Enhancing agriculture recovery of Phaseolus vulgaris L. and Cucurbita pepo L. with Olea europaea L. plant growth promoting rhizobacteria
R. Hadjouti A * , H. Mohand Kaci A , F. Benzina A and J. N. Furze B C D
A * , H. Mohand Kaci A , F. Benzina A and J. N. Furze B C D
A Laboratoire de Valorisation et Conservation des Ressources Biologiques (VALCOR), Faculté des Sciences, Département de Biologie, Université M’hamed Bougara de Boumerdes, BP35000 Boumerdes, Algeria.
B Royal Geographical Society (with the Institute of British Geographers), 1 Kensington Gore, SW7 2AR, London, UK.
C Laboratory of Biotechnology and Valorization of Natural Resources, Faculty of Sciences of Agadir, Department of Biology, Ibn Zohr University, BP 8106, 80000 Agadir, Morocco.
D Control and Systems Engineering Department, University of Technology, Alsinaah Street, P.O. Box: 19006, 10066 Baghdad, Iraq.
Soil Research 60(8) 850-863 https://doi.org/10.1071/SR21320
Submitted: 27 December 2021 Accepted: 2 April 2022 Published: 6 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Context: The rhizosphere is an environment created by interactions between root exudates and microorganisms. Interactions are beneficial due to certain components having a plant growth promoting rhizobacteria (PGPR) effect.
Aims: This study consists of the isolation, screening of PGPR from the rhizosphere of Olea europaea L. of a Mediterranean climatic region in Algeria and the study of their effects on growth of two agronomic vegetables Phaseolus vulgaris L. and Cucurbita pepo L.
Methods: Based on their ability to produce the PGPR molecules indole-3-acetic acid (IAA), phosphatase and siderophores, three rhizobacteria (S25, S75, and S79) were chosen for in vivo tests and capacity to produce the cell wall degrading enzymes chitinase, lipase, protease, glucanase, cellulase, and and phospholipase. They were also examined using scanning electron microscopy (SEM) and analysed using matrix-assisted laser desorption/ionisation time of flight mass spectrometry (MALDI-TOF MS) for identification.
Key results: Bacterial strains identified as Bacillus cereus and Bacillus thuringiensis were able to enhance significantly germination of the two vegetables at P < 0.001. Vegetative parameters of C. pepo were significantly affected by the bacterial inoculation. We noted increases in stem length (P < 0.05), number of flowers (P < 0.01), and root length (P < 0.001).
Conclusion: The bacterial isolates of this study provide biological options in treatments originating from alternate hosts.
Implications: They provide hope for companion/intercrop planting schemes, leading to optimisation of agricultural yields in agroecological blends.
Keywords: Bacillus, cell wall degrading enzymes, indole-3-acetic acid, matrix-assisted laser desorption/ionisation time of flight mass spectrometry, phosphatase, plant growth promoting rhizobacteria, siderophores.
Introduction
The principle imperative facing us today is the feeding of a human population, which may reach in excess of 8.9 billion by 2050, with major global increased demand for food in developing countries such as those in Asia and Africa. Agricultural practise optimisation serves to meet our expanding nutritional requirements with limits to agricultural production; catastrophic scenarios are eminent. Conventional modern agriculture increases productivity through external chemical inputs, including fertilisers, pesticides, fungicides and herbicides (Mokrani et al. 2019; Fadiji and Babalola 2020). However, excessive chemical application causes environmental disorders that affect both soil quality and plant health. In addition, chemical application promotes resistant pathogen emergence and decreases beneficial organism populations in the edaphic environment (Pandey et al. 2019; Sabaté et al. 2020). Crucial interactions between plant soil and microfauna occur in edaphic settings. Three distinct components are recognised in plant rhizospheres; the rhizosphere, the rhizoplane, and the root itself. Rhizospheric soil zones are influenced by root exudates that effect microbial relation (activity). The rhizoplane is the root surface, including adhering root components. Herein, endophytic microorganisms are able to colonise inner root tissues (Compant et al. 2019). In the dynamic environment of the rhizosphere, microorganisms develop and interact (Rabbee et al. 2019; Chandra et al. 2020). Rhizospheric microorganisms have essential roles in plant–host ecological fitness. They complement plant growth and improve pathogenic resistance. Microorganisms uphold growth of plants and thereby have effects on soil and crop qualities (Zhu et al. 2020; Azizoglu et al. 2021). Plant growth promoting rhizobacteria (PGPR) are soil bacteria present around/on the root domain and are involved in promoting plant growth and development via secretion of various regulatory analogues predominant in the rhizospheric zone (da Silva et al. 2018; Rodriguez et al. 2019). Hence, they may decrease our dependence on agricultural chemicals (Ahemad and Kibret 2014; Pereira et al. 2019). The mode of action of PGPR that promotes plant growth comprises: (1) abiotic stress tolerance in plants; (2) nutrient fixation and uptake; (3) growth regulation; and (4) siderophores, volatile organic compounds, and protecting enzymes production such as chitinase, cellulase, glucanase, and 1-aminocyclopropane-1-carboxylic acid (ACC)-deaminase for the prevention of plant diseases (Minotto et al. 2014; Vejan et al. 2016; Duca et al. 2018).
Recently, consumers are exhibiting an increased interest in the relationship between their health and the nutritional aspects of their food (including vitamin content, mineral elements and antioxidants, etc.) (Chekanai et al. 2018; Neves et al. 2019). Common bean (Phaseolus vulgaris L.) and zucchini (Cucurbita pepo L.) are fresh grown vegetables with important nutritional and economic value. Common bean is the world’s most important food legume for human consumption and is in great demand in Africa and Latin America (Myers and Kmiecik 2017). Beans provide a source of protein, dietary fibre, starch and minerals (including potassium, thiamine, vitamin B6 and folic acid) (Chekanai et al. 2018). Bean cultures are characterised by sensitivity to environmental factors such as sub-optimal availability of mineral nutrients. Low phosphorus (P) availability is considered to be the principal limiting factor for legume growth (Neila et al. 2014). Zucchini is an important crop of Mediterranean origin. Beneficial characteristics of zucchini include its nutrient content, short growing period, ease of storage and transportation and medicinal value (Liu et al. 2020). It contains a number of beneficial micronutrients such as minerals, carotenoids, vitamin C and phenolic compounds (Martínez-Valdivieso et al. 2017). Olive (Olea europaea L.) displays strong growth and productivity across northern Africa (Atrouz et al. 2021). This crop is not irrigated or chemically treated in Algeria, hence the need to preserve and valorise their rhizosphere, which constitutes a reservoir of biodiversity and provides a candidate for rhizobacterial flora with PGPR benefits to complement bean and zucchini growth.
The aim of this study to isolate and screen for new rhizobacteria from olive (O. europaea) with a PGPR effect in C. pepo and P. vulgaris. The objective of the current study is to demonstrate bacterial isolates production of plant growth molecules (IAA, siderophores, phosphatase) and to show acceleration of germination and vegetative development of the two plants. Further, we hope to confirm identification of isolates using basic laboratory testing, scanning electron microscopy (SEM) and matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectroscopy.
