Nitrous oxide emissions from grain production systems across a wide range of environmental conditions in eastern Australia
Henrike Mielenz A F I , Peter J. Thorburn A , Robert H. Harris B G , Sally J. Officer B H , Guangdi Li C , Graeme D. Schwenke D and Peter R. Grace EA CSIRO Agriculture, Queensland Bioscience Precinct, 306 Carmody Road, St Lucia, Qld 4067, Australia.
B Agriculture Victoria, Department of Economic Development, Jobs, Transport and Resources, Hamilton Centre, Post Office Box 105, Hamilton, Vic. 3300, Australia.
C NSW Department of Primary Industries, Wagga Wagga Agricultural Institute, Pine Gully Road, Wagga Wagga, NSW 2650, Australia.
D NSW Department of Primary Industries, Tamworth Agricultural Institute, 4 Marsden Park Road, Calala, NSW 2340, Australia.
E Institute for Future Environments, Queensland University of Technology, Level 7, P Block, Gardens Point Campus, 2 George Street, Brisbane, Qld 4000, Australia.
F Present address: Julius Kühn-Institut (JKI), Institute for Plant and Soil Science, Bundesallee 50, 38116 Braunschweig, Germany.
G Present address: 70 Martin Street, Dunkeld, Vic. 3294, Australia.
H Deceased.
I Corresponding author. Email: henrike.mielenz@julius-kuehn.de; h.mielenz@outlook.com
Soil Research 54(5) 659-674 https://doi.org/10.1071/SR15376
Submitted: 19 December 2015 Accepted: 19 April 2016 Published: 18 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Nitrous oxide (N2O) emissions from Australian grain cropping systems are highly variable due to the large variations in soil and climate conditions and management practices under which crops are grown. Agricultural soils contribute 55% of national N2O emissions, and therefore mitigation of these emissions is important. In the present study, we explored N2O emissions, yield and emissions intensity in a range of management practices in grain crops across eastern Australia with the Agricultural Production Systems sIMulator (APSIM). The model was initially evaluated against experiments conducted at six field sites across major grain-growing regions in eastern Australia. Measured yields for all crops used in the experiments (wheat, barley, sorghum, maize, cotton, canola and chickpea) and seasonal N2O emissions were satisfactorily predicted with R2 = 0.93 and R2 = 0.91 respectively. As expected, N2O emissions and emissions intensity increased with increasing nitrogen (N) fertiliser input, whereas crop yields increased until a yield plateau was reached at a site- and crop-specific N rate. The mitigation potential of splitting N fertiliser application depended on the climate conditions and was found to be relevant only in the southern grain-growing region, where most rainfall occurs during the cropping season. Growing grain legumes in rotation with cereal crops has great potential to reduce mineral N fertiliser requirements and so N2O emissions. In general, N management strategies that maximise yields and increase N use efficiency showed the greatest promise for N2O mitigation.
Additional keywords: Agricultural Production Systems sIMulator (APSIM), grain legumes, mitigation, model calibration, N2O, splitting fertiliser.
Introduction
Nitrous oxide (N2O) is an important greenhouse gas with a global warming potential approximately 300-fold greater than that of carbon dioxide (CO2) within a 100-year period (Intergovernmental Panel on Climate Change (IPCC) 2006). Globally, approximately 60% of anthropogenic N2O emissions originate from agricultural crop and livestock production (Syakila and Kroeze 2011). In Australia, agricultural soils contribute 55% of national N2O emissions (Commonwealth of Australia 2015). Currently, 24.2 Mha of land is used for cropping in Australia, with wheat being the most important crop (12.8 Mha in 2012–13; Australian Bureau of Agricultural and Resource Economics and Sciences (ABARES) 2013). Over 30% of N2O emissions from agricultural land are attributed to nitrogen (N) fertilisers (Dalal et al. 2003). Of the one million tonnes of fertiliser N used every year in Australia in this sector (ABARES 2013), approximately 70% is applied to cereals. These figures highlight the importance of N2O emissions from agricultural land in Australia and the importance of finding suitable options for reducing N2O production.
N2O emissions measured in Australian grain systems have generally ranged from <0.1 to 2.0 kg N ha–1 year–1 (Wang et al. 2011; Barton et al. 2013; De Antoni Migliorati et al. 2015; Jamali et al. 2015; Officer et al. 2015; Schwenke et al. 2016). Higher emissions (up to 45 kg N ha–1 year–1) have been found in experiments conducted in wheat and canola crops after conversion from pasture to cropping (Harris et al. 2013; Mielenz et al. 2016a) or in irrigated maize and barley (Wallace et al. 2015) in south-eastern Australia. In general, N2O emissions have been found to be higher in grain systems in subtropical and temperate zones of eastern Australia (Wang et al. 2011; Scheer et al. 2012; De Antoni Migliorati et al. 2014; Jamali et al. 2015; Schwenke et al. 2015) compared with the particularly low N2O emissions measured in semi-arid rain-fed grain cropping in south-eastern and south-western Australia (Barker-Reid et al. 2005; Barton et al. 2008, 2010, 2013). Mitigation of N2O emissions is therefore particularly important in Australia’s eastern grain cropping systems.
To design N2O mitigation strategies, the processes involved in N2O formation and emission need to be well understood. The two main processes during which N2O is formed are nitrification (nitrifier nitrification, nitrifier denitrification and nitrification-coupled denitrification) and denitrification (heterotrophic denitrification; Zhu et al. 2013). These processes are controlled by the availability of mineral N (ammonium or nitrate) and regulated by environmental factors such as soil water content as proxy for soil oxygen levels, soil organic carbon (SOC), soil temperature and pH (Stehfest and Bouwman 2006; Hénault et al. 2012). Most of these factors, in turn, are determined by climatic conditions and crop management practices, such as fertilisation, irrigation, residue management and tillage practices. As a result, N2O emissions are highly variable in space and time under Australia’s diversity of climate, soils and farming practices. Observations of N2O emissions with high temporal resolution and the simultaneous characterisation of environmental variables are important for characterising N2O emissions from agricultural systems across Australia (Yao et al. 2009).
Recent use of automated chambers has significantly improved the understanding of N2O emissions from grain systems, and associations between N2O emissions and specific management strategies have been identified. Generally, N2O emissions have been higher in fertilised than unfertilised treatments, increasing with increasing rates of N fertiliser (e.g. Wang et al. 2011; De Antoni Migliorati et al. 2014, 2015; Officer et al. 2015). Although nitrification inhibitors such as 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) have reduced N2O emissions in subtropical (De Antoni Migliorati et al. 2014) and temperate (Harris et al. 2013) environments, their current high cost and negligible yield improvement provide limited financial incentive for on-farm adoption (Harris et al. 2013). No clear differences in N2O emissions have been found between tilled and untilled treatments (Li et al. 2016; Mielenz et al. 2016a). In subtropical Australia, N2O emissions from unfertilised N-fixing legumes were significantly lower than from fertilised crops such as canola (Schwenke et al. 2015). In irrigated systems, N2O emissions were reduced either by applying irrigation more frequently, to avoid large irrigation applications (Jamali et al. 2015), or by avoiding excessive N fertilisation (Mielenz et al. 2016b). However, the abovementioned studies tended to confine measurements to crop seasons only, neglecting emissions during fallow periods, or they represent emissions during a single year, thereby disregarding interannual variability. In addition, the diversity in climate, soils and farming practices exacerbates the quantitative assessment of long-term effects of agricultural management practices on N2O emissions in different environments. Long-term simulations with process-based models can potentially capture the spatial and temporal variability of N2O emissions and explore the effect of management options across different environments.
The aim of the present study was to simulate N2O emissions and crop yield from a range of cropping systems across Australia’s eastern grain-growing regions to identify N2O mitigation options that do not limit, or may even improve, grain yields under different climatic environments and soils in eastern Australia. For this analysis, we used the Agricultural Production Systems sIMulator (APSIM), an agricultural systems model well tested for simulating Australian farming systems and soil N and C dynamics (Keating et al. 2003; Holzworth et al. 2014). To ensure credibility of the model output, the performance of APSIM in simulating yields and N2O emissions was evaluated against experimental measurements taken from six field sites, each of which was representative of typical grain-growing regions of eastern Australia. Model calibration was kept to a minimum to confirm the suitability of the model for a wide range of environments, crops and management practices, and to enable model application at other sites. Long-term scenarios with various management practices were then simulated for each experimental site using historical climate data to identify specific mitigation options for each environment.
Material and methods
The present study consisted of three parts: (1) site-specific model parameters in APSIM were calibrated against a subset of data from six field sites across a diverse range of farming systems in the eastern grain-growing regions of Australia; (2) the performance of the calibrated APSIM model in modelling N2O emissions and crop yields was evaluated by validating it against the remaining datasets from the six field sites; and (3) long-term (40 years) scenarios with simple crop rotations and varying management strategies representative of the respective cropping regions were calculated for six sites. These were then analysed for management effects on yield and N2O emissions to assess possible N2O mitigation strategies without compromising yield. Management factors were N fertiliser rate and strategy, inclusion of legumes in the crop rotation and winter or summer cropping.
