Microbial community diversity and enzyme activity varies in response to long-term fertilisation in a continuous potato (Solanum tuberosum L.) cropping system
Haotian Yuan A B , Meilian Meng A * , Youjun Chen C , Shenghui Yang A , Tingting Zhang B , Chunlei Xue B and Jiangan Guo B
A B , Meilian Meng A * , Youjun Chen C , Shenghui Yang A , Tingting Zhang B , Chunlei Xue B and Jiangan Guo B
A College of Agronomy, Inner Mongolia Agricultural University, Hohhot 010018, People’s Republic of China.
B Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences, Hohhot 010030, People’s Republic of China.
C College of Life Science, Inner Mongolia Agricultural University, Hohhot 010018, People’s Republic of China.
Soil Research 61(3) 224-240 https://doi.org/10.1071/SR22015
Submitted: 21 January 2022 Accepted: 21 October 2022 Published: 18 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The misuse of chemical fertilisers is still prevalent in potato cultivation. However, the large-scale application of manure is increasingly being accepted by growers to improve soil health.
Aim: To clarify what effect manure and chemical fertilisers have on the development of soil microbes in potato fields.
Methods: The status of soil microbial community abundance and structure were determined by analysing soil metagenomes, which were assessed by applying high through-put sequencing technology. The potato field received one of the following treatments for 9 years: manure (M); manure plus nitrogenous (N), phosphatic (P), and potassic (K) fertiliser (MNPK); NPK fertiliser; NP fertiliser; NK fertiliser; PK fertiliser; and no fertiliser.
Key results: The application of manure significantly increased soil organic matter, and this increase was one of the main factors in reducing bacterial Shannon and Heip indices and increasing the Simpson index. The MNPK treatment significantly reduced the relative abundance of α-Proteobacteria and β-Proteobacteria in the soil, which are essential microorganisms involved in nitrogen cycling. The M treatment significantly increased the abundance of Actinobacteria.
Conclusions: In summary, applying manure increases Actinobacteria’s abundance, and using MNPK fertilisers decreases the abundance of Proteobacteria, whereas chemical fertilisers are detrimental to soil fungal diversity.
Implications: Applying MNPK fertilisers has a complex effect on soil microorganisms. It is not simply a combination of the effects of manure and chemical fertilisers on microbes; the interaction mechanism of microbial succession needs to be further explored.
Keywords: bacteria, community diversity, enzyme activity, fertilisation, fungi, microbial abundance, potato, soil microbes.
Introduction
Soil is the natural medium in which crops are grown, and the microorganisms within the soil play an important role in promoting the cycling of nutrients and energy in farmland ecosystems. Improving the ability of crops to obtain nutrients by exploiting the role of soil microorganisms has been the focus of global sustainable agricultural development in recent decades (Freitas et al. 2007). The Food and Agriculture Organization (FAO) of the United Nations promotes sustainable soil management using means that increase soil organic matter (OM) content in the surface layer of farmland soil and create a suitable environment for the survival and reproduction of beneficial microorganisms, which in turn promote the better utilisation of soil nutrients by crops (FAO 2016). In resource-poor developing countries, low soil fertility remains the most important factor limiting increase in crop yield (Mohammadi and Sohrabi 2012). Thus, growers often use large amounts of nitrogen and phosphate fertilisers to promote yield, but this can lead to soil acidification and consequently reduce crop yield and quality over time (Liang et al. 2013).
Physical, chemical, and biological characteristics of fertile soil and the chemical fertility of soil are affected by biological processes (Abbott and Murphy 2007). Soil biological fertility involves direct or indirect activities of microorganisms, such as organic compound mineralisation and mineral dissolution facilitated by microorganisms. Soil biological fertility can be quantified by determining the abundance, diversity, and level of activities of microbial communities (Lemanceau et al. 2015). Studies have shown that the microbes that promote plant growth are members of common bacterial and fungal genera found in soils. Bacillus, Enterobacter, Rhizobium, Bradyrhizobium, Pantoea, Erwinia, and Pseudomonas are the common bacterial genera, and Aspergillus, Trichoderma, and Penicillium are the common fungal representatives found in soil (Marschner 2008; Saharan and Nehra 2011; Ahemad and Kibret 2014; Yadav 2017). Maintaining the diversity and activity of beneficial microorganisms in soil promotes the continuous absorption of nitrogen and phosphorus by the crops present (Barea et al. 2007; Richardson et al. 2009; Lugtenberg et al. 2013; De Bruijn 2015).
The application of nitrogen and phosphate fertilisers can greatly affect soil microorganisms in farmland, and thus microbial activity in farmland ecosystems is often limited by unsuitable fertilisation management (Fan et al. 2020). Fungi often play a more important role in the decomposition and transformation of soil nutrients when fertiliser supplies are scarce (Bardgett et al. 1999). Changes in soil-available nitrogen and phosphorus contents can alter the structure and function of the microbial community (Cruz et al. 2009; Das et al. 2017). Mainly, available nitrogen content strongly influences changes in the microbial community (Liu et al. 2020a). Ammonium-nitrogen fertiliser had a more significant negative influence on microbial community structure than manure application; the abundance ratio of fungi to bacteria in manure treatment was significantly higher than that in chemical fertiliser treatment, and the ratio in the no fertiliser treatment was similar to that in manure treatment (Suzuki et al. 2005). Liu et al. (2020b) and Zhang et al. (2019) found that soil fertility was positively correlated with the α-diversity of fungi in agricultural soils. Compared with abundances in abandoned farmland, long-term chemical fertiliser application significantly reduced the abundance of bacteria and fungi (Li et al. 2005).
As one of the most important food crops, potato (Solanum tuberosum L.) is widely cultivated and produced worldwide. Continuous progress in potato production requires sustained soil health and high microbial activity. Rational application of fertilisers can improve soil microbial diversity and community structure (Gu et al. 2019), and potato’s growing environment. However, the effects of long-term application of different fertilisers on soil microbial diversity and community structure in potato fields have rarely been reported. Hence, this study aims to explore the changes in soil microbial species abundance and community structure in a continuous potato cropping system exposed to long-term fertilisation with different organic and chemical sources of nutrients.
Materials and methods
Experimental design and soil sampling
The long-term fertilisation experiment was conducted in a potato field from 2010 to 2018 in the northern foothills of Yinshan Mountains (41°10′57″N, 111°36′17″E, 1606 m above sea level), which is one of the main potato-producing areas in China (Fig. 1). The area has a mid-temperate continental climate with abundant sunshine and large temperature differences during the day and night. The average annual temperature is 2.4°C; the active accumulated temperature is about 2000°C from mid-May to mid-September. The annual average precipitation is about 350 mm, mostly concentrated in summer, and the annual evaporation is 2068 mm, which is almost six times the annual average precipitation. The annual frost-free period varies in the range of 110–120 days. Soils here belong to the group Calcic Kastanozem.
The cropping system was continuous summer potato cultivation (variety Kexin 1) followed by winter fallow. We established seven fertiliser treatments: (1) manure (M), (2) inorganic nitrogen, phosphorus, and potassium fertilisers (NPK), (3) manure combined with NPK (MNPK), (4) inorganic nitrogen and phosphorus fertilisers (NP), (5) inorganic nitrogen and potassium fertilisers (NK), (6) inorganic phosphorus and potassium fertilisers (PK), and (7) a no-fertiliser control (CK). Annual NPK fertilisation comprised 150 kg N hm−2 as urea, 75 kg P2O5 hm−2 as calcium superphosphate, and 270 kg K2O hm−2 as potassium sulfate. Annual sheep manure (0.6%N, 0.5%P2O5, 0.2%K2O) application rate was 22 500 kg hm−2. The treatments, each with four replicate plots, were set up using a randomised block design (the design was unchanged throughout the entire period). The potato seed tubers with a diameter of about 2 cm were sown in a ditch at a depth of 8 cm, the proposed fertiliser was applied all at once. The experimental fields were manually weeded and watered by trickle irrigation. Field management practices were the same in all treatments throughout the experimental period.
