Responses of soil nutrients and microbial activity to the mill-mud application in a compaction-affected sugarcane field
Xiangyu Liu A , Rob Milla B , Terry Granshaw B , Lukas Van Zwieten A C , Mehran Rezaei Rashti A , Maryam Esfandbod A and Chengrong Chen
A C , Mehran Rezaei Rashti A , Maryam Esfandbod A and Chengrong Chen  A *
A *
A Australian Rivers Institute and School of Environment and Science, Griffith University, Brisbane, Qld 4111, Australia.
B Burdekin Productivity Services, 210 Old Clare Road, Ayr, Qld 4807, Australia.
C NSW Department of Primary Industries, Wollongbar Primary Industries Institute, Wollongbar, NSW 2477, Australia.
Soil Research 60(4) 385-398 https://doi.org/10.1071/SR21162
Submitted: 15 June 2021 Accepted: 25 October 2021 Published: 29 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Compaction removal and organic amendment application are commonly used to mitigate the compaction-induced declines in crop yield, soil carbon (C) and soil health. However, the response of microbial activities and nutrient pools to the combination of mill-mud amendments and decompaction in the soil profile are not fully understood.
Aims: A field trial was conducted at Burdekin, Australia, to investigate the effects of different decompaction managements on soil nutrient cycling, associated biological activities and sugarcane yield.
Methods: This experiment included four treatments: control (CK, without mill-mud), mill-mud shallow furrow (MS), deep trenching without mill-mud (DT) and deep trenching mill-mud application (MD).
Key results: The MD treatment increased concentrations of hot water extractable organic C by 30–70% and hot water extractable total nitrogen (N) by 30–90% at the application depth. Soil microbial biomass C and N were also higher in mill-mud applied layers. Mill-mud applied treatments increased plant cane yield by 7% (MS treatment) and 14% (MD treatment) compared to CK. The DT treatment also increased plant cane yield by 11% compared to CK.
Conclusion: The MD treatment increased the supply of organic C and nutrients to the microbial community within the entire soil profile, enhanced nutrient cycling processes, improved soil environmental conditions and soil health for sugarcane growth and thus increased sugarcane productivity.
Implications: Further research should focus on microbial community composition shifts to further explore the mechanisms responsible for soil microorganism regulation of nutrient cycling in sugarcane farming systems.
Keywords: Colwell P, compaction removal, deep trench, enzyme activities, labile organic C and N, microbial biomass, mineral N, sugarcane yield.
Introduction
Compaction, a type of soil disturbance that commonly occurs with over use of heavy machinery in fields, leads to severe crop yield loss and soil health decline worldwide (Shah et al. 2017). Compaction may substantially impact soil functions, such as restricting root growth and limiting soil water and nutrient availability. For example, it has been reported that 20% of sugarcane yield losses were due to compaction in Bundaberg, Australia (Bell et al. 2002), 12–23% wheat grain yield decline due to compaction was reported in a field study conducted in Morocco (Oussible et al. 1992) and an average of 16% sugarbeet yield decline was reported in Hungary by Lipiec et al. (2003). The effect of compaction on crop productivity, soil carbon (C) and nutrient pools and dynamics has been assessed in a range of field studies (George et al. 2011; Colombi and Keller 2019; Shukla et al. 2020). It has been reported that compaction, excessive tillage and the depletion of soil organic matter are the main contributors to sugarcane (Saccharum officinarum) (Garside et al. 2005), maize (Zea mays) (Chen and Weil 2011) and spring barley (Hordeum vulgare) (Czyż 2004) yield decline. However, the response of soil microbial activity to compaction removal practices has been less explored. Such studies could provide some insight into the underlying mechanisms and improve the knowledge of field managers to enhance their decision-making in applying appropriate field management practices to minimise the effects of compaction.
Adequate soil bulk density, organic C and nutrient pools support soil microbial performance and further determine soil health conditions (Magdoff and Weil 2004; Flavel and Murphy 2006). High soil bulk density (>1.47 g cm−3 for clay soil and >1.8 g cm−3 for sandy soil) affects soil health condition by reducing soil porosity, which decreases soil microbial activity and restricts root growth (Li et al. 2002). Labile C, which is largely produced by organic matter decomposition processes, is considered the main food source of soil microbes (Valenzuela-Solano and Crohn 2006). Labile C limitation or organic matter depletion may result in soil health decline (Liu et al. 2018). While the importance of mineral nutrients for crop growth has been recognised, organic nutrients are also strongly related to crop yield and soil health condition (e.g. Liu et al. 2018). Brackin et al. (2015) highlighted that the organic form of N was more suitable for plant root uptake, while inorganic nitrogen (N) concentration in soil was, to some extent, overestimated for plant nutrition due to its extraction method. Organic phosphorus (P) may not be directly utilised by plants; however, enhancing microbial activities would increase P mineralisation, thus enhancing P availability (Gilbert et al. 2008). In addition to other physical (e.g. soil bulk density) and chemical indicators (e.g. soil nutrient pools and availability), soil microbial biomass C and N, microbial activities and the bacterial-to-fungal ratio are also considered important and sensitive soil health indicators because these parameters can change rapidly in response to shifts in environmental conditions (Bhandari et al. 2018; Bünemann et al. 2018). As a key driver of nutrient cycling, the soil microbial community plays a vital role in soil C mineralisation (Nicolardot et al. 1994). A measure of overall microbial activity was suggested to be an important indicator of the impacts of field management practices on soil health (Schloter et al. 2003; Arias et al. 2005).
In recent decades, sugarcane yield decline has occurred in many sugarcane growing regions in Australia due to compaction and inappropriate field practices. Depletion of soil organic matter and overuse of heavy machinery in the field are reportedly responsible for declines in soil structure and sugarcane yield, particularly in monoculture sugarcane-growing areas (Pankhurst et al. 2003; Garside et al. 2005). To prevent further yield loss and soil health decline, the application of organic amendments such as mill-mud (a primary by-product from sugar production) or compost has been suggested to increase soil organic matter, reduce soil bulk density and improve soil structure (Qureshi et al. 2007; Pattison et al. 2011; Ishak and Brown 2018). Mill-mud application is an efficient way to increase sugarcane yield by improving soil physical, chemical and biological properties (Naidu and Syers 1992; Morris et al. 2007; Gilbert et al. 2008). McCray et al. (2015) reported that broadcast application of mill-mud significantly increased sugarcane yield via boosting soil nutrient concentrations in a Florida sandy soil. Several studies worldwide have indicated that mill-mud application substantially increased soil organic C and nutrient transformation by providing substrates for microbial utilisation (Fauci and Dick 1994; Lima et al. 2009; Tan 2009). It has been suggested that the mill-mud can also modify soil microbial pool size and microbial C use efficiency in a poorly structured soil (Fang et al. 2020). Hence, mill-mud application is considered one of the most suitable field practices to ameliorate or remove compaction impacts. Based on the above discussion, more attention should be paid to the response of microbial activities and changes in soil nutrient pools to organic amendments used for removing compaction. This will enable better predictions of soil health condition in the context of boosting crop yield and developing sustainable agriculture.
This study aimed to investigate and compare the responses of soil microbial activity and nutrient pools to different compaction removal practices, such as deep trenching mill-mud application and shallow furrow application, in sugarcane fields. The following hypotheses were tested: (a) the application of mill-mud would increase soil nutrient pools including total and labile pools, (b) different application methods (shallow furrow application and deep trench application) would lead to different distribution patterns of organic C and nutrients at different depths in the soil profile and (c) mill-mud application and soil compaction stress removal would increase the size of the microbial community and microbial activity, which would further increase soil health condition and sugarcane yield.