Field experiments
Data were sourced from field experiments conducted at six locations in eastern Australia’s main grain-growing regions, namely Kingaroy, Kingsthorpe, Tamworth, Wagga Wagga, Horsham and Hamilton (Fig. 1). The eastern grain-growing region of Australia is subdivided into the northern and southern grain-growing regions (Fig. 1). The site characteristics and experiments are briefly summarised below and in Table 1. Further details can be found in the original publications listed in Table 1. At each site, experiments had been conducted over a period of 1–3 years, involving sequences of up to three consecutive crops. Each experiment comprised two to four treatments with different irrigation, fertilisation or tillage intensities, crop histories and/or crop rotations. The experiments included wheat, barley, sorghum, maize, cotton, canola and chickpea crops, as well as short and long fallow periods.

|
Kingaroy
At Kingaroy, different fertilisation intensities were applied to a wheat–maize rotation (De Antoni Migliorati et al. 2014). The fertilisation treatments used in that study were a control with minimal N fertiliser applied to allow crop establishment (N0), reduced N rate (N1) and conventional N rate similar to farm practice (N2; Table 1). Wheat (Triticum aestivum, var. Hartog) was sown in July 2011 and harvested in November, with all residues left on the field and later incorporated into the soil. In December 2011, maize (Zea mays L., var. 32P55) was sown and harvested in June 2012. To prevent water stress, the field trial was sprinkler irrigated occasionally during both cropping seasons, with all treatments receiving the same amount of water at the same time.
On an adjacent plot, from December 2012 to September 2013, the effects of different crop histories (one season of sulla (Hedysarum coronarium L.) pasture, a legume, or wheat) and two fertilisation intensities on N2O emissions and yield were tested in a sorghum crop followed by fallow (Table 1; De Antoni Migliorati et al. 2015). Both sites had been managed without fertiliser application since 2009. Sulla and wheat were direct drilled in August 2012, managed as forage crops and terminated in November 2012, with all residues left on the field and incorporated into the soil. Sorghum (Sorghum bicolor L., var. Pioneer G22) was sown in December 2012 and harvested in June 2013; the experiment then continued under fallow until September 2013. To avoid water stress, the whole field trial was irrigated occasionally with a total of 125 mm applied during the sorghum season.
Kingsthorpe
At Kingsthorpe, N2O emissions were measured during a wheat–cotton rotation from three irrigation treatments (Table 1; Scheer et al. 2012, 2013). Irrigation was scheduled based on plant-available water capacity (PAWC), determined by neutron probe soil water content measurements, which were made approximately weekly at 10 cm depth increments to a depth of 150 cm in each plot. The wheat variety ‘Lang’ was sown in June 2009 and harvested in late October, with residues removed and stubble burnt. In November 2009, cotton (Gossypium hirsutum L., var. Bollgard Sicala 60 BRF) was sown and harvested in May 2010. All treatments received a total of 200 kg N ha–1 fertiliser, applied in three applications as urea to each crop.
On an adjacent field, N2O emissions were measured in a sorghum crop (var. MR Buster) from October 2012 to May 2013, managed with conventional fertiliser application (173 kg urea-N ha–1 applied at sowing). Grain yields were also imposed in a zero fertiliser treatment at this trial, but N2O emissions were not measured (Table 1).
Tamworth
At Tamworth, a 3-year field experiment (May 2009–May 2012) was conducted comparing N2O emissions from a range of dryland crops and crop rotations (Table 1; Schwenke et al. 2015, 2016). Crops grown in the experiment were canola (Brassica napus, var. Hyola 50), chickpea (Cicer arietinum, var. PBA Hatrick), the wheat variety ‘Crusader’, the sorghum variety ‘MR43’ and barley (Hordeum vulgare, var. Shepherd). After grain harvest, all plant residues were left on the soil surface.
Wagga Wagga
From May 2012 to March 2013, the effects of fertilisation rate (0, 25, 50 and 100 kg urea-N ha–1) and tillage (conventional tillage (CT) vs no tillage (NT)) were observed in wheat (var. Lincoln) and the following fallow period. From April 2013 to April 2014, the effects of fertilisation rate (0, 25, 50 and 100 kg urea-N ha–1) and tillage (CT vs NT) were observed in the growing season of canola (var. Hyola 555) and the following fallow period (Table 1; Li et al. 2016). The previous crop was wheat (var. Lincoln) grown from May 2012 to March 2013, managed with the same treatments. All treatments (including zero N treatments) received 5 kg N ha–1 at sowing, with the respective N rates for each treatment applied at tillering. Plots in the tillage treatments (T) were scarified twice and harrowed twice in two opposite directions 1–2 days before planting. Crop residues in the tillage treatments (T) were incorporated to 0.07–0.1 m depth with the cultivation operation, whereas they were left on the ground in the NT treatments. N2O emissions were measured with automated chambers only in the unfertilised and highest N-fertilised treatments (NT_N0, NT_N100, T_N0 and T_N100) during the 2013–14 experiment. However, yield data were measured from all 16 treatments, and so all treatments were simulated.
Horsham
The effects of N fertilisation and supplementary irrigation on N2O emissions were observed in the wheat variety ‘Caliph’ and the following fallow from April 2007 to February 2008 (Table 1; Officer et al. 2015). Wheat was sown into broad bean (Vicia faba) stubble in June 2007 and harvested in December 2007, with residues left on the soil surface. Treatments included no N fertiliser (N0), 50 kg N ha–1 (N50) and 50 kg N ha–1 and 50 mm irrigation (N50I). N fertiliser was applied side-banded at sowing, and irrigation was applied once in September at mid-tillering.
Hamilton
During 2010 and 2011, N2O emissions were monitored for 1 year during wheat and fallow after conversion from long-term improved pasture to cropland on adjacent plots (Mielenz et al. 2016a). Soils had been under long-term pasture, consisting of subterranean clover (Trifolium subterraneum L.) and perennial ryegrass (Lolium perenne L.). Therefore, the soil was rich in stored organic carbon (Table 1). In each case, in the year before the start of the experiment, a selective herbicide was used to reduce the pasture grasses and encourage a sward dominated by subterranean clover. In late summer before cropping, the site was completely sprayed out with glyphosate. Winter wheat variety ‘Revenue’ was sown in April in each of the two years and harvested in January the following year. The effect of tillage on N2O emissions was assessed by comparing zero tillage (ZT) by direct drilling the seed into sprayed out pasture with CT, where cultivation with several passes of discs, tynes and harrows preceded sowing (Table 1).
Measurements
At all sites, N2O emissions were monitored continuously with fully automated gas measurement chambers (Scheer et al. 2012; Officer et al. 2015). N2O emissions were measured from three replicate plots within a randomised split-plot design for each treatment at each site. The chambers were closed for measurement for 30–60 min, during which time the air was sampled four to 10 times sequentially. The gas samples were automatically pumped to a gas chromatograph or tuneable diode laser (TDL) for N2O analysis. N2O fluxes were calculated from the slope of the linear change in N2O concentration during chamber closure. In this way, up to 16 N2O flux measurements per day were collected from each chamber.
At all sites, soil water contents were monitored during the field experiments with different methods and at different depth and time resolutions. At Kingaroy, soil moisture was measured at 30-min intervals at three depths (0–0.1, 0.1–0.2 and 0.2–0.4 m) with frequency domain reflectometers (FDR; EnviroScan probes; Sentek Sensor Technologies). At Kingsthorpe, soil moisture was measured at least weekly at 0.1–0.2 and 0.2–0.4 m depth with neutron probes (503DR Hydroprobe; CPN International). At Tamworth, Wagga Wagga, Horsham and Hamilton, soil moisture was measured sub-daily at 0–0.06 m depth with site-calibrated theta probes (Theta-Probe MK2x; Delta-T Devices; 60 mm probe length). In addition, at all sites, topsoil samples were taken regularly (weekly to monthly) for measuring ammonium (NH4+) and nitrate (NO3–) concentrations. Crop yields were recorded at harvest. Further details on the measurement techniques are detailed in the original publications (Table 1).
Model description
APSIM version 7.5 (Holzworth et al. 2014), with alterations made to the denitrification submodel as proposed by Mielenz et al. (2016b), was used in the present study. APSIM is a modular modelling framework that simulates biophysical processes in farming systems. Soil and crop processes are simulated by separate modules that interact on a daily time step and are driven by climate data and crop management activities. The dynamics of soil water, N and C, and plant growth and development are comprehensively simulated in APSIM. The APSIM-Manager module allows specification of flexible management options, such as crop and residue management, tillage, fertilisation and irrigation, and the implementation of crop rotations. Therefore, APSIM has been used extensively to explore options for climate change mitigation and adaptation, as well as food security (Hochman et al. 2014; Holzworth et al. 2015), including N2O emissions (Thorburn et al. 2010; Mielenz et al. 2016a, 2016b).
