One new endemic plant species on average per month in New Caledonia, including eight more new species from Île Art (Belep Islands), a major micro-hotspot in need of protection
Gildas Gâteblé A G , Laure Barrabé
A G , Laure Barrabé  B , Gordon McPherson C , Jérôme Munzinger
B , Gordon McPherson C , Jérôme Munzinger  D , Neil Snow E and Ulf Swenson
D , Neil Snow E and Ulf Swenson  F
F
A Institut Agronomique Néo-Calédonien, Equipe ARBOREAL, BP 711, 98810 Mont-Dore, New Caledonia.
B Endemia, Plant Red List Authority, 7 rue Pierre Artigue, Portes de Fer, 98800 Nouméa, New Caledonia.
C Herbarium, Missouri Botanical Garden, 4344 Shaw Boulevard, Saint Louis, MO 63110, USA.
D AMAP, IRD, CIRAD, CNRS, INRA, Université Montpellier, F-34000 Montpellier, France.
E T.M. Sperry Herbarium, Department of Biology, Pittsburg State University, Pittsburg, KS 66762, USA.
F Department of Botany, Swedish Museum of Natural History, PO Box 50007, SE-104 05 Stockholm, Sweden.
G Corresponding author. Email: gateble@iac.nc
Australian Systematic Botany 31(6) 448-480 https://doi.org/10.1071/SB18016
Submitted: 30 March 2018 Accepted: 10 September 2018 Published: 12 December 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
The New Caledonian biodiversity hotspot contains many micro-hotspots that exhibit high plant micro-endemism, and that are facing different types and intensities of threats. The Belep archipelago, and especially Île Art, with 24 and 21 respective narrowly endemic species (1 Extinct, 21 Critically Endangered and 2 Endangered), should be considered as the most sensitive micro-hotspot of plant diversity in New Caledonia because of the high anthropogenic threat of fire. Nano-hotspots could also be defined for the low forest remnants of the southern and northern plateaus of Île Art. With an average rate of more than one new species described for New Caledonia each month since January 2000 and five new endemics for the Belep archipelago since 2009, the state of knowledge of the flora is steadily improving. The present account of eight new species from Île Art (Bocquillonia montrouzieri Gâteblé & McPherson, Cleidion artense Gâteblé & McPherson, Endiandra artensis Munzinger & McPherson, Eugenia belepiana J.W.Dawson ex N.Snow, Eugenia insulartensis J.W.Dawson ex N.Snow, Macaranga latebrosa Gâteblé & McPherson, Planchonella serpentinicola Swenson & Munzinger and Psychotria neodouarrei Barrabé & A.Martini) further demonstrates the need both to recognise the Belep Islands as a major New Caledonian micro-hotspot and to formulate concrete conservation programs for the archipelago.
Introduction
Since the concept of biodiversity hotspots was first proposed (Myers 1988; Myers et al. 2000), the recognition of the more recent concept of hotspots within hotspots sensu Cañadas et al. (2014) has become crucial for effective conservation. Fenu et al. (2010) proposed recognition of micro- and nano-hotspots for small regions with high levels of endemism and exemplified this with biodiversity-rich areas in Sardinia (Italy), one of 335 km2 (micro-hotspot), and others less than 3 km2 (nano-hotspots). This idea may challenge older concepts such as ‘key biodiversity areas’ sensu Eken et al. (2004), but we believe that small oceanic islands rich in biodiversity are easy to recognise as micro- or nano-hotspots.
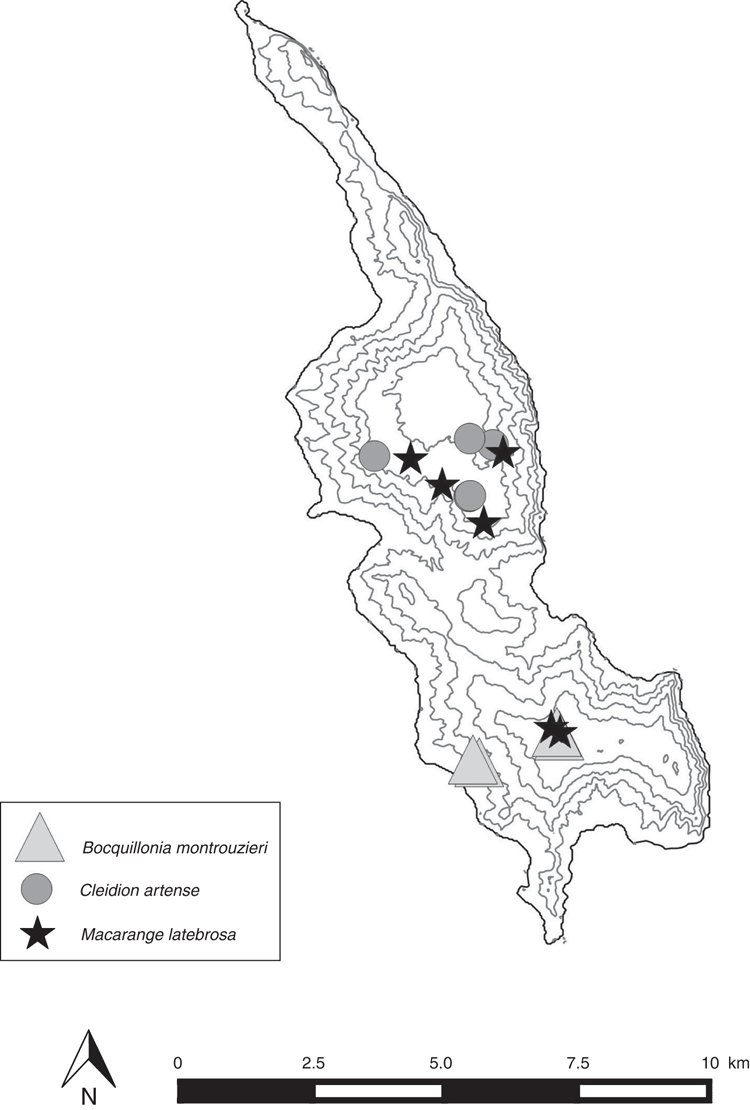
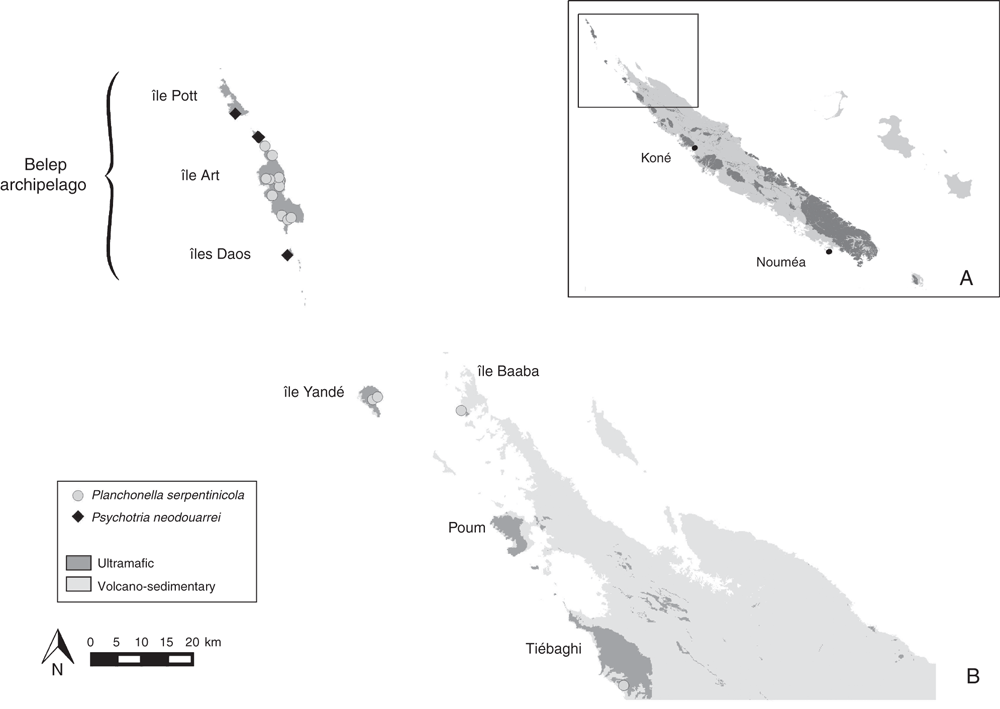
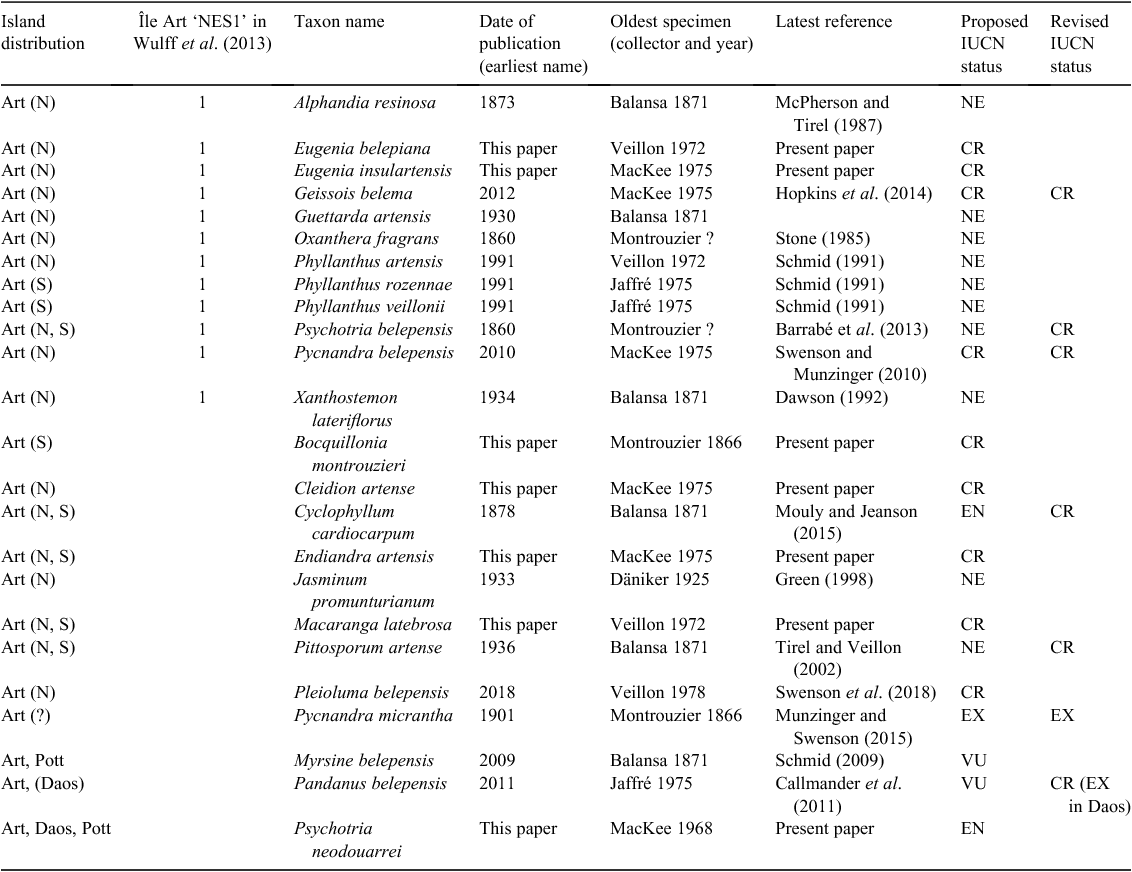