On 24 August 2018, three intact soil samples were randomly collected at the tillage horizon (0–20 cm) in each plot using a stainless-steel soil sampler with a diameter of 5 cm. All samples from each plot were carefully mixed to form a composite sample and transported to the laboratory in a constant temperature box containing ice. Each sample was divided into two parts: one part was air-dried for determination of the physicochemical properties and the other part was sealed in a plastic bag with Drikold® (−78.5°C) for determination of the metagenome of soil microorganisms.
Soil properties analysis
Soil pH was measured by using a glass electrode (FE20-FiveEasy™ pH; Mettler Toledo, Weilheim, Baden-Württemberg, Germany) with a 1:1 soil-to-water ratio. Soil humidity was measured by using a soil water content meter (Diviner2000; Sentek, Adelaide, Australia). Soil available potassium (AK) was extracted using 1 M ammonium acetate and measured using flame photometry (FP640 Flame Photometer; INESA, Shanghai, China). Soil available nitrogen (AN) was calculated as ammonia-nitrogen using the methods described in detail in Bremner and Shaw (1955). Soil available phosphorus (AP) was extracted using 0.5 M NaHCO3, and its concentration determined using the molybdenum blue method with colorimetry (660 nm) (Holman 1943). Soil OM was determined using dilution heat method with a 1:0.2 K2Cr2O7-to-H2SO4 ratio, and direct titration with 0.1 M FeSO4 (Bisutti et al. 2004).
DNA extraction and metagenome sequencing analysis
Bacterial and fungal community genomic DNA was extracted from each soil sample using the DNeasy® PowerSoil® DNA Kit (QIAGEN, Redwood City, CA, USA) according to the manufacturer’s instructions. The quality, purity, and concentration of the extracted DNA were determined by 1% agarose gel electrophoresis and NanoDrop™ 2000 UV–vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The hypervariable region V3–V4 of the bacterial 16S rRNA gene was amplified with primer pairs 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′). The internal transcribed spacer (ITS) region of rRNA gene was amplified with primer pairs V43NDF (5′-GGCAAGTCTGGTGCCAG-3′) and EukV4R (5′-ACGGTATCTRATCRTCTTCG-3′). All rRNA genes were amplified using an ABI GeneAmp® 9700 PCR thermocycler (ABI, Carlsbad, CA, USA). The purifying and quantifying of the extracted PCR product were performed with 2% agarose gel, AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and a Quantus™ Fluorometer (Promega, Madison, WI, USA).
Library construction was performed using the NEXTFLEX® Rapid DNA-Seq Kit (Bioo Scientific, Austin, TX, USA). Purified amplicons were pooled in equimolar and paired-end sequenced on a MiSeq PE300 platform platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw rRNA gene sequencing reads were deposited into the Nucleotide Sequence Database. The raw reads were demultiplexed, quality-filtered by fastp ver. 0.20.0 (Chen et al. 2018) and merged by FLASH ver. 1.2.7 (Magoč and Salzberg 2011). Using UPARSE ver. 7.1 (Edgar 2013), operational taxonomic units (OTUs) with 97% similarity cut-off were clustered and chimeric sequences were identified and removed. Representative sequences were annotated by RDP Classifier ver. 2.2 (Wang et al. 2007) against the Silva v138 (http://www.arb-silva.de).
Soil enzymatic activity analysis
Enzyme activities were determined using the following methods. The soil urease (EC 3.5.1.5) activity was determined using 10% urea solution as the substrate and citrate buffer (pH 6.7); the ammonium released from urea hydrolysis was quantified using absorbance at 578 nm (Evolution™ One Thermo Scientific™, Waltham, MA, USA). The activity level was expressed as NH4+ per gram of dry weight of soil per 24 h (Guan 1986; Nannipieri et al. 2012). The activity of soil invertase (EC 3.2.1.26) was determined, using 8% sucrose solution as the substrate and phosphate buffer (pH 5.5), calorimetrically according to the reaction of glucose with 3,5-dinitrosalicylic acid, taking absorbance at 508 nm (Evolution™ One Thermo Scientific™, Waltham, MA, USA). The value was expressed as glucose per gram of dry weight of soil per 24 h (Guan 1986). Soil alkaline phosphatase (EC 3.1.3.1) activity was measured with disodium phenyl phosphate solution as a substrate and borate buffer (pH 9.6), and absorbance was measured at 660 nm (Evolution™ One Thermo Scientific™, Waltham, MA, USA). The value was expressed as phenol per gram of dry weight of soil per 2 h (Guan 1986). Soil catalase (EC 1.11.1.6) activity was measured with H2O2 (0.3%) as a substrate, the mixture was shaken for 30 min, and then 5 mL of H2SO4 at 1.5 mol L−1 was added. Its filtrate was titrated with 0.002 mol L−1 KMnO4. The reacted amount of KMnO4 was calculated per gram of the sample to represent catalase activity (Roberge 1978).
Statistical analysis
The SPSS Statistics package for Windows (SPSS, ver. 25.0, Chicago, IL, USA) was used for one-way ANOVA of abundances of bacterial and fungal communities and soil enzyme activities. Multiple comparisons were performed using the S–N–K method (Hilton and Armstrong 2006) and a confidence interval of 95%. Because the sequencing depth varied across samples, we used a sub-sampling procedure to normalise the number of reads to the minimum observed across all samples (19 461 reads in bacterial data and 4245 reads in fungal data). The α-diversity indices of microbes were calculated using Mothur (ver. 1.30.2) (Xu et al. 2018). The community abundances of microbes and the calculation of β-diversity were performed by QIIME (ver. 1.9.1) (Caporaso et al. 2010). Using the vegan package of the R language (Dixon 2003), clustering was carried out according to the similarity of species abundance. The results are displayed on the community heatmap map, which clusters high- and low-abundance species in blocks through colour change and similarity degree to reflect the similarities and differences in community composition of different treatments at the genus level. Based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Kanehisa 2002), the PICRUSt software package was used for functional prediction analysis on the 16S and ITS sequencing data. Through the GreenGene ID corresponding to the measured OTU, the Clusters of Orthologous Groups (COG) information and KEGG Orthology (KO) information of the OTU were obtained, and its abundance was calculated. Information about enzymes and enzymatic reactions could be annotated on each OTU. By analysing the OTU quantity data annotated by the target enzyme in each fertiliser treatment, the activity of the target enzyme-catalysed reaction in the corresponding soil was judged.
Results
Soil properties
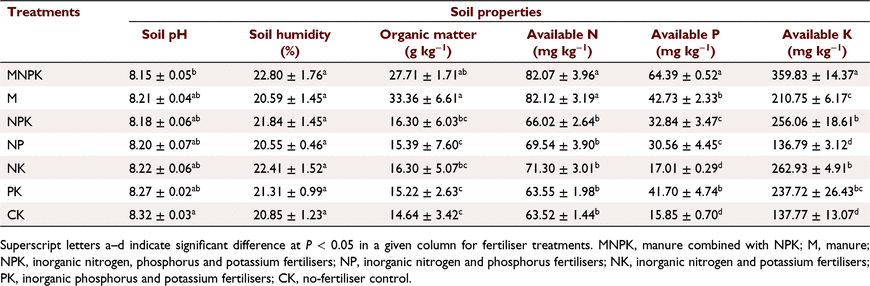
Compared with CK, soil pH was lower in the MNPK soil, but was similar to the soils with the other treatments. The highest OM (33.36 g kg−1) and AN contents (82.12 mg kg−1) were in the M soil, and were similar to the MNPK soil. Application of MNPK also significantly increased AP (64.39 mg kg−1) and AK contents (359.83 mg kg−1) compared to the other treatments (Table 1).
|
Microbial diversity
When analysing the diversity of bacterial and fungal communities, the corresponding α-diversity index value of each sample was used as a Y-axis, and the extracted data volume as a X-axis to draw the distribution rarefaction curves. All curves reached a smooth state, indicating that the sequencing data volume was sufficient to accurately represent the communities in the field soil.
