Taxonomic revision of Australian Erythrophleum (Fabaceae: Caesalpinioideae) including description of two new species
Russell L. Barrett A B C D E * and Matthew D. Barrett
A B C D E * and Matthew D. Barrett  C D E F
C D E F
A
B
C
D
E
F
Australian Systematic Botany 36(5) 401-426 https://doi.org/10.1071/SB23007
Submitted: 2 March 2023 Accepted: 6 September 2023 Published: 27 September 2023
Abstract
The genus Erythrophleum Afzel ex R.Br. is revised for Australia and three species are recognised, all previously included in E. chlorostachys (F.Muell.) Baill. Erythrophleum arenarium R.L.Barrett & M.D.Barrett is described as a new species to accommodate populations from desert sands of the Great Sandy Desert and Dampier Botanical Districts in north-western Australia, parapatric to the remaining species. Erythrophleum pubescens R.L.Barrett & M.D.Barrett is described as a new species spanning tropical Australia, and is sympatric with E. chlorostachys sensu stricto in the Northern Territory and adjacent areas of Western Australia and Queensland. Morphological examination has shown these three taxa to be consistently distinct across their respective ranges. Analyses of the nuclear ribosomal ITS1 region recovered three well-supported clades corresponding to the three morphologically defined species, and ITS1 has utility as a marker to separate sterile specimens. Erythrophleum pubescens is widespread in the Australian Monsoon Tropics, from the coast of Western Australia, near Derby, to Cape York Peninsula in northern Queensland. Erythrophleum chlorostachys is also widespread, from the eastern Kimberley Region, in Western Australia, to the Gulf of Carpentaria, in northern Queensland. A lectotype is chosen for Laboucheria chlorostachya F.Muell. Full descriptions, illustrations of key features and identification keys are provided for the three Australian species. A summary of the significant utilisation of Erythrophleum species is presented.
Keywords: Australian monsoon tropics, Dimorphandra, Dinizia, Fabaceae, forestry timber, Indigenous utilisation, ironwood, new species, Ordeal tree, savanna, typification.
Introduction
Ten species of Erythrophleum Afzel ex R.Br. are traditionally recognised, with six species being found in Africa (Ross 1977; Coates Palgrave and Drummond 2002; Lewis et al. 2005) and Madagascar (Du Puy et al. 2002), three species in Cambodia (Larsen et al. 1980), Thailand (Larsen et al. 1984), Vietnam and China (Chen et al. 2010), and a single species in northern Australia (Ross 1998). Interestingly, the genus is absent from Malesia (Ding Hou et al. 1996). Although this disjunct pattern of distribution may seem unusual, similar disjunctions are found in a number of other angiosperm clades, including Causonis Raf. (Parmar et al. 2021), Cyperaceae subtribe Tricostulariinae (Barrett et al. 2021), Diplacrum R.Br. (Wilson and Barrett 2023), Schoenus L. (Elliott et al. 2021), and Terminalia L. (Maurin et al. 2023), with dispersals between Africa and Australia–Asia having been inferred in both directions within these clades. There is no modern global revision of the genus and the last new species to be described was Erythrophleum lasianthum Corbishley (1922, in Anonymous 1922). A number of Asian species previously included in Erythrophleum are now placed in Cynometra L., Gymnocladus Lam. or Sympetalandra Stapf., including E. angustifolium Gagnep. (Gagnepain 1952). In Australia, Erythrophleum is widespread and locally common, but most other species in the genus are threatened to varying degrees by exploitation for medicinal or timber resources, or by development (Okeyo 2012; Missanjo et al. 2017; Maroyi 2019; Wang et al. 2019; Chauke and Kritzinger 2020; Delporte et al. 2021; De Meyer 2023).
Phylogenetic relationships
The genus Erythrophleum is placed in subfamily Caesalpinioideae, where it is broadly related to Bussea Harms, Campsiandra Benth., Dimorphandra Schott, Dinizia Ducke, Jacqueshuberia Ducke, Pachyelasma Harms and Parkinsonia L. (Bruneau et al. 2001; Herendeen et al. 2003; Luckow et al. 2003; Duminil et al. 2013; Lewis et al. 2013; Azani et al. 2017; Koenen et al. 2020). Determining its closest allies is hampered by uneven sampling of both species and genetic markers. The phylogenetic reconstruction by Duminil et al. (2013) found a strong sister relationship with Dinizia; however, this relationship changes depending on the markers used (Manzanilla and Bruneau 2012). On the basis of nearly 1000 loci, Koenen (2019) and Ringelberg et al. (2022) recovered Pachyelasma as the sister clade to Erythrophleum, but with only moderate support. Analysis of transcriptome data by Zhao et al. (2021) demonstrated that Erythrophleum is sister to all of Mimoseae and Ingeae (although Pachyelasma was not included in their analyses), where it occupies an interesting position in the transition of nitrogen fixation syndromes (de Faria et al. 2022). On the basis of a sampling of five species utilising chloroplast non-coding sequences, Erythrophleum chlorostachys sensu lato was found to be sister to E. fordii Oliver from Asia, with an estimated mean divergence date of 12.2 (2.4–24.1) Ma between the two species (Duminil et al. 2013). However, later analyses based on more data by Duminil et al. (2015) reduced this mean divergence age estimate significantly, to 1.6 (0.5–2.8) Ma. Pollen studies have shown that Erythrophleum has tricolporate monads, common to several related genera in the Dimorphandra group (Banks and Lewis 2009; https://apsa.anu.edu.au/), but differing from the unique tricolporate tetrads of Dinizia (Banks et al. 2010).
Taxonomic history in Australia
Ferdinand Mueller (1859) described the new genus Laboucheria F.Muell., which he considered to be related to Adenanthera L., for a single species (L. chlorostachya F.Muell.), from northern Australia. Mueller (1863) subsequently recognised that the species belonged in Erythrophleum, but used the illegitimate name E. laboucheri, which was followed by Bentham (1864). Baillon (1870) recognised that Mueller’s specific epithet from 1859 had priority and made the formal combination Erythrophleum chlorostachys. Regional treatments of Erythrophleum in Australia have been published by Symon (1981), Wheeler (1992), Dunlop et al. (1995) and Kenneally et al. (1996) (all as a single species, E. chlorostachys). Ross (1998) specifically documented a variant of E. chlorostachys (here recognised as E. arenarium), but did not suggest that it warranted taxonomic recognition. Fieldwork in northern Australia by the present authors suggested that further study was warranted and a taxonomic revision is presented here.
Morphological examination of herbarium specimens, supported by phylogenetic analyses of the nrDNA ITS1 region, has enabled the recognition of three species endemic to northern Australia, including two species that are newly described. Erythrophleum pubescens R.L.Barrett & M.D.Barrett is the most widespread species, from the western Kimberley Region of Western Australia to Cape York Peninsula, in northern Queensland, being sympatric with E. chlorostachys for much of its range. Where the two species have been observed in relative proximity, E. chlorostachys has occupied rockier substrates with relatively shallow soils than has E. pubescens. Erythrophleum chlorostachys has a more restricted distribution, from the eastern Kimberley, through the northern third of the Northern Territory (the Top End), into north-western Queensland. Erythrophleum arenarium R.L.Barrett & M.D.Barrett is restricted to the Dampier Botanical District and Great Sandy Desert in Western Australia. Erythrophleum arenarium joins a growing number of species recognised as endemic to the sand communities of the Pindan and Great Sandy Desert, and distinct from their savanna counterparts (Barrett and Barrett 2015; Barrett and Telford 2015; Barrett 2015, 2016, 2019; Barrett et al. 2015a, 2015b). The flora of northern Australia remains poorly studied and new species from the region, including shrubs and trees, are still being described on a regular basis (e.g. Harrington et al. 2012; Barrett 2006, 2007, 2013; Maslin et al. 2013; Jobson 2014; Barrett and Barrett 2015; Cowie and Guymer 2015; Barrett et al. 2015c; Craven et al. 2016; Jackes 2017; Nicolle and Barrett 2018; Dillon et al. 2020; Callmander et al. 2021; Ford and Wilson 2021; Ford et al. 2021; Cooper 2022; Cooper and Lamei 2023).
Utilisation and cultural significance of Erythrophleum
The genus Erythrophleum has a significant place in the northern Australian tree flora (Addicott et al. 2018; Hunter et al. 2022), and specific notes on its importance are provided here. Because Erythrophleum was previously considered monotypic in Australia, no distinction among the three species recognised here has been made in the literature. In some cases, published records can be linked to one or other of the revised species’ concepts, but in many cases the record is insufficient to discriminate between the sympatric species E. chlorostachys and E. pubescens. The disjunct distribution of E. arenarium allows the relevant literature records to be reliably attributed to that species. Specific utilisation of the three Australian species recognised here should be reviewed to determine whether usage may have been species-specific.
Chemistry and toxicity
Erythrophleum as a genus includes the African ordeal trees (Maroyi 2019; Chauke and Kritzinger 2020; De Meyer 2023), so it is not surprising that the Australian species are highly toxic and are also utilised for medicinal purposes (Quattrocchi 2016). Australian Erythrophleum is well known as a stock poison (Hall 1964; Cribb and Cribb 1981; Harborne and Baxter 1996). The first detailed chemical investigations based on leaves and seeds provided by Walter Hill in Darwin were undertaken by James Petrie (1921a, 1921b), who confirmed the presence of erythrophleine and demonstrated its highly toxic effects. Griffin et al. (1971) recognised only a single species of Erythrophleum in Australia, but identified at least two chemical varieties differing markedly in their alkaloidal constituents. A voucher from Mareeba (W. Griffin in V.K. Moriarty HN 557; CANB 335549) is E. pubescens. Definite vouchers for the other samples cited by Griffin et al. (1971) from near Darwin and Cooktown could not be traced at CANB or BRI for lack of detailed information, but the Darwin samples may represent E. chlorostachys sensu stricto, whereas the Cooktown samples will belong to E. pubescens. This suggests regional variation in chemical constituency, something also noted for African species (Fernández-Marín et al. 2017; Delporte et al. 2021).
Erythrophleine has also been isolated from populations here included in E. arenarium (Gardner and Bennetts 1956). Loder et al. (1972) further documented the chemical structure of alkaloids in Australian Erythrophleum. Bisby et al. (1994) listed numerous terpenoids and alkaloids recorded from E. chlorostachys (sensu lato). The complex chemistry associated with toxic compounds in Australian taxa has been further refined by Qu et al. (2006) and Sim (2023), with there being similar studies on African (Armah et al. 2015; Kablan et al. 2020; Imolede et al. 2022) and Asian (Yu et al. 2005; Huang et al. 2018) species. The diterpinoid alkaloids, particularly erythrophleine, which are found in wood dust, wood, bark and leaves, are highly toxic, and contact can cause nausea, headaches, asthma, blindness, skin irritations and dermatitis (McKenzie 2012). The extreme toxicity of erythrophleine, which can cause heart failure if ingested, has been responsible for large numbers of stock deaths following consumption of even small quantities of the foliage, particularly from root suckers, but also from fallen crown leaves (Bailey 1900; Everist 1974; Milson 2000; McKenzie 2012). Cattle, sheep, goats, horses, donkey and camels are all known to have been poisoned by this species, often fatally, whereas several native possums and parrots such as little corellas appear to be immune to the poison, at least within their native ranges where they co-occur (Cribb and Cribb 1981; McKenzie 2012; Carmelet-Rescan et al. 2022). Caterpillars of the large saturn moth, Neodiphthera excavus (Lane), a species that is unusual in that the caterpillars pupate underground, do feed on the leaves (Lane 1995); however, no butterfly larvae is known to feed on Erythrophleum.
Utilisation
All plant parts of Australian Erythrophleum species have been extensively utilised by First Nations Australians or post-colonial humans, including timber, gum, pigments, and various parts for ceremonial and medicinal purposes, making it one of the most utilised native taxa in tropical Australia (Si 2020; Thompson 2020). Early colonial records of wooden utensils include spears, spear-point and prongs, spear-throwers, digging-sticks, throwing sticks, mallets, clubs, and as a handle for a hafted elouera (scraper knife) (Palmer 1883, 1884; Blackman 1904; Roth 1909; Tindale 1925, 1926; Spencer 1928; Thomson 1936, 1939; Setzler and McCarthy 1950). In northern Queensland, bark was used to cover huts (Roth 1910). In some regions, trees were culturally modified, reflecting their traditional utilisation, especially cutting into the cambium to retrieve honey from native bees (Morrison et al. 2010; Cole 2022). Because utilisation of the genus is so significant in northern Australia, a review of each usage is provided here.
The commercial timber use of E. chlorostachys (sensu lato, including E. pubescens), usually referred to by the common name of Cooktown Ironwood, has been documented by Boland et al. (1984), Bootle (2010) and Lake (2015). An English author commented on Australia that ‘You could laugh at the idea of wooden weapons until you saw the kind of wood that grew here’ (Pratchett 1998). Erythrophleum chlorostachys sensu lato has a density of 1220–1300 kg m−2 (at 12% moisture), being one of the densest native timbers in Australia (Boland et al. 1984; Cause et al. 1989; Bootle 2010). In fact, it is one of the densest timbers in the world, with 7 of the 10 densest woods globally having density very similar to that of E. chlorostachys sensu lato, with only Acacia cambagei R.T.Baker, Guaiacum officinale L. and Schinopsis Engl. species having significantly higher density (Meier 2015). The strength of the timber is a key reason that many trees were uprooted, rather than having most branches broken off, from the intense winds of Cyclone Tracey, which devastated Darwin on 25 December 1974 (Stocker 1976). Missing branches are probably more commonly associated with lightning strikes, and large scars and hollows are also commonly attributable to past cultural modification. Australian Erythrophleum species are long-lived, and large trees may be over 500 years old (Taylor 2002).
A tightly interlocking grain, good termite resistance, and a smooth finish enable use for fencing, railway sleepers, boat building, firewood, charcoal, decorative turning and joinery, balls for lawn bowls and replacement of worn-out machine bearings, with potential for musical instrument manufacturing (Swain 1928; Boland et al. 1984; Bootle 2010; Lake 2015; Zich et al. 2020). The exceptional qualities of the timber mean that it was one of the earliest timber products exported from Australia, being utilised by Macassan fishermen since about the 17th century for both masts and anchors (MacKnight 1976; Clarke 2007, 2008), and some export of wild-harvested timber continues today. A private catalogue from 2019 listed the timber at A$20 kg−1 (∼A$25 000 m−3). It was also utilised as a favoured timber in the earliest construction of European settlements in the Northern Territory (Woinarski et al. 2002). Commercial wild-harvesting continues today (Taylor 2002; Cook et al. 2005). It is noted that dried timber can be susceptible to lyctine beetles (Cookson et al. 2009). The tightly interlocking grain does make Erythrophleum species generally resistant to white-rot fungi (Nguyen et al. 2018), although some brown and white rot (particularly Truncospora Pilát) fungi have been observed on Australian Erythrophleum (M. D. Barrett, pers. obs.).
Australian Erythrophleum species have been extensively used by First Nations Australians for medicinal purposes, for manufacturing canoes, tools and ceremonial artefacts (Brock 1988; Aboriginal Communities of the Northern Territory 1993; Lazarides and Hince 1993; Dunlop et al. 1995; Kenneally et al. 1996; Clarke 2012). Some specific examples identifiable to species are listed here, with more extensive records presented in Table 1. Erythrophleum pubescens has been used to make boomerangs and ceremonial clap-sticks in the north Kimberley (Karadada et al. 2011) and also harpoon points and axe handles on the Cobourg Peninsula (Blake et al. 1998). It is interesting to note that the wood is of sufficient density that it was historically used to make flat fighting swords by the Alawa people, from south-east of Katherine in the Northern Territory (Wightman et al. 1991). Stems of young saplings have been utilised for spear shafts (Wightman et al. 1992). Many uses are recorded for E. pubescens on Groote Eylandt, the dense wood being used for woomera pegs, the heads of hooked spears, bamboo spears and harpoons, for roasting sticks and for grinding food (Levitt 1981).
| Language group or location (local names) | Use | References | |
|---|---|---|---|
| Alawa (Marlbamba), Marra (Malbamba), Marra (Wirlwirl) | Timber used to make boomerangs, woomeras, flat fighting swords, clap-sticks, and for building; resin used to affix spear heads, axe heads; leaves used to make smoke for funeral ceremonies | Wightman et al. (1991), Roberts et al. (2018), Yugul Mangi Rangers (2023) | |
| Bardi (Joonggoomarr) | Timber used to make clap-sticks | Kenneally et al. (1996) | |
| Batjamal (Melhe), Emi (Mawuny) | Timber used to make clap-sticks and for building | Smith and Wightman (1989) | |
| Belaa (Winjabarr minya, Winjabarr) | Wood for long-lasting fires, timber for clap-sticks (limburr), fighting-sticks (waalu), leaves used in various cleansing smoking ceremonies | Cheinmora et al. (2017) | |
| Ngarinyin (Unggarrun) | Smoke (Bijagun) from leaves used for cleansing ceremonies | Wilinggin Aboriginal Corporation (2012) | |
| Dalabon (Kirdidjdjirrh, Mulyurrunj, Murutilla) | Gum (Marnû) eaten or boiled to make a drink, or eaten for stomach upsets; inner bark used as antiseptic wash for skin sores; resin (Kabbay, Kalanjan) from roots used to affix spear heads and woomera hooks; smoke from green leaves used for cleansing ceremonies; timber used for clap-sticks, digging-sticks, woomeras, fighting-sticks, heads of shovel-spears | Smith (1991), Bordulk et al. (2012) | |
| Djambarrpuyngu (Maypiny), Kunwinjku (Mandubang) | Leaves, inner bark boiled for antiseptic wash for skin lesions; muscle and bone pain; inner bark from roots used to make smoke to stop lactation; timber used for building | Smith (1991), Aboriginal Communities of the Northern Territory (1993) | |
| Gija (Berawooroony) | Timber used to make fighting-sticks and digging-sticks | Purdie et al. (2018) | |
| Iwaidja (Gardunggun/Kartungkun) | Bark used to treat skin sores and boils; timber used for fighting sticks (Murrgan), harpoon point, clap-sticks (Arrilil); axe handles; resin from roots used to fasten spear heads; sticks used to pierce nasal septum; green leaves used in smoking ceremonies for cleansing | Aboriginal Communities of the Northern Territory (1993), Blake et al. (1998) | |
| Jamindjung (Jirrwili), Jawoyn (Marukkal), Kunwok (Mandubang) | Leaves heated and applied to body to relieve pain; timber used for spear-tips | Thompson (2020) | |
| Jingulu, Mudburra (Mandarrngarra) | Bark of roots used to treat skin sores; leaves used to make smoke to cleanse houses during funeral ceremonies; timber used to make clap-sticks, nulla-nullas, boomerangs | Raymond et al. (2018) | |
| Kriol (Ainud tri); Ritharrŋu/Wägilak (Maypiny); Wubuy (Yirrbara); Rembarrnga (Mirniyarrh) | Timber used for many purposes because of strength and density; leaves used to make smoke for cleansing ceremonies; bark boiled and applied to skin sores | Yugul Mangi Rangers (2023) | |
| Kuku Thaypan (Ku Morteall) | Leaves used to make smoke for ceremonial purposes and cleansing ceremonies; an important tree for sugarbag nests | Standley (2019) | |
| Mangarrayi (Yangarr); Yangman (Yarramala) | Timber used for spear shafts, spear heads, clap-sticks (Gundarl), fighting-sticks (Barrgu), boomerangs (Barlgan); sin from roots (Gabay) used to affix spear heads; boiled bark produces red dye and treats skin ailments; leaves used to make smoke for cleansing ceremonies; gum eaten | Roberts et al. (2011) | |
| Marri Ngarr, Magati Ke (Nandji Tjiwi) | Timber used for clap-sticks, boomerangs, shields, heads of double-sides hooked spears, large war swords, making hammers (Nanji Pamuri) for crushing cycad seeds, firewood, carved fish-hooks; saplings used for digging sticks; smoke from leaves used in cleansing ceremonies; resin from roots used to affix spear heads and woomera shafts; leaves warmed on fire and held to head for headache relief | Nambatu et al. (2009) | |
| Mudburra (Mandarrngarra) | Bark of roots used to treat skin sores; leaves used to make smoke to cleanse houses during funeral ceremonies; timber used to make clap-sticks, nulla-nullas, boomerangs | Wightman et al. (1992) | |
| Ngalkbun (Murutilla) | Leaves, inner bark and root bark used for skin lesions; muscle and bone pain; to end lactation | Aboriginal Communities of the Northern Territory (1993) | |
| Ngandi (Ma-mirniyarrh); Ngalakgan (Malbah) | Timber used to make clap sticks, fighting swords | Daniels et al. (2019) | |
| Ngan’gikurunggurr (Mawuny); Ngan’giwumirri (Kinimannggini) | Leaves used as a skin wash; stems heated to pierce nasal cavity; timber used to make boomerangs, fighting sticks, clap-sticks, digging sticks, firewood (but not when cooking); roots for spear heads; sap eaten; resin from roots used to affix spear heads, woomera pegs, plugging holes in dugout canoes; leaves used to make smoke for cleansing ceremonies | Aboriginal Communities of the Northern Territory (1993), Marrfurra et al. (1995) | |
| Ngarunyman (Dilwirli) | Timber used to make nulla-nullas, clap-sticks, woomeras | Smith et al. (1993) | |
| Rirratjiŋu (Buwatji, Maypiny) | Timber used to make clap-sticks (Bilma), boomerangs (Galiwali); roots used to make woomera hooks and spear points and as a source of resin; leaves used during funeral ceremonies | Yunupinŋu et al. (1994) | |
| Tiwi (Kartukunu, Pijitinga, Tumpurama) | Timber used for clap-sticks, fighting-sticks, axe handles; dry logs used to carve Pukamani poles and ornamental carvings; sharpened sticks used pierce nasal septum and as needles for sewing; smoke from leaves used in cleansing ceremonies; inner bark used for skin sores; young babies waved in smoke to make them strong | Puruntatameri et al. (2001), Thompson (2020) | |
| Uunguu (Winjabarr) | Timber used to make fighting sticks, clap-sticks, digging-sticks and spear-heads; root-stem joints used to make number-7 boomerangs | Karadada et al. (2011) | |
| Yawuru (Jun’ju and Bilamana) | Timber used to make clap-sticks | Kenneally et al. (1996) | |
| Darwin region (–) | Medicinal purposes; ceremony; utilitarian artefacts including shovel-nosed spear heads; bark gum eaten; red dye from bark | Clark and Traynor (1987), Dunlop et al. (1995) | |
| Kakadu (–) | Leaves steamed at childbirth and death; tannin-rich bark infused in water and used for skin sores; smoke used to deter mosquitoes | Low (1990) |
In Australia, Erythrophleum bark produces a red dye, the extruded gum contains a tanning agent and can be eaten raw and a resin from the roots has been used to affix spearheads to shafts and pegs to spear throwers (Blake et al. 1998; Clarke 2007; Beasley 2009). A gum from the roots of young E. pubescens plants has also been used to affix spear and axe heads (Powell et al. 2013); a gum from saplings being used as a poison; and leaves being used in cleansing ceremonies on Groote Eylandt (Levitt 1981). The unique chemistry of these gums allows identification of Erythrophleum products associated with historical First Nations artefacts using spectroscopy, confirming their long-term use in First Nations Australians societies (Georgiou et al. 2022). Smoke from burning bark can cause female sterility, whereas burning leaves repel mosquitos and sandflies (Beasley 2009). Smoke from burning leaves is also used in cleansing ceremonies (Blake et al. 1998; Clarke 2007). Crushed bark is effective as a fish poison and in African species; crushed seeds have been used to poison arrows (Lewis et al. 2005). In northern Queensland, medicinal properties from bark of E. pubescens are reported as effective for the treatment of sores, skin lesions, wounds, cuts, pain, and sprains (Turpin et al. 2022). Numerous traditional medicinal uses have been documented (e.g. Devanesen and Henshall 1982; Aboriginal Communities of the Northern Territory 1993), and bark material is known to be active against tumour cell cultures (Collins et al. 1990). African species have been reported to have medicinal and toxic properties similar to those of the Australian species (Dongmo et al. 2001; Coates Palgrave and Drummond 2002; Okeyo 2012; Son 2019; Teclaire et al. 2019).
Although published records probably represent only a small portion of actual utilisation, identified uses are summarised in Table 1 to reflect the current state of recorded knowledge. Most of these records may apply to either E. chlorostachys or E. pubescens, but it is likely that most apply (at least primarily) to E. pubescens as the more common and widespread species, especially on plains. A few records can be categorically assigned to E. pubescens or E. arenarium on the basis of distribution.
Ecology
All species are trees, usually growing in savanna or seasonally dry forests, but sometimes in tropical wet forests or rainforests. Members of Erythrophleum species are semi-deciduous, maintaining only a sparse canopy in spring, when new foliage is produced (Williams et al. 1997). Reproduction can occur from seeds, root suckers, or lignotubers (Lacey and Whelan 1976; Fensham and Bowman 1992). Germination is reasonably straightforward for Erythrophleum species because the seeds behave like most legumes (Missanjo et al. 2017). Stem survival following fire is low (often <10%), although survival of lignotubers is high (88%; Williams et al. 1999b) because greater resource allocation is made to root mass relative to co-occurring eucalypts (Paramjyothi et al. 2020). Interestingly, both smaller (DBH < 20 cm) and larger (DBH > 30 cm) trees are most likely to die following fire, with mid-aged trees being most resilient (Williams et al. 1999b). This is directly related to bark thickness (Lawes et al. 2011), so they may be useful indicators of recent fire history (Paramjyothi et al. 2020). Whitau et al. (2018) provided evidence for the fluctuation in abundance of Erythrophleum related to long-term fire regimes in the northern Kimberley on the basis of campfire deposits spanning a 45 000-year occupation sequence. In Australia, Erythrophleum species increase significantly in abundance when fire is excluded (Bowman et al. 1988; Fensham 1990; Bowman and Panton 1995).
Erythrophleum species provide important habitat hollows for animals, with a high density of hollows in the landscape compared with other northern savanna trees, although not uniformly across the landscape (Braithwaite et al. 1985; Taylor and Chisholm 2005). The flaky bark of Erythrophleum arenarium provides habitat for at least one species of pseudoscorpion (Harvey 1987). Although flowering can be infrequent (Williams et al. 1999a), Erythrophleum species also provide important nectar resources for birds and insects (Woinarski et al. 2000) and to some extent also for fruit bats (Mickleburgh et al. 1992; Fleming et al. 2009). Extrafloral nectaries found on the leaf rachis are likely to provide an important food resource to ants (Pascal et al. 2000; Marazzi et al. 2019). Erythrophleum chlorostachys sensu lato has been reported to form vesicular-arbuscular mycorrhizae, although the specific fungal species involved was not identified (Brundrett et al. 1995; Wang and Qiu 2006). A number of different Bradyrhizobium species are associated with Erythrophleum across the range of the genus, often being unique to distinct species (and multiple Bradyrhizobium Jordan species are commonly present; Yao et al. 2015).
Materials and methods
Specimens
Herbarium specimens have been examined at AD, BRI, CANB, DNA, MEL, NSW and PERTH by using light microscopy. Images of specimens at E (http://elmer.rbge.org.uk/bgbase/vherb/bgbasevherb.php), K (http://apps.kew.org/herbcat/) and P (https://science.mnhn.fr/institution/mnhn/collection/p/item/search) were examined in March 2018. Field photographs were taken with a 60-mm Sigma Macro lens (Sigma Corporation of America, New York). All three species have been examined in the field by the authors.
DNA extraction and polymerase chain reaction (PCR)
All taxa newly sampled for DNA sequencing are represented by voucher specimens in Australian herbaria. Voucher specimens sampled for this paper are detailed in Table 2. All Sanger-derived sequences deposited to GenBank contain ITS1 and partial 5.8S only, except for Dauncey H666, PERTH 08422060 (indicated with an asterisk), for which a full-length internal transcribed spacer (ITS) sequence (including ITS1, 5.8S and ITS2) was obtained. Gene complements assembled from short-read archives are described under each sample in Table 2.
| Species | Voucher | GenBank and ENA accession numbers | |
|---|---|---|---|
| Erythrophleum arenarium R.L.Barrett & M.D.Barrett | Western Australia (WA), Barrett 9080 (NSW, PERTH) | MT581272 | |
| WA, Bean 25079 (BRI) | MT581273 | ||
| WA, Byrne 1271 (PERTH 07148453) | MT581270 | ||
| WA, Forbes 2465 (PERTH 01958534) | MT581269 | ||
| WA, Sweedman 8997 (PERTH 08786607) | MT581271 | ||
| Erythrophleum chlorostachys (F.Muell.) Baill. | WA, Byrne 3693 (PERTH 08793034) | MT581275 | |
| Northern Territory (NT), Lazarides 8845 (CANB 295340) | MT581276 | ||
| WA, Weston 12284 (PERTH 02211750) | MT581274 | ||
| NT, Larcombe 2 (DNA D0057605) | MT581277 | ||
| NT, Wightman 5205 (DNA D0051920) | MT581278 | ||
| Erythrophleum fordii Oliv. | China, no voucher | ITS1 only, consensus assembly from SRR8191117 (Wang et al. 2019) A | |
| China, no voucher | Contiguous nearly complete (with gaps) 18S–ITS1–5.8S–ITS2–28S sequence, consensus assembly from SRR8191118 (Wang et al. 2019) | ||
| Erythrophleum ivorense A.Chev. | Gabon, Wieringa 5487 (WAG) | OQ572325; contiguous complete 18S–ITS1–5.8S–ITS2–28S sequence, consensus assembly from ERR4363217 (Koenen et al. 2020) | |
| Erythrophleum pubescens R.L.Barrett & M.D.Barrett | WA, Barrett MDB5902 (plant 1) (PERTH) | MT581285 | |
| WA, Barrett MDB5902 (plant 2) (PERTH) | MT581286 | ||
| WA, Byrne 3721 (PERTH 08760632) | MT581282 | ||
| WA, Coate 224 (PERTH 02888580) | MT581279 | ||
| WA, Dauncey H666 (PERTH 08422060) B | MT581297 | ||
| WA, Foulkes 340 (PERTH 02523922) | MT581280 | ||
| NT, Brennan 4583 (DNA D0146433) | MT581290 | ||
| NT, Clark 1670 (DNA D0034313) | MT581291 | ||
| NT, Cowie 5303 (DNA D0121980) | MT581292 | ||
| NT, Dunlop 7145 (NSW 451601) | MT581293 | ||
| NT, Egan 2873 (DNA D0077644) | MT581294 | ||
| NT, Evans 3270 (NSW 451600) C | MT581289 | ||
| NT, Smith 101 (DNA D0044224) | MT581295 | ||
| NT, Smith 128 (DNA D0029224) | MT581296 | ||
| NT, Whaite 3979 & Whaite (NSW 415299) | MT581283 | ||
| Queensland (Qld), Blake 23184 (PERTH 02211556) | MT581281 | ||
| Qld, Leitch QDA003815 (BRI AQ854110) | MT581288 | ||
| Qld, McDonald KRM9767 (BRI AQ846978) | MT581287 | ||
| Qld, Wannan 213 & Lynch (NSW 396373) | MT581284 | ||
| Qld, McDonald KRM17554 (MEL 2416964A) | OQ471964 (dominant copy, contiguous complete 18S–ITS1–5.8S–ITS2–28S sequence) and OQ396764 (minor copy, ITS1–5.8S–ITS2 only), consensus assembly from ERR7599610 | ||
| Pachyelasma tessmannii (Harms) Harms | Gabon, Wieringa 5229 (WAG) | OQ572326; contiguous complete 18S–ITS1–5.8S–ITS2–28S sequence, consensus assembly from ERR4363236 (Koenen et al. 2020) |
DNA extraction, PCR and sequence alignment followed the protocol for sequencing the ITS region of ribosomal DNA (rDNA) described in Anderson et al. (2016), with the exception of primers used. Initially, amplification of the full ITS region was attempted by using a range of primers and PCR reaction conditions, but only a single sample (Dauncey H666) could be amplified, by using the primers ITS S3 (5′-AACCTGCGGAAGGATCATTG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). Subsequently, ITS amplification was attempted for two smaller fragments (ITS1 and ITS2 respectively); however, only the ITS1 region could be reliably amplified from herbarium samples, using the primers S3 and ITS2-R (5′-GCTGCGTTCTTCATCGATGC-3′), despite a wide range of conditions and nested primers being applied. Attempts to perform PCR for chloroplast matK and trnF–trnL regions by using a range of primers also failed for Erythrophleum. Consequently, only the ITS1 dataset was used for phylogenetic analyses. The high failure rate using standard primers could be due to the concentration of tannins, terpenoids and alkaloids in the leaves (Bisby et al. 1994; Blake et al. 1998), which are known inhibitors of PCR amplification (Katterman and Shattuck 1983).
Ribosomal assembly from short-read archives
Four Erythrophleum short-read archives (Table 2) generated for other studies were downloaded from European Nucleotide Archive (ENA) and mined for ribosomal sequences. The mined archives were generated by hybrid-capture (Erythrophleum pubescens and Pachyelasma tessmannii (Harms) Harms) or RADseq (two E. fordii Oliv. accessions), but all proved to have sufficient bycatch of nuclear ribosomal DNA for confident assembly (>20× coverage) of the full ribosomal region, including 18S, ITS1, 5.8S, ITS2 and 28S in all samples except for the E. fordii samples, for which there were some short gaps in the full ribosomal assembly of SRR8191118, and only ITS1 could be recovered from SRR8191118. Sanger-derived ITS sequences were used as a reference to map reads in Geneious Prime (ver. 2023.0.1), using the default Map to Reference module, setting 5% maximum mismatches per read, three iterations of fine tuning, maximum gaps per read = 15%, word length = 14, maximum gap size = 50, index word length = 12, and maximum ambiguity = 4. The mapped reads were then de-novo assembled using the De Novo Assemble module in Geneious, using the Geneious Assembler set to medium sensitivity, and merging homopolymer variants, saving the consensus sequence of assembled reads calling heterozygous positions when variants present at greater than 35% (threshold 65%). Assembled contigs were then re-mapped to the original reference, and contigs covering the ITS region at depth greater than five times the coverage were treated as independent ribosomal copies. Only a single ribosomal contig was assembled for the E. ivorense A.Chev. sample, whereas two very similar contigs were assembled for the E. pubescens sample. For the dominant copy in each case, the contigs were extended to include the 18S and 26S genes by mapping to reference with 1% similarity and 20–30 iterations in Geneious, saving the consensus sequence and calling heterozygous positions when variants present at greater than 35% (threshold 65%).
All recovered Australian ITS haplotypes were verified as genuine ITS homologs by their ability to form plausibly folding ITS1 (and a single ITS2) RNA molecules with free energy ∆G comparable to that of other Erythrophleum species, using the web server for m-fold (Zuker 2003; http://unafold.rna.albany.edu/?q=mfold), and that 5.8S and ends of 18S and 28S genes present in the transcripts could be aligned to related Fabaceae, and contained no indels.
Outgroups
Two other non-Australian Erythrophleum samples of ITS are available on GenBank (E. fordii MH844617 and E. suaveolens KY306573). Unfortunately, both of the samples on GenBank were highly divergent in ITS from all Erythrophleum samples used here (including newly assembled ITS of E. fordii), with few regions that could be readily aligned, and almost no parsimony-informative sites relevant to resolving relationships between the Australian species. Consequently, these GenBank sequences of E. fordii and E. suaveolens were excluded from further analyses. We were unable to amplify ITS from a recent collection of Erythrophleum africanum Harms located in an Australian herbarium. The final alignment, therefore, included ribosomal assemblies only from E. fordii and E. ivorense as the only non-Australian Erythrophleum representative. A ribosomal assembly of Pachyelasma tessmannii (Harms) Harms, one of the closest relatives to Erythrophleum in previous analyses, was used to root the tree.
Alignment and phylogenetic analyses
The four full-length ribosomal sequences, and 30 ITS sequences were assembled using the MAFFT (ver. 7.490, see https://mafft.cbrc.jp/alignment/server/index.html) module in Geneious Prime, using default settings (Katoh and Standley 2013).
Partitions were grouped using the program PartitionFinder2 (ver. 2.1.1, see https://www.robertlanfear.com/partitionfinder/; Lanfear et al. 2016), using the corrected Akaike information criterion (AICc), greedy search (Lanfear et al. 2012) with PhyML (ver. 3.0, see http://www.atgc-montpellier.fr/phyml/; Guindon et al. 2010), and the models limited to those accommodated by MrBayes (ver. 3.2.6, see https://nbisweden.github.io/MrBayes/; Huelsenbeck and Ronquist 2001). The best partition scheme found three partitions (18S, 5.8S) (ITS1, ITS2) (26S), with the best substitution models being GTR, GTR and GTR+G respectively.
Two methods of generating phylogenetic trees were used, namely, maximum likelihood (ML) by using RAxML (ver. 8.2.11, see https://github.com/stamatak/standard-RAxML; Stamatakis 2014), and Bayesian inference (BI) in MrBayes (ver. 3.2.6; Huelsenbeck and Ronquist 2001), both being implemented in Geneious Prime (ver. 2023.0.1, see http://www.geneious.com/; Kearse et al. 2012). RAxML was run using partition and substitution models described above, by using 10 searches for the best maximum-likelihood tree, and support values were calculated from 100 bootstrap replicates. MrBayes was run using four heated chains over 1 100 000 generations with a burn-in length of 100 000 generations, under partition and substitution models described above. MrBayes analyses were evaluated by confirming that all runs converged on similar log-likelihood scores, traces had reached stationarity, and all run parameters had effective sample sizes greater than 500. Trees were visualised and edited using FigTree (ver. 1.4.4, see http://tree.bio.ed.ac.uk/software/figtree/).
Results
The ribosomal alignment consisted of four full-length ribosomal sequences, covering all three non-Australian outgroups, plus one representative of the Australian clade (E. pubescens). In total, 30 ITS sequences (all but two consisting of ITS1 only), represented all three Australian morphological species, plus an additional sample of the Asian E. fordii.
The alignment consisted of 5840 and 1479 total aligned residues and variable residues respectively, with 1809 aligned bases in 18S, 276 in ITS1, 159 in 5.8S, 207 in ITS2 and 3389 in 26S. The ITS1 dataset, when restricted to the Australian samples only, had 11 parsimony-informative sites. Four indels were not coded, but were diagnostic for (1) E. arenarium (n = 1, 1-bp insertion, A at Position 1818), (2) E. chlorostachys (n = 1, C insertion at Position 1900), (3) E. pubescens (n = 1, 8-bp deletion relative to E. arenarium and E. chlorostachys at Positions 2026–2033), and (4) E. arenarium + E. chlorostachys (n = 1, GAA insertion at Positions 2076–2078); indels are labelled as per Fig. 1, whereas alignment positions refer to aligned residues in the full ribosomal alignment (see File S1.nex in the Supplementary material).
Maximum-likelihood tree of aligned ribosomal sequences of Erythrophleum, rooted with Pachyelasma tessmannii. Nodal support values are bootstrap support values (RAxML) followed by posterior probabilities (MrBayes). Indel positions in ITS1 are indicated by grey bars, and numbered as in the text (see Results). Three clades (E. arenarium, E. chlorostachys and E. pubescens) match species designations from morphology, as described in the taxonomy section. Within the sampled Erythrophleum taxa, the three Australian species form a clade, and there is weak support for a sister relationship between E. arenarium and E. chlorostachys. The intraspecific variation within E. pubescens lacks support, and is not considered taxonomically informative. Sample names indicate species, state, location, collector and number, and GenBank or ENA accession number.
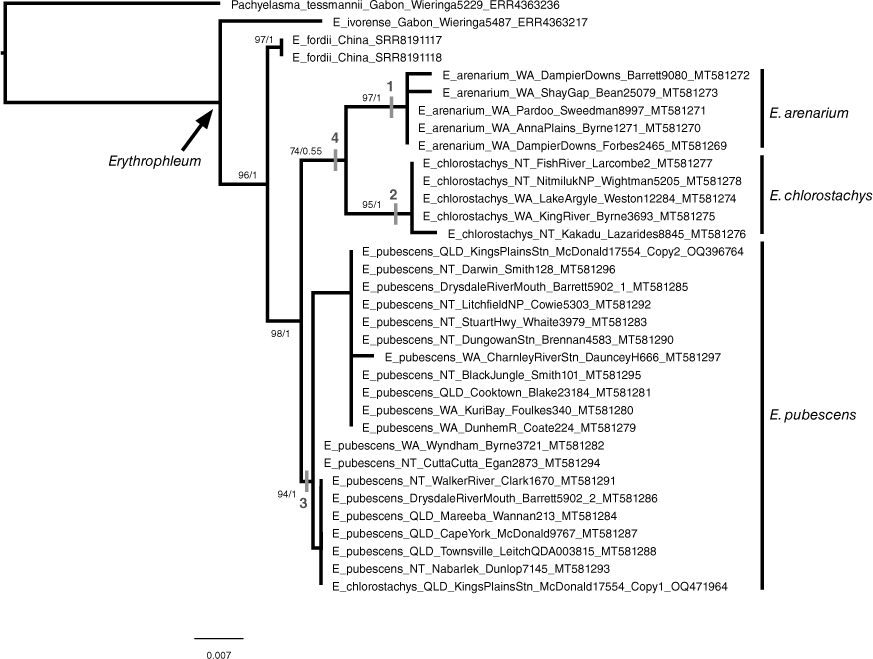
Sampling within Australia covered all three morphological species, and all broad geographic regions were represented for each species (although no Queensland representatives of E. chlorostachys sensu stricto were included). The RAxML tree is shown in Fig. 1, and bootstrap (BS) and posterior probability (PP) support values from the RAxML and MrBayes analyses respectively are indicated on relevant nodes. The tree rooted with Pachyelasma tessmannii recovered the African species, E. ivorense, sister to the remaining sampled Erythrophleum (96% BS, 1.00 PP), and the Asian E. fordii sister to a monophyletic Australian clade (98% BS, 1.00 PP). Within the Australian clade, all samples clustered into one of three strongly supported clades (94–97% BS, 1.00 PP), corresponding to the three morphologically defined species, namely, E. arenarium, E. chlorostachys or E. pubescens. E. arenarium and E. chlorostachys were weakly supported (74% BS, 0.55 PP) as sister species, with E. pubescens being the most divergent lineage within the Australian clade.
One short-read archive (E. pubescens McDonald 17554) allowed assembly of two similar ITS haplotypes (OQ471964 and OQ396764), both of which fell within the clade interpreted here as E. pubescens (Fig. 1).
Discussion
Phylogeny of Erythrophleum
Although only two non-Australian Erythrophleum species were represented in the ribosomal tree, the Australian clade was strongly supported as sister to the Asian species E. fordii, as recovered in chloroplast phylogenies (Duminil et al. 2013, 2015). Denser sampling of African Erythrophleum is required to further elucidate relationships and biogeography of the genus.
Although most samples used for this study were represented by ITS1 only, careful phylogenetic selection of full-length ribosomal sequences enabled robust phylogenetic reconstruction. Because all Australian samples were recovered as a well-supported monophyletic Australian clade (Fig. 1), the sampled taxon–locus combination enabled the full-length ribosomal sequences to resolve relationships of the Australian clade to the outgroups, for which ITS1 alone proved too variable to resolve with high support (data not shown), whereas the ITS1 region alone was sufficient to resolve relationships within the more densely sampled Australian clade, with high support values.
The division of Australian Erythrophleum into three species was supported by the moderately to well-supported clustering of ITS1 sequences into three discrete clades, matching morphological designations. The greatest intra-specific variation in ITS1 sequences was found within the most widely distributed species, E. pubescens; however, clades within this species were not supported by maximum-likelihood bootstrap or Bayesian analyses, and there is no geographical trend to the clades. Indeed, two plants from the same population (Drysdale River Mouth, Barrett MDB 5902) were placed divergently, one each in the two most numerous clades. Similarly, two ITS assemblages representing each clade were reconstructed from the single available E. pubescens short-read archive, indicating that these copies can both be present within individuals; it is possible that this is a fixed polymorphism in E. pubescens, through duplication of the ribosomal locus. Consequently, we interpret both variants as intraspecific variation within a single species, E. pubescens.
Within the three Australian species, E. arenarium and E. chlorostachys were recovered as sister taxa in the RAxML tree (74% BS), but not in the MrBayes tree (0.55 PP). This node was supported by a diagnostic 3-bp (GAA) insertion in the ITS1 alignment (Indel 4, Fig. 1), as described under results, providing additional evidence for this sister relationship in the ribosomal phylogeny. The sister relationship of these two species is a plausible result, with the two species sharing (relative to E. pubescens) non-corky branchlets, fewer leaflets per pinna, not or slightly asymmetric leaflets, glabrous raceme axis and pedicels, and longer calyx, anthers and pod stipe, and generally shorter aril, which are possible apomorphies for the clade. However further multi-locus data are required to conclusively demonstrate this relationship.
Species concepts in Erythrophleum
Taxonomy of the genus Erythrophleum has not been revised as a whole, but several regional treatments with adequate descriptions for all species are available (Larsen et al. 1980, 1984; Ross 1977, 1998; Coates Palgrave and Drummond 2002; Du Puy et al. 2002; Lewis et al. 2005; Chen et al. 2010); however, consistently distinguishing among some of the African species across their respective ranges is not without its challenges (Gorel 2019). The Australian species are probably most closely related to E. fordii, which is morphologically most similar to E. pubescens (see Chen et al. 2010). Erythrophleum fordii differs most obviously from E. pubescens in having rough (but not corky) bark, larger (5–8 cm long v. 2–6 cm long), acute (v. obtuse) leaflets, longer inflorescences (15–25 cm v. 7–16 cm) and shortly pedicellate flowers.
The Australian species have probably been historically maintained as a single taxon, largely owing to the variability of leaf morphology within each species (and even a single plant), confusing the boundaries between them. Plants can demonstrate three different leaf types at various growth stages, as seedling leaves, resprouting leaves, or an adult crown, similar to the ontogenetic stages seen in Corymbia (Hill and Johnson 1995; Myrtaceae). Careful field observations of each taxon by the authors have shown that three distinct leaf forms are present on each taxon at these stages (and two leaf forms can be present on any individual, depending on life stage or regeneration phase), and comparison of leaves at the same stage makes identification quite simple, whereas comparing alternate stages between species can lead to occasional confusion (such as herbarium specimens consisting entirely of juvenile leaves). Leaves of Erythrophleum species are bipinnate, with two to five pairs of opposite pinnae in the Australian species, but sometimes one or two pinnae abort, so they can appear alternate, and the leaflets are alternate. Seedling and adult crown leaves tend to be small, whereas resprouting leaves (both from lignotubers and trunks) can be much larger. Resprouting leaves (following disturbance such as fire) are often more symmetrical than are juvenile or adult crown leaves, so resprout leaves of E. pubescens can look similar to adult crown leaves of E. chlorostachys in some cases, making occasional sterile collections difficult to place in either species where their distributions overlap. When present, cork on the upper branchlets is diagnostic for E. pubescens, but younger branchlets sometimes cannot reliably be distinguished from E. chlorostachys if leaflet shape is also ambiguous. There is also regional variation in the size of crown leaves within E. pubescens in particular, largely correlating with a rainfall gradient, with very small (~1 cm long) leaflets in the northern Kimberley, and very large (up to 6 cm long) leaflets on Cape York. Misinterpretation of leaf-stage variation has led to similar taxonomic confusion in Corymbia (see Nicolle 2014).
Once leaf-stage variation was understood, correlating significant differences in floral morphology could be identified in each of the three taxa recognised here, and the combined differences are considered sufficient to justify recognition at species rank. Past confusion has generally led to this variation being described under a single entity. On the basis of ITS1 data and comprehensive geographic sampling, three monophyletic phylogenetic lineages were recovered, perfectly matching morphological distinctions, and consistent with the phylogenetic species concept. Two of the species overlap broadly, through the Top End of the Northern Territory and adjacent parts of Western Australia and Queensland, while maintaining morphological and genetic distinction, consistent with reproductive isolation. Consequently, three taxa are recognised at species rank, with two species being newly described.
Taxonomy
Erythrophleum Afzel. ex R.Br. in D.Denham, H.Clapperton & W.Oudney, Narr. Travels Africa 235 (1826)
Type: Erythrophleum suaveolens (Guill. & Perr.) Brenan.
Fillaea Guill. & Perr. in Guillemin, Perrottet & A.Richard, Fl. Seneg. Tent. t. 55 (1832). Type: F. suaveolens Guill. & Perr.
Mavia Bertol.f., Mem. Accad. Bologna 2: 570, t. 39 (1850). Type: M. judicialis Bertol.f.
Laboucheria F.Muell., J. Proc. Linn. Soc., Bot. 3: 158 (1859). Type: L. chlorostachya F.Muell.
Brenan (1960) nominated Fillaea Guill. & Perr. for rejection on the basis of a later publication date for Erythrophleum Afzel. ex G.Don. (1832); however, Brown (1826) is considered to have validated the name Erythrophleum, so rejection was deemed unnecessary by the nomenclature committee.
A genus of 12 species in the tropical regions of Africa, Madagascar, Asia, and Australia; three species endemic to northern Australia.
Erythrophleum arenarium R.L.Barrett & M.D.Barrett, sp. nov.
Type: Frome Rocks Road, 18 miles [~29 km] south-east of Manguel Creek Station Homestead, SW Kimberley, Western Australia, 21 October 1984, D.Fell 297 (holo: PERTH 02211947; iso: BRI AQ444796).
Tree up to 6(–8) m tall, sometimes a shrub, few-branched with a compact crown, resprouting after fire; bark dark grey to blackish, roughly tessellated; branchlets usually glabrous, sometimes a little corky, sometimes slightly glaucous at the nodes. Leaves with petiole 42–55 mm long; rachis 14–40 mm long; pinnae usually 2(3) pairs, sometimes 1 or 2 pinna aborted so appearing alternate; secondary rachises 40–110 mm long; leaflets alternate, mostly 2–5(–7) per pinna, orbicular to somewhat deltoid, mostly 29–74 mm long, 27–83 mm wide, slightly larger on resprout growth (up to 87 mm long, up to 97 mm wide), obtuse to cordate, ±symmetric or slightly asymmetric, rounded, obtuse or emarginate apically, glabrous or slightly glaucous; petiolules 4–9(–11) mm long; venation conspicuous. Racemes mostly 80–140 mm long; axis 1.0–2.1 mm thick below the flowers, glabrous. Flowers 72–88; distinctly pedicellate, pedicels 3.0–5.5 mm long at anthesis; cream to greenish-yellow. Floral bracts 1.5–2.5 mm long, margins fimbriate, lamina glabrous. Calyx 3.6–5.0 mm long; lobes shorter than the tube, 1.1–2.1 mm long, pubescent only on margins. Petals 4.2–6.4 mm long, with pubescent margins and scattered hairs on the adaxial surface. Stamens alternately long and short, filaments 4.6–9.7 mm long, glabrous. Anthers 1.0–1.3 mm long. Ovary densely pubescent, 3.6–5.3 mm long. Style 1.0–2.2 mm long. Pod often slightly curved, dehiscing along both sutures, (1–)3–6-seeded, (85–)145–175 mm long, 30–38 mm wide, apex acute to apiculate; dark reddish-brown, glabrous; stipe of pod often asymmetric, 11–33 mm long. Seeds brown, suborbicular, 12–14 mm long, 10–13 mm wide, 4.0–5.0 mm thick; aril 3–6 mm long. (Fig. 2)
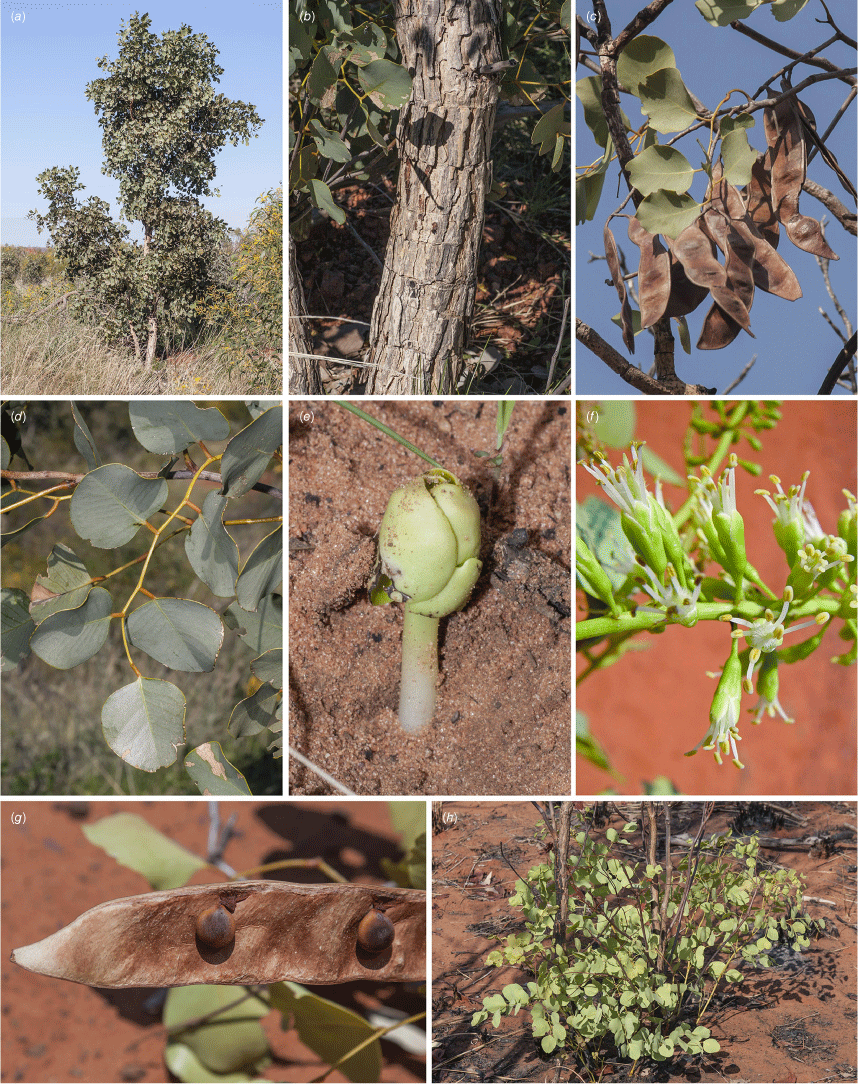
Erythrophleum arenarium: (a) habitat and habit; (b) bark; (c) fruiting branchlet; (d) slightly glaucous leaf; (e) seedling; (f) portion of inflorescence; (g) inside view of legume with attached seeds; (h) young sapling with main stems killed by fire and multiple stems resprouting. Vouchers: north of Pardoo Roadhouse, WA, not vouchered (a, b, d); north of Dampier Downs Station, WA, R.L.Barrett & M.Gresser RLB 9080, PERTH (c, e, g, h); Anna Plains, WA, G.Byrne 1271, PERTH (f). Images by R. L. Barrett (a–e, g, h), and G. Byrne (f).
D. E. Symon in J. P. Jessop (Ed.), Fl. Centr. Australia 104, fig. 127 (1981); K. F. Kenneally et al., Broome & Beyond. Pl. & People Dampier Peninsula 76, [lower pl. only] (1996); B. Kane, Broome’s Natural Environment (2023); http://wkfl.asn.au/nature/ironwood.html (accessed 9 August 2023), (all as E. chlorostachys).
Endemic in northern Australia, from the northern edge of the Pilbara, north to the Dampier District in the southern Kimberley and east through most of the Great Sandy Desert.
Flowering recorded for August–November and fruiting recorded for April, May, August, October and November.
The epithet is derived from the Latin arenarius (pertaining to sand), in reference to the specific habitat of this species on sands associated with the Great Sandy Desert (see McKenzie et al. 1983).
The following combination of characters is diagnostic: tree up to 6(–8) m tall, few-branched with a compact crown, branchlets usually glabrous, sometimes glaucous. Leaves with pinnae usually in 2(3) pairs; leaflets mostly 2–5(–7) per pinna, orbicular to somewhat deltoid, not or slightly asymmetric; glabrous or slightly glaucous; petiolules 4–9(–11) mm long; raceme axis glabrous. Calyx 3.6–5.0 mm long; lobes 1.1–2.1 mm long, pubescent only on margins; pedicels 3.0–5.5 mm long at anthesis; stipe of pod 11–33 mm long.
Ross (1998) noted that plants from the south-western Kimberley differed from the majority of the species’ range in their almost glabrous, pedicellate flowers that are also larger. Field observations have found these characteristics to be consistent, with plants readily identifiable as either E. arenarium or E. pubescens in a narrow zone of overlapping distribution in adjacent habitats between Willare Bridge and Blina, in Western Australia.
Known as camel poison. The common name of desert ironwood is suggested here as a more definitive name. First Nations names include joonggoomarr (Bardi), jun’ju, and bilamana (Yawuru) (Kenneally et al. 1996).
Although reasonably widespread and not currently threatened, it is noted that some populations may be affected by mining for mineral sands on the Dampier Peninsula (https://www.epa.wa.gov.au/1080-thunderbird-mineral-sands-project).
WESTERN AUSTRALIA. 1 km NW of Dampier Downs Station northern gate, 1 Nov. 2014, R.L.Barrett & M.Gresser RLB 9080 (CANB, DNA, NSW, PERTH); 23.3 km along pipeline track, ESE of Shay Gap, and NE of Marble Bar, 23 Apr. 2006, A.R.Bean 25079 (BRI); Great Sandy Desert, 25 Apr. 1964, J.S.Beard 3249 (PERTH); Anna Plains Road, 3 km from Great Northern Highway, 28 Sep. 2004, G.Byrne 1271 (PERTH); between De Grey River and La Grange Bay, Carey s.n. (MEL); near Racecourse, Broome, 31 Aug. 1991, B.J.Carter 484 (DNA, n.v., PERTH); 55 km SW of Derby, 12 Oct. 1985, H.Demarz 10906 (PERTH); 25 km E of Port Hedland Road on Dampier Downs Road, 26 June 1984, S.J.Forbes 2465 (HO n.v., MEL, PERTH); Millijiddee Homestead, St George Ranges, 5 May 1960, C.A.Gardner 12389 (PERTH); McLarty Hills, Great Sandy Desert, 5 Aug. 1977, A.S.George 14639 (PERTH); 94 km ESE of Telegraph Line, Anketell Ridge Road, Great Sandy Desert, 13 Aug. 1977, A.S.George 14819 (PERTH); [near] Beagle Bay, 1879, A.Forrest & Carey (MEL); 58 km SSW of No. 1 McHugh Bore on Dampier Downs Station, 26 Sep. 1980, S.D.Hopper 1733 (PERTH); near Roebuck Bay, Nov. 1967, F.Lullfitz s.n. (PERTH); Dampier’s Land near Broome, July 1911, E.M.Mjöberg s.n. (NSW); ~32 km E of Yeeda Homestead on the Great Northern Highway, 27 May 1967, E.A.Shaw 810 (AD); 12 km N of Pardoo Roadhouse on North West Coastal Highway, 23 Oct. 2015, L.S.J.Sweedman 8997 (PERTH).
Erythrophleum chlorostachys (F.Muell.) Baill., Hist. Pl. 2: 150 (1870)
Laboucheria chlorostachya F.Muell., J. Proc. Linn. Soc., Bot. 3: 159 (1859). Erythrophleum laboucheri F.Muell., Ann. Rep. Gov. Bot. & Dir. Bot. Gard. 1862–1863: 12 (1863), nom. illeg., nom. superfl., as ‘E. laboucherii’. Erythrophleum laboucheri F.Muell. ex Benth., Fl. Austral. 2: 297 (1864), nom. nov., isonym, as ‘E. laboucherii’. Erythrophleum chlorostachys (F.Muell.) Hennings ex Taub., Nat. Pflanzenfam. [Engler & Prantl] 3(3): 127 (1892), isonym.
Type citation: ‘A plagis boreali-occidentalibus Australiae usque ad flumen Burdekin tractus orientalis, tam in solo fertiliore quam steriliore planitierum montiumque satis frequenter obvia.’
Type: Victoria River, [Northern Territory], September 1855, F.Mueller s.n. (lecto, here designated: MEL 1524198; isolecto: MEL 1524197).
Residual syntypes (all representing E. chlorostachys): Northern Territory: Arnhem Land, F.Mueller s.n. (K 000756965, K 000756966 (photos at MEL)); Victoria River, June 1856, F.Mueller s.n. (K 000756961, K 000756963, K 000756967); Gulf of Carpentaria, F.Mueller s.n. (MEL 1524187); seen at the Burdekin River, Queensland, and Port Essington, Northern Territory, 1849, F.W.L.Leichhardt s.n. (NSW 415300, P 02939477).
Possible syntypes: Australia: location, collector and date unknown (K 000756964); northern coast of Arnhem Land, J.M’Kinlay s.n. (MEL 1524178); scrub towards the Gulf of Carpentaria, lat. 17°S, J.M.Stuart (MEL 1524189).
Excluded syntype (representing E. pubescens): Strangways River, n.d., F.Mueller (AD 97813219, MEL 1524186).
Tree up to (4–)6–9 m tall, many-branched with a spreading crown, resprouting after fire; bark dark grey to blackish, roughly tessellated and irregularly, shallowly furrowed; branchlets smooth, rarely with any cork, usually glabrous, not glaucous. Leaves with petiole 23–72 mm long; rachis 24–115 mm long; pinnae usually (1)2(3) pairs, sometimes 1 or 2 pinna aborted so appearing alternate; secondary rachises 50–170 mm long; leaflets alternate, mostly 3–6(–7) per pinna, orbicular to somewhat deltoid, mostly 31–87 mm long, 31–80 mm wide, slightly larger on resprout growth (up to 90 mm wide), obtuse to cordate, slightly asymmetric, rounded, obtuse or emarginate apically, glabrous or rarely very finely pubescent when very young, soon glabrescent; petiolules 2.5–7 mm long; venation conspicuous. Racemes usually simple, rarely once-branched, mostly 60–105 mm long; axis 0.9–1.6 mm thick below the flowers, glabrous. Flowers 55–100; distinctly pedicellate, pedicels 0.5–1.2 mm long at anthesis (occasionally sessile in bud); cream to greenish-yellow. Floral bracts 0.7–0.9 mm long, margins ciliate, lamina glabrous. Calyx 2.3–3.8 mm long; lobes shorter than the tube, 0.9–1.2 mm long, ciliate only on margins. Petals 2.8–3.8 mm long, exserted by 0.8–2.3 mm, with ciliate margins and glabrous adaxial surface. Stamens alternately long and short, filaments 3.2–6.4 mm long, glabrous. Anthers 0.7–1.0 mm long. Ovary densely pubescent, 1.8–2.7 mm long. Style 1.9–2.8 mm long. Pod often slightly curved, dehiscing along both sutures, (2–)3–8-seeded, (90–)120–210 mm long, 23–32 mm wide, apex acute to apiculate; dark reddish-brown, glabrous; stipe of pod often asymmetric, 12–20 mm long. Seeds brown, suborbicular, 10–11 mm long, 9–10 mm wide, 2.5–5.1 mm thick; aril 2–4.8(–7) mm long. (Fig. 3)
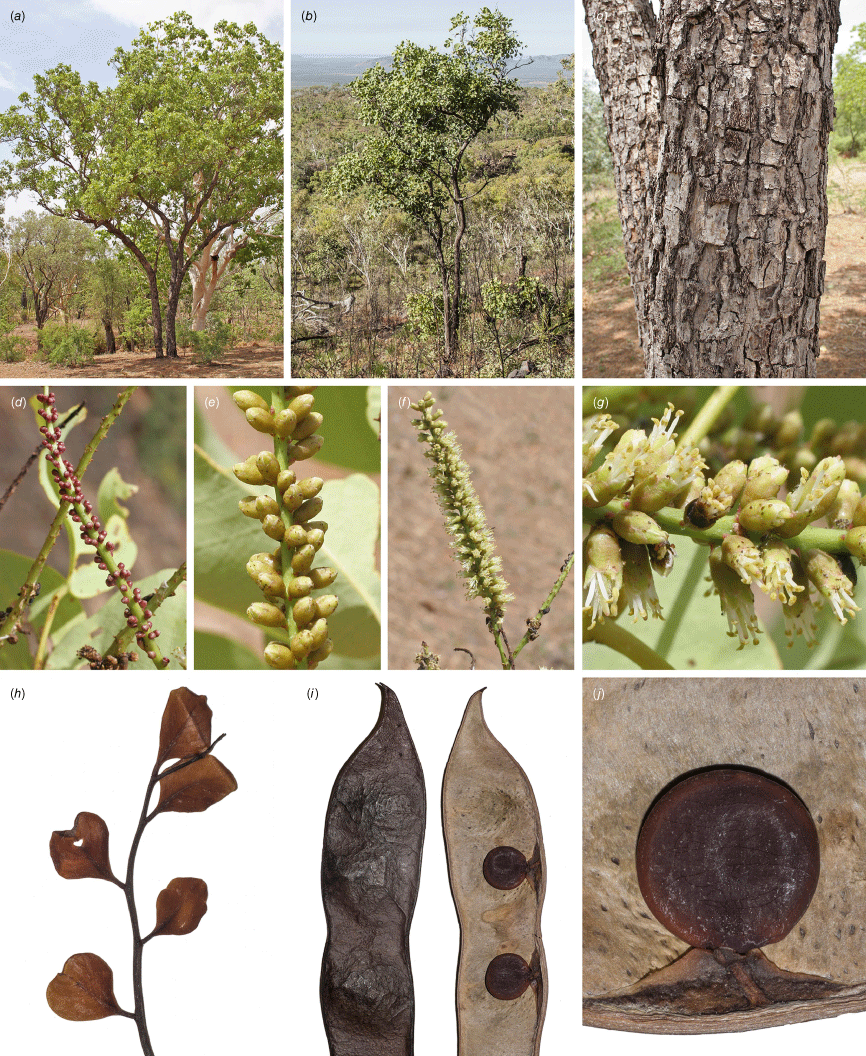
Erythrophleum chlorostachys: (a, b) habit and habitat; (c) bark; (d) inflorescence with very young buds; (e) flower buds; (f) flowering inflorescence (g) portion of flowering inflorescence showing shortly pedicellate flowers, glabrous axis and glabrous calyx; (h) glabrous new adult growth; (i) pod; (j) seed. Vouchers: King River, Kununurra, WA, G.Byrne 3693, PERTH (a, c–g); Ragged Range, WA, not vouchered (b); Katherine, NT, L.G.Adams 1653, NSW (h); ‘Katherine National Park’, NT, M.D.Tindale 6040 & C.R.Dunlop, NSW (i, j). Images by G. Byrne (a, c–g), and R. L. Barrett (b, h–j).
K. Brennan, Wildfl. Kakadu 52, pl. 83 (1986); M. Clark & S. Traynor, Pl. Trop. Woodl. 50, fig. (1987); J. Brock, Top End Native Pl. 150, pl. (1988); J. H. Ross in A. E. Orchard (Ed.), Fl. Australia 12: 70, fig. 28 (1998); P. Moore, Guide Pl. Inland Australia 392–3, pl. (2005).
Endemic in northern Australia, from Wyndham and along the Ord River, through central Northern Territory to the Gulf of Carpentaria, Queensland.
The epithet is derived from the Greek chloro- (green) and -stachys (spike), in reference to the greenish flower spikes.
The following combination of characters is diagnostic: tree (4–)6–9 m tall, many-branched with a spreading crown, branchlets glabrous, not glaucous; leaves with pinnae usually in (1)2(3) pairs; leaflets mostly 3–6(–7) per pinna, orbicular to somewhat deltoid, slightly asymmetric; glabrous or rarely very finely pubescent when very young, soon glabrescent; petiolules 2.5–7 mm long; raceme axis glabrous; pedicels 0.5–1.2 mm long; calyx 2.3–3.8 mm long; lobes 0.9–1.2 mm long, ciliate only on margins; stipe of pod 12–20 mm long.
Close examination of specimens at MEL and images of the remaining syntypes of Laboucheria chlorostachys showed that most of the specimens are referable to the taxon defined here as E. chlorostachys. Of the six possible syntypes identified above, three possible syntypes were likely to be available to Mueller at the time of description, but cannot be verified as original material. One syntype is referable to a different taxon than the majority, namely E. pubescens. Of the remaining two specimens, the more complete specimen was selected as lectotype.
The lectotype bears a label with the name ‘Laboucheria chlorostachys Ferd. Mueller.’ This collection is in fruit, so the distinguishing floral characteristics cannot be determined on this specimen, but the leaf characters and location clearly distinguish it from E. arenarium and E. pubescens.
Ferdinand Mueller (1859) described Laboucheria chlorostachya on the basis of material that mostly belonged to the entity here typified; however, material later illustrated for the Iconography of Australian Species of Acacia and Cognate Genera (Mueller 1888) actually represents E. pubescens. The excellent lithograph from that work is reproduced here to illustrate the new species. In the treatment by Ross (1998), fig. 28 represents E. chlorostachys, whereas fig. 31 and 43 represent E. pubescens.
Known as Top End ironwood.
WESTERN AUSTRALIA. Cambridge Gulf, 1886, H.S.Banford & E.W.Nyulasy s.n. (MEL); Buttons Gap, Ord River, 17 Apr. 1956, N.T.Burbidge 5196 (CANB, MEL); E bank of the King River, Kununurra, 12 Dec. 2009, G.Byrne 3693 (MEL, PERTH); Wyndham, 24 May 1944, C.A.Gardner 7257 (PERTH, 2 sheets); W of Cambridge Gulf, 1887, A.J.Keiller s.n. (MEL); Five Rivers Lookout, Wyndham, 20 Aug. 2001, R.C.H.Shepherd 76 (MEL); ~1 km uphill from Camp Nicholas, Smoke Creek, SW of Lake Argyle, 5 May 1980, A.S.Weston 12284 (PERTH). NORTHERN TERRITORY. Stuart Hwy, 6 miles [~9.6 km] NW of Katherine, L.G.Adams 811 (BRI, CANB); 4-mile farm, CSIRO Research Station, Katherine, 7 Dec. 1966, L.G.Adams 1653 (BRI, CANB, E, MEL, NSW); 3 km N of Dunmurra, Stuart Highway, 2 July 1974, A.C.Beauglehole 46481 & G.W.Carr 2702 (BRI n.v., MEL); U.D.P. Falls, ±80 km NE of Pine Creek, 12 Aug. 1978, A.C.Beauglehole 58566 & E.G.Errery (MEL); Weimoor Springs, 9 Aug. 1962, M.Cole 132 & D.Provan (BRI); Fitzmaurice River basin, 13 May 1994, C.R.Dunlop 9979 & P.K.Latz (DNA n.v., MEL); Maningrida, 1961, L.Gressitt 2668 (BRI); Fish River, 11 Feb. 1991, D.Larcombe 2 (DNA); Kakadu National Park, 22 km SSW of Cooinda on Pine Creek Road, 19 May 1980, M.Lazarides 8845 (CANB, DNA); Pine Creek, 6 June 1973, M.Parker 113 (BRI, DNA, NT); Tortilla Flats, 4 Nov. 1974, M.Parker 528 (CANB, DNA, n.v., NE, n.v.); No. 7 Stock Route Bore (S of Dunmarra), undated, A.L.Rose s.n. (BRI); 40 miles [~64.4 km] S of Katherine, 7 Sep. 1961, N.H.Speck 1643 (AD, BRI, CANB, MEL, NSW, PERTH, 2 sheets); 17 mi. W of Katherine on road to Willaroo, 17 July 1967, D.E.Symon 5188 (AD, CANB, K, NT); Katherine National Park, 8 July 1979, M.D.Tindale 6040 & C.R.Dunlop (CANB, DNA n.v., NSW); Ranger HQ, Nitmiluk National Park, 13 Dec. 1990, G.Wightman 5205 (DNA); Mary River Camp, June 1955, M.White M.R. 18 (CANB). QUEENSLAND. Etheridge River, Armit 679 (MEL, 2 sheets); 14 miles [~22.6 km] SE of Carpentaria Downs Station, 12 July 1954, N.H.Speck 4702 (AD, CANB, NSW, PERTH).
Erythrophleum pubescens R.L.Barrett & M.D.Barrett, sp. nov.
Type: Carson Escarpment, N of Coucal Gorge, Drysdale River National Park, Western Australia, 17 August 1975, A.S.George 13961 (holo: PERTH 2211939; iso: CANB 267799, K, n.v.).
Tree 8–20(–30) m tall, many-branched with a spreading canopy, resprouting after fire, rarely flowering as a shrub when frequently burnt; bark dark grey to blackish, tessellated; branchlets usually prominently fissured, corky, pubescent when young, newest growth with rusty hairs, eventually becoming glabrous. Leaves with petiole 18–51 mm long; rachis 35–138 mm long; pinnae usually 2 or 3 pairs; secondary rachises 35–155 mm long; leaflets alternate, mostly 5–11 per pinna, obliquely elliptic, ovate or obovate, mostly 19–62 mm long, 9–45 mm wide, resprout growth often markedly larger; attenuate, distinctly asymmetric, rounded, obtuse or emarginate apically, discolourous, with scattered hairs basally and on the petiolules, eventually becoming glabrous; petiolules 1.5–4 mm long; venation conspicuous. Racemes simple to 5-branched, (45–)70–160 mm long; axis 1.0–1.7 mm thick below the flowers, moderately to densely pubescent, hairs white or rusty. Flowers 62–125; sessile; cream to greenish-yellow. Floral bracts 0.5–0.7 mm long, margins ciliate and lamina pubescent. Calyx 1.9–2.5 mm long; lobes shorter than the tube, 0.5–0.9 mm long, pubescent on surface and margins. Petals 2.3–2.9 mm long, pubescent at the margins, otherwise glabrous. Stamens alternately long and short, filaments 3.4–8.9 mm long, glabrous. Anthers 0.6–0.7 mm long. Ovary densely pubescent, 2.1–3.4 mm long. Style 0.7–1.7 mm long. Pod often slightly curved, dehiscing, at least initially, along one suture only, (1–)2–7-seeded, 97–120 mm long, 26–46 mm wide, apex acute to apiculate; dark reddish-brown, pubescent when young, glabrescent; stipe of pod often lateral, 8–10 mm long. Seeds dark brown, suborbicular, 12–15 mm long, 9–12 mm wide, 4.5–6.0 mm thick; aril 5–8 mm long. (Fig. 4, 5.)
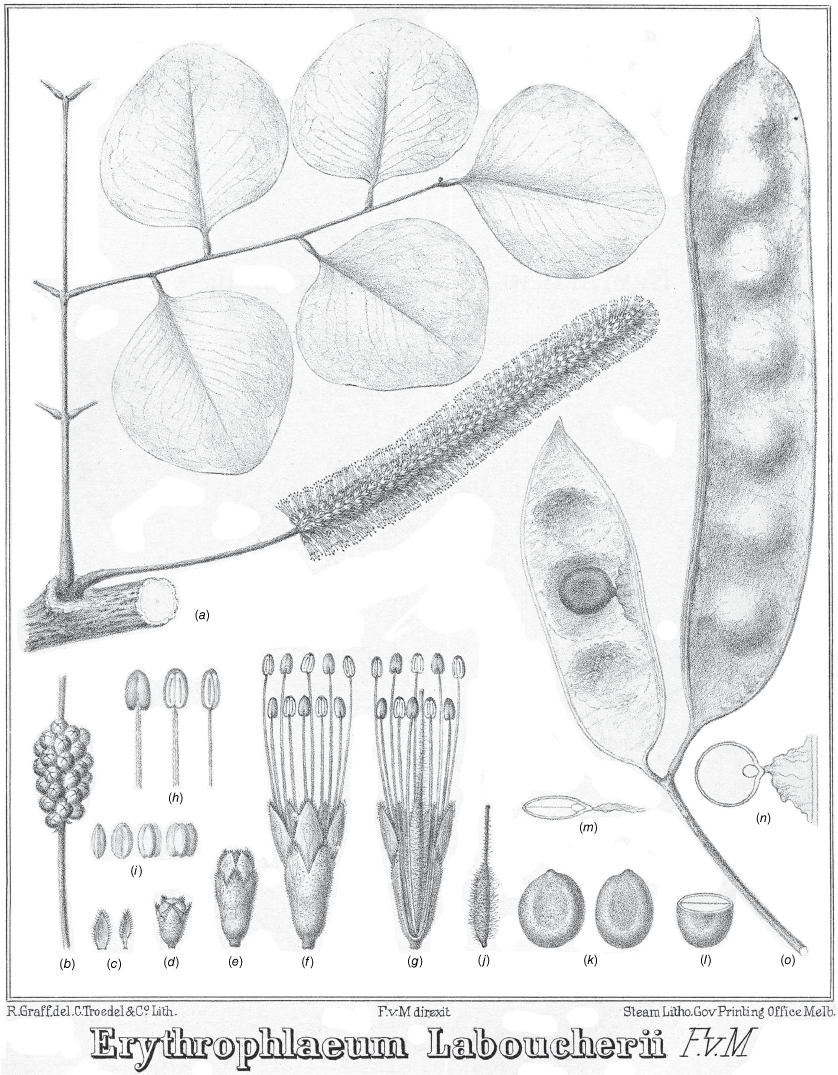
Erythrophleum pubescens (published as ‘Erythrophlaeum Laboucherii’). (a) Flowering branchlet and portion of a bipinnate leaf (probably resprout leaf growth, or possibly leaflets representing E. chlorostachys); (b) flower buds on inflorescence; (c) floral bracts; (d) flower bud; (e) flower just prior to anthesis; (f) staminate flower at anthesis; (g) partially dissected bisexual flower; (h) back, front and lateral view of a stamen; (i) pollen grains; (j) ovary and style; (k) two seeds; (l) transverse section of a seed; (a, b) longitudinal sections of seeds; (o) pods, left hand pod opened to show seed and aril (voucher: not specified). Original lithograph by R. Graff under supervision of F. von Mueller. Originally published in Mueller (1888, t. 9).
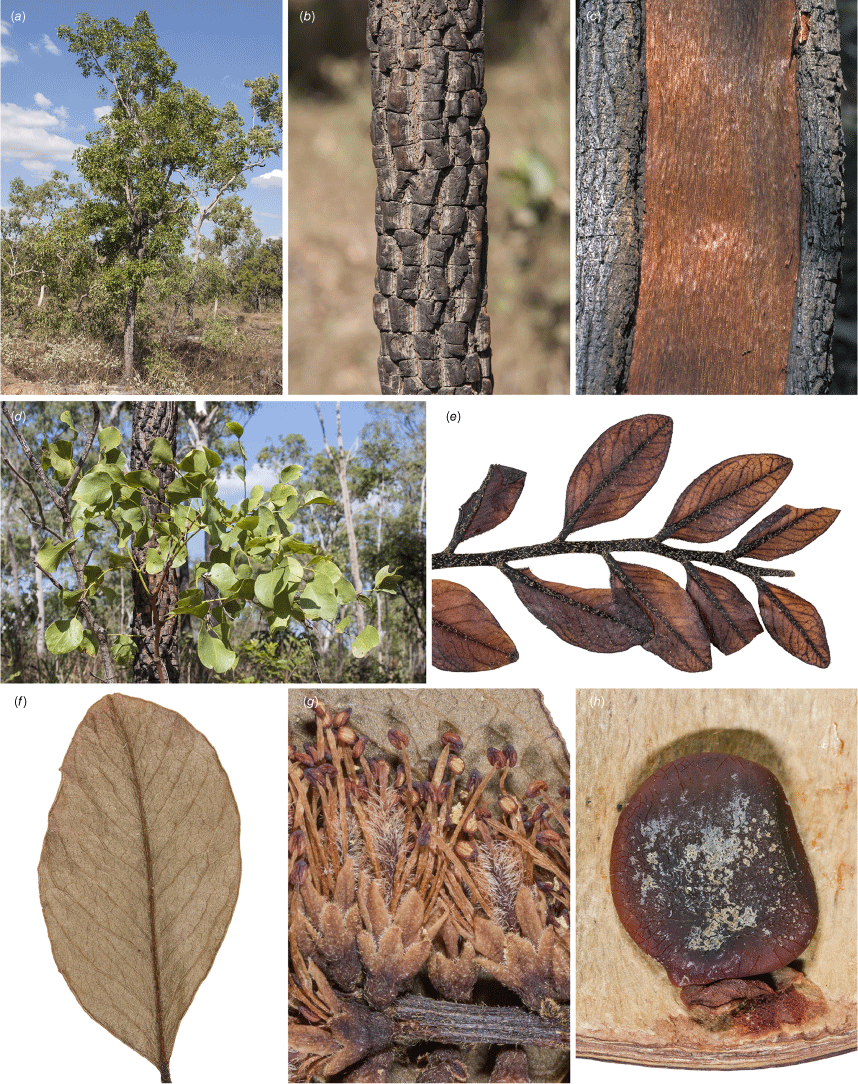
Erythrophleum pubescens: (a) habit and habitat; (b) bark; (c) natural blaze on trunk showing red heartwood prized for woodworking; (d) juvenile, asymmetric pinnae; (e) pubescence on new adult growth; (f) asymmetric pinna with sparse hairs; (g) portion of inflorescence showing sessile flowers, pubescent axis and pubescent calyx; (h) seed. Vouchers: King Edward River, WA, not vouchered (a, b, d); Mount Fyfe, Drysdale River Station, WA; not vouchered (c); Prince Regent Nature Reserve, WA, A.S.George 12300, PERTH (e, h); Drysdale River National Park, WA, A.S.George 13961, PERTH (f, g). Images by R. L. Barrett.
F. Mueller, Iconogr. Austral. Acacia 13: t. 9 (1888). C. A. Gardner & H. W. Bennetts, Poison. Pl. West. Australia, pl. 3 (1956); D. Levitt, Pl. People pl. 8 (1981); J. R. Wheeler in J. R. Wheeler (Ed.), Fl. Kimberley Region 347, fig. 104a (1992); C. R. Dunlop et al., Fl. Darwin Region 2: 32, fig. 13 (1995); K. F. Kenneally et al., Broome & Beyond. Pl. & People Dampier Peninsula 76, [upper pl. only] (1996); J. H. Ross in A. E. Orchard (Ed.), Fl. Australia 12: 70, fig. 31, 43 (1998); J. Milson, Trees Shrubs NW Queensl. 18–19, pl. (2000); W. Cooper, Fruits Austral. Trop. Rainfor. 102, fig. (2004); J. Beasley, Pl. Trop. N. Queensl. 156, pls (2006); J. Beasley, Pl. Cape York 43, pls (2012), (all as E. chlorostachys).
Endemic in northern Australia, from near Derby in the western Kimberley, WA, through NT and islands of the Gulf of Carpentaria to north-eastern Qld, Cape York and some Torres Strait islands.
Usually found in open Eucalyptus or mixed forest, woodland or savanna on sandstone- or basalt-derived soils.
Flowering mostly July–November, but also recorded for March and May. Fruiting mostly recorded for April–August.
The epithet is from the Latin pubescens (with short, soft hairs), in reference to the presence of hairs on most parts at least when young, diagnostically always present on the raceme axis and outer surfaces of the calyx.
The following combination of characters is diagnostic: tree 8–20 m tall, many-branched with a spreading canopy, branchlets pubescent when young; leaves with pinnae usually in 2 or 3 pairs; leaflets mostly 5–11 per pinna, attenuate, distinctly asymmetric, with scattered hairs basally and on the petiolules; petiolules 1.5–4 mm long; raceme axis moderately to densely pubescent; calyx 1.9–2.5 mm long; lobes 0.5–0.9 mm long, pubescent on surface and margins; flowers sessile; stipe of pod 8–10 mm long.
Although the majority of the syntypes represent the taxon here recognised as E. chlorostachys sensu stricto, the most-used concept in the literature, and apparently the main source for Mueller’s original description and his illustration in Iconogr. Austral. Acacia 13: t. 9 (1888) is the taxon described here as E. pubescens.
Resprout foliage of E. pubescens is large, similar in size to that of E. arenarium, but it can be separated by the presence of scattered to dense hairs on the branchlets, petioles and petiolules. Juvenile pinnae also remain distinctly asymmetric in most cases, although not always as distinctly as the adult pinnae.
Widely known as Cooktown ironwood under the name E. chlorostachys, a species that is not known to occur near Cooktown; so, this common name must logically be transferred to E. pubescens, or its use discontinued. There are many First Nations names, which may also include E. chlorostachys, as detailed in Table 1.
The most useful characters for distinguishing the three species are presented in Table 3.
| Item | E. arenarium | E. chlorostachys | E. pubescens | |
|---|---|---|---|---|
| Habit | (2–)4–6(–8) m tall, few-branched with a compact crown | (4–)6–9 m tall, many-branched with a spreading crown | 8–20(–30) m tall, many-branched with a spreading crown | |
| Branchlet bark | Smooth, sometimes a little corky | Smooth, rarely any cork | Prominently fissured-corky | |
| Branchlets & leaves | Usually glabrous, sometimes glaucous | Glabrous, not glaucous | Pubescent when young, not glaucous | |
| Pinnae | 2(3) pairs | (1)2(3) pairs | 2 or 3 pairs | |
| Leaflets | 29–74 mm long, 27–83 mm wide, 2–5(–7) per pinna, orbicular to somewhat deltoid, not or slightly asymmetric; glabrous or slightly glaucous | 31–87 mm long, 31–80 mm wide, 3–6(–7) per pinna, orbicular to somewhat deltoid, slightly asymmetric; glabrous | 19–62 mm long, 9–48 mm wide, 5–11 per pinna, attenuate, distinctly asymmetric with scattered hairs on and near the petiolule | |
| Petiolules | 4–9(–11) mm long | 2.5–7 mm long | 1.5–4 mm long | |
| Inflorescence | Simple | Simple or once branched | Simple or once branched | |
| Raceme axis | Glabrous | Glabrous | Pubescent | |
| Floral bracts | 1.5–2.5 mm long, margins fimbriate, lamina glabrous | 0.7–0.9 mm long, margins ciliate, lamina glabrous | 0.5–0.7 mm long, margins ciliate and lamina pubescent | |
| Pedicel | 3.0–5.5 mm long | 0.5–1.2 mm long | Absent | |
| Calyx | 3.6–5.0 mm long, glabrous | 2.3–3.8 mm long, glabrous | 1.9–2.5 mm long, pubescent | |
| Calyx lobes | 1.1–2.1 mm long | 0.9–1.2 mm long | 0.5–0.9 mm long | |
| Petals | 4.2–6.4 mm long (exserted by 2.3–4.5 mm) | 2.8–3.8 mm long (exserted by 0.8–2.3 mm) | 3.1–3.3 mm long (exserted by 1.6–1.9 mm) | |
| Anthers | 1.0–1.3 mm long | 0.7–1.0 mm long | 0.60–0.7 mm long | |
| Stipe of pod | 11–33 mm long | 12–20 mm long | 8–10 mm long | |
| Aril | 3–6 mm long | 2–4.8(–7) mm long | 5–8 mm long | |
| Distribution | Great Sandy Desert, Dampier Peninsula | Wyndham to Cape York | Derby to Cape York |
WESTERN AUSTRALIA. Drysdale River, S of river mouth on Carson River Station, 24 June 2018, M.D.Barrett MDB5902 (PERTH); ‘Prince Regent’ [Roe] River, 1891, Bradshaw & Allen s.n. (MEL, NSW); Five Rivers Lookout, 7 Feb. 2010, G.Byrne 3721 (PERTH); Gorge in Saw Ranges, NW of Dunham River, 6 Nov. 1992, K.Coate 224 (PERTH); Careening Bay, 1820, A.Cunningham 227 (BM, n.v., K, NSW); Charnley River [Station], 1 km N of Potts Camp, 26 June 2012, H.Dauncey H 666 (PERTH); Cone Mountain, on peninsula between Vansittart Bay and Napier Broome Bay, ±25 km WNW of Kalumburu, 22 may 1984, S.J.Forbes 2085a (MEL); Kuri Bay, Bonaparte Archipelago, 2 Sep. 1985, P.R.Foulkes 340 (PERTH); Lawley River, 30 July 1921, C.A.Gardner 997 (PERTH); Bushfire Hill, Prince Regent [Nature] Reserve, 15 Aug. 1974, A.S.George 12300 (PERTH); Kununurra, 15 Sep. 1982, C.Glover 112 (MEL, PERTH); Boiga Falls, Drysdale River National Park, 4 Aug. 1975, K.F.Kenneally 3046 (CANB, PERTH); Loc. 4.0, SE of Beverley Springs Homestead, 19–26 May 1979, B.G.Muir et al. 734 (PERTH); Jameson Arch, Bachsten Camp track, Mount Elizabeth Station, 15 Sep. 2003, G. & N.Sankowsky 2249 (PERTH); Adcock Gorge, 13 Apr. 1980, D.E.Symon 12095 (AD, K, PERTH). NORTHERN TERRITORY. Darwin, Nov. 1929, F.A.K.Bleeser 503 (MEL); Sturt Plateau, Dungowan Station, 12 Oct. 2000, K.Brennan 4583 (DNA); Black Point, 29 Sep. 1968, N.Byrnes 1043 (AD, NT, PERTH); 77.3 miles [~124.4 km] S of Katherine, 9 Sep. 1957, G.Chippendale NT 3732 (AD, NSW, NT, PERTH); Arnhem land 8.6 km S of Walker River airstrip, 11 Oct. 1987, M.Clark 1607 (DNA); Kapalga Ref. 1211, 15 Dec. 1976, R.Collins BC172 (CANB, DNA, n.v., MEL, NSW); Litchfield NP, Walker Creek area, 15 Mar. 1995, I.Cowie 5303 (DNA); Narbalek, 25 Oct. 1987, C.R.Dunlop 7145 (DNA, n.v., MEL, NSW); Cutta Cutta, 13 July 1990, M.Evans 3270 (CANB, DNA, MEL, NSW); Cutta Cutta Reserve, 23 Nov. 1993, J.Egan 2873 (DNA); Newcastle Waters, 17 July 1911, G.F.Hill 473 (MEL); Port Darwin, 1883, Holtze (MEL); Black Jungle area, 17 Sep. 1986, N.M.Smith 101 (DNA); Lameroo Beach, Darwin, 8 Oct. 1986, N.Smith 128 (DNA); on Stuart Highway 42 km N of Carpentaria Highway junction, 5 Sep. 1981, T.Whaite 3979 & J.Whaite (CANB, DNA, n.v., K, n.v., MO, n.v., NSW). QUEENSLAND. Georgetown, n.d., Armit 717 (MEL); Cooktown, 13 May 1970, S.T.Blake 23184 (BRI, CNS, n.v., MEL, NSW, PERTH); Laura sandstone area N of Laura River near Early Man site, 16 May 1975, N.B.Byrnes 3365 (BRI, MEL, QRS, n.v.); Croydon, 24 Aug. 1913, R.H.Cambage 3920 (NSW, 2 sheets); Dutton River Station, ~46 km NW of homestead and 110 km E of Richmond, 15 June 2012, E.Leitch QDA003815 (BRI); Emu Creek Station, NE of Petford, 12 Nov. 2005, K.R.McDonald KRM4610, L.J.Roberts & J.A.Covacevich (BRI, MEL); creek flowing into Emu Lagoon, Errk Oykangand National Park, 1 Sep. 2010, K.R.McDonald KRM9767 (BRI); 42.9 km by road W of Georgetown, near Blancourt Station turnoff, 19 Sep. 2010, K.R.McDonald KRM9840 (BRI, MEL); Kings Plain Station, near Lily Dam, 5 Nov. 2015, K.R.McDonald KRM17554 (BRI, MEL); Weipa North township, State School oval, Jan. 1981, A.Morton 1183 (AD, MEL, 2 sheets); Endeavour River, 1882, W.A.Persieh 719 (MEL); Granite Creek, 12 km SW of Mareeba, 16 Nov. 1995, B.S.Wannan 213 & L.Lynch (NSW); Mareeba, road from Kennedy Highway to Springmount, 27 June 2001, J.J.Wieringa 4178, L.J.G. van der Maesen, A.Bruneau & P.Herendeen (NSW, WAG, n.v.); Gamboola, Mitchell River, 22 Sep. 1882, Wools s.n. (MEL).
| 1. | Branchlets commonly with fissured, corky bark; petiolules, leaf bases and inflorescence axis pubescent, at least when young; pinnae regularly in 2 or 3 pairs (i.e. both mixed on branchlets); leaflets 5–11 per pinnae, distinctly asymmetric; calyx 1.9–2.5 mm long; stipe of pod 8–10 mm long; flowers sessile...E. pubescens Branchlets smooth, rarely with corky bark, and then poorly developed; petiolules, leaf bases and inflorescence axis usually glabrous, sometimes glaucous; pinnae mostly in 2 pairs; leaflets 2–6(–7) per pinnae, usually ±symmetric or only slightly asymmetric; calyx 2.3–5.0 mm long; stipe of pod 11–33 mm long; flowers pedicellate (pedicels <0.5 mm long)...2 |
| 2. | Leaflets usually ±symmetric; floral bracts 1.5–2.5 mm long, margins fimbriate; pedicels 3.0–5.5 mm long; petals 4.2–6.4 mm long; anthers 1.0–1.3 mm long...E. arenarium Leaflets usually slightly asymmetric; floral bracts 0.7–0.9 mm long, margins pubescent; pedicels 0.5–1.2 mm long; petals 2.8–3.8 mm long; anthers 0.75–0.95 mm long...E. chlorostachys |
Supplementary material
Nexus alignment file of the ribosomal sequence data. Supplementary material is available online.
Conflicts of interest
Dr Russell Barrett is an editor for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Acknowledgements
Directors and staff at AD, BRI, CANB, DNA, MEL, NSW and PERTH are thanked for access to collections and facilities. Helen Barnes is thanked for checking specimens and for providing images of types at MEL. Geoff and Ruth Byrne are thanked for permission to use photographs. Miguel Garcia is thanked for scanning the original illustration from Mueller’s Iconography of Australian Species of Acacia and Cognate Genera. Brendan Lepschi is thanked for discussions on taxon concepts. Numerous people have supported fieldwork in the Kimberley region over a period of many years, including Buru Energy and Outback Ecology. Fieldwork in the Pilbara region was supported by BHP Iron Ore (Pilbara Seed Atlas Project). Mark Gresser (Outback Ecology) is thanked for assistance in the field. We acknowledge the contribution of the Genomics for Australian Plants (GAP) Framework Initiative consortium (https://www.genomicsforaustralianplants.com/consortium/), in particular Todd McLay and Daniel Murphy of the Royal Botanic Gardens Victoria, in the generation of data used in this publication. The GAP Initiative is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia. Peter Jobson, Michelle Waycott, Dan Murphy and four anonymous reviewers are thanked for constructive comments that improved an earlier version of the paper.
References
Addicott E, Newton M, Laurance S, Neldner J, Laidlaw M, Butler D (2018) A new classification of savanna plant communities on the igneous rock lowlands and Tertiary sandy plain landscapes of Cape York Peninsula bioregion. Cunninghamia 18, 29-72.
| Crossref | Google Scholar |
Anderson BM, Barrett MD, Krauss SL, Thiele K (2016) Untangling a species complex of arid zone grasses (Triodia) reveals patterns congruent with co-occurring animals. Molecular Phylogenetics and Evolution 101, 142-162.
| Crossref | Google Scholar | PubMed |
Anonymous (1922) Diagnoses africanae: LXXV. Bulletin of Miscellaneous Information (Royal Gardens, Kew) 1922(1), 27-32.
| Crossref | Google Scholar |
Armah FA, Annan K, Mensah AY, Amponsah IK, Tocher DA, Habtemariam S (2015) Erythroivorensin: a novel anti-inflammatory diterpene from the root-bark of Erythrophleum ivorense (A.Chev.). Fitoterapia 105, 37-42.
| Crossref | Google Scholar | PubMed |
Azani N, Babineau M, Bailey CD, Banks H, Barbosa AR, Pinto RB, Boatwright JS, Borges LM, Brown GK, Bruneau A, Candido E, Cardoso D, Chung K-F, Clark RP, Conceição AdS, Crisp M, Cubas P, Delgado-Salinas A, Dexter KG, Doyle JJ, Duminil J, Egan AN, De La Estrella M, Falcão MJ, Filatov DA, Fortuna-Perez AP, Fortunato RH, Gagnon E, Gasson P, Rando JG, Azevedo Tozzi AMG, Gunn B, Harris D, Haston E, Hawkins JA, Herendeen PS, Hughes CE, Iganci JRV, Javadi F, Kanu SA, Kazempour-Osaloo S, Kite GC, Klitgaard BB, Kochanovski FJ, Koenen EJM, Kovar L, Lavin M, Roux M, Lewis GP, de Lima HC, López-Roberts MC, Mackinder B, Maia VH, Malécot V, Mansano VF, Marazzi B, Mattapha S, Miller JT, Mitsuyuki C, Moura T, Murphy DJ, Nageswara-Rao M, Nevado B, Neves D, Ojeda DI, Pennington RT, Prado DE, Prenner G, de Queiroz LP, Ramos G, Ranzato Filardi FL, Ribeiro PG, Rico-Arce ML, Sanderson MJ, Santos-Silva J, São-Mateus WMB, Silva MJS, Simon MF, Sinou C, Snak C, de Souza ÉR, Sprent J, Steele KP, Steier JE, Steeves R, Stirton CH, Tagane S, Torke BM, Toyama H, de Cruz DT, Vatanparast M, Wieringa JJ, Wink M, Wojciechowski MF, Yahara T, Yi T, Zimmerman E (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: the Legume Phylogeny Working Group (LPWG). Taxon 66, 44-77.
| Crossref | Google Scholar |
Bailey FM (1900) Plants reputed to be poisonous to stock. Ironwood tree, or Leichhardt’s leguminous ironbark (Erythrophleum laboucherii, F.v.M.). Queensland Agricultural Journal 7, 153.
| Google Scholar |
Banks H, Lewis G (2009) Pollen morphology of the Dimorphandra group (Leguminosae, Caesalpinioideae). Grana 48, 19-26.
| Crossref | Google Scholar |
Banks H, Himanen I, Lewis GP (2010) Evolution of pollen, stigmas and ovule numbers at the caesalpinioid–mimosoid interface (Fabaceae). Botanical Journal of the Linnean Society 162, 594-615.
| Crossref | Google Scholar |
Barrett RL (2006) A review of Planchonia (Lecythidaceae) in Australia. Australian Systematic Botany 19, 147-153.
| Crossref | Google Scholar |
Barrett RL (2007) Corymbia cadophora subsp. polychroma (Myrtaceae): a new subspecies from the east Kimberley region of Western Australia. Nuytsia 17, 31-36.
| Crossref | Google Scholar |
Barrett RL (2013) Solanum zoeae (Solanaceae), a new species of bush tomato from the North Kimberley, Western Australia. Nuytsia 23, 5-21.
| Crossref | Google Scholar |
Barrett RL (2015) Examining range disjunctions in Australian Terminalia (Combretaceae) with taxonomic revision of the T. canescens and T. cunninghamii species complexes. Australian Systematic Botany 28, 23-45.
| Crossref | Google Scholar |
Barrett RL (2016) Three new species from the Kimberley region of Western Australia from the families Caryophyllaceae, Convolvulaceae and Poaceae. Nuytsia 27, 287-298.
| Crossref | Google Scholar |
Barrett RL (2019) Three new species of Corchorus L. and Grewia L. (Sparmanniaceae / Malvaceae subfamily Grewioideae) from northern Australia, an earlier name in Grewia, and recircumscription of Triumfetta kenneallyi Halford. Austrobaileya 10, 458-472.
| Crossref | Google Scholar |
Barrett RL, Barrett MD (2015) Twenty-seven new species of vascular plants from Western Australia. Nuytsia 26, 21-87.
| Crossref | Google Scholar |
Barrett RL, Telford IRH (2015) Two new species of Phyllanthus from northern Australia and notes on Phyllanthus, Sauropus and Synostemon (Phyllanthaceae) in Western Australia. Nuytsia 26, 149-166.
| Crossref | Google Scholar |
Barrett RL, Barrett MD, Kenneally KF, Lowrie A (2015a) Four new species of Stylidium (Stylidiaceae) from the Kimberley region of Western Australia. Nuytsia 26, 127-141.
| Crossref | Google Scholar |
Barrett RL, Hopper SD, Macfarlane TD, Barrett MD (2015b) Seven new species of Haemodorum (Haemodoraceae) from the Kimberley region of Western Australia. Nuytsia 26, 111-125.
| Crossref | Google Scholar |
Barrett RL, Barrett MD, Duretto MF (2015c) Four new species of Boronia (Rutaceae) from the Kimberley region of Western Australia. Nuytsia 26, 89-109.
| Crossref | Google Scholar |
Barrett RL, Bruhl JJ, Wilson KL (2021) Revision of generic concepts in Schoeneae subtribe Tricostulariinae (Cyperaceae) with a new genus Ammothryon and new species of Tricostularia. Telopea 24, 61-169.
| Crossref | Google Scholar |
Blackman LG (1904) Aboriginal wooden weapons of Australia: illustrative of a collection in the B.P.B. Museum. Bernice P. Bishop Museum Papers 2, 173-191.
| Google Scholar |
Bordulk D, Dalak N, Tukumba M, Bennett L, Tingey RB, Katherine M, Cutfield S, Pamkal M, Wightman G (2012) Dalabon (Ngalkbon/Dangbon) plants and animals: Aboriginal biocultural knowledge from southern Arnhem Land, North Australia. Northern Territory Botanical Bulletin Number 41, Department of Land Resource Management, Darwin, NT, Australia; and Diwurruwurru-jaru AC and Mimi Aboriginal Art & Craft, Katherine, WA, Australia.
Bowman DMJS, Panton WJ (1995) Munmarlary revisited: response of a north Australian Eucalyptus tetrodonta savanna protected from fire for 20 years. Australian Journal of Ecology 20, 526-531.
| Crossref | Google Scholar |
Bowman DMJS, Wilson BA, Hooper RJ (1988) Response of Eucalyptus forest and woodland to four fire regimes at Munmarlary Northern Territory Australia. Journal of Ecology 76, 215-232.
| Crossref | Google Scholar |
Brenan JPM (1960) (72) Proposal to conserve the generic name 3471, Erythrophleum Afzel. ex G. Don (Leguminosae) against Fillaea Guill. & Perr. (also Leguminosae). Taxon 9, 193-194.
| Crossref | Google Scholar |
Brundrett M, Abbott L, Jasper D, Malajczuk N, Bougher N, Brennan K, Ashwath N (1995) ‘Mycorrhizal associations in the Alligator Rivers Region. Part II Results of Experiments’. Final Report. (Office of the Supervising Scientist: Jabiru, NT, Australia) 10.13140/RG.2.1.2191.7043
Bruneau A, Forest F, Herendeen PS, Klitgaard BB, Lewis GP (2001) Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Systematic Botany 26, 487-514.
| Crossref | Google Scholar |
Callmander MW, Buerki S, Zich FA, Field AR, Gallaher T (2021) Pandanus grayorum (Pandanaceae), a new species endemic to north-eastern Queensland (Australia). Australian Systematic Botany 34, 327-335.
| Crossref | Google Scholar |
Carmelet-Rescan D, Morgan‐Richards M, Pattabiraman N, Trewick SA (2022) Time‐calibrated phylogeny and eco-logical niche models indicate Pliocene aridification drove intraspecific diversification of brushtail possums in Australia. Ecology and Evolution 12, e9633.
| Crossref | Google Scholar | PubMed |
Chauke SH, Kritzinger Q (2020) Erythrophleum lasianthum. In ‘Underexplored medicinal plants from Sub-Saharan Africa: plants with therapeutic potential for human health’. (Ed. N Lall) pp. 105–110. (Elsevier) 10.1016/B978-0-12-816814-1.00015-6
Cheinmora D, Charles A, Karadada T, Bernadette W, Nyalerin F, Waina L, Punchi M, Chalarimeri A, Unghango D, Saunders T, Sefton M, Vigilante T, Wightman G (2017) Belaa plants and animals: biocultural knowledge of the Kwini people of the far north Kimberley, Australia. Northern Territory Botanical Bulletin Number 46, Department of Environment and Natural Resources, Palmerston, NT, Australia; and Kwini people through Balanggarra Aboriginal Corporation, Wyndham, WA, Australia.
Cole N (2022) Aboriginal rock art of the Laura valleys: one landscape, many stories. In ‘Histories of Australian rock art research’. (Eds PSC Taçon, SK May, UK Frederick, J McDonald) pp. 257–275. (ANU Press: Canberra, ACT, Australia) 10.22459/TA55.2022.13
Cook GD, Taylor RJ, Williams RJ, Banks JCG (2005) Sustainable harvest rates of ironwood, Erythrophleum chlorostachys, in the Northern Territory, Australia. Australian Journal of Botany 53, 821-826.
| Crossref | Google Scholar |
Cookson LJ, Carr J, Chew N, Creffield JW (2009) Lyctine susceptibility of selected hardwoods. Australian Forestry 72, 20-24.
| Crossref | Google Scholar |
Cooper W (2022) Diospyros venablesii W.E.Cooper (Ebenaceae), a new and endemic species from the Iron Range area, Cape York Peninsula. Telopea 25, 75-79.
| Crossref | Google Scholar |
Cooper WE, Lamei PA (2023) Anacolosa australis W.E.Cooper (Aptandraceae), a new species from Cape York Peninsula, Queensland. Australian Journal of Taxonomy 23, 1-4.
| Crossref | Google Scholar |
Cowie ID, Guymer GP (2015) A new, rare species of Brachychiton from Fish River Station, Northern Territory. Australian Systematic Botany 27, 462-468.
| Crossref | Google Scholar |
Craven LA, Barrett RL, Barrett MD (2016) Three new species and one new combination in Hibiscus (Malvaceae). Muelleria 35, 3-14.
| Crossref | Google Scholar |
Daniels CW, Mardulpu S, Thompson SM, Nyuluk E, Daniels HJ, George LW, Murray L, Mardawurrngh D, Jaranajiny B, Duncan DN, James SG, James RG, Woods P, Baker B, Thompson GM, Wightman G (2019) Ngandi and Ngalakgan plants and animals: biocultural knowledge of flora and fauna from the Rose, Wilton, Roper and Phelp rivers, north Australia. Northern Territory Botanical Bulletin Number 50, Department of Environment and Natural Resources: Palmerston, NT, Australia; and Ngukurr Language Centre, Ngukurr, NT, Australia.
de Faria SM, Ringelberg JJ, Gross E, Koenen EJM, Cardoso D, Ametsitsi GKD, Akomatey J, Maluk M, Tak N, Gehlot HS, Wright KM, Teaumroong N, Songwattana P, de Lima HC, Prin Y, Zartman CE, Sprent JI, Ardley J, Hughes CE, James EK (2022) The innovation of the symbiosome has enhanced the evolutionary stability of nitrogen fixation in legumes. New Phytologist 235, 2365-2377.
| Crossref | Google Scholar | PubMed |
De Meyer E (2023) The spiritual valuation of Erythrophleum suaveolens, a cultural keystone species in the Democratic Republic of Congo. Economic Botany 77, 103-109.
| Crossref | Google Scholar |
Delporte C, Noret N, Vanhaverbeke C, Hardy OJ, Martin J-F, Tremblay-Franco M, Touboul D, Gorel A, Faes M, Stévigny C, Van Antwerpen P, Souard F (2021) Does the phytochemical diversity of wild plants like the Erythrophleum genus correlate with geographical origin? Molecules 26, 1668.
| Crossref | Google Scholar | PubMed |
Devanesen D, Henshall TS (1982) A study of plant medicines in Central Australia. Transactions of the Menzies Foundation 1, 161-166.
| Google Scholar |
Dillon SJ, Barrett RL, Shepherd KA (2020) Corchorus fitzroyensis (Malvaceae: Grewioideae), a new, poorly known species from Western Australia’s Kimberley region. Nuytsia 31, 83-87.
| Crossref | Google Scholar |
Ding Hou, Larsen K, Larsen SS (1996) Caesalpiniaceae. In ‘Flora Malesiana. Series 1 – Spermatophyta. Flowering Plants. Vol. 12, part 2: Caesalpiniaceae, Geitonoplesiaceae, Hernandiaceae, Lowiaceae’. (Eds Ding Hou, SS Larsen, JE Laferrière, BEE Duyfjes) pp. 409–784. (Leiden University: Leiden, Netherlands)
Dongmo AB, Kamanyi A, Anchang MS, Chungag-Anye Nkeh B, Njamen D, Nguelefack TB, Nole T, Wagner H (2001) Anti-inflammatory and analgesic properties of the stem bark extracts of Erythrophleum suaveolens (Caesalpiniaceae), Guillemin & Perrottet. Journal of Ethnopharmacology 77, 137-141.
| Crossref | Google Scholar | PubMed |
Duminil J, Brown RP, Ewédjè E-EBK, Mardulyn P, Doucet J-L, Hardy OJ (2013) Large-scale pattern of genetic differentiation within African rainforest trees: insights on the roles of ecological gradients and past climate changes on the evolution of Erythrophleum spp. (Fabaceae). BMC Evolutionary Biology 13, 195.
| Crossref | Google Scholar | PubMed |
Duminil J, Mona S, Mardulyn P, Doumenge C, Walmacq F, Doucet J-L, Hardy OJ (2015) Late Pleistocene molecular dating of past population fragmentation and demographic changes in African rain forest tree species supports the forest refuge hypothesis. Journal of Biogeography 42, 1443-1454.
| Crossref | Google Scholar |
Elliott TL, van Mazijk R, Barrett RL, Bruhl JJ, Joly S, Muthaphuli N, Wilson KL, Muasya AM (2021) Global dispersal and diversification of the genus Schoenus (Cyperaceae) from the Western Australian biodiversity hotspot. Journal of Systematics and Evolution 59, 791-808.
| Crossref | Google Scholar |
Fensham RJ (1990) Interactive effects of fire frequency and site factors in tropical Eucalyptus forest. Austral Ecology 15, 255-266.
| Crossref | Google Scholar |
Fensham RJ, Bowman DMJS (1992) Stand structure and the influence of overwood on regeneration in tropical eucalypt forest on Melville Island. Australian Journal of Botany 40, 335-352.
| Crossref | Google Scholar |
Fernández-Marín B, Míguez F, Méndez-Fernández L, Agut A, Becerril JM, García-Plazaola JI, Kranner I, Colville L (2017) Seed carotenoid and tocochromanol composition of wild Fabaceae species is shaped by phylogeny and ecological factors. Frontiers in Plant Science 8, e01428.
| Crossref | Google Scholar | PubMed |
Fleming TH, Geiselman C, Kress WJ (2009) The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104, 1017-1043.
| Crossref | Google Scholar | PubMed |
Ford AJ, Wilson PG (2021) A new species of Rhodomyrtus (Myrtaceae) with brochidodromous venation from north-eastern Queensland, Australia. Telopea 24, 351-357.
| Crossref | Google Scholar |
Ford AJ, Halford DA, van der Merwe M (2021) A new and substrate specific species of Ilex (Aquifoliaceae) from north-eastern Queensland, Australia. Telopea 24, 233-239.
| Crossref | Google Scholar |
Gagnepain F (1952) Cæsalpiniées nouvelles d’Indochine. Bulletin du Muséum d’Histoire Naturelle, sér. 2 24, 317-320 Available at https://biostor.org/reference/271170 [In French].
| Google Scholar |
Georgiou R, Popelka-Filcoff RS, Sokaras D, Beltran V, Bonaduce I, Spangler J, Cohen SX, Lehmann R, Bernard S, Rueff J-P, Bergmann U, Bertrand L (2022) Disentangling the chemistry of Australian plant exudates from a unique historical collection. Proceedings of the National Academy of Sciences 119, e2116021119.
| Crossref | Google Scholar |
Griffin WJ, Phippard JH, Culvenor CCJ, Loder JW, Nearn R (1971) Alkaloids of the leaves of Erythrophleum chlorostachys. Phytochemistry 10, 2793-2797.
| Crossref | Google Scholar |
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307-321.
| Crossref | Google Scholar | PubMed |
Hall WTK (1964) Plant toxicoses of tropical Australia. Australian Veterinary Journal 40, 176-182.
| Crossref | Google Scholar |
Harrington MG, Jackes BR, Barrett MD, Craven LA, Barrett RL (2012) Phylogenetic revision of Backhousieae (Myrtaceae): Neogene divergence, a revised circumscription of Backhousia and two new species. Australian Systematic Botany 25, 404-417.
| Crossref | Google Scholar |
Harvey M (1987) A revision of the genus Synsphyronus Chamberlin (Garypidae: Pseudoscorpionida: Arachnida). Australian Journal of Zoology Supplementary Series 35, 1-99.
| Crossref | Google Scholar |
Herendeen PS, Bruneau A, Lewis GP (2003) Phylogenetic relationships in caesalpinioid legumes: a preliminary analysis based on morphological and molecular data. In ‘Advances in Legume Systematics 10, Higher Level Systematics’. (Eds BB Klitgaard, A Bruneau) pp. 37–62. (Royal Botanic Gardens, Kew: London, UK)
Hill K, Johnson L (1995) Systematic studies in the eucalypts. 7. A revision of the bloodwoods, genus Corymbia (Myrtaceae). Telopea 6, 185-504.
| Crossref | Google Scholar |
Huang X, Chen Z, Zhou S, Huang P, Zhuo Z, Zeng S, Wang L, Wang Y, Xu C, Tian H (2018) Cassaine diterpenoids from the seeds of Erythrophleum fordii and their cytotoxic activities. Fitoterapia 127, 245-251.
| Crossref | Google Scholar | PubMed |
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754-755.
| Crossref | Google Scholar | PubMed |
Hunter JT, Lewis D, Addicott E, Luxton S, Cowie I, Sparrow B, Leitch E (2022) A plot-based analysis of the vegetation of the Northern Territory, Australia: a first assessment within the International Vegetation Classification framework. Vegetation Classification and Survey 3, 161-174.
| Crossref | Google Scholar |
Imolede IO, Asomugha RN, Erhirhie EO (2022) Phytochemical and acute toxicity evaluations of aqueous stem bark extract of Erythrophleum guineense (G.Don). Journal of Applied Sciences 22, 370-377.
| Crossref | Google Scholar |
Jackes BR (2017) A revision of Dolichandrone (Bignoniaceae) in Australia. Australian Systematic Botany 30, 26-37.
| Crossref | Google Scholar |
Jobson P (2014) Two rare new species of Isotropis (Fabaceae: Faboideae: Mirbelieae) from tropical northern Australia. Telopea 17, 347-354.
| Crossref | Google Scholar |
Kablan ACL, Konan JD, Komlaga G, Kabran FA, Daouda B, N’Tamon AD, Kouamé T, Jagora A, Leblanc K, Seon-Méniel B, Beniddir MA, Attioua KB, Le Pogam P, Champy P (2020) Five new cassane diterpenes from the seeds and bark of Erythrophleum suaveolens. Fitoterapia 146, 104700.
| Crossref | Google Scholar | PubMed |
Karadada J, Karadada L, Goonack W, Mangolomara G, Bunjack W, Karadada L, Djanghara B, Mangolomara S, Oobagooma J, Charles A, Williams D, Karadada R, Saunders T, Wightman GM (2011) Uunguu plants and animals. Aboriginal biological knowledge from Wunambul Gaambera Country in the north-west Kimberley, Australia. Northern Territory Botanical Bulletin Number 35, Wunambal Gaambera Aboriginal Corporation, Wyndham, WA, Australia.
Katoh K, Standley DM (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Katterman FRH, Shattuck VI (1983) An effective method of DNA isolation from the mature leaves of Gossypium species that contain large amounts of phenolic terpenoids and tannins. Preparative Biochemistry 13, 347-359.
| Crossref | Google Scholar | PubMed |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647-1649.
| Crossref | Google Scholar | PubMed |
Koenen EJM, Kidner C, de Souza ÉR, Simon MF, Iganci JR, Nicholls JA, Brown GK, de Queiroz LP, Luckow M, Lewis GP, Pennington RT, Hughes CE (2020) Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. American Journal of Botany 107, 1710-1735.
| Crossref | Google Scholar | PubMed |
Lacey CJ, Whelan PI (1976) Observations on the ecological significance of vegetative reproduction in the Katherine–Darwin region of the Northern Territory. Australian Forestry 39, 131-139.
| Crossref | Google Scholar |
Lane DA (1995) A new species of Opodiphthera Wallengren (Lepidoptera: Saturniidae) from northern Australia. Australian Entomologist 22, 115-122 Available at https://biostor.org/reference/274050.
| Google Scholar |
Lanfear R, Calcott B, Ho SY, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29, 1695-1701.
| Crossref | Google Scholar | PubMed |
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2016) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34, 772-773.
| Crossref | Google Scholar |
Lawes MJ, Richards A, Dathe J, Midgley JJ (2011) Bark thickness determines fire resistance of selected tree species from fire-prone tropical savanna in north Australia. Plant Ecology 212, 2057-2069.
| Crossref | Google Scholar |
Lewis GP, Schrire BD, MacKinder BA, Rico L, Clark R (2013) A 2013 linear sequence of legume genera set in a phylogenetic context – a tool for collections management and taxon sampling. South African Journal of Botany 89, 76-84.
| Crossref | Google Scholar |
Loder JW, Culvenor CCJ, Nearn RH, Russell GB, Stanton DW (1972) Isolation of norcassamidide and authentic norcassamidine from Erythrophleum chlorostachys. Structural revision of the alkaloids previously known as norcassamidine, norcassamine, norethythrosuamine and dehydro-norerythrosuamine. Tetrahedron Letters 13, 5069-5072.
| Crossref | Google Scholar |
Manzanilla V, Bruneau A (2012) Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on duplicated copies of the sucrose synthase gene and plastid markers. Molecular Phylogenetics and Evolution 65, 149-162.
| Crossref | Google Scholar | PubMed |
Marazzi B, Gonzalez AM, Delgado-Salinas A, Luckow MA, Ringelberg JJ, Hughes CE (2019) Extrafloral nectaries in Leguminosae: phylogenetic distribution, morphological diversity and evolution. Australian Systematic Botany 32, 409-458.
| Crossref | Google Scholar |
Maroyi A (2019) A review of botany, medicinal uses, phytochemistry and biological activities of Erythrophleum africanum. Journal of Pharmaceutical Sciences and Research 11, 3559-3564.
| Google Scholar |
Marrfurra P Akanburru M, Wawui M, Kurnunerrin T, Adya H, Kamarrama K, Kanintyanyu M, Waya T, Kannyl M, Wightman G, Williams L (1995) Ngan’gikurunggurr and Ngangiwumirri ethnobotany: Aboriginal plant use from the Daly River area, Northern Australia. Northern Territory Botanical Bulletin Number 22, Conservation Commission of the Northern Territory, Palmerston, NT, Australia.
Maslin BR, Barrett MD, Barrett RL (2013) A baker’s dozen of new wattles highlights significant Acacia (Fabaceae: Mimosoideae) diversity and endemism in the north-west Kimberley region of Western Australia. Nuytsia 23, 543-587.
| Crossref | Google Scholar |
Maurin O, Anest A, Forest F, Turner I, Barrett RL, Cowan RC, Wang L, Tomlinson KW, Charles-Dominique T (2023) Drift in the tropics: phylogenetics and biogeographical patterns in Combretaceae. Global Ecology and Biogeography 32(10), 1790-1802.
| Crossref | Google Scholar |
McKenzie NL, Burbidge AA, George AS, Mitchell AS (1983) Part 1. Environment. In ‘Wildlife of the Great Sandy Desert, Western Australia’. (Eds Burbidge AA, McKenzie NL) Wildlife Research Bulletin Western Australia Number 12, pp. 1–37. (Western Australian Wildlife Research Centre, Department of Fisheries and Wildlife: Perth, WA, Australia)
Meier E (2015) ‘Wood! Identifying and using hundreds of woods worldwide.’ (The Wood Database) Available at https://www.wood-database.com/
Missanjo E, Ziba M, Munthali C (2017) Seed biology of Erythrophleum suaveolens (Guill. and Perr.) Brenan: a threatened medicinal plant. SDRP Journal of Plant Science 2, 53-58.
| Crossref | Google Scholar |
Morrison M, McNaughton D, Shiner J (2010) Mission-based Indigenous production at the Weipa Presbyterian Mission, Western Cape York Peninsula (1932–66). International Journal of Historical Archaeology 14, 86-111.
| Crossref | Google Scholar |
Mueller F (1859) Dennisonia, Barklya, et Laboucheria; genera floræ Australiæ nondum cognita. Journal of the Proceedings of the Linnean Society of London, Botany 3(11), 157-159 [In Latin].
| Crossref | Google Scholar |
Nambatu NJ, Nudjulu PP, Nama LJ, Munar KJ, Kungul DA, Munar LR, Tchinburrurr KB, Jongmin NJ, Kungul YB, McTaggart PM, Crocobe M, Wightman G (2009) Marri Ngarr and Magati Ke plants and animals: Aboriginal knowledge of flora and fauna from the Moyle River and Neninh areas, north Australia. Northern Territory Botanical Bulletin Number 32, Department of Natural Resources, Environment, the Arts and Sport, Darwin, NT, Australia.
Nguyen TD, Nishimura H, Imai T, Watanabe T, Kohdzuma Y, Sugiyama J (2018) Natural durability of the culturally and historically important timber: Erythrophleum fordii wood against white-rot fungi. Journal of Wood Science 64, 301-310.
| Crossref | Google Scholar |
Nicolle D (2014) The scantily collected Corymbia punkapitiensis (Myrtaceae) is not distinct from the widespread arid-zone species C. aparrerinja. Nuytsia 24, 263-267.
| Crossref | Google Scholar |
Nicolle D, Barrett RL (2018) Eucalyptus revelata, a rare new species related to E. mooreana (Myrtaceae) from the Kimberley region of Western Australia. Nuytsia 29, 109-118.
| Crossref | Google Scholar |
Okeyo JM (2012) Erythrophleum suaveolens (Guill. & Perr.) Brenan. In ‘Plant Resources of Tropical Africa 7: Timbers 2’. (Eds Lemmens RHMJ, D Louppe, AA Oteng-Amoako) pp. 340–343. (PROTA Foundation: Wageningen, Netherlands) Available at https://uses.plantnet-project.org/en/Erythrophleum_suaveolens_(PROTA) [Verified 7 August 2023]
Palmer E (1883) On plants used by the natives of North Queensland, Flinders and Mitchell Rivers, for food, medicine, etc., etc. Journal and Proceedings of the Royal Society of New South Wales 17, 93-113.
| Google Scholar |
Palmer E (1884) Notes on some Australian tribes. The Journal of the Anthropological Institute of Great Britain and Ireland 13, 276-334.
| Crossref | Google Scholar |
Paramjyothi H, Murphy BP, Lawes MJ, Rossiter-Rachor NA, Richards AE (2020) Does rapid utilization of elevated nutrient availability allow eucalypts to dominate in the tropical savannas of Australia? Ecology and Evolution 10, 4021-4030.
| Crossref | Google Scholar | PubMed |
Parmar G, Dang V-C, Rabarijaona RN, Chen Z-D, Jackes BR, Barrett RL, Zhang Z-Z, Niu Y-T, Trias‐Blasi A, Wen J, Lu L-M (2021) Phylogeny, character evolution and taxonomic revision of Causonis, a segregate genus from Cayratia (Vitaceae). Taxon 70, 1188-1218.
| Crossref | Google Scholar |
Pascal LM, Motte-Florac EF, McKey DB (2000) Secretory structures on the leaf rachis of Caesalpinieae and Mimosoideae (Leguminosae): implications for the evolution of nectary glands. American Journal of Botany 87, 327-338.
| Crossref | Google Scholar | PubMed |
Petrie JM (1921a) The active principle of Erythrophloeum Laboucherii. Proceedings of the Linnean Society of New South Wales 46, 333-348.
| Crossref | Google Scholar |
Petrie JM (1921b) Societies and Academies. Nature 108, 230-231.
| Crossref | Google Scholar |
Powell O, Fensham RJ, Memmott P (2013) Indigenous use of spinifex resin for hafting in north-eastern Australia. Economic Botany 67, 210-224.
| Crossref | Google Scholar |
Purdie, S, Patrick P, Nyadbi L, Thomas P, Fletcher D, Barrett G, Ramsey M, Watbi D, Martin M, Thomas M, Thomas M, Widalji P, Kofod F, Thomas S, Mung Mung P, Peters R, Blythe J, Wightman G (2018) Gija plants and animals: Aboriginal flora and fauna knowledge from the East Kimberley, North Australia. Northern Territory Botanical Bulletin Number 47, Batchelor Press, Batchelor, NT, Australia; and Department of Environment and Natural Resources, Palmerston, NT, Australia.
Puruntatameri J, Puruntatameri R, Pangiraminni A, Burak L, Tipuamantymirri C, Tipakalippa M, Puruntatameri J, Puruntatameri P, Pupangamirri JB, Kerinaiua R, Tipiloura D, Orsto M-M, Kantilla B, Kurrupuwu M, Puruntatameri PF, Puruntatameri TD, Puruntatameri L, Kantilla K, Wilson J, Cusack J, Jackson D, Wightman GM (2001) Tiwi plants and animals. Aboriginal flora and fauna knowledge from Bathurst and Melville Islands, northern Australia. Northern Territory Botanical Bulletin Number 24, Parks and Wildlife Commission of the Northern Territory and Tiwi Land Council, Darwin, NT, Australia.
Qu J, Yu S, Tang W, Liu Y, Liu Y, Liu J (2006) Progress on cassaine-type diterpenoid ester amines and amides (Erythrophleum alkaloids). Natural Product Communications 1, 1934578X0600101.
| Crossref | Google Scholar |
Raymond PD, Dixon PD, Dixon SM, Dixon SK, Dixon RD, Dixon JM, Dixon JWL, Dixon E, Raymond MM, Dalywaters HI, Collins JK, Woods RY, Peterson-Cooper EM, Meakins F, Pensalfini R, Wightman G (2018) Jingulu and Mudburra plants and animals: biocultural knowledge of the Jingili and Mudburra people of Murranji, Marlinja, Warranganku (Beetaloo) and Kulumindini (Elliott) Northern Territory, Australia. Northern Territory Botanical Bulletin Number 49, Papulu Apparr-Kari Aboriginal Corporation, Tennant Creek, NT, Australia; Batchelor Institute Press, Batchelor, NT, Australia; and Department of Environment and Natural Resources, Palmerston, NT, Australia.
Ringelberg JJ, Koenen EJM, Iganci JR, de Queiroz LP, Murphy DJ, Gaudeul M, Bruneau A, Luckow M, Lewis GP, Hughes CE (2022) Phylogenomic analysis of 997 nuclear genes reveals the need for extensive generic re-delimitation in Caesalpinioideae (Leguminosae). PhytoKeys 205, 3-58.
| Crossref | Google Scholar | PubMed |
Roberts JG, Conway SY, Morgan R, Dirn.gayg A, Harris S, Farrar EB, Roberts FB, Merlan F, Collyer E, Calnan T, Wightman G (2011) Mangarrayi and Yangman plants and animals: Aboriginal biocultural knowledge from Elsey and the Roper River, north Australia. Northern Territory Botanical Bulletin Number 39, Diwurruwurrujaru Aboriginal Corporation, Katherine, WA, Australia; Mimi Aboriginal Art & Craft, Katherine, WA, Australia; and Northern Territory Department of Natural Resources, Environment, the Arts and Sport, Darwin, NT, Australia.
Roberts, BN, Daniels CW, Roberts FM, Joshua I, Farrell H, Roberts S, Roberts R, Wilfred C, Wilfred E, Gathawuy F, Numamurdirdi T, Harris S, Daniels A, Juluba H, Wightman G (2018) Alawa, Marra and Warndarrang plants and animals: biocultural knowledge of flora and fauna from Ngukurr, Numbulwar, Minyerri and Limmen National Park, north Australia. Northern Territory Botanical Bulletin Number 48, Northern Territory, Department of Environment and Natural Resources, Palmerston, NT, Australia.
Roth WE (1909) North Queensland ethnography. Bulletin No. 13. Fighting weapons. Records of the Australian Museum 7, 189-211.
| Crossref | Google Scholar |
Roth WE (1910) North Queensland Ethnography. Bulletin No. 16. Huts and shelters. Records of the Australian Museum 8, 55-66.
| Crossref | Google Scholar |
Setzler FM, McCarthy FD (1950) A unique archeological specimen from Australia. Journal of the Washington Academy of Sciences 40, 1-5.
| Google Scholar |
Si A (2020) Patterns in the transmission of traditional ecological knowledge: a case study from Arnhem Land, Australia. Journal of Ethnobiology and Ethnomedicine 16, 52.
| Crossref | Google Scholar | PubMed |
Sim CMD (2023) Isolation and characterization of bioactive Australian natural products, PhD thesis, The University of Queensland, Brisbane, Qld, Australia. 10.14264/3cd7a71
Smith NM (1991) Ethnobotanical field notes from the Northern Territory, Australia. Journal of the Adelaide Botanic Garden 14, 1-65.
| Google Scholar |
Son NT (2019) Genus Erythrophleum: botanical description, traditional use, phytochemistry and pharmacology. Phytochemistry Reviews 18, 571-599.
| Crossref | Google Scholar |
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312-1313.
| Crossref | Google Scholar | PubMed |
Standley P-M (2019) The importance of campfires to effective conservation. PhD thesis, James Cook University, Townsville, Qld, Australia. 10.25903/e3ff-8844
Taylor RJ, Chisholm RA (2005) The occurrence of hollows in eucalypts and ironwood Erythrophleum chlorostachys in the Gulf region of the Northern Territory and its implications for timber harvesting. Pacific Conservation Biology 11, 57-63.
| Crossref | Google Scholar |
Teclaire NF, Brice MOP, Marguerite ELG, Jacques Bruno NB, De Matha NJ, Armel T, Duplex WJ, Jules PR, Siegfri D (2019) Ethnobotany and floral characterization of plants used in three major ethnic groups in Cameroon to treat sinusitis. Saudi Journal of Medical and Pharmaceutical Sciences 5, 1067-1082.
| Crossref | Google Scholar |
Thomson DF (1936) Notes on some bone and stone implements from north Queensland. Journal of the Royal Anthropological Institute 36, 71-74.
| Google Scholar |
Thomson DF (1939) The seasonal factor in human culture. Proceedings of the Prehistoric Society 5, 209-221.
| Crossref | Google Scholar |
Tindale NB (1925) Natives of Groote Eylandt and of the west coast of the Gulf of Carpentaria. Records of the South Australian Museum 3, 61-102.
| Google Scholar |
Tindale NB (1926) Natives of Groote Eylandt and of the west coast of the Gulf of Carpentaria. Records of the South Australian Museum 3, 103-134.
| Google Scholar |
Turpin G, Ritmejerytė E, Jamie J, Crayn D, Wangchuk P (2022) Aboriginal medicinal plants of Queensland: ethnopharmacological uses, species diversity, and biodiscovery pathways. Journal of Ethnobiology and Ethnomedicine 18, 54.
| Crossref | Google Scholar | PubMed |
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299-363.
| Crossref | Google Scholar | PubMed |
Wang Z, Liu H, Dai S, Cao H, Wang R, Wang Z (2019) Endangered but genetically stable − Erythrophleum fordii within Feng Shui woodlands in suburbanized villages. Ecology and Evolution 9(19), 10950-10963.
| Crossref | Google Scholar |
Whitau R, Vannieuwenhuyse D, Dotte-Sarout E, Balme J, O’Connor S (2018) Home is where the hearth is: anthracological and microstratigraphic analyses of Pleistocene and Holocene combustion features, Riwi Cave (Kimberley, WA, Australia). Journal of Archaeological Method and Theory 25, 739-776.
| Crossref | Google Scholar | PubMed |
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a north Australian tropical savanna. Ecology 78, 2542-2558.
| Crossref | Google Scholar |
Williams RJ, Myers BA, Eamus D, Duff GA (1999a) Reproductive phenology of woody species in a north Australian tropical savanna. Biotropica 31, 626-636.
| Crossref | Google Scholar |
Williams RJ, Cook GD, Gill AM, Moore PHR (1999b) Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia. Australian Journal of Ecology 24, 50-59.
| Crossref | Google Scholar |
Wilson KL, Barrett RL (2023) Revision of the tropical genus Diplacrum (Cyperaceae: Bisboeckelereae) in Australia. Australian Systematic Botany 36, 143-156.
| Crossref | Google Scholar |
Woinarski JCZ, Connors GT, Franklin DC (2000) Thinking honeyeater: nectar maps for the Northern Territory, Australia. Pacific Conservation Biology 6, 61-80.
| Crossref | Google Scholar |
Woinarski JCZ, Beggs K, Hempel C, Price OF, Fisher A (2002) Ironwood: an ecological summary. In ‘Ironwood Erythrophleum chlorostachys in the Northern Territory: aspects of its ecology in relation to timber harvesting. Report to Agriculture, Fisheries and Forestry, Australia’. (Ed. R Taylor) pp. 10–21. (Parks and Wildlife Commission: Palmerston, NT, Australia)
Yao Y, Sui XH, Zhang XX, Wang ET, Chen WX (2015) Bradyrhizobium erythrophlei sp. nov. and Bradyrhizobium ferriligni sp. nov., isolated from effective nodules of Erythrophleum fordii. International Journal of Systematic and Evolutionary Microbiology 65, 1831–1837. 10.1099/ijs.0.000183
Yu F, Li N, Yu S-S (2005) A new diterpenoid glucopyranoside from Erythrophleum fordii. Journal of Asian Natural Products Research 7, 19-24.
| Crossref | Google Scholar | PubMed |
Yugul Mangi Rangers (2023) South East Arnhem Land plants and animals. Available at https://profiles.ala.org.au/opus/southeastarnhemland [Verified 25 July 2023]
Zhao Y, Zhang R, Jiang K-W, Qi J, Hu Y, Guo J, Zhu R, Zhang T, Egan AN, Yi T-S, Huang C-H, Ma H (2021) Nuclear phylotranscriptomics and phylogenomics support numerous polyploidization events and hypotheses for the evolution of rhizobial nitrogen-fixing symbiosis in Fabaceae. Molecular Plant 14, 748-773.
| Crossref | Google Scholar | PubMed |
Zich F, Hyland BPM, Whiffin T, Kerrigan RA (2020) Australian tropical rainforest plants. Trees, shrubs, vines, herbs, grasses, sedges, palms, pandans & epiphytes. Edn 8. Available at https://apps.lucidcentral.org/rainforest/ [Verified 9 August 2023]
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31, 3406-3415.
| Crossref | Google Scholar | PubMed |


