Nitrification (DMPP) and urease (NBPT) inhibitors had no effect on pasture yield, nitrous oxide emissions, or nitrate leaching under irrigation in a hot-dry climate
Warwick J. Dougherty A D , Damian Collins A , Lukas Van Zwieten B and David W. Rowlings CA NSW Department of Primary Industries, Elizabeth Macarthur Agricultural Institute, PMB 4008, Narellan, NSW 2567, Australia.
B NSW Department of Primary Industries, Wollongbar Agricultural Institute, 1243 Bruxner Highway, Wollongbar, NSW 2477, Australia.
C Queensland University of Technology, Institute for Future Environments, GPO Box 2434 Brisbane, Qld 4001, Australia.
D Corresponding author. Email: warwick.dougherty@dpi.nsw.gov.au
Soil Research 54(5) 675-683 https://doi.org/10.1071/SR15330
Submitted: 9 November 2015 Accepted: 9 March 2016 Published: 25 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Modern dairy farming in Australia relies on substantial inputs of fertiliser nitrogen (N) to underpin economic production. However, N lost from dairy systems represents an opportunity cost and can pose several environmental risks. N-cycle inhibitors can be co-applied with N fertilisers to slow the conversion of urea to ammonium to reduce losses via volatilisation, and slow the conversion of ammonium to nitrate to minimise leaching of nitrate and gaseous losses via nitrification and denitrification. In a field campaign in a high input ryegrass–kikuyu pasture system we compared the soil N pools, losses and pasture production between (a) urea coated with the nitrification inhibitor 3,4-dimethyl pyrazole phosphate (b) urea coated with the urease inhibitor N-(n-butyl) thiophosphoric triamide and (c) standard urea. There was no treatment effect (P > 0.05) on soil mineral N, pasture yield, nitrous oxide flux or leaching of nitrate compared to standard urea. We hypothesise that at our site, because gaseous losses were highly episodic (rainfall was erratic and displayed no seasonal rainfall nor soil wetting pattern) that there was a lack of coincidence of N application and conditions conducive to gaseous losses, thus the effectiveness of the inhibitor products was minimal and did not result in an increase in pasture yield. There remains a paucity of knowledge on N-cycle inhibitors in relation to their effective use in field system to increase N use efficiency. Further research is required to define under what field conditions inhibitor products are effective in order to be able to provide accurate advice to managers of N in production systems.
Additional keywords: autochamber, dairy, diurnal, kikuyu, ryegrass, temporal.
Introduction
Nitrogen (N) is a critical input in Australian dairy farming enterprises, and there is a very strong relationship between N input and milk production (Gourley et al. 2012). The major source of N input for most Australian farms is as inorganic fertiliser, and the median contribution to total farm N inputs is 53% (Gourley et al. 2012). Typically dairy farmers apply 40–50 kg N ha–1 as urea per application, with the number of applications per year being highly variable. The 2012 ‘Dairying for Tomorrow NRM Survey’ (Watson and Watson 2012) found that that the national average N application rate was 155 kg N ha–1, suggesting that on average Australian dairy farmers are making 3–5 applications of N fertiliser per annum. There is often a substantial quantity of applied N in dairy systems that is not recovered in pasture (Rowlings et al. 2016) nor exported in milk (Gourley et al. 2012). It is generally considered that the greater the excess of N in soil–plant systems, the greater its loss to the environment (Jarvis et al. 2011). Increasing intensification of livestock production systems in the future is likely to further exacerbate this situation unless remedial actions are implemented (Gourley and Weaver 2012).
Losses of N represent a potential economic and environmental cost. Dissipation pathways include ammonia (NH3) volatilisation, gaseous losses of nitric oxide, nitrous oxide (N2O) and dinitrogen via nitrification and denitrification, and leaching of nitrate (NO3–). In New Zealand dairying systems, it has been estimated that 30–40% of N is lost from the farm via leaching, runoff, NH3 volatilisation and denitrification (Edmeades 2004). Similar losses were reported by Prasertsak et al. (2001) for southern Queensland in Australia. The agricultural sector is the dominant source of anthropogenic N2O, accounting for 78.6% of the net national N2O emissions (Commonwealth of Australia 2014). Leaching losses are also of interest because of their potential impact on surface and ground water quality (Thorburn et al. 2003).
Soils under dairy pastures typically have relatively high soil organic carbon (C) contents of 3–6% (Dougherty 2007), compared with 1–2% in soils used for cropping. Because of the intensive nature of dairy production, farms are typically located either in high rainfall zones or rely on irrigation. The combination of these two factors (high organic C and high soil moisture) with substantial applications of N predisposes dairy soils to potentially high N2O emissions (Burford and Bremner 1975; Phillips et al. 2007).