We analysed the diversity of microbial communities from the perspectives of richness and evenness. Fig. 2 shows the α-diversity boxplots of the bacterial community. The Chao1 values for the different treatments showed no significant differences, but the medians of Chao1 values of M and MNPK were lower than those of the lower quartiles of the other treatments, indicating reductions in the richness of the bacterial communities in M and MNPK (Fig. 2a). Analysis of the Shannon index values showed that the CK, NP, and NPK treatments had values higher than the other treatments (Fig. 2c). The range of Heip values for NP was wider than that of the other treatments (Fig. 2b). The Shannon values of M were narrower in range than those of the other treatments, and the medians of Heip and Shannon values of M were lower than those of the other treatments. Compared with M, the medians of Heip and Shannon values for MNPK were higher and wider ranging. The Simpson index represents species richness and evenness, where a greater numerical value indicates that some microbes are more represented than others in samples. The median Simpson’s index value of M was significantly higher than those of NPK and PK. Additionally, MNPK’s upper quartile was also higher than those of the chemical fertiliser treatments (Fig. 2d). All four index values indicated that the application of manure substantially promoted the dominance of particular soil bacterial populations, and reduced evenness of the bacterial community.
Fig. 3 shows the α-diversity indices of fungal communities. Compared with the CK, only the upper quartile of M was higher for the Chao1 index values of the fungal communities, while the upper quartiles of other treatments were lower. The medians and upper quartiles of NPK and NK were much lower than the respective values of the CK (Fig. 3a). These data suggest that manure helped to maintain the richness of the soil fungal community, while NPK fertiliser had the opposite effect. Results of the Heip and Shannon indices suggested that fungal evenness of MNPK and NK was substantially lower compared to those of CK, and the Heip index value of M was closer to that of CK (Fig. 3b). Moreover, the Shannon index value of M was significantly higher than those of MNPK and NK (Fig. 3c). The Simpson index value of MNPK was significantly larger than those of M and CK. The Simpson index values of all fertiliser treatments were higher than that of CK to different degrees, except for that of M (Fig. 3d). Overall, the results showed that soil fungal communities were more sensitive to inorganic fertilisers, while manure had little to no effect on altering the diversity of the fungal communities in potato field soil.
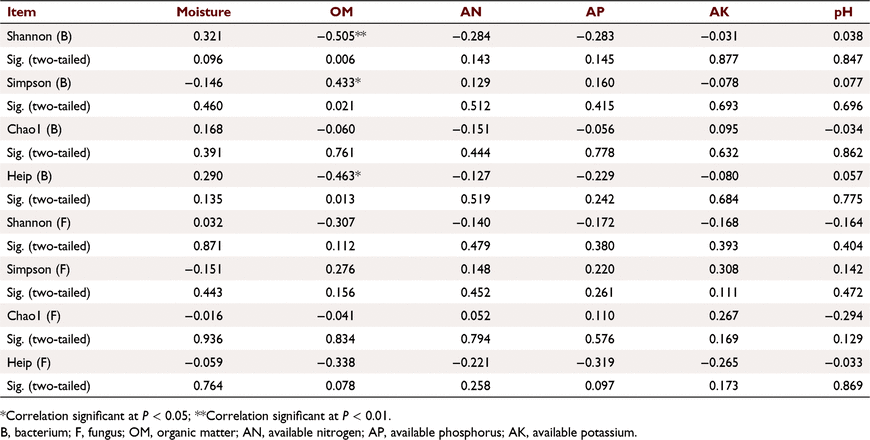
Further analysis of the Pearson correlations between the α-diversity index and soil properties showed that bacterial Shannon index was significantly negatively correlated with OM content (P < 0.01), bacterial Simpson index was significantly positively correlated with OM content (P < 0.05), and the Heip index of bacteria was significantly negatively correlated with OM content (P < 0.05) (Table 2).
|
Microbial community composition and abundance
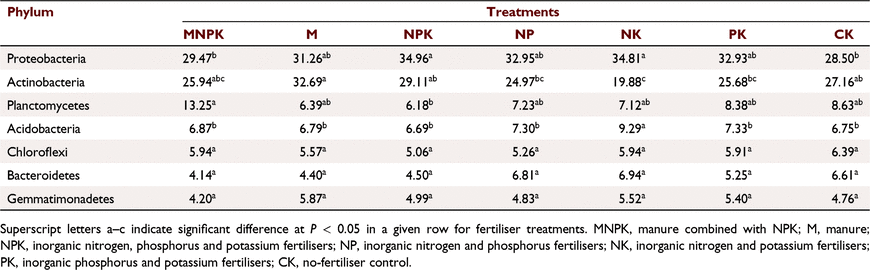
Seven phyla (with relative abundance >5%) made up a core community of bacteria, comprising Proteobacteria, Actinobacteria, Planctomycetes, Acidobacteria, Chloroflexi, Bacteroidetes, and Gemmatimonadetes. Among the different fertilisation treatments, Proteobacteria and Actinomycetes were the dominant groups (Table 3). The relative abundance of Proteobacteria in NPK was the highest (34.96%), followed by that of NK; both were significantly higher than those of CK and MNPK. The Proteobacteria abundance in CK was the lowest (28.50%). All treatments with only chemical fertilisers were higher in Proteobacteria abundance than M. The relative abundance of Actinobacteria was the highest in M (32.69%) and lowest in NK (19.88%). The relative abundance of Actinobacteria was significantly higher in M than in NK, NP, and PK, while those for NPK and CK were significantly higher than in NK. Different fertilisation treatments also produced significant differences in relative abundances of Planctomycetes and Acidobacteria. The MNPK had the highest relative abundance of Planctomycetes (13.25%), which was significantly higher than in NPK. The NK had the highest relative abundance of Acidobacteria (9.29%), significantly higher than in the other treatments.
