Rapid colorimetric methods for analysis of pH, extractable aluminium and Colwell phosphorus in soils
Chandrakumara Weligama A * , Anton Wasson A , Gilbert Permalloo A and Emmanuel Delhaize B
A * , Anton Wasson A , Gilbert Permalloo A and Emmanuel Delhaize B
A CSIRO Agriculture and Food, Canberra, ACT, Australia.
B Australian National University, Canberra, ACT, Australia.
Soil Research 61(2) 126-135 https://doi.org/10.1071/SR22012
Submitted: 19 January 2022 Accepted: 27 July 2022 Published: 23 August 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Analytical procedures and technologies for soil analyses can be prohibitively expensive for small laboratories and researchers in developing countries. There is a need for low cost and high-throughput methods for assaying pH, extractable aluminium and phosphorus when conducting field trials on acid soils.
Aims: We investigated methods to develop rapid yet inexpensive colorimetric assays for the assay of pH, extractable aluminium and Colwell phosphorus in soil extracts.
Methods: We developed a colorimetric method to measure soil pH enabling pH to be quantified in a high-throughput assay. Similarly, two existing methods for extractable aluminium and Colwell P were modified for high throughput assays also using microtiter plates.
Key results: All three methods yielded linear relationships when using absorbance to quantify the parameters with the high throughput methods. Furthermore, there was a strong correlation between pH values of the soil samples obtained with the colorimetric assay and pH values measured with a glass electrode.
Conclusions: We demonstrated that the rapid assays for all three methods can be implemented to characterise field sites through the mapping of distributions for extractable Al, Colwell P and pH.
Implications: The high-throughput methods described here will be useful for researchers who conduct field trials to map variations in soil pH, soluble Al and Colwell P. Although the focus of the current work was on acid soils, the colorimetric pH and Colwell P methods can also be applied to non-acid soils.
Keywords: acid soils, aluminium toxicity, colorimetric soil analysis, colorimetric soil pH, Colwell-P, pyrocatechol violet method, soil acidity, soil tests, stratified pH.
Introduction
Soil analysis is an important component of farm management and research in agriculture as virtually all calories consumed by humans are derived from the land (Pimentel and Burgess 2013). Soils have been evolving for millions of years under various weather, bedrock, chemical and physical conditions. Natural and human processes have created soil constraints on plant production in agriculture. Soil acidity is one of the major constraints world-wide causing aluminium (Al) toxicity and impeding the uptake of the phosphorus (P) required for effective plant growth (von Uexküll and Mutert 1995). Soil analyses are essential for conducting effective field trials with the aim of developing solutions to mitigate acidity.
Analytical procedures and technologies for soil analyses have improved in recent years. However, many technologies and methods are prohibitively expensive for small laboratories and researchers in developing countries. Furthermore, some analytical procedures require special training and equipment that are beyond the budget of small laboratories. Soil analyses of many samples, as is required for large field trials, can be prohibitively costly even for well-funded projects.
Soil pH is one of the most common and useful parameters to characterise soils used in agriculture and is commonly measured using water or CaCl2 extractions. The effect of soil to water ratio on soil pH was extensively investigated in the development of methods for measuring soil pH (Wilcox 1936). Among others, an important factor in measuring soil pH is the salt content of soil (Thomas 1996). Today, a 1:5 ratio of soil to solution has been adopted by many researchers as a standard for the measurement of soil pH (Rayment and Lyons 2011). Currently, the most widespread method to measure pH is electrometrically/potentiometrically using glass electrodes. However, measuring soil pH with an electrode can be time-consuming. Although automated pH metres are available (see for example: https://hudsonrobotics.com/products/other-products/ph-meter-probe/) high initial and ongoing costs are prohibitive for small laboratories.
The notion of measuring pH in a solution using colorimetric methods with indicator dyes as an alternative to the electrode method has been proposed by numerous researchers (Pierre 1925; Snyder 1935; Wilcox 1936; Raupach and Tucker 1959). Snyder (1935) provided a comprehensive review of early colorimetric methods of soil pH measurements and their applications. The assays were based on estimation of pH by visual assessment of dye colours and were not quantified methods. In more recent years, there has been a renewed interest in colorimetric methods to measure pH in a wide range of media (Newman et al. 2012; Kirkwood et al. 2014; Mito et al. 2016). These methods were developed for samples such as ground water and protein buffers and are not suitable for soil suspensions (Kirkwood et al. 2014; Mito et al. 2016). Finding a suitable indicator or mix of indicators for soil suspensions can be difficult. Dyes can be absorbed by soil particles and colour reactions can vary in different soils as observed by Raupach and Tucker (1959). Raupach and Tucker (1959) were successful in identifying indicator dyes that were consistent in their reactions with various soils and could be used to estimate soil pH. However, their assay was based on a visual assessment of colours and was not based on a quantified measurement of absorbance.