It has been suggested that fertilisers with enhanced efficiency have the potential to reduce losses of N from agricultural systems (Chen et al. 2008). Two commonly used inhibitors in the Australian dairy industry are the nitrification inhibitor 3,4-dimethyl pyrazole phosphate (DMPP) and the urease inhibitor N-(n-butyl) thiophosphoric triamide (NBPT). DMPP acts to slow the microbial conversion of ammonium (NH4+) to NO3– and production of gaseous forms of N via nitrification and denitrification. There is a body of research that indicates that a range of inhibitors can retard this conversion and thus lower N2O emissions (Chen et al. 2010; Menéndez et al. 2012). The use of NBPT seeks to inhibit the urease enzyme which is particularly abundant in high-C soils under pasture (Chen et al. 2008), thus slowing the conversion of urea to NH4+ and minimising losses of N via volatilisation (Watson et al. 1994) but exposing the NH4+ to nitrification and denitrification. Urea can be readily purchased with either of these active ingredients applied to it ready for on-farm use.
Inputs of N via fertiliser constitute an important component of the whole farm N-cycle on Australian dairy farms (Gourley et al. 2012) and recovery of this N in pasture even when livestock are absent is only modest (Rowlings et al. 2016). Any attempt to optimise production and environmental outcomes on dairy farms requires an understanding of the effect of inhibitors applied with fertilisers on not only N loss pathways but also on pasture production. Although there is some knowledge on the effect of inhibitor products on N cycling and production under Australian conditions, there is little comprehensive data where both production and N2O emissions data have been collected simultaneously. The need to increase production to achieve economies of scale on dairy farms will place pressure on farmers to increase N application rates and thus increase the risk of losses to the environment. Thus opportunities to increase fertiliser N use efficiency will be important for both economic and environmental reasons.
The objectives of this research were to evaluate the effect of the nitrification (DMPP) and urease (NBPT) inhibitors on pasture yield, N2O emissions and N leaching relative to standard urea.
Materials and methods
The research was undertaken 50 km south-west of Sydney (34.1°S, 150.7°E) on a site with an average annual rainfall of 788 mm (BOM 2015). The soil is a Eutrophic Red Chromosol (Isbell 2002), or Haploxeralf (Soil Survey Staff 1999). The site had been under permanent pasture used for dairy production for over 20 years, and over this period received regular irrigation and fertiliser N inputs. The soil had moderately high soil C and N and contained at least adequate concentrations of other macro-nutrients. Key physical and chemical characteristics of the soil at the commencement of the trial are described in Table 1. Twelve plots were laid out in a randomised complete block design with four replicates of three treatments (described below). Each plot measured 5 m × 5 m, with the whole plot being fertilised, harvested and irrigated. Measurements were only taken within the central 4 m × 4 m area of each plot, thus creating a 1-m buffer between each measurement area.

|
Site management
The pasture at the site was a mixed ryegrass (Lolium perenne and L. multiflorum L.) and kikuyu (Pennisetum clandestinum) system. Kikuyu has summer active growth at the experimental site during approximately December–April at which point it is over-sown with ryegrass.
The N was typically applied immediately after every simulated grazing (harvest) in spring and autumn and every other harvest in summer and winter. N was applied as urea at a rate of 46 kg N ha–1 per application (100 kg ha–1 of urea). There were four replicates of each of three treatments: (1) standard urea, (2) urea + nitrification inhibitor (0.16% w/w DMPP) and (3) urea + urease inhibitor (0.045% w/w NBPT). The concentrations of the active ingredients were as per commonly available commercial urea formulations. In the results section, the N application rate is described as average annualised N application rate (kg N ha–1 year–1) because the N rates varied slightly between the years of the experiment.
During the course of our trial, 19 pasture harvests to simulate grazing were performed over two years with fertiliser applied a total of 17 times. Irrigation was applied on an ‘as needed’ basis determined by a combination of visual inspection of the pasture and soil moisture data. Formal scheduling of irrigation using the soil moisture data was not undertaken because of the potential bias it may introduce to the effect of fertiliser formulations.
Pasture yield and quality measurements
Pasture production was measured by collecting and compositing pasture which was cut to a height of 5 cm from four quadrats (50 cm × 50 cm) per plot at the appropriate ‘grazing’ time. Pasture harvests were nominally taken at the three-leaf stage for ryegrass and four-leaf stage for kikuyu. The pasture samples were then dried in a forced draught oven at 60°C and weighed to calculate pasture yield as tonnes of dry matter (DM) ha–1. The remainder of each of the plots was mown and all mown pasture was removed off-site. Pasture samples were then analysed for total N and protein estimated by multiplying total N by 6.25 (AOAC International 2012).
N2O fluxes
Fluxes of N2O from the soil were determined using an automated gas sampling system as described in detail by Rowlings et al. (2012). This system consisted of pneumatically operated static chambers, linked to an automated sampling system and subsequently an in situ gas chromatograph for analysis of N2O. The clear acrylic glass chambers covered a surface area of 0.25 m2 (0.5 m × 0.5 m), had a height of 0.5 m and a volume of 0.125 m3 and were secured to stainless steel bases inserted permanently into the soil to a depth of 0.1 m. Each plot had two bases and the chambers were alternated between these on a weekly basis to minimise any effects on pasture growth. In the warmer months of October–March, a reflective foil was placed on the north-facing side of the chambers and the lids to reduce heating in the chambers during their closure. A tipping bucket rain gauge connected to the system allowed for automated opening of the lids during rainfall events to ensure all chambers received the same rainfall as the larger plots.
