There’s gold in them thar hills! Morphology and molecules delimit species in Xerochrysum (Asteraceae; Gnaphalieae) and reveal many new taxa
Timothy L. Collins A , Alexander N. Schmidt-Lebuhn
A , Alexander N. Schmidt-Lebuhn  B * , Rose L. Andrew A , Ian R. H. Telford
B * , Rose L. Andrew A , Ian R. H. Telford  A and Jeremy J. Bruhl
A and Jeremy J. Bruhl  A
A
A School of Environmental and Rural Science, University of New England, Trevenna Road, Armidale, NSW 2351, Australia.
B CSIRO, Centre for Australian National Biodiversity Research, Clunies Ross Street, Canberra, ACT 2601, Australia.
Australian Systematic Botany 35(2) 120-185 https://doi.org/10.1071/SB21014
Submitted: 14 April 2021 Accepted: 14 March 2022 Published: 9 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Golden everlasting paper daisies in the genus Xerochrysum Tzvelev are iconic Australian native plants grown worldwide. The X. bracteatum species complex has been regarded as taxonomically confusing and in need of revision for over 60 years. We applied morphological and molecular analyses to delimit species, detect common ancestry among populations, and identify putative hybrids in the genus Xerochrysum (Asteraceae: Gnaphalieae). Multiple lines of evidence provided strong support for the recognition of new taxa. Here we describe the following 11 new species: X. andrewiae T.L.Collins & J.J.Bruhl, X. berarngutta T.L.Collins & I.Telford, X. copelandii J.J.Bruhl & I.Telford, X. frutescens J.J.Bruhl & I.Telford, X. gudang T.L.Collins & J.J.Bruhl, X. hispidum T.L.Collins & I.Telford, X. macsweeneyorum T.L.Collins, X. murapan T.L.Collins & I.Telford, X. neoanglicum J.J.Bruhl & I.Telford, X. strictum T.L.Collins, and X. wilsonii T.L.Collins, reinstate Helichrysum banksii A.Cunn. ex DC. (as X. banksii (A.Cunn. ex DC.) T.L.Collins & I.Telford), lectotypify X. banksii and X. papillosum (Labill.) R.J.Bayer, and recircumscribe X. bicolor (Lindl.) R.J.Bayer to include X. halmaturorum Paul G.Wilson and some populations of X. bracteatum sens. lat. from mainland South Australia and Victoria. We also provide revised descriptions of all taxa in the genus, their conservation status, a dichotomous key, tables distinguishing closely related taxa and distribution maps.
Keywords: Australia, biodiversity, Bracteantha, Compositae, endemic, Helichrysum bracteatum, integrated taxonomy, paper daisy, systematics.
Introduction
Background to the study
Golden everlasting paper daisies in the genus Xerochrysum Tzvelev are iconic Australian native plants grown worldwide. The X. bracteatum (Vent.) Tzvelev species complex has been regarded as taxonomically confusing and in need of revision for over 60 years. Thirteen species are currently recognised in Xerochrysum, with an additional four species being added by Wilson (2017; endorsed by the Council of Heads of Australasian Herbaria for inclusion in the Australian Plant Census, but not yet available in that dataset) since the studies by Schmidt-Lebuhn et al. (2015), and species are found in all Australian states and mainland territories, as well as the highlands of Papua New Guinea (CHAH 2020a; B. J. Conn, R. Banka, L. L. Lee, ‘Plants of Papua New Guinea’, see http://www.pngplants.org). Xerochrysum includes some of the showiest and most widely grown of Australia’s native flora, yet taxonomic questions have persisted, alongside the widespread cultivation of many different cultivars, ‘varieties’ and ‘forms’, for over 60 years (Curtis 1956; Harden 1991; Flann 1998). First grown in England and Europe from the late 1700s (Andrews 1805), the type of Xerochrysum (as Xeranthemum bracteatum Vent.) was described by Ventenat (1803) from plants cultivated in the Empress Joséphine’s orangerie at Malmaison. Ventenat expressed reservations in his prelude (Ventenat 1803) that X. bracteatum was enlarging Linnaeus’ concept of Xeranthemum, and also saw taxonomic issues in the inclusion of the new species in Helichrysum Mill.; however, the species was soon transferred to this genus by Andrews (1805). The publication of Flora Australiensis (Bentham 1867) recognised a broadly circumscribed H. bracteatum Vent. subsuming H. viscosum Sieber ex Spreng. (Sprengel 1826), H. bicolor Lindl. (Lindley 1835), H. acuminatum (Link) Sweet (Sweet 1826), H. papillosum Labill. (Labillardiere 1806), H. banksii A. Cunn. ex DC. (de Candolle 1838), and H. macrocephalum A. Cunn. ex DC., a species described as growing on ‘the sandy shores of Moreton Bay’ (de Candolle 1838, p. 188). Bentham’s work (1867) remained the standard reference for over 120 years despite increasing taxonomic uncertainty (Curtis 1956; Burbidge 1970; Willis 1973). Anderberg (1991) drew on the prominent involucral bracts, hereafter referred to as phyllaries, and the large glabrous cypselae with barbellate pappus bristles to erect Bracteantha Anderb. & Haegi., but this was shown to be illegitimate (Bayer 2001) owing to the publication of Xerochrysum (Tzvelev 1990, cited in Bayer 2001) the previous year.
Phyllary indumentum characters have been informative when delimiting species of Xerochrysum (Flann 1998; Buchanan 2004; Wilson 2017) and, combined with root-system characters, have been used to separate Xerochrysum into two subgeneric groups (Wilson 2008, 2017). Initially, the two groups comprised the ‘X. bracteatum group’ with a multi-veined claw, and the single-veined ‘X. leucopsideum group’ (Wilson 2008). In the recent partial revision of Xerochrysum, Wilson (2017) modified the concept by defining the ‘X. milliganii (Hook.f.) Paul G.Wilson group’ to contain those species with a single-veined claw, removing Helichrysum leucopsideum DC., which had been shown to be more closely related to some species of Coronidium Paul G. Wilson (Schmidt-Lebuhn et al. 2015). Wilson (2017) recognised four new species previously included in X. bracteatum sens. lat. and raised further taxonomic questions. Currently, X. bracteatum is a variable species, possibly including several unrecognised taxa, and distributed in a broad arc from the highlands of Papua New Guinea to the Eyre Peninsula in South Australia (Wilson 2017; Australia’s Virtual Herbarium, see http://avh.chah.org.au, accessed 12 September 2020).
Taxonomic uncertainty
Most specimens of Xerochrysum in Australian herbaria have been determined to species or an accepted phrase-name taxon, yet limits of many of them remain unclear with consequent inconsistent application of names. Past species limits applied to Xerochrysum have drawn exclusively on morphological differences, and this approach has been unable to find disjunctions among some populations of polymorphic X. bracteatum (the X. bracteatum complex) occurring across over 8000 km2 in eastern and southern Australia. For example, collections from the Barrington Tops National Park in NSW, occurring in distinct populations with white or yellow phyllaries, are treated as a separate entity by herbarium NE but have been variously determined by other Australian herbaria as either X. bracteatum sens. lat., X. sp. Glencoe (M. Gray 4401) NE Herbarium, X. sp. New England (L. M. Copeland 3731) NE Herbarium, or X. bracteatum subsp. barringtonense Paul G. Wilson MS (Australia’s Virtual Herbarium, see http://avh.chah.org.au; New South Wales Flora Online, see http://plantnet.rbgsyd.nsw.gov.au/floraonline.htm).
Taxonomic uncertainty is not limited to the X. bracteatum species complex. Xerochrysum boreale Paul G.Wilson is a recently described perennial from northern Australia, with a scattered distribution across the northern Pilbara and the Kimberley in Western Australia and the northern end of the Northern Territory (the ‘Top End’), occurring in a variety of habitats including coastal sands, seasonal swamps and sandstone escarpments. Not all specimens of X. boreale were considered distinguishable from Pilbara populations of X. interiore Paul G.Wilson (Wilson 2017; FloraBase – the Western Australian Flora, see https://florabase.dpaw.wa.gov.au/, accessed 29 August 2019), and one specimen cited as X. boreale came from mulga–eucalypt woodland in southern Queensland, well beyond its stated distribution (Wilson 2017).
Morphological variation has also been reported between the mainland populations and the Tasmanian populations of X. subundulatum (Sch.Bip.) R.J.Bayer, a subalpine to alpine species with disjunctions in distribution in Tasmania, Victoria and New South Wales (Wilson 2017). Collections from wetlands in Tasmania, Victoria and New South Wales have scabridulous outer abaxial phyllary surfaces but otherwise share similar morphological and ecological characteristics with X. palustre (Flann) R.J.Bayer, a slender, erect perennial occurring in lowland wetlands (Flann 1998). The scabridulous phyllary indumentum character has seen these populations included in X. subundulatum by herbaria HO, CANB and NSW.
Putative new taxa
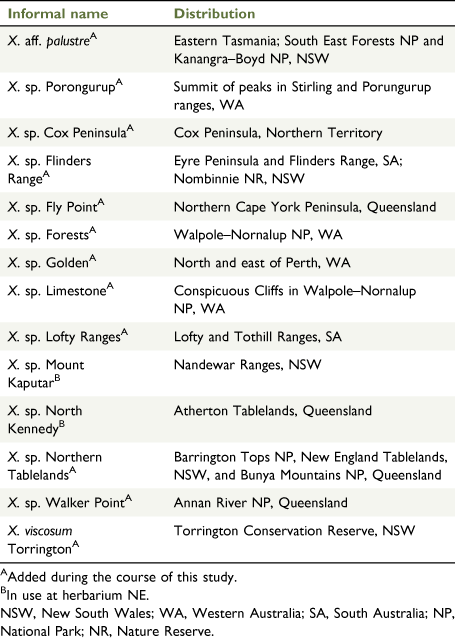
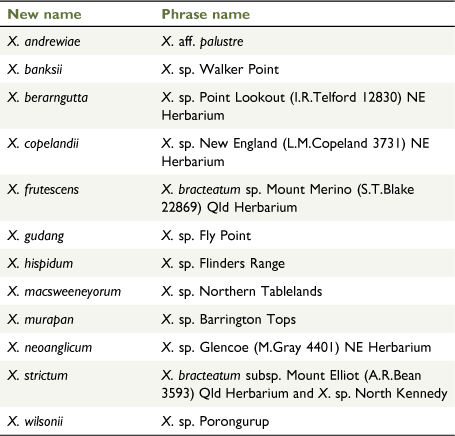
In the 1970s, Nicholas Lander applied informal groupings (e.g. ‘inland form’, ‘coastal ecotype’, ‘radical leaves’) on determination slips and specimen annotations, to some populations of X. bracteatum sens. lat. [as Helichrysum bracteatum] but did not proceed to publication. Currently, Australian herbaria recognise phrase names for the following six putative new taxa in Xerochrysum (CHAH 2020b; Australia’s Virtual Herbarium, see http://avh.chah.org.au): (1) X. bracteatum subsp. Mount Elliot (A.R.Bean 3593) Qld Herbarium; (2) X. sp. Glencoe (M.Gray 4401) NE Herbarium; (3) X. sp. Mount Merino (S.T.Blake 22869) NE Herbarium (synonym: X. bracteatum subsp. Mount Merino (S.T.Blake 22869) Qld Herbarium); (4) X. sp. New England (L.M.Copeland 3731) NE Herbarium; (5) X. sp. North Stradbroke Island (L.Durrington 675) NE Herbarium (synonym: X. bracteatum subsp. North Stradbroke Island (L.Durrington 675) Qld Herbarium); and (6) X. sp. Point Lookout (I.R.Telford 12830) NE Herbarium. Examination of specimens at NSW and CANB suggest Lander’s ‘inland form’ may represent the recently described X. interiore (Wilson 2017), whereas the ‘coastal ecotype’ corresponds to X. sp. North Stradbroke Island and possibly de Candolle’s (1838) H. macrocephalum, whereas ‘radical leaves’ corresponds to X. sp. Glencoe.
Xerochrysum has been the subject of many unpublished taxonomic concepts, such as the proposed splitting of the genus and erection of Diemenica to include the ‘X. milliganii group’ (annotated on some specimens of CANB; T. L. Collins, pers. obs., 2017). Within Xerochrysum, unpublished taxa and combinations include X. leucopsideum ined. (Wilson 2008), X. bracteatum subsp. barringtonense ined. (New South Wales Flora Online, see http://plantnet.rbgsyd.nsw.gov.au/floraonline.htm) and X. bracteatum subsp. dromedariense ined., X. bracteatum subsp. eriophyllum ined., and X. bracteatum subsp. lanatum ined. (the last three annotated on some specimens at AD, BRI, CANB, MEL, and NSW; T. L. Collins, pers. obs., 2017–2019).
Wilson (2017) did not attempt to resolve the putative new taxa of Xerochrysum that have been under study for more than 10 years at the University of New England (Duley 2007; N.C.W. Beadle Herbarium (NE) database, see https://ncw-beadleherbarium.une.edu.au/). Phenetic analyses using continuous and discrete characters had suggested that there were up to seven distinct entities in the X. bracteatum sens. lat. species complex from eastern Australia (Duley 2007). The discovery of the putative new entity X. sp. Mount Kaputar, not included in the phenetic analyses, suspended publication of the results.
The Western Australian endemic X. macranthum (Benth.) Paul G.Wilson has a distribution from Geraldton to Albany and occurs in diverse habitats from dry woodland to forest swamps, mountain peaks and coastal limestone heath (Wilson 2017; Australia’s Virtual Herbarium, see http://avh.chah.org.au). Most populations of X. macranthum have white phyllaries, yet, in the north of the range, yellow-phyllaried populations occur. Populations of X. macranthum restricted to mountain tops in the Stirling and Porungurup ranges in south-western Western Australia, an area known for very high endemism (Crisp et al. 2001), have broader leaves than do those at lower altitudes and latitudes, and morphological differences have been maintained under cultivation (G. J. Keighery, pers. comm., cited in Wilson 2017).
Putative hybrids
Species delimitation in complex groups can be complicated by the presence of hybrids, apomixes and polyploidy (Depypere et al. 2009; Schilling 2011). Wilson speculated that some populations of X. bracteatum sens. lat. were intergrading or hybridising with either X. papillosum (Labill.) R.J.Bayer, Xerochrysum halmaturorum Paul G.Wilson, X. interiore or X. boreale, and were morphologically similar to X. bicolor (Lindl.) R.J.Bayer and X. macranthum, but these hypotheses were not tested with molecular data (Wilson 2017). Xerochrysum sp. Blackfellows Gap (N.T.Burbidge 6926; currently treated as a synonym of X. subundulatum; CHAH 2020a), has been applied to specimens from the Brindabella Ranges on the Australian Capital Territory–New South Wales border. Collections assigned to this entity have unusual leaf and stem indumentum, and have been suggested to be hybrids between X. subundulatum and X. viscosum (Sieber ex DC.) R.J.Bayer (Burbidge 1970), or a putative new species (Wilson 2017).
Xerochrysum viscosum is a woodland species broadly distributed in south-eastern Queensland, central and eastern New South Wales, the Australian Capital Territory and Victoria. The presence of viscid glands and narrow leaves has been regarded as a distinctive feature of X. viscosum (Harden 1991; Wilson 2017). Some collections with leaf width intermediate between X. viscosum and X. bracteatum sens. lat. have been treated as hybrids between these two species (Wilson 2017).
Populations of X. bracteatum sens. lat. in the Barrington Tops New South Wales have been recorded with either white, white and pink, or yellow phyllaries. Intermediates with pale yellow or orange phyllaries, suspected to be hybrids, have been seen where these differently coloured populations co-occur (J. R. Hosking, pers. comm., 2018).
The final hypothesis of hybridisation that should be mentioned is that various cultivars of X. bracteatum (with slightly curved phyllaries coloured pink, orange, purple and red) are widely thought to have been developed in Germany in the 1800s and 1900s, from hybridisation with South African species of Helichrysum (Rymer 2006; Russell 2015). Evolutionary analyses have suggested that breeding compatibility between Xerochrysum and African Helichrysum is unlikely because of the large phylogenic distance between them (Galbany-Casals et al. 2014; Nie et al. 2016). A recent study of gene flow and ancestry analyses showed that some colourful cultivars are hybrids between eastern Australian X. bracteatum and Western Australian X. macranthum (Collins et al. 2021).
Resolution of the longstanding taxonomic uncertainty in Xerochrysum will facilitate study of evolutionary relationships and ecology of members of the genus, provide a sound basis for assessment of conservation status, allow accurate curation of herbarium collections and identification within the genus, and highlight taxa with particular horticultural potential. The present study applies molecular and morphological approaches to test species limits and find evidence of common ancestry or recent gene flow among populations of Xerochrysum from across Australia. Identification of corresponding molecular and morphological discontinuities would support taxon boundaries indicative of separately evolving lineages, consistent with the unified species concept (De Queiroz 2007). Specifically, this study aims to test the status of formal and informal (phrase name) taxa and putative hybrids within Xerochrysum. Taxa recognised here will be described and diagnosed using morphology, congruent with the molecular data.
Materials and methods
Definition and usage of morphological terms, including those describing stylar appendage shape, leaf shape and indumentum, follow the ‘Flora of Australia’ glossary (Orchard and Thompson 1999). Trichome terminology draws on Payne (1978).
Study group
The current study uses the species of Xerochrysum defined by Wilson (2017) and other formally accepted phrase name and manuscript taxa (Table 1; CHAH 2020a), but also includes informal phrase names in use at NE (Table 2; N.C.W. Beadle Herbarium (NE) database, see https://ncw-beadleherbarium.une.edu.au/). This approach ensured testing of the current species limits against multiple hypotheses of putative new entities. During the course of fieldwork, populations in the Sydney Basin and New South Wales South Coast bioregions were seen to have leaf indumentum and habit distinct and different from populations in the New England Tablelands Bioregion, and the former are hereafter being referred to as X. bracteatum sens. str., and the latter as X. sp. Northern Tablelands. Populations currently included in the X. bracteatum complex and of uncertain grouping are referred to as X. bracteatum sens. lat. Additional informal phrase names were used to group populations outside the Sydney Basin and New South Wales South Coast, and they were included in analyses under these informal phrase names (Table 2). Populations similar to Xerochrysum palustre but with scabridulous phyllaries were tentatively referred to as X. aff. palustre.

|
|
Field collection
Field collection was essential to gather high-quality specimens, as well as propagules, and tissue for DNA extraction with sufficient sampling density. Many gatherings of Xerochrysum in Australian herbaria, including many type specimens, are fragments and comprise just the inflorescence and upper cauline leaves and do not have the base of the plants, lower leaves, root-system, or cypselae (T. L. Collins, pers. obs., 2017–2020). Sites were prioritised to collect topotypes, and other sites known from specimen collections <20 years old (Australia’s Virtual Herbarium, see http://avh.chah.org.au) to increase the likelihood of relocating sites. Fieldwork was conducted between November 2017 and March 2019. Owing to record-low rainfall and extreme drought (Bureau of Meteorology 2020), no fieldwork was conducted in western NSW and western Queensland. Descriptions of habit, habitat and associated plants, topography, number of plants in the population, and soil were recorded at each collection site. Herbarium specimens, leaf material for DNA extraction, cypselae and cuttings were collected when found, and when permit conditions allowed. When available, leaf material from at least five plants spaced ~5 m apart was placed immediately in nylon bags and then in sealed containers with silica gel desiccant (Si gel). Herbarium specimens were pressed in the field, and air-dried at ~16°C and 16% relative humidity before freezing for 1 week at ~−30°C for herbarium biosecurity. A complete list of gatherings used in the present study can be found in Supplementary Table S1.
To broaden sampling, additional sources were accessed. Small quantities of leaf were taken from Si gel collections at the N.C.W. Beadle Herbarium (NE) or from herbarium specimens. Si gel collections of X. bracteatum sens. lat. and X. subundulatum were made available for analysis by the Terrestrial Ecosystem Research Network (TERN). Collections of cypselae were accessed from the South Australian Seed Conservation Centre, Australian PlantBank, Royal Tasmanian Botanical Gardens, and the Australian National Botanic Gardens. An extended network of colleagues, friends and volunteers collected material when available and permit conditions allowed.
Collections were made under the following permits and licences: Queensland Government permit number WITK18639817; New South Wales permit number SL100305; Northern Land Council permit number 81511; Northern Territory Government permit number 62139; South Australia permit number G25787-3; Tasmania permit number TFL17334; Western Australia permit number SW019464 and Regulation 4 number CE005748.
Plant propagation and cultivation
Fruits obtained during field collection and from seedbank collections were sown in a commercial seed-raising medium (Searles Seed Raising Mix, JC & AT Searle Pty Ltd, Kilcoy, Qld, Australia) and germinated in a refrigerated incubator (Model: RI250SG, Thermoline Scientific Equipment Pty Ltd, Sydney, NSW, Australia), set at 22°C day, 12°C night and 10-h–14-h day–night with fluorescent lighting. Germinated seedlings were transplanted to a soil-less potting medium (Searles Professional Potting Mix, JC & AT Searle Pty Ltd, Kilcoy, Qld, Australia). Seedlings and plants were grown in an air-conditioned glasshouse maintaining the maximum temperature below 25°C.
Small numbers of seedling transplants were collected when fruits were unavailable. Seedlings were removed from soil, wrapped in damp paper inside a plastic bag and kept cool. On return to the university, seedlings were potted in soil-less potting media and kept under intermittent mist with bottom heat for ~2 weeks before transferring to the air-conditioned glasshouse.
Phenetic analyses of morphology
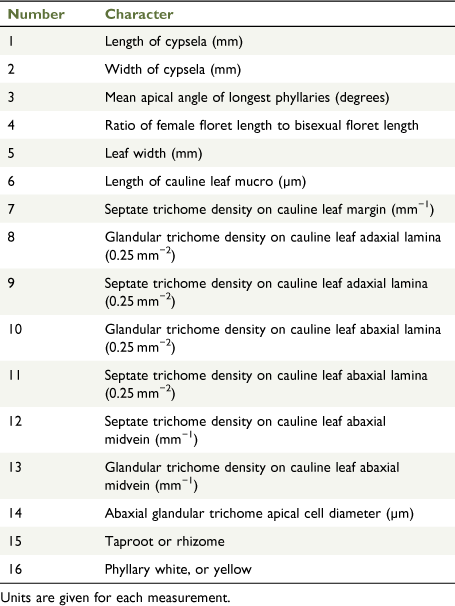
Fourteen quantitative and two qualitative characters (Table 3) were recorded from 97 herbarium specimens representing all study group taxa (Table 4) except for the ‘X. milliganii group’. The ‘X. milliganii group’ was excluded on the basis of the distinctive phyllary morphology, thereby focussing the phenetic analysis on the X. bracteatum complex. Live plants were grown to provide fresh material for comparison with dried specimens. Use of herbarium specimens for phenetic analyses gave access to a broader range of populations and putative entities. The choice of characters was based on preliminary examinations of herbarium specimens, cultivated plants, plants in the field, and published characters applied to species of Xerochrysum (Flann 1998; Wilson 2017). Quantitative characters were used in preference over qualitative characters, to avoid imposing artificial or arbitrary boundaries on any morphological variability. Means were calculated from at least three measurements per specimen for all characters except length of cauline leaf mucro (Table 3, Character 6). Cypselae length and width, not including pappus, were measured using the eyepiece graticule in a Leica 7.5 stereomicroscope, calibrated using a ruler with a half-millimetre scale. Leaf-width dimensions on mature leaves were measured using a ruler with a half-millimetre scale. Leaf length was not measured because of the imprecision of measuring folded and curved leaf specimens. Phyllary apex angle was measured using the angle tool in ImageJ (ver. 1.52a, see https://imagej.nih.gov/ij/download.html; Schneider et al. 2012) on images taken on an iPhone 6 camera (Apple Inc., Cupertino, CA, USA). Stereome and phyllary lamina vascular bundles were observed using a Leica 7.5 stereomicroscope with transmitted light. Scanning electron microscopy (SEM) was used to examine sections of mature leaves mounted onto 12.5-mm aluminium SEM stubs with double-sided carbon tape, and included the margin, apex and midvein for both adaxial and abaxial surfaces. Mounted samples were placed on an angled stage and gold-coated using a JEOL MP-19020NCTR NeoCoater (JEOL Ltd, Tokyo) for 6 min, rotating the stage 60° laterally every 2 min. Specimens were examined and images captured with a JEOL JSM-6010LA Analytical SEM (JEOL Ltd, Tokyo) at 10 kV. A series of images was taken at 75× and 100× magnifications. Trichome density was recorded from SEM using a stencil template representing 0.25-mm2 leaf area and the cell-counter tool in ImageJ (ver. 1.52a; Schneider et al. 2012). Apical-cell diameter and length of cauline leaf-tip mucro were measured on SEM micrographs with a ruler with a half-millimetre scale calibrated against the micrograph scale bar.
|

|
Data were entered into PATN (ver. 3.12, see https://patn.org/; Belbin 1993). All characters were weighted equally for cluster and ordination analysis to examine patterns of similarity and difference among individuals. Three-dimensional ordination fusing semi-strong hybrid multidimensional scaling (SSH MDS) was conducted using the Gower metric association measure (Gower 1971), a widely used method that involves range-standardisation of data for each character. SSH MDS has a demonstrated effective recovery of phenetic clusters (Faith et al. 1987; Holman et al. 2003; Plunkett et al. 2013). The ordination analyses were produced with 250 random starts and 500 iterations. The principal component correlation (PCC) analysis in PATN uses multiple linear regressions to fit each selected variable independently into the ordination space (1, 2 or 3 dimensions). The result is a set of coordinates that represents the tip of the vector of the variable. An r-squared value provides some estimate on how good the fit was. Variables with PCC values >0.7 were considered to contribute strongly. Phenograms were produced from classification analysis using the unweighted pair-group method with arithmetic mean (UPGMA) agglomerative hierarchical fusion (β = −0.1). Subsets of the morphometric data (Table 4) representing broad distribution patterns of Xerochrysum across Australia (i.e. north, south, east and west) were analysed using PATN as above, to assess the extent to which co-located species and putative entities can be distinguished by morphometric analysis. Ordinations were plotted in R (ver. 3.6.1, R Foundation for Statistical Computing, Vienna, Austria) through RStudio (ver. 1.0.153, RStudio PBC, Boston, MA, USA, see https://rstudio.com/) with species and putative entities being designated unique colours.
Sample selection for molecular analyses
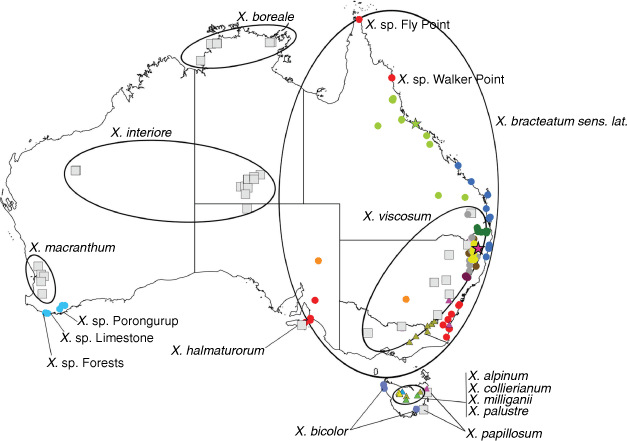
Sampling density was not applied uniformly across all collection sites. For example, the long-standing phrase name entity X. sp. Mount Merino, currently included in X. bracteatum, has a perennial life form, shrub-like habit, very large capitula, dense tomentose leaf indumentum, and is known only from cliff-edge habitats on the margins of Nothofagus moorei closed forests in northern New South Wales and south-eastern Queensland. The distinct morphology and limited distribution of X. sp. Mount Merino suggests a rare and restricted ‘narrow endemic’, and because of finite resources, only two samples were sequenced. The morphologically distinctive Tasmanian alpine endemics X. collierianum and X. milliganii were limited to three samples each. Greater sampling density was applied to test species limits, shared common ancestry and recent gene flow between populations and putative hybrids with less certain boundaries occurring sym- or parapatrically. Sampling locations for all species and putative entities is shown in Fig. 1. Details of the rationale, used to include the populations representing species and putative entities examined with molecular data, can be found in the Supplementary material under the subheading, ‘Rationale for sample selection in molecular analyses’.
|
DArTseq genotyping, population genetic structure and ordination
DNA extraction and DArTseq reduced representation sequencing were conducted by Diversity Arrays Technology Ltd, using the DArTseq approach with medium marker density and proprietry DNA purification (Kilian et al. 2012). This method can be optimised for detecting gene flow in organisms lacking a reference genome (Al-Beyroutiová et al. 2016; Egea et al. 2017; Alam et al. 2018). Details of the DArTseq approach can be found in the Supplementary material under the subheading, ‘DArTseq genotyping’.
Exploratory filtering of DArTseq loci at different levels of stringency confirmed only minor effects on the clustering of samples in PCoA. Filtering loci and individual call rate using the function gl.filter.callrate (Gruber et al. 2018) excluded samples with >20 and >30% missing data respectively, ensuring minimal missing data, inclusion of most samples, and maximal computational efficiency.
Genetic divergence and diversity were estimated for each taxon in R (ver. 3.6.1). Expected heterozygosity and inbreeding estimation FIS were estimated using the adegenet package HS function and inbreeding function respectively (ver. 0.5-11, see https://cran.r-project.org/package=adegenet; Jombart and Ahmed 2011). Observed heterozygosity was calculated using the dartR function gl.report.heterozygosity (ver. 2.0.3, see https://CRAN.R-project.org/package=dartR; Gruber et al. 2018) and means were calculated. Nei’s genetic divergence coefficient GST is analogous to FST for bi-allelic loci (Nei 1973, p. 3322). FST for species and cultivars was calculated using the hierfstat package fstat function (ver. 0.5-11, J. Goudet and T. Jombart, see https://CRAN.R-project.org/package=hierfstat, accessed 12 May 2022).
Underlying data structure and visualisation of genetic differences for all Xerochrysum samples was undertaken by principal coordinate analysis (PCoA) using the function gl.pcoa in the dartR package (Gruber et al. 2018). Principal coordinate analysis (Gower 1966) produces low-dimensional coordinates from a derived distance matrix that summarises as much as possible the variance in the original data (Faraway 2012). Exploratory filtering of DArTseq loci at different levels of stringency confirmed only minor effects on the clustering of samples in PCoA. Filtering loci and individual call rate using the function gl.filter.callrate (Gruber et al. 2018) excluded samples with >20 and >30% missing data respectively, ensuring minimal missing data, inclusion of most samples, and maximal computational efficiency. Subsets of the DArTseq samples (Table 5) representing large clusters in the PCoA (Fig. 2) were produced from the raw data, filtered to exclude samples with loci call rates missing >40% and individual call rates missing >40%, to allow examination of finer-scale genetic differences (Rutherford et al. 2018). Individual samples were identified in each PCoA by using the function gl.pcoa.plot, with the label argument set to ‘interactive’ (Gruber et al. 2018).