In the APSIM-SoilN module (Probert et al. 1998), the two main processes during which N2O is formed, nitrification and denitrification, are simulated (Thorburn et al. 2010). Nitrification follows Michaelis–Menten kinetics and is modified by pH, soil moisture and temperature. A fixed proportion of nitrified N (the default value being 0.2%) is emitted as N2O. Denitrification is simulated as a first-order reaction dependent on the amount of nitrate (kg ha–1) in each layer with a default denitrification rate coefficient of 0.0006 kg mg–1 day–1 (Rolston et al. 1984). It is further multiplied by functions describing the effect of active carbon, temperature and soil moisture. The soil moisture factor (Fsw,z) in the modified version of APSIM increases linearly from 0 to 1 for water-filled pore space (WFPS) between the dimensionless threshold parameter (dnitlim) and saturation (SAT) and is equal to zero below WFPS at dnitlim (Mielenz et al. 2016b):
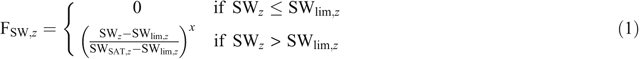

where z refers to the soil layer, SWz (m3 m–3) is the actual water content, SWSAT,z (m3 m–3) is the water content at saturation and SWlim,z (m3 m–3) is the water content above which denitrification starts in each soil layer. The dimensionless threshold parameter dnitlim is defined as SWlim,z normalised by porosity and hence defined as a fraction of WFPS (WFPS = volumetric water content/total porosity). During denitrification, N2 and N2O are produced with an N2 : N2O ratio depending on gas diffusivity of the soil at field capacity, the quotient of NO3– concentration to heterotrophic carbon dioxide (CO2) respiration and WFPS, following the approach of Del Grosso et al. (2000). Detailed description of the soil N processes can be found in Probert et al. (1998) and Thorburn et al. (2010).
Model parameterisation and initialisation
Parameterisations of APSIM for Kingaroy, Kingsthorpe and Hamilton used in the present study have been described previously by Mielenz et al. (2016a, 2016b), but are briefly described here again.
Daily weather data, rainfall, solar radiation and daily minimum and maximum temperature were obtained from the nearest weather stations of the Australian Bureau of Meteorology (Jeffrey et al. 2001). These were: Kingaroy Prince Street, number 040112 for Kingaroy; Oakey-Aero, number 041359 for Kingsthorpe; Tamworth Airport, number 055054 for Tamworth; Wagga Wagga AMO, number 072150 for Wagga Wagga; Horsham Polkemet Road, number 079023 for Horsham; and Hamilton Research Station, number 090103 for Hamilton. In some cases, rainfall data came from measurements at the field sites.
Most soil physical and chemical input parameters were adapted from measurements during the experiments and laboratory studies conducted at the same or nearby sites (Table 1; Fig. 2).
All crop sequences within one experiment were modelled in one simulation without reinitialising the model. For experiments conducted on adjacent field sites (in Kingaroy, Kingsthorpe and Hamilton), soil properties were assumed to be the same. In cases where bulk density and SOC contents were measured before each experiment, these were updated. Where available, water contents and mineral N pools were initialised according to measurements made before or at the beginning of the respective experiments. Otherwise, they were set to reasonable values or, in case of mineral N, estimated by an N balance from the measurements.
Model calibration and simulation of experiments
Site-specific parameters were developed from measured data where possible so that a minimum number of parameter values was derived from calibration. To enable extrapolation of the model to other soil and climate conditions, rate and efficiency constants in the model, such as nitrification rates, crop radiation use efficiency etc., were generally not altered, with only a few exceptions described below.
Several soil properties had been measured at the experimental sites (Table 1; Fig. 2). An exception was the parameter KLz (day–1), which describes the maximum proportion of plant-available water that can be extracted from each soil layer, z, per day. KLz is a depth-dependent, plant- and soil-specific parameter that cannot be measured directly. To optimise simulation of crop yields, values for KLz were calibrated for each crop at each site using crop yield and, where possible, water content data from one treatment in each case.
It is known that the potential rate of mineralisation is not necessarily constant across soils, but can depend on several environmental factors, including soil texture (Asseng et al. 1998) and substrate quality (Benbi and Khosa 2014), among others. The default potential mineralisation rate in APSIM was developed for medium- and fine-textured soils. At Wagga Wagga and Hamilton, higher mineralisation rates were used that are more appropriate for coarser-textured soils (Asseng et al. 1998). At Hamilton, the higher rate was further justified by the conversion from pasture to cropping, where substrate of a different quality was provided (Angus et al. 2006).
In the topsoil at Hamilton, pH values were very low (4.4) and just below the lower limit of pH values where nitrification ceases in APSIM (default pH lower limit: 4.5; Table 1). However, according to the measured NO3– and NH4+ concentrations in the top 0.1 m, nitrification must have occurred. Therefore, the limit of pH below which nitrification ceases was estimated from NO3– and NH4+ measurements in the topsoil from the CT2010 treatment. The data indicated that nitrification at the site was limited below pH 4.32.
After calibrating the model for adequate prediction of yield and mineral N concentrations, the parameter dnitlim, determining the water content at which denitrification starts (Mielenz et al. 2016b), was calibrated against cumulative N2O emissions over at least 1 year from one treatment at each site. The calibration phase of dnitlim ran for at least 1 year at each site to make sure important denitrification events were captured.
Finally, the calibrated model was validated against the remaining datasets at each site. The validation datasets comprised a total of 38 crop–treatment combinations for yield and 56 crop–treatment combinations (including fallows) for seasonal N2O emissions.
Scenario analysis
After testing the performance of APSIM to simulate N2O emissions, we assessed the sensitivity of N2O emissions and yields to different management practices for each site. Management options included N fertiliser rate, single or split N applications and growing grain legumes in rotation with cereals. This analysis aimed to identify optimal management strategies to reduce N2O emissions and maintain yields. For each site, crop rotations were selected that were representative of the respective farming systems and met our purpose of exploring mitigation opportunities. In the northern and southern grain-growing regions of eastern Australia, wheat is a commonly grown winter crop. In the northern grain-growing region, sorghum is additionally commonly grown as a summer crop. Chickpea (winter crop) and mung bean (Vigna radiata; summer crop) are typical grain legumes grown in eastern Australia. Often cropping intensity in rain-fed cropping systems in eastern Australia is one crop per year (Gobbett et al. 2016) and, accordingly, in all simulated crop rotations one crop was grown per year. A spring wheat monoculture and a wheat–chickpea rotation were simulated for all sites except Hamilton. Kingaroy, Kingsthorpe and Tamworth represented the northern grain-growing region, where a sorghum monoculture and a sorghum–mung bean rotation were also simulated. Sowing rules were defined based on local expertise, driven by rainfall and soil plant-available water (Gobbett et al. 2016).
At Hamilton, a winter wheat monoculture and a winter wheat–chickpea rotation were simulated. Results of the scenario analysis for the site at Hamilton are presented separately because the crop rotations differed from those at the other sites. In addition, the high emissions measured at this site are unlikely to be representative of typical N2O emissions in southern Australian grain production systems. The site had atypically high N2O emissions because: (1) conversion from pasture to cropping led to accelerated mineralisation and large quantities of mineral N to accumulate (Mielenz et al. 2016a); (2) organic carbon contents were >4% in the top 0.1 m, much higher than in most cropping soils of southern Australia (Dalal and Chan 2001); and (3) the site is situated in the high-rainfall zone, which currently represents only a small area of the southern grain-growing region of Australia (Gobbett et al. 2016).
For each rotation, seven rates of N fertiliser (0, 30, 60, 90, 120, 150 and 180 kg N ha–1) were applied to the non-legume crops. An additional three rates of N fertiliser were applied for Hamilton: 210, 240 and 270 kg N ha–1. N was applied to the non-legume crops using two different application strategies: (1) total amount applied at sowing (Single); and (2) splitting fertiliser into two applications (Split), where 20 kg N ha–1 was applied at sowing (except in the zero fertiliser treatment) and the remainder was applied 30 days after sowing for sorghum and 40 days after sowing for wheat.
For each simulation, the model was run for 40 years using weather data from 1971 to 2010 from the local weather stations (Jeffrey et al. 2001), with the first 10 years being used as run-in to avoid effects of unrepresentative initial conditions and data from the last 30 years used for data analyses, to study the effect of interannual weather variability. In the case of Hamilton, data from 20 years were used for the run-in simulation to reduce the effect of conversion from pasture to cropping.
Data analysis
For crop yields and daily N2O emissions, the arithmetic mean ± s.d. was calculated from the available replicates. For the calculation of cumulative N2O emissions, daily averages of three replicates were summed. The s.d. for cumulative values was calculated from daily s.d. by Gaussian error propagation. Data gaps in measurements of daily N2O emissions were left out in calculation of cumulative (seasonal) N2O emissions for both measurements and simulations.
