Storage of soil samples leads to over-representation of the contribution of nitrate to plant-available nitrogen
Taleta Bailey A B * , Nicole Robinson A , Mark Farrell
A B * , Nicole Robinson A , Mark Farrell  B , Ben Macdonald
B , Ben Macdonald  C , Tim Weaver D , Diogenes L. Antille C , Aidan Chin A and Richard Brackin
C , Tim Weaver D , Diogenes L. Antille C , Aidan Chin A and Richard Brackin  A
A
A School of Agriculture and Food Sciences, The University of Queensland, Brisbane, Qld, Australia.
B CSIRO Agriculture and Food, Waite Campus, Adelaide, SA, Australia.
C CSIRO Agriculture and Food, Black Mountain, Canberra, ACT, Australia.
D CSIRO Agriculture and Food, Myall Vale, Narrabri, NSW, Australia.
Soil Research 60(1) 22-32 https://doi.org/10.1071/SR21013
Submitted: 14 January 2021 Accepted: 15 June 2021 Published: 4 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Delays between soil sampling and processing for analysis are common in both research and agronomy, but the effects of storage conditions on measurements of plant-available nitrogen (N) are rarely considered. With increasing recognition of organic N pools in soils, such as amino acids and peptides, it is necessary to determine how sample handling impacts the outcomes of soil N quantification. In this study, we used in situ microdialysis to approximate plant availability of amino acids, ammonium and nitrate, then compared to both potassium chloride (KCl) extract and microdialysis samples taken from excavated soil samples when in the field, after 24 h refrigerated storage, and after storage for 1 month, either refrigerated or air-dried. Nitrate levels measured with microdialysis and KCl extracts increased immediately after soil sampling and continued to accumulate in the next day and 1 month stored samples. Amino acid and ammonium measurements remained more constant; however, microdialysis showed a decline in amino acid-N between in situ and next day samples. The proportional representation of N pools in the in-field extracts was most similar to in situ microdialysis. Soil samples should be processed for N analysis as close to sampling as possible, and the storage duration and conditions reported. The influence of storage must be considered in interpreting soil test results.
Keywords: air-dry, amino acids, ammonium, in situ soil sampling, microdialysis, nitrogen flux, organic nitrogen, refrigerated storage, soil testing.
Introduction
Nitrogen (N) in soils is a highly dynamic nutrient, rapidly converting between different forms and moving through the soil profile. Nitrogen pool dynamics are driven by the chemical, physical and biological characteristics of the soil, and can drastically change with a stress applied to any of these conditions, such as during waterlogging and anoxia (Kuypers et al. 2018; Wooliver et al. 2019). This presents a considerable challenge for quantification of soil N pools, where disturbance and removal from the in situ conditions are often unavoidable.
Soil N analysis is an essential undertaking of agronomy and field research in agriculture, particularly when investigating nitrogen use efficiency (NUE) in production systems. Typical analyses include measurement of nitrate, ammonium and, increasingly, dissolved organic N (DON), using water or salt extracts (Mulvaney 1996; Rayment and Lyons 2011). In agronomy settings, testing for soil mineral-N (nitrate and ammonium) can be a key determinant of the amount of fertiliser applied for the subsequent crop. However, inconsistency in sampling methods and data interpretation makes test results less reliable for growers (Schwenke et al. 2019). In research trials comparing N fertiliser forms, application rates, management techniques or loss prevention strategies, gaining accurate measurements of nitrate, ammonium and DON is critical to evaluating plant N availability, susceptibility to loss and ultimately NUE (Shaw et al. 2014; Rochester and Bange 2016; Macdonald et al. 2017a; Allen et al. 2019). Ensuring routine sampling and analysis procedures give accurate representations of N form distribution is crucial to both developing effective strategies for improving NUE and informing improved fertiliser management.
Given the dynamic nature of soil N, it is expected that transformations will continue after a sample is removed from the field (Li et al. 2012). To minimise changes in N pools, samples are stored in either refrigerated or air-dried conditions to slow or limit microbial activity (Stenberg et al. 1998; Verchot 1999; Lee et al. 2007; Černohlávková et al.2009). Studies comparing storage treatments have shown a large increase in nitrate with drying and sieving (Jones and Willett 2006; Ros et al. 2011; Inselsbacher 2014; Gütlein et al. 2016). Fewer studies have investigated the effects of refrigerated sample storage, despite often being the recommended alternative to air drying (Jones and Willett 2006; Lee et al. 2007; Černohlávková et al.2009). Even fewer have compared refrigerated samples to in situ analysis. One study covering both these aspects found a large increase in nitrate and decrease in ammonium concentration, however it did not include measurements of organic N (Arnold et al. 2008).
Determining the importance of DON, such as amino acids, in-field settings is hindered by the low concentration and rapid flux through microbial consumption and production (Warren 2014). This effect is exacerbated during soil sampling and extraction. A rapid decline in added amino acids has been observed across a range of soil processing and extracting methods, including sieving and during extraction with water, potassium chloride (KCl) or potassium sufate (K2SO4) (Rousk and Jones 2010; Inselsbacher 2014), illustrating the continued cycling of soil N after sampling. Yet, these observations lack comparison to in situ amino acid levels. To circumvent these problems, microdialysis has been developed as a minimally disruptive technique for measuring fluxes (rather than concentration) of small N molecules in soil (reviewed by Buckley et al. 2020).
Microdialysis samples solutes from the soil solution via diffusion across a semi-permeable membrane into a probe inserted into the soil. The probe sampling surface is small (e.g. 30 mm length, 0.5 mm diameter), and the membrane has a specific molecular weight cut-off (e.g. 20 or 100 kDa), so will exclude larger molecules or microorganisms (Inselsbacher et al. 2011; Buckley et al. 2020). Microdialysis is considered to provide a better representation of plant-available N due to being minimally disruptive, measuring a flux rather than pool size (Hobbie and Hobbie 2012), and being analogous to plant roots in the size of the probe and in sampling by diffusion (Brackin et al. 2017). Microdialysis sampling has been used in situ on undisturbed soils, and demonstrated that amino acid-N can comprise a greater proportion of the soluble N pool compared to traditional extracts (Inselsbacher and Näsholm 2012a; Brackin et al. 2015; Leitner et al. 2017). However, microdialysis samples collected from soils that have been stored tend to show a dominance of nitrate or ammonium over amino acids (Shaw et al. 2014). This suggests a need to re-evaluate both soil sampling and storage methods for measurement of small organic N compounds in soils.
Soil sample storage conditions and sampling technique evidently affect observations of soil N pools. Using minimally disruptive soil sampling techniques like microdialysis, it is possible to directly evaluate the deviation from in-field soil N status occurring during sample storage and processing (Inselsbacher and Näsholm 2012a; Brackin et al. 2015; Warren 2018; Buckley et al. 2020). In this study, we consider in situ microdialysis sampling of soil N flux as the measurement most representative of plant-available N (Brackin et al. 2015), and compare how sample storage duration and condition (refrigerated at 4°C or dried) affects levels of available N measured in traditional KCl extracts and microdialysis samples. These sampling procedures were applied in the field and to soil collected from a cotton field trial comparing high and low N fertiliser rates, giving direct relevance to a typical soil sampling scenario. We hypothesise that microdialysis will show a greater availability of amino acids relative to inorganic N than KCl extracts, and that organic N levels will decrease with duration of soil sample storage.