|
|
Bayesian genetic clustering
To assess putative hybridisation and account for fine-scale variation, ‘Bracteatum’ and ‘Boreale’ subsets of the sequence data identified using PCoA (Table 5, Fig. 2) were investigated using the Bayesian Monte Carlo Markov-chain (MCMC) procedure implemented in STRUCTURE (ver. 2.3.4, see https://web.stanford.edu/group/pritchardlab/structure_software/release_versions/v2.3.4/html/structure.html; Pritchard et al. 2000; Beaumont et al. 2001; Falush et al. 2003). The program optimises the allele frequencies that characterise K genetic clusters based on a model assuming random mating and linkage disequilibrium within ancestral populations. If a model allowing admixture is used, each individual is assigned a proportion of ancestry derived probabilistically from each ancestral population. This approach is appropriate because it does not assume ancestry a priori. We used the default setting for RECESSIVEALLELES = 0, treating all samples as diploid, as diploids comprised the majority of samples (Laport et al. 2016). STRUCTURE has previously been tested in several diploid–polyploid species complexes (D’hoop et al. 2010; Stöck et al. 2010; Moore et al. 2014), with little effect of the RECESSIVEALLELES flag observed for codominant markers (Moore et al. 2014; Meirmans 2019). Simulations have shown STRUCTURE to be robust in detecting population structure in mixed-ploidy samples (Stift et al. 2019). Estimation of ancestry clusters was performed on 10 replications using K = 1–16 for the ‘Bracteatum’ group, and 10 replications using K = 1–10 for the ‘Boreale’ group and species and putative entities associated with X. sp. Blackfellows Gap. Higher values of K were tested for the ‘Bracteatum’ group because of the greater number of putative entities. Values of K best supported by the data were identified by plotting the natural logarithm of the likelihood and were used to assess the support for each value of K following the reasoning outlined by Pritchard et al. (2000). Caution must be taken when interpreting large STRUCTURE barplots because the algorithm parsimoniously explains variation among individuals and does not provide a parametric model of divergence and admixture (Lawson et al. 2018). To address this issue, we examined a further four subsets of the data, including (1) X. sp. Barrington Tops, X. sp. Glencoe, X. sp. New England and X. sp. Point Lookout, (2) X. bicolor, X. halmaturorum and X. sp. Lofty Ranges, (3) X. boreale, X. sp. Mount Elliott, X. sp. North Kennedy, X. sp. Fly Point and X. sp. Walker Point, and (4) X. bracteatum sens. lat. (putative weedy populations), X. sp. Northern Tablelands, X. sp. North Stradbroke, and X. sp. Chinchilla. Violations of the model assumptions can occur by inclusion of samples that are close relatives, population divergence owing to isolation-by-distance, subtle subdivisions nested within diverged groups, and recent genetic bottlenecks, potentially leading to misinterpretation of the results (Lawson et al. 2018). We also applied an alternative to estimating the optimal number of K clusters, by cautiously describing the clustering results over several values of K in relation to known natural history data (Funk et al. 2020).
Results
Field collections
Collections were made at 116 sites, with 89 vouchers bearing mature cypselae. Photographs of habit and habitat were taken, and notes on associated species and soil substrate were collected.
Phenetic analyses of morphology
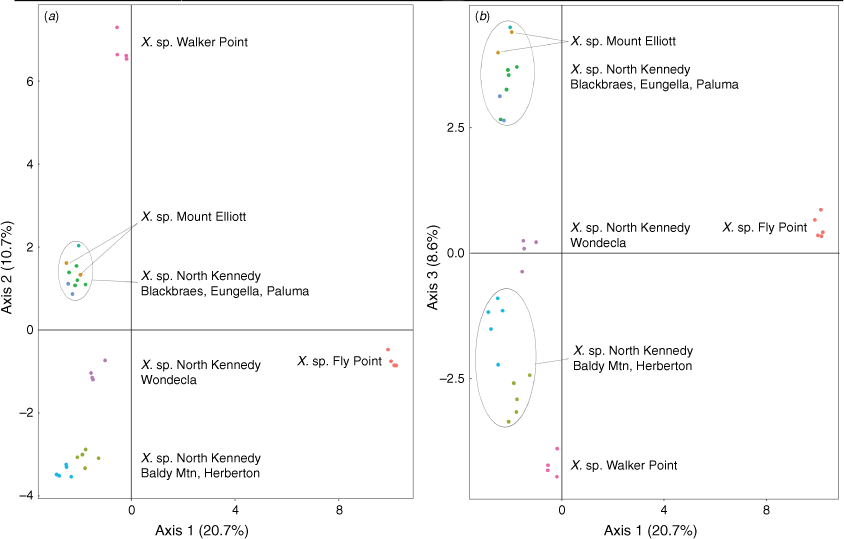
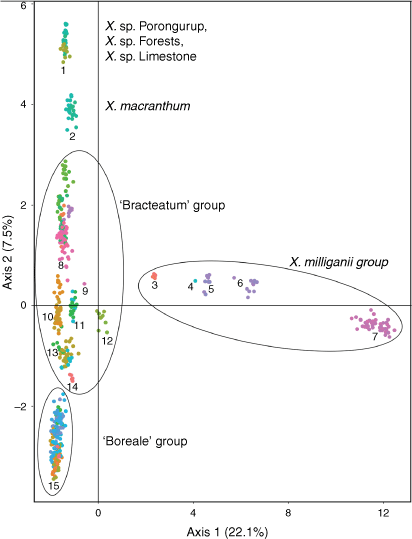
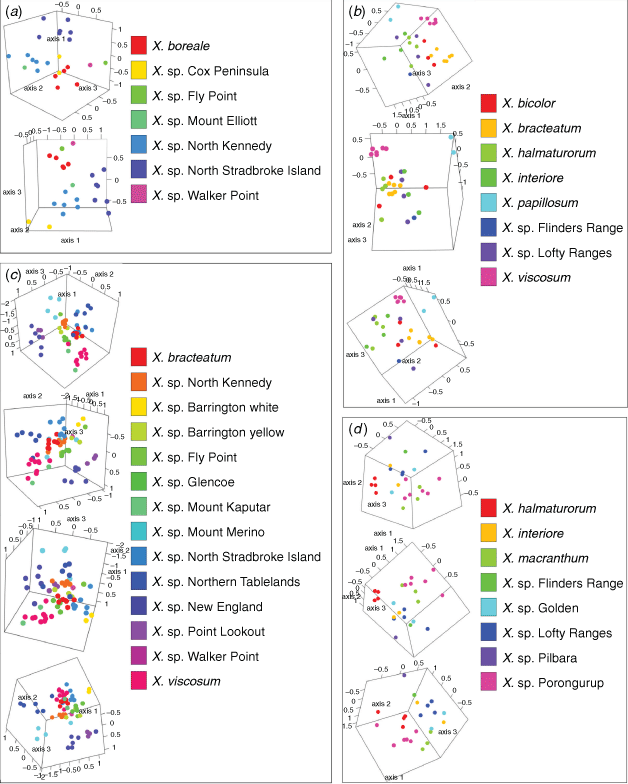
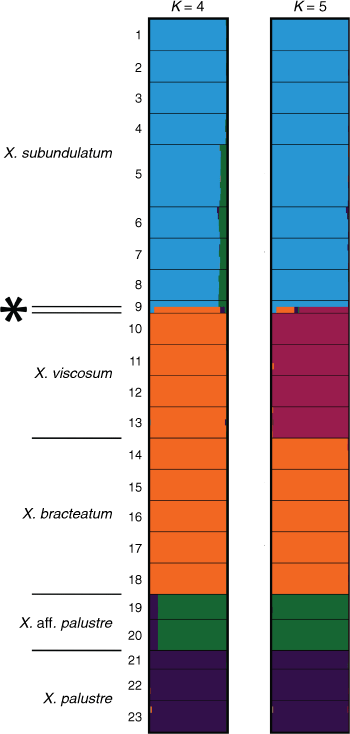
Indumentum and trichome length observed on cultivated plants was consistent with that of dried herbarium specimens, although spreading trichomes on live plants became appressed on the dried specimens, an artefact that was likely due to the pressing and drying process. Indumentum on cauline leaves was observed to vary among species and entities, with differing states being described as cobwebby, hispidulous, hispid, hirsute, felted, pilose, tomentose, and woolly, composed of septate trichomes (Fig. 3), sometimes with flagelliform apices (see Fig. 4), or with sessile or stipitate glands. Phenetic analysis of all 97 specimens of Xerochrysum using 16 characters produced mixed groups loosely corresponding to named species and putative entities in most cases (Supplementary Fig. S1); viz, X. halmaturorum, X. viscosum, X. sp. Glencoe, X. sp. North Stradbroke Island, X. bracteatum, X. sp. Mount Elliott, X. sp. North Kennedy, X. boreale, X. sp. Point Lookout, X. sp. New England, and X. sp. Northern Tablelands. Entities not forming clusters included X. bicolor, X. sp. Mount Kaputar, X. sp. Lofty Ranges, X. sp. Barrington Tops, X. sp. Mount Merino, X. macranthum, X. sp. Porongurup, and X. papillosum. Ordinations based on subsets of the data broadly corresponding to northern, southern, eastern and western distribution showed tighter clustering, but again groups were incomplete and somewhat mixed (Fig. 5).
|
|
Leaf trichome density, in particular the density of glands on the leaf adaxial surface, had the highest PCC values, indicating that they were the most informative characters in the phenetic analyses (Supplementary Tables S2–S7). Absence or near absence of non-glandular multicellular trichomes on the leaf abaxial surface was recorded on X. bracteatum sens. str., X. sp. Barrington Tops with white phyllaries (yellow-phyllaried plants have multicellular trichomes), X. bicolor, X. halmaturorum, X. sp. Lofty Ranges, X. interiore, X. macranthum, X. sp. Walker Point and X. viscosum (Tables 6–8). Absence or near absence of glands on the leaf abaxial midvein was recorded on X. boreale, X. sp. Fly Point, X. sp. North Kennedy, and X. sp. Walker Point (Tables 6–8). A summary of the morphometric data is available as Supplementary material to this paper (Supplementary Table S8).

|

|

|
Examination of 15 specimens of X. bracteatum sens. lat. collected from the eastern and western highlands of Papua New Guinea and held at CANB showed large variations in leaf and stem indumentum, but overall similarity with populations of X. sp. North Kennedy and X. sp. Mount Elliott. A densely tomentose leaf indumentum, composed of septate trichomes and stipitate glands, was seen on both abaxial and adaxial surfaces. Six collections dating from 1954 to 1968 seemingly have no glands, possibly because of use of EtOH during specimen preservation.
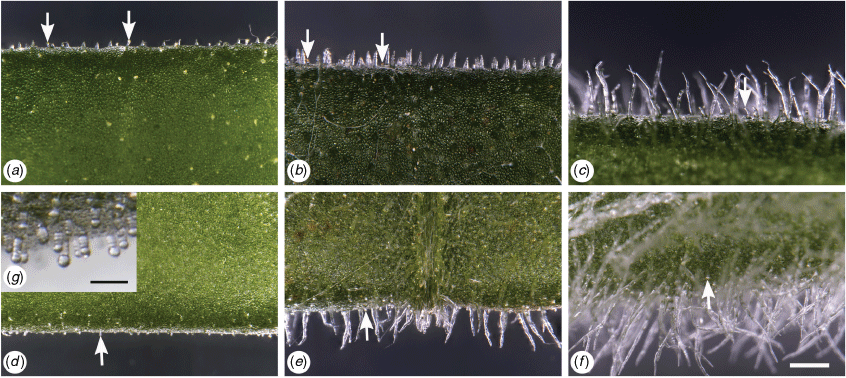
Scanning electron micrographs
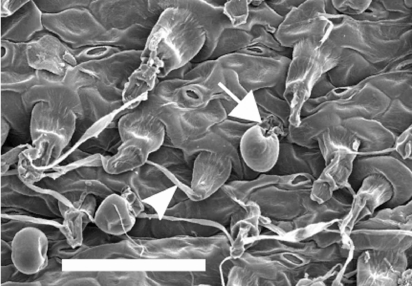
Stipitate or sessile glands were seen on most leaves dried for SEM (Fig. 4, S2–S9). Flagelliform trichomes, with filaments extending from the apical cell, were seen on many species and putative entities (Fig. 4, S10, S11). The filament, and eventually the trichome, appeared to readily abrade away to retain the trichome base and forming a hispid indumentum. Complete removal of the trichome base was observed on some species (e.g. X. sp. North Stradbroke Island, Supplementary Fig. S4b) causing a ‘trichome-scar’.
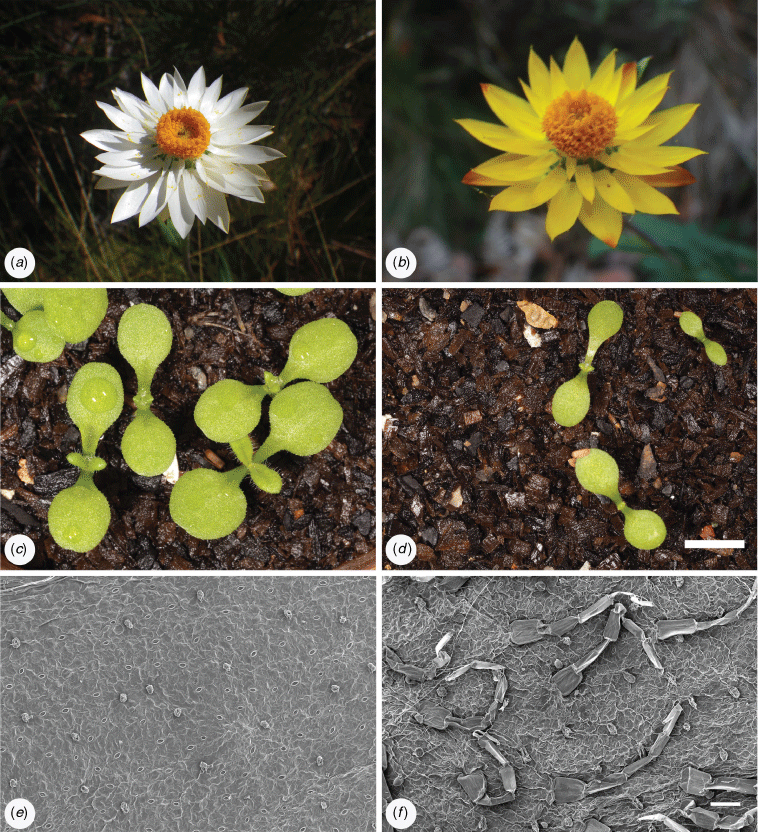
Examination of fresh leaves from cultivated plants confirmed that stipitate or sessile glands occur on all Xerochrysum either on adaxial or abaxial surfaces, or on both surfaces. Presence or absence of multicellular trichomes on cauline leaf abaxial surfaces varied between species and putative entities (Tables 7, 8). White phyllary colour seen in some X. sp. Barrington Tops populations covaried with the near absence of septate trichomes on the cauline leaf abaxial surface (Fig. 6e,f), as well as the much larger cotyledon size than in X. sp. Barrington Tops populations with yellow phyllaries.
DArTseq genotyping, population genetic structure and ordination
A total of 555 Xerochrysum samples were sequenced using the DArTseq method (Kilian et al. 2012). Fourteen of these failed to sequence and provided no data, including all X. collierianum samples. The sequence data partition contained 541 samples, with 47 207 loci representing all named species of Xerochrysum (except X. collierianum) and putative entities (hereafter: ‘allXerochrysum’). Filtering ‘allXerochrysum’ on locus and individual call rate >20 and >30% missing data respectively reduced the dataset to 524 samples and 2486 loci.
Observed heterozygosity was slightly lower than expected for most taxa and populations (Supplementary Table S9). All taxa had a positive FIS indicative of reduced heterozygosity, with less densely sampled populations having both higher expected and observed heterozygosities than did the larger groups. Very high values of FST (>0.4) were obtained for X. bicolor, X. boreale, X. halmaturorum, X. palustre, X. aff. palustre, X. papillosum, X. sp. Lofty Ranges, and X. sp. New England (Supplementary Table S9). Pairwise FST comparisons among X. bicolor, X. halmaturorum and X. sp. Lofty Ranges indicated a gradual increase in genetic divergence between populations as distance between populations increased (Supplementary Table S11). Populations of X. halmaturorum on Kangaroo Island showed the greatest level of divergence (0.593–0.599) from Tasmanian X. bicolor (0.222–0.313). Pairwise FST comparisons among populations of X. sp. North Kennedy, X. sp. Mount Elliott, X. sp. weedy, and X. sp. North Stradbroke Island indicated similar levels of genetic divergence among populations of X. sp. North Kennedy on the Atherton Tablelands (0.182–0.201), and between Atherton Tablelands populations and those in the Paluma Ranges and X. sp. Mount Elliott at Mount Elliott (0.137–0.214; Supplementary Table S13).
The first two axes in the PCoA of ‘allXerochrysum’ (Fig. 2, Table 9) explained 29.6% of the genetic variation and showed distinct separation of some taxa. Axis 1 clearly separates X. subundulatum (Fig. 2, Cluster 7) and the rest of X. milliganii group from all other species and putative entities of Xerochrysum. Xerochrysum aff. palustre (Cluster 6) is disjunct and separate from X. palustre sens. str. (Cluster 5), X. milliganii (Cluster 4) and X. alpinum (Cluster 3). Axis 1 also separates X. sp. Glencoe (Cluster 12) from other species and putative entities of Xerochrysum. Axis 2 of ‘allXerochrysum’ (Fig. 2) shows X. sp. Porungurup, X. sp. Forests and X. sp. Limestone as a distinct group (Cluster 1) separated from X. macranthum (Cluster 2).

