Mitigation of N2O emissions from surface-irrigated cropping systems using water management and the nitrification inhibitor DMPP
Hizbullah Jamali A B E , Wendy Quayle A B , Clemens Scheer C and Jeff Baldock DA CSIRO Agriculture, PMB 3, Griffith, NSW 2680, Australia.
B Centre for Regional and Rural Futures (CeRRF), Deakin University, Griffith, NSW 2680, Australia.
C Queensland University of Technology, Brisbane, Qld 4000, Australia.
D CSIRO Agriculture, PMB 2, Glen Osmond, SA 5064, Australia.
E Corresponding author. Email: hiz.jamali@csiro.au; hizbjamali@gmail.com
Soil Research 54(5) 481-493 https://doi.org/10.1071/SR15315
Submitted: 29 October 2015 Accepted: 11 March 2016 Published: 21 June 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Soils under irrigated agriculture are a significant source of nitrous oxide (N2O) owing to high inputs of nitrogen (N) fertiliser and water. This study investigated the potential for N2O mitigation by manipulating the soil moisture deficit through irrigation scheduling in combination with, and in comparison to, using the nitrification inhibitor, 3,4-dimethylpyrazole phosphate (DMPP). Lysimeter cores planted with wheat were fitted with automated chambers for continuous measurements of N2O fluxes. Treatments included conventional irrigation (CONV), reduced deficit irrigation (RED), CONV-DMPP and RED-DMPP. The total seasonal volume of irrigation water applied was constant for all treatments but the timing and quantity in individual irrigation applications varied among treatments. 15N-labelled urea was used to track the source of N2O emissions and plant N uptake. The majority of N2O emissions occurred immediately after irrigations began on 1 September 2014. Applying RED and DMPP individually slightly decreased N2O emissions but when applied in combination (RED-DMPP) the greatest reductions in N2O emissions were observed. There was no effect of treatments on plant N uptake, 15N recovery or yield possibly because the system was not N limited. Half of the plant N and 53% to 87% of N2O was derived from non-fertiliser sources in soil, highlighting the opportunity to further exploit this valuable N pool.
Additional keywords: irrigation, leaching, nitrogen-15 isotope, nitrous oxide, wheat.
Introduction
It is predicted that irrigated agricultural systems will produce two-thirds of future global food demand because of their potential for higher productivity (FAO 2010). In 2007–08, irrigated agricultural land comprised less than 0.5% of total agricultural land in Australia but produced 28% of the total gross value of agricultural production (ABS 2010). Higher inputs of water and fertiliser that drive higher productivity in irrigated systems also result in a higher potential for emissions of the highly potent greenhouse gas, nitrous oxide (N2O). Nitrification and denitrification are the main biological processes responsible for N2O emissions from soils although several other processes may also contribute (Butterbach-Bahl et al. 2013; Firestone and Davidson 1989; Mosier et al. 1998). Nitrification is the oxidation of ammonium to nitrite (NO2–) or nitrate (NO3–) under aerobic conditions, which not only results in N2O emissions but also provides substrate (nitrate) for denitrification. Denitrification is the biological reduction of NO3– to NO2–, nitric oxide (NO), N2O and dinitrogen (N2) under anaerobic conditions (Bouwman 1998).
In irrigated arable systems, the cumulative N2O emissions from soil during a crop season are dominated by episodes of high N2O emissions observed after irrigation (Jamali et al. 2015; Liu et al. 2011; Scheer et al. 2012). These elevated N2O peaks observed after water application may contribute up to 90% of total N2O emissions (Scheer et al. 2008). Such episodes of high N2O emissions are mainly derived from denitrification, which occurs under low soil oxygen levels, generally corresponding with high soil water contents, and results in denitrifying bacteria using NO3– as an alternate electron acceptor (Firestone and Davidson 1989). As soil redox potential and oxygen levels are regulated by soil water status, the irrigated systems may provide an opportunity to mitigate N2O emissions by adjusting the timing and quantity of irrigation water (Scheer et al. 2008).
The total amount of irrigation applied to a crop primarily depends on the season, soil type, rainfall, and plant species. In the southern Murray–Darling Basin, Australia, two to three spring irrigations of ~100 mm are generally recommended to ensure good yields from irrigated wheat (Dunn 2014). Such large irrigation applications have resulted in significant losses of N and water through leaching, and large N2O emissions in an irrigated sorghum crop (Jamali et al. 2015). In an irrigated wheat crop, the amount of NO3– lost through denitrification was directly proportional to the time span of waterlogged conditions following irrigation (Humphreys et al. 1991). Waterlogged soil conditions following irrigation can also affect crop growth depending on the duration and timing of waterlogging (Grieve et al. 1986; Melhuish et al. 1991; North 2012). In a previous field study located locally to this study on a similar soil type, flooded soil conditions for ≥48 h resulted in a significant decline in wheat yield despite sufficient N availability (Melhuish et al. 1991). Such decline in yield of irrigated wheat has been linked to the detrimental effects of low oxygen levels created by prolonged waterlogged conditions on root growth (Meyer et al. 1985). Thus, minimising the duration of waterlogged conditions through improved irrigation practices may provide an opportunity to improve yield (Melhuish et al. 1991) and decrease N losses from leaching and denitrification, including N2O emissions. The period of waterlogged conditions can be minimised by improving irrigation layouts or applying irrigation in smaller quantities, with reduced deficits. In a recent study, a significant decrease in N2O emissions and leaching losses was achieved when irrigation was applied in smaller, frequent events compared with conventional practice of fewer, larger irrigation events while keeping total seasonal water application constant for a grain sorghum summer crop (Jamali et al. 2015). However, this approach has not been used in partially-irrigated winter crops, such as wheat, which can be an important component of irrigated double cropping systems in the southern Murray–Darling Basin.
Urea (CH4N2O) is the most common nitrogen (N) fertiliser used for topdressing agricultural crops. It hydrolyses to form ammonium (NH4+) which is available for plant uptake. The NH4+, however, is rapidly oxidised by nitrifying bacteria to form nitrate (NO3–), increasing the risk of N loss through leaching and gaseous N losses as N2 and N2O. Thus, nitrification of NH4+ derived from ammonium-based fertilisers can decrease the amount of N potentially available to plants through leaching losses. It also causes environmental concern by increasing the NO3– levels in the hydrosphere available for transformation to N2O and further loss to the atmosphere. Nitrification following urea hydrolysis may be decreased by applying nitrification inhibitors to soil. These are chemicals containing compounds known for depressing the activity of Nitrosomas bacteria that is responsible for oxidation of NH4+ to nitrite (NO2–) during nitrification (Zerulla et al. 2001). Nitrification inhibitors can decrease N2O emissions directly by slowing nitrification, and indirectly by limiting the NO3– substrate available for denitrification (Bremner and Blackmer 1978; de Klein et al. 1996; Linzmeier et al. 2001). Benefits to crop yield are also expected as inhibiting nitrification may lessen the risk of NO3– losses via leaching (Wu et al. 2007) and N2 losses via denitrification, especially following irrigation and rain events, therefore ensuring more N remains available to plants (Freney et al. 1992; Pasda et al. 2001). Consequently, it has been hypothesised that using nitrification inhibitors may improve fertiliser use efficiency and provide the opportunity to apply fertiliser earlier (Linzmeier et al. 2001). However, the effectiveness of nitrification inhibitors can vary with the type of inhibitor used, soil type, region, cropping system, pH and temperature (Liu et al. 2015; Menéndez et al. 2012; Suter et al. 2014).