Within the New Caledonian biodiversity hotspot, the Belep archipelago (Fig. 1) is a striking example combining high endemism and high threat level that could meet the definitions of micro- or nano-hotspots of Fenu et al. (2010). The Belep archipelago, ~35 km north-west of the main island of Grande Terre (Fig. 1), is formed by Île Art (51.08 km2), Île Pott (11.69 km2) just north of Île Art, and some other small islands in the south (Dau Âc and Daos), totaling 1.96 km2 (Gouvernement de la Nouvelle-Calédonie 2018). Île Art has two low elevation plateaus, plateau Nord (~200–280 m high) and plateau Sud (~200–250 m high) and is the only permanently inhabited island. These quite isolated ultramafic islands are (or were) covered by typical low-elevation maquis (a shrubby sclerophyll vegetation) and by one of the most endangered ecosystems in New Caledonia, i.e. low-elevation rainforest on ultramafic substrate (Isnard et al. 2016). According to Wulff et al. (2013), Île Art is the fourth-most diverse area in New Caledonia (equaling the Dogny–Rembai–Table Unio–Amieu area) in terms of narrow endemic species richness (NES1) with 12 micro-endemic species (Table 1). Under the New Caledonian Mining Code, 18 mining concessions in Île Art and Île Pott are presently assigned to mining companies, and these mining concessions represent 72% (45.16 km2 of 62.77 km2) of the total surface (Gouvernement de la Nouvelle-Calédonie 2009, 2018). However, there is no active nickel mining at the moment because of a memorandum of understanding between the mining company Société Le Nickel and the (local) tribal custom authorities. The Belep Islands also are currently free of a major threat to the mainland flora, the rusa deer (Rusa timorensis), which does not seem to have been introduced (Endemia Red List Authority 2017). Presently, anthropogenic fires are the main threat to the biodiversity in this archipelago. Deliberately set fires are of great concern because they occur regularly between August and January (OEIL 2018) and are usually difficult to fight because of a limited fire brigade and lack of roads in this quite rugged terrain.
|
|
New Caledonian biodiversity is facing major threats that led to its early recognition as one of the 10 original world biodiversity hotspots (Myers 1988); however, unfortunately, these threats have evolved and increased since then. Although the understanding and quantification of the main threats have improved greatly, the need to better assess and undertake concrete and urgent actions to dramatically lower these threats remains. For instance, the preliminary results of the local IUCN Red List Authority, established in 2014 to evaluate the risk of extinction of New Caledonian species, are particularly of concern. Of the 1040 plant species assessed thus far (30.7% of the total New Caledonian native flora), 42% are threatened across all categories, of which 100 are listed as Critically Endangered (CR), 183 as Endangered (EN) and 153 as Vulnerable (VU; Endemia Red List Authority 2017). The main threats associated with these 436 species are anthropogenic fires (62% of the species potentially being affected), nickel mining activities (42%) and invasive species (41%), especially the exotic rusa deer.
The purpose of this paper is to describe eight new species from the Belep Islands and provide IUCN Red List assessments. We also draw attention to the Belep Islands as a micro-hotspot area within the New Caledonian hotspot, and provide a current account of our knowledge of the New Caledonian flora and especially in the Belep archipelago. We emphasise how rich but vulnerable the biodiversity can be in micro- or nano-hotspots.