|
The large subset ‘Bracteatum’ PCoA Axes 1 and 2 (Fig. 7a) showed 23.4% of the genetic variation and all species and putative entities formed distinct clusters except for X. papillosum and X. viscosum. ‘Bracteatum’ PCoA Axes 1 and 3 (Fig. 7b) showed separation of X. papillosum from X. viscosum and also separated the X. interiore central Australian populations from X. sp. Pilbara. Unexpectedly, X. sp. New England separated into two clusters, with one representing the Henry River Falls population clustering close to populations of X. viscosum, and the other Round Waterhole Creek and Jeogla populations clustering near samples of X. sp. Glencoe and X. sp. Mount Merino. Also, X. bracteatum sens. str. forms three groups, comprising (1) introduced plants on St Helena, (2) naturally occurring populations in New South Wales, and (3) the cultivars (Fig. 7, labelled a, b, and c respectively). Tasmanian and South Australian populations of X. bicolor, X. halmaturorum, and X. sp. Lofty form a single cluster in both Axes 1 and 2, and 1 and 3.
The smaller subset ‘Boreale’ PCoA Axes 1 and 2 (Fig. 8a) explained 30.4% of the genetic variation of these samples, and clearly separated the Gheebulum Kunungai (Moreton Island) National Park population of X. sp. North Stradbroke Island from all other samples. Other populations of this putative entity formed separate clusters; the Iluka population was nested within X. sp. Northern Tablelands as was the naturalised X. bracteatum sens. lat. from Tasmania; the Noosa NP, Town of 1770 and K’gari (Fraser Island) populations clustered very close to Gloucester Tops plants of X. sp. Northern Tablelands as well as Chinchilla and Narrabri plants of X. bracteatum sens. lat.; and the Byfield plants clustered as a distinct group nearby. Samples of X. sp. North Kennedy clustered with X. sp. Fly Point, X. sp. Walker Point and X. sp. Mount Elliott, except for the single sample from Eungella, west of Mackay, which was placed either intermediate to the Yeppoon plants (Fig. 8a), or the Byfield plants (Fig. 8b). All samples of X. boreale formed a distinct and well-separated cluster. ‘Boreale’ group PCoA Axes 1 and 3 (Fig. 8b) explained 28.5% of the genetic variation and showed distinct separation of X. sp. Fly Point, X. boreale and the Gheebulum Kunungai (Moreton Island) National Park population of X. sp. North Stradbroke Island from all other samples. Clustering of remaining samples from the ‘Boreale’ group PCoA Axes 1 and 3 was in accordance with Axes 1 and 2.
PCoA of the X. sp. North Kennedy and closely associated entities, Axes 1 and 2 (Fig. 9a), contained 29.7% of the genetic variation of these samples. Axes 1 and 3 (Fig. 9b) contained 23.7% of the genetic variation. Populations of X. sp. North Kennedy formed three clusters congruent with a north–south distribution. Both Axes 1 and 2, and Axes 1 and 3 showed X. sp. Fly Point and X. sp. Walker Point as separate clusters from X. sp. North Kennedy.
Population genetic structure Bayesian genetic clustering
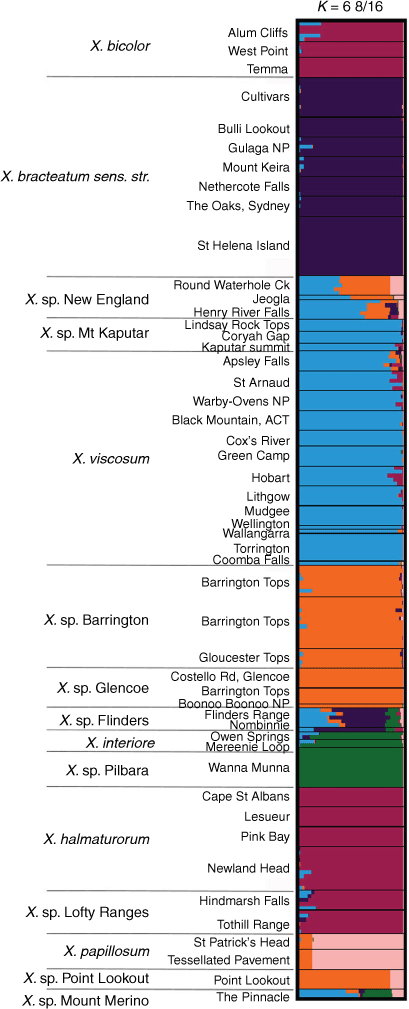
For the ‘Bracteatum’ group, the log-likelihood of the model suggested K lay in the range K = 6–9 (Supplementary Fig. S12). There was very little increase in K from K = 6, and, at K = 7, large variations in mean log-likelihood made interpretation uncertain. This indicated that the single-nucleotide polymorphism data may represent between six and nine distinct entities. STRUCTURE bar plots in the range K = 6–9 suggested that there is no shared ancestry or recent gene flow between X. bracteatum sens. str. and all of the putative entities except a small proportion with X. sp. Flinders Range populations, which contained a complex mix of allele frequencies (Fig. 10, S13). Xerochrysum papillosum was distinct from all other entities K = 6–9. The putative entities Xerochrysum sp. Mount Merino and X. sp. Point Lookout were different mixtures of ancestry clusters. Bar plots between K = 6–9 also suggest that X. viscosum and X. sp. Mount Kaputar share a common ancestry, with only slight variations between populations, as do X. sp. Barrington Tops–X. sp. Glencoe, and Xerochrysum interiore–X. sp. Pilbara.
|
Common ancestry was also suggested among X. bicolor, X. halmaturorum and X. sp. Lofty Ranges (Fig. 10). At K = 7–8, different allele frequencies were suggested between X. bicolor and X. halmaturorum, with X. sp. Lofty Ranges sharing proportions of both (Supplementary Fig. S13 and Table S10). At K = 9, X. bicolor was again similar to X. sp. Lofty Ranges, with small differences in ancestry in X. halmaturorum.
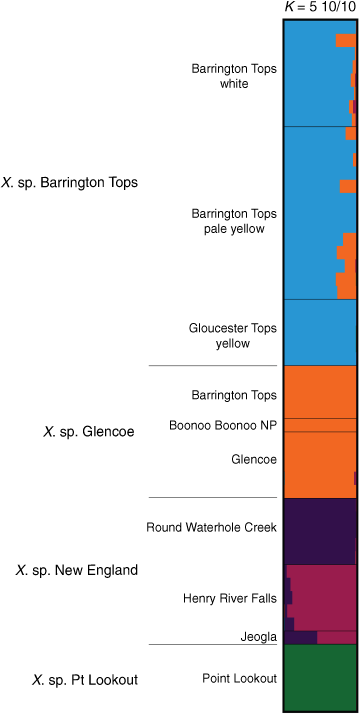
Bayesian analysis on a subset of the ‘Bracteatum’ data examining allele frequencies shared among X. sp. Barrington Tops, X. sp. Glencoe, X. sp. New England and X. sp. Point Lookout suggested K = 5 (Supplementary Fig. S14). Results confirmed separate ancestry of X. sp. Barrington Tops, X. sp. Glencoe, and X. sp. Point Lookout (Fig. 11), with X. sp. New England diverged into two lineages, with the population at Round Waterhole Creek being largely distinct from those at Henry River Falls and Jeogla.
|
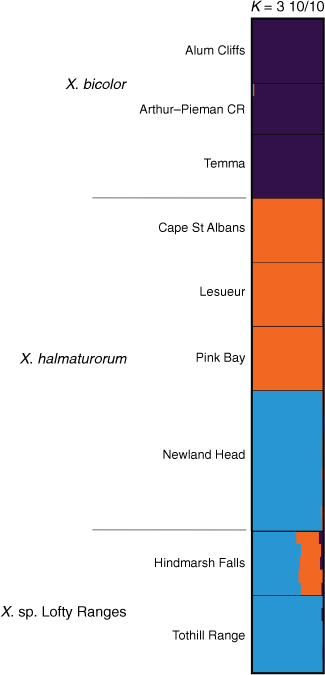
A subset containing Xerochrysum bicolor, X. halmaturorum and X. sp. Lofty Ranges suggested K = 3 (Supplementary Fig. S15), with X. bicolor being distinct from both other entities (Fig. 12). This subset suggested greater genetic complexity than did the analysis of ‘Bracteatum’. Populations of X. halmaturorum on Kangaroo Island at Cape St Albans, Lesueur and Pink Bay shared ancestry distinct and separate from plants at Newland Head, which had shared ancestry with the Tothill Range populations. Plants of X. sp. Lofty Ranges at Hindmarsh Falls were suggested to have ~30% ancestry shared with Kangaroo Island populations and the remainder with plants at Newland Head and Tothill Range (Fig. 12).
|
Bayesian analysis of a subset containing populations of X. viscosum and X. sp. Mount Kaputar was largely congruent with ‘Bracteatum’ data, suggesting X. viscosum and X. sp. Mount Kaputar to be conspecific (Supplementary Fig. S13, S16, S17). Populations at the northern extent of the distribution of X. viscosum, at Torrington, Wallangara and Coomba Falls, were suggested to have different ancestry (Supplementary Fig. S17). The plants at Apsley Falls, previously thought to be a putative new entity conspecific with X. sp. Mount Kaputar, share ~36% ancestry with the Torrington plants.
The log-likelihood of the model of the STRUCTURE analysis for the ‘Boreale’ group (Supplementary Fig. S18), suggested K = 6. However, at K = 7, large variations in mean log probability scores made assessment of K difficult. The main differences between clusters at K = 6 and those at K = 7 (Supplementary Fig. S19) were the suggested divergence of X. bracteatum sens. lat. populations from X. sp. North Stradbroke Island, and the suggested divergence of X. sp. Fly point and X. sp. Walker Point, both from each other, and from X. sp. North Kennedy. These results can also be seen in the major and minor clusters at K = 6 (Fig. 13). Ancestry of all populations of X. boreale, including X. sp. Cox Peninsula, are shared at K = 6 (Fig. 13). Most populations of X. sp. Northern Tablelands shared a common ancestry with the Iluka population of X. sp. North Stradbroke Island. Exceptions were plants in southern Queensland, at The Head and Bunya Mountains NP, which had ~12–23% of X. sp. North Stradbroke Island ancestry. At K = 6, differences between major and minor clusters suggested varying proportions of shared ancestry among X. sp. North Stradbroke Island from Byfield NP, 1770, Noosa National Park and K’gari (Fraser Island) and X. bracteatum sens. lat. from Narrabri and Chinchilla. The Gheebulum Kunungai (Moreton Island) National Park population of X. sp. North Stradbroke Island was distinct from all other samples in the analysis. Bar plots of major and minor clusters at K = 6 showed that all populations sampled of X. sp. North Kennedy clustered with X. sp. Mount Elliott and suggested similar ancestry to populations at Fly Point and Walker Point, except for differing proportions of a maroon-coloured cluster (Fig. 13).
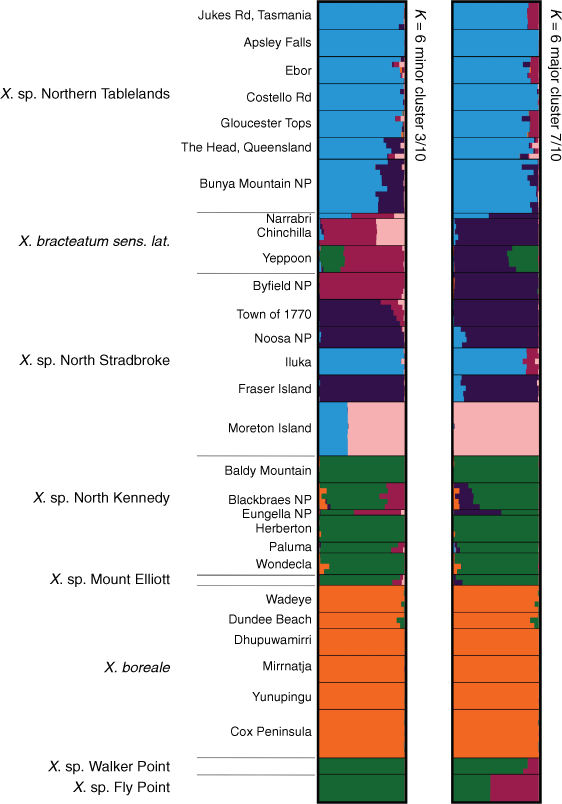
|
Bayesian analysis of a subset of the ‘Boreale’ data examining allele frequencies shared between X. boreale, X. sp. Fly Point, X. sp. Mount Elliott, X. sp. North Kennedy and X. sp. Walker Point suggested K = 6 (Supplementary Fig. S20). Results confirmed the unique ancestry of both X. sp. Fly Point and X. sp. Walker Point, and suggested population genetic structure between eastern and western populations of X. boreale, and between northern and southern populations of X. sp. North Kennedy, with the southern populations at Paluma and Blackbraes National Park having ancestry similar to that of X. sp. Mount Elliott (Fig. 14). Plants at Wondecla were suggested to have ~56% ancestry shared with Herberton and Baldy Mountain, and ~43% with Blackbraes National Park. The Paluma Range and Mount Elliott populations were suggested to have a complex ancestry; however, examination of the raw output showed both clustering with the Blackbraes population in 8 of the 10 runs at K = 6. A subset analysing allele frequencies shared between X. sp. North Stradbroke Island, X. sp. Northern Tablelands, and X. bracteatum sens. lat. (including X. sp. Chinchilla and X. sp. weedy) suggested K = 4 (Supplementary Fig. S21), and STRUCTURE bar plots (Supplementary Fig. S22) were congruent with ‘Boreale’ results (Fig. 13).
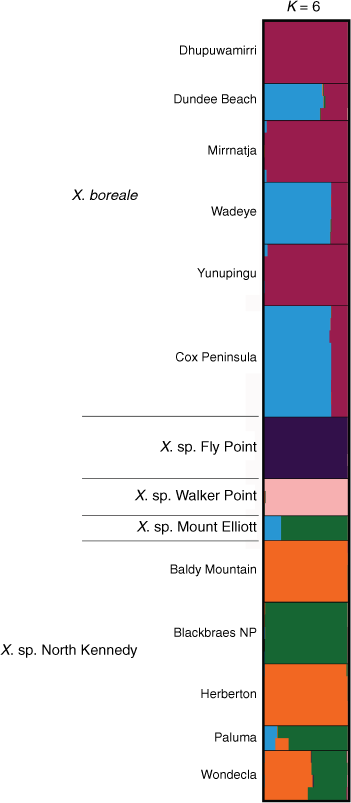
|
For the analysis of putative relatives of X. sp. Blackfellows Gap, the log-likelihood of the model suggested K = 5 (Supplementary Fig. S23); however, biologically relevant clustering occurred up to K = 6. STRUCTURE bar plots at K = 4 did not distinguish between X. bracteatum sens. str., X. viscosum and X. sp. Blackfellows Gap, but did separate X. subundulatum, X. palustre and X. aff. palustre (Fig. 15). At K = 5, 9 of the 10 runs separated X. bracteatum sens. str. and X. viscosum, whereas X. sp. Blackfellows Gap appeared to share a complex mix of ancestry with both of these species and small proportion of X. subundulatum.
|
Discussion
This study is the first to apply molecular and quantitative morphological approaches to attempt to resolve species limits in Xerochrysum. Multiple data sources are critical to delimit species in taxonomically difficult groups (Dayrat 2005; Anderson et al. 2017). Although phenetic analysis of morphological characters did not produce distinct clusters corresponding with named species and putative entities, indumentum characters were found to be informative in delimiting the phrase name entities. The differences observed here support most species recognised in past revisions that applied intuitive approaches (i.e. without quantitative analysis) to species delimitation in Xerochrysum (e.g. Wilson 2017). Molecular analyses of populations of X. aff. palustre and populations currently included in the X. bracteatum complex, indicated genetic disjunctions, and the diagnostic morphology presented here (Tables 6–8) strongly supports the long-standing hypothesis that many of the recognised and putative entities merit consideration as taxonomic groups.
Molecular data indicated that X. aff. palustre is distinct and separate from both X. subundulatum and X. palustre (Fig. 2, Table 9). Bayesian clustering clearly distinguished this entity at low values of K (Fig. 15). Morphologically X. aff. palustre is distinguished from X. subundulatum by the sessile glands on the adaxial leaf surface (stipitate glands in X. subundulatum) and narrower leaves, and from X. palustre by the scabridulous outer phyllaries (Table 8). These multiple lines of evidence support the recognition of X. aff. palustre as a distinct species.
The Western Australian perennial, X. sp. Porongurup, clustered in the PCoA (Fig. 2) with X. sp. Forests and X. sp. Limestone, and morphological comparisons were also unable to find differences to separate these entities. However, these entities are genetically distinct from the annual or occasionally biennial herb X. macranthum and all other species and putative entities of Xerochrysum. Morphological comparisons from herbarium specimens and field observations indicated that X. sp. Porongurup, X. sp. Forests and X. sp. Limestone comprise a single entity that is a long-lived perennial, with broader, hairier leaves and slightly larger inflorescences than those of the annual X. macranthum (Table 7), and should be recognised as a distinct entity.
Analyses of subsets of molecular data (Fig. 7, 8) were able to detect and visualise finer-scale genetic differences. Molecular data supported the hypotheses formed during field work that X. bracteatum sens. str. occurs only in the Sydney Basin, and South East Corner bioregions (Fig. 7 and Collins et al. 2021). The molecular data also showed distinct clusters corresponding to X. sp. Flinders Range, X. sp. Mount Merino, X. sp. Northern Tablelands, and X. sp. Point Lookout, corroborating the morphological differences seen in the field and on herbarium specimens. An unexpected outcome was X. sp. New England forming two genetic clusters, with Henry River Falls and Jeogla plants being intermediate between the Round Waterhole Creek samples and the cluster of X. viscosum (Fig. 7). Bayesian analysis suggested that the Henry River Falls plants have mixed ancestry and share ~56% of their ancestry with X. viscosum, ~31% with X. sp. New England, and ~13% with X. sp. Northern Tablelands (T. L. Collins, J. J. Bruhl, R. L. Andrew, I. R. H. Telford and A. N. Schmidt-Lebuhn, unpubl. data). This putative hybrid has leaf shape and size similar to those of X. sp. New England; however, cauline leaf abaxial surface and margin indumentum are very similar to X. viscosum (Supplementary Table S8). None of the putative parental species has been collected from Henry River Falls, but X. viscosum is common at Torrington State Conservation Area (~75 km away), and X. sp. New England was collected from Round Waterhole Creek ~30 km away.
Xerochrysum sp. Mount Elliot and X. sp. North Kennedy were genetically similar (Fig. 9), with populations of both exhibiting separation along a geographic cline, possibly owing to genetic drift (Mitchell-Olds and Schmitt 2006), although genetic divergence was observed to be small (Supplementary Table S13). Bayesian clustering suggested shared ancestry (Fig. 13) and morphological comparisons (Table 8) were unable to detect distinct characters that separate these entities. Both these lines of evidence supported the conclusion that X. sp. Mount Elliot and X. sp. North Kennedy are a single entity, hereafter referred to as X. strictum, which is formally described in the Taxonomy section (below). Specimens of Xerochrysum collected in Papua New Guinea in the eastern and western highlands at altitudes of ~1000 m were morphologically similar to X. strictum, supporting the hypothesis that this species also occurs there.
The molecular data showed X. strictum, X. sp. Fly Point and X. sp. Walker Point as having distinct genetic differences (Fig. 9). Comparisons of habit, capitula arrangement, leaf indumentum (on both adaxial and abaxial surfaces), and leaf width showed differences that were maintained in cultivation among the three entities (Table 8, Supplementary Fig. S5, S6). Genetic and morphological differentiation outlined above supported the recognition of X. strictum, X. sp. Fly Point and X. sp. Walker Point as distinct species.
Xerochrysum sp. New England, X. sp. Barrington Tops and X. sp. Glencoe were genetically and morphologically distinct in multivariate ordinations, and Bayesian clustering did not distinguish between X. sp. Barrington Tops populations with white and yellow phyllaries. Most Australian herbaria include these three putative entities in the unpublished subspecies X. bracteatum subsp. barringtonense Paul G.Wilson MS. At Barrington Tops NP, X. sp. Barrington Tops and X. sp. Glencoe populations occur sympatrically and have similar flowering phenology (Australia’s Virtual Herbarium, see http://avh.chah.org.au; T. L. Collins, pers. obs., 2018). Some plants of X. sp. Barrington Tops with intermediate pale yellow phyllary colour were seen at one site where yellow and white phyllary populations co-occurred with X. sp. Glencoe (T. L. Collins, pers. obs., 2018). The intermediate plants with pale yellow phyllaries shared similar leaf abaxial indumentum, with plants with white phyllaries, whereas plants with golden yellow phyllaries are hirsute abaxially.
Comparisons of morphology among X. sp. Barrington Tops, X. sp. New England and X. sp. Glencoe (Table 7) demonstrated that the crown and root system, arrangement of capitula, and leaf-width characters separate them, both from each other, and from X. bracteatum sens. str. Recognising these three putative entities as distinct groups, separate from X. bracteatum sens. str. and all other entities studied here, is supported by the genetic differentiation (Fig. 2, 7) and the diagnosable morphological differences (Table 7). The population of putative hybrids between X. sp. New England and X. viscosum at Henry River Falls can also be distinguished using leaf morphology, from among X. sp. Barrington Tops, X. sp. Glencoe, and their parents (Table 7).
Wilson’s (2017) formal description of X. halmaturorum is very similar to his description of X. bicolor. The only clear distinguishing character in the descriptions is the presence of ‘tuberous roots’ on X. bicolor (Wilson 2017). We did not observe tuberous roots on any species or putative entities of Xerochrysum throughout the fieldwork or on herbarium specimens. Morphological comparisons were unable to find distinct differences to separate the group of X. bicolor, X. halmaturorum and X. sp. Lofty Ranges (Table 7), and the molecular data did not consistently identify distinct ancestry clusters (Fig. 7, 12). Pairwise comparisons of FST indicated similar genetic divergence between Tasmanian populations of X. bicolor, and between X. bicolor and X. sp. Lofty Ranges (Supplementary Table S11). Populations of X. halmaturorum on Kangaroo Island had the greatest level of divergence from both Tasmanian X. bicolor and X. sp. Lofty Ranges in the Tothill Ranges. This indicated genetic similarity and may reflect continuous gradations or admixture between some populations (Rosenberg et al. 2002). These multiple sources of data supported the inclusion of X. halmaturorum and X. sp. Lofty Ranges in an expanded X. bicolor, which has nomenclatural precedence (Lindley 1835).
A long-standing putative natural hybrid between X. subundulatum and X. viscosum (Burbidge 1970), X. sp. Blackfellows Gap, is currently included in X. subundulatum (CHAH 2020a). First collected in 1961, additional collections were made in 1987 (~10 km south of Blackfellows Gap at Mount Murray, P. Gilmore 6209), and in 2007 (3.5 km north-east of Mount Murray at Cotter Hut, J.J. Bruhl 2596). Xerochrysum viscosum and X. subundulatum are both common in Namadgi National Park (Australia’s Virtual Herbarium, see http://avh.chah.org.au). Molecular data showed X. sp. Blackfellows Gap to be genetically more similar to X. viscosum and X. bracteatum sens. str. than to X. subundulatum (Fig. 2). Bayesian clustering suggested a complex mixture of ancestry, mainly comprising X. viscosum but with traces of X. bracteatum, X. subundulatum, X. palustre and X. aff. palustre (Fig. 15). Confident conclusions cannot be formed (Rosenberg et al. 2002); however, the single sample was neither intermediate nor nested in X. subundulatum or any of the X. milliganii group (Fig. 2). The three disjunct collections made over the past 46 years enclose an area over 1500 ha (Australia’s Virtual Herbarium, see http://avh.chah.org.au), indicating a persistent presence. Superficially, X. sp. Blackfellows Gap is similar to X. subundulatum with a low-growing clonal habit and rhizomatous root system; however, glabrous and obtuse phyllaries (v. scabridulous and acuminate), a deciduous pappus (v. persistent), and sessile glands on leaf abaxial surfaces (v. stipitate glands) distinguish it from that species. Increased sampling would be necessary to confirm that X. sp. Blackfellows Gap is of hybrid origin, or a distinct entity.
Samples grouped under the phrase name X. sp. North Stradbroke Island were not a single morphological and genotypic entity. The data indicated at least three distinct groups that are not corroborated by variations in morphology. The Iluka population clustered in the PCoA with X. sp. Northern Tablelands (Fig. 8), and samples from Byfield National Park formed a distinct cluster separate from all others in the ‘Boreale’ subset. Populations from Gheebulum Kunungai (Moreton Island) National Park were genetically widely diverged from other populations of X. sp. North Stradbroke Island, with little indication of shared common ancestry (Fig. 8, 13). Comparisons of morphology between mainland populations on headlands and the Gheebulum Kunungai (Moreton Island) National Park population showed differences in leaf length and width, and leaf indumentum. Plants on K’gari (Fraser Island; from habitat similar to those on Gheebulum Kunungai) were genetically similar to those on headlands at Noosa National Park and the Town of 1770, and also shared a similar leaf indumentum with headland populations. The failure to clarify the species limits in X. sp. North Stradbroke Island populations could be due to insufficient sampling, or historical and ongoing disturbance.
Naturalised populations of Xerochrysum seem to have contributed to the taxonomic confusion in other locations. During fieldwork, several populations of Xerochrysum bracteatum sens. lat. appeared to be naturalised or spreading outside their natural distribution, including near Queenstown in Tasmania (cited as X. bicolor in Wilson 2017), near Woodford and Yeppoon in Queensland. Molecular data placed the Tasmanian population with X. sp. Northern Tablelands (Fig. 8), and examination of morphology supported this finding. Xerochrysum sp. Tin Can Bay from Yeppoon was suggested to have ancestry shared with X. bracteatum sens. lat. and X. sp. North Kennedy (Fig. 13) and could be the product of a recent hybridisation event. All putative naturalised populations of Xerochrysum seen during this study were in highly disturbed roadside habitats and had been first recorded in the past 20 years or were new records, and they did not seem to occur in native vegetation nearby.
Conclusions
Multiple lines of evidence, including distinct genotypic differences, common ancestry and distinct morphology, support the recognition of 12 additional species of Xerochrysum (Table 10).
|
In all cases, distinct morphology on herbarium specimens and cultivated plants, corroborated by the molecular data, was observed among these entities, and it is on the combination of morphology and genetic differences that we propose taxonomic recognition at species rank. The parapatric populations within X. murapan that differ in multiple morphological traits were shown to be genetically similar, indicating recent divergence (see De Queiroz 2005, 2007).
Confounding results prevented confident assignment of coastal populations of Xerochrysum from Diamond Head, New South Wales, to Mackay, Queensland. They are treated here as either X. sp. North Stradbroke Island or X. sp. Tin Can Bay (includes X. sp. weedy). Limited sampling of populations west of the Great Dividing Range in New South Wales and Queensland prevented confident conclusions on the taxonomy of populations of Xerochrysum at Narrabri and Chinchilla. Denser sampling may indicate these populations to be part of X. hispidum or another entity. They are treated here as X. sp. Chinchilla.
Molecular and morphological data indicated the recently described X. halmaturorum to be conspecific with X. bicolor and the putative entity X. sp. Lofty Ranges. A taxonomic revision of an expanded concept for X. bicolor is provided in the following section.
Recognition and description of these 12 new taxa in Xerochrysum, together with basic information on ecology, distribution and conservation status, provide a taxonomic service to conservation and land managers, enabling future detailed assessment of conservation status and facilitating targeted conservation management where required.
This study has shown previously unrecognised species diversity in a much-admired and widely grown genus. Most species of Xerochrysum are perennial, with habits varying across shrub-like (e.g. X. frutescens), prostrate (e.g. X. banksii) and compact growth forms (e.g. X. neoanglicum). There are distinctly tropical (e.g. X. boreale, X. strictum, X. gudang), arid zone (e.g. X. interiore), coastal (e.g. X. bicolor, X. papillosum, X. sp. North Stradbroke Island), temperate (e.g. X. bracteatum sens. str., X. macranthum, X. macsweeneyorum), wetland (e.g. X. palustre) and alpine species (e.g. X. subundulatum, X. alpinum, X. milliganii, and X. collierianum). Application of this knowledge to plant breeding and development could expand the range of ornamental cultivars.
Taxonomy
Arrangement of this section follows that of Wilson (2008, 2017) and recognises the morphologically distinct X. bracteatum and X. milliganii groups. Characters in species descriptions follows Flann (1998) and Wilson (2017). Taxa are sorted alphabetically within groups. Type specimens for the names treated below have been examined either directly (indicated by ‘!’) or as images from CANB and on JSTOR Global Plants (indicated by ‘*’, see https://plants.jstor.org/). Distribution data are based on selected specimens seen and use IBRA regions (Department of the Environment and Energy 2016). Conservation status is based on International Union for Conservation of Nature Red List Categories and Criteria, Version 3.1. (IUCN 2019).
Xerochrysum Tzvelev, Novosti Sist. Vyssh. Rast. 27: 151 (1990)
Annual or perennial herbs, usually taprooted, sometimes rhizomatous. Indumentum cobwebby, felted, hirsute, hispid, pilose, tomentose, villous, or woolly, with septate trichomes, often flagelliform, and with sessile or stipitate glands. Leaves alternate; lamina flat or with margin recurved. Capitula terminating branches or branchlets, homogamous or heterogamous, disciform. Phyllaries multiseriate, medial phyllaries longest; lamina rigidly chartaceous, often spreading at junction with claw when mature; claw coriaceous, broadly oblong, flat; stereome broad, that of inner phyllaries fenestrate, veins numerous and extending into lamina, veins each of equal thickness in the X. bracteatum group, or central vein thickest in the X. milliganii group. Receptacle ± flat or concave, epaleate. Outermost florets usually sterile or sometimes female; corolla very narrowly tubular, shorter than bisexual florets, 3-, 4- or 5-lobed. Inner (or all) florets bisexual; corolla narrowly tubular, 5-lobed, lobes ovate, yellow to orange; anthers with apical appendage ovate and outwardly concave, tails slender, ±equal to collar, pollen pale yellow; style arms slender, with rounded, clavate, ovate, deltoid, triangular or narrowly triangular stylar appendage, yellow. Cypsela cylindrical or oblong, ~2.5–3.5 mm long; pericarp thick, finely striated, glabrous, surface with linear idioblasts, brown or straw- or bronze-coloured; stipe hollow, carpopodium of one row of thickened cells; apex patelliform when mature. Testa free from pericarp, cells ±equilateral, without thickening; vein passing to apex. Pappus uniseriate; bristles slender, equal to or exceeding corolla, white or yellow, consistent with the colour of the medial phyllaries, barbellate with apical cells acute and occasionally coloured red, bristles very shortly united at base and eventually deciduous as a whole or in pieces in the X. bracteatum group, or persistent in the X. milliganii group.
Key to species of Xerochrysum|
1. Herbs, or shrub-like, taprooted or sometimes rhizomatous; claw of medial phyllaries with several vascular bundles that terminate at its apex; cypselae with deciduous pappus, or if persisting, then with a distinct pappus–pericarp abscission line |
|
2. Phyllaries yellow |
|
3. Stem indumentum hirsute with stipitate glands; leaves cobwebby to woolly on margin, adaxially hirsute, otherwise glands both sides |
|
4. Stem indumentum cobwebby and glandular with glands towards apex |
|
5. Outer phyllaries scabridulous abaxially |
|
6. Cauline leaves 25–90 mm long and 5–20 mm wide, hispid or scabrid with stipitate glands adaxially |
|
7. Cauline leaves abaxially without septate trichomes |
|
8. Most cauline leaves 10 mm wide or greater |
|
9. Cauline leaves abaxially hirsute to hispid or tomentose |
|
10. Cauline leaves with fragile stipitate or sessile glands on cauline leaf adaxial surface; erect habit; not in far northern Queensland |
|
11. Cauline leaves with stipitate glands, fragile and usually lost but retaining persistent stipes and not appearing varnished; Top End, Northern Territory, and Kimberley, Western Australia |
|
12. Basal rosette usually present at flowering, flowering stems unbranched, capitula solitary |
|
13. Perennial; short rhizome or taproot present; basal leaf rosette absent; leaves hirsute abaxially with flagelliform trichomes on septate bases up to 0.1 mm long; restricted to gorge rim habitats on skeletal metasedimentary and granitic soils |
|
14. Cauline leaf margin woolly with septate trichomes; habitat mostly on margins of rainforest or Nothofagus moorei forest |
|
15. Populations restricted to northern Queensland |
|
16. Cauline leaf abaxial lamina pilose with septate flagelliform trichomes; lanceolate stylar appendages; restricted to high-altitude escarpment in New England National Park, New South Wales |
|
17. Stylar appendage ovate with obtuse apex; cauline leaf adaxial indumentum hispid, with stout septate trichomes arising from thickened basal cells, stipitate glands few or absent; decumbent habit |
|
18. Most mid-cauline leaves greater than 10 mm wide |
|
19. Phyllaries narrow and lanceolate |
|
20. Compact habit; perennial; stylar appendage narrow triangular; coastal headlands in New South Wales, and southern and central Queensland |
|
21. Perennial; foliaceous bract subtending capitulum greater than 10 mm long; Tasmania and Victoria |
|
22. Rhizomatous; alpine habitats |
|
23. Stylar appendage broad triangular or ovate |
|
24. Foliaceous bract subtending capitulum less than 10 mm long; cauline leaves sparsely hirsute adaxially; arid Northern Territory, northern South Australia, and arid Western Australia |
|
25. Stems hispid or with flagelliform trichomes below capitulum |
|
26. Phyllaries white or pink; Western Australia |
|
27. Cauline leaf adaxial indumentum hispid with minute flagelliform trichomes, older leaves glabrescent; south-eastern New South Wales and eastern Victoria |
|
28. Cauline leaves 5–30 mm wide and 15–130 mm long, cauline leaf adaxial indumentum hirsute with septate trichomes |
Xerochrysum bracteatum group
Xerochrysum banksii (A.Cunn. ex DC.) T.L.Collins & I.Telford, comb. nov.
Prostrate, tap-rooted, perennial herb. Stems and branches hirsute, scabrid, glabrescent and with glands; internode length 5–60 mm. Flowering stems branched or unbranched. Basal leaf rosette present at flowering in first year, later absent. Basal leaves oblanceolate to spathulate, 50–120 mm long and 10–25 mm wide, base amplexicaul, apex mucronate, margin hirsute; basal leaf abaxial indumentum with glands and occasionally hirsute with septate trichomes, midvein indumentum pilose with septate trichomes; basal leaf adaxial indumentum hirsute with septate trichomes. Cauline leaves oblanceolate, 20–50 mm long and 5–10 mm wide, base subauriculate and amplexicaul, apex mucronate, margin hirsute; abaxial indumentum with glands, midvein indumentum hirsute with septate trichomes; adaxial indumentum hirsute, scabrid and with glands. Foliaceous bracts subtending capitula 6–12 mm long, margin hispid. Capitula 30–40 mm wide, terminal, solitary. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex cuspidate. Stylar appendages triangular. Cypsela oblong, ~2.5 mm long and 0.9 mm wide, cross-section squarish or circular; pericarp straw- or brass-coloured, idioblasts present. Pappus deciduous, ~6 mm long.
Distribution
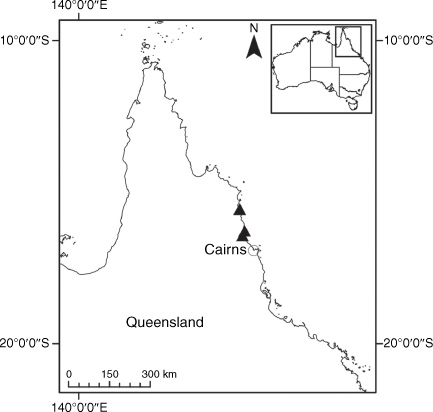
Endemic to Queensland where it occurs in the Wet Tropics Bioregion on the eastern coast between Cairns and Cooktown (Fig. 16).
Phenology
Inflorescences recorded from June–December. Mature cypselae collected in July and October (Fig. 17).
Habitat
The species inhabits grassy herblands on rocky coastal headlands. Associated species include Heteropogon triticeus, Themeda triandra, Myoporum boninense and Santalum lanceolatum.
Conservation status
Only two collections are known from the past 15 years from a single population of unknown size in Annan River (Yuku Baja-Muliku) National Park. Populations recorded in the 1800s in Cooktown and Trinity Bay near Cairns may now be extinct because of intense coastal development. On the basis of only one known extant population of unknown size, we suggest that a ‘Data Deficient’ status is appropriate under the IUCN (2019). Confirmation of the loss of populations in Cairns, Mossman River and Cooktown may qualify X. banksii as ‘Endangered’ or ‘Critically Endangered’.
Notes
Some variation in leaf indumentum was seen on specimens collected in the 1800s from Trinity Bay (MEL 61211, and MEL 61314), with a hispid to hirsute leaf indumentum both abaxially and adaxially with septate trichomes. Recent collections from Annan River National Park have no septate trichomes abaxially, only sessile glands. A specimen from Mossman’s [=Mossman] River (W.A. Sayer s.n. MEL 61192) has hispid leaf indumentum with much shorter septate trichomes. Xerochrysum banksii retains a prostrate habit in cultivation. The informal phrase name X. sp. Walker Point NE Herbarium has been used at NE for curatorial purposes and this study.
Alan Cunningham’s specimen at G-DC is here selected as the lectotype, as this specimen would have been used by de Candolle in preparing the protologue.
Selected specimens examined
QUEENSLAND: Cook: Endeavour River, June 1770, J. Banks & D. Solander s.n. (MEL 1591810!); Cooktown, 1877, W. Persieh s.n. (MEL 0061318A!); Endeavour River, 1882, W. Persieh s.n. (MEL 0061336A!); Annan River National Park, Walker Point, 22 Oct. 2018, A.J. Saunders 1 (BRI!, CANB!, CNS!, NE 110024!); Walker Point, S of Cooktown, 5 Dec. 2005, B.S. Wannan 4156 (BRI!, BSW, NSW!); Mossman’s [=Mossman] River, 1886, W.A. Sayer s.n. (MEL 0061192A!); Trinity Bay, 1881, G. Karsten s.n. (MEL 0061211A!); Trinity Bay, E. Fitzalan s.n. (MEL 0061314A!).
|

