Benchmarking nitrous oxide emissions in deciduous tree cropping systems
Nigel Swarts A C , Kelvin Montagu B , Garth Oliver A , Liam Southam-Rogers A , Marcus Hardie A , Ross Corkrey A , Gordon Rogers B and Dugald Close AA Tasmanian Institute of Agriculture, University of Tasmania, Sandy Bay Campus, Tas. 7005, Australia.
B Applied Horticulture Research, Level 3 Biomedical Building, Australian Technology Park, 1 Central Avenue, Eveleigh, NSW 2015, Australia.
C Corresponding author. Email: nigel.swarts@utas.edu.au
Soil Research 54(5) 500-511 https://doi.org/10.1071/SR15326
Submitted: 4 November 2015 Accepted: 17 March 2016 Published: 12 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Nitrous oxide (N2O) emissions contribute 6% of the global warming effect and are derived from the activity of soil-based microorganisms involved in nitrification and denitrification processes. There is a paucity of greenhouse gas emissions data for Australia’s horticulture industry. In this study we investigated N2O flux from two deciduous fruit tree crops, apples and cherries, in two predominant growing regions in eastern Australia, the Huon Valley in southern Tasmania (Lucaston – apples and Lower Longley – cherries), and high altitude northern New South Wales (Orange – apples and Young – cherries). Estimated from manual chamber measurements over a 12-month period, average daily emissions were very low ranging from 0.78 g N2O-N ha–1 day–1 in the apple orchard at Lucaston to 1.86 g N2O-N ha–1 day–1 in the cherry orchard in Lower Longley. Daily emissions were up to 50% higher in summer (maximum 5.27 g N2O-N ha–1 day–1 at Lower Longley) than winter (maximum 2.47 g N2O-N ha–1 day–1 at Young) across the four trial orchards. N2O emissions were ~40% greater in the inter-row than the tree line for each orchard. Daily flux rates were used as a loss estimate for annual emissions, which ranged from 298 g N2O-N ha–1 year–1 at Lucaston to 736 g N2O-N ha–1 year–1 at Lower Longley. Emissions were poorly correlated with soil temperature, volumetric water content, water filled porosity, gravimetric water content and matric potential – with inconsistent patterns between sites, within the tree line and inter-row and between seasons. Stepwise linear regression models for the Lucaston site accounted for less than 10% of the variance in N2O emissions, for which soil temperature was the strongest predictor. N2O emissions in deciduous tree crops were among the lowest recorded for Australian agriculture, most likely due to low rates of N fertiliser, cool temperate growing conditions and highly efficient drip irrigation systems. We recommend that optimising nutrient use efficiency with improved drainage and a reduction in soil compaction in the inter-row will facilitate further mitigation of N2O emissions.
Additional keywords: apples, cherries, fertiliser, global warming, greenhouse gas, nitrogen.
Introduction
Nitrous oxide (N2O) is a potent greenhouse gas (GHG) with 310 times the global warming potential of carbon dioxide (Ramaswamy et al. 2001). Despite comprising only 3.0 × 10–6 % of the earth’s atmosphere, N2O contributes ~6% of the warming effect caused by increased GHG emissions (Dalal et al. 2003). While the Australian Greenhouse Office (AGO 2001) estimates that agriculture is the second largest contributor of GHG emissions, agriculture contributes ~78% of all N2O emissions. Furthermore, N2O emissions from agricultural activities are expected to increase by 50% in the near future, due to greater fertiliser demand to meet the global food production needs (US EPA 2006).
An estimated two-thirds of global N2O emissions are derived from the activity of soil-based microorganisms involved in nitrification and denitrification processes (Prather et al. 1995; Dalal et al. 2003). These processes and thus N2O emissions are strongly influenced by both environmental factors, such as soil moisture, oxygen availability and soil and air temperature, as well as management practices, specifically fertiliser and irrigation use, but also inter-row weed management, soil compaction and organic matter flux due to pruning and thinning (Davidson 1992; Tiedje 1994).
Uncertainty as to the relative contribution of different agricultural sectors to N2O emissions is in part due to scarcity of data, which is compounded by considerable variance between studies due to differences in soils, climate, and management practices (Barton et al. 2008; Denmead et al. 2010).
The horticulture sector of Australia’s agriculture industries contributes approximately $8.7 billion to the national economy and is the third largest agricultural industry (Australian Bureau of Statistics 2012). The horticultural sector is diverse and comprises many different commodities, each requiring its own unique production system including evergreen and deciduous fruit tree production, vegetable production, nuts, table grapes, turf and cut flowers. Consequently determining the N2O emissions from horticulture is extremely complex. Despite the economic importance of the sector, emissions data for production horticulture are almost non-existent. Within the horticultural sector, apple and cherry production is estimated to occupy 10 600 ha (George 1999) or 37% of the total area for deciduous tree fruit crops. Apples and cherries are produced from higher altitude areas of southern Queensland and central New South Wales (NSW), to inland Victoria, Adelaide hills, Perth hills and south-western Western Australia and southern Tasmania.
Deciduous trees by their nature are highly effective utilisers of nitrogen (N) as they withdraw N from their leaves before leaf senescence into storage organs (roots, trunk and branches) during winter dormancy (Millard 1996). This stored N is remobilised for the current season’s growth, which for some crops can contribute up to 50% of the total N requirement for the season (Millard and Neilsen 1989). Deciduous fruit tree crops are strong sinks for N, in which a large portion of N (30–60 kg ha–1) is removed on an annual basis depending on crop load (Neilsen et al. 1997). As developing fruit have a strong demand for N over a short growth stage, growers tend to apply high rates of fertiliser to the soil over a short period pre-harvest to meet tree demands and/or post-harvest to facilitate storage of N by trees during winter dormancy. Similarly, deciduous fruit tree crops have a significant water requirement due to high crop water content (up to 85%) and transpiration losses during the summer months. N fertiliser application for mature apple trees broadly ranges from 30 to >200 kg N ha–1, while irrigation requirements range from zero in rainfed areas to 1–5 ML ha–1 in cold climates and 5–9 ML ha–1 in Mediterranean climates (APAL 2013).
Given the paucity of GHG emissions data for the horticulture sector (Huang et al. 2012; Rowlings et al. 2013) there is an ongoing need to determine the spatial and temporal variations in N2O fluxes from typical production systems within the sector. In response this study was established to determine annual N2O emissions from two deciduous fruit tree crops, apples and cherries, in two predominant growing regions in eastern Australia: the Huon Valley, Tasmania and high altitude northern NSW. Furthermore, we sought to improve our understanding of the influence of environmental factors such as soil temperature, volumetric water content (VWC), water filled porosity (WFP), gravimetric water content (GWC) and matric potential (MP) on N2O emissions in two deciduous fruit tree production systems. Results are discussed in the context of potential management strategies for mitigating N2O emissions within the deciduous fruit tree crop industries.