|
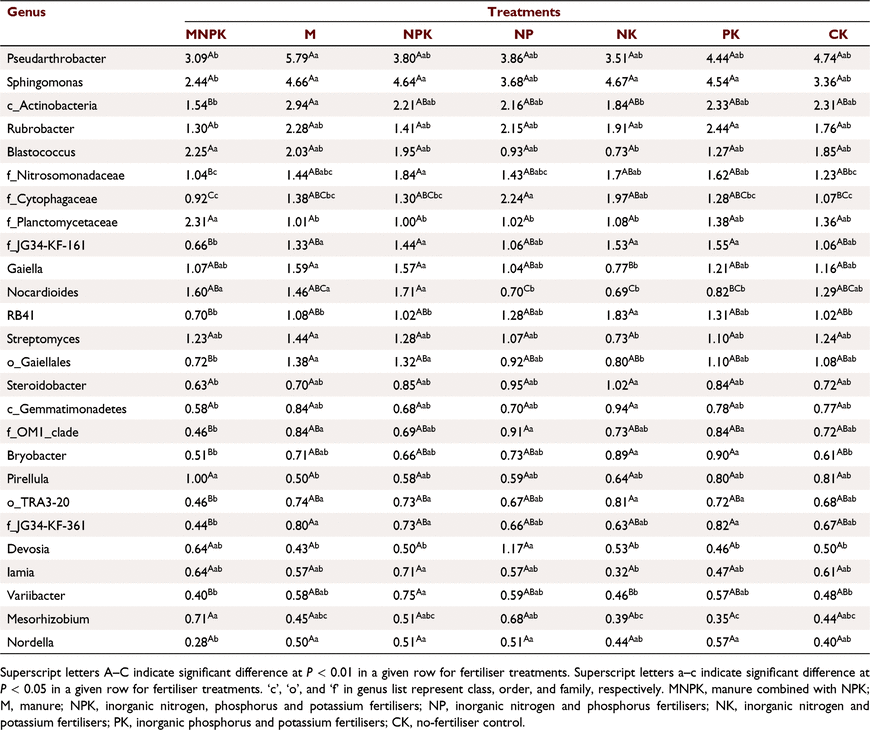
We analysed the relative abundance data of the top 50 OTUs in total that were each at least 0.5% of the total abundance. Among the top 50 bacterial communities in the sum of relative abundances of all treatments, there were 26 communities with significant differences in relative abundance between treatments (Table 4). Firstly, there were 11 communities with significant changes in the relative abundance of bacteria under MNPK treatment. Seven bacterial genera were from Proteobacteria, and the relative abundance of Sphingomonas in MNPK was 2.44%, which was significantly lower than for M, NPK, NK, and PK. The relative abundance of Nitrosomonadaceae (Family) in MNPK was 1.04%, significantly lower than those of NPK, NK, and PK. The relative abundance of JG34-KF-161 (Family) in MNPK was 0.66%, significantly lower than those of PK, NK, NPK, and M; the relative abundance of JG34-KF-361 (Family) in MNPK was 0.44%, significantly lower than that of PK, M, and NPK. The relative abundance of TRA3-20 (Order) of MNPK was 0.46%, significantly lower than those of NK, M, and NPK. The relative abundance of Nordella in MNPK was 0.28%, significantly lower than those of PK, NPK, NP, and M. The relative abundance of Mesorhizobium in MNPK was 0.71%, significantly higher than those of PK and NK. Two bacterial genera were derived from Actinobacteria. The relative abundance of Blastococcus in MNPK was 2.25%, which was significantly higher than that of NK; the relative abundance of OM1-clade (Family) in MNPK was 0.46%, significantly lower than those of NP, M, and PK. Two bacterial genera were derived from Planctomycetes. The relative abundance of Planctomycetaceae (Family) in MNPK was 2.31%, which was significantly higher than those of M, NPK, NP, and NK; and the relative abundance of Pirellula in MNPK was 1.00%, significantly higher than that of M.
|
Secondly, there were nine communities with significant changes in the relative abundance under partial chemical fertiliser treatment. Two bacterial genera were from Proteobacteria, and the relative abundance of Steroidobacter in NK was 1.02%, significantly higher than that of MNPK; the relative abundance of Devosia in NP was 1.17%, significantly higher than for M, PK, CK, NPK, and NK. The three bacterial genera were from Actinobacteria. The relative abundance of Rubrobacter in PK was 2.44%, significantly higher than that of MNPK. The relative abundance of Gaiella in NK was 0.77%, significantly lower than those of M and NPK; the relative abundance of Nocardioides in NK, NP, and PK was 0.69–0.82%, significantly lower than for NPK, MNPK, and M. One bacterial genus was derived from Bacteroidetes. The relative abundances of Cytophagaceae (Family) in NP and NK were 2.24% and 1.97%, respectively, significantly higher than those in MNPK and CK. Two bacterial genera were from Acidobacteria. The relative abundance of RB41 in NK was 1.83%, significantly higher than those in MNPK, NPK, CK, and M; the relative abundance of Bryobacter in PK and NK was 0.90% and 0.89%, respectively, significantly higher than those in MNPK and CK. One bacterial genus was derived from Gemmatimonadetes, and the relative abundance of Gemmatimonadetes (Class) in NK was 0.94%, significantly higher than that in MNPK.
Thirdly, the relative abundance of four bacteria from Actinobacteria increased significantly under M treatment. The relative abundance of Pseudarthrobacter in M was 5.79%, significantly higher than that of MNPK. The relative abundance of Actinobacteria (Class) in M was 2.94%, significantly higher than those of MNPK and NK. The relative abundance of Streptomyces in M was 1.44%, significantly higher than that of NK. The relative abundance of Gaiellales (Order) in M was 1.38%, significantly higher than those of MNPK and NK. The relative abundances of two bacteria belonging to Proteobacteria and Actinobacteria increased significantly under NPK treatment. The relative abundance of Variibacter was 0.75%, which was significantly higher than those of MNPK and NK. The relative abundance of Iamia was 0.71%, which was significantly higher than that of NK.
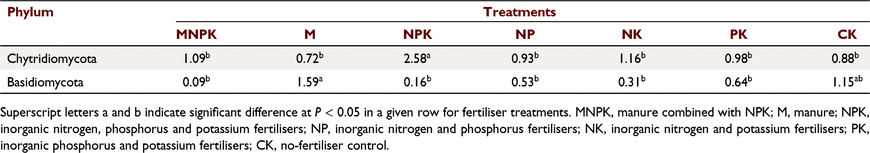
Three fungal phyla (Ascomycota, Chytridiomycota, and Basidiomycota) had relative abundances >1%. Ascomycota clearly dominated in abundance across all treatments, with relative abundances with range of 88–92%, and the relative abundances of other fungal phyla were <3% with the exception of the unclassified fungi. The relative abundance of Chytridomycota in NPK reached 2.58%, which was significantly higher than in the other treatments. Furthermore, the Chytridomycota abundance was 0.72% in M, the lowest among all treatments. The abundance of Basidiomycota was highest in CK (1.15%) followed by that in M (1.59%), which were significantly higher than abundances in the other treatments. Among the treatments with chemical fertilisers, the range of relative abundances of Basidiomycota was 0.09–0.64%, suggesting an inhibitory effect of chemical fertilisers on Basidiomycota (Table 5). In Fig. 4, the abundance data of all 63 genus-level communities of Ascomycota are shown along with the cluster analysis by treatments. The M, PK, and CK treatments were more similar than the others, while MNPK, NK, and NPK could be classified as one lineage. Overall, the data indicate that fungal community changes of Ascomycota were greatly affected by nitrogen fertiliser.
|
Microbial community structure affected by different fertilisers
Based on an unweighted UniFrac distance algorithm, species evolutionary relationships and relative abundances of microbes were examined using principal coordinate analysis (PCoA) and non-metric multidimensional scaling (NMDS) analysis. The amounts of variance explained by axes one and two in the PCoA of bacteria were 37.75% and 19.45%, respectively (Fig. 5a). The amounts of variance explained by axes one and two of the PCoA of fungi were 71.44% and 7.07%, respectively (Fig. 5c). The NMDS analysis (Fig. 5b, d) showed that the stress values of bacteria and fungi were 0.111 and 0.041, respectively. The bacterial community results (OTU level) for the samples from NPK, NP, NK, and PK were clustered around the coordinate origin (Fig. 5a). The samples from NPK and PK were most similar, clustering closer to the origin than samples of other treatments. The MNPK and M samples clustered beyond the origin and were located on both sides of the origin along axis one. The CK samples were also located far from the origin, as well as from samples of the other treatments. The fungal community results (OTU level) for the NPK and M samples clustered nearer to the coordinate origin than samples from the other treatments (Fig. 5c). The NK and MNPK samples were also clustered close to the origin, but the positions of the two groups of samples were not similar. Samples of NP and PK clustered far from the origin and had obvious differences.