Materials and methods
Soil sampling and isolation of rhizobacteria
Olive rhizospheric soil and root samples were collected from an olive field (in January 2019) in Bir Khadem region, Algiers, Algeria (36°42′59.99″N, 3°03′60.00″E). Samples were taken 0.5–1 m around plants at 30–50 cm depth in the soil. Extracted root systems were carefully shaken by hand to remove soil. Roots and rhizospheric soil were placed in sterilised plastic bags. In the laboratory, serial dilutions were used to isolate bacteria from the three parts of the rhizosphere: (1) rhizospheric soil (RS); (2) rhizoplane (RP); and (3) endorhizosphere (E). A total of 10 g of soil and 10 g of roots were added separately to 90 mL of sterilised saline solution (0.9% NaCl) for isolation from RS and RP respectively. Whereas in endospheric isolation 10g of roots were sterilised with 2% sodium hypochlorite solution for 3 min, then washed five times with sterile distilled water in order to eliminate bacteria residing in the root surface which belong to RP. The roots were ground with a sterile mortar to free the inside of the roots and then added to 90 mL of sterilised saline solution (0.9% NaCl). The three resulting saline solutions were used for preparation of serial dilutions for isolation of rhizobacteria using nutrient agar and King B medium. After incubation for 24–72 h at 30°C, all morphologically different colonies according to their macro and microscopic characteristics (shape and texture of colonies, Gram colouration, spore presence, motility) were isolated, purified and tested for phytopathogenicity and PGPR traits.
Determination of gram strains
Thin microbial smears were air dried and fixed by heat. Smears were held using a slide rack then covered with crystal violet for 1 min. Each slide was washed with distilled water for a few seconds, then covered with an iodine solution for 30 s. The iodine was washed off with 95% ethyl alcohol solution. Fushine was applied to each smear for 1 min. Smears were washed with distilled water. The stained slides were air dried and observed (Aneja 2007).
Scanning electron microscopy
Bacteria were cryofixed and rapidly examined at very low temperatures (below −120°C) by cryo-SEM and metalised by cathodic spraying with gold alloy according to Kaláb et al. (2008).
Determination of phytopathogenic rhizobacteria
Isolated rhizobacteria were tested for their pathogenicity on plants by their hypersensitive reaction. A volume of 1 mL of bacterial suspension was injected in tobacco (Nicotiana tabacum L.) leaves at 20–25°C. A control was maintained with sterile physiologic water injection. After 24 h, the appearance of a collapse at the injection site indicated a positive hypersensitive reaction (Cooksey et al. 1990).
Phytopathogenic isolates were eliminated and were not subjected to determination of plant growth promoting attributes.
Determination of plant growth promoting attributes
The isolated rhizobacteria were screened for PGPR attributes by assessing the production of IAA, siderophore and phosphate solubilisation as follows.
IAA production
IAA production was estimated calorimetrically with the standard method described by Bric et al. (1991) with some modifications. In the current study, Luria–Bertani (LB) broth (g/L) was prepared with yeast extract (5 g/L), NaCl (5 g/L) in distilled water (1000 mL) and supplemented with tryptone (10 g/L). pH was adjusted to 7.5 and supplemented with 5 mM of l-tryptophan (LBT: LB supplemented with l-tryptophan), 0.06% of SDS and 1% glycerol. Bacterial suspensions (106 CFU mL−1) were incubated for 3 days under continuous stirring at 180 rpm at 28 ± 2°C, pelleted through centrifugation at 10 000g for 10 min at 4°C). A volume of 1 mL of the supernatant was incubated with 2 mL of Salkowski reagent: HClO4 (150 mL), distilled water (250 mL), and 0.5 M FeCl3·6H2O (7.5 mL) for 30 min in the dark at room temperature (30°C). The concentration of IAA produced was calculated using a standard curve. Optical density was recorded at 535 nm with known amounts of commercial IAA in Salkowski reagent and sterile LBT broth.
Siderophore production
Siderophore production was revealed using King B solid medium: (peptone 20 g); (K2HPO4 1.5 g); (MgSO4 1.5 g/L); glycerol (15 mL/L) and agar agar (15 g/L). After inoculation and incubation during 24–96 h at 30°C of bacteria isolates, florescent pigmentation was observed in siderophore producing bacteria with the naked eye and under ultraviolet (UV) light at wavelengths 254 and 366 nm (King et al. 2009).
Siderophores were also detected on Chrome Azurol-S (CAS) agar medium. Isolates were streaked on Petri plates containing CAS medium and incubated at 30°C for 48 h. A positive result was revealed by formation of orange halos (Alexander and Zuberer 1991).
Phosphate solubilisation
Phosphate solubilisation was indicated following Pikovskaya (1948). Bacteria were inoculated in Pikovskaya medium (PVK: 10 g/L glucose, 5 g/L, Ca3(PO4)2, 0.2 g/L of KCl, 0.1 g/L of MgSO4·7H2O, 0.2 g/L of NaCl, 0.5 g/L of (NH4)2SO4, 0.02 g/L of FeSO4·7H2O, 0.002 g/L of MnSO4·2H2O, 20 g/L of agar agar), then incubated at 30°C during 72 h. Positive reactions were shown by the presence of a clear halo surrounding bacterial colonies.
Cell wall degrading enzymes production
The three bacterial isolates with greatest capacity to produce PGPR molecules were subjected to extracellular enzyme production tests.
Chitinase production
The ability of bacterial isolates to produce chitinase was ascertained by spotting them on a basal medium mixed with 2.4% chitin suspension (El-Masry et al. 2002).
Protease production
A growing bacterial culture was inoculated in the form of a line in skim milk agar for 48 h at 30°C. A clear zone around the line indicated a positive result (Smibert and Krieg 1994).
Lipase production
Spots of isolates were deposited in the surface of LB medium supplemented with 1% Tween 80 and incubated from 1 to 5 days at 28°C (Sierrea 1957). An opaque halo around colonies indicated a positive result.
Glucanase production
A specific agar medium was used composed of peptone (5 g/L), yeast extract (5 g/L) and barley (Hordrum vulgare L.) flour 10 (g/L). A colony of each strain was inoculated following Zouari et al. (2020).
Cellulase production
Cellulase production was determined with the method described by Prasad et al. (2012) using M9 agar (Miller 1972), supplemented with 10 g/L cellulose and 1.2 g/L yeast extract. Isolates were inoculated and incubated for 8 days at 28°C. Development of a clear halo around colonies indicated a positive response (Verma et al. 2007).
Phospholipase production
Nutrient agar was supplemented with 10 mL of sterile egg yolk emulsion in physiological water. Spots of isolates were deposited in the surface of the prepared medium, then incubated at 30°C for 24 h. The appearance of a clear halo around the spots indicated a positive result (Thaler et al. 1998).
In vivo assays
Three of the best rhizobacteria from isolates with the highest level of IAA production and/or the highest number of positive result for production of PGP molecules were chosen for in vivo testing according to Lwin et al. (2012), including germination tests and vegetative growth assays. Seeds of zucchini and common bean were used for each treatment. The seeds used in the current experiment were supplied by the Algerian National Centre for Seed and Plant Control and Certification.
Germination test
Zucchini and common bean seeds were sterilised in 2% sodium hypochlorite solution for 3 min, then washed five times with sterile distilled water. Sterilised seeds were incubated in 50 mL of bacterial suspension (106 CFU mL−1) at 28 °C for 24 h. Control preparation of seeds was maintained in sterile distilled water. After incubation, seeds were transferred into Petri plates containing sterile humid cotton and incubated at normal room temperature for 10 days (Lwin et al. 2012).
