Using RADseq to resolve species boundaries in a morphologically complex group of yellow-flowered shrubs (Geleznowia, Rutaceae)
Benjamin M. Anderson A * , Rachel M. Binks A , Margaret Byrne A , Andrew D. Crawford A and Kelly A. Shepherd A
A * , Rachel M. Binks A , Margaret Byrne A , Andrew D. Crawford A and Kelly A. Shepherd A
A Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, Bentley, WA 6983, Australia.
Australian Systematic Botany 36(4) 277-311 https://doi.org/10.1071/SB23010
Submitted: 31 March 2023 Accepted: 8 July 2023 Published: 3 August 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
The morphologically complex and charismatic genus Geleznowia (Rutaceae) is endemic to south-western Australia and faces existing and potential conservation issues associated with land clearing, climate change and commercial harvesting. Two species are currently recognised in the genus, but horticulturally recognised forms and phrase-named taxa reflect additional suspected species diversity. The genus exhibits complicated and subtle patterns of morphological variation that have historically inhibited delimitation of taxonomic entities and, as a result, precluded effective conservation assessments. Here we used ddRAD data from 25 populations across the range of Geleznowia to elucidate genomic diversity in the group in conjunction with morphological re-assessment so as to delimit species and revise the taxonomy. Our analyses consistently identified seven entities that maintain genomic distinctiveness even in sympatry with other entities, supporting the inference of reproductive barriers and lineage divergence. Morphological assessment of more than 300 specimens corroborated these seven taxa. Consequently, we recognise seven species of Geleznowia in Western Australia, retaining G. amabilis K.A.Sheph. & A.D.Crawford, recircumscribing G. verrucosa Turcz., reinstating G. calycina (J.Drumm. ex Harv.) Benth., and naming four new species as G. eximia K.A.Sheph. & A.D.Crawford, G. narcissoides K.A.Sheph. & A.D.Crawford, G. occulta K.A.Sheph. & A.D.Crawford, and G. uberiflora K.A.Sheph. & A.D.Crawford.
Keywords: angiosperm, conservation genomics, ddRAD, new species, reduced representation, SNP, species delimitation, taxonomy, Western Australia.
Introduction
The hyperdiverse Southwest Australian Floristic Region (SWAFR) harbours ~7700 native species of angiosperms (see the Western Australian Herbarium’s Florabase, https://florabase.dbca.wa.gov.au/) in an area approximately the size of Italy (see Hopper and Gioia 2004; Department of Agriculture, Water and the Environment 2020). Patterns of diversity in the south-west include both geographically restricted species and widespread species, often with strong geographic structuring of genetic diversity that has been suggested to reflect complex patterns of localised extinction and persistence in response to climatic fluctuations (Byrne 2008). The contrasting narrow and broad structuring of diversity and variable levels of genetic connectivity make taxonomic resolution of species boundaries and identification of distinct genetic groups crucial for effective conservation decisions amid large-scale human impacts in the region (Coates 2000).
Restricted to the northern sandplains of south-western Western Australia (WA), yellow-flowered shrubs in the genus Geleznowia Turcz. (Rutaceae) show indications of this varied and complicated geographic structuring of diversity. These plants often depend on disturbance for recruitment, and are frequently observed after fire, in gravel pits and along road verges. Despite often occurring in large numbers following disturbance, the plants appear to be transient in the landscape, with most senescing within a decade and only a few persisting longer. As their floral displays are stunning (Fig. 1), wild populations are targeted for harvesting by the cut-flower industry. Geleznowia has not been brought into commercial cultivation, despite work demonstrating horticultural potential (Plummer et al. 2000), because propagating them from cuttings in sustainable numbers has been shown to be challenging (D. Growns, pers. comm.). Some taxa appear to be narrow-range endemics because they are known only from a few populations. With their scarcity and patchy distribution, these plants are potentially under threat from over-harvesting and habitat decline through climate change, weed invasion, altered fire regimes, and further development in the region, but taxonomic uncertainties prevent formal conservation assessments and development of appropriate management plans. Clarifying the taxonomy of Geleznowia is therefore essential to guide the conservation of this charismatic genus.
Floral variation within Geleznowia (Rutaceae), compared with two allied outgroups included in this study (Philotheca and Drummondita). Identifications at the outset of this study (names are not necessarily correct following taxonomic changes presented in this paper): (a) Geleznowia amabilis; (b) G. verrucosa; (c) G. sp. Binnu; (d) G. sp. Marchagee; (e) G. ‘White Peak’; (f) Philotheca sericea; (g) Drummondita ericoides. Vouchers: A. Crawford ADC 1384 (PERTH 07828659) (a), A. Crawford ADC 1357 (PERTH 07752180) (b), K.A. Shepherd KS 1728 (PERTH 09508031) (c), J.A. Wege JAW 2139 (CANB, MEL, PERTH 09507825) (d), A. Crawford ADC 588 (PERTH 07118252) (e), K.A. Shepherd KS 1735 (PERTH 09508678) (f), and A. Chant 4007 (PERTH 09508708) (g). Photos by A. Crawford (a, b, e), J. A. Wege (d), K. A. Shepherd (c, f), and A. Chant (g).

The taxonomic history of Geleznowia reflects some of the uncertainty about species boundaries. The genus was described by Turczaninow (1849) with a single species, G. verrucosa Turcz. Later, Bentham (1863) also recognised G. calycina (J.Drumm. ex Harv.) Benth. and G. macrocarpa Benth. Taxonomic usage of the names since then has synonymised G. calycina and G. macrocarpa under G. verrucosa, retaining Geleznowia as a monotypic genus (Wilson 1970; Council of Heads of Australian Herbaria 2007). Genetic and morphological work by Broadhurst (Broadhurst et al. 1999, 2001; Broadhurst 2000) suggested the presence of at least two subspecies, with a third form hypothesised to have resulted from hybridisation between the other two; however, no taxonomic changes were published. At approximately the same time, a horticultural study (Plummer et al. 2000) recognised five distinct morphological forms that retained differences when grown together in a common garden. To recognise and provide legislative protection for some of these taxa, informal phrase names (see Barker 2005) were created in 2010 for three of the forms, namely, G. sp. Red Bluff (A. Crawford ADC 597), G. sp. Marchagee (A. Crawford ADC 1353) and G. sp. Binnu (K.A. Shepherd & J. Wege KS 1301). Although G. verrucosa and G. sp. Marchagee were reasonably widespread, G. sp. Red Bluff and G. sp. Binnu were range restricted and respectively given Priority Two and Priority Three statuses, indicating that they may warrant listing as Threatened but currently do not meet the criteria owing to insufficient data, and thus require further survey. Noting considerable variation in indumentum, leaf size and flowers in his Flora of Australia treatment, Wilson (2013) still recognised a single variable species, G. verrucosa, suggesting infraspecific variation might eventually be delimited. Although published in 2013, it is likely that his treatment pre-dated the erection of the phrase-named taxa and did not refer to them.
More recently, Geleznowia sp. Red Bluff was recognised as G. amabilis K.A.Sheph. & A.D.Crawford, retaining its Priority Two conservation status because it is currently known only from a few populations in the Kalbarri region (Shepherd and Crawford 2020). However, the resolution of G. sp. Binnu and G. sp. Marchagee, as well as a geographically restricted and morphologically divergent population designated here as ‘White Peak’ (Fig. 1), has faced challenges. It has proven difficult to confidently separate entities within what appears to be a species complex on the basis of the examination of herbarium specimens alone, partly because of subtle character differences that sometimes overlap because of variation during plant growth stages. Given the current and historical uncertainty about morphological taxa and putative hybridisation within this complex, genomic data represent an ideal tool to shed light on evolution in the group and help delimit species. We undertook a large field-based study to collect specimens and material for genomic analysis and applied a method based on a reduced representation of the genome through sequencing of restriction site-associated DNA (RAD: Baird et al. 2008; ddRAD: Peterson et al. 2012). The method provides thousands of single-nucleotide polymorphisms (SNPs) useful for both population genetic and phylogenetic analyses. RAD methods have been used to effectively evaluate species boundaries in other plant genera, e.g. Diospyros L. (Linan et al. 2021), Leptospermum J.R.Forst. & G.Forst. (Binks and Byrne 2022), Rhodanthemum B.H.Wilcox, K.Bremer & Humphries (Wagner et al. 2020), Seringia J.Gay (Binks et al. 2020), Triodia R.Br. (Anderson et al. 2017) and Viburnum L. (Spriggs et al. 2019).
In approaching species delimitation in a complex, we adopt a species concept following ideas developed by de Queiroz (1998, 2007) that species represent evolutionarily distinct lineages that acquire distinguishing features (e.g. reciprocal monophyly, fixed morphological differences) over time since speciation. Lineage recognition is then based on detecting one or a combination of these distinguishing features, with greater confidence for a given delimitation when more lines of evidence agree. We approach species delimitation as an integrative task (e.g. Dayrat 2005; Padial et al. 2010), comparing genomic evidence with morphological and ecological evidence to support the distinction of taxa.
This study aims to use genomic data from populations across the range of morphological forms in Geleznowia (G. amabilis, G. verrucosa, G. sp. Binnu, G. sp. Marchagee and G. ‘White Peak’) (Fig. 2) in combination with morphological measurements and field observations to delimit species and revise the taxonomy, highlighting patterns of evolution in the group and informing their conservation.
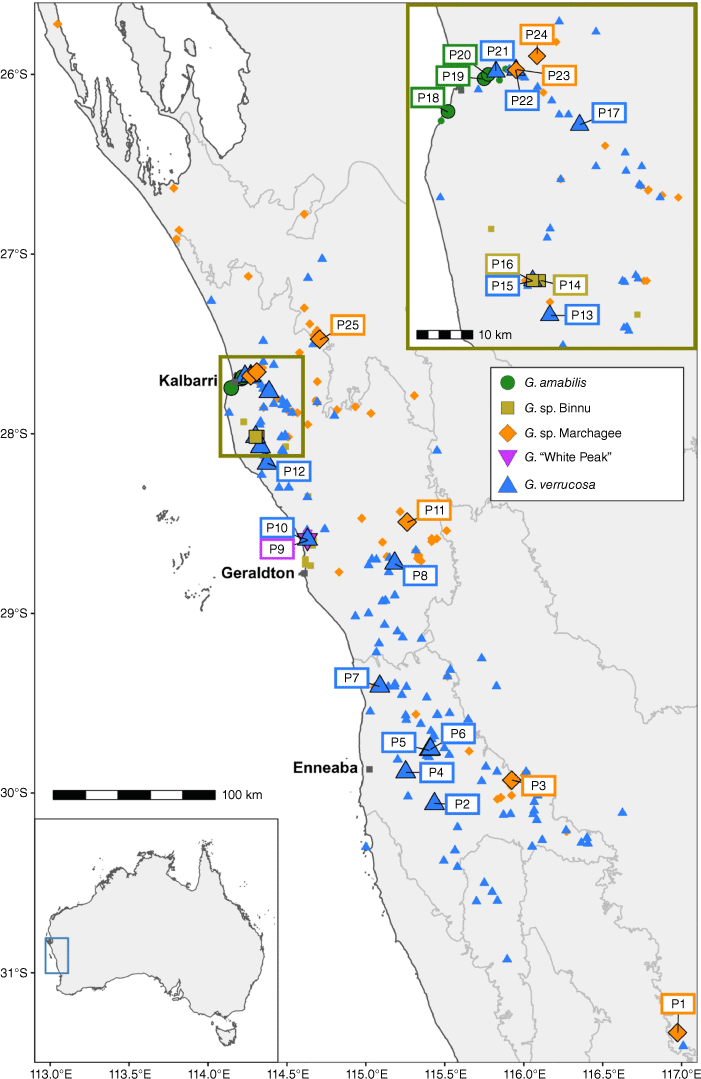
Distribution of sampled populations of Geleznowia in Western Australia. Rectangles surrounding population labels and symbols are coloured by initial morphological identification corresponding to forms recognised at the outset of this study. Populations are numbered, increasing south to north. Smaller symbols correspond to herbarium specimens and are coloured similarly. The base map is sourced from GADM (ver. 3.6, see https://gadm.org), with grey borders showing the Interim Biogeographic Regionalisation for Australia (IBRA) subregions (Department of Agriculture, Water and the Environment 2020).
Materials and methods
Sampling
Field work focused on surveying the area between Perth and Kalbarri in September 2020 and 2021 to collect herbarium vouchers, spirit material, and tissue samples of the five Geleznowia forms recognised at the start of this study (Fig. 1, 2, Supplementary Table S1). For DNA sampling, fresh leaf material was collected from 142 plants in 2020, with six individuals per form per population except in two cases (P9 and P10) where only two individuals were present. Molecular data indicated that Geleznowia is closely related to Philotheca Rudge & Drummondita Harv. (Bayly et al. 2013; Appelhans et al. 2021), so these were chosen as outgroups. We included six fresh collections each of two populations of Drummondita ericoides Harv. (Fig. 1g), two of Philotheca spicata (A.Rich.) Paul G.Wilson, one of P. pinoides (Paul G.Wilson) Paul G.Wilson, and one of P. sericea (Paul G.Wilson) Paul G.Wilson (Fig. 1f). Fresh material was collected into silica gel for immediate dehydration and freeze-dried on return to the laboratory. At least one voucher specimen per taxon per population was submitted to PERTH (Table S1).
Library preparation, sequencing, processing and assembly
Extraction of DNA followed a modified CTAB protocol (Doyle and Dickson 1987), with the addition of polyvinylpyrrolidone (PVP) to the extraction buffer. Approximately 40 mg of dried leaf material was used. Aliquots were taken twice from 13 samples to serve as technical replicates (bringing the total to 191 samples). DNA was sent to the Australian Genome Research Facility (AGRF) in Melbourne for library preparation and sequencing. Library preparation was based on a modified double-digest restriction site-associated DNA (ddRAD) protocol (Peterson et al. 2012). Modifications included slight alterations to digestion conditions, moving clean-up steps to after ligation, and including a column clean-up step. Initial screening of eight digestion enzyme combinations on three samples was followed by library generation for 191 samples by using the optimum combination (PstI/MspI) chosen based on the amount of amplified product and the absence of repeat regions. Following enzyme digestion and adapter ligation (including a barcode), samples were pooled in groups of 48 for size selection (280–375 bp) and 11 cycles of indexing polymerase chain reaction (PCR) for seven replicates (separated then re-pooled) of the group. All samples were then pooled into a single sequencing run (an entire SP200 flow cell = 2 lanes) in a 150 bp single-end configuration on an Illumina NovaSeq 6000 (ver. 1.0, see https://sapac.illumina.com/systems/sequencing-platforms/novaseq.html). Raw reads were returned in multiplexed files based on four indices.
Demultiplexing of reads was performed using ipyrad (ver. 0.9.81, see https://github.com/dereneaton/ipyrad; Eaton and Overcast 2020), including filtering for adapter contamination and low-quality base pairs, allowing a maximum barcode mismatch of one, a minimum quality score of 30 for trimming the end of reads, and a maximum number of low-quality bases per trimmed read of two. Any reads less than 50 bp long after trimming were removed. After demultiplexing and quality filtering, samples were compared for the number of retained reads. If the number of retained reads was less than two standard deviations below the average number of retained reads across samples or if it was less than 800 000, the sample was removed from the full dataset. This resulted in the removal of four samples (including two of the technical replicates).
To choose optimal assembly parameters in ipyrad, we ran assemblies for a subset of samples (two samples per population, including all original technical replicates, for a total of 73 samples) for each hundredth of the clustering threshold from 0.85 to 0.99. The resulting assemblies were then compared for a range of parameters suggested by earlier studies (Ilut et al. 2014; Mastretta-Yanes et al. 2015; Paris et al. 2017; McCartney‐Melstad et al. 2019). Based on this, a clustering threshold of 0.93 was selected for assembling the full dataset.
The full assembly used ipyrad parameters corresponding to a minimum read depth for base calls of six, maximum alleles in a consensus sequence of two (diploids; n = 14; Smith-White 1954), maximum proportion of uncertain base calls and maximum proportion of heterozygous base calls in consensus sequences of 0.05, maximum proportion of SNPs in a locus of 0.2, and maximum proportion of samples sharing a heterozygous site of 0.5. We kept all SNPs that were present in at least three samples to allow flexibility in downstream filtering. The output variant call format file (VCF; containing SNPs) and loci file (full alignments) were used for downstream filtering and analyses.
Filtering
To estimate SNP error rate in relation to read depth, we used Tiger (ver. 1.0, see https://bitbucket.org/wegmannlab/tiger; Bresadola et al. 2020) and SNPs from 11 technical replicates. The heterozygous error rate was used to set minimum (17) and maximum (100) mean depth cut-offs for retained loci to avoid higher error rates at low and high depths. Following error-rate estimation, replicates were removed in downstream filters, typically keeping the sample with more retained loci in the dataset.
To assess whether there were any samples that could not be distinguished genomically (effective clones), ingroup samples were filtered for SNPs present in at least 50% of samples, and pairwise SNP comparisons were made using a custom Python script (ver. 3.8, see https://docs.python.org/3/reference/). The proportion of SNPs (called in both) differing between samples for each of the nine technical replicate pairs was determined. These proportions were averaged for all replicates and doubled to give a conservative approximate lower limit for distinguishing between samples (~0.8% difference). Pairwise comparisons with fewer differences than this limit (i.e. <0.8% different) represent effective clones, and these were randomly reduced to a single representative per clone. This resulted in the removal of 57 ingroup samples, in some cases reducing populations to a single individual or, in one case, two populations (P21 and P22) to a single individual. Consequently, downstream analysis focused on individual-based metrics of genomic relationships, rather than population-based metrics.
Custom scripts (see Data availability) were then used to generate four datasets for various downstream analyses on the basis of differing filtering and sample combinations (Table 1). For ordination and allele-frequency analyses (Dataset 1), stricter filter settings were used, requiring SNPs to be present in at least 90% of samples, to be bi-allelic and to have a minimum minor allele count of three. A single SNP was retained per locus (chosen on the basis of maximum sample coverage, and randomly in the case of ties) to reduce the potential for linkage. For analyses less sensitive to missing data (genomic distances and phylogenetic analyses; Datasets 2–4), laxer filter settings were used, requiring SNPs to be present in at least 25 or 50% of samples (see Table 1), and without restrictions on allele count.
| Dataset | Analysis | Sampling | Number of samples | Minimum sample cover (%) | Minimum minor allele count | SNPs per locus | SNPs | Missing per sample (%) | Average depth per site |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PCA, STR | Ingroup | 84 | 90 | 3 | 1 | 1683 | 2.2 (0.8–4.3) | 58.9 (19–100) |
| 2 | DIST | Ingroup | 84 | 25 | 1 | 1 | 7392 | 40.8 (23.4–54.9) | 42.6 (17–100) |
| 3 | CONCAT | Phylo | 97 | 50 | 1 | all | 22 929 | 23.3 (9.2–73.6) | 51.7 (17–100) |
| 4 | SVD | Phylo | 97 | 50 | 1 | 1 | 3338 | 24.9 (8.2–82.3) | 51.3 (17–100) |
PCA, principal-component analysis; STR, Structure; DIST, distance-based SplitsTree4 network; CONCAT, concatenated maximum-likelihood phylogenetic analysis; SVD, SVDquartets coalescent phylogenetic analysis. Sampling included all populations of Geleznowia (ingroup) and all populations of Geleznowia plus Drummondita ericoides and Philotheca sericea, excluding putative hybrid individuals (phylo).
