Middle to Late Paleocene Leguminosae fruits and leaves from Colombia
Fabiany Herrera A B D , Mónica R. Carvalho B , Scott L. Wing C , Carlos Jaramillo B and Patrick S. Herendeen A
A B D , Mónica R. Carvalho B , Scott L. Wing C , Carlos Jaramillo B and Patrick S. Herendeen A
A Chicago Botanic Garden, 1000 Lake Cook Road, Glencoe, IL 60022, USA.
B Smithsonian Tropical Research Institute, Box 0843-03092, Balboa, Ancón, Republic of Panamá.
C Department of Paleobiology, NHB121, PO Box 37012, Smithsonian Institution, Washington, DC 20013, USA.
D Corresponding author. Email: fherrera@chicagobotanic.org
Australian Systematic Botany 32(6) 385-408 https://doi.org/10.1071/SB19001
Submitted: 10 January 2019 Accepted: 5 April 2019 Published: 30 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Leguminosae are one of the most diverse flowering-plant groups today, but the evolutionary history of the family remains obscure because of the scarce early fossil record, particularly from lowland tropics. Here, we report ~500 compression or impression specimens with distinctive legume features collected from the Cerrejón and Bogotá Formations, Middle to Late Paleocene of Colombia. The specimens were segregated into eight fruit and six leaf morphotypes. Two bipinnate leaf morphotypes are confidently placed in the Caesalpinioideae and are the earliest record of this subfamily. Two of the fruit morphotypes are placed in the Detarioideae and Dialioideae. All other fruit and leaf morphotypes show similarities with more than one subfamily or their affinities remain uncertain. The abundant fossil fruits and leaves described here show that Leguminosae was the most important component of the earliest rainforests in northern South America c. 60–58 million years ago.
Additional keywords: diversity, Fabaceae, fossil plants, legumes, Neotropics, South America.
Introduction
Leguminosae, the third-largest family of flowering plants with ~770 genera and ~19 600 species, has a cosmopolitan distribution and an extraordinary ecological (e.g. symbiosis with nitrogen-fixing bacteria) and economic importance (e.g. Doyle and Luckow 2003; Lewis et al. 2005; Legume Phylogeny Working Group 2013a, 2013b; Epihov et al. 2017). The family is one the most dominant plant groups in the Neotropics (Phillips et al. 2002), from lowland rainforests to deciduous and semi-arid forests and savannas (Schrire et al. 2005a, 2005b; Yahara et al. 2013).
Six subfamilies are currently recognised within Leguminosae on the basis of plastid matK gene sequences obtained from a large number of genera (~91.2% of all currently recognised genera; Legume Phylogeny Working Group 2017) and include the following: Caesalpinioideae, Cercidoideae, Detarioideae, Dialioideae, Duparquetioideae and Papilionoideae. The relationships among the three early branching subfamilies (i.e. Cercidoideae, Detarioideae and Duparquetioideae) are still unresolved, whereas the other three subfamilies form a strongly supported clade (i.e. Caesalpinioideae, Papilionoideae and Dialioideae; Lavin et al. 2005; Bruneau et al. 2008; Legume Phylogeny Working Group 2017). Stem-group Leguminosae has been inferred to originate c. 92.1 million years ago (Magallón et al. 2015) and many other studies have inferred dates for the crown clades ranging from the Cretaceous to the Early Paleogene, c. 90–50 million years ago (e.g. Wikström et al. 2004; Lavin et al. 2005; Bruneau et al. 2008; Bell et al. 2010). Tremendous efforts have been made to understand the relationships and rates of molecular evolution within Leguminosae. Although these studies have been very helpful in understanding the origin and patterns of relationships of extant legumes, the only direct evidence we have of diversity through time and evolutionary history comes from the fossil record.
Here, we report abundant and well preserved Middle to Late Paleocene fossils from Colombia on the basis of fruits and leaves that can be confidently assigned to the Leguminosae. The purposes of this study are to present the descriptions of legume diversity observed in the Paleocene of northern South America, and to assess their overall phylogenetic affinity to the extent possible. This report on fossil legumes from Colombia is significant because there are very few records of fossil plants from low-latitude tropics, where the family is most diverse today. The fossils document important additional evidence about three extant legume subfamilies, diversity and abundance in Paleocene Neotropical rainforests.
Materials and methods
The fossil fruits and leaves reported here were collected from the Cerrejón and Bogotá Formations in Colombia. These two stratigraphic sequences are geographically separated but they are approximately coeval. The Cerrejón Formation crops out in the open cast Cerrejón coalmine, near the base of the Guajira Peninsula, ~1000 km north-east of Bogotá (GPS: ~11.1°N, 72.5°W; see also Wing et al. 2009). The Bogotá Formation is exposed in open clay pits of central Colombia, Sabana de Bogotá (GPS: Nemocón locality: ~5°08′146″N, 73°50′820″W; Cogua locality: ~5°04′605″N, 73°57′318″W), between 2700 and 3000 m abve sea level along the Eastern Cordillera.
Approximately 300 legume specimens (fruits and leaves) were collected from the middle and upper levels of the Cerrejón Formation, a ~700-m-thick stratigraphic sequence composed of abundant and thick coals, sandstones and siltstones (Bayona et al. 2008). This formation was deposited in a complex system of coastal to fluvial floodplains. The Cerrejón strata have been dated as Middle to Late Paleocene (c. 60–58 million years ago) on the basis of pollen zonation, correlations with stable carbon isotopic data, and marine microfossils (Jaramillo et al. 2007, 2011).
Approximately 200 legume specimens (fruits and leaves) were collected from the Bogotá Formation. The stratigraphic sequence is composed of extensive and thick siltstones, claystones, paleosols, inter-bedded sandstones, sporadic conglomerates and breccias (Morón et al. 2013). This formation was deposited predominantly in fluvial environments, and characteristically lacks coal deposits. During the Paleocene, the Andes had not been uplifted and the Bogotá Formation was deposited in lowland settings (Bayona et al. 2010). Pollen assemblages from the same sites where the megafossils were collected belong to the zone T-03b-Foveotricolpites perforatus of Jaramillo et al. (2011), indicating Middle to Late Paleocene age (c. 60–58 million years ago).
Fossil specimens are deposited at the paleontological collections of the Colombian Geological Survey (SGC) and the Colombian Petroleum Institute (ICP). Additional information about samples and localities can be accessed through the Smithsonian Tropical Research Institute (STRI) sample database (http://biogeodb.stri.si.edu/jaramillosdb/web/login, accessed 30 April 2019). The leaf morphological descriptions are based on the terminology of shape and venation characters of Ellis et al. (2009). The terminology of the fruit morphological descriptions is mostly based on Gunn (1984, 1991), Kirkbride et al. (2003) and Legume Phylogeny Working Group (2017).
Results
All fossil fruits and leaves are described here as ‘morphotypes’, an informal taxonomic category, rather than identified to formal taxonomic groups. Eight fruit and six leaf morphotypes referable to the Leguminosae are recognised from the Cerrejón and Bogotá Formations (Table 1).
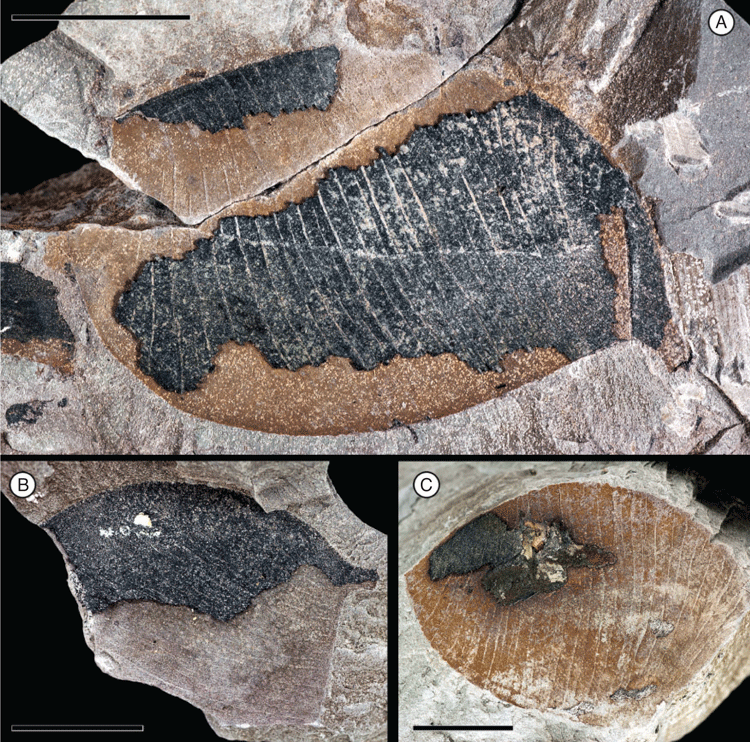
Fruit morphotype 1
(Fig. 1A–C.)
Fruits incomplete, ~14 cm long and ~6 cm wide. Placental margin conspicuously winged, ~0.7–1.1 cm wide. Non-placental margin inconspicuous, not winged. Fragment of pedicel ~2.2 cm long. Base outline obtuse, rounded; apex not preserved. Seeds 6–10+, attached along the placental margin. Seed chambers distinct, strongly septate, more or less symmetrical near the centre (arranged in a columnar-like pattern) of the fruit to asymmetrical near the base, ~0.7–1.8 cm wide, transverse to slightly oblique near the base of the fruit.