Vegetative growth assays
Sterilisation and inoculation of seeds for vegetative growth tests was carried out following the same steps described as for germination. After 24 h of incubation, inoculated seeds were sown in plastic pots (16 cm high and 19 cm diameter) containing sterilised soil (three times autoclaved for 20 min at 120°C with an interval of 24 h) in greenhouse conditions. The experiment was carried out three times with nine replicates per treatment. All pots were watered three times a week with 200 mL of sterile water. After 40 days, plants were separated and transferred. Measurements of root length, stem length, number of lateral roots, number of leaves, and number of flowers were recorded (Lwin et al. 2012).
Characterisation of efficient bacteria
MALDI-TOF MS (matrix-associated laser desorption/ionisation-time of flight mass spectrometry)
Matrix preparation was carried out by diluting a saturated solution of α-cyano-4-hydroxycinnamic acid (HCCA) in 500 μL of 50% acetonitrile, 250 μL of 10% trifluoroacetic acid (TFA) and 250 μL of HPLC water. After vigorous stirring, sonication was carried out for 10 min, followed by centrifugation (13 000g, for 5 min). Samples were transferred to clean polypropylene tubes.
Each bacterial colony obtained from young cultures (18–24 h) was deposited in duplicate on the MALDI-TOF target plate and covered with 1.5 μL of the matrix solution. The matrix and target plate were dried at room temperature (28°C) for 5 min and analysed (Pfleiderer et al. 2013). A Microflex LT MALDI-TOF mass spectrometer was used for bacterial identification. The spectra of the bacteria obtained were compared with the Bruker computer database using the flex Analysis ver. 3.3 and MALDI-Biotyper ver. 3.0 software for data analysis. Isolates were assumed to be correctly identified at the species level when the logarithmic score value (LSV) was greater than or equal to 1.9 (Seng et al. 2009).
Statistical analysis
Germination and vegetative assays data were subjected to one-way and two-way ANOVA and the Tukey Test, using SPSS ver. 16. Differences were considered to be significant at P < 0.05.
Results
Isolation of rhizobacteria and observation of phenotypic characteristics
A total of 113 rhizobacteria were isolated from the three different part of the olive rhizophere. A total of 28.32% of the rhizobacteria were endophytes; 36.28% are from the rhizoplane and 35.4% are from the rhizosphere.
The majority of the obtained colonies were white or beige, others were orange. The texture was opaque for the majority, sometimes shiny, flat or convex, mucoid or dry. The contour was regular or irregular.
Gram colouration and scanning electron microscopy
The isolates were divided into four groups: (1) Gram-positive rod-shaped (46.90%); (2) Gram-positive spherical-shaped (8%); (3) Gram-negative rod-shaped (39.82%); and (4) Gram-negative spherical-shaped (4.42%) (Fig. 1b) based on the Gram staining analysis. Most of the Gram-positive bacteria were able to grow at 40°C and 55°C with presence of spores while Gram-negative isolates, which were mostly isolated in King B medium did not form endospores.
The three selected rhizobacteria for in vivo tests had rod-shaped form under photonic microscopy and scanning electron microscopy (Fig. 2). S75 was Gram-negative, whereas S25 and S79 were Gram-positive (Fig. 1a) with the presence of endospores.
|
|
Phytopathogenicity traits
Only two of the isolated rhizobacteria showed positive pathogenicity, they were eliminated from the study. The three selected rhizobacteria (S25, S75 and S79) were chosen according to their PGPR traits and showed negative reactions in the phytopathogenicity test.
Plant growth promoting attributes
IAA production
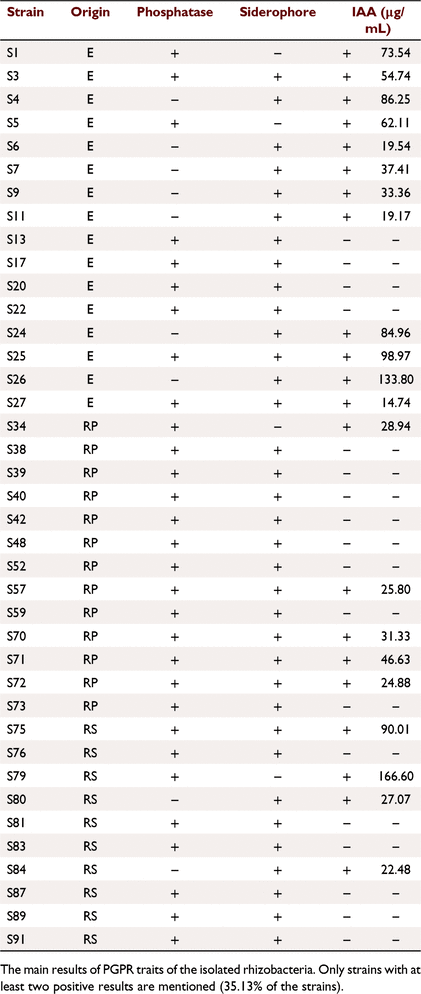
A total of 47.72% of bacteria tested for IAA production revealed positive results (Fig. 3). According to the realised standard curve, quantitative IAA production on LB broth supplemented with (0.5%) of l-tryptophan gave a varying level of IAA production. The highest level recorded was by strains S1, S4, S24, S25, S26, S75 and S79 (Table 1).
|
Siderophore production
After 72 h of incubation, 79.54% of bacterial isolates developed a fluorescent pigmentation on King B medium indicating the production of siderophores (Fig. 3), they also showed positive reaction on CAS medium represented by the appearance of an orange halo around colonies.
Phosphate solubilisation
A total of 68.18% of tested bacteria induced solubilisation of tri-calcium phosphate on Pikovskaya’s medium by forming clear zones around the colonies where the highest production level according to the clear zones was recorded by strains S26 and S72. However, 43% of isolates did not show any halo around their colonies, indicating that no inorganic phosphate solubilisation (Fig. 3).
Cell wall degrading enzymes
The three selected bacteria were tested for extracellular enzyme production and produced chitinase, protease, lipase, phospholipase, and cellulase. Strain S79 was the only producer of glucanase (Fig. 4 and Table 2).

|
Germination test
Three bacterial strains (S25, S75 and S79), were chosen according to their PGPR efficacy and were inoculated in common bean and zucchini seeds. These isolates showed significant effects at P < 0.001. Best effect on zucchini and bean seed germination was recorded with the strain S79 (Fig. 5).
Vegetative growth assays
Strain S79 had the highest effect on stem length (84.12%), number of leaves (85.71%) and number of flowers (100%), though the lowest effect on roots length and lateral roots. Strains with S25 and S75 enhanced roots length and lateral roots number. One-way ANOVA tests showed a significant effect at P < 0.05 on stem length and flowers number, and a significant effect at P < 0.001 on roots length, while a non-significant effect (n.s.) was shown on lateral roots number (Fig. 6).
|
|
MALDI-TOF MS
MALDI-TOF MS analysis of the three selected bacteria revealed that the strains S25 and S79 are both Bacillus genus, whereas S75 logarithmic score value was below the recommended value for identification (Table 3). The mass spectrometry profiles characteristics of each strain are mentioned in Fig. 7. Each peak in the respective profiles identifies specific bacterial proteins.

|
Discussion
In this work, we demonstrated that the three parts of olive rhizosphere (rhizosphere, rhizoplane and endorhizoplane) from a Mediterranean region (north of Algeria) are rich in rhizobacteria with high capacity for producing plant growth molecules (auxins, siderophores and phosphatase). The findings concerning the ability of rhizobacterial strains producing these molecules, indicate that they can be used to enhance crop growth. This concurs with Muñoz et al. (2020) in which rhizobacteria originating from cultivated crops from extreme environments were used.