Materials and methods
Site
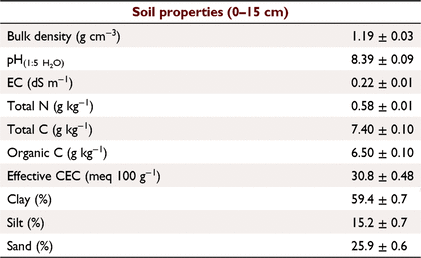
The study site was located at Riverview (28°35′56″S, 149°49′37″E; 192 m asl), near Toobeah, Queensland, Australia. The soil is classified as a Grey Vertosol (Vertisol in the USDA NRCS Soil Taxonomy) with the dominant clay mineral being Montmorillonite (Soil Survey Staff 1999; Isbell 2016). Selected soil properties are summarised in Table 1. Samples were collected from a skip-row furrow-irrigated field trial with two varieties of cotton (Sicot 714 B3F and Sicot 746 B3F) and two N fertiliser rates (172 and 309 kg N ha−1). The field was arranged into plots of 24 rows wide with 1.02 m row spacing and 14 plants m−1. Each fertiliser rate was applied to four replicate plots, arranged in randomised blocks, with the two varieties as subplots (12 rows). All plots received 147 kg N ha−1 as urea before planting, and 25 kg N ha−1 with irrigation 88 days after planting. The high N rate plots received an additional 137 kg N ha−1 50 days after planting. Samples were collected on 22 January 2019 (mid-growing season, 99 days after planting, 3 days after irrigation).
|
Field in situ microdialysis
Microdialysis sampling was performed using probes with a 30 mm membrane length, 0.5 mm outer diameter and 20 kDa membrane cut-off (CMA 20, CMA Microdialysis). Probes were attached to a four-channel syringe pump (CMA 4004) set to a flow rate of 5 μL min−1 with deionised water as the perfusate (Inselsbacher et al. 2011). All dialysate samples were collected over 60 min for microdialysis sampling in both the field and laboratory microcosms. Probe calibration and recoveries of nitrate, ammonium and amino acids under these sampling conditions can be found in Buckley et al. (2017).
In situ sampling of nitrate, ammonium and amino acid diffusive flux using microdialysis was conducted on the shoulder of the cotton hill. A shovel was used to dig a vertical cut into the shoulder and the microdialysis probe inserted horizontally into the soil face at 100 mm depth. One sampling location was used in each field replicate plot. Four probes were inserted at each sampling location (separated by approximately 100 mm to avoid interference), resulting in four technical replicates which were analysed separately for concentration of each N form, then averaged to form a single sample per field plot. Soil temperature was measured at 100 mm depth by inserting a probe thermometer during sample collection (see Supplementary Table S1, available at the journal website).
Soil sampling and processing
Soil samples were collected from each field plot as soon as microdialysis sampling ended. Soil was collected to 100 mm depth and adjacent to where microdialysis probes were placed to avoid any possible confounding effects of in situ microdialysis sampling. One soil sample was collected by trowel, placed in a plastic zip-lock bag, and mixed. Sub-samples were used in ‘disturbed microdialysis’ and ‘field extractions’ described below. The remaining soil sample was kept in a portable refrigerator at 4°C while stored overnight and during transport to the laboratory the following day. A second soil sample was collected using a bulk density ring (diameter, 48 mm; length, 38 mm) and placed into a second zip-lock bag. This was immediately weighed on a balance in the field to determine current soil fresh bulk density.
Disturbed microdialysis
To evaluate the effects of disturbance alone (without storage delays) on soil N measurements, microdialysis samples were taken in the field, immediately after soil sampling to compare to samples collected in situ. Mixed soil was placed into 50 mL microcosms (adjusted method from Inselsbacher et al. 2009) and tapped to the correct bulk density for its plot of origin (soil height, ∼970 mm). Microcosms were returned to the field and buried vertically with the opening level with the soil surface to maintain soil temperature. Microdialysis was then conducted using the setup described earlier, with one microdialysis probe inserted from the top per microcosm.
In-field extractions
Soil extracts were conducted in the field using 5 g fresh, homogenised soil mixed with 25 mL of 1 M KCl in a 50 mL centrifuge tube. The tubes were laid horizontally on an orbital shaker and mixed for 30 min, then left to stand until settled. One mL of supernatant was removed by pipette and stored in the portable refrigerator for transport to the laboratory, where it was stored frozen until analysis.
Soil storage treatments
The next day after field sampling, soil samples stored at 4°C overnight were prepared for microdialysis in microcosms and extractions as described above. Soil was returned to the original in-field temperature before microdialysis was performed in order to prevent temperature induced artefacts in diffusion rates (Inselsbacher and Näsholm 2012b).
The remaining soil samples were split into two halves: one half was stored at 4°C; and the other half was air dried at 25°C until constant weight. After 1 month, soil extracts and microdialysis perfusate samples were collected using the same microdialysis microcosms and KCl extract procedures. Air-dried soils were rehydrated to the original moisture content due to the soil moisture limitations of microdialysis sampling and the potential influence on solute recovery (Miró et al. 2010). Immediately before microdialysis and extraction, deionised water was added to return the soil samples to their mass at the time of storage. Soil water content at the time of sampling was estimated by measuring the drained upper limit (DUL) at 100 cm suction (DUL100) using a pressure plate, and was equal to 0.43 ± 0.01 g cm−3, assuming the soil was near field capacity 3 days after irrigating (Dalgliesh et al. 2016). DUL is the highest water content of a soil after it has been thoroughly wetted and allowed to drain until drainage becomes negligible (Ratliff et al. 1983). When DUL is measured under controlled laboratory conditions using a suction plate, it is taken to be equivalent to soil water retained at a suction of 100 cm and is referred to as DUL100 (Marshall 1959).
Sample analysis
All KCl extract and microdialysis samples were stored frozen at −20°C until analysis. Samples collected in the field were stored at 4°C during transport to the laboratory, then transferred to a freezer. Samples were analysed for nitrate using the colorimetric vanadium (III) chloride method (as per Miranda et al. 2001), and ammonium and amino acids via ultra-performance liquid chromatography (UPLC) (as per Holst et al. 2012).
The soil properties listed in Table 1 were obtained using standard methods described by Rayment and Lyons (2011): pH and electrical conductivity via 1:5 soil:water suspension; total C, total N and organic C (after pre-treatment with sufurous acid (H2SO3) to remove carbonate) using a LECO TruMac CN analyser (LECO Corporation); effective cation exchange capacity as sum of exchangeable bases measured by leaching with alcoholic 1 M ammonium chloride (NH4Cl) at pH 8.5; and particle size analysis via hydrometer.
Data analysis
Data analysis was performed using R software (R Core Team 2020). Data for amino acid-N, ammonium-N and nitrate-N concentrations in KCl extracts and flux in microdialysis samples were analysed separately. Samples from plots growing the two cotton varieties were used as replicates, giving eight replicate samples of each N fertiliser rate. All data were log10 transformed to meet the model assumptions of normally distributed residuals. The effect of storage treatment and N fertiliser rate on N species concentration or flux was analysed using a mixed-effects linear model using the nlme package (Pinheiro et al.2020). Field plot was included as a random effect for the slope in the model. Tukey pairwise comparisons and P-values were determined using the emmeans package (Lenth et al.2020).