This study aimed to investigate the effect of irrigation management and the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on controlling N2O emissions, leaching losses and plant N uptake in an irrigated wheat crop using automated chambers and a lysimeter core system. The experimental design allowed quantification of potential N2O mitigation through irrigation schedule manipulation compared with chemical inhibitors and their interactions. It was hypothesised that applying irrigation in smaller, frequent applications will decrease N2O emissions and leaching losses. Similarly, it was hypothesised that DMPP application will decrease N2O emissions by suppressing nitrification and thereby decreasing the available NO3– substrate for denitrifying bacteria. By decreasing N losses via leaching and denitrification, gains in plant N uptake can be expected. The results may be used for more informed decision making by farmers and agronomists to improve nitrogen use efficiency and gross margin analysis.
Materials and methods
Climate
The average daily temperature, maximum temperature and minimum temperature during the wheat growing season, as measured by an on-site weather station, were 13.1°C, 20.9°C and 5.5°C, respectively (Fig. 1). Of 116 mm total rainfall received during the growing season, 65 mm was received prior to irrigations beginning on 1 September 2014 (Fig. 1). Most of the rainfall received after irrigations began was excluded using the lysimeter roof to isolate the effects of irrigation on N2O emissions and other related processes.

|
Experimental design
This study was conducted at the drainage lysimeter facility (Fig. 2) in Griffith, New South Wales as described in detail by Jamali et al. (2015). Sixteen intact lysimeter cores (0.7 m diameter × 1.2 m height) were collected from Willbriggie (34°28ʹ48ʹʹS, 145°56ʹ60ʹʹE), ~20 km south of Griffith, New South Wales, Australia in November 2013. The soil type, locally known as Willbriggie clay loam, is classified as a Chromosol (Isbell 2002) and chemical properties are given in Table 1. The bulk density at 0–10 cm depth is 1.45 g cm–3 increasing to 1.74 g cm–3 at 40–50 cm depth, which corresponds with the highest clay content (60.4%) at this depth (Table 1). The bulk density and clay content decrease to 1.45 g cm–3 and 44.7%, respectively, at 90–100 cm depth. Soil pH was 6.83 at 0–10 cm depth increasing slightly with depth to a maximum of 7.37 at 90–100 cm depth (Table 1). Grain sorghum was grown in these cores in the preceding summer (December 2013 to April 2014) using the same irrigation and fertiliser rates in all cores. The cores were left fallow between the sorghum harvest in April 2014 and wheat sowing (this experiment) in June 2014. Sorghum was harvested at ground level resulting in minimal stubble and roots were not removed from the soil.

|

|
EnviroPro® probes (MEA, Magill, SA, Australia) linked to data loggers (Campbell Scientific Inc.) were installed in 12 of the lysimeter cores allowing hourly measurements of soil volumetric water content (VWC) and soil temperature at 0.1 m intervals throughout the profile to a depth of 1.2 m. The remaining four cores were used for temporal destructive soil sampling.
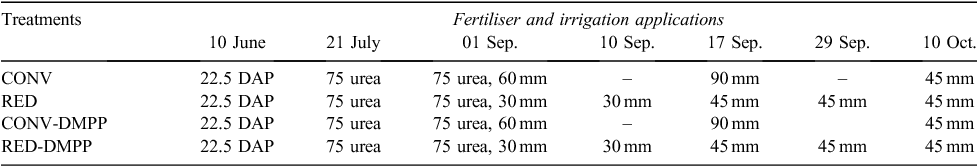
Following conventional practice in the region, all cores were irrigated in May 2014 when irrigation water was still available and allowed to drain to ensure a full soil profile at sowing. In the commercial setting, irrigation water becomes unavailable during June, July and August and crops rely solely on rainfall during this period. Typically a spring irrigation may be applied in early September depending upon irrigation water availability and soil moisture conditions. Pre-sowing irrigation of the lysimeter cores and resultant leaching also allowed the soils to equilibrate before the start of the experiment. Prior to sowing, 22.5 kg N ha–1 of diammonium phosphate (DAP) was incorporated in the 0–5 cm soil depth in all cores (Table 2). A dwarf variety (Maringa Rht3) of wheat (Triticum aestivum) was sown on 10 June 2014 and harvested on 19 November 2014. Wheat was sown at 5 cm depth at the recommended rate of 100 kg seed ha–1 resulting in ~180 plants m–2 after accounting for 95% germination and an establishment rate of 80%. Treatments included conventional irrigation (CONV), reduced deficit irrigation (RED), conventional irrigation with DMPP (CONV-DMPP) and reduced deficit irrigation with DMPP (RED-DMPP). Each treatment had three replicate cores. The cumulative amount of irrigation water for the entire wheat season was kept constant for all cores. However, the timing and amount of water in irrigation events varied among treatments (Table 2). In the post-sowing period, 75 kg N ha–1 of 15N-labelled urea (60% enriched) was applied at six-leaf stage (21 July 2014) and a second application of 75 kg N ha–1 at flag-leaf stage on 1 September 2014 (Table 2). Urea was dissolved in 1 L of irrigation water and sprinkled on the soil surface using a watering can. For the nitrification inhibitor treatments, a 17.6% dimethyl pyrazole (DMP) solution, provided by Incitec Pivot®, Australia, was mixed with urea and 1 L of water and sprinkled on the soil surface.
|
Automated N2O measurements
Twelve lysimeter cores were fitted with fully automated chambers covering the entire surface area of the cores for measuring N2O fluxes from the soil surface as described by Jamali et al. (2015). The original height of the chambers was 0.3 m which was increased to 0.6 m using an extension made from the same material (acrylic) to accommodate the growing plants. The automated chambers were connected to a sampling unit and a gas chromatograph (GC, SRI 8610, Torrance, CA, USA) fitted with an electron capture detector (ECD) and a flame ionisation detector (FID) for analysing N2O and CH4, respectively. Chambers were divided into three sets of four chambers each, with each set closing for 60 min during a measurement cycle. Each chamber was sampled sequentially at 15 min intervals thus collecting four samples per hour per chamber. Three hours were required to complete samples from all 12 chambers. Nitrous oxide fluxes were calculated using the slope of linear change in concentration of N2O in four gas samples, and corrected for chamber pressure and temperature using the ideal gas law.