The N2O concentrations were determined using a gas chromatograph (SRI GC8610, Torrance, CA, USA) equipped with 63Ni Electron Capture Detector (ECD). To minimise interference from moisture vapour and carbon dioxide on N2O measurement, a pre-column filled with silica coated by sodium hydroxide was installed ahead of the analytical column and changed weekly. A full measurement cycle for flux determination commenced with lid closure, and finished when the lids were opened 60 min later. During this time, each chamber was sequentially sampled for 3 min followed by a known calibration standard (1.5 ppm N2O). This process was repeated at 20-min intervals, sampling each chamber four times over the closure period. The lids remained open for a further 120 min before the commencement of the next cycle, allowing eight flux measurements for each chamber to be obtained per day.
Hourly N2O fluxes were calculated from the slope of the linear regression of N2O concentration vs time during the chamber lid closure, corrected for air temperature, atmospheric pressure and the ratio of chamber volume to surface area as described by Schwenke and Haigh (2016). The raw data were processed using an Auto GHG System Flux Calculator (Flux.net3.3) developed by the Queensland University of Technology (D. W. Rowlings, pers. comm.). The Pearson’s correlation coefficient (r) for the linear regression was calculated and used as a quality check for each regression. Flux rates were set as missing values if r2 < 0.8. Daily N2O emission for each chamber was calculated by averaging the eight emission measurements for that day.
Soil moisture was monitored continuously at the site using theta (Delta-T, UK) probes connected to a data logger. The probes were calibrated for the soil at this site. Water-filled pore space (WFPS) was calculated as
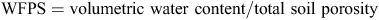
where
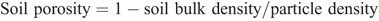
The particle density is 2.65 g cm–3 and the soil bulk density (0–0.1 m) at the site was determined as 1.36 g cm–3. Rainfall, temperature, humidity and wind speed and direction were all logged using an automated weather station (Measurement Engineering Australia, Magill, Australia).
Soil mineral N
Soil samples (0–0.1 m) were collected from every plot approximately monthly by taking 10 cores from each plot and compositing the cores from each plot. Soils samples were then dried in a forced draught oven at 40°C before grinding to <2 mm. Mineral N was then extracted using 2 M potassium chloride and shaking samples for 30 min on an end-over-end shaker. The extracts were then filtered (<0.45 µm) and analysed for NO3– and NH4+ on a flow injection analyser.
N leaching
Intact lysimeter cores were collected for quantifying the volume of drainage and NO3– contained in this drainage based on the design of Cameron et al. (1992). The cores were 0.3 m in diameter and 0.8 m deep (0.8 m was estimated to approximate the maximum effective base of the rooting zone). A single intact core was taken from each plot and encased in 0.3-m diameter polyvinyl chloride (PVC) tubing. Petroleum jelly was poured down the small gap between the soil and the PVC tube to prevent edge flow effects. The cores were then housed in a lysimeter pit adjacent to the plots with at least 0.5 m of soil surrounding each lysimeter on all sides (to minimise any temperature effects) and sown to pasture as per the plots. The lysimeters were treated in all respects exactly as the plots including the timing and rate of fertiliser, irrigation, pasture harvest and management. Leachate samples were collected and measured to determine volume of leachate, and the concentration of NO3– was determined on a sub-sample as per the method previously described.
Statistical methods
Analysis of data to determine existence of treatment effects was undertaken using mixed model analyses performed in ASReml-R (Butler et al. 2009). For the soil mineral N and pasture yield responses, a linear mixed model was fitted with fixed effects of treatment and time (as a factor) and their interaction, and random effects for block, block by time and plot. For these response variates there was little indication of autocorrelation in the data, so a simple equal correlation model was assumed. For N2O flux, a mixed-model smoothing spline framework was used (Verbyla et al. 1999), with fixed effects of treatment and linear time, and random effects of spl(time), ran(time), block and plot and their interactions with linear, spline and random time, and interactions of treatment with spline and factor time. Flux was square-root transformed before analyses. Means in the text are reported with ± standard error of the mean shown in parentheses.
Results and discussion
N2O emissions were monitored continuously for 15 months between November 2012 and January 2014 (inclusive). For the period November 2012 to January 2014, total rainfall was 892 mm compared with the long-term average of 1000 mm. During this period, 710 mm of irrigation was applied and the average annualised N application rate was 442 kg N ha–1 year–1. Other parameters such as pasture production were monitored for 24 months between November 2012 and October 2014. For the two-year period during which pasture production was monitored, the total rainfall was 1425 mm compared with the long-term average of 1764 mm. Over the two-year period, 952 mm of irrigation was applied and the average annualised N application rate was 391 kg N ha–1 year–1.