Remarks
Both specimens are from the Cerrejón Fm. (locality SW0317). This is the largest legume fruit morphotype described here. The fruits appear to have been thin and membranous, not woody, and were probably indehiscent wind-dispersed samaras.
Comments on affinity
Broadly winged fruits are known from several legume genera, including Mezoneuron (Caesalpinioideae), Barnebydendron (Detarioideae), Zenia, Storckiella and Dicorynia (Dialioideae). This type of samara has evidently evolved multiple times within the family. Venation patterns on the valves and wing can be useful in distinguishing among the genera (Herendeen and Dilcher 1990); however, venation details are not preserved in these fossil fruits. The fruits are perhaps most similar to those of Barnebydendron (occurs today in Central and South America) in their overall size and presence of multiple seeds; however, without venation details, it is not possible to investigate relationships beyond the superficial resemblance.
Fruit morphotype 2
(Fig. 1D.)
Fruit nearly complete, ~2.2 cm long and 1.3 cm wide. Placental margin narrowly winged, ~1.7–2.1 mm wide. Non-placental margin prominent, ~0.5 mm thick. Fragment of pedicel ~2.4 mm long. Base obtuse, rounded; apex not preserved. Seeds 2–3(?), attached along the placental margin. Seed chambers indistinct, ~5.0–5.7 mm wide, and oblique. Fruit margin slightly constricted near the centre.
Remarks
Only one specimen has been found in the Cerrejón Fm. (locality SW0318). This is the smallest legume fruit morphotype from the Cerrejón flora discovered so far. The fruit is likely to be indehiscent because the coalified matter in the compression seems to indicate complete closed valves.
Comments on affinity
Similar to Fruit morphotype 1, but it is distinguished by its small size, presence of a narrow wing on the placental margin, a well-developed non-placental margin, indistinct seed chambers, and a slightly constricted margin. Narrowly winged fruits are known from numerous legume genera in multiple subfamilies. No venation details are preserved, which precludes evaluation of possible relationships.
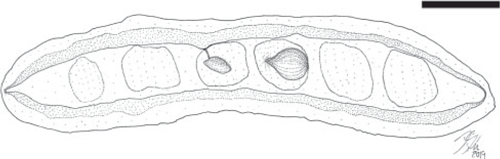
Fruit morphotype 3
(Fig. 2–4.)
Fruits ~3.0–7.9 cm long and 0.9–1.7 cm wide. Placental and non-placental margins conspicuously winged, ~1.0–3.8 mm wide. Fragment of pedicel ~5 mm long. Base outline acute to obtuse, sometimes tapered; apex obtuse, rounded. Seeds 3–9, ~4.0–9.0 mm wide and 1.5–4.0 mm high, attached along the placental margin, oriented parallel to the margin or longest axis of the fruit; seed outlines somewhat reniform, ovate to elliptical; funiculus thin and short. Seed chambers indistinct, ~0.6–1.0 cm wide, transverse to slightly oblique. Margin straight to slightly constricted between the seeds. In some specimens, the constriction corresponds to the presence of an abortive ovule (Fig. 2A, 3A). Outer and inner surfaces of the two valves with faint, oblique striations.
Remarks
This is the most abundant fruit morphotype recovered in the Bogotá flora (locality FH0903). It co-occurs with Fruit morphotype 8. Only one specimen (Fig. 3G) of this fruit morphotype has been collected from the Cerrejón flora (locality FH0711). One immature (likely abortive) ovule is present in the specimen shown in Fig. 2A and 3A, which corresponds to the position of the constriction in the fruit valves. The fruit is likely to be indehiscent because most recovered impressions seem to indicate complete closed valves.
Comments on affinity
Legume fruits bearing wings on both margins are quite uncommon in the family. They are known in Cladrastis platycarpa (Papilionoideae), and Acrocarpus and Peltophorum (Caesalpinioideae). This morphology is also present in, for example, Derris, Paraderris and Xeroderris (Papilionoideae), although the seed outline is clearly reniform. Orientation of the seeds parallel to the long axis of the fruit is unusual in legumes, but does occur for example in C. platycarpa and several species of Peltophorum, however, these extant fruits posses distinct venation patterns that are not present in the Bogotá fossils. Fruit morphotype 3 is likely to be indehiscent, similar to C. platycarpa and Peltophorum fruits (like most samaroid legume fruits).
|
|
|
Fruit morphotype 4
(Fig. 5A–C.)
Fruits ~3.3–4.1 cm long and 0.8–1.1 cm wide. Placental and non-placental margins distinct, narrow and non-winged; approximately of equal thickness, ~0.7 mm wide. Fruits stipitate, stipe ~0.4 mm long. Fragment of pedicel ~5 mm long. In one specimen remnants of the calyx are present. Base outline acute, tapering; apex obtuse, slightly asymmetrical and with a short remnant of the style. Seeds 2+, attached along the placental margin; seed outline poorly preserved, possibly rounded. Seed chambers not evident. Margin straight to slightly constricted between the seeds.
Remarks
Only two specimens have been recovered from the Cerrejón flora (locality FH0708). The fruit is likely to be indehiscent because the thick coalified matter in the compressions seems to indicate complete closed valves.
Affinity
The fossils are distinct from other fruit morphotypes, but characteristic features that would help identify relationships within the family must await additional specimens. Unfortunately, the calyx remnants are not adequately preserved to help in assessing relationships.
Fruit morphotype 5
(Fig. 5D.)
Fruit incomplete, ~5.6 cm long and 3.4 cm wide. Placental and non-placental margins inconspicuous and non-winged; placental margin slightly wider than the non-placental one, ~2.0 mm v. 1.0 mm wide. Base and apex not preserved. Seeds 1+, ~1.0 cm long and 0.7 cm wide, attached along the placental margin; seed outline more or less elliptical; funiculus thin and long, ~7.0 mm, apically attached to the seed; Seed chambers not evident. Margin straight to slightly constricted between the seeds.
Remarks
Only one specimen has been recovered from the Bogotá flora (locality FH0918).
Affinity
This incomplete fossil fruit is different from the other fruit morphotypes, but distinctive features that would help identify relationships within the family must await additional specimens. Fruits of many extant legume genera are similarly non-distinctive and are very difficult to identify to genus- or higher-level taxon without additional information from leaves, flowers or other organs.
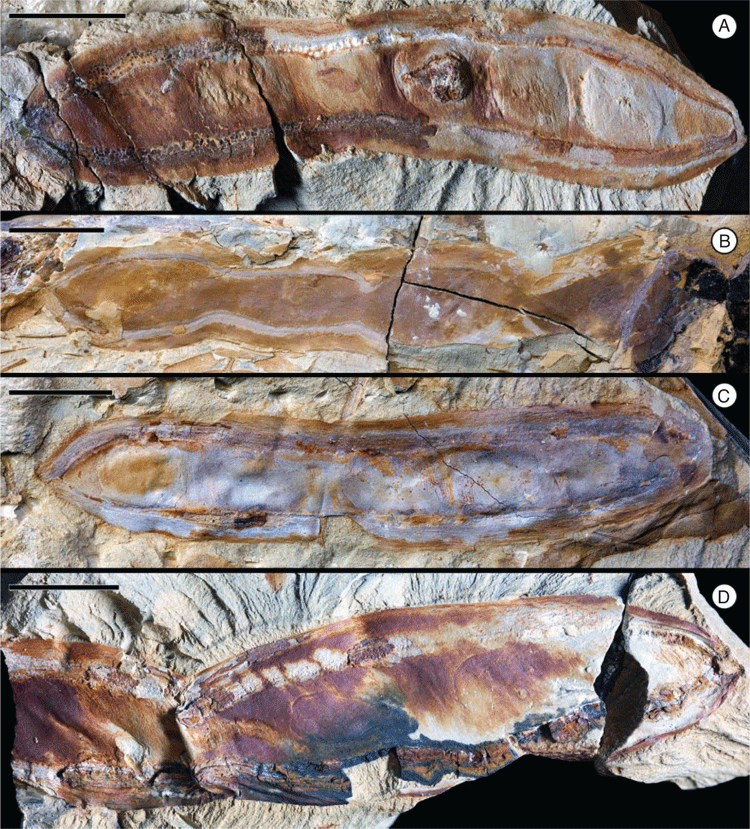
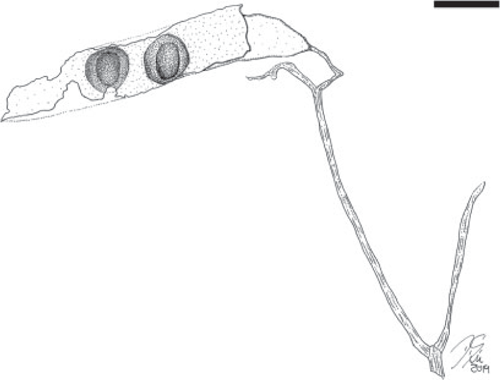
Fruit morphotype 6
(Fig. 6–8.)