Model performance was evaluated by root mean square error (RMSE), a measure of the average deviation of the model output from the measurements, coefficient of determination (R2), which describes the proportion of variance in the data explained by the model, and Nash–Sutcliffe model efficiency (ME), which measures the performance of a model relative to the mean of the observations. ME ranges from –∞ to 1.0, with the model being perfect at ME = 1 and for ME < 0 the observed mean is a better predictor than the model. These quality criterions were calculated as an integrative measure considering yields and seasonal N2O emissions of all calibration and validation datasets.
From the yearly outputs of the long-term scenarios, arithmetic means for yields and yearly N2O emissions were calculated. In addition, N2O intensities (kg N t–1) were calculated for the non-legume crops by dividing average yearly N2O-N emissions by average crop yields. To quantify interannual variability of annual N2O emissions and yields, the CV was calculated as the ratio of s.d. to the mean. ‘optimum’ N fertiliser rates were determined from the scenarios as the rate above which cereal crop yield increased by <2% to the next higher N rate. This was done for each cereal crop at each site in both the monoculture and in rotation with a legume.
To determine the effects of environmental factors on N2O emissions, N2O emissions were correlated with selected soil and climate parameters. For this, N2O emissions at the ‘optimum’ N fertiliser rates from the spring wheat monocultures and wheat–chickpea rotations were used as a comparable dataset. The Hamilton site was not included in this analysis.
Results
Field experiments
In the fertilised treatments of the experiments used in the present study, N2O-N emissions during the crop-growing seasons were equivalent to 0.1–1.4% of applied fertiliser N. N2O emissions from fertilised crops in the experiments ranged from 0.1 to 1.6 kg N ha–1 during the growing season, and from 0.0 to 0.4 kg N ha–1 during fallow periods, which ranged from 1 to 12 months in duration. Only at the Hamilton site were N2O emissions much larger, with maximum N2O emissions of 46 kg N ha–1 measured during a wheat season (9 months) after terminating long-term pasture. Emissions at Hamilton were extremely high because conditions for N2O emissions from nitrification and denitrification were favourable: measurements were made in the first year after converting long-term pasture to cropping, where large amounts of soil mineral N accumulated from accelerated mineralisation in soil with very high concentrations of organic C, which, in combination with very wet soil conditions, led to the extraordinarily high emissions (Mielenz et al. 2016a).
Model calibration and validation
Calibration
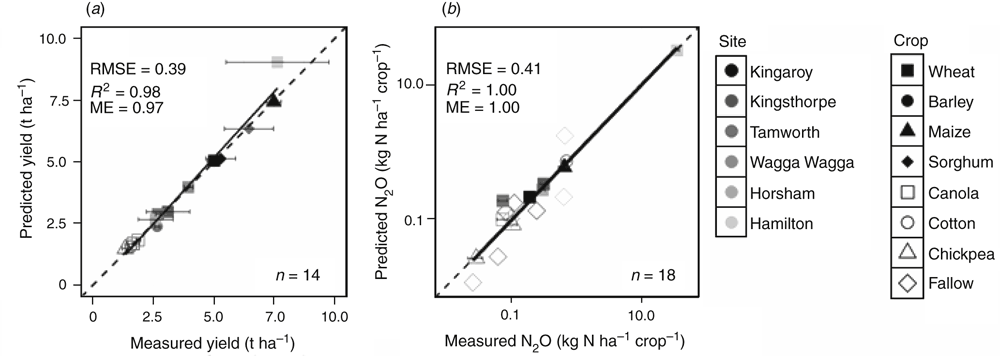
A small subset of the experimental data from each site was used for model calibration. After adjusting KLz for each crop at each site, yields of the calibration treatments were adequately simulated, where predictions lay within the standard deviations of the measured yield in all but one case (Fig. 3a).
Cumulative N2O emissions proved to be very sensitive to dnitlim, the parameter that determines the WFPS above which denitrification commences. Calibration of this parameter for each site (using a subset of treatments in the experiments) resulted in adequate prediction of seasonal N2O emissions for all crops and fallows in the calibration datasets (Fig. 3b). Calibrated dnitlim values were between 0.80 and 0.93 and showed a direct relationship with WFPS at the drained upper limit (DUL). At each of the six experimental sites, dnitlim was between 0.99- and 1.11-fold (mean 1.05-fold) WFPS at the drained upper limit (WFPSDUL; Fig. 4). For the sites at Kingaroy, Kingsthorpe and Hamilton, detailed results of model calibration and validation have been provided by Mielenz et al. (2016a, 2016b). Goodness-of-fit measures for the simulations of N2O emissions, soil mineral N and soil water contents for the experiments at Tamworth, Wagga Wagga and Horsham were in a similar range as those for the published data and are therefore not presented here.

|
Validation
The model was validated with the datasets from the remaining crop seasons. These comprised 38 crop–treatment combinations for yield and 54 crop–treatment combinations (including fallows) for seasonal N2O emissions across the six sites. Yields for the 38 validation data were well predicted (R2 = 0.93), and predictions lay within the standard deviation of the measured values in 24 cases (Fig. 5a). Seasonal N2O emissions were also well predicted for all 54 validation datasets, with R2 = 0.91 (Fig. 5b).
Simulation of mitigation options
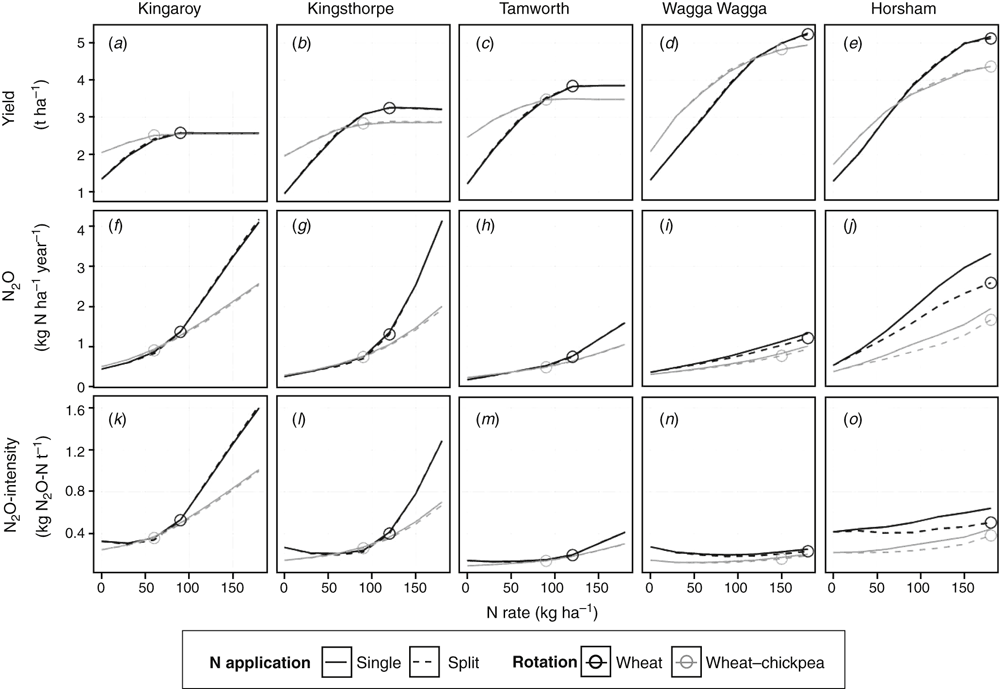
Crop yields for both spring wheat and sorghum increased from north to south (Figs 6a–e, 7a–c). Simulated average N2O emissions varied from 0.2 to 4.2 kg N ha–1 year–1 (Figs 6f–j, 7d–f), equivalent to emission factors of 0.2–2.2% (data not shown). We show long-term averages but acknowledge that annual N2O emissions and crop yields were highly variable between years, with CVs of between 20% and 100% for annual N2O emissions and between 10% and 60% for crop yields.
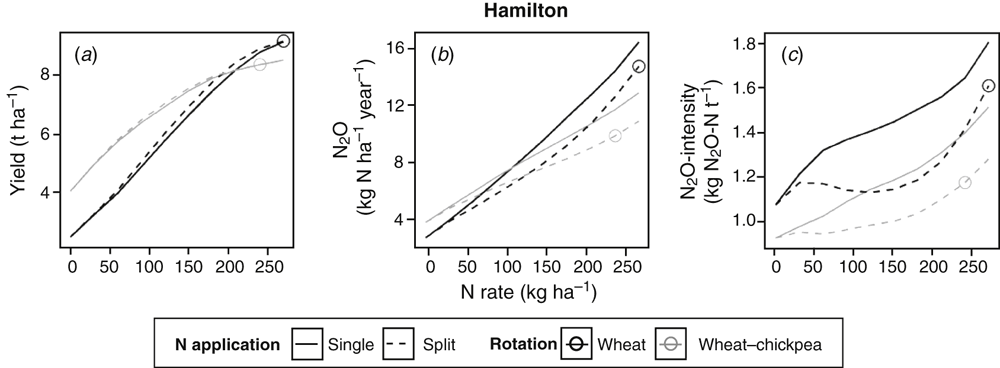
At Hamilton, where conditions for both crop growth and N2O emissions are favourable, average winter wheat yields of up to 9.1 t ha–1 were simulated (Fig. 8a). Average N2O emissions were higher than at the other sites and ranged from 2.6 to 16.7 kg N ha–1 year–1.