Soil mineral N
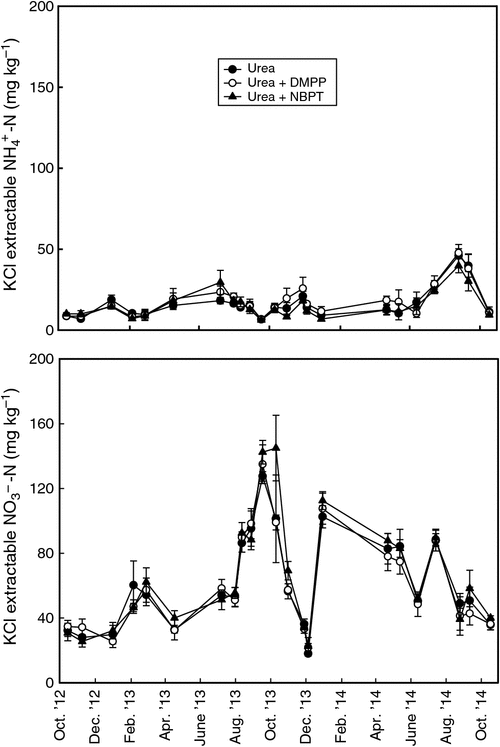
There was no significant effect (P > 0.05) of treatment on total mineral N (NO3– + NH4+), NO3– and NH4+ nor on their relative proportions, although there was a highly significant effect (P < 0.001) of time on mineral N. Mineral N was highly variable between sampling dates with a range of 32–158 mg kg–1 (Fig. 1). NO3–-N was the dominant form of N, accounting on average for 78% of the mineral N fraction. There was an accumulation of soil mineral N until October 2013 whereupon it declined rapidly. Following this, the concentrations of soil mineral N were more variable and displayed no particular patterns. The lack of an effect of treatment on mineral N contrasts with laboratory incubations and other field experiments in which differences in the forms of mineral N have been reported that are consistent with the mechanism of the respective inhibitor products (Irigoyen et al. 2003; Chen et al. 2010; Dawar et al. 2010). Dawar et al. (2010) observed that NBPT resulted in a lower NH4+ concentration for the first 5 days following fertiliser application only, beyond which NH4+ concentration did not differ from the urea control. Chen et al. (2010) observed that under moist (60% WFPS) and mild conditions (15°C), the effect of nitrification inhibitor declined substantially after 14 days. In our soils, mean annual temperature at 0.05 m was 17°C. We sampled only approximately monthly and always at least several weeks after fertiliser application and this may have hampered our ability to detect differences in mineral N between treatments. However, our observed lack of treatment effects on mineral N may also have implications for the effectiveness of the inhibitors on N2O production as discussed below.
|
Pasture yield and N content
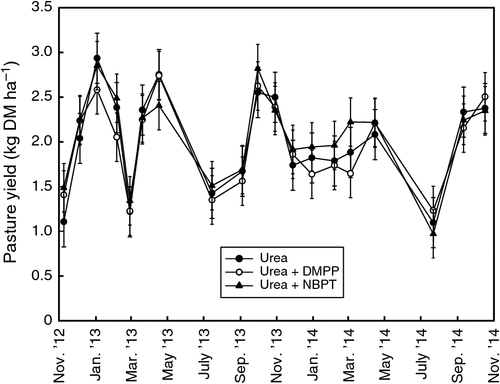
Pasture harvests were undertaken on 19 occasions over the two-year experimental period (Fig. 2). There was no effect (P > 0.05) of treatment on yield or on protein (protein data not shown) although there was a highly significant effect (P < 0.001) of time on pasture yield. The large differences in yield between harvests reflect differences in growth patterns because of stage of growth, temperature, day length and soil moisture. Small harvest yields typically occurred during periods of pasture species transition when pasture was often being managed to re-establish the new species for the forthcoming season. Total cumulative pasture production was 38.1 (±0.94), 37.4 (±1.29) and 39.3 (±0.58) t ha–1 for the urea, urea + DMPP and urea + NBPT treatments respectively; and corresponding protein contents on average were 17.0 (±0.7), 17.2 (±1.7) and 17.1 (±0.9)%. There were large differences in protein contents between harvests with low protein content in early summer. The differences are attributable in part to a change from a ryegrass dominated sward to a kikuyu sward, which typically has lower protein content (Fulkerson 2007). The lack of a treatment effect on pasture yields is consistent with production trials in the nearby Hunter Valley where only small non-significant differences in yields were found when the same inhibitor-coated urea products were used on ryegrass–kikuyu pastures (Neil Griffiths, NSW DPI, pers. comm.). Similarly, Suter et al. (2015) reported that neither DMPP- nor NBPT-coated urea resulted in increased pasture yields. However, NBPT has been shown to increase pasture yields in several other studies in New Zealand: Dawar et al. (2010) reported that the use of NBPT increased pasture yield by 20% and Zaman et al. (2008) reported that NBPT increased pasture yield by 17%. One possible explanation for our observed lack of treatment effects on yields is that maximum yields were already reached at the high rates of N we used and thus any N ‘saved’ by the use of inhibitors could not translate into higher yields. However, data from a companion N rates trial undertaken at our site (W. J. Dougherty, unpubl. data) indicated that this was not the case. This companion trial showed a linear response (P < 0.001, r2 = 0.97) to increasing N rates up to a maximum rate of 667 kg N ha–1 year–1 (application rates of N were 0, 25, 38, 50, 75 and 100 kg N ha–1 per application). Furthermore, there were little differences in the key N loss pathways (and they were small) between treatments, suggesting that pasture responses to inhibitors would be unlikely. Although we used only a single concentration of each of the active inhibitor ingredients as dictated by the commonly available commercial urea coated products that we used, it is worth noting that the rate of the active ingredient can affect the quantity of N lost via volatilisation and denitrification (Rawluk et al. 2001; Zerulla et al. 2001).