Manual 15N2O measurements
Gas samples were collected manually for 15N2O determination following 15N-labelled urea application on 21 July 2014. Chambers were closed for three hours and gas samples collected at 0 and 3 h after chamber closure. Manual chambers were closed for a longer period (three hours) than automated chambers (one hour) to capture the expected low fluxes of 15N2O as observed during pre-testing. A rainout shelter was drawn across during manual sampling as it offered some shading and decreased overheating within the chambers.
Nitrous oxide emissions derived from 15N-labelled urea were determined using a SERCON 20–22 Isotope Ratio Mass Spectrometer with a CryoPrep Trace Gas Module and calculated as below:
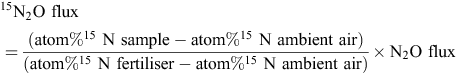
where N2O flux is that measured by the automated system. Cumulative 15N2O emissions from 12 to 26 August 2014 were calculated by integrating daily 15N2O fluxes and using averages, separately calculated for pre-irrigation and post-irrigation phases, for the days when 15N2O flux was not measured.
Temporal soil sampling
Four additional cores (i.e. one per treatment), not fitted with chambers, were used to monitor the temporal changes in soil chemistry. Soil was sampled from the 0–10 cm depth fortnightly or on strategic occasions using a steel corer (2.5 diameter cm × 10 cm height) and analysed for NH4+, NO3–, dissolved organic carbon (DOC) and pH. A plastic tube of the same dimensions as the extracted soil core was inserted in to the holes created by soil sampling and filled with soil collected from the same site to minimise the effect on soil hydrological properties that could alter temporal N and C dynamics.
Plant N uptake and 15N recovery
At the end of the growing season, wheat plants were harvested from all chambers, dried at 60°C for 48 h and separated into grain and straw. To measure the residual 15N in soil at the end of the growing season, three soil cores of 10 cm diameter were collected from all lysimeters at 10 cm intervals to full core depth. The soil collected from each depth was homogenised by mixing and larger roots were excluded from these samples. The plant and soil samples were ground and analysed for total N using a Shimadzu® TOC-L analyser and for 15N using a Thermo-Finnigan Delta V Plus Isotope Ratio Mass Spectrometer. The recovery of 15N in wheat plant samples was calculated using standard methods (IAEA 2001) as below:
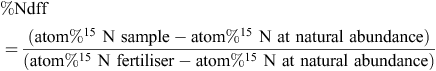
where %Ndff is the fraction of N derived from fertiliser. The recovery of 15N was calculated separately for grain and straw and integrated to calculate the total 15N in recovery in wheat shoots.
Leachate measurements
Leachate was collected at least once a week using 20 L buckets that were attached to the drainage outlets of the cores using an L-shaped PVC pipe. For each measurement, a 50 mL leachate sample was filtered through a Whatman® 42 filter paper and frozen for mineral N analysis using the same method as for KCl soil extracts.
Calculations and statistical analyses
Daily N2O flux for a treatment was calculated by taking the average of hourly flux measurements from all chambers within that treatment. Total N2O emissions for the whole season were calculated by integrating the daily N2O fluxes. For statistical analyses, the N2O flux data were transformed using log10 to improve the residual normality. The significant difference among treatments was analysed using one-way analysis of variance (ANOVA). Tukey’s post-hoc test was used to check the significant difference among individual treatments. The differences in yield, biomass, plant N uptake and leaching were also analysed using ANOVA.
Results
Temporal N2O fluxes
From sowing to six-leaf stage (21 July 2014)
Although all lysimeter cores were treated in the same way from sowing until six-leaf stage (Table 2), there were significant differences (P < 0.001) in N2O fluxes among treatments (Table 3). Highest cumulative N2O emissions were observed from RED treatment, which significantly exceeded the other treatments by factors of 1.7 to 5.5 (Table 3). The variability in N2O emissions in this period was mainly a result of high emissions from one replicate core in each of the RED and CONV-DMPP treatments from sowing until the first week of July as shown by the large standard deviations (Fig. 3). However, the N2O fluxes were similar among all treatments from the first week of July until the six-leaf stage.

|
From six-leaf stage to flag-leaf stage
In this period, DMPP was the only variable as irrigation applications commenced from 1 September 2014, while urea was applied at the same rate to all cores (Table 2). Thus, treatments were divided into two groups, with or without DMPP, for this period of measurements. Daily N2O emissions generally remained low (≤4.2 g N2O-N ha–1 day–1) in all treatments in this period despite ample mineral N availability because of urea or urea + DMPP application on 21 July 2014 (Fig. 3; Table 4). The treatments with DMPP showed a decrease in N2O emissions compared with those without DMPP although differences were relatively smaller, with the exception of RED-DMPP that continued to show significantly (P < 0.001) lower N2O emissions than other treatments (Table 3).
From flag-leaf stage to harvest
All of the irrigation water was applied in the period 1 September to 18 November 2014. The first irrigation also coincided with the second urea or urea + DMPP application of 75 kg N ha–1 applied at flag-leaf stage (1 September 2014). The N2O emissions in this period represented 47% (RED) to 70% (CONV) of total N2O emitted during the entire growing season (Table 3). The average N2O emission (i.e. cumulative N2O emissions in this period divided by the number of days) was also highest in this period, with the exception of the RED treatment where high N2O emissions were observed in the first two weeks of the growing season (Fig. 3). The greatest and lowest amount of cumulative N2O emissions in this period were observed from the CONV and RED-DMPP treatments, respectively (Table 3). The total N2O emissions in the CONV treatment in this period were greater than those in the RED treatment by a factor of 1.3, although this differences was not significant (Table 3). The N2O emissions in the CONV and RED treatments exceeded the N2O emissions from the corresponding treatments with DMPP by a factor of 1.6, but the differences again were not significant (Table 3).
In the CONV treatment, the 60 mm irrigation on 1 September 2014, which coincided with the urea application (75 kg N ha–1), resulted in a slight increase in N2O emissions to a maximum of 4.5 ± 2.9 g N2O-N ha–1 day–1 at a corresponding VWC of 37% (Figs 3a, 4a). The irrigation of 90 mm on 17 September 2014 resulted in the highest average flux of 55 ± 73 g N2O-N ha–1 day–1 from the CONV treatment, corresponding with the highest VWC of 45% (Figs 3a, 4a). The high N2O emissions dropped to ≤6 g N2O-N ha–1 day–1 from 21 September 2014 with a corresponding drop in VWC to ≤35% (Figs 3a, 4a).

|
In the CONV-DMPP treatment, the irrigation application of 60 mm, coinciding with the application of urea and DMPP, resulted in a short-lived N2O peak of 13 ± 17 g N2O-N ha–1 day–1 at the corresponding VWC of 32% (Figs 3c, 4c). The irrigation of 90 mm on 17 September 2014 resulted in emissions of 23 ± 25 g N2O-N ha–1 day–1 at a corresponding VWC of 37% (Figs 3c, 4c).
