Biological quality of a podzolic soil after 19 years of irrigated minimum-till kikuyu–ryegrass pasture
P. A. Swanepoel A F , J. Habig B , C. C. du Preez C , P. R. Botha D and H. A. Snyman EA Western Cape Department of Agriculture, Outeniqua Research Farm, PO Box 249, George 6530, South Africa.
B Agricultural Research Council – Plant Protection Research Institute, Private Bag X134, Queenswood, Pretoria 0121, South Africa.
C Department of Soil, Crop and Climate Sciences, University of the Free State, PO Box 339, Bloemfontein 9300, South Africa.
D Western Cape Department of Agriculture, Outeniqua Research Farm, PO Box 249, George 6530, South Africa.
E Department of Animal, Wildlife and Grassland Sciences, University of the Free State, PO Box 339, Bloemfontein 9300, South Africa.
F Corresponding author. Email: pieters@elsenburg.com
Soil Research 52(1) 64-75 https://doi.org/10.1071/SR13237
Submitted: 2 May 2013 Accepted: 21 September 2013 Published: 5 February 2014
Abstract
Conversion of natural rangeland to minimum-tillage kikuyu (Pennisetum clandestinum) based pastures for dairy production in the southern Cape of South Africa, may be beneficial to soil biological quality. The objective was to evaluate whether 19 years of minimum-till kikuyu-ryegrass pasture had altered the distribution and quality of biological properties formerly developed under natural rangeland. An irrigated minimum-till kikuyu-ryegrass pasture soil was compared to virgin soil with natural rangeland. Soil organic matter, soil organic C, active C, microbial biomass C, total N and enzymatic activities (β-glucosidase, urease and alkaline phosphatase) behaved similarly by having higher values in the surface layers of the cultivated pasture soil than in virgin soil, decreased with depth until they become similar at the 200–300 mm depth. Acid phosphatase activity was similar (P > 0.05) between soils. Vertical distribution of potentially mineralizable N was similar (P > 0.05) at 0–100 mm soil depth, but higher (P ≤ 0.01) in the cultivated pasture soil than in the virgin soil. The microbial indicated along with stratification ratios for different biological indicators that the cultivated pasture soil’s ecosystem functionality improved. Soil microbial functional diversity and carbon source utilisation patterns of the cultivated pasture soil and virgin soil was influenced by plant species present and root exudate composition. The soil microbial diversity, as shown by the Shannon-Weaver and Enrichment Indices, was significantly altered between cultivated pasture and the virgin soil, especially at different soil depths. A general appraisal of biological soil properties indicated that conversion of natural fynbos vegetation to irrigated minimum-till kikuyu-ryegrass pasture after 19 years of cultivation on a podzolic soil beneficial.
Additional keywords: organic carbon, enzyme activities, microbial biomass, microbial functional diversity, organic matter, total nitrogen.
Introduction
Conversion of large areas of natural rangeland to cultivated pastures in the southern Cape region of South Africa is driven by the demand for sustainable fodder production within dairy farm systems (Botha 2003). Various management techniques have evolved to increase productivity from pastures on dairy farms. Such techniques include irrigation, fertilisation, reduced tillage, maintaining a permanent groundcover with perennial forage species, intensive grazing and incorporation of legumes (Botha 2003; Van der Colf 2011). The benefit of these management techniques resulted mainly from an increase in soil organic matter (SOM) stocks (Swanepoel and Botha 2012a).
Minimum-tillage systems with kikuyu (Pennisetum clandestinum) as a pasture base, over-sown annually with different ryegrass species (Lolium spp.) and varieties, have been adopted by most dairy farmers in the southern Cape region of South Africa (Van der Colf 2011). It has been reported that minimum-tillage systems may alter biological soil properties and change the distribution patterns of SOM (Müller-Stöver et al. 2012). Such distribution patterns are usually associated with a build-up of immobile constituents at the surface layer of minimum-tillage systems (Carter and Rennie 1982), and therefore the soil surface is mostly enriched with nutrients and SOM (López-Garrido et al. 2011). The distribution of SOM is of particular interest since SOM supplies nutrients to plants and microbes, maintains soil structure, and increases water-holding capacity and cation exchange capacity; SOM is therefore an important factor to ensure agro-ecosystem health and sustainability of pastures (Conant et al. 2001). Changes in SOM and mineralised nitrogen (N) are associated with changes in the below-ground microbial community structure and functionality (Bossio et al. 2005). This is because the rate of SOM turnover is dependent on microbial functioning (Müller-Stöver et al. 2012), which is vital, because the microbial community safeguards ecosystem health and soil quality. Apart from changes in the distribution pattern of SOM and related parameters, the kind of vegetation grown on soil may also have a direct influence on the microbial community below the soil surface (Wardle et al. 2004).
Conversion of natural rangeland to permanent kikuyu-based pastures, under a minimum-tillage regime, may be beneficial to soil quality and create potential carbon (C) sinks (Conant et al. 2001; Desjardins et al. 2004). The objective of this study was to evaluate whether 19 years of irrigated, minimum-till kikuyu–ryegrass pasture on a podzolic soil altered the distribution and quality of biological properties formerly developed under undisturbed natural fynbos rangeland. In a preceding paper the effects on the physical condition of this podzolic soil were reported (Swanepoel et al. 2013).
Materials and methods
Site description
Two study sites which are 800 m from one another were selected on the Outeniqua Research Farm (33°58′38″S, 22°25′16″E; 204 m above sea level) near George in the southern Cape region of South Africa. These sites enabled us to compare biological indicators of soil quality between different land uses. The first site comprised an established kikuyu-based pasture and the second was characterised by fynbos vegetation in its native state. Both sites are on a Podzol (IUSS Working Group 2006) or Spodosol (Soil Survey Staff 2003) and locally known as a Witfontein soil form (Soil Classification Working Group 1991). This is one of the major soil forms in the region consisting of three diagnostic horizons: an orthic A horizon (0–200 mm), followed by a podzol B horizon (200–300 mm), which is underlain by unconsolidated material with signs of wetness (300–600 mm).
The region has a mild climate with long-term (45 years) mean ambient temperatures fluctuating in winter between 7°C and 18°C and in summer between 15°C and 25°C. No frost occurs. The long-term mean annual precipitation of the sites is 728 mm, evenly distributed throughout the year (ARC-ISCW 2012).