|
Currently in the Australian dairy industry, testing for soil mineral N or plant N status is not routinely used for refining N requirements. Emerging technologies to measure plant tissue N remotely and soil mineral N rapidly may be an effective means of refining N inputs to better match plant demand and thus reduce N losses and maximise N use efficiency. These approaches to refining N management require further development and evaluation of their cost effectiveness.
N2O emissions
The N2O emissions were monitored for 15 months. N2O fluxes are shown in Fig. 3 along with average WFPS (0–0.1 m). The effect of treatment on N2O-N emissions was not significant (P > 0.05). The emissions were highly episodic and were often associated with periods of high soil moisture content as would be expected (Weier et al. 1993). Cumulative emissions of N2O-N (Fig. 4) for the monitoring period were 2976 (±242), 2675 (±307) and 2999 (±380) g ha–1 for the urea, urea + DMPP and urea + NBPT treatments respectively (over a 15-month period), during which 598 kg N ha–1 was applied. Of the total N2O emissions, 50% occurred in just 34 days of the total 431 days of monitoring of emissions, illustrating the highly episodic nature of emissions. In a simple regression of WFPS against average daily N2O emissions for the urea treatment, WFPS explained 33% of the variation in emissions. The relatively low emissions for the last 7 months of monitoring occurred despite high mineral N concentrations (see Fig. 1). We hypothesise that the lack of emissions during this period was the result of WFPS remaining <80% at all times – averaging ~60% WFPS due to low rainfall during this period. Even the application of irrigation only resulted in short periods of high soil moisture and even then WFPS rarely approached 80%. Bulk measures of WFPS (such as those made by moisture probes) fail to accurately estimate microsites of high moisture (and subsequent low oxygen status) and moisture is elevated for such short periods that oxygen does not become depleted and thus denitrification is limited. This is supported by the conclusion of Rowlings et al. (2015) that N2O emissions were more related to magnitude and duration of rainfall events than WFPS per se. Major emissions events occurred after heavy rain resulting in episodic waterlogging, indicating that manipulating irrigation (e.g. smaller applications more frequently) is unlikely to result in a reduction of already small losses of N2O.
Rowlings et al. (2015) reported N2O emissions of 1.83 kg ha–1 over two years in an unfertilised pasture system. Carran et al. (1995) reported annual N2O-N emissions of 3–5 kg ha–1 year–1, whereas Scheer et al. (2011) reported a range of 2.7–3.1 kg ha–1 year–1 in an intensively managed subtropical pasture. The presence of livestock and the associated excretion of high concentrations of N into urine patches may have been responsible for the emissions reported by Rowlings et al. (2015) and Carran et al. (1995) being higher than would occur if only fertiliser N was added.
As previously noted, we detected no treatment effect on mineral N, although our soil sampling was typically undertaken at least several weeks after fertiliser application, which is consistent with the lack of treatment effects on N2O emissions. The effectiveness of DMPP can be substantially reduced at high temperatures (Irigoyen et al. 2003; Suter et al. 2010). Over summer at this site, soil temperatures at 0.1 m reached ~35°C (data not presented) and although not measured, the temperature in the very surface of the soil would reasonably be expected to approach ambient air temperatures which commonly were ~40°C during the day. Irigoyen et al. (2003) showed significant declines in the efficacy of DMPP when soil temperatures increased from 10°C to 20 or 30°C. Furthermore, the efficacy of DMPP is affected by soil properties such as clay content that influences its adsorption (Barth et al. 2001). The combination of these factors may all contribute to the apparent lack of effect of nitrification inhibitors when monitored in field situations such as ours.
Although there was no significant effect of treatment on N2O emissions, Fig. 4 suggests an apparent lowering in N2O-N emissions from the DMPP treatment of ~10%. The majority of the apparent differences in cumulative N2O emissions between the urea and the DMPP treatments occurred during two large emission events in February and June 2013. One of these events occurred in summer and the other in winter, suggesting no clear influence of environmental factors on effectiveness. The pattern of rainfall distribution at our site was evenly distributed compared with other dairying locations such as south-east Victoria, which have a strongly winter dominant rainfall resulting in periodic waterlogging. Furthermore, N is mostly used in winter and spring in south-east Victoria and so the use of inhibitor-coated urea may be more easily targeted to key times of the year when emissions would be expected to be high.