Incomplete branching infructescence bearing opposite fruits. The infructescence is composed of bifurcating axes; only a small portion of a central ‘primary’ axis is preserved (~0.8 cm long), this central axis bifurcates into two long and diverging ‘secondary’ axes (~9.2 cm long and 0.2 cm wide) that bifurcate two more times into ‘tertiary’ (~2.1 cm long) and ‘quaternary’ axes. Fruits ~4.5–13 cm long and 0.9–2.0 cm wide. Placental and non-placental margins very narrow and non-winged, approximately of equal thickness, ~1 mm wide. Pedicel ~1.0–1.6 mm long. Base outline convex to slightly cuneate (tapered); apex not preserved. Seeds 6+, occupying most of the seed chamber, attached along the placental margin; seed outline elliptical. Seed chambers possibly septate, ~0.7–0.9 cm wide. Margin straight. Surface of outer valves with faint, oblique striations.
Remarks
The most common fruit morphotype in the Cerrejón flora (localities SW0318, FH0705, FH0708). Fruit is likely to be dehiscent because most recovered specimens seem to be impressions and compressions of only one valve.
Affinity
This is a very ‘generic’ fruit morphology lacking distinctive features, which is seen in many extant groups of legumes. However, the incomplete but distinctive organisation of the infructescence is very similar to the branched thyrsoid inflorescences and infructescences that characterise the subfamily Dialioideae (Legume Phylogeny Working Group 2017).
|
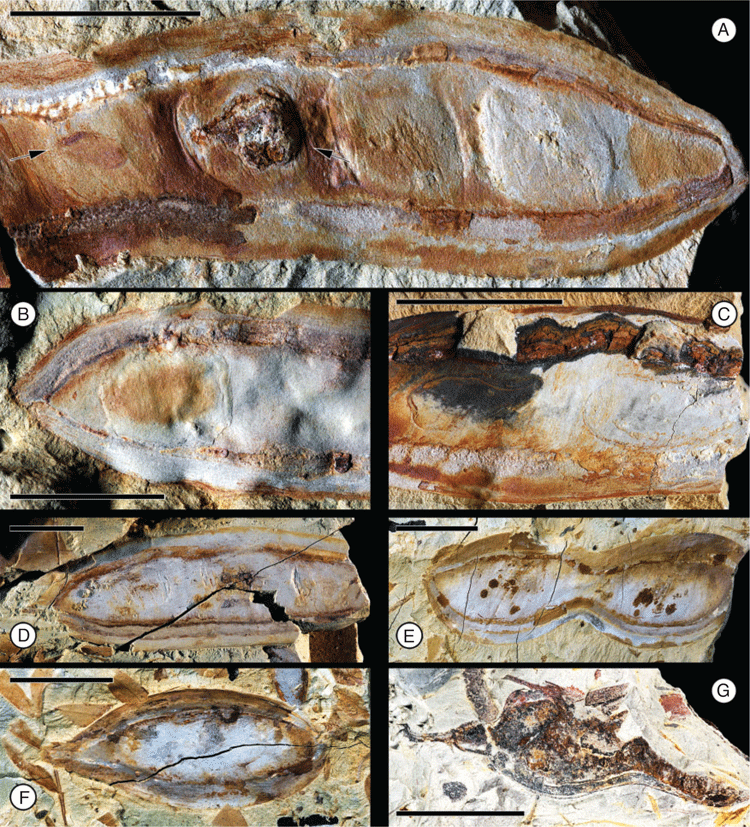
Fruit morphotype 7
(Fig. 9.)
Fruits nearly complete, ~3.8–4.1 cm long and 2.0–2.2 cm wide; strongly asymmetrical. Placental and non-placental margins very narrow and non-winged; approximately of equal thickness, ~1.0 mm wide. Pedicel short and stout, ~4.0–5.0 mm long, perpendicular to long axis of fruit. Base outline obtuse, rounded; apex not preserved. Number of seeds unclear. Seed chambers not evident. Margins straight. Fruit venation transverse to oblique, veins closely spaced, evenly distributed (~1–2 mm apart), more or less recurved at the margins.
Remarks
Three specimens have been recovered from the Cerrejón flora (locality SW0318).
Affinity
There is some similarity to some members of Detarioideae (e.g. Macrolobium, Peltogyne), but diagnostic features that would place it in a subfamily are lacking.
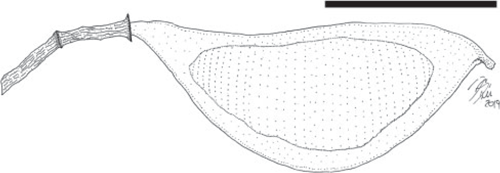
Fruit morphotype 8
Fruits small, ~10–18 mm long and 3–5 mm wide; strongly asymmetrical. Placental and non-placental margins narrow and non-winged; approximately of equal thickness, ~0.5 mm wide. Fruits stipitate, stipe ~0.4 mm long. Pedicel short, ~1.8–2.0 mm long. Base outline acute, tapered; apex obtuse to acute with a prominent persistent style base. Seeds 1(2?), oblique, transverse, to more or less parallel to the margin or longest axis of the fruit. Seed chambers not evident. Placental margin straight to slightly concave, non-placental margin curved, convex. Fruit venation straight and oblique, veins equally distant (~80–140 μm apart). Fruit surface covered with small, rounded resinous bodies (~65–96 μm in diameter).
Remarks
The second most common and smallest fruit morphotype from the Bogotá flora (locality FH0903). It co-occurs with Fruit morphotype 3. The fruit is likely to be indehiscent because the thick impressions seem to indicate complete closed valves.
Affinity
Detarioideae. Several genera of Detarioideae produce resin and have resin bodies present in the fruit walls (e.g. Hymenaea, Peltogyne, Guibourtia, Copaifera).
|
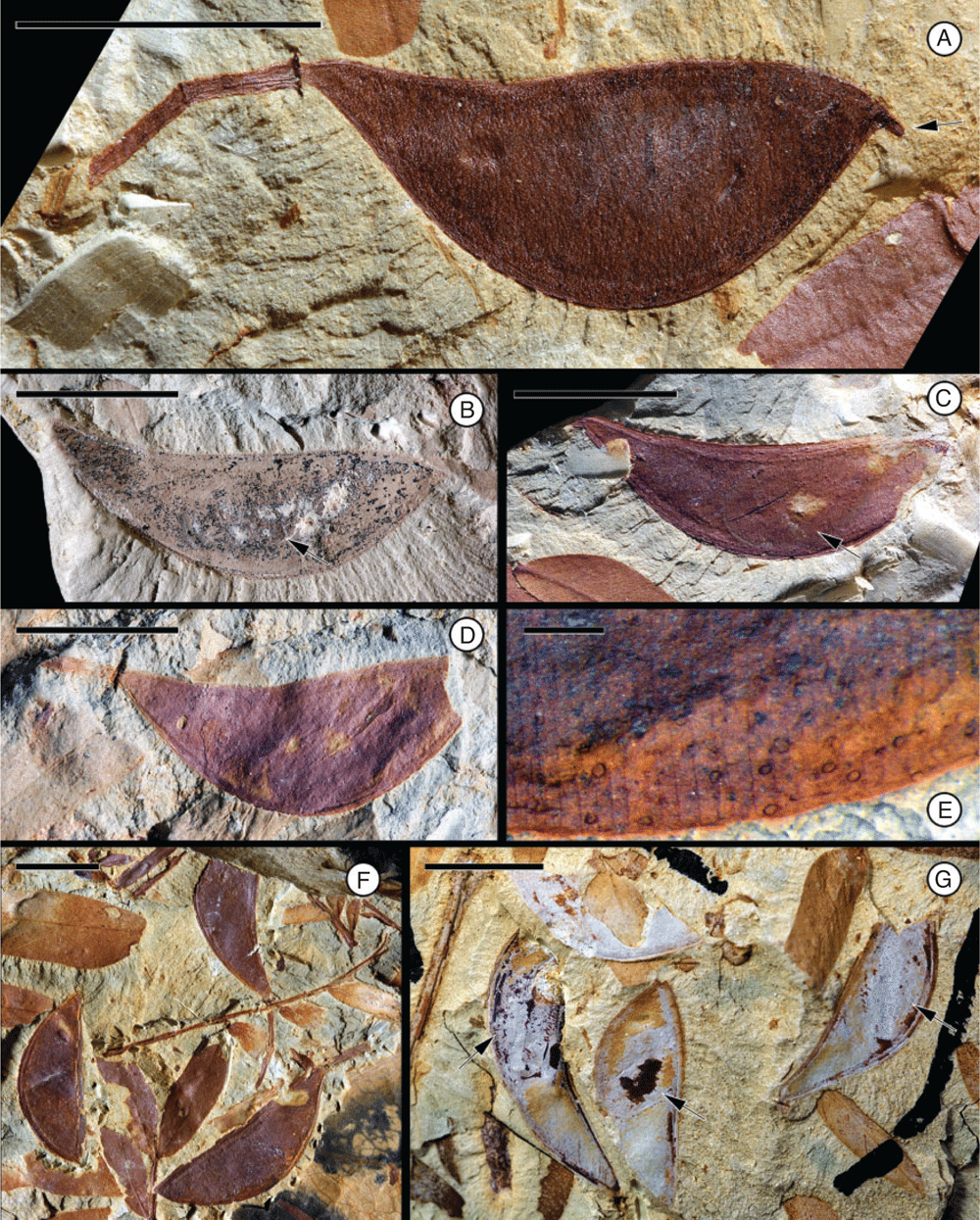
Leaf morphotype 1
(Fig. 12.)