Materials and methods
Species count
A list of putative endemics from the Belep archipelago was compiled using the series Flore de Nouvelle-Calédonie, various taxonomic papers, unpublished data and personal communications. The herbarium specimens of each putative Belep endemic were reviewed to crosscheck that each is a true micro-endemic and were mapped according to their label data (Appendix 1). To provide a current overview of the New Caledonian flora, a second robust list of new species described from New Caledonia since 2000 was compiled (Appendix 2), primarily using the International Plant Names Index (see http://www.ipni.org, accessed September 2018) and complemented by bibliographic data gathered by the authors during the past 18 years. The new species awaiting description are listed as ‘ined.’ and were extracted from Munzinger et al. (2016). Various publications were examined to check species names and entities. We have excluded doubtful species (seven), hybrids (four), taxa of lower taxonomic rank (subspecies and varieties) as well as nomenclatural novelties (comb. nov. and nom. nov.).
Taxonomy
For the descriptions of the eight new species, careful examination and measurements of morphological characters were taken from fresh material as well as herbarium specimens, and character terminology follows that of previous authors, for example, McPherson and Tirel (1987) for Euphorbiaceae, Kostermans (1974) and Munzinger and McPherson (2016) for Lauraceae, Snow et al. (2016a, 2016b) for Eugenia L. (Myrtaceae), Barrabé (2014) and Barrabé et al. (2014) for Psychotria L. (Rubiaceae), Munzinger and Swenson (2009) and Swenson et al. (2007) for Planchonella Pierre (Sapotaceae). The species concept used here is based on morphological characters, usually in combination with molecular phylogenetic analyses, as previously used by the authors. Voucher specimens are deposited primarily in BRI, G, MO, MPU, NOU, P and S (abbreviations follow Index Herbariorum, see http://sweetgum.nybg.org/science/ih/, accessed 4 June 2018). Numbers given after herbaria abbreviations are barcode numbers, except for MO and S where they represent accession numbers.
Conservation assessment
The IUCN Standards and Petitions Subcommittee (2017) bases threat analyses on a variety of criteria and subcriteria involving population size and its reduction (Criteria A, C and D), population geographic range, including fragmentation and decline (Criterion B), decline or fragmentation of small to very small populations (Criteria C, D), and quantitative analyses of the probability of extinction (E). Geographic range is measured as extent of occurrence (EOO) and area of occupancy (AOO), where EOO is the minimum convex polygon containing all points of occurrence and AOO is the area estimated by superimposing a grid (2 × 2 km) onto occurrence points and calculating the cumulative area of the cells occupied by the species. Herbarium specimens were geo-referenced (Appendix 1), as much as possible, according to label information (locality, coordinates, elevation and type of vegetation). For our assessments, we used the data gathered in Appendix 1 to calculate EOO and AOO with the online ‘GeoCAT’ software (ver. 9.1, S. Bachman and J. Moat, Botanic Gardens Conservation International, see http://geocat.kew.org, accessed September 2018; Bachman et al. 2011). Criterion B was also applied by characterising the nature and level of the threats.