In the RED treatment, the N2O fluxes were generally smaller compared with the CONV treatments with the highest N2O peaks of ≤ 8 g N2O-N ha–1 day–1 observed following irrigation (Fig. 3b). The highest VWC of 35% was observed following irrigations of 45 mm on 17 and 29 September 2014 (Fig. 4). In the RED-DMPP treatment, the N2O fluxes remained low with a maximum flux of 5 ± 2 g N2O-N ha–1 day–1 (Fig. 3).
Total N2O emissions
The total N2O emissions over the entire growing season were in the order RED > CONV > CONV-DMPP > RED-DMPP (Table 3). The total N2O emissions in the RED-DMPP treatment were significantly lower than other treatments by factors of 1.7–2.3, although differences among other treatments were not significant (Table 3).
N2O emissions derived from 15N-labelled urea
All the results presented in this section are for the period starting 21 July 2014 when 15N-labelled urea or urea + DMPP was applied to the lysimeter cores. The fluxes of 15N-N2O were measured on 18 occasions in this period. The daily 15N-N2O fluxes showed large temporal variations and were generally elevated in the period immediately after beginning irrigation i.e. from 1 September 2014 (Fig. 5). In the conventional irrigation treatments, the highest daily 15N-N2O emissions (averaged for three replicate cores ± standard deviation) were observed on 17 September 2014 after 90 mm irrigation resulting in fluxes of 6.6 ± 6.5 g N2O-N ha–1 in CONV and 4.1 ± 5.8 g N2O-N ha–1 day–1 in CONV-DMPP treatment (Fig. 5). In the reduced deficit irrigation treatments, the highest 15N-N2O emissions were observed on 29 September 2014 following an irrigation of 45 mm which generated 15N-N2O peaks of 3.5 ± 4.3 g N2O-N ha–1 day–1 in RED and 0.9 ± 0.8 g N2O-N ha–1 day–1 in the RED-DMPP treatment (Fig. 5).

|
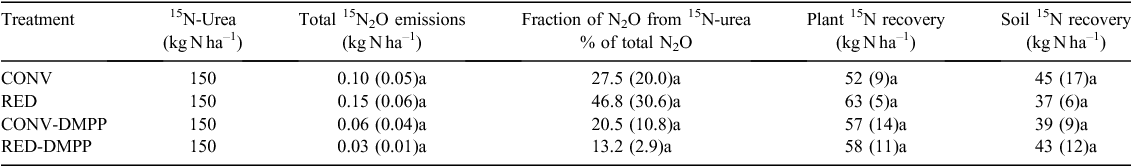
The fraction of N2O derived from 15N-labelled urea (average ± standard deviation of three chambers in a treatment) varied temporally and ranged from 1 ± 0.1% to 45 ± 9% of the total N2O emissions on different measurement days. Greatest cumulative 15N-N2O emissions were measured from the treatments that did not receive DMPP, contributing 27.5 ± 20.0% (CONV) and 46.8 ± 30.6% (RED) to the total N2O emitted from all sources (Table 5). In the treatments that received DMPP, the N2O emissions derived from 15N-labelled urea contributed 20.5 ± 10.8% (CONV-DMPP) and 13.2 ± 2.9% (RED-DMPP) to the total N2O emissions derived from all sources (Table 5). The differences in 15N-N2O fluxes among treatments were not significant as a large variability was observed among cores within a treatment.
Plant N uptake, 15N recovery and leaching losses
A total of 172.5 kg N ha–1 fertiliser was applied during the wheat growing season, of which 150 kg N ha–1 was 15N-labelled urea while the remaining 22.5 kg N ha–1 was applied as DAP (non-labelled) at sowing (Table 2). Plant N uptake and 15N recovery were slightly lower in the CONV treatment compared with the other three treatments, although differences were not significant (Tables 4 and 5). The N uptake in the wheat shoots ranged from 127 ± 11 kg N ha–1 (CONV) to 135 ± 13 kg N ha–1 (RED-DMPP) with no significant differences among treatments (Table 4). The 15N results showed a recovery of 35 ± 5% (CONV) to 42 ± 3% (RED) of the 150 kg N ha–1 that was applied as 15N-labelled urea in the aboveground parts of wheat plants (Table 5). Most of the 15N recovered from the soil profile at the end of the growing season resided in the 0–10 cm soil depth (Fig. 6). The residual 15N in the soil profile ranged from 37 ± 6 kg N ha–1 (RED) to 45 ± 17 kg N ha–1 (CONV) accounting for 25 ± 4% to 30 ± 12%, respectively, of the total 15N-labelled urea with no significant differences among treatments (Table 5). Leaching losses of water and mineral N were negligible and less than 0.7 mm and 0.2 kg N ha–1, respectively (Table 5). Thus, 33% to 36% of 15N-labelled urea was unaccounted for given negligible leaching losses.

|
Average grain yield ranged from 2.87 to 3.23 Mg ha–1 (Table 5), which is at the low end of expected yield from irrigated wheat in this region (i.e. 4 to 8 Mg ha–1). This is likely caused by the elevated temperatures experienced in the chambers, particularly during the reproductive and grain filling stages that resulted in pinched grain. This was further confirmed by the higher yields recorded in the additional four cores without chambers (>4 Mg ha–1) used to monitor temporal changes in soil nutrients and pH compared with cores fitted with chambers (<3 Mg ha–1; data not shown).
Discussion
Effect of irrigation management and DMPP on N2O emissions
Nitrous oxide fluxes (average of eight measurements per chamber per day) ranged from –3.6 to 139.6 g N2O-N ha–1 day–1 during the wheat gowning season. Most of the N2O emissions during the season occurred in the period following irrigation that commenced on 1 September 2014 (Table 3).