Microbial abundance under soil enzyme annotation and soil enzyme activity
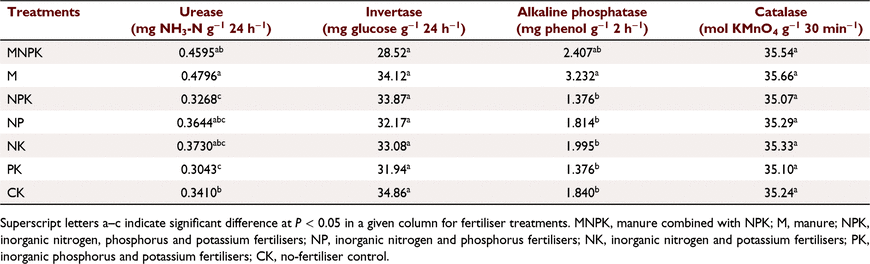
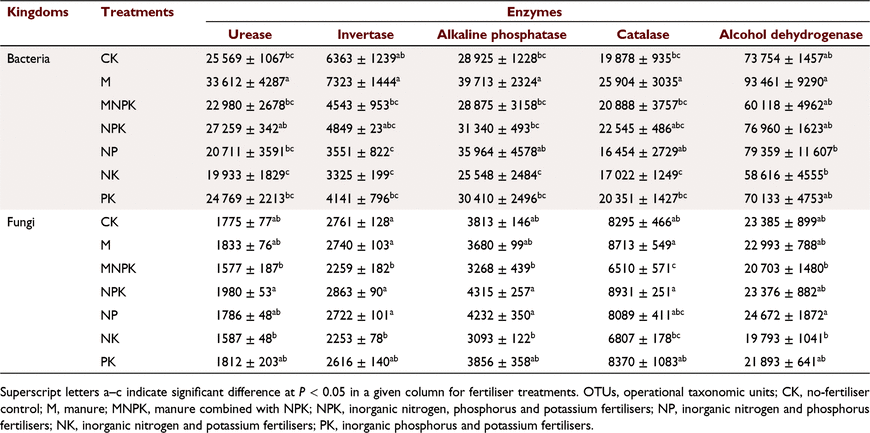
Five soil enzymes, urease, invertase, alkaline phosphatase, catalase, and alcohol dehydrogenase, are considered to be the key enzymes associated with plant nutrient absorption, and so their activities were measured (Table 6). Facilitated by the information on microbial genes and their associated five enzymes annotated in the KEGG database, the abundances of these soil enzyme-associated microbes were obtained (Table 7). By analysing these OTU quantity data annotated by the five soil enzymes, we appraised the activity of the target enzyme-catalysed reaction in the corresponding soil. The bacterial and fungal communities associated with alcohol dehydrogenase had the highest abundances. Among the bacterial communities, the group associated with invertase had the lowest abundance, and the group associated with urease had a higher abundance of 4–5 times that of invertase-associated bacteria. The fungal data exhibited the lowest abundance in the community associated with urease, which was significantly lower than that associated with invertase.
|
|
Among the seven fertiliser treatments, the differences in abundances of the bacteria and fungi associated with each of these five enzymes were similar. The bacterial abundance associated with each enzyme in the M treatment was the highest among all treatments, and the bacterial community abundances associated with urease, invertase, and alkaline phosphatase were significantly higher in M than in NK and PK. In the soil treated with MNPK, except for the bacterial abundance associated with alcohol dehydrogenase, the abundances of bacterial communities associated with other enzymes were significantly lower than those of the M treatment. The bacterial community abundance associated with alkaline phosphatase under the NPK treatment was significantly lower than that of the M treatment, while the differences in abundances between the NPK and M treatments associated with the other enzymes were not significant. The abundances of bacterial communities associated with these five enzymes under the NK treatment were lower than those in the other treatments.
Regarding the fungal communities associated with each of the five enzymes (Table 7), except for that associated with alcohol dehydrogenase, the highest values of fungal community abundance associated with the other enzymes were all obtained from the NPK treatment. The differences in abundance between the M treatment and the treatments with chemical fertiliser application were lower than the same comparisons of the bacterial community. Moreover, the differences in abundances of fungal communities associated with each of the five soil enzymes among the CK, M, and NPK treatments were not significant. Among the abundances associated with urease, those in the MNPK and NK treatments were significantly lower than those in the NPK treatment. The abundances associated with invertase under the MNPK and NK treatments were significantly lower than those under the M, NPK, NP, and CK treatments. The abundances of fungal communities associated with alkaline phosphatase under the MNPK and NK treatments were significantly lower than those under the NPK and NP treatments. The abundances of communities associated with catalase under the MNPK treatment were significantly lower those those under the CK, M, NPK, and PK treatments. Among the fungal community abundances associated with alcohol dehydrogenase, MNPK and NK treatments had significantly lower abundance than the NP treatment. The abundances of fungal communities associated with all five enzymes under the NK and MNPK treatments were significantly lower than those under the other treatments.
Soil enzyme activities determined by a microbial inactivation treatment and substrate culture are presented in Table 6. Urease activity in the M treatment was 50.1% higher than that in NPK, 62.0% higher than PK, and 43.4% higher than CK. Urease activity of the MNPK treatment was also 43.5% higher than that of the NPK and 54.9% higher than PK. The M and MNPK treatments had relatively higher alkaline phosphatase activity, and that of the M treatment was significantly higher than those of the NPK, NP, NK, PK, and CK treatments. Activity levels in the NPK and PK treatments were the lowest. There were no significant differences in the activities of soil invertase and catalase among all fertilisation treatments.
Discussion
Effect of different fertilisers on microbial community diversity
The diversity of microbial communities is affected by many factors, such as different land-use types and farmland soil management methods (Zhong and Cai 2004). The type of applied fertiliser also has significant effects on soil microbial diversity. Research by Kamaa et al. (2011) suggests that the application of chemical fertilisers negatively influences the diversity of soil bacterial communities. Moreover, the effects of manure versus chemical fertiliser treatment on the diversity of fungal communities differ greatly. A study in Chernozem soils of northeastern China showed that the α-diversity of bacterial communities in soils treated with the combined application of manure and chemical fertiliser was higher than that of communities in soils treated with NPK fertiliser (Ding et al. 2016). In our study, the Chao1 and Shannon indices of soil bacterial communities were higher in the NPK than the MNPK treatment. Nie et al. (2018) found in paddy soils that the Chao1 and ACE indices of bacterial communities in soils supplied with N, P, and K fertilisers were lower than those without fertilisers, and there were no significant differences in other α-diversity indices between the two fertiliser treatments. These inconsistencies with our results may be due to differences in soil types in the experimental areas.
Zhou et al. (2016) showed that the diversity of soil fungi somewhat depends on pH of the soil. They reported that with the greater amount of chemical fertiliser applied, there was greater decline in soil pH and greater decrease in fungal community diversity. However, the adverse effects of chemical fertilisers on soil fungal communities can be alleviated by addition of manure (Ding et al. 2017). The PCoA results of this study showed that the composition of the soil fungal community in the plots applied with a combination of manure and chemical fertiliser was similar to that of plots that did not receive fertiliser. Murphy et al. (2007) showed that this phenomenon is due to the slow decomposition of manure and release of nutrients in the soil, which helps maintain populations of all kinds of soil microorganisms.
This study showed that the diversity of soil bacterial and fungal communities varied in response to the different long-term fertilisation treatments. Chao1 and Shannon indices of soil bacteria decreased under the MNPK treatment compared to the CK. However, this is contrary to the results of Lu et al. (2015), who found that the Shannon and richness indices of soil bacteria increased significantly under the MNPK treatment compared to the CK, while there were no changes in the two indices of fungi. The Pearson correlations between bacterial α-diversity index and soil properties showed a significant negative correlation between OM content and bacterial Shannon index (P < 0.01); however, the correlation between OM content and the bacterial Chao1 index was not significant. This result indicated that the increased OM of manure plus chemical fertilisers provided more assimilable carbon sources for fungi, significantly reducing soil bacterial richness. In addition, the soil pH range in the experimental area of Lu et al. (2015) was 4.46–4.67, and the dominant population of soil bacteria clearly differed from that in our study, which may be one reason for the inconsistent results.
Consistent with the results of Xia et al. (2015), the α-diversity indices of bacterial and fungal communities in treatment with no fertilisation and application of NPK fertilisers were relatively similar. Based on the Pearson correlation results, the increase of soil OM content significantly decreased the bacterial Shannon index (richness) and Heip index (evenness), while the increase of soil OM content significantly increased the bacterial Simpson index (dominance of the main community). These indices for fungi also had similar correlations with OM content, but were less significant than for bacteria. In addition, the AN, AP, and AK contents had low correlations with the α-diversity index of the above bacteria and fungi, indicating that only chemical fertilisers or no fertilisers had little effect on soil bacterial and fungal communities. Hence, the community structure of bacteria and fungi under the CK treatment was more similar to that of NPK than MNPK. This result is also consistent with PCoA and NMDS results.