Materials and methods
Trial sites
Tasmania
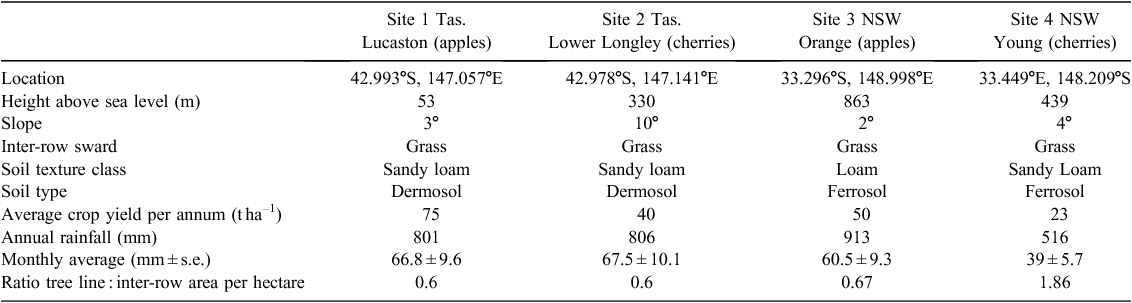
Two greenhouse gas monitoring sites were established in the Huon Valley, Tasmania, at Lucaston in an apple orchard, and Lower Longley in a cherry orchard. The Huon Valley has a cool temperate climate with an average daily maximum temperature of 21°C in summer and 12°C in winter. Over the study period, Lucaston and Lower Longley received a total of 801 and 806 mm of rainfall respectively (Table 1). Rainfall patterns were similar at both sites with fairly uniform monthly totals, reflected in the low standard errors over the study period, representing a typical Huon Valley rainfall scenario (Fig. 1). Soils used for orchards in the Huon Valley mostly consist of texture contrast Kurosols and Chromosols (Isbell 2002) derived from Quaternary alluvium.
|
|
The Lucaston site consisted of 12-year-old ‘Royal Gala/Galaxy’ trees on M26 rootstocks. Trees were grown in mounded rows running north-west–south-east trained as a central leader and spaced at ~1 m along rows and 4 m between rows. The orchard was irrigated during October–March with inline drippers (2.3 L h–1) at 500-mm spacings along the tree line. Fertiliser was applied by a spreader along the mound shoulders as calcium nitrate at an annual rate of 40 kg N ha–1 split into a 15 kg N ha–1 pre-harvest (10 October 2013) and 25 kg N ha–1 post-harvest (3 April 2014). The Lower Longley site consisted of 12-year-old ‘Simone’ trees, grown on Colt rootstocks, pruned to a Spanish bush training system with tree spacing of 2 m and row spacing of 4.5 m. Row orientation was north-west–south-east with a considerable slope down from the northern end of the row. The orchard was drip-irrigated as described above and N was fertigated as six split fortnightly 25 kg N ha–1 of calcium nitrate and potassium nitrate along the tree line, commencing on 25 October 2013 at an annual rate of 150 kg N ha–1.
NSW
Two greenhouse gas monitoring sites were established in NSW: an apple orchard at Orange and a cherry orchard at Young. The Orange site has a continental climate with a notable difference in mean maximum temperature between summer and winter (25.7°C and 10.6°C respectively). Rainfall in the region is generally uniform across the year, averaging 859 mm. The climate at the Young site is classified as cool temperate with uniform precipitation across the year, averaging 591 mm. The annual maximum mean temperature (1991–2015) is 30.1°C in summer and 13.2°C in winter. Over the study period, the orchards at Orange and Young received total rainfall of 913 and 516 mm respectively with uniform monthly totals reflected in low standard errors between the monthly averages (Table 1).
The Orange site consisted of 8-year-old ‘Pink Lady’ or ‘Sundowner’ apple cultivars on M106 rootstocks, planted in double rows (1.5 m), spaced at 2.1 m along rows and 6 m between rows. The orchard was irrigated with under tree drip irrigation from November to May (harvest). The N fertiliser was applied via three fertigation events (1 February 2014, 10 February 2015 and 28 February 2015). At each fertigation, 4.75 kg N ha–1 was applied as potassium nitrate and mono ammonium phosphate along the tree line, resulting in annual rate of 14.3 kg N ha–1. The Young site consisted of 10-year-old ‘Van’ or ‘Lapins’ cherry cultivars on Mahaleb rootstocks planted in single rows, spaced at 4.65 m apart and 6 m between rows. The orchard was irrigated by under tree micro-sprinklers from October to December (harvest). A total of 58.5 kg N ha–1 of N fertiliser was applied during the 2014–15 sample period. This was applied by hand as 21.7 kg N ha–1 of ammonium nitrate to the tree row on the 8 August 2014, by fertigation as 9.2 kg N ha–1 of urea and calcium nitrate on the 15 October 2014 or as a post-harvest foliar spray at 27.6 kg N ha–1 on the 12 April 2015.
At all trial sites, herbicides were used to keep the tree line free of weeds. Pests and diseases were managed by the grower in accordance with commercial practices.
Soil N2O emissions
N2O emissions were measured using static non-flow through chambers, comprising a mounting collar and a detachable chamber. At the two Tasmanian field sites, chambers were ~9 L (240 mm diameter × 200 mm in height) with 0.046 m2 surface area. Chambers were lined with Mylar to reflect UV and fitted with air-stones for passive pressure compensation to minimise the influence of heating from solar insulation. Collars were inserted 100 mm into the soil and remained in place throughout the trial. The collars each had three rows of 3.6-mm holes to allow for root penetration, and projected 50 mm above the soil surface. At Lucaston, eight static chambers were installed in October 2013, four in the tree line and four in the inter-row of the orchard. At Lower Longley, six chambers were installed, three in the tree line and three in the inter-row. At both sites, where chambers were installed in the tree line, 16-mm holes were drilled (then sealed) into the collar sidewall to allow the dripper irrigation line to pass through the collar. Each chamber top was fitted with an iButton (Maxim) temperature sensor that logged air temperature inside the chamber at 5-min intervals. For both sites, N2O was sampled weekly during the peak growing season (November–April) and once monthly during the winter period (May–August). More frequent (every second day) sampling events coincided with fertiliser application in November and March and a large rainfall event in July 2014. During collection events, the chamber top was secured to the collar with clips and a rubber O-ring to prevent air movement. The atmosphere in each chamber was sampled sequentially over a one-hour period at 0, 30 and 60 min intervals, starting at ~10 a.m. to represent the average daily temperature. Samples were extracted through a butyl rubber septa located centrally on the chamber top using a 25-mL glass gas-tight syringe (SGE, 25MDR-LL-GT,Melbourne Australia); the sample was then injected into a 12-mL Exetainer (Labco, High Wycombe, Buckinghamshire, UK) to ~2 atm.
At the two NSW field sites, the static non-flow through chambers were slightly smaller than those used in Tasmania (diameter 243 mm, height 205 mm and installed volume of 7.3 L) but otherwise the same. At each of the NSW sites, eight collars were installed in the tree line and eight randomly located in the inter-rows. Sampling was conducted every two weeks, and more frequently during the growing season (October–March). Chamber air samples were collected from the static chambers at 0, 30 and 45 min after lids were sealed using a 25-mL gas-tight syringe (SGE, 25MDR-LL-GT), and introduced into pre-evacuated 12-mL Exetainer (Labco) vials with grey silicon septa. Chamber temperature was measured during sampling using a TP3001 digital thermometer.