In contrast to soil pH, there are established methods for quantifying soluble Al (Al3+) and phosphate in soils using colorimetric methods. A colorimetric method using pyrocatechol violet termed the ‘PCV method’ to estimate Al3+ in solution was developed by Anton (1960). The PCV method was subsequently modified to suit various applications and situations (Henriksen and Bergmann-Paulsen 1975; Samaritan et al. 1993) including the estimation of Al3+ in soil solutions (Ares 1986; Bromfield 1987; Kerven et al. 1989; Conyers et al. 1991; Barton and Carr 1996).
The plant-available P in soil comprises mainly of orthophosphate species (H2PO4− and HPO42−). Orthophosphate can form a blue phosphomolybdenum complex with molybdate reagent in a colorimetric reaction. However, the amount of ‘available P’ in soil solutions can vary according to the extractant used. Olsen et al. (1954) introduced a P extraction method using 0.5 M NaHCO3 solution at pH 8.5. The sodium bicarbonate method was modified by Colwell (1963) to determine P fertiliser requirements in Australian soils. This method can be applied to a wide range of acid and alkaline soils (Irving and McLaughlin 1990). The colour development of the phosphomolybdenum complex in soil solutions can be assayed with various reagents. One such reagent is malachite green and a method describing the use of malachite green is described by Irving and McLaughlin (1990).
Here, we present three low-cost and high-throughput methods to assay pH, Colwell P and extractable Al3+ in soil using small volumes of extracts in microtiter plates. A colorimetric method to measure soil pH was developed based on a commercially available kit that relies on a visual assessment of soil pH. We adapted the method to enable pH to be quantified in a high-throughput assay while existing methods for extractable Al and Colwell P were modified for high throughput assays also using microtiter plates. We show that these methods can be applied to map the distribution of pH, soluble Al3+ and Colwell P as is required for field trials. Although the methods were developed primarily for analysis of acid soils, they are applicable to a range of soil types.
Materials and methods
Soil samples
All soil samples were air dried, crushed and passed through a 2 mm sieve before they were used for the various analyses.
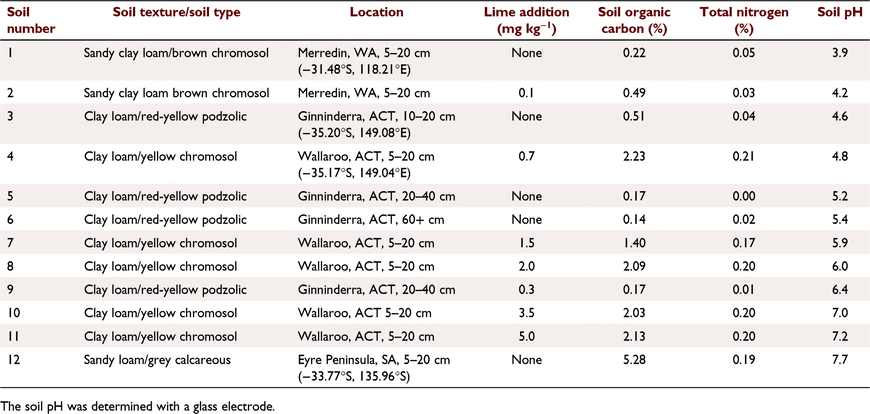
Soils used as standards for the colorimetric pH method were collected from four locations in Australia. The soils were either unmodified or limed to obtain particular pH values (Table 1). As a part of soil characterisation, soil organic carbon and total nitrogen content were analysed by dry combustion with a Leco-CNS 895 analyser (Leco Corporation, MI, USA),.
|
To test the colorimetric method for soil pH, samples were taken in August 2020 from a field trial that had been limed by deep ripping 5 T of lime ha−1 to 15–25 cm after surface application in late May 2020 at the Boorowa Agriculture Research Station, NSW (−34.46°S, 148.70°E). A total of 69 soil samples were collected at 0–5, 5–10, 10–20, 20–30 and 30–40 cm depths from 15 locations on the trial.