Materials and methods
Site description, field treatments and cane yield measurement
The experimental site was located in a sugarcane producing area, Burdekin (19°30′S, 147°20′E), Queensland, Australia. The soil is a Mesonatric Brown Sodosol in Australia (Isbell 2016) which is also classified as a Solonchak based on the FAO world reference base (WRB) for soil resources (IUSS Working Group WRB 2014). The mean annual temperature is 29.2°C and mean annual precipitation is 741.0 mm (Australia Bureau of Meteorology 2021). The experimental field had been cropped with sugarcane for the past 15 years. As a common practice, fine agricultural gypsum was applied to a depth of ∼10 cm using the broadcasting method. Gypsum was applied to improve soil structure and calcium (Ca) concentration (5 t ha−1). Soil at this field site has been affected by compaction due to routine use of heavy machinery (e.g. harvester) for management and harvesting. Mean values of surface soil properties from six composite samples (>30 soil cores) taken prior to the study across the paddock (c. 50 ha) include pH (6.01), EC (29.1 μS cm−1), clay (4.5%), silt (37.3%), magnesium (Mg; 477 mg kg−1), lead (Pb; 7.7 mg kg−1), copper (Cu; 9.5 mg kg−1), nickel (Ni; 4.7 mg kg−1), titanium (Ti; 398 mg kg−1), manganese (369 mg kg−1) and iron (Fe; 5.9 g kg−1) (Supplementary Table S1a, b). Overall variations among these composite samples were relatively small with coefficients of variation <15% for most parameters measured (e.g. pH, EC, particle size, Mg, Pb, Cu, Ni, Ti and Fe) at various soil depths (Table S1a, b). This provided a basis for comparing the field treatments. In this study, there were four treatments: (a) control treatment (CK, without application of mill-mud), (b) mill-mud shallow furrow (c. 20 cm depth) addition treatment (MS, mill-mud shallow furrow), (c) deep trench treatment (DT, deep trench without mill-mud) and (d) deep trench mill-mud application (c. 40 cm depth) treatment (MD, deep trench with mill-mud application). Mill-mud was applied in January 2018 at a rate of 35 t ha−1 (dry weight based) and sugarcane (S. officinarum) harvested in July 2019. Amendments were applied to the targeted subsoil levels (c. 20 and 40 cm depths) in each trench 2 weeks before mung bean (Vigna radiata (L.) Wilczek) planting using a large trencher and conveyer spreader to drop mill-mud into trenched slots in the area where the cane row would be planted. Mill-mud materials (a mixture of mill-mud and mill-ash) were slightly acidic (pH 6.4) and mill-mud nutrients included C (9.1% of dry matter), N (0.42% of dry matter), P (0.73% of dry matter) and potassium (0.61% of dry matter). Soil sampling took place in July 2019 with six replicates from each treatment. Adjacent CK (26.05 ha), MS (26.05 ha), DT (8 ha) and MD (8 ha) treatments were divided into six subplots. Further description of soil sampling is shown in Fig. S1. The soil cores (five) were randomly sampled with a 5-cm diameter auger and divided into four layers of 0–10, 10–20, 20–40 and 40–60 cm depth in each subplot and bulked together to make a composite sample. Fresh soil samples were sieved (<2 mm) and stored at 4°C prior to chemical and biochemical analyses (within 1 week).
Sugarcane yield was measured by harvesting the entire plot of four treatments, and yield was calculated based on weight of harvested cane divided by its entire treatment area (t ha−1). Therefore, unfortunately, there was no replicate of the sugarcane yield result. This has been the common practice in the sugarcane farming due to the short window for harvesting in Burdekin. It was acknowledged that the yield data (without statistical analysis) could only be used as indication of impacts of mill-mud amendments.
Soil physicochemical analysis
The soil sand, silt and clay contents were measured using a Maxing sizer (Malvern Panalytical, UK). Soil bulk density was estimated by soil weight divided by its volume using the method described by Maynard and Curran (2007). Soil pH and EC values were measured in a 1:5 volumetric suspension of soil to distilled water (Rayment and Lyons 2011). Soil mineral N (NH4+-N and NO3−-N) was extracted by 2 M KCl at a 1:5 ratio of soil to extractant using an end-over-end shaker for 1 h, filtered by a Whatman 42 filter paper (Rayment and Lyons 2011) with concentrations of NH4+-N and NO3−-N determined by a SEAL AA3 Continuous Segmented Flow Analyser (SEAL Analytical Limited, USA). Soil Colwell P was extracted by 0.5 M NaHCO3 at a 1:5 ratio of soil to extractant using an end-over-end shaker for 16 h, filtered by a Whatman 42 filter paper (Rayment and Lyons 2011) with concentrations of Colwell P measured by a SEAL AA3 Continuous Segmented Flow Analyser. Soil total C (TC) and N (TN) contents were measured by the combustion method using a LECO CNS-2000 analyser (LECO Corporation, MI, USA). Soil total P was measured using an inductively coupled plasma optical emission spectrometer after digestion (ICP-OES; Perkin Elmer; Optima 8300). Hot water extractable organic C (HWEOC) and hot water extractable total N (HWETN) were measured using the method described by Chen et al. (2000). Briefly, 4.0 g (oven-dry equivalent) of fresh soil was incubated with 20 mL of water in a capped Falcon tube at 70°C for 18 h. After incubation, the tubes were shaken on an end-over-end shaker for 5 min and filtered through a Whatman 42 filter paper, followed by a 0.45-μm filter membrane. Concentrations of dissolved organic C and total extractable N in the filtrate were determined using a SHIMADZU TOC-VCPH (Shimadzu, Kyoto, Japan) TOCN analyser. All results were expressed on an oven-dry basis.
Soil biological analysis
Soil microbial biomass C (MBC) and N (MBN) contents were measured by fumigation-extraction method using an Ec conversion factor of 2.64 (Vance et al. 1987) and an En conversion factor of 2.22 (Wilson 1988). Concentrations of soluble C and N of the fumigated and nonfumigated soil samples were determined using a SHIMADZU TOC-VCSH/CSN TOCN analyser. Fluorescein diacetate hydrolysis, widely accepted as an accurate and simple method for measuring the overall microbial activity in a range of environmental samples, was used to measure soil microbial activity in this study (e.g. Green et al. 2006). The activities of soil β-glucosidase (hydrolysing cellulose to glucose) at pH 6.0 and soil phosphatase (hydrolysing phosphomonoesters) at pH 6.5 were measured according to methods described by Tabatabai (1994).
Statistical analysis
Univariate ANOVA was used for soil properties data using the IBM SPSS Statistics 23 software package (IBM Corp., Armonk, NY, USA). Differences at P ≤ 0.05 between treatments were considered significant and all variables were tested for normality of distribution using the Kolmogrov–Smirnov test. Pearson linear correlations were used to describe the relationships between soil properties. In addition, data on all soil properties were analysed using principal component analysis (PCA) to distinguish the effects of mill-mud addition and deep trench application on soil.