Data from KCl extracts and microdialysis samples cannot be directly compared since the former measured a concentration (mg N kg soil−1) while the latter measured a flux (nmol N cm−2 h−1) of N compounds. To allow for comparison, the proportion of N in each form was calculated by dividing the concentration or flux of amino acid-N, ammonium-N and nitrate-N by the sum of N in all three forms (total measured-N) in each sample. This was analysed for the effect of sampling method and storage treatment using a mixed-model using the nlme package, including an interaction term for the two fixed effects. Proportional data were averaged across N fertiliser rates and cotton varieties, as no significant effect was found (P > 0.05), and plot was included as random slope in the model. The emmeans package was used to calculate P-values for Tukey pairwise comparisons across sampling method and storage treatments.
Results
Effect of storage on 1 M KCl extracted N
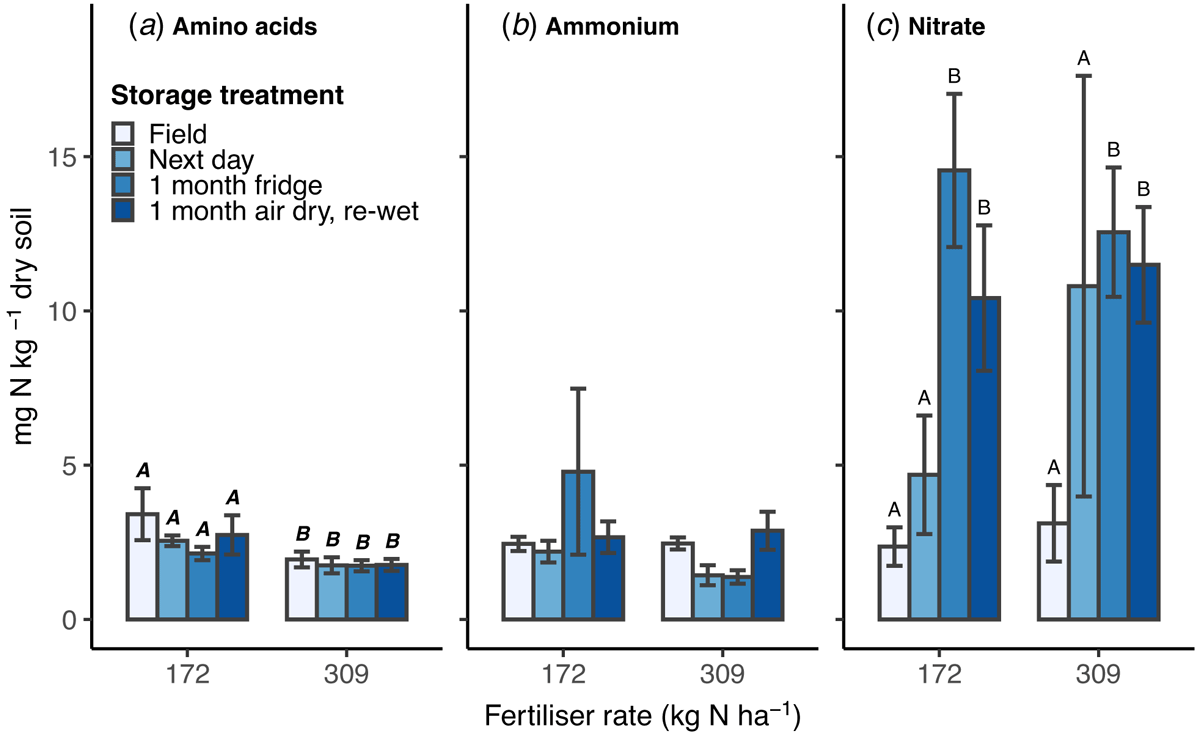
Overall, there was no difference between the 172 (low) and 309 (high) kg N ha−1 fertiliser rates in soil ammonium-N and nitrate-N concentration measured in 1 M KCl extracts (Fig. 1b, c). In contrast, amino acid-N concentration was higher in extracts from the low N rate plots (2.7 ± 0.3 mg N kg−1; mean ± s.e.) than the high (1.8 ± 0.1 mg N kg−1) (P = 0.007; Fig. 1a). No interaction between N rate and storage treatment was found in either the KCl extract or microdialysis data (P > 0.05).
Within each N rate, there was no statistically significant change in the extracted amino acid-N or ammonium-N concentration between the storage treatments (Fig. 1a, b). In soil from the low N rate plots, amino acid-N slightly decreased from soil extracted in the field to stored soils, although this effect was not significant. In soil from the high N rate plots, ammonium-N was similar between the field and air-dried treatments, and slightly lower in the next day and refrigerated soils, but no statistically significant differences were found.
Nitrate-N concentration measured using KCl extracts showed the greatest difference between storage treatments, with a similar pattern across the two N rates (P < 0.001; Fig. 1c). Nitrate-N content was 2 and 3.5 times higher in soil extracted the next day than in the field, in both low and high N rate plots, respectively; although there was high variation in the soils processed the next day and therefore the difference was not statistically significant (P = 0.49). Soil samples stored for 1 month, either refrigerated or air-dried, had nitrate levels significantly higher than soil extracted in the field or the next day. Nitrate in refrigerated soil was 6.2 and 4.0 (low and high N rate) times higher than field extracted (P < 0.001); air-dried soil was 4.4 and 3.7 (low and high N rate) times higher than soil extracted in the field (P < 0.001). Soil samples stored for 1 month showed no difference in nitrate levels measured in KCl extract between refrigerated or air-dried treatments (P = 0.48). The increase in nitrate with storage contributed an overall increase in the cumulative measured-N detected in the KCl extracts (data not shown).