In the period following the first urea + DMPP application on 21 July 2014, the reduced deficit irrigation applications decreased N2O emissions by factors of 1.2 (RED) and 1.4 (RED-DMPP) compared with conventional irrigation treatments. The reduction in N2O emissions in the reduced deficit irrigation treatments during this time were mainly achieved by avoiding episodes of high N2O emissions observed in the conventional irrigation treatments following irrigation. For example, on 17 September 2014 the 90 mm irrigation (CONV) resulted in N2O peaks of 55 ± 73 g N2O-N ha–1 day–1. However, the 45 mm irrigation (RED) applied on the same day resulted in much smaller N2O emissions of just 7 ± 0.2 g N2O-N ha–1 day–1. Similarly, in the treatments with DMPP, the 90 mm irrigation (CONV-DMPP) resulted in N2O peaks of 23 ± 25 g N2O-N ha–1 day–1 compared with the 45 mm irrigation (RED-DMPP) that resulted in emissions of 3 ± 2 g N2O-N ha–1 day–1. These results agree with Scheer et al. (2012) who reported that decreasing the quantity of water in an irrigation event resulted in a reduction in the maxima of the N2O flux peak. In a summer grain sorghum crop, applying irrigation in ≤60 mm events resulted in large reductions in N2O emissions compared with applications of ≥90 mm (Jamali et al. 2015). The high N2O emissions observed following irrigation are likely caused by denitrification under anaerobic conditions. Smith et al. (1989) reported that most of the nitrate losses in irrigated wheat occurred after the first two irrigations following urea application, most likely through denitrification. The effect of irrigation management on N2O mitigation may be accentuated further under conventional farm conditions compared with the results in this experimental study. This may be because plant development and the lysimeter core system conditions only permitted the application of one irrigation event (i.e. 90 mm) that simulated the timing and amount that is typically applied in on-farm conditions. In conventional practice, where water can be applied and drained relatively quickly, it is more typical for two to three irrigations of ~100 mm to be applied in the spring to ensure a good wheat yield (Dunn 2014). If these irrigation events were to be applied in smaller applications, as proposed in this study, it is postulated that a larger overall total N2O mitigation effect may be achieved than was demonstrated here.
In the period following urea + DMPP application, DMPP application decreased N2O emissions by factors of 1.5 and 1.7 in conventional and reduced deficit irrigation, respectively (Table 3). These results are in agreement with other studies from irrigated wheat that reported decreased N2O emissions by factors of 1.6 (Liu et al. 2013) and 1.3 (De Antoni Migliorati et al. 2014) compared with treatments that did not have DMPP applied.
The effect of DMPP on N2O mitigation was further confirmed by lower urea-derived N2O emissions in the treatments applied with DMPP compared with those that did not receive DMPP. The contribution of urea-derived N2O emissions to daily average N2O fluxes ranged from 1% to 45% on different measurement days, which is comparable with the 10% to 40% emissions derived from the 15N-labelled ammonium sulfate nitrate with or without DMPP in wheat observed in Germany (Linzmeier et al. 2001). Large contributions of soil-derived N2O emissions, despite DMPP application, suggests nitrification of mineral N was not inhibited effectively by DMPP. DMPP has been shown to degrade quickly under higher temperatures and was effective for 7 weeks at 20°C (Zerulla et al. 2001). In our study, maximum temperatures in the post-DMPP period (122 days) were ≥20°C for 78 days and ≥30°C for 24 days (Fig. 1). It is likely that the efficacy of DMPP decreased in such high temperatures especially in the post-irrigation phase when microbial activity was also potentially high due to high soil moisture and temperature.
Significant differences in N2O fluxes among different treatments were also observed at the onset of the wheat growing season although all cores were treated the same in this period, partially overriding the effects of DMPP and irrigation management on N2O emissions when considered for the whole season (Table 3). The variability among treatments in the period between sowing and the first urea + DMPP application (i.e. 10 June to 20 July 2014) in soil NH4+ (7.7 to 20.8 mg NH4+-N kg soil–1) and NO3– (0.4 to 12.1 mg NO3–-N kg soil–1) levels at 0–10 cm depth despite similar N application suggests natural variability in the potential of soils for one or more of the N-transformation processes such as mineralisation, nitrification, denitrification and immobilisation.
The soil NH4+ levels in the four soil sampling cores did not show a clear trend in the effectiveness of DMPP to inhibit nitrification (Fig. 7b). In the pre-irrigation phase, when DMPP was the only treatment variable, the soil cores applied with DMPP showed lower average NH4+ levels (13–14 mg NH4+-N kg-soil–1) than corresponding non-DMPP treatments (31–73 mg NH4+-N kg-soil–1). It is not clear what caused this variability as DMPP usually results in either elevated NH4+ levels by delaying nitrification or has no effect if soil conditions are not favourable. Of the two cores that did not receive DMPP (CONV and RED), RED had the highest NH4+ levels and the highest NO3– levels of ≤ 37 mg NO3–-N kg-soil–1 (Fig. 7) in the pre-irrigation phase, indicating simultaneous higher mineralisation and nitrification rates. However, NO3– build-up was not observed in the CONV treatment core in this period despite exactly the same management suggesting natural variability rather than a DMPP effect as the reason for variable NH4+ concentrations. In contrast, in the post-irrigation phase, the treatments with DMPP showed slightly elevated NH4+ levels (19–46 mg NH4+-N kg-soil–1) compared with the corresponding non-DMPP treatments (13–32 mg NH4+-N kg-soil–1) which supports the N2O flux data where lower emissions were observed from DMPP treatments in this period (Fig. 7). Given the low replication in soil sampling cores (n = 1) and observed large variability among cores, the soil chemistry data should be interpreted with caution.
Total N2O emissions and emission factors
Total N2O emissions (average of three cores) over the wheat growing season ranged from 0.25 kg N ha–1 (RED-DMPP) to 0.57 kg N ha–1 (RED). These results are comparable to the emissions of 0.25 kg N ha–1 (with DMPP) to 0.40 kg N ha–1 (without DMPP) reported in an irrigated wheat crop in south-east Queensland (Kingaroy), Australia (De Antoni Migliorati et al. 2014). Another study from an irrigated wheat crop in south-east Queensland (Toowoomba), Australia, reported emissions of 0.43 to 0.75 kg N ha–1 using different irrigation intensities (Scheer et al. 2012). Ding et al. (2007) reported emissions of 0.39 to 0.77 kg N ha–1 from a rain-fed wheat crop on the North China Plain. Earlier work using this lysimeter core system showed much higher N2O emissions (1.7 kg N2O-N ha–1) from an irrigated (summer) sorghum crop using conventional farmer practice (Jamali et al. 2015). Although considered less effective at higher temperatures (Merino et al. 2005; Zerulla et al. 2001), DMPP effectively decreased N2O emissions in summer crops where N2O emissions were also significantly greater than in the winter wheat crops because of higher N and irrigation inputs together with warm, moist soil conditions suitable for nitrification and denitrification activity (Liu et al. 2013; De Antoni Migliorati et al. 2014). Thus, the scope for N2O mitigation through irrigation and DMPP may be greater in higher N2O-emitting summer irrigated crops and needs further research.