Leaf bipinnate, incomplete; largest leaf fragment ~5.4 cm long and 3.5 cm wide; petiole base and pulvinus not preserved, apex even pinnate (ending with two opposite pinnae). Primary rachis incomplete, ~2.5 cm long. Pinnae opposite, at least 3 pairs; interval between pinnae is 1–1.3 cm. Pinnae ~3.5–3.9 cm long, rachis ~0.8 mm wide at base, first pair of leaflets ~1.4 mm from base, with well-developed basal pulvinus at base of pinna (~1 mm long), pulvinus with transverse striations. Pinnae bear at least 62 pairs of small leaflets; apex of pinnae ending with two opposite leaflets. Leaflets opposite, ~2.4–5.8 mm long and 0.8–1.7 mm wide (leptophyll to nanophyll); petiolule and pulvinus at base of leaflet very short, ~0.2–0.3 mm long; interval between leaflets is ~1.2–2.5 mm. Leaflet margin entire. Leaflet shape elliptical to ovate; apex acute, convex to rounded; base asymmetrical (slightly broader on the proximal side than on the distal side of the primary vein). Leaflet venation pinnate, primary vein ~0.1 mm thick; secondary venation not preserved. Extrafloral nectaries apparently absent.
Remarks
This fossil leaf morphotype is known from the Cerrejón flora (localities FH075, FH0708, FH0711). Most specimens are represented by isolated leaflets.
Affinity
Bipinnate leaves are restricted to the Caesalpinioideae (Legume Phylogeny Working Group 2017). They are found in several clades including the mimosoid clade, Dimorphandra group genera, the Umtiza clade (or grade), and the Caesalpinia group. Extrafloral nectaries are present on the leaves of most members of the mimosoid clade, whereas they appear to be lacking in the fossil. Similarities seem to be greatest with the Dimorphandra group genera with bipinnate leaves, but preservation of morphological details is not adequate to make detailed comparisons.
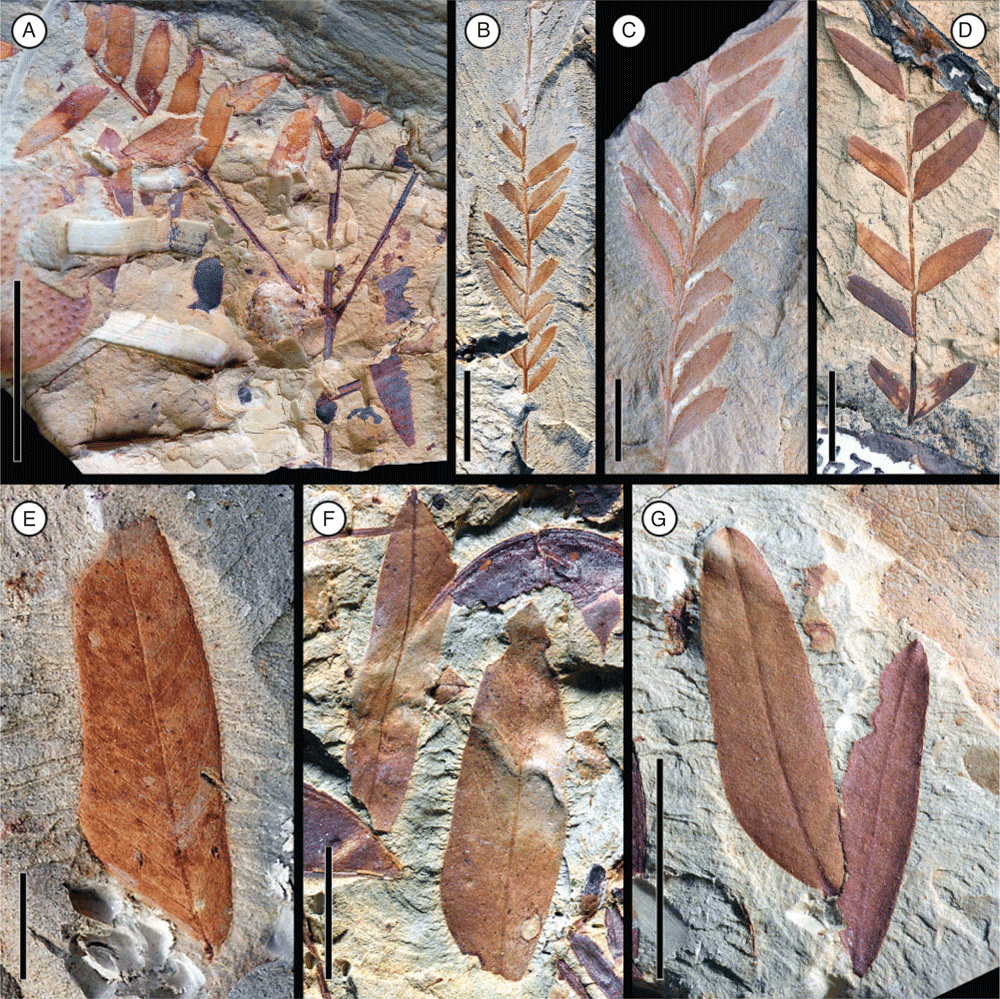
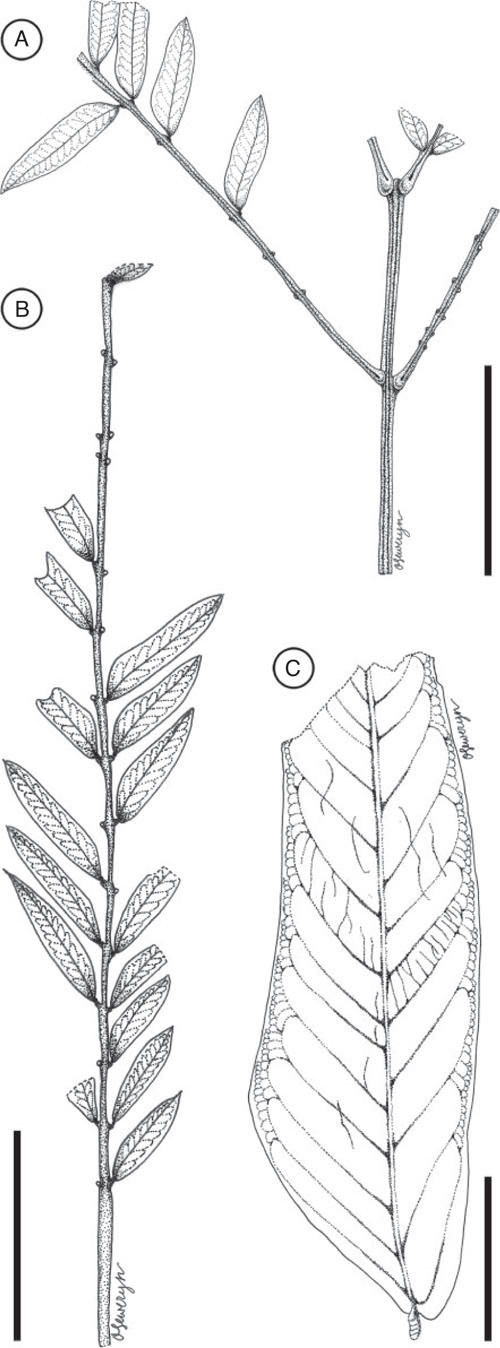
Leaf morphotype 2
Leaf bipinnate; largest leaf fragment ~2.5 cm long and 3.0 cm wide; basal pulvinus and petiole not preserved, apex even pinnate (ending with two opposite pinnae). Primary rachis incomplete, ~1.7 cm long. Pinnae opposite, at least 4 pairs; interval between pinnae is ~1.0 cm. Pinnae at least ~4.4 cm long, rachis ~0.8 mm wide at base, with well-developed basal pulvinus at base of pinna (~2 mm long), pulvinus with transverse striations. Pinnae bear at least 20 pairs of small leaflets; apex of pinnae ending with two opposite leaflets. Leaflets opposite, ~5.5–7.9 mm long and 1.0–1.6 mm wide (leptophyll to nanophyll); petiolule and pulvinus at base of leaflet very short, ca. 0.4 mm long; interval between leaflets is 2.6–3.5 mm. Leaflet margin entire. Leaflet shape oblong, elliptical to ovate; apex acute, convex to rounded with a mucronate tip; base asymmetrical (slightly broader on the proximal side than on the distal side of the primary vein). Leaflet venation pinnate, primary vein ~1.0 mm thick. Secondary venation eucamptodromous, 10–15 pairs of veins. Tertiary venation opposite percurrent. Extrafloral nectaries apparently absent.
Remarks
Most abundant leaf morphotype recovered in the Bogotá flora (locality FH0903). It co-occurs with Fruit morphotypes 3 and 8. Leaf morphotype 2 can be distinguished from Leaf morphotype 1 by the smaller number of leaflets per pinna (20 pairs v. 62), the more oblong and asymmetrical shape of leaflets, their larger size (up to 7.9 mm long v. 5.8 mm long), and the greater interval between leaflets (up to 3.5 mm v. 2.5 mm).
Affinity
Bipinnate leaves are restricted to the Caesalpinioideae (Legume Phylogeny Working Group 2017).
|
|
Leaf morphotype 3
(Fig. 15.)