Effect of disturbance and storage on microdialysis N flux
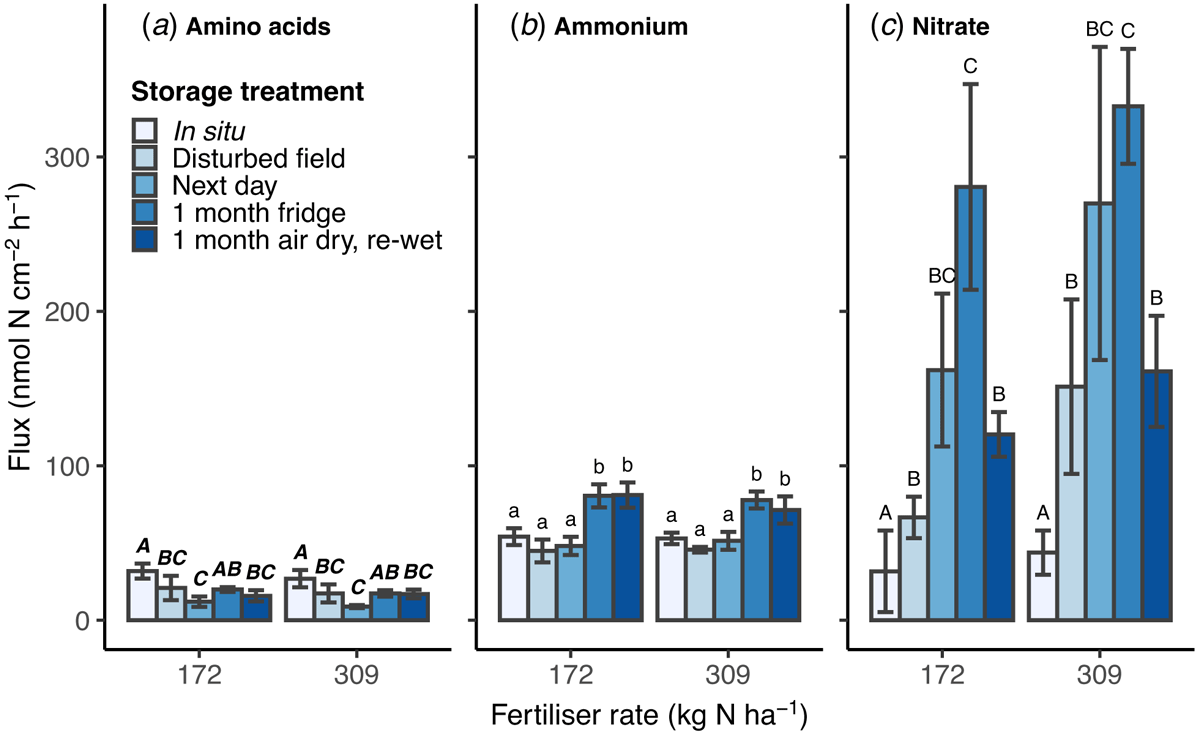
Fluxes of N as amino acids, ammonium or nitrate measured using microdialysis were not statistically different between the high and low N fertiliser rates (Fig. 2a–c). The storage treatments had differing effects on the flux of each N form. In situ microdialysis showed a higher flux of amino acid-N than all storage treatments, except 1 month refrigerated (Fig. 2a). Amino acid-N flux decreased between disturbed-field and next day stored soil, then increased in the soil samples refrigerated for 1 month.
Ammonium-N flux was higher than amino acid-N flux. There was no difference between soil sampled in situ, disturbed-field or stored until the next day, but ammonium-N flux was significantly higher after 1 month of storage both refrigerated and air-dried (P < 0.05; Fig. 2b).
Nitrate-N flux in all disturbed and stored soil samples was at least double in situ flux and increased with time in storage (P < 0.01; Fig. 2c). Soil refrigerated for 1 month had the greatest nitrate-N flux (average 306.6 ± 37.4 nmol-N cm−2 h−1) and was significantly higher than in situ measurement, and from disturbed-field and air-dried stored soils. Nitrate-N flux was on average 5.6-times higher than in situ microdialysis in soil stored for 1 day (next day), and 8.2-times higher in soil refrigerated for 1 month.
In situ vs ex situ samples
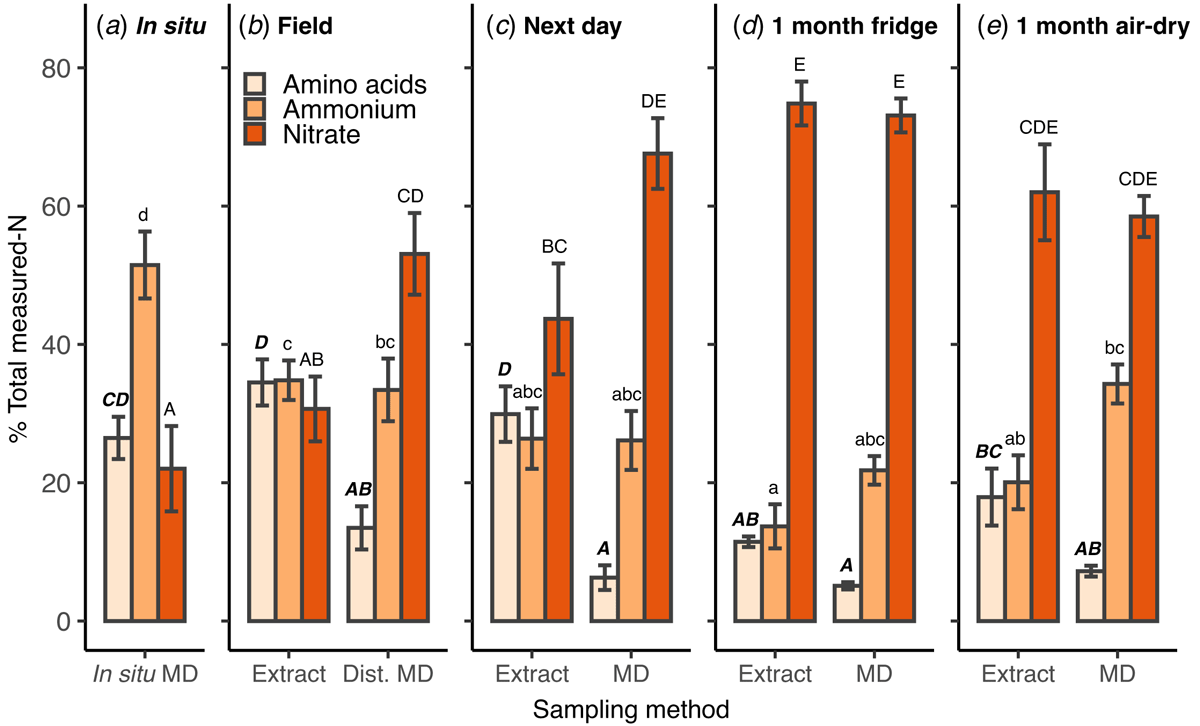
No samples taken from disturbed soil (field, next day, fridge and air-dry), with either microdialysis or KCl extract, had the same relative proportions of amino acid-N, ammonium-N and nitrate-N as measured with in situ microdialysis. The in situ samples had the highest proportion of N as ammonium (51%) and the lowest as nitrate (22%; Fig. 3a). Across microdialysis measurements, the proportion of amino acid-N was immediately lower in the disturbed-field soil (P < 0.01; Fig. 3b), then showed no difference in all other storage treatments (Fig. 3c–e). The proportion of amino acid-N in extracts conducted in the field (Fig. 3b) was similar to in situ microdialysis and declined after 1 month of storage (Fig. 3d, e). The proportion of nitrate-N follows an increasing trend with delay in soil processing. It is the dominant N form measured with KCl extracts and microdialysis from the next day after field sampling (Fig. 3c), with only a slight increase after 1 month (Fig. 3d).
Comparing microdialysis to 1 M KCl extracts
Comparing KCl extracts to microdialysis (Fig. 3b–e) shows a different distribution of N in the field and next day stored soils, and a more similar distribution in the 1 month refrigerated and air-dried soils. Microdialysis generally showed a lower proportion of amino acid-N, but this was only statistically significant in the samples taken in the field and the next day (P < 0.01; Fig. 3b, c). The proportion of ammonium-N was not significantly different between the two sampling techniques in all storage treatments, although it was slightly higher in the microdialysis samples taken from 1 month refrigerated and air-dried soils (Fig. 3d, e). The proportion of nitrate-N was higher in microdialysis samples taken from disturbed-field and the next day treatments (Fig. 3b, c) and was not different between KCl and microdialysis in the 1 month stored soil.