Experimental layout and treatments
The two sites had different land uses and hence species composition which served as treatments. At each site there were six plots. Plot size was 15 m by 15 m.
Site 1 was a 19-year-old kikuyu–ryegrass pasture on a minimum-tillage regime with permanent sprinkler irrigation. Kikuyu formed a permanent pasture base and annual Westerwold ryegrass (Lolium multiflorum var. westerwoldicum) was established into the base annually. It was over-sown once a year during March or April to increase the pasture productivity during winter and spring. Pasture was grazed to a height of 50 mm aboveground level before establishment of ryegrass. The kikuyu stubble was mulched to ground level and an Aitchison Seedmatic-3116C seeder was subsequently used to drill the seed into the mulched layer (Botha 2003; Van der Colf 2011). A strip-grazing system at a grazing intensity of ~5.7 Jersey cows ha–1 was used at a mean grazing cycle of roughly 30 days (Van der Colf 2011). The soil nutrient levels were maintained according to recommendations for a kikuyu–ryegrass pasture. Limestone ammonium nitrate was topdressed at an approximate rate of 55 kg N ha–1 on a monthly basis. Traffic intensity with a 50–55 kW tractor was restricted to the centre of a strip after grazing for N fertilisation and once a year for over-sowing ryegrass. Soil temperatures were monitored and soil water content was maintained at levels higher than ~80% of field capacity by continuous-logging soil probes (DFM Software Solutions CC 2012).
The second site remained historically undisturbed and soil was conserved in its virgin state. Natural fynbos rangeland was dominated by Helichrysum spp. and Pentaschistis spp. (Mucina and Rutherford 2006). Animals were not allowed to graze the area, which was not subject to fire.
Sampling and analyses
Annual aboveground herbage production [kg dry matter (DM) per ha] was determined on a monthly basis for the cultivated pasture before grazing by dairy cattle and that of the natural rangeland was measured seasonally. The assumption was made that the aboveground phytomass production of the natural vegetation was in equilibrium and remained stable throughout the year. Production was determined by cutting herbage within the border of quadrats (0.25 m2) to a height of 30 mm aboveground level and dried at 60°C for 72 h.
In October 2011, representative soil samples were taken from 0–100, 100–200 and 200–300 mm depth increments in each plot for determination of particle size distribution with the hydrometer method (Day 1965) and extractable phosphorus (P) with the citric acid method (Non-Affiliated Soil Analysis Work Committee 1990). Then bulk density was measured with a hammer-driven cylindrical sampler with an inner volume of 288.8 cm3 (Blake 1965). The measured bulk densities enabled the calculation of soil parameter stocks, which were useful to compare sites with regard to pedological significance of soil biological properties (Arshad and Martin 2002). For analysis of biological soil properties, 20 samples were taken aseptically per depth interval (0–100, 100–200 and 200–300 mm) from each plot. The SOM content was determined by gravimetric measurement of CO2 loss during ignition at 550°C for 3 h (Broadbent 1965) and soil organic C (SOC) by dichromatic digestion using the Walkley–Black procedure (Nelson and Sommers 1982). Active C was measured by oxidation of 1.0 g oven-dry equivalent of air-dried soil with 0.02 m KMnO4 in 1 m CaCl2 (pH 7.2) and colourimetric measurement of non-reduced Mn at 550 nm (Weil et al. 2003). The microwave irradiation method was used to determine microbial biomass C (MBC) (Islam and Weil 1998) and the anaerobic incubation procedure for potential mineralisable N (PMN) determination (Drinkwater et al. 1996). Total soil N (total N) was analysed by Kjeldahl digestion (Bremner 1960). Soil samples destined for biological analyses were composited and stored at 4°C, except those destined for enzyme assays. These samples were dried for 48 h at 40°C before storage.
From the measured data, microbial quotients (MBC/SOC) and C/N ratios (SOC/total N) were calculated. Stratification ratios were calculated by dividing relevant soil property values of the 0–100 mm depth interval by those of the 200–300 mm depth interval.
Enzymes involved in the C (β-glucosidase), N (urease) and P (alkaline and acid phosphatase) nutrient cycles were assayed in this study. β-Glucosidase and phosphatase activities were calculated according to methods described by Dick et al. (1996), determining the release of p-nitrophenyl moiety after incubation of soil with p-nitrophenyl glucoside and p-nitrophenyl phosphate, respectively. Urease activity was assayed by incubating the soil with urea according to the method of Kandeler and Gerber (1988). Residual ammonia was measured after incubation and urease activities were calculated with reference to a calibration curve.
Qualitative community level physiological profiles (CLPP) were assessed by measuring the amount of substrates and the speed of substrate utilisation (Arias et al. 2005). Soil samples were diluted in sterile distilled water (1 : 3000) (Buyer and Drinkwater 1997) to allow for the recovery of several types of bacteria, and to retain numerically abundant organisms while eliminating fast-growing competitors (De Fede et al. 2001). The soil suspensions were inoculated into the Biolog EcoPlates™ (Biolog Inc., Hayward, CA, USA) containing 31 sources of C and a control well, in triplicate. The plates were incubated at 28°C. Respiration of C sources by microbial populations reduced the tetrazolium dye within each EcoPlate well, causing a colour change. This colour change was spectrophotometrically determined twice daily over 7 days at 590 nm to determine average well colour development (Winding and Hendriksen 1997). The optical density (OD) values obtained from each plate were analysed using the average well colour development (AWCD) technique as described by Garland (1996). Standardised patterns were obtained by blanking the absorbance values for the wells with C sources against the absorbance value of the control well without a C source. Any negative values were converted to zero, and any variance in the inoculum density was accounted for by dividing the absorbance of each well by the average absorbance for the whole plate, giving the standardised OD. Instead of using the absolute values, standardised patterns were subsequently compared (Habig 2003).
The functional diversity of the soil microbial populations was determined using the amount and equitability of C substrates metabolised as indicators of richness and evenness, respectively (Garland and Mills 1991). Biodiversity was determined using the Shannon–Weaver diversity index (H′) and substrate Evenness index (E), which indicates species richness and the variation between species within the local soil microbial community, respectively (Magurran 1988).