To test the soluble Al3+ and Colwell P rapid methods, we collected soil samples from two field trials. One trial had 210 plots of wheat located at Little Billabong ‘Warrenboo’ farm, NSW (−35.68°S, 147.57°E) and samples were collected in September 2017. The other trial had 220 plots of wheat in Merredin, WA (−31.48°S, 188.21°E) and samples were collected in July 2018. Both sites were initially selected because of the acidic nature of their soils. Phosphorous fertiliser was supplied as triple super phosphate at sowing with treatment rates of 0, 5, 10, 20 and 40 P kg ha−1 at the Little Billabong trial and 0, 7, 14, and 30 P kg ha−1 at the Merredin trial. A total of 630 (Little Billabong) and 660 (Merredin) samples were collected at three depths (0–5, 5–10 and 10–20 cm) from the trials.
Colorimetric soil pH assay
Four grams of subsamples of well-mixed samples (~50 g) were weighed into 50 mL centrifuge tubes (Cellstar #227 261, Greiner Bio-One). Soils were extracted with a 0.01 M CaCl2 solution (1:5 w/v; soil: solution) by mixing for 1 h on an end-over-end rotary mixer. Extractants in 50 mL centrifuge tubes were then centrifuged for 10 min at 2093g to pellet the soil particles.
The indicator dye mix used in the method was purchased from a local hardware store as part of a soil pH measuring kit (Soil pH test kit, Model No: MTO8000, Manutec Pty Ltd, SA; available on eBay). The indicator dye was diluted to 25% by adding deionised water on the day of use. Dilution of the dye on the day of use was found to be necessary as absorbances of standard solutions would change if the diluted solution was stored for several days.
Soil pH was measured with a Mettler Toledo Seven Compact pH metre with three calibration points that was attached to a Mettler Toledo InLab Exper Pro-ISM probe (Order No. 30014096). Prior to measuring a set of soil extractants, the metre was calibrated daily with pH buffers of 4.01, 7.01 and 10.01 (Hanna HI7004, 7007 and 7010). The stability criterion of the metre was set to the standard level. Values of pH were recorded when measurements had fully stabilised and the metre had locked in. It took an average of three and a half minutes per extracted soil sample to measure the pH with this instrument.
For the colorimetric pH assay, 200 μL of soil extractant in 0.01 M CaCl2 was pipetted into the wells of a 96-well clear flat-bottomed microtiter plate (Greiner Bio-One # 655101). The first row of each plate (A1 to A12) was allocated to extractants of soil standards. Diluted indicator dye (10 μL) was then pipetted into each well with colour development occurring essentially instantaneously. Absorbance of the solutions in the wells of the microtiter plates were measured at 590 nm using a Bio Tek Power Wave HT microplate spectrophotometer with KC4 V3.0 software (Millennium Science Pty Ltd, Vic, Australia). A calibration curve was constructed using the soil standards by plotting the measured soil pH using the pH metre against the absorbances using the colorimetric method. Soil pH values of test samples were obtained from the linear relationship between measured pH and absorbance as obtained from the calibration curve.
Extractable Al3+ and Colwell P
The same CaCl2 extractant as used for pH measurements was used for Al3+ assay by the PCV method based on that described by Conyers et al. (1991).
For Colwell P analysis, a subsample of 0.5 g of soil was weighed into a 50 mL centrifuge tube to which 50 mL of 0.5 M NaHCO3 (pH 8.5) was added. The soil suspension was mixed on an end-over-end rotary mixer for 16 h at 25°C before tubes were centrifuged at 2093g for 10 min (Colwell 1963).
PCV assay
The working standard of Al3+ solution 5 μg mL−1 (185 μM) was prepared by dilution with deionised water of a 10 mg mL−1 (370 mM) of metal Al in 10% HNO3 (Choice Analytical, Cat # 10M1-1) stock solution. An Al3+ standard curve was constructed to cover the range 30.9–185.2 μM by addition of the standard Al3+ solutions in 60 μL final volume into each well. Blank wells comprised of 60 μL of 0.01 M CaCl2. It was convenient to use the first row of the microtiter plate for the standards and the soil extractants (60 μL) were added to the remaining wells.