Discussion
The dynamic nature of N in soils creates a considerable challenge for gaining accurate measurement of N pools, particularly plant-available forms of nitrate, ammonium and DON, such as amino acids and peptides. Correctly determining how much N is in a soil sample and the forms that it exists in is central to outcomes for both agricultural research and efficient fertiliser use in production systems.
The present study used an established cotton N fertiliser rate trial to determine the effect of soil sampling technique and handling processes on outcomes of soil N measurement. Despite the considerable gap between the N rates (172 and 309 kg-N ha−1), there were no significant differences in the concentration or flux of ammonium or nitrate at the time of sampling. This was likely due to high N losses immediately after fertiliser application and furrow-irrigation (Macdonald et al. 2017a, 2017b). Contrary to trends in inorganic N forms, amino acid-N concentrations measured using KCl extracts were significantly higher in the low N rate, while the flux showed no difference between fertiliser rates. This inconsistency between extracts and microdialysis is not unexpected since amino acids may be displaced from exchange sites or released from disrupted microbial cells during extraction (Jones and Willett 2006; Rousk and Jones 2010). The lower amino acid level in the high fertiliser rate could be related to suppression of microbial activity and lower microbial biomass (Zhang et al. 2018). The similarity in inorganic N availability, but disparity in amino acid levels between the low and high N rates highlights the need to better understand soil N dynamics for improved N management, including changes in availability over the season and effects of N fertiliser additions. Ensuring soil N measurement protocols yield data which accurately reflects the in situ soil N pools is pivotal to research in this area.
It is known that soil handling processes affect N pools between sampling and analysis, but little work has evaluated current soil handling practices and how this influences interpretation of N availability. Our findings show an increase in the amount of nitrate-N within 1 day of sampling and a continued increase after 1 month of refrigerated or air-dried storage measured by both extraction and microdialysis (Figs 1c and 2c). Surprisingly, this did not appear to be related to changes in ammonium- or amino acid-N levels, and suggests that ammonification and nitrification were not adequately inhibited by storage at 4°C. This is consistent with the findings of Arnold et al. (2008), and contradicts the notion that chilled transportation and storage is effective for preserving the state of the soil as it was sampled in the field (Rayment and Lyons 2011). Freezing soil samples to preserve inorganic N pools has been tested in previous studies; however, variability in the duration of thawing, temperature of freezing, and between soils has given inconsistent conclusions for the effectiveness of frozen storage (Nelson and Bremner 1972; Crouse et al. 1994; Ma et al. 2005). Further, the effect of freezing on soil organic N pools has received little investigation, but changes in microbial activity, particularly N mineralisation, after frozen storage suggest that organic N levels would likely change during thawing (Stenberg et al. 1998). Performing soil extracts in the field, or as close to sampling as possible, should be adopted to avoid inflated measurements of nitrate-N.
Comparing observations from microdialysis and KCl extracts allows separation of the differences due to disturbance, storage, and the extraction process. More pronounced differences between storage treatments were seen in samples collected via microdialysis, possibly indicating confounding N transformations during extraction (Fig. 2). Further, extract samples may be expected to have a higher proportion of ammonium, since microdialysis does not include the fixed pool (Inselsbacher et al. 2011; Brackin et al. 2015). However, this was not observed, suggesting that ammonium may have been nitrified during extraction or is not effectively exchanged from the clay interlayer in this vertic soil (Fig. 3; Leinweber et al. 2013). The immediate decrease in amino acids and increase in nitrate between in situ and disturbed microdialysis is further evidence that the disturbance caused by sampling accelerates mineralisation and nitrification. Other studies examining N recoveries have demonstrated exacerbated loss of amino acids and concomitant increase in inorganic N due to disturbance caused by sieving, and a rapid decline in added isotope labelled amino acids within 1 h of extracting (Rousk and Jones 2010; Inselsbacher 2014).
Here, we show that KCl extracts and microdialysis have complementary characteristics for measuring soil N pools. While KCl extracts are a highly disruptive, but industry standard technique, microdialysis provides a direct and minimally invasive measure of native N pools. We show that microdialysis has a greater sensitivity for determining changes in amino acid levels, such as during soil storage, than traditional extracts. The necessity for such sensitivity is evident in Leitner et al. (2017), in which microdialysis sampling detected temporal changes in in situ N flux while extracts failed to show any significant differences between sampling times over a 20 h period. Overall, prompt processing of soil for N measurement appears more influential than the sampling method used for preserving N pools (Fig. 3).
Techniques for measuring amino acids in soils have received considerable research attention, but it is still uncertain which is most representative of plant-available N. Microdialysis is considered a more appropriate technique for measuring soil amino acids because absolute concentrations in the soil solution are low, but the pool turnover is high. Its strengths include being minimally invasive and capable of high spatial and temporal resolution, and measuring a flux rather than pool size (Warren 2014, 2018; Buckley et al. 2020). In contrast, extracts using a salt solution disintegrates the soil structure and collects amino acids that were protected in soil aggregates, bound to exchange sites, or contained within microbial cells (Jones and Willett 2006; Rousk and Jones 2010). While this does not exactly reflect the quantity of organic N immediately available for plant uptake, it provides broader measurement of potentially available N. In situ microdialysis often shows a much higher proportion of amino acid-N when compared to soil extracts (Inselsbacher and Näsholm 2012a; Brackin et al. 2015; Buckley et al. 2017). Our results provide an interesting caveat: extracts performed immediately after soil sampling in the field had a higher proportion of amino acid-N than in situ microdialysis. This is likely due to displacement from exchange sites or disruption of microsites with increased microbial activity in extraction, and the inherent bias of microdialysis toward nitrate due to faster diffusion (Inselsbacher et al. 2011; Hill et al. 2019). Contrarily, microdialysis of a disturbed soil sample in the field gave lower relative proportion of amino acid-N and higher nitrate-N; this pattern persisted to the day after field sampling. An alternative explanation to the typically higher amino acids seen in microdialysis samples could be that delays before soil extracts increase soil nitrate levels, overshadowing the amount of amino acid-N. Considering the shortfalls of either extracts or microdialysis discussed, in situ soil solution collection such as with small tension lysimeters could provide an intermediate approach. Use of lysimeters is less destructive than extracts, capable of in situ deployment and measures the N pools immediately available to the roots. Previous studies tend to show relatively more nitrate and ammonium than amino acids in soil solution samples collected via lysimeter, suggesting it may not reflect flux of N compounds well (Andersson and Berggren 2005; Jämtgård et al. 2010; Inselsbacher et al. 2011). Comparing microdialysis to extracts highlights the need to consider the combined effects of soil handling, storage conditions and measurement techniques on perceived importance of soil N pools, and to ensure that the sampling technique used matches the goals of N pool interpretation.