|
Xerochrysum berarngutta T.L.Collins & I.Telford, sp. nov.
Diagnosis
Distinguished from X. bracteatum sens. str. by a perennial life form (annual or biennial in X. bracteatum), abaxial leaf surface pilose with septate and glandular trichomes and glands (only with glands in X. bracteatum), cuspidate to apiculate phyllary apices (obtuse in X. bracteatum), and foliaceous bracts subtending capitula 10–20 mm long (8–10 mm in X. bracteatum); and from all other species in the genus by a long, thick rhizome, a densely pilose leaf indumentum of septate trichomes, and stipitate glands both abaxially and adaxially.
Decumbent, rhizomatous, perennial herb. Stems and branches with glands and villous with septate trichomes, internode length 40–55 mm. Basal leaf rosette absent at flowering. Basal leaves spathulate, 70–200 mm long and 15–30 mm wide, base amplexicaul, margin villous with septate trichomes, apex obtuse and mucronate; abaxial indumentum densely pilose with septate trichomes and with glands, midvein indumentum villous with septate trichomes; adaxial indumentum densely pilose with septate trichomes. Radical leaves arising from a rhizome. Cauline leaves oblanceolate to obovate, 40–150 mm long and 4–30 mm wide, leaf base attenuate, margin villous with septate trichomes, apex apiculate and mucronate; abaxial indumentum densely pilose with septate trichomes and sessile glands, midvein indumentum villous with septate trichomes; adaxial indumentum hirsute to densely pilose with septate trichomes, and sessile glands. Foliaceous bracts subtending capitula 10–20 mm long, margin villous. Capitula 40–60 mm wide, terminal, in panicles or solitary. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate, apex apiculate, abaxial surface smooth. Medial phyllaries ovate to lanceolate, abaxially orange or yellow, apex cuspidate to apiculate. Stylar appendages narrowly triangular to ovate. Cypsela oblong, ~2.7 mm long and 0.9 mm wide, cross-section squarish or circular; pericarp straw- or brass-coloured, idioblasts present. Pappus deciduous, ~7 mm long.
Distribution
Endemic to the high-altitude escarpment on the eastern edge of the New England Tablelands Bioregion, where it is known only from the vicinity of Point Lookout, ~70 km east of Armidale, New South Wales (Fig. 18).
Phenology
Recorded flowering from February–April (Fig. 19). Late-stage infructescence containing small numbers of cypselae collected in April.
Habitat
Occurring in small openings in the canopy at ~1400-m altitude, growing in humic sediments on and between basalt boulders, on steep slopes and broken cliffs. Associated with Acacia melanoxylon, Banksia integrifolia subsp. monticola, Eucalyptus pauciflora, Lomatia fraseri, Cassinia telfordii, Lomandra longifolia, Solanum and Plectranthus. Other associated species include the localised cliff-line endemics Coronidium elatum subsp. minus, Gingidia rupicola, Gaultheria viridicarpa and Wahlenbergia telfordii.
Conservation status
Only known from effectively one population in the New England National Park, New South Wales, where 17 plants were recorded scattered along ~170 m of escarpment in 2017. On the basis of only one known extant population of less than 50 individuals, we suggest a ‘Critically Endangered’ status is appropriate under the IUCN (2019), fulfilling Criteria C2 and D.
Etymology
The species epithet is the traditional name of the type locality, a place considered sacred to traditional owners (Steven Ahoy, pers. comm., 2020), as a noun in apposition (ICN Art. 23.5, Shenzhen Code, Turland et al. 2018).
Selected specimens examined
NEW SOUTH WALES: Northern Tablelands: New England National Park, Point Lookout, 12 Apr. 2017, T.L. Collins 958 & J.J. Bruhl (BRI!, NE!, NSW!); New England National Park, Eagles Nest Lookout, 11 Mar. 2006, G.P. Duley 69, J.J. Bruhl & I.R. Telford (NE!).
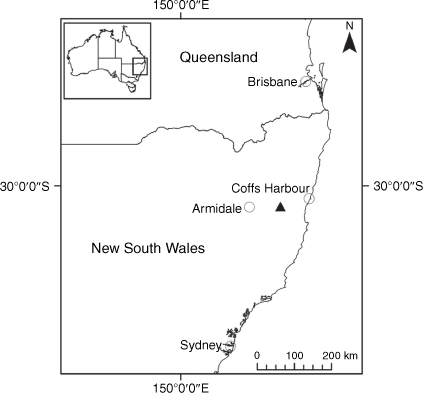
|
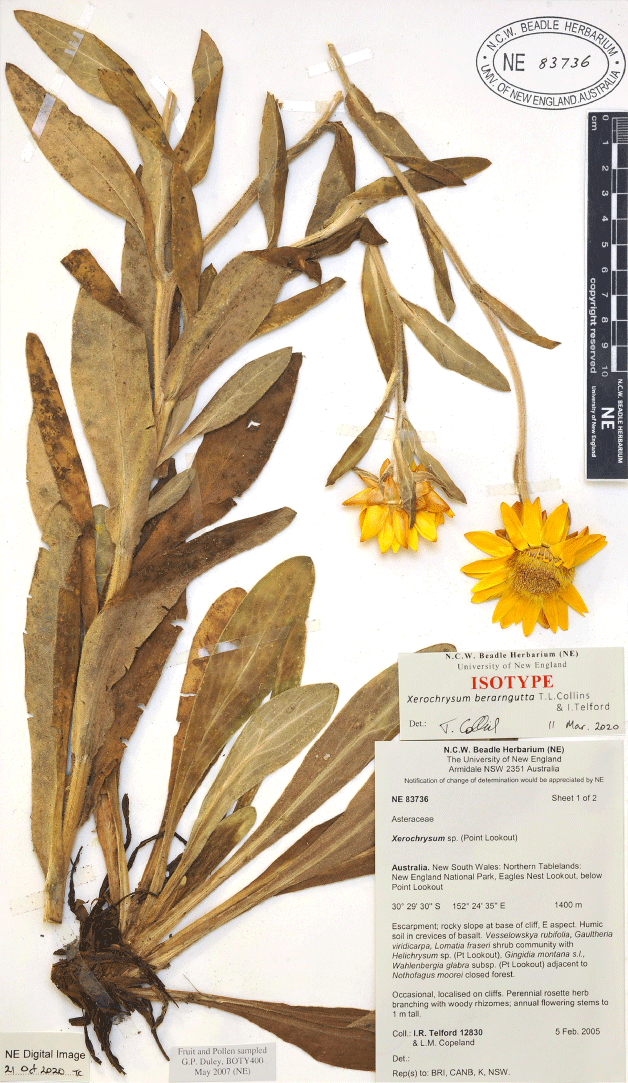
|
Xerochrysum bicolor (Lindl.) R.J.Bayer, Kew Bulletin 56: 1014 (2001)
Erect, perennial, taprooted herb. Stems and branches with glands, and scabrid or hispid (prominent raised ridges along stems), internode length 10–35 mm. Basal leaf rosette absent at flowering. Basal leaves obovate or spathulate, 50–150 mm long and 10–30 mm wide, base auriculate or attenuate and amplexicaul, margin hirsute, pilose or hispid with septate trichomes, apex obtuse to apiculate and mucronate; abaxial indumentum with glands, midvein indumentum hirsute to hispid with septate trichomes and with glands; adaxial indumentum scabrid or hispid with septate trichomes, and with glands. Cauline leaves obovate, 40–120 mm long and 10–35 mm wide, base auriculate and amplexicaul, margin hispid or scabrid with dense, shorter septate trichomes as well as scattered septate trichomes, 2–4 times longer, and with stipitate glands; apex acuminate to apiculate and mucronate; abaxial indumentum with glands, midvein indumentum hispid and scabrid with septate trichomes; adaxial indumentum hispid or scabrid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 5–15 mm long, margin hispid with stipitate glands. Capitula 30–60 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate or hispid, abaxial surface smooth, apex acuminate. Medial phyllaries narrow ovate to lanceolate, abaxially brown, white or yellow, apex acuminate to cuspidate. Stylar appendages deltoid to ovate. Cypsela oblong, ~2.5 mm long and 0.75 mm wide, cross-section squarish; pericarp brown, idioblasts present. Pappus deciduous ~4–8 mm long.
Distribution
Occurs sporadically over a broad area in southern South Australia in the Flinders Lofty Block and Kanmantoo bioregions, in south-western Victoria in the Victorian Midlands Bioregion, and coastal Tasmania in the King, Tasmanian West, and Tasmanian South East bioregions (Fig. 20).
Phenology
Flowers recorded from November–January, and mature cypselae in February.
Habitat
Coastal heath, low shrubland grassland mosaic and Allocasuarina–Eucalyptus woodland, on sandy or skeletal gravelly loam soils, among low rock outcrops or on cliffs.
Conservation status
Occurs over a large area, including public and private conservation reserves. Populations on Kangaroo Island and in the Tothill Ranges estimated in the hundreds and thousands of plants respectively. Listed as ‘rare’ under the Tasmanian Threatened Species Protection Act 1995. Not listed under the Commonwealth of Australia Environment Protection and Biodiversity Conservation Act 1999. Monitoring and assessment of response and recovery after the 2019–2020 bushfires would inform conservation status (Keelty et al. 2020). With the inclusion of populations in South Australia and Victoria, we recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
The majority of collections are from populations with yellow phyllaries; however, a population with white phyllaries from the western coast of Tasmania near Temma was found during fieldwork and in the analyses.
‘Eastcoast paperdaisy’ is used in Tasmania for X. bicolor (State of Tasmania, ‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au, accessed 22 March 2020).
Selected specimens examined
SOUTH AUSTRALIA: Goyder: Tothill Range, Mollers Gap Road, 26 Nov. 2017, T.L. Collins 988 & A.N. Schmidt-Lebuhn (AD!, CANB!, NE!). Adelaide Hills: South Australia, 10 Nov. 1879, R. Tate s.n. (AD!). Victor Harbor: South Australia, 1 Dec. 1909, E.H. Ising s.n. (AD!); Hindmarsh Falls, 23 Nov. 2017, T.L. Collins 982 & A.N. Schmidt-Lebuhn (AD!, CANB!, NE!); Newland Head Conservation Park, 23 Nov. 2017, T.L. Collins 981 & A.N. Schmidt-Lebuhn (AD!, CANB!, NE!). Yankalilla: South Australia, Jan. 1926, J.B. Cleland s.n. (AD!). Kangaroo Island: Cape St Albans, 20 Nov. 2017, T.L. Collins 973 & A.N. Schmidt-Lebuhn (AD!, CANB!, NE!). VICTORIA: Southern Grampians: Victorian Midlands, 29 Dec. 1988, R.M. King 9703 & F.E. Heinz (MEL!). Horsham: Victorian Midlands, 8 Nov. 1987, S.T.W. Parfett 134 (MEL!). TASMANIA: Little Badger Island, s. dat., J.S. Whinray 8605 (CANB, HO, MEL!); Alum Cliffs, S of Taroona, Hobart, 28 Feb. 2018, T.L. Collins 1016 & R.L. Andrew (NE!, HO!, CANB!); Arthur Pieman Conservation Reserve, 4 Mar. 2018, T.L. Collins 1033 & R.L. Andrew (NE!, HO!, CANB!); S of Temma on coastal track, 4 Mar. 2018, T.L. Collins 1034 & R.L. Andrew (NE!, HO!, CANB!).
|
Xerochrysum boreale Paul G.Wilson, Nuytsia 28: 17 (2017)
Erect, annual or perennial, taprooted herb. Stems and branches cobwebby or pilose, internode length 5–30 mm. Basal leaf rosette present in first year at flowering, later absent. Basal leaves oblanceolate to obovate, 30–70 mm long and 5–12 mm wide, base amplexicaul, margin cobwebby or pilose with septate trichomes, apex mucronate; abaxial indumentum hirsute with septate trichomes and with glands, midvein indumentum cobwebby or hirsute with septate trichomes; adaxial indumentum hirsute with septate trichomes. Cauline leaves oblanceolate, 20–80 mm long and 4–9 mm wide, base amplexicaul, margin cobwebby or hirsute with septate trichomes, apex mucronate; abaxial indumentum hirsute with septate trichomes, and with glands, midvein indumentum cobwebby to hirsute; adaxial indumentum cobwebby to hirsute with septate trichomes. Foliaceous bracts subtending capitula 5–8 mm long, margin cobwebby. Capitula 25–35 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin hispid, abaxial surface smooth, apex apiculate. Medial phyllaries ovate to narrow ovate, abaxially yellow, apex cuspidate. Stylar appendages narrowly triangular. Cypsela oblong, ~2.3 mm long and 0.75 mm wide, cross-section circular; pericarp grey–brown, idioblasts present. Pappus deciduous, ~6–7 mm long.
Distribution
Endemic to the northern end (‘Top End’) of the Northern Territory in the Darwin Coastal and Arnhem Coast bioregions (Fig. 21).
Phenology
Recorded flowering August–October, with cypselae being recorded in October.
Habitat
Populations in the Darwin Coastal Bioregion commonly occur in eucalypt and Pandanus woodlands on sandy coastal plains with rare occurrences further inland on sandstone plateaux in the Mount Tolmer and Bundy Station areas, and on sandy clay seasonal swamps on the Cox Peninsula. Occurs on clay soils on the margins of the Arafura Swamp and gravelly loams in eastern Arnhem Land.
Notes
The largest populations seen during the course of this study were in areas that were patchily burnt as part of traditional land management practices in the early dry season (June–July) near the communities of Wadeye (U. Crocombe, pers. comm., 2018) and Mirrnatja (S. Guyula, pers. comm., 2018). Two populations from sandy clay seasonal swamps on Cox Peninsula have some individuals appearing to have rhizomatous stems, possibly adventitious roots in response to partial burial, and some with taproots. Molecular data indicated no genotypic differences within or between any X. boreale populations included in this study, and for this reason, we are not recognising rhizomatous plants as a different taxon.
Conservation status
Occurs over a large area, with some populations in the thousands recorded in 2018. Changes to land management and absence of traditional early dry season burning may be affecting some populations that could not be located in 2018 (T. L. Collins and J. J. Bruhl, unpubl. data). Plants at Dundee Beach in the Darwin Coastal Bioregion were affected by habitat loss as a result of coastal development and grassland mowing. We recommend a status of ‘Least Concern’ (IUCN 2019).
Selected specimens examined
NORTHERN TERRITORY: Darwin and Gulf: Dundee Beach, S of Dunheved Road, 2 Oct. 2018, T.L. Collins 1088 & J.J. Bruhl (CANB!, DNA!, NE!); Dhupuwamirri Road, on road to Mirrnatja, 9 Oct. 2018, T.L. Collins 1091 & J.J. Bruhl (CANB!, DNA!, NE!); Mirrnatja, ~2.2 km N of village, 10 Oct. 2018, T.L. Collins 1092 & J.J. Bruhl (CANB!, DNA!, NE!); Central Arnhem Road, Yunupingu Cattle Farm, 10 Oct. 2018, T.L. Collins 1093 & J.J. Bruhl (CANB!, DNA!, NE!); Wadeye, Old Mission Road, 5 Oct. 2018, T.L. Collins 1089 & J.J. Bruhl (CANB!, DNA!, NE!); Wadeye, on unnamed coast track, 5 Oct. 2018, T.L. Collins 1090 & J.J. Bruhl (CANB!, DNA!, NE!). WESTERN AUSTRALIA: West Kimberley: West Kimberley, 1901, F.M. House s.n. (PERTH!). Wyndham–East Kimberley: Head of King Edward River, 7 Sep. 1921, C.A. Gardner 1565 (PERTH!).
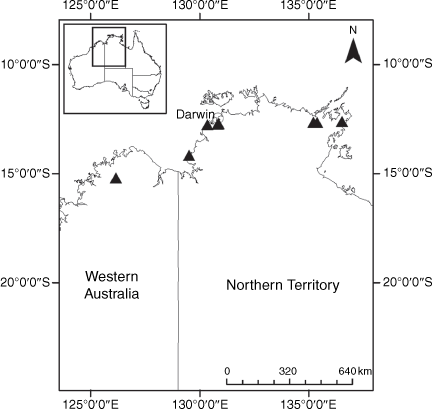
|
Xerochrysum bracteatum (Vent.) Tzvelev, Novosti Sist. Vyssh. Rast. 27: 151 (1990)
Erect, annual or sometimes short-lived perennial (dependant on season), tap-rooted herb, 0.5–1.8 m tall. Stems and branches cobwebby, hirsute to pilose with septate trichomes, or glabrescent, and with glands; internode length 10–35 mm. Flowering stems branched, or unbranched (becoming branched with maturity, occasionally single-stemmed). Basal leaf rosette usually absent at flowering. Basal leaves elliptic to spathulate, 50–150 mm long, and 10–30 mm wide, base amplexicaul and attenuate, margin woolly to hirsute with septate trichomes, apex obtuse to apiculate; abaxial indumentum hispidulous with septate trichomes, and with glands; adaxial indumentum hispidulous with septate trichomes. Cauline leaves oblanceolate, 50–180 mm long, 5–25 mm wide, base attenuate and subamplexicaul, margin hispid with septate trichomes, apex acuminate to acute; abaxial indumentum with glands, midvein indumentum hispid with septate trichomes; adaxial indumentum hispidulous with septate trichomes and with glands. Foliaceous bracts subtending capitula ~8–10 mm long, margin hispid with septate trichomes. Capitula terminal, 30–50 mm wide, in panicles; outer phyllaries broad-ovate, yellow or brown, basal margin hispid, abaxial surface smooth, apex apiculate. Medial phyllaries oblong, narrow ovate or lanceolate, abaxially brown or yellow, apex cuspidate. Stylar appendages deltoid to acute. Cypsela oblong, ~2.2 mm long and 0.75 mm wide, cross-section squarish; pericarp grey–brown, idioblasts present. Pappus deciduous 5–6 mm long.
Distribution
Endemic to south-eastern New South Wales and far-eastern Victoria in the Sydney Basin, South East Corner, and South East Coastal Plain bioregions (Fig. 22). Naturalised in Saint Helena and many countries owing to widespread cultivation (Missouri Botanical Garden 2020).
Phenology
Recorded flowering in Australia August–February and fruiting December–February.
Habitat
Most commonly recorded from eucalypt woodland and forest on a wide variety of soils including ones derived from granite and basalt, from sea level to ~1000-m altitude. Common on roadsides where disturbance and water shed from the road may favour dispersal, germination and establishment.
Conservation status
Although there have been relatively few collections in the past few decades in the Hawkesbury–Nepean region, X. bracteatum has been collected from ~20 populations on the New South Wales South Coast in the past 30 years, including in conservation reserves. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Xerochrysum bracteatum is illustrated in Fairley and Moore (1989), plate 1158 on page 317, as Helichrysum bracteatum, yellow paper daisy or golden everlasting.
Selected specimens examined
NEW SOUTH WALES: Central Tablelands: Mount Tomah, Lithgow Road, 25 Jan. 1959, B.R. Paterson s.n. (CANB*). Central Coast: The Oaks, on road to Penrith (Silverdale Road), 15 Dec. 2018, T.L. Collins 1005 (CANB!, NE!, NSW!); Hopetoun Park, Panorama House Motel, Bulli Lookout, 7 Jan. 2019, T.L. Collins 1168 (CANB!, NE!, NSW!); Mount Keira Road, 7 Jan. 2019, T.L. Collins 1169 (CANB!, NE!, NSW!). South Coast: Nowra Road ~4 km E of intersection with Gretas Road, 26 Jan. 2019, T.L. Collins 1174 (CANB!, NE!, NSW!); Central Tilba, Paradise Hill, 27 Jan. 2019, T.L. Collins 1175 (CANB!, NE!, NSW!); Gulaga National Park, Mount Dromedary Trail, 27 Jan. 2019, T.L. Collins 1177 (CANB!, NE!, NSW!); Nullica State Forest, track to Nethercote Falls, 3 Feb. 2019, T.L. Collins 1181 (CANB!, NE!, NSW!). VICTORIA: East Gippsland: on track to summit of Mount Ellery, 22 Feb. 1984, D.E. Albrecht 212 (AD, MEL!); Mount Elizabeth Nature Conservation Reserve, 26 Oct. 2019, J.J. Bruhl 3643, S. Dema, & H.T. Kennedy (CANB!, MEL!, NE 109790!, NSW!).
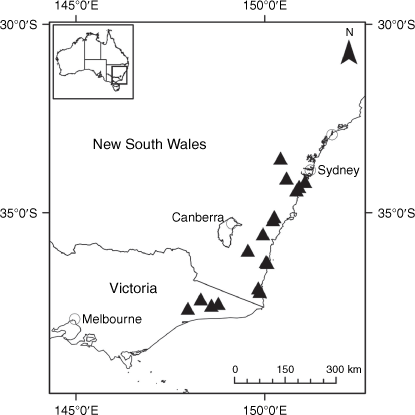
|
Xerochrysum copelandii J.J.Bruhl & I.Telford, sp. nov.
Diagnosis
Distinguished from X. bracteatum by a perennial life form (v. annual or sometimes short-lived perennial), septate trichomes on leaf abaxial surface (v. with glands), and acuminate phyllary apices (v. apiculate). Distinguished from X. murapan by foliaceous bracts subtending capitula 8–10 mm long (v. 10–25 mm long in X. murapan), acuminate to cuspidate phyllary apex (v. apiculate), and cauline leaves 5–10 mm wide (v. 10–25 mm wide in X. murapan).
Erect, shortly rhizomatous or taprooted, perennial herb, up to ~1 m tall. Stems and branches cobwebby, hirsute, or glabrescent, and with glands; internode length 15–30 mm. Basal leaf rosette absent at flowering. Basal leaves spathulate, 80–130 mm long and 20–35 mm wide, base subamplexicaul, margin hirsute with septate trichomes, apex apiculate; abaxial indumentum hirsute to hispid with septate trichomes, and with glands; abaxial midvein indumentum villous with septate trichomes; adaxial indumentum hispid with septate trichomes, and with glands. Cauline leaves oblanceolate or lanceolate, 20–90 mm long and 5–10 mm wide, base subauriculate and amplexicaul, margin hispid and scabrid with septate trichomes, apex mucronate; abaxial indumentum hirsute with septate trichomes, to glabrous, and with glands; abaxial midvein indumentum cobwebby and hispid with septate trichomes, and with glands; adaxial indumentum cobwebby and hispid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 8–10 mm long or sometimes absent, margin glabrous or hispid. Capitula 25–50 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex acuminate. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex acuminate to cuspidate. Stylar appendages deltoid to ovate (female florets have clavate to rounded stylar appendages). Cypsela oblong, ~2.3 mm long and 0.75 mm wide, cross-section squarish to circular; pericarp brown to brass- or straw-coloured, idioblasts present. Pappus deciduous, ~6 mm long.
Distribution
Endemic to north-eastern New South Wales where it is known only from the New England Tablelands Bioregion (Fig. 23). Mostly occurring along the eastern escarpment of the plateau from the Great Dividing Range south-east of Tenterfield, New South Wales, south to the gorges of the Macleay River catchment east of Armidale.
Phenology
Recorded flowering January–February and fruiting in February (Fig. 24).
Habitat
The species inhabits ridge tops and gorge rims, often in rocky sites, at 900–1500-m altitude on skeletal or gravelly soils derived mostly from metasediments or basalt, rarely from granite. The species grows in grassy open forest or woodland with Eucalyptus laevopinea, E. nobilis, E. obliqua, E. pauciflora, E. retinens or E. caliginosa recorded as dominants. Other associated species include Acacia melanoxylon, Allocasuarina torulosa, Coprosma quadrifida, Pimelea neoanglica and Poa sieberiana.
Conservation status
Recorded as common, although localised at most sites. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Cauline leaf lamina abaxial indumentum is variable: populations at Round Waterhole Creek, Metz Gorge, Werrikimbe National Park, Cathedral Rock National Park, and New England National Park, have an hirsute indumentum of scattered septate trichomes; populations at Washpool National Park, Styx River, Round Mountain, and the putative hybrid X. copelandii x viscosum at Henry River Falls have sessile glands.
Etymology
The species epithet recognises the work of outstanding field-botanist and taxonomist Lachlan Mackenzie Copeland (1973–) of Coffs Harbour.
Selected specimens examined
NEW SOUTH WALES: Northern Tablelands: Guy Fawkes River National Park, Henry River Falls, 24 Aug. 2017, T.L. Collins 969, R.L. Andrew, J.J. Bruhl & J.K. Janes (NE!); Round Waterhole Creek, 11 Feb 2018, T.L. Collins 1013 & B. Wright (CANB!, BRI!, NE!, NSW!); Hillgrove Gorge, 28 Feb. 1999, J.J. Bruhl 1840 & I.R. Telford (NE!); Washpool National Park, S of summit of Mount Bajimba, 25 Feb. 2011, L.M. Copeland 4502 (BRI, NE!, NSW); Great Dividing Range, Washpool National Park, 25 Jan. 2014, I.R. Telford 13440 & T. Vollbon (NE!).
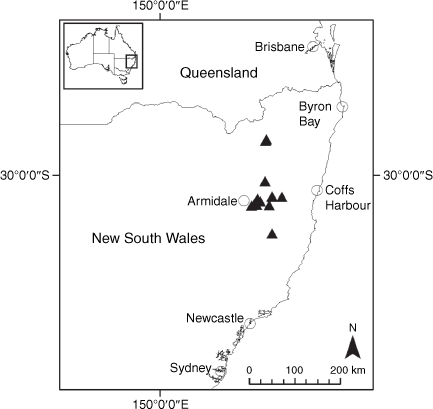
|