Phytohormones have regulatory and signalling functions in growth and development. They are additionally produced by rhizobacteria supplementing cell division, cell elongation, and differentiation. Auxins are represented by IAA and analogues, which increase the surface area, length of the root and root exudation, providing the plant with better access to soil nutrients (Dastager et al. 2011; Park et al. 2013; Cecagno et al. 2015; Koua et al. 2020). Phosphate is the second most important macronutrient after nitrogen, effecting plant growth. Even in soil enriched with phosphate, only 0.1% is soluble and assimilated by plants. Paucity of phosphate severely limits global crop production (Mezaache-Aichour et al. 2012; Anzuay et al. 2013). Microbial solubilisation of phosphate is a significant factor in the conversion of insoluble phosphate to soluble phosphate (Koua et al. 2020).
Low molecular weight molecules, siderophores have high affinity to Fe3+ ions. Siderophores therefore facilitate iron availability, and are secreted under conditions of iron deficiency. On formation of the siderophore ion Fe3+ complex, a microbial cell’s external membrane produces siderophores catalysing internalisation. Under conditions of iron deficiency, a siderophore-Fe3+ complex is formed, and the external membrane of the microbial cell producing siderophores catalyses the internalisation of these complexes (Gupta and Gopal 2008). The results of IAA production on LB supplemented with l-tryptophan showed that 47.72% of the tested bacteria produced IAA. 68.18% of isolates solubilised phosphate on PVK solid medium. 79.54% of the tested isolates were able to produce siderophores. These PGPR molecules have been reported in different rhizobacteria (Bhattacharyya and Jha 2012; Nabti et al. 2013; Zennouhi et al. 2018; Mokrani et al. 2019; Qessaoui et al. 2019; Oulebsir-Mohandkaci et al. 2021).
Three strains of the best producers of PGPR molecules (S25, S75 and S79) were selected and tested for their capacity of producing extracellular enzymes: chitinase, protease, glucanase, lipase, phospholipase and cellulase. Enzyme activities in soil are of increasing interest, breaching soil microbiology and biochemistry research. Bacterial and fungal microorganisms secrete extracellular enzymes with a major role in biogeochemical cycles (Bonnet et al. 2017). PGPR enzyme production insures sustainable plant disease management. The aforementioned enzymes break down the cell wall of fungal phytopathogens causing cell death; additionally they are lethal to nematodes and insects (Gow et al. 2017; Jadhav et al. 2017; O’Brien 2017). S25, S75 and S79 produced the mentioned enzymes apart from S25 and S75, which did not produce glucanase.
Chitin is the major component of fungi and insects exteriors. Previous research shows the chitinase from Chitinophaga spp. to have antifungal and nematicidal activity against Fusarium oxysporum, Alternaria alternate, Cladosporium spp. and root knot nematode, Meloidogyne incognita, a major pest responsible for economic losses in agriculture (Sharma et al. 2020). Chitinase from a biocontrol fungus, Trichoderma asperellum prevent anthracnose caused by Colletotrichum spp. on both mango (Mangifera indica L.) and chilli (Capsicum frutescens L.) fruits up to 72 h after enzyme pre-treatment at 40 U/mL (Loc et al. 2020). The study conducted by (Arora et al. 2007) showed inhibition of Rhizoctonia solani peaks when the synthesis of chitinase and glucanase is at maximum by a fluorescent pseudomonad.
The relationship between Beauveria bassiana producing enzymes, including proteases, against Helicoverpa armigera has been demonstrated (Kaur and Padmaja 2009). Protease purified from Streptomyces flavogriseus in the study conducted by Mostafa et al. (2019) showed inhibition against different phytopathogenic fungi, especially F. oxysporium and R. solani.
It is also important to note that cellulase acts against phytopathogenic fungi, Phytophtora and Pythium, whose cellulose content in cell walls is between 17% and 35% (Minotto et al. 2014). Sheetal et al. (2019) reported phospholipase from entomopathogenic Xenorhabdus spp. has a proven efficacy against filarial vector Culex quinquefasciatus.
Bacterial chemotaxonomy of S25, S75 and S79 using MALDI-TOF MS revealed S25 and S79 to be B. cereus and B. thuringiensis, respectively, while S75 could not be identified. MALDI-TOF MS profiling enables the identification of bacteria by the detection of proteins profiles, given that its spectrum is available in the mass spectrometer’s database; giving the same result as 16S rRNA and rDNA gene sequence analysis but at a rapid rate and a lower cost (Rahi et al. 2016; Grégory et al. 2018). However, its application on environmental bacteria is limited due to a lack of data on non-clinical microorganisms (Rahi et al. 2016; Kostrzewa and Maier 2017). Additionally, the culture medium effects the mass spectra, notably when the former does not sustain optimal growth. Growth medium compounds impede with the ionisation of the bacterial biomolecules, as the bacteria have a tendency to adhere to the culture medium surface (Wieme et al. 2014). Bacterial identification is related to cell concentration, various methods determine the minimum concentration of cell material needed for identification of bacteria using MALDI-TOF MS. A study showed Escherichia coli could be identified at species level at 8 × 104 viable cell count (VCC) per mL using a Bruker Autoflex, whereas Enterococcus faecalis did not reach consistently high identification scores even at 5 × 105 VCC per mL. Further, the detection limit may optimally be identified using diafiltration and specific extraction methods, combined with improved algorithms for spectral analyses (Mörtelmaier et al. 2019). As an example, the creation of a custom reference library make it possible to distinguish between E. coli and Shigella species, which were difficult to discriminate. Similarly, ClinProTools make it possible to identify Streptococcus pneumoniae and Streptococcus mitis/oralis despite the fact that these species are frequently confused (Grégory et al. 2018). Precedent issues, call for specific data analysis such as machine learning algorithms which have been leveraged to maximally exploit the information contained in MALDI-TOF MS, with the ultimate goal of refining species identification (Weis et al. 2020), otherwise, the use of machine learning techniques for microbial species identification purposes remains limited and is dominated by the application of adaptive artificial neural networks (ANNs). Research on ANN analysis of MALDI-TOF MS for bacterial identification is limited due to lack of resolution/reporting into the respective knowledge bases used. The evaluation of two other popular machine learning techniques: random forests and support vector machines by De Bruyne et al. (2011) proved to be very successful. Combinatorial of ANN and Fuzzy Logic Systems enables the representation of real-world. Hybrids of these methods increase their advantages and decrease their shortcomings. For example, in fuzzy neural systems, the fuzzy system can provide an input vector to a multi-layer neural network as a response to linguistic statements. Subsequently, the neural network is trained to generate required outputs or decisions (Vlamou and Papadopoulos 2019). Fuzzy logic is extremely useful for many people involved in research and development including environmental engineers, natural scientists (biology and agriculture), and medical researchers (Singh et al. 2013). Recently, Neuro-Fuzzy systems have gained more attention from research communities than other types of fuzzy expert systems since it combines the advantages of the learning ability of neural network and the reasoning ability of fuzzy logic to solve many non-linear and complex real world problems with high accuracy (Salleh et al. 2018). The concept of the fuzzy set operats via intelligent controllers formed through Mamdani and Takagi-Sugeno-Kang (TSK) systems. The TSK-type fuzzy model has advantages over the Mamdani-type in terms of computational efficiency. The defined input of TSK enables precise output ensuring accurate prevision of otimazation of adaptive techniques. The TSK system rationale enables further higher methods such as searching algorithm techniques, which can theoretically identify anything (optimisation by Hybrid Genetic Algorithm; optimisation by particle swarm, artificial bee colony. These searching optimisation techniques can then lead to functional distributes and expressions (for expansion of taxonomic information) (Furze et al. 2017; Yazid et al. 2019; Furze and Mayad 2021, 2022).