Fertilisation rate
In our simulations, at all sites, increasing N inputs increased yield, N2O emissions and N2O intensities. At a certain N rate (depicted by circles in Figs 6a–e, 7a–c, 8a), crop yields ceased responding to additional N inputs (reaching a ‘yield plateau’), whereas the increase in N2O emissions and N2O intensities continued or, in some cases, even accelerated (Figs 6f–o, 7d–i, 8b, c). The yield plateau was reached at varying N rates depending on site and crop rotation. We propose these rates as the ‘optimal’ average N supply (circles in Figs 6–8) specific for the respective crop and site, although we acknowledge that the ‘optimal’ N rate depends on starting soil water, soil mineral N and growing season rainfall, and will therefore vary from year to year.
The yield plateau for spring wheat increased from north to south, being 2.6 t ha–1 at Kingaroy and 5.2 t ha–1 at Horsham (Fig. 6a–e). Accordingly, the simulated average optimal N rate increased with increasing yield potential from 90 kg N ha–1 at Kingaroy to 180 kg N ha–1 at Horsham. In the cereal monocultures (wheat or sorghum) at all sites (except Hamilton), average yearly N2O emissions at the N rate where maximum yield was obtained were between 0.5 and 2.6 kg N ha–1 year–1, with the highest emissions at Horsham and the lowest at Tamworth. In the winter wheat monoculture at Hamilton, the yield plateau was reached at an N rate of 270 kg N ha–1.
According to our simulations, average maximum yield in sorghum grown in monoculture was obtained with 90, 120 and 150 kg N ha–1 at Tamworth, Kingaroy and Kingsthorpe respectively (Fig. 7a–c). At all three sites, maximum average sorghum yields were approximately 5 t ha–1. In the monocultures, average annual N2O emissions at the optimal N rate decreased from north to south (Kingaroy > Kingsthorpe > Tamworth) from 1.9 to 0.5 kg N ha–1 year–1 (Fig. 7d–f).
Splitting N fertiliser
Splitting N fertiliser did not affect yields or annual N2O emissions in either wheat (Fig. 6a–c, f–h) or sorghum (Fig. 7a–f) crops at the northern grain region sites (Kingaroy, Kingsthorpe and Tamworth). In contrast, splitting N fertiliser reduced N2O emissions at the optimal N rate by 0.1 and 0.7 kg N ha–1 year–1 (or 8% and 22%) at Wagga Wagga and Horsham respectively (Fig. 6i–j) and by 1.7 kg N ha–1 year–1 (or 10%) in winter wheat at Hamilton (Fig. 8b).
Inclusion of legumes in the crop rotation
At all sites, the inclusion of legumes into summer and winter crop rotations changed the shape of the simulated N response curve for wheat and sorghum yields, with the yield plateau being reached at lower N rates compared with the monocultures (Figs 6a–e, 7a–c, 8a). In both spring and winter wheat, this resulted in the optimal N rate being, on average, 30 kg N ha–1 less in the wheat–chickpea rotations than the wheat monocultures (Figs 6a–e, 8a). In sorghum, the inclusion of mung bean into the crop rotation resulted in the optimal N rate being 60 kg N ha–1 (Kingsthorpe and Tamworth) or 90 kg N ha–1 (Kingaroy) less than in the monoculture (Fig. 7a–c). The shapes of the N response curves of N2O emissions and N2O intensities in wheat and sorghum became flatter with the inclusion of legumes (Figs 6f–o, 7d–i, 8b, c). In spring wheat, N2O emissions (Fig. 6f–j) and N2O intensities (Fig. 6k–o) were the same or lower in the wheat–chickpea rotation than in the monoculture. However, in sorghum, N2O emissions (Fig. 7d–f) and N2O intensities (Fig. 7g–i) were higher in the sorghum–mung bean rotations than in the sorghum monocultures at N rates of up to approximately 150 kg N ha–1, where the response curves intersected. This differing effect between the summer and winter crops is likely to be explained by the higher surplus of available N in the summer crop rotation caused by greater N supply from the mung bean compared with chickpea residues.
N2O emissions at the optimal N rate were smaller when cereals (wheat or sorghum) were grown in rotation with legumes compared with those in the monocultures in all cases except for sorghum at Tamworth, where emissions were very small in both cases (Figs 6f–j, 7d–f, 8b). This corresponds to the smaller ‘optimal’ N rates applied when legumes were included in the crop rotation. Note that these figures show average emissions for years in which non-legume crops were grown. Emissions during years in which legumes were grown were generally smaller. Consequently, with the inclusion of legumes in the crop rotation, N2O emissions could be reduced by up to 0.9 kg N ha–1 year–1 in both sorghum and spring wheat and by up to 4.8 kg N ha–1 year–1 (30%) in winter wheat at Hamilton.
Maximum sorghum yields when grown in rotation with mung bean were at the same level as when grown in monoculture. However, maximum yields were lower by 7–30% or by 9% for spring and winter wheat respectively when grown in rotation with chickpea than in monoculture at all sites except for Kingaroy (Figs 6a–e, 8a). The yield plateau was lower compared with cereal crops grown in monoculture, because simulated chickpea growth left less residual soil water than wheat, leading to more water stress in wheat following chickpea than following wheat when grown in monoculture.
Summer or winter crops
Average N2O emissions at the optimal N rate were 0.2 and 0.5 kg N ha–1 year–1 smaller from winter (wheat; Fig. 6f, g) compared with summer cropping (sorghum; Fig. 7d, e) at Kingsthorpe and Kingaroy respectively. This is associated with the higher optimal N rates in wheat than in sorghum at these sites. At Tamworth, N2O emissions from winter cropping were 0.2 kg N ha–1 year–1 higher than from summer cropping at the optimal N rate between sorghum and wheat (Figs 6h, 7f). At Kingaroy and Kingsthorpe, at the highest N rates applied in our calculations (180 kg N ha–1), annual N2O emissions were higher in wheat than in sorghum (Figs 6f, g, 7d, e), whereas at Tamworth it was the other way round. Emissions were higher in the rotation where more excess mineral N accumulated in the soil profile. N2O intensities at the optimal N rates were higher in wheat than in sorghum, because wheat yields were smaller than sorghum yields by 1.4–2.2 t ha–1.
Factors driving N2O emissions
Although SOC is a driver of N2O emissions, we found no relationship between these two parameters in the simulated results (Fig. 9a; R2 = 0.00). PAWC, drainable porosity (i.e. the difference between soil water contents at saturation and at DUL) and rainfall affect soil water content, which, in turn, affects N2O emissions. However, there was no relationship between N2O emissions and PAWC (Fig. 9b; R2 = 0.02) and only a weak relationship between drainable porosity and N2O emissions (Fig. 9c; R2 = 0.22). Contrary to our expectations, we found that N2O emissions decreased with increasing annual rainfall (Fig. 9d; R2 = 0.37). We also found decreasing N2O emissions with increasing average maximum temperatures (Fig. 9e; R2 = 0.30), which is counterintuitive. At the same time, the optimal N-rate applied to maximise crop yield at the different sites was strongly related in the same way as N2O emissions to drainable porosity (Fig. 9h; R2 = 0.67), rainfall (Fig. 8i; R2 = 0.80) and maximum temperature (Fig. 8j; R2 = 0.78). Therefore, the relationships between N2O and these soil and climate variables appear to be artefacts, and are instead caused by the applied N rate. There were no relationships between the optimal N rate and SOC (Fig. 9f; R2 = 0.12) or PAWC (Fig. 9g; R2 = 0.14).
Discussion
Model evaluation
Crop yields for seven different crops (wheat, barley, maize, canola, chickpea, sorghum and cotton) were accurately predicted under a wide range of environmental conditions and management practices (R2 = 0.93; Fig. 5a). After calibrating the parameter dnitlim, seasonal N2O emissions in 54 crop and fallow validation datasets were also accurately predicted, with R2 = 0.91 (Fig. 5b). These excellent predictions were achieved by keeping parameters of the nitrogen and carbon cycles constant and only calibrating the site-specific parameter dnitlim. However, at Hamilton and Wagga Wagga, a few other parameters needed to be adjusted with reasons justified above. Therefore, the model can be reliably extrapolated to other areas within the eastern Australian grain growing regions with different soil and climate conditions. Our extensive validation substantiates the ability of APSIM to reliably predict yields and seasonal N2O emissions for various crops under diverse management practices and soil and climate conditions. Therefore, the calibrated APSIM model can be used as a valid tool for exploring N2O mitigation options by simulating long-term scenarios for the experimental field sites representative of typical grain growing areas.