In situ microdialysis has value for directly measuring a root’s perspective of N in soil, so may be a better indicator of plant-available N (Inselsbacher and Näsholm 2012a; Brackin et al. 2017; Randewig et al. 2019). In the present study, we have considered in situ microdialysis as the ‘gold standard’ of available soil N sampling techniques because we were specifically interested in how other methods deviated. We could conclude that most N available to the crop was as ammonium (Fig. 3a). However, microdialysis has considerable uncertainties for making generalised statements about the status of soil N. Some of these have already been alluded to, including the small volume of soil sampled and collection of only free solutes. It remains unclear how well microdialysis can be scaled to larger areas of soil, or the impact of factors such as soil moisture, mass flow of soil water, soil texture or temperature on recovery of N molecules (Buckley et al. 2020).
These findings illustrate that common steps in soil analysis procedures, principally the delay between soil sampling and processing for analysis, can cause an overstated representation of nitrate in soil. This has likely contributed to the historically nitrate-centric plant nutrition models and prevailing dominance of nitrate measurements in agronomic fertiliser recommendations (Robinson et al. 2011; Rochester and Bange 2016; Schwenke et al. 2019). For research, this raises a need to improve soil sampling practices to better characterise soil N, particularly if concerned with field-relevant measurements. In-field extracts with refrigeration (or freezing) of extract samples are evidently the best practice, but time constraints and additional equipment make this difficult. Taking intact cores may reduce disruption, but prolonged storage is still not advisable (Arnold et al. 2008). Further research should evaluate if the effects of storage observed here are universal across soils of contrasting characteristics, including land use, texture, pH and carbon content. At a minimum, the duration of soil storage should be reported, and interpretations of N data weighted appropriately.
The over-representation of nitrate in soil samples does not necessarily undermine its historic use as a soil test in agronomy. High nitrate levels in stored samples are indicative of the mineralisation and nitrification rates. This is consistent with the observed correlation between pre-planting nitrate and crop N uptake, and the large supply of N from mineralisation (Rochester and Bange 2016; Macdonald et al. 2017a; Brackin et al. 2019). Even though a soil test may not reflect in-field nitrate levels, it may still indicate overall N availability. This suggests that formalised assessment of mineralisable N, as has been expanding in other cropping industries such as sugarcane, may have greater value for predicting fertiliser requirements (Allen et al. 2019; Brackin et al. 2019). As with research applications, details of soil sample handling and storage, such as time and temperature, should be considered when interpreting commercial soil test results.
The work reported here did not capture the various other forms dissolved N exist as, nor the myriad of N transformation process in soils (Kuypers et al. 2018). It is difficult to confidently describe changes to N conversion processes from looking at the size of pools and fluxes of amino acids, ammonium and nitrate. For instance, the lower nitrate flux measured by microdialysis in the air-dried samples (but not KCl extracts) is possibly caused by changes in N transformations on rewetting, such as denitrification or rapid immobilisation of nitrate (Fig. 2c; Leitner et al. 2017). Given the low temperature used for air-drying, N transformation are expected to have continued during drying, also limiting the assessment of this method. Further investigation of changes to N transformation process using 15N pool dilution techniques (Barraclough and Puri 1995) would determine if nitrate accumulates at a normal rate, or if it is enhanced during soil sampling and storage.
Conclusions
In this study, we have used both conventional KCl soil extracts and an in situ microdialysis technique to examine the influence of soil disturbance during sampling, soil sample storage conditions and storage duration on the measurement of plant-available N forms. In-field extracts showed a distribution of nitrate, ammonium and amino acids most similar to the in situ N flux measured with microdialysis. Delaying processing of soil samples, even until the following day, tended to disproportionately increase measured nitrate, biasing the perceived importance of this pool of plant-available N. Storage of soil samples before analysis should be avoided or minimised where possible, instead favouring in-field extracts immediately after soil collection. In situ sampling techniques such as microdialysis or soil solution sampling can provide a valuable tool for minimally disruptive sampling with samples bearing direct relevance to plant-available N. However, applying these techniques outside of research in commercial soil testing requires evaluation for how to translate results to field-scale fertiliser applications. Conventional soil sampling and extracts are currently the best option for measuring available N in agronomic settings, However, there needs to be details of the soil handling recorded to aid interpretation of measured N levels, particularly high levels of nitrate.
Supplementary material
Supplementary material is available online.
Data availability
Data and code available on request.
Conflicts of interest
MF is an Associate Editor for Soil Research. He had no editorial involvement with this paper, which was handled independently by other members of the Editorial Board. The authors declare no other conflicts of interest.
Declaration of funding
This work was funded by the Australian Cotton Research and Development Corporation (CRDC) (Grant number CSP1904 awarded to BM and MF at CSIRO, and RB and NR from UQ). TB’s PhD is supported by an Australian Government RTP scholarship and a CSIRO Postgraduate Scholarship.
References
Allen DE, Bloesch PM, Orton TG, Schroeder BL, Skocaj DM, Wang W, Masters B, Moody PM (2019) Nitrogen mineralisation in sugarcane soils in Queensland, Australia: I. Evaluation of soil tests for predicting nitrogen mineralisation. Soil Research 57, 738–754.| Nitrogen mineralisation in sugarcane soils in Queensland, Australia: I. Evaluation of soil tests for predicting nitrogen mineralisation.Crossref | GoogleScholarGoogle Scholar |
Andersson P, Berggren D (2005) Amino acids, total organic and inorganic nitrogen in forest floor soil solution at low and high nitrogen input. Water, Air, and Soil Pollution 162, 369–384.
| Amino acids, total organic and inorganic nitrogen in forest floor soil solution at low and high nitrogen input.Crossref | GoogleScholarGoogle Scholar |
Arnold J, Corre MD, Veldkamp E (2008) Cold storage and laboratory incubation of intact soil cores do not reflect in-situ nitrogen cycling rates of tropical forest soils. Soil Biology and Biochemistry 40, 2480–2483.
| Cold storage and laboratory incubation of intact soil cores do not reflect in-situ nitrogen cycling rates of tropical forest soils.Crossref | GoogleScholarGoogle Scholar |
Barraclough D, Puri G (1995) The use of 15N pool dilution and enrichment to separate the heterotrophic and autotrophic pathways of nitrification. Soil Biology and Biochemistry 27, 17–22.
| The use of 15N pool dilution and enrichment to separate the heterotrophic and autotrophic pathways of nitrification.Crossref | GoogleScholarGoogle Scholar |
Brackin R, Näsholm T, Robinson N, Guillou S, Vinall K, Lakshmanan P, Schmidt S, Inselsbacher E (2015) Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertilizer supply and sugarcane root uptake capacity. Scientific Reports 5, 15727
| Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertilizer supply and sugarcane root uptake capacity.Crossref | GoogleScholarGoogle Scholar | 26496834PubMed |
Brackin R, Atkinson BS, Sturrock CJ, Rasmussen A (2017) Roots-eye view: using microdialysis and microCT to non-destructively map root nutrient depletion and accumulation zones. Plant Cell and Environment 40, 3135–3142.
| Roots-eye view: using microdialysis and microCT to non-destructively map root nutrient depletion and accumulation zones.Crossref | GoogleScholarGoogle Scholar |
Brackin R, Buckley S, Pirie R, Visser F (2019) Predicting nitrogen mineralisation in Australian irrigated cotton cropping systems. Soil Research 57, 247–256.