|
Xerochrysum frutescens J.J.Bruhl & I.Telford, sp. nov.
Diagnosis
Distinguished from X. bracteatum by the perennial life form and shrub-like habit (v. annual or sometimes short-lived perennial, and erect habit), acuminate to cuspidate phyllary apices (v. apiculate), and foliaceous bracts subtending capitula 10–40 mm long (8–10 mm long in X. bracteatum). Distinguished from X. berarngutta by the taproot (v. rhizome), and the hispid leaf lamina adaxial indumentum (v. hirsute to pilose).
Erect, taprooted, perennial shrub-like herb, up to ~80 cm tall. Stems and branches cobwebby, to felted, tomentose, villous, or woolly with septate trichomes, and with glands; internode length 5–40 mm. Basal leaf rosette absent at flowering. Basal leaves oblanceolate to obovate or spathulate; 40–100 mm long and 10–25 mm wide, base amplexicaul and attenuate, margin cobwebby to villous with septate trichomes, apex obtuse and mucronate; abaxial indumentum cobwebby to tomentose or villous with septate trichomes, and with glands; abaxial midvein indumentum cobwebby and villous with septate trichomes, or glabrous; adaxial indumentum cobwebby to hirsute with septate trichomes, and with glands. Cauline leaves oblanceolate to lanceolate, 50–200 mm long and 5–20 mm wide, leaf base amplexicaul and attenuate, margin cobwebby, hispid, or woolly with septate trichomes, apex acute and mucronate; abaxial indumentum cobwebby to villous or tomentose with septate trichomes, and with glands; abaxial midvein indumentum cobwebby, hirsute or woolly with septate trichomes, and with glands; adaxial indumentum cobwebby to hirsute or hispid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 10–40 mm long, margin felted to woolly. Capitula 50–90 mm wide, terminal, in panicles or solitary. Outer phyllaries ovate to broad-ovate, brown, basal margin fimbriate and hispid, abaxial surface smooth, apex cuspidate, or acute to acuminate. Medial phyllaries lanceolate, abaxially yellow, apex acuminate to cuspidate. Stylar appendages deltoid to ovate. Cypsela oblong, ~2.5 mm long and 0.9 mm wide, cross-section squarish to circular; pericarp straw- or brass-coloured, idioblasts present. Pappus deciduous, ~8 mm long.
Distribution
Restricted to South Eastern Queensland, and New South Wales North Coast bioregions along the Main Range (Great Dividing Range) from Cunninghams Gap, Queensland, south to Acacia Plateau, eastward along the McPherson Range to Springbrook and southward along the Tweed Range, New South Wales (Fig. 25).
Phenology
Recorded flowering November–March (Fig. 26) with mature cypselae collected in May.
Habitat
Xerochrysum frutescens inhabits rocky slopes and cliff tops at 1000–1150-m altitude in skeletal loamy soils mostly on trachyte and basalt cliff edges of the Main Range and Tweed volcanoes. The species grows in open forest or shrub communities, mostly adjacent to rainforest including Nothofagus moorei closed forest. Associated taxa at Main Range sites include Leptospermum polygalifolium subsp. montanum, Cuttsia viburnea, Pimelea umbratica, Doryanthes palmeri and at McPherson Range sites, Cassinia straminea, Coronidium telfordii, Podolepis monticola, Prostanthera lanceolata and Leucopogon sp. Lamington (G. Leiper AQ633386).
Conservation status
Most populations occur in conservation reserves but there are no precise data on population sizes. Specimen label data indicate fewer than 10 known populations that are localised or restricted in extent, and most occur at altitudes of >1000 m. Without population data it is not possible to confidently evaluate conservation status. Ongoing threats associated with anthropogenic climate change including heatwaves, extreme drought and intense fires potentially threaten X. frutescens and the associated vegetation. Given the very limited geographic range and estimated small population sizes, we suggest a ‘Vulnerable’ status is appropriate under the IUCN (2019) because it fulfils the criteria of D1 and D2. A precise assessment of population size and estimated stability would clarify whether X. frutescens should be listed as ‘Vulnerable’, ‘Endangered’ or ‘Critically Endangered’.
Notes
The two collections from Mount Merino Lookout (I.R. Telford 12886 and I.R. Telford 2632) have relatively long, scattered septate trichomes compared with the shorter, closely spaced septate trichomes seen on specimens from other populations.
Etymology
The specific epithet is from the Latin frutex (a bush or shrub) and refers to the shrub-like habit of this species.
Selected specimens examined
QUEENSLAND: Moreton: Main Range National Park, ~200 m S of summit of Mount Cordeaux, 11 Mar. 2005, L.M. Copeland 3904 & A.J. Lynch (NE!, PERTH); Main Range National Park, Goomburra Section, 28 Oct. 2015, P.I. Forster 43151 (BRI!); Mount Mitchell, Cunninghams Gap, 18 Aug. 1992, P.I. Forster 11105 (BRI!); Mount Lindesay, base of trachyte cliffline, 15 Nov. 1990, P.I. Forster 7562 (BRI!). NEW SOUTH WALES: North Coast: McPherson Range, Limpinwood Nature Reserve, Mount Merino, 6 Nov. 2005, I.R. Telford 12886 (NE!); Koreelah National Park, 28 Mar. 2009, L.M. Copeland 4353 (CANB, NE!, NSW); Lamington Plateau, Hunter’s Lookout, 12 Mar. 2014, P.I. Forster 40802 (BRI!).
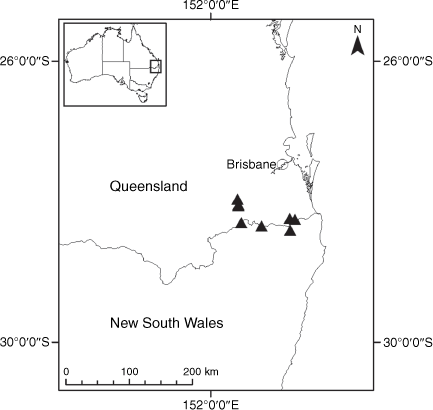
|
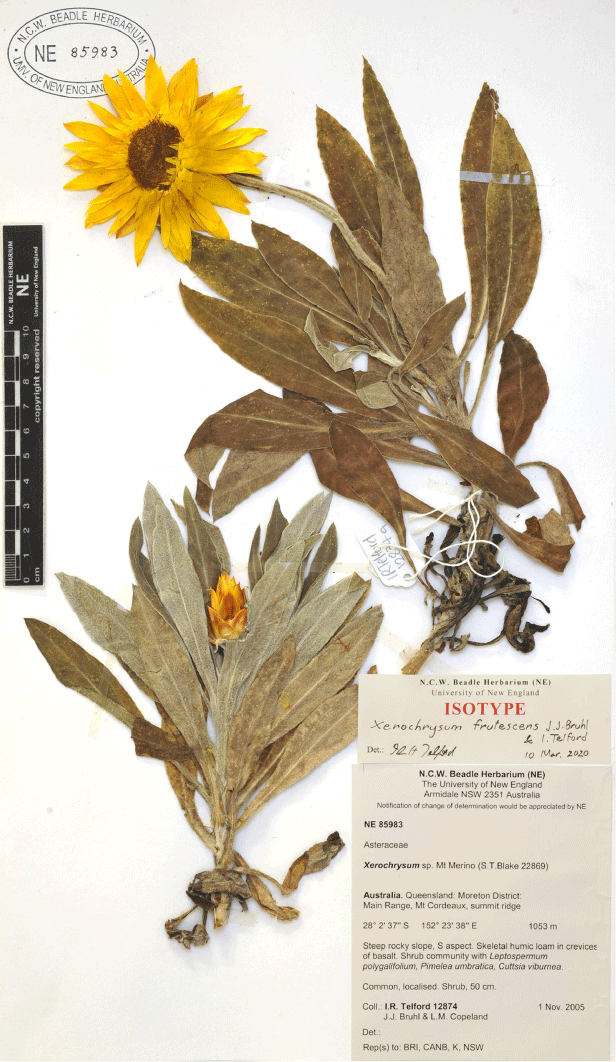
|
Xerochrysum gudang T.L.Collins & J.J.Bruhl, sp. nov.
Diagnosis
Distinguished from X. bracteatum by a perennial life form (v. annual or sometimes short-lived perennial), the presence of septate trichomes on leaf lamina abaxial surface (v. with glands), and the absence of glands on stems and leaf lamina adaxial surface (v. with glands). Distinguished from X. strictum, X. banksii and X. boreale by the absence of glands on stems and leaf adaxial surfaces (present on X. strictum, X. banksii and X. boreale).
Decumbent to erect, taprooted, perennial herb. Stems and branches up to 70 cm long, cobwebby, hirsute, scabrid, or woolly, to glabrescent; internode length 10–20 mm. Previous season’s flowering stems marcescent. Basal leaf rosette absent at flowering. Basal leaves oblanceolate to spathulate, 70–120 mm long and 5–15 mm wide, base subamplexicaul, margin villous with septate trichomes, apex mucronate; abaxial indumentum villous with septate trichomes, and with glands, midvein indumentum pilose with septate trichomes; adaxial indumentum villous with septate trichomes. Cauline leaves oblanceolate to lanceolate, 50–100 mm long and 6–15 mm wide, base auriculate and amplexicaul, margin cobwebby and hirsute with septate trichomes, apex mucronate; abaxial indumentum hispid with septate trichomes and with glands, midvein indumentum cobwebby and hispid with septate trichomes; adaxial indumentum cobwebby and hirsute with septate trichomes. Foliaceous bracts subtending capitula 6–8 mm long, margin woolly to cobwebby, and hispid. Capitula 30–40 mm wide, terminal, in panicles. Outer phyllaries ovate to broad-ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex cuspidate. Stylar appendages deltoid. Cypsela oblong, ~2.7 mm long and 0.75 mm wide, cross-section circular; pericarp straw- or brass-coloured, idioblasts present. Pappus deciduous, ~8–9 mm long.
Distribution
Known only from the Somerset area of northern Cape York and nearby islands in the Cape York Peninsula Bioregion (Fig. 27). Extensive survey of similar habitat on Cape York Peninsula has not recorded populations of Xerochrysum between Jardine River National Park and Cooktown, except for X. strictum near Coen (J. R. Clarkson, pers. comm., 2018).
Phenology
Recorded in flower June–October and fruiting in June (Fig. 28).
Habitat
Windswept grassy herbfields among shrubland on low rocky headlands.
Conservation status
Population-size data are scant, with 50–100 plants being estimated at Somerset Lookout in 2018, and other specimen label data describing populations as ‘sporadic’ (L.J. Brass 18778), and ‘infrequent’ (K.R. Thiele 905). Given the very limited geographic range and estimated small population sizes, we suggest a ‘Vulnerable’ status is appropriate under the IUCN (2019) because it fulfils the criteria of D1 and D2. A precise assessment of population size and estimated stability would clarify whether X. gudang should be listed as ‘Endangered’ or ‘Critically Endangered’.
Notes
The decumbent habit observed in the field was retained on glasshouse-grown plants. The informal phrase name X. sp. Fly Point NE Herbarium has been used at NE for curatorial purposes and this study.
Etymology
The species epithet is the traditional name of the type locality, a place important to traditional owners (Christo Lifu, pers. comm., 2020), as a noun in apposition (Shenzhen Code art. 23.5; Turland et al. 2018).
Selected specimens examined
QUEENSLAND: Cook: Albany Island, s.dat., W. Hill 71 (K000899118*); Fly Point, near Albany Pass, Cape York Peninsula, 25 June 1973, S. Powell 9 (CANB!); Fly Point, ~11 km SE of Cape York, 30 Oct. 1965, L.S. Smith 12636 (BRI!); Newcastle Bay, Cape York Peninsula, 15 Feb. 1986, D.L. Jones s.n. (BRI!); Newcastle Bay, 2.5 miles S of Somerset, 2 May 1948, L.J. Brass 18778 (A, CANB!); Headland above Nanthau Beach, 4.5 km direct line SSW of Somerset, 30 June 1985, K.R. Thiele 905 (CANB!).
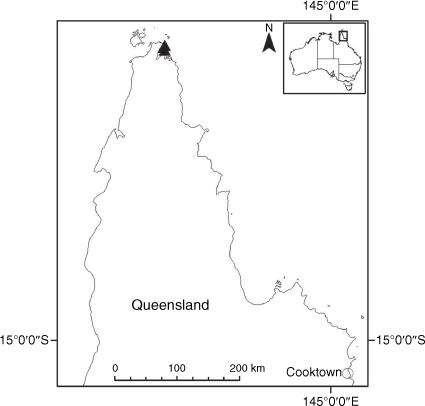
|

|
Xerochrysum hispidum T.L.Collins & I.Telford, sp. nov.
Diagnosis
Distinguished from X. bracteatum and X. bicolor by the presence of foliaceous bracts subtending capitula 15–35 mm long (8–10 mm long in X. bracteatum; 5–6 mm long in X. bicolor), and the prominent, robust, hispid septate trichomes scattered on the leaf lamina adaxial surface, leaf margin and abaxial midvein. Further distinguished from X. bicolor by a strictly annual life form (v. perennial), and the attenuate leaf base (v. auriculate).
Erect, taprooted, annual herb, 10–80 cm tall, depending on rainfall. Stems and branches cobwebby and hispid with septate trichomes, and with glands; internode length 15–30 mm. Basal leaf rosette may be present or absent at flowering. Basal leaves obovate to spathulate, 50–150 mm long and 10–40 mm wide, base amplexicaul, margin hispid and pilose with septate trichomes, apex mucronate and apiculate; abaxial indumentum with glands, midvein indumentum pilose with septate trichomes; adaxial leaf surface glabrous (with scattered septate trichomes). Cauline leaves oblanceolate to obovate, 60–160 mm long and 10–30 mm wide, base amplexicaul and attenuate, margin cobwebby and hispid with septate trichomes, apex acute and mucronate; abaxial indumentum with glands, midvein indumentum hispid or pilose with septate trichomes; adaxial indumentum hispid with septate trichomes, and with additional scattered large septate trichomes, and with glands. Foliaceous bracts subtending capitula 15–35 mm long, margin hispid. Capitula 35–50 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries oblong to ovate, abaxially yellow, apex acuminate to cuspidate, or apiculate. Stylar appendages narrowly triangular to deltoid. Cypsela oblong, ~3.3 mm long and 1 mm wide, cross-section squarish; pericarp brown, idioblasts present. Pappus deciduous, ~8.5 mm long.
Distribution
Occurs sporadically following winter rainfall over a broad area of South Australia, Victoria and New South Wales, in the Eyre Yorke Block, Gawler, Flinders Lofty Block, Murray–Darling Depression, and Riverina bioregions (Fig. 29).
Phenology
Recorded flowering August–October (Fig. 30).
Habitat
Shrubby and grassy eucalypt woodlands and Acacia shrublands, on orange–red sandy loams and red, gravelly, clay soils.
Conservation status
Occurs over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
The informal phrase name Xerochrysum sp. Flinders Range NE Herbarium has been used at NE for curatorial purposes and this study.
Etymology
The specific epithet is from the Latin, hispidus (rough, shaggy, hairy), in reference to the indumentum, comprising scattered, large, spreading septate trichomes, occurring abaxially along the leaf midrib and adaxially over the lamina, which are particularly helpful in identifying specimens.
Selected specimens examined
SOUTH AUSTRALIA: unincorporated: Balcanoona, near Nudlamutana Well, 26 Oct. 1967, H. Eichler 19646 (AD!). Elliston: Hambidge Flora and Fauna Reserve, 18 Sep. 1966, C.R. Alcock 1023 (AD!). Cleve: Hincks National Park, summit of Verran Hill, 6 Oct. 1968, J.R. Wheeler 733 (AD!). NEW SOUTH WALES: unincorporated: Scotia Sanctuary, 22 May 2011, D. Wood 240 (CANB!). Lachlan: 2.5 miles [~4 km] E from Euabalong turn-off from Lake Cargelligo, 23 May 1969, P.N. Martensz 178 (CANB*). Cobar: 10.5 miles [~16.8 km] S of Cobar on Nymagee road, 30 Sep. 1966, C.W.E. Moore 4487 (CANB*). Coolamon: Near Ariah Park, ~40 km WNW of Temora, 28 Oct. 1978, C.J. Shepherd 870 (CANB*). VICTORIA: Mildura: Murray-Sunset National Park, 10 Oct. 2014, V. Stajsic 7611 (MEL!); Hattah Lakes National Park, 1 Oct. 1948, A.C. Beauglehole 1101 (MEL!); ~10.5 miles [~16.8 km] NW of Warooka, 25 Oct. 1967, T. Smith 774 (AD!).
|

|
Xerochrysum interiore Paul G.Wilson, Nuytsia 28: 19 (2017)
Erect, annual (never biennial), taprooted herb. Stems and branches hispid with septate trichomes, or glabrescent, and with glands; internode length 15–20 mm. Basal leaf rosette often present at flowering, or absent (will flower with loose basal rosette in drier seasons). Basal leaves obovate to spathulate, 70–110 mm long and 15–35 mm wide, base auriculate and attenuate, apex apiculate; abaxial indumentum with glands, midvein indumentum pilose with septate trichomes; adaxial indumentum with scattered septate trichomes. Cauline leaves spathulate to obovate, 50–200 mm long and 10–30 mm wide, leaf base attenuate and auriculate, margin hirsute with septate trichomes, apex mucronate; abaxial indumentum with glands, midvein indumentum hispid with septate trichomes, and with glands; adaxial indumentum hirsute with septate trichomes, and with glands. Foliaceous bracts subtending capitula ~2–4 mm long or absent, margin fimbriate or hispid. Capitula 25–40 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, straw-coloured, basal margin fimbriate, abaxial surface smooth, apex obtuse or apiculate. Medial phyllaries ovate to narrow ovate, abaxially yellow, apex cuspidate. Stylar appendages narrowly triangular or rounded. Cypsela ~3.5 mm long and 0.9 mm wide, cross-section squarish or circular; pericarp grey–brown, idioblasts present. Pappus deciduous, ~10–11 mm long.
Distribution
Occurs sporadically following winter rainfall over a broad area in central Australia, including the Finke, MacDonnell Ranges, Great Sandy Desert, and Central Ranges bioregions, with a disjunct distribution in the Pilbara Bioregion (Fig. 31).
Phenology
Recorded flowering July–September and fruiting September–December.
Habitat
Acacia shrublands on red–orange sandy loam and gravelly loam soils.
Notes
A specimen from Balladonia, Western Australia (B.L.Turner 5251, MEL 602769!) is noteworthy in that it has cobwebby trichomes below the capitulum (v. short, stiff, septate trichomes) and immediately noticeable, much shorter (15–30 mm long) obovate cauline leaves (v. spathulate to obovate cauline leaves, 50–200 mm long). The area in which this collection was made is markedly disjunct from all other populations of X. interiore and other species of Xerochrysum in Western Australia
Conservation status
Occurs over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Selected specimens examined
NORTHERN TERRITORY: MacDonnell: 1.4 km N of Erldunda Roadhouse, ~50 m E of Stuart Highway, 25 Sep. 2016, J.J. Bruhl 3446 (NE!, NT!); Alice Springs, 500 m NE of the end of Baldissera Drive, 19 Oct. 2014, T.L. Collins 713 (NT!, NE!); S of Alice Springs, 17 km along Maryvale Road, 2 Jul. 2014, T.L. Collins 706 (NT!, NE!). SOUTH AUSTRALIA: unincorporated: De Rose Hill Station ~9 km NE of Homestead, 31 Aug. 1989, H.P. Vonow 1288 (AD!). WESTERN AUSTRALIA: Fortescue: Wanna Munna Road, Pilbara, 22 Aug. 2018, T.L. Collins 1073 & J.J. Bruhl (CANB!, NE!, NT!, PERTH!). Ngaanyatjarraku: ~49 km N of Mount Fanny on Giles–Mount Davies road, 11 May 1977, M. Lazarides 8341 (CANB, NSW, PERTH!). Dundas: 87.7 km E of Norseman on Eyre Highway, 16 Aug. 1995, R.J. Cranfield 10058 (PERTH!).
|
Xerochrysum macranthum (Benth.) Paul G.Wilson, Taxon 64(1): 105 (2015)
Erect, taprooted, annual to short-lived perennial herb up to ~70 cm. Stems and branches cobwebby to hirsute and hispid with septate trichomes, or glabrescent, and with glands; internode length 20–40 mm. Basal leaf rosette usually absent at flowering. Basal leaves spathulate, 70–150 mm long and 15–25 mm wide, base amplexicaul, margin cobwebby to hirsute with septate trichomes, apex apiculate; abaxial indumentum with glands, midvein indumentum pilose with septate trichomes and with glands; adaxial indumentum with scattered septate trichomes. Cauline leaves oblanceolate to elliptic, 30–100 mm long and 5–12 mm wide, base subauriculate and amplexicaul, margin hispid with septate trichomes, apex acute; abaxial indumentum with glands, midvein indumentum hirsute with septate trichomes and with glands; adaxial indumentum hispid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 5–7 mm long, margin hispid. Capitula 30–50 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, straw-coloured, pink, white, or occasionally yellow or brown; basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially white or yellow, apex cuspidate. Stylar appendages deltoid. Cypsela oblong, ~2.6 mm long and 0.8 mm wide, cross-section squarish; pericarp brown, idioblasts present. Pappus deciduous, ~7–8 mm long.
Distribution
Endemic to south-western Western Australia in the Geraldton Sandplains, Jarrah Forest, and Swan Coastal Plain bioregions (Fig. 32).
Phenology
Recorded flowering September–December and fruiting in November–December.
Habitat
Shrubby and grassy eucalypt woodland on gravelly sandy loam soils.
Conservation status
Occurs over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Some populations to the north and east of Perth consist entirely of plants with golden-yellow phyllaries. Molecular data confirmed that these are genetically similar to X. macranthum plants with white phyllaries. The informal phrase name X. sp. Golden was applied to populations with yellow phyllaries in the analyses.
Selected specimens examined
WESTERN AUSTRALIA: Coorow: north heading track 7.0 km E of intersection of Coastal Road [Indian Ocean Drive] and Eneabba–Coolimba road, 20 Sep. 1994, E.D. Kabay 655 (PERTH!). Chittering: Wannamal (127 km N of Perth), 13 Dec. 2018, T.L. Collins 1148 (CANB!, K!, NE!, PERTH!). Mundaring: Government Road (Chidlow Road), ~1.9 km S of Wooroloo, 13 Dec. 2018, T.L. Collins 1150 (CANB!, NE!, PERTH!, US!); junction Turkey Farm track and York–Chidlow road, 2 Nov. 1996, B.J. Lepschi 3164 & T.R. Lally (AD, CANB, MEL, PERTH!); Wandoo National Park, Talbot West Road, 13 Dec. 2018, T.L. Collins 1153 (CANB!, NE!, PERTH!). Kalamunda: Kalamunda National Park, 1 Nov. 2000, K. Macey 258 (PERTH!); Lesmurdie Falls National Park, 14 Sep. 1992, S. Patrick 1213 (PERTH!). West Arthur: ~23.5 km E of Collie, 19 Dec. 2018, T.L. Collins 1164 (CANB!, NE!, NSW!, PERTH!). Boyup Brook: Donnybrook–Boyup Brook road and intersection with Camballan Road, 19 Dec. 2018, T.L. Collins 1163 (CANB!, MO!, NE!, PERTH!).
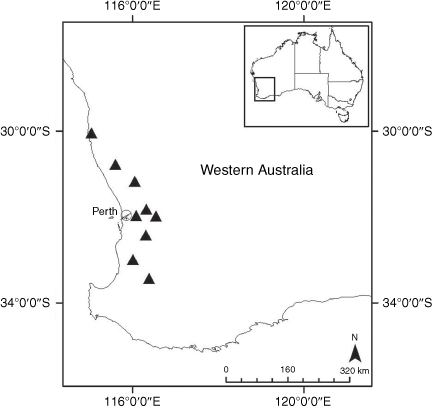
|
Xerochrysum macsweeneyorum T.L.Collins, sp. nov.
Diagnosis
Distinguished from X. bracteatum by presence of a dense covering of very small septate flagelliform trichomes on leaf abaxial surface (X. bracteatum has no septate trichomes abaxially), cauline leaves 3–10 mm wide (5–25 mm wide in X. bracteatum); and from other similar species by the annual or biennial life form (v. perennial life form in X. copelandii and X. murapan), cauline leaf width (10–25 mm wide in X. murapan), foliaceous bracts subtending capitula 4–9 mm long (10–25 mm long in X. murapan), obtuse phyllary apices (acuminate in X. copelandii).
Erect, taprooted, annual to short-lived perennial herb, up to ~1.3 m tall. Stems and branches cobwebby or glabrescent, and with glands; internode length 10–40 mm. Basal leaf rosette may be present but usually marcescent at flowering. Basal leaves spathulate, 80–150 mm long and 15–30 mm wide, base subamplexicaul, margin pilose with septate trichomes, apex mucronate; abaxial indumentum hirsute to hispidulous with septate trichomes, and with glands; abaxial midvein indumentum pilose with septate trichomes; adaxial indumentum hirsute with septate trichomes. Cauline leaves oblanceolate to lanceolate, 30–110 mm long and 3–10 mm wide, leaf base subauriculate, margin hispid with septate trichomes, apex mucronate; abaxial indumentum cobwebby and hispid with septate trichomes, and with glands; abaxial midvein indumentum cobwebby and hispid with septate trichomes, and with glands; adaxial indumentum hispid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 4–9 mm long or absent, margin with glands, cobwebby, fimbriate, and hispid. Capitula 30–45 mm wide, terminal, in panicles. Outer phyllaries broad-ovate to rounded, brown or straw-coloured, basal margin fimbriate to hispid, abaxial surface smooth, apex apiculate. Medial phyllaries oblong, narrow ovate or elliptic, abaxially yellow, apex acuminate to apiculate. Stylar appendages narrowly triangular to triangular. Cypsela oblong, ~2 mm long and 0.7 mm wide, cross-section squarish to circular; pericarp straw- or brass-coloured, idioblasts present. Pappus ~6–8 mm long.
Distribution
Occurs in the New England Tablelands and South Eastern Queensland bioregions, with disjunct populations on grassy balds at Bunya Mountains National Park in Queensland, and at Copeland Tops and in Barrington Tops National Park in New South Wales (Fig. 33).
Phenology
Recorded flowering November–May and fruiting December–May (Fig. 34).
Habitat
Grassy herblands and woodlands, often on basalt-derived clay soils, usually at altitudes of >450 m.
Conservation status
Occurs over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Often with persistent, desiccated (marcescent) basal rosette leaves or leaf bases. A population introduced to Jukes Road, Queenstown, Tasmania, has become naturalised there (M. Baker, pers. comm., 2018). The informal phrase name Xerochrysum sp. Northern Tablelands NE Herbarium has been used at NE for curatorial purposes and this study.
Etymology
Honouring my partner’s grandfather and mother, Eddie and Geraldine (Gerry) McSweeney of Dublin and Randwick respectively, both growers and lovers of plants. Orthography follows ICN Rec. 60C.4(a) (Shenzhen Code, Turland et al. 2018).
Selected specimens examined
QUEENSLAND: Darling Downs: Bunya Mountains National Park, track to Barker Creek Lookout from Paradise, 21 Nov. 2018, T.L. Collins 1141 (BRI!, CANB!, NE!); Spring Creek Road, W of Condamine River, 20 Mar. 2018, T.L. Collins 1039 & B. Wright (BRI!, CANB!, NE!); Eukey, Anderson Lane, 21 Nov. 2018, I.R. Telford 13531 (BRI!, CANB!, NE!, NSW!); Stanthorpe, 5 Nov. 1963, W.T. Jones s.n. (CANB 266087.1*). NEW SOUTH WALES: Northern Tablelands: E of Glencoe, Costello Road, 8 Dec. 2018, T.L. Collins 1147 (CANB!, NE!, NSW!); Ben Lomond Range, N of Guyra, 24 Feb. 1970, I.R. Telford 1415 (CANB*); ~20 miles [~32 km] from Guyra towards Glen Innes on New England Highway, 23 Feb. 1961, M.E. Phillips s.n. (CANB 14159.1*, NSW 518529!); junction to New England National Park, 4 Feb. 1996, M. Ito 96011, T. Nishino & Y. Kita (AD, CANB*, NSW, TI); Waterfall Way, ~1.5 km E of Ebor, 22 Apr. 2018, T.L. Collins 1049 & D.T. Collins (AD!, CANB!, NE!, NSW!); near Meldrum between Dorrigo and Ebor, 16 May 1978, B. Barnsley 191 (CANB*); Oxley Wild Rivers National Park, Apsley Falls, 17 Feb. 2018, T.L. Collins 1015 (BRI!, CANB!, HO!, NE!, NSW!); Thunderbolts Way, ~4.96 km S of Nowendoc Road intersection, 22 Dec. 2017, T.L. Collins 1009 & P.L. Collins (BRI!, CANB!, E!, NE!, NSW!); Barrington Tops National Park, Gloucester Tops, 9 Apr. 2018, T.L. Collins 1042 (CANB!, MO!, NE!, NSW!, PERTH!). TASMANIA: Mount Jukes Road, 4.9 km from King River crossing, 20 Feb. 2005, M.L. Baker 1539 (CANB!, HO!, PERTH!); Mount Jukes Road, 21.4 km SSE of Queenstown, 23 Jan. 2018, J.R. Nevin 154 (NE!); Queenstown area, Mount Jukes Road, 4.85 km after the King River bridge, 1 Mar. 2018, T.L. Collins 1023 & R.L. Andrew (CANB!, HO!, NE!).
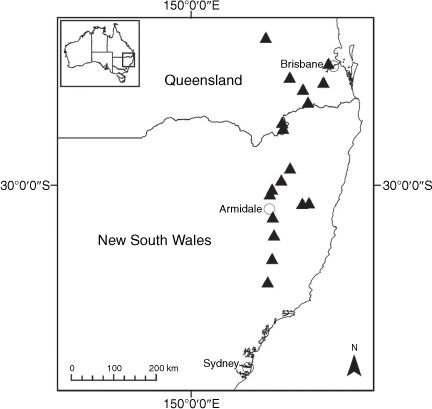
|

