricultural Research 55, 321–334.
| Crossref | GoogleScholarGoogle Scholar |

Leach LD
(1947) Growth rates of host and pathogen as factors determining the severity of preemergence damping-off. Journal of Agricultural Research 75, 161–179.

Lekberg Y, Koide RT
(2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytologist 168, 189–204.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Letey J,
Sojka RE,
Upchurch DR,
Cassel DK,
Olson KR,
Payne WA,
Petrie SE,
Price GH,
Reginato RJ,
Scott HD,
Smethurst PJ, Triplett GB
(2003) Deficiencies in the soil quality concept and its application. Journal of Soil and Water Conservation 58, 180–187.

Liljeroth E,
Burgers SLGE, van Veen JA
(1991) Changes in bacterial populations along roots of wheat (Triticum aestivum L.) seedlings. Biology and Fertility of Soils 10, 276–280.
| Crossref | GoogleScholarGoogle Scholar |

Marschner P,
Yang CH,
Lieberei R, Crowley DE
(2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biology and Biochemistry 33, 1437–1445.
| Crossref | GoogleScholarGoogle Scholar |

Matiru VN, Dakora FD
(2005) The rhizosphere signal molecule lumichrome alters seedling development in both legumes and cereals. New Phytologist 166, 439–444.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Matthiessen JN,
Warton B, Shackleton MA
(2004) The importance of plant maceration and water addition in achieving high Brassica-derived isothiocyanate levels in soil. Agroindustria 3, 277–280.

Mazzola M,
Funnell DL, Raaijmakers JM
(2004) Wheat cultivar-specific selection of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas species from resident soil populations. Microbial Ecology 48, 338–348.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

McCully ME
(1999) Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50, 695–718.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

McCully ME
(2001) Niches for bacterial endophytes in crop plants: a plant biologist’s view. Australian Journal of Plant Physiology 28, 983–990.

McCully ME, Canny MJ
(1985) Localisation of translocated 14C in roots and root exudates of field-grown maize. Physiologia Plantarum 65, 380–392.
| Crossref |

Merbach W,
Mirus E,
Knof G,
Remus R,
Ruppel S,
Russow R,
Gransee A, Schulze J
(1999) Release of carbon and nitrogen compounds by plant roots and their possible ecological importance. Journal of Plant Nutrition and Soil Science 162, 373–383.
| Crossref | GoogleScholarGoogle Scholar |

Miki NK,
Clarke KJ, McCully ME
(1980) A histological and histochemical comparison of the mucilages on the root tips of several grasses. Canadian Journal of Botany 58, 2581–2593.

Miller HJ.,
Liljeroth E,
Henken G, van Veen JA
(1990) Fluctuations in the fluorescent pseudomonad and actinomycete populations of the rhizosphere and rhizoplane during the growth of spring wheat. Canadian Journal of Microbiology 36, 254–258.

Morra MJ, Kirkegaard JA
(2002) Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biology and Biochemistry 34, 1683–1690.
| Crossref | GoogleScholarGoogle Scholar |

Nagahashi G, Douds DD
(2004) Isolated root caps, border cells, and mucilage from host roots stimulate hyphal branching of the arbuscular mycorrhizal fungus, Gigaspora gigantea. Mycological Research 108, 1079–1088.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Neal JL,
Larson RI, Atkinson TG
(1973) Changes in rhizosphere populations of selected physiological groups of bacteria related to substitution of specific pairs of chromosomes in spring wheat. Plant and Soil 39, 209–212.
| Crossref | GoogleScholarGoogle Scholar |

Nelson DR, Mele PM
(2006) The impact of crop residue amendments and lime on microbial community structure and nitrogen-fixing bacteria in the wheat rhizosphere. Australian Journal of Soil Research 44, 319–329.

Newman EI, Watson A
(1977) Microbial abundance in the rhizosphere: A computer model. Plant and Soil 48, 17–56.
| Crossref | GoogleScholarGoogle Scholar |

van Noordwijk M,
de Ruiter PC,
Zwart KB,
Bloem J,
Moore JC,
van Faassen HG, Burgers SLGE
(1993) Synlocation of biological activity, roots, cracks and recent organic inputs in a sugar beet field. Geoderma 56, 265–276.
| Crossref | GoogleScholarGoogle Scholar |

O’Connell KP,
Goodman RM, Handelsman J
(1996) Engineering the rhizosphere: expressing a bias. Trends in Biotechnology 170, 6–10.