Results
Soil physical and chemical properties
Soil contained around 5% clay, 38% silt and 57% sand in topsoil (0–10 cm), and clay content increased with soil depth. Soil bulk density in MS was significantly (P < 0.05) lower than other treatments at 0–10 cm (1.07 g cm−3) and 10–20 cm depths (1.38 g cm−3), while there were no significant differences in bulk density among CK, DT and MD treatments at these two depths (1.47–1.60 g cm−3 at 0–10 cm and 1.54–1.71 g cm−3 at 10–20 cm) (Table 1). At 20–40 cm depth, MD bulk density (1.49 g cm−3) was significantly lower than MS (1.78 g cm−3) (Table 1). The CK had the highest bulk density (2.32 g cm−3) among all treatments at 40–60 cm depth, while DT had the lowest bulk density (1.67 g cm−3) (Table 1). All soil samples were slightly acidic. Soil pH values in CK (5.72) and MS (5.92) were significantly (P < 0.05) higher than MD (5.09) at 0–10 cm depth (Table 1). A similar trend in soil pH was observed at 10–20 cm depth (Table 1). For the deeper soil profiles (20–60 cm), soil pH in CK and MS was significantly higher than DT and MD (P < 0.05). In addition, the treatments that received mill-mud (MS and MD) inputs had higher pH than treatments without mill-mud (CK and DT) for deeper soil profiles (20–60 cm). Total C and N contents in the MS treatment were generally higher than for other treatments at 0–20 cm depth. Additionally, the MD treatment had significantly higher TC and TN concentrations than other treatments at depth of 20–40 cm. Interestingly, at 40–60 cm depth, DT had the highest TC content (0.18%) and MD had the highest TN content (0.058%) (Table 1).
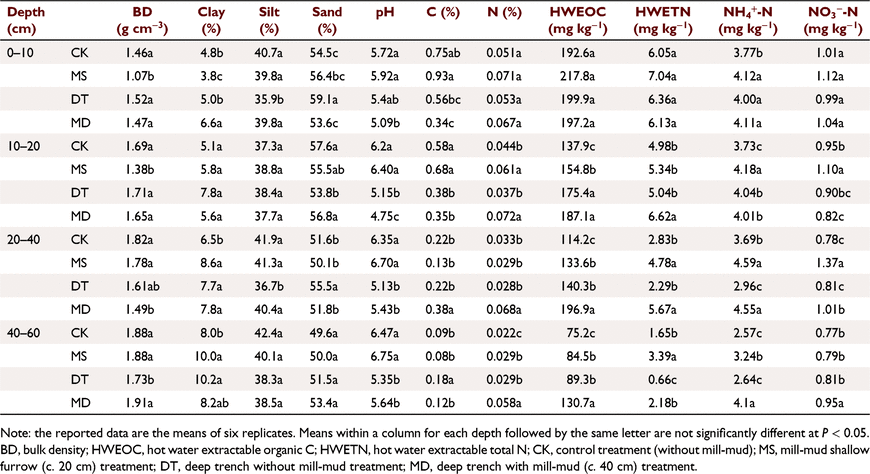
|
Labile organic C and N pool
There were no significant differences in concentrations of HWEOC among all treatments for surface soil (0–10 cm), despite MS having relatively higher HWEOC concentration (Table 1). For the 10–20 cm depth, the DT and MD treatments had significantly greater HWEOC than MS and CK, with the lowest HWEOC in CK. There were no significant differences in HWEOC between the two deep trench treatments (DT and MD). At 20–40 cm depth, the MD treatment had the highest HWEOC concentration (196.9 mg kg−1, P < 0.05), followed by DT and MS treatments, with the lowest value in CK (Table 1). A similar trend was found for the 40–60 cm depth (Table 1).
There were no significant differences in HWETN concentrations among all treatments for surface soil (0–10 cm). At 10–20 cm depth, HWETN concentrations were significantly greater for the MD than the other three treatments, while there were no significant differences among CK, MS and DT treatments (Table 1). At 20–40 cm depth, as expected, HWETN concentration in the treatments receiving mill-mud (MS and MD) were at least 70% higher (P < 0.05) than the two treatments without mill-mud. At the 40–60 cm depth, mill-mud application significantly increased concentrations of HWETN in MS and MD treatments (3.39 and 2.18 mg kg−1, respectively), compared to CK and DT (1.65 and 0.66 mg kg−1, respectively). Interestingly, surface applied mill-mud did not increase labile C store in the entire soil profile (0–60 cm) while deep trench applied mill-mud significantly increased labile C store in the entire soil profile (Table 1).
Inorganic N pools
Concentrations of soil NH4+-N were in the range of 4.00–4.12 mg kg−1 among MS, DT and MD treatments, being significantly (P < 0.05) higher than in CK (3.77 mg kg−1) (Table 1) for the surface soil (0–10 cm). However, there were no significant differences in NH4+-N concentrations among MS, DT and MD. At the 10–20 cm depth, the MS treatment increased NH4+-N concentrations (4.18 mg kg−1) while CK had the lowest NH4+-N concentrations (3.73 mg kg−1). Concentrations of NH4+-N in MS and MD were significantly higher (P < 0.05) than in the other treatments at 20–40 cm depth. In addition, we found MD had the highest (P < 0.05) NH4+-N concentration (4.10 mg kg−1) at 40–60 cm depth, followed by MS (3.24 mg kg−1). No significant differences in soil NO3−-N concentration were found in the 0–10 cm depth among all treatments. The MS had significantly higher (P < 0.05) NO3−-N concentration (1.10 mg kg−1) than the other treatments at 10–20 cm depth. The treatments receiving mill-mud (MS and MD) had significantly (P < 0.05) higher NO3−-N concentration than the other treatments at 20–40 cm depth. At 40–60 cm depth, MD had the highest NO3−-N concentration (0.95 mg kg−1, P < 0.05), while there were no significant differences among the CK, MS and DT treatments.
P pools
Total P concentrations in MS and MD significantly increased (P < 0.05) after mill-mud application at 10–20 cm (380.8 mg kg−1 in MS) and 20–40 cm depths (294.5 mg kg−1 in MD) compared with CK and DT at the same soil depth (Fig. 1a). As expected, MS treatment increased total P concentration at the targeted soil level (10–20 cm) but also increased total P for the surfac soil (0–10 cm) (Fig. 1). The MD treatment had the highest soil total P for the deep layers (20–40 and 40–60 cm) (Fig. 1). However, total P concentration decreased with soil depth (20–60 cm) in the CK treatment (Fig. 1a).
Colwell P concentration decreased with soil depth (20–60 cm) in the treatments without mill-mud application (CK and DT) (Fig. 1b). The mill-mud application treatments (MS and MD) exhibited significantly greater amounts of Colwell P in soil compared with CK and DT. The MS treatment increased Colwell P concentration in the surface soil (0–20 cm) but had no impact on the deeper soil (20–60 cm) (Fig. 1b). However, the MD treatment significantly increased Colwell P levels in deeper soil (20–60 cm) but had no effects on levels in surface soil (0–20 cm) (Fig. 1b).
Microbial C and N pools
Mill-mud application significantly (P < 0.05) increased levels of MBC in the MS (0–20 cm) and MD (0–60 cm) treatments, compared with CK and DT treatments (Fig. 2a). Notably, the DT treatment increased the concentration of MBC in the lower soil profile (20–60 cm) compared to CK. Increases in MBC were mainly in the upper layers (0–20 cm) for the MS treatment (Fig. 2a). As expected, increases in MBC were in deeper layers (20–60 cm) for the MD treatment (Fig. 2a). In terms of MBN, the MS treatment increased the concentration of MBN, similar to the pattern for MBC (Fig. 2b). However, the MD treatment had the lowest MBN level in nearly all layers (Fig. 2b). In general, MBC levels decreased with soil depth, but this trend was not clear for MBN. Interestingly, mill-mud application (MS and MD) significantly (P < 0.05) increased the microbial C:N ratio in comparison to CK, although this pattern became unclear in the MS treatment in the 40–60 cm layer (Fig. 2c). Notably, DT treatment had no impact on the microbial C:N ratio in the 0–40 cm soil profile, but significantly increased microbial C:N ratio at 40–60 cm depth (Fig. 2c).