Once-pinnately compound leaves, whether paripinnate or imparipinnate unknown (apex not preserved). Largest leaf fragment ~5 cm long and 5.3 cm wide. Apex, pulvinus and petiole not preserved. Rachis incomplete, ~4.6 cm long and 0.16 cm wide, with central groove and two parallel longitudinal ridges running close to the edge of the rachis on the adaxial surface. Leaflets opposite, ~1.3–5.4 cm long and 0.9–1.8 cm wide (microphyll to notophyll). Pulvinus at base of leaflet well developed, ca. 2.3–2.7 mm long and 0.8–1.1 mm wide. Leaflet base asymmetrical with lamina on inferred proximal side attached near base of petiolule, creating a nearly ‘sessile’ attachment. At least 6 pairs of leaflets; interval between leaflets is 0.6 to 1.3 cm. Leaflet margin entire. Leaflet shape oblong, elliptical to slightly ovate; apex convex, sometimes emarginate; base cordate and strongly asymmetrical (broader on the proximal side than on the distal side of the primary vein). Leaflet venation pinnate, primary vein ~0.8 mm thick. Secondary venation strongly brochidodromous, a few basal veins eucamptodromous; 13–16 pairs of secondary veins, with ~4–6 basal veins strongly crowded on the asymmetrical proximal side of the leaflet blade. Marginal secondary vein present. One to two inter-secondary veins present that run parallel to major secondaries. Tertiary and quaternary veins reticulate.
Remarks
This leaf morphotype is known from the Bogotá flora (localities FH0806, FH0808, FH0914). Specimens show diverse insect damage marks.
Affinity
The strongly unequal leaflet base, multiple eucamptodromous basal veins, and well developed secondary and tertiary venation are characteristic of many Detarioideae genera.
Leaf morphotype 4
(Fig. 16.)
Once-pinnately compound leaves, likely imparipinnate. Largest leaf fragment ~4.7 cm long. Petiole and basal pulvinus not preserved. Rachis incomplete, ~1.1. mm wide. Leaflets opposite, ~1.8–9.5 cm long and 1.0–4.6 cm wide (microphyll to mesophyll). Pulvinus at base of leaflet well developed, ca. 1.0 mm long and 1.6 mm wide. Leaflet base asymmetrical with lamina on inferred proximal side attached near base of petiolule, creating a nearly ‘sessile’ attachment. At least 4 pairs of leaflets; interval between leaflets is ~2.3–2.6 cm. Leaflet margin entire. Leaflet shape elliptical to slightly ovate; apex not well preserved; base asymmetrical, strongly unequal (broader on the proximal side than on the distal side of the primary vein). Leaflet venation pinnate, primary vein ~2 mm thick. Secondary venation eucamptodromous becoming brochidodromous distally; 8–12 pairs of secondary veins, with ~2 or 3 basal veins strongly crowded on the asymmetrical proximal side of the leaf blade. Marginal secondary vein absent. One to three inter-secondary veins present which run parallel to major secondaries. Tertiary and quaternary veins reticulate.
Remarks
This leaf morphotype is known from the Cerrejón flora (localities SW0317, FH0410). Specimens show diverse insect damage marks.
Affinity
Possibly Detarioideae. The strongly unequal leaflet base with two or three veins originating at the apex of the petiolule on the proximal side of the leaflet is characteristic of several genera in the Detarioideae.
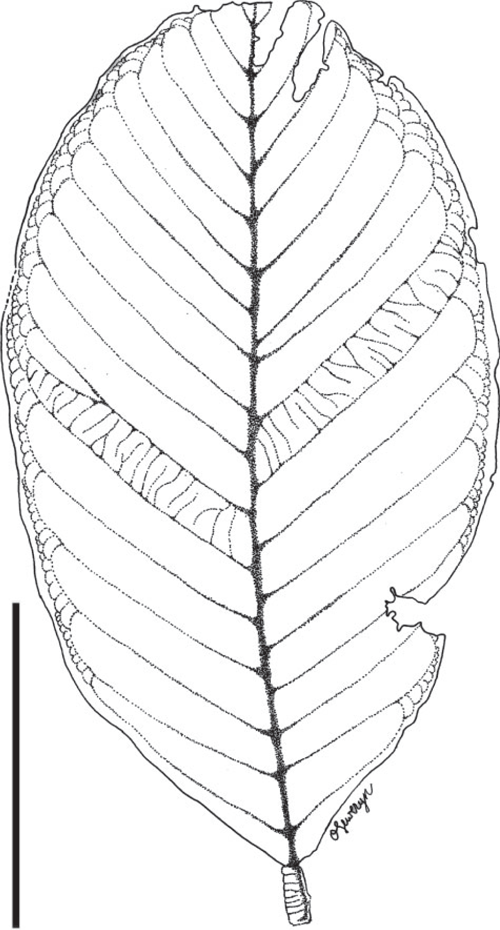
Leaf morphotype 5
(Fig. 17.)
Isolated leaflets, ~9.3–17 cm long and 3.0–5.4 cm wide (microphyll to mesophyll). Petiolule and pulvinus at base of leaflet well developed, ~5.1 mm long and 3.4 mm wide. Leaflet margin entire. Leaflet shape ovate to oblong; apex and base symmetrical, apex acute; base rounded to convex. Leaflet venation pinnate, primary vein ~3–5 mm thick. Secondary venation eucamptodromous becoming brochidodromous distally; 13–15 pairs of crowded secondary veins; secondaries strongly decurrent on mid-vein. Marginal secondary vein absent. One to two inter-secondary veins present, which run parallel to major secondaries. Tertiary and quaternary veins reticulate.
Remarks
This leaf type is known from the Cerrejón flora (localities SW0315, SW0318, FH0708).
Affinity
The leaflet morphology is very generalized within the family and can be observed in numerous extant legume genera. There are no distinctive features that would help narrow possible relationships.
Leaf morphotype 6
Mostly isolated leaflets; one specimen with a leaflet still attached to a fragment of a rachis. Fragment of rachis ~1.5 cm long and 6 mm wide. Leaflets ~13–28 cm long and 5–10 cm wide (notophyll to macrophyll). Pulvinus on petiolule well developed, strongly transversely striated, ~9.5–15 mm long and 4–8 mm wide. Leaflet margin entire. Leaflet shape elliptic, obovate to oblong; apex and base symmetrical, apex acuminate (with and without drip tip) to rounded; base obtuse to rounded. Leaflet venation pinnate, primary vein ~4–6 mm thick. Secondary venation eucamptodromous becoming brochidodromous distally; 19–22 pairs of secondary veins; secondaries excurrent on mid-vein; minor secondary veins simple brochidodromous, looping at low angles near the leaf margin. Marginal secondary vein absent. Inter-secondary veins absent. Tertiary veins percurrent, opposite straight to opposite convex. Quaternary veins alternate percurrent. Quinternary veins irregular reticulate. Areolation present.
Remarks
The most abundant leaf morphotype recovered in the Cerrejón flora (localities SW0315, SW0317, SW0319, SW0322, FH0410).
Affinity
Legume genera with large leaflets are known from the Dialioideae, Detarioideae, Caesalpinioideae, and Papilionoideae. The symmetrical, equal base is more frequently found in the latter two groups, whereas the leaflet base is typically asymmetrical in Detarioideae. Insufficient details are preserved to evaluate relationships more precisely.
|
|
Discussion
Comparison with other Paleocene fruits
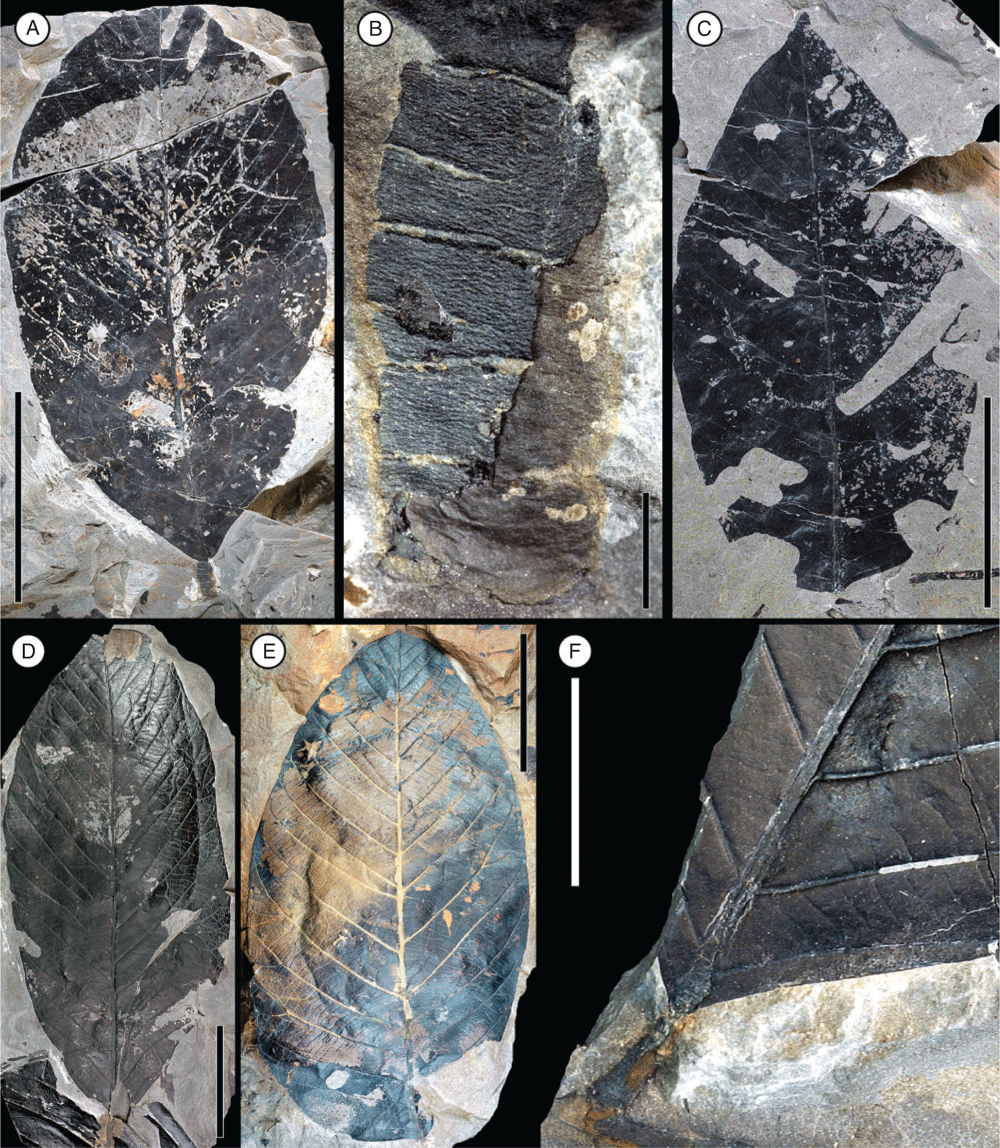
A few reliable legume fossils of Paleocene age have been reported from southern South America, North America, Europe and India (Fig. 20). Although some of these fossils show similarities with the Cerrejón and Bogotá fruits and leaves, those described here are distinct from all other Paleocene records.