Olsson S, Persson P
(1999) The composition of bacterial populations in soil fractions differing in their degree of adherence to barley roots. Applied Soil Ecology 12, 205–215.
| Crossref | GoogleScholarGoogle Scholar |

Pahlavian AM, Silk WK
(1988) Effect of temperature on spatial and temporal aspects of growth in the primary maize root. Plant Physiology 87, 529–532.
| PubMed |

Pankhurst CE,
McDonald HJ,
Hawke BG, Kirby CA
(2002) Effect of tillage and stubble management on chemical and microbiological properties, and the development of suppression towards cereal root disease, in soils from two sites in NSW, Australia. Soil Biology and Biochemistry 34, 833–840.
| Crossref | GoogleScholarGoogle Scholar |

Pierret A,
Moran CJ, Pankhurst CE
(1999) Differentiation of soil properties related to the spatial association of wheat roots and soil macropores. Plant and Soil 211, 51–58.
| Crossref | GoogleScholarGoogle Scholar |

Pietikäinen J,
Pettersson M, Baath E
(2005) Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiology Ecology 52, 49–58.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Prosser JI
(2002) Molecular and functional diversity in soil micro-organisms. Plant and Soil 244, 9–17.
| Crossref | GoogleScholarGoogle Scholar |

Refshauge S,
Watt M,
McCully ME, Huang CX
(2006) Frozen in time: A new method using cryo-SEM to visualise root-fungal interactions. New Phytologist In press ,
| Crossref | GoogleScholarGoogle Scholar |

Rengel Z, Marschner P
(2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytologist 168, 305–312.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rumberger A, Marschner P
(2003) 2-phenylethylisothiocyanate concentration and microbial community composition in the rhizosphere of canola. Soil Biology and Biochemistry 35, 445–452.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH, Angus JF
(2003) Arbuscular mycorrhizae in wheat and field pea crops on low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant and Soil 250, 225–239.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH, Graham JH
(2002) Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant and Soil 244, 263–271.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH,
van Herwaarden AF,
Angus JF, Kirkegaard JA
(2005) Colonisation by arbuscular mycorrhizal fungi is associated with reductions in biomass of wheat in a low-P soil under field conditions. Plant and Soil 270, 275–286.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH,
Kirkegaard JA, Angus JF
(2006) Nitrogen mineralisation in relation to previous crops and pasture. Australian Journal of Soil Research 44, 367–377.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH,
McCully ME, Huang CX
(2003) Location and quantification of phosphorus and other elements in fully-hydrated, soil-grown arbuscular mycorrhizas: a cryo-analytical scanning electron microscopy study. New Phytologist 160, 429–441.
| Crossref | GoogleScholarGoogle Scholar |

Ryan MH,
Norton RM,
Kirkegaard JA,
McCormick KM,
Knights SE, Angus JF
(2002) Increasing mycorrhizal colonisation does not improve growth and nutrition of wheat on Vertosols in south-eastern Australia. Australian Journal of Agricultural Research 53, 1173–1181.
| Crossref | GoogleScholarGoogle Scholar |

Sarwar M,
Kirkegaard JA,
Wong PTW, Desmarchelier JM
(1998) Biofumigation potential of brassicas. III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant and Soil 201, 103–112.
| Crossref | GoogleScholarGoogle Scholar |

Schönhammer A, Fischbeck G
(1987) Investigations on cereal crop rotations and monocultures. III Changes in soil properties. Bayerisches Landwirtschaftliches Jahrbuch 64, 681–694.

Schroth MN, Hildebrand DC
(1964) Influence of plant exudates on root-infecting fungi. Annual Review of Phytopathology 2, 101–132.
| Crossref | GoogleScholarGoogle Scholar |

Scott EM,
Rattray EAS,
Prosser JI,
Killham K,
Glover LA,
Lynch JM, Bazin MJ
(1995) A mathematical model for dispersal of bacterial inoculants colonizing the wheat rhizosphere. Soil Biology and Biochemistry 27, 1307–1318.
| Crossref | GoogleScholarGoogle Scholar |

Sharma A,
Sahgal M, Johri BN
(2003) Microbial communication in the rhizosphere: operation of quorum sensing. Current Science 85, 1164–1172.