Fungal richness was significantly greater with a single application of manure than without any fertiliser, while fungal richness was significantly lower with applications of NPK and NK fertilisers than without fertiliser. Together, the Shannon and Simpson index data also showed that the single application of manure increased the quantity and diversity of fungi in Calcic Kastanozem, while the single application of chemical fertilisers or combined application of manure and chemical fertilisers had the opposite effect. This effect may be because the fungal abundance of Basidiomycota decreased significantly under the combined action of manure and chemical fertilisers, which in turn reduced the fungal richness (Shannon index). The ecological niche left by Basidiomycota was occupied by other dominant fungi, which increased the Simpson index of fungi. The Heip index results indicated that manure alone did not affect the evenness of the fungal community in Calcic Kastanozem, while the application of NK and combined application of manure and NPK fertilisers significantly reduced the evenness of fungi. This is basically consistent with the results of Nie et al. (2018). Xu et al. (2015) found that the Shannon indices of the soil fungal community subjected to a treatment of manure in combination with NPK fertilisers and a treatment of only NK fertilisers were higher than those of N, NP, PK, and NPK treatments. In addition, they reported that a single application of NPK fertilisers or combined application of manure and NPK fertilisers could improve diversity of the soil fungal community in grey desert soil, which is inconsistent with our results. Their results may be related to the low content of AN and AP in the grey desert soil. A large amount of nitrogen and phosphorus provided by NPK fertiliser or manure plus NPK fertiliser increased the abundance of various microorganisms, thus enhancing the diversity of fungi. Other results from red paddy soils showed that the Shannon and Chao1 indices of the fungal community of soil that did not receive fertiliser did not differ from those of fertilisation treatments (Lu et al. 2015). Altogether, the results of all these studies differed from our results to varying degrees, possibly due to the different soil types, crops, and soil pH in the experimental areas. In this study, the community diversities of soil bacteria and fungi varied among treatments with no clear pattern, regardless of whether the addition of nutrients was withheld in the CK or the addition of a sufficient amount of nutrients was present in the MNPK treatment. The variability in our results is consistent with the results of the long-term fertilisation experiment of Kamaa et al. (2011).
Effects of different fertilisers on microbial community abundances
Under different fertilisation treatments, the relative abundances of Proteobacteria and Actinobacteria changed the most, followed by Acidobacteria, Planctomycetes, Bacteroidetes, and Gemmatimonadetes. In the soil with the combined application of manure and NPK fertiliser, the relative abundance of Sphingomonas bacteria decreased significantly, which can produce catalase and have a solid ability to decompose aromatic hydrocarbons (Janbandhu and Fulekar 2011). Declining abundance is not conducive to purifying contaminated farmland soils. The relative abundance of Nitrosomonadaceae and various Rhizobiales decreased significantly, and these microorganisms can promote the soil nitrogen cycle (Kowalchuk and Stephen 2001) and decomposition of AP and AK (Zai et al. 2021). The decrease in their abundance may be related to the plentiful nitrogen in the soil. However, abundance of Mesorhizobium significantly increased, and it was more suitable for MNPK treatment than other Rhizobia bacteria. In addition, the abundance of Planctomycetaceae, which are aquatic bacteria and are obligately aerobic, increased significantly, indicating a significant improvement in soil water content and aeration. Due to the presence of a large number of easily metabolised carbon sources in the soil with a single manure application, the relative abundance of Actinobacteria increased significantly (Chessa et al. 2016), which have a better ability to decompose lignin (Wang et al. 2014) and can promote soil carbon and nitrogen cycles. The relative abundance of Iamia (Acidimicrobiales) was the highest in the NPK-fertilised soil. This was significantly higher than in the NK treatment, indicating that the lack of phosphorus fertiliser was not conducive to its community development. This may be because phosphates lower the soil pH appropriately to a range of 7.0–8.0, which is optimum for Iamia (Kurahashi et al. 2009). In the soil with unbalanced fertilisation, the relative abundance of soil bacteria in the NK treatment changed the most, and the relative abundances of Cytophagaceae and Bryobacter were significantly higher than those in the MNPK and CK treatments, which may be beneficial to the development of cellulose-degrading microorganisms.
The dominant phyla of fungi were Ascomycota, Chytridiomycota, and Basidiomycota. The fertilisers differentially affected the relative abundances of the different fungi in the soil. Depending on the fertiliser, different phyla may dominate the fungal community as demonstrated by research on changes in dominant communities of fungi in tea field soil in response to various fertilisers (Ji et al. 2018). In this study, Ascomycota was the dominant group in the Calcic Kastanozem fungal community, followed by Chytridiomycota and Basidiomycota. The single application of manure reduced the abundance of Chytridiomycota and increased that of Basidiomycota. The combined application of NPK fertilisers significantly increased the abundance of Chytridiomycota and decreased the abundance of Basidiomycota. This is consistent with results from tobacco field soils (Chen et al. 2014) and continuous-cropping experiments with cucumber (Zhang 2016). Milkereit et al. (2021) found that soils with more complex organic compounds present can provide soil nutrients for fungal growth and reproduction, while the soil bacteria are limited in using these complex organic compounds. Therefore, long-term application of manure not only helps to maintain and stabilise development of the soil fungal community, it also alters composition of the soil bacterial community. This facilitates the utilisation of nutrients by crops during the growth process.
Effects of different fertilisers on soil enzyme activities
Soil enzyme activity is composed of the activity of the enzymes that have accumulated in soil from cells that collapse after the death of some organism and the enzymes released into the soil by microorganisms. Our long-term fertilisation treatments significantly affected soil enzyme activity. The activities of the accumulated urease and alkaline phosphatase in the soil treated with manure were significantly higher than those of the chemical fertiliser and no-fertilisation treatments, consistent with the microbial abundances associated with the corresponding soil enzymes. Long-term application of manure significantly increased the microbial abundance associated with the major soil enzymes: urease, invertase, alkaline phosphatase, catalase, and alcohol dehydrogenase. Only the NPK application had positive effects on the fungal abundances associated with the major soil enzymes, while the other chemical fertiliser treatments reduced the abundances of bacteria and fungi associated with the major soil enzymes. This result is similar to those of studies by Tan et al. (2007), Jin et al. (2012), Li et al. (2015), and Zhang et al. (2018).
Conclusions
This study revealed the response of microorganisms to sheep manure and nitrogen, phosphorus, and potassium fertilisers in terms of community structure and relative abundance in Calcic Kastanozem. The application of manure significantly increased soil OM, and the increase of OM content was one of the main factors in reducing soil bacterial richness and evenness and increasing bacterial dominance. The combined application of manure and chemical fertiliser significantly reduced the relative abundance of Proteobacteria in the soil, mainly due to α-Proteobacteria and β-Proteobacteria, which are essential microorganisms involved in nitrogen cycling. The single application of manure significantly increased the abundance of Actinobacteria and effectively enhanced the soil’s ability to decompose OM. In North China, we conducted a 9-year continuous positioning fertilisation experiment in potato fields for the first time. We found that the combined application of manure and NPK fertilisers had a complex effect on soil microorganisms and was not simply a combination of the effects of manure and chemical fertilisers on microbes. The interaction mechanism between the two on microbial succession changes needs further exploration.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The study was funded by the Agriculture Research System of China (CARS-09-P10).
Acknowledgements
The authors would like to acknowledge the Ministry of Agriculture and Rural Affairs of the People’s Republic of China for the financial support provided through the Agriculture Research System of China.