Gas analysis
Samples were analysed on an Agilent 7890A gas chromatograph fitted with a Gilson (GX 271) auto sampler. The system had two channels leading to μ-ECD and FID detectors. N2O was analysed by μ-ECD. The sample was loaded onto a 1000-mL sample loop, and then injected onto a 1-m Porapak Q pre-column. Gases were then passed onto a 2-m Porapak Q column for further separation. The pre-column was back-flushed to remove moisture after the analytes had passed onto the analytical column. Relative standard deviation (based on seven replicate injections) for N2O was <2%. For each batch of samples, a range of standards, controls and blanks were included for quality control purposes (van Zwieten et al. 2010).
Soil properties
At all sites, soil temperature was measured at 10 cm depth every hour using iButtons (DS1920, Maxim, San Jose, USA) temperature logger. In the Tasmanian sites, soil moisture probes (Decagon,10HS, Pullman, USA) were installed at 5–10 cm below the soil surface to record VWC (cm3 cm–3) at hourly intervals in the inter-row and tree line under the irrigation dripper in two locations at Lucaston and one location at Lower Longley. In the NSW sites, VWC was measured in the tree line at 10, 20, 30 and 50 cm depth using a continuously logging capacitance soil moisture probe (EnviroSCAN, Sentek Sensor Technologies, Stepney, Australia).
In the Tasmanian field sites, soil was sampled along the tree line and inter-row of three experimental rows at three locations in November 2013 and July 2014. Soil samples were taken from 0–10 cm depth adjacent to the drippers in the tree line and next to the installed gas chambers in the inter-row. Samples were collected by ‘pogo stick’ in which four plugs were collected ~20 cm apart and mixed from which a representative subsample was obtained. Soil subsamples were air-dried, then gently disaggregated in a mortar and pestle and sieved to <2 mm. The NO3– content was determined by placing 8 g of dried soil in a 50-mL centrifuge tube which was extracted in 40 mL of 2 M potassium chloride over 18 h. The tubes were centrifuged at 12.6 g for 10 min and a 10-mL subsample was filtered through a 0.45-µm syringe filter. The NO3– analysis was performed on a Westco Smartchem 200 discrete analyser, following the US EPA Method 353.2 (US EPA 1993).
For all sites, total N (%) and total carbon (C, %) were also determined from a fine-ground sample (Retsch MM200 ball mill, Dusseldorf, Germany) of 20–30 mg using an oxidative combustion analyser (Perkin Elmer CHN-S 2400, Waltham, USA) (Table 2). Soil bulk density of the A1 horizon was determined at all sites using three 60 mm × 61 mm cores following Cresswell and Hamilton (2002). GWC was determined by drying the entire core at 105°C for 24 h. Total porosity (TP) and WFP were calculated from bulk density, assuming a particle density of 2.65 g cm–3 and 98% saturation (Table 2). At the two Tasmanian sites, infiltration was measured in triplicate using a 20 mm diameter, single ring, constant head, infiltrometer in both the tree line and inter-row areas. The infiltration ring was inserted 3 cm and sealed on the outside with compacted soil slurry, water was maintained at head of 0.5–1.5 cm (variation due to surface elevation) for ~20–40 min to ensure establishment of steady-state conditions. Saturated hydraulic conductivity and sorptivity were estimated (Reynolds and Elrick 1990; Reynolds 2008) in which α (macroscopic capillary length equivalent to the wetting front suction) was estimated to be 0.12 (Table 1). At Lucaston, MP was calculated from the van Genuchten–Mualem equation (Mualem 1976; van Genuchten 1980). The soil water retention and conductivity parameters as input for the equation were determined using the extended HYPROP evaporative flux approach (Peters and Durner 2008). In the Tasmanian sites, a subsample of soil from each chamber location was sent to AgVita Analytical (Devonport, Tasmania) for labile C analysis using the permanganate method based on Weil et al. (2003). Potential N mineralisation was performed following the anaerobic incubation method of Curtin and Campbell (2008).

|
Data analysis
The flux rate, FN2O, was calculated using Eqns 1 and 2 (Scheer et al. 2014). All N2O flux rates were corrected for the actual air temperature during the measurement and recorded as μg N2O-N m2 h–1:

where ACH is basal area of the measuring chamber (m2), b is increase in concentration (ppb min–1), MWN2O-N is molecular weight of N2O-N (28 g mol–1), MVcorr is temperature-corrected molecular volume (m3 mol–1) and VCH is volume of the measuring chamber (m3)

where MVcorr is defined above, T is air temperature during the measurement (°C) and 0.02241 m3 is the molar volume of an ideal gas at 1 atm and 273.15K (Aylward and Findlay 1974).
A linear regression (r) was computed using the Pearson’s correlation coefficient to determine the quality of the slope relationship and flux rates were discarded if r < 0.8. Daily N2O fluxes from each site were calculated by averaging hourly flux from each replicate and multiplying the data by 24. Cumulative seasonal fluxes for each site were calculated by integrating daily N2O fluxes via linear interpolation over the study period.
Associations among N2O emissions and soil environmental variables (soil temperature, VWC, WFP, GWC and MP) were assessed using Pearson’s correlation in SPSS Version 22. Correlations were performed for each site, sampling location within site (tree line and inter-row) and for each season using the combined dataset from all sites. A multiple linear regression using a stepwise approach was used to develop a model to predict N2O emissions from soil environmental variables using SPSS. This was undertaken in SPSS using the dataset from Lucaston, as this was the only site where data from all soil environmental variables were complete over the study period.
Auxiliary measurements
Emission factors were calculated uncorrected for background emission over the 12 months and expressed as the percentage of the total fertiliser N applied that was emitted as N2O-N. The emissions intensity of each treatment was calculated as the ratio of N2O emissions in relation to crop yield and relates to how much N2O was emitted per tonne of fruit harvested. Direct N2O emissions were converted to carbon dioxide equivalents (CO2eq) within a 100-year horizon by multiplying by a radiative forcing potential equivalent to CO2 of 310 (IPCC 2001). Yield-scaled emissions for this perennial system (CO2eq Mg−1) were calculated by dividing annual N2O emissions (CO2eq ha−1 year−1) for the annual production cycle by total apple or cherry yield (Mg ha−1). In each orchard, fruit yield was measured at harvest using Millennium Mechatronics pallet scales or by harvesting a random sample of whole trees and multiplying yield by orchard tree number.
Results
Site conditions
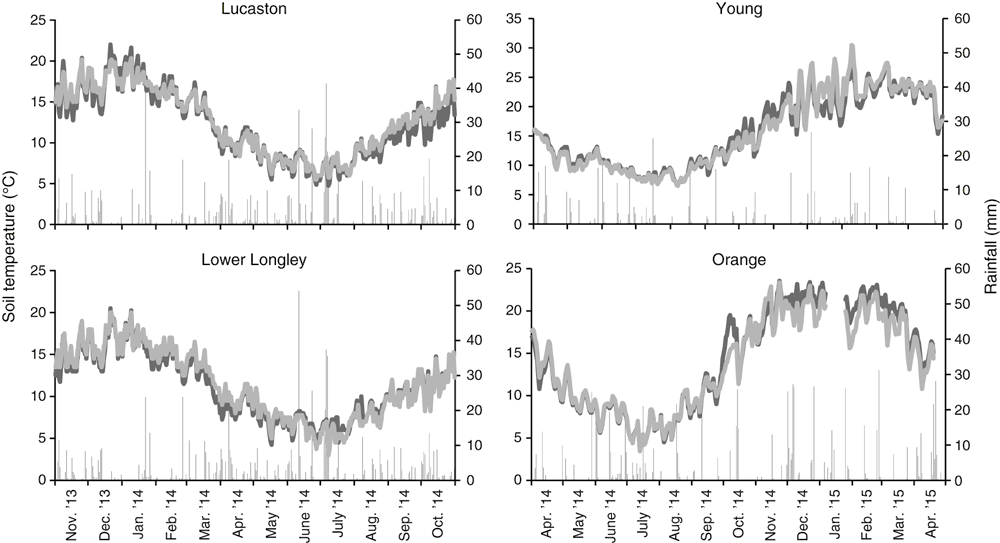
Average daily soil temperature ranged from 4°C in winter to 21°C in summer in the Tasmanian orchards and 5 to 26°C in the NSW orchards (Fig. 1). In Tasmania, daily soil temperature was consistently >13°C from early November to mid-April; whereas in NSW, daily soil temperature was >13°C between late September to mid-April (Fig. 1).