Genomic analyses
Overall genomic differences among ingroup samples were assessed using principal-component analysis (PCA) of Dataset 1. The filtered VCF was imported and converted to a genlight object with the vcfR package (ver. 1.12.0, see https://cran.r-project.org/package=vcfR; Knaus and Grünwald 2017) for R (ver. 4, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/), and the PCA was run using the ‘glPca’ function from the package adegenet (ver. 2.1.5, see https://cran.r-project.org/package=adegenet; Jombart 2008).
Genetic clustering was assessed using a Bayesian approach in the program Structure (ver. 2.3.4, see https://web.stanford.edu/group/pritchardlab/structure.html; Pritchard et al. 2000). Dataset 1 was run under an admixture model with no priors, and 40 runs per K value for K1–15. Each run included a burn-in of 100 000 generations of the Markov-chain Monte Carlo (MCMC) and sampling for a further 100 000 generations. The optimal K value corresponding to the highest level of structure in the data was evaluated with the top 10 runs for each K on the basis of likelihood by using the Evanno delta K method (Evanno et al. 2005). The top 10 runs were submitted to the online web service for CLUMPAK (see http://clumpak.tau.ac.il/index.html; Kopelman et al. 2015) to obtain the ancestry proportions for major and minor modes in the runs.
Genomic distances between samples were calculated using Dataset 2. The filtered VCF was imported and converted using vcfR into a DNAbin object for the package ape (ver. 5.6.1, see https://cran.r-project.org/package=ape; Paradis and Schliep 2019). Distances between samples were calculated using the MATCHSTATES method in the R package pofadinr (ver. 1.0, see https://github.com/simjoly/pofadinr). The method uses matches in nucleotides between two samples at each position (Joly et al. 2015) and can account for missing data. The distance matrix was visualised as a network in SplitsTree4 (ver. 4.17.1, see https://software-ab.cs.uni-tuebingen.de/download/splitstree4/welcome.html; Huson and Bryant 2006), using the Neighbour-Net method (Bryant and Moulton 2003), with splits displayed using the Equal Angle algorithm (Dress and Huson 2004).
Phylogenetic relationships among samples were estimated using Datasets 3 and 4 as input to concatenation and coalescent approaches respectively. For concatenation, loci from the filtered VCF were extracted from the full-loci file, samples were filtered from those loci, and alignments were combined and filtered for a maximum 50% missing data per site. The resulting alignment was used in a maximum-likelihood analysis in IQ-TREE (ver. 2.1.3, see http://www.iqtree.org/; Nguyen et al. 2015; Minh et al. 2020a). IQ-TREE was run with a search for the optimal model (Kalyaanamoorthy et al. 2017), 1000 ultrafast bootstrap (UFB) replicates (Hoang et al. 2018), and 1000 bootstrap replicates of the Shimodaira–Hasegawa-like (SH) approximate likelihood-ratio test (Guindon et al. 2010).
For a coalescent approach, a single SNP per locus was run in SVDquartets (Chifman and Kubatko 2014) as implemented in PAUP* (ver. 4.0a, build 168; see https://paup.phylosolutions.com/; Swofford 2003). The SNPs were concatenated into a single sequence per sample, with ambiguity codes being used for heterozygous sites. Two analyses were run, namely (1) individual lineages (i.e. each sample), and (2) samples grouped by putative species on the basis of results from PCA, genomic distances and Structure. Each SVDquartets analysis was run to sample quartets exhaustively, with ambiguous counts being distributed over compatible site patterns, with quartets assembled using QFM (Reaz et al. 2014), and with 100 bootstrap replicates. To evaluate conflicting phylogenetic signal, site concordance factors (Minh et al. 2020b) were calculated for the lineages tree by using the full alignment in IQ-TREE and sampling 100 000 random quartets around each internal branch. Site concordance factors indicate the percentage of informative sites for a given branch that support that branch in the tree. Trees were plotted using the R package ape. To visualise the conflict among bootstrap replicates, bootstrap trees were used to construct consensus networks (Holland and Moulton 2003) in SplitsTree4, including splits found in at least 10 of the 100 replicates.
Morphological measurements
Thirteen morphological characters were used for taxon comparison (see Results), with additional characters measured for taxonomic descriptions (see Taxonomic treatment) by using dried herbarium specimens (380 sheets held at PERTH including 172 vouchers collected in 2020 and 2021). Specimens were assessed to ensure that they fit the character ranges for a given taxon, but individual measurements were not recorded for all specimens. Detailed floral measurements (at anthesis) were made from field material preserved in 70% ethanol or rehydrated herbarium material by using boiling water with a drop of detergent. Assessment of the whorls of yellow petaloid bracts subtending each flower (Fig. 1) can be challenging because of a gradual transition from green leaves to yellow bracts (with some structures being both green and yellow). Each bract was scored as such only when there was an increase in size from the leaves below and the yellow colour extended for more than half its length. Seed characters (see Supplementary Fig. S1) were measured using SmartGrain (ver. 1.2, see https://www.naro.affrc.go.jp/archive/nias/qtl/SmartGrain/; Tanabata et al. 2012) on seed collections held in the Western Australian Seed Centre (Kensington) for which the identity at the collecting locality could be verified by a specimen lodged at PERTH.
Examination of type material from MEL was facilitated through a loan to PERTH. Scanned images of type material were also viewed in Global Plants (http://plants.jstor.org/) and the Museum National d’Histoire Naturelle (P) online database (http://science.mnhn.fr/institution/mnhn/search).
Results
Assembled ddRAD dataset
The fully assembled dataset for 187 samples resulted in 298 399 loci and 897 344 SNPs, with an average of 18 701 (6238–65 590) loci per sample. Of the 193 446 loci that had SNPs, 3024 were present in at least 80% of samples. In addition to the 57 ingroup samples removed because of effective clonality, two of the outgroup taxa (P. spicata and P. pinoides) had excessive levels of missing data and were removed from the phylogenetic analyses (17 samples). The 11 technical replicates were removed after clonality and error-rate assessment. This left 84 ingroup samples and 18 outgroup samples. For phylogenetic analysis, five more ingroup samples were removed, given indications from the genomic distance and Structure analyses that they were hybrids (see below).
Genomic analyses
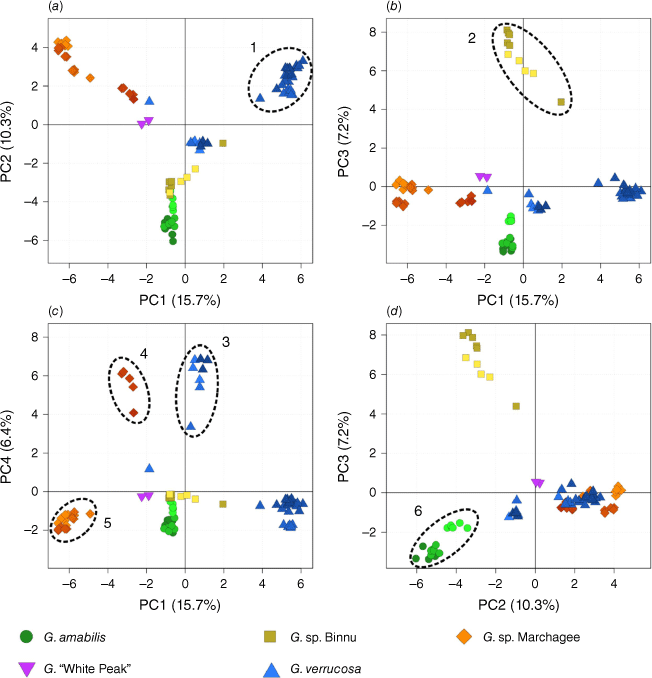
The PCA (Fig. 3) generally showed continuous variation across the samples, but distinct clusters were evident (sometimes corresponding with morphology) in some dimensions. The first four principal components of the PCA explained similarly substantive variation (~40% of the total genomic variation) and allowed for the identification of six cohesive genomic clusters. The first and second components separated a group of G. verrucosa samples (Group 1) from the rest (including other G. verrucosa samples). In the third principal component, the G. sp. Binnu samples formed a distinct cluster (Group 2) from everything else. The fourth principal component more clearly demarcated a second group of G. verrucosa samples (Group 3) and separated two groups of G. sp. Marchagee samples (Groups 4 and 5) as distinct. Finally, the second and third components separated G. amabilis samples as a distinct cluster (Group 6). The morphologically divergent G. ‘White Peak’ (P9; two samples) and a single population of G. verrucosa (P4; one sample) consistently clustered near other groups and did not fall out as clearly distinct. For the remaining analyses, we refer to these six genomic groups and two divergent populations (P4 and P9).
Principal-component analysis of 1683 SNPs for Geleznowia. The first four principal components are plotted against each other: (a) 2 v. 1; (b) 3 v. 1; (c) 4 v. 1; (d) 3 v. 2. Note that (a), (b) and (c) share the same x-axis, so only the vertical positions of the samples change. Colours and shapes correspond to initial morphological identification, with shading by population. Distinct genomic clusters are indicated with dashed circles and numbered from 1 to 6 (referred to in the text).
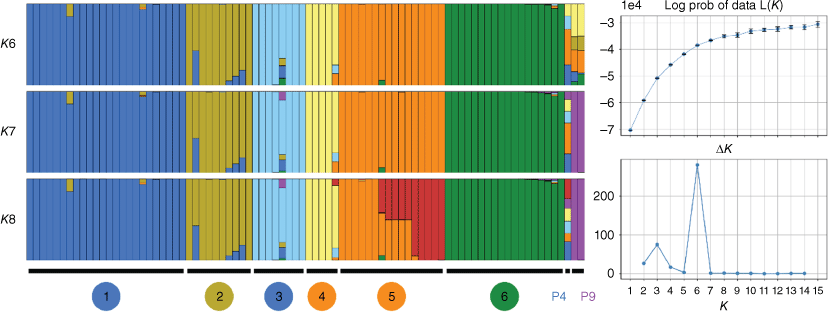
Structure output (Fig. 4) suggested K6 as optimal or the highest level of structure on the basis of the Evanno method. At K6, the same six genomic groups were evident as in the PCA, with the majority of samples showing no admixture among them. Some admixture was evident in a few individuals in Groups 1 and 2, with one sample of Group 2 that co-occurs with plants from Group 1 showing almost half of the assigned ancestry to Group 1, consistent with hybridisation. Other samples of Group 2 showed a smaller proportion of shared ancestry, and one individual from the Group 1 population P15 showed a small proportion of admixture with Group 2. One sample from Group 3 showed indications of possible mixed ancestry, and the divergent population P4 was unresolved with ancestry assigned to multiple groups. At K6, the divergent population P9 showed admixture with Groups 2, 4 and 5, yet, at K7, it was resolved as a distinct cluster. At K8, further population structure was shown within Group 5, consistent with more continuous variation.
Results of Structure runs for Geleznowia on the basis of 1683 unlinked SNPs. Sample ancestry assignments for major modes of K6–8 are shown as vertical bars for each sample. Numbered groups (1–6) at the bottom correspond to the six genomic groups identified from the PCA, whereas P4 and P9 refer to divergent populations. The estimated log probability of the data and delta K are shown at the right for K1–15. Values are from the top 10 runs for each K, and error bars show ±s.d.
Whereas major modes detected by CLUMPAK are shown in Fig. 4, minor modes were also detected (see Fig. S2), although they did not largely differ from the overall pattern recovered and showed the same six groups at K6.
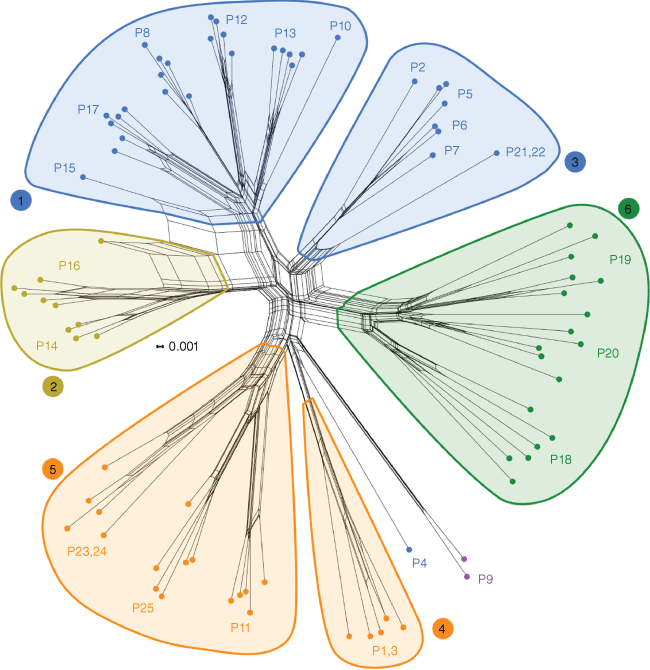
The SplitsTree4 network (Fig. 5) showed separation of the same six genomic clusters as those present in the PCA and Structure results, along with the two divergent populations (P4 and P9, both on isolated branches). Populations typically grouped distinctly within each of the six clusters, but not in all cases. Again, the G. verrucosa groups (1 and 3) were separated, and there was a deep division within the G. sp. Marchagee sampling (Groups 4 and 5). There were long splits connecting co-occurring populations P15 (in Group 1) and P16 (in Group 2), consistent with hybridisation.
SplitsTree4 Neighbour-Net network constructed from MATCHSTATES distances between samples of Geleznowia on the basis of 7392 unlinked SNPs. Numbered groups supported by indicated splits correspond with genomic groups from the PCA. Colours correspond to initial morphological identification. Tip labels indicate population numbers. The scale bar indicates distance. Parallel lines show splits in the data, which separate all the samples into two groups.
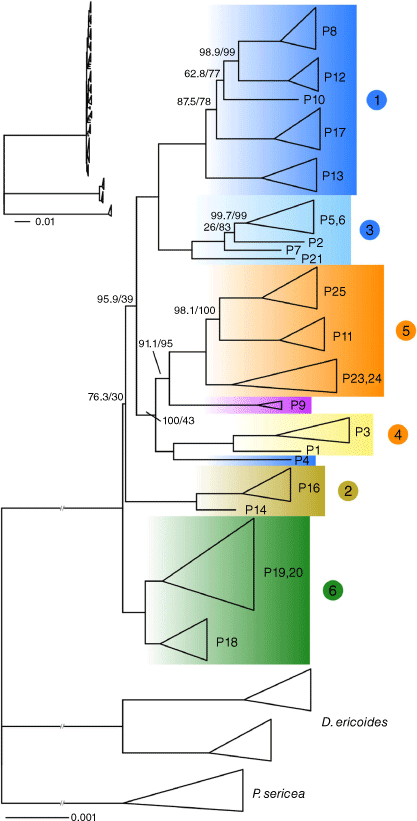
The concatenated alignment was 442 161 bp, with 13 369 parsimony informative sites. Samples had an average of 24.6% (8.2–82.0%) gaps or ambiguities; outgroup samples all had >75% and ingroup samples all had <20%. The best-fit model chosen by IQ-TREE on the basis of the Bayesian information criterion was TPM3 + F + R7 (AC = CG, AG = CT, AT = GT + empirical base frequencies + seven free rate categories). In the resulting tree (Fig. 6), all six of the genomic groups from the PCA were fully supported as monophyletic (100/100 SH/UFB). Relationships between groups were generally poorly supported for a dataset of this size, except for a sister relationship between Groups 1 and 3. The divergent population P4 was supported (100/100) as sister to Group 4, whereas P9 had some support (91.1/95) as sister to Group 5. Branching from the backbone was typically short compared with divergence from outgroups and reflected a lack of information about relationships between groups in the ingroup.
Concatenation maximum-likelihood phylogenetic tree of Geleznowia and two outgroup taxa. Monophyletic groups are collapsed for clarity and labelled with population numbers. Coloured numbers correspond to genomic groups in the PCA and are coloured by initial morphological identification, whereas colours of clades match colours in the Structure figure. Support values are Shimodaira–Hasegawa-like approximate likelihood-ratio test values and ultrafast bootstrap values, in that order. All branches have full support (100/100) except where shown. The scale bar indicates the average number of substitutions per site. Branches are cut in the main figure for clarity, but full branch lengths are provided at the top left.
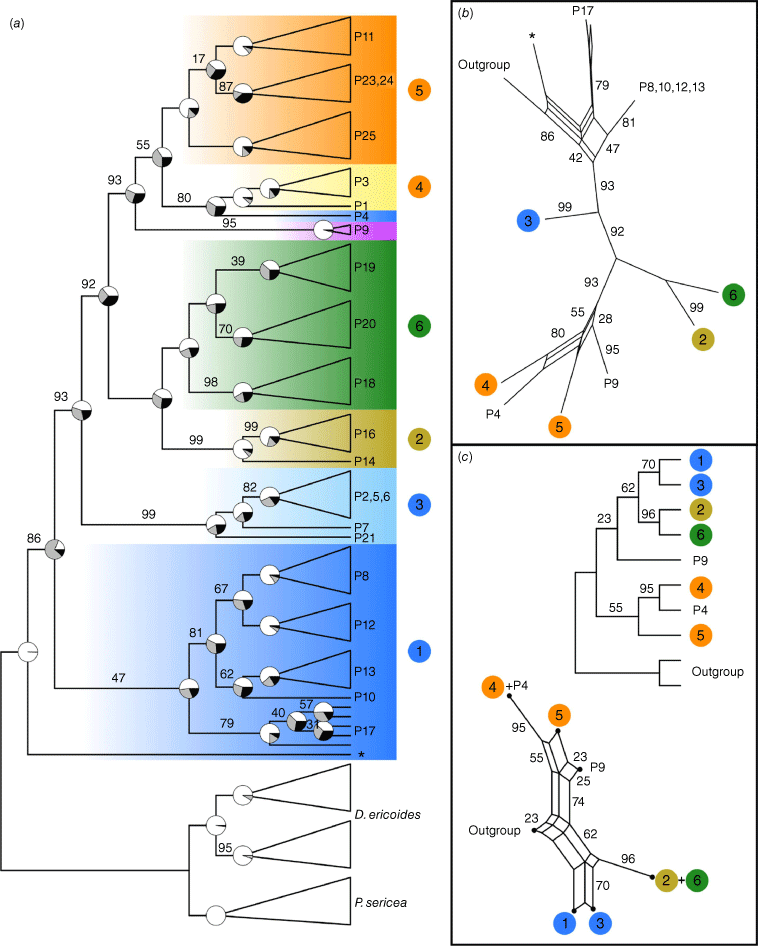
For the SVDquartets coalescent analyses, the lineages and species trees (Fig. 7) had 16.7 and 26.7% of quartets respectively, incompatible with the resulting trees. The individual lineages analysis recovered good support for the monophyly of most of the genomic groups from the PCA, as evident in high bootstrap values and concordance factors indicating most sites supported that grouping. Groups 2 and 3 had 99% rather than 100% bootstrap support, whereas Group 1 was not recovered as monophyletic because of the odd placement of one sample from P17 (asterisk in Fig. 7), which grouped with the outgroup, although with significant conflicting signal (see the concordance pie chart at the node subtending the remainder of the ingroup). Relationships among groups had some support. Groups 2 and 6 were supported as sister (100% bootstrap support), whereas Groups 4 and 5 formed a clade together with P4 and P9 (93%). The divergent population P4 was recovered as sister to Group 4 (80%). In the species analysis, there was strong support for the sister relationships of Groups 2 and 6 (96%), and low support for a sister relationship between Groups 1 and 3 (70%). On the basis of the bootstrap tree network, support for Groups 1 and 3 as sister was potentially affected by signal linking Group 1 and the outgroup, as in the lineages tree. The relationship between Groups 4 and 5 as belonging to a single clade was poorly supported (55%), and it was likely that this was affected by the placement of P9. Groups 4 and 5 were recovered in a single clade with P4 and P9 in 74% of the bootstrap trees.