The emission factor (EF) i.e. the percentage of applied fertiliser emitted as N2O (uncorrected for background emissions) in different treatments were 0.29 ± 0.13% (CONV), 0.33 ± 0.20 (RED), 0.24 ± 0.08% (CONV-DMPP), and 0.14 ± 0.07% (RED-DMPP). The EF is generally corrected for background emissions using N2O emissions from a zero N fertiliser treatment (IPCC 2006). However, this study did not include a zero fertiliser treatment. Considering that 53% to 87% of total N2O emissions were derived from non-fertiliser sources (Table 5), it can be expected that the emission factors reported here are conservative. The recommended default emissions factor for N2O emissions derived from fertiliser in cropping soils is 1% as per the IPCC (2006) guidelines, which is considered significantly overestimated for irrigated wheat crops of the southern Murray–Darling Basin grown on soil types similar to that used in this study. Since this study was conducted using a lysimeter core system over one season, further field-based seasonal data are needed to confirm these emission factors. Nitrous oxide losses observed in the current work are similar to the 0.2% to 0.5% losses reported for irrigated, fertilised wheat crops (without nitrification inhibitors) in Queensland, Australia (De Antoni Migliorati et al. 2014; Scheer et al. 2012). Applying DMPP-coated urea (ENTEC®) resulted in N2O losses similar to those from a no fertiliser treatment in De Antoni Migliorati et al. (2014). Ding et al. (2007) reported 0.25% of fertiliser emitted as N2O in a rain-fed wheat crop in the North China Plain, and in a study from the Indo-Gangetic plains of India, N2O losses of 0.33% and 0.21% were observed when an irrigated wheat crop was fertilised with urea and urea + DCD (dicyandiamide), respectively (Pathak et al. 2002). These previous estimates from different environments and under different conditions are in close agreement with the results reported here.
Leaching Losses
The leaching losses of mineral N (<0.2 kg N ha–1) and water (<0.7 mm) were negligible in all treatments (Table 4) as leaching only occurred once after the first irrigation in early September 2014 when plants were in the early growth stage. Later in the season when plant water uptake increased, application of 90 mm irrigation did not result in any leaching. In soils where leaching losses of water are high, DMPP has been shown to decrease NO3– losses through leaching (Li et al. 2008; Wu et al. 2007). Because water leaching was negligible during the wheat growing season, our data is insufficient to make conclusions regarding the effectiveness of DMPP in decreasing N leaching losses. It should be noted that lower boundary conditions of lysimeter core systems such as those used in this study can differ from field conditions, which may also affect the leaching capacity of soils.
In surface irrigated systems like the irrigation districts of the Murray–Darling Basin, DMPP may be more effective in summer cropping where relatively large pre-watering and irrigation applications of 1–2 ML ha–1 can result in quite large losses of mineral N through leaching (Jamali et al. 2015). Although the effect of this chemical on nitrification inhibition has been shown in laboratory studies to be temperature-dependent, the relatively higher summer soil temperatures may also decrease the efficacy of DMPP (Merino et al. 2005; Zerulla et al. 2001). This is an area that also requires further research.
Effect of DMPP on plant N uptake and 15N balance
DMPP has been most effective in inhibiting nitrification in soils with neutral pH (Liu et al. 2015), similar to pH values used in this study. However, the effect of DMPP on plant N uptake and yield was negligible in our study. Our results are in agreement with other studies, which did not see a yield response of DMPP in wheat and maize crops in south-east Queensland, Australia (De Antoni Migliorati et al. 2014), in wheat crops in Spain (Huérfano et al. 2015), or in rye grass seed crop in southern Australia (Suter et al. 2014). This is caused by N being non-limiting for plant development because of high fertiliser rates in all treatments, thereby limiting the scope of DMPP in having an effect on plant N uptake and yield (De Antoni Migliorati et al. 2014). For example, DMPP application with a 50% decreased fertiliser application rate (23 kg N ha–1) resulted in similar dry matter yield as full fertiliser application rate (45 kg N ha–1) in a subtropical dairy pasture in Queensland, Australia (Rowlings et al. 2016).
Similar 15N recoveries in wheat plant shoots suggest no effect of treatments on fertiliser use efficiency. The recovery of 15N in wheat shoots in this study (35–42%) is comparable with the 40% to 54% recovery in other field studies conducted in irrigated wheat crops in Australia although fertiliser rate and timings were different among these studies (Humphreys et al. 1991; Smith et al. 1989; Smith and Whitfield 1990). In contrast to the above studies, roots were not included in recoveries in the present study, but have been shown to recover 2% of the 15N-labelled nitrate in wheat (Van Cleemput et al. 1981). Assuming a similar plant N uptake of DAP as that measured for 15N-labelled urea, the total plant N derived from applied inorganic fertiliser (i.e. 15N-labelled urea and unlabelled DAP) sources was 47% (CONV), 55% (RED), 51% (CONV-DMPP) and 50% (RED-DMPP) of total fertiliser applied (data not shown). Thus, the remaining 45% to 53% of plant N was derived from non-fertiliser sources in soil. These results are consistent with Humphreys et al. (1991) who reported that 46% of N in irrigated wheat was derived from urea when it was applied at the end of tillering compared with this study where urea was applied in split applications starting earlier in the growing season. In irrigated cotton, only 32% of the total N uptake was derived from applied fertiliser (Rochester et al. 1993). Estimates of approximately ≤ 42% 15N recoveries in plants in this study suggest there is an opportunity to improve the fertiliser use efficiency in irrigated wheat crops.
A large proportion (33–36%) of 15N-labelled urea could not be accounted for in the plant shoot and soil recoveries at the end of the wheat growing season. The major mechanisms of such N loss are ammonia (NH3) volatilisation and denitrification (i.e. as N2). Ammonia losses of zero to 35 kg N ha–1 within five days of fertilisation have been reported from irrigated wheat depending on management practices and soil type (Bacon et al. 1986; Smith et al. 1989). In this study, the application of urea pre-dissolved in water and low soil pH (i.e. ≤6.9) would result in negligible losses via NH3 volatilisation (Freney et al. 1983; Smith et al. 1989). Losses via denitrification would be negligible in the pre-irrigation phase because of drier soil conditions, which is also supported by lower N2O emissions in this period compared with the post-irrigation period. Thus, it can be inferred that most N losses would occur in the post-irrigation phase through denitrification. This postulation is in agreement with Smith et al. (1989) who observed significant NO3–-N losses in irrigated wheat after irrigation following fertiliser addition.
Conclusions
This study investigated the effect of irrigation management and the nitrification inhibitor DMPP on N2O mitigation in irrigated wheat using a lysimeter core and automated chamber system. Most of the N2O mitigation opportunity was in the period following onset of irrigations in early spring. The highest decrease in N2O emissions was achieved when DMPP was applied in combination with smaller irrigations. In isolation, DMPP tended to be more efficient than optimised irrigation management in mitigating N2O emissions. There was a negligible effect of treatments on yield and plant N uptake. Given the extra cost of DMPP, farmers would be less likely to adopt such fertiliser strategies without evidence of proportionate gains in yield. However, as DMPP helps to provide improved N use efficiency there may be scope for using DMPP-coated fertiliser entirely applied up front at lower N rates, which may offset extra costs associated with DMPP application and the labour and machinery cost associated with in-crop side dressing applications. Similarly, applying irrigation in smaller, frequent events decreases N losses through denitrification and possibly through leaching in well-draining soils, increasing N use efficiency and enabling decreased fertiliser rates without yield penalty. It is recommended that further research should focus on the interaction between optimised irrigation, N rate and DMPP in research field trials.