|
Xerochrysum murapan T.L.Collins & I.Telford, sp. nov.
Diagnosis
Distinguished from other species, with which it has been confused in the past, by the perennial life form (v. X. bracteatum and X. macsweeneyorum annual to biennial), foliaceous bracts subtending capitula 10–25 mm long (v. 8–10 mm long on X. bracteatum and X. copelandii), inflorescences in panicles (v. X. neoanglicum inflorescence solitary), cuspidate to apiculate medial phyllary apices (v. X. neoanglicum obtuse), and cauline leaves 10–25 mm wide (v. X. neoanglicum leaves 2–12 mm wide; X. copelandii leaves 5–10 mm wide).
Erect, rhizomatous or taprooted, perennial herb up to ~70 cm tall. Stems and branches becoming purple–red with age, cobwebby and hirsute with septate trichomes, or glabrescent, and with glands; internode length 10–75 mm. Basal leaf rosette present or absent at flowering. Basal leaves spathulate, 60–180 mm long and 15–35 mm wide, base amplexicaul, margin hirsute with septate trichomes, apex apiculate; abaxial indumentum hirsute with septate trichomes, midvein with scattered septate trichomes; adaxial indumentum hirsute with septate trichomes. Cauline leaves oblanceolate to obovate, 30–200 mm long and 10–25 mm wide, base subauriculate and amplexicaul, margin cobwebby and hirsute with septate trichomes; apex mucronate; abaxial indumentum cobwebby and hirsute with septate trichomes, and with glands; midvein indumentum hirsute with septate trichomes or hispid with scattered glands; adaxial indumentum hispid with septate trichomes, and with glands. Foliaceous bracts subtending capitula 10–25 mm long, margin woolly, or cobwebby, and hispid. Capitula 30–45 mm wide, terminal, in panicles or occasionally solitary. Outer phyllaries broad-ovate, brown, basal margin fimbriate or hispid, abaxial surface smooth, apex apiculate. Medial phyllaries ovate to lanceolate, abaxially yellow, apex cuspidate to apiculate. Stylar appendages ovate. Cypsela oblong, ~2.3 mm long and 1 mm wide, cross-section squarish to circular; pericarp brown, idioblasts present. Pappus deciduous, ~8 mm long.
Distribution
Restricted to the Barrington Tops National Park in the New England Tablelands Bioregion, with known occurrences restricted to Barrington Tops itself and Gloucester Tops, ~22 km to the south-east, in New South Wales (Fig. 35).
Phenology
Recorded flowering February–April with mature cypselae recorded in February and April (Fig. 36).
Habitat
Occurs in eucalypt forest and woodland at altitudes of >1000 m.
Conservation status
All existing collections come from either Barrington Tops National Park, including the Gloucester Tops area, or the adjoining Barrington Tops State Forest, with specimen label data estimating some populations comprising thousands of plants. Issues associated with anthropogenic climate change including heatwaves, extreme drought and intense fires are likely to present a threat to X. murapan and the associated vegetation in the future. We recommend a status of ‘Least Concern’ (IUCN 2019), with the need for reassessment in the future if climate changes rapidly.
Notes
The informal phrase name Xerochrysum sp. Barrington Tops has been used at NE for curatorial purposes and this study.
Etymology
The specific epithet, in reference to the phyllary colour on the type specimen, is the colour yellow in the Gathang and Wonaruah languages of the traditional owners of Barrington Tops (Stephen Brereton, pers. comm., 2020), and is used as a noun in apposition.
Selected specimens examined
NEW SOUTH WALES: Northern Tablelands: Moonan State Park [Barrington Tops State Forest], Cobark Lookout, 5 Feb. 1996, M. Ito 96029, T. Nishino & Y. Kita (CANB, MEL!, NSW, TI); Barrington Trail, 0.6 km south of the Barrington Tops Forest Road, 27 Feb. 2009, J.R. Hosking 3204 (CANB, MEL, NE!, NSW, PERTH); Barrington Tops National Park, Gloucester Tops, 9 Apr. 2018, T.L. Collins 1041 (CANB!, BRI!, NE!, NSW!); Barrington Tops National Park, Polblue Swamp, 10 Apr. 2018, T.L. Collins 1044 (CANB!, BRI!, NE!, NSW!); Barrington Tops National Park, Bull Ridge Road, 10 Apr. 2018, T.L. Collins 1046 (CANB!, BRI!, NE!, NSW!); Careys Peak, Barrington Tops, 12 Feb. 1971, I.R. Telford 2729 (CANB!).
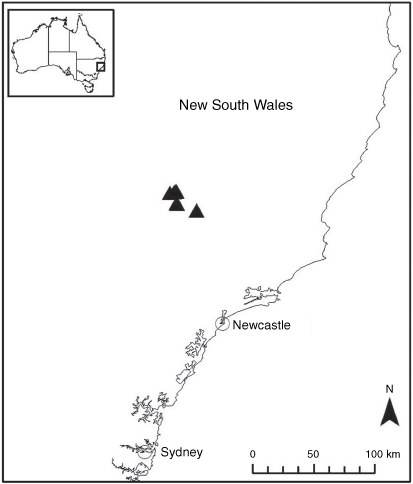
|

|
Xerochrysum neoanglicum J.J.Bruhl & I.Telford, sp. nov.
Diagnosis
Distinguished from other species, with which it has been confused in the past, by the perennial life form (v. annual or biennial in X. bracteatum), solitary inflorescence (v. paniculate in X. bracteatum, X. copelandii, and X. murapan), presence of septate trichomes on leaf abaxial surface (v. absent in X. bracteatum), leaves 2–12 mm wide (5–25 mm wide in X. bracteatum and 10–25 mm wide in X. murapan, and 5–10 mm wide in X. copelandii), and foliaceous bracts subtending capitula 10–20 mm long (8–10 mm long in X. bracteatum and 8–10 mm long or sometimes absent in X. copelandii).
Erect, taprooted, perennial herb annually reshooting from a crown, up to ~50 cm tall. Stems and branches becoming reddish with age, cobwebby, hirsute to woolly with septate trichomes, and with glands; internode length 15–30 mm. Basal leaf rosette usually present at flowering. Basal leaves oblong to obovate, 30–100 mm long and 10–20 mm wide, base amplexicaul, margin cobwebby to villous with septate trichomes, apex mucronate and apiculate; abaxial indumentum with glands, midvein indumentum cobwebby or villous with septate trichomes, and with glands; adaxial indumentum hirsute to villous with septate trichomes, and with glands. Cauline leaves lanceolate, 25–100 mm long and 2–12 mm wide, base attenuate, margin cobwebby or villous with septate trichomes, and hispid, apex mucronate; abaxial indumentum hirsute with septate trichomes, and with glands, midvein indumentum cobwebby with septate trichomes, and with glands; adaxial indumentum hispid to pilose with septate trichomes, and with glands. Foliaceous bracts subtending capitula 10–20 mm long, margin woolly or cobwebby. Capitula 35–60 mm wide, terminal, solitary (never branched). Outer phyllaries broad-ovate to ovate, brown or straw-coloured, basal margin fimbriate and hispid (extending to the apex), abaxial surface smooth, apex apiculate. Medial phyllaries oblong to narrow ovate, abaxially yellow, apex apiculate. Stylar appendages clavate to ovate. Cypsela oblong, ~2.2 mm long and ~1 mm wide, cross-section squarish to circular; pericarp straw- or brass-coloured, idioblasts absent. Pappus deciduous, ~8 mm long, apical cells often tinted red.
Distribution
Xerochrysum neoanglicum is widespread through the New England Tablelands Bioregion (NETB) from the eastern edge of the Granite Belt near Wallangarra, Queensland, south through the higher eastern edge of the NETB to Werrikimbe National Park, New South Wales, and in the New South Wales North Coast Bioregion at Barrington Tops National Park (Fig. 37).
Phenology
Recorded flowering November–February (Fig. 38).
Habitat
Xerochrysum neoanglicum grows in frost hollows, gullies and on swamp margins at 850–1350-m altitude. The vegetation is usually herbfield or Eucalyptus pauciflora grassy woodland, soils are mostly clay loam on basalt, occasionally on humic silt on granite (Granite Belt and Boonoo Boonoo). Other taxa recorded at sites include Eucalyptus acaciiformis, Poa sieberiana and Carex sp. Majors Point (L.M. Copeland 1812).
Conservation status
Invasive grass species and their management on road reserves, land clearing and improved pastures, small population sizes and potential in-breeding depression, and issues associated with anthropogenic climate change, in particular extreme drought, present threats to X. neoanglicum. Unregulated seed collection and over-grazing could also threaten populations by depleting seedbanks, trampling habitat, and introducing invasive species and pathogens. We recommend a status of ‘Vulnerable’ under the (IUCN 2019), as it fulfils the criteria for VU D1.
Notes
This species has horticultural potential with large inflorescences borne on single-stems and a naturally compact habit. Native plant nurseries and societies on the Northern Tablelands have been growing and selling this species for several years; however, plants can be difficult to maintain because they appear to be drought sensitive (T. L. Collins, pers. obs., 2016 and 2021; J. Nevin, pers. comm., 2020). The unpublished name X. bracteatum subsp. barringtonense Paul G.Wilson MS has been applied to this species.
Etymology
The specific epithet recognises the New England Tablelands from where this species is mostly found.
Selected specimens examined
QUEENSLAND: Darling Downs: Mount Norman, Wallangarra, 3 Oct. 1998, D. Hockings s.n. (BRI-AQ 663705!); Racecourse Creek Swamp, 25 km E of Mount Norman picnic area, 24 Dec. 1999, F.D. Hockings 1003 (BRI!). NEW SOUTH WALES: Northern Tablelands: Boonoo Boonoo, Resurrection Creek, at Mount Lindesay Road crossing, 8 Nov. 2010, I.R. Telford 13334 & T. Vollbon (BRI!, CANB!, K!, MEL!, MO!, NE!, NSW!, NY!, US!); E of Glencoe, Costello Road, 8 Dec. 2018, T.L. Collins 1146 (CANB!, NE!, NSW); 5.4 km N of Llangothlin along road to Ben Lomond, 12 Nov. 2005, L.M. Copeland 4006 (CANB, NE!, NSW, US); Little Llangothlin Nature Reserve, Billy Bung Lagoon, 20 Nov. 2005, I.R. Telford 12914 (CANB!, CHR!, MEL!, NE!, NSW!, US!); NE of Ebor along Waterfall Way, 17 Dec. 2016, J.J. Bruhl 3511 & I.R. Telford (NE!); Werrikimbe National Park, SW side of Racecourse Swamp, 13 Feb. 2003, L.M. Copeland 3561, J. Hodgon & I.R. Telford (CANB!, MEL!, NE!, NSW!, PERTH!); alongside Edwards Swamp Trail, north of Barrington River, 23 Feb. 2005, J.R. Hosking 2586 (CANB, MEL, NE!, NSW).
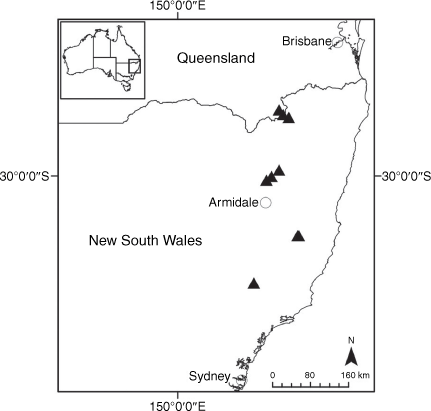
|

|
Xerochrysum papillosum (Labill.) R.J.Bayer, Kew Bull. 56(4): 1015 (2001)
Erect, taprooted, perennial herb. Stems and branches cobwebby, hirsute, hispid, or glabrescent, and with glands; internode length 1–40 mm. Basal leaf rosette absent at flowering. Basal leaves spathulate, 50–120 mm long and 10–20 mm wide, base amplexicaul, margin cobwebby or hispid, apex apiculate and mucronate; basal leaf abaxial indumentum with glands, midvein glabrous (occasionally with scattered septate trichomes); adaxial indumentum hispid and with glands. Cauline leaves oblanceolate to lanceolate, 35–150 mm long and 3–10 mm wide, base subauriculate, margin cobwebby, hispid, scabrid, and with glands, apex apiculate and mucronate; abaxial indumentum with scattered septate trichomes and glands, midvein indumentum cobwebby, hispid, and with glands; adaxial indumentum hispid and with glands. Foliaceous bracts subtending capitula 10–35 mm long, margin cobwebby, hispid and with glands. Capitula 35–55 mm wide, terminal, in panicles or solitary. Outer phyllaries broad-ovate, pink, brown, or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex acuminate to apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially white or yellow, apex cuspidate or apiculate. Stylar appendages clavate to ovate. Cypsela ~2.8 mm long and 1 mm wide, cross-section squarish or circular; pericarp brown, idioblasts present. Pappus deciduous, ~7 mm long.
Distribution
Occurs sporadically in Tasmania, the Bass Strait islands and on Wilsons Promontory, Victoria, in the Tasmanian South East, Furneaux and South East Coastal Plain bioregions (Fig. 39).
Phenology
Flowering recorded August–March.
Habitat
Occurs in coastal heath and margins of eucalypt forests on sandy soils from sea level to ~650-m altitude.
Conservation status
Many populations occur in conservation reserves; however, there are no data on population sizes. Currently considered ‘Not Threatened’ (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au). We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Labillardière’s specimen at FI (006315) is here selected as the lectotype, because it is the most complete of the available specimens here considered to comprise original material, and has been extensively annotated by Labillardière. Material at FI, G-DC and MEL, here treated as probable isolectotypes, appear to have been collected on the same voyage as the isolectotype to judge from annotations on the specimens.
The common names ‘cliff paperdaisy’ and ‘cliff everlasting’ are used for X. papillosum in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au).
Selected specimens examined
VICTORIA: South Gippsland: Wilsons Promontory National Park, 10 Nov. 1983, A.C. Beauglehole 75352 (MEL!); South Cape Bay, 6 Apr. 1930, H.F. Comber 2278 (E 00231983!, HO). TASMANIA: Little Dog Island, Furneaux Group, 28 Aug. 1973, J.S. Whinray 186 (CANB!); Mount Chappell Island, Furneaux Group, 8 Feb. 1972, J.S. Whinray 222 (CANB!); Sea Lion Island, near its centre, 29 Aug. 1985, J.S. Whinray 8758 (CANB!); Flinders Island, slopes of Strzelecki Peaks, 28 Feb. 1977, B.C. Crisp 460 (CANB!); Mount Chappell Island, S end, 1 Nov. 1992, R. Burns 444 (CANB!); Mount Chappell Island, Mount Chappell, N face, 1 Nov. 1992, R. Burns 436 (CANB!); St Patricks Head State Reserve, upper slopes and summit, 6 Mar. 2018, T.L. Collins 1037 & R.L. Andrew (CANB!, HO!, NE!); Eaglehawk Neck, Tessellated Pavement, 28 Feb. 2018, T.L. Collins 1018 & R.L. Andrew (CANB!, HO!, NE!).
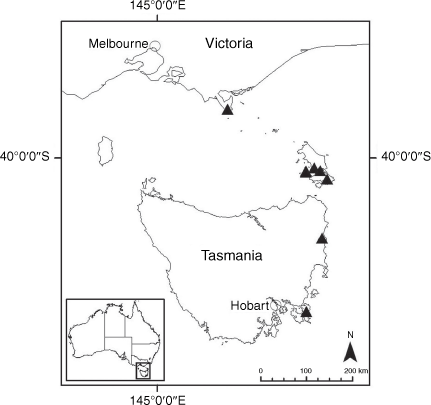
|
Xerochrysum strictum T.L.Collins & J.J.Bruhl, sp. nov.
Diagnosis
Distinguished from other species growing in northern Queensland or those with which it has been confused in the past, by the perennial life form (v. X. bracteatum annual or biennial), presence of septate trichomes and stipitate glands on leaf abaxial surface (v. X. bracteatum and X. banksii with glands only), hirsute leaf indumentum adaxially (v. X. bracteatum hispid), erect habit (v. X. banksii prostrate; X. gudang decumbent to erect), acuminate medial phyllary apices (v. X. bracteatum and X. banksii obtuse, X. boreale cuspidate), leaves 5–25 mm wide (leaves 5–10 mm wide in X. banksii, 4–9 mm wide in X. boreale, and 6–15 mm wide in X. gudang), and presence of persistent glands on leaf adaxial surface (v. fragile and readily lost in X. boreale and X. gudang).
Erect, taprooted, perennial herb, up to ~1.3 m tall, readily resprouting from taproot. Stems and branches cobwebby or woolly, hirsute, and with glands; internode length 15–25 mm. Basal leaf rosette absent at flowering unless resprouting. Basal leaves obovate, 80–120 mm long and 20–50 mm wide, base subauriculate and amplexicaul, margin woolly, apex mucronate; abaxial indumentum villous with septate hairs and stipitate glands, midvein indumentum hirsute; adaxial indumentum villous. Cauline leaves oblanceolate to obovate, 50–200 mm long and 5–25 mm wide, base subauriculate and amplexicaul, margin cobwebby to cottony, or hirsute, apex mucronate; abaxial indumentum cobwebby to hirsute, and with glands; abaxial midvein indumentum cobwebby to hirsute; adaxial indumentum hirsute and with glands. Foliaceous bracts subtending capitula 5–10 mm long, margin with glands, cobwebby, and hispid. Capitula 30–40 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, yellow, brown, or straw-coloured; basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex acuminate. Stylar appendages narrowly triangular to obovate. Cypsela oblong, ~2.2 mm long and ~0.7 mm wide, cross-section circular; pericarp straw- or brass-coloured, idioblasts present. Pappus deciduous, ~8 mm long.
Distribution
Endemic to Queensland, occurring in the Brigalow Belt North, Einasleigh Uplands and Cape York Peninsula Bioregions and as disjunct populations in the Brigalow Belt South Bioregion (Fig. 40).
Phenology
Recorded flowering June–November and fruiting recorded in July (Fig. 41).
Habitat
Grassy eucalypt woodland at altitudes of >500 m in the northern distribution and >300-m altitude in the Brigalow Belt South Bioregion.
Conservation status
Occurs over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
Xerochrysum strictum is a morphologically variable species distributed along a geographic cline, with some populations having distinctly narrow leaves, such as, for example, S.A. Morain 197 and R.J. Fensham 253, similar to a population included in this study from Blackbraes National Park (T.L. Collins 1067) in the western part of the distribution. Specimens from Mount Fox seemingly have no glands on the uppermost cauline leaf margins, and no glands on the stems, for example, M.S. Clemens s.n. (BRI 248110!), M.S. Clemens s.n. (BRI 362638!). The informal phrase name Xerochrysum sp. North Kennedy has been used at NE for curatorial purposes and this study.
Etymology
The specific epithet is from the Latin, strictus (tight, close, straight, drawn together), and refers to the erect habit seen in the field and in cultivation.
Selected specimens examined
QUEENSLAND: Cook: McIlwraith Range, edge of scrub, north-west of campsite, 25 May 2003, D.L. Jones 18897 (CANB!); Herberton Range Ridge Road, 27 June 2018, T.L. Collins 1051 & J.J. Bruhl (BRI!, CANB!, CNS!, NE!); Herberton–Irvinebank road, 27 June 2018, T.L. Collins 1058 & J.J. Bruhl (BRI!, CANB!, CNS!, NE!); SSW of Wondecla, 1 July 2018, T.L. Collins 1063 & J.J. Bruhl (BRI!, CANB!, CNS!, NE!); Blackbraes National Park, 2 July 2018, T.L. Collins 1067 & J.J. Bruhl (BRI!, CANB!, CNS!, NE!). North Kennedy: ~17 km W of Paluma along road to Hidden Valley, 11 Nov. 2006, J.J. Bruhl 2473 (BRI!, NE!); ~700 m SSE of Mount Zero along high-tension power-line access track, 11 Nov. 2006, J.J. Bruhl 2477 & I.R. Telford (BRI!, NE!, NSW!); base of Roma Peak, ~40 km S of Bowen, 30 June 1991, A.R. Bean 3347 (BRI!); 21 km NW of Pentland on road to Lolworth Station, Hughenden, 24 July 1975, A.D. Chapman 1355 (BRI!, CANB). South Kennedy: Clarke Range, ~8 km NW of Eungella, 24 June 2014, I.R. Telford 13489, J.J. Bruhl & C.J. Prychid (BRI!, CANB!, NE!, US!). Leichardt: W lower slopes of Wolgang [Wolfgang] Peak, 17 Oct. 1983, R.J. Henderson H2912 & G.P. Guymer (CANB!).
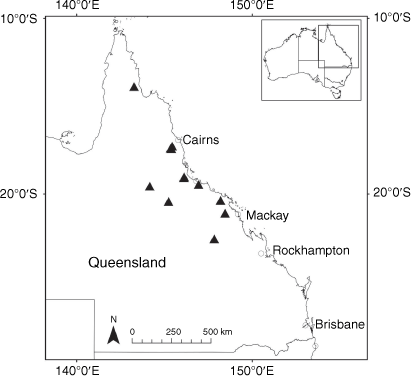
|
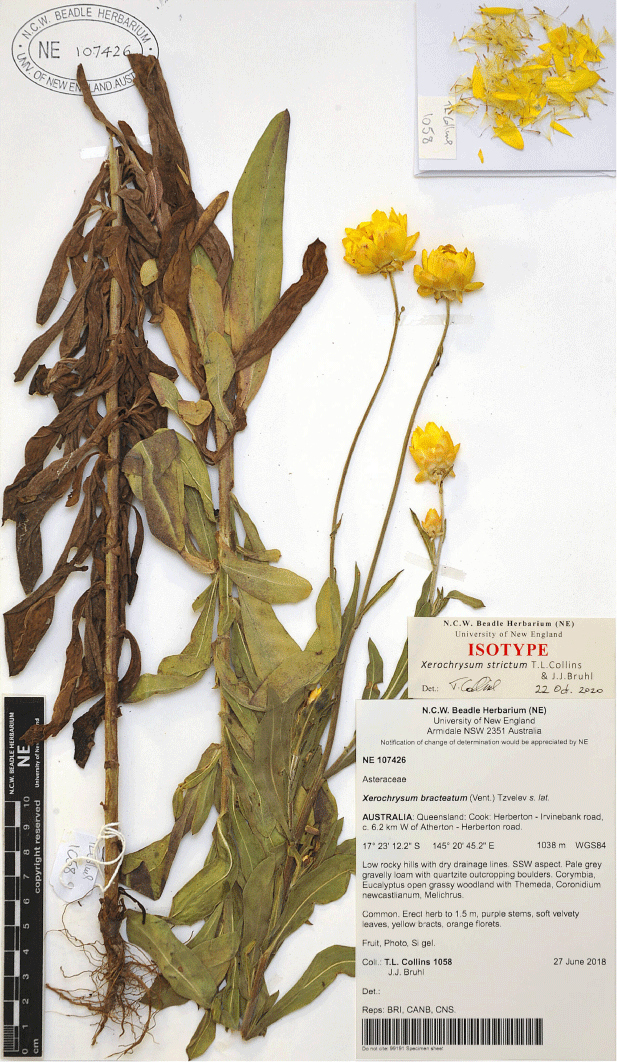
|
Xerochrysum viscosum (Sieber ex DC.) R.J.Bayer, Kew Bull. 56(4): 1015 (2001)
Erect, taprooted, perennial herb, up to ~60 cm tall. Stems and branches scabrid and with glands, internode length 5–20 mm. Basal leaf rosette absent at flowering. Basal leaves oblanceolate to spathulate, 50–120 mm long and 3–15 mm wide, base amplexicaul, margin hispid and with glands, apex apiculate; abaxial indumentum with glands, midvein glabrous (occasionally with scattered septate trichomes); adaxial indumentum pilose. Cauline leaves linear to oblanceolate, 20–80 mm long and 1–5 mm wide, base truncate, apex acute or apiculate and mucronate, margin hispid and with glands; abaxial indumentum with glands, midvein indumentum with glands (occasionally with scattered septate trichomes); adaxial indumentum hispid and with glands. Foliaceous bracts subtending capitula 4–8 mm long, margin with glands. Capitula 20–30 mm wide, terminal, in panicles or solitary. Outer phyllaries broad-ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex acuminate or apiculate. Medial phyllaries oblong, ovate or narrow ovate, abaxially yellow, apex cuspidate or apiculate. Stylar appendages narrowly triangular or deltoid. Cypsela ~2.2 mm long and 0.7 mm wide, cross-section squarish or circular; pericarp brown, idioblasts present. Pappus deciduous, ~7–8 mm long.
Distribution
Occurs over a broad area from Victoria to south-eastern Queensland. Recorded in the Murray–Darling Depression, Riverina, New South Wales South Western Slopes, South Eastern Highlands, Brigalow Belt South, Nandewar, New England Tablelands, and South Eastern Queensland bioregions (Fig. 42).
Phenology
Recorded flowering October–March.
Habitat
Occurs in woodlands and open forests on gravelly and sandy soils.
Notes
Lower cauline leaves on primary shoots are longer and broader than those higher and on axillary branches. Glands on leaves and stems are fragile and commonly leak resin, coating leaves and stems and leaving a shiny or varnished appearance. Populations in the Torrington State Conservation Area have slightly broader and more scabrid leaves, for example, P.J. Clarke s.n. (NE 85889!), C.E. Nano 48 (NE 66184!).
‘Sticky everlasting’ is in use in New South Wales (New South Wales Flora Online, see http://plantnet.rbgsyd.nsw.gov.au/floraonline.htm) and Victoria (Royal Botanic Gardens Victoria, VicFlora, see https://vicflora.rbg.vic.gov.au/, accessed 22 March 2020).
Conservation status
Recorded over a wide geographical area, including in several conservation reserves and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Selected specimens examined
QUEENSLAND: Burnett: Coomba Falls, near Maidenwell, 25 Mar. 1997, P.I. Forster 20583 (BRI!). Darling Downs: 5 km from Wallangarra along New England Highway towards Stanthorpe, 21 Jan. 2009, I.R. Telford 13281 & J.J. Bruhl (BRI, CANB, NE!). NEW SOUTH WALES: Northern Tablelands: Torrington SRA, 16 Jan. 1997, C.E. Nano 48 (NE!); Oxley Wild Rivers National Park, Apsley Falls, 17 Feb. 2018, T.L. Collins 1014 (CANB!, NE!, NSW!). Central Western Slopes: Mudgee–Muswellbrook road, 16 Dec. 2017, T.L. Collins 1008 (CANB!, NE!, NSW!); Wellington, 2 km NE from summit of Mount Arthur, 10 May 2017, M.R. Thomas 28 (NE!). Central Tablelands: Western Highway, W of Lithgow, 16 Dec. 2017, T.L. Collins 1007 (CANB!, NE!, NSW!, US!). AUSTRALIAN CAPITAL TERRITORY: Black Mountain ACT, lower slope, 6 Dec. 2017, T.L. Collins 991 (CANB!, NE!, NSW!). VICTORIA: St Arnaud, 2 km SW of T-junction of Wimmera Highway with Dundas Street, 22 Oct. 2018, J.R. Nevin 164 (BRI!, CANB!, CHR!, MEL!, NE!, NSW!, US!); Warby-Ovens National Park, below road to Pine Gully Walk, 20 Dec. 2018, J.R. Hosking 4060 (AD!, CANB!, MEL!, NE!, NSW!).
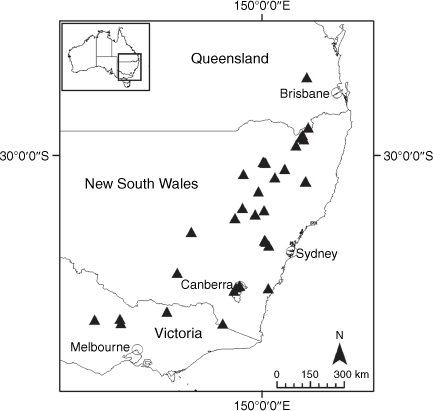
|
Xerochrysum wilsonii T.L.Collins, sp. nov.
Diagnosis
Distinguished from X. macranthum by a perennial life form (v. annual or biennial), cauline leaves 10–20 mm wide (v. 5–12 mm), capitula 40–60 mm wide (v. 30–50 mm wide).
Erect or occasionally decumbent, taprooted, perennial shrub-like herb, 30–180 cm in length. Stems and branches cobwebby to hispid, scabrid, and with glands; internode length 15–50 mm. Basal leaf rosette absent at flowering. Basal leaves spathulate, 80–160 mm long and 20–30 mm wide, base amplexicaul, margin cobwebby to pilose, and hispid; apex apiculate; abaxial indumentum pilose and with glands, midvein indumentum pilose and with glands; adaxial indumentum hispid, pilose, and with glands. Cauline leaves oblanceolate, 60–110 mm long and 10–20 mm wide, base amplexicaul, margin cobwebby, hispid, and scabrid, apex mucronate; abaxial indumentum with sessile glands and scattered septate trichomes; midvein indumentum hispid, scabrid, and with glands; adaxial indumentum hispid, scabrid, and with glands. Foliaceous bracts subtending capitula ~10 mm long, margin cobwebby and hispid. Capitula 40–60 mm wide, terminal, in panicles. Outer phyllaries broad-ovate, pink, white, or straw-coloured; basal margin fimbriate and hispid, abaxial surface smooth, apex apiculate. Medial phyllaries narrow ovate to elliptic, abaxially white, apex cuspidate. Stylar appendages ovate. Cypsela oblong, ~2.1 mm long and 0.9 mm wide, cross-section squarish or circular; pericarp brown, idioblasts present. Pappus deciduous, ~7–8 mm long.
Distribution
Endemic to the far south-west of Western Australia and recorded in the Esperance, Jarrah Forest and Warren bioregions (Fig. 43).
Phenology
Recorded flowering November–January and fruiting December–February (Fig. 44).
Habitat
Occurring in diverse habitats from montane heath and scree slopes at ~1000-m altitude to coastal heath near sea level, on skeletal sandy soils.
Conservation status
All known populations occur in conservation reserves, but appear to be restricted to specific habitats. We recommend a status of ‘Data Deficient’ (IUCN 2019) and suggest that a detailed analysis of the area of extent be conducted to determine appropriate conservation status.
Notes
At Toolbrunup in Stirling Range National Park, large plants up to 1.8 m in length were seen decumbent across the scree-slope below the summit, with stem diameters up to ~100 mm. Occasional plants have been recorded at lower elevations (e.g. J.R. Wheeler 4022 (PERTH!)), possibly owing to flower picking and subsequent discarding by bushwalkers; however, these lowland populations in Stirling Range National Park do not appear to persist (T. L. Collins, pers. obs., 2018). The informal phrase-names X. sp. Porongurup, X. sp. Limestone and X. sp. Forests have been used at NE for curatorial purposes and this study.
Etymology
The specific epithet honours the work of botanist Paul Graham Wilson (1928–), of the Western Australian Herbarium (PERTH), who has contributed greatly to Australian daisy taxonomy.
Selected specimens examined
WESTERN AUSTRALIA: Darling: Stirling Range National Park, summit of Bluff Knoll, 16 Dec. 2018, T.L. Collins 1157 (CANB!, K!, NE!, PERTH!, US!); Stirling Range National Park, summit of Toolbrunup, 16 Dec. 2018, T.L. Collins 1158 (CANB!, NE!, PERTH!); Walpole–Nornalup National Park, Conspicuous Cliffs, 18 Dec. 2018, T.L. Collins 1161 (CANB!, K!, NE!, NSW!, PERTH!, US!); Walpole–Nornalup National Park, Delta Road, 18 Dec. 2018, T.L. Collins 1162 (CANB!, NE!, PERTH!); Collier Peak, Porongurup Range, 20 Nov. 1987, G.J. Keighery 8722 (PERTH!); Yallerungup Peak, Porongurup Range, 15 Dec. 1986, G.J. Keighery 8419 (PERTH!); Mount Many Peaks, 4 Oct. 1994, S. Barrett 24 (PERTH!).
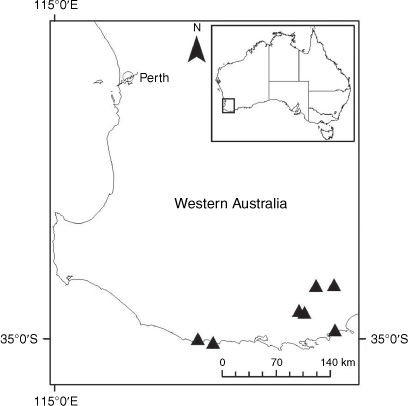
|