Microbial and enzyme activities
Overall, microbial activity for CK, MS and DT treatments decreased with soil depth, but MD tended to have increased microbial activity down to 40 cm and then declined in the 40–60 cm layer. It is notable that the DT treatment increased microbial activity in the deeper soil profile (20–60 cm) compared to CK, which was confirmed by the MBC result. The MS treatment had the highest microbial activity in the surface soil (0–20 cm) while the MD treatment had the highest MBC at 20–40 cm depth (Fig. 3a).
Activities of β-glucosidase and phosphatase enzymes were highest at 10–20 cm depth for the MS treatment, and highest at the 20–40 cm depth for MD, which corresponded well with the target depth of mill-mud application for the MS and MD treatments. In the CK treatment, activities of both enzymes decreased with soil depth, while in the DT treatment, β-glucosidase activity tended to decline with soil depth, but phosphatase increased in the 20–40 and 40–60 cm layers (Fig. 3b, c).
Cane and sugar yield
Fig. 4 shows clearly that field management of MS, DT, and MD obviously increased plant cane yield. It is noteworthy that the DT treatment increased plant cane yield (157.4 t ha−1) more than for the MS treatment (152.1 t ha−1). As expected, field management also increased sugar yield; however, there was only a slight increase for the MS treatment while DT and MD treatments boosted sugar yield.
Relationships between soil physiochemical and microbial properties and PCA
Soil bulk density was significantly negatively correlated with many biological properties such as microbial activity, enzyme activity, MBC and MBN (r = 0.557–0.768, P < 0.01) (Table 2). Soil pH was also negatively correlated with microbial activity, phosphatase activity, MBC and microbial C:N ratio (r = 0.308–0.519, P < 0.05) although there was no significant correlation between pH and β-glucosidase activity (Table 2). In addition, TC, TN and total P were positively correlated with microbial activity, enzyme activities, MBC and MBN (r = 0.285–0.880, P < 0.05). As expected, soil Colwell P was positively correlated with microbial activity, enzyme activities, MBC and MBN (r = 0.505–0.696, P < 0.01). Soil NH4+-N was positively correlated with microbial activity, enzyme activities, MBC and microbial C:N ratio (r = 0.334–0.566, P < 0.05) while NO3−-N only showed a positive correlation with enzyme activities (r = 0.354–0.542, P < 0.05). Notably, soil labile pools also showed positive correlations with microbial activity, enzyme activities, MBC, MBN and microbial C:N ratio (r = 0.364–0.846, P < 0.05), while labile N showed no correlation with microbial C:N ratio (Table 2).
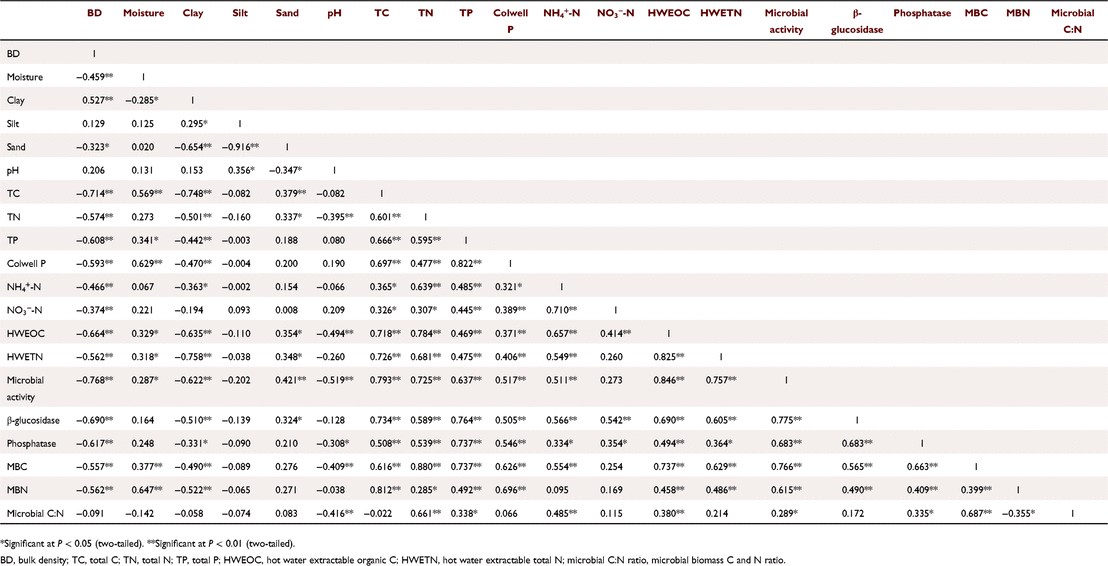
|
The PCA results showed that principal components (PCs) PC1 and PC2 explained 57.0% and 12.2% of the data variance, respectively (Fig. 5). Treatments were clearly separated from each other based on PC1 and PC2. At the 0–10 and 10–20 cm depths, the MS treatment was clearly separated from the other three treatments along PC2. The CK, DT and MD treatments could be separated from each other along PC2 at different soil depths (Fig. 5). At the 20–40 and 40–60 cm depths, the MD treatment was separated from the other treatments along PC1, while the DT treatment was separated from the CK and MS along PC2 at these depths (Fig. 5). The parameters with the highest correlation coefficients (>0.85) for PC1 were total C, labile C, MBC and microbial and β-glucosidase activities; and the parameter showing the highest correlation coefficient (>0.85) for PC2 was pH (Table S2a, b).
Discussion
In the present study, it was assumed that adjacent soils (under different treatments) within a paddock were similar in their origin and parent materials. This assumption has been generally accepted and applied as the basis of many paired-site studies (e.g. Chen et al. 2004). This has been supported by similar results of particle size and elemental analyses among composite samples across the paddock prior to the treatment (Table S1a, b; Materials and methods section). In addition, the paddock was uniform due to the same cropping, ploughing and management history. We acknowledge the limitation of this study, as for many other paired-site studies, conducted using pseudo-replication. Soil compaction and the decrease in soil organic matter are key issues that limit the continuous increase of sugarcane yield (Liu et al. 2018). The compaction is brought about by the overuse of heavy machinery in harvesting while lower organic matter results from long-term monoculture and cultivation. To address sugarcane yield decline due to deterioration of soil health (Garside et al. 2005), mill-mud has been widely applied to the surface soil of sugarcane fields, but this application is limited to within 20 km of the sugar mill due to the freight cost. Mill-mud application to soil can enhance soil health and fertility through an increase in soil organic matter and supply of nutrients (Orndorff et al. 2018), improved soil physical structure and retention of water (Fang et al. 2020), increased soil microbial activity (Ishak and Brown 2018) and enhanced soil pH buffering capacity (Medina et al. 2015).
It has been reported that deep trench application of composted municipal-biosolid to sugarcane fields can help plant growth and increase sugarcane yield (Viator et al. 2002). However, soil nutrient pool and microbial activity responses to compaction removal may vary with different depths of mill-mud application, soil type and environmental conditions such as soil temperature and moisture content. The PCA results showed a clear separation of soil samples under different treatments (Fig. 5), indicating that overall, mill-mud application methods had diverse and significant impacts on soil properties and function. Changes in the key soil parameters (including soil pH, total soil C, labile C, MBC and microbial and β-glucosidase activities) induced by the different treatments contributed to the separation of samples.
Impact of different compaction removal approaches on soil physiochemical properties
Mill-mud application significantly reduced soil bulk density at targeted levels (c. 20 cm soil depth in MS and c. 40 cm soil depth in MD) compared to CK. In addition, DT treatment also reduced bulk density down to the 60 cm depth (Table 1). This indicated that deep trench and mill-mud application could be considered compaction removal methods to reduce soil compaction stress. Early research conducted on a clay soil showed that deep trench field practices would reduce soil bulk density down to 102 cm depth to improve soil water infiltration and cotton root growth (Heilman and Gonzalez 1973). In the present study, deep trenching did not have a significant impact on soil pH while mill-mud application slightly increased soil pH which concurs with results of other studies (Morris et al. 2007; Liu et al. 2018). Notably, increased soil pH via mill-mud application will provide better environmental conditions for soil nutrient turnover which would further increase crop yield (Morris et al. 2007).