At Lucaston and Lower Longley, a substantially higher infiltration rate was observed in the tree line (13.77 and 11.66 cm h–1 respectively) than the inter-row (0.49 and 1.19 cm h–1 respectively) most likely due to mounding of the sandy loam topsoil at both sites for drainage purposes (Table 2). This was also reflected in an increased bulk density of the inter-row (1.32 and 1.18 g cm–3 respectively) at both sites compared with the tree line (1.21 and 1.03 g cm–3 respectively). Consequently, WFP was consistently higher along the inter-row than the tree line but never exceeded 80% or dropped below 40% over the study period (data not shown). Due to logger failure, only 60 days of soil moisture data was recorded at Lower Longley, where a similar trend was found for the period recorded. At Orange, bulk density of the inter-row was 1.53 g cm–3 and was higher than the tree line (1.42 g cm–3); however, at Young, bulk density of the inter-row (1.49 g cm–3) was almost identical to the tree line (1.48 g cm–3). Considerable variation in WFP was measured at the wetter Orange site, ranging from 15% in summer to 85% during winter. At Young, WFP ranged from 15% in summer to 65% in winter (data not shown).
Soil NO3– levels measured in November 2014 at Lucaston and Lower Longley were higher along the tree line (5.44 and 4.3 mg kg–1 respectively) than in the inter-row (2.53 and 4.14 mg kg–1 respectively). There was a similar trend for row and inter-row NO3–1 levels at Orange (6.9 and 5.7 mg kg–1 respectively) and Young (6.5 and 5.7 mg kg–1 respectively) (Table 1). Total N (%), total organic C (%) and the C : N ratio were substantially higher in the Tasmanian than the NSW orchards. At Lucaston and Lower Longley respectively, labile C was substantially higher in the inter-row (442.3 and 394.3 ppm) than the tree line (400 and 344.7 ppm).
Temporal and spatial variability of N2O emissions
Mean daily N2O emissions over the 12-month period were very low, ranging from 0.78 g N2O-N ha–1 day–1 in the apple orchard at Lucaston to 1.86 g N2O-N ha–1 day–1 at the cherry orchard in Lower Longley. Mean daily N2O emissions from the tree line were low (<1.31 g N2O-N ha–1 day–1) but similar across all orchards (Table 3). Mean daily emissions from the inter-row were consistently higher for each orchard but did not exceed 2.44 g N2O-N ha–1 day–1. The highest daily flux recorded (irrespective of sampling location) was 13.86 g N2O-N ha–1 day–1 in the cherry orchard at Lower Longley, whereas the lowest daily flux of 0.09 g N2O-N ha–1 day–1 was in the apple orchard at Lucaston. Mean daily emissions within seasons followed a similar trend of higher inter-row emissions than the tree line with few exceptions (Fig. 2). N2O emissions were almost double in summer compared with other seasons for all orchards, with the exception of the cherry orchard at Young. Greatest variation in N2O emissions within orchards was also observed in summer with daily inter-row fluxes twice that of the tree line at each site. N2O emissions during winter (<0.9 g N2O-N ha–1 day–1) were consistently the lowest recorded for the Tasmanian orchards. In NSW, N2O emissions were lowest (<0.91 g N2O-N ha–1 day–1) during autumn for Orange and summer for Young.

|
Cumulative emissions reached 890 g N2O-N ha–1 year–1 in the inter-row at Lower Longley but were as low as 230 g N2O-N ha–1 year–1 in the tree line at Lucaston (Table 3). The substantial spatial variation in daily N2O fluxes within the tree line and inter-row static chamber replicates were reflected in the high standard errors. When added and corrected for the actual area within the orchard that the tree line and inter-row occupied, maximum cumulative emissions (736 g N2O-N ha–1 year–1) were recorded at Lower Longley with the least (298 g N2O-N ha–1 year–1) for Lucaston (Fig. 3).

|
Associations between daily N2O fluxes and soil environmental variables for each orchard, sampling location and season are described in Table 4. Soil temperature showed a weak positive but significant correlation with N2O emissions for all sites, with the exception of a negative correlation for Young (r = –0.157). Overall, N2O emissions were negatively correlated with summer soil temperatures (r = –0.211). Indeed, at all sites, daily N2O fluxes >5 g N2O-N ha–1 day–1 were only observed for soil temperature >13°C. There were relatively weak, contrasting associations between soil temperature and N2O emissions between the tree line and inter-row for all sites with the exception of Lower Longley. N2O emissions decreased as VWC and WFP increased at all sites, except Young in the tree line with an opposite effect (r = 0.372). The strongest association between N2O emissions and WFP was during winter (r = –0.506), which matched the trends found within sites. N2O emissions were consistently associated with decreasing GWC at all sites and within the tree line and inter-row. The strongest association was again in winter (r = –0.743), largely driven by data for Young. A very weak positive yet significant association between N2O emissions and MP was found in the Lucaston tree line (r = 0.159).

|
The model produced from the stepwise regression analysis (Table 5), accounted for only 9% of the variability in the measured data driven by soil temperature (partial r2 = 0.072) and WFP (partial r2 = 0.021). Splitting the data into sampling location within the orchard improved the predictive capacity of the regression model to 24.5% for the tree line but reduced it to 3.7% for the inter-row, driven largely by soil temperature.

|
Discussion
Temporal and spatial variability of N2O emissions
Average daily N2O emissions averaged across the four orchards representing a temperate deciduous tree cropping system was 1.47 g N2O-N ha–1 day–1. Considerable variability in N2O emissions was found between sites, within orchards and treatment replicates and between seasons, reflected in the high standard errors of the means (Table 3). The magnitude of the maximum daily values more closely followed N2O emissions from Australian rainforests (Rowlings et al. 2012) than perennial tree crops receiving N fertiliser and irrigation (Pang et al. 2009; Rowlings et al. 2013; Xia et al. 2014; Ge et al. 2015). Pang et al. (2009) reported an average of 9.35 g N2O-N ha–1 day–1 and Ge et al. (2015) reported an average of a much higher 36 g N2O-N ha–1 day–1 for apple orchards in China – both were orders of magnitude above the average reported here. In another study of horticulture crops from sub-tropical soils, Huang et al. (2012) reported average daily N2O emissions of 4.6, 5.9 and 3.3 g N2O-N ha–1 day–1 for mango, custard apple and pineapple respectively.