If the extractable Al3+ concentration was too high (absorbance exceeded highest standard), samples were diluted with deionised water. Ten μL of mix of hydroxylamine (0.1% w/v) plus ortho-phenanthroline (10% w/v) was added to each well followed by 10 μL of pyrocatechol violet (0.1% w/v) and finally 120 μL of ammonium acetate (pH 7.0, 10% w/v). After addition of each reagent, solutions were pipetted in and out to ensure adequate mixing. The use of multi-well pipettors enabled rapid addition and mixing of the various reagents. The plate was left for 30 min for colour development and absorbance was measured at 580 nm (Conyers et al. 1991) using the microtiter plate spectrophotometer described above.
Colwell assay
A working standard of 2 μg mL−1 (64.5 μM) of NH4H2PO4 for the Colwell P assay was prepared from a 10 mg mL−1 (322.6 mM) stock solution of NH4H2PO4 in 0.05% HNO3 (Choice Analytical Cat# 10M39-1). A P calibration curve was developed by adding 100 μL of solutions prepared to cover the range of 1.6–29.0 μM and included 100 μL of NaHCO3 as the matrix common to all samples. Absorbance of the blank was corrected by subtracting the value obtained when using the extractant solution of NaHCO3.
For soil extractants, the pH was adjusted to values between 5 and 7 by adding a required amount of 1.8 M H2SO4. The amount of acid to add was determined by measuring the pH values of a subset of samples. The addition of H2SO4 was carried out using 96 well plates (Greiner Bio-One Cat#780201) in order to make it easier to take multiple sub-samples if required with multi-well pipettors for colorimetric P analysis.
A previously described method (Irving and McLaughlin 1990) was slightly modified as the basis for the assay with volumes adjusted to be suitable for a microtiter plate. Using the nomenclature of Irving and McLaughlin (1990), 40 μL of Reagent 1 was added to all sample wells and left for 20 min. Reagent 2 (40 μL) was then added to all sample wells and left for a further 20 min before absorbance at 630 nm was measured using the microplate spectrophotometer described above.
Reagent 1 was prepared by adding 17.55 g L−1 of ammonium molybdate (Sigma-Aldrich #A7302) to 3.1 M H2SO4 and Reagent 2 prepared by adding 3.5 g L−1 of polyvinyl alcohol (Sigma-Aldrich #341583, Mw 89000–98000, 99+% hydrolysed) to 0.25 g L−1 of malachite green carbinol hydrochloride (Sigma-Aldrich #213020) all dissolved in DI water.
Results
Colorimetric assay for pH
We assessed the dye reagent from a commercially available kit describing a colorimetric assay for soil pH. The kit provides a visual estimate of pH using a soil paste and we adapted the dye reagent to develop a quantified assay to measure pH values of a soil extracted with a 0.01 M CaCl2 solution. We determined the absorbance spectrum of the dye reagent that had reacted with soil extractions over this pH range, which was of primary interest to our research. Fig. 1a shows that absorbance measured at about 590 nm gave the greatest discrimination between the various pH values of the soil extracts and we subsequently used 590 nm for routine measurements. Fig. 1b shows the colour development and absorbance at 590 nm by extracted soils that had pH values over the range of pH 4.0–7.0. Fig. 1c shows there was a strong linear relationship between pH measured with a glass electrode and the absorbance of solutions that had reacted with the dye reagent. These data indicated that the dye reagent was suitable for quantifying the pH of soil extracts over the range of pH 4.0–7.5. The data also show that the dye mix included in the commercial kit was robust in quantifying pH in a range of soils that varied in their properties.
|
|
To demonstrate the utility of the method, we measured pH values of soil extracts using the colorimetric assay and compared it to pH values measured with a glass electrode. The soil samples were collected from a field site on an acid soil that had been treated with lime by incorporation to about 20 cm. Soil samples were taken down the profile to determine if the liming had increased the pH relative to soil deeper down the profile. The two methods showed the same pattern down the profile (Fig. 2) and indicated that the pH of the surface layers were increased (0–5 cm layer and 5–10 cm layer both pH 5.7 ± 0.17; n = 15 at each depth, mean ± s.e.) compared to samples taken from the surface layers of an adjacent un-limed site (0–5 cm layer pH 5.1 ± 0.14; 5–10 cm layer pH 4.5 ± 0.25; n = 3 at each depth, mean ± s.e.). The pH values of the soil samples obtained with the colorimetric assay were strongly correlated with the pH values measured with a glass electrode (Fig. 3).