N loss through NO3– leaching
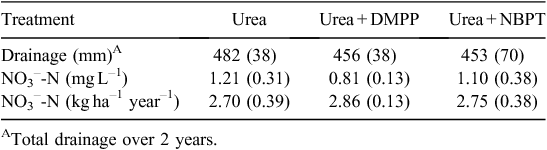
There was little or no drainage from the lysimeters until the large rainfall events of late January 2013. Of the total precipitation received of 2200 mm (comprising 1425 mm rainfall and 775 mm irrigation), on average 232 mm year–1 of drainage occurred with no effect of treatment on drainage (P > 0.05). There was no significant effect (P > 0.05) of treatment on the quantities of NO3– leached. NO3–-N was the dominant form of inorganic N leached. The quantities of NO3– leached are shown in Table 2, and represented only a small proportion of the N applied. Eckard et al. (2004) reported NO3– leaching of 6.2–22 kg N ha–1 in a three-year study applying 200 kg N ha–1 year–1. The losses we measured of <3 kg N ha–1 year–1 are low relative to those reported for New Zealand summarised by Burkitt (2014) and are at the low end of the ranges reported by Eckard et al. (2004) in Victoria. Our lysimeters operated under a zero tension approach – widely recognised to underestimate the volume of drainage – and so our NO3– leaching fluxes may be underestimated. However, the proportion of precipitation estimated to be leached was substantial (20%) and the estimates of volume of drainage were similar to those reported by Eckard et al. (2004). It should be noted that leaching under urine patches is often the key pathway or source of N leaching from pasture systems (Silva et al. 1999; Di and Cameron 2002), although this was not tested in the current study. The small quantities of N leached most likely reflect the high demand for soil N by plants in our systems – while we only applied 782 kg N ha–1, on average 996 kg N ha–1 was removed in harvested pasture over 2 years. Clearly, in this non-grazed system, leaching was not a major loss pathway despite these soils being apparently moderately well drained. Further research is required to examine what effect the rate of N application has on N use efficiency and thus losses via various pathways, with and without other N sources such as urine.
|
Conclusions
Substantial losses of N2O can occur when high rates of N are applied, although at our site the losses of N as N2O were relatively low compared with other studies in pasture systems in Australasia. When soil mineral N concentrations were high, the N2O emissions were relatively large if soil moisture conditions were conducive to denitrification. We observed no effect of nitrification or urease inhibitor (DMPP and NBPT respectively) coated urea on soil mineral N, pasture yield, N2O flux or NO3– leaching. Based on our observations we hypothesise that better matching plant demand with N supply from fertiliser and from mineralisation of organic N in order to minimise soil mineral N, may provide an effective means of minimising the risk of N2O emissions. On several occasions we observed a large build-up of mineral N in late summer or late autumn. At such times, we propose that N inputs could be reduced without compromising soil fertility and subsequent pasture production. Currently in the dairy industry, soil testing for mineral N is not used for determining N requirements. Emerging technologies to measure plant tissue N remotely and rapidly may be an effective means of refining N inputs to better match plant demand and thus reduce N losses and maximise N use efficiency. These approaches to refining N management require further development and an evaluation of their cost effectiveness.
Acknowledgements
The authors would like to thank Kulan Turton, Kate Wright, Deirdre Harvey, Brett Enman and Lyn Muirhead for their assistance in maintenance of the site and processing and analysis of samples and data. Clemmens Scheer from the Queensland University of Technology provided valuable advice and assistance with the setup and maintenance of the automated N2O monitoring system. Thanks are also extended to Charlie Walker of Incitec Pivot for valuable advice and the provision of fertiliser products for the trial. This project was supported by NSW DPI Agriculture through funding from Dairy Australia and the Australian Government Department of Agriculture’s Carbon Farming Futures Filling the Research Gap program.
References
AOAC International (2012) ‘Official methods of analysis.’ 19th edn. (AOAC International: MD, USA)Barth G, von Tucher SV, Schmidhalter U (2001) Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor. Biology and Fertility of Soils 34, 98–102.
| Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7Y%3D&md5=c0c19b1ef5f6c3ea8612e3a5468c3a0bCAS |
Bureau of Meteorology (BOM) (2015) Monthly rainfall, Camden AWS. (Bureau of Meteorology, Australian Government: Australia). Available at www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=068192
Burford JR, Bremner JM (1975) Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biology and Biochemistry 7, 389–394.
| Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XhtlCls74%3D&md5=79bb62266b9c6a7490a2ac59f832d035CAS |
Burkitt LL (2014) A review of nitrogen losses due to leaching and surface runoff under intensive pasture management in Australia. Soil Research 52, 621–636.
| A review of nitrogen losses due to leaching and surface runoff under intensive pasture management in Australia.Crossref | GoogleScholarGoogle Scholar |
Butler DG, Cullis BR, Gilmour AR, Gogel BJ (2009) ‘ASReml-R reference manual.’ Release 3 edn. (VSN International, Hemel Hempstead, UK)
Cameron KC, Smith NP, McLay CDA, Fraser PM, McPherson RJ, Harrison DF, Harbottle P (1992) Lysimeters without edge flow - an improved design and sampling procedure. Soil Science Society of America Journal 56, 1625–1628.
