Can fruit ontogenetic features prove to be an important tool in the circumscription of Psychotrieae alliance?
Anderson F. Santos A D , Amanda A. O. do Carmo A , Vanessa C. Harthman B , Mariza B. Romagnolo A C and Luiz A. Souza A
A D , Amanda A. O. do Carmo A , Vanessa C. Harthman B , Mariza B. Romagnolo A C and Luiz A. Souza A
A Plant Histotechnical Laboratory, Graduate Program of Comparative Biology, Maringá State University (UEM), 87020-900, Maringá, Paraná, Brazil.
B Federal University of Mato Grosso do Sul (UFMS), Pantanal Campus, 79304-902, Corumbá, Mato Grosso do Sul, Brazil.
C Center of Biological Sciences, Research Nucleus in Limnology, Ichthyology and Aquaculture (Nupélia), UEM, 87020-900, Maringá, Paraná, Brazil.
D Corresponding author. Email: andersonf.santos@hotmail.com
Australian Systematic Botany 34(6) 527-540 https://doi.org/10.1071/SB20020
Submitted: 17 July 2020 Accepted: 3 July 2021 Published: 8 September 2021
Abstract
The Rubiaceae tribe Psychotrieae sensu lato and its two largest genera, Psychotria L. and Palicourea Aubl., have been considered taxonomically controversial for a long time. We aimed to identify structural features of the ontogeny of the fruits and seeds with taxonomic potential for the tribe by using species of these two genera, and Rudgea jasminoides (Cham.) Müll.Arg. The samples were obtained from a herbarium and from Brazilian state parks, and sectioned by using a rotation microtome. The fruits were found to be derived from an inferior ovary, and were characterised by a fleshy mesocarp and sclerenchymatic sinuate pyrene. The seeds were pachychalazal and arillate. The fruit was classified as a pomaceous drupoid nuculanium. The investigation showed the utility of some fruit features to discriminate species. Our study also showed that ontogenetic features of fruits and seeds are very homogeneous in Palicourea and Psychotria, which supports the inclusion of both genera in the tribe Psychotrieae.
Keywords: aril, ontogeny, pachychalazal seed, pomaceous fruit, pyrene.
Introduction
The Psychotrieae alliance belongs to the subfamily Rubioideae and is a species-rich pantropical group of Rubiaceae (Razafimandimbison et al. 2008). According to Razafimandimbison et al. (2014), the group is composed of nine tribes, namely, Craterispermeae Verdc., Gaertnereae Bremek. ex Darwin, Mitchelleae Razafim. & B.Bremer, Morindeae Miq., Palicoureeae Robbr. & Manen, Prismatomerideae Ruan, Psychotrieae Cham. & Schltdl., Schizocoleeae C.Rydin & B.Bremer and Schradereae Bremek. The sister groups Psychotrieae sensu stricto and Palicoureeae contain 91% of the species diversity of the Psychotrieae alliance and 24% of all Rubiaceae species (Razafimandimbison et al. 2014). The Psychotrieae alliance has a cosmopolitan distribution and high species diversity, with probably 3000 species and 54 genera (Razafimandimbison et al. 2008).
The wide variety of genera in the Psychotrieae alliance (Psychotrieae sensu lato) has led to different reclassification proposals, such as those by Robbrecht and Manen (2006) and Razafimandimbison et al. (2014), who, on the basis of molecular studies, proposed Psychotrieae sensu stricto and Palicoureeae as tribes within Psychotrieae sensu lato. This classification differs from that by Bremer and Eriksson (2009), who adopted the traditional view of Palicourea Aubl. and Psychotria L. as genera within Psychotrieae sensu lato, and not as distinct tribes, arguing that their analyses do not support a division by groups. According to Bremer and Eriksson (2009) and Razafimandimbison et al. (2014), Psychotrieae sensu lato has many morphological features of potential taxonomic significance, such as the presence of bacterial nodules, structural diversity of reproductive organs and morphology of fleshy and schizocarpic fruits. It must be pointed out that fleshy fruits and schizocarps with persistent calyx have systematic value (Barroso et al. 1999).
Psychotrieae sensu stricto is mainly paleotropical and, although the tribe is well circumscribed (Robbrecht and Manen 2006; Razafimandimbison et al. 2008), the generic limits are uncertain. Razafimandimbison et al. (2014) indicated that the tribe is paraphyletic, with many small genera being nested within Psychotria. These authors also suggested the synonymisation of these small genera into a broadly circumscribed Psychotria sensu lato.
The Psychotria sensu lato has gone through various delimitations, and several satellite taxa have been variably excluded from or included in Psychotria; the delimitation of this genus is still in a state of flux (Delprete and Jardim 2012).
In the most recent molecular study, Psychotrieae is shown as highly paraphyletic, but, considering the group as Psychotria sensu lato, seven delimited lineages can be observed. These are the Pacific Psychotria clade, the Indian–Sri Lankan Psychotria clade, the Western Indian Ocean region (WIOR) Psychotria clade, the Australasian Psychotria clade, the Afro–neotropical Psychotria clade, the Afro–WIOR Psychotria clade or the leaf-nodulated Psychotria clade, and the Afro–Asian–WIOR–neotropical Psychotria clade (Razafimandimbison et al. 2014).