Systematic progress, endemic species and threats
New Caledonia
Some studies suggest that New Caledonia may be the region with the world’s highest vascular plant endemism density, with an estimated 1350 species per 10 000 km2 (Kier et al. 2009). A calculation based on the checklist of the indigenous vascular flora of New Caledonia (Morat et al. 2012; Munzinger et al. 2016) leads to a similar value of 1341 endemic species per 10 000 km2. With a complete identification key of the Flora known at that time made by Guillaumin (1948), and an ongoing Flore de Nouvelle-Calédonie (et Dépendances) that started in 1967, one might consider that the flora is fairly well known. However, we believe that a significant amount of work still needs to be conducted on the basis of both existing herbarium specimens and on discoveries made in the field (see, for example, the series Novitates neocaledonicae, Munzinger 2015). Supporting this idea are recently published studies estimating that 9% of New Caledonian plant and 12% of New Caledonian animal species have very narrow distributions, i.e. plant species being restricted to one locality (Wulff et al. 2013) and where the total distribution area is less than 5.2 km2 for animals (Caesar et al. 2017).
Systematic studies since the year 2000 have shown many novelties for New Caledonia (Appendix 2). Between January 2000 and December 2017, no fewer than four endemic genera (McPherson and Lowry 2004; Snow 2009; Schmid 2012; Hopkins et al. 2015) and 217 new endemic plant species have been described across 38 families and 65 genera (Table 2). This is equivalent to an average description rate of slightly more than one new endemic species per month. According to Munzinger et al. (2016), this rate is likely to be maintained in the near future, given that 71 new species were then listed as ‘ined.’ and are now at various stages of being described. These data bear on the question of how many additional new plant species are to be expected within the New Caledonian biodiversity hotspot and partly address the ‘impossible prediction’ formulated by Joppa et al. (2011) who were unable to predict the percentage of the New Caledonian flora remaining to be described. Earlier, this question was also considered by Morat (1993), who tentatively concluded that 5–10% of the vascular plant flora remained to be described. Significantly, in light of the study cited above (Wulff et al. 2013), 40% of the new species described since 2000 have very narrow distributions (data not shown), a trend that we predict will be even more pronounced in the future (e.g. Meve et al. 2018, for Île des Pins) and one that clearly applies to the Belep archipelago, for which 12 micro-endemic (5 between 2009 and 2018 and 7 in the present paper) species have been described during the past 10 years (Table 1).

|
Belep archipelago
The French naturalist and priest Jean Xavier Hyacinthe Montrouzier (1820–1897) was the first to document the flora of Belep in his Flore de l’Île Art (Montrouzier 1860). Later, the Frenchman Gaspard Joseph Benedict Balansa (1825–1891) and the Swiss Albrecht Ulrich Däniker (1894–1957) visited and collected specimens in 1871 and 1925 respectively. Thereafter, the Belep flora was neglected until the 1960s and 1970s when botanists such as Jean-Pierre Blanchon, Dominique Bourret (1940–present), Pierre Cabalion (1947–present), Tanguy Jaffré (1942–present), Hugh Shaw MacKee (1912–1995), Philippe Morat (1937–present), Christiane Tirel (1939–present) and Jean-Marie Veillon (1939–present) visited the islands. A few more collections have been made in the 21st Century by four of the authors (L. Barrabé, G. Gâteblé, J. Munzinger and U. Swenson) and by Jean-Pierre Butin (DDEE) and Antony Pain (IAC). The flora of Île Art is the most thoroughly collected, whereas only some hundred specimens have been collected on Île Pott. Specimens from the smaller islands in the south are even fewer, partly because of inaccessibility and partly because of their degraded vegetation.
From the data presented here, the number of species restricted to the Belep archipelago is 17, and we herein add another seven Belep endemic new species (Table 1). Putative Île Art endemics linked to old dubious names that are most probably synonyms or taxa published in Montrouzier (1860), Beauvisage (1894; 1901) and so on, such as Acrostichum forsteri Montrouz., Canavalia bouquete Montrouz., Gardenia artensis Montrouz. and Korthalsella amentacea (Tiegh.) Engl., were considered but rejected. Codia belepensis H.C.Hopkins is excluded because it also occurs in Île Yandé (Poum). Oxanthera (Citrus) fragrans Montrouz. and Guettarda artensis Guillaumin may not be endemic to the Belep Islands, but according to Stone (1985) for the former and the determination of specimens in the Paris herbarium for the latter, these two are here considered endemic to Île Art. Myrsine belepensis (M.Schmid) Ricketson & Pipoly and Pandanus belepensis Callm. & Munzinger also are known from Pott and Daos islands respectively, but the latter is considered extinct on Daos (Table 1). The present account raises the number of strictly Belep endemic species from 17 to 24 (Table 1) and the number of Île Art micro-endemics from 15 (16 with Pandanus belepensis extinct on Daos and now restricted to Île Art) to 21 (22 with Pandanus belepensis). Even more significantly, 12 Île Art micro-endemics are restricted to the northern part of the island and three to the southern plateau, whereas five occur on both parts of the island (Table 1). Half of Belep endemic species have been described since 2009, but all are known from collections that date back at least to the 1970s (Table 1).