Labile pools of soil organic matter are key fractions in terms of soil quality as they are available for microbial consumption and play an important role in soil nutrient cycling (Hu et al. 1997). Chen and Xu (2005) suggested that the hot water extractable soil organic C and total N measured as soil labile C and N pools are some of the best indicators of the impact of field management practices on soil fertility. It has been reported that compost application can increase soil organic matter content (Pankhurst et al. 2002; Rahman et al. 2007; Luo et al. 2010) and provide the bioavailable organic C and N for soil microbial growth and for stimulating soil nutrient recycling (Mendham et al. 2003). In this study, deep trench application of mill-mud significantly increased concentrations of HWEOC and HWETN in the deep soil layer due to the inputs of labile organic matter from mill-mud (Table 1). However, DT treatment alone also increased HWEOC concentrations throughout the soil profile compared with the CK, which might be ascribed to the increased mineralisation of organic matter because of the disturbance effects induced by deep trenching. As expected, mill-mud application substantially increased soil total C and total N concentrations in the present study due to the presence of high C and N in the mill-mud. This result indicates that application of mill-mud increases soil C and N stock and, with the deep trench application method, mill-mud can significantly increase soil total C and N concentrations in deep soils (40–60 cm depth). Eghball et al. (2004) suggested that soil mineral N concentrations increased following organic amendment application to soil. Our study also showed an increase in concentrations of soil NH4+-N and NO3−-N following mill-mud application; however, this occurred at different depths for the MS and MD treatments. The MS treatment slightly increased mineral N in the 10–20 cm layer while the MD treatment slightly increased NH4+-N and NO3−-N in the 20–40 cm layer.
Mill-mud application significantly increased soil P concentration (MS and MD) in the applied layer mainly due to the substantial amount of P in the mill-mud (c. 0.73% P of dry matter). Increased P availability (Cowell P) following mill-mud application could also contribute to (i) enhanced competitive sorption between low molecular-weight aliphatic acid (via mill-mud application) and P for soil sorption sites and (ii) increased metal complexation and dissolution reactions by organic matter, resulting in increased release of sorbed P (Bolan et al. 1994; Hue et al. 1994; Maurice 1995). Additionally, mill-mud application may improve soil moisture conditions (Parmar and Sharma 1996) and cause structural shifting of soil microaggregates which decrease the number of potential P sorption sites (Wang et al. 2001). Importantly, mill-mud application increases soil mineralisable C which boosts microbial incorporation of P, resulting in increased available P (Chen et al. 2000; Guppy et al. 2005). In the present study, we also found that mill-mud application increased soil labile C and available P simultaneously. However, the DT treatment alone had no effect on soil P content.
Impact of different compaction removal approaches on soil biological properties
Mill-mud application to sugarcane fields can supply available organic C and reduce compaction stress for microbial activities. Liu et al. (2018) indicated that organic amendment application might not only increase soil MBC, but also enhance soil MBN content. In the present study, soil MBC contents in the mill-mud applied layers (0–10 cm in MS and 20–40 and 40–60 cm in MD) were significantly higher compared with other treatments. This may be ascribed to the organic C inputs to the targeted and adjacent soil layers in the mill-mud applied treatment, and subsequently more litter, which may increase MBC contents in the soil profile. Lima et al. (2009) confirmed that presence of carbohydrates from organic amendment influenced synthesis of humic substances and biological properties in general. Higher concentrations of MBN in MS at the 0–20 cm depth were observed compared with other treatments, which was similar to the trend in MBC. However, the DT treatment had the highest MBN for deep layers (20–60 cm), which may contribute to leaching and improved porosity due to decreased bulk density. Leaching of soluble organic C and N provides an available energy source for utilisation by soil microbial communities, leading to an increase in soil microbial activity (Mendham et al. 2003). This is supported by the positive correlations between soil microbial biomass content and HWEOC and HWETN contents in the present study. Li et al. (2002) reported that decreased bulk density increased microbial pools as it is negatively correlated with bulk density. This statement supports our finding that the DT treatment increased MBC and MBN content in deep soil layers (20–60 cm), which suggests that compaction removal would increase soil microbial pools. In the present study, we found that MD had the highest MBC concentration of all treatments, indicating that the deep trench method could help mill-mud boosting of soil MBC content.
Field application of soil organic amendments has been reported to significantly increase soil enzyme activity (Albiach et al. 2000). In the present study, overall microbial, β-glucosidase and phosphatase activities tended to be higher in the mill-mud application than the other treatments (10–20 cm in MS and 20–40 cm in MD). This was likely due to the higher organic inputs from the mill-mud as shown by the significant and positive correlations between enzyme activities and soil total C and N as well as soil HWEOC and HWETN contents. Phalke et al. (2016) showed that soil organic C content had a significant correlation with soil β-glucosidase activity after organic amendment of a soybean–maize cropping system. In addition, it has been reported that the increase in soil enzyme activities can be related to decreased soil bulk density (Kaiser and Heinemeyer 1993). In this study, microbial activity showed a significant negative correlation with soil bulk density and thus the improved enzyme activities in the mill-mud and deep-trench applied treatments, suggesting a partial alleviation of constraint due to soil compaction removal practices.
Impact of different compaction removal methods on sugarcane yield
Organic amendment field application can significantly improve crop yield (Goswami et al. 2017). Tejada and Gonzalez (2003) argued that the significantly increased grain yield after organic amendment application could be attributed to increased soil organic matter. Liu et al. (2018) demonstrated a significantly increased cumulative sugarcane yield over 4 years in an organic amendment applied treatment, which linked improved soil chemical properties to the resulting yield. In the present study, increases in plant cane yield and labile C took place in deep trench and mill-mud applied treatments. This indicated that soil labile C from slow decomposition of mill-mud plays a vital role in increasing sugarcane production. A possible explanation could be lignin-like products from decomposition of plant tissue benefiting the formation of soil humus, which further benefits plant growth (Tan 2009), and carbohydrates released from mill-mud being a very active soil organic component and ready energy source for improving microbial activities (Pascual et al. 1999). Ghimire et al. (2017) also suggested that soil labile C pools played important roles in maintaining soil fertility. In addition, soil labile C, which is extracted by hot water, is biologically active and highly related to soil microbial activity (Chen et al. 2004). Mill-mud would also increase soil P and mineral N to further improve crop yield by overcoming P deficit (Balemi and Negisho 2012) and balancing the soil N:P ratio (Dai et al. 2020). In the present study, labile C, mineral N, total P and Colwell P were significantly positively correlated with MBC, MBN, enzyme activities and microbial activity. Therefore, the labile organic C and nutrients derived from the mill-mud decomposition enhanced soil microbial activity and nutrient cycling and ameliorated soil compaction stress for sugarcane growth. This subsequently improved soil chemical and physical conditions and increased the plant cane yield. In the present study, surface applied mill-mud slightly increased cane yields but there was no significant increase in soil labile C stores and microbial activity in comparison to CK. Interestingly, the DT treatment significantly increased plant cane yield and moderately increased soil labile C store and microbial activity. As there was no organic matter input in the DT treatment, the significantly reduced soil bulk density resulting from this practice increased soil aerobic condition (Drew 1983), availability of nutrients (Jusoff 1991) and decomposition of native organic matter which may increase nutrient cycling (De Neve and Hofman 2000), labile C store, microbial activity (Li et al. 2002) and crop yield (Wallace and Terry 1998). However, this field compaction removal practice may not be good for sustainable farming as huge soil organic C losses would occur in a long run without extra organic matter input. This study clearly demonstrated that mill-mud deep trench application would simultaneously increase plant cane yield and maintain soil health by removing soil compaction, increasing soil labile C store and boosting microbial activity.
Conclusions
It was clearly demonstrated that application of mill-mud improved soil physiochemical properties including bulk density, nutrient availability and labile organic C and N (HWEOC and HWETN) contents, thereby stimulating nutrient cycling, and enhancing soil health and crop yield. Deep trench application of mill-mud stratified nutrients supplied the soil microbial community in all soil layers, enhanced nutrient cycling processes and thus increased soil health and sugarcane productivity. In contrast, surface applied mill-mud only increased surface nutrients and organic matter content with a limited crop yield improvement. Surface mill-mud application would also result in C loss from soil. It is noteworthy that the DT treatment unexpectedly increased sugarcane yield, which was mainly attributed to increased mineralisation of native organic matter, increased nutrient availability and subsequent ameliorated soil compaction, improved soil structure and redistribution of soil organic C through the entire soil profile. Further research should focus on microbial community composition shifts and functional genes involved in C and N cycling in response to compaction removal to further explore the mechanisms responsible for soil microorganism regulation of nutrient cycling in sugarcane farming systems.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The corresponding author, Chengrong Chen, is an Associate Editor of Soil Research, but was blinded from the peer review process for this paper. The other authors declare no conflicts of interest.
Declaration of funding
This work has been supported by the Cooperative Research Centre for High Performance Soils whose activities are funded by the Australian Government’s Cooperative Research Centre Program. Moreover, it is acknowledged that Xiangyu Liu is financially supported by a Soil CRC Top-up Scholarship and a Griffith University Postgraduate Research Scholarship.
Acknowledgements
We would like to thank Danielle Skocaj and other staff members of Sugar Research Australia for providing access to the field sampling site. Also, we would like to give special thanks to staff members of Burdekin Productivity Services Pty Ltd for their assistance in the field sampling. We would also like to thank Clare Morrison for her English editing throughout the manuscript.
References
Albiach R, Canet R, Pomares F, Ingelmo F (2000) Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresource Technology 75, 43–48.| Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil.Crossref | GoogleScholarGoogle Scholar |
Arias ME, Gonzalez-Perez JA, Gonzalez-Vila FJ, Ball AS (2005) Soil health – a new challenge for microbiologists and chemists. International Microbiology 8, 13–21.
| Soil health – a new challenge for microbiologists and chemists.Crossref | GoogleScholarGoogle Scholar | 15906257PubMed |
Australia Bureau of Meteorology (2021). Average annual, seasonal and monthly rainfall. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=033287. [Verified 7 February 2021]
Balemi T, Negisho K (2012) Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. Journal of Soil Science and Plant Nutrition 12, 547–562.
| Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review.Crossref | GoogleScholarGoogle Scholar |
Bell MJ, Garside AL, Moody PW, Pankhurst C, Halpin NV, Berthelsen J (2002) Nutrient dynamics and root health in sugarcane soils. In ‘Proceedings of the 2002 conference of the Australian Society of Sugar Cane Technologists’, 29 April–2 May 2002, Cairns, Qld, Australia. pp. 92–98. (PK Editorial Services Pty Ltd)
Bhandari KB, West CP, Acosta-Martinez V, Cotton J, Cano A (2018) Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Applied Soil Ecology 132, 179–186.
| Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures.Crossref | GoogleScholarGoogle Scholar |
Bolan NS, Naidu R, Mahimairaja S, Baskaran S (1994) Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biology and Fertility of Soils 18, 311–319.
| Influence of low-molecular-weight organic acids on the solubilization of phosphates.Crossref | GoogleScholarGoogle Scholar |
Brackin R, Näsholm T, Robinson N, Guillou S, Vinall K, Lakshmanan P, Schmidt S, Inselsbacher E (2015) Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertiliser supply and sugarcane root uptake capacity. Scientific Reports 5, 15727
| Nitrogen fluxes at the root-soil interface show a mismatch of nitrogen fertiliser supply and sugarcane root uptake capacity.Crossref | GoogleScholarGoogle Scholar | 26496834PubMed |
Bünemann EK, Bongiorno G, Bai Z, Creamer RE, De Deyn G, de Goede R, Fleskens L, Geissen V, Kuyper TW, Mäder P, Pulleman M, Sukkel W, van Groenigen JW, Brussaard L (2018) Soil quality – a critical review. Soil Biology and Biochemistry 120, 105–125.
| Soil quality – a critical review.Crossref | GoogleScholarGoogle Scholar |
Chen CR, Condron LM, Davis MR, Sherlock RR (2000) Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant and Soil 220, 151–163.
| Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil.Crossref | GoogleScholarGoogle Scholar |
Chen CR, Xu ZH (2005) Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: impacts of harvest residue management. Forest Ecology and Management 206, 237–247.
| Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: impacts of harvest residue management.Crossref | GoogleScholarGoogle Scholar |
Chen CR, Xu ZH, Mathers NJ (2004) Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Science Society of America Journal 68, 282–291.
| Soil carbon pools in adjacent natural and plantation forests of subtropical Australia.Crossref | GoogleScholarGoogle Scholar |
Chen G, Weil RR (2011) Root growth and yield of maize as affected by soil compaction and cover crops. Soil and Tillage Research 117, 17–27.
| Root growth and yield of maize as affected by soil compaction and cover crops.Crossref | GoogleScholarGoogle Scholar |
Colombi T, Keller T (2019) Developing strategies to recover crop productivity after soil compaction—a plant eco-physiological perspective. Soil and Tillage Research 191, 156–161.
| Developing strategies to recover crop productivity after soil compaction—a plant eco-physiological perspective.Crossref | GoogleScholarGoogle Scholar |
Czyż EA (2004) Effects of traffic on soil aeration, bulk density and growth of spring barley. Soil and Tillage Research 79, 153–166.
| Effects of traffic on soil aeration, bulk density and growth of spring barley.Crossref | GoogleScholarGoogle Scholar |
Dai Z, Liu G, Chen H, Chen C, Wang J, Ai S, Wei D, Li D, Ma B, Tang C, Brookes PC, Xu J (2020) Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. The ISME Journal 14, 757–770.
| Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems.Crossref | GoogleScholarGoogle Scholar | 31827246PubMed |
De Neve S, Hofman G (2000) Influence of soil compaction on carbon and nitrogen mineralization of soil organic matter and crop residues. Biology and Fertility of Soils 30, 544–549.
| Influence of soil compaction on carbon and nitrogen mineralization of soil organic matter and crop residues.Crossref | GoogleScholarGoogle Scholar |
Drew MC (1983) Plant injury and adaptation to oxygen deficiency in the root environment: a review. Plant and Soil 75, 179–199.
| Plant injury and adaptation to oxygen deficiency in the root environment: a review.Crossref | GoogleScholarGoogle Scholar |
Eghball B, Ginting D, Gilley JE (2004) Residual effects of manure and compost applications on corn production and soil properties. Agronomy Journal 96, 442–447.
| Residual effects of manure and compost applications on corn production and soil properties.Crossref | GoogleScholarGoogle Scholar |
Fang Y, Singh BP, Collins D, Armstrong R, Van Zwieten L, Tavakkoli E (2020) Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil. Biology and Fertility of Soils 56, 219–233.
| Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil.Crossref | GoogleScholarGoogle Scholar |
Fauci MF, Dick RP (1994) Soil microbial dynamics: short- and long-term effects of inorganic and organic nitrogen. Soil Science Society of America Journal 58, 801–806.
| Soil microbial dynamics: short- and long-term effects of inorganic and organic nitrogen.Crossref | GoogleScholarGoogle Scholar |
Flavel TC, Murphy DV (2006) Carbon and nitrogen mineralization rates after application of organic amendments to soil. Journal of Environmental Quality 35, 183–193.
| Carbon and nitrogen mineralization rates after application of organic amendments to soil.Crossref | GoogleScholarGoogle Scholar | 16391289PubMed |
Garside AL, Bell MJ, Robotham BG, Magarey RC, Stirling GR (2005) Managing yield decline in sugarcane cropping systems. International Sugar Journal 107, 16–26.
George TS, Brown LK, Newton AC, Hallett PD, Sun BH, Thomas WTB, White PJ (2011) Impact of soil tillage on the robustness of the genetic component of variation in phosphorus (P) use efficiency in barley (Hordeum vulgare L.). Plant and Soil 339, 113–123.
| Impact of soil tillage on the robustness of the genetic component of variation in phosphorus (P) use efficiency in barley (Hordeum vulgare L.).Crossref | GoogleScholarGoogle Scholar |
Ghimire R, Lamichhane S, Acharya BS, Bista P, Sainju UM (2017) Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: a review. Journal of Integrative Agriculture 16, 1–15.
| Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: a review.Crossref | GoogleScholarGoogle Scholar |
Gilbert RA, Morris DR, Rainbolt CR, McCray JM, Perdomo RE, Eiland B, Powell G, Montes G (2008) Sugarcane response to mill mud, fertilizer, and soybean nutrient sources on a sandy soil. Agronomy Journal 100, 845–854.
| Sugarcane response to mill mud, fertilizer, and soybean nutrient sources on a sandy soil.Crossref | GoogleScholarGoogle Scholar |
Goswami L, Nath A, Sutradhar S, Bhattacharya SS, Kalamdhad A, Vellingiri K, Kim K-H (2017) Application of drum compost and vermicompost to improve soil health, growth, and yield parameters for tomato and cabbage plants. Journal of Environmental Management 200, 243–252.
| Application of drum compost and vermicompost to improve soil health, growth, and yield parameters for tomato and cabbage plants.Crossref | GoogleScholarGoogle Scholar | 28582747PubMed |
Green VS, Stott DE, Diack M (2006) Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples. Soil Biology and Biochemistry 38, 693–701.
| Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples.Crossref | GoogleScholarGoogle Scholar |
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Soil Research 43, 189–202.
| Competitive sorption reactions between phosphorus and organic matter in soil: a review.Crossref | GoogleScholarGoogle Scholar |
Heilman MD, Gonzalez CL (1973) Effect of narrow trenching in harlingen clay soil on plant growth, rooting depth, and salinity. Agronomy Journal 65, 816–819.
| Effect of narrow trenching in harlingen clay soil on plant growth, rooting depth, and salinity.Crossref | GoogleScholarGoogle Scholar |
Hu S, Coleman DC, Carroll CR, Hendrix PF, Beare MH (1997) Labile soil carbon pools in subtropical forest and agricultural ecosystems as influenced by management practices and vegetation types. Agriculture, Ecosystems & Environment 65, 69–78.
| Labile soil carbon pools in subtropical forest and agricultural ecosystems as influenced by management practices and vegetation types.Crossref | GoogleScholarGoogle Scholar |
Hue NV, Ikawa H, Silva JA (1994) Increasing plant-available phosphorus in an Ultisol with a yard-waste compost. Communications in Soil Science and Plant Analysis 25, 3291–3303.
| Increasing plant-available phosphorus in an Ultisol with a yard-waste compost.Crossref | GoogleScholarGoogle Scholar |
Isbell R (2016) ‘The Australian soil classification’. (CSIRO Publishing)
Ishak L, Brown PH (2018) Changes in microbiacommunity as affected by soil compaction and organic matter amendment. International Journal on Advanced Science Engineering and Information Technology 8, 2349–2354.
| Changes in microbiacommunity as affected by soil compaction and organic matter amendment.Crossref | GoogleScholarGoogle Scholar |
IUSS Working Group WRB (2014) World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome
Jusoff K (1991) Effect of compaction of soils on growth of Acacia mangium Willd. under glasshouse conditions. New Forests 5, 61–66.
| Effect of compaction of soils on growth of Acacia mangium Willd. under glasshouse conditions.Crossref | GoogleScholarGoogle Scholar |
Kaiser E-A, Heinemeyer O (1993) Seasonal variations of soil microbial biomass carbon within the plough layer. Soil Biology and Biochemistry 25, 1649–1655.
| Seasonal variations of soil microbial biomass carbon within the plough layer.Crossref | GoogleScholarGoogle Scholar |
Li CH, Ma BL, Zhang TQ (2002) Soil bulk density effects on soil microbial populations and enzyme activities during the growth of maize (Zea mays L.) planted in large pots under field exposure. Canadian Journal of Soil Science 82, 147–154.
| Soil bulk density effects on soil microbial populations and enzyme activities during the growth of maize (Zea mays L.) planted in large pots under field exposure.Crossref | GoogleScholarGoogle Scholar |
Lima DLD, Santos SM, Scherer HW, Schneider RJ, Duarte AC, Santos EBH, Esteves VI (2009) Effects of organic and inorganic amendments on soil organic matter properties. Geoderma 150, 38–45.
| Effects of organic and inorganic amendments on soil organic matter properties.Crossref | GoogleScholarGoogle Scholar |
Lipiec J, Medvedev VV, Birkas M, Dumitru E, Lyndina TE, Rousseva S, Fulajtár E (2003) Effect of soil compaction on root growth and crop yield in Central and Eastern Europe. International Agrophysics 17, 61–69.
Liu X, Rezaei Rashti M, Dougall A, Esfandbod M, Van Zwieten L, Chen C (2018) Subsoil application of compost improved sugarcane yield through enhanced supply and cycling of soil labile organic carbon and nitrogen in an acidic soil at tropical Australia. Soil and Tillage Research 180, 73–81.
| Subsoil application of compost improved sugarcane yield through enhanced supply and cycling of soil labile organic carbon and nitrogen in an acidic soil at tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Luo Z, Wang E, Sun OJ (2010) Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: a review and synthesis. Geoderma 155, 211–223.
| Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Magdoff F, Weil RR (2004) ‘Soil organic matter in sustainable agriculture’. (CRC Press)
Maurice PA (1995) Evolution of hematite surface microtopography upon dissolution by simple organic acids. Clays and Clay Minerals 43, 29–38.
| Evolution of hematite surface microtopography upon dissolution by simple organic acids.Crossref | GoogleScholarGoogle Scholar |
Maynard DG, Curran MP (2007) Bulk density measurement in forest soils. In ‘Soil sampling and methods of analysis’. 2nd edn. (Eds MR Carter, EG Gregorich) (CRC Press)
McCray JM, Ji S, Baucum LE (2015) Sugarcane yield response to furrow-applied organic amendments on sand soils. International Journal of Agronomy 2015, 426387
| Sugarcane yield response to furrow-applied organic amendments on sand soils.Crossref | GoogleScholarGoogle Scholar |
Medina J, Monreal C, Barea JM, Arriagada C, Borie F, Cornejo P (2015) Crop residue stabilization and application to agricultural and degraded soils: a review. Waste Management 42, 41–54.
| Crop residue stabilization and application to agricultural and degraded soils: a review.Crossref | GoogleScholarGoogle Scholar | 25936555PubMed |
Mendham DS, O’Connell AM, Grove TS, Rance SJ (2003) Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. Forest Ecology and Management 181, 357–372.
| Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts.Crossref | GoogleScholarGoogle Scholar |
Morris DR, Gilbert RA, Rainbolt CR, Perdomo RE, Powell G, Eiland B, Montes G (2007) Sugarcane yields and soil chemical properties due to mill mud application to a sandy soil. In ‘XXVI congress, International Society of Sugar Cane Technologists, 29 July–2 August 2007, ICC, Durban, South Africa’. pp. 444–448. (International Society of Sugar Cane Technologists)
Naidu R, Syers JK (1992) Influence of sugarcane millmud, lime, and phosphorus, on soil chemical properties and the growth of Leucaena leucocephala in an Oxisol from Fiji. Bioresource Technology 41, 65–70.
| Influence of sugarcane millmud, lime, and phosphorus, on soil chemical properties and the growth of Leucaena leucocephala in an Oxisol from Fiji.Crossref | GoogleScholarGoogle Scholar |
Nicolardot B, Fauvet G, Cheneby D (1994) Carbon and nitrogen cycling through soil microbial biomass at various temperatures. Soil Biology and Biochemistry 26, 253–261.
| Carbon and nitrogen cycling through soil microbial biomass at various temperatures.Crossref | GoogleScholarGoogle Scholar |
Orndorff SG, Lang TA, Bhadha JH, McCray JM, Daroub SH (2018) Sugarcane by-products used as soil amendments on a sandy soil: effects on sugarcane crop nutrition and yield. Journal of Plant Nutrition 41, 928–942.
| Sugarcane by-products used as soil amendments on a sandy soil: effects on sugarcane crop nutrition and yield.Crossref | GoogleScholarGoogle Scholar |
Oussible M, Crookston RK, Larson WE (1992) Subsurface compaction reduces the root and shoot growth and grain yield of wheat. Agronomy Journal 84, 34–38.
| Subsurface compaction reduces the root and shoot growth and grain yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Pankhurst CE, Magarey RC, Stirling GR, Blair BL, Bell MJ, Garside AL (2003) Management practices to improve soil health and reduce the effects of detrimental soil biota associated with yield decline of sugarcane in Queensland, Australia. Soil and Tillage Research 72, 125–137.
| Management practices to improve soil health and reduce the effects of detrimental soil biota associated with yield decline of sugarcane in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Pankhurst CE, McDonald HJ, Hawke BG, Kirkby CA (2002) Effect of tillage and stubble management on chemical and microbiological properties and the development of suppression towards cereal root disease in soils from two sites in NSW, Australia. Soil Biology and Biochemistry 34, 833–840.
| Effect of tillage and stubble management on chemical and microbiological properties and the development of suppression towards cereal root disease in soils from two sites in NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Parmar DK, Sharma PK (1996) Phosphorus and mulching effects on nutrient uptake and grain yield of wheat at different growth stages. Tropical agriculture 73, 196–200.
Pascual JA, García C, Hernandez T (1999) Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biology and Fertility of Soils 30, 1–6.
| Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil.Crossref | GoogleScholarGoogle Scholar |
Pattison AB, Badcock K, Sikora RA (2011) Influence of soil organic amendments on suppression of the burrowing nematode, Radopholus similis, on the growth of bananas. Australasian Plant Pathology 40, 385–396.
| Influence of soil organic amendments on suppression of the burrowing nematode, Radopholus similis, on the growth of bananas.Crossref | GoogleScholarGoogle Scholar |
Phalke DH, Patil SR, Pharande AL, Manna MC, Sahu A, Kaur S (2016) Effect of in situ recycling of sugarcane crop residues on soil enzyme activities under soybean–maize system. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 86, 299–307.
| Effect of in situ recycling of sugarcane crop residues on soil enzyme activities under soybean–maize system.Crossref | GoogleScholarGoogle Scholar |
Qureshi ME, Qureshi SE, Wegener MK (2007) Economic implications of alternative mill mud management options in the Australian sugar industry. Agricultural Economics 36, 113–122.
| Economic implications of alternative mill mud management options in the Australian sugar industry.Crossref | GoogleScholarGoogle Scholar |
Rahman L, Chan KY, Heenan DP (2007) Impact of tillage, stubble management and crop rotation on nematode populations in a long-term field experiment. Soil and Tillage Research 95, 110–119.
| Impact of tillage, stubble management and crop rotation on nematode populations in a long-term field experiment.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia’. (CSIRO Publishing)
Schloter M, Dilly O, Munch JC (2003) Indicators for evaluating soil quality. Agriculture, Ecosystems & Environment 98, 255–262.
| Indicators for evaluating soil quality.Crossref | GoogleScholarGoogle Scholar |
Shah AN, Tanveer M, Shahzad B, Yang G, Fahad S, Ali S, Bukhari MA, Tung SA, Hafeez A, Souliyanonh B (2017) Soil compaction effects on soil health and cropproductivity: an overview. Environmental Science and Pollution Research 24, 10056–10067.
| Soil compaction effects on soil health and cropproductivity: an overview.Crossref | GoogleScholarGoogle Scholar | 28108925PubMed |
Shukla SK, Jaiswal VP, Sharma L, Pathak AD, Singh AK, Gupta R, Awasthi SK, Gaur A, Zubair A, Tiwari R (2020) Sugarcane yield using minimum tillage technology through subsoiling: beneficial impact on soil compaction, carbon conservation and activity of soil enzymes. Sugar Tech 22, 987–1006.
| Sugarcane yield using minimum tillage technology through subsoiling: beneficial impact on soil compaction, carbon conservation and activity of soil enzymes.Crossref | GoogleScholarGoogle Scholar |
Tabatabai MA (1994) Soil enzymes. In ‘Methods of soil analysis. Part 2: Microbiological and biochemical properties’. (Eds RW Weaver, S Angle, P Bottomley, D Bezdicek, S Smith, A Tabatabai, A Wollum) pp. 775–833. (Soil Science Society of America)
| Crossref |
Tan KH (2009) ‘Environmental soil science’. (CRC Press)
Tejada M, Gonzalez JL (2003) Effects of the application of a compost originating from crushed cotton gin residues on wheat yield under dryland conditions. European Journal of Agronomy 19, 357–368.
| Effects of the application of a compost originating from crushed cotton gin residues on wheat yield under dryland conditions.Crossref | GoogleScholarGoogle Scholar |
Valenzuela-Solano C, Crohn DM (2006) Are decomposition and N release from organic mulches determined mainly by their chemical composition? Soil Biology and Biochemistry 38, 377–384.
| Are decomposition and N release from organic mulches determined mainly by their chemical composition?Crossref | GoogleScholarGoogle Scholar |
Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biology and Biochemistry 19, 689–696.
| Microbial biomass measurements in forest soils: determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils.Crossref | GoogleScholarGoogle Scholar |
Viator RP, Kovar JL, Hallmark WB (2002) Gypsum and compost effects on sugarcane root growth, yield, and plant nutrients. Agronomy Journal 94, 1332–1336.
| Gypsum and compost effects on sugarcane root growth, yield, and plant nutrients.Crossref | GoogleScholarGoogle Scholar |
Wallace A, Terry RE (Eds) (1998) Introduction: soil conditioners, soil quality and soil sustainability. In ‘Handbook of soil conditioners: substances that enhance the physical properties of soil’. pp. 1–41. (CRC Press)
Wang X, Yost RS, Linquist BA (2001) Soil aggregate size affects phosphorus desorption from highly weathered soils and plant growth. Soil Science Society of America Journal 65, 139–146.
| Soil aggregate size affects phosphorus desorption from highly weathered soils and plant growth.Crossref | GoogleScholarGoogle Scholar |
Wilson JR (1988) Advances in nitrogen cycling in agricultural ecosystems. In ‘Proceedings of the symposium on advances in nitrogen cycling in agricultural ecosystems, 11th–15th May 1987, Brisbane, Australia’. (CAB International)