| Lysimeters without edge flow - an improved design and sampling procedure.Crossref | GoogleScholarGoogle Scholar |
Carran RA, Theobald PW, Evans JP (1995) Emissions of nitrous oxide from some grazed pasture soils in New Zealand. Australian Journal of Soil Research 33, 341–352.
| Emissions of nitrous oxide from some grazed pasture soils in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtFWrt74%3D&md5=b1296105d4e94a964511633e3c87a27aCAS |
Chen D, Suter H, Islam A, Edis R, Freney JR, Walker CN (2008) Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers. Australian Journal of Soil Research 46, 289–301.
| Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1OktLw%3D&md5=fec61f7dd15fe4ea2a2bc5ea7113200cCAS |
Chen D, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biology & Biochemistry 42, 660–664.
| Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Sgtrw%3D&md5=cb0fbf1d12401ca061462b81b933ca1fCAS |
Commonwealth of Australia (2014) Agriculture. In ‘National inventory report 2012. Vol. 1’. pp. 257–351. (Commonwealth of Australia: Canberra, ACT)
Dawar K, Zaman M, Rowarth JS, Blennerhassett J, Turnbull MH (2010) The impact of urease inhibitor on the bioavailability of nitrogen in urea and in comparison with other nitrogen source in ryegrass (Lolium perenne L.). Crop and Pasture Science 61, 214–221.
| The impact of urease inhibitor on the bioavailability of nitrogen in urea and in comparison with other nitrogen source in ryegrass (Lolium perenne L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivV2ku7g%3D&md5=264464dfe75a296e531f4b88b02897f1CAS |
Di HJ, Cameron KC (2002) The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use and Management 18, 395–403.
| The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland.Crossref | GoogleScholarGoogle Scholar |
Dougherty WJ (2007) What is the dairy industry is doing to ensure it sustains its soils? In ‘Healthy soils: can Australian soils sustain our agricultural systems?’ (Ed. E Price). (Land and Water Australia: Sunshine Coast, Qld)
Eckard RJ, White RE, Edis R, Smith A, Chapman DF (2004) Nitrate leaching from temperate perennial pastures grazed by dairy cows in south-eastern Australia. Australian Journal of Agricultural Research 55, 911–920.
| Nitrate leaching from temperate perennial pastures grazed by dairy cows in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvVKiurY%3D&md5=f2dcc03fff34786b05fdf1c22eeded8cCAS |
Edmeades DC (2004) ‘Nitrification and urease inhibitors: a review of the national and international literature on their effects on nitrate leaching, greenhouse gas emissions and ammonia volatilisation from temperate legume-based pastoral systems.’ (Environment Waikato: Hamilton East, New Zealand).
Fulkerson B (2007) ‘Perennial ryegrass.’ (Future Dairy, University of Sydney: Camden, NSW).
Gourley CJP, Weaver D (2012) Nutrient surpluses in Australian grazing systems: management practices, policy approaches, and difficult choices to improve water quality. Crop and Pasture Science 63, 805–818.
| Nutrient surpluses in Australian grazing systems: management practices, policy approaches, and difficult choices to improve water quality.Crossref | GoogleScholarGoogle Scholar |
Gourley CJP, Dougherty WJ, Weaver DM, Aarons SR, Awty IM, Gibson D, Hannah MC, Smith AP, Peverill KI (2012) Farm-scale nitrogen, phosphorus, potassium and sulphur balances and use efficiencies on Australian dairy farms. Animal Production Science 52, 929–944.
| Farm-scale nitrogen, phosphorus, potassium and sulphur balances and use efficiencies on Australian dairy farms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1GrsL7O&md5=dfe21dce7f75abd049127b621fbfe355CAS |
Irigoyen I, Muro J, Azpilikueta M, Aparicio-Tejo P, Lamsfus C (2003) Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Australian Journal of Soil Research 41, 1177–1183.
| Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1Cgt70%3D&md5=92d1b1fadc60b4d0482344e6dd14bd3cCAS |
Isbell RF (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Jarvis S, Hutchings N, Brentrup F, Olsen JE, Van der Hoek KW (2011) Nitrogen flows in farming systems across Europe. In ‘The European nitrogen assessment: sources, effects and policy perspectives’. (Eds MA Sutton, CM Howard, JW Erisman, G Billen, A Bleeker, P Greennfelt, H van Grinsven, B Grizzetti). (Cambridge University Press: Cambridge)
Menéndez S, Barrena I, Setienb I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biology & Biochemistry 53, 82–89.
| Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions.Crossref | GoogleScholarGoogle Scholar |
Phillips FA, Leuning R, Baigent R, Kelly KB, Denmead OT (2007) Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques. Agricultural and Forest Meteorology 143, 92–105.
| Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques.Crossref | GoogleScholarGoogle Scholar |
Prasertsak P, Freney JR, Denmead OT, Saffigna PG, Prove BG (2001) Signficance of gaseous nitrogen loss from a tropical dairy pasture fertilised with urea. Australian Journal of Experimental Agriculture 41, 625–632.