Our addition of seven micro- or narrowly endemic species increases the density of micro-endemic species per square kilometre for the Belep archipelago, and for Île Art in particular, from 0.26 to 0.37 and from 0.29 (0.31) to 0.41 (0.43) respectively. Compared with the data presented by Wulff et al. (2013), Île Art now can be considered the richest area of narrow endemism in New Caledonia, ahead of Mount Panié with its 16 narrowly endemic species (NES1). By itself, the northern plateau of Île Art hosts 12 very narrowly endemic species and could easily qualify as a nano-hotspot. However, it is possible that some Île Art species here considered as micro-endemics are indeed present on the other plateaus, or even on Pott or Daos, as future fieldwork may show. Moreover, we predict that the number of species endemic to the Belep archipelago will increase as future field trips, phylogenetic analyses, population-genetics studies and revisions are conducted, for example, in Alyxia Banks ex R.Br., Arthropodium R.Br., Gynochthodes Blume, Ochrosia Juss., Meryta J.R.Forst. & G.Forst., Pittosporum Banks ex Gaertn., Stigmaphyllon A.Juss. and Vitex L.
Threats to the Belep Islands flora
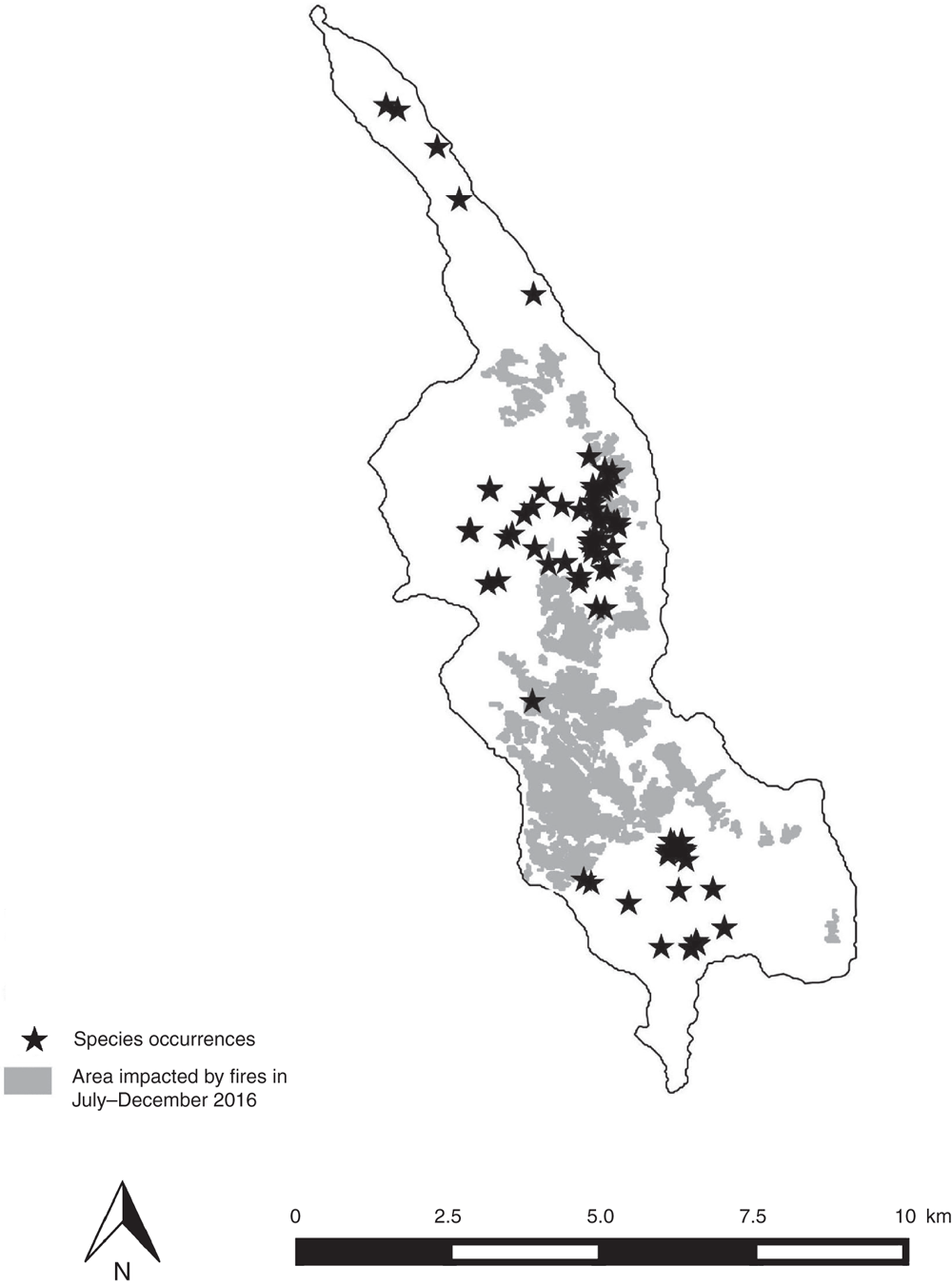
The most serious threat to the flora in the Belep archipelago, especially in Île Art, is deliberately set fire. For instance, during July–December 2016, more than 1000 ha (20% of the entire surface) of Île Art was burnt, and much of the maquis and remnant forest of this area succumbed to the flames (Fig. 2). Île Art is indeed recognised as one of the worst places in New Caledonia for the number and intensity of fires detected by satellites (see http://geoportail.oeil.nc/AlerteIncendies, accessed September 2018). According to the study of Curt et al. (2015) on Grande Terre, the total area of the ultramafic maquis could be burnt within a 34-year period. Gomez et al. (2015, their fig. 3) also considered the potential biodiversity loss as high in the central parts of the southern and northern plateaus of Art and Pott islands.
|
Nickel mining is also a potential threat to the Belep flora, because three mining concessions (three more being expired) are recorded on Île Pott and no less than 15 (two more being expired) on Île Art (Gouvernement de la Nouvelle-Calédonie 2018). Presently, a status quo exists between the local tribal custom authorities and nickel mining companies, and no mining is expected to occur in the near future. Other potential threats to the biodiversity include introduced deer and pigs, feral cattle and horses, exotic and invasive plants, and land clearings for agriculture and infrastructures, but these problems must be ranked as minor in the Belep archipelago.