Simons M,
van der Bij AJ,
Brand I,
de Weger LA,
Wijffelman CA, Lugtenberg BJJ
(1996) Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Molecular Plant-Microbe Interactions 9, 600–607.
| PubMed |

Simpfendorfer S,
Kirkegaard JA,
Heenan DP, Wong PTW
(2001) Involvement of root inhibitory Pseudomonas spp. in the poor early growth of direct drilled wheat: studies in intact cores. Australian Journal of Agricultural Research 52, 845–853.
| Crossref | GoogleScholarGoogle Scholar |

Simpfendorfer S,
Kirkegaard JA,
Heenan DP, Wong PTW
(2002) Reduced early growth of direct drilled wheat in southern New South Wales—role of root inhibitory pseudomonads. Australian Journal of Agricultural Research 53, 323–331.
| Crossref | GoogleScholarGoogle Scholar |

Sivasithamparam K, Parker CA
(1979) Rhizosphere micro-organisms of seminal and nodal roots of wheat grown in pots. Soil Biology and Biochemistry 11, 155–160.
| Crossref | GoogleScholarGoogle Scholar |

Smiley RW, Wilkins DE
(1992) Impact of sulfonylurea herbicides on Rhizoctonia root rot, growth, and yield of winter wheat. Plant Disease 76, 399–404.

Smith BJ, Kirkegaard JA
(2002) In vitro inhibition of soil microorganisms by 2-phenylethyl isothiocyanate. Plant Pathology 51, 585–593.
| Crossref | GoogleScholarGoogle Scholar |

Smith BJ,
Kirkegaard JA, Howe GN
(2004) Impacts of Brassica break crops on soil biology and yield of following wheat crops. Australian Journal of Agricultural Research 55, 1–11.
| Crossref | GoogleScholarGoogle Scholar |

Steidle A,
Sigl K,
Schuhegger R,
Ihring A,
Schmid M,
Gantner S,
Stoffels M,
Riedel K,
Givskov M,
Hartmann A,
Langebartels C, Eberl L
(2001) Visualization of N-acylhomoserine lactone-mediated cell–cell communication between bacteria colonizing the tomato rhizosphere. Applied and Environmental Microbiology 67, 5761–5770.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Stewart A
(2001) Commercial biocontrol—reality or fantasy? Australasian Plant Pathology 30, 127–131.
| Crossref | GoogleScholarGoogle Scholar |

Stirzaker RJ,
Passioura JB, Wilms Y
(1996) Soil structure and plant growth: Impact of bulk density and biopores. Plant and Soil 185, 151–162.
| Crossref | GoogleScholarGoogle Scholar |

Teplitski M,
Robinson JB, Bauer WD
(2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviours in associated bacteria. Molecular Plant-Microbe Interactions 13, 637–648.
| PubMed |

Thompson JP
(1987) Decline of vesicular-arbuscular mycorrhizas in long fallow disorder of field crops and its expression in phosphorus deficiency in sunflower. Australian Journal of Agricultural Research 38, 847–867.
| Crossref | GoogleScholarGoogle Scholar |

Wang Y-J, Leadbetter JR
(2005) Rapid acyl-homoserine lactone quorum signal biodegradation in diverse soils. Applied and Environmental Microbiology 71, 1291–1299.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Watt M,
Hugenholtz P,
White R, Vinall K
(2006a) Numbers and locations of native bacteria on field grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environmental Microbiology 8, 871–884.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Watt M,
Kirkegaard JA, Rebetzke GJ
(2005) A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Functional Plant Biology 32, 695–706.
| Crossref | GoogleScholarGoogle Scholar |

Watt M,
McCully ME, Canny MJ
(1994) Formation and stabilisation of maize rhizosheaths: Effect of soil water content. Plant Physiology 106, 179–186.
| PubMed |

Watt M,
McCully ME, Jeffree CE
(1993) Plant and bacterial mucilages of the maize rhizosphere: Comparison of their soil binding properties and histochemistry in a model system. Plant and Soil 151, 151–165.
| Crossref | GoogleScholarGoogle Scholar |

Watt M,
McCully ME, Kirkegaard JA
(2003) Soil strength and rate of rot elongation alter the accumulation of Pseudomonas spp. and other bacteria in the rhizosphere of wheat. Functional Plant Biology 30, 483–491.
| Crossref | GoogleScholarGoogle Scholar |

Watt M,
Silk WK, Passioura JP
(2006b) Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Annals of Botany 97, 839–855.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

West ES
(1952) A study of the annual soil temperature wave. Australian Journal of Scientific Research 5, 303–314.
| PubMed |

Wheal MS,
Rengel Z, Graham RD
(1998) Chlorsulfuron reduces extension of wheat root tips in low-zinc solution culture. Annals of Botany 81, 385–389.
| Crossref | GoogleScholarGoogle Scholar |

Wright SF, Anderson RL
(2000) Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biology and Fertility of Soils 31, 249–253.
| Crossref | GoogleScholarGoogle Scholar |

Zelenev VV,
van Bruggen AHC, Semenov AM
(2000) ‘BACWAVE’, a spatial-temporal model for travelling waves of bacterial populations in response to a moving carbon source in soil. Microbial Ecology 40, 260–272.
| PubMed |