SVDquartets trees for Geleznowia and two outgroup taxa on the basis of 3338 SNPs. Coloured numbers correspond to genomic groups in the PCA and are coloured by initial morphological identification, and colours of clades match colours in the Structure figure. (a) Lineage tree with individuals represented by a single sequence. Monophyletic groups are collapsed for clarity and labelled with population numbers. Bootstrap support values less than 100 are shown above branches. Pie charts at nodes of interest show site concordance factors: the proportion of informative sites supporting the given branch topology (white) and the two alternative topologies (grey and black). One sample from Group 1 that is placed strangely is indicated with an asterisk (*) in (a) and (b). (b) Consensus network of bootstrap trees for the lineage analysis, showing splits found in at least 10 of the 100 trees. Branch lengths are proportional to bootstrap value; values are omitted for 100 and <20%. (c, top) Species tree with individuals grouped into putative ‘species’ (genomic groups from the PCA plus the divergent populations P4 and P9). Bootstrap support values less than 100 are shown above branches. (c, bottom) Consensus network of bootstrap trees for the species analysis, showing splits found in at least 10 of the 100 trees. Branch lengths are proportional to bootstrap value; values are omitted for <20%.
Morphological measurements
Floral and vegetative characters were compared for each genomic group identified in the genomic analyses and similar associated specimens (see Table 2). The comparisons showed unique sets of characters that could be used to demarcate each of the six genomic groups, plus the morphologically divergent population P9 (see the key in the Taxonomic treatment). Diagnostic characters included the number, shape, size and indumentum of the yellow petaloid bracts subtending each flower (see Fig. 1), the total number of flowers per inflorescence, and the size and shape of the sepals.
| Genomic group | 1 | 2 | 3 | 4 | 5 | 6 | P4 | P9 |
|---|---|---|---|---|---|---|---|---|
| Plant height (m) | 0.3–0.75 | 0.4–1.2 | 0.25–0.8 | 0.2–0.4 | 0.2–0.7 | 1–2 | 0.35 | 0.6–0.75 |
| Leaf colour | Glaucous to dull green | Dull green | Glaucous to dull green | Glaucous or pale green | Glaucous or pale green | Silvery grey–green | Dull green | Silvery grey–green |
| Flower number | 5–7, sometimes 1–4 | (5)7–10 | 1–3(4–5) | 1–3(5) | 1–6 | 5–17 | 1 | 5–7 |
| Flower colour | Yellow | Pale lemon yellow | Pale greenish-yellow to yellow | Yellow | Yellow | Vivid yellow | Yellow | Vivid yellow |
| Bract number | (7)10–14 | 7–11 | (5)6–8 | 0–3(4) | (4)5–7 | 8–10 | 7 | 6–7 |
| Bract length (mm) | 8.2–16.9 | 7.2–22 | 6.8–14 | 3.7–6.3 | 4.1–8.6 | 7–16 | 6–11.1 | 10.2–16 |
| Bract width (mm) | 5–10.3 | 4.6–11.5 | 4–10 | 1.8–2.9 | 2.4–5.5 | 3.6–13 | 4.2–6.7 | 6.4–10 |
| Bract abaxial surface indumentum | Glabrous or sometimes with scattered hairs 0.05–0.1 mm long | Moderately dense to dense hairs 0.2–1.2 mm long | Glabrous | Glabrous or with scattered hairs 0.05 mm long | Glabrous or with scattered to moderately dense hairs 0.05 mm long | Glabrous or with hairs 0.04 mm long | Glaborous or with few scattered hairs 0.05 mm long | Glabrous or with scattered hairs 0.05 mm long |
| Bracteole number | 8–12 | 9–18 | 0–6 | 0–2(4) | 0–8 | 4–36 | 0 | 10 |
| Sepal length (mm) | 9–12.3 | 8.5–15 | 9–12.8 | 6.3–6.9(7.3) | 5–9.3 | 8.5–14 | 9.3–10.4 | 3.5–10.2 |
| Sepal width (mm) | 4.7–8 | 4.1–7 | (4)5–8.3 | 2.5–3.1 | 3.2–5.2(5.7) | 4.3–9 | 4.8–6.4 | 4–5.2 |
| Petal length (mm) | 5.2–7.6 | 4–8.5 | 5–8.8 | 4.6–7 | 4.2–5.9 | 4.8–8 | 5–5.3 | 6.6–7 |
| Stigma length (mm) | 0.4–0.5 | 0.4–0.5 | 0.5–0.6 | 0.5 | 0.6–1 | 0.1–0.3 | 0.4 | 0.4–0.5 |
Discussion
Our analyses of Geleznowia have demonstrated again the potential of genomic data to distinguish separate lineages within morphologically variable and taxonomically challenging groups of plants. Population sampling of ddRAD genomic data across the genus enabled the identification of multiple distinct entities that largely maintain their distinctiveness when in sympatry. Detailed morphological review and comparison of those entities uncovered sets of characters that can be used to distinguish them. Together, our results point to unrecognised lineage diversity in the group that warrants taxonomic recognition and conservation assessment.
Species delimitation
Consistent patterns across ordination, distance, Bayesian and phylogenetic analyses, coupled with morphological measurements, point to seven morphologically recognisable and genomically distinct groups within Geleznowia. The challenge for understanding species complexes is to distinguish the fuzzy boundary where population differentiation has transitioned to effective reproductive isolation and independent evolution that we expect for species (e.g. de Queiroz 2007). One of the strongest indicators of this is co-occurrence of taxa with the maintenance of morphological, genomic or ecological differences (Harrison and Larson 2014; Rannala and Yang 2020). Within Geleznowia, this is reflected in the region near Kalbarri, where three of the groups (3, 5 and 6) occur within short distances of each other and sometimes at the same location. Field observations also include members of Group 1 co-occurring with Groups 3 and 5 in this area (the closest sampled population is P17). These groups were recovered as distinct in all analyses and represent the extremes of morphological and genomic variation in the group. Their close occurrence without admixture points to established reproductive barriers and independent evolution.
Co-occurrence or nearby populations and a lack of admixture is likely to be caused by processes that result in reproductive isolation, e.g. flowering-time differences, different pollinators, and reproductive incompatibilities, among others (Grant 1971). The pattern might also result from a breeding system that reduces outcrossing (e.g. Martin and Willis 2007). Little is known about what pollinates Geleznowia, with only a few observations of moths, ants and beetles having been reported on the flowers, none of which appeared to result in pollination (Broadhurst and Tan 2001). The showy flowers are superficially alike and do not appear to exhibit differing specialisations for specific pollinators among the groups. Work on the breeding system and genetic diversity in morphological forms of Geleznowia (Broadhurst et al. 1999; Broadhurst and Tan 2001) suggested facultative selfing but also some level of inter-form compatibility (sometimes with lower seed set). Although some forms showed more self-compatibility than others, none was completely selfing (Broadhurst and Tan 2001). Given that genomic groups in Geleznowia retain some level of outcrossing and genomic diversity, genetic cohesion across large distances (hundreds of kilometres in some cases; see Fig. 8) without mixing with overlapping and sometimes co-occurring groups seems unlikely to be solely due to selfing or the apomictic spread of clones. However, even if the patterns are driven or reinforced by selfing, there is an arguably effective barrier to gene flow leading to relative evolutionary independence among the groups, compatible with calling them species under the concept we are using.
Distributions of all seven Geleznowia species in Western Australia. The base map is sourced from GADM (ver. 3.6, see https://gadm.org), with grey borders showing IBRA regions and subregions (Department of Agriculture, Water and the Environment 2020).
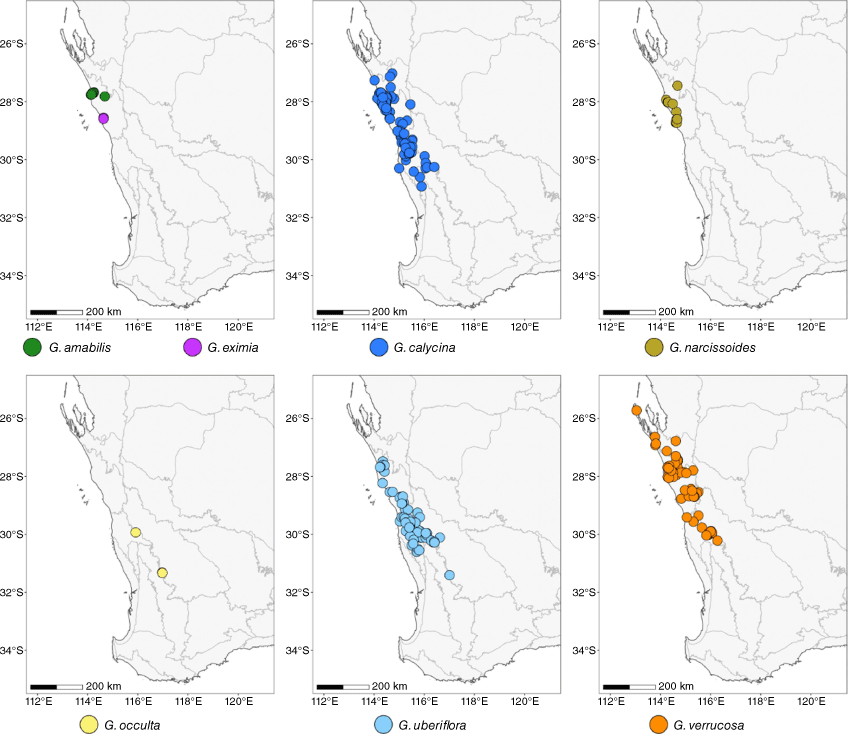
Other examples of co-occurrence and retained genomic differences are found south of Kalbarri, where Groups 1 and 2 are found growing together at the same location (P15 and P16). Maintenance of morphological distinction between the two at the same location suggests distinction, even when some individuals show evidence of introgression (see below). Further south, P9 co-occurs with Group 1 (P10) without good evidence for current admixture or morphological ambiguity. And finally, Groups 1 and 3 flower at different times at the same location (P5; Group 3 sampled the first year, both observed the second year). The field observations of co-occurrence between Groups 1 and 3 and the broad overlap in their distributions (see Fig. 8), while maintaining genomic distinctiveness point to reproductive isolation.
Differentiating species boundaries from population divergence becomes more difficult when taxa are allopatric (Rannala and Yang 2020), and this was largely the case for our sampling of Groups 4 and 5. These taxa are morphologically similar and were considered one entity during sampling (G. sp. Marchagee). Although specimen distributions may partly overlap (Group 4 has a patchy and limited distribution, but P3 is ~3 km from an unsampled population of plants with morphology matching Group 5), we did not observe them co-occurring at the same location. Genomic evidence from the PCA, SplitsTree4 network and Structure analysis indicates a close relationship but with consistent differences. The phylogenetic analyses suggest that they may be closely related, but support values for this were variable (43% in the concatenation tree, 93% in the lineages tree and 74% in the species tree) and were potentially complicated by the inclusion of the divergent populations P4 and P9. Given that the two groups are not widely geographically separated on the basis of field observations and herbarium specimens and that there is no strong evidence for admixture, coupled with consistent morphological differences (see Taxonomic treatment), we recognise them as separate species. Future work may help map the distributions of the two more clearly, broaden sampling and refine understanding of their relationship.
Our analyses point to complex patterns of divergence for populations P4 and P9. They are found on isolated branches in the distance network and concatenation tree but show indications of admixture in the Structure analysis and in the conflict and concordance factors in the lineages tree that could possibly represent ancient hybrid origins. As mentioned above, P9 is morphologically and genomically distinct from co-occurring Group 1 and can be recognised as a distinct species. However, the P4 population is less morphologically distinct, with characters similar to Group 3 (see Table 2). Since it is genomically divergent but associated with Group 4, it is unclear how to treat the population. Given Groups 3, 4 and 5 occur in the region and are geographically close to P4, a hybrid origin seems possible, although perhaps not recently enough to show clear genomic admixture. We provisionally retain the population as a single genomically divergent lineage of possible hybrid origin between ancestors of Groups 3 and 4.
Taxonomic implications
On the basis of agreement between genomic and morphological differences that are typically maintained in sympatry, we recognise seven species in Geleznowia. We retain the currently circumscribed G. amabilis and provide new circumscriptions for the six remaining species. For clarity, a summary is provided in Table 3, so as to clearly link the genomic groups used within this paper to previous identifications and the new taxonomy.
| Genomic group: taxonomic identification at the outset of this study | Taxonomy proposed herein |
|---|---|
| 1: G. verrucosa Turcz., p.p. | G. calycina (J.Drumm. ex Harv.) Benth. (syn. G. macrocarpa Benth.) |
| 2: G. sp. Binnu (K.A. Shepherd & J. Wege KS 1301) | G. narcissoides K.A.Sheph. & A.D.Crawford, sp. nov. |
| 3: G. verrucosa Turcz. | G. uberiflora K.A.Sheph. & A.D.Crawford, sp. nov. |
| 4: G. sp. Marchagee (A. Crawford ADC 1353) | G. occulta K.A.Sheph. & A.D.Crawford, sp. nov. |
| 5: G. sp. Marchagee (A. Crawford ADC 1353) | G. verrucosa Turcz. |
| 6: G. amabilis K.A.Sheph. & A.D.Crawford | G. amabilis K.A.Sheph. & A.D.Crawford |
| P4: G. verrucosa Turcz., p.p. | Provisional identification as G. uberiflora × occulta |
| P9: G. ‘White Peak’ | G. eximia K.A.Sheph. & A.D.Crawford, sp. nov. |
It became evident through the examination of high-resolution scans of type specimens recently made available via Global Plants (http://plants.jstor.org/), particularly of the holotype of G. verrucosa (KW 001001059) held in the M.G. Kholodny Institute of Botany, National Academy of Sciences of Ukraine, that the current concept of G. verrucosa had been misapplied in Western Australia. The type instead matched the morphology of Genomic group 5, originally treated in WA as the phrase-named G. sp. Marchagee. Specimens previously considered to be typical G. verrucosa are recognised here as two different and distinct species. The first, corresponding to Genomic group 1, represents one of Bentham’s previously recognised species, G. calycina, which is reinstated here (and includes his G. macrocarpa as a synonym). The second, corresponding to Genomic group 3, is newly described here as G. uberiflora K.A.Sheph. & A.D.Crawford.
Evolution in the group
One pattern evident in both the SplitsTree4 network and the Structure analysis was admixture between some individuals of Groups 1 and 2 in the co-occurring populations of P15 and P16. The location of these populations matches that of a putative hybrid population from earlier work by Broadhurst et al. (2001), who found genetic and morphological evidence of hybridisation between their putative taxa. At the time, Broadhurst and Tan (2001) considered Geleznowia to consist of just two subspecies, so matching their concepts to ours is imprecise. Regardless, we also found hybridisation in that population, though we interpret our results at the species rather than subspecies level (see below). Our results show strong evidence for current admixture between groups 1 and 2, with one individual of group 2 having a genomic composition consistent with an F1 or early-generation hybrid. Other individuals showed evidence of some introgressed genomic background, including the individual of group 1. Broadhurst et al. (2001) found evidence that introgression in the hybrid population was asymmetrical. Although we found some indication of introgression in both directions, more admixture was detected in individuals from Group 2 (e.g. Fig. 4), in line with asymmetric introgression. Although the admixture was evident in our genomic results, individuals were readily assigned to either morphological group without ambiguity, suggesting that the morphological signal of hybridisation was weak. This is consistent with previous observations of only a few morphological intermediates and the absence of an obvious ‘hybrid zone’ (Broadhurst et al. 2001).
The presence of some rare admixture suggests that reproductive isolation between Groups 1 and 2 is not complete. This is not incompatible with species divergence, because species boundaries are known to sometimes be leaky or allow genetic exchange, especially in hybrid zones (e.g. Martin and Willis 2007; see Harrison and Larson 2014). Broadhurst (2000) suggested that their two putative taxa were distinct, but because there were additional ‘intermediate’ form(s) possibly derived from hybridisation, the two should be recognised as subspecies (however, this was not formally done). The topic of using the rank of subspecies and its evolutionary relevance has been and continues to be contentious (de Queiroz 2020, 2021; Hillis 2021; Burbrink et al. 2022). It has been suggested that the rank of subspecies may be used to denote species that are incompletely separated (de Queiroz 2020). In practice, evidence of genomic mixing between divergent lineages has been used to justify keeping them as single species (e.g. Georges et al. 2018), whereas lack of clear evolutionary independence has led to suggestions to sink some morphologically recognised subspecies (e.g. Prates et al. 2023). The choice remains partly subjective with regards to how much divergence or evidence of evolutionary independence is sufficient to recognise taxa. We choose to recognise Groups 1 and 2 at the level of species, despite the evidence of admixture, for a number of reasons, including the following: (1) morphological divergence between the two groups is strong, without indication that it is breaking down in the population where the two co-occur and undergo introgression; (2) observation of admixture is rare and highly localised (no admixture in the widespread Group 1 was clearly evident outside of the hybrid population); and (3) the degree of genomic divergence between them is similar to that between other genomic groups that co-occur without admixture (e.g. Groups 1 and 3) that we have interpreted as separate species. The P4 population showed indications of potential hybrid origin in the Structure and SVDquartets analyses, which may help explain why it is morphologically less distinct but genomically divergent. In the SVDquartets lineages tree (Fig. 7), there is indication that a substantial proportion of sites are in conflict and point to affinities elsewhere, although it is supported (80%) as sister to Group 4. Morphologically, these plants are closest to those in Group 3. The flowers of the P4 plants have a number of bracts similar to that of the flowers of Group 3 and a lack of bracteoles similar to some Group 3 plants (and Group 4 plants). Flower size is more similar to flowers in Group 3 than flowers in Group 4. On the basis of the SplitsTree4 network, there is no indication of recent introgression, suggesting that the ambiguity may be the result of an ancient hybridisation event. The P4 population is geographically close to the ranges of both Group 3 and Group 4, so a hybrid origin is possible. Further sampling in the region around P4 may help clarify whether there are any other similarly anomalous populations.
Our phylogenetic results indicated little support for tree-like relationships among species of Geleznowia, with lower support and conflict along the backbone of the group. One possible explanation for this pattern is a rapid radiation and the fixation of ancestral variation in small populations. In line with previous work by Broadhurst et al. (1999), we detected extremely low genetic diversity within and sometimes among populations (results not shown), in some cases resulting in effective clonality. Given the ephemeral nature of populations of Geleznowia and their response to disturbance, population bottlenecks could have substantial effects on their genetic diversity (Broadhurst et al. 1999). The presence of essentially identical genomic samples is also consistent with previous breeding system work, indicating facultative selfing in the group, which varied in degree between putative taxa (Broadhurst and Tan 2001). Diversification within Geleznowia may be dominated by these kinds of processes, where morphological and genetic variation is rapidly fixed and conversely rapidly lost in small populations.
Conservation implications
Our proposed new taxonomy of Geleznowia highlights species of immediate conservation concern, because some of these species are highly range restricted and under threat from land clearing. For example, G. eximia (P9) and G. occulta (Group 4) are known from only one or two locations and are therefore in urgent need of further survey to determine whether there are additional populations. The situation is particularly dire for G. eximia, which is currently known from only two plants in a single population and has not been previously conservation listed given the lack of taxonomic recognition. It will be listed as a Priority One conservation species (T. Llorens, pers. comm.) but is likely to warrant consideration for assessment as Threatened. Geleznowia amabilis (Group 6) remains known from a small area near Kalbarri, and G. narcissoides (Group 2) is currently difficult to locate and in need of survey. Herbarium records suggest G. narcissoides was potentially more widespread; however, it is unclear whether all recorded populations are persisting. Given G. narcissoides was a primary target for the cut flower industry, review of the conservation management of this species is needed.
The observation of low genomic diversity (unexpected clonality) for most species of Geleznowia (all except G. amabilis) is notable in that it applies to both localised and widespread species. Conservation assessment and management would be improved by a more extensive assessment of clonality and diversity within species of Geleznowia. Given these species maintain a soil seed bank and appear to depend on disturbance such as fire to regenerate populations, populations are often transient. This complicates survey efforts to assess diversity and full geographic distributions and means that the genetic diversity represented by the soil seed bank is largely unmeasured. The provision of formal species names and their descriptions will greatly aid survey efforts and allow conservation assessments for these poorly known species.
Taxonomic treatment
Geleznowia Turcz., Bull. Soc. Imp. Naturalistes Moscou 22(3): 12–13 (1849)
Type: G. verrucosa Turcz.
Erect subshrubs to shrubs 0.15–2 m high, branchlets terete, glandular–verrucose, glabrous or with an indumentum of simple hairs. Leaves sessile or with a short petiole, overlapping and crowded towards terminal branches, coriaceous, elliptic to obovate, glandular–verrucose adaxial surface, with or without short, simple hairs. Flowers 1–17 in terminal heads. Bracts 0–14 petaloid, yellow, surrounding flowers. Sepals 5, free, 3.5–14 mm long, imbricate and resembling bracts. Petals 5, yellow, elliptic, thicker than sepals, 4–8.8 mm long. Stamens 10, free, 2.5–5.5 mm long, glabrous. Carpels 5, free, thickened at the apex, glandular–verrucose, with two ovules per carpel; style glabrous, with a narrow to club-shaped stigma. Fruit obovoid with a single seed per locule. Seeds dark brown to black 3.6–5 mm long, with a pale aril.
A genus of seven species endemic to Western Australia (Fig. 8).
Named for Nikolai Ivanovich Zheleznov (1816–1877), an agronomist at Moscow University (Turczaninow 1849).
| 1. | Moderate to tall shrubs 0.6–2 m high; leaves silvery green; inflorescence of dense heads 15–33 mm long, each with 5–17 flowers; bracts and sepals vivid yellow |
| 2. | Habit 1–2 m high; maximum 17 flowers per inflorescence; 8–10 bracts; stigma narrower than the style, 0.1–0.3 mm wide (Kalbarri National Park area)... G. amabilis |
| 2: | Habit 0.6–0.75 m high; maximum 7 flowers per inflorescence; 6 or 7 bracts; stigma broader than the style, 0.4–0.5 mm wide (north Geraldton)... G. eximia |
| 1: | Moderate shrubs 0.2–1.2 m high; leaves glaucous, pale green to dull dark green; inflorescence of solitary flowers or dense heads 5.2–23 mm long, each with 1–10 flowers; bracts and sepals pale lemon yellow, yellow or greenish-yellow (some of which may be strongly tinged red post-pollination) |
| 3. | Inflorescence with (5)7–10 flowers; bracts, bracteoles and sepals pale lemon yellow with moderately dense to dense long hairs up to 1.2 mm (north Geraldton to north Kalbarri)... G. narcissoides |
| 3: | Inflorescence with 1–7 flowers; bracts, bracteoles and sepals pale greenish-yellow to yellow, glabrous or with scattered to moderately dense short hairs up to 0.1 mm |
| 4. | Inflorescence 5.2–15 mm long; bracts 3.7–8.6 mm long, 1.8–5.5 mm wide; sepals 5–9.3 mm long, equal to or slightly longer than petals (ratio 1.09–1.24) |
| 5. | Inflorescence 12–15 mm long; few bracts (0–3(4)) surrounding 1–6 flowers; bracts 2.4–5.5 mm wide; bracteoles variable 0–8 (east Watheroo to Dirk Hartog Island)... G. verrucosa |
| 5: | Inflorescence 5.2–10 mm long; numerous bracts ((4)5–7) surrounding 1–3(5) flowers; bracts 1.8–2.9 mm wide; bracteoles infrequent 0–2(4) (west Coorow to east Goomalling)... G. occulta |
| 4: | Inflorescence 12–22 mm long; bracts 6.8–16.9 mm long, 4–10.3 mm wide; sepals 9–12.8 mm long, obviously longer than petals (ratio 1.45–1.8) |
| 6. | Flowers usually 5–7 (sometimes 1–4) per inflorescence; numerous bracts (7)10–14; numerous bracteoles 8–12; peak flowering August–September (west Gillingarra to south Shark Bay)... G. calycina |
| 6: | Flowers usually 1–3 per inflorescence; fewer bracts (5)6–8; fewer bracteoles 0–6; peak flowering May–July (south-eastern Goomalling to Kalbarri area)... G. uberiflora |
Geleznowia amabilis K.A.Sheph. & A.D.Crawford, Nuytsia 31: 89–93, fig. 1a–c (28 April 2020)
Type: Western Australia, Kalbarri [precise locality withheld for conservation reasons], 23 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1305 (holo: PERTH 08152012; iso: CANB 721134, NSW).
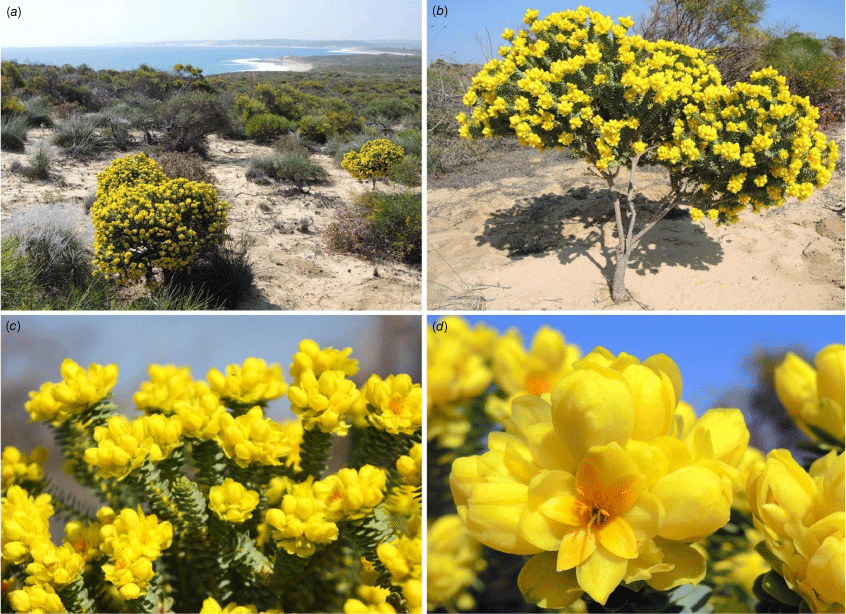
Erect shrub 1–2 m high; older branches cream to light brown and glabrous, younger branches pale yellowish-green with an indumentum of sparse, simple hairs up to 0.05 mm long. Leaves silvery green, elliptic to obovate, 4.6–11.5 mm long, 2.5–7 mm wide, adaxial surface slightly concave and glabrous, abaxial surface glandular–verrucose and glabrous. Flowers 5–17, terminal inflorescences 15–33 mm long. Pedicel central flower 3.8–7 mm long, with scattered to moderately dense hairs 0.2–0.6 mm long. Bracts 8–10, vivid golden-yellow, rarely becoming tinged with red in fruit, elliptic to obovate, 7–16 mm long, 3.6–13 mm wide, sessile or shortly stalked; adaxial surface glabrous; abaxial surface glandular–verrucose, glabrous or with minute hairs to 0.04 mm long towards the base. Bracteoles 4–36, usually paired below each flower except central flower, narrowly obovate, narrowly elliptic or oblanceolate, 7–17 mm long, 1.7–6 mm wide, sometimes with an attenuate base, glabrous or both surfaces with hairs to 0.04 mm long. Sepals elliptic to oblong, longer than petals, 8.5–14 mm long, 4.3–9 mm wide, glabrous or sometimes with hairs at the point of attachment. Petals bright orange–yellow, cupped, coriaceous, narrowly elliptic, 4.8–8 mm long, 1.7–3.5 mm wide, glabrous. Stamens 10; filaments 3–4.4 mm long, broadening at base up to 0.3–0.5 mm wide, glabrous; anthers oblong, 1.4–2.3 mm long, 0.4–0.8 mm wide. Carpels 5, free, with two ovules per carpel, total length 1.4–3.5 mm, total width 1.6–2.3 mm wide, verrucose, glabrous. Style glabrous, 4.5–7.3 mm long; 0.2–0.3 mm wide; stigma narrower than style apex, 0.1 mm long, 0.1–0.3 mm wide. Fruit obovoid, 5.5–5.7 mm long, 8–10 mm wide. Seeds dark brown to black 3.8–5.5 mm long, 2.1–3.3 mm wide, aril pale cream 1.9–4.0 mm long (Fig. 1a, 9).
Geleznowia amabilis: (a) habitat; (b) single-stemmed habit; (c) dense head of vivid yellow flowers above whorls of silvery grey–green leaves; (d) open flower showing the large, vivid yellow sepals surrounding broad, orange–yellow petals and narrow stigma. Voucher: K.A. Shepherd & B.M. Anderson KS 1840. Photos: K. A. Shepherd.
Currently known only from a few populations in or near Kalbarri National Park (Fig. 8) in the Geraldton Sandplains bioregion (Department of Agriculture, Water and the Environment 2020). This species is found growing in yellow or brown sand over sandstone, or red–brown sandy loam with laterite, in coastal scrubland, dense heath, low mallee or Acacia shrubland with Calytrix, Grevillea, Calothamnus and Melaleuca.
This species flowers from July to October, with fruits forming in October to November. The distinctive vivid golden-yellow colour of the bracts and sepals is maintained throughout flowering, although some outer bracts rarely become tinged with red towards the apex as fruits develop.
This species is listed as Priority Two under Conservation Codes for Western Australian Flora. Although some populations are found within a National Park, the extent of the distribution of this species remains poorly known and further survey is required.
Geleznowia amabilis is unique in the genus by virtue of the following combination of characters: a tall shrub 1–2 m high with silvery grey–green leaves; 5–17 flowers per inflorescence, surrounded by 8–10 vivid golden-yellow bracts, 7–16 mm long, 3.6–13 mm wide, abaxial surface glabrous or sometimes with minute hairs 0.04 mm long; 4–36 bracteoles; sepals 8.5–14 mm long, 4.3–9 mm wide; and a narrow stigma 0.1–0.3 mm wide. Geleznowia amabilis is morphologically most similar to G. eximia but can be distinguished from it by its larger habit (shrub 1–2 m high 0.6–0.75 m high), generally larger numbers of flowers per inflorescence (5–17 cf. 5–7), which are subtended by more bracts (8–10 cf. 6–7), and the stigma being narrower than the style and 0.1–0.3 mm wide (cf. broader than the style and 0.4–0.5 mm wide).
WESTERN AUSTRALIA. [localities withheld for conservation reasons] 6 Sep. 1990, D.E. Albrecht & B.A. Fuhrer DEA 4235 (MEL 2013859, PERTH 02933330); 6 Aug. 1967, A.M. Ashby 2209 (AD 97849315, AD 968071301, MEL 2101785, PERTH 0968072); 1 Sep. 2012, G.N. Brand 351 (PERTH 08713146); 14 July 1994, L. Broadhurst 3 (PERTH 05496624); 18 Oct. 1996, L. Broadhurst 18 (PERTH 05599016); 11 Oct. 1996, M.G. Corrick & B.A. Fuhrer MGC 11388 (MEL 2037214, PERTH 05876540); 29 Nov. 1995, A. Crawford s.n. (PERTH 04398947); 30 Nov. 2001, A. Crawford ADC 118 (PERTH 06118933); 3 Oct. 2004, A. Crawford ADC 597 (PERTH 07118090); 3 Oct. 2007, A. Crawford ADC 1383 (PERTH 07828705); 3 Oct. 2007, A. Crawford ADC 1384 (PERTH 07828659); 11 Sep. 2008, A. Crawford ADC 1850/1 (PERTH 08201161); 22 July 2004, M. Harding 7 (PERTH 06947972); 28 Sep. 1985, N. Hoyle 520 (CANB 364776, PERTH 0971227); 24 Oct. 2000, B.J. Lepschi & L.A. Craven 4343 (CANB 638268, MEL 2213143, PERTH 06755348); 31 Aug. 2021, K.A. Shepherd & B.M. Anderson KS 1840 (PERTH 09514732); 23 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1306 (DNA D0273574, PERTH 08152020); 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1730 (AD, CANB, K, MEL, NSW, NY, PERTH 09508058); 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1732 (BRI, CANB, MEL, NSW, PERTH 09508007); 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1733 (CANB, DNA, MEL, MO, NSW, PERTH 09508015).
Geleznowia calycina (J.Drumm. ex Harv.) Benth., Fl. Austral. 1: 348 (30 May 1863)
Sanfordia calycina J.Drumm. ex Harv. in W.J.Hooker (ed.), Hooker’s J. Bot. Kew Gard. Misc. 7: 54 (1855); Eriostemon sanfordii F.Muell., Fragm. 1(5): 107 (Apr. 1859), nom. nov., as ‘sandfordii’.
Type citation: ‘On sand plains to the east and west of the southern branch of the Hill River, and in similar situations to the south of the Irwin.’ Type: Western Australia, between Moore and Murchison Rivers, s. dat., J. Drummond 6: 83 (lecto, here designated: MEL 232716! [left-hand fragment]; isolecto: K 000717327 image!; PERTH 00967556! [ex Herbario Musei Britannici]; possible isolecto: TCD 0013332 image!).
Geleznowia macrocarpa Benth., Fl. Austral. 1: 347 (30 May 1863). Type citation: ‘W. Australia. Murchison river, Oldfield.’ Type: W[estern] Aust[ralia], Murchison R[iver], s. dat., A.F. Oldfield s.n. (lecto, here designated: K 000717328 image!; isolecto: MEL 232721!; MEL 232723!).
Erect single- or multiple-stemmed subshrub or shrub 0.3–0.75(1) m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of scattered to moderately dense, simple hairs up to 0.1 mm long. Leaves glaucous green to dark green, elliptic to obovate, 3.8–7.2 mm long, 2.1–4.7 mm wide, adaxial surface glabrous or rarely with scattered minute hairs to 0.05 mm long, abaxial surface glandular–verrucose and glabrous or rarely with scattered minute hairs up to 0.05 mm long. Flowers 5–7 (rarely 1–4), yellow, terminal inflorescences 15–22 mm long. Pedicel of central flower 2.8–3.7 mm long, with dense hairs 0.2–0.6 mm long. Bracts (7)10–14, pale yellow to yellow, usually tinged red post-pollination, elliptic to broadly obovate, 8.2–16.9 mm long, 5–10.3 mm wide; adaxial surface with scattered to moderately dense hairs 0.05–0.1 mm long; abaxial surface glandular–verrucose, glabrous or sometimes with scattered hairs 0.05–0.1 mm long. Bracteoles 8–12, usually paired below each flower except central flower, narrowly elliptic to obovate, 8.5–12.7 mm long, 2.5–6.7 mm wide, sometimes with an attenuate base, adaxial surface with scattered to moderately dense hairs 0.05–0.1 mm long; abaxial surface scattered to moderately dense hairs 0.05–0.3 mm long. Sepals pale yellow to yellow, elliptic to broadly elliptic, longer than petals, 9–12.3 mm long, 4.7–8 mm wide, glabrous or sometimes with hairs 0.05–0.1 mm long. Petals yellow, narrowly elliptic, cupped, coriaceous, 5.2–7.6 mm long, 2–3.7 mm wide, glabrous. Stamens 10; filaments 3–4.8 mm long, broadening at base up to 0.4–0.7 mm wide, glabrous; anthers oblong, 1.1–1.9 mm long, 0.3–0.7 mm wide. Carpels 5, free, with two ovules per carpel, total length 1.3–2 mm, total width 2–2.9 mm wide, verrucose, glabrous or with scattered to rarely moderately dense hairs up to 0.1 mm long. Style glabrous, 4–4.7 mm long, 0.4–0.5 mm wide; stigma obovoid, broader than style apex, 0.3–0.4 mm long, 0.4–0.5 mm wide. Fruit obovoid, 6–10 mm long, 8–9.8 mm wide. Seeds dark brown to black, 4.2–5.3 mm long, 2.7–3.5 mm wide; aril pale cream 2.3–3.3 mm long (Fig. 10).
Geleznowia calycina: (a) single-stemmed habit; (b) flowering heads showing the large pale yellow bracts subtending the inflorescence (some tinged red at the apex) with whorls of grey–green leaves below; (c) dense flowering head post-pollination, highlighting the red bracts and sepals; (d) open flower showing the broad pale yellow sepals with bracts below, bright yellow petals and obovoid stigma at the apex of the style; (e) mixed population of G. calycina (left) with a multiple-stemmed habit and clusters of flowers tinged red, compared with G. uberiflora (right) with single pale yellow terminal flowers. Vouchers: K.A. Shepherd & B.M. Anderson KS 1848 (a); K.A. Shepherd & C.F.Wilkins KS 1723 (b); K.A. Shepherd & J.A. Wege KS1300 (c); A.D. Crawford ADC 1344 (d); A.D. Crawford ADC 1840 (right) and ADC 1839 (left) (e). Photos: K. A. Shepherd (a–c, e); A. D. Crawford (d).

Widespread through the northern sandplains from west of Gillingarra to south of Shark Bay (Fig. 8) in the Avon Wheatbelt, Geraldton Sandplains, Swan Coastal Plain and Yalgoo bioregions (Department of Agriculture, Water and the Environment 2020). This species grows on flats, gentle slopes, or steeper lateritic slopes, in yellow, white or brown sand over limestone or with laterite. G. calycina is a disturbance opportunist and plants can be found growing in scrapes on the edge of gravel pits or road verges. Often found growing in low heath or shrubland associated with Allocasuarina, Acacia, Banksia, Hibbertia, Conospermum and Adenanthos.
Early flowering species with flowers at anthesis from May to early August, and fruits forming in late August to September. The yellow petaloid bracts usually become distinctly tinged with red post-pollination (Fig. 10c).
In April 1854, William Harvey met James Drummond in Perth and they discussed ‘several new and curious genera which he had recently discovered in the newly opened country 300 miles [~480 km] to the Northwards… One of the Rue family [Rutaceae] is a very curious & beautiful plant & quite a new type in the order’ (Harvey 1854a, p. 110). Drummond had briefly described this new species, without providing a name, as ‘a stiff upright-growing shrub, about 2 ft [~0.61 m] high; the flowers are borne in corymbs from nine inches to a foot in diameter; they are not very conspicuous, but are accompanied by numerous large bracts of a golden-yellow colour, which render this one of the most showy of our native plants; it appears on sand-plains to the east and west of the southern branch of the Hill River, and in other similar situations to the south of the Irwin River’ (Drummond 1853, p. 122). Harvey offered to describe this species by using the name Sanfordia calycina proposed by Drummond (Harvey 1854b, 1855); however, it is unclear what specimens he may have examined to refine his description, which was published while he was still overseas prior to his return to Trinity College in Dublin in 1856. Harvey may have examined material with Drummond. Alternatively, Harvey could have used material sent to Melbourne while visiting in 1854, because he received other specimens from Western Australia during that time (Harvey 1854c). Of the several available syntypes for this name, Wilson (2013) treated the Drummond 83 specimen at the Royal Botanic Gardens, Kew (K 000717327) as the ‘holotype’ and listed three sheets as ‘isotypes’ (MEL 232716; PERTH 00967556; TCD 0013332). The sheet at Kew (K 000717327) with a blue ‘83’ label has a ‘Herbarium Hookerianum 1867’ stamp and a note ‘between Moore and Murchison Rivers, W. Australia, J. Drummond 1853’, suggesting that it was received at Kew in 1853 before Harvey met Drummond, so he is unlikely to have seen this material. Similarly, the PERTH 00967556 specimen that was previously lodged at BM is dated 1854, again presumably when it was received there, and is also unlikely to have been seen by Harvey. Therefore, the mounted specimen on the left-hand side of the MEL 232716 sheet with a Drummond ‘83’ tag attached and a corresponding grey Mueller label on the right with ‘Geleznowia verrucosa Turcz. var calycina, W.A. J.Dr. 83’ in his handwriting is selected here as the lectotype of G. calycina, whereas the packet of fragments on the right is isolectotype material of G. verrucosa (see typification under that name). The specimen of G. calycina at the Trinity College Herbarium (TCD 0013332) was collected by Drummond and is not numbered, and the locality is given only as the Swan River Colony. The specimen was not annotated by Harvey, and the name under Geleznowia verrucosa is ‘Sanfordia floribunda JDr’ not S. calycina as Drummond had proposed, so it is unclear whether this material was used by Harvey to form the protologue and, subsequently, lodged at TCD. Paul Wilson treated this specimen as a ‘probable isotype’ (in sched. 30 June 1993); however, this cannot be confirmed, so it is treated as a possible isolectotype here. A specimen at the Muséum National d’Histoire Naturelle (P06615735 image!), with a blue Mueller label and identified in his hand as Geleznowia verrucosa var. calycina, is another J. Drummond collection but lacks a collection number, date or locality, other than ‘W.A.’. This specimen along with several other Drummond collections at MEL without collection dates or locality information are therefore excluded from consideration as type material of Sanfordia calycina.
Of the available syntypes for Geleznowia macrocarpa, Wilson treated the Kew specimen from Hooker’s Herbarium (K 000717328) as the ‘holotype’. This specimen is here designated as the lectotype for Geleznowia macrocarpa. This sheet is an Oldfield collection from the Murchison River with a ‘Herbarium Hookerianum 1867’ stamp and was therefore available to Bentham. Similarly, the MEL 232723 specimen is an Oldfield collection from the same locality and is initialled with Bentham’s characteristic ‘B’, so it was also seen by him. The fragment on the MEL 232721 specimen also has a note in Bentham’s hand, indicating that the material is from the specimen sent to Hooker and as such is treated here as an isolectotype, even though there is no information to suggest it was an Oldfield collection. Overall, the quality of the type material is very poor, consisting of bare branchlets with a few loose leaves and flowers with the subtending bracts having fallen off, thus the total number of flowers or bracts within each inflorescence cannot be confirmed. Distinguishing features mentioned by Bentham (1863) in his protologue include narrow sepals and the ‘[c]occi (not yet fully ripe) more than twice as long as broad’. There are a few specimens at PERTH that have sepals narrower than typical (3.2–4.2 mm wide cf. 4.5–8.5(9.6) mm wide) and an ovary that is longer than broad (e.g. C.A. Gardner 7744 PERTH 0968617), corresponding to the flowers observed on the type. These specimens are intact and are currently identified as G. calycina because of the size and number of flowers and bracts within each inflorescence. Therefore, G. macrocarpa is currently retained herein as a synonym of G. calycina. It should be noted that within this ‘narrow sepal’ group there are a few specimens that have quite dense hairs and two specimens where the fruits also have simple hairs and unusual finger-like protrusions extending from the verrucose glands at the apex (e.g. A.M. Ashby 2172 PERTH 00969206; R. Bates 4052 PERTH 04274695).
This species can be distinguished from other Geleznowia by the following character combination: a low subshrub 0.3–0.75 mm high with dark green leaves; usually with 5–7 (sometimes 1–4) flowers per inflorescence, surrounded by (7)10–14 pale yellow to yellow petaloid bracts (often tinged red post-pollination), 8.2–16.9 mm long, 5–10.3 mm wide, abaxial surface glabrous or sometimes with scattered hairs 0.05–0.1 mm long; 8–12 bracteoles; sepals 9–12.3 mm long, 4.7–8 mm wide; and a broad stigma 0.4–0.5 mm wide. Geleznowia calycina most closely resembles G. uberiflora but can be distinguished from it by its maximum flower number of 5–7 flowers per inflorescence (cf. usually 1–3 flowers), with more bracts ((7)10–14 cf. (5)6–8) and more bracteoles (8–12 cf. 0–6). G. calycina is also an early flowering species with flowers appearing as early as May and fruits forming in late August to September (cf. peak flowering in August–September in G. uberiflora). Moreover, the bracts and sepals in G. calycina usually turn red post-pollination (Fig. 10c), which readily distinguishes this species when it is found growing with the later-flowering G. uberiflora (Fig. 10e).
Geleznowia calycina is also somewhat morphologically similar to G. narcissoides but is readily distinguished by its fewer flowers, with 5–7 flowers per inflorescence (cf. (5)7–10 flowers), greenish-yellow to yellow petaloid bracts (cf. pale lemon yellow), and bracts, bracteoles and sepals being glabrous or with an indumentum of scattered to moderately dense hairs up to 0.1 mm long (cf. an indumentum of moderately dense to dense hairs up to 1.2 mm long). G. calycina also flowers earlier than G. narcissoides, often showing the distinctive red blush to bracts post-pollination when co-occurring with G. calycina when its flowers are in bud or reaching anthesis in spring.
The type nominated by Broadhurst for her unpublished G. verrucosa subsp. formosa ms R.V. Smith 66/370 (PERTH 0967580) (in sched.) falls within Wilson’s (2013) broad concept of G. verrucosa, but examination of this sheet confirms that it is a match for G. calycina. Other specimens determined by Broadhurst as G. verrucosa subsp. formosa ms represent various taxa, for example L. Broadhurst 11 (PERTH 05645298) is G. narcissoides, whereas L. Broadhurst 3 (PERTH 05496624) and L. Broadhurst 18 (PERTH 05599016) are G. amabilis.
WESTERN AUSTRALIA. 93 miles [~150 km] N of Geraldton on North West Coastal Highway, [60 km N of Binnu], 30 July 1967, A.M. Ashby 2172 (PERTH 00969206); Kalbarri National Park, 30 Aug. 1984, R. Bates 4052 (PERTH 04274695); 1.4 km from turnoff along Hawks Head Road, 31 May 1996, L. Broadhurst 13 (PERTH 05496667); corner West Binnu Road and turnoff to Hutt River Province, 18 Oct. 1996, L. Broadhurst 17 (PERTH 05599032); Yerina Springs Road, 8.2 km S of West Ogilvie Road, 3 Oct. 2004, A. Crawford ADC 590 (PERTH 07118279); eastern verge of the Midlands Road ~4.6 km N of the Buntine–Marchagee Road (N of Marchagee), 7 Sep. 2007, A. Crawford ADC 1344 (PERTH 07752318); 2.2 km E of the turnoff to the Loop and Z Bend gorges, Kalbarri NP on Kalbarri–Ajana Road, E of Kalbarri, 3 Oct. 2010, A. Crawford ADC 1386 (PERTH 07828748, NSW); Gravel Reserve E of White Gums Nature Reserve, 9 Sep. 2008, A. Crawford ADC 1839 (PERTH 08202095); Strawberry–Walkaway Road, 28.7 miles (~46 km) S of Walkaway, 1976, Hj. Eichler 22002 (CANB, NSW, PERTH 08280614); near Eradu, 9 Oct. 1945, C.A. Gardner 7744 (PERTH 00968617); Strawberry–Walkaway Road, ~46 km (~28.7 miles) S of Walkaway, 28 Sep. 1976, R.W. Johnson 3366 (AD, PERTH 00970808); 9.6 km W of the Hawkes Head Lookout Road on the Ajana–Kalbarri Road, E of Kalbarri, 1 Sep. 2021, K.A. Shepherd & B.M. Anderson KS 1848 (PERTH 09508120); 10.7 km from Natta Road on Tomkins Road, E of Brand Highway, 22 Sep. 2009, K.A. Shepherd & J. Wege KS 1290 (PERTH 08159882); 12.6 km N from the Port Gregory Road on Yerina Springs Road, SSE of Kalbarri, 23 Sep. 2009, K.A. Shepherd & J. Wege KS 1300 (PERTH 08159998); 5.8 km S of Eneabba Drive on the Brand Highway, E side of the road, 19 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1721 (AD, BRI, CANB, K, MEL, NSW, NY, PERTH 09508066); 100 m N on Wells Road on the North West Coastal Highway on W side of road, N of Geraldton, 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1722 (PERTH 09508074); 8 km N on Yerina Springs Road from Port Gregory Road, N of Northampton, 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1723 (BM, CANB, MEL, NSW, P, PERTH 09508082); 100 m S of Ogilvie West Road on Yerina Spring Road, NW of Northampton, 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1726 (CANB, DNA, HO, MEL, MSB, NSW, PERTH 09508104); 3.2 km W on Binnu West Road from Yerina Spring Road, N of Northampton, 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1729 (CANB, MEL, NSW, PERTH 09508090); 9.6 km W of the Hawkes Head Lookout Road on the Ajana–Kalbarri Road, E of Kalbarri, 22 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1739 (AD, BM, CANB, MEL, NSW, PERTH 09508112).
Geleznowia eximia K.A.Sheph. & A.D.Crawford, sp. nov.
Type: Western Australia, Brand Highway [North of Geraldton] [precise locality withheld for conservation reasons], 10 Sep. 2008, A. Crawford ADC 1844 (holo: PERTH 08298858).
Erect single-stemmed shrub up to 0.6–0.75 m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of moderately dense, simple hairs to 0.2–0.25 mm long. Leaves silvery grey–green to green, elliptic to obovate, 5.3–8.5 mm long, 2.8–6 mm wide, adaxial surface slightly concave and glabrous or minutely scabrous with scattered hairs <0.05 mm long, abaxial surface glandular–verrucose, glabrous or minutely scabrous with scattered hairs <0.05 mm long. Flowers 5–7, vivid golden-yellow, terminal inflorescences 16–25 mm long. Pedicel central flower up to 4.5 mm long, with dense hairs up to 0.5 mm long. Bracts 6–7, vivid yellow, obovate to broadly obovate sometimes with an attenuate base, 10.2–16 mm long, 6.4–10 mm wide; adaxial surface glabrous or with scattered hairs up to 0.05–0.2 mm long; abaxial surface faintly glandular–verrucose, glabrous or with scattered hairs 0.05 mm long. Bracteoles 10 usually paired below each flower except central flower, elliptic to obovate, 3.5–10.2 mm long, 4–5.2 mm wide, adaxial surface glabrous or with scattered to moderately dense hairs 0.05–0.3 mm long; abaxial surface glabrous or with scattered hairs up to 0.1 mm long. Sepals yellow, oblong to broadly obovate, longer than petals, 10.6–12.2 mm long, 6–7.6 mm wide, glabrous or with scattered hairs up to 0.1 mm long. Petals deep yellow, elliptic, cupped, coriaceous, 6.6–7 mm long, 2.3–3 mm wide, glabrous. Stamens 10; filaments 3–3.4 mm long, broadening at base up to 0.4–0.6 mm wide, glabrous; anthers oblong, 1.5–1.8 mm long, 0.6–0.8 mm wide. Carpels 5, free, with 2 ovules per carpel, total length 2.6 mm, total width 2.8 mm, verrucose, glabrous or with scattered hairs up to 0.05 mm long. Style glabrous, 3.3–5.3 mm long, 0.3 mm wide; stigma obovoid, 0.3 mm long, 0.4–0.5 mm wide. Fruit obovoid, 8 mm long, 9.5 mm wide. Seeds dark brown to black, 3.8–5.0 mm long, 2.4–3.3 mm wide, aril pale cream 2.1–2.9 mm long (Fig. 1e, 11).
Geleznowia eximia: (a) habitat; (b) habit; (c) flowering stem showing yellowish-green stems and whorls of green leaves; (d) dense flowering heads with up to six flowers; (e) open flower showing broad petals and sepals and style with a distinct obovoid stigma at the apex. Vouchers: K.A. Shepherd & B.M. Anderson KS 1832 and KS 1833 (PERTH). Photos: K. A. Shepherd.
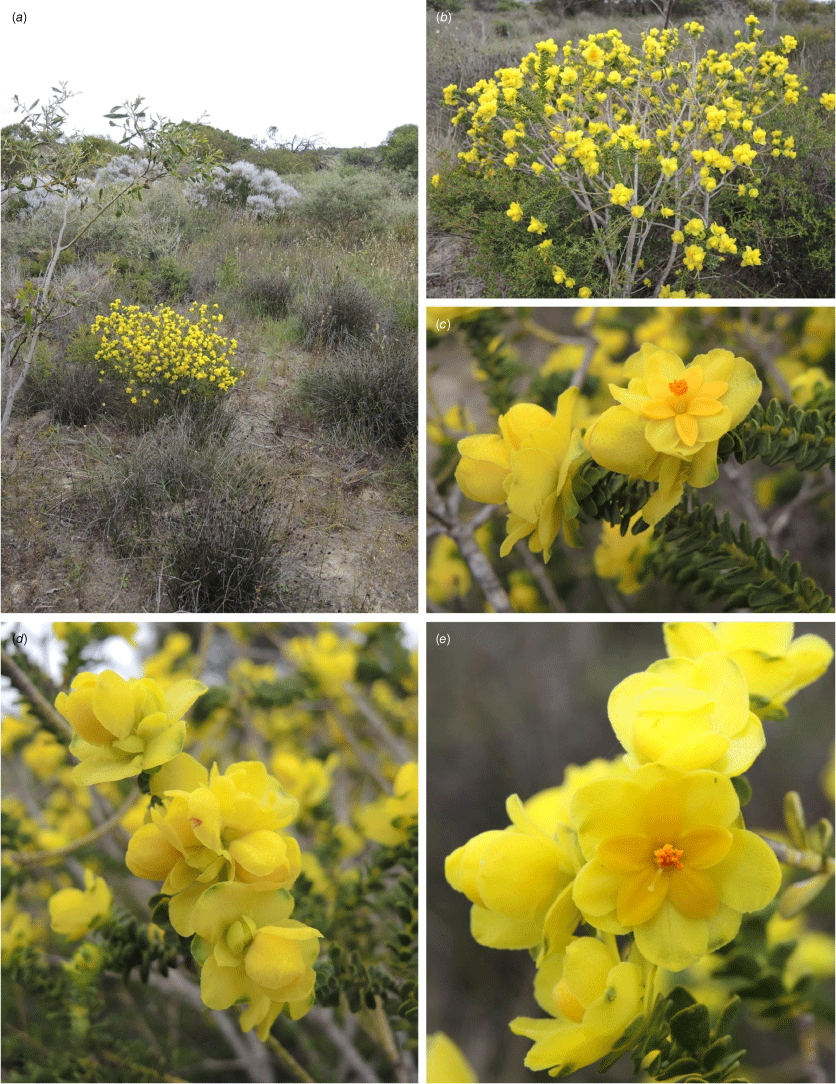
Known only from one location (Fig. 8). Found growing in deep yellow sand in low shrubland with Grevillea, Acacia, Hibbertia and Conospermum.
Flowering specimens collected in September and October, with fruits forming in late October.
This species is currently known only from a single population with two extant plants. As such, it is likely to meet criteria for listing as Threatened (Critically Endangered); however, further survey is urgently needed to confirm this. It is to be listed as Priority One under Conservation Codes for Western Australian Flora (T. Llorens, pers. comm.).
From the Latin eximius, meaning exceptional or uncommon, in reference to the attractiveness and rarity of this new species.
Geleznowia eximia can be recognised within the genus by the following combination of characters: a shrub 0.6–0.75 m high with silvery grey–green to green leaves; 5–7 flowers per inflorescence, surrounded by 6 or 7 vivid yellow petaloid bracts, 10.2–16 mm long, 6.4–10 mm wide, abaxial surface glabrous or with scattered hairs 0.05 mm long; 10 bracteoles; sepals 10.6–12.2 mm long, 6–7.6 mm wide; and a broad stigma 0.4–0.5 mm wide. For comparison, see notes under G. amabilis.
WESTERN AUSTRALIA. [localities withheld for conservation reasons] 23 Aug. 1997, P.G. Armstrong s.n. (PERTH 05982073); 3 Oct. 2004, A. Crawford ADC 588 (PERTH 07118252); 28 Aug. 2020, D. Growns DGRO 162 (MEL, PERTH 09508724); 21 July 2004, M. Harding 2 (PERTH 06947956); 31 Aug. 2021, K.A. Shepherd & B.M. Anderson KS 1832 (PERTH 09507949); 31 Aug.2021, K.A. Shepherd & B.M. Anderson KS 1833 (PERTH 09507930).
Geleznowia narcissoides K.A.Sheph. & A.D.Crawford, sp. nov.
Type: Western Australia, south–south-east of Kalbarri [precise locality withheld for conservation reasons], 23 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1301 (holo: PERTH 08151970; iso: BM).
Geleznowia sp. Binnu (K.A. Shepherd & J. Wege KS 1301), Western Australian Herbarium, in Florabase, https://florabase.dbca.wa.gov.au [accessed 10 May 2022].
Erect single-stemmed subshrub to shrub 0.4–1.2 m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of scattered to moderately dense, simple hairs 0.05–0.3 mm long. Leaves dull green, elliptic to obovate, 7.5–10 mm long, 4–6.8 wide, adaxial surface slightly concave and glabrous or with a few simple hairs 0.05–0.1 mm long, abaxial surface glandular–verrucose, glabrous. Flowers (5)7–10, terminal inflorescences 12–23 mm long. Pedicel of central flower 5–6 mm long, with dense to tomentose hairs 1.2–2.5 mm long. Bracts 7–11, pale lemon yellow, sometimes tinged with red post-pollination, broadly obovate with an attenuate base or spathulate, 7.2–22 mm long, 4.6–11.5 mm wide, adaxial surface with moderately dense to dense hairs 0.2–1.2 mm long, abaxial surface glandular–verrucose with moderately dense to dense hairs 0.2–1.2 mm long. Bracteoles 9–18, usually paired below each flower except central flower, narrowly obovate to spathulate, 8.8–17 mm long, 2.8–8 mm wide, adaxial surface moderately dense hairs 0.2–0.3 mm long, abaxial surface with moderately dense hairs 0.2–0.5 mm long. Sepals pale lemon yellow, elliptic to obovate, longer than petals, 8.5–15 mm long, 4.1–7 mm wide, adaxial surface with dense hairs 0.1–0.6 mm long and up to 1.2 mm long towards the base and margin, abaxial surface with scattered to dense hairs 0.05–0.5 mm long. Petals yellow, elliptic, cupped, coriaceous, 4–8.5 mm long, 1.8–3.5 mm wide, glabrous. Stamens 10; filaments 2.5–4.1 mm long, broadening at base to 0.3–0.5 mm wide, glabrous; anthers oblong, 1–1.5 mm long, 0.6–0.9 mm wide. Carpels 5, free, with 2 ovules per carpel, total length 1.6–7 mm, total width 1.8–7.8 mm, verrucose, glabrous or sometimes with scattered hairs 0.1 mm long. Style glabrous, 3.7–5.5 mm long; stigma obovoid, 0.2–0.3 mm long, 0.4–0.5 mm wide. Fruit obovoid, 5–7 mm long, 7–12 mm wide. Seeds dark brown to black, 4.3–5.2 mm long, 2.6–3.4 mm wide; aril pale cream 2.3–3.0 mm long (Fig. 1c, 12).
Geleznowia narcissoides: (a) single-stemmed habit; (b) inflorescence with eight young buds surrounded by pale lemon-yellow bracts; (c) flowering branchlet highlighting the obovate, sessile, dark green leaves below the large inflorescence subtended by a number of broad bracts and sepals; (d) open flower with a long style and obovoid stigma surrounded by large sepals covered in dense hairs 0.1–0.6 mm long; (e) G. narcissoides (left) co-occurring with G. calycina (right), which has smaller and few flowers and bracts that become strongly flushed in red post-pollination. Vouchers: A.D. Crawford ADC 1846 (a); A.D. Crawford ADC 1381 (b); K.A. Shepherd & C.F. Wilkins KS 1727 (c); K.A. Shepherd & C.F. Wilkins KS 1728 (d, e left); K.A. Shepherd & C.F. Wilkins KS 1729 (e right). Photos: A. D. Crawford (a, b); K. A. Shepherd (c–e).
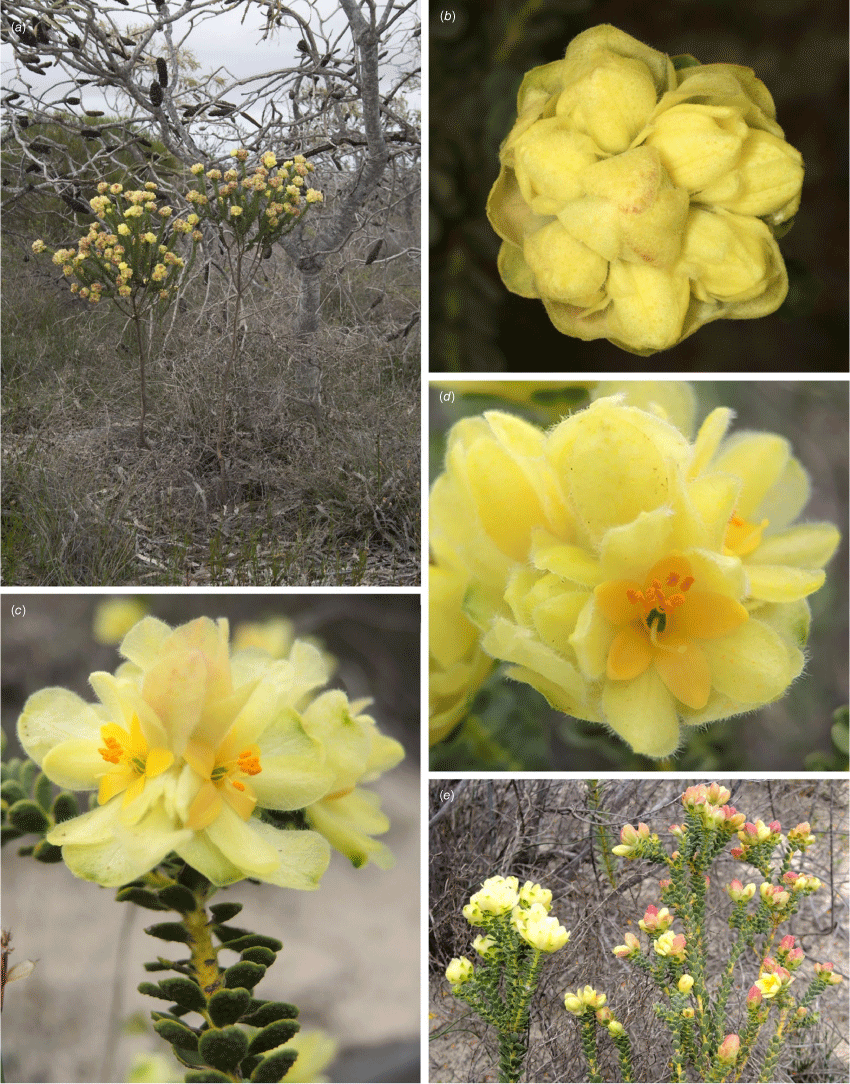
This species is known from a few widespread populations from north of Geraldton to north of Kalbarri (Fig. 8) in the Geraldton Sandplains bioregion (Department of Agriculture, Water and the Environment 2020). However, recent field work has failed to relocate this species in some areas where it has been previously collected, and it is unclear how many populations are currently persisting. Found growing on gentle slopes or flats in white–grey or yellow sand or brown sand over laterite in Banksia woodlands with Grevillea, Hibbertia, and small-flowered myrtaceous species or in low heath with Acacia, Banksia attenuata, Allocasuarina, Grevillea, Calothamnus, Calytrix and Stirlingia.
Flowering commences in August and continues through to September. Fruiting from October to November. The bracts in G. narcissoides may become tinged with red post-pollination.
This range-restricted species is known from only a few populations outside the conservation estate. It is listed as Priority Three under Conservation Codes for Western Australian Flora as G. sp. Binnu (K.A. Shepherd & J. Wege KS 1301).
From Narcissus L. and the Latin -oides (like), alluding to the showy flowers of this species being reminiscent of a double-headed daffodil.
Differs from morphologically similar species in Geleznowia by the following features: subshrub to shrub 0.4–1.2 m high with dull green leaves; (5)7–10 flowers per inflorescence, surrounded by 7–11 pale lemon-yellow bracts 7.2–22 mm long, 4.6–11.5 mm wide, abaxial surface with moderately dense to dense hairs 0.2–1.2 mm long; 9–18 bracteoles; sepals 8.5–15 mm long, 4.1–7 mm wide; and a broad stigma 0.4–0.5 mm wide. For comparison, see notes under G. calycina.
WESTERN AUSTRALIA. [localities withheld for conservation reasons] 27 June 1995, L. Broadhurst 11 (Curtin University Herbarium, PERTH 05645298); 30 Aug. 1965, A.C. Burns 31 (PERTH 00967017); 28 Nov. 2001, A. Crawford ADC 108 (PERTH 06118771); 3 Oct. 2004, A. Crawford ADC 593 (PERTH 07118287); 2 Oct. 2007, A. Crawford ADC 1381 (AD, PERTH 07828691); 11 Sep. 2008, A. Crawford ADC 1846/1 (PERTH 08201242); 11 Sep. 2008, A. Crawford ADC 1848/1 (PERTH 08201145); 6 Sep. 1997, G. Flowers & S. Donaldson GF 202 (CBG, PERTH 05920485); 14 July 2004, M. Harding s.n. (PERTH 06949851); 23 July 2004, M. Harding 11 (PERTH 06947999); 23 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1302 (AD, PERTH 08151989); 31 Aug. 2021, K.A. Shepherd & B.M Anderson KS 1837 (PERTH 09514716); 31 Aug. 2021, K.A. Shepherd & B.M Anderson KS 1838 (PERTH 09514724); 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1727 (AD, BRI, CANB, DNA, K, MEL, MSB, NSW, NY, PERTH 09508023); 20 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1728 (CANB, MEL, NSW, P, PERTH 09508031); 16 Aug. 2016, R. Simkin RSM 02 (PERTH 09236880); 26 Aug. 2016, R. Simkin RS 1621(PERTH 09236872).
Geleznowia occulta K.A.Sheph. & A.D.Crawford, sp. nov.
Type: Western Australia, south-west of Coorow [precise locality withheld for conservation reasons], 23 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1743 (holo: PERTH 09507787; iso: AD, CANB, K, MEL, NSW).
Erect, single-stemmed subshrub up to 0.2–0.4 m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of scattered to moderately dense, simple hairs up to 0.05–0.1 mm long. Leaves glaucous green or pale green, elliptic, 2.7–5.3 mm long, 1.1–2.8 mm wide, adaxial surface slightly concave and glabrous or rarely with scattered hairs up to 0.05 mm long, abaxial surface glandular–verrucose, glabrous or with scattered hairs to 0.05 mm long. Flowers 1–3(5), yellow, terminal inflorescences 5.2–10 mm long. Pedicel of central flower 1.9–2.7 mm long, with dense hairs 0.1–0.3 mm long. Bracts 0–3(4), yellow, sometimes tinged red post-pollination, elliptic, 3.7–6.3 mm long, 1.8–2.9 mm wide; adaxial surface glabrous or with scattered hairs up to 0.05 mm long; abaxial surface glandular–verrucose, glabrous or with scattered hairs 0.05 mm long. Bracteoles 0–2(4) usually paired below each flower except central flower, narrowly obovate, 5.2–7.2 mm long, 2–2.6 mm wide, both surfaces glabrous or with scattered hairs up to 0.05 mm long. Sepals yellow, oblong to elliptic, longer than petals, 6.3–6.9(7.3) mm long, 2.5–3.1 mm wide, glabrous or with scattered hairs up to 0.05 mm long. Petals yellow, cupped, coriaceous, narrowly elliptic, 4.2–5.9 mm long, 1.7–2.8 mm wide, glabrous. Stamens 10; filaments 1.2–3.2 mm long, broadening at base up to 0.3–0.5 mm wide, glabrous; anthers oblong, 1.1–1.6 mm long, 0.5–0.8 mm wide. Carpels 5, free, with two ovules per carpel, 1.5–1.6 mm long, 1.6–2.4 mm wide, verrucose, glabrous or with scattered hairs up to 0.05 mm long. Style glabrous, 1.7–3.5 mm long, 0.2–0.3 mm wide; stigma obovoid, 0.2–0.4 mm long, 0.5 mm wide. Fruit obovoid, 5.5–7 mm long, 5.2–8.5 mm wide. Seeds dark brown to black, 3.3–4.7 mm long, 2.2–3.1 mm wide; aril pale cream 2.0–2.8 mm long (Fig. 13).
Geleznowia occulta: (a) habitat; (b) habit; (c) flowering stems with young buds showing solitary flowers with few bracts; (d) single flower showing petals as long as sepals and obovoid stigma; (e) old fruit. Vouchers: K.A. Shepherd & C.F. Wilkins KS 1744 (a, d); K.A. Shepherd & B.M. Anderson KS 1853 (b); K.A. Shepherd & C.F. Wilkins KS 1743(PERTH) (c, e). Photos: K. A. Shepherd.
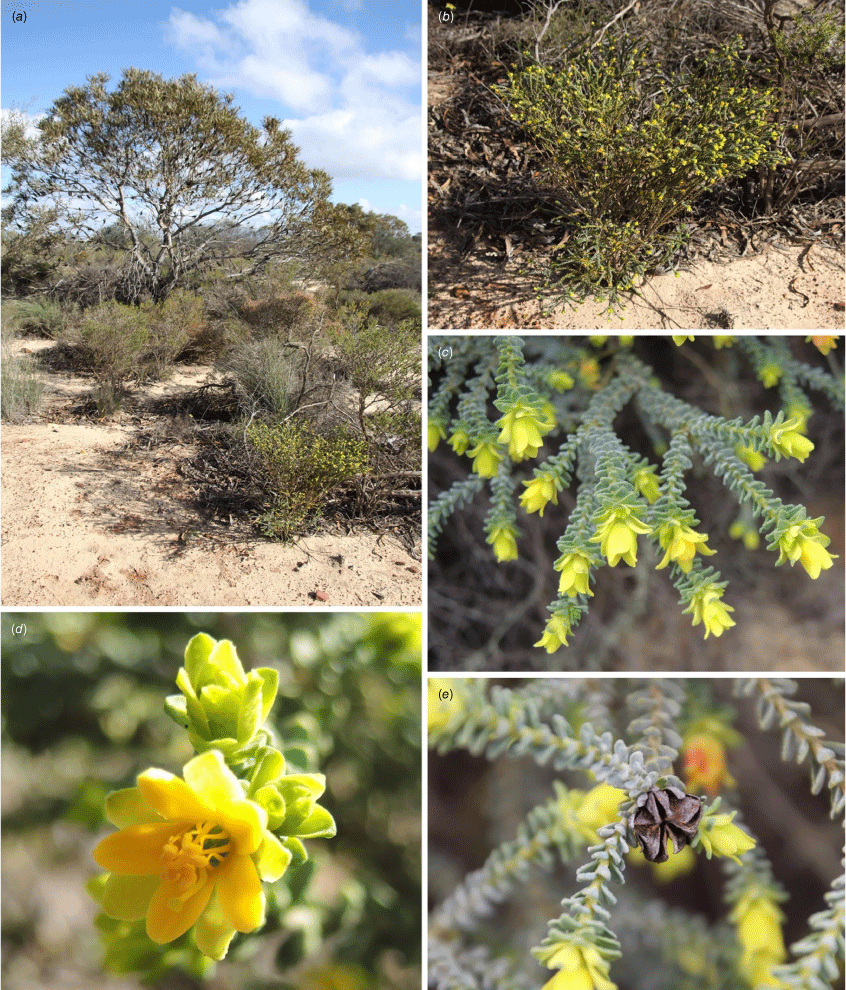
This species is currently known from only two populations west of Coorow and east of Goomalling (Fig. 8) in the Geraldton Sandplains and Avon Wheatbelt bioregions (Department of Agriculture, Water and the Environment 2020). Found growing in deep yellow sand, sometimes in disturbed areas such as firebreaks, in low Banksia woodlands associated with Xylomelum, Grevillea and myrtaceous shrubs.
Flowering in September and October with fruiting specimens observed in November and December.
This species is currently known from only two populations, one of which is on private property. The second population, although occurring in a nature reserve, has been observed only in low numbers. It is to be listed as Priority Two under Conservation Codes for Western Australian Flora (T. Llorens, pers. comm.).
From the Latin occultus (hidden, concealed) in reference to the cryptic nature of this species, because it was initially recognised as distinct through this molecular study, and that it is currently known from only two localities.
Geleznowia occulta can be distinguished from related Geleznowia by the following characters: a small subshrub 0.15–0.2 m high with glaucous green or pale green leaves; 1–3(5) flowers per inflorescence, surrounded by 0–3(4) yellow bracts, 3.7–6.3 mm long, 1.8–2.9 mm wide, abaxial surface glabrous or with scattered hairs 0.05 mm long; 0–2(4) bracteoles; sepals 6.3–6.9(7.3) mm long, 2.5–3.1 mm wide; and a broad stigma 0.5 mm wide. This species is very morphologically similar to the more widespread G. verrucosa, but can be recognised as distinct from it by its shorter inflorescences 5.2–10 mm long (cf. 12–15 mm long) usually with few flowers 1–3(5) per inflorescence (cf. 1–6 flowers per inflorescence), fewer bracts (0–3(4) cf. (4)5–7) that are narrower (1.8–2.9 mm wide cf. 2.4–5.5 mm wide) and fewer bracteoles (0–2(4) cf. 0–8).
WESTERN AUSTRALIA. [localities withheld for conservation reasons] 8 Jan. 1999, L. Broadhurst 14 (PERTH 05547822); 27 Nov. 1990, S. Patrick 505 (PERTH 01165348); 4 Dec. 2000, J. Schmidberger JS 038 (PERTH 05814855); 2 Sep. 2021, K.A. Shepherd & B.M. Anderson KS 1853 (PERTH 9507760); 25 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1314 (PERTH 08152136); 23 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1744 (BM, BRI, CANB, HO, MEL, NSW, PERTH 09507779).
Geleznowia uberiflora K.A.Sheph. & A.D.Crawford, sp. nov.
Type: Western Australia, 1.3 km W of Murchison House Access Road on the Ajana–Kalbarri Road, E of Kalbarri, 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1734 (holo: PERTH 09507914; iso: CANB, K, MEL, NSW, NY, P).
Erect single- or multiple-stemmed subshrub or shrub 0.25–0.8 m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of scattered to moderately dense, simple hairs up to 0.1 mm long. Leaves glaucous green to dull green, elliptic, 3–9 mm long, 2–5.8 mm wide, adaxial surface slightly concave and glabrous or rarely with scattered hairs 0.05 mm long, abaxial surface glandular–verrucose, glabrous. Flowers 1–3 (rarely 4–5), yellow, terminal inflorescences 12–19 mm long. Pedicel of central flower (2)3–4 mm long with dense hairs 0.2–0.5 mm long. Bracts (5)6–8, pale greenish-yellow to yellow, rarely tinged red at the apex post-pollination, elliptic to broadly obovate, 6.8–14 mm long, 4–10 mm wide, adaxial surface glabrous or with scattered hairs up to 0.05 mm long; abaxial surface glandular–verrucose, glabrous. Bracteoles 0–6, usually paired below each flower except central flower, narrowly elliptic to narrowly obovate, 5.1–10.1 mm long, 2.4–4.2 mm wide, adaxial surface glabrous or with scattered hairs up to 0.05 mm long; abaxial surface glabrous or with scattered hairs up to 0.05 mm long. Sepals elliptic, 9–12.8 mm long, (4)5–8.3 mm wide, adaxial surface glabrous or with scattered hairs up to 0.05 mm long; abaxial surface faintly glandular–verrucose, glabrous or with scattered hairs up to 0.05 mm long. Petals yellow, narrowly elliptic to elliptic, cupped, coriaceous, 5–8.8 mm long, 2.2–3.5 mm wide, glabrous or with a few scattered hairs up to 0.05 mm long. Stamens 10; filaments 3.1–5.5 mm long, 0.3–0.5 mm wide at the base, glabrous; anthers oblong, 1.3–1.7 mm long, 0.4–0.7 mm wide. Carpels 5, free, with two ovules per carpel, total length 3–5 mm, total width 3.2–5.8 mm; verrucose, glabrous or with hairs 0.1–0.25 mm long. Style glabrous, 3.5–4.5 mm long, 0.2–0.4 mm wide; stigma obovoid, broader than style apex, 0.2–0.3 mm long, 0.5–0.6 mm wide. Fruit obovoid, 6–7.3 mm long, 7–10.5 mm wide. Seeds dark brown to black 4.6–5.2 mm long, 2.7–3.3 mm wide; aril pale cream 1.6–3.2 mm long (Fig. 1b, 14).
Geleznowia uberiflora: (a) habitat; (b) habit, highlighting the prolific flowers observed in some plants; (c) solitary flower before anthesis, subtended by a few pale yellow bracts tinged with green at the apex; (d) side view of flowers showing the ovate green leaves and the young yellowish-green branchlet (red arrow); (e) flower beginning to open, showing the large obovoid stigma (red arrow); (f) open flower showing the large pale yellow sepals hiding the bracts below, and the bright yellow elliptic petals. Vouchers: A.D. Crawford ADC 1839 (b, c); K.A. Shepherd & B.M. Anderson KS 1828 (d, f); K.A. Shepherd & B.M. Anderson KS 1824 (e). Photos: A. D. Crawford (b, c); K. A. Shepherd (a, d–f).
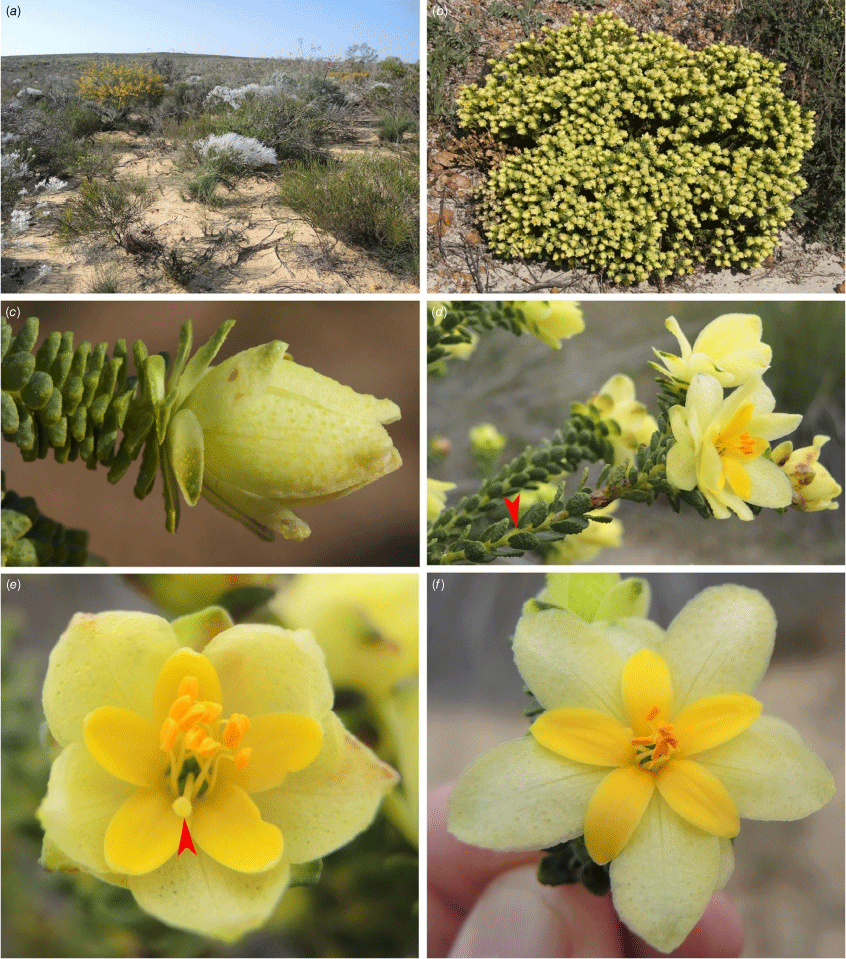
This species is reasonably widespread through the northern sandplains from south-east of Goomalling and west of Dalwallinu to the Kalbarri region (Fig. 8) in the Avon Wheatbelt, Swan Coastal Plain, and Geraldton Sandplains bioregions (Department of Agriculture, Water and the Environment 2020). Found on flat sandplains or gentle slopes growing in yellow, brown or white sand sometimes with laterite. This species also responds to disturbance because young plants can be found on graded road verges and in recently burnt habitats. Occurring in open mallee, woodland, shrublands or dense low heath, associated with Allocasuarina, Banksia, Acacia, Callitris, Conospermum, Grevillea, Synaphea and Verticordia.
Peak flowering from August to September with fruits beginning to form in late September through to October.
This species is widespread and represented within the conservation estate and therefore is not currently considered to be under threat.
From the Latin uber (fruitful, abundant) and -florus (-flowered), in reference to the numerous showy yellow flowers typical of this species.
Geleznowia uberiflora can be morphologically delineated from other members of the genus by virtue of the following combination of characters: subshrub or shrub 0.25–0.8 m high with glaucous green to dull green leaves; 1–3 (rarely 4 or 5) flowers per inflorescence, surrounded by (5)6–8 pale greenish yellow to yellow bracts, 6.8–14 mm long, 4–10 mm wide, abaxial surface glabrous; sepals elliptic, 9–12.8 mm long, (4)5–8.3 mm wide; and a broad stigma 0.5–0.6 mm wide. For comparison, see notes under G. calycina.
One population from south of Eneabba (K.A. Shepherd & C.F. Wilkins KS 1712 (PERTH 09507817)) was detected as genomically divergent (Group P4) and is provisionally identified here as a hybrid G. uberiflora × occulta.
WESTERN AUSTRALIA. Gravel Reserve E of White Gums Nature Reserve, 2 Oct. 2004, A. Crawford ADC 580 (PERTH 07118244); Simpson Road 7.2 km E of the Morawa–Three Springs Road, 30 Sep. 2007, A. Crawford ADC 1357 (PERTH 07752180); Burma Road NR, 5.7 km E of the western firebreak along central track, E of Walkaway, 3 Oct. 2007, A. Crawford ADC 1391 (PERTH 07752202); Masons Road, 5.7 km N of Carot Well Road, NE of Watheroo, 12 Nov. 2007, A. Crawford 1564 (PERTH 07752326); Gravel Reserve E of White Gums Nature Reserve, 9 Sep. 2008, A. Crawford ADC 1839 (PERTH 08202095); E of White Gums Nature Reserve, ~0.7 km W of First North Road on the Eneabba–Three Springs Road (E of Eneabba), 9 Sep. 2008, A. Crawford ADC 1840 (PERTH 08201129); Nature Reserve, E of intersection of Nambling South Road and Hagboom West Road, 22 Nov. 2011, A. Crawford ADC 2110 (CANB 823253, PERTH 08397201); ~400 m E of creek, Depot Hill Reserve, ~10 km NW of Mingenew, 7 Sept. 2007, R. Davis 11223 (DNA D0190324, PERTH 07748744, PERTH 07748752); on N side of Nabbewa–Yetna Road, 3.2 km NE of Nanson–Howatharra Road, UCL, ~5 km SW of Nabawa, 30 Sep. 1998, N. Gibson 4333 (PERTH 07055501); 11.1 km E of Brand Highway on Coorow–Greenhead Road, SE of Eneabba, 30 Aug. 2021, K.A. Shepherd & B.M. Anderson KS 1824 (MEL, PERTH 09507892); White Gums Nature Reserve, 1 km E of Brimson Road on the Eneabba–Three Springs Road, E of Eneabba then 700 m along track through the gravel pit, 30 Aug. 2021, K.A. Shepherd & B.M. Anderson KS 1828 (NSW, PERTH 09507906); 11.1 km E of Brand Highway on Coorow–Greenhead Road, SE of Eneabba, 17 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1710 (CANB, MEL, NSW, PERTH 09507957); White Gums NR, 1 km E of Brimson Road on the Eneabba–Three Springs Road, E of Eneabba, 18 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1715 (CANB, MEL, NSW, P, PERTH 09507965); Wotto NR, near the corner of First North Road and the Eneabba–Three Springs Road, E of Eneabba, 18 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1716 (CANB, MEL, NSW, PERTH 09507973); 7.6 km E on Mt Adams Road from Brand Highway, S of Dongara, 18 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1719 (CANB, MEL, NSW, PERTH 09507981); 800 m E on Gorges Road from the Ajana–Kalbarri Road, E of Kalbarri, 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1738 (CANB, MEL, NSW, PERTH 09507922).
Geleznowia verrucosa Turcz., Bull. Soc. Imp. Naturalistes Moscou 22(3): 13, t. I. (1849)
Type citation: ‘Nova Hollandia. Swan River. Drum. Coll. III n. 8.’ Type: [Western Australia], Swan River, [1845], J. Drummond 3: 8 (holo: KW 001001059 image!; iso: BM 001015536 image!; CANB 386497 image!; K 000717329 image! [top and left fragments] image!; K 000717330 [bottom right fragments] image!; MEL 232716! [fragments in right-hand packet]; P06615731 image!; TCD 0013330 image!; TCD 0013331 image!; possible iso: MEL 232725!).
Geleznowia sp. Marchagee (A. Crawford ADC 1353), Western Australian Herbarium, in Florabase, https://florabase.dbca.wa.gov.au/ [accessed 10 May 2022].
Erect single-stemmed subshrub 0.2–0.7 m high; older branches light brown to light grey–brown and glabrous, younger branches yellowish-green with an indumentum of scattered to moderately dense, simple hairs up to 0.05–0.1 mm long. Leaves glaucous green or pale green, elliptic, 2.2–6.8 mm long, 1.3–3.4 mm wide, adaxial surface slightly concave and glabrous or with scattered to moderately dense, simple hairs up to 0.05–0.2 mm long, abaxial surface glandular–verrucose, glabrous or with scattered to moderately dense, simple hairs up to 0.05–0.2 mm long. Flowers 1–6, yellow, terminal inflorescences 12–15 mm long. Pedicel of central flower 2–3.3 mm long, with dense hairs 0.1–0.3 mm long. Bracts (4)5–7, yellow, sometimes tinged red post-pollination, elliptic to obovate, 4.1–8.6 mm long, 2.4–5.5 mm wide; adaxial surface glabrous or with scattered to moderately dense hairs up to 0.05 mm long; abaxial surface glandular–verrucose, glabrous or with scattered to moderately dense hairs 0.05 mm long. Bracteoles 0–8, usually paired below each flower except central flower, narrowly elliptic, 5–6.6 mm long, 1.7–3 mm wide, adaxial surface glabrous or with scattered hairs up to 0.05 mm long, abaxial surface glabrous or with scattered hairs up to 0.05 mm long. Sepals yellow, oblong to elliptic, equal to or longer than petals, 5–9.3 mm long, 3.2–5.2(5.7) mm wide, glabrous or with scattered to moderately dense, simple hairs up to 0.05–0.1 mm long. Petals yellow, narrowly elliptic, 4.6–7 mm long, 2.4–2.7 mm wide, glabrous. Stamens 10; filaments 3.7–4.1 mm long, broadening at base up to 0.4–0.8 mm wide, glabrous; anthers oblong, 1.1–1.7 mm long, 0.5–0.9 mm wide. Carpels 5, free, with two ovules per carpel, total length 1.5–2 mm, total width 2.2–2.5 mm, verrucose, glabrous or with scattered to moderately dense, simple hairs up to 0.05–0.1 mm long. Style glabrous, 4.2–5.4 mm long, 0.3–0.5 mm wide; stigma obovoid, 0.3–0.8 mm long, 0.6–1 mm wide. Fruit obovoid, 7–7.5 mm long, 8.3–9.2 mm wide. Seeds dark brown to black, 3.6–4.4 mm long, 2.2–3.1 mm wide; aril pale cream 1.9–2.7 mm long (Fig. 1d, 15).
Geleznowia verrucosa: (a) habitat; (b) single-stemmed habit; (c) multi-stemmed habit showing large numbers of flowers; (d) yellowish-green branchlet with small elliptic grey–green leaves and inflorescences with four or five flowers; (e) flower showing petals almost as long as the sepals and the obovoid stigma; (f) mature fruits surrounded by sepals tinged red. Vouchers: K.A. Shepherd & C.F. Wilkins KS 1736 (a); K.A. Shepherd & J.A. Wege KS 1312 (b); K.A. Shepherd & B.M. Anderson KS 1845 (c, e); A.D. Crawford ADC 1369 (d); K.A. Shepherd & J.A. Wege KS 1309 (f). Photos: K. A. Shepherd (a, e, f); A. D. Crawford (b, d); B. M. Anderson (c).
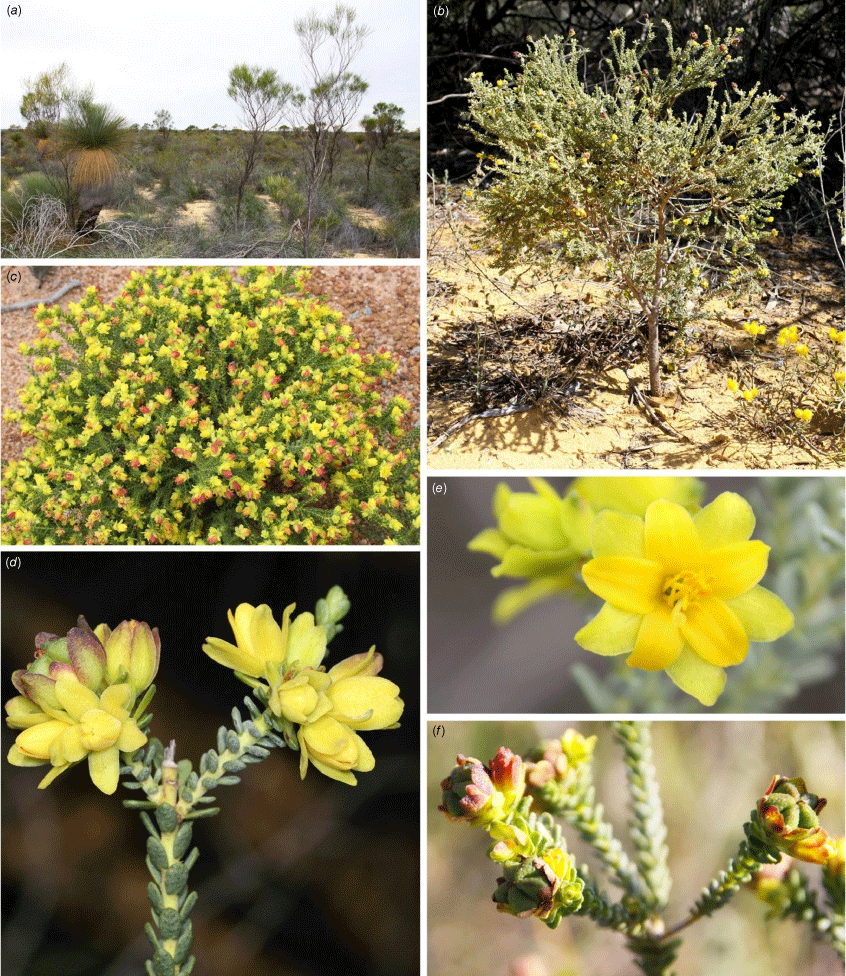
This widespread species occurs from east of Watheroo to Dirk Hartog Island (Fig. 8) in the Geraldton Sandplains and Yalgoo bioregions (Department of Agriculture, Water and the Environment 2020). Found growing in flat or gently sloping areas, sometimes in disturbed sites such as road verges or fire breaks, in white to brown or yellow sand, sometimes over laterite or sandstone, in low, dense heath or shrublands associated with Allocasuarina, Acacia, Banksia, Grevillea and Callitris.
Flowering commences in July and extends through to early spring with fruits forming in September to October.