In vivo tests were useful to ensure experimental conditions similar to the conditions of definitive application of rhizobacteria. PGPR can use different mechanisms to improve seed germination, root development or to improve mineral nutrition and water use (Dobbelaere et al. 2003; Mitter et al. 2013). Most Bacillus spp. have PGPR characteristics (Jin et al. 2019). Germination tests on common bean and zucchini seeds showed both significant effects at P < 0.001. Concerning vegetative growth, several studies have shown that rhizobacteria stimulate root development, such as research done by Cassán and Diaz-Zorita (2016); Agapit et al. (2020). Other studies have shown that rhizobacteria stimulate flowering, increase leaf and stem length compared to plants, which were not inoculated with rhizobacteria (El Habbasha et al. 2013; Mouradi et al. 2016) in agreement with our results on zucchini crops but with variable enhancement of growth. In spite of the latter, there are still no commercial B. thuringiensis-based PGPR products on the biofertiliser market (Azizoglu 2019), though B. thuringiensis-based commercial biopesticides are available. It should be noted that the resistance of Bacillus spores enables them to enhance plant growth in extreme environments, an advantageous feature compared to non-sporulating PGPR.
Conclusion
Rhizobacterial isolates of B. cereus and B. thuringiensis (S25, S75 and S79) can find their place in biotechnological applications such as crop production enhancement and environment protection. The strains provide a base for biofertilisers and biostimulants, in production of industrially important enzymes, and to produce pesticides.
Further work should study the biocontrol activity of the strains against different phytopathogens to evaluate their activity. It is also preferable to study the efficacy of beneficial strains combined to find the best matching mixture and investigate the mechanism of actions using biochemistry and molecular biology such as in gene expression analysis, amino acid, protein analysis and gene knockout studies.
Data availability
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was supported by the Algerian Ministry of Higher Education (DGRST) and PRFU project program (Code: D00L05UN350120180004).
Acknowledgements
We acknowledge the Algerian National Centre for Seed and Plant Control and Certification for kindly supplying seeds of zucchini and common bean.
References
Agapit C, Gigon A, Girin T, et al. (2020) Split-root system optimization based on the survival, growth and development of the model Poaceae Brachypodium distachyon. Physiologia Plantarum 168, 227–236.| Split-root system optimization based on the survival, growth and development of the model Poaceae Brachypodium distachyon.Crossref | GoogleScholarGoogle Scholar | 30950064PubMed |
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King saud University-science 26, 1–20.
| Mechanisms and applications of plant growth promoting rhizobacteria: current perspective.Crossref | GoogleScholarGoogle Scholar |
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biology and Fertility of Soils 12, 39–45.
| Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria.Crossref | GoogleScholarGoogle Scholar |
Aneja KR (2007) ‘Experiments in microbiology, plant pathology and biotechnology.’ (New Age International)
Anzuay MS, Frola O, Angelini JG, et al. (2013) Genetic diversity of phosphate-solubilizing peanut (Arachis hypogaea L.) associated bacteria and mechanisms involved in this ability. Symbiosis 60, 143–154.
| Genetic diversity of phosphate-solubilizing peanut (Arachis hypogaea L.) associated bacteria and mechanisms involved in this ability.Crossref | GoogleScholarGoogle Scholar |
Arora NK, Kim MJ, Kang SC, et al. (2007) Role of chitinase and β-1,3-glucanase activities produced by a fluorescent pseudomonad and in vitro inhibition of Phytophthora capsici and Rhizoctonia solani. Canadian Journal of Microbiology 53, 207–212.
| Role of chitinase and β-1,3-glucanase activities produced by a fluorescent pseudomonad and in vitro inhibition of Phytophthora capsici and Rhizoctonia solani.Crossref | GoogleScholarGoogle Scholar | 17496968PubMed |
Atrouz K, Bousba R, Marra FP (2021) Algerian olive germplasm and its relationships with the Central-Western Mediterranean varieties contributes to clarify cultivated olive diversification. Plants 10, 678
| Algerian olive germplasm and its relationships with the Central-Western Mediterranean varieties contributes to clarify cultivated olive diversification.Crossref | GoogleScholarGoogle Scholar | 33916098PubMed |
Azizoglu U (2019) Bacillus thuringiensis as a biofertilizer and biostimulator: a mini-review of the little-known plant growth-promoting properties of Bt. Current Microbiology 76, 1379–1385.
| Bacillus thuringiensis as a biofertilizer and biostimulator: a mini-review of the little-known plant growth-promoting properties of Bt.Crossref | GoogleScholarGoogle Scholar | 31101973PubMed |
Azizoglu U, Yilmaz N, Simsek O, Ibal JC, Tagele SB, Shin JH (2021) The fate of plant growth-promoting rhizobacteria in soilless agriculture: future perspectives. 3 Biotech 11, 382 (1–13)
| The fate of plant growth-promoting rhizobacteria in soilless agriculture: future perspectives.Crossref | GoogleScholarGoogle Scholar |
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology and Biotechnology 28, 1327–1350.
| Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture.Crossref | GoogleScholarGoogle Scholar | 22805914PubMed |
Bonnet SAF, Maxfield PJ, Hill AA, et al. (2017) Biogeochemistry in the scales. In ‘Mathematical advances towards sustainable environmental systems’. (Eds JN Furze, K Swing, AK Gupta, RH Mc Clatchey, DM Reynolds). (Springer International)
| Crossref |
Bric JM, Bostock RM, Silverstones SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Applied and Environmental Microbiology 57, 535–538.
| Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane.Crossref | GoogleScholarGoogle Scholar | 16348419PubMed |
Cassán F, Diaz-Zorita M (2016) Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biology and Biochemistry 103, 117–130.
| Azospirillum sp. in current agriculture: from the laboratory to the field.Crossref | GoogleScholarGoogle Scholar |
Cecagno R, Fritsch TE, Schrank IS (2015) The plant growth-promoting bacteria Azospirillum amazonense: genomic versatility and phytohormone pathway. BioMed Research International 2015, 898592
| The plant growth-promoting bacteria Azospirillum amazonense: genomic versatility and phytohormone pathway.Crossref | GoogleScholarGoogle Scholar | 25866821PubMed |
Chandra AK, Kumar A, Bharati A, Joshi R, Agrawal A, Kumar S (2020) Microbial-assisted and genomic-assisted breeding: a two way approach for the improvement of nutritional quality traits in agricultural crops. 3 Biotech 10, 2
| Microbial-assisted and genomic-assisted breeding: a two way approach for the improvement of nutritional quality traits in agricultural crops.Crossref | GoogleScholarGoogle Scholar | 31824813PubMed |
Chekanai V, Chikowo R, Vanlauwe B (2018) Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agriculture, Ecosystems & Environment 266, 167–173.
| Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe.Crossref | GoogleScholarGoogle Scholar |
Compant S, Samad A, Faist H, et al. (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. Journal of Advanced Research 19, 29–37.