Acknowledgements
This research was funded by the Australian Federal Department of Agriculture through Filling the Research Gap and the CSIRO Office of the Chief Executive. We are thankful to Roy Zandona and Alisson Fattore of CSIRO for their excellent technical support. We are also thankful to Dr Henrike Mielenz of CSIRO for her feedback, which helped improve the manuscript.
References
ABS (2010) Experimental Estimates of the Gross Value of Irrigated Agricultural Production, 2000–01 - 2007–08. (Australian Bureau of Statistics: Belconnen, Australia)Bacon PE, Hoult EH, McGarity JW (1986) Ammonia volatilization from fertilisers applied to irrigated wheat soils. Fertiliser Research 10, 27–42.
| Ammonia volatilization from fertilisers applied to irrigated wheat soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xmt1GhsLc%3D&md5=4246178aace67d6af7406b2ecfe82926CAS | [in English]
Bouwman AF (1998) Environmental science: Nitrogen oxides and tropical agriculture. Nature 392, 866–867.
| Environmental science: Nitrogen oxides and tropical agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtFekur4%3D&md5=ecb3cf3e9c2d2f31ad2914d5bad394c9CAS |
Bremner J, Blackmer A (1978) Nitrous oxide: emission from soils during nitrification of fertiliser nitrogen. Science 199, 295–296.
| Nitrous oxide: emission from soils during nitrification of fertiliser nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXntlGlsw%3D%3D&md5=e9c12a6d65dc54bbff6f9a7ff12c4946CAS | 17759663PubMed |
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society, B: Biological Sciences 368, 1–13.
| Nitrous oxide emissions from soils: how well do we understand the processes and their controls?Crossref | GoogleScholarGoogle Scholar |
De Antoni Migliorati M, Scheer C, Grace PR, Rowlings DW, Bell M, McGree J (2014) Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N 2 O emissions from a subtropical wheat–maize cropping system. Agriculture, Ecosystems & Environment 186, 33–43.
| Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N 2 O emissions from a subtropical wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVCjtbo%3D&md5=0844cd1e106bc3431f689d097fcc0b36CAS |
de Klein CAM, van Logtestijn RSP, van de Meer HG, Geurink JH (1996) Nitrogen losses due to denitrification from cattle slurry injected into grassland soil with and without a nitrification inhibitor. Plant and Soil 183, 161–170.
| Nitrogen losses due to denitrification from cattle slurry injected into grassland soil with and without a nitrification inhibitor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlsVahuw%3D%3D&md5=ec4f08a2db2edd634e7374078b5cbab3CAS |
Ding W, Cai Y, Cai Z, Yagi K, Zheng X (2007) Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain. The Science of the Total Environment 373, 501–511.
| Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlams7c%3D&md5=b010245589f09b14fffa4c936dd60cdeCAS | 17229455PubMed |
Dunn B (2014) Wheat after rice - How many irrigations? In ‘IREC Farmers’ Newsletter. Vol. 190’. (Irrigation Research and Extension Committee: Griffith, NSW)
Firestone MK, Davidson EA (1989) O production and consumption in soil. Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere 47, 7–21.
Food and Agriculture Organisation (FAO) (2010) ‘Statistical Yearbook.’ (Food and Agriculture Organisation of the United Nations: Rome).
Freney JR, Simpson JR, Denmead OT (1983) Volatilization of ammonia. In ‘Gaseous Loss of Nitrogen from Plant-Soil Systems. Vol. 9’. (Eds JR Freney and JR Simpson) pp. 1–32. (Springer: Netherlands)
Freney JR, Smith CJ, Mosier AR (1992) Effect of a new nitrification inhibitor (wax coated calcium carbide) on transformations and recovery of fertiliser nitrogen by irrigated wheat. Fertiliser Research 32, 1–11.
| Effect of a new nitrification inhibitor (wax coated calcium carbide) on transformations and recovery of fertiliser nitrogen by irrigated wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmtFWmt7g%3D&md5=cc86d01ba347af0464727c56c05ba6c3CAS | [in English]
Grieve A, Dunford E, Marston D, Martin R, Slavich P (1986) Effects of waterlogging and soil salinity on irrigated agriculture in the Murray Valley: a review. Animal Production Science 26, 761–777.
| Effects of waterlogging and soil salinity on irrigated agriculture in the Murray Valley: a review.Crossref | GoogleScholarGoogle Scholar |
Huérfano X, Fuertes-Mendizábal T, Duñabeitia MK, González-Murua C, Estavillo JM, Menéndez S (2015) Splitting the application of 3, 4-dimethylpyrazole phosphate (DMPP): Influence on greenhouse gases emissions and wheat yield and quality under humid Mediterranean conditions. European Journal of Agronomy 64, 47–57.
| Splitting the application of 3, 4-dimethylpyrazole phosphate (DMPP): Influence on greenhouse gases emissions and wheat yield and quality under humid Mediterranean conditions.Crossref | GoogleScholarGoogle Scholar |
Humphreys E, Melhuish F, Xi Z, White R, Muirhead W (1991) Flood irrigation of wheat on a transitional red-brown earth. II. Effect of duration of ponding on availability of soil and fertiliser nitrogen. Australian Journal of Agricultural Research 42, 1037–1051.
| Flood irrigation of wheat on a transitional red-brown earth. II. Effect of duration of ponding on availability of soil and fertiliser nitrogen.Crossref | GoogleScholarGoogle Scholar |
IAEA (2001) ‘Use of isotope and radiation methods in soil and water management and crop nutrition.’ (International Atomic Energy Agency (FAO): Vienna).
IPCC (2006) ‘2006 IPCC guidelines for national greenhouse gas inventories.’ (Eds HS Eggleston, L Buendia, K Miwa, T Ngara, K Tanabe). (National Greenhouse Gas Inventories Programme, IGES: Japan) Available at http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/0_Overview/V0_0_Cover.pdf [verified 31 May 2016]
Isbell R (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Jamali H, Quayle WC, Baldock J (2015) Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling. Agricultural and Forest Meteorology 208, 32–39.
| Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling.Crossref | GoogleScholarGoogle Scholar |
Li H, Liang X, Chen Y, Lian Y, Tian G, Ni W (2008) Effect of nitrification inhibitor DMPP on nitrogen leaching, nitrifying organisms, and enzyme activities in a rice-oilseed rape cropping system. Journal of Environmental Sciences (China) 20, 149–155.
| Effect of nitrification inhibitor DMPP on nitrogen leaching, nitrifying organisms, and enzyme activities in a rice-oilseed rape cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFSisrw%3D&md5=d9d88575c1c3131b78e75ad7180e8c15CAS |
Linzmeier W, Gutser R, Schmidhalter U (2001) Nitrous oxide emission from soil and from a nitrogen-15-labelled fertiliser with the new nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP). Biology and Fertility of Soils 34, 103–108.