The single specimen lodged at the M.G. Kholodny Institute of Botany, National Academy of Sciences of Ukraine (KW 001001059) was used by Turczaninow to write the protologue and is therefore recognised as the holotype (Mosyakin et al. 2019). The bottom two fragments on the specimen at the Natural History Museum London (BM 001015536) labelled ‘J. Drummond 3: 8’ (pencil marked ‘a’) represent isotype material, whereas the top three E. Pritzel 644 fragments (pencil marked ‘b’) do not. Five fragments mounted on the top and bottom left (K 000717329) of a sheet stamped ‘Herbarium Hookerianum 1867’ and two fragments (K 000717330) mounted on blue paper affixed to the bottom right and stamped ‘Herbarium Benthamianum 1845’ are both labelled as ‘Drummond n. 8’ collections and are recognised as isotypes. On the bottom right of the MEL 232716 sheet is a blue Mueller label with ‘G. verrucosa Turcz, W.A. J.Dr. 8’ in his handwriting, above are two ‘8’ tags and a packet of fragmented material mounted on the top right that is a match for the protologue of G. verrucosa. As such, this material is also treated as an isotype. The mounted specimen on the left-hand side of this MEL 232716 sheet has a Drummond ‘83’ tag and is an isolectotype of G. calycina (see typification section under that name). The MEL 232725 sheet has a blue Mueller label with ‘Geleznowia verrucosa Turcz. W.A. J.Dr.’ in his handwriting but a note in pencil in another hand at the bottom says ‘evidently, no 8’. Paul Wilson noted that this specimen was a ‘probable isotype’ (in sched., 21/9/1999); however, here it is treated as a possible isotype.
Geleznowia verrucosa is distinguished from other species in the genus by the following characters: subshrub 0.2–0.7 m high with glaucous green or pale green leaves; 1–6 flowers per inflorescence, surrounded by (4)5–7 yellow bracts, 4.1–8.6 mm long, 2.4–5.5 mm wide, abaxial surface glabrous or with scattered to moderately dense hairs 0.05 mm long; sepals 5–9.3 mm long, 3.2–5.2(5.7) mm wide; and a broad stigma 0.6–1 mm wide. For comparison, see notes under G. occulta.
WESTERN AUSTRALIA. Kalbarri NP, Ajana–Kalbarri Road, 20 km SE of the Z-bend turnoff, 5 Sep. 1990, D.E. Albrecht & B.A. Fuhrer DEA 4195 (MEL, PERTH 04204395); 24.1 km W of Kalbarri turnoff from North West Coastal Highway, 18 Oct. 1996, L. Broadhurst 8 (Curtin University Herbarium, PERTH 05599040); 3.1 km W of T-junction along Coorow–Green Head Road, 31 May 1996, L. Broadhurst 15 (PERTH 05496659); Indarra Springs Nature Reserve, Moore Road, 5.6 km S of Geraldton–Mullewa Road, SW of Mullewa, 1 Oct. 2007, A.D. Crawford ADC 1369 (PERTH 07828713); Binnu West Road, 1.8 km E of Telegraph Road, S side of road in drainage ditch, W of Binnu, 2 Oct. 2007, A. Crawford ADC 1378 (CANB, PERTH 07828721); alongside E side of track alongside railway line running through Marchagee NR, 29 Sep. 2007, A. Crawford ADC 1353 (AD, PERTH 07828616); Coorow–Green Head Road, 3.5 km E of Carger Road (SW of Coorow), 29 Sept. 2007, A. Crawford ADC 1355 (PERTH 07752261); Yuna–Tenindewa Road, 9.7 km W of Wheeldon–Hosking Road, 11 Sep. 2008, A. Crawford ADC 1855-2 (PERTH 08249849); 12 km E of Mullewa on the Geradlton Road, 18 Sep. 1982, L.A. Craven 7618 (CANB, PERTH 01881361); in fruticetis arenosis inter flumina Moor et Murchison, Sep. 1901, E.G. Pritzel 644 (BM 000099961 ‘b’ image!; P 06615732 image!); 16 miles [~26 km] SSE of Tamala Station Homestead, 5 Sep. 1972, A.S. George 11557 (BH, CANB, PERTH 00969656); Kalbarri NP, 5.5 km along road to the Gorges from the Kalbarri–Ajana Road, K.A. Shepherd & J. Wege KS 1308 (HO, K, MEL, PERTH 08152039); 5.4 km NE on Gorges Road from the Ajana–Kalbarri Road, E of Kalbarri, 1 Sep. 2021, K.A. Shepherd & B.M. Anderson KS 1845 (PERTH 09507884); Bindoo Hill Nature Reserve, 1.7 km W of Byron North Road on Williams Road, 24 Sep. 2009, K.A. Shepherd & J.A. Wege KS 1312 (PERTH 08152098; US); 5.8 km S of Eneabba Drive on the Brand Highway, E side of the road, 17 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1712 (CANB, MEL, NSW, PERTH 09507817); 5.4 km NE on Gorges Road from the Ajana–Kalbarri Road, E of Kalbarri, 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1736 (BRI, CANB, MEL, NSW, PERTH 09507833); 800 m E on Gorges Road from the Ajana–Kalbarri Road, E of Kalbarri, 21 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1737 (AD, CANB, MEL, NSW, PERTH 09507841); 41.4 km N of the Murchison River Galeana Bridge on the North West Coastal Highway, 22 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1741 (CANB, MEL, NSW, NY, PERTH 09507868); 5.6 km E on Williamson Road from the Yuna–Tenindewa Road, NW of Mullewa, 22 Aug. 2020, K.A. Shepherd & C.F. Wilkins KS 1742 (CANB, MEL, NSW, PERTH 09507876).
Data availability
Sequencing data produced from the ddRAD library is available on the Bioplatforms Australia, Genomics for Australian Plants website (https://data.bioplatforms.com/organization/bpa-plants) under Dataset 83666. Sequence data are also available at the NCBI Sequence Read Archive (SRA) under Project PRJNA947349. Scripts and steps used for filtering and analyses are available on a Github repository (https://github.com/bmichanderson/Geleznowia). Alignments for concatenation and SVDquartets analyses are available as Supplementary files on the journal website (https://www.publish.csiro.au/sb).
Declaration of funding
We acknowledge the contribution of the Genomics for Australian Plants Framework Initiative consortium (https://www.genomicsforaustralianplants.com/consortium/) in the generation of data used in this publication. The Initiative is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia. The funders did not participate in the analysis of data or preparation of the paper. The Department of Biodiversity, Conservation and Attractions, Western Australia approved the content of the paper and the decision to submit for publication.
Acknowledgements
We thank Bronwyn Macdonald and Nicola Delnevo for laboratory expertise and DNA extractions. Isaac Overcast provided support using ipyrad through online discussion (https://gitter.im/dereneaton/ipyrad). We also thank Mabel Lum, Lalita Simpson and the Genomics for Australian Plants team for project support. This work was supported by resources provided by the Pawsey Supercomputing Centre with funding from the Australian Government and the Government of Western Australia. Julie Plummer is acknowledged for introducing Andrew Crawford to Geleznowia many years ago, sowing the seed of interest that has culminated in this work. Carol Wilkins and Juliet Wege are acknowledged for their excellent company and support during field work and Wendy Thompson for early work on morphological analyses. Sergei Mosyakin and Natalia Shiyan at KW kindly supplied information on the 1849 Turczaninow publication and Nikolai Zheleznov. Juliet Wege, Terry Macfarlane and Brendan Lepschi provided typification advice, and Mike Bayly is thanked for early discussions about the generic status of Geleznowia relative to Philotheca. We sincerely thank the PERTH curation team for specimen support and Julia Percy-Bower for providing database information. We are also grateful for a loan of specimens from MEL.
References
Anderson BM, Thiele KR, Krauss SL, Barrett MD (2017) Genotyping-by-sequencing in a species complex of Australian hummock grasses (Triodia): methodological insights and phylogenetic resolution. PLoS One 12, e0171053.
| Crossref | Google Scholar |
Appelhans MS, Bayly MJ, Heslewood MM, Groppo M, Verboom GA, Forster PI, Kallunki JA, Duretto MF (2021) A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. TAXON 70, 1035-1061.
| Crossref | Google Scholar |
Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3, e3376.
| Crossref | Google Scholar |
Barker B (2005) Standardising informal names in Australian publications. Australian Systematic Botany Society Newsletter 122, 11-12.
| Google Scholar |
Bayly MJ, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2013) Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences. PLoS One 8, e72493.
| Crossref | Google Scholar |
Bentham G (1863) Geleznowia. Flora Australiensis 1, 347-348.
| Google Scholar |
Binks RM, Byrne M (2022) Species delimitation, hybridization and possible apomixis in a rapid radiation of Western Australian Leptospermum (Myrtaceae). Botanical Journal of the Linnean Society 200, 378-394.
| Crossref | Google Scholar |
Binks RM, Wilkins CF, Markey AS, Lyons MN, Byrne M (2020) Genomic data and morphological re‐assessment reveals synonymy and hybridisation among Seringia taxa (Lasiopetaleae, Malvaceae) in remote north‐western Australia. TAXON 69, 307-320.
| Crossref | Google Scholar |
Bresadola L, Link V, Buerkle CA, Lexer C, Wegmann D (2020) Estimating and accounting for genotyping errors in RAD‐seq experiments. Molecular Ecology Resources 20, 856-870.
| Crossref | Google Scholar |
Broadhurst L (2000) Morphometric analysis of variation in Geleznowia verrucosa Turcz. (Rutaceae). Australian Systematic Botany 13, 479-490.
| Crossref | Google Scholar |
Broadhurst LM, Tan BH (2001) Floral biology of the Western Australian endemic ‘yellow bells’, Geleznowia verrucosa Turcz. (Rutaceae). Journal of the Royal Society of Western Australia 84, 83-89.
| Google Scholar |
Broadhurst LM, Coates DJ, Tan BH (1999) Genetic diversity in the monospecific Western Australian endemic, Geleznowia verrucosa Turcz. (Rutaceae). Heredity 82, 292-299.
| Crossref | Google Scholar |
Broadhurst LM, Coates DJ, Tan BH (2001) Patterns of morphological variation and allelic distribution across a zone of overlap between two subspecies of Geleznowia verrucosa (Rutaceae). Australian Journal of Botany 49, 451-458.
| Crossref | Google Scholar |
Bryant D, Moulton V (2003) Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution 21, 255-265.
| Crossref | Google Scholar |
Burbrink FT, Crother BI, Murray CM, Smith BT, Ruane S, Myers EA, Pyron RA (2022) Empirical and philosophical problems with the subspecies rank. Ecology and Evolution 12, e9069.
| Crossref | Google Scholar |
Byrne M (2008) Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews 27, 2576-2585.
| Crossref | Google Scholar |
Chifman J, Kubatko L (2014) Quartet inference from SNP data under the coalescent model. Bioinformatics 30, 3317-3324.
| Crossref | Google Scholar |
Coates DJ (2000) Defining conservation units in a rich and fragmented flora: implications for the management of genetic resources and evolutionary processes in south-west Australian plants. Australian Journal of Botany 48, 329-339.
| Crossref | Google Scholar |
Council of Heads of Australian Herbaria (2007) Vascular Plants. In ‘Australian Plant Census’. Available at https://id.biodiversity.org.au/reference/apni/44408
Dayrat B (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society 85, 407-415.
| Crossref | Google Scholar |
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879-886.
| Crossref | Google Scholar |
de Queiroz K (2020) An updated concept of subspecies resolves a dispute about the taxonomy of incompletely separated lineages. Herpetological Review 51, 459-461.
| Google Scholar |
de Queiroz K (2021) Response to criticisms of an updated species concept. Herpetological Review 52, 773-776.
| Google Scholar |
Department of Agriculture, Water and the Environment (2020) Australia’s bioregions (IBRA). Available at http://www.environment.gov.au/land/nrs/science/ibra
Doyle JJ, Dickson EE (1987) Preservation of plant samples for DNA restriction endonuclease analysis. TAXON 36, 715-722.
| Crossref | Google Scholar |
Dress AWM, Huson DH (2004) Constructing splits graphs. IEEE/ACM Transactions on Computational Biology and Bioinformatics 1, 109-115.
| Crossref | Google Scholar |
Drummond J (1853) On the botany of the north-western district of Western Australia. Hooker’s Journal of Botany and Kew Garden Miscellany 5, 115-122.
| Google Scholar |
Eaton DAR, Overcast I (2020) ipyrad: interactive assembly and analysis of RADseq datasets. Bioinformatics 36, 2592-2594.
| Crossref | Google Scholar |
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14, 2611-2620.
| Crossref | Google Scholar |
Georges A, Gruber B, Pauly GB, White D, Adams M, Young MJ, Kilian A, Zhang X, Shaffer HB, Unmack PJ (2018) Genomewide SNP markers breathe new life into phylogeography and species delimitation for the problematic short-necked turtles (Chelidae: Emydura) of eastern Australia. Molecular Ecology 27, 5195-5213.
| Crossref | Google Scholar |
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307-321.
| Crossref | Google Scholar |
Harrison RG, Larson EL (2014) Hybridization, introgression, and the nature of species boundaries. Journal of Heredity 105, 795-809.
| Crossref | Google Scholar |
Harvey WH (1855) Extracts from Australian letters of Dr. Harvey. Hooker’s Journal of Botany and Kew Garden Miscellany 7, 47-51.
| Google Scholar |
Hillis DM (2021) New and not-so-new conceptualizations of species and subspecies: a reply to the ‘it’s species all the way down’ view. Herpetological Review 52, 49-50.
| Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar |
Holland B, Moulton V (2003) Consensus networks: a method for visualising incompatibilities in collections of trees. In ‘Algorithms in Bioinformatics’. (Eds G Benson, RDM Page) Lecture Notes in Computer Science. pp. 165–176. (Springer) 10.1007/978-3-540-39763-2_13
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623-650.
| Crossref | Google Scholar |
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23, 254-267.
| Crossref | Google Scholar |
Ilut DC, Nydam ML, Hare MP (2014) Defining loci in restriction-based reduced representation genomic data from nonmodel species: sources of bias and diagnostics for optimal clustering. BioMed Research International 2014, 675158.
| Crossref | Google Scholar |
Joly S, Bryant D, Lockhart PJ (2015) Flexible methods for estimating genetic distances from single nucleotide polymorphisms. Methods in Ecology and Evolution 6, 938-948.
| Crossref | Google Scholar |
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403-1405.
| Crossref | Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar |
Knaus BJ, Grünwald NJ (2017) VCFR: a package to manipulate and visualize variant call format data in R. Molecular Ecology Resources 17, 44-53.
| Crossref | Google Scholar |
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Molecular Ecology Resources 15, 1179-1191.
| Crossref | Google Scholar |
Linan AG, Lowry II PP, Miller AJ, Schatz GE, Sevathian J-C, Edwards CE (2021) RAD-sequencing reveals patterns of diversification and hybridization, and the accumulation of reproductive isolation in a clade of partially sympatric, tropical island trees. Molecular Ecology 30, 4520-4537.
| Crossref | Google Scholar |
Martin NH, Willis JH (2007) Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61, 68-82.
| Crossref | Google Scholar |
Mastretta-Yanes A, Arrigo N, Alvarez N, Jorgensen TH, Piñero D, Emerson BC (2015) Restriction site-associated DNA sequencing, genotyping error estimation and de novo assembly optimization for population genetic inference. Molecular Ecology Resources 15, 28-41.
| Crossref | Google Scholar |
McCartney‐Melstad E, Gidiş M, Shaffer HB (2019) An empirical pipeline for choosing the optimal clustering threshold in RADseq studies. Molecular Ecology Resources 19, 1195-1204.
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020a) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar |
Minh BQ, Hahn MW, Lanfear R (2020b) New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37, 2727-2733.
| Crossref | Google Scholar |
Mosyakin SL, McNeill J, Boiko GV (2019) Comments on proper type designation for names of taxa validated by Turczaninow in his Animadversiones, with case studies. Ukrainian Botanical Journal 76, 379-389.
| Crossref | Google Scholar |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar |
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Frontiers in Zoology 7, 16.
| Crossref | Google Scholar |
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526-528.
| Crossref | Google Scholar |
Paris JR, Stevens JR, Catchen JM (2017) Lost in parameter space: a road map for stacks. Methods in Ecology and Evolution 8, 1360-1373.
| Crossref | Google Scholar |
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One 7, e37135.
| Crossref | Google Scholar |
Plummer JA, Crawford A, Hall D, Watkins P, Growns D (2000) Taming yellow bells: floricultural diversity within Geleznowia verrucosa (Rutaceae). Acta Horticulturae 541, 153-157.
| Crossref | Google Scholar |
Prates I, Doughty P, Rabosky DL (2023) Subspecies at crossroads: the evolutionary significance of genomic and phenotypic variation in a wide-ranging Australian lizard (Ctenotus pantherinus). Zoological Journal of the Linnean Society 197, 768-786.
| Crossref | Google Scholar |
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155, 945-959.
| Crossref | Google Scholar |
Rannala B, Yang Z (2020) Species delimitation. In ‘Phylogenetics in the Genomic Era’. (Eds C Scornavacca, F Delsuc, N Galtier) p. 5.5:1–5.5:18. (Published by the authors) Available at https://hal.archives-ouvertes.fr/hal-02536468/document
Reaz R, Bayzid MS, Rahman MS (2014) Accurate phylogenetic tree reconstruction from quartets: a heuristic approach. PLoS One 9, e104008.
| Crossref | Google Scholar |
Shepherd KA, Crawford AD (2020) Worthy of love: Geleznowia amabilis (Rutaceae), a stunning new species of ‘yellow bells’ from Kalbarri in Western Australia. Nuytsia 31, 89-93.
| Crossref | Google Scholar |
Smith-White S (1954) Chromosome numbers in the Boronieae (Rutaceae) and their bearing on the evolutionary development of the tribe in the Australian flora. Australian Journal of Botany 2, 287-303.
| Crossref | Google Scholar |
Spriggs EL, Eaton DAR, Sweeney PW, Schlutius C, Edwards EJ, Donoghue MJ (2019) Restriction-site-associated DNA sequencing reveals a cryptic Viburnum species on the North American Coastal Plain. Systematic Biology 68, 187-203.
| Crossref | Google Scholar |
Tanabata T, Shibaya T, Hori K, Ebana K, Yano M (2012) SmartGrain: high-throughput phenotyping software for measuring seed shape through image analysis. Plant Physiology 160, 1871-1880.
| Crossref | Google Scholar |
Turczaninow NS (1849) Decas sexta generum plantarum hucusque non descriptorum adjectis descriptionibus specierum nonnullarum. Bulletin de la Societe Imperiale des Naturalistes de Moscou 22, 12-13.
| Google Scholar |
Wagner F, Ott T, Schall M, Lautenschlager U, Vogt R, Oberprieler C (2020) Taming the red bastards: hybridisation and species delimitation in the Rhodanthemum arundanum-group (Compositae, Anthemideae). Molecular Phylogenetics and Evolution 144, 106702.
| Crossref | Google Scholar |
Wilson PG (1970) A taxonomic revision of the genera Crowea, Eriostemon and Phebalium (Rutaceae). Nuytsia 1, 3-155.
| Crossref | Google Scholar |
Wilson PG (2013) Geleznowia. Flora of Australia 26, 415-416.
| Google Scholar |