From the Early Paleocene (~63–64 million years) of Patagonia, Argentina, a medium-size leaflet from the Salamanca Formation (Palacio de los Loros; Iglesias et al. 2007; Brea et al. 2008) slightly resembles three of the leaf morphotypes from Colombia, Morphotypes 3–5 (Fig. 15–17). Leaf morphotypes 3 and 4 differ from the Salamanca specimen because of their strongly asymmetrical bases, whereas Morphotype 5 has a large amount of inter-secondary veins that appear to be absent in the Patagonian fossil.
Four morphotypes of fossil leaflets and a similar number of fossil fruits of Leguminosae have been reported from Paleocene–Eocene Thermal Maximum (PETM) strata in Wyoming (~56 million years ago; Wing et al. 2005; Wing and Currano 2013). Among those leaflets are some that are similar to Leaf morphotypes 1 and 2 (Fig. 12–14), although no bipinnate leaves have been recovered from Wyoming. One Wyoming leaflet type of similar size has strongly eucamptodromous venation unlike Morphotypes 1 and 2 described here, and the other has larger (1.9–2.3 cm), more symmetrical leaflets. Other PETM fabaceous leaflet morphotypes from Wyoming have venation that is distinctly different from the larger leaflets described here. Fossil fruits and leaves from the Late Paleocene Willwood Formation in the Bighorn Basin of Wyoming are referable to the Papilionoideae (P. S. Herendeen and S. L. Wing, unpubl. data). The fruits are samaras with a narrow wing on the placental margin that are clearly distinct from the fruit morphotypes from Cerrejón and Bogotá floras. Likewise, the associated pinnately compound leaves are distinct from the leaf morphotypes described in the present paper.
Fruits of Leguminocarpon gardneri (Chandler) Herendeen & Crane from the Late Paleocene Reading Formation of England are to some extent similar to Fruit morphotypes 7 and 8 (Fig. 9–11; Herendeen and Crane 1992). Those fossils range from symmetrical to asymmetrical, ~8–22 mm long and 7–13 mm wide, are stipitate with a narrow wing along the placental margin, and the valve venation appears transverse and strongly reticulate (Herendeen and Crane 1992). However, fruits of Morphotype 7 are wingless, not stipitate, and the valve venation is not reticulate (Fig. 9). The pods of Fruit morphotype 8 are stipitate, but differ from the England fossils by the lack of a wing along the placental margin and the absence of reticulate venation (Fig. 10). Fruits of Morphotype 8 are also much smaller (~10–18 mm long and 3–5 mm wide) than those of L. gardneri.
Five species of Leguminocarpon were described from compression fossils of the Tura Formation in the Garo Hills, Meghalaya, India (Bhattacharyya 1985). The age of the Tura Formation was initially reported as Early Eocene (Bhattacharyya 1985; Tripathi et al. 2000), but it is now considered Late Paleocene on the basis of several palynological studies in the Garo Hills region (Saxena et al. 1996; Ambwani and Kar 2000; Mehrotra 2000; Agarwal 2008; Monga et al. 2014). From the Indian fossils, fruits of L. desmodioides and L. albizioides are more or less similar to Fruit morphotype 6 (Fig. 6–8), but they differ from the Colombian fossils because of the strongly segmented pods and apparent two series of alternate seeds respectively. Pods of L. derrisoides appear to be inconspicuously winged on the placental side, being, to some extent, similar to Fruit morphotypes 1 and 2 (Fig. 1), but the placental wing in the Colombian fossils is much wider and the fruits are also larger in size. Other potential legume fruits and wood have been reported from the Late Cretaceous to the Paleocene of the Deccan Intertrappean beds in India (e.g. Srivastava 2011); however, the legume affinity and age of those fossils remain poorly documented.

|
Implications for legume evolution
The Cerrejón and Bogotá floras preserve a remarkable assemblage of fruits and leaves of the legume family from the Middle to Late Paleocene of northern South America. The morphological variety of these fossil fruits and leaves is also a reflection of the floristic legume diversity seen in the earliest Neotropical rainforests of Colombia. Two morphotypes of bipinnate leaves (Morphotypes 1 and 2; Fig. 12–13) can be placed with confidence in the Caesalpinioideae subfamily. Fruit morphotype 6, with its branching thyrsoid inflorescence, is placed in the subfamily Dialioideae (Fig. 6–8), and the resinous Fruit morphotype 8 (Fig. 10–11) shows a strong similarity with several genera in the resin-producing clade of Detarioideae. Although all other Cerrejón and Bogotá fruits and leaves show clear and definitive legume characteristics, most of those fossils show morphological similarities with more than one subfamily (e.g. Fruit morphotypes 1, 2, 3 and 7; Leaf types 3, 4 and 6; Table 1) and cannot be placed confidently within any single group. Nevertheless, sufficient details are preserved to compare these fossil taxa with previously published fossils. All Cerrejón and Bogotá leaves and fruits can be clearly distinguished from other Paleocene records from southern South America, North America, Europe and India.
The Cerrejón and Bogotá floras represent the earliest lowland rainforests in the Neotropical region (Wing et al. 2009; Herrera et al. 2011) and the new abundant fossils described here also show that the Leguminosae was the most important component of this biome (Wing et al. 2009). Like extant lowland rainforests in the Neotropics, these fossil floras were also composed of Annonaceae, Araceae, Arecaceae, Lauraceae, Malvaceae, Menispermaceae, Ulmaceae, Zingiberales, and several unidentified ferns, among others (Jaramillo et al. 2007; Doria et al. 2008; Herrera et al. 2008, 2014; Gomez-Navarro et al. 2009; Wing et al. 2009; Carvalho et al. 2011). These forests also included families and genera that are characterised by lianas and woody climbers that have been extirpated from the Neotropical region, such as Icacinaceae and Stephania in Menispermaceae (Herrera et al. 2011; Stull et al. 2012). The Cerrejón and Bogotá fossils contribute to our understanding of the extraordinary diversification of Leguminosae during the Middle to Late Paleocene tropics of South America. Contrary to most molecular-dating analyses that suggest a Cretaceous age for the family (e.g. Wikström et al. 2004; Lavin et al. 2005; Bruneau et al. 2008; Bell et al. 2010; Magallón et al. 2015), no reliable legume fossils have been discovered prior to the Paleocene.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this work was provided by National Science Foundation grants EAR-1829299 (to F. Herrera, M. R. Carvalho and C. Jaramillo), DEB-1748286 (to P. S. Herendeen and F. Herrera), the Oak Spring Garden Foundation (to F. Herrera), STRI-Tupper Postdoctoral Fellowship (to M. R. Carvalho), and STRI, the Anders Foundation, 1923 Fund and Gregory D. and Jennifer Walston Johnson (to C. Jaramillo).
Acknowledgements
The authors thank the editors of the Advances in Legume Systematics Vol. 13, C. E. Hughes, A. Egan, T. Kajita and D. Murphy; G. Lewis for comments on the affinity of the fossils; L. Paganucci de Queiroz and two additional anonymous reviewers for providing constructive comments on the manuscript; Cerrejón S. A. and the Ochoa Brothers for permission to work in their mines; E. Cadena, L. Oviedo, J. Moreno and A. Rincon for assistance with fieldwork in Colombia; O. Seweryn and D. Sanín for the drawings in Fig. 14, 19 and Fig. 4, 8, 11 respectively; F. Herrera thanks B. Himschoot for constant support.