Past, present and future threats to the biodiversity in Belep archipelago have led to the Red Listing of seven micro-endemic species by the local Red List Authority (Endemia Red List Authority 2017), six as Critically Endangered (CR), and one as Extinct (EX, Table 1). Another nine Belep endemics have not yet been assessed against the IUCN criteria by the local Red List Authority (Table 1), but on the basis of the previous evaluations, eight are likely to be Critically Endangered (CR) and one (Myrsine belepensis) Endangered (EN). The seven new Île Art endemic species (six published in the present paper and one in Swenson et al. 2018) also are all assessed as being CR. Once all endemic species of Île Art have been assessed by the local Red List Authority, 21 CR and one EX species, in total, are expected to be listed. Île Art would then be the place with the highest number of Critically Endangered species compared with other areas in New Caledonia. Given its exceptional concentration of micro-endemism and the number of CR and EX taxa, the Belep archipelago, and Île Art in particular, deserves recognition as the hottest plant micro-hotspot within the New Caledonian biodiversity hotspot. The southern and northern plateaus of Île Art could further be considered each as a nano-hotspot because their total areas above 200 m are just 3.45 and 7.22 km2 respectively. Indeed, 88% of occurrences and 20 of the 21 Île Art’s CR endemic species would be protected if a reserve were established to include all the area above 200 m (data calculated from Appendix 1, Fig. 2–4). So as to fit exactly to the definition by Fenu et al. (2010) of a nano-hotspot of less than 3 km2, it would require being above 209 m for the southern plateau and 247 m for the northern plateau, but this would not be adequate to protect the rainforest remnants of both plateaus.
Eight new species
Euphorbiaceae
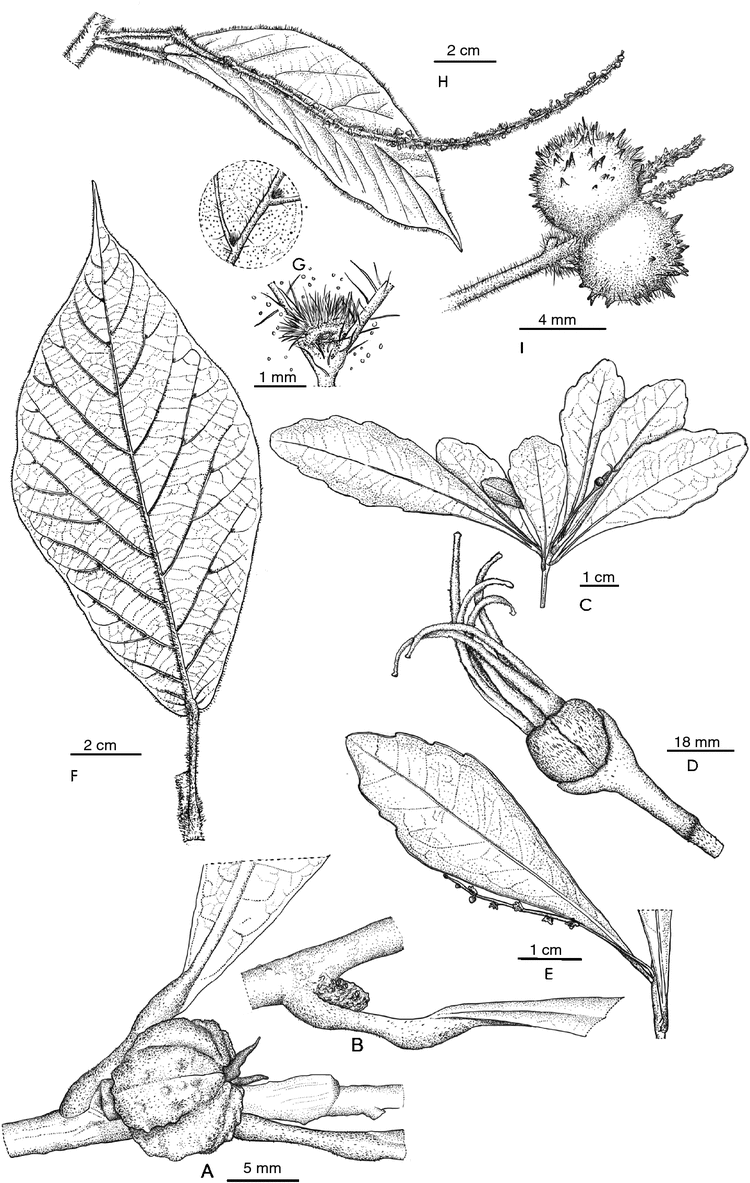
Bocquillonia montrouzieri Gâteblé & McPherson, sp. nov.
Diagnosis: Bocquillonia montrouzieri resembles B. brachypoda Baill. in having small leaves with short petioles and condensed ♂ inflorescences, but differs most notably from the latter species in having caducous stipules 1.5–2.0 mm long (v. persistent, 1.5–4.0 mm long), petioles typically with a prominent distal pulvinus (v. without apparent distal pulvinus), one ♀ flower per pistillate inflorescence (v. several), and ovaries and fruits that bear many short, spine-like appendages or crests (v. ovaries and fruits smooth).
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Sud, 24.viii.1978, J.-M.Veillon 3685 [♀] (holo: P 00160297!; iso: NOU 046131!).