|
|
Rapid assay for Al3+
Here, we adapted an existing PCV colorimetric assay to a high throughput assay using microtiter plates and a multi-well pipetteman as described for the colorimetric pH assay. Fig. 4a shows there was a strong relationship between colour development and Al3+ even though the volumes of reagents and samples for the assay had been scaled down to suit the microtiter plates.
To assess the utility of the PCV assay, we assayed soil samples obtained from two field trials established on acid soils. The distribution of Al3+ across the site at various depths for both the Little Billabong ‘Warrenboo’ and Merredin sites indicated the presence of Al3+ down the profiles (Fig. 5).
Rapid assay for Colwell P
As for the PCV assay, we adapted an existing Colwell P assay for high throughput assay of soil extracts using microtiter plates and multi-well pipettors. Again, a strong correlation of absorbance at 630 nm and P was obtained with reduced volumes of reagents (Fig. 4b).
Fig. 6 shows the Colwell P distribution of soil sampled to a depth of 20 cm for two contrasting field sites.
Discussion
Soil pH
Suitable indicator dyes to measure soil pH colorimetrically should ideally absorb over a wide range of pH values and be stable regardless of soil type. The commercially available single indicator dyes typically provide good colour development over a narrow pH range for either the acid or alkaline range (data not shown). Furthermore, colour development of dyes with pH buffers can differ to the dye reactions with solutions extracted from soil even when they have similar pH values when measured with a glass electrode. We tested several pH indicator dyes including phenol red, nitrazine yellow, chlorophenol red, bromophenol blue and bromothymol blue for their suitability. We found several problems with the use of these indicators for a colorimetric assay. Apart from being only suitable for a narrow pH range, the colour development with soil standards was inconsistent (data not shown). As discussed by Raupach and Tucker (1959), this could be due to reactions of indicator dyes with soil particles.
Raupach and Tucker (1959) surveyed a wide range of acid–base indicators to identify a combination that could be used in a simple colorimetric assay of soil pH that they termed ‘soil reaction’. The assay was based on mixing a sample of soil with a reagent comprising of a mixture of acid–base indicators to yield a coloured paste. The pH was estimated by comparing the colour developed to a colour chart, which allowed the pH to be estimated. Here, we developed a quantified colorimetric assay for pH that has several advantages over the use of a pH electrode. First, the assay is considerably quicker than a pH electrode when measuring many samples. For instance, it is conceivable that hundreds to thousands of samples need to be collected from field trials that aim to assess germplasm for tolerance of acid soils. Burns et al. (2017) proposed that samples should be assayed for pH down a soil profile and that the resolution of the soil collections in some instances require the collection of samples at 2.5 cm depth increments. Collection of samples throughout the trial in a 2Dl pattern including sampling at variable depths can quickly generate large numbers. For instance, using an accurate pH probe it took 3–10 min for the pH to stabilise at each reading, which becomes a limiting factor when measuring many samples. By contrast, there is no such delay using the colorimetric assay, which based on the strong linear correlation shown in Fig. 3, has a similar level of accuracy as the pH electrode. Although soil collection, drying and preparation is unchanged with this method, time for the preparation of soil can also be curtailed by reducing the sample volumes.
Soil Al3+
The activity of Al3+ increases when the soil pH drops below 5 (Parfitt et al. 1995). Soluble Al as Al3+ is the major limiting factor for crop and pasture production on acid soils (Dolling et al. 1991; Tang et al. 2002; Anderson and Bell 2019). This contrasts with plant species such as legumes that are sensitive to low pH and can be limited at soil pH values where Al3+ would be absent (Burns et al. 2017). Extractable monomeric Al, largely as the Al3+ ion in soil solution, is a good indicator of likely Al toxicity in plants grown on acid soil (Pavan et al. 1982; Kerven et al. 1989; Conyers et al. 1991). Although pH can be a good indicator of acid soils, it does not always correlate with the concentration of Al3+ in soil solution or crop yield (Conyers and Poile 2018; Anderson and Bell 2019). Furthermore, it is important to know if toxic Al3+ is present at soil pH values that are near the limit where Al is solubilised by the acidity. For these reasons, it is valuable to couple Al3+ with pH assays when surveying field sites.