Cumulative N2O emissions proved to be very sensitive to the newly introduced parameter dnitlim, which determines the WFPS above which denitrification commences. The values derived for dnitlim ranged from 0.80 to 0.93 (Fig. 4), which are at the upper end of the range 0.5–0.9 that was found for this threshold in reviews of denitrification models (Heinen 2006) and observations (Barton et al. 1999). In all cases, dnitlim was close to WFPSDUL, although generally higher (average 5%) than WFPSDUL (Fig. 4). Because five of six data points in Fig. 4 lie very close together, a regression to determine a quantitative relationship between dnitlim and WFPSDUL is not sensible. However, the relationship between dnitlim and WFPSDUL indicated by the data suggests that dnitlim is a site-specific variable that can potentially be predicted from DUL. The generality of this relationship or the relationship with other measurable soil properties should be tested over a wider range of soil types. If a relationship between measurable soil properties and dnitlim existed, values of dnitlim could be determined and N2O emissions readily predicted at other sites.
Simulation of mitigation options
The satisfactory agreement between predicted and observed N2O emissions and crop yields provides greater confidence in long-term simulations. The results from field experiments can be extrapolated to explore longer-term effects of management practices across a range of cropping environments in eastern Australia.
Applying N fertiliser at rates above where crop yields plateaued increased N2O emissions. Predicted maximum spring wheat yields were in accordance with potential water-limited wheat yields proposed for Australia (Gobbett et al. 2016). The yields for winter wheat at Hamilton were at the higher end of what can be expected in the high-rainfall zone. There are many risks associated with managing N fertiliser in rain-fed cropping systems in the northern and southern grain-growing regions of Australia due to high variability in rainfall and prices. Grain growers often make risk-adverse decisions, resulting in N applied at lower rates than optimal for maximising yield (Monjardino et al. 2015). Although these decisions are partly responsible for the high yield gaps in Australia’s cereal cropping (Gobbett et al. 2016), they are beneficial for mitigating N2O emissions and N2O emissions intensity. Should N applications be increased to reduce the yield gap, decision makers should be aware of the implications for N2O emissions, particularly if excessive N rates above the ‘optimum’ are applied.
Splitting N fertiliser application has been proposed as an option for reducing N2O emissions (Burton et al. 2008; Schwenke et al. 2016). In our simulations, splitting N showed no effect on yield (Figs 6a–e, 7a–c), with a minor effect only found for winter wheat at Hamilton (Fig. 8a). A later application of the N top dressing, as done under normal practice in the southern region, did not show any yield increase either (not shown). Splitting N was found to be an effective option to reduce N2O emissions (by up to 22%) compared with single N application in the southern but not northern grain-growing region (Figs 6f–j, 7d–f, 8b). One reason for the difference between regions may be annual rainfall and associated N2O emission patterns. In wheat monocultures at Wagga Wagga, Horsham and Hamilton, an average of 80–90% of yearly N2O emissions occurred during the crop season when 60–80% of annual rain fell, with only 10–20% of yearly N2O emissions occurring during the fallow season where rainfall was low. In contrast, at the northern grain-growing sites, only 40–60% of yearly N2O emissions occurred during the wheat cropping season where 30–40% of annual rain fell, with the majority of N2O emitted during the wet summer fallow season. Consequently, splitting N applications has the potential to better synchronise fertiliser N supply to meet crop needs and so reduces the potential for denitrification (and associated N2O emissions). This is more important in the southern grain region, where rainfall-induced soil moisture conditions are more conducive for denitrification and associated N2O emissions early in the cropping season compared with the northern grain region, where wheat is grown during the dry winter season. Varying effects of splitting N fertiliser on N2O emissions have been found in previous studies. Some authors found no effects or reduced N2O emissions (Burton et al. 2008; Allen et al. 2010; Schwenke et al. 2016), depending on the timing of significant rainfall events in relation to N applications. In contrast, Venterea and Coulter (2015) found splitting N fertiliser increased N2O emissions compared with a single application. The results of the present and previous studies show that timing of N fertiliser application is important, and so soil moisture conditions and rainfall patterns should inform the decision making regarding the timing of N fertiliser applications.
Legumes are increasingly being proposed as break crops in grain cropping systems to control weeds and diseases (Angus et al. 2015) and, particularly in the case of high-value pulses, as a valuable commodity in their own right. Including a legume in the crop rotation increased yields at lower N rates and reduced the N rate at which the yield plateau was reached compared with monoculture cereals. Thus growing legumes has the potential to save considerable amounts of N fertiliser and so to reduce N2O emissions and N2O intensities in the subsequent cereal crops (Figs 6–8), as demonstrated in previous studies (De Antoni Migliorati et al. 2015; Mielenz et al. 2016b). Simulated N2O emissions were generally smaller when unfertilised grain legumes were grown compared with fertilised cereal crops, which supports the experimental findings at the Tamworth field site (Schwenke et al. 2015). However, if N fertiliser inputs to cereal crops are not reduced when legumes are included in rotations, N2O emissions may increase (Fig. 7; Huth et al. 2010).
Although the simulation results indicated that including a legume in the crop rotation increased yields at lower N rates compared with monoculture cereals, the maximum yields of cereal crops were similar in rotations with or without legumes. In previous studies, the yield of wheat after legumes generally exceeded the yield of wheat after wheat (Angus et al. 2015), partly due to increased N availability after legumes. This discovery supports our finding of a reduced N rate at which the yield plateau was reached for wheat and sorghum in rotation with legumes than in monoculture. However, in our simulations the maximum yield of wheat crops (i.e. at high N rates) grown in rotation with chickpea was lower than in monoculture. This appears contradictory to the findings of Angus et al. (2015). However, Angus et al. (2015) also found that yield response to break crops was smaller when less rain fell during the grain cropping season, because then yield may have been limited by water as well as N. Although in the studies reviewed by Angus et al. (2015) N availability instead of water availability may have been the main factor for the positive yield response to legumes, in our simulations water stress was the main limiting factor. Indeed, in our simulations chickpea left less residual water in the soil than wheat, which led to more water stress in wheat following chickpea than when grown in monocultures. This contradicts results from Miller et al. (2003), who found that some legumes left more water in the soil than cereals, but they conducted their experiments in a different climate. Also in the grain-growing regions of Australia, residual water contents after legume break crops were reported to contribute to increases in subsequent wheat yields (Kirkegaard et al. 2014). Another reason that cereal yields after break crops may be higher than cereals in monoculture is that legumes are beneficial in controlling root diseases in cereals. This effect is not accounted for in our simulations because disease effects are not simulated in APSIM. Therefore, our finding of maximum wheat yields in monocultures being higher than when in rotation with chickpea should be interpreted with caution.
In conclusion, using grain legumes in rotation with cereal crops is an effective measure to mitigate N2O emissions when N fertiliser applications to cereals in the rotations are reduced to account for the legume N inputs. Their effect as disease break crops for cereals makes the integration of legumes into crop rotations an even better mitigation strategy. The mitigation potential of using grain legumes depends on the rate of fertiliser N application otherwise used in the absence of the legume in rotation. Therefore, the contribution of legume residues to plant-available N should be carefully considered when deciding the extent to which fertiliser N application can be reduced. Water availability should also be included in the decision making for N fertiliser management, especially when legumes are included in the crop rotation.
In the northern grain-growing region, both winter and summer crops are typically grown, although generally only one crop per year is grown in rain-fed cropping environments unless excessive rainfall occurs. Our simulations showed negligible differences in N2O emissions between growing a winter or a summer crop rotation. Therefore, the choice of growing summer or winter crops is unlikely to represent a significant mitigation option.
N2O emissions are driven by soil mineral N content, SOC, water and temperature. Mineral N concentrations are strongly influenced by N fertiliser application rates and legume residues, which can be managed by farmers. Our simulations of N2O emissions at different N rates (Figs 6f–j, 7d–f, 8b) show the extent to which N2O emissions can be reduced by N fertiliser management. Soil carbon, water and temperature are influenced less easily by management in a growing crop under rain-fed conditions. It is important to determine how these factors affect N2O emissions in the different grain cropping environments represented in the present study. Therefore, we related N2O emissions at the ‘optimum’ N-rate in wheat and wheat–chickpea rotations (circles in Fig. 6) to relevant environmental variables (Fig. 9a–e). Contrary to our expectations, there was no relationship between SOC and N2O emissions (Fig. 9a), possibly because of the relatively small range of carbon concentrations in the soils simulated. The post-pasture cropping site at Hamilton was excluded from this analysis. However, the high N2O emissions observed at Hamilton (Figs 3b, 5b), where SOC in the topsoil is two- to threefold higher than at the other sites (Table 1), shows that SOC does have an important effect on N2O emissions. The effect of parameters influencing soil water content (PAWC, drainable porosity and rainfall) or temperature (average maximum temperature) was overridden by the different N application rates applied at the different sites. This suggests that mineral N concentration largely determines the level of N2O emissions, whereas the other drivers provide the conditions conducive for N2O production.