Psychotria is traditionally divided into the following two subgenera: Psychotria, with caducous, round, ovate or obovate stipules, red mature fruits, and grayish to pinkish to reddish-brown dry vegetative organs; and Heteropsychotria Steyerm., with persistent bilobed stipule, red mature fruits, blue, purple to black, yellow, orange or white, and green dry vegetative organs (Delprete and Kirkbride 2016; Taylor 2007). Taylor (2007) states that Palicourea and Psychotria subgenus Heteropsychotria are morphologically similar and distinguished only by the shape of the corolla. Recent molecular studies (Robbrecht and Manen 2006; Razafimandimbison et al. 2008; Razafimandimbison et al. 2014) showed that Palicourea and Psychotria, subgenus Heteropsychotria, form a neotropical clade. According to Delprete and Kirkbride (2016), although many species of Psychotria subgenus Heteropsychotria were transferred to Palicourea (Taylor et al. 2010; Delprete and Kirkbride 2016), the inclusion of all species in the latter genus would require a detailed morphological analysis of the group.
Palicoureeae, delimited by Robbrecht and Manen (2006), is mainly neotropical and composed of ~1500 species, within the following eight genera: Carapichea Aubl., Chassalia Comm. ex Poir., Geophila D.Don, Hymenocoleus Robbr., Margaritopsis C.Wright, Notopleura (Hook.f.) Bremek., Palicourea Aubl. and Rudgea Salisb. (Razafimandimbison et al. 2014). Palicourea is the biggest genus of the tribe, with ~800 species, and it includes many species that previously belonged to Psychotria subgenus Heteropsychotria Steyerm. and Cephaelis Sw. (Taylor et al. 2010). In a molecular analysis of Palicoureeae, Razafimandimbison et al. (2014) found that Palicourea sensu lato, the Notopleura–Rudgea clade, Carapichea and Margaritopsis sensu lato formed a basal grade, sister to a group formed by Hymenocoleus, Geophila gerrardii Baker and Chassalia sensu lato (including the remaining Geophila).
Traditional studies on the structure of fruits (Roth 1977; Spjut 1994; Barroso et al. 1999) and seeds (Martin 1946; Singh 1964; Corner 1976) have shown many anatomical features that are useful in the assessment of the phylogenetic and systematic position of some plant groups. However, the literature lacks extensive studies on the ontogeny of Rubiaceae fruits and seeds, with only a few exceptions, such as Dedecca (1957), Pietrobom (2008) and De Toni and Mariath (2011). Species of Psychotrieae sensu lato typically exhibit fleshy subglobose drupoid fruits (Barroso et al. 1999), commonly red, purple, black, blue or white (Taylor 2017), with ruminate seeds (Corner 1976).
The present paper aimed to identify the structural features of fruit and seed development of some species of Palicourea and Psychotria, and a related species Rudgea jasminoides (Cham.) Müll.Arg., that have significant taxonomic potential. The Rudgea Salisb. species was added because it is commonly confused with sympatric Palicoureeae because of similarities in their reproductive organs and their phylogenetic proximity (Robbrecht and Manen 2006; Taylor 2015).
Materials and methods
Fresh and dried herbarium samples of ovaries and developing fruits from Palicourea croceoides Ham., Palicourea marcgravii A.St.-Hil., Palicourea sessilis (Vell.) C.M.Taylor., Psychotria carthagenensis Jacq., Psychotria suterella Müll.Arg. and Rudgea jasminoides were examined. The source of material is shown in Table 1.
The dried material was rehydrated with boiling water, placed in potassium hydroxide, and subjected to 10, 30, 50 and 70% ethyl series according to the technique of Smith and Smith (1942). The fresh material was fixed in 50% formalin–acetic acid alcohol (Johansen 1940) and stored in 70% ethyl alcohol (Johansen 1940).
The samples were dehydrated in ethanolic series and embedded in Historesin (Leica Biosystems Nussloch GmbH, Heidelberg, Germany), according to the manufacturer’s instructions. The samples were sectioned transversally and longitudinally (sections of 6–8 μm) with a rotation microtome (American Optical 820), and stained with toluidine blue (O’Brien et al. 1964), using acetate buffer (pH 4.7). Light-microscope photographs were taken with a Leica ICC50 digital camera and, subsequently, processed using the software Leica Application Suite LAS EZ (ver. 3.1.0, Leica Microsystems (Switzerland) Limited).