Statistical analyses
Soil parameter data were analysed using a two sample Student’s t-test for independent samples per depth. The data were acceptably normally distributed, but data had heterogeneous treatment variances. Data were analysed using the statistical program Genstat (Payne et al. 2011). Data on C source utilisation and enzymatic activity were subjected to non-parametric statistical analyses using Statistica 6.1 (StatSoft Inc. Tulsa, OK, USA). Substrate utilisation patterns were compared from the intermediate phase of the Biolog incubation and an AWCD value of 0.25 absorbance units was used as the reference point for multivariate statistical analysis of the data. Carbon substrate utilisation profiles were statistically analysed by principal component analysis (PCA) (Palojärvi et al. 1997) and cluster analyses (vertical hierarchical tree plots). Homogenous grouping was determined with Fisher’s least significant difference (l.s.d.) at P = 0.05.
Results and discussion
Herbage production
The annual herbage production of kikuyu–ryegrass pasture was 20.33 kg DM ha–1 (s.e.m. 0.60) which was high and concurrent to the findings of Botha (2003) and Van der Colf (2011) for similar pastures in the region. However, the annual herbage yield of the natural fynbos vegetation was only 8.79 kg DM ha–1 (s.e.m. 0.86) and differed (P ≤ 0.001) from that of the cultivated pasture.
Physical soil parameters
Particle size distribution for the cultivated pasture soil and virgin soil is displayed in Table 1. In both soils, clay content increased with depth, but per depth interval, the clay content was similar across land uses, except in the 200–300 mm layer. Thus, although soil biological activity may be linked to clay content (Bronick and Lal 2005; Van Antwerpen et al. 2009), interpretation of biological indicators should be unbiased in this study.

|
In both the cultivated pasture soil and the virgin soil, bulk density increased from low values in the 0–100 mm layer to high values in the 100–200 and 200–300 mm layers (Table 2). The bulk density of cultivated pasture soil was similar to that of virgin soil (P > 0.01).

|
Organic matter indicators
Stock of organic matter indicators (Table 3) in cultivated pasture soil was higher (P ≤ 0.01) in the upper two layers than in virgin soil, with the exceptions of MBC and PMN in the 0–100 mm layer and SOM and active C in the 100–200 mm layer, which was similar (P > 0.01).
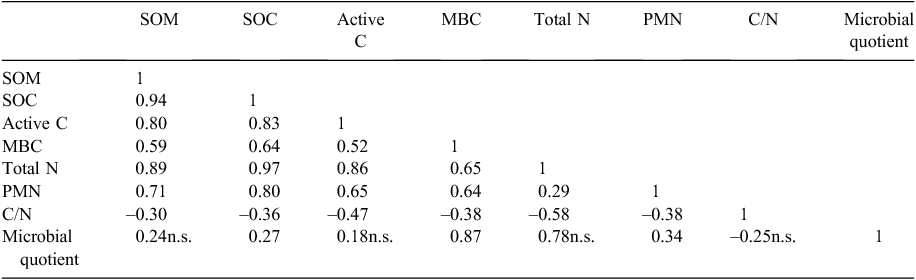
In the 200–300 mm layer stock values for organic matter indicators were mostly similar (P > 0.01), except MBC and PMN, which were higher (P ≤ 0.05) in cultivated pasture soil than in virgin soil. Bulk density increased as SOM related indicators decreased which was concurrent to the findings of Haynes and Tregurtha (1999). The content of SOC comprised a lower concentration of SOM (Allison 1965; Conyers et al. 2011) and therefore recovery of SOC for the cultivated pasture soil and virgin soil was only 50.4% and 41.4% of SOM, respectively. Conversion of virgin soil to cultivated pasture soil improved conditions supporting the build-up of SOM levels and, therefore, the soil’s microbial component and soil health. Kikuyu with its high density of rhizomes and stolons is in particular a good pasture crop to enhance SOM levels (Skjemstad et al. 1990). Although the virgin soil had lower SOC stocks than the cultivated pasture, it maintained very high levels of SOC in its native state. The SOC is mainly regarded by agriculturalists in the southern Cape as the single most important indicator of soil quality, since there is a lack of any other indicators providing information about the condition of soil. Neither SOM nor SOC is probably the most suitable measurement to estimate biological activity for soil quality assessments, since concurrent changes to adapted management may be very slow to detect. The highly labile proportion of SOM, i.e. active C, comprised only 1.96% and 0.03% of SOM in cultivated pasture soil and virgin soil, respectively, and may be a more useful and sensitive measurement to detect subtle changes in the SOM pool than SOC concentration or stock (Karlen et al. 1999). The active C concentration in the cultivated pasture soil was ~530 times higher than that of the virgin soil at 0–100 mm depth, reflecting the importance of the soil surface as biologically active interface and entry point for additions of readily available organic material (López-Garrido et al. 2011). In these intensively grazed dairy pastures, high volumes of labile organic matter are added in forms of manure, moribund forage material and forage wastage. Active C provided additional information to that of SOC, by proving that cultivated pasture soil improved the system by introducing high volumes of vital energy substrates for microbial metabolism in the surface layer of the soil. These microbes play an important role in organic matter decomposition and nutrient cycling (Granatstein et al. 1987) and MBC was used to investigate the action of soil microbes within the SOM cycle (Carter 1986). Mean MBC comprised 0.41% and 0.26% of SOM in cultivated pasture soil and virgin soil, respectively. The higher MBC content in the cultivated pasture soil than in the virgin soil could be ascribed to the vigorous and large root systems of the kikuyu–ryegrass pasture with improved external environmental conditions such as water supply by irrigation, and nutrient supply by fertilisation, liming and manure from grazing animals (Carter 1986). These conditions create an environment that supports microbes by providing active C by means of root exudates and organic nutrients from manure.
The PMN revealed the capacity of the soil to supply mineralised N from SOM reserves with the aid of microbes (Edenborn et al. 2011). Due to low productivity of natural fynbos species and the associated low grazing capacity of 77 ha per large stock unit (Boshoff et al. 2001), the virgin soil required relatively low N levels to function in a sustainable manner, rendering the potential of the microbes to mineralise N higher in cultivated pasture soil than in the virgin soil. This was supported by the C/N ratios, which were narrower than 25 : 1 within all sampling layers in both soils (Table 4), a value considered the threshold for rapid mineralisation (Miles and Manson 2000).