References
Agarwal A (2008) Angiospermous fossil fruits/seeds during Tertiary in India. Palaeobotanist 57, 165–175.Ambwani K, Kar RK (2000) Occurrence of Anonidium-like pollen in the Tura formation (Palaeocene) of Meghalaya, India. Palaeobotanist 49, 219–223.
Bayona G, Cortes M, Jaramillo C, Ojeda G, Aristizabal JJ, Reyes-Harker A (2008) An integrated analysis of an orogeny–sedimentary basin pair: latest Cretaceous–Cenozoic evolution of the linked eastern Cordillera orogen and the Llanos foreland basin of Colombia. Geological Society of America Bulletin 120, 1171–1197.
| An integrated analysis of an orogeny–sedimentary basin pair: latest Cretaceous–Cenozoic evolution of the linked eastern Cordillera orogen and the Llanos foreland basin of Colombia.Crossref | GoogleScholarGoogle Scholar |
Bayona G, Montenegro O, Cardona A, Jaramillo CA, Lamus F, Morón S, Quiroz L, Ruíz MC, Valencia V, Parra M, Stockli D (2010) Estratigrafía, procedencia, subsidencia y exhumación de las unidades Paleógenas en el Sinclinal de Usme, sur de la zona axial de la Cordillera Oriental. Geología Colombiana 35, 5–35.
Bell CD, Soltis DE, Soltis PS (2010) The age and diversification of the angiosperms re‐revisited. American Journal of Botany 97, 1296–1303.
| The age and diversification of the angiosperms re‐revisited.Crossref | GoogleScholarGoogle Scholar | 21616882PubMed |
Bhattacharyya B (1985) Leguminous fruits from the Eocene of Garo Hills, Meghalaya. Quarterly Journal of the Geological, Mining and Metallurgical Society of India 57, 215–225.
Brea M, Zamuner AB, Matheos SD, Iglesias A, Zucol AF (2008) Fossil wood of the Mimosoideae from the early Paleocene of Patagonia, Argentina. Alcheringa 32, 427–441.
| Fossil wood of the Mimosoideae from the early Paleocene of Patagonia, Argentina.Crossref | GoogleScholarGoogle Scholar |
Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008) Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86, 697–718.
| Phylogenetic patterns and diversification in the caesalpinioid legumes.Crossref | GoogleScholarGoogle Scholar |
Cantrill DJ, Bamford MK, Wagstaff BE, Sauquet H (2013) Early Eocene fossil plants from the Mwadui kimberlite pipe, Tanzania. Review of Palaeobotany and Palynology 196, 19–35.
| Early Eocene fossil plants from the Mwadui kimberlite pipe, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Carvalho MR, Herrera F, Jaramillo CA, Wing SL, Callejas R (2011) Paleocene Malvaceae from northern South America and their biogeographical implications. American Journal of Botany 98, 1337–1355.
| Paleocene Malvaceae from northern South America and their biogeographical implications.Crossref | GoogleScholarGoogle Scholar | 21821594PubMed |
Collinson ME, Manchester SR, Wilde V (2012) Fossil fruits and seeds of the middle Eocene Messel biota, Germany. Abhandlungen Senckenberg Gesellschaft für Naturforschung 570, 1–251.
Crepet WL, Herendeen PS (1992) Papilionoid flowers from the Early Eocene of southwestern North America. In ‘Advances in Legume Systematics: Part 4, the Fossil Record’. (Eds PS Herendeen, DL Dilcher) pp. 45–55. (Royal Botanic Gardens, Kew: London, UK)
Crepet WL, Taylor DW (1985) The diversification of the Leguminosae: first fossil evidence of the Mimosoideae and Papilionoideae. Science 228, 1087–1089.
| The diversification of the Leguminosae: first fossil evidence of the Mimosoideae and Papilionoideae.Crossref | GoogleScholarGoogle Scholar | 17737903PubMed |
Crepet WL, Taylor DW (1986) Primitive mimosoid flowers from the Paleocene–Eocene and their systematic and evolutionary implications. American Journal of Botany 73, 548–563.
| Primitive mimosoid flowers from the Paleocene–Eocene and their systematic and evolutionary implications.Crossref | GoogleScholarGoogle Scholar |
Dilcher DL, Lott TA, Gibson MA, Dudley C (2014) An extinct caesalpinoid flower from the Eocene of western Tennessee. In ‘Paleobotany and Biogeography, A Festschrift for Alan Graham in His 80th Year’. (Eds WD Stevens, OM Montiel, PH Raven) pp. 51–63. (Missouri Botanical Garden Press: Saint Louis, MO, USA)
Doria G, Jaramillo CA, Herrera F (2008) Menispermaceae from the Cerrejón Formation, middle to late Paleocene, Colombia. American Journal of Botany 95, 954–973.
| Menispermaceae from the Cerrejón Formation, middle to late Paleocene, Colombia.Crossref | GoogleScholarGoogle Scholar | 21632418PubMed |
Doyle JJ, Luckow MA (2003) The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiology 131, 900–910.
| The rest of the iceberg. Legume diversity and evolution in a phylogenetic context.Crossref | GoogleScholarGoogle Scholar | 12644643PubMed |
Ellis B, Daly DC, Hickey LJ, Johnson KR, Mitchell JD, Wilf P, Wing SL (2009) ‘Manual of Leaf Architecture.’ (Cornell University Press: Ithaca, NY, USA)
Epihov DZ, Batterman SA, Hedin LO, Leake JR, Smith LM, Beerling DJ (2017) N2-fixing tropical legume evolution: a contributor to enhanced weathering through the Cenozoic? Proceedings of the Royal Society of London – B. Biological Sciences 284, 20170370
| N2-fixing tropical legume evolution: a contributor to enhanced weathering through the Cenozoic?Crossref | GoogleScholarGoogle Scholar |
Gomez-Navarro C, Jaramillo CA, Herrera F, Wing SL, Callejas R (2009) Palms (Arecaceae) from a Paleocene rainforest of northern Colombia. American Journal of Botany 96, 1300–1312.
| Palms (Arecaceae) from a Paleocene rainforest of northern Colombia.Crossref | GoogleScholarGoogle Scholar | 21628279PubMed |
Gunn CR (1984) Fruits and seeds of genera in the subfamily Mimosoideae (Fabaceae). USDA Technical Bulletin 1681, Beltsville, MD, USA.
Gunn CR (1991) Fruits and seeds of genera in the subfamily Caesalpinioideae (Fabaceae). USDA Technical Bulletin 1755, Beltsville, MD, USA.
Herendeen PS (1992) The fossil history of the Leguminosae from the Eocene of southeastern North America. In ‘Advances in Legume Systematics: Part 4, the Fossil Record’. (Eds PS Herendeen, DL Dilcher) pp. 85–160. (Royal Botanic Gardens, Kew: London, UK)
Herendeen PS, Crane PR (1992) Early caesalpinioid fruits from the Paleogene of southern England. In ‘Advances in Legume Systematics: Part 4, the Fossil Record’. (Eds PS Herendeen, DL Dilcher) pp. 57–68. (Royal Botanic Gardens, Kew: London, UK)
Herendeen PS, Dilcher DL (1990) Fossil mimosoid legumes from the Eocene and Oligocene of southeastern North America. Review of Palaeobotany and Palynology 62, 339–361.
| Fossil mimosoid legumes from the Eocene and Oligocene of southeastern North America.Crossref | GoogleScholarGoogle Scholar |
Herendeen PS, Jacobs BF (2000) Fossil legumes from the middle Eocene (46.0 Ma) Mahenge flora of Singida, Tanzania. American Journal of Botany 87, 1358–1366.
| Fossil legumes from the middle Eocene (46.0 Ma) Mahenge flora of Singida, Tanzania.Crossref | GoogleScholarGoogle Scholar | 10991905PubMed |
Herendeen PS, Crepet WL, Dilcher DL (1992) The fossil history of the Leguminosae: phylogenetic and biogeographic implications. In ‘Advances in Legume Systematics: Part 4, the Fossil Record’. (Eds PS Herendeen, DL Dilcher) pp. 303–316. (Royal Botanic Gardens, Kew: London, UK)
Herrera F, Jaramillo CA, Dilcher DL, Wing SL, Gómez-Navarro C (2008) Fossil Araceae from a Paleocene neotropical rainforest in Colombia. American Journal of Botany 95, 1569–1583.
| Fossil Araceae from a Paleocene neotropical rainforest in Colombia.Crossref | GoogleScholarGoogle Scholar | 21628164PubMed |
Herrera F, Manchester SR, Hoot SB, Wefferling K, Carvalho MR, Jaramillo CA (2011) Phytogeographic Implications of fossil endocarps of Menispermaceae from the Paleocene of Colombia. American Journal of Botany 98, 2004–2017.
| Phytogeographic Implications of fossil endocarps of Menispermaceae from the Paleocene of Colombia.Crossref | GoogleScholarGoogle Scholar | 22114219PubMed |
Herrera F, Manchester SR, Carvalho MR, Jaramillo CA, Wing SL (2014) Fossil wind-dispersed fruits and seeds from the Paleocene of Colombia and their implications for early Neotropical rainforests. Acta Palaeobotanica 54, 197–229.
| Fossil wind-dispersed fruits and seeds from the Paleocene of Colombia and their implications for early Neotropical rainforests.Crossref | GoogleScholarGoogle Scholar |
Iglesias A, Wilf P, Johnson KR, Zamuner AB, Cúneo NR, Matheos SD, Singer BS (2007) A Paleocene lowland macroflora from Patagonia reveals significantly greater richness than North American analogs. Geology 35, 947–950.
| A Paleocene lowland macroflora from Patagonia reveals significantly greater richness than North American analogs.Crossref | GoogleScholarGoogle Scholar |
Jacobs BF, Herendeen PS (2004) Eocene dry climate and woodland vegetation in tropical Africa reconstructed from fossil leaves from northern Tanzania. Palaeogeography, Palaeoclimatology, Palaeoecology 213, 115–123.
| Eocene dry climate and woodland vegetation in tropical Africa reconstructed from fossil leaves from northern Tanzania.Crossref | GoogleScholarGoogle Scholar |
Jaramillo CA, Bayona G, Pardo-Trujillo A, Rueda M, Torres V, Harrington GJ, Mora G (2007) The palynology of the Cerrejón Formation (upper Paleocene) of northern Colombia. Palynology 31, 153–189.