Results
Fruit
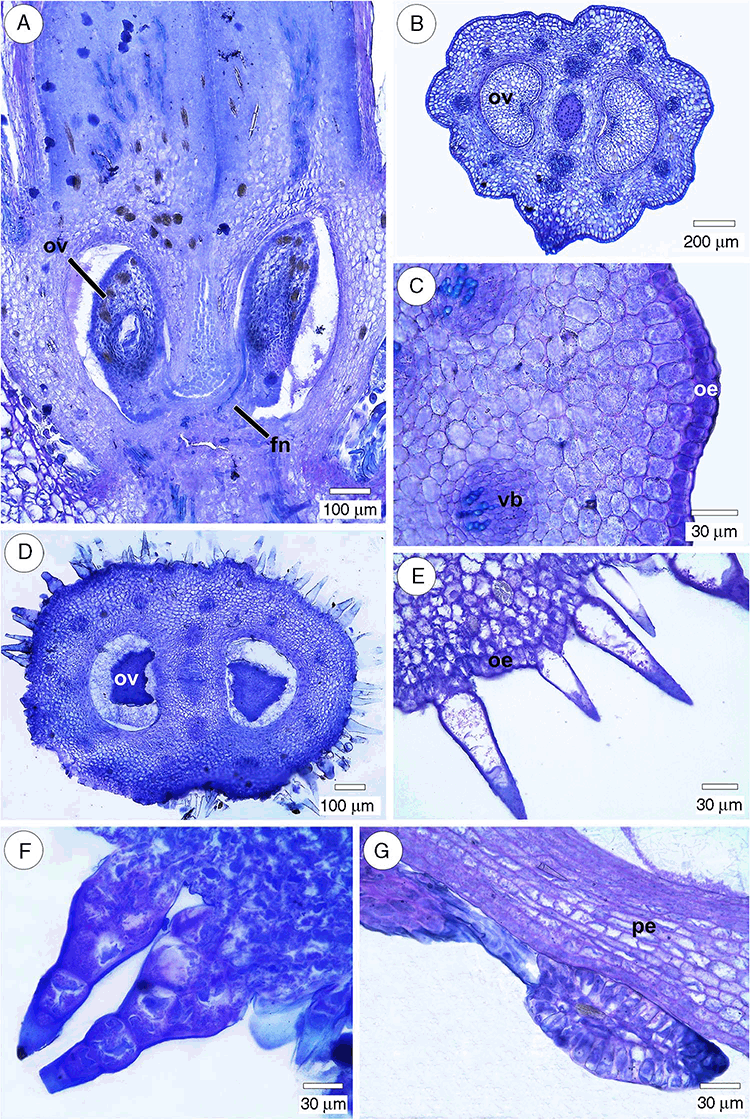
All species had a single ovule per locule, and placentation was basal to sub-basal (Fig. 1A). The fruits of all taxa are derived from an inferior, bicarpellary and bilocular ovary (Fig. 1B, D).
The ovary (Table 2) was rounded in cross-section, with prominent lobes, in Palicourea sessilis and Palicourea croceoides (Fig. 1B). The outer epidermis was uniseriate, and the cells were square, rectangular, rounded or slightly radially elongated in transversal view (Fig. 1C). The outer epidermis was glabrous in Palicourea croceoides and P. sessilis (Fig. 1B), and pubescent in P. marcgravii, Psychotria carthagenensis, P. suterella and Rudgea jasminoides (Fig. 1D–F). Trichomes were sparse in Psychotria suterella and Rudgea jasminoides, but numerous in Palicourea marcgravii and Psychotria carthagenensis (Table 2).

|
The outer epidermis in Palicourea marcgravii consisted of ovate and lanceolate unicellular trichomes (Fig. 1E), and multicellular trichomes with globose base, tapering gradually from the widest part near the base to the point (Fig. 1F). The trichomes of Psychotria suterella were glandular and multicellular. Unicellular trichomes, ovate glandular and non-glandular, with a tapered end, were observed in Psychotria carthagenensis. Colleters (Fig. 1G) were observed in the perianth base, in the vicinity of the ovary.
The ovarian mesophyll (Table 2) consisted of homogeneous parenchyma in Palicourea marcgravii and P. sessilis (Fig. 2A). The mesophyll of Palicourea croceoides and Psychotria carthagenensis also consisted of parenchyma, but the parenchyma located below the epidermis usually showed radially elongated cells, whereas the inner parenchyma had more or less rounded cells (Fig. 2B). Collenchyma and parenchyma occurred in the mesophyll of Psychotria suterella (Fig. 2C) and Rudgea jasminoides. Idioblasts with raphides (Fig. 2D) occurred in the parenchyma and collenchyma. Sclereidic idioblasts (Fig. 2D) were particularly common in Psychotria suterella.
The precursory tissue of the pyrene was especially striking, because it was already formed in the ovary wall of the flower in pre-anthesis. In all species, it was composed by cell-layers of the inner mesophyll, owing to the activity of an adaxial meristem, and the inner epidermis (Fig. 3A, Table 2).
The ovarian vasculature (Table 2) was composed of two rings of collateral vascular bundles in the mesophyll, as seen in Psychotria carthagenensis and P. suterella (Fig. 3B), and a single ring in the other species (Fig. 3C). The septum (Fig. 3D) consisted of parenchyma, precursory tissue of the pyrene, and a strand of transmitting tissue (Table 2). In all species, two marginal bundles were located in the septa adjacent to the ovary wall (Fig. 3B–D). The collateral bundles had cambium (the largest) and, in some of them, the phloem almost completely surrounds the xylem (U-shaped bundle; Fig. 3E).
The calyx and nectariferous ring persist in the mature fruit. In Palicourea croceoides and P. marcgravii, the nectariferous ring is especially prominent, but the calyx is reduced (Fig. 4A) and it exhibits colleters on the adaxial surface (Fig. 4B). In contrast, in Psychotria suterella, the calyx is prominent and the nectariferous ring is reduced (Fig. 4C). The calyx and nectariferous ring of the other species are reduced (Table 3).

|
The pericarp (Table 3) was derived from the ovary wall and was composed of epidermal exocarp, collenchymatous and parenchymatous mesocarp in most of the species and sclerenchymatous pyrene. The exocarp was uniseriate, hairy in Palicourea marcgravii, sparsely hairy in Psychotria carthagenesnis, and glabrescent or glabrous in the other species.
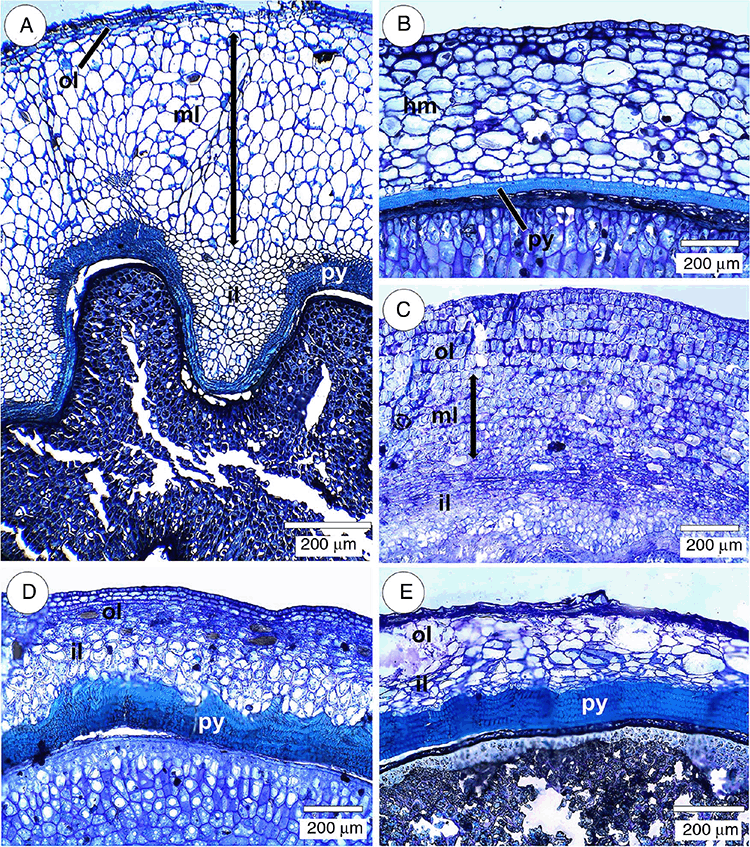
Similarly to the ovarian mesophyll, the mesocarp (Table 3) was composed of collenchyma and parenchyma, or only parenchyma, as seen in Psychotria carthagenensis (Fig. 5A). In this species, the parenchyma had three layers, including (1) the three-cell-thick outer layer, with cells being mostly elongated tangentially, (2) the middle layer, composed of rounded or radially elongated cells and (3) the inner layer, composed of rounded and tangentially elongated cells.
In the mesocarp (Table 3) with collenchyma and parenchyma, the parenchymatous tissue can be more or less homogeneous, as in Palicourea sessilis and Rudgea jasminoides (Fig. 5B), or it can consist of some different regions of this tissue. In Psychotria suterella (Fig. 5C), there were three layers of parenchyma, namely (1) an outer layer with cells more or less rounded and radially elongated, where sclereid and idioblasts with raphids stand out, (2) a middle layer with relatively smaller cells and (3) the inner layer consisting of tangentially elongated cells. Palicourea croceoides and P. marcgravii mesocarps showed two layers of parenchyma (Fig. 5D, E). Palicourea croceoides (Fig. 5D) had an outer layer of parenchyma with radially elongated cells, and an inner layer with rounded and tangentially elongated cells. Palicourea marcgravii (Fig. 5E) had an outer layer composed of rounded cells and an inner layer of tangentially elongated cells.
The pyrene (Table 3) consisted of a hard sclerenchymatous tissue that delimits the inner surface of each locule and surrounds the seed cavity (Fig. 6A, 7A–F). The pyrene was composed of fibres and macrosclereids that were differently oriented within the tissue (Fig. 6B). It had a mixed origin, i.e. it consisted of mesocarp and endocarp cells (Fig. 6C, F). Most of the species had a pluriseriate and sinuate pyrene (Fig. 6C, 7A–E), whereas in Rudgea jasminoides, it was commonly biseriate and not sinuate (Fig. 6D, 7F). Outer pyrene protuberances (Fig. 6F) were observed in some species. Pyrene and parenchyma together comprised the septum (Fig. 6D, E, G). The vascular supply pattern was similar to that of the ovary (Table 3).
Seed
The seeds originated from anatropous, unitegmic, tenuinucellate ovules (Fig. 1A, 8A, B). The ovules were elongated in Palicourea croceoides, P. marcgravii and Rudgea jasminoides (Fig. 8A), and short in Palicourea sessilis, Psychotria carthagenensis and P. suterella (Fig. 8B). The short funiculus consisted of a prominence (Fig. 8B, detail) that acts as an obturator. This structure undergoes repeated cell divisions to form an aril that surrounds the entire developing seed (Fig. 8C). The aril is composed of a few layers of parenchymatic cells that can collapse at more mature stages of the seed.
The most notable distinction between the ovule and the developing seed was the formation of the pachychalaza (Fig. 8D). Cells of the chalazal region in the ovule phase (Fig. 8A) multiply in all directions and build an intercalary growth (Fig. 8D, detail), making the single integument more or less vestigial. The embryo (Fig. 8E) in the developed seed was straight, small, with two cotyledons, plumule not evident, and a hypocotyl–radicle axis. The endosperm was reduced in mature seed.
Discussion
The fruit derived from an inferior ovary, as observed in all species in the present study, is a prominent feature within Rubiaceae (Robbrecht 1988). The question of the origin and organisation of the inferior ovary of flowers is very important from a phylogenetic standpoint (Dickison 2000). It is usually admitted that the inferior ovary can have either an appendicular or a receptacular nature, although the former condition is more frequent (Roth 1977). The arrangement of the vascular bundles can be used to interpret the nature of the inferior ovary. Inversely oriented recurrent bundles (inverted bundles) present inside the floral tube can indicate the receptacular nature of the inferior ovary (Roth 1977), although there is no consensus among researchers on this interpretation. Inversely oriented vascular bundles are not found in the species of Palicourea, Psychotria and Rudgea, nor in Psychotria suterella and P. carthagenensis, which have two rings of bundles. In view of this consideration, and assuming that most of inferior ovaries are appendicular (Douglas 1944; Roth 1977), it is possible that the inferior ovary of the three genera is appendicular.
The pyrene precursor of all studied species results from the meristematic activity of a ventral or adaxial meristem, which is installed in the subepidermal layer of the ovary inner mesophyll, in pre-anthesis; the ovary inner epidermis is also part of the pyrene precursor. The sclerenchyma seems to be common in the pericarp of Rubiaceae (Pietrobom 2008), but the author named it leathery tissue. Instead of using the term pyrene, Roth (1977) uses endocarp, which, according to the author, can be considered the most characteristic part of the drupe, which is very heterogeneous in terms of origin and differentiation. The term endocarp should be used for drupes only when it originates exclusively from the ovary inner epidermis (Roth 1977). Therefore, the most suitable term for drupe or drupoid fruit is pyrene, which may originate from the mesophyll cell layers and inner epidermis of the ovary.
Rudgea jasminoides also possesses a pyrene, but it develops by sclerification and lignification of the subepidermal layer of the inner mesophyll and the inner epidermis of the ovary.
During fruit growth in Psychotria, Palicourea and Rudgea jasminoides, the pericarp and seed increase considerably in size, but the embryo develops slowly. Roth (1977) reported that the division of growth periods is very characteristic of the growth cycle of drupes, and there are particularly three periods well defined, in which nutrient competition is likely to occur. According to the author, after the hardening of the endocarp, the embryo develops rapidly.
The folding of the surface of the pyrene of Palicourea and Psychotria causes rumination in the seeds (unverified in Rudgea jasminoides). Rumination in seeds of some Rubiaceae (including Psychotria) was reported by Corner (1976). Periasamy (1962, sensu Werker 1997) distinguished seven types of rumination in angiosperm seeds, indicating the Spigelia type for Rubiaceae; in this type, meristematic activity occurs in the unitegmic ovule. According to De Block (1995), Periasamy’s (1962) Spigelia-type rumination is characterised by unitegmic ovules, a one-layered seed coat, folds, undulations in the seed coat, late endosperm development, and chalazal hypertrophy. It is notable that these features occur in the Palicourea and Psychotria species investigated here, but seed ruminations originate from the folds and undulations of the pyrene and not from the seed coat, which is very reduced in these species. Thus, Periasamy’s (1962) classification of rumination does not apply to Palicourea and Psychotria seeds.
The seeds of Psychotria, Palicourea and Rudgea jasminoides were previously reported by Corner (1976) for Psychotria. In these pachychalazal species, the chalaza forms the greatest part of the seed coat (Werker 1997), and the integument remains more or less vestigial. All species also develop a funicular aril that collapses at more mature stages of seed development. Corner (1976) reported that condition in Rubiaceae as exarillate, probably because the aril is exhibited only in the early stages of the seeds.
In conclusion, the fruits of Psychotria, Palicourea and Rudgea jasminoides can be classified as pomaceous and drupoid, subtype nuculanium, according to the classification by Souza (in press). This fruit type is derived from an inferior ovary and contains two pyrenes. Spjut (1994) considers the fruit of Psychotria lauracea (K.Schum.) E.M.A.Petit to be a drupe, which may consist of one or more stones (a stone is a shell that encloses one or more seeds). Barroso et al. (1999) recognised the fruit of Psychotria, Palicourea and Rudgea as drupoid, presenting more than one pyrene. It must be stated that the term drupoid fruit is not suitable for Rubiaceae if the inferior ovary origin of the fruit is not analysed. It seems logical, therefore, to distinguish between drupoid fruit originated from a superior or inferior ovary. The terminology applied by Souza (in press), ‘pomaceous drupoid’, used to describe a drupe derived from an inferior ovary, appears to be the most suitable.
There are probably two major evolutionary trends in the fruits of Rubiaceae. The first was shown by the phylogenetic studies of Bremer and Eriksson (1992), which indicated that the common ancestor of the family had capsular dry fruit with many seeds. This character was considered as a plesiomorphic state for the family, later evolving to fleshy fruits at least 12 times during the entire evolutionary line of Rubiaceae, and at least four times in Rubioideae (Bremer and Eriksson 1992). The second evolutionary trend is based on molecular investigations performed by Razafimandimbison et al. (2014), which suggested that schizocarp fruits evolved independently at least twice from ancestors with fleshy and drupaceous fruits in Psychotrieae, a group that ancestrally has fleshy drupes.
Molecular studies, such as those conducted by Bremer and Eriksson (2009), identified Psychotrieae sensu lato as a basal group within the subfamily Rubioideae. Razafimandimbison et al. (2008) explained that, in the recently designated tribe Psychotrieae, a carpel with one single seed was interpreted as ancestral, and carpels with multiple seeds were interpreted as derived. All the studied species have carpels with single seeds.
Among the characteristics with taxonomic potential for Psychotrieae sensu lato, there is the occurrence of floral parts in the fruit, such as the prominent or reduced development of the nectariferous ring and the larger or smaller size of the persistent sepals, the ovary outline in cross section, with or without lobes on the surface, the indumentum type of the outer epidermis of the ovary, which can be glabrous or hairy, the vasculature of the ovary and the number of vascular bundles in the ovary wall, the number and shape of the trichome cells, which can be unicellular or multicellular, and the structural differentiation and display of the mesocarp tissues, differing in the number of collenchyma and parenchyma cell layers that are associated with the pyrene morphology. The features described above cannot support reclassification proposals, but they are important diagnostic characters, because the species have very similar morphology. Additional noteworthy features are the thickness of the pericarp and septum, and the pyrene shape in Palicourea and Psychotria (Fig. 7), which also have taxonomic value. The reduced number of cell layers and low sinuosity of the pyrene separates Rudgea jasminoides from the investigated species of Palicourea and Psychotria. The pyrene shape seems to have a taxonomic value at the specific level of Rubiaceae, as emphasised by Cortés and Delprete (2003) in a study of the genus Retiniphyllum Bonpl.
Studies, such as that by Petit (1964), have highlighted that different genera of Psychotrieae may be distinguished by fruit characters. The author was a pioneer in using pyrene structural characters, such as dehiscence type and rumination, in the classification of African Psychotria. On the basis of the combination of molecular and fruit data with the characteristics of the fruit, Nepokroeff et al. (1999) suggested reclassification of the species of the section Notopleura, which is closely related to several tropical and African lineages of the tribe Psychotrieae, including Neotropical Rudgea. In the analyses of Nepokroeff et al. (1999), characters such as the pyrene strongly flattened dorsally and presenting two marginal germination slits, were considered highly diagnostic and derived, thus justifying the elevation of the section Notopleura to the level of genus in the tribe Psychotrieae.
The pyrene present in ripe fruit of Psychotrieae may have taxonomic potential in the circumscription of the group. Fleshy fruit and drupe with endocarp differentiated into two bone thick structures, dorsally flattened and with hemispheric shape in cross-section with a seed in each of them, are reported as the most common in Psychotrieae (Nepokroeff et al. 1999). Such information, when allied to the ontogenetic origin of the structure (with its action as the precursor of rumination along the fruit maturation stages), the thickness and sinuosity of this structure may indicate other diagnostic characters with potential to be studied. Nevertheless, the pyrene composed of fibres and macrosclereids oriented in a different way as shown in our results refers to a character previously highlighted by Robbrecht (1989), who defined this composition as ‘preformed germination slits’ (PGS). Such slits, which indicate weakness spots in the pyrene structure, help the embryo in the process of germination and may be useful to define germination patterns in different groups.
Our study showed that the fundamental ontogenetic features of fruits and seeds are very homogeneous in Palicourea and Psychotria, because there were no significant differences in the anatomy of the species of the different subgenus of Psychotria. Features such as fleshy mesocarp, formation and structure of the pyrene, type of ovule, pachychalaza in the seeds, presence of funicular aril, and pomaceous fruit type are found in all investigated species.
Our interpretation favourable to keeping both genera in the tribe Psychotrieae is supported by studies of Brazilian Rubiaceae reported by Delprete and Jardim (2012). However, these authors emphasised that the delimitation of the genus Psychotria is still in a state of flux, in which there is, for example, the transference of several species from Psychotria to Palicourea. In conclusion, current generic delimitations are not yet completely clear, particularly between Palicourea and Psychotria. Nevertheless, they are associated with morphological similarity, especially of fruits and seeds of both genera, thus reinforcing our conception of a unique tribe.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
We thank CAPES (Higher Education Personnel Improvement Coordination, Brazil), CNPq (National Council of Scientific and Technological Development, Brazil), FA (Araucaria Foundation of the Paraná, Brazil) for the support granted to the accomplishment of this study. These institutions contributed to the payment of scholarships, the purchase of laboratory materials used to prepare the paper, and travel expenses for the collection of botanical material.
Acknowledgements
We thank the herbarium of the State University of Maringá (HUEM) and the Botanical Museum of Curitiba (MBM) for authorising the collection of botanical material.
References
Barroso GM, Morim MP, Peixoto AL, Ichaso CLF (1999) ‘Frutos e sementes: morfologia aplicada à sistemática de dicotiledôneas.’ (Editora UFV: Viçosa, Brazil)Bremer B, Eriksson O (1992) Evolution of fruit characters and dispersal modes in the tropical family Rubiaceae. Biological Journal of the Linnean Society. Linnean Society of London 47, 79–95.
| Evolution of fruit characters and dispersal modes in the tropical family Rubiaceae.Crossref | GoogleScholarGoogle Scholar |
Bremer B, Eriksson T (2009) Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes. International Journal of Plant Sciences 170, 766–793.
| Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes.Crossref | GoogleScholarGoogle Scholar |
Corner EJH (1976) ‘The Seeds of Dicotyledons.’ (Cambridge University Press: Cambridge, UK)
Cortés BR, Delprete PG (2003) Estudio morfológico de los pirenos en el género Retiniplyllum (Rubiaceae). Colombia Forestal 8, 21–30.
De Block P (1995) Ovary, seed and fruit of Rutidea (Rubiaceae, Pavetteae). Plant Systematics and Evolution 196, 1–17.
| Ovary, seed and fruit of Rutidea (Rubiaceae, Pavetteae).Crossref | GoogleScholarGoogle Scholar |
De Toni KLG, Mariath JEA (2011) Developmental anatomy and morphology of the flowers and fruits of species from Galium and Relbunium (Rubieae, Rubiaceae). Annals of the Missouri Botanical Garden 98, 206–225.
| Developmental anatomy and morphology of the flowers and fruits of species from Galium and Relbunium (Rubieae, Rubiaceae).Crossref | GoogleScholarGoogle Scholar |
Dedecca DM (1957) Anatomia e desenvolvimento ontogenético de Coffea arabica L. var. typica Cramer. Bragantia 16, 315–366.
| Anatomia e desenvolvimento ontogenético de Coffea arabica L. var. typica Cramer.Crossref | GoogleScholarGoogle Scholar |
Delprete PG, Jardim JG (2012) Systematics, taxonomy and floristics of Brazilian Rubiaceae: an overview about the current status and future challenges. Rodriguésia 63, 101–128.
| Systematics, taxonomy and floristics of Brazilian Rubiaceae: an overview about the current status and future challenges.Crossref | GoogleScholarGoogle Scholar |
Delprete PG, Kirkbride JH (2016) New combinations and new names in Palicourea (Rubiaceae) for species of Psychotria subgenus Heteropsychotria occurring in the Guianas. Journal of the Botanical Research Institute of Texas 10, 409–442.
Dickison WC (2000) ‘Integrative Plant Anatomy.’ (Harcourt Academic Press: San Diego, CA, USA)
Douglas GE (1944) The inferior ovary. Botanical Review 10, 125–186.
| The inferior ovary.Crossref | GoogleScholarGoogle Scholar |
Govaerts R (2020). World Checklist of Rubiaceae. Facilitated by the Royal Botanic Gardens, Kew. Available at http://wcsp.science.kew.org/ [Verified 18 December 2020].
Johansen DA (1940) ‘Plant microtechnique.’ (McGraw-Hill Book Company: New York, NY, USA)
Martin AC (1946) The comparative internal morphology of seeds. American Midland Naturalist 36, 513–660.
| The comparative internal morphology of seeds.Crossref | GoogleScholarGoogle Scholar |
Nepokroeff M, Bremer B, Sytsma KJ (1999) Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data. Systematic Botany 24, 5–27.
| Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data.Crossref | GoogleScholarGoogle Scholar |
O’Brien TP, Feder N, Mccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59, 368–373.
| Polychromatic staining of plant cell walls by toluidine blue O.Crossref | GoogleScholarGoogle Scholar |
Periasamy K (1962) The ruminate endosperm: development and type of rumination. In ‘Plant Embryology: a Symposium’, 11–14 November 1960, New Delhi, India. pp. 62–74. (Council of Scientific & Industrial Research: New Delhi, India)
Petit E (1964) Les especes africaines du genre Psychotria L. (Rubiaceae): I. Bulletin du Jardin botanique de l’État a Bruxelles 34, 1–229.
| Les especes africaines du genre Psychotria L. (Rubiaceae): I.Crossref | GoogleScholarGoogle Scholar |
Pietrobom RCV (2008) Morfoanatomia do pericarpo, semente, folha de Psychotria hoffmannseggiana (Willd. ex Roem. & Schult.) Müll. Arg. e Psychotria trichophora Müll. Arg., e desenvolvimento morfológico da plântula de Psychotria hoffmannseggiana (Willd. ex Roem. & Schult.) Müll. Arg. (Rubiaceae, Rubioideae, Psychotrieae). PhD thesis, Postgraduate Program in Plant Biology, Universidade Estadual Paulista, Rio Claro, Brazil. Available at https://repositorio.unesp.br/handle/11449/100652 [Verified 8 February 2020].
Razafimandimbison SG, Rydin C, Bremer B (2008) Evolution and trends in the Psychotrieae alliance (Rubiaceae): a rarely reported evolutionary change of many-seeded carpels from one-seeded carpels. Molecular Phylogenetics and Evolution 48, 207–223.
| Evolution and trends in the Psychotrieae alliance (Rubiaceae): a rarely reported evolutionary change of many-seeded carpels from one-seeded carpels.Crossref | GoogleScholarGoogle Scholar | 18499483PubMed |
Razafimandimbison SG, Taylor CM, Wikström N, Pailler T, Khodabandeh A, Bremer B (2014) Phylogeny and generic limits in the sister tribes Psychotrieae and Palicoureeae (Rubiaceae): evolution of schizocarps in Psychotria and origins of bacterial leaf nodules of the Malagasy species. American Journal of Botany 101, 1102–1126.
| Phylogeny and generic limits in the sister tribes Psychotrieae and Palicoureeae (Rubiaceae): evolution of schizocarps in Psychotria and origins of bacterial leaf nodules of the Malagasy species.Crossref | GoogleScholarGoogle Scholar | 25049266PubMed |
Robbrecht E (1988) Tropical woody Rubiaceae. Opera Botanica Belgica 1, 1–271.
Robbrecht E (1989) A remarkable new Chazaliella (African Psychotrieae), exemplifying the taxonomic value of pyrene characters in the Rubiaceae. Bulletin du Muséum National d’Histoire Naturelle 4 série, Section B, Adansonia, Botanique. Phytochimie 11, 341–349.
Robbrecht E, Manen JF (2006) The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Systematics and Geography of Plants 76, 85–146.
Roth I (1977) Fruits of angiosperms. In ‘Encyclopedia of Plant Anatomy. Vol. 10: Fruits of Angiosperms’. (Ed. K Linsbauer) pp. 1–675. (Gebrüder Borntraeger: Berlin, Germany)
Singh B (1964) Development and structure of angiosperms seed: I. review of the Indian work. Bulletin of the National Botanic Gardens 89, 1–115.
Smith FH, Smith EC (1942) Anatomy of the inferior ovary of Darbya. American Journal of Botany 29, 464–471.
| Anatomy of the inferior ovary of Darbya.Crossref | GoogleScholarGoogle Scholar |
Souza LA (in press) ‘Botânica estrutural: morfologia e anatomia de traqueófitas.’ (Ed. UEPG: Ponta Grossa, Brazil)
Spjut RW (1994) A systematic treatment of fruit types. Memoirs of the New York Botanical Garden 70, 1–181.
Taylor CM (2007) Psychotria (Rubiaceae). In ‘Flora Fanerogâmica do Estado de São Paulo. Vol. 5: Rubiaceae’. (Ed. SL Jung-Mendaçolli) pp. 389–412. (Hucitec: São Paulo, Brazil)
Taylor CM (2015) Rubiacearum americanarum magna hama pars XXXIV: the new group Palicourea sect. Tricephalium with eight new species and a new subspecies (Palicoureeae). Novon 24, 55–95.
| Rubiacearum americanarum magna hama pars XXXIV: the new group Palicourea sect. Tricephalium with eight new species and a new subspecies (Palicoureeae).Crossref | GoogleScholarGoogle Scholar |
Taylor CM (2017) Rubiacearum americanarum magna hama pars XXXVI: new species and taxonomic changes in Palicourea (Palicoureeae). Novon 25, 238–258.
| Rubiacearum americanarum magna hama pars XXXVI: new species and taxonomic changes in Palicourea (Palicoureeae).Crossref | GoogleScholarGoogle Scholar |
Taylor CM, Lorence DH, Gereau RE (2010) Rubiacearum americanarum magna hama pars XXV: the nocturnally flowering Psychotria domingensis–Coussarea hondensis group plus three other Mesoamerican Psychotria species transfer to Palicourea. Novon 20, 481–492.
| Rubiacearum americanarum magna hama pars XXV: the nocturnally flowering Psychotria domingensis–Coussarea hondensis group plus three other Mesoamerican Psychotria species transfer to Palicourea.Crossref | GoogleScholarGoogle Scholar |
Werker E (1997) Seed anatomy. In ‘Encyclopedia of Plant Anatomy. Vol. 10: Seed Anatomy’. (Ed. K Linsbauer) pp. 1–424. (Gebrüder Borntraeger: Berlin, Germany)