We modified a pre-existing PCV assay for soluble Al3+ (Conyers et al. 1991) and showed that it could be successfully used to profile two contrasting acid soils. The peak Al3+ concentration for ‘Warrenboo’ occurred in the 5–10 cm layer (Fig. 5a–c) whereas for Merredin, the peak Al3+ concentration occurred in the 10–20 cm layer (Fig. 5d–f). These findings are consistent with the Merredin site being a deep acid sand (Scanlan et al. 2017) whereas Little Billabong ‘Warrenboo’ typifies sites that have an Al3+-toxic acid ‘throttle’ closer to the surface with pH increasing as the depth increases. Both pH and Al3+ could be assayed in the same CaCl2 extract enabling sites to be rapidly profiled for both parameters.
Colwell P
Colwell P values provide a good estimate of the P status of soil (Bolland et al. 1995). Studies have shown that there can be a linear relationship of soil Colwell P with plant leaf tissue P concentration of the crop (Kirchhof et al. 2008). When screening germplasm for phosphorus use efficiency (PUE), there is a need to measure the soil P status of every plot and at multiple depths. The ‘Warrenboo’ site (Fig. 6a–c) shows a strong stratification of P down the profile with the majority of the P located in the surface soil (0–5 cm). This pattern is typical of many soils where the P remains near the surface unless it is incorporated and is a consequence of the tight binding of phosphate to positively charged soil particles. By contrast, although the P down the profile of the Merredin site was also stratified, a larger proportion leached down the profile as would be expected for a sandy soil (Fig. 6e, f).
Concluding remarks
Here, we describe high throughput assays for soil pH, Al3+ and Colwell P using microtiter plates and multi-well pipettors. We developed a quantified colorimetric assay for pH, adapted existing methods for monomeric Al3+ and Colwell P and showed the utility of the assays in characterising field sites. The Al3+ and pH assays have the advantage of using the same soil extract, which is valuable for assessing field trials aimed at screening germplasm for tolerance of acid soil. Extensive soil sampling is required if researchers aim to use pH and Al3+ concentrations as covariates when analysing data based on plant productivity.
Foregoing soil pH electrodes means this methodology is high throughput, and comparatively accessible. However, microplate readers are expensive equipment and are also likely to be inaccessible to some laboratories. Fortunately, there has been progress in the development of ‘open source’ microplate readers, which can be constructed for a tenth of the cost of commercially available units (Szymula et al. 2019).
Although we described high throughput methods, the assays can be adapted for single-sample assays where a microplate reader is not available by adjusting reagent and sample volumes to suit different size cuvettes. The reagents for the various assays are relatively cheap and the small volumes of the assays further reduces costs. The small volume of dye reagent used in the pH assay enables many thousands of samples to be assayed from a single kit. Using these colorimetric assays, it is feasible to scale down the required soil sampled to 1 g or less if adequate mixing of a larger sample is undertaken to ensure that the subsample is representative of the total.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by the Australian Grains and Research Development Council (Project Number CFF00009).
Acknowledgements
We acknowledge the support of the Australian Grains and Research Development Council through project CFF00009 ‘Molecular markers for root hair traits and enhanced phosphorus use efficiency (PUE) in wheat’.
References
Anderson G, Bell R (2019) Wheat grain-yield response to lime application: relationships with soil pH and aluminium in Western Australia. Crop & Pasture Science 70, 295–305.| Wheat grain-yield response to lime application: relationships with soil pH and aluminium in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Anton A (1960) Colorimetric estimation of aluminum with pyrocatechol violet. Analytical Chemistry 32, 725–726.
| Colorimetric estimation of aluminum with pyrocatechol violet.Crossref | GoogleScholarGoogle Scholar |
Ares J (1986) Identification of aluminum species in acid forest soil solutions on the basis of Al: F reaction kinetics: 1. Reaction paths in pure solutions. Soil Science 141, 399–407.
| Identification of aluminum species in acid forest soil solutions on the basis of Al: F reaction kinetics: 1. Reaction paths in pure solutions.Crossref | GoogleScholarGoogle Scholar |
Barton L, Carr SJ (1996) Development and verification of a quick test for the diagnosis of aluminium toxicity on the deep yellow earths of Western Australia. Soil Research 34, 781–788.