|
Xerochrysum milliganii group
Xerochrysum alpinum Paul G.Wilson, Nuytsia 28: 36 (2017)
Erect, perennial, rhizomatous herb up to 20 cm. Stems and branches cobwebby and with glands, internode length 10–35 mm. Basal leaf rosette present at flowering. Basal leaves obovate, 20–60 mm long and 10–25 mm wide, base attenuate, margin cottony and with glands, apex apiculate; abaxial indumentum with glands, midvein glabrous; adaxial indumentum hispid and with glands (hispid indumentum on midvein basally). Cauline leaves obovate, 20–50 mm long and 1–3 mm wide, base attenuate, margin cobwebby or cottony, apex apiculate; abaxial indumentum with glands, midvein indumentum with glands; adaxial indumentum with glands. Foliaceous bracts subtending capitula 10–12 mm long (with fimbriate apical hairs), margin cobwebby and with glands. Capitula 30–45 mm wide, terminal, solitary. Outer phyllaries ovate, straw-coloured, basal margin fimbriate, extending to apex, abaxial surface scabridulous towards apex, apex fimbriate. Medial phyllaries lanceolate, abaxially yellow, apex cuspidate. Stylar appendages clavate. Cypsela 2.3 mm long and 0.75–1 mm wide, cross-section with oblique angles; pericarp grey–brown, idioblasts absent. Pappus persistent, ~5–6 mm long.
Distribution
Occurring in the Tasmanian Central Highlands and Ben Lomond bioregions (Fig. 45).
Phenology
Recorded flowering February–March and fruiting in March.
Habitat
Alpine herbfields and shrublands at >900-m altitude.
Conservation status
Considered widespread (Wilson 2017), and recorded in several conservation reserves, currently listed as ‘Data Deficient’ in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au). We recommend a status of ‘Data Deficient’ (IUCN 2019) and suggest that a detailed analysis of area of extent, population sizes and health be conducted to determine appropriate conservation status.
Selected specimens examined
TASMANIA: Vale of Belvoir Conservation Reserve, Lake Lee Road, 2 Mar. 2018, T.L.ttp://anpsa.org.au/APOL2006/ju Collins 1024 & R.L. Andrew (CANB!, HO!, NE!); Cradle Mountain National Park, Face Track, 3 Mar. 2018, T.L. Collins 1030 & R.L. Andrew (CANB!, HO!, NE!), summit of Blue Tier, 23 Feb. 1878, A. Simson 1101 (MEL 0061148A).
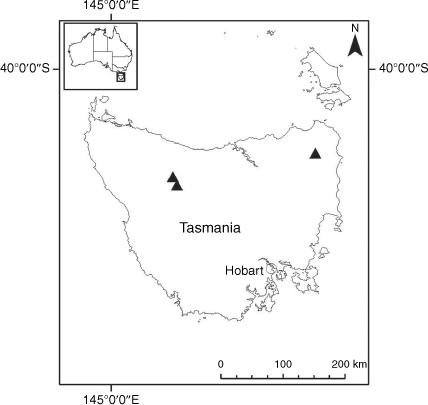
|
Xerochrysum andrewiae T.L.Collins & J.J.Bruhl, sp. nov.
Diagnosis
Distinguished by the sessile glands on the adaxial leaf surface (v. stipitate glands in X. subundulatum; absent in X. palustre), foliaceous bracts subtending capitula up to 15 mm long (v. 5–10 mm long on X. palustre), leaves 2–10 mm wide (v. 5–20 mm in X. subundulatum), outer phyllary abaxial surface scabridulous (v. smooth in X. palustre).
Erect, rhizomatous, perennial herb, 30–60 cm tall. Stems and branches cobwebby to glabrescent, internode length 10–20 mm. Basal leaf rosette absent at flowering. Seedling and basal leaves not seen. Cauline leaves oblanceolate to lanceolate, 20–60 mm long and 2–10 mm wide, base attenuate, margin cobwebby, hispid, or glabrous, apex acute and apiculate; abaxial indumentum with glands, midvein glabrous or indumentum hispid; adaxial indumentum cobwebby, scattered hispid, and with glands. Foliaceous bracts subtending capitula 15 mm long, margin cobwebby. Capitula 35–40 mm wide, terminal, solitary. Outer phyllaries ovate, orange, brown, or straw-coloured; basal margin fimbriate and hispid, abaxial surface scabridulous, apex acuminate to apiculate. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex cuspidate or apiculate. Stylar appendages ovate. Cypsela oblong, 2.3 mm long and 0.8 mm wide, cross-section squarish to circular; pericarp brass-coloured, idioblasts present. Pappus persistent.
Distribution
Recorded from New South Wales, Victoria and Tasmania, occurring in the Sydney Basin, South East Corner, Australian Alps, and Ben Lomond bioregions (Fig. 46).
Phenology
Flowering February–April and fruiting March (Fig. 47).
Habitat
Lowland wetlands in Tasmania to wetlands up to ~1400-m altitude on the mainland in New South Wales and Victoria.
Conservation status
Population sizes in Tasmania have been estimated in the hundreds to thousands. Ongoing threats associated with anthropogenic climate change; in particular, extreme drought, and intense wildfire, potentially threaten X. andrewiae across the distribution. Given the past taxonomic confusion, we recommend a status of ‘Data Deficient’ (IUCN 2019) and suggest that a detailed analysis of the area of extent, population sizes and health be conducted to determine appropriate conservation status.
Notes
At Tantawangalo State Forest populations of X. palustre sens. str. and X. andrewiae are sympatric and mixed collections have been made (e.g.: D.L. Jones 17164, MEL 2101319!, CANB 616790). The informal phrase name Xerochrysum aff. palustre has been used at NE for curatorial purposes and this study.
Etymology
The specific epithet recognises the significant contributions of botanist, supervisor and co-collector of the type, Rose Lorien Andrew (1978–), of the University of New England Armidale, New South Wales.
Selected specimens examined
NEW SOUTH WALES: Central Tablelands: Kanangra Walls, 22 Mar. 2011, R. Johnstone 2906 (BRI, CANB, MEL!, NSW!); Kanangra Boyd National Park, 19 Apr. 2015, J. Miles 15–50 (NSW!); Kanangra Walls Road, 12 Jan. 2001, D.L. Jones 17798 (CANB*, MEL, NSW); Jensens Swamp, 1 Mar. 1985, D.H. Benson 2330 (NSW!). South Coast: Coolumbooka Nature Reserve, 30 Apr. 2002, I. Crawford 7018 (CANB!); on Outskirt Creek tributary arm in NW corner of Bondi or Mountain Top Travelling Stock Reserve, 16 Feb. 2015, J. Miles 15–27 (NSW!); Bega Swamp at the head of Brogo River, 6 May 1976, R. Pullen 10268 (CANB!, NSW); ~9.8 km E of Cathcart towards Pambula, 8 Feb. 2000, D.L. Jones 17157 (MEL!); Badja State Forest, 12 Feb. 2004, N.G. Walsh 6006 (MEL!); 17.8 km E of Braidwood–Clyde Mountain, 19 Mar. 2006, D.L. Jones 19352 (CANB, MEL!). VICTORIA: East Gippsland: Alpine National Park, Playgrounds, 10 Feb. 2005, N.G. Walsh 6260 (MEL!); Alpine National Park, Cowombat Flat Track, 10 Feb. 2005, N.G. Walsh 6258 (MEL!); Snowy River National Park, 11 Jan. 1993, I.R. Telford 11782 (CANB*, MEL); Bidwell, Upper Delegate River, 19 Jan. 1953, R. Melville 2959 (MEL!, NSW). TASMANIA: North East: Break O’Day: Bells Marsh, 13 Apr. 2009, M. Wapstra 714 (HO!); Powers Rivulet Marsh, 12 May 2009, M. Wapstra 717 (HO!); unnamed marsh, W of Ansons Bay Road, 13 Apr. 2009, M. Wapstra 715 (HO!).
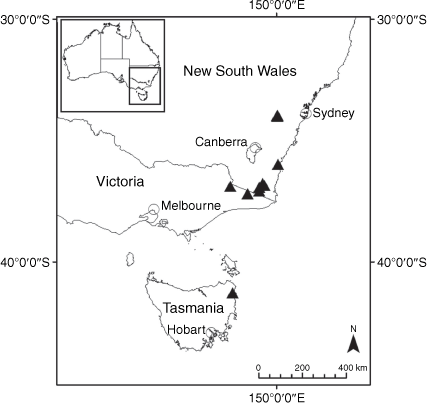
|

|
Xerochrysum collierianum A.M.Buchanan, Muelleria 20: 49 (2004)
Erect, perennial, fibrous-rooted herb up to ~25 cm. Stems and branches hirsute or glabrescent, and with glands; internode length 10–35 mm. Basal leaf rosette present or absent at flowering. Basal leaves obovate to spathulate, 20–80 mm long and 5–10 mm wide, base amplexicaul, margin cobwebby or hirsute, apex mucronate or apiculate; abaxial indumentum with glands, midvein indumentum with glands; adaxial indumentum hirsute and with glands. Cauline leaves oblanceolate, 20–40 mm long and 3–7 mm wide, leaf base attenuate, margin cobwebby or woolly, and hispid; apex mucronate; abaxial indumentum with glands, midvein indumentum with glands; adaxial indumentum hirsute and with glands. Foliaceous bracts subtending capitula 7–12 mm long, margin cobwebby or hispid. Capitula 30–40 mm wide, terminal, solitary. Outer phyllaries ovate, white, basal margin fimbriate or hispid, abaxial surface smooth, apex acute. Medial phyllaries narrow ovate to lanceolate, abaxially white, apex apiculate. Stylar appendages ovate. Cypsela ~2.5 mm long and 0.75 mm wide, cross-section with oblique angles; pericarp brown, idioblasts indistinct. Pappus persistent, ~6 mm long.
Distribution
Endemic to Tasmania in the Central Highlands Bioregion (Fig. 48).
Phenology
Fruiting recorded in March.
Habitat
Steep rocky ridges and outcrops on mountain sides, growing in skeletal gravelly soils and cracks in rocks.
Conservation status
Listed as of conservation significance in Tasmania because of occurrence in only one bioregion and being endemic to Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au). We recommend a status of ‘Data Deficient’ (IUCN 2019) and suggest that a detailed analysis of the area of extent, population sizes and health be conducted to determine appropriate conservation status.
Notes
The common name ‘quartzite paperdaisy’ is in use in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au).
Selected specimens examined
TASMANIA: Central Highlands: Mount Claude Lookout track from Olivers Road, 2 Mar. 2018, T.L. Collins 1026 & R.L. Andrew (CANB!, HO!, NE!); on rocky outcrop at intersection of Mount Claude Lookout track and Olivers Road, 2 Mar. 2018, T.L. Collins 1027 & R.L. Andrew (CANB!, HO!, NE!); Cradle Mountain National Park, 3 Mar. 2018, T.L. Collins 1032 & R.L. Andrew (CANB!, HO!, NE!).
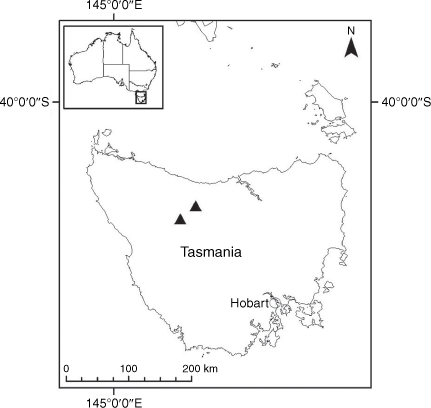
|
Xerochrysum milliganii (Hook.f.) Paul G.Wilson, in A.N.Schmidt-Lebuhn et al., Taxon 64: 106 (2015)
Erect, fibrous-rooted, perennial herb, up to 20 cm. Stems and branches cobwebby, or woolly, or glabrescent; internode length 5–20 mm. Flowering stems unbranched. Basal leaf rosette present at flowering. Basal leaves obovate, 10–15 mm long and 3–6 mm wide, base amplexicaul, apex acute, margin cobwebby and with glands; abaxial leaf surface glabrous, midvein glabrous; adaxial indumentum with scattered glands. Leaf arrangement cauline or radical. Cauline leaves oblanceolate or lanceolate, 15–25 mm long and 3–5 mm wide, base truncate, apex acute, margin cobwebby, or glabrous, or woolly; abaxial indumentum cobwebby, midvein indumentum cobwebby; adaxial indumentum with scattered glands. Foliaceous bracts subtending capitula 5–7 mm long, margin cobwebby or fimbriate. Capitula 30–40 mm wide, terminal, solitary. Outer phyllaries white, basal margin cobwebby or fimbriate, abaxial surface smooth, apex acute. Medial phyllaries abaxially white, apex cuspidate or apiculate. Stylar appendages clavate. Cypsela ~3 mm long and ~0.75 mm wide, cross-section squarish; pericarp grey–brown, idioblasts absent. Pappus persistent, ~6 mm long.
Distribution
Endemic to Tasmania in the Central Highlands Bioregion (Fig. 49).
Phenology
Recorded flowering January–March and fruiting in March.
Habitat
Alpine herbfields and shrublands at >900-m altitude.
Conservation status
Listed as of conservation significance in Tasmania and endemic to Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au). We recommend a status of ‘Data Deficient’ (IUCN 2019) and suggest that a detailed analysis of the area of extent, population sizes and health be conducted to determine appropriate conservation status.
Notes
The common name ‘snow paperdaisy’ is a name in use in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au).
Selected specimens examined
TASMANIA: Central Highlands: Cradle Mountain National Park, upper slope of Marions Lookout, 3 Mar. 2018, T.L. Collins 1028 & R.L. Andrew (CANB!, HO!, NE!); Cradle Mountain National Park, Face Track, 3 Mar. 2018, T.L. Collins 1029 & R.L. Andrew (CANB!, HO!, NE!); Mount Rufus, 25 Jan. 1949, N.T. Burbidge 3341 (CANB*). Huon Valley: Moonlight Ridge, hill one, 31 Jan. 1983, P.S. Short 1873 (CANB*, MEL); Adamsons Peak, 7 Feb. 1969, I.R. Telford EMC2480 (CANB!, PERTH).
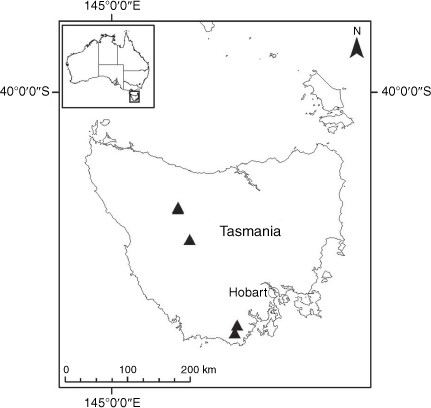
|
Xerochrysum palustre (Flann) R.J.Bayer, Kew Bull. 56(4): 1014 (2001)
Erect, rhizomatous, perennial herb, ~30–60 cm tall. Stems and branches cobwebby or woolly to glabrescent, and with glands; internode length 15–35 mm. Basal leaf rosette absent at flowering. Seedling and basal leaves not seen. Cauline leaves lanceolate, 20–120 mm long and 2–10 mm wide, base attenuate, margin cobwebby, hispid, and scabrid, apex acute and apiculate; abaxial indumentum with glands, midvein glabrous (occasionally with scattered septate trichomes and glands); adaxial indumentum cobwebby or glabrous, and with glands. Foliaceous bracts subtending capitula 5–10 mm long, margin cobwebby and fimbriate. Capitula 35–45 mm wide, terminal, solitary. Outer phyllaries ovate, brown or straw-coloured, basal margin fimbriate and hispid, abaxial surface smooth, apex acute to apiculate. Medial phyllaries lanceolate, abaxially brown or yellow, apex apiculate to acute. Stylar appendages clavate to ovate. Cypsela oblong, ~3 mm long and ~0.75 mm wide, in cross-section with oblique angles to circular; pericarp brass-coloured, idioblasts present. Pappus persistent, ~8 mm long.
Distribution
Occurring in New South Wales, Victoria and Tasmania in the South East Corner, South East Coastal Plain and Tasmanian Southern Ranges bioregions (Fig. 50).
Phenology
Recorded flowering December–January and fruiting in February.
Habitat
Swamps typically below 500-m altitude.
Conservation status
Listed as ‘Vulnerable’ under the Tasmanian Threatened Species Protection Act 1995, the Victorian Flora and Fauna Guarantee Act 1988, and under the Commonwealth of Australia Environment Protection and Biodiversity Conservation Act 1999.
Notes
At Tantawangalo State Forest populations of X. palustre and X. andrewiae are sympatric and mixed collections have been made (e.g. D.L. Jones 17164 MEL 2101319!, CANB 616790).
The common name ‘swamp paperdaisy’ is in use in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au), and ‘swamp everlasting’ in New South Wales and Victoria (New South Wales Flora Online, see http://plantnet.rbgsyd.nsw.gov.au/floraonline.htm; VicFlora, see https://vicflora.rbg.vic.gov.au/).
Selected specimens examined
NEW SOUTH WALES: South Coast: corner Tin Mine Road and Tantawangalo Mountain Road, 17 Jan. 2000, D.L. Jones 17111 (CANB, NE!); ~9.8 km E of Cathcart towards Pambula, 8 Feb. 2000, D.L. Jones 17157 (CANB, MEL!). VICTORIA: East Gippsland: Gisborne Racecourse Marshlands Reserve, 12 Dec. 1996, C. Flann 5 & N.G. Walsh (MEL!). Western Plains: Lower Glenelg River area, Red Gum Flat, 25 Jan. 1970, A.C. Beauglehole 33395 (MEL!). TASMANIA: Central Highlands: Bronte Lagoon, 1 Mar. 2018, T.L. Collins 1019 & R.L. Andrew (CANB!, HO!, NE!). Northern Midlands: Smiths Lagoon, 7 Mar. 2018, T.L. Collins 1038 & R.L. Andrew (CANB!, HO!, NE!).
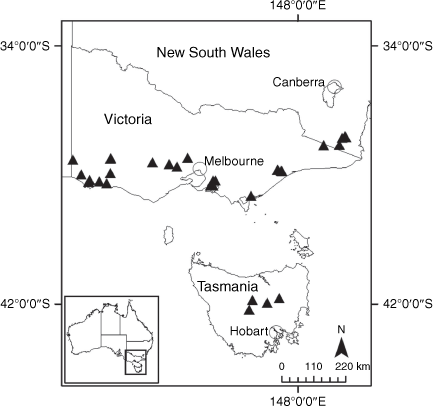
|
Xerochrysum subundulatum (Sch.Bip.) R.J.Bayer, Kew Bull. 56(4): 1015 (2001)
Procumbent or erect, rhizomatous, perennial herb. Stems and branches cobwebby, woolly, or glabrescent, and with glands; internode length 10–30 mm. Basal leaf rosette present or absent at flowering. Basal leaves obovate to spathulate, 20–60 mm long and 6–20 mm wide, base amplexicaul, margin cobwebby or villous, apex apiculate; abaxial indumentum with glands, midvein indumentum cobwebby or villous; adaxial indumentum cobwebby, hispid, and with glands. Cauline leaves oblanceolate to obovate, 25–90 mm long and 5–20 mm wide, base attenuate and amplexicaul, margin cobwebby or hispid, apex apiculate and mucronate; abaxial indumentum hirsute, glabrescent and with glands; abaxial midvein indumentum cobwebby, hispid, and with glands; adaxial indumentum hispid, scabrid, and with glands. Foliaceous bracts subtending capitula 12–15 mm long, margin cobwebby, hispid and with glands. Capitula 35–50 mm wide, terminal, solitary. Outer phyllaries ovate to broad-ovate, orange, brown, or straw-coloured; basal margin hispid, abaxial surface scabridulous, apex acute. Medial phyllaries narrow ovate to lanceolate, abaxially yellow, apex acute. Stylar appendages ovate. Cypsela ~3 mm long and 1 mm wide, cross-section with oblique angles; pericarp brown, idioblasts present. Pappus persistent, ~7.5 mm long.
Distribution
Occurs in New South Wales, Victoria and Tasmania, in the South Eastern Highlands, Australian Alps, and Central Highlands and Ben Lomond bioregions respectively (Fig. 51).
Phenology
Recorded flowering January–March and fruiting in March.
Habitat
Alpine and subalpine herblands, shrublands and woodlands with good moisture availability.
Notes
See notes above for X. sp. Blackfellows Gap collections from Namadgi National Park and Kosciuszko National Park that may represent hybrids between X. subundulatum and X. viscosum.
Conservation status
Occurs over a wide geographical area, including in several conservation reserves, and is not considered to be rare or threatened. We recommend a status of ‘Least Concern’ (IUCN 2019).
Notes
The common names ‘orange paperdaisy’ or ‘orange everlasting’ are in use in Tasmania (‘Natural Values Atlas’, see www.naturalvaluesatlas.tas.gov.au) and ‘alpine everlasting’ is in use in New South Wales and Victoria (New South Wales Flora Online, see http://plantnet.rbgsyd.nsw.gov.au/floraonline.htm; VicFlora, see https://vicflora.rbg.vic.gov.au/).
Selected specimens examined
AUSTRALIAN CAPITAL TERRITORY: Namadgi National Park, Cotter Hut Road, 3 Jan. 2007, J.J. Bruhl 2595 & I.L. Crawford (CANB!, NE!, NSW!). NEW SOUTH WALES: Southern Tablelands: Kosciuszko National Park, alongside Tooma Road, 25 Mar. 2014, J.R. Hosking 3746 (CANB, MEL, NE!, NSW, US). VICTORIA: unincorporated: Panorama Hill, Falls Creek, near the top of the Panorama Poma, 11 Mar. 1984, D.E. Albrecht 263 (MEL!). Alpine: Mount Buffalo National Park, 19 Jan. 1988, A.C. Beauglehole 92582 (MEL!). East Gippsland: Bogong High Plains, Buckety Plain, 20 Jan. 1988, N.G. Walsh 2021 (MEL!). TASMANIA: Ben Lomond: Ben Lomond National Park, 5 Mar. 2018, T.L. Collins 1035 & R.L. Andrew (CANB!, HO!, NE!). Central Highlands: Central Highlands Highway, Little Pine Lagoon, 1 Mar. 2018, T.L. Collins 1020 & R.L. Andrew (CANB!, HO!, NE!); Liaweeni, 1 Mar. 2018, T.L. Collins 1021 & R.L. Andrew (CANB!, HO!, NE!); Lake Augusta, 1 Mar. 2018, T.L. Collins 1022 & R.L. Andrew (CANB!, HO!, NE!); Cradle Mountain National Park, Face Track, 3 Mar. 2018, T.L. Collins 1031 & R.L. Andrew (CANB!, HO!, NE!).
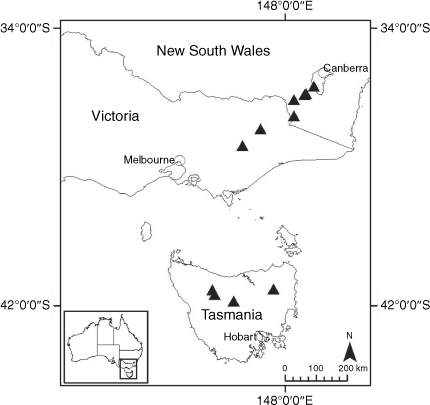
|
Entities of the Xerochrysum bracteatum group requiring further study
Xerochrysum sp. Blackfellows Gap (N.T.Burbidge 6926) Qld Herbarium
Low-growing, rhizomatous, perennial herb. Cauline leaves lanceolate, margin revolute and hispid with stipitate glands, apex acuminate to acute, and mucronate. Abaxial lamina indumentum with sessile glands, midvein indumentum hispid; adaxial lamina indumentum hispid and with glands. Outer phyllaries smooth, medial phyllaries oblong to ovate, abaxially yellow. Cypsela ~3 mm long and 1 mm wide; pericarp brown, idioblasts absent. Pappus deciduous, ~9 mm long.
Habitat and distribution
Collections from open grassy herbfields and woodlands in the Namadgi and Kosciuszko national parks, in the Australian Capital Territory and adjacent New South Wales in the Australian Alps Bioregion, are tentatively assigned to this entity.
Notes
Collections referred to Xerochrysum sp. Blackfellows Gap from Namadgi National Park and Kosciuszko National Park, labelled by herbaria as X. subundulatum (e.g.: J.J. Bruhl 2596, L.A. Craven 10074) have broad-ovate, smooth, outer phyllaries with apiculate apices, and broad medial phyllaries with acuminate apices. These collections also have sessile glands on cauline leaves abaxially and cypsela with a deciduous pappus, whereas typical X. subundulatum has narrow acute phyllaries, cauline leaves abaxially bearing glands on stalks, scattered septate trichomes, and the cypsela with a persistent pappus.
Species limits among X. sp. Blackfellows Gap, X. viscosum and X. subundulatum are uncertain because limited sampling and molecular data were unable to resolve genetically distinct entities or confirm hybrid ancestry; however, informative morphological characters separating these entities deserve further study. The historical legacy of colonialisation and dispossession of the Ngunnawal, Ngunawal and Ngambri people in Namadgi National Park is reflected in the phrase name derived from the location of the first herbarium collection.
Xerochrysum sp. Chinchilla (A.N.Schmidt-Lebuhn 1745) NE Herbarium
Erect, annual or biennial, taprooted herb. Cauline leaves oblanceolate, margin cobwebby and hispid, apex acute and mucronate. Abaxial indumentum with glands, midvein indumentum hispid and with glands; adaxial indumentum cobwebby, hispidulous and with glands. Medial phyllaries oblong to ovate, abaxially yellow.
Habitat and distribution
Collections from open grassy woodlands from central western Queensland to Narrabri, New South Wales in the Brigalow Belt South Bioregion are tentatively assigned to this entity.
Notes
Usually a short-lived annual following winter rainfall. Species limits between X. sp. Chinchilla and X. hispidum remain uncertain, despite the limited molecular data suggesting genetically distinct entities. Informative morphological characters distinguishing these entities could not be defined and require further study.
Xerochrysum sp. North Stradbroke Island (L.Durrington 675) Qld Herbarium
Erect, perennial, tap-rooted herb. Cauline leaves obovate, margin cobwebby, hispid and scabrid, apex obtuse and mucronate. Abaxial indumentum with glands, midvein indumentum hispid with scattered glands; adaxial indumentum hispid and scabrid. Medial phyllaries ovate to narrow ovate, abaxially yellow.
Habitat and distribution
Collections from coastal sand dunes and dune swales on Minjerribah (North Stradbroke Island), K’gari (Fraser Island) and Mulgumpin (Moreton Island), and from coastal headlands from the Whitsunday Islands south to Diamond Head, in the Central Mackay Coast, South Eastern Queensland and New South Wales North Coast bioregions are tentatively assigned to this entity.
Notes
Seedling leaves seen on Mulgumpin (Moreton Island) can be up to 35 cm long and 8 cm wide, the largest in the genus. Xerochrysum sp. North Stradbroke Island may be conspecific with H. macrocephalum but molecular and morphological data were inconclusive. Further research is underway at the University of New England.
Xerochrysum sp. Tin Can Bay (T.L.Collins 1116) NE Herbarium
Erect, perennial, tap-rooted herb. Cauline leaves oblanceolate to lanceolate, margin cobwebby and hispid, apex acuminate to acute, and mucronate. Abaxial indumentum with glands, midvein indumentum hispid with scattered glands; adaxial indumentum cobwebby, hispid, and with glands. Medial phyllaries ovate to narrow ovate, abaxially orange, brown, or yellow.
Distribution and habitat
Collections in woodlands on sandy soils east of the Great Dividing Range in Queensland, from Mackay to Tin Can Bay in the Central Mackay Coast, South Eastern Queensland bioregions are tentatively assigned to this entity.
Notes
Requires further sampling to test species limits. Treated in the analyses as X. sp. weedy.
Data availability
Morphological data are available in the article and in the accompanying Supplementary material. SNP matrices are available from the first author upon request.
Conflicts of interest
Jeremy Bruhl is an associate editor of Australian Systematic Botany but played no editorial role for the journal for this paper, as is standard practice when handling manuscripts submitted by an editor to this journal. The other authors declare that they have no conflicts of interest.
Declaration of funding
The project ‘Integrative taxonomic revision of the everlasting daisy genera Xerochrysum and Coronidium (Asteraceae, Gnaphalieae)’ is supported through funding from the Australian Government’s Australian Biological Resources Study National Taxonomy Research Grant Programme. T.L. Collins received a Linnean Society of New South Wales Joyce W. Vickery Scientific Research Fund grant to travel to the Pilbara; a Keith and Dorothy McKay Travelling Scholarship for work at CANB; Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Programme (NTRGP) Student Travel Grants to attend the 5th National Postgraduate Training Workshop in Systematics in Adelaide, Australia, and the joint conference of the Australasian Systematic Botany Society and the New Zealand Plant Conservation Network in Wellington, New Zealand. T. L. Collins was supported by a University of New England Strategic Scholarship, and funding from the School of Environmental and Rural Science.
Supplementary material
Supplementary material is available online.
Acknowledgements
Access to the collection and facilities of the N.C.W. Beadle Herbarium (NE) have been crucial to this study. We thank the following herbaria for loans of their specimens and access to their collections: AD, BM, BRI, CANB, CNS, DNA, E, K, HO, G, MEL, NSW, PERTH. We also thank the South Australian Seed Conservation Centre, the Australian PlantBank, Royal Tasmanian Botanical Gardens, and the Australian National Botanic Gardens for access to seedbank and living collections. We thank the following Aboriginal Corporations for assistance accessing Aboriginal land: Quandamooka Yoolooburrabee Aboriginal Corporation, Jabalbina Yalanji Aboriginal Corporation, Darumbal Enterprises Pty Ltd, and Arafura Swamp Ranger Aboriginal Corporation. Thanks go to Stephen Brereton, Steven Ahoy, and Christo Lifu for cultural advice and consultation with elders on aboriginal language names used as specific epithets. Ilse Breitwieser, Mercè Galbany-Cassals, and Nicola Bergh are thanked for constructive comments on the thesis-chapter version of the manuscript. Field assistance was generously provided by Yuku Baja-Muliku Indigenous Land and Sea Rangers, Thamarrurr Rangers Wadeye, Arafura Swamp Rangers, Mark Harris, Matthew Bashford, Michelle Haby, Bev Overton, John Hosking, John Nevin, Tanya Howard, Dieuwer Reynders, Peter Latz, Peter Jobson, and Amy-Jayne Dutton and The St Helena National Trust. Field collection planning assistance was generously provided by Ian Cowie, Matthew Baker, Darren Crayn, Paul Forster, Andrew Mitchell, Stephen van Leeuwen, Gareth Catt, John Clarkson, and Keith McDonald. Thanks go to Lachlan Copeland for helpful discussions on field observations. Thanks go to Norman Gaywood for help accessing UNE mathematics servers, Malcolm Lambert for help with SEM, Ian Simpson and Rhyan Gorman for help with glasshouse management, and Audrey Larsen for digital enhancement of type images. Two anonymous reviewers and David Cantrill provided constructive feedback that greatly improved the paper. Selected specimens of X. hispidum (as X. bracteatum sens. lat.), X. interiore and X. subundulatum were collected by the Ecosystem Surveillance subfacility and all rights pertaining to these specimens are claimed by the University of Adelaide. Specimens were made available for analysis by the Terrestrial Ecosystem Research Network (TERN), supported by the Australian Government through the National Collaborative Research Infrastructure Strategy and the Super Science Initiative. Data were provided under the TERN Attribution-Share Alike Data Licence v1.0, which allows sharing of the data provided a user shares their new data or products in return. Specimens were provided under equivalent terms.
References
Al-Beyroutiová M, Sabo M, Sleziak P, Dušinský R, Birčák E, Hauptvogel P, Kilian A, Švec M (2016) Evolutionary relationships in the genus Secale revealed by DArTseq DNA polymorphism. Plant Systematics and Evolution 302, 1083–1091.| Evolutionary relationships in the genus Secale revealed by DArTseq DNA polymorphism.Crossref | GoogleScholarGoogle Scholar |
Alam M, Neal J, O’Connor K, Kilian A, Topp B (2018) Ultra-high-throughput DArTseq-based silicoDArT and SNP markers for genomic studies in Macadamia. PLoS One 13, e0203465
| Ultra-high-throughput DArTseq-based silicoDArT and SNP markers for genomic studies in Macadamia.Crossref | GoogleScholarGoogle Scholar | 30169500PubMed |
Anderberg AA (1991) Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Botanica 104, 1–195.
Anderson BM, Thiele KR, Barrett MD (2017) A revision of the Triodia basedowii species complex and close relatives (Poaceae: Chloridoideae). Australian Systematic Botany 30, 197–229.
| A revision of the Triodia basedowii species complex and close relatives (Poaceae: Chloridoideae).Crossref | GoogleScholarGoogle Scholar |
Andrews HC (1805) ‘The Botanist’s Repository for New, and Rare Plants. Vol. 6.’ (Published by the author: London, UK)
| Crossref |
Australian Cultivar Registration Authority (1977) Xerochrysum ‘Diamond Head’; Xerochrysum bracteatum ‘Diamond Head’. In ‘Descriptions of Registered Cultivars’. Available at https://www.anbg.gov.au/acra/descriptions/acc107.html [Verified 21 February 2020]
Bayer RJ (2001) Xerochrysum Tzvelev, a pre-existing generic name for Bracteantha Anderb. & Haegi (Asteraceae: Gnaphalieae). Kew Bulletin 56, 1013–1015.
Beaumont M, Barratt EM, Gottelli D, Kitchener AC, Daniels MJ, Pritchard JK, Bruford MW (2001) Genetic diversity and introgression in the Scottish wildcat. Molecular Ecology 10, 319–336.
| Genetic diversity and introgression in the Scottish wildcat.Crossref | GoogleScholarGoogle Scholar | 11298948PubMed |
Belbin L (1993) ‘PATN – pattern analysis package.’ (CSIRO Division of Wildlife and Ecology: Canberra, ACT, Australia)
Bentham G (1867) ‘Flora Australiensis.’ (Lovell Reeve & Co.: London, UK)
Buchanan AM (2004) A new species of Xerochrysum (Gnaphalieae: Asteraceae) from western Tasmania, Australia. Muelleria 20, 49–52.
Burbidge NT (1970) Compositae (Asteraceae). In ‘Flora of the Australian Capital Territory.’ (Ed. M Gray) (Australian National University Press: Canberra, ACT, Australia)
Bureau of Meteorology (2020) Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/averages/tables/ [Verified 15 June 2020]
CHAH (2020a) Vascular plants. In ‘Australian Plant Census’. Available at https://biodiversity.org.au/nsl/services/APC [Verified 20 February 2020]
CHAH (2020b) Usage of a name (Instance). Helichrysum leucopsideum DC. tax. nov. In ‘Australian Plant Name Index’. Available at https://id.biodiversity.org.au/instance/apni/503733 [Verified 4 January 2020]
Collins TL, Bruhl JJ, Schmidt-Lebuhn AN, Telford IRH, Andrew RL (2021) Tracing the origins of hybrids through history: monstrous cultivars and Napoléon Bonaparte’s exiled paper daisies (Asteraceae; Gnaphalieae). Botanical Journal of the Linnean Society 197, 277–289.
| Tracing the origins of hybrids through history: monstrous cultivars and Napoléon Bonaparte’s exiled paper daisies (Asteraceae; Gnaphalieae).Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Laffan S, Linder HP, Monro A (2001) Endemism in the Australian Flora. Journal of Biogeography 28, 183–198.
| Endemism in the Australian Flora.Crossref | GoogleScholarGoogle Scholar |
Curtis WM (1956) ‘The student’s flora of Tasmania.’ (Government Printer: Hobart, Tas., Australia)
Dayrat B (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society. Linnean Society of London 85, 407–415.
| Towards integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
de Candolle AP (1838) ‘Prodromus Systematis Naturalis Regni Vegetabilis Pars sexta.’ (Sumptibus Sociorum Treuttel et Würtz, viâ dictà de Lille, n° 17. Venitque in eorundem bibliopolio Argentorati: Parisiis)
De Queiroz K (2005) A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences 4, 196–215.
De Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar | 18027281PubMed |
Department of the Environment and Energy (2016) ‘Interim Biogeographical Regionalisation for Australia (IBRA) version 7.’ (Commonwealth of Australia: Canberra, ACT, Australia)
Depypere L, Chaerle P, Breyne P, Vander Mijnsbrugge K, Goetghebeur P (2009) A combined morphometric and AFLP based diversity study challenges the taxonomy of the European members of the complex Prunus L. section Prunus. Plant Systematics and Evolution 279, 219–231.
| A combined morphometric and AFLP based diversity study challenges the taxonomy of the European members of the complex Prunus L. section Prunus.Crossref | GoogleScholarGoogle Scholar |
D’hoop BB, Paulo MJ, Kowitwanich K, Sengers M, Visser RGF, van Eck HJ, van Eeuwijk FA (2010) Population structure and linkage disequilibrium unravelled in tetraploid potato. Theoretical and Applied Genetics 121, 1151
| Population structure and linkage disequilibrium unravelled in tetraploid potato.Crossref | GoogleScholarGoogle Scholar | 20563789PubMed |
Duley G (2007) Species limits in Xerochrysum (Asteraceae). Honours Thesis, University of New England.
Egea LA, Mérida-García R, Kilian A, Hernandez P, Dorado G (2017) Assessment of genetic diversity and structure of large garlic (Allium sativum) germplasm bank, by Diversity Arrays Technology ‘genotyping-by-sequencing’ platform (DArTseq). Frontiers in Genetics 8, 1–9.
| Assessment of genetic diversity and structure of large garlic (Allium sativum) germplasm bank, by Diversity Arrays Technology ‘genotyping-by-sequencing’ platform (DArTseq).Crossref | GoogleScholarGoogle Scholar |
Fairley A, Moore P (1989) ‘Native plants of the Sydney district: an identification guide.’ (Kangaroo Press: Sydney, NSW, Australia)
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69, 57–68.
| Compositional dissimilarity as a robust measure of ecological distance.Crossref | GoogleScholarGoogle Scholar |
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587.
| Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies.Crossref | GoogleScholarGoogle Scholar | 12930761PubMed |
Faraway JJ (2012) Backscoring in principal coordinates analysis. Journal of Computational and Graphical Statistics 21, 394–412.
| Backscoring in principal coordinates analysis.Crossref | GoogleScholarGoogle Scholar |
Flann C (1998) Bracteantha palustris (Asteraceae: Gnaphalieae), a new species in Victoria and Tasmania. Muelleria 11, 97–100.
Funk SM, Guedaoura S, Juras R, Raziq A, Landolsi F, Luís C, Amparo Martínez M, Abubakar Musa M, Mujica F, Maria do Mar O, Ouragh L, Yves‐Marie S, Jose Luis VP, Cothran EG (2020) Major inconsistencies of inferred population genetic structure estimated in a large set of domestic horse breeds using microsatellites. Ecology and Evolution 10, 4261–4279.
| Major inconsistencies of inferred population genetic structure estimated in a large set of domestic horse breeds using microsatellites.Crossref | GoogleScholarGoogle Scholar | 32489595PubMed |
Galbany-Casals M, Unwin M, Garcia-Jacas N, Smissen RD, Susanna A, Bayer RJ (2014) Phylogenetic relationships in Helichrysum (Compositae: Gnaphalieae) and related genera: Incongruence between nuclear and plastid phylogenies, biogeographic and morphological patterns, and implications for generic delimitation. Taxon 63, 608–624.
| Phylogenetic relationships in Helichrysum (Compositae: Gnaphalieae) and related genera: Incongruence between nuclear and plastid phylogenies, biogeographic and morphological patterns, and implications for generic delimitation.Crossref | GoogleScholarGoogle Scholar |
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53, 325–338.
| Some distance properties of latent root and vector methods used in multivariate analysis.Crossref | GoogleScholarGoogle Scholar |
Gower JC (1971) A general coefficient of similarity and some of its properties. Biometrics 27, 857–871.
Gruber B, Unmack PJ, Berry OF, Georges A (2018) dartr: an r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18, 691–699.
| dartr: an r package to facilitate analysis of SNP data generated from reduced representation genome sequencing.Crossref | GoogleScholarGoogle Scholar | 29266847PubMed |
Harden GJ (1991) ‘Flora of New South Wales.’ (NSW University Press: Sydney, NSW, Australia)
Holman JE, Hughes JM, Fensham RJ (2003) A morphological cline in Eucalyptus: a genetic perspective. Molecular Ecology 12, 3013–3025.
| A morphological cline in Eucalyptus: a genetic perspective.Crossref | GoogleScholarGoogle Scholar | 14629382PubMed |
IUCN (2019) ‘IUCN Red List categories and criteria.’ (International Union for Conservation of Nature: Gland, Switzerland)
Jombart T, Ahmed I (2011) adegenet 1.3–1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27, 3070–3071.
| adegenet 1.3–1: new tools for the analysis of genome-wide SNP data.Crossref | GoogleScholarGoogle Scholar | 21926124PubMed |
Keelty M, Dilag L, Loughlin B, Howard A, McDaid S, Gardner L, Boddington D (2020) The independent review into South Australia’s 2019–2020 bushfire season. (Government of South Australia) Available at https://www.lga.sa.gov.au/__data/assets/pdf_file/0036/739287/ECM_714111_v12_Summary-of-the-Independent-Review-into-South-Australia-s-2019-2020-Bushfire-Season-the-Keelty-Revie.pdf
Kilian A, Wenzl P, Huttner E, Carling J, Xia L, Blois H, Caig V, Heller-Uszynska K, Jaccoud D, Hopper C (2012) Diversity arrays technology: a generic genome profiling technology on open platforms. In ‘Data production and analysis in population genomics’. (Eds F Pompanon, A Bonin) pp. 67–89. (Humana Press: Totowa, NJ, USA)
Labillardière JJH (1806) ‘Novae Hollandiae Plantarum Specimen.’ (Ex typographia Dominæ Huzard: Paris, France)
Laport RG, Minckley RL, Ramsey J (2016) Ecological distributions, phenological isolation, and genetic structure in sympatric and parapatric populations of the Larrea tridentata polyploid complex. American Journal of Botany 103, 1358–1374.
| Ecological distributions, phenological isolation, and genetic structure in sympatric and parapatric populations of the Larrea tridentata polyploid complex.Crossref | GoogleScholarGoogle Scholar | 27440793PubMed |
Lawson DJ, van Dorp L, Falush D (2018) A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots. Nature Communications 9, 3258
| A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots.Crossref | GoogleScholarGoogle Scholar | 30108219PubMed |
Lindley J (1835) ‘Edwards’s Botanical register v.21.’ (James Ridgway and Sons: London, UK)
Meirmans PG (2019) Subsampling reveals that unbalanced sampling affects Structure results in a multi-species dataset. Heredity 122, 276–287.
| Subsampling reveals that unbalanced sampling affects Structure results in a multi-species dataset.Crossref | GoogleScholarGoogle Scholar | 30026534PubMed |
Missouri Botanical Garden (2020) !Xerochrysum Tzvelev. In ‘Tropicos’. (Missouri Botanical Garden) Available at http://www.tropicos.org/Name/50007766 [Verified 18 August 2019]
Mitchell-Olds T, Schmitt J (2006) Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature 441, 947–952.
| Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 16791187PubMed |
Moore AJ, Moore WL, Baldwin BG (2014) Genetic and ecotypic differentiation in a Californian plant polyploid complex (Grindelia, Asteraceae). PLoS One 9, e95656
| Genetic and ecotypic differentiation in a Californian plant polyploid complex (Grindelia, Asteraceae).Crossref | GoogleScholarGoogle Scholar | 24755840PubMed |
Nei M (1973) Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the United States of America 70, 3321–3323.
| Analysis of gene diversity in subdivided populations.Crossref | GoogleScholarGoogle Scholar | 4519626PubMed |
Nie Z-L, Funk VA, Meng Y, Deng T, Sun H, Wen J (2016) Recent assembly of the global herbaceous flora: evidence from the paper daisies (Asteraceae: Gnaphalieae). New Phytologist 209, 1795–1806.
| Recent assembly of the global herbaceous flora: evidence from the paper daisies (Asteraceae: Gnaphalieae).Crossref | GoogleScholarGoogle Scholar | 26528674PubMed |
Orchard AE, Thompson HS (1999) ‘Flora of Australia. Vol. 1. 2nd edn. Introduction.’ (AGPS: Canberra, ACT, Australia)
Payne WW (1978) A glossary of plant hair terminology. Brittonia 30, 239–255.
| A glossary of plant hair terminology.Crossref | GoogleScholarGoogle Scholar |
Plunkett GT, Wilson KL, Bruhl JJ (2013) Sedges in the mist: a new species of Lepidosperma (Cyperaceae, Schoeneae) from the mountains of Tasmania. PhytoKeys 28, 19–59.
| Sedges in the mist: a new species of Lepidosperma (Cyperaceae, Schoeneae) from the mountains of Tasmania.Crossref | GoogleScholarGoogle Scholar |
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155, 945–959.
| Inference of population structure using multilocus genotype data.Crossref | GoogleScholarGoogle Scholar | 10835412PubMed |
Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW (2002) Genetic structure of human populations. Science 298, 2381–2385.
| Genetic structure of human populations.Crossref | GoogleScholarGoogle Scholar | 12493913PubMed |
Russell J (2015) The story of ‘Xerochrysum bracteatum’. Journal (Australian Native Plants Society, Canberra Region) 18, 16–17.
Rutherford S, Rossetto M, Bragg JG, McPherson H, Benson D, Bonser SP, Wilson PG (2018) Speciation in the presence of gene flow: population genomics of closely related and diverging Eucalyptus species. Heredity 121, 126–141.
| Speciation in the presence of gene flow: population genomics of closely related and diverging Eucalyptus species.Crossref | GoogleScholarGoogle Scholar | 29632325PubMed |
Rymer B (2006) Australian daisies. In ‘Australian Plants Online’. (Association of Societies for Growing Australian Plants) Available at http://anpsa.org.au/APOL2006/jun06-4.html [Verified 4 October 2019]
Schilling EE (2011) Systematics of the Eupatorium album complex (Asteraceae) from eastern North America. Systematic Botany 36, 1088–1100.
| Systematics of the Eupatorium album complex (Asteraceae) from eastern North America.Crossref | GoogleScholarGoogle Scholar |
Schmidt-Lebuhn AN, Bruhl JJ, Telford IRH, Wilson PG (2015) Phylogenetic relationships of Coronidium, Xerochrysum and several neglected Australian species of “Helichrysum” (Asteraceae: Gnaphalieae). Taxon 64, 96–109.
| Phylogenetic relationships of Coronidium, Xerochrysum and several neglected Australian species of “Helichrysum” (Asteraceae: Gnaphalieae).Crossref | GoogleScholarGoogle Scholar |
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675.
| NIH Image to ImageJ: 25 years of image analysis.Crossref | GoogleScholarGoogle Scholar | 22930834PubMed |
Sprengel K (1826) ‘Systema vegetabilium.’ (Dieterichianae: New York, NY, USA)
Stift M, Kolář F, Meirmans PG (2019) Structure is more robust than other clustering methods in simulated mixed-ploidy populations. Heredity 123, 429–441.
| Structure is more robust than other clustering methods in simulated mixed-ploidy populations.Crossref | GoogleScholarGoogle Scholar | 31285566PubMed |
Stöck M, Ustinova J, Lamatsch DK, Schartl M, Perrin N, Moritz C (2010) A vertebratestandard reference reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup). Evolution 64, 944–959.
| A vertebratestandard reference reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup).Crossref | GoogleScholarGoogle Scholar | 19863582PubMed |
Sweet R (1826) ‘Sweet’s Hortus Britannicus.’ (J. Ridgway: London, UK)
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) Regnum Vegetabile’, vol. 159. (Koeltz Botanical Books: Glashütten, Germany)
| Crossref |
Tzvelev NN (1990) Zametki o nekotorykh slozhnotsvetnykh (Asteraceae) evropeiskoi hasti SSSR. [Notae de Asteraceis nonnullis partis europaeae URSS.]. Novosti sistematiki vysshikh rastenii 27, 145–152.
Ventenat EP (1803) ‘Jardin de la Malmaison.’ (De l’imprimerie de Crapelet: Paris, France)Willis
Willis JH (1973) ‘A handbook to plants in Victoria.’ (Melbourne University Press: Melbourne, Vic., Australia)
Wilson PG (2008) Coronidium, a new Australian genus in the Gnaphalieae (Asteraceae). Nuytsia 18, 295–329.
Wilson PG (2017) An examination of the Australian genus Xerochrysum (Asteraceae: Gnaphalieae). Nuytsia 28, 11–38.