|
The C/N ratio was markedly lower (P ≤ 0.01) in the cultivated pasture soil than in the virgin soil in all sampling layers. Cultivated pasture soil had C/N ratios of ~11–12, which was in the range normally reported for agricultural soil (Karlen et al. 1999; Ernst and Siri-Prieto 2009). The recommended C/N ratio of a healthy SOC turnover rate in well-managed pasture is 10–12 (Miles and Manson 2000). The higher C/N ratio in virgin soil is indicative of the undisturbed and sustainable state of the ecosystem, but also reflects effects from the very low grazing capacity.
The microbial quotient, shown in Table 5, indicates the substrate-use efficiency of the microbial community and its importance in regulating SOM transformations (Moore et al. 2000). Insam and Domsch (1988) stated that the microbial quotient serves as an indicator of C accumulation or release. The microbial quotient of the 0–100 and 200–300 mm depths was higher (P ≤ 0.05) in the cultivated pasture soil than in the virgin soil, but it did not differ (P > 0.05) between sites in the 100–200 mm depth. Therefore, more microorganisms were sustained per unit SOM in the cultivated pasture soil than in the virgin soil.

|
The microbial quotient should be used as a reference point during steady-state conditions (Martens 1995). Thus, the assumption was made that the virgin soil is in equilibrium and sustainable for its relevant land use. The microbial quotient of the cultivated pasture soil was higher than (P ≤ 0.05) or similar to (P > 0.05) that of the virgin soil and indicated that the cultivated soil’s living component was improved. Sudden deviations from this level should indicate that the system is changing and C is being released or accumulated. It is therefore a valuable tool to predict C sequestration actions.
The degree of ecosystem functionality to 300 mm depth of the two land uses is indicated by stratification ratios (Franzluebbers 2002) in Fig. 1. A stratification ratio of 1 indicates a uniform distribution to 300 mm deep, and when >2, it generally indicates an improvement in the system (López et al. 1996). Stratification ratios of organic matter indicators of virgin soil remained <2, except for active C and PMN, but those of cultivated pasture soil were >2. The stratification ratios of cultivated pasture soil were higher for all organic matter indicators except for PMN. It can therefore be reasoned that the ecosystem quality and functionality were improved by enhancing organic matter indicators at the soil surface when soil is converted to cultivated pastures. Maintaining soil quality at the surface is important, since this is the interface supporting infiltration of water, gaseous exchange and organic materials from manure and forage (López-Garrido et al. 2011).
There was a very high positive correlation (r = 0.94) between SOM and SOC (Table 6), which was concurrent to the findings of Swanepoel and Botha (2012b). The close relationship between organic-matter related indicators was stressed by the high correlation values between SOM or SOC and the other organic-matter related indicators, except for MBC.
Enzyme activities
The activities of four soil enzymes were assayed to evaluate ecosystem functioning and are shown in Table 7. β-Glucosidase, urease and alkaline phosphatase activities were higher (P ≤ 0.01) in the cultivated pasture soil than in the virgin soil in all layers, except for β-glucosidase at 200–300 mm depth. The enzymatic activities rapidly declined with soil depth in the cultivated pasture soil, which is the trend usually observed (Dick et al. 1988; Curci et al. 1997; Green et al. 2007) and is concomitant with the decline in organic matter indicators observed, also noted by Verhulst et al. (2010). Acid phosphatase activity had a different distribution pattern than the other soil enzymatic activities. The virgin soil and the cultivated pasture soil had similar (P > 0.05) activities in all depths. Soil pH(KCl) of the virgin soil was significantly lower (P ≤ 0.05) at all soil depths than that of the cultivated pasture soil, which rendered the efficiency of acid phosphatase higher in virgin soil (Table 8).

|

|
This agreed with the findings of Dick (1992), who reported a decrease in acid phosphatase activity when native soil is cultivated. This effect was also visible from the negative but strong correlation between acid phosphatase and SOC, β-glucosidase, alkaline phosphatase and urease activity (Table 9).

|
The patterns of SOM and SOC distribution and β-glucosidase, alkaline phosphatase and urease activity were similar (P > 0.01), but stratification ratios were generally higher (P ≤ 0.01) than those of organic matter indicators (Fig. 2). β-Glucosidase activity had a very high correlation (r = 0.94) with SOC, since it is involved in the C cycle. Although urease and alkaline phosphatase are not directly involved in C turnover, the correlations between them and SOC were also high (r = 0.90 and 0.88, respectively), proving the important indirect functions of SOM in the P and N cycles. Correlation between urease and total N was very high (r = 0.95), but a correlation between alkaline phosphatase and P content was moderate (r = 0.60).

|
Stratification ratios >2 were reported for β-glucosidase, urease and alkaline phosphatase activities in the virgin soil, which was not the case of the organic matter indicators. However, for β-Glucosidase and urease, the cultivated pasture soil had much higher stratification ratios (P ≤ 0.01) than the virgin soil. It could therefore be speculated that the thresholds for stratification ratios of enzymatic activities should be higher to indicate improvement of the system. Availability of C sources at the various depths and, therefore, microbial activity should influence the different enzymatic activities. The correlation between enzymatic activities and MBC were, however, moderate to low (r = 0.59, Table 9).
Functional diversity
The mechanism of colour development in Biolog EcoPlates is associated with differences in C-source utilisation relating to the number of viable microorganisms with the ability to utilise the substrates within the EcoPlate wells as a sole C source. The results of this research also confirm findings by Garland and Mills (1991) that the direct incubation of environmental samples in EcoPlates produced patterns of metabolic response useful in the characterisation of soil microbial communities. From Fig. 3, it is clear that the functional diversity differed between the virgin soil and the cultivated pasture soil.