Shrubs dioceous 50 cm to 2.5 m tall, usually well branched, young stems sparsely appressed-puberulent, quickly glabrescent. Leaf simple, blade narrowly elliptic to oblanceolate, 5.0–11.5 × 1.5–3.0 cm, base narrowly obtuse to cuneate, apex acuminate, margin dentate, teeth (3–)6–12 on each side, chartaceous to subcoriaceous, adaxial surface glabrous, abaxial surface sparsely and obscurely appressed-puberulent to glabrous, with many minute, circular gland-like inclusions often visible under the microscope, and with 0–2 larger sunken laminar glands on each side of the midvein, ~0.2 mm in diameter, secondary veins ~11–16 on each side of the midrib below the acumen, not or barely raised adaxially, prominent abaxially, tertiary veins not or barely raised on both surfaces. Petiole 4–12 mm long, ~1 mm in diameter, prominently pulvinate at both ends, flat or shallowly channeled adaxially, sparsely appressed-puberulent or glabrous. Stipules subulate, 1.5–2.0 mm long, caducous. Staminate inflorescence reduced to axillary sessile glomerules 1–3 mm in diameter and up to 5 mm long; bracts minute; staminate flower subsessile when immature (mature flowers unknown). Pistillate inflorescence reduced to axillary, solitary flowers; bracts ~1 mm long, pubescent, glands not evident; pistillate flowers sessile on stout, bracteate bases up to 2 mm long; sepals 1.0–1.5 mm long; ovary spherical, ~2 mm in diameter, verruculose, pubescent; stylodia 2–3 mm long, red. Fruit capsule 3-lobed, ~10 mm in diameter, ~8 mm high, bearing many short, spine-like appendages or crests, pubescent; column 5–6 mm long; seeds 5–6 × 4 mm, covered with low, blister-like swellings.
Recognition
The reduced inflorescences of Bocquillonia montrouzieri, glomerulate in staminate and one-flowered in pistillate specimens, recall those of B. grandidens Baill., B. sessiliflora Baill., B. castaneifolia Guillaumin and B. brachypoda Baill. However, the first three have much longer and wider leaves than those of the new species, which do not surpass 11.5 × 3.0 cm, as well as smooth fruit. The similarly small-leaved B. montrouzieri can be most easily distinguished from B. brachypoda by its petioles, which lack a distal pulvinus, as well as by its persistent, longer stipules (1.5–4.0 mm in B. brachypoda v. caducous and 1.5–2.0 mm in B. montrouzieri) and smooth (v. ornamented) fruit. Of these four somewhat similar species, only B. castaneifolia is known to occur on Île Art.
Distribution
Bocquillonia montrouzieri is known only from the southern plateau of Île Art in the remnants of rainforests between 80 and 230 m. This new species is locally common in the forest surrounding the antenna located at the summit, but was not seen at lower elevations during fieldwork in 2017.
Etymology
The species is named after its first collector, the Marist missionary and naturalist Xavier Montrouzier, who wrote an early account of the Île Art flora (Montrouzier 1860) and who spent the last years of his life and is buried in the tribal area of Saint-Louis, Mont-Dore, where both authors have also lived and worked.
Conservation status
Bocquillonia montrouzieri has been found in only one locality, namely, in the rainforest remnants of the southern plateau, which represents a single location (sensu IUCN) with respect to the main threat. It is highly threatened by recurrent anthropogenic fires that progressively reduce the surface of the remaining patches of forests, resulting in an observed and projected decline in EOO, AOO, habitat quality and number of mature individuals. The calculated EOO being smaller than the AOO, the AOO value of 8 km2 also applies to the EOO. On the basis of IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, B. montrouzieri is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v)+2ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. s.loc., Montrouzier 342, (MPU 312123); Île Art, partie Sud, 80 m, C.Tirel 1277 (P 00160305); Île Art, forêt de Païromé en bordure de marais, sommet du plateau, D.Bourret 1910 (NOU 046110); Île Art, plateau Sud, forêt autour de l’antenne, 19°44′59.19″S, 163°40′40.30″E, 230 m, G.Gâteblé 904 (NOU, P).

|
Cleidion artense Gâteblé & McPherson, sp. nov.
Diagnosis: Cleidion artense resembles C. verticillatum Baill. and C. marginatum McPherson in that all three species have subverticillate, small (less than 10 × 4 cm) leaves with acute bases and pistillate inflorescences typically reduced to a solitary flower. However, C. artense has longer staminate inflorescences (2.0–5.5 v. 0.1–0.8 cm), staminate flowers with fewer stamens (~30–35 v. 50–60) and longer pistillate inflorescences (1.5–2.0 v. 0.5–1.5 cm) than does C. verticillatum, and it has wider leaves (1.0–3.5 v. 0.8–2.2 cm) with attenuate bases (v. cuneate) and fewer marginal teeth (2–5 v. 5–9 per side) than does C. marginatum, as well as lacking the broad whitish band typical of C. marginatum, having shorter staminate inflorescences (up to 5.5 v. up to 10 cm) and longer pistillate inflorescences (1.5–2.0 v. up to ~0.6 cm) .
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Nord, 10.xii.1975, T.Jaffré 1657 [♂] (holo: P 00066651!; iso: NOU 024370!, P 00066652!).