The high interannual variability in crop yields and annual N2O emissions we simulated using long-term weather data (CV = 20–100% for annual N2O emissions and CV = 10–60% for crop yields) shows that climate factors are important in controlling N2O emissions and yields. N2O emissions were often higher in wet years than in dry years, because denitrification is responsible for large proportions of N2O emissions from wet soils. In wet years, higher crop yields were obtained than in dry years because plant-available water is the main limiting factor for crop growth in Australian dryland cropping systems. Therefore, in dry years, less N fertiliser is needed because water limited yields and associated N requirements are much lower. Consequently, there is no one ‘optimum’ N rate that applies across seasons to achieve maximum yield and ensure low N2O emissions. Instead, the N rate will be highly influenced by soil mineral N at planting and subsequent supply through the growing season, along with expected yield based on soil water stored at planting and forecast weather. Soil testing for mineral N and crop yield forecasting tools in combination with reliable long-term weather forecasts would provide great certainty in managing N fertiliser applications.
Conclusions
APSIM proved to be a reliable tool for predicting seasonal N2O emissions and crop yields for various crops across different soil and climatic conditions of eastern Australia’s northern and southern grain-growing regions. The parameter dnitlim (Eqns 1, 2), determining the WFPS threshold above which denitrification occurs, was found to be very important for predicting seasonal N2O emissions. The estimates of dnitlim for the six soils used in the present study indicate a potential relationship between dnitlim and the WFPS at DUL (Fig. 4). This and other potential relationships of dnitlim with measurable soil properties should be further investigated to be able to predict this parameter and so to improve the ability of APSIM to predict seasonal N2O emissions. Because only site-specific parameters needed calibration, we propose that APSIM could be readily used to predict yields and N2O emissions in other regions of the world with similar climates and soils.
N2O mitigation options are consistent with N management strategies that maximise crop yields without exceeding plant demand, thereby increasing N use efficiency and simultaneously minimising N losses to the environment. Timing of N application was also important, splitting N applications at planting and again in season was found to be an effective measure for mitigating N2O emissions in the southern, but not in the northern grain region of eastern Australia. Including legumes in crop rotations has great potential in reducing N fertiliser inputs and so mitigating N2O emissions.
Acknowledgements
This research was undertaken as part of the National Agricultural Nitrous Oxide Research Program (NANORP) funded by the Australian Grains Research and Development Corporation (GRDC) and the Australian Federal Government Department of Agriculture and Water.
References
Allen DE, Kingston G, Rennenberg H, Dalal RC, Schmidt S (2010) Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agriculture, Ecosystems & Environment 136, 209–217.| Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFentb8%3D&md5=3d8ca5935f97bbe807b7a0c7de966c8dCAS |
Angus JF, Bolger TP, Kirkegaard JA, Peoples MB (2006) Nitrogen mineralisation in relation to previous crops and pastures. Australian Journal of Soil Research 44, 355–365.
| Nitrogen mineralisation in relation to previous crops and pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFKgsL4%3D&md5=207176ac04c451abea23869fcfad29ffCAS |
Angus JF, Kirkegaard JA, Hunt JR, Ryan MH, Ohlander L, Peoples MB (2015) Break crops and rotations for wheat. Crop and Pasture Science 66, 523–552.
| Break crops and rotations for wheat.Crossref | GoogleScholarGoogle Scholar |
Asseng S, Fillery IRP, Anderson GC, Dolling PJ, Dunin FX, Keating BA (1998) Use of the APSIM wheat model to predict yield, drainage, and NO3 – leaching for a deep sand. Australian Journal of Agricultural Research 49, 363–377.
| Use of the APSIM wheat model to predict yield, drainage, and NO3 – leaching for a deep sand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFGqsbY%3D&md5=d9644aef49bbe9e08a91e950d07fd975CAS |
Australian Bureau of Agricultural and Resource Economics and Sciences (ABARES) (2013) Agricultural commodity statistics 2013. (ABARES: Canberra). Available at www.agriculture.gov.au/abares/publications [verified 7 Jun 2016]
Barker-Reid F, Gates WP, Wilson K, Baigent R, Galbally IE, Meyer CP, Weeks IA, Eckard RJ (2005) Soil nitrous oxide emissions from rainfed wheat in SE Australia. In ‘Proceedings of the Fourth International Symposium on Non-CO2 Greenhouse Gases: Science Control, Policy and Implementation (NCGG-4)’, Utrecht, The Netherlands, 4–6 July 2005. (Ed. AR van Amstel) pp. 25–32. (Millpress: Rotterdam)
Barton L, McLay C, Schipper L, Smith C (1999) Annual denitrification rates in agricultural and forest soils: a review. Australian Journal of Soil Research 37, 1073–1093.
| Annual denitrification rates in agricultural and forest soils: a review.Crossref | GoogleScholarGoogle Scholar |
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
| Nitrous oxide emissions from a cropped soil in a semi-arid climate.Crossref | GoogleScholarGoogle Scholar |
Barton L, Murphy DV, Kiese R, Butterbach-Bahl K (2010) Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate. GCB Bioenergy 2, 1–15.
| Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1antb0%3D&md5=d1c7d24d4264220dbb973917781d5336CAS |
Barton L, Murphy DV, Butterbach-Bahl K (2013) Influence of crop rotation and liming on greenhouse gas emissions from a semi-arid soil. Agriculture, Ecosystems & Environment 167, 23–32.
| Influence of crop rotation and liming on greenhouse gas emissions from a semi-arid soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktlGjt7c%3D&md5=87dba4171e0b58f15a0517d8d0d73e56CAS |
Benbi DK, Khosa MK (2014) Effects of temperature, moisture, and chemical composition of organic substrates on C mineralization in soils. Communications in Soil Science and Plant Analysis 45, 2734–2753.
| Effects of temperature, moisture, and chemical composition of organic substrates on C mineralization in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslyktr3J&md5=dc86a20706dfc84ddfcc0b03d9216eb5CAS |
Burton DL, Zebarth BJ, Gillam KM, MacLeod JA (2008) Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Canadian Journal of Soil Science 88, 229–239.
| Effect of split application of fertilizer nitrogen on N2O emissions from potatoes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvV2lsbk%3D&md5=d5cb42fbee71113248eb75d89580932fCAS |
Commonwealth of Australia (2015) National inventory report 2013 volume 1: Australian national greenhouse accounts, Commonwealth of Australia, Canberra.
Dalal RC, Chan KY (2001) Soil organic matter in rainfed cropping systems of the Australian cereal belt. Australian Journal of Soil Research 39, 435–464.
| Soil organic matter in rainfed cropping systems of the Australian cereal belt.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1Kqt7c%3D&md5=6b67fb64f99b99ee47918e96418af5f7CAS |
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
De Antoni Migliorati M, Scheer C, Grace PR, Rowlings DW, Bell M, McGree J (2014) Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system. Agriculture, Ecosystems & Environment 186, 33–43.
| Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVCjtbo%3D&md5=0844cd1e106bc3431f689d097fcc0b36CAS |
De Antoni Migliorati M, Bell M, Grace PR, Scheer C, Rowlings DW, Liu S (2015) Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems. Agriculture, Ecosystems & Environment 204, 27–39.
| Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjtFegsL4%3D&md5=1e18334b81692abfbeea6425732375d5CAS |
Del Grosso SJ, Parton WJ, Mosier AR, Ojima DS, Kulmala AE, Phongpan S (2000) General model for N2O and N2 gas emissions from soils due to denitrification. Global Biogeochemical Cycles 14, 1045–1060.
| General model for N2O and N2 gas emissions from soils due to denitrification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVGlsw%3D%3D&md5=e80412f670b691166db8e2c055a1faa8CAS |
Gobbett DL, Hochman Z, Horan H, Navarro Carcia J, Grassini P, Cassman KG (2016) Yield gap analysis of rainfed wheat demonstrates local to global relevance. The Journal of Agricultural Science, in press.