| Nitrous oxide emission from soil and from a nitrogen-15-labelled fertiliser with the new nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7c%3D&md5=21711d3e685d6e48d1960feab869e19aCAS |
Liu C, Wang K, Meng S, Zheng X, Zhou Z, Han S, Chen D, Yang Z (2011) Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agriculture, Ecosystems & Environment 140, 226–233.
| Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlWitro%3D&md5=42657d28bf4a58ed9cb855ad6e059733CAS |
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat–maize cropping system. Biogeosciences 10, 2427–2437.
| Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltlGktLg%3D&md5=7ae6f766dbe0c2a108f66906ecb09266CAS |
Liu R, Hayden H, Suter H, He J, Chen D (2015) The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils. Journal of Soils and Sediments 15, 1113–1118.
| The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXisl2qtLk%3D&md5=072bc00be70d22828a3fb561a3dbf373CAS | [in English]
Melhuish F, Humphreys E, Muirhead W, White R (1991) Flood irrigation of wheat on a transitional red-brown earth. I. Effect of duration of ponding on soil water, plant growth, yield and N uptake. Australian Journal of Agricultural Research 42, 1023–1035.
| Flood irrigation of wheat on a transitional red-brown earth. I. Effect of duration of ponding on soil water, plant growth, yield and N uptake.Crossref | GoogleScholarGoogle Scholar |
Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biology & Biochemistry 53, 82–89.
| Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions.Crossref | GoogleScholarGoogle Scholar |
Merino P, Menéndez S, Pinto M, González‐Murua C, Estavillo J (2005) 3, 4-imethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use and Management 21, 53–57.
| 3, 4-imethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application.Crossref | GoogleScholarGoogle Scholar |
Meyer W, Barrs H, Smith R, White N, Heritage A, Short D (1985) Effect of irrigation on soil oxygen status and root and shoot growth of wheat in a clay soil. Australian Journal of Agricultural Research 36, 171–185.
| Effect of irrigation on soil oxygen status and root and shoot growth of wheat in a clay soil.Crossref | GoogleScholarGoogle Scholar |
Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, van Cleemput O (1998) Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. Nutrient Cycling in Agroecosystems 52, 225–248.
| Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnsVSmtLk%3D&md5=5ff8b6168c8281761fd7b99d2fdcd290CAS | [in English]
North S (2012) Waterlogging, anoxia and wheat growth in surface irrigated soils. In ‘Proceedings of the 16th Australian Agronomy Conference’. Armidale, NSW. (Ed. I Yunusa) Available at http://www.regional.org.au/au/asa/2012/soil-water-management/8144_northsh.htm#TopOfPage [verified 31 May 2016]
Pasda G, Hähndel R, Zerulla W (2001) Effect of fertilisers with the new nitrification inhibitor DMPP (3, 4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biology and Fertility of Soils 34, 85–97.
| Effect of fertilisers with the new nitrification inhibitor DMPP (3, 4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7k%3D&md5=bafb362aac112d94738dc506d8e7e4ffCAS |
Pathak H, Bhatia A, Prasad S, Singh S, Kumar S, Jain M, Kumar U (2002) Emission of nitrous oxide from rice-wheat systems of Indo-Gangetic plains of India. Environmental Monitoring and Assessment 77, 163–178.
| Emission of nitrous oxide from rice-wheat systems of Indo-Gangetic plains of India.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvVemsbg%3D&md5=f09345a81a37178678eda942fddf7b62CAS | 12180654PubMed |
Rochester I, Constable G, MacLeod D (1993) Cycling of fertiliser and cotton crop residue nitrogen. Soil Research 31, 597–609.
| Cycling of fertiliser and cotton crop residue nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhs1altL0%3D&md5=bcfeb40749f25b101bfc0a528e3a5924CAS |
Rowlings DW, Scheer C, Liu S, Grace PR (2016) Annual nitrogen dynamics and urea fertiliser recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates. Agriculture, Ecosystems & Environment 216, 216–225.
| Annual nitrogen dynamics and urea fertiliser recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1yjtb7K&md5=96447a2be2ec2cdc3b0046fcba10d9a5CAS |
Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R (2008) Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices. Soil Biology & Biochemistry 40, 290–301.
| Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlajs7bN&md5=632f84bcfab0a6dead24d4cc75a89447CAS |
Scheer C, Grace P, Rowlings D, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant and Soil 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS | [in English]
Smith C, Whitfield D (1990) Nitrogen accumulation and redistribution of late applications of 15 N-labelled fertiliser by wheat. Field Crops Research 24, 211–226.
| Nitrogen accumulation and redistribution of late applications of 15 N-labelled fertiliser by wheat.Crossref | GoogleScholarGoogle Scholar |
Smith C, Freney J, Chapman S, Galbally I (1989) Fate of urea nitrogen applied to irrigated wheat at heading. Australian Journal of Agricultural Research 40, 951–963.
| Fate of urea nitrogen applied to irrigated wheat at heading.Crossref | GoogleScholarGoogle Scholar |
Suter H, Chen D Turner D (2014) Stabilized nitrogen fertilisers to reduce greenhouse gas emissions and improve nitrogen use efficiency in Australian agriculture. In ‘proceedings of the International Symposium on Managing Soils for Food Security and Climate Change Adaptation and Mitigation’. (Eds LK Heng, K Sakadevan, G Dercon, ML Nguyen) pp. 33–36. (Food and Agriculture Organisation of the United Nations: Rome) Available at http://www-naweb.iaea.org/Nafa/swmn/public/ManagingSoilFoodSecurityProceedingsFAO-1.pdf [verified 31 May 2016]
Van Cleemput O, Hofman G, Baert L (1981) Fertiliser nitrogen balance study on sandy loam with winter wheat. Fertilizer Research 2, 119–126.
| Fertiliser nitrogen balance study on sandy loam with winter wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkvFKitr8%3D&md5=73858362332b1b7da30894536b231892CAS |
Wu SF, Wu LH, Shi QW, Wang ZQ, Chen XY, Li YS (2007) Effects of a new nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) on nitrate and potassium leaching in two soils. Journal of Environmental Sciences 19, 841–847.
| Effects of a new nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) on nitrate and potassium leaching in two soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptVSnurw%3D&md5=03da0388fec8098aa45adb75047293e4CAS |
Zerulla W, Barth T, Dressel J, Erhardt K, von Locquenghien KH, Pasda G, Rädle M, Wissemeier A (2001) 3, 4-Dimethylpyrazole phosphate (DMPP)–a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils 34, 79–84.
| 3, 4-Dimethylpyrazole phosphate (DMPP)–a new nitrification inhibitor for agriculture and horticulture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |