Taxonomy of the Australian Nicotiana benthamiana complex (Nicotiana section Suaveolentes; Solanaceae): five species, four newly described, with distinct ranges and morphologies
Mark W. Chase A B * , Luiz A. Cauz-Santos C , Steven Dodsworth
A B * , Luiz A. Cauz-Santos C , Steven Dodsworth  D and Maarten J. M. Christenhusz A
D and Maarten J. M. Christenhusz A
A Department of Environment and Agriculture, Curtin University, Bentley, WA 6102, Australia.
B Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3DS, UK.
C Department of Botany and Biodiversity Research, University of Vienna, Rennweg 14, A-1030 Vienna, Austria.
D School of Biological Sciences, University of Portsmouth, King Henry 1 Street, King Henry Building, Portsmouth, PO1 2DY, UK.
Australian Systematic Botany 35(5) 345-363 https://doi.org/10.1071/SB22009
Submitted: 5 March 2022 Accepted: 26 August 2022 Published: 29 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Although some authors previously questioned the circumscription of Nicotiana benthamiana, it has never been treated taxonomically as more than a single widespread, variable species. A recent study employing phylogenetic and population genetic analyses has demonstrated that N. benthamiana comprises five species that are geographically and morphologically distinct. Here, we describe four new species in the N. benthamiana complex, namely, N. bilybara M.W.Chase & Christenh., N. candelabra M.W.Chase & Christenh., N. rupestris M.W.Chase & Christenh. and N. scopulorum M.W.Chase & Christenh., and illustrate all five. We provide descriptions, a diagnostic key and a table of morphological characters distinguishing these closely related species. The major morphological feature that distinguishes N. benthamiana from the other four species is its petiolate leaves that become sessile only near the apex of the inflorescence; N. candelabra is distinct in its bifacial branching, N. bilybara in its commonly winged petiole with an auriculate base, N. rupestris in the leafy apices of its calyx lobes, and N. scopulorum is the least morphologically divergent species, conforming most closely to the general description of the N. benthamiana species complex.
Keywords: Australian flora, inbreeding, integrative taxonomy, model species, plant viruses, river drainage basins, tropical Australian flora, widespread species.
Introduction
Nicotiana benthamiana Domin, 1929 has long been considered one of the most widespread species of Nicotiana section Suaveolentes Goodsp. (Chase and Christenhusz 2018), which includes all native members of the genus in Australia (Chase et al. 2022). Nicotiana benthamiana occurs across the tropical and subtropical zones with summer monsoons, extending south into the winter-rain regions as far south as the Northern Territory–South Australia border and adjacent Western Australia (Fig. 1). In all areas, it occurs in sheltered sites, usually on the south-facing sides of rock outcrops and cliffs and in gorges of various compositions. Despite growing in some of the driest regions of the continent, its preferred habitats protect these plants from experiencing the climate extremes typical in these regions. It has been labelled as an ‘extremophile’ (Bally et al. 2015), but this characterisation is inaccurate (Cauz-Santos et al. 2022) because it has strategies to avoid the extremes.

|
It was clear to us from the beginning of this project on Nicotiana section Suaveolentes (Chase and Christenhusz 2018; Chase et al. 2022) that the broad ranges exhibited by some species were likely to represent more than a single biological entity, despite sharing broadly similar morphologies. Although they possess attractive and potentially chasmogamous flowers, we knew that nearly all species of N. section Suaveolentes were likely to be naturally highly inbred because most flowers set capsules without manipulation in the absence of pollinators in the greenhouse. We often speculated about how such highly inbred species maintain genetic and phenotypic cohesion across great distances because a large range increases the possibilities for fragmentation and population divergence (cf. Rosenzweig 1995; Pigot et al. 2012). We do not know which vectors distribute the seeds of species such as N. benthamiana, or how broadly, but we have observed evidence (faeces) that wallabies and kangaroos, among other mammals, rest at the favoured sites of these species (Fig. 2). It thus seems possible that the small seeds of Nicotiana stick to the fur of these mammals while sheltering under rock ledges and are carried from one resting site to the next, perhaps guided in some areas by river drainages, given that we found these new species being largely confined to a single broad drainage region (topographic drainage divisions and river regions, as defined in www.bom.gov.au/water; Cauz-Santos et al. 2022).

|
Gene flow by pollen is likely to be rare in this species complex and others that depend on suitable rock outcrops, and therefore the distances of gene flow are likely to be short. These habitats are nearly continuous in the Pilbara region of north-western Western Australia, and here we, indeed, find some evidence of gene flow and lower levels of divergence among populations, but even in the Pilbara we did not detect widespread admixture when the populations were close to each other. In general, most populations of the N. benthamiana complex are geographically isolated, highly inbred and genetically distinct (Cauz-Santos et al. 2022).
It had been noted previously that there is substantial morphological variation in plants considered to represent N. benthamiana (Burbidge 1960; Chase and Christenhusz 2018). Burbidge (1960) concluded that a ‘considerable range of variation is at present accepted under this name, as can be seen by a comparison of Domin’s figure [Fig. 3] with that published by Goodspeed’ (i.e. Goodspeed 1954, p. 484, fig. 117). However, she concluded that ‘population variations show characters occurring in different combinations and there is too little evidence on which to attempt any subdivision’. Of course, since the time of Burbidge’s (1960) revision, many more collections of this taxon have accumulated, which is especially apparent if her distribution map for the species (Burbidge 1960, p. 346) is compared with that of collections now held in Australian herbaria (Fig. 1).

|
Taxonomy, as it is often practiced, can be haphazard, with different authors treating regional sets of species at different times, and the situation in Nicotiana section Suaveolentes has never had a rigorous overall evaluation, resulting in the naming of many fewer species than exist (Chase et al. 2018, 2021a). A recent genetic study (Cauz-Santos et al. 2022) confirmed our suspicions (Chase and Christenhusz 2018) that despite their shared unusual habit, namely, plants entirely comprising one or more upright inflorescences with cordate leaves, we are in fact dealing with a species complex in N. benthamiana. The former broad concept of N. benthamiana comprises five geographically and genetically distinct species that show little evidence of recent gene flow and diverged from each other, starting with the type group of N. benthamiana sens. str. c. 1.1 Ma and others as recently as 75 000 years ago (Cauz-Santos et al. 2022).
The original type of the name Nicotiana benthamiana was the Bynoe collection (Fig. 3) from the coastal region of north-western Western Australia, and thus the name would normally have been retained for that entity when naming the other entities in this species complex. However, N. benthamiana is one of the most cited model plant species (Goodin et al. 2008; Bally et al. 2018), and a recent internet search recorded 145 000 hits, compared with 335 000 hits for Arabidopsis thaliana (L.) Heynh. The reason for this high level of attention is that one accession of the species, called LAB in recent literature, is a near-universal host for plant viruses, making it the plant of choice for the study of plant–viral interactions (Goodin et al. 2008). The origin of LAB has been traced (Bally et al. 2015, 2018) to a gift of seeds collected by Cleland at the Waite Agricultural Research Institute in Adelaide and sent to Goodspeed at the University of California at Berkeley, who published the most recent monograph of Nicotiana (Goodspeed 1954). The material of N. benthamiana in question was collected in 1936 at the Granites Goldmine, Tanami, Northern Territory, and included LAB and other genotypes (Cauz-Santos et al. 2022; Wylie and Li 2022), which are related to other accessions, mostly further to the north of this site in the Northern Territory and the Kimberley region of Western Australia (Cauz-Santos et al. 2022). Its widespread use as a model species and high rates of literature citations have led us to propose conservation of the name with a new type (Chase et al. 2021b), in this case a Cleland specimen at AD (AD 97219292) from the original Granites Goldmine site, to keep the name consistent with its current widespread use in literature.
Domin’s figure and Bynoe’s specimen represent the species described below as N. candelabra, whereas the figure in Goodspeed (1954) is N. benthamiana (type conservation proposed; Chase et al. 2021b). Burbidge (1960) illustrated plants of both types (Fig. 4), namely (i) the north-western coastal species, N. candelabra, with large flowers arranged distichously and little basal branching, and (ii) LAB, obtained from the United States Department of Agriculture, which is N. benthamiana sens. str., with smaller flowers spirally arranged and abundant basal branching. The USDA material was undoubtedly obtained from Goodspeed (who, in turn, received it from Cleland in 1939) and was illustrated in his monograph (Goodspeed 1954). Comparing the two plants illustrated in Burbidge (1960; Fig. 4), one can easily make a case on morphological grounds for this being more than one species. Some of the other species described below are less easily distinguished, but they, nevertheless, can all be differentiated genetically and morphologically (Table 1, Fig. 5).

|

|
The first description below is based on that in Chase and Christenhusz (2018) and is a summary of the features of this species complex relative to the other species in N. section Suaveolentes. The five that follow will be based on this but do not repeat the shared details (i.e. these descriptions focus on the differences among the five species and are, in this respect, extended diagnoses). When grown together under the same conditions in the greenhouse with ample water and light, it is easy to see that these plants form five morphologically distinct groups, but many of these differences are not obvious when dealing with plants in the field and many herbarium specimens. The differences (Table 1, Fig. 5) can be difficult to observe if the specimen is immature, has suffered from drought, damage or exposure or is fragmentary owing to the collector not making a complete specimen of these sometimes-large plants (up to 2 m tall). When trying to identify an unknown member of the N. benthamiana group, the best attribute is provenance because these species never appear to be sympatric. If provenance is known, identification is simple.
The response of these plants to drought is surprisingly plastic, and if the rains are enough to induce germination but insufficient to permit full development, growth and typical flowering can be aborted, although capsule development typically continues, so that seeds are formed. The first flowers produced, even in wet seasons, are smaller than those generated later in the season and are often cleistogamous, sometimes corolla-less or with a highly reduced corolla (Fig. 2). The photograph in Fig. 2 was taken in 2018, a severe drought year, and the plants of N. scopulorum are difficult to spot because they are so small. The material used in the study of Cauz-Santos et al. (2022) was derived from cultivated plants grown from the seeds collected at that location, so despite the poor development of the wild plants, we were, nonetheless, able to sample them for genetic study, but reliable identification of such inadequate material is possible only by knowing its provenance or, subsequently, growing plants from the seeds collected in better conditions.
Morphological description of the species of the Nicotiana benthamiana group
Plants up to 2.0 m tall, never forming a rosette, effectively all inflorescence, with few basal leaves, highly reduced in dry years, perhaps only producing 3–5 small leaves and 1–3 cleistogamous flowers. Leaves cordiform, nearly as long as wide, 1.0–16.5 × 1.8–16.8 cm (excluding petiole), arranged loosely along one main stem that branches variously, some basally and others only in the upper half, the petioles unwinged to only weakly winged, if wings are present, then usually with an auriculate leaf base. Vestiture of dense, multicellular, gland-tipped hairs of various lengths, those on stems and veins longer, those on the lower lamina less dense, peduncle and calyx densely glandular–hairy with numerous short glandular hairs interspersed with long multicellular glandular hairs with swollen bases (e.g. pustulate hairs), especially obvious on the leaf lamina. Basal leaves petiolate, base often unequal, cordate, apex acute, curving, the upper leaves ovate, sessile, with a rounded base and acuminate apex, leaves bract-like near the apex of the inflorescence, the margins erose–dentate, undulating. Calyx lobes unequal, one longer, all strongly reflexed, some larger and leaf-like, most becoming larger in fruit. Corolla tube 2.0–6.5 cm long (from the end of the calyx), 0.2–0.3 cm in diameter, with a slight throat cup in most cases. In nearly all cases, stigma of the same length as or protrudes slightly beyond the 4 longer stamens, which are the same length or of 2 lengths and positioned near the mouth of the floral tube on short filaments; a fifth shorter stamen is located lower in the floral tube but with a longer filament than for the other 4 stamens. Fruit a capsule that splits into 2 valves containing numerous dark-brown seeds, 500–805 µm long, 56–100 µm in diameter, slightly flattened and curved with undulate-serpentine to honeycomb reticulation.
For these five species, we provide a key below, which works best on complete specimens in good condition. Fragmentary specimens will be difficult to determine, but if provenance is known, then identification is easy because all five are geographically disjunct.
Key to the species of the Nicotiana benthamiana species complex|
1 Main stem with petiolate leaves to at least the middle, base of stems with straight hairs, distribution not as above |
|
2 Main stem with little basal branching |
|
3 Calyx lobes linear, not leafy, 0.2–0.4 cm wide, Queensland and north-eastern NT |
|
4 Inflorescence branching on all sides, Pilbara Craton (north-western WA, excluding the coastal plain |
Nicotiana candelabra M.W.Chase & Christenh., sp. nov.
Diagnosis
Nicotiana candelabra is closely related and is similar morphologically to N. bilybara M.W.Chase & Christenh., but it has a different inflorescence structure with sparse major branching in the upper half of the stem and the shorter branches and flowers arranged distichously rather than on all sides of the stems (more or less spirally) as in N. bilybara. The four longer stamens are of the same length, whereas those in N. bilybara are of two, slightly different lengths.
Plants up to 2 m tall, with large branches up to 30 cm long in the upper half of the stem, lower-most portions of stems covered in straight gland-tipped hairs. Basal-most 3–5 leaves with a pronounced petiole, up to 5 cm long, that rapidly becomes shorter, the leaves in the upper 2/3 of stems sessile, with bases that partially encircle the stems. In younger stages, flowers and short inflorescences are distichously arranged, although at later stages of flowering or branching some of this distinctive arrangement becomes distorted. Flower tubes are 4.0–5.5 × 0.2–0.3 cm (from the end of the calyx) and the limbs are 2.4–3.8 cm in diameter with a sinus up to 0.7 cm deep, the lobe apices acute to acuminate; a stamen cup is located immediately below the tube opening. Four of the anthers are of the same length and positioned at the mouth of the floral tube, with filaments 0.1–0.2 cm long, the 5th stamen is 0.6–1.0 cm further down the tube with a filament 1.0–1.2 cm long. Stigmas 0.1–0.2 cm longer than the 4 stamens. Chromosome number: n = 18, 19 (Chase et al. 2022).
Etymology
Named for the resemblance of the stem to a candlestick, candelabra in Latin, with distichously arranged branches and flowers in the upper half of the stem. The species epithet is a noun in apposition.
Phenology
This species typically germinates during the summer monsoon but also opportunistically during the winter if there has been rain. Flowering collections have been made April–January. Capsules mature ~10–12 weeks post-anthesis.
Notes
This species is restricted to the coastal regions adjoining the larger Pilbara Craton (including the Hamersley Range; Fig. 8), where it occurs on granite outcrops, in some cases within 30 km of N. bilybara populations. Along the WA coast between Karratha and Port Hedland, it occurs on the East Pilbara Craton on the same substrate conditions as those of N. bilybara. No admixture with N. bilybara was observed in N. candelabra by Cauz-Santos et al. (2022) despite their geographical proximity, a result we think might be due to a high level of inbreeding and low pollen flow via an unknown vector in both species, which is likely to be a hawkmoth given their flower morphology and phenology, namely, long, white tubular flowers that open at dusk with a carnation- or clove-like scent. The difference in stamen length between N. candelabra and N. bilybara, four of the same length versus one pair longer than the other respectively, is subtle and difficult to observe in herbarium specimens, and for that reason we did not include this feature in the key. This region of the Pilbara is large, and suitable sites for these plants are nearly continuous (except for the Fortescue Marsh, mentioned below), in contrast to the isolated populations of N. benthamiana, N. rupestris and N. scopulorum. This species is the most frequently collected of the five species in the N. benthamiana complex.
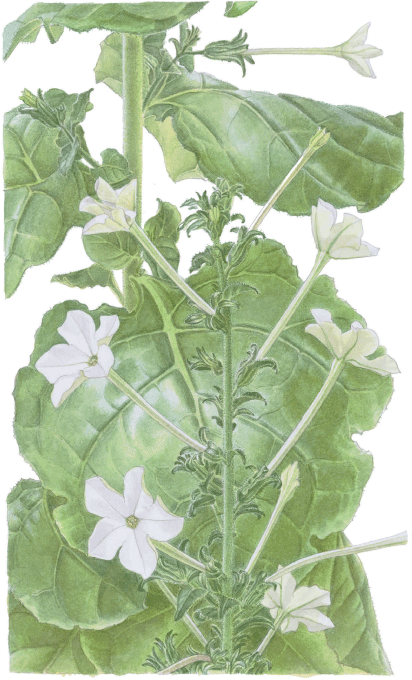
|

|

|
Selected specimens examined
WESTERN AUSTRALIA: Jarman Island, off Cossack, 20.65°S, 117.22°E, June 1978, Abbott 39 (PERTH 1985655); NW coast of Australia, Bynoe s.n. (K, type of N. benthamiana proposed for rejection by Chase et al. 2021b); Robe River between Onslow and Roebourne, 21.61666667°S, 115.9130556°E, 27 Aug. 1966, Butler 19 (PERTH 3691713); Python Pool, Chichester National Park, Fortescue District, 21.33166667°S, 117.2333333°E, 12 Aug. 1974, Carr/Beauglehole C5046/B 48824 (PERTH 1430491); Millstream–Chichester National Park, Palm Pool, cliff faces along northern edge of Fortescue River (Palm Pool), 290 m, 21°34′11″S, 117°03′08″E, 13 Aug. 2015, Chase & Christenhusz 68194 (PERTH, CANB); WSW facing rocky outcrop along road from Roebourne to Harding Dam. 20°50′22″S, 117°08′13′E, 14 Aug. 2015, Chase & Christenhusz 68199 (PERTH, CANB); North West Coastal Hwy, ~5 km E of Whim Creek, 65 m, 20°50′16″S, 117°53′21″E, 17 Aug. 2015, Chase & Christenhusz 68199 (PERTH, CANB); Millstream–Chichester National Park, Python Pool, 175 m, 21°20′02″S, 117°14′18″E, 13 Aug. 2015, Chase & Christenhusz 68289 (PERTH, CANB); Karratha, 20.75°S, 116.87°E, 11 Aug. 1982, Conrick 1027 (AD 98250148); Dolphin Island, Dampier Archipelago, 20.48°S, 116.85°E, 20 June 1970, Fisheries Department 174 (PERTH 3691764); Karratha, rear sewerage tanks, above Bullgarra cell, 20.73972222°S, 116.8480556°E, Jan. 1987, Glennon 300 (PERTH 3691497); Croydon Repeater Site, N of Whim Creek, 0.826555°S, 117.924155°E, 14 July 1999, Murfet 3569 (AD 107471); Yaburara trail, in valley 750–1000 m from water tanks, Karratha, 20.74315°, 116.84212°, 10 July 2004, Wajon 1004 (PERTH 7336756); Pilbara, Millstream–Chichester National Park, ~300 m upstream from Palm Pool on Fortescue River, 21.57111111°, 117.0530556°, 26 Sep. 2006, Walsh et al. 6566 (PERTH 8183082); 1.9 km WNW of Wittenoom turn off, North West Coastal Hwy, Fortescue, granite hill to NE of road, 20.88°S, 117.3310°E, Sep. 1991, Wilson et al. 1009 (AD 99220161; NSW 248734; PERTH 2116456).
Nicotiana bilybara M.W.Chase & Christenh., sp. nov.