References
Abbott LK, Murphy DV (2007) What is soil biological fertility? In ‘Soil biological fertility-a key to sustainable land use in agriculture’. (Eds LK Abbott, DV Murphy) pp. 1–15. (Springer Publishing: Berlin/Heidelberg)Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science 26, 1–20.
| Mechanisms and applications of plant growth promoting rhizobacteria: current perspective.Crossref | GoogleScholarGoogle Scholar |
Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC (1999) Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biology and Biochemistry 31, 1021–1030.
| Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands.Crossref | GoogleScholarGoogle Scholar |
Barea JM, Toro M, Azcón R (2007) The use of 32P isotopic dilution techniques to evaluate the interactive effects of phosphate-solubilizing bacteria and mycorrhizal fungi at increasing plant P availability. In ‘First international meeting on microbial phosphate solubilization series: developments in plant and soil sciences’. (Eds E Velázquez, C Rodríguez-Barrueco) pp. 223–227. (Springer Publishing: Dordrecht)
Bisutti I, Hilke I, Raessler M (2004) Determination of total organic carbon – an overview of current methods. TrAC Trends in Analytical Chemistry 23, 716–726.
| Determination of total organic carbon – an overview of current methods.Crossref | GoogleScholarGoogle Scholar |
Bremner JM, Shaw K (1955) Determination of ammonia and nitrate in soil. The Journal of Agricultural Science 46, 320–328.
| Determination of ammonia and nitrate in soil.Crossref | GoogleScholarGoogle Scholar |
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7, 335–336.
| QIIME allows analysis of high-throughput community sequencing data.Crossref | GoogleScholarGoogle Scholar |
Chen DM, Duan YQ, Yang YH, Jin Y, Huang JG, Yuan L (2014) Effects of long-term fertilization on flue-cured tobacco soil nutrients and microorganisms community structure. Scientia Agricultura Sinica 47, 3424–3433.
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890.
| fastp: an ultra-fast all-in-one FASTQ preprocessor.Crossref | GoogleScholarGoogle Scholar |
Chessa L, Pusino A, Garau G, Mangia NP, Pinna MV (2016) Soil microbial response to tetracycline in two different soils amended with cow manure. Environmental Science and Pollution Research 23, 5807–5817.
| Soil microbial response to tetracycline in two different soils amended with cow manure.Crossref | GoogleScholarGoogle Scholar |
Cruz AF, Hamel C, Hanson K, Selles F, Zentner RP (2009) Thirty-seven years of soil nitrogen and phosphorus fertility management shapes the structure and function of the soil microbial community in a Brown Chernozem. Plant and Soil 315, 173–184.
| Thirty-seven years of soil nitrogen and phosphorus fertility management shapes the structure and function of the soil microbial community in a Brown Chernozem.Crossref | GoogleScholarGoogle Scholar |
Das S, Jeong ST, Das S, Kim PJ (2017) Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Frontiers in Microbiology 8, 1702
| Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy.Crossref | GoogleScholarGoogle Scholar |
De Bruijn FJ (2015) Biological nitrogen fixation. In ‘Principles of plant-microbe interactions’. (Ed. FJ De Bruijin) pp. 215–224. (Springer Publishing: Cham)
Ding JL, Jiang X, Guan DW, Ma MC, Zhao BS, Zhou BK, et al. (2016) Responses of micropopulation in black soil of Northeast China to long-term fertilization and crops. Scientia Agricultura Sinica 49, 4408–4418.
Ding J, Jiang X, Guan D, Zhao B, Ma M, Zhou B, et al. (2017) Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Applied Soil Ecology 111, 114–122.
| Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols.Crossref | GoogleScholarGoogle Scholar |
Dixon P (2003) VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14, 927–930.
| VEGAN, a package of R functions for community ecology.Crossref | GoogleScholarGoogle Scholar |
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods 10, 996–998.
| UPARSE: highly accurate OTU sequences from microbial amplicon reads.Crossref | GoogleScholarGoogle Scholar |
Fan R, Du J, Liang A, Lou J, Li J (2020) Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry. Biology and Fertility of Soils 56, 1109–1120.
| Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry.Crossref | GoogleScholarGoogle Scholar |
FAO (2016) Soil fertility. Available at www.fao.org. [Retrieved 18 June 2019]
Freitas ADSd, Vieira CL, Santos CEdRS, Stamford NP, Lyra MdCCPd (2007) Caracterização de rizóbios isolados de Jacatupé cultivado em solo salino no Estado de Pernanbuco, Brasil. Bragantia 66, 497–504.
| Caracterização de rizóbios isolados de Jacatupé cultivado em solo salino no Estado de Pernanbuco, Brasil.Crossref | GoogleScholarGoogle Scholar |
Gu S, Hu Q, Cheng Y, Bai L, Liu Z, Xiao W, et al. (2019) Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil and Tillage Research 195, 104356
| Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils.Crossref | GoogleScholarGoogle Scholar |
Guan SY (1986) Determination of soil enzyme activity. In ‘Soil enzyme and its research methods’. (Eds SY Guan, DS Zhang) pp. 260–344. (Agriculture Press: Beijing, China)
Hilton A, Armstrong RA (2006) Statnote 6: post-hoc ANOVA tests. Microbiologist 2006, 34–36.
Holman WIM (1943) A new technique for the determination of phosphorus by the molybdenum blue method. Biochemical Journal 37, 256–259.
| A new technique for the determination of phosphorus by the molybdenum blue method.Crossref | GoogleScholarGoogle Scholar |
Janbandhu A, Fulekar MH (2011) Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. Journal of Hazardous Materials 187, 333–340.
| Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment.Crossref | GoogleScholarGoogle Scholar |
Ji LF, Ni K, Ma LF, Chen ZJ, Zhao YY, Ruan JY, et al. (2018) Effect of different fertilizer regimes on the fungal community of acidic tea-garden soil. Acta Ecologica Sinica 38, 8158–8166.
Jin Z, Tai J, Pan G, Li L, Song X, Xie T, et al. (2012) Comparison of soil organic carbon, microbial diversity and enzyme activity of wetlands and rice paddies in Jingjiang area of Hubei, China. Scientia Agricultura Sinica 45, 3773–3781.
Kamaa M, Mburu H, Blanchart E, Chibole L, Chotte J-L, Kibunja C, Lesueur D (2011) Effects of organic and inorganic fertilization on soil bacterial and fungal microbial diversity in the Kabete long-term trial, Kenya. Biology and Fertility of Soils 47, 315–321.
| Effects of organic and inorganic fertilization on soil bacterial and fungal microbial diversity in the Kabete long-term trial, Kenya.Crossref | GoogleScholarGoogle Scholar |
Kanehisa M (2002) The KEGG database. In ‘‘In silico’ simulation of biological processes. (Eds G Bock, JA Goode) pp. 91–100. (John Wiley Press: New York)
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annual Review of Microbiology 55, 485–529.
| Ammonia-oxidizing bacteria: a model for molecular microbial ecology.Crossref | GoogleScholarGoogle Scholar |
Kurahashi M, Fukunaga Y, Sakiyama Y, Harayama S, Yokota A (2009) Iamia majanohamensis gen. nov., sp. nov., an actinobacterium isolated from sea cucumber Holothuria edulis, and proposal of Iamiaceae fam. nov. International Journal of Systematic and Evolutionary Microbiology 59, 869–873.
| Iamia majanohamensis gen. nov., sp. nov., an actinobacterium isolated from sea cucumber Holothuria edulis, and proposal of Iamiaceae fam. nov.Crossref | GoogleScholarGoogle Scholar |
Lemanceau P, Maron PA, Mazurier S, Mougel C, Pivato B, Plassart P, et al. (2015) Understanding and managing soil biodiversity: a major challenge in agroecology. Agronomy for Sustainable Development 35, 67–81.
| Understanding and managing soil biodiversity: a major challenge in agroecology.Crossref | GoogleScholarGoogle Scholar |
Li XY, Zhao BQ, Li XH, Li YT, Sun RL, Zhu LS, et al. (2005) Effects of different fertilization systems on soil microbe and its relation to soil fertility. Scientia Agricultura Sinica 38, 1591–1599.