| Development and verification of a quick test for the diagnosis of aluminium toxicity on the deep yellow earths of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA, Yeates JS, Clarke MF (1995) Effect of fertilizer type, sampling depth, and years on Colwell soil test phosphorus for phosphorus leaching soils. Fertilizer Research 44, 177–188.
| Effect of fertilizer type, sampling depth, and years on Colwell soil test phosphorus for phosphorus leaching soils.Crossref | GoogleScholarGoogle Scholar |
Bromfield SM (1987) Simple tests for the assessment of aluminium and manganese levels in acid soils. Australian Journal of Experimental Agriculture 27, 399–404.
| Simple tests for the assessment of aluminium and manganese levels in acid soils.Crossref | GoogleScholarGoogle Scholar |
Burns H, Norton M, Tyndall P (2017) Reduced nodulation of faba bean on acidic soils; the role of topsoil pH stratification. In ‘Doing more with less. Proceedings of the 18th Australian Agronomy Conference’ 24–28 September 2017, Ballarat, Australia. (Eds GJ O’Leary, RD Armstrong, L Hafner). (Australian Society of Agronomy). Available at http://agronomyaustraliaproceedings.org/images/sampledata/2017/53_ASA2017_Burns_Helen_Final.pdf
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry 3, 190–198.
| The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis.Crossref | GoogleScholarGoogle Scholar |
Conyers M, Poile G (2018) Simultaneous measurement of exchangeable Al and other cations in acidic soils. Soil Research 56, 503–508.
| Simultaneous measurement of exchangeable Al and other cations in acidic soils.Crossref | GoogleScholarGoogle Scholar |
Conyers MK, Poile GJ, Cullis BR (1991) Lime responses by barley as related to available soil aluminium and manganese. Australian Journal of Agricultural Research 42, 379–390.
| Lime responses by barley as related to available soil aluminium and manganese.Crossref | GoogleScholarGoogle Scholar |
Dolling PJ, Porter WM, Robson AD (1991) Effect of soil acidity on barley production in the south-west of Western Australia. 1. The interaction between lime and nutrient application. Australian Journal of Experimental Agriculture 31, 803–810.
| Effect of soil acidity on barley production in the south-west of Western Australia. 1. The interaction between lime and nutrient application.Crossref | GoogleScholarGoogle Scholar |
Henriksen A, Bergmann-Paulsen I-M (1975) An automatic method for determining aluminium in natural waters. Vatten 31, 339–342.
Irving GCJ, McLaughlin MJ (1990) A rapid and simple field test for phosphorus in Olsen and Bray No. 1 extracts of soil. Communications in Soil Science and Plant Analysis 21, 2245–2255.
| A rapid and simple field test for phosphorus in Olsen and Bray No. 1 extracts of soil.Crossref | GoogleScholarGoogle Scholar |
Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S (1989) Aluminum determination in soil solution. 2. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminum in the presence of organic acid ligands. Australian Journal of Soil Research 27, 91–102.
| Aluminum determination in soil solution. 2. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminum in the presence of organic acid ligands.Crossref | GoogleScholarGoogle Scholar |
Kirchhof G, Ramakrishna A, Bailey JS (2008) An evaluation of Colwell-P as a measure of plant-available phosphorus in soils of volcanic and non-volcanic origins in the highlands of Papua New Guinea. Soil Use and Management 24, 331–336.
| An evaluation of Colwell-P as a measure of plant-available phosphorus in soils of volcanic and non-volcanic origins in the highlands of Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Kirkwood J, Wilson J, O’Keefe S, Hargreaves D (2014) A high-throughput colourimetric method for the determination of pH in crystallization screens. Acta Crystallographica. Section D, Biological Crystallography 70, 2367–2375.
| A high-throughput colourimetric method for the determination of pH in crystallization screens.Crossref | GoogleScholarGoogle Scholar |
Mito S, Okamura K, Kimoto H (2016) Colorimetric pH measurement of pressurized groundwater containing CO2. Analytical Sciences 32, 437–442.