(Fig. 9)
Diagnosis
Nicotiana bilybara is closely related and morphologically similar to N. candelabra, but it differs in inflorescence structure, with the major branching in the upper half of the stem and the stems being shorter and flowers arranged more or less spirally around the stem. The leaves of most but not all accessions have winged petioles with auriculate bases. The four longer stamens in N. bilybara are in two pairs, one being slightly longer than the other, whereas in N. candelabra these four stamens are all the same length. Nicotiana bilybara is restricted to the interior of the larger Pilbara Craton, whereas the latter occurs on the north-western coastal shelf of the Pilbara (Fig. 8).

|
Plants up to 2 m tall with large branches up to 20 cm long in the upper half of the stem, lower-most portions of stems covered in straight gland-tipped hairs. In general, these plants are coarser than the other 4 members of this species complex, with a denser covering of the pustulate hairs. Basal-most 3–5 leaves petiolate, petiole 1.0–3.0 cm long, quickly becoming sessile in top 2/3 of the stem, often winged and then with an auriculate base, those in the upper 2/3 of stem sessile, often with the base partially encircling the stems. Flowers and short inflorescences are arranged on all sides of the stems. Calyx lobes 1.4–2.5 cm × 0.3–0.8 cm, up to 2.6 × 1.0 cm in fruit. Flower tubes (2.8) 3.2–5.5 long (from the end of the calyx), 0.2–0.4 cm wide, stamen cup present, limb 2.4–3.8 cm in diameter, with a sinus up to 0.7 cm deep, the lobe apices acute to acuminate. Stamens with a slightly longer pair, 0.1–0.2 cm longer than the shorter pair, the shorter pair nearly sessile, all 4 at the mouth of the floral tube, the 5th 0.5–1.3 cm deeper in the floral tube, 0.5–1.5 cm long. Chromosome number: n = 18, 19 (Chase et al. 2022).
Etymology
In the Nyamal and Banyjima Aboriginal languages, bilybara means ‘dry’, and this word is the probable origin of Pilbara, the English name given to the larger region of north-western Western Australia (Sharpe and Thieberger 1992). It is a noun in apposition.
Phenology
Germination occurs generally during the summer months, giving this species a winter–early spring flowering period, in April–September. Capsules mature roughly 10–12 weeks post-anthesis.
Notes
The difference is stamen arrangement between this species and N. candelabra is difficult to detect on herbarium specimens, especially if the flowers dry poorly. Plants in the East Pilbara Craton have petioles that are typically unwinged and leaves that are less coarse than those of plants in the Hamersley Range. Accessions of N. bilybara are found in the East Pilbara Craton on granite outcrops scattered among the greenstone in this ancient piece of continental crust and in the Hamersley Range on granite outcrops and banded ironstone gorges (Fig. 8). The Fortescue Marsh has no suitable sites and divides this range, and these two disjunct areas are clearly reflected in genetic groupings and morphological differences (those with unwinged petioles occur only in the East Pilbara Craton). There was some admixture detected by Cauz-Santos et al. (2022) in N. bilybara from N. candelabra (but not the reverse), which is understandable, given how close populations of these two species are and the lack of clear geological differences between the areas. It seems likely that the pollen vector would be the same for these two species, possibly a hawkmoth. This region is large, and suitable sites for these plants are nearly continuous (with the exception of the Fortescue Marsh, mentioned above), in contrast to the situation for N. benthamiana, N. rupestris and N. scopulorum. This species is the most frequently collected of the five species in the N. benthamiana complex.
Selected specimens examined
WESTERN AUSTRALIA: Oakover River crossing of Marble Bar–Telfer road, 21.3119°S, 121.0511°E, 25 Aug. 2004, Albrecht 11071 (DNA D0193472; NT D0193472); NT A109653; NT A0109653; PERTH 7025335); Site 2, 2.5 km SW of Silver Grass Peak, 30 km NNE of Mount Farquhar, West Hamersley Range, 22.07034626°S, 116.8255165°E, 22 July 1999, Backhouse et al. BEM 19 (PERTH 5462835); 61 km SE of Port Hedland on Marble Bar Road, ‘Black-topped Range’, 20.558053 S, 119.091470 E, 11 Aug. 1965, Beauglehole 11348 (PERTH 3691780); near Belvedere Mine, 9 miles (~14.5 km) NE from Wyloo Station, 22.59111111°S, 116.3513889°E, 27 Sep. 1968, Blockley 989 (PERTH 3691748; Mount Edgar Station, SE from Marble Bar, 21.3°S, 120.1°E, 13 June 1941, Burbidge 1191 (PERTH 3691675); Great Northern Highway (95), 11 km from Newman, 790 m, 23°09′05″S, 119°20′05″E, 9 Aug. 2015, Chase & Christenhusz 68172 (PERTH, CANB, K); Mount Robinson, along trail into gorge leading from parking and picnic area, 790 m, 23°02′27″S, 118°51′04″E, 9 Aug. 2015, Chase & Christenhusz 68174 (PERTH, CANB, K); Karijini National Park, Hancock Gorge, 650 m, 22°21′30″S, 118°17′04″E, 11 Aug. 2015, Chase & Christenhusz 68183 (PERTH, CANB, K); ~2 km WNW of Wittenoom turn off, North West Coastal Highway, 30 m, 20°53′19″S, 117°20′26″E, 14 Aug. 2015, Chase & Christenhusz 68200 (PERTH, CANB, K); rock outcrop along Great Northern Highway, 200 m, 21°21′42″S, 118°42′55″E, 15 Aug. 2015, Chase & Christenhusz 68207 (PERTH, CANB, K); Woodstock–Marble Bar road, rocky outcrop 240 m, 21°33′44″S, 119°19′14″, 15 Aug. 2015, Chase & Christenhusz 68209 (PERTH, CANB, K); Woodstock–Marble Bar road, 250 m, 21°19′54″S, 119°35′28″E, 15 Aug. 2015, Chase & Christenhusz 68212 (PERTH, CANB, K); Pannawonica Road, 135 m, 21°38′04″S, 116°00′35″E, 18 Aug. 2015, Chase & Christenhusz 68223 (PERTH, CANB, K); Pannawonica Road, W of Pannawonica, 200 m, 21°39′39″S, 116°16′30″E, 18 Aug. 2015, Chase & Christenhusz 68224 (PERTH, CANB, K); Mount Augustus, ~195 km NE of Gascoyne Junction, 24.33°S, 116.85°E, 19 Aug. 1983, Hopper 3185 (PERTH 4918215); Barlee Range, Henry River, 23.67305556°S, 116.2413889°E, 17 Aug. 1961, Royce 6508 (PERTH 1438980); near pool on S side of Mount Barricade, Hamersley Range National Park, 22.85638889, 118.2205556, 8 May 1980, Trudgen 2470 (PERTH 6089739); Pilbara, Hamersley Station, 3.4 km NW of intersection of Mount Brockman Road and power line access track, ~15 km ENE of Mount Brockman homestead, 22.27416667, 117.4566667, 24 Sep. 2006, Walsh et al. 6533 (MEL 2296126A; PERTH 8183317); N of Turee Creek on track heading for ranges, 23.332°S, 117.863°E, 7 June 2006, Wilson 1774 (NSW 780044).
Nicotiana rupestris M.W.Chase & Christenh., sp. nov.
(Fig. 10)
Diagnosis
Nicotiana rupestris is most similar morphologically to N. scopulorum, which is found in north-western Queensland and north-eastern NT, whereas the former is found in eastern WA, south-western-most NT and the north-eastern corner of SA. Nicotiana rupestris is distinguished from the other members of the N. benthamiana species complex by its basal branching and enlarged, leaf-like calyx lobes. It differs from the geographically close N. bilybara in its basal branching and shorter floral tubes. From the adjacent N. benthamiana sens. str., it differs in its sessile leaves in the upper 2/3 of the stem.

|
Plants up to 1.5 m tall, with large branches up to 20 cm long in the lower half of the stem, lower-most portions of stems covered in straight gland-tipped hairs. Basal-most 3–5 leaves petiolate, rarely winged, whereas those in the upper 2/3 of stem are sessile, often with the base partially encircling the stems. Flowers and short inflorescences are arranged on all sides in the upper half of stems. Calyx lobes 1.4–2.5 × 0.4–1.0 cm, up to 3.2 × 1.3 cm in fruit. Flower tubes are from 2.2–2.9 × 0.2 cm (from the end of the calyx) and the limbs, 1.4–2.6 cm in diameter, with a sinus up to 0.7 cm deep, the lobe apices rounded to acute; stamen cup highly reduced to absent; 4 stamens at the mouth of the floral tube with the upper pair 0.1–0.2 cm longer than the lower pair, filaments 0.1–0.2 cm long, the 5th stamen 0.5–0.7 cm deeper in the tube with a filament 0.5–1.0 cm long; stigma 0.1–0.3 cm longer than the four stamens at the mouth of the floral tube. Chromosome number: n = 19 (Chase et al. 2022).
Etymology
From Latin rupes, rock or cliff, referring to the preferred habitat of this species on rocky outcrops. It is a third-declension, two-termination adjective.
Phenology
Flowering has been recorded throughout the winter and early spring,April–September, indicating that germination takes place during the summer monsoon, which is much less pronounced in the southern part of the distribution of this species. Capsules mature in 8–12 weeks after anthesis.
Notes
Nicotiana rupestris occurs on sandstone outcrops throughout the Little Desert, Great Sandy Desert and Gibson Desert from the eastern edge of the Pilbara Craton in WA to the western-most NT and north-western SA, where in the Petermann and Rawlinson ranges it occurs on granite and gneiss outcrops (Fig. 8). The arrangement of the stamens (2 + 2 + 1) differs from that in N. scopulorum (4 + 1), but this is impossible to detect in pressed, dried material, which is why we omitted this feature from the key. The first flowers on stems typically have wider apices on their calyx lobes, and these become much more leafy (longer and wider) in fruit. In older flowers, this leafiness is less pronounced, and, in this condition, N. rupestris and N. scopulorum become difficult to distinguish. However, their ranges are highly disjunct, and N. benthamiana sens. str., which occupies the region between them, is readily distinguished from plants of both these species by its mostly petiolate leaves.
The material studied by Cauz-Santos et al. (2022) was Chase & Christenhusz 18087, which was derived from viable seeds removed from the holotype. An Aboriginal name, tjuntiwari, is provided on Gould s.n. (PERTH 3691683), which was collected near Karlamilyi National Park, which is in Martu country.
Selected specimens examined
WESTERN AUSTRALIA: 115.3 to 118.5 km due W of Kiwirrkurra, Gary Junction Road, 430 m, 22°44′39″S, 126°36′27′′E, 11 Sep. 2015, Butcher & Albrecht 2048 (PERTH 8855234); 55.6 km SE of Glen Ayle Homestead on the Carnegie–Glen Ayle Station road, 25.61666667°S, 122.4833333°E, 9 Sep. 1973, Chinnock 894 (AD 97347327; PERTH 3685098); Gibson Desert. Gunbarrel Hwy, ridge on E side of Mount Beadell, 25.5321°S, 125.284439°E, 13 July 1985, Copely 1175 (AD 98549147); McLarty Hills, Great Sandy Desert, 19.5°S, 123.5°E, 6 Aug. 1977, George 14660 (PERTH 3691454); Picture Hill, 130 miles NW of Well 35 (Canning Stock Route), 21.667°S, 123.825°E, 24 April 1967, Gould s.n. (PERTH 3691683); Glen Cummings Gorge, Rawlinson Ranges, 25.01666667°S, 128.3833333°E, 13 Sep. 1983, Kalotas 1599 (DNA A0076176; PERTH 3691691); Mimbi, Emmanuel Range (E end) on Christmas Creek Station, near Ngumban Cliff, 490 m, 18.75°S, 126.05°E, 5 July 1989, Kenneally 11004 (CANB 474868; PERTH 1477528); 50 km SE of Parnngurr, Little Sandy Desert, 23.07168648°S, 123.0255061°E, 18 July 2001, Latz 17831 (NT A0109412; PERTH 7317484); Mount Everard, Gibson Desert Nature Reserve, Gunbarrel Hwy, 25.1747°S, 125.0622°E, 21 July 2001, Latz 17913 (NT A0109420; PERTH 7317522); Grid 521181, Rudall River, −22.6°S, 122.1°E, 9 Sep. 1971, Maslin 2219 (PERTH 6507565); Biella Springs, W side of Durba Hills, Canning Stock Route, 23.76858302°S, 122.465111°E, 25 Aug. 2004, Muir 848 (PERTH 8761019); Alfred and Marie Range, Gibson Desert Nature Reserve, 24.4°S, 125.8°E, 12 July 1988, Pearson 486 (PERTH 1477536); Lake Alec, Tanami Downs, hill, 20.48192994°S, 129.0512787°E, 10 May 1985, Strong 707 (DNA A0077569); Ngaanyatjarraku Shire, Karrku outstation, ~700 m NE of settlement buildings, beside bore, 48 km NW of Giles Meteorological Station, 24.40111111°S, 127.9402778°E, 15 Aug. 2012, Walsh 7590 (MEL 2361860A).
NORTHERN TERRITORY: Dean Range, 24.9°S, 129.1°E, 26 Aug. 1973, Latz 4187 (CANB 244693; DNA A0040325; PERTH 3685799); Mongrel Downs, 20.66526747°S, 129.4512731°E, 2 Aug. 1976, Latz 6524 (AD 98565384; DNA A0063390); 14 km E of Lake Mackay, Sandford Cliff, 380 M, 22°02′32″S, 129°20′08″E, 7 Sep. 2001, Latz 18092 (NT A0104670; PERTH 8305579); 12 km SE of Docker River Settlement, Learmonth Park, 640 m, 24°58′00″S, 129°08′52″E, 21 Aug. 2007, Latz 22902 (NT D0182743).
SOUTH AUSTRALIA: Mann Ranges, near Mount Whinham, S side, 26.065406°S, 130.417749°E, without collection date, Bates 58720 (AD 137278).
Nicotiana scopulorum M.W.Chase & Christenh., sp. nov.
(Fig. 11)
Diagnosis
Nicotiana scopulorum is most similar morphologically to N. rupestris. The former is found in eastern WA, south-western NT and the north-western corner of SA, whereas the latter is found in north-western Queensland and central to north-eastern NT. They differ principally in the enlarged, leaf-like calyx lobes of N. rupestris, but there are also subtle differences in stamen arrangement (noted below).

|
Plants up to 1.5 m tall with large branches up to 20 cm long in the lower half of the stem, lower-most portions of stems covered in straight gland-tipped hairs. Basal-most 3–5 leaves petiolate, rarely winged, whereas those in the upper 2/3 of stem are sessile, often with the base partially encircling the stems. Flowers and short inflorescences are arranged on all sides of the upper half of stems. Calyx lobes 1.2–1.6 × 0.3–0.4 cm, up to 1.8 × 0.5 cm in fruit. Flower tubes are 2.4–3.4 × 0.2 cm (from the end of the calyx) and the limbs, starry, 1.5–2.5 cm in diameter, sinus up to 0.6 cm deep, apices rounded to acute; stamen cup absent to slight. Four stamens of equal length at the mouth of the floral tube, filaments 0.2–0.3 cm long, 5th stamen 0.5–0.6 cm deeper in the floral tube, filament 0.5–0.6 cm long. Chromosome number: unknown.
Etymology
From Latin scopulus, rock or crag, referring to the preferred habitat of this species on rocky outcrops. It is a noun in the genitive plural, meaning ‘of the rocks’.
Phenology
Flowering specimens have been recorded in April–October, indicating that germination takes place in the summer monsoon. Capsules mature in 8–10 weeks post-anthesis.
Notes
This species is most easily confused with N. rupestris, but their ranges are widely disjunct, with N. benthamiana sens. str. occurring in the intervening area (Fig. 8). Plants of these three species are all shorter, in general, than are N. candelabra and N. bilybara, with abundant basal branching, and have shorter floral tubes than do N. bilybara and N. candelabra (Table 1). The petiolate leaves in the lower 2/3 of the stems in N. benthamiana make it easily distinguishable from both N. rupestris and N. scopulorum, and the generally more leaf-like calyx distinguishes N. rupestris among this trio.
Selected specimens examined
NORTHERN TERRITORY. Mount Tietkens, North Simpson Desert, 275 m, 23°03′20″S, 136°58′58″E, 12 July 2007, Albrecht 12441 (NT D0182096); near Alcoora Spring, Tobermorey, 22.2667°S, 137.9667°E, 10 Oct. 1955, Chippendale 1799 (BRI AQ038310; CANB 98167; DNA A0001799; NSW 60705); Nicholson River area, 17.86521427°S, 137.4845027°E, 11 June 1974, Henshall 383 (AD 98565392; BRI AQ086505; DNA D0009040; CANB 475351; NSW 580027; PERTH 3691810); Dulcie Ranges, 22.4833°S, 135.5167°E, 25 July 1983, Langford 1 (DNA A0071479; MEL 0301543A); 17 km SSE of Lucy Creek Homestead, 22.56522081°S, 136.3845544°E, 2 May 1984, Latz 9870 (BRI AQ368774; DNA A0077170); Old Huckitta Homestead, 22.5667°S, 135.5833°E, 20 July 1970, Latz 642 (CANB 40379; DNA A0027625); 22.71°S, 37.9667°E, 22 May 1972, Latz 2548 (CANB 234040; DNA A0035065); Neutral Junction Station, 21.7°S, 133.95°E, 4 July 1974, Latz 5608 (AD 98141181; CANB 8112221; BRI AQ360615; DNA A0065383); 30 km W of Mittiebah Homestead, 18.8°S, 136.9°E, 23 April 2012, Leitch & Whitton s.n. (DNA D0215567); 10 miles SW of Tarlton Downs Station, 22.7°S, 136.6833°E, 2 Oct. 1955, Perry 5556 (BRI AQ038307; CANB 82439; NSW 48721; PERTH 3691802).
QUEENSLAND. May Downs Station, W of Mount Isa, 390 m, 20°47′41″S, 139°17′05″E, 21 July 2004, Booth 3555 (BRI AQ0610671; DNA D0174260); Duchess–Cloncurry Road, SSW of Cloncurry, 265 m, 20°53′07″S, 140°20′59″E, 22 Sep. 2018, Chase & Christenhusz 18178 (BRI, CANB); Duchess–Mount Isa Road, 55 km SE of Mount Isa, 440 m, 21°06′47″S, 139°48′54″E, 22 Sep. 2018, Chase & Christenhusz 18183 (BRI, CANB); Glenormiston, Toko Range, S-Bend Gorge on Mulligan River; Simpson Desert, W Of Boulia, 23.06162173°S, 138.3543817°E, 20 June 2010, Forster PIF37402 (BRI AQ0816272); near Amethyst Castle, 142 km SE of Mount Isa (by air), 355 m, 21°37′28″S, 140°27′36″E, 30 Apr. 2000, Fraser 324 (CANB 636218); amphitheatre 42.5 km N of Musselbrook Mining Camp, ~210 km N of Camooweal, 18.3569496°S, 138.1592615°E, 4 May 1995, Johnson MRS823 (BRI AQ0489559); 9.75 km N of Silver Star Mine Magazine Hill, 18.67361458, 138.5092594, 19 Apr. 1991, Jones 96 (BRI AQ0542905); Lillyvale Hills lookout, ENE of Boulia on road to Winton, 22.6402664°S, 141.1759167°E, 20 July 1997, Milson JM1359 (BRI AQ0656748); ‘Cravens Peak’, Painted Gorge, Toomba Range, 3.24666667°S, 138.1005556°E, 11 June 2010, Silcock JLS51(BRI AQ0788712); beside tributary of Elizabeth Creek, Bellevue, 310 m, 16°40′43″S, 144°12′29″E, 14 July 2010, Wannan 5860 (BRI AQ0855406).
Nicotiana benthamiana Domin, Biblioth. Bot. 22(89): 591 (1929), sens. str., emend. M.W.Chase & Christenh
(Fig. 12)
Etymology
Named for English botanist George Bentham, author of the Flora Australiensis (the first flora of Australia), in which he described this taxon as a variety of Nicotiana suaveolens Lehm. When recognised as a species, the varietal name is not available for potential use because of the older N. cordifolia Phil., a species from the Juan Fernandez Islands, and hence Domin (1929) renamed this taxon for Bentham.

|
Phenology
Flowering specimens have been collected throughout the year, but most of these are confined to April–September, indicating the germination typically occurs during the summer monsoon. Fruit maturation occurs 10–12 weeks post-anthesis.
Notes
The original type (Bynoe s.n., K) is now nominated for rejection (Chase et al. 2021b) and that specimen is included in N. candelabra here. Viable seeds were removed from Willing s.n. (AD 216824; PERTH 8557993), and this material was referred to as Chase & Christenhusz 18083 in the genetic study of Cauz-Santos et al. (2022); it was demonstrated to be an admixed individual with morphology atypical for a collection of N. benthamiana sens. str. The seeds of N. benthamiana are larger than those of the other species (Cauz-Santos et al. 2022), especially if they have the Rdr1 gene insert.
This species diverged first from the common ancestor with the rest of the N. benthamiana complex, c. 1.5 Ma (Cauz-Santos et al. 2022), and is the most easily distinguished on morphological grounds. The leaves have much longer petioles and are petiolate in most of the stem, becoming sessile only in the upper third to quarter of the stem. The base of the main stem has lanate, gland-tipped hairs, whereas in the other four the hairs are straight and gland-tipped. In some accessions, e.g. Fish River Station (Cowie 13343, CANB 595117), the four longer stamens are located much further down the floral tube and in others the stamens are nearly sessile at the mouth of the floral tube. Nicotiana benthamiana is confined to the Tanami–Timor Sea Coast drainage basin (Fig. 8; www.bom.gov.au/water).
Selected specimens examined
WESTERN AUSTRALIA: Piccaninny Creek Gorge, 15 km SE of Bungle Bungle Outcamp, Bungle Bungle Range, NE Kimberley, 17.45°S, 128.42°E, 4 April 1985, Blackwell BB24 (PERTH 3691543); Kimberleys, Napier Range, N side, 3 km E of Yammera Gap, 17.337901°S, 124.85023°E, 27 July 1974, Carr/Beauglehole 4292/48070 (AD 99203054; BRI AQ515896; PERTH 1584464); N end, Windjana Gorge, Napier Range, Kimberleys, 17.33166667°S, 124.8258333°E, 27 July 1974, Carr/Beauglehole 4284/48062 (CANB 9104011; PERTH 1430483); Wanjana National Park, rocky ledges, 17.4°S, 125°E, 18 Aug. 1982, Conrick 1069 (AD 98323377); along Red Rock Creek from Fowl House, Osmond Range, E Kimberley, 17.27023444°S, 128.4962594°E, 5 May 1999, Edinger 1369 (PERTH 5370620); Craticus Falls, Drysdale River National Park, Kimberley, 14.78333333°S, 127.0833333°E, 10 Aug. 1975, Kenneally 4181 (PERTH 3691551); 11 miles ESE of Halls Creek township, Kimberley, 18.28416667°S, 127.8505556°E, 9 July 1959, Lazarides 6292 (AD 96032074; BRI AQ038311; CANB 78355.2; CANB 78356; MEL 2236534A; NSW 51775; PERTH 3691632); PERTH 3691705); ~80 km N of Kununurra on road to Ningbing, 15.27916667°S, 128.6841667°E, 25 July 1995, Mitchell 4000 (CANB 544230; PERTH 4347765); N of Lissadel Station Homestead, 101 km from Kununurra Post Office on a bearing of 190 degrees, 16.6639°S, 128.5233°E, 28 Feb. 2002, Mitchell 7073 (AD 132902; DNA D0161830, DNA D0161831; PERTH 6195814); SE Kimberley region, Bungle Bungle, Muwundulngi, ~6 km ENE of Bungle Bungle outcamp, near Red Rock Creek, 17.3167°S, 128.3833°E, 12 July 1984, Scarlett 354 (AD 98528184; CANB 355633; DNA D0056016; MEL 0671676A; S-facing cliffs above King George River tidal estuary, N Kimberley, 17 April 2008, Willing s.n. (AD 216824; PERTH 8557993).
NORTHERN TERRITORY: Judbarra National Park, Joe Creek, along trail next to cliff face, 140 m, 15°36′22″S, 131°05′03″E, 26 July 2016, Chase & Christenhusz 16006 (NT); Judbarra National Park, Joe Creek, 130 m, 15°36′26″S, 131°05′09″E, 27 July 2016, Chase & Christenhusz 16007 (NT); Judbarra National Park, Hwy 1, just east of Victoria River Crossing, 150 m, 15°36′93″S, 131°08′59″E, 27 July 2016, Chase & Christenhusz 16008 (NT); Fish River Station, ~9 km NE of Collah Waterhole, 14.3114°S, 130.9714°E, 2 May 2012, Cowie 13343 (CANB 595117); 64 miles SSW of The Granites, 21.1667°S, 130°E, 31 July 1970, Latz 703 (CANB 223157; CANB 35696; DNA A0027711); Mount Doreen, 22.33191559°S, 131.3012722°E, 14 Jan. 1972, Latz 2662 (DNA A0038542); Crown Hill, 21 km SSW of Coniston Homestead, 22.2172°S, 132.4156°E, 19 Sep. 2006, Latz 22341 (NT A0112090); Jasper Gorge, ~10 km by road NW Jasper Creek bridge, 16.02165099°S, 130.7151176°E, 18 April 1996, Albrecht 7506 (NT A0092974); 12 km NE of Mistake Creek Homestead (abandoned), 17°2′15″S, 129°7′34″E, 30 April 2016, Latz 30488 (NT D0272062); 40 miles (~64 km) W of Wave Hill, 17.54859398°S, 130.2679133°E, 27 June 1949, Perry 2269 (AD 97551380; BRI AQ038312; CANB 82440; NSW 48723; PERTH 3691829; PERTH 3691837).
Data availability
No data other than those presented in the text were used in production of this paper.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Financial support to prepare the RAD library and analyse data was provided by research grants P26548-B22 and P33028-B of the Austrian Science Fund (FWF) to Rose Samuel and Luiz Cauz-Santos.
Acknowledgements
We thank the managers and rangers of the national parks and reserves of Western Australia, the Northern Territory and Queensland for providing collecting permits, 2015–2019, to M. W. Chase and M. J. M. Christenhusz and providing assistance on the ground, especially, but not limited to, Judbarra National Park and Karijini National Park. Fieldwork was funded by honorary and visiting professorships from The University of Western Australia and Curtin University to M. W. Chase. Removal of still viable seeds from herbarium specimens resulted in a much more representative sampling of these species over their ranges, and we thank the curators of the following herbaria for permission to do this: AD, BRI, CANB, NSW, NT and PERTH. Seeds were imported to the UK and living material was grown at the Royal Botanic Gardens, Kew, in the Quarantine House, with the help and advice of Sara Redstone, who is gratefully acknowledged. We also thank Russell Barrett and an anonymous reviewer, who provided helpful suggestions and critique.
References
Bally J, Nakasugi K, Jia F, Jung H, Ho SYW, Wong M, Paul CM, Naim F, Wood CC, Crowhurst RN, Hellens RP, Dale JL, Waterhouse PM (2015) The extremophile Nicotiana benthamiana has traded viral defence for early vigour. Nature Plants 1, 15165| The extremophile Nicotiana benthamiana has traded viral defence for early vigour.Crossref | GoogleScholarGoogle Scholar |
Bally J, Jung H, Mortimer C, Naim F, Philips JG, Hellens R, Bombarely A, Goodin MM, Waterhouse PM (2018) The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annual Review of Phytopathology 56, 405–426.
| The rise and rise of Nicotiana benthamiana: a plant for all reasons.Crossref | GoogleScholarGoogle Scholar |
Burbidge NT (1960) The Australian species of Nicotiana L. (Solanaceae). Australian Journal of Botany 8, 342–380.
| The Australian species of Nicotiana L. (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Cauz-Santos LA, Dodsworth S, Samuel R, Christenhusz MJM, Patel D, Shittu T, Jakob A, Paun O, Chase MW (2022) Genomic insights into recent species divergence in Nicotiana benthamiana and natural variation in Rdr1 gene controlling viral susceptibility. The Plant Journal 111, 7–18.
| Genomic insights into recent species divergence in Nicotiana benthamiana and natural variation in Rdr1 gene controlling viral susceptibility.Crossref | GoogleScholarGoogle Scholar |
Chase MW, Christenhusz MJM (2018) 890. Nicotiana benthamiana. Curtis’s Botanical Magazine 35, 286–294.
| 890. Nicotiana benthamiana.Crossref | GoogleScholarGoogle Scholar |
Chase MW, Christenhusz MJM, Conran JG, Dodsworth S, Medeiros de Assis FN, Felix LP, Fay MF (2018) Unexpected diversity of Australian tobacco species (Nicotiana section Suaveolentes, Solanaceae). Curtis’s Botanical Magazine 35, 212–227.
| Unexpected diversity of Australian tobacco species (Nicotiana section Suaveolentes, Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Chase MW, Christenhusz MJM, Palsson RL, Fay MF, Dodsworth S, Conran JG, Cauz‐Santos LA, Nollet F, Samuel R, Paun O (2021a) Species delimitation in Nicotiana sect. Suaveolentes (Solanaceae): reciprocal illumination leads to recognition of many new species. Curtis’s Botanical Magazine 38, 266–286.
| Species delimitation in Nicotiana sect. Suaveolentes (Solanaceae): reciprocal illumination leads to recognition of many new species.Crossref | GoogleScholarGoogle Scholar |
Chase MW, Knapp S, Cauz‐Santos LA, Christenhusz MJM (2021b) (2845) Proposal to conserve the name Nicotiana benthamiana (N. suaveolens var. cordifolia) (Solanaceae) with a conserved type. TAXON 70, 1146–1147.
| (2845) Proposal to conserve the name Nicotiana benthamiana (N. suaveolens var. cordifolia) (Solanaceae) with a conserved type.Crossref | GoogleScholarGoogle Scholar |
Chase MW, Samuel R, Leitch AR, Guignard MS, Conran JG, Nollet F, Fletcher P, Jakob A, Cauz-Santos LA, Vignolle G, Dodsworth S, Christenhusz MJM, Buril MT, Paun O (2022) Down, then up: non-parallel genome size changes and a descending chromosome series in a recent radiation of the Australian allotetraploid plant species, Nicotiana section Suaveolentes (Solanaceae). Annals of Botany mcac006
| Down, then up: non-parallel genome size changes and a descending chromosome series in a recent radiation of the Australian allotetraploid plant species, Nicotiana section Suaveolentes (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Domin K (1929) Nicotiana. Bibliotheca Botanica 84, 1144–1148.
Goodin MM, Zaitlin D, Naidu RA, Lommel SA (2008) Nicotiana benthamiana: its history and future as a model for plant–pathogen interactions. Molecular Plant-Microbe Interactions 21, 1015–1026.
| Nicotiana benthamiana: its history and future as a model for plant–pathogen interactions.Crossref | GoogleScholarGoogle Scholar |
Goodspeed TH (1954) ‘The genus Nicotiana; origins, relationships and evolution of its species in the light of their distribution, morphology and cytogenetics.’ (Chronica Botanica: New York, NY, USA)
Pigot AL, Owens IPF, Orme CDL (2012) Speciation and extinction drive the appearance of directional range size evolution in phylogenies and the fossil record. PLoS Biology 10, e1001260
| Speciation and extinction drive the appearance of directional range size evolution in phylogenies and the fossil record.Crossref | GoogleScholarGoogle Scholar |
Rosenzweig ML (1995) ‘Species diversity in space and time.’ (Cambridge University Press) Available at link.gale.com/apps/doc/A18475502/AONE
Sharp J, Thieberger N (1992) ‘Bilybara: the Aboriginal languages of the Pilbara Region of Western Australia.’ (Wangka Maya Pilbara Aboriginal Language Centre: Port Hedland, WA, Australia)
Wylie S, Li H (2022) Historical and scientific evidence for the origin and cultural importance to Australia’s first-nations peoples of the laboratory accession of Nicotiana benthamiana, a model for plant virology. Viruses 14, 771
| Historical and scientific evidence for the origin and cultural importance to Australia’s first-nations peoples of the laboratory accession of Nicotiana benthamiana, a model for plant virology.Crossref | GoogleScholarGoogle Scholar |