Li F, Xin XL, Zhang CZ, Ning Q, Zhao JH, Wu QC, et al. (2015) Soil enzyme levels in fluvo-auic soil with different long-term fertilization in North China plain. Ecology and Environmental Sciences 24, 984–991.
Liang LZ, Zhao XQ, Yi XY, Chen ZC, Dong XY, Chen RF, et al. (2013) Excessive application of nitrogen and phosphorus fertilizers induces soil acidification and phosphorus enrichment during vegetable production in Yangtze River Delta, China. Soil Use and Management 29, 161–168.
| Excessive application of nitrogen and phosphorus fertilizers induces soil acidification and phosphorus enrichment during vegetable production in Yangtze River Delta, China.Crossref | GoogleScholarGoogle Scholar |
Liu X, Lamb EG, Zhang S (2020a) Nitrogen addition impacts on soil microbial stoichiometry are driven by changes in plant resource stoichiometry not by the composition of main microbial groups in an alpine meadow. Biology and Fertility of Soils 56, 261–271.
| Nitrogen addition impacts on soil microbial stoichiometry are driven by changes in plant resource stoichiometry not by the composition of main microbial groups in an alpine meadow.Crossref | GoogleScholarGoogle Scholar |
Liu J, Zhang J, Li D, Xu C, Xiang X (2020b) Differential responses of arbuscular mycorrhizal fungal communities to mineral and organic fertilization. MicrobiologyOpen 9, e920
| Differential responses of arbuscular mycorrhizal fungal communities to mineral and organic fertilization.Crossref | GoogleScholarGoogle Scholar |
Lu HF, Zheng JW, Yu XC, Zhou HM, Zheng JF, Zhang XH, et al. (2015) Microbial community diversity and enzyme activity of red paddy soil under long-term combined inorganic-organic fertilization. Journal of Plant Nutrition and Fertilizers 21, 632–643.
Lugtenberg BJJ, Malfanova N, Kamilova F, Berg G (2013) Plant growth promotion by microbes. In ‘Molecular microbial ecology of the rhizosphere’. (Eds FJ De Bruijn) pp. 561–573. (Wiley Publishing: Hoboken)
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963.
| FLASH: fast length adjustment of short reads to improve genome assemblies.Crossref | GoogleScholarGoogle Scholar |
Marschner P (2008) The role of rhizosphere microorganisms in relation to P uptake by plants. In ‘The ecophysiology of plant-phosphorus interactions series: plant ecophysiology. Vol. 7’. (Eds PJ White, J Hammond) pp. 165–176. (Springer Publishing: Dordrecht)
Milkereit J, Geisseler D, Lazicki P, Settles ML, Durbin-Johnson BP, Hodson A (2021) Interactions between nitrogen availability, bacterial communities, and nematode indicators of soil food web function in response to organic amendments. Applied Soil Ecology 157, 103767
| Interactions between nitrogen availability, bacterial communities, and nematode indicators of soil food web function in response to organic amendments.Crossref | GoogleScholarGoogle Scholar |
Mohammadi K, Sohrabi Y (2012) Bacterial biofertilizers for sustainable crop production: a review. ARPN Journal of Agricultural and Biological Science 7, 307–316.
Murphy DV, Stockdale EA, Brookes PC, Goulding KW (2007) Impact of microorganisms on chemical transformations in soil. In ‘Soil biological fertility’. (Eds LK Abbott, DV Murphy) pp. 37–59. (Springer Publishing: Dordrecht)
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, et al. (2012) Soil enzymology: classical and molecular approaches. Biology and Fertility of Soils 48, 743–762.
| Soil enzymology: classical and molecular approaches.Crossref | GoogleScholarGoogle Scholar |
Nie S, Zhao L, Wang Y, Lei X, Wang F, Xing S (2018) Effects of long-term fertilizations on microbial community structure and diversity in yellow clayey paddy soil. Research of Agricultural Modernization 39, 689–699.
| Effects of long-term fertilizations on microbial community structure and diversity in yellow clayey paddy soil.Crossref | GoogleScholarGoogle Scholar |
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil 321, 305–339.
| Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms.Crossref | GoogleScholarGoogle Scholar |
Roberge MR (1978) Methodology of enzymes determination and extraction. In ‘Soil enzymes’. (Ed. RG Burns) pp. 341–352. (Academic Press: New York)
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sciences and Medicine Research 21, 30
Suzuki C, Kunito T, Aono T, Liu C-T, Oyaizu H (2005) Microbial indices of soil fertility. Journal of Applied Microbiology 98, 1062–1074.
| Microbial indices of soil fertility.Crossref | GoogleScholarGoogle Scholar |
Tan ZJ, Zhou WJ, Zhang YZ, Zeng XB, Xiao NQ, Liu Q (2007) Effect of fertilization systems on microbes in the paddy soil. Plant Nutrition and Fertilizer Science 13, 430–435.
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology 73, 5261–5267.
| Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy.Crossref | GoogleScholarGoogle Scholar |
Wang C, Guo X, Deng H, Dong D, Tu Q, Wu W (2014) New insights into the structure and dynamics of actinomycetal community during manure composting. Applied Microbiology and Biotechnology 98, 3327–3337.
| New insights into the structure and dynamics of actinomycetal community during manure composting.Crossref | GoogleScholarGoogle Scholar |
Xia X, Shi K, Huang QR, Li DM, Liu MQ, Li HX, et al. (2015) The changes of microbial community structure in red paddy soil under long-term fertilization. Acta Pedologica Sinica 52, 697–705.
Xu WL, Tang GM, Ge CH, Wang XH, Liu H (2015) Effects of long-term fertilization on diversities of soil microbial community structure and function in grey desert soil of Xinjiang. Acta Ecologica Sinica 35, 468–477.
Xu J, Liu S, Song S, Guo H, Tang J, Yong JWH, et al. (2018) Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biology and Biochemistry 120, 181–190.
| Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability.Crossref | GoogleScholarGoogle Scholar |
Yadav AN (2017) Beneficial role of extremophilic microbes for plant health and soil fertility. Journal of Agricultural Science and Botany 1, 9–12.
| Beneficial role of extremophilic microbes for plant health and soil fertility.Crossref | GoogleScholarGoogle Scholar |
Zai X, Luo W, Bai W, Li Y, Xiao X, Gao X, et al. (2021) Effect of root diameter on the selection and network interactions of root-associated bacterial microbiomes in Robinia pseudoacacia L. Microbial Ecology 82, 391–402.
| Effect of root diameter on the selection and network interactions of root-associated bacterial microbiomes in Robinia pseudoacacia L.Crossref | GoogleScholarGoogle Scholar |
Zhang Y (2016) Analysis on soil microbial diversity of cucumber continuous cropping under different fertilization. Master dissertation, Yangzhou University.
Zhang EP, Tian YY, Li M, Shi M, Jiang YH, Ren RB, et al. (2018) Effects of various long-term fertilization regimes on soil microbial functional diversity in tomato rhizosphere soil. Acta Ecologica Sinica 38, 5027–5036.
Zhang T, Wang Z, Lv X, Li Y, Zhuang L (2019) High-throughput sequencing reveals the diversity and community structure of rhizosphere fungi of Ferula sinkiangensis at different soil depths. Scientific Reports 9, 6558
| High-throughput sequencing reveals the diversity and community structure of rhizosphere fungi of Ferula sinkiangensis at different soil depths.Crossref | GoogleScholarGoogle Scholar |
Zhong W-H, Cai Z-C (2004) Effect of soil management practices and environmental factors on soil microbial diversity: a review. Biodiversity Science 12, 456–465.
| Effect of soil management practices and environmental factors on soil microbial diversity: a review.Crossref | GoogleScholarGoogle Scholar |
Zhou J, Jiang X, Zhou B, Zhao B, Ma M, Guan D, et al. (2016) Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biology and Biochemistry 95, 135–143.
| Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China.Crossref | GoogleScholarGoogle Scholar |