|
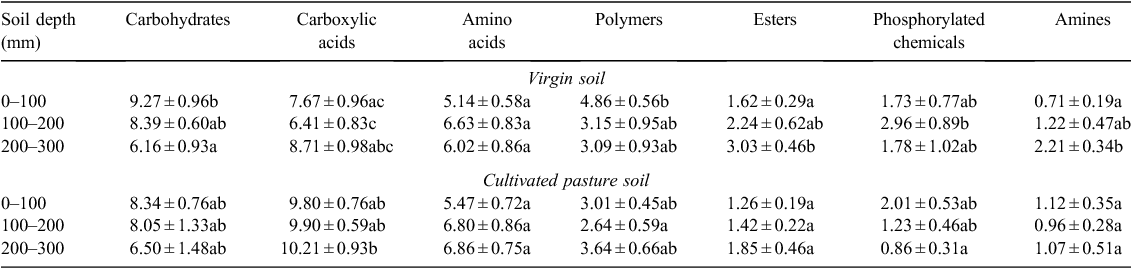
In accordance with results found by Bissett et al. (2011), results obtained in this study indicated no significant (P > 0.05) differences in overall CLPP between sites or sampling depths. Observed changes in overall CLPP can be attributed to the very different plant community composition, as well as the quality and quantity of SOM available with increased soil depth (Wardle et al. 2004) in the two sites. The composition of pasture crops on cultivated soil altered the functional composition of the responsive soil microbial community, as determined by the composition of root exudates, which is greatly influenced by the crop present (Bardgett et al. 1999; Stephan et al. 2000). The difference in root-exudate composition between crops thus contributed to the difference in physiological profiles of soil microbial populations between the sites. The released root exudates attract microbial populations that are especially well adapted to utilise the specific compounds very rapidly (Garbeva et al. 2004). Utilisation of substrate guilds by soil microbial communities in the virgin soil and cultivated pasture soil at three depths is illustrated in Table 10.
|
No significant differences (P > 0.05) in utilisation patterns of all substrate guilds were observed at the same depths between the two sites, except for carboxylic acid utilisation at 100–200 mm and esters and amines at the 200–300 mm depth. Results clearly indicated that carboxylic acids and amino acids were more readily utilised in the cultivated pasture soil, whereas the remainder of the substrate guilds were more readily utilised in the virgin soil. It appears that substrate guilds were equally utilised in the cultivated pasture soil, irrespective of depth. Virgin soil, on the other hand, showed significant (P ≤ 0.05) differences in carbohydrate, ester and amine utilisation at increased soil depth.
Cluster analysis for the soil microbial functional diversity in the cultivated pasture soil and the virgin soil is illustrated in Fig. 4. Cluster analysis assigns treatments into groups, so that treatments in the same cluster are more similar to each other than to treatments in other clusters. Distinctive clusters could be observed between CLPP in the cultivated pasture soil and in the virgin soil. Carbon source utilisation in the cultivated pasture soil was more similar at 0–100 and 100–200 mm depths, compared with 200–300 mm depth. The virgin soil demonstrated a slightly different pattern, in which substrate utilisation was more similar at 100–200 and 200–300 mm depths than at 0–100 mm depth. The distinctive difference in clustering between the two sites could largely be attributed to higher SOM, SOC, active C, MBC and total N values in cultivated pasture soil, which decreased with depth until values became comparable in the 200–300 mm soil layer.

|
Biodiversity indices
Soil microbial diversity was determined by the Shannon–Weaver diversity index, which distinguished between the two sites’ soil microbial communities based on the number of different C sources utilised in Biolog EcoPlates. Values of this index typically range from 1.5 to 3.5, and rarely increase to >4.5 (Magurran 1988). A moderate to high percentage of C sources were utilised, with average values of 2.7 and 2.6 in cultivated pasture soil and virgin soil, respectively (Tables 11 and 12).

|

|
From Table 11 and Table 12, it is clear that a higher soil microbial diversity was present in the cultivated pasture soil than the virgin soil. Since the Shannon–Weaver index is based on the amount of C sources utilised, it is clear that the amount of available C sources declined with depth, i.e. a decline in microbial diversity. No difference (P ≤ 0.05) in microbial diversity was observed between depths in the cultivated pasture soil (Table 11). A significant difference (P ≤ 0.05), however, could be observed in the virgin soil between the 0–100 mm depth with the highest microbial diversity, and the 100–200 and 200–300 mm depths with lower diversity. This observation might be attributed to the absence of any soil disturbance to distribute the top-layer SOM to the lower depths to make it more readily available to the microbial communities at levels deeper than 100 mm.
The Evenness (equitability) index (E), a derivative of the Shannon–Weaver index, was used as an indication of equality of abundance of species within a soil microbial population. Substrate Evenness indices ranged between 0.8 and 0.9 (Table 11). According to Magurran (1988), substrate evenness assumes a value between 0 and 1, with 1 presenting a situation in which all species are equally abundant. This results in less variation in microbial populations between species, therefore, less dominance and higher diversity. Soil microbial species in the cultivated pasture soil were more equally abundant within the microbial community, compared with the virgin soil. The significant decrease (P ≤ 0.05) in species abundance, resulting in increased species dominance, at different depths in the virgin soil, as well as between the various depths in the virgin soil compared with corresponding depths in the cultivated pasture soil (Table 12), suggested an increase in particular microbial species within the virgin soil specialising in the utilisation of specific C sources released by the high diversity of fynbos vegetation present at the site. This observation is also supported by the lowest soil microbial diversity present in the virgin soil, i.e. different soil microbial species within microbial populations within the virgin soil became less equally abundant, but more specialised, thus dominating the virgin soil. The difference in microbial diversity between the two sites might also be attributed to the irrigation of the cultivated pasture soil, whereas the fynbos site is solely dependent on rainfall.
Soil health appraisal
Figure 5 represents a star plot of the virgin soil and cultivated pasture soil. The area of the stars may be interpreted as the biological activity and vigour of the soil. It is clear that the cultivated pasture soil had higher biological activity and vigour than the virgin soil, and conversion of natural vegetation to cultivated pasture improved soil health and markedly modified the biological component of the soil.

|
Conclusion
The importance of SOM to maintain soil health of a sandy podzolic soil and a balance between environmental sustainability and agricultural production is emphasised by biological soil properties. Conversion of virgin soil to irrigated cultivated pasture soil improved environmental conditions, supported the build-up of SOM levels and, in effect, enhanced the soil’s microbial component, ecosystem functionality and soil health. This was reflected by the distribution patterns, stratification and degree of organic-matter indicator accumulation and enzymatic activities. Soil microbial functional diversity in the cultivated pasture soil and virgin soil was greatly influenced by plant species present and root exudate composition. The soil microbial diversity between cultivated pasture soil and the virgin soil was significantly altered, especially at different soil depths. The plant diversity present influenced the composition of root exudates, thus contributing to the difference in microbial diversity. The released root exudates attracted microbial species that were well adapted to utilise the specific compounds very rapidly. A general appraisal of biological soil properties indicated that conversion of natural fynbos vegetation to irrigated, minimum-till, kikuyu–ryegrass pasture after 19 years of cultivation on a podzolic soil is beneficial. The indicator values reported may also serve as baseline values, since such reference values for the southern Cape region of South Africa are not available; however, the initial data from soil quality studies on the Outeniqua Research Farm near George in South Africa should offer the best reference values and can be amended with future research.
Acknowledgements
The Western Cape Department of Agriculture in South Africa is gratefully acknowledged for funding this project. Mr Brian Zulu and his team of farm aids need special reference for their hard work while executing the project. Appreciation is extended to Mrs M.F. Smith from stats-4-science in Pretoria, South Africa for valuable assistance with statistical analyses.
References
Allison LE (1965) Organic carbon. In ‘Methods of soil analysis. Part 2. Chemical and microbiological properties’. (Eds CA Black, DD Evans, JL White, LE Ensminger, FE Clark, RC Dinauer) pp. 1367–1378. (American Society of Agronomy: Madison, WI)ARC-ISCW (2012) ‘Agro-climatology database.’ Agricultural Research Council’s Institute for Soil Climate and Water. (Agricultural Research Council: Pretoria, South Africa)
Arias ME, Gonzalez-Perez JA, Gonzalez-Vila FJ, Ball AS (2005) Soil health—a new challenge for microbiologists and chemists. International Microbiology 8, 13–21.
Arshad M, Martin S (2002) Identifying critical limits for soil quality indicators in agro-ecosystems. Agriculture, Ecosystems & Environment 88, 153–160.
| Identifying critical limits for soil quality indicators in agro-ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bardgett RD, Mawdsley JL, Edwards S, Hobbs PJ, Rodwells JS, Davies WJ (1999) Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Functional Ecology 13, 650–660.
| Plant species and nitrogen effects on soil biological properties of temperate upland grasslands.Crossref | GoogleScholarGoogle Scholar |
Bissett A, Richardson AE, Baker G, Thrall PH (2011) Long-term land use effects on soil microbial community structure and function. Applied Soil Ecology 51, 66–78.
| Long-term land use effects on soil microbial community structure and function.Crossref | GoogleScholarGoogle Scholar |
Blake GR (1965) Bulk density. In ‘Methods of soil analysis: Part 1, Physical and mineralogical properties, including statistics of measurement and sampling’. (Ed. CA Black) pp. 374–390. (American Society of Agronomy: Madison, WI)
Boshoff AF, Kerley GIH, Cowling RM (2001) A pragmatic approach to estimating the distributions and spatial requirements of the medium- to large-sized mammals in the Cape Floristic Region, South Africa. Diversity & Distributions 7, 29–43.
| A pragmatic approach to estimating the distributions and spatial requirements of the medium- to large-sized mammals in the Cape Floristic Region, South Africa.Crossref | GoogleScholarGoogle Scholar |
Bossio DA, Girvan MS, Verchot L, Bullimore J, Borelli T, Albrecht A, Scow KM, Ball AS, Pretty JN, Osborn AM (2005) Soil microbial community response to land use change in an agricultural landscape of Western Kenya. Microbial Ecology 49, 50–62.
| Soil microbial community response to land use change in an agricultural landscape of Western Kenya.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslyis70%3D&md5=27120c211d7ce37c3dbb74752474976bCAS | 15690227PubMed |
Botha PR (2003) Die produksiepotensiaal van oorgesaaide kikoejoeweiding in die gematigde kusgebied van die Suid-Kaap. PhD Thesis, University of the Free State, Bloemfontein, South Africa.
Bremner J (1960) Determination of nitrogen in soil by the Kjeldahl method. The Journal of Agricultural Science 55, 11–33.
| Determination of nitrogen in soil by the Kjeldahl method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXms12isQ%3D%3D&md5=00999fc45c464c94ac275548b49699e0CAS |
Broadbent FE (1965) Organic matter. In ‘Methods of soil analysis. Part 2. Chemical and microbiological properties’. (Eds CA Black, DD Evans, JL White, LE Ensminger, FE Clark, RC Dinauer) pp. 1397–1400. (American Society of Agronomy: Madison, WI)
Bronick C, Lal R (2005) Soil structure and management: a review. Geoderma 124, 3–22.
| Soil structure and management: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOru7jP&md5=13b1caddb93603f12795c258ca7ad29aCAS |
Buyer JS, Drinkwater LE (1997) Comparison of substrate utilization assay and fatty acid analysis of soil microbial communities. Journal of Microbiological Methods 30, 3–11.
| Comparison of substrate utilization assay and fatty acid analysis of soil microbial communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlslersLo%3D&md5=b574544c68747106bb84917148ad1461CAS |
Carter M (1986) Microbial biomass as an index for tillage-induced changes in soil biological properties. Soil & Tillage Research 7, 29–40.
| Microbial biomass as an index for tillage-induced changes in soil biological properties.Crossref | GoogleScholarGoogle Scholar |
Carter MR, Rennie DA (1982) Changes in soil quality under zero tillage farming systems: Distribution of microbial biomass and mineralizable C and N potentials. Canadian Journal of Soil Science 62, 587–597.
| Changes in soil quality under zero tillage farming systems: Distribution of microbial biomass and mineralizable C and N potentials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXovVWgtQ%3D%3D&md5=f270b01b565e6890a76d0f1068065338CAS |
Conant RT, Paustian K, Elliott ET (2001) Grassland management and conversion into grassland: effects on soil carbon. Ecological Applications 11, 343–355.
| Grassland management and conversion into grassland: effects on soil carbon.Crossref | GoogleScholarGoogle Scholar |
Conyers MK, Poile GJ, Oates AA, Waters D, Chan KY (2011) Comparison of three carbon determination methods on naturally occurring substrates and the implication for the quantification of ‘soil carbon’. Soil Research 49, 27–33.
| Comparison of three carbon determination methods on naturally occurring substrates and the implication for the quantification of ‘soil carbon’.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXit1Wgu78%3D&md5=d5a49129fa4dcdf6c8184879b1439e75CAS |
Curci M, Pizzigallo MDR, Crecchio C, Mininni R, Ruggiero P (1997) Effects of conventional tillage on biochemical properties of soils. Biology and Fertility of Soils 25, 1–6.
| Effects of conventional tillage on biochemical properties of soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXktV2isbg%3D&md5=a1aee1bc4c80b60539e2522e6bfa7570CAS |
Day PR (1965) Particle fractionation and particle-size analysis. In ‘Methods of soil analysis: Part 1, Physical and mineralogical properties, including statistics of measurement and sampling’. (Ed. CA Black) pp. 545–567. (American Society of Agronomy: Madison, WI)
De Fede KL, Panaccione DG, Sexstone AJ (2001) Characterization of dilution enrichment cultures obtained from size-fractioned soil bacteria by BIOLOG® community-level physiological profiles and restriction analysis of 16S rRNA genes. Soil Biology & Biochemistry 33, 1555–1562.
| Characterization of dilution enrichment cultures obtained from size-fractioned soil bacteria by BIOLOG® community-level physiological profiles and restriction analysis of 16S rRNA genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFSlsL8%3D&md5=743a0d9a3f0d4aac7a702862af006f1cCAS |
Desjardins T, Barros E, Sarrazin M, Girardin C, Mariotti A (2004) Effects of forest conversion to pasture on soil carbon content and dynamics in Brazilian Amazonia. Agriculture, Ecosystems & Environment 103, 365–373.
| Effects of forest conversion to pasture on soil carbon content and dynamics in Brazilian Amazonia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVOgs7g%3D&md5=406e30df10d5a45e9f193f6690e4be28CAS |
Dick RP (1992) A review: long-term effects of agricultural systems on soil biochemical and microbial parameters. Agriculture, Ecosystems & Environment 40, 25–36.
| A review: long-term effects of agricultural systems on soil biochemical and microbial parameters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltFSlurk%3D&md5=6d3da0e6f52c3269d44adb775ad9b591CAS |
Dick RP, Myrold DD, Kerle EA (1988) Microbial biomass and soil enzyme-activities in compacted and rehabilitated skid trail soils. Soil Science Society of America Journal 52, 512–516.
| Microbial biomass and soil enzyme-activities in compacted and rehabilitated skid trail soils.Crossref | GoogleScholarGoogle Scholar |
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In ‘Methods for assessing soil quality’. SSSA Special Publication No. 49. (Eds JW Doran, AJ Jones) pp. 247–271. (Soil Science Society of America: Madison, WI)
Drinkwater L, Cambardella C, Reeder J, Rice CW, Doran J, Jones AJ (1996) Potentially mineralizable nitrogen as an indicator of biologically active soil nitrogen. In ‘Methods for assessing soil quality’. SSSA Special Publication No. 49. (Eds JW Doran, AJ Jones) pp. 217–229. (Soil Science Society of America: Madison, WI)
Edenborn S, Sexstone A, Sutanto Y, Chapman J (2011) Relationships among contrasting measurements of microbial dynamics in pasture and organic farm soils. Applied and Environmental Soil Science 2011, 1–10.
| Relationships among contrasting measurements of microbial dynamics in pasture and organic farm soils.Crossref | GoogleScholarGoogle Scholar |
Ernst O, Siri-Prieto G (2009) Impact of perennial pasture and tillage systems on carbon input and soil quality indicators. Soil & Tillage Research 105, 260–268.
| Impact of perennial pasture and tillage systems on carbon input and soil quality indicators.Crossref | GoogleScholarGoogle Scholar |
Franzluebbers A (2002) Soil organic matter stratification ratio as an indicator of soil quality. Soil & Tillage Research 66, 95–106.
| Soil organic matter stratification ratio as an indicator of soil quality.Crossref | GoogleScholarGoogle Scholar |
Garbeva P, Van Veen JA, Van Elsas JD (2004) Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology 42, 243–270.
| Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotFyrtbk%3D&md5=6c563d0bd593276017603ffbeecec495CAS | 15283667PubMed |
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biology & Biochemistry 28, 213–221.
| Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtlClurs%3D&md5=3222b9519dc236c59a0dd637eb5c6538CAS |
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Applied and Environmental Microbiology 57, 2351–2359.
Granatstein DM, Bezdicek D, Cochran V, Elliott L, Hammel J (1987) Long-term tillage and rotation effects on soil microbial biomass, carbon and nitrogen. Biology and Fertility of Soils 5, 265–270.
| Long-term tillage and rotation effects on soil microbial biomass, carbon and nitrogen.Crossref | GoogleScholarGoogle Scholar |
Green VS, Stott DE, Cruz JC, Curi N (2007) Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil & Tillage Research 92, 114–121.
| Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol.Crossref | GoogleScholarGoogle Scholar |
Habig J (2003) Soilborne disease suppressiveness/conduciveness: analysis of microbial community dynamics. MSc (Environmental Science) Thesis, School of Environmental Sciences and Development, North-West University, Potchefstroom, South Africa.
Haynes RJ, Tregurtha R (1999) Effects of increasing periods under intensive arable vegetable production on biological, chemical and physical indices of soil quality. Biology and Fertility of Soils 28, 259–266.
| Effects of increasing periods under intensive arable vegetable production on biological, chemical and physical indices of soil quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnslKjur8%3D&md5=882774ebe9c8a68744d8771ba0967077CAS |
Insam H, Domsch K (1988) Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microbial Ecology 15, 177–188.
| Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c7ktVCktA%3D%3D&md5=279e541ff49105a275ecef2b46431c59CAS | 24202999PubMed |
Islam K, Weil R (1998) Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biology and Fertility of Soils 27, 408–416.
| Microwave irradiation of soil for routine measurement of microbial biomass carbon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlvFensro%3D&md5=251d8796a3f725e4a47c38465f8fd766CAS |
IUSS Working Group (2006) ‘World reference base for soil resources.’ 2nd edn. World Soil Resources Report No. 103. (FAO: Rome)
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biology and Fertility of Soils 6, 68–72.
| Short-term assay of soil urease activity using colorimetric determination of ammonium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXktlOitb8%3D&md5=0bf1bcd37622de104028e33cfe1ba67eCAS |
Karlen D, Rosek M, Gardner J, Allan D, Alms M, Bezdicek D, Flock M, Huggins D, Miller B, Staben M (1999) Conservation Reserve Program effects on soil quality indicators. Journal of Soil and Water Conservation 54, 439–444.
López M, Arrúe J, Sánchez-Girón V (1996) A comparison between seasonal changes in soil water storage and penetration resistance under conventional and conservation tillage systems in Aragon. Soil & Tillage Research 37, 251–271.
| A comparison between seasonal changes in soil water storage and penetration resistance under conventional and conservation tillage systems in Aragon.Crossref | GoogleScholarGoogle Scholar |
López-Garrido R, Madejón E, Murillo JM, Moreno F (2011) Soil quality alteration by mouldboard ploughing in a commercial farm devoted to no-tillage under Mediterranean conditions. Agriculture, Ecosystems & Environment 140, 182–190.
| Soil quality alteration by mouldboard ploughing in a commercial farm devoted to no-tillage under Mediterranean conditions.Crossref | GoogleScholarGoogle Scholar |
Magurran AE (1988) ‘Ecological diversity and its measurement.’ (Princeton University Press: Princeton, NJ)
Martens R (1995) Current methods for measuring microbial biomass C in soil: potentials and limitations. Biology and Fertility of Soils 19, 87–99.
| Current methods for measuring microbial biomass C in soil: potentials and limitations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkvVCkt7Y%3D&md5=695f99facae8bc7b6e2976a340340f2fCAS |
Miles N, Manson AD (2000) Nutrition of cultivated pastures. In ‘Pasture management in South Africa’. (Ed. NM Tainton) pp. 180–232. (University of Natal Press: Pietermaritzburg, South Africa)
Moore J, Klose S, Tabatabai M (2000) Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biology and Fertility of Soils 31, 200–210.
| Soil microbial biomass carbon and nitrogen as affected by cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsFKjur8%3D&md5=f96c884da3c8645a1cf55d5c486b05daCAS |
Mucina L, Rutherford MC (2006) ‘The vegetation of South Africa, Lesotho and Swaziland.’ Strelitzia 19. (South African National Biodiversity Institute: Pretoria, South Africa)
Müller-Stöver D, Hauggaard-Nielsen H, Eriksen J, Ambus P, Johansen A (2012) Microbial biomass, microbial diversity, soil carbon storage, and stability after incubation of soil from grass–clover pastures of different age. Biology and Fertility of Soils 48, 371–383.
| Microbial biomass, microbial diversity, soil carbon storage, and stability after incubation of soil from grass–clover pastures of different age.Crossref | GoogleScholarGoogle Scholar |
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In ‘Methods of soil analysis: Part 2, Chemical and microbiological properties’. (Ed. AL Page) pp. 539–579. (American Society of Agronomy: Madison, WI)
Non-Affiliated Soil Analysis Work Committee (1990) ‘Handbook of standard soil testing methods for advisory purposes.’ (Soil Science Society of South Africa: Pretoria, South Africa)
Palojärvi A, Sharma S, Rangger A, Von Lutzow M, Insam H (1997) Comparison of BIOLOG® and Phospholipid Fatty acid patterns to detect changes in microbial community. In ‘Microbial communities—Functional versus structural approaches’. (Eds H Insam, A Rangger) pp. 37–48. (Springer-Verlag: Berlin, Heidelberg)
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2011) ‘An introduction to Genstat for Windows (14th edn),’ (VSN International: Hemel Hempstead, UK)
Skjemstad JO, Lefeuvre RP, Prebble RE (1990) Turnover of soil organic matter under pasture as determined by 13C natural abundance. Australian Journal of Soil Research 28, 267–276.
| Turnover of soil organic matter under pasture as determined by 13C natural abundance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtFSlurw%3D&md5=7c458cc3211acb6fe18128c0c815634eCAS |
Soil Classification Working Group (1991) ‘Soil Classification: a taxonomic system for South Africa.’ Memoirs on the Agricultural Natural Resources of South Africa No. 15. (Department of Agricultural Development: Pretoria, South Africa)
Soil Survey Staff (2003) ‘Keys to Soil Taxonomy.’ (Department of Agriculture: Washington, DC)
Stephan A, Meyer AH, Schmid B (2000) Plant diversity affects culturable soil bacteria in experimental grassland communities. Journal of Ecology 88, 988–998.
| Plant diversity affects culturable soil bacteria in experimental grassland communities.Crossref | GoogleScholarGoogle Scholar |
Swanepoel PA, Botha PR (2012a) Sustainability of cultivated pastures in a no-till system. In ‘Proceedings of the 47th Congress of the Grassland Society of Southern Africa’. p. 123. (Grassland Society of Southern Africa: Langebaan, South Africa)
Swanepoel PA, Botha PR (2012b) Predicting soil organic carbon content from loss-on-ignition. In ‘Proceedings of the Combined Congress’. 16–19 January 2012. p. 254. (NWU-Potchefstroom: South Africa)
Swanepoel PA, Botha PR, du Preez CC, Snyman HA (2013) Physical quality of a podzolic soil following 19 years of irrigated minimum-till kikuyu-ryegrass pasture. Soil & Tillage Research 133, 10–15.
| Physical quality of a podzolic soil following 19 years of irrigated minimum-till kikuyu-ryegrass pasture.Crossref | GoogleScholarGoogle Scholar |
Van Antwerpen R, Berry S, Van Antwerpen T, Sewpersad C, Cadet P (2009) Indicators of soil health for use in the South African sugar industry: a work in progress. Proceedings of the South African Sugar Technologists’ Association 82, 551–563.
Van der Colf J (2011) The production potential of kikuyu (Pennisetum clandestinum) pastures over-sown with ryegrass (Lolium spp.). MSc Thesis, University of Pretoria, Pretoria, South Africa.
Verhulst N, Govaerts B, Verachtert E, Castellanos-Navarrete A, Mezzalama M, Wall P, Chocobar A, Deckers J, Sayre K (2010) ‘Conservation agriculture, improving soil quality for sustainable production systems. Advances in soil science: food security and soil quality.’ (CRC Press: Boca Raton, FL)
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633.
| Ecological linkages between aboveground and belowground biota.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXks1Cgsrs%3D&md5=eb1efe1da381917d703ba1c4c3e9dd8bCAS | 15192218PubMed |
Weil RR, Islam KR, Stine MA, Gruver JB, Samson-Liebig SE (2003) Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. American Journal of Alternative Agriculture 18, 3–17.
| Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use.Crossref | GoogleScholarGoogle Scholar |
Winding A, Hendriksen NB (1997) BIOLOG® substrate utilisation assay for metabolic fingerprints of soil bacteria: incubation effects. In ‘Microbial communities: Functional versus structural approaches’. (Eds I Insam, A Ranger) pp. 195–205. (Springer-Verlag: Berlin)