Harris RH, Officer SJ, Hill PA, Armstrong RD, Fogarty KM, Zollinger RP, Phelan AJ, Partington DL (2013) Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in south eastern Australia? Nutrient Cycling in Agroecosystems 95, 269–285.
| Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in south eastern Australia?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru70%3D&md5=dfcb3a9185e64759c620914c4cfd4732CAS |
Heinen M (2006) Simplified denitrification models: overview and properties. Geoderma 133, 444–463.
| Simplified denitrification models: overview and properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtlOnt78%3D&md5=0da9e4bcea6fe943fd5d5c92e7a445c4CAS |
Hénault C, Grossel A, Mary B, Roussel M, Léonard J (2012) Nitrous oxide emission by agricultural soils: a review of spatial and temporal variability for mitigation. Pedosphere 22, 426–433.
| Nitrous oxide emission by agricultural soils: a review of spatial and temporal variability for mitigation.Crossref | GoogleScholarGoogle Scholar |
Hochman Z, Prestwidge D, Carberry PS (2014) Crop sequences in Australia’s northern grain zone are less agronomically efficient than implied by the sum of their parts. Agricultural Systems 129, 124–132.
| Crop sequences in Australia’s northern grain zone are less agronomically efficient than implied by the sum of their parts.Crossref | GoogleScholarGoogle Scholar |
Holzworth DP, Huth NI, DeVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, Moore AD, Brown H, Whish JPM, Verrall S, Fainges J, Bell LW, Peake AS, Poulton PL, Hochman Z, Thorburn PJ, Gaydon DS, Dalgliesh NP, Rodriguez D, Cox H, Chapman S, Doherty A, Teixeira E, Sharp J, Cichota R, Vogeler I, Li FY, Wang E, Hammer GL, Robertson MJ, Dimes JP, Whitbread AM, Hunt J, van Rees H, McClelland T, Carberry PS, Hargreaves JNG, MacLeod N, McDonald C, Harsdorf J, Wedgwood S, Keating BA (2014) APSIM: evolution towards a new generation of agricultural systems simulation. Environmental Modelling & Software 62, 327–350.
| APSIM: evolution towards a new generation of agricultural systems simulation.Crossref | GoogleScholarGoogle Scholar |
Holzworth DP, Snow V, Janssen S, Athanasiadis IN, Donatelli M, Hoogenboom G, White JW, Thorburn P (2015) Agricultural production systems modelling and software: current status and future prospects. Environmental Modelling & Software 72, 276–286.
| Agricultural production systems modelling and software: current status and future prospects.Crossref | GoogleScholarGoogle Scholar |
Huth NI, Thorburn PJ, Radford BJ, Thornton CM (2010) Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: a simulation study. Agriculture, Ecosystems & Environment 136, 351–357.
| Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: a simulation study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFenurk%3D&md5=dc420d89770e39f6151f19212e083ea0CAS |
Intergovernmental Panel on Climate Change (IPCC) (2006) N2O emissions from managed soils, and CO2 emissions from lime and urea application. In ‘2006 IPCC guidelines for national greenhouse gas inventories. Volume 4: agriculture, forestry and other land use’. (Eds S Eggleston, L Buendia, K Miwa, T Ngara, K Tanabe) pp. 11.1–11.54. Available at www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf [verified 17 November 2015]
Isbell RF (2002) ‘The Australian soil classification.’ Revised edn. (CSIRO Publishing: Melbourne)
Jamali H, Quayle WC, Baldock J (2015) Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling. Agricultural and Forest Meteorology 208, 32–39.
| Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling.Crossref | GoogleScholarGoogle Scholar |
Jeffrey SJ, Carter JO, Moodie KB, Beswick AR (2001) Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environmental Modelling & Software 16, 309–330.
| Using spatial interpolation to construct a comprehensive archive of Australian climate data.Crossref | GoogleScholarGoogle Scholar |
Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z, McLean G, Verburg K, Snow V, Dimes JP, Silburn M, Wang E, Brown S, Bristow K, Asseng S, Chapman S, McCown RL, Freebairn DM, Smith CJ (2003) An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288.
| An overview of APSIM, a model designed for farming systems simulation.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Hunt JR, McBeath TM, Lilley JM, Moore A, Verburg K, Robertson M, Oliver Y, Ward PR, Milroy S, Whitbread AM (2014) Improving water productivity in the Australian grains industry: a nationally coordinated approach. Crop and Pasture Science 65, 583–601.
| Improving water productivity in the Australian grains industry: a nationally coordinated approach.Crossref | GoogleScholarGoogle Scholar |
Li GD, Conyers MK, Schwenke GD, Hayes RC, Liu DL, Lowrie AJ, Poile GJ, Oates AA, Lowrie RJ (2016) Tillage does not increase nitrous oxide emissions under dryland canola (Brassica napus L.) in a semiarid environment of south-eastern Australia. Soil Research 54, 512–522.
| Tillage does not increase nitrous oxide emissions under dryland canola (Brassica napus L.) in a semiarid environment of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Mielenz H, Thorburn PJ, Harris RH, Grace PR, Officer SJ (2016a) Mitigating N2O emissions from cropping systems after conversion from pasture: a modelling approach. European Journal of Agronomy.
| Mitigating N2O emissions from cropping systems after conversion from pasture: a modelling approach.Crossref | GoogleScholarGoogle Scholar |
Mielenz H, Thorburn PJ, Scheer C, De Antoni Migliorati M, Grace PR, Bell MJ (2016b) Opportunities for mitigating nitrous oxide emissions in subtropical cereal and fiber cropping systems: a simulation study. Agriculture, Ecosystems & Environment 218, 11–27.
| Opportunities for mitigating nitrous oxide emissions in subtropical cereal and fiber cropping systems: a simulation study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvVyrsLrM&md5=ddb67a695e0091c598ced47fde73f2cfCAS |
Miller PR, Gan Y, McConkey BG, McDonald CL (2003) Pulse crops for the northern great plains: I. Grain productivity and residual effects on soil water and nitrogen. Agronomy Journal 95, 972–979.
| Pulse crops for the northern great plains: I. Grain productivity and residual effects on soil water and nitrogen.Crossref | GoogleScholarGoogle Scholar |
Monjardino M, McBeath T, Ouzman J, Llewellyn R, Jones B (2015) Farmer risk-aversion limits closure of yield and profit gaps: a study of nitrogen management in the southern Australian wheatbelt. Agricultural Systems 137, 108–118.
| Farmer risk-aversion limits closure of yield and profit gaps: a study of nitrogen management in the southern Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Officer SJ, Phillips F, Kearney G, Armstrong R, Graham J, Partington D (2015) Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models. Soil Research 53, 227–241.
| Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGntL0%3D&md5=1f110bcfecd3b8de81e4f80e9df39fffCAS |
Probert ME, Dimes JP, Keating BA, Dalal RC, Strong WM (1998) APSIM’s water and nitrogen modules and simulation of the dynamics of water and nitrogen in fallow systems. Agricultural Systems 56, 1–28.
| APSIM’s water and nitrogen modules and simulation of the dynamics of water and nitrogen in fallow systems.Crossref | GoogleScholarGoogle Scholar |
Rolston DE, Rao PSC, Davidson JM, Jessup RE (1984) Simulation of denitrification losses of nitrate fertilizer applied to uncropped, cropped, and manure-amended field plots. Soil Science 137, 270–279.
| Simulation of denitrification losses of nitrate fertilizer applied to uncropped, cropped, and manure-amended field plots.Crossref | GoogleScholarGoogle Scholar |
Scheer C, Grace PR, Rowlings DW, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant and Soil 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2016) Greenhouse gas (N2O and CH4) fluxes under nitrogen-fertilised dryland wheat and barley on sub-tropical Vertosols: risk, rainfall, and alternatives. Soil Research 54, 634–650.
| Greenhouse gas (N2O and CH4) fluxes under nitrogen-fertilised dryland wheat and barley on sub-tropical Vertosols: risk, rainfall, and alternatives.Crossref | GoogleScholarGoogle Scholar |
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutrient Cycling in Agroecosystems 74, 207–228.
| N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVynur8%3D&md5=2d10bf2076458bc6ed7561f6877e810bCAS |
Syakila A, Kroeze C (2011) The global nitrous oxide budget revisited. Greenhouse Gas Measurement and Management 1, 17–26.
| The global nitrous oxide budget revisited.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsValsr%2FL&md5=3e1418c4c151854072e535a43f7a40baCAS |
Thorburn PJ, Biggs JS, Collins K, Probert ME (2010) Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems. Agriculture, Ecosystems & Environment 136, 343–350.
| Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFenurg%3D&md5=94287091d4343e47b5666b535f246724CAS |
Venterea RT, Coulter JA (2015) Split application of urea does not decrease and may increase nitrous oxide emissions in rainfed corn. Agronomy Journal 107, 337–348.
| Split application of urea does not decrease and may increase nitrous oxide emissions in rainfed corn.Crossref | GoogleScholarGoogle Scholar |
Wallace A, Jones D, Pay R, Armstrong R (2015) High nitrous oxide emissions from irrigated maize and barley in northern Victoria. In ‘Proceedings of the 17th Australian Society of Agronomy Conference’, 20–24 September 2015, Hobart, Australia. (Australian Society of Agronomy: Hobart). Available at www.agronomy2015.com.au/1226 [verified 9 June 2016]
Wang W, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Yao Z, Zheng X, Xie B, Liu C, Mei B, Dong H, Butterbach-Bahl K, Zhu J (2009) Comparison of manual and automated chambers for field measurements of N2O, CH4, CO2 fluxes from cultivated land. Atmospheric Environment 43, 1888–1896.
| Comparison of manual and automated chambers for field measurements of N2O, CH4, CO2 fluxes from cultivated land.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitFChtbg%3D&md5=4bc4e9b5b6f29b2f93ba0251c16e2b32CAS |
Zhu X, Vurger M, Doane TA, Horwath WR (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proceedings of the National Academy of Sciences of the United States of America 110, 6328–6333.
| Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvVOltbk%3D&md5=e6595e76a6f8266c666327a9d5dbe70cCAS | 23576736PubMed |