| Signficance of gaseous nitrogen loss from a tropical dairy pasture fertilised with urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvVers7w%3D&md5=7c80389420988444a65c348ea47c6660CAS |
Rawluk CDL, Grant CA, Racz GJ (2001) Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Canadian Journal of Soil Science 81, 239–246.
| Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVGqsrc%3D&md5=28fd754abc7da7993c5dedc04fa3f362CAS |
Rayment GE, Lyons DJ (2010) Soil chemical methods - Australasia. (CSIRO Publishing: Melbourne)
Rowlings DW, Grace PR, Kiese R, Weier KL (2012) Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Global Change Biology 18, 726–738.
| Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Rowlings DW, Grace PR, Scheer C, Liu S (2015) Rainfall variability drives interannual variation in N2O emissions from a humid, subtropical pasture. The Science of the Total Environment 512–513, 8–18.
| Rainfall variability drives interannual variation in N2O emissions from a humid, subtropical pasture.Crossref | GoogleScholarGoogle Scholar | 25613765PubMed |
Rowlings DW, Scheer C, Liu S, Grace PR (2016) Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates. Agriculture, Ecosystems & Environment 216, 216–225.
| Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1yjtb7K&md5=96447a2be2ec2cdc3b0046fcba10d9a5CAS |
Scheer C, Grace PR, Rowlings DW, Kimber S, Van Zwieten L (2011) Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant and Soil 345, 47–58.
| Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFymt7o%3D&md5=035eaf908fa5f45f19e185ef59a541bbCAS |
Schwenke GD, Haigh BM (2016) The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols. Soil Research 54, 604–618.
| The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols.Crossref | GoogleScholarGoogle Scholar |
Silva RG, Cameron KC, Di HJ, Hendry T (1999) A lysimeter study of the impact of cow urine, dairy shed effluent, and nitrogen fertiliser on nitrate leaching. Australian Journal of Soil Research 37, 357–369.
| A lysimeter study of the impact of cow urine, dairy shed effluent, and nitrogen fertiliser on nitrate leaching.Crossref | GoogleScholarGoogle Scholar |
Soil Survey Staff (1999) ‘Soil taxonomy: a basic system of classification for making and interpreting soil surveys.’ 2nd edn. (U.S Government Printing Office: Washington DC)
Suter H, Chen D, Li H, Edis R, Walker C (2010) Comparison of the ability of the nitrification inhibitors DCD and DMPP to reduce nitrification and N2O emissions from nitrogen fertilisers. In ‘Proceedings of the 19th World Congress of Soils Science’, 1–6 August 2010, Brisbane, Australia. (DVD). (Eds R Gilkes, N Prakongkep) pp. 24–27. (International Union Soil Science)
Suter H, Lam SK, Walker C, Chen D (2015) Nitrogen use efficiency for pasture production – impact of enhanced efficiency fertilisers and N rate. In ‘Agronomy 2015’. (Agronomy Society of Australia: Hobart, Australia)
Thorburn PJ, Biggs JS, Weier KL, Keating BA (2003) Nitrate in groundwaters of intensive agricultural areas in coastal Northeastern Australia. Agriculture, Ecosystems & Environment 94, 49–58.
| Nitrate in groundwaters of intensive agricultural areas in coastal Northeastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptFGqsrc%3D&md5=4f140239f3e4b151fc458f38a41b7be5CAS |
Verbyla AP, Cullis BR, Kenward MG, Welham SJ (1999) The analysis of designed experiments and longitudinal data using smoothing splines (with discussion). Applied Statistics 48, 269–311.
| The analysis of designed experiments and longitudinal data using smoothing splines (with discussion).Crossref | GoogleScholarGoogle Scholar |
Watson P, Watson D (2012) Dairying for Tomorrow survey of natural resource management on dairy farms report May 2012, Dairy Australia, Melbourne.
Watson C, Miller H, Poland P, Kilpatrick DJ, Allen MDB, Garrett MK, Christianson CB (1994) Soil properties and the ability of the urease inhibitor NBPT to reduce ammonia volatilization from surface-applied urea. Soil Biology & Biochemistry 26, 1165–1171.
| Soil properties and the ability of the urease inhibitor NBPT to reduce ammonia volatilization from surface-applied urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmtVCiurg%3D&md5=bbeee6a8063533eebcb21197fb167017CAS |
Weier K, Doran J, Power J, Walters D (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon and nitrate. Soil Science Society of America Journal 57, 66–72.
| Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeqtLk%3D&md5=fd56bf11bee9b78d91071f533d1e8f08CAS |
Zaman M, Nguyen ML, Blennerhassett JD, Quin BF (2008) Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biology and Fertility of Soils 44, 693–705.
| Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsFCmtLg%3D&md5=a337cf6eba31daf4ad7dfe7e7c4f5c54CAS |
Zerulla W, Barth T, Dressel J, Erhardt K, von Locquenghien K, Pasda G, Radle P, Wissemeier A (2001) 3-4 dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture. An introduction. Biology and Fertility of Soils 34, 79–84.
| 3-4 dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture. An introduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |




