Impacts of hydrological conditions on the activities of soil enzymes in temperate floodplain forest sites
Anna Frymark-Szymkowiak A * and Leszek Karliński B
A * and Leszek Karliński B
A Department of Environmental Biology, Kazimierz Wielki University, Ossolinskich 12, 85-093 Bydgoszcz, Poland.
B Institute of Dendrology, Polish Academy of Sciences, Parkowa 5, 62-035 Kórnik, Poland.
Soil Research 60(7) 637-647 https://doi.org/10.1071/SR21156
Submitted: 10 June 2021 Accepted: 11 January 2022 Published: 15 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The development and survival of floodplain forests are dependent on the occurrence of seasonal flooding that provides soil moisture and nutrient availability suitable for the development of soil microorganisms, which are the main sources of soil enzymes involved in the decomposition and mineralisation of soil organic matter.
Aims: We compared the soil enzyme activities of a floodplain forest in the Lower Vistula Valley, Poland, cut off for 80 years from the river by artificial embankment, and of two other floodplain forests located in close vicinity and subjected to seasonal flooding. We hypothesised that inhibiting the inflow of the river water to the floodplain forest will alter the soil water and mineral conditions, decreasing the activity of extracellular soil enzymes.
Methods: Enzyme activity, soil moisture content, and pH were monitored for 3 years in the upper soil level (0–30 cm). The activities of soil β-glucosidase, acid phosphatase, alkaline phosphatase, and nonspecific dehydrogenase were determined by spectrophotometry.
Key results: Inhibition of floods decreased soil moisture, the concentrations of carbon, nitrogen, and phosphorus, as well as the activity of soil enzymes.
Conclusions: Forest site and soil depth, but not season, had significant effects on soil chemical and biological features. Soil enzyme activities were significantly positively correlated with soil moisture and the levels of total and organic carbon, total nitrogen, and available phosphorus, as well as the carbon/nitrogen ratio.
Implications: This information is essential for the protection and restoration of riverine habitats destroyed by human activity.
Keywords: flooding, forest soils, Populus alba, riparian zones, soil biology, soil depth, soil enzyme activity, soil water.
Introduction
The valleys of large rivers are highly dynamic systems, in which periodic flooding provides the specific hydrological and mineral conditions of the soils. Riparian forests are among the most complex, diverse, and dynamic ecological systems. The frequency and duration of flooding, groundwater level fluctuations, and the type of riverbed material determine soil chemistry, biogeochemical cycles, plant species richness, as well as the composition and distribution in the river valley (Corenblit et al. 2007; Timm et al. 2009). Natural riparian forests are the most threatened woodland type. In Europe, about 88% of the riparian forests have disappeared from their potential ranges (Bravard et al. 1986). The main threats to alluvial hardwood communities from human activities are deforestation and land use changes, decrease in river flow because of dam construction and loss of wetland areas (Nilsson and Berggren 2000), increased frequency of flooding due to human-induced climate change, and invasion of alien species (Karrenberg et al. 2002). Decreases or increases in the frequency and magnitude of river flooding affect the spread and development of vegetation, including the dominating trees, i.e. willows and poplars, because for proper germination, their seeds require soils of high moisture with readily decomposable alluvial materials deposited by the river (Shafroth et al. 1998).
Riparian soils are highly heterogeneous due to the periodically added alluvial materials and suspended sediments. Soil microorganisms (fungi, bacteria, and algae) are involved in key biogeochemical cycling processes as they are the main sources of soil enzymes, both cell-associated and extracellular, that regulate the course and rate of decomposition and transformation of organic compounds as well as the circulation of chemical elements in the ecosystems (Nannipieri et al. 2017). Factors such as climatic conditions, soil type, site fertility, vegetation type, as well as air and soil pollution influence microbial biomass and nutrient mobilisation in soil. Therefore, soil enzymes are used as important soil quality indicators (Nannipieri 1994; Rossel et al. 1997). Soil dehydrogenases, cell-associated enzymes, are exclusively intracellular, active only in viable cells, and involved in aerobic respiration, making them suitable indicators of overall microbial oxidative activities in soil (Skujiņš 1973; Casida 1977). Dehydrogenase activity (DHA) is positively correlated with microbial biomass carbon (C), organic matter content, and basal soil respiration (García and Hernández 1997; Rossel et al. 1997) and is a rapid and sensitive measure of microbial vitality in soils. It has been used to evaluate the microbial activity in forest soils under various environmental conditions, such as the growth of different tree species (Błońska et al. 2016), geographically diverse populations (Kieliszewska-Rokicka et al. 2003), forest management practices (Quilchano and Marañón 2002), or the contamination of soils with toxic metals (Chaperon and Sauvé 2007; Bojarczuk and Kieliszewska-Rokicka 2010).
The construction of flood embankments causes fragmentation of the riverside forest and creates different hydrological conditions of the soils on both sides of the embankment, which in turn leads to the transformation of the plant communities occurring there (Jagodziński and Maciejewska-Rutkowska 2005). Dams are used to regulate the river flow and reduce the extent of flooding in the downstream area, which contributes to the loss of hydrological conditions appropriate for riparian habitats (Karrenberg et al. 2002). Although numerous studies have investigated soil enzymes, the impacts of environmental factors, including climate and seasonality, on such enzymes are still a subject of debate (Brockett et al. 2012; Baldrian et al. 2013). The chemical and microbiological features of soils in riparian forests have been investigated by several authors (e.g. Wright et al. 2001; Wilson et al. 2011; Geng et al. 2017); however, little is known regarding the changes in soil properties as a result of drastic hydrological transformations caused by the separation of alluvial habitats from the riverbed. Although prolonged periods of flood can decrease soil microbial activity and reduce C and nitrogen (N) cycling (Chambers et al. 2014), increased activities of soil enzymes in riparian locations with long periods of flooding have also been reported (Geng et al. 2017).
To explore the role of the availability of river water on riparian forest soil biogeochemistry, we investigated the interactions between soil chemical and microbial properties at a floodplain forest site cut off from seasonal flooding for 80 years by a flood embankment and compared them to those of occasionally flooded sites. Riparian forests located in the valley of the Lower Vistula River, in close proximity to each other, albeit with varying hydrological conditions, offer a unique opportunity to address these questions, especially against the background that alluvial forests in Europe have been almost completely destroyed.
We hypothesised that the absence of flooding, an essential element in the functioning of riverside forests, may reduce C inputs and decrease the vitality and specific physiological activities of soil microorganisms. Our study has the following objectives: (1) to determine the respiratory activity of soil microorganisms (based on DHA), (2) to compare the activities of selected extracellular soil enzymes that play important roles in essential nutrient cycles, β-glucosidase (β-Glu) related to the C cycle and two enzymes involved in the phosphorus (P) cycle (acid phosphatase (AcP) and alkaline phosphatase (AlP)), and (3) to evaluate the influences of soil depth, soil moisture, season, and essential nutrient concentrations on microbial respiratory and specific physiological activity.
Materials and methods
Study sites
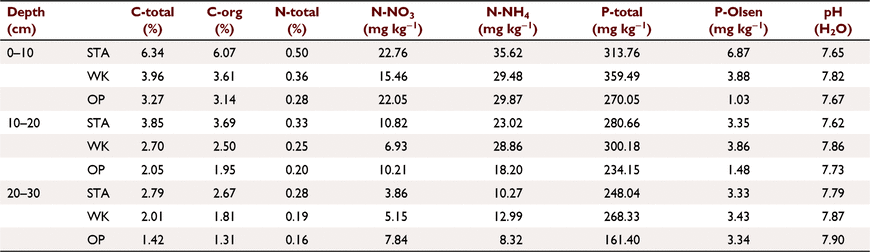
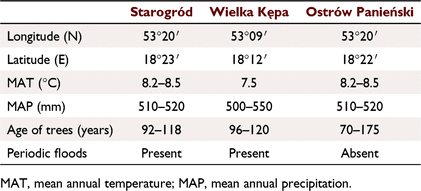
This study was conducted under canopies of mature white poplars grown in semi-natural floodplains of the Vistula River, the largest river of Poland and the longest river draining into the Baltic Sea. In terms of catchment area, the Vistula River is the tenth largest river in Europe. The high water levels in the study area are mainly related to the melting of the snow cover and the possibility of ice blockages at the turn of winter and spring. Summer floods, if they occur, are short-lived and last for an average of 4 days. In the long-term cycles, years without the occurrence of emergency conditions also occur (Gorączko 2008). We selected three forests located on the lowest floodplain terrace at a distance of 300–400 m from the river bank: Ostrów Panieński (OP), Wielka Kępa (WK), and Starogród (STA). They are characterised by alluvial soils with an organic layer of approximately 30 cm and similar climatic conditions because of the small distance between them (within 30 km). The characteristics of the study sites are presented in Table 1. The OP Nature Reserve was cut off from periodic flooding from the Vistula River following construction of a flood embankment in 1935. At present, OP represents a transition community between willow–poplar forest and oak–hornbeam forest and mainly consists of pedunculate oak (Quercus robur), elm (Ulmus minor), and ash (Fraxinus excelsior), with some specimens of field maple (Acer campestre), European white elm (U. laevis), white poplar (Populus alba), and black poplar (P. nigra). The WK Nature Reserve includes willow–poplar and elm–ash alluvial forests and is characterised by the presence of numerous monumental, nearly 200-year-old white and black poplar trees and about 150-year-old oak, elm, ash, and willow trees. The STA forest site is dominated by white poplars of different ages, with individuals being older than 100 years (authors’ observation). Study sites WK and STA were periodically flooded, with a greater flood intensity and duration in STA.
|
Sampling procedure
Soil sampling for enzyme analyses was done in 2009–2014, in autumn (October 2009–2011) and spring (May 2011, 2012, and 2014), using a core sampler with a diameter of 5.5 cm, from depths of 0–10, 10–20, and 20–30 cm. Due to extremely difficult weather conditions and above-average floods, sampling was not possible in spring 2010 throughout the research area, and in May 2012 in WK.
To avoid autocorrelation, the distance between sampling points was 8–10 m. The soil samples were placed in plastic bags and stored at −20°C until further analyses. In 2011, the research was expanded to include analyses of β-Glu activity. Additionally, in October 2013, soil samples from each soil layer were taken for analysis of macronutrients; four subsamples were combined to provide one composite soil sample for each depth and plot and sieved (Ø = 2 mm) to remove roots. Prior to chemical analyses, subsamples were air-dried for 24 h.
Soil properties
The total and organic C contents were determined by dry combustion, and the concentration of total N was measured according to Kjeldahl (1883). Ammonium (NH4)- and nitrate (NO3)-N were extracted in 0.03 N acetic acid (soil:solution 1:10) and analysed with ion-selective electrodes. Bioavailable ortho-phosphate (PO4-P) was analysed using the colorimetric method developed by Olsen et al. (1954). Soil pH was measured in a 1:2 (w/v) soil-deionised water suspension. The soil moisture content was determined by the standard drying technique (Hausenbuiller 1975).
Enzyme analyses
Soil DHA was measured according to Thalmann (1968), with modifications (Rossel et al. 1997). Soil samples were incubated in 0.5 M Tris (hydroxymethyl) aminomethane buffer pH 8.0, containing 1% 2,3,5-triphenyltetrazolium chloride (TTC) as an electron acceptor, for 24 h at 30°C in darkness. Two reference samples were analysed to eliminate the influence of non-enzymatic absorbance: (1) soil + Tris buffer and (2) TTC + Tris buffer. The coloured product of the enzymatic reaction (triphenyltetrazolium formazan, TTF) was extracted with 96% ethanol and measured spectrophotometrically at 480 nm. The TTF content was calculated by referring to a calibration curve. Soil DHA was calculated as nkatal g−1 dry soil.
Activities of phosphatases AcP and AlP were determined according to Tabatabai and Bremner (1969), with modifications. We used 0.1 M phosphate buffer (pH 6.5) for analysis of AcP and a modified universal buffer (MUB, pH 11.0) to determine the activity of AlP, with p-nitrophenyl phosphate as substrate. To the incubation mixture, we added the bacteriostatic substance toluene to neutralise soil microorganisms and eliminate intracellular enzymes without affecting the extracellular phosphatases. The samples were incubated in a water bath (at 37°C) with shaking for 1 h. The reaction was terminated by adding 0.5 M CaCl2 in 0.1 M Tris buffer. The intensity of the product of the enzymatic reaction, p-nitrophenol (p-NP), was measured spectrophotometrically at 405 nm. Enzyme activity was expressed as nkatal g−1dry soil.
The activity of β-Glu was measured according to Eivazi and Tabatabai (1988). Briefly, soil samples were incubated with p-nitrophenyl-β-d-glucoside (pNPG) and toluene in MUB buffer (pH 6.0) at 37°C in a water bath with shaking for 1 h. To stop the reaction, 0.5 M CaCl2 in 0.1 M Tris buffer was added. The concentration of p-NP released by the enzyme was measured spectrophotometrically at 405 nm, and β-Glu activity was expressed as nkatal g−1 dry soil. All enzymatic analyses were performed in triplicate with one control without substrate.
Hydrologic data
Data on water levels in the Vistula River during the research period were collected thanks to the website run by the Institute of Meteorology and Water Management – National Research Institute (https://danepubliczne.imgw.pl). The readings included the water levels on the water gauges in Fordon and Chełmno at the dates corresponding to the sampling of soil samples.
Statistical analysis
Hierarchical analysis of variance was used to examine the impacts of site, soil depth, and season on the activity of soil enzymes. In our model, site and soil depth (both factors nested in season) and season were treated as fixed effects. Prior to the analyses, normal distribution and variance homogeneity were checked using, respectively, Shapiro–Wilk’s and Bartlett’s tests. Variant means were separated using Tukey’s honestly significant difference test for unequal sample size. Pearson’s correlation coefficients were calculated to estimate associations between enzyme activities and the soil water content. Statistical analyses were performed using the software package Statistica12 StatSoft. Redundancy analysis (RDA) of enzyme activity and soil nutrients was carried out using CANOCO (Version 4.5). Data were log-transformed (log(n + 1)) for RDA and correlation coefficient calculation.
Results
Soil properties and soil moisture
The water content in the soil was significantly lower (on average about 20%) in the forest without seasonal flooding (OP) than in the two other sites (WK and STA). This concerned all soil levels studied in the spring season; whereas, in autumn, a significantly lower water content was observed only in the shallowest soil layer (0–10 cm). Soil moisture decreased with soil depth at the study locations subjected to periodical flooding and did not differ between the soil levels at the unflooded study site. Fluctuations in soil moisture at all study sites showed a strong positive relationship with changes in the water level of the Vistula River, measured on the water gauge in Fordon (for WK) and Chełmno (for STA and OP) (Fig. 1). Soil pH values were similar across the riparian area with range 7.62–7.90, indicating that the soils at the three study sites were slightly alkaline. The chemical soil properties varied with the study site and soil depth (Table 2). With increasing soil depth, the contents of C, total N, N-NO3, N-NH4, and total P decreased. The differences between the study sites were more pronounced in the upper (0–10 cm) than in the lower soil level. The unflooded forest site (OP) was characterised by lower levels of C, total N, and total and bioavailable P in the soil compared to the two periodically flooded sites (WK and STA) (Table 2).
Enzyme activities
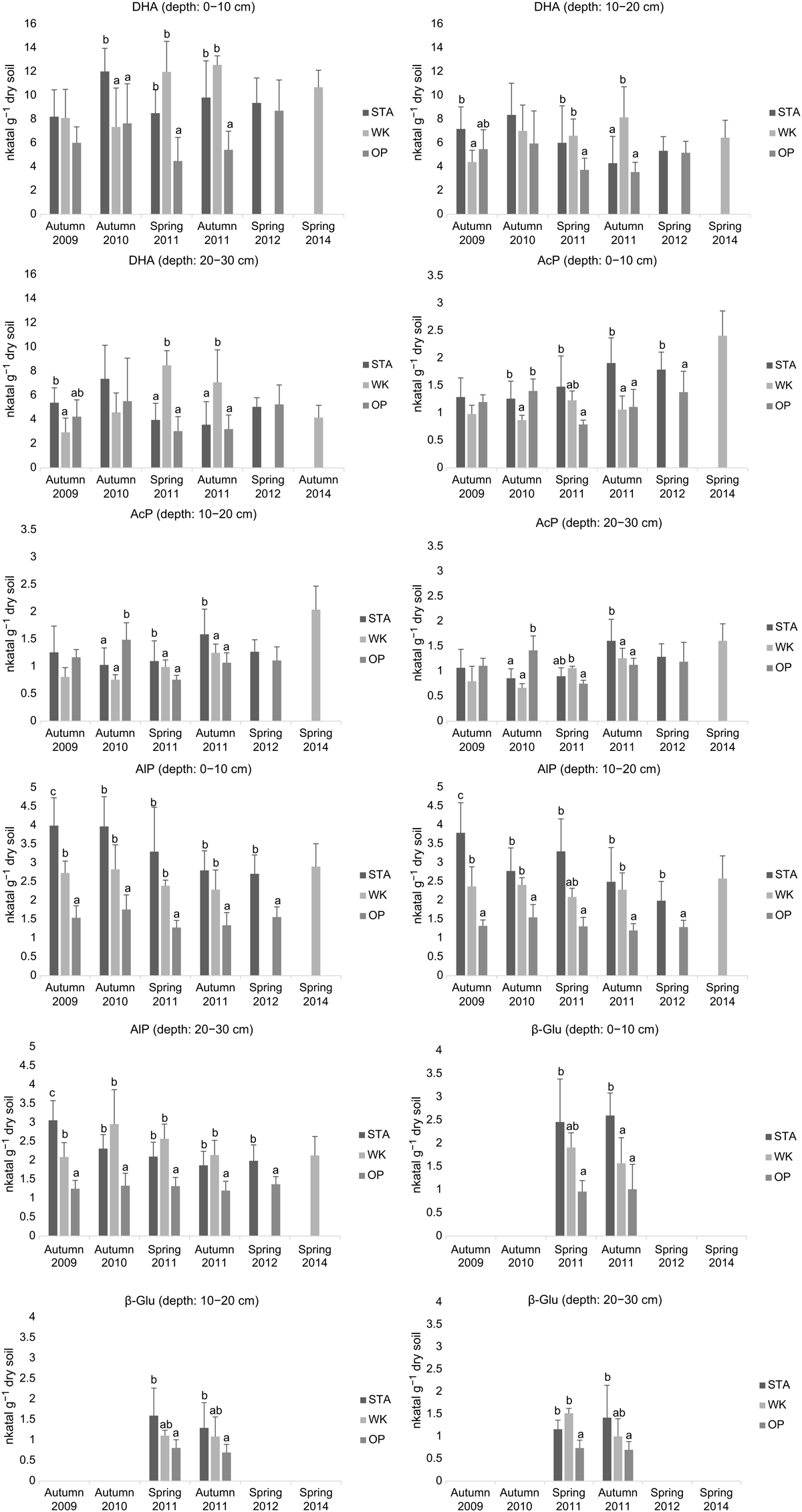
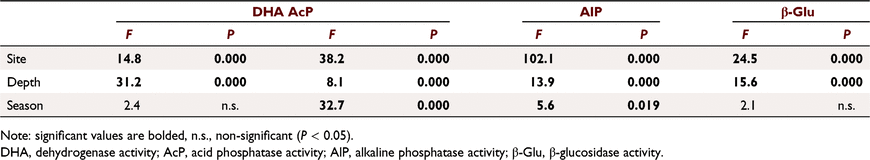
Soil DHA values ranged from 3.04 nkatal g−1 dry soil in the 20–30 cm soil layer in OP (spring 2011) to 12.57 nkatal g−1 dry soil in the 0–10 cm soil layer in WK (autumn 2011). The DHA differed (P < 0.001) among soil depths within the study sites, whereas season (spring and autumn) had no significant influence (Table 3). At all study sites, DHA was highest in the surface soil layer (0–10 cm) and decreased with soil depth. The most significant differences in the study sites were observed in the subsurface soil layer (0–10 cm) (Fig. 2).
|
The AcP activity ranged from 0.67 nkatal g−1 dry soil in the 20–30 cm soil at WK in autumn 2010 to 2.41 nkatal g−1 dry soil in the upper layer of soil (0–10 cm) taken from the same site in spring 2014. The AlP activity varied from 1.2 nkatal g−1 dry soil in soil from the OP site at soil levels 10–20 and 20–30 cm (autumn 2011), to 3.99 nkatal g−1 dry soil in the 0–10 cm level in the STA forest (autumn 2009). The AcP and AlP activities were significantly influenced (P < 0.05) by study site, soil depth, and season (Table 3). Study site was the main factor affecting AlP activity (Table 3). At the unflooded site (OP), AlP activity was, on average, two times lower in each of the soil layers studied than at the two seasonally flooded sites.
The β-Glu activity varied between 0.7 nkatal g−1 dry soil at OP in autumn 2011 in the 20–30 cm soil layer and 2.6 nkatal g−1 dry soil at STA in the 0–10 cm layer (autumn 2011). Study site and soil depth had a significant impact (P < 0.05) on enzyme activity, but sampling time (spring and autumn) did not (Table 3). Enzyme activity decreased with soil depth at each of the study sites; however, the most pronounced differences between the surface and the deeper soil layers were at the periodically flooded forest sites (STA and WK). The flooded forests were also characterised by higher β-Glu activity in each of the examined soil layers compared to the forest site without flooding (OP) (Fig. 2).
Correlations between soil characteristics and activity
Pearson’s correlation coefficient showed a significant positive relationship between the activities of all analysed enzymes and soil moisture (Table 4).

|
The outcome of the RDA is provided as a biplot that contains the environmental data on soil conditions (soil pH, moisture, and soil nutrients) and mean enzyme activities (AlP, AcP, DHA, and β-Glu) analysed for different sampling dates in three soil layers (0–10, 10–20, and 20–30 cm) of the three study sites (Fig. 3). The first two axes of the RDA explained 83.4% of the enzyme activities and soil parameter relationship. The forward selection procedure of the soil variables showed that soil moisture (55%), N-tot (12%), and P-availability (11%) significantly contributed to the variance in soil enzyme activities in different periods of time in the upper soil layer (0–30 cm) at the three sites. The lower pH values tended to favour an increase in AcP and DHA activities at STA.
Discussion
Periodic floods can influence soil conditions, such as moisture, oxygen availability, redox potential, and organic matter content, thereby directly affecting floodplain vegetation and soil microbial community biomass and functioning (Kozlowski 2002; Wilson et al. 2011). Both increases and decreases of soil enzymatic activities have been reported in the literature depending on the degree and duration of flooding (e.g. Geng et al. 2017; Zhang et al. 2020). Our research showed a strong relationship between the water level in the river and the water content of the soil at the study sites in the Lower Vistula Valley (Fig. 1). The availability of water is considered to be the main factor limiting the growth of soil microorganisms and the activity of their enzymes (Borowik and Wyszkowska 2016; Gomez et al. 2020). Current work confirms the key role of water in soil microbial activity. The forest site that was cut off from the Vistula River by a flood embankment (OP) had lower soil moisture of up to 40% on average, and a significantly lower activity of soil enzymes compared to the two other floodplain forests nearby that were seasonally flooded (WK and STA) (Fig. 2). Significant positive correlations between soil water content and the activities of soil enzymes (DHA, AcP, β-Glu, and AlP) were demonstrated as Pearson’s correlation coefficients (Table 4) and RDA results (Fig. 3).
Flood waters can influence soil microbiota directly by maintaining cell turgor, influencing respiration via oxygen diffusion, and redox potential (Stępniewski et al. 2000; Wolińska and Bennicelli 2010) and indirectly as an important source of organic matter and available nutrients, as reported by Tockner et al. (1999) and confirmed by our research. We revealed significantly higher concentrations of total C, organic C, total N, and total and bioavailable P in the two periodically flooded forests (WK and STA) than in the unflooded forest site (OP) (Table 2).
Soil moisture has a positive effect on non-specific DHA, considered a semiquantitative estimate of the total biomass of physiologically active soil microorganisms, as we found in the floodplain forests (Fig. 2, Table 4), and has also been reported for different natural ecosystems (Quilchano and Marañón 2002; Subrahmanyam et al. 2011) and in laboratory experiments (Brzezińska et al. 1998). Flooding can reduce the oxygen availability and increase DHA activity, which was confirmed by the negative correlation between the soil redox potential and DHA (Wolińska and Bennicelli 2010). However, an extremely high soil moisture level can suppress microbial activity, which has been recorded for the humus layer in a coastal Norway spruce forest located along the Finnish and Swedish coasts (Merilä and Ohtonen 1997), for waterlogged soil in lowland depressions (Fedorov-Davydov 1998), and after prolonged excessive rainfall in a Scots pine field experiment (Kieliszewska-Rokicka et al. 2003). Soil moisture also significantly influenced the soil AcP and AlP activities in riparian forests, especially at the STA site, which was characterised by high intensity and duration of floods (Fig. 2). Stimulating effects of water on soil DHA have been found in forests of different biogeographic regions (e.g. Huang et al. 2011; Brockett et al. 2012), but not in cold mountain forests (Brockett et al. 2012), indicating that temperature can be important factor influencing the soil microbial community.
The results concerning the influence of temperature on soil enzymes were ambiguous.
Our results showed that DHA did not differ between seasons (spring/autumn), which indicates that soil temperature did not influence soil microbial vitality. Similar results were reported by Kieliszewska-Rokicka et al.(2003), who found in a field experiment that cold temperatures in autumn and early winter had no negative effect on soil DHA. However, a marked increase in soil DHA with increasing soil temperature in laboratory experiments was reported by Brzezińska et al. (1998).
Our research showed, however, a significant overall impact of different seasons (spring/autumn), which can be characterised by different soil temperatures, on AcP and AlP activity (Table 3).
Soil pH can directly affect enzyme activities and indirectly by impacting the viability of microorganisms. In our research, the slightly alkaline pH of all floodplain forest soils was responsible for the higher AlP activity compared to AcP activity (Fig. 2), as AcP predominates in acidic soils, whereas AlP is more common in alkaline soils (Stępniewski et al. 2000). Our results indicated a significant negative trend for correlation between pH (although in a narrow range of values) and AcP and AlP activities (Fig. 3), which has also been identified by other authors (Dick et al. 2000); however, a lack of correlation has also been reported (Brockett et al. 2012).
Although in our research all soil samples were taken under canopies of mature P. alba trees, the forest sites investigated differed in tree species composition. The seasonally flooded WK site and the unflooded OP site represented mixed forests, and the STA was a single-species forest. No relationship was found between tree species diversity at the study sites and soil biological activity. Relatively high respiratory activity of soil microorganisms (DHA) was recorded in the two periodically flooded sites: the monoculture of white poplar (STA) and in the stand containing numerous mature white and black poplar trees and other tree species (oak, elm, ash, and willow) (WK). Significantly lower activity of soil enzymes was demonstrated at the OP site, with composition of different tree species (pedunculate oak, elm, ash, field maple, European white elm, white poplar, and black poplar), but a lower water level in the soil (Fig. 1) and lower content of nutrients, than at the flooded sites (Table 2). Generally, it is well accepted that different tree species differentially affect the physical and chemical properties of the soil under the tree cover, including direct effects from their above- and below-ground litter and root exudates, and indirectly by the understory vegetation (Reich et al. 2005; Hobbie et al. 2006, 2007). However, the results of such studies are ambiguous. Most distinct differences in soil microbial communities were reported between broadleaf and coniferous species (Prescott and Graystone 2013). In mature forest stands, higher activity of AcP and DHA was observed in soils of mixed forests compared to single-species stands (Olszowska 2018); however, seedlings of different broadleaf tree species grown in rhizoboxes in mixtures or as monocultures caused variable activities of some soil enzymes, with significantly higher enzyme activity under Populus and Salix monocultures, than in soils inhabited by a species mixture. The response to tree species may also depend on the enzymes (Fang et al. 2013). Tree productivity and size may have great impact on soil biological activity diversity (Kieliszewska-Rokicka et al. 2003; Fang et al. 2013). In addition, forest sites can differ in some factors such as mycorrhizal symbionts associated with plant roots and earthworms, which may influence soil microbial communities and enzyme activities (Prescott and Graystone 2013).
Soil depth is an important factor influencing microbial biomass and activity. The highest amount of microbial biomass is located in the surface soil layer (0–10 cm), which corresponds both to the higher availability of water and the occurrence of plant roots, important sources of soluble C. Our results indicating significant effects of soil depth on activity of extracellular enzymes (AcP, AlP, and β-Glu) are in agreement with those of Brockett et al. (2012), who found the highest activities of these enzymes in the forest floors compared to the mineral soil and showed a gradual decrease in phosphatases with soil depth in the range of 0–100 cm (Ma et al. 2010). We observed a positive correlation between AcP and AlP activities and organic C content, as previously reported (Krämer and Green 2000; Cenini et al. 2016). Higher β-Glu activity in the soil of periodically flooded sites than in soil of the riverside forest without flooding (OP) (Fig. 2), associated with different soil C levels (Fig. 3), indicates a contribution of this enzyme in C turnover. Strong positive correlations of β-Glu activity with contents of organic substances have been found for various land uses (Ma et al. 2010); however, the absence of correlations between soil C and β-Glu activity was also reported (Cenini et al. 2016).
For respiration, soil microorganisms primarily use easily accessible C present in root exudates. Any deficiency in available C in the rhizosphere, such as a seasonal decrease in the concentration of non-structural carbohydrates in roots (Kieliszewska-Rokicka et al. 2003) or the elimination of below-ground C allocation by stripping the stem bark of trees to the depth of the xylem (Edwards and Ross-Todd 1979), decreases soil respiration. Periodic flooding is essential for optimal growth of floodplain vegetation, which influences the below-ground biota. The disruption of river flow by dams leads to decreases in forest production because of deficiencies in soil water and mineral nutrient levels, which decreases the allocation of photosynthesis products to below-ground plant parts (Kozlowski 2002). Research conducted in diverse ecosystems, where different environmental conditions such as forest type, soil pH and humidity, and temperature, could strongly modify the enzymatic processes in soil, may produce ambiguous results (Brockett et al. 2012). Also, in the present study, factors other than water and C, such as total N and total and bioavailable P, influenced β-Glu activity (Fig. 3).
Decreased soil moisture causes water deficiency in trees, which in turn decreases photosynthetic activity and mineral nutrition of the plants, consequently inhibiting plant growth and leading to mortality (Kozlowski 2002). Due to the inhibition of flooding in the OP site, the water conditions have become inappropriate for the riparian community. Phytosociological studies in the years 1956–2002 in OP forest have shown that the number of riparian tree species has decreased, whereas that of oak–hornbeam species has increased (Jagodziński and Maciejewska-Rutkowska 2005). Significant changes in plant species composition in floodplain forests in the Bzura River valley (Poland) as a result of long-term anthropogenic modifications of the river valley (melioration) have been reported (Kopeć et al. 2014). Because the soil microbial community depends on the species and age of the plant community, mainly trees (Dukunde et al. 2019), the differences in soil microbial composition, biomass, and activity can be a result of the changed tree composition following alterations in water availability. In addition to the river regulation effects, the climatic changes in Central Europe contribute to the increase in the frequency of warm snowless winters and thus the lack of natural conditions for the formation of seasonal floods. In addition, droughts, which appeared almost annually in recent years in spring and summer, reduce the groundwater level and alter the riverside ecosystems.
Conclusion
Our results demonstrate that inhibition of the inflow of river water and organic matter into a floodplain ecosystem, as a result of flood embankment, significantly modified the soil physico-chemical properties. Consequences included decreased soil moisture, soil nutrient depletion (C, N, and P), and reduced metabolic activity. The activities of extracellular soil enzymes, especially AlP and β-Glu, were reduced. Water is the main driver of these properties, and with decreasing soil moisture over decades and the deficiency of mineral nutrients, mainly C and P, the vegetation structure and biomass were altered, with indirect impacts on the microbial community. Our results provide important information on soil microbial community functioning in floodplain forests of diverse environmental conditions, showing the consequences of disrupting natural processes in riparian forests. Our study can be used as a scientific basis for the protection and restoration of these most threatened woodland ecosystems.
Data availability
All data underlying the result are available as part of the article and no additional source data are required.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by the National Science Center, Poland (Grant No. NN 304 0689 33) and by the Polish Minister of Science and Higher Education under the programme ‘Regional Initiative of Excellence’ in 2019–2022 (Grant No. 008/RID/2018/19).
References
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2013) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biology and Biochemistry 56, 60–68.| Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change.Crossref | GoogleScholarGoogle Scholar |
Błońska E, Lasota J, Gruba P (2016) Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecological Research 31, 655–664.
| Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand.Crossref | GoogleScholarGoogle Scholar |
Bojarczuk K, Kieliszewska-Rokicka B (2010) Effect of ectomycorrhiza on Cu and Pb accumulation in leaves and roots of silver birch (Betula pendula Roth.) seedlings grown in metal-contaminated soil. Water, Air, and Soil Pollution 207, 227–240.
| Effect of ectomycorrhiza on Cu and Pb accumulation in leaves and roots of silver birch (Betula pendula Roth.) seedlings grown in metal-contaminated soil.Crossref | GoogleScholarGoogle Scholar |
Borowik A, Wyszkowska J (2016) Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant, Soil and Environment 62, 250–255.
| Soil moisture as a factor affecting the microbiological and biochemical activity of soil.Crossref | GoogleScholarGoogle Scholar |
Bravard J-P, Amoros C, Pautou G (1986) Impact of civil engineering works on the successions of communities in a fluvial system: a methodological and predictive approach applied to a section of the Upper Rhône River, France. Oikos 47, 92–111.
| Impact of civil engineering works on the successions of communities in a fluvial system: a methodological and predictive approach applied to a section of the Upper Rhône River, France.Crossref | GoogleScholarGoogle Scholar |
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biology and Biochemistry 44, 9–20.
| Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada.Crossref | GoogleScholarGoogle Scholar |
Brzezińska M, Stępniewska Z, Stępniewski W (1998) Soil oxygen status and dehydrogenase activity. Soil Biology and Biochemistry 30, 1783–1790.
| Soil oxygen status and dehydrogenase activity.Crossref | GoogleScholarGoogle Scholar |
Casida LE (1977) Microbial metabolic activity in soil as measured by dehydrogenase determinations. Applied and Environmental Microbiology 34, 630–636.
| Microbial metabolic activity in soil as measured by dehydrogenase determinations.Crossref | GoogleScholarGoogle Scholar | 339829PubMed |
Cenini VL, Fornara DA, Mcmullan G, Ternan N, Carolan R, Crawley MJ, Clément J-C, Lavorel S (2016) Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biology and Biochemistry 96, 198–206.
| Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils.Crossref | GoogleScholarGoogle Scholar |
Chambers LG, Davis SE, Troxler T, Boyer JN, Downey-Wall A, Scinto LJ (2014) Biogeochemical effects of simulated sea level rise on carbon loss in an Everglades mangrove peat soil. Hydrobiologia 726, 195–211.
Chaperon S, Sauvé S (2007) Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biology and Biochemistry 39, 2329–2338.
| Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils.Crossref | GoogleScholarGoogle Scholar |
Corenblit D, Tabacchi E, Steiger J, Gurnell AM (2007) Reciprocal interactions and adjustments between fluvial landforms and vegetation dynamics in river corridors: a review of complementary approaches. Earth-Science Reviews 84, 56–86.
| Reciprocal interactions and adjustments between fluvial landforms and vegetation dynamics in river corridors: a review of complementary approaches.Crossref | GoogleScholarGoogle Scholar |
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biology and Biochemistry 32, 1915–1919.
| Soil acid and alkaline phosphatase activity as pH adjustment indicators.Crossref | GoogleScholarGoogle Scholar |
Dukunde A, Schneider D, Schmidt M, Veldkamp E, Daniel R (2019) Tree species shape soil bacterial community structure and function in temperate deciduous forests. Frontiers in Microbiology 10, 1519
| Tree species shape soil bacterial community structure and function in temperate deciduous forests.Crossref | GoogleScholarGoogle Scholar | 31338079PubMed |
Edwards NT, Ross-Todd BM (1979) The effects of stem girdling on biogeochemical cycles within a mixed deciduous forest in eastern Tennessee. I. Soil solution chemistry, soil respiration, litterfall and root biomass studies. Oecologia 40, 247–257.
| The effects of stem girdling on biogeochemical cycles within a mixed deciduous forest in eastern Tennessee. I. Soil solution chemistry, soil respiration, litterfall and root biomass studies.Crossref | GoogleScholarGoogle Scholar | 28309609PubMed |
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biology and Biochemistry 20, 601–606.
| Glucosidases and galactosidases in soils.Crossref | GoogleScholarGoogle Scholar |
Fang S, Liu D, Tian Y, Deng S, Shang X (2013) Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: a rhizobox approach. PLoS ONE 8, e61461
| Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: a rhizobox approach.Crossref | GoogleScholarGoogle Scholar | 23637838PubMed |
Fedorov-Davydov DG (1998) Respiration activity in tundra biocenoses and soils of the Kolyma Lowland. Eurasian Soil Science 31, 263–273.
García C, Hernández T (1997) Biological and biochemical indicators in derelict soils subject to erosion. Soil Biology and Biochemistry 29, 171–177.
| Biological and biochemical indicators in derelict soils subject to erosion.Crossref | GoogleScholarGoogle Scholar |
Geng Y, Wang D, Yang W (2017) Effects of different inundation periods on soil enzyme activity in riparian zones in Lijiang. CATENA 149, 19–27.
| Effects of different inundation periods on soil enzyme activity in riparian zones in Lijiang.Crossref | GoogleScholarGoogle Scholar |
Gomez EJ, Delgado JA, Gonzalez JM (2020) Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecology Evolution 10, 10105–10115.
| Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature.Crossref | GoogleScholarGoogle Scholar | 33005367PubMed |
Gorączko M (2008) The Vistula River floods in the Bygdoszcz Region – genesis, course and influence for hazard in the city. The Problems of Landscape Ecology 22, 7–17.
Hausenbuiller RL (1975) ‘Soil science principles and practice 4th printing.’ (Wm. C. Brown Co.: Dubuque, Iowa)
Hobbie SE, Ogdahl M, Chorover J, Chadwick OA, Oleksyn J, Zytkowiak R, Reich PB (2007) Tree species effects on soil organic matter dynamics: the role of soil cation composition. Ecosystems 10, 999–1018.
| Tree species effects on soil organic matter dynamics: the role of soil cation composition.Crossref | GoogleScholarGoogle Scholar |
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87, 2288–2297.
| Tree species effects on decomposition and forest floor dynamics in a common garden.Crossref | GoogleScholarGoogle Scholar | 16995629PubMed |
Huang W, Liu J, Zhou G, Zhang D, Deng Q (2011) Effects of precipitation on soil acid phosphatase activity in three successional forests in southern China. Biogeosciences 8, 1901–1910.
| Effects of precipitation on soil acid phosphatase activity in three successional forests in southern China.Crossref | GoogleScholarGoogle Scholar |
Jagodziński AM, Maciejewska-Rutkowska I (2005) Flora naczyniowa i roślinność rezerwatu “Ostrów Panieński” koło Chełmna. Parki Narodowe i Rezerwaty Przyrody 24, 61–87.
Karrenberg S, Edwards PJ, Kollmann J (2002) The life history of Salicaceae living in the active zone of floodplains. Freshwater Biology 47, 733–748.
| The life history of Salicaceae living in the active zone of floodplains.Crossref | GoogleScholarGoogle Scholar |
Kieliszewska-Rokicka B, Oleksyn J, Zytkowiak R, Reich PB (2003) Links between root carbohydrates and seasonal pattern of soil microbial activity of diverse European populations of Pinus sylvestris grown in a provenance plantation. Acta Societatis Botanicorum Poloniae 72, 167–173.
| Links between root carbohydrates and seasonal pattern of soil microbial activity of diverse European populations of Pinus sylvestris grown in a provenance plantation.Crossref | GoogleScholarGoogle Scholar |
Kjeldahl J (1883) New method for the determination of nitrogen. Chemistry News 48, 101–102.
Kopeć D, Ratajczyk N, Wolańska-Kamińska A, Walisch M, Kruk A (2014) Floodplain forest vegetation response to hydroengineering and climatic pressure – a five decade comparative analysis in the Bzura River valley (Central Poland). Forest Ecology and Management 314, 120–130.
| Floodplain forest vegetation response to hydroengineering and climatic pressure – a five decade comparative analysis in the Bzura River valley (Central Poland).Crossref | GoogleScholarGoogle Scholar |
Kozlowski TT (2002) Physiological-ecological impacts of flooding on riparian forest ecosystems. Wetlands 22, 550–561.
| Physiological-ecological impacts of flooding on riparian forest ecosystems.Crossref | GoogleScholarGoogle Scholar |
Krämer S, Green DM (2000) Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biology and Biochemistry 32, 179–188.
| Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland.Crossref | GoogleScholarGoogle Scholar |
Ma XZ, Chen LJ, Chen ZH, Wu ZJ, Zhang LL, Zhang YL (2010) Soil glycosidase activities and water soluble organic carbon under different land use type. Revista de la ciencia del suelo y nutrición vegetal 10, 93–101.
| Soil glycosidase activities and water soluble organic carbon under different land use type.Crossref | GoogleScholarGoogle Scholar |
Merilä P, Ohtonen R (1997) Soil microbial activity in the coastal Norway spruce [Picea abies (L.) Karst.] forests of the Gulf of Bothnia in relation to humus-layer quality, moisture and soil types. Biology and Fertility of Soils 25, 361–365.
| Soil microbial activity in the coastal Norway spruce [Picea abies (L.) Karst.] forests of the Gulf of Bothnia in relation to humus-layer quality, moisture and soil types.Crossref | GoogleScholarGoogle Scholar |
Nannipieri P (1994) The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In ‘Soil biota: management in sustainable farming systems’. (Eds CE Pankhurst, BM Double, VVSR Gupta, PR Grace) pp. 238–244. (CSIRO Information Services: East Melbourne, Vic)
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2017) Microbial diversity and soil functions. European Journal of Soil Science 68, 12–26.
| Microbial diversity and soil functions.Crossref | GoogleScholarGoogle Scholar |
Nilsson C, Berggren K (2000) Alterations of riparian ecosystems caused by river regulation. BioScience 50, 783–792.
| Alterations of riparian ecosystems caused by river regulation.Crossref | GoogleScholarGoogle Scholar |
Olsen S, Cole C, Watanabe F, Dean L (1954) ‘Estimation of available phosphorus in soils by extraction with sodium bicarbonate.’ (U.S. Dept. of Agriculture: Washington, D.C.)
Olszowska G (2018) Denoting the intensity of soil biochemical transition according to stand species composition. Leśne Prace Badawcze/Forest Research Papers 79, 327–334.
| Denoting the intensity of soil biochemical transition according to stand species composition.Crossref | GoogleScholarGoogle Scholar |
Prescott CE, Graystone SJ (2013) Tree species influence on microbial communities in litter and soil: current knowledge and research needs. Forest Ecology and Management 309, 19–27.
| Tree species influence on microbial communities in litter and soil: current knowledge and research needs.Crossref | GoogleScholarGoogle Scholar |
Quilchano C, Marañón T (2002) Dehydrogenase activity in Mediterranean forest soils. Biology and Fertility of Soils 35, 102–107.
| Dehydrogenase activity in Mediterranean forest soils.Crossref | GoogleScholarGoogle Scholar |
Reich PB, Oleksyn J, Modrzynski J, Hobbie SE, Eissenstat DM, Chadwick OA, Hale CM, Tjoelker MG (2005) Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecology Letters 8, 811–818.
| Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species.Crossref | GoogleScholarGoogle Scholar |
Rossel D, Tarradellas J, Bitton G, Morel J-L (1997) Use of enzymes in ecotoxicology: a case for dehydrogenase and hydrolytic enzymes. In ‘Soil ecotoxicology’. (Eds J Tarradellas, G Bitton, D Rossel) pp. 179–206. (Levis Publishers CRC Press: Boca Raton, Florida)
Shafroth PB, Auble GT, Stromberg JC, Patten DT (1998) Establishment of woody riparian vegetation in relation to annual patterns of streamflow, Bill Williams River, Arizona. Wetlands 18, 577–590.
| Establishment of woody riparian vegetation in relation to annual patterns of streamflow, Bill Williams River, Arizona.Crossref | GoogleScholarGoogle Scholar |
Skujiņš J (1973) Dehydrogenase: an indicator of biological activities in arid soils. Bulletins from the Ecological Research Committee 17, 235–241.
Stępniewski W, Stępniewska Z, Gliński J, Brzezińska M, Włodarczyk T, Przywara G, Varallyay G, Rajkai J (2000) Dehydrogenase activity of some hungarian soils as related to their water and aeration status. International Agrophysics 14, 341–354.
Subrahmanyam G, Archana G, Chamyal LS (2011) Soil microbial activity and its relation to soil indigenous properties in semi-arid alluvial and estuarine soils of Mahi River basin, Western India. International Journal of Soil Science 6, 224–237.
| Soil microbial activity and its relation to soil indigenous properties in semi-arid alluvial and estuarine soils of Mahi River basin, Western India.Crossref | GoogleScholarGoogle Scholar |
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatate activity. Soil Biology and Biochemistry 1, 301–307.
| Use of p-nitrophenyl phosphate for assay of soil phosphatate activity.Crossref | GoogleScholarGoogle Scholar |
Thalmann A (1968) Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtschaftliche Forschung 21, 249–258.
Timm H, Olšauskytė V, Druvietis I, Ģe GS, Aleksandrov JV, Łapińska M, et al. (2009) Baltic and eastern continental rivers. In ‘Rivers of Europe’. (Eds K Tockner, U Uehlinger, CT Robinson) p. 728. (Elsevier/Academic Press: San Diego, USA).
| Crossref |
Tockner K, Schiemer F, Baumgartner C, Kum G, Weigand E, Zweimüller I, Ward JV (1999) The Danube restoration project: species diversity patterns across connectivity gradients in the floodplain system. Regulated Rivers: Research and Management 15, 245–258.
Wilson JS, Baldwin DS, Rees GN, Wilson BP (2011) The effects of short-term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil. River Research and Applications 27, 213–225.
| The effects of short-term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil.Crossref | GoogleScholarGoogle Scholar |
Wolińska A, Bennicelli R (2010) Dehydrogenase activity response to soil reoxidation process described as varied condition of water potential, air porosity and oxygen availability. Polish Journal of Environmental Studies 19, 651–657.
Wright RB, Lockaby BG, Walbridge MR (2001) Phosphorus availability in an artificially flooded southeastern floodplain forest soil. Soil Science Society of America Journal 65, 1293–1302.
| Phosphorus availability in an artificially flooded southeastern floodplain forest soil.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Cui D, Yang H, Kasim N (2020) Differences of soil enzyme activities and its influencing factors under different flooding conditions in Ili Valley, Xinjiang. PeerJ 8, e8531
| Differences of soil enzyme activities and its influencing factors under different flooding conditions in Ili Valley, Xinjiang.Crossref | GoogleScholarGoogle Scholar | 32201637PubMed |