| Predicting nitrogen mineralisation in Australian irrigated cotton cropping systems.Crossref | GoogleScholarGoogle Scholar |
Buckley S, Brackin R, Näsholm T, Schmidt S, Jämtgård S (2017) Improving in situ recovery of soil nitrogen using the microdialysis technique. Soil Biology and Biochemistry 114, 93–103.
| Improving in situ recovery of soil nitrogen using the microdialysis technique.Crossref | GoogleScholarGoogle Scholar |
Buckley S, Brackin R, Jämtgård S, Näsholm T, Schmidt S (2020) Microdialysis in soil environments: current practice and future perspectives. Soil Biology and Biochemistry 143, 107743
| Microdialysis in soil environments: current practice and future perspectives.Crossref | GoogleScholarGoogle Scholar |
Černohlávková J, Jarkovský J, Nešporová M, Hofman J (2009) Variability of soil microbial properties: effects of sampling, handling and storage. Ecotoxicology and Environmental Safety 72, 2102–2108.
| Variability of soil microbial properties: effects of sampling, handling and storage.Crossref | GoogleScholarGoogle Scholar | 19477519PubMed |
Crouse KK, Varco JJ, Jones WF (1994) Evaluation of methods for soil inorganic nitrogen analysis. Communications in Soil Science and Plant Analysis 25, 419–433.
| Evaluation of methods for soil inorganic nitrogen analysis.Crossref | GoogleScholarGoogle Scholar |
Dalgliesh N, Hochman Z, Huth N, Holzworth D (2016) ‘Field protocol to APSoil characterisations’. (CSIRO Agriculture and Food: Canberra, ACT, Australia) https://publications.csiro.au/rpr/download?pid=csiro:EP166550&dsid=DS4
Gütlein A, Dannenmann M, Kiese R (2016) Gross nitrogen turnover rates of a tropical lower montane forest soil: impacts of sample preparation and storage. Soil Biology and Biochemistry 95, 8–10.
| Gross nitrogen turnover rates of a tropical lower montane forest soil: impacts of sample preparation and storage.Crossref | GoogleScholarGoogle Scholar |
Hill EJ, Jones DL, Paterson E, Hill PW (2019) Hotspots and hot moments of amino acid N in soil: real-time insights using continuous microdialysis sampling. Soil Biology and Biochemistry 131, 40–43.
| Hotspots and hot moments of amino acid N in soil: real-time insights using continuous microdialysis sampling.Crossref | GoogleScholarGoogle Scholar |
Hobbie JE, Hobbie EA (2012) Amino acid cycling in plankton and soil microbes studied with radioisotopes: measured amino acids in soil do not reflect bioavailability. Biogeochemistry 107, 339–360.
| Amino acid cycling in plankton and soil microbes studied with radioisotopes: measured amino acids in soil do not reflect bioavailability.Crossref | GoogleScholarGoogle Scholar |
Holst J, Brackin R, Robinson N, Lakshmanan P, Schmidt S (2012) Soluble inorganic and organic nitrogen in two Australian soils under sugarcane cultivation. Agriculture, Ecosystems and Environment 155, 16–26.
| Soluble inorganic and organic nitrogen in two Australian soils under sugarcane cultivation.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E (2014) Recovery of individual soil nitrogen forms after sieving and extraction. Soil Biology and Biochemistry 71, 76–86.
| Recovery of individual soil nitrogen forms after sieving and extraction.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E, Näsholm T (2012a) The below-ground perspective of forest plants: soil provides mainly organic nitrogen for plants and mycorrhizal fungi. New Phytologist 195, 329–334.
| The below-ground perspective of forest plants: soil provides mainly organic nitrogen for plants and mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E, Näsholm T (2012b) A novel method to measure the effect of temperature on diffusion of plant-available nitrogen in soil. Plant and Soil 354, 251–257.
| A novel method to measure the effect of temperature on diffusion of plant-available nitrogen in soil.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E, Ripka K, Klaubauf S, Fedosoyenko D, Hackl E, Gorfer M, Hood-Novotny R, Von Wirén N, Sessitsch A, Zechmeister-Boltenstern S, Wanek W, Strauss J (2009) A cost-effective high-throughput microcosm system for studying nitrogen dynamics at the plant-microbe-soil interface. Plant and Soil 317, 293–307.
| A cost-effective high-throughput microcosm system for studying nitrogen dynamics at the plant-microbe-soil interface.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E, Öhlund J, Jämtgård S, Huss-Danell K, Näsholm T (2011) The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil. Soil Biology and Biochemistry 43, 1321–1332.
| The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil.Crossref | GoogleScholarGoogle Scholar |
Isbell RF (2016) ‘The Australian soil classification’. (CSIRO Publishing: Clayton, Vic., Australia)
Jämtgård S, Näsholm T, Huss-Danell K (2010) Nitrogen compounds in soil solutions of agricultural land. Soil Biology and Biochemistry 42, 2325–2330.
| Nitrogen compounds in soil solutions of agricultural land.Crossref | GoogleScholarGoogle Scholar |
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry 38, 991–999.
| Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil.Crossref | GoogleScholarGoogle Scholar |
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nature Reviews Microbiology 16, 263–276.
| The microbial nitrogen-cycling network.Crossref | GoogleScholarGoogle Scholar | 29398704PubMed |
Lee YB, Lorenz N, Dick LK, Dick RP (2007) Cold storage and pretreatment incubation effects on soil microbial properties. Soil Science Society of America Journal 71, 1299–1305.
| Cold storage and pretreatment incubation effects on soil microbial properties.Crossref | GoogleScholarGoogle Scholar |
Leinweber P, Kruse J, Baum C, Arcand M, Knight JD, Farrell R, Eckhardt K-U, Kiersch K, Jandl G (2013) Chapter two – Advances in understanding organic nitrogen chemistry in soils using state-of-the-art analytical techniques. In ‘Advances in agronomy’. (Ed. DL Sparks) pp. 83–151. (Academic Press: San Diego, CA, USA)
| Crossref |
Leitner S, Homyak PM, Blankinship JC, Eberwein J, Jenerette GD, Zechmeister-Boltenstern S, Schimel JP (2017) Linking NO and N2O emission pulses with the mobilization of mineral and organic N upon rewetting dry soils. Soil Biology and Biochemistry 115, 461–466.
| Linking NO and N2O emission pulses with the mobilization of mineral and organic N upon rewetting dry soils.Crossref | GoogleScholarGoogle Scholar |
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2020) emmeans: estimated marginal means, aka least-squares means. R package version 1.7.0. https://CRAN.R-project.org/package=emmeans
Li KY, Zhao YY, Yuan XL, Zhao HB, Wang ZH, Li SX, Malhi SS (2012) Comparison of factors affecting soil nitrate nitrogen and ammonium nitrogen extraction. Communications in Soil Science and Plant Analysis 43, 571–588.
| Comparison of factors affecting soil nitrate nitrogen and ammonium nitrogen extraction.Crossref | GoogleScholarGoogle Scholar |
Ma BL, Ying J, Balchin D (2005) Impact of sample preservation methods on the extraction of inorganic nitrogen by potassium chloride. Journal of Plant Nutrition 28, 785–796.