| Colorimetric pH measurement of pressurized groundwater containing CO2.Crossref | GoogleScholarGoogle Scholar |
Newman J, Sayle RA, Fazio VJ (2012) A universal indicator dye pH assay for crystallization solutions and other high-throughput applications. Acta Crystallographica. Section D, Biological Crystallography 68, 1003–1009.
| A universal indicator dye pH assay for crystallization solutions and other high-throughput applications.Crossref | GoogleScholarGoogle Scholar |
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) ‘Estimation of available phosphorus in soils by extraction with sodium bicarbonate.’ Circular 939. (U.S. Department of Agriculture)
Parfitt RL, Percival HJ, van der Lee G (1995) Aluminium species and pH in soil solution under different crops. In ‘Plant-soil interactions at low pH’. (Eds RA Dates, NJ Grundon, GE Rayment, ME Probert) pp. 817–822. (Kluwer Academic Publishers: Dordrecht). https://doi.org/10.1007/978-94-011-0221-6_131
Pavan MA, Bingham FT, Pratt PF (1982) Toxicity of aluminum to coffee in ultisols and oxisols amended with CaCO3, MgCO3, and CaSO4·2H2O. Soil Science Society of America Journal 46, 1201–1207.
| Toxicity of aluminum to coffee in ultisols and oxisols amended with CaCO3, MgCO3, and CaSO4·2H2O.Crossref | GoogleScholarGoogle Scholar |
Pierre WH (1925) The H-ion concentration of soils as affected by carbonic acid and the soil-water ratio, and the nature of soil acidity as revealed by these studies. Soil Science 20, 285–306.
| The H-ion concentration of soils as affected by carbonic acid and the soil-water ratio, and the nature of soil acidity as revealed by these studies.Crossref | GoogleScholarGoogle Scholar |
Pimentel D, Burgess M (2013) Soil erosion threatens food production. Agriculture 3, 443–463.
| Soil erosion threatens food production.Crossref | GoogleScholarGoogle Scholar |
Raupach M, Tucker BM (1959) The field determination of soil reaction. The Journal of the Australian Institute of Agriculture Science 25, 129–133.
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australia.’ (CSIRO Publishing: Collingwood, Vic.)
Samaritan JM, Wehr JD, Buccafuri A, Sahn M (1993) Automated measurement of aqueous aluminum by the pyrocatechol violet method. International Journal of Environmental Analytical Chemistry 50, 173–182.
| Automated measurement of aqueous aluminum by the pyrocatechol violet method.Crossref | GoogleScholarGoogle Scholar |
Scanlan CA, Brennan RF, D’Antuono MF, Sarre GA (2017) The interaction between soil pH and phosphorus for wheat yield and the impact of lime-induced changes to soil aluminium and potassium. Soil Research 55, 341–353.
| The interaction between soil pH and phosphorus for wheat yield and the impact of lime-induced changes to soil aluminium and potassium.Crossref | GoogleScholarGoogle Scholar |
Snyder EF (1935) ‘Methods for determining hydrogen-ion concentration of soils.’ Circular 56. (U. S. Department of Agriculture)
Szymula KP, Magaraci MS, Patterson M, Clark A, Mannickarottu SG, Chow BY (2019) An open-source plate reader. Biochemistry 58, 468–473.
| An open-source plate reader.Crossref | GoogleScholarGoogle Scholar |
Tang C, Rengel Z, Abrecht D, Tennant D (2002) Aluminium-tolerant wheat uses more water and yields higher than aluminium-sensitive one on a sandy soil with subsurface acidity. Field Crops Research 78, 93–103.
| Aluminium-tolerant wheat uses more water and yields higher than aluminium-sensitive one on a sandy soil with subsurface acidity.Crossref | GoogleScholarGoogle Scholar |
Thomas GW (1996) Soil pH and soil acidity. In ‘Methods of soil analysis, Part 3. Chemical methods’. SSSA Book Series No. 5. (Eds D Sparks, A Page, P Helmke, R Loeppert, PN Soltanpour, MA Tabatabai, CT Johnston, ME Sumner) pp. 475–490. (Soil Science Society of America and American Society of Agronomy: Madison, WI 53711, USA)
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant and Soil 171, 1–15.
| Global extent, development and economic impact of acid soils.Crossref | GoogleScholarGoogle Scholar |
Wilcox JC (1936) Some factors involved in the colorimetric determination of the pH of soils. Scientific Agriculture 16, 225–232.