| A review on the plant microbiome: ecology, functions, and emerging trends in microbial application.Crossref | GoogleScholarGoogle Scholar | 31341667PubMed |
Cooksey DA, Azad HR, Cha JS, et al. (1990) Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Applied and Environmental Microbiology 56, 431–435.
| Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato.Crossref | GoogleScholarGoogle Scholar | 16348118PubMed |
da Silva CF, Vitorino LC, Soares MA, et al. (2018) Multifunctional potential of endophytic and rhizospheric microbial isolates associated with Butia purpurascens roots for promoting plant growth. Antonie van Leeuwenhoek 111, 2157–2174.
| Multifunctional potential of endophytic and rhizospheric microbial isolates associated with Butia purpurascens roots for promoting plant growth.Crossref | GoogleScholarGoogle Scholar | 29850967PubMed |
Dastager SG, Deepa CK, Pandey A (2011) Potential plant growth-promoting activity of Serratia nematodiphila NII-0928 on black pepper (Piper nigrum L.). World Journal of Microbiology and Biotechnology 27, 259–265.
| Potential plant growth-promoting activity of Serratia nematodiphila NII-0928 on black pepper (Piper nigrum L.).Crossref | GoogleScholarGoogle Scholar |
De Bruyne K, Slabbinck B, Waegeman W, et al. (2011) Bacterial species identification from MALDI-TOF mass spectra through data analysis and machine learning. Systematic and Applied Microbiology 34, 20–29.
| Bacterial species identification from MALDI-TOF mass spectra through data analysis and machine learning.Crossref | GoogleScholarGoogle Scholar | 21295428PubMed |
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. Critical Reviews in Plant Sciences 22, 107–149.
| Plant growth-promoting effects of diazotrophs in the rhizosphere.Crossref | GoogleScholarGoogle Scholar |
Duca DR, Rose DR, Glick BR (2018) Indole acetic acid overproduction transformants of the rhizobacterium Pseudomonas sp. UW4. Antonie van Leeuwenhoek 111, 1645–1660.
| Indole acetic acid overproduction transformants of the rhizobacterium Pseudomonas sp. UW4.Crossref | GoogleScholarGoogle Scholar | 29492769PubMed |
El Habbasha SF, Tawfik MM, El Kramany MF (2013) Comparative efficacy of different bio-chemical foliar applications on growth, yield and yield attributes of some wheat cultivars. World Journal of Agricultural Sciences 9, 345–353.
| Comparative efficacy of different bio-chemical foliar applications on growth, yield and yield attributes of some wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
El-Masry MH, Khalil AI, Hassouna MS, et al. (2002) In situ and in vitro suppressive effect of agricultural composts and their water extracts on some plant pathogenic fungi. World Journal of Microbiology and Biotechnology 18, 551–558.
| In situ and in vitro suppressive effect of agricultural composts and their water extracts on some plant pathogenic fungi.Crossref | GoogleScholarGoogle Scholar |
Fadiji AE, Babalola OO (2020) Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi Journal of Biological Sciences 27, 3622–3633.
| Exploring the potentialities of beneficial endophytes for improved plant growth.Crossref | GoogleScholarGoogle Scholar | 33304173PubMed |
Furze JN, Mayad EH (2021) Harmonics, evolutionary generators, DANCE, and HEAR – functional dimensions. Environmental Science and Pollution Research 28, 64181–64190.
| Harmonics, evolutionary generators, DANCE, and HEAR – functional dimensions.Crossref | GoogleScholarGoogle Scholar | 33846914PubMed |
Furze JN, Mayad EH (2022) Generators, harmonics and evolutionary emergence. In: ‘Earth systems protection and sustainability. Vol 1’. (Eds Furze JN, Eslamian S, Raafat SM, Swing K) pp. 17–34. (Springer: Cham)
| Crossref |
Furze JN, Zhu Q, Hill J, Qiao F (2017) Biological modelling for sustainable ecosystems. In ‘Mathematical advances towards sustainable environmental systems’. (Eds JN Furze, K Swing, AK Gupta, et al.) pp. 9–42. (Springer: Cham).
| Crossref |
Gow NAR, Latge J-P, Munro CA (2017) The fungal cell wall: structure, biosynthesis, and function. Microbiology Spectrum 5, 267–292.
| The fungal cell wall: structure, biosynthesis, and function.Crossref | GoogleScholarGoogle Scholar |
Grégory D, Chaudet H, Lagier JC, et al. (2018) How mass spectrometric approaches applied to bacterial identification have revolutionized the study of human gut microbiota. Expert Review of Proteomics 15, 217–229.
| How mass spectrometric approaches applied to bacterial identification have revolutionized the study of human gut microbiota.Crossref | GoogleScholarGoogle Scholar | 29336192PubMed |
Gupta A, Gopal M (2008) Siderophore production by plant growth promoting rhizobacteria. Indian Journal of Agricultural Research 42, 153–156.
Jadhav HP, Shaikh SS, Sayyed RZ (2017) Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: an overview. In ‘Rhizotrophs: plant growth promotion to bioremediation’. (Ed. S Mehnaz) pp. 183–203. (Springer: Singapore)
| Crossref |
Jin Y, Zhu H, Luo S, et al. (2019) Role of maize root exudates in promotion of colonization of Bacillus velezensis strain S3-1 in rhizosphere soil and root tissue. Current Microbiology 76, 855–862.
| Role of maize root exudates in promotion of colonization of Bacillus velezensis strain S3-1 in rhizosphere soil and root tissue.Crossref | GoogleScholarGoogle Scholar | 31073734PubMed |
Kaláb M, Yang AF, Chabot D (2008) Conventional scanning electron microscopy of bacteria. Infocus magazine 10, 42–61.
| Conventional scanning electron microscopy of bacteria.Crossref | GoogleScholarGoogle Scholar |
Kaur G, Padmaja V (2009) Relationships among activities of extracellular enzyme production and virulence against Helicoverpa armigera in Beauveria bassiana. Journal of Basic Microbiology 49, 264–274.
| Relationships among activities of extracellular enzyme production and virulence against Helicoverpa armigera in Beauveria bassiana.Crossref | GoogleScholarGoogle Scholar | 19025880PubMed |
King D, Larsen R, Pogliano K, et al. (2009) Two simple media for the demonstration of pyocyanin and fuorescens. Journal of Laboratory and Clinical Medicine 44, 301–307.
Kostrzewa M, Maier T (2017) Criteria for development of MALDI-TOF mass spectral database. In ‘MALDI-TOF and tandem MS for clinical microbiology’. (Eds HN Shah, SE Gharbia) pp. 39–54. (John Wiley & Sons, Ltd)
| Crossref |
Koua SH, N’golo DC, Alloue-Boraud WM, et al. (2020) Bacillus subtilis strains isolated from Cocoa Trees (Theobroma cacao L.) rhizosphere for their use as potential plant growth promoting rhizobacteria in Côte d’Ivoire. Current Microbiology 77(9), 2258–2264.
| Bacillus subtilis strains isolated from Cocoa Trees (Theobroma cacao L.) rhizosphere for their use as potential plant growth promoting rhizobacteria in Côte d’Ivoire.Crossref | GoogleScholarGoogle Scholar |
Liu J, Wang B, Li Y, et al. (2020) RNA sequencing analysis of low temperature and low light intensity-responsive transcriptomes of zucchini (Cucurbita pepo L.). Scientia Horticulturae 265, 109263
| RNA sequencing analysis of low temperature and low light intensity-responsive transcriptomes of zucchini (Cucurbita pepo L.).Crossref | GoogleScholarGoogle Scholar |
Loc NH, Huy ND, Quang HT, et al. (2020) Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 11, 38–48.
| Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34.Crossref | GoogleScholarGoogle Scholar | 32128280PubMed |
Lwin KM, Myint MM, Tar T, et al. (2012) Isolation of plant hormone (indole-3-acetic acid-IAA) producing rhizobacteria and study on their effects on maize seedling. Engineering Journal 16, 137–144.
| Isolation of plant hormone (indole-3-acetic acid-IAA) producing rhizobacteria and study on their effects on maize seedling.Crossref | GoogleScholarGoogle Scholar |
Martínez-Valdivieso D, Font R, Fernández-Bedmar Z, et al. (2017) Role of zucchini and its distinctive components in the modulation of degenerative processes: genotoxicity, anti-genotoxicity, cytotoxicity and apoptotic effects. Nutrients 9, 755
| Role of zucchini and its distinctive components in the modulation of degenerative processes: genotoxicity, anti-genotoxicity, cytotoxicity and apoptotic effects.Crossref | GoogleScholarGoogle Scholar |
Mezaache-Aichour S, Guechi A, Nicklin J, et al. (2012) Isolation, identification and antimicrobial activity of pseudomonads isolated from the rhizosphere of potatoes growing in Algeria. Journal of Plant Pathology 94, 89–98.
| Isolation, identification and antimicrobial activity of pseudomonads isolated from the rhizosphere of potatoes growing in Algeria.Crossref | GoogleScholarGoogle Scholar |
Miller JH (1972) ‘Experiment in molecular genetics.’ (Cold Spring Harbor Laboratory: Cold Spring Harbor, New York)
Minotto E, Milagre LP, Oliveira MT, et al. (2014) Enzyme characterization of endophytic actinobacteria isolated from tomato plants. Journal of Advanced Scientific Research 5, 16–23.
Mitter B, Brader G, Afzal M, et al. (2013) Advances in elucidating beneficial interactions between plants, soil, and bacteria. In ‘Advances in agronomy’. Vol. 121 (Ed. DL Sparks) pp. 381–445. (Academic Press)
| Crossref |
Mokrani S, Rai A, Belabid L, et al. (2019) Pseudomonas diversity in western Algeria: role in the stimulation of bean germination and common bean blight biocontrol. European Journal of Plant Pathology 153, 397–415.
| Pseudomonas diversity in western Algeria: role in the stimulation of bean germination and common bean blight biocontrol.Crossref | GoogleScholarGoogle Scholar |
Mörtelmaier C, Panda S, Robertson I, et al. (2019) Identification performance of MALDI-ToF-MS upon mono-and bi-microbial cultures is cell number and culture proportion dependent. Analytical and Bioanalytical Chemistry 411, 7027–7038.
| Identification performance of MALDI-ToF-MS upon mono-and bi-microbial cultures is cell number and culture proportion dependent.Crossref | GoogleScholarGoogle Scholar | 31486868PubMed |
Mostafa EE, Saad MM, Hassan HM, et al. (2019) Purification and characterization of alkaline protease produced by Streptomyces flavogriseus and its application as a biocontrol agent for plant pathogens. Egyptian Pharmaceutical Journal 18, 332
| Purification and characterization of alkaline protease produced by Streptomyces flavogriseus and its application as a biocontrol agent for plant pathogens.Crossref | GoogleScholarGoogle Scholar |
Mouradi M, Bouizgaren A, Farissi M, et al. (2016) Osmopriming improves seeds germination, growth, antioxidant responses and membrane stability during early stage of Moroccan alfalfa populations under water deficit. Chilean Journal of Agricultural Research 76, 265–272.
| Osmopriming improves seeds germination, growth, antioxidant responses and membrane stability during early stage of Moroccan alfalfa populations under water deficit.Crossref | GoogleScholarGoogle Scholar |
Muñoz PA, Arismendi MJ, Cárdenas SF, et al. (2020) Diversity of culturable bacteria isolated from ancestral crops of Arica and Parinacota Region, Atacama Desert. Antonie van Leeuwenhoek 113, 2123–2137.
| Diversity of culturable bacteria isolated from ancestral crops of Arica and Parinacota Region, Atacama Desert.Crossref | GoogleScholarGoogle Scholar | 33136285PubMed |
Myers JR, Kmiecik K (2017) Common bean: economic importance and relevance to biological science research. In ‘The common bean genome’. (Eds M Pérez de la Vega, M Santalla, F Marsolais) pp. 1–20. (Springer: Cham)
| Crossref |
Nabti EH, Mokrane N, Ghoul M, et al. (2013) Isolation and characterization of two halophilic Bacillus (B. licheniformis and Bacillus sp.) with antifungal activity. Journal of Ecology of Health & Environment 1, 13–17.
| Isolation and characterization of two halophilic Bacillus (B. licheniformis and Bacillus sp.) with antifungal activity.Crossref | GoogleScholarGoogle Scholar |
Neila A, Adnane B, Mustapha F, et al. (2014) Phaseolus vulgaris-rhizobia symbiosis increases the phosphorus uptake and symbiotic N2 fixation under insoluble phosphorus. Journal of Plant Nutrition 37, 643–657.
| Phaseolus vulgaris-rhizobia symbiosis increases the phosphorus uptake and symbiotic N2 fixation under insoluble phosphorus.Crossref | GoogleScholarGoogle Scholar |
Neves FIG, Silva CLM, Vieira MC (2019) Combined pre-treatments effects on zucchini (Cucurbita pepo L.) squash microbial load reduction. International Journal of Food Microbiology 305, 108257
| Combined pre-treatments effects on zucchini (Cucurbita pepo L.) squash microbial load reduction.Crossref | GoogleScholarGoogle Scholar | 31276954PubMed |
O’Brien PA (2017) Biological control of plant diseases. Australasian Plant Pathology 46, 293–304.
| Biological control of plant diseases.Crossref | GoogleScholarGoogle Scholar |
Oulebsir-Mohandkaci H, Benzina-Tihar F, Hadjouti R (2021) Exploring biofertilizer potential of plant growth-promoting rhizobacteria Bacillus clausii strain B8 (MT305787) on Brassica napus and Medicago sativa. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49, 12484–12484.
| Exploring biofertilizer potential of plant growth-promoting rhizobacteria Bacillus clausii strain B8 (MT305787) on Brassica napus and Medicago sativa.Crossref | GoogleScholarGoogle Scholar |
Pandey S, Gupta S, Ramawat N (2019) Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter. 3 Biotech 9, 277
| Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter.Crossref | GoogleScholarGoogle Scholar | 31245241PubMed |
Park K, Park JW, Lee SW, et al. (2013) Disease suppression and growth promotion in cucumbers induced by integrating PGPR agent Bacillus subtilis strain B4 and chemical elicitor ASM. Crop Protection 54, 199–205.
| Disease suppression and growth promotion in cucumbers induced by integrating PGPR agent Bacillus subtilis strain B4 and chemical elicitor ASM.Crossref | GoogleScholarGoogle Scholar |
Pereira LB, Andrade GS, Meneghin SP, et al. (2019) Prospecting plant growth-promoting bacteria isolated from the rhizosphere of sugarcane under drought stress. Current Microbiology 76, 1345–1354.
| Prospecting plant growth-promoting bacteria isolated from the rhizosphere of sugarcane under drought stress.Crossref | GoogleScholarGoogle Scholar | 31372732PubMed |
Pfleiderer A, Lagier J-C, Armougom F, et al. (2013) Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. European Journal of Clinical Microbiology & Infectious Diseases 32, 1471–1481.
| Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample.Crossref | GoogleScholarGoogle Scholar |
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17, 362–370.