| Impact of sample preservation methods on the extraction of inorganic nitrogen by potassium chloride.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Chang YF, Nadelko A, Tuomi S, Glover M (2017a) Tracking fertiliser and soil nitrogen in irrigated cotton: uptake, losses and the soil N stock. Soil Research 55, 264–272.
| Tracking fertiliser and soil nitrogen in irrigated cotton: uptake, losses and the soil N stock.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Ringrose-Voase AJ, Nadelko AJ, Farrell M, Tuomi S, Nachimuthu G (2017b) Dissolved organic nitrogen contributes significantly to leaching from furrow-irrigated cotton–wheat–maize rotations. Soil Research 55, 70–77.
| Dissolved organic nitrogen contributes significantly to leaching from furrow-irrigated cotton–wheat–maize rotations.Crossref | GoogleScholarGoogle Scholar |
Marshall TJ (1959) ‘Relations between water and soil’. (Commonwealth Agricultural Bureaux: Harpenden, UK)
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5, 62–71.
| A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite.Crossref | GoogleScholarGoogle Scholar | 11178938PubMed |
Miró M, Fitz WJ, Swoboda S, Wenzel WW (2010) In-situ sampling of soil pore water: evaluation of linear-type microdialysis probes and suction cups at varied moisture contents. Environmental Chemistry 7, 123–131.
| In-situ sampling of soil pore water: evaluation of linear-type microdialysis probes and suction cups at varied moisture contents.Crossref | GoogleScholarGoogle Scholar |
Mulvaney RL (1996) Nitrogen – inorganic forms. In ‘Methods of soil analysis: Part 3. Chemical methods, 5.3’. SSSA book series. (Eds DL Sparks, AL Page, PA Helmke, RH Loeppert, PN Soltanpour, MA Tabatabai, CT Johnston, ME Sumner) pp. 1123–1184. (John Wiley & Sons, Ltd: Madison, WI, USA)
| Crossref |
Nelson DW, Bremner JM (1972) Preservation of soil samples for inorganic nitrogen analyses1. Agronomy Journal 64, 196–199.
| Preservation of soil samples for inorganic nitrogen analyses1.Crossref | GoogleScholarGoogle Scholar |
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2020) nlme: linear and nonlinear mixed effects models. R package version 3. 1–152. https://CRAN.R-project.org/package=nlme
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. Available at https://www.R-project.org/
Randewig D, Marshall JD, Näsholm T, Jämtgård S (2019) Combining microdialysis with metabolomics to characterize the in situ composition of dissolved organic compounds in boreal forest soil. Soil Biology and Biochemistry 136, 107530
| Combining microdialysis with metabolomics to characterize the in situ composition of dissolved organic compounds in boreal forest soil.Crossref | GoogleScholarGoogle Scholar |
Ratliff LF, Ritchie JT, Cassel DK (1983) Field-measured limits of soil water availability as related to laboratory-measured properties. Soil Science Society of America Journal 47, 770–775.
| Field-measured limits of soil water availability as related to laboratory-measured properties.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods – Australasia’. (CSIRO Publishing: Collingwood, Vic., Australia)
Robinson N, Brackin R, Vinall K, Soper F, Holst J, Gamage H, Paungfoo-Lonhienne C, Rennenberg H, Lakshmanan P, Schmidt S (2011) Nitrate paradigm does not hold up for sugarcane. PLoS One e19045
| Nitrate paradigm does not hold up for sugarcane.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ, Bange M (2016) Nitrogen fertiliser requirements of high-yielding irrigated transgenic cotton. Crop and Pasture Science 67, 641–648.
| Nitrogen fertiliser requirements of high-yielding irrigated transgenic cotton.Crossref | GoogleScholarGoogle Scholar |
Ros GH, Temminghoff EJM, Hoffland E (2011) Nitrogen mineralization: a review and meta-analysis of the predictive value of soil tests. European Journal of Soil Science 62, 162–173.
| Nitrogen mineralization: a review and meta-analysis of the predictive value of soil tests.Crossref | GoogleScholarGoogle Scholar |
Rousk J, Jones DL (2010) Loss of low molecular weight dissolved organic carbon (DOC) and nitrogen (DON) in H2O and 0.5 M K2SO4 soil extracts. Soil Biology and Biochemistry 42, 2331–2335.
| Loss of low molecular weight dissolved organic carbon (DOC) and nitrogen (DON) in H2O and 0.5 M K2SO4 soil extracts.Crossref | GoogleScholarGoogle Scholar |
Schwenke G, Beange L, Cameron J, Bell M, Harden S (2019) What soil information do crop advisors use to develop nitrogen fertilizer recommendations for grain growers in New South Wales, Australia? Soil Use and Management 35, 85–93.
| What soil information do crop advisors use to develop nitrogen fertilizer recommendations for grain growers in New South Wales, Australia?Crossref | GoogleScholarGoogle Scholar |
Shaw R, Williams AP, Jones DL (2014) Assessing soil nitrogen availability using microdialysis-derived diffusive flux measurements. Soil Science Society of America Journal 78, 1797–1803.
| Assessing soil nitrogen availability using microdialysis-derived diffusive flux measurements.Crossref | GoogleScholarGoogle Scholar |
Soil Survey Staff (1999) ‘Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys’. (United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA) https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051232.pdf
Stenberg B, Johansson M, Pell M, Sjödahl-Svensson K, Stenström J, Torstensson L (1998) Microbial biomass and activities in soil as affected by frozen and cold storage. Soil Biology and Biochemistry 30, 393–402.
| Microbial biomass and activities in soil as affected by frozen and cold storage.Crossref | GoogleScholarGoogle Scholar |
Verchot LV (1999) Cold storage of a tropical soil decreases nitrification potential. Soil Science Society of America Journal 63, 1942–1944.
| Cold storage of a tropical soil decreases nitrification potential.Crossref | GoogleScholarGoogle Scholar |
Warren CR (2014) Organic N molecules in the soil solution: what is known, what is unknown and the path forwards. Plant and Soil 375, 1–19.
| Organic N molecules in the soil solution: what is known, what is unknown and the path forwards.Crossref | GoogleScholarGoogle Scholar |
Warren CR (2018) Development of online microdialysis-mass spectrometry for continuous minimally invasive measurement of soil solution dynamics. Soil Biology and Biochemistry 123, 266–275.
| Development of online microdialysis-mass spectrometry for continuous minimally invasive measurement of soil solution dynamics.Crossref | GoogleScholarGoogle Scholar |
Wooliver R, Pellegrini AFA, Waring B, Houlton BZ, Averill C, Schimel J, Hedin LO, Bailey JK, Schweitzer JA (2019) Changing perspectives on terrestrial nitrogen cycling: the importance of weathering and evolved resource-use traits for understanding ecosystem responses to global change. Functional Ecology 33, 1818–1829.
| Changing perspectives on terrestrial nitrogen cycling: the importance of weathering and evolved resource-use traits for understanding ecosystem responses to global change.Crossref | GoogleScholarGoogle Scholar |
Zhang T, Chen HYH, Ruan H (2018) Global negative effects of nitrogen deposition on soil microbes. The ISME Journal 12, 1817–1825.
| Global negative effects of nitrogen deposition on soil microbes.Crossref | GoogleScholarGoogle Scholar | 29588494PubMed |