The maximum daily flux from any of the four sites was 13.9 g N2O-N ha–1 day–1, which occurred in the inter-row where no N fertiliser or irrigation was applied. For all sites the maximum daily flux of the tree line, where N fertiliser and irrigation was applied, was in the range of 3.8–6.0 g N2O-N ha–1 day–1 (Table 3).The absence of large N2O emission pulses in this study is not considered to be an artefact of the sampling systems. While it is possible that static chamber sampling can miss pulse events, the frequency of sampling and reactive sampling around specific events (such as increased sampling in the spring and summer months following fertiliser application) in this study should have captured pulse events if they occurred. Furthermore, under the same sampling conditions, N2O pulses exceeding 20 g N2O-N ha–1 day–1 were observed in a companion study (N. Swarts unpubl. data) related to high fertiliser applications carried out by our research team. Therefore, we consider that the reported values for N2O emissions from the four orchards of this study accurately reflected the processes occurring in this land-use.
The estimated annual soil N2O emissions from four commercial perennial tree crop orchards in temperate south-east Australia varied within 0.30–0.74 kg N2O-N ha–1 year–1 (Fig. 3). These values represent very low annual N2O emissions compared with other cropping systems (Scheer et al. 2012, 2013; Shi et al. 2013; De Antoni Migliorati et al. 2014). In contrast, N2O emissions from apple orchards were reported as 3.22–44.30 kg N2O-N ha–1 year–1 in China (Pang et al. 2009; Xia et al. 2014). Furthermore, the results from this study are at the low end reported for grapes (0.56–3.92 kg N2O-N ha–1 year–1; Garland et al. 2014), and similar to those reported for almonds (0.53–0.80 kg N2O-N ha–1 year–1; Schellenberg et al. 2012).
Given the limitations of integrated scaling from manual chamber sampling (Smith and Dobbie 2001) as recognised above, this study suggests that Australian tree orchard systems are making relatively minor contributions to overall GHG emissions of the country; however, testing over multiple seasons and in a variety of orchard systems is required to further validate this finding. We propose that the low emissions found in this study were due to judicious management of irrigation and N fertiliser application through tree line drippers with regimes well suited to the local soils and tree uptake requirements.
The four commercial orchards, on which this study focussed, used frequent low-intensity irrigation via under tree drip or micro-sprinkler systems with fertiliser applied as multiple split applications. In this study, the VWC of the soil rarely exceeded field capacity as determined by the van Genuchten retention function at Ψ = –10 kPa, suggesting that the upper soil layers in the tree line would have remained aerobic for all but very short periods. Thus the irrigation management used across the four sites, which was based on objective soil water measures, would have minimised anaerobic soil conditions. This reduces the likelihood of denitrification being a major pathway for N2O emissions (Russow et al. 2000; Rashti et al. 2015). Instead, it is likely that nitrification is the main pathway for N2O emissions as oxygen availability is generally not limiting for the soil microorganisms when WFP is below 60–70% (Bollmann and Conrad 1998). This has additional significance in this study as 50–100% of the applied N was as NO3– (Table 1) and was not susceptible to loss via the nitrification pathway.
Across the four sites in this study, the rate of N fertiliser application was not a strong determinant of annual N2O emissions. For example, the NSW apple orchard received only 10% of the N fertiliser (15 kg N ha–1) applied to the Tasmania cherry orchard (150 kg N ha–1) but the N2O emissions (552 g N2O-N ha–1 year–1) were 75% of those from the Tasmania cherry orchard (736 g N2O-N ha–1 year–1). The use of multiple split N-fertiliser applications would have more closely matched the N requirement of the trees during the growing season (Tagliavini et al. 1996). The lack of correlation between the rate of N fertiliser application and the magnitude of subsequent N2O emissions in perennial crops was highlighted by Rowlings et al. (2013). The rate of N fertiliser clearly increases the risk of N2O emissions, but the actual emissions are more closely associated with soil environmental conditions than the quantity of fertiliser.
N2O emissions are biologically driven (Dalal et al. 2003) and it was expected that relatively low soil temperatures (<14°C for half the year) experienced at all four sites (i.e. temperate climate) and consistently distributed rainfall between seasons were likely to have played an important role in reducing N2O production. Soil temperature, well known to be a major determinant of emissions timing and intensity and consequently, was significantly (although weakly) associated with N2O emissions over the study period. Increased emissions were observed as expected, when soil temperatures were higher in three sites, with the exception of Young. At these three sites, the cherry crop at Lower Longley and the apple crops at Orange and Lucaston were not harvested until mid-February and late March respectively. This required considerable irrigation inputs that maintained relatively moist conditions in the orchard. In contrast, the cherry orchard at Young was harvested in late November and irrigation ceased completely at this time to conserve the limited water resource in the region. Although soil temperatures were the highest in Young in summer, soil moisture (VWC) was lowest, substantially limiting capacity for N2O production.
Spatial variation in N2O emissions is attributed to soil aeration as governed by gas diffusion (van der Weerden et al. 2012). This parameter is difficult to measure and so WFP is used as a proxy in field studies investigating N2O emissions (Pang et al. 2009; Rowlings et al. 2012); however, gas diffusion in soils depends on the relative volumetric fractions of water and air. Furthermore, volumes of air and water in soil at given values of WFP will be influenced by the bulk density of the soil (Castellano et al. 2010). As such, Farquharson and Baldock (2008) proposed that VWC is more appropriate for estimating the spatial and temporal variability of N2O emissions for different soils, and further supported by van der Weerden et al. (2012). In our study, relationships between VWC and WFP and N2O emissions were consistently weak but almost identical for each site, sampling location and season with the exception of winter, when VWC was more strongly correlated. This was not surprising, as the TP and bulk density used to calculate WFPS from VWC were similar between the tree line and inter-row for each site. VWC and WFP for the Lower Longley, Lucaston and the inter-row at Young showed an atypical significant negative (weak) influence on N2O emissions. Elevated emissions are linked to WFP > 60% (Chantigny et al. 1998; Helgason et al. 2005) and VWC > 0.55 cm3 cm–3 (van der Weerden et al. 2012), conditions rarely seen in the orchards during the study period. For these sites, during winter when WFP and VWC were highest, soil temperatures were extremely low and accounted for the negative correlation observed and the moderately negative correlation between VWC and N2O emissions in winter (r = –0.506). In stark contrast to this was the moderate positive correlation of VWC (r = 0.372) and WFP (r = 0.362) with N2O emissions in the tree line at Young, most likely due to the early application of fertiliser and generally warmer soil temperatures of the site during the winter–spring periods.