Prasad P, Bedi S, Singh T (2012) In vitro cellulose rich organicmaterial degradation by cellulolytic Streptomyces albospinus (MTCC 8768). Malaysian Journal of Microbiology 8, 164–169.
| In vitro cellulose rich organicmaterial degradation by cellulolytic Streptomyces albospinus (MTCC 8768).Crossref | GoogleScholarGoogle Scholar |
Qessaoui R, Bouharroud R, Furze JN, et al. (2019) Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Scientific Reports 9, 12832
| Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth.Crossref | GoogleScholarGoogle Scholar | 31492898PubMed |
Rabbee MF, Ali MS, Choi J, et al. (2019) Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24, 1046
| Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes.Crossref | GoogleScholarGoogle Scholar |
Rahi P, Prakash O, Shouche YS (2016) Matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS) based microbial identifications: challenges and scopes for microbial ecologists. Frontiers in Microbiology 7, 1359
| Matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS) based microbial identifications: challenges and scopes for microbial ecologists.Crossref | GoogleScholarGoogle Scholar | 27625644PubMed |
Rodriguez PA, Rothballer M, Chowdhury SP, et al. (2019) Systems biology of plant-microbiome interactions. Molecular Plant 12, 804–821.
| Systems biology of plant-microbiome interactions.Crossref | GoogleScholarGoogle Scholar | 31128275PubMed |
Sabaté DC, Petroselli G, Erra-Balsells R, et al. (2020) Beneficial effect of Bacillus sp. P12 on soil biological activities and pathogen control in common bean. Biological Control 141, 104131
| Beneficial effect of Bacillus sp. P12 on soil biological activities and pathogen control in common bean.Crossref | GoogleScholarGoogle Scholar |
Salleh MNM, Talpur N, Talpur KH (2018) A modified neuro-fuzzy system using metaheuristic approaches for data classification. In ‘Artificial intelligence–emerging trends and applications’. (Ed. MAA Fernandez) pp. 29–45. (IntechOpen: London, UK)
Seng P, Drancourt M, Gouriet F, et al. (2009) Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clinical Infectious Diseases 49, 543–551.
| Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 19583519PubMed |
Sharma S, Kumar S, Khajuria A, et al. (2020) Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167. World Journal of Microbiology and Biotechnology 36, 1–15.
| Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167.Crossref | GoogleScholarGoogle Scholar |
Sheetal BP, Geetha P, Vaidehi D, et al. (2019) Mosquitocidal efficacy of lecithinase derived from entomopathogenic bacteria Xenorhabdus sp. strain PBU1755 against filarial vector Culex quinquefasciatus. Biocatalysis and Agricultural Biotechnology 17, 492–498.
| Mosquitocidal efficacy of lecithinase derived from entomopathogenic bacteria Xenorhabdus sp. strain PBU1755 against filarial vector Culex quinquefasciatus.Crossref | GoogleScholarGoogle Scholar |
Sierrea G (1957) A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie van Leeuwenhoek 23, 15–22.
| A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates.Crossref | GoogleScholarGoogle Scholar |
Singh H, Gupta MM, Meitzler T, et al. (2013) Real-life applications of fuzzy logic. Advances in Fuzzy Systems 2013, 581879
| Real-life applications of fuzzy logic.Crossref | GoogleScholarGoogle Scholar |
Smibert RM, Krieg NR (1994) Phenotypic characterization. In ‘Methods for general and molecular bacteriology’. (Eds P Gerharelt, RGE Murray, WA Wood, NR Krieg) pp. 607–654. (American Society of Microbiology: Washington DC)
| Crossref |
Thaler J-O, Duvic B, Givaudan A, et al. (1998) Isolation and entomotoxic properties of the Xenorhabdus nematophilus F1 Lecithinase. Applied and Environmental Microbiology 64, 2367–2373.
| Isolation and entomotoxic properties of the Xenorhabdus nematophilus F1 Lecithinase.Crossref | GoogleScholarGoogle Scholar | 9647801PubMed |
Vejan P, Abdullah R, Khadiran T, et al. (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability—a review. Molecules 21, 573
| Role of plant growth promoting rhizobacteria in agricultural sustainability—a review.Crossref | GoogleScholarGoogle Scholar |
Verma M, Brar SK, Tyagi RD, et al. (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochemical Engineering Journal 37, 1–20.
| Antagonistic fungi, Trichoderma spp.: panoply of biological control.Crossref | GoogleScholarGoogle Scholar |
Vlamou E, Papadopoulos B (2019) Fuzzy logic systems and medical applications. AIMS Neuroscience 6, 266–272.
| Fuzzy logic systems and medical applications.Crossref | GoogleScholarGoogle Scholar | 32341982PubMed |
Weis CV, Jutzeler CR, Borgwardt K (2020) Machine learning for microbial identification and antimicrobial susceptibility testing on MALDI-TOF mass spectra: a systematic review. Clinical Microbiology and Infection 26, 1310–1317.
| Machine learning for microbial identification and antimicrobial susceptibility testing on MALDI-TOF mass spectra: a systematic review.Crossref | GoogleScholarGoogle Scholar | 32217160PubMed |
Wieme AD, Spitaels F, Aerts M, et al. (2014) Effects of growth medium on matrix-assisted laser desorption–ionization time of flight mass spectra: a case study of acetic acid bacteria. Applied and Environmental Microbiology 80, 1528–1538.
| Effects of growth medium on matrix-assisted laser desorption–ionization time of flight mass spectra: a case study of acetic acid bacteria.Crossref | GoogleScholarGoogle Scholar | 24362425PubMed |
Yazid E, Garratt M, Santoso F (2019) Position control of a quadcopter drone using evolutionary algorithms-based self-tuning for first-order Takagi–Sugeno–Kang fuzzy logic autopilots. Applied Soft Computing 78, 373–392.
| Position control of a quadcopter drone using evolutionary algorithms-based self-tuning for first-order Takagi–Sugeno–Kang fuzzy logic autopilots.Crossref | GoogleScholarGoogle Scholar |
Zennouhi O, El Mderssa M, Ibijbijen J, et al. (2018) Caractérisation génotypique de bactéries solubilisant le phosphate isolées de nodules racinaires d’Adenocarpus boudyi (Maire), endémique du Moyen Atlas central marocain. Journal of Applied Biosciences 126, 12630–12637.
Zhu Z, Zhang H, Leng J, et al. (2020) Isolation and characterization of plant growth-promoting rhizobacteria and their effects on the growth of Medicago sativa L. under salinity conditions. Antonie van Leeuwenhoek 113, 1263–1278.
| Isolation and characterization of plant growth-promoting rhizobacteria and their effects on the growth of Medicago sativa L. under salinity conditions.Crossref | GoogleScholarGoogle Scholar | 32564275PubMed |
Zouari I, Masmoudi F, Medhioub K, et al. (2020) Biocontrol and plant growth-promoting potentiality of bacteria isolated from compost extract. Antonie van Leeuwenhoek 113, 2107–2122.
| Biocontrol and plant growth-promoting potentiality of bacteria isolated from compost extract.Crossref | GoogleScholarGoogle Scholar | 33156472PubMed |