Jaramillo CA, Rueda M, Torres V (2011) A palynological zonation for the Cenozoic of the Llanos and Llanos Foothills of Colombia. Palynology 35, 46–84.
| A palynological zonation for the Cenozoic of the Llanos and Llanos Foothills of Colombia.Crossref | GoogleScholarGoogle Scholar |
Jia H, Manchester SR (2014) Fossil leaves and fruits of Cercis L.(Leguminosae) from the Eocene of western North America. International Journal of Plant Sciences 175, 601–612.
| Fossil leaves and fruits of Cercis L.(Leguminosae) from the Eocene of western North America.Crossref | GoogleScholarGoogle Scholar |
Kirkbride JH, Gunn CR, Weitzman AL (2003) Fruits and seeds of genera in the subfamily Faboideae (Leguminosae). USDA Technical Bulletin 1890 Vol. 1, 2, Beltsville, MD, USA.
Lavin M, Herendeen PS, Wojciechowski MF (2005) Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Systematic Biology 54, 575–594.
| Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary.Crossref | GoogleScholarGoogle Scholar | 16085576PubMed |
Legume Phylogeny Working Group (2013a) Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 62, 217–248.
| Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades.Crossref | GoogleScholarGoogle Scholar |
Legume Phylogeny Working Group (2013b) Towards a new classification system for legumes: progress report from the 6th international legume conference. South African Journal of Botany 89, 3–9.
| Towards a new classification system for legumes: progress report from the 6th international legume conference.Crossref | GoogleScholarGoogle Scholar |
Legume Phylogeny Working Group (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66, 44–77.
| A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny.Crossref | GoogleScholarGoogle Scholar |
Lewis G, Schrire B, Mackinder B, Lock M (2005) ‘Legumes of the World.’ (Royal Botanic Gardens, Kew: London, UK)
Magallón S, Gómez‐Acevedo S, Sánchez‐Reyes LL, Hernández‐Hernández T (2015) A metacalibrated time‐tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist 207, 437–453.
| A metacalibrated time‐tree documents the early rise of flowering plant phylogenetic diversity.Crossref | GoogleScholarGoogle Scholar | 25615647PubMed |
Mehrotra RC (2000) Study of plant megafossils from the Tura Formation of Nangwalbibra, Garo Hills, Meghalaya, India. Palaeobotanist 49, 225–237.
Monga PR, Srivastava GA, Kumar M, Mehrotra RC (2014) Further palynological investigation of coaliferous sequences of Tura Formation of Nangwalbibra, East Garo Hills, Meghalaya: inferences on palaeovegetation and palaeoclimate. Palaeobotanist 63, 79–85.
Morón S, Fox DL, Feinberg JM, Jaramillo C, Bayona G, Montes C, Bloch JI (2013) Climate change during the Early Paleogene in the Bogotá Basin (Colombia) inferred from paleosol carbon isotope stratigraphy, major oxides, and environmental magnetism. Palaeogeography, Palaeoclimatology, Palaeoecology 388, 115–127.
| Climate change during the Early Paleogene in the Bogotá Basin (Colombia) inferred from paleosol carbon isotope stratigraphy, major oxides, and environmental magnetism.Crossref | GoogleScholarGoogle Scholar |
Phillips O, James M, Gentry A (2002) ‘Global Patterns of Plant Diversity: Alwyn H. Gentry’s Forest Transect Data Set.’ (Missouri Botanical Garden Press: Saint Louis, MO, USA)
Saxena RK, Tripathi SKM, Prasad V (1996) Palynofloral investigation of the Tura Formation (Palaeocene) in Nongwal Bibra area, East Garo Hills, Meghalaya. Geophytology 26, 19–31.
Schrire BD, Lavin M, Lewis GP (2005a) Global distribution patterns of the Leguminosae: insights from recent phylogenies. Biologiske Skrifter 55, 375–422.
Schrire BD, Lewis GP, Lavin M (2005b) Biogeography of the Leguminosae. In ‘Legumes of the World’. (Eds G Lewis, B Schrire, B Mackinder, M Lock) pp. 21–54. (Royal Botanic Gardens, Kew: London, UK)
Scotese CR (2001) ‘Paleogeographic Atlas. Earth System History Geographic Information System, Version 02b.’ (PALEOMAP Project: Arlington, TX, USA)
Senesse S, Gruas-Cavagnetto C (1990) Caesalpinieaepollenites (Caesalpinioideae, Légumineuse), une nouvelle forme de genre dans l’Eocène inférieur du Bassin de Paris. Position systématique et phylogénétique. Review of Palaeobotany and Palynology 66, 13–24.
| Caesalpinieaepollenites (Caesalpinioideae, Légumineuse), une nouvelle forme de genre dans l’Eocène inférieur du Bassin de Paris. Position systématique et phylogénétique.Crossref | GoogleScholarGoogle Scholar |
Shukla A, Mehrotra RC (2016) Early Eocene (~50 My) legume fruits from Rajasthan. Current Science 111, 465–467.
Srivastava RA (2011) Indian upper Cretaceous–Tertiary flora before collision of Indian Plate: a reappraisal of central and western Indian flora. Memoir of the Geological Society of India 77, 281–292.
Stull GW, Herrera F, Manchester SR, Jaramillo C, Tiffney BH (2012) Fruits of an ‘Old World’ tribe (Phytocreneae; Icacinaceae) from the Paleogene of North and South America. Systematic Botany 37, 784–794.
| Fruits of an ‘Old World’ tribe (Phytocreneae; Icacinaceae) from the Paleogene of North and South America.Crossref | GoogleScholarGoogle Scholar |
Tripathi SKM, Saxena RK, Prasad V (2000) Palynological investigation of the Tura Formation (Early Eocene) exposed along the Tura–Dalu road, West Garo Hills, Meghalaya, India. Palaeobotanist 49, 239–251.
Wikström N, Savolainen V, Chase MW (2004) Angiosperm divergence times: congruence and incongruence between fossils and sequence divergence estimates. Systematics Association Special Volume 66, 142–165.
Wilf P, Cúneo NR, Johnson KR, Hicks JF, Wing SL, Obradovich JD (2003) High plant diversity in Eocene South America: evidence from Patagonia. Science 300, 122–125.
| High plant diversity in Eocene South America: evidence from Patagonia.Crossref | GoogleScholarGoogle Scholar | 12677065PubMed |
Wing SL, Currano ED (2013) Plant response to a global greenhouse event 56 million years ago. American Journal of Botany 100, 1234–1254.
| Plant response to a global greenhouse event 56 million years ago.Crossref | GoogleScholarGoogle Scholar | 23825133PubMed |
Wing SL, Harrington GJ, Smith FA, Bloch JI, Boyer DM, Freeman KH (2005) Transient floral change and rapid global warming at the Paleocene–Eocene boundary. Science 310, 993–996.
| Transient floral change and rapid global warming at the Paleocene–Eocene boundary.Crossref | GoogleScholarGoogle Scholar | 16284173PubMed |
Wing SL, Herrera F, Jaramillo CA, Gómez-Navarro C, Wilf P, Labandeira CC (2009) Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proceedings of the National Academy of Sciences of the United States of America 106, 18627–18632.
| Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest.Crossref | GoogleScholarGoogle Scholar | 19833876PubMed |
Xu Q, Qiu J, Zhou Z, Jin J (2015) Eocene Podocarpium (Leguminosae) from South China and its biogeographic implications. Frontiers of Plant Science 6, 938
| Eocene Podocarpium (Leguminosae) from South China and its biogeographic implications.Crossref | GoogleScholarGoogle Scholar |
Yahara T, Javadi F, Onoda Y, de Queiroz LP, Faith D, Prado DE, Akasaka M, Kadoya T, Ishihama F, Davies S, Slik JWF, Yi T, Ma K, Bin C, Darnaedi D, Pennington RT, Tuda M, Shimada M, Ito M, Egan AN, Buerki S, Raes N, Kajita T, Vatanparast M, Mimura M, Tachida H, Iwasa Y, Smith GF, Victor JE, Nkonki T (2013) Global legume diversity assessment: concepts, key indicators, and strategies. Taxon 62, 249–266.
| Global legume diversity assessment: concepts, key indicators, and strategies.Crossref | GoogleScholarGoogle Scholar |