Low N2O emission rates were observed between late autumn through to early spring irrespective of the WFP and VWC (Table 4.). We attribute these emissions to the low soil temperatures which were in the range (on average) of 13−18°C over the four sites during the study period. Across the four sites, N2O emissions classified as moderate (10–100 g N2O-N ha–1 day–1) from European cool temperate soils (Conen et al. 2000) or 16–160 g N2O-N ha–1 day–1 from Australian tropical soils (Wang and Dalal 2010) were only observed for soil temperatures >14°C. However, as observed with other studies, single parameter correlations of soil environmental variables, while significant, only explained a small amount of the variation in N2O emissions due to the preconditions required before N2O emissions were observed (e.g. Ding et al. 2007; Schellenberg et al. 2012). Indeed, in this study, no consistent pattern emerged in the relationship between daily N2O flux and soil temperature and soil water measurements either in the tree line or inter-row, between seasons or sites. This may be due in part to the overall very low emissions captured during the course of the study period, the high spatial and temporal variability within the dataset and that there were multiple interacting factors in this complex biogeochemical process. The stepwise regression analysis captured <10% of the total variability of the Lucaston dataset. Splitting the analysis between the tree line and inter-row improved the predictive capacity up to 24% for the tree line but reduced it to <4% for the inter-row. Soil temperature was a significant key predictor in all three models; however, partial coefficients were still very weak with r2 < 0.072. The VWC was the best predictor in the tree line regression model, most likely due to the influence of irrigation via dripper lines over the apple growing season.
Tree line v. inter-row emissions
The 40% greater average N2O emissions from the grassed inter-row at all sites, compared with the tree line were unexpected (Fig. 2, Table 2). In all orchards in this study, N fertiliser and irrigation were applied only to the tree line. As a result, the higher substrate availability and soil moisture would have been expected to be more conducive to N2O emissions from the tree line. This should have been most pronounced in the cherry orchard where the equivalent of 150 kg N ha–1 was applied on the tree line along with irrigation, while no fertiliser or irrigation was applied to the inter-row. Despite this, average N2O emission in the inter-row was almost double that of the tree line at this site (2.44 and 1.31 g N2O-N ha–1 day–1 respectively).
Large N2O emissions from the inter-row were observed in a vineyard with a legume cover crop but not when the inter-row was bare fallowed (Garland et al. 2014). Increased N2O emissions are often observed due to the addition of C and N from plants, which stimulates nitrification and denitrification (Bouwman et al. 2002). Consequently, it is speculated that the grass sward in the orchard inter-rows would have increased the input of C into the soil and so stimulated greater microbial activity, particularly in the top 10 cm where the bulk of the grass root mass exists. This is consistent with the inter-rows having higher labile C in the Tasmanian orchards and rates of N mineralisation at the Lucaston orchard (Table 2). At the Tasmanian sites, the orchards were heavily mounded (up to 40 cm) by scraping the sandy loam topsoil from the inter-row into the mound, to facilitate drainage and soil volume in the tree line, while leaving a very shallow, bleached, low-C A2 horizon above a deep clay subsoil within the inter-row. These soils had little macroporosity, which with compaction by tractor traffic means that they had little capacity to drain or store soil water, as demonstrated by the 10-fold reduction in infiltration compared with topsoils in the tree line. Consequently, during rainfall or irrigation soils in the inter-row rapidly approached field capacity compared with the more porous high C soils in the tree line.
Implications for management
Overall, the contribution of the apple and cherry orchards of the current study to global warming potential associated with N2O emissions was very low on an area basis. When expressed on a yield basis, the emissions intensity was in the range of 1.6–3.2 and 4.6–6.4 kg CO2eq Mg−1 for apples and cherries respectively. By comparison the N2O derived emissions intensity of the major grain crops of wheat and maize were reported at 166 and 185 kg CO2eq Mg−1 respectively (Linquist et al. 2012), or 80.8 CO2eq Mg−1 for almond kernel (Schellenberg et al. 2012). The order of magnitude lower values estimated for apples and cherries were maintained even when the values were expressed on a dry matter basis, principally due to the low annual emissions from the orchard crops in this study. On a dry weight yield basis, the emissions intensity was in the range of 11.6–46.8 and 22.7–66.6 kg CO2eq Mg−1 for apples and cherries respectively. The benefits of yield-scaling to produce emissions intensity is that it takes into account both the need for food production and environmental impacts, in this case contribution to global warming. Clearly, comparing fresh fruit to grains and other more dried products is complicated by the differing moisture contents. At present, there is no overarching system to allow different food products to be compared. However, the use of emissions intensity will be useful for comparing across differing production systems for the same food product. As an example, the Cool Farm Tool (Hillier et al. 2011) is used across the processing tomato industry globally to consider the global warming potentials of differing production systems. Extension of this approach more widely in the fresh food sector is still in its infancy but should be encouraged, as exclusive use of area-based impacts can lead to low-yielding management practices in the pursuit of reducing environmental impacts.
Based on the findings of this study, perennial cropping systems such deciduous fruit tree cropping have substantially lower N2O emissions compared with other agricultural systems. It is highly likely that N2O emissions would be lower in these systems generally given the length of time soil temperatures are <13°C, as these crops require winter chill to break dormancy in spring (Greer et al. 2006). However, given that fertiliser application in deciduous tree cropping systems is generally post-harvest during the warmer summer–autumn months when soil temperatures are at their highest, potential remains to mitigate emissions through increased fertiliser use efficiency. Although not tested here, optimising N fertiliser inputs to meet tree demand will facilitate a reduction in both emissions production and N leaching below the root zone, as more N is utilised by the trees. Further research to test emissions production under optimised fertiliser regimes will enable better determination of mitigation strategies based on fertiliser management.
Improved management of the inter-row areas, where consistently higher N2O emissions were recorded, is possible – for example, bare fallow, but this will require consideration of the trade-off against potential increases in erosion and loss of soil C associated with bare fallow. Additionally, investigating the effect of growing cover crops, composting and side throwing of mower clippings and prunings to the tree line to increase soil C on N2O emissions is necessary. Improved drainage in areas of the orchard prone to waterlogging is likely to mitigate N2O emissions as well as using standard width machinery to minimise compacted areas of the inter-row.
Acknowledgements
The authors would like to thank the owners of the orchards for their considerable assistance during the monitoring. Andrew Griggs of Lucaston Park Orchards at Lucaston, Ross Affleck from Ridgy Didge Cherries at Lower Longley, Peter Darley from Day Dawn Orchard at Young and Geoff Hall from Geoff Hall Orchard in Orange. The project was funded under the Carbon Farming Futures program of the Federal Department of Agriculture, Fisheries and Forestry. Funding was also provided by Horticulture Innovation Australia Limited with co-investment from the Tasmanian Institute of Agriculture, Applied Horticulture Research and funds from the Australian Government. We acknowledge the two anonymous reviewers for their helpful comments on the earlier version of this manuscript.
References
Apple & Pear Australia Ltd (APAL) (2013) Guidelines for irrigation management for apple & pear growers. Available at http://apal.org.au/wp-content/uploads/2013/04/Apple_pear_guidelines_Irrigation_Management.pdf [verified 25 October 2015]Australian Bureau of Statistics (2012) Agriculture production. Available at http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/1301.0~2012~Main%20Features~Agricultural%20production~260 [verified 29 October 2015].
Australian Greenhouse Office (AGO) (2001) ‘National greenhouse gas inventory 1999 with methodology supplements.’ (AGO: Australia)
Aylward GH, Findlay TJV (1974) ‘SI chemical data.’ 2nd edn. (John Wiley and Sons: New York, NY)
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
Bollmann A, Conrad R (1998) Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biology 4, 387–396.
| Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils.Crossref | GoogleScholarGoogle Scholar |
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Global Biogeochemical Cycles 16, 1058
| Emissions of N2O and NO from fertilized fields: Summary of available measurement data.Crossref | GoogleScholarGoogle Scholar |
Castellano MJ, Schmidt JP, Kaye JP, Walker C, Graham CB, Lin H, Dell CJ (2010) Hydrological and biogeochemical controls on the timing and magnitude of nitrous oxide flux across an agricultural landscape. Global Change Biology 16, 2711–2720.
| Hydrological and biogeochemical controls on the timing and magnitude of nitrous oxide flux across an agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Chantigny MH, Prevost D, Angers DA, Simard RR, Chalifour FP (1998) Nitrous oxide production in soils cropped to corn with varying N fertilization. Canadian Journal of Soil Science 78, 589–596.
| Nitrous oxide production in soils cropped to corn with varying N fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXls1Smug%3D%3D&md5=fafdf240b1b1888708db8651d1e7a81eCAS |
Conen F, Dobbie K, Smith K (2000) Predicting N2O emissions from agricultural land through related soil parameters. Global Change Biology 6, 417–426.
| Predicting N2O emissions from agricultural land through related soil parameters.Crossref | GoogleScholarGoogle Scholar |
Cresswell H, Hamilton G (2002) Bulk density and pore space relations. In ‘Soil physical measurement and interpretation for land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 35–58. (CSIRO Publishing: Melbourne)
Curtin D, Campbell C (2008) Mineralizable nitrogen. In ‘Soil sampling and methods of analysis’. 2nd edn. (Eds MR Carter, EG Gregorich) pp. 599–606. (CRC Press: Boca Raton, FL)
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
Davidson EA (1992) Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Science Society of America Journal 56, 95–102.
| Sources of nitric oxide and nitrous oxide following wetting of dry soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XitFemsLw%3D&md5=2879aa5abd2dd8835f4de3772c798c33CAS |
De Antoni Migliorati M, Bell MJ, Grace PR, Rowlings DW, Scheer C, Strazzabosco A (2014) Assessing agronomic and environmental implications of different N fertilisation strategies in subtropical grain cropping systems on Oxisols. Nutrient Cycling in Agroecosystems 100, 369–382.
| Assessing agronomic and environmental implications of different N fertilisation strategies in subtropical grain cropping systems on Oxisols.Crossref | GoogleScholarGoogle Scholar |
Denmead O, Macdonald B, Bryant G, Naylor T, Wilson S, Griffith DW, Wang W, Salter B, White I, Moody P (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agricultural and Forest Meteorology 150, 748–756.
| Emissions of methane and nitrous oxide from Australian sugarcane soils.Crossref | GoogleScholarGoogle Scholar |
Ding WX, Meng L, Cai ZC, Han FX (2007) Effects of long-term amendment of organic manure and nitrogen fertilizer on nitrous oxide emission in a sandy loam soil. Journal of Environmental Sciences (China) 19, 185–193.
| Effects of long-term amendment of organic manure and nitrogen fertilizer on nitrous oxide emission in a sandy loam soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjt1SmtLk%3D&md5=f7b36d274c35702a03f977d24e21b7c0CAS |
Farquharson R, Baldock J (2008) Concepts in modelling N2O emissions from land use. Plant and Soil 309, 147–167.
| Concepts in modelling N2O emissions from land use.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVyhs7w%3D&md5=b5d35e5e7b068d830cdd8d26705d8cc2CAS |
Garland GM, Suddick E, Burger M, Horwath WR, Six J (2014) Direct N2O emissions from a Mediterranean vineyard: event-related baseline measurements. Agriculture, Ecosystems & Environment 195, 44–52.
| Direct N2O emissions from a Mediterranean vineyard: event-related baseline measurements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVyhsb3K&md5=6ecb5a8ce6a58dbf429e50094d5fc376CAS |
Ge S, Jiang Y, Wei S (2015) Gross nitrification rates and nitrous oxide emissions in an apple orchard soil in northeast China. Pedosphere 25, 622–630.
| Gross nitrification rates and nitrous oxide emissions in an apple orchard soil in northeast China.Crossref | GoogleScholarGoogle Scholar |
George AP (1999) Deciduous fruit production in Australia. Available at http://www.fao.org/docrep/004/ab985e/ab985e04.htm#bm04 [verified 25 October 2015]
Greer DH, Wünsche JN, Norling CL, Wiggins HN (2006) Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburne’™ (Malus domestica) apple trees. Tree Physiology 26, 105–111.
| Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburne’™ (Malus domestica) apple trees.Crossref | GoogleScholarGoogle Scholar | 16203720PubMed |
Helgason B, Janzen H, Chantigny M, Drury C, Ellert B, Gregorich E, Lemke R, Pattey E, Rochette P, Wagner-Riddle C (2005) Toward improved coefficients for predicting direct N2O emissions from soil in Canadian agroecosystems. Nutrient Cycling in Agroecosystems 72, 87–99.
| Toward improved coefficients for predicting direct N2O emissions from soil in Canadian agroecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKitrfK&md5=5e7500a1f945cf1f942399fdd0978636CAS |
Hillier J, Walter C, Malin D, Garcia-Suarez T, Mila-i-Canals L, Smith P (2011) A farm-focused calculator for emissions from crop and livestock production. Environmental Modelling & Software 26, 1070–1078.
| A farm-focused calculator for emissions from crop and livestock production.Crossref | GoogleScholarGoogle Scholar |
Huang X, Grace P, Weier K, Mengersen K (2012) Nitrous oxide emissions from subtropical horticultural soils: a time series analysis. Soil Research 50, 596–606.
| Nitrous oxide emissions from subtropical horticultural soils: a time series analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12jsrjM&md5=8a983a7399826bf47f3be4d630623e54CAS |
IPCC (2001) Climate change 2001: the scientific basis. In ‘The climate change contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change’. (Eds J Houghton, Y Ding, D Griggs, M Noguer, P Van der Linden, X Dai, K Maskell, C Johnson) p. 881. (Cambridge University Press: Cambridge)
Isbell RF (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Linquist B, van Groenigen KJ, Adviento-Borbe MA, Pittelkow C, van Kessel C (2012) An agronomic assessment of greenhouse gas emissions from major cereal crops. Global Change Biology 18, 194–209.
| An agronomic assessment of greenhouse gas emissions from major cereal crops.Crossref | GoogleScholarGoogle Scholar |
Millard P (1996) Ecophysiology of the internal cycling of nitrogen for tree growth. Zeitschrift für Pflanzenernährung und Bodenkunde 159, 1–10.
Millard P, Neilsen GH (1989) The influence of nitrogen supply on the uptake and remobilization of stored N for the seasonal growth of apple trees. Annals of Botany 63, 301–309.
Mualem Y (1976) A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resources Research 12, 513–522.
| A new model for predicting the hydraulic conductivity of unsaturated porous media.Crossref | GoogleScholarGoogle Scholar |
Neilsen D, Millard P, Neilsen GH, Hogue EJ (1997) Sources of N for leaf growth in a high-density apple (Malus domestica) orchard irrigated with ammonium nitrate solution. Tree Physiology 17, 733–739.
| Sources of N for leaf growth in a high-density apple (Malus domestica) orchard irrigated with ammonium nitrate solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmvFKgu7Y%3D&md5=c1fb33934435da49484c10f21b90f6dcCAS | 14759898PubMed |
Pang JZ, Wang XK, Mu YJ, Ouyang ZY, Liu WZ (2009) Nitrous oxide emissions from an apple orchard soil in the semiarid Loess Plateau of China. Biology and Fertility of Soils 46, 37–44.
| Nitrous oxide emissions from an apple orchard soil in the semiarid Loess Plateau of China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlymu7nE&md5=c0fbd62f9b90f71950810ed9b051a221CAS |
Peters A, Durner W (2008) Simplified evaporation method for determining soil hydraulic properties. Journal of Hydrology 356, 147–162.
| Simplified evaporation method for determining soil hydraulic properties.Crossref | GoogleScholarGoogle Scholar |
Prather M, Derwent R, Ehhalt D, Fraser P, Sanhueza E, Zhou X (1995) Other trace gases and tmospheric. In ‘Climate change, 1994: radiative forcing of climate change and an evaluation of the IPCC IS92 emission scenarios’. (Eds J Houghton, LG Meira Filho, BJ Hoesung Lee, BA Callander, E Haites, N Harris, K Maskrell) p. 73. (University of Cambridge: Cambridge)
Ramaswamy V, Boucher O, Haigh J, Hauglustine D, Haywood J, Myhre G, Nakajima T, Shi G, Solomon S (2001) Radiative forcing of climate change. In ‘Climate change 2001: the scientific basis: contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change’. (Eds J Houghton, Y Ding, D Griggs, M Noguer, P Van der Linden, X Dai, K Maskell, C Johnson) pp. 349–416. (Cambridge University Press: Cambridge, UK)
Rashti MR, Wang WJ, Harper SM, Moody PW, Chen CR, Ghadiri H, Reeves SH (2015) Strategies to mitigate greenhouse gas emissions in intensively managed vegetable cropping systems in subtropical Australia. Soil Research 53, 475–484.
| Strategies to mitigate greenhouse gas emissions in intensively managed vegetable cropping systems in subtropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtl2qurnO&md5=6c8b449de2ffbef6e20cd50cf258c3d4CAS |
Reynolds WD (2008) Saturated hydraulic properties: Ring infiltrometer. In ‘Soil sampling and methods of analysis’. 2nd edn. (Eds MR Carter, EG Gregorich) Ch. 77, pp. 1043–1056. (Canadian Society of Soil Science, CRC Press: Boca Raton)
Reynolds WD, Elrick DE (1990) Ponded infiltration from a single ring: I. Analysis of steady flow. Soil Science Society of America Journal 54, 1233–1241.
| Ponded infiltration from a single ring: I. Analysis of steady flow.Crossref | GoogleScholarGoogle Scholar |
Rowlings DW, Grace PR, Kiese R, Weier KL (2012) Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Global Change Biology 18, 726–738.
| Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Rowlings D, Grace P, Scheer C, Kiese R (2013) Influence of nitrogen fertiliser application and timing on greenhouse gas emissions from a lychee (Litchi chinensis) orchard in humid subtropical Australia. Agriculture, Ecosystems & Environment 179, 168–178.
| Influence of nitrogen fertiliser application and timing on greenhouse gas emissions from a lychee (Litchi chinensis) orchard in humid subtropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1WiurzM&md5=1f09e92e7c0b3b52f1ec5767e8704567CAS |
Russow R, Sich I, Neue H-U (2000) The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: results of kinetic 15N tracer investigations. Chemosphere. Global Change Science 2, 359–366.
| The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: results of kinetic 15N tracer investigations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXos1egs7g%3D&md5=da95549dade311e86742a6147ce1d53bCAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant and Soil 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=ee89302220febfea18060a4a6d021cc2CAS |
Schellenberg DL, Alsina MM, Muhammad S, Stockert CM, Wolff MW, Sanden BL, Brown PH, Smart DR (2012) Yield-scaled global warming potential from N2O emissions and CH4 oxidation for almond (Prunus dulcis) irrigated with nitrogen fertilizers on arid land. Agriculture, Ecosystems & Environment 155, 7–15.
| Yield-scaled global warming potential from N2O emissions and CH4 oxidation for almond (Prunus dulcis) irrigated with nitrogen fertilizers on arid land.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xnt12ktr0%3D&md5=10dc93769e6370fbdc41419e54d16b13CAS |
Shi Y, Wu W, Meng F, Zhang Z, Zheng L, Wang D (2013) Integrated management practices significantly affect N2O emissions and wheat–maize production at field scale in the North China Plain. Nutrient Cycling in Agroecosystems 95, 203–218.
| Integrated management practices significantly affect N2O emissions and wheat–maize production at field scale in the North China Plain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru7Y%3D&md5=0311ed4e8785febde4c5cd8844eb10a3CAS |
Smith KA, Dobbie KE (2001) The impact of sampling frequency and sampling times on chamber‐based measurements of N2O emissions from fertilized soils. Global Change Biology 7, 933–945.
| The impact of sampling frequency and sampling times on chamber‐based measurements of N2O emissions from fertilized soils.Crossref | GoogleScholarGoogle Scholar |
Tagliavini M, Scudellazi D, Marangoni B, Toselli M (1996) Nitrogen fertilization management in orchards to reconcile productivity and environmental aspects. Fertilizer Research 43, 93–102.
| Nitrogen fertilization management in orchards to reconcile productivity and environmental aspects.Crossref | GoogleScholarGoogle Scholar |
Tiedje JM (1994) Denitrifiers. In ‘Methods of soil analysis: Part 2. Microbiological and biochemical properties’. (Eds RW Weaver, A J. S. and PS Bottomley) pp. 245–267. (Soil Science Society of America: Madison, WI)
US EPA (1993) ‘Method 353.2: determination of nitrate-nitrite nitrogen by automated colorimetry.’ Revision 2. (US Environmental Protection Agency: Cincinnati, Ohio)
US EPA (2006) ‘Global anthropogenic non-CO2 greenhouse gas emissions: 1990–2020.’ (United States Environmental Protection Agency, EPA 430-R-06–003: Washington DC)
van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Science Society of America Journal 44, 892–898.
| A closed-form equation for predicting the hydraulic conductivity of unsaturated soils.Crossref | GoogleScholarGoogle Scholar |
van der Weerden TJ, Kelliher FM, de Klein CAM (2012) Influence of pore size distribution and soil water content on nitrous oxide emissions. Soil Research 50, 125–135.
van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010) Influence of biochars on flux of N2O and CO2 from Ferrosol. Australian Journal of Soil Research 48, 555–568.
| Influence of biochars on flux of N2O and CO2 from Ferrosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Sru7bP&md5=0443a82a78f914d6c5394c63056979b5CAS |
Wang WJ, Dalal RC (2010) Assessment of the boundary line approach for predicting N2O emission ranges from Australian agricultural soils. In ‘19th World Congress of Soil Science, Soil Solutions for a Changing World’. (N2O Network: Brisbane, Australia)
Weil RR, Islam KR, Stine MA, Gruver JB, Samson-Liebig SE (2003) Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use. American Journal of Alternative Agriculture 18, 3–17.
| Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use.Crossref | GoogleScholarGoogle Scholar |
Xia Y, She D, Li Y, Yan X (2014) Impact of sampling time on chamber-based measurements of riverine nitrous oxide emissions using relative difference analysis. Geoderma 214–215, 197–203.
| Impact of sampling time on chamber-based measurements of riverine nitrous oxide emissions using relative difference analysis.Crossref | GoogleScholarGoogle Scholar |