Shrubs apparently dioecious (only 5 collections known, all unisexual) up to 1.5 m tall, well branched; young stems drying brown, pubescent at first, eventually glabrescent. Leaf simple, loosely clustered near the ends of twigs, blade obovate, 2.5–8.5 × 1.0–3.5 cm, base attenuate, apex shortly acuminate or rounded, margin dentate in distal part, the teeth 2–5 on each side; subcoriaceous, adaxial surface glabrous, abaxial surface thinly pubescent when immature, glabrescent, laminar glands 0–4 in the basal half, subcircular to narrowly elliptic, up to 1.0 × 0.5 mm, secondary veins 6–8 on each side of the midvein, essentially flush adaxially, slightly raised abaxially as are the higher-order veins. Petiole 2.0–3.5 mm long, ~1 mm in diameter, shallowly channeled, pubescent at first then glabrescent. Stipules ovate to subulate, 3 mm long, pubescent, deciduous. Staminate inflorescence spiciform, 2.0–5.5 cm long, the axis ~0.8 mm in diameter, pubescent, bearing 5–11 glomerules of flowers; bracts 1.5 mm long, pubescent, pedicels 1–2 mm long, pubescent; calyx in bud typically glabrous to slightly pubescent except for a tuft of hairs at the apex and around the base, splitting into 4 lobes 1.5 mm long; stamens ~30–35. Pistillate inflorescence reduced to a single axillary flower, the axes totalling 1.5–2.0 cm long, consisting of the peduncle ~1.3–1.7 cm long and the pedicel 2.0–2.5 mm long; peduncle sparsely pubescent; pedicel almost glabrous, articulation between peduncle and pedicel densely pubescent; bracts not seen; sepals ~1 mm long; ovary spherical, ~2.5 mm in diameter, sparsely pubescent, hairs ~0.1 mm long; 4–6 stylodia, 4–5 mm long, densely pubescent abaxially. Fruit unknown.
Recognition
Cleidion artense resembles C. verticillatum Baill. and C. marginatum McPherson in that all three species have subverticillate, small (less than 10 × 4 cm) leaves with acute bases, and pistillate inflorescences typically reduced to a solitary flower. However, C. artense has longer staminate inflorescences (2.0–5.5 v. 0.1–0.8 cm), staminate flowers with fewer stamens (~30–35 v. 50–60), and longer pistillate inflorescences (1.5–2.0 v. 0.5–1.5 cm) than does C. verticillatum, and it has wider leaves (1.0–3.5 v. 0.8–2.2 cm) with attenuate bases (v. cuneate) and fewer marginal teeth (2–5 v. 5–9 per side) than does C. marginatum, and lacking the broad whitish band typical of C. marginatum, as well as shorter staminate inflorescences (up to 5.5 v. up to 10 cm) and longer pistillate inflorescences (1.5–2.0 v. up to ~0.6 cm). Cleidion vieillardii Baill., which has much longer leaves and inflorescences, also occurs on Île Art.
Distribution
Cleidion artense is known only from the northern plateau of Île Art in the remnants of rainforests and dense shrubby maquis between 200 and 250 m. The new species is not common at this locality.
Etymology
This species is named after Île Art, where it is a newly recognised micro-endemic.
Conservation status
Cleidion artense has been found in only one locality, in the rainforest and shrubby maquis remnants of the northern plateau, which represents a single location (sensu IUCN) with respect to the main threat. The main threats to it are the frequent anthropogenic fires in the area that progressively reduce the surface of the remaining patches of forests resulting in an observed and projected decline in EOO, AOO, habitat quality and number of mature individuals. The calculated EOO being smaller than the AOO, the AOO value of 8 km2 also applies to the EOO. From IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, C. artense is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v)+2ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau Nord (rebord Est), 250 m, H.S.MacKee 30383 (MO 3603593, P 00166146); Île Art, plateau Nord, 200 m, H.S.MacKee 30523 (NOU 024369, P00166147); Île Art, plateau Nord, J.-P.Butin 255 (NOU 084489); Île Art, sentier du plateau au dessus de chez Donatienne, 19°41′58.91″S, 163°38′41.26″E, 200 m, G.Gâteblé, E.Bourguet & G.Templier 885 (NOU, P).
Macaranga latebrosa Gâteblé & McPherson, sp. nov.
Diagnosis: among New Caledonian members of the genus, Macaranga latebrosa is most similar to M. corymbosa (Müll.Arg.) Müll.Arg., with which it shares basally obtuse to cordate and domatia-bearing leaves as well as fruits with soft, spine-like appendages, but from which it can be most easily distinguished by its long-pubescent stems (v. puberulence appressed-scurfy) and its longer pistillate inflorescences (5.5–15.0 v. 1–3 cm).
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Sud, forêt autour de l’antenne, 19°44′55.68″S, 163°40′36.30″E, 230 m, 26.iv.2017, G.Gâteblé 901 [♀, ♂] (holo: P!; iso: NOU!).
Shrubs dioecious or monoecious, the individual inflorescences unisexual, 1.0–2.5 m tall, multicaulous, with reddish sap; young stems abundantly pubescent with hairs of two lengths, the shorter ones erect, straight, ~0.5 mm long, the longer ones erect, straight or somewhat bent, 1.5–2.2 mm long. Leaf simple, blade elliptic to ovate or oblanceolate, 7.0–18.5 × 2.5–8.5 cm, base narrowly obtuse to narrowly cordate; apex acuminate, the acumen typically 1.0–1.5 cm long, margin entire to somewhat irregularly and coarsely dentate, chartaceous, adaxial surface evenly and openly pubescent with long hairs, abaxial surface similarly but more densely pubescent, abundantly granulose-glandular the midvein and secondary veins also bearing some short hairs, secondary veins (6–)10–15 on each side of the midvein, barely raised adaxially, prominent abaxially and many of them abmedially forked 1–4 times, tertiary veins arranged in a scalariform pattern, raised abaxially, proximal embedded laminar glands occasionally present, 0–4 pairs, often more apparent adaxially than abaxially, up to 0.5 mm long, domatia often present in the axils of the midrib and distal secondary veins as well as in the axils at the abmedial forkings of the secondaries, each domatium formed by a triangular to rounded flap of tissue and densely pubescent (medium hair length) within. Petiole 1.0–3.7 cm long, 0.8–1.8 mm in diameter, nearly terete, pubescent like the stem. Stipules ovate, acute, 1.0–1.5 mm long, pubescent, caducous. Staminate inflorescence spiciform or often with a pair of branches arising at the lowest node, 8–17 cm long, the axis ~0.5 mm in diameter, pubescent like the stem; peduncle up to ~0–3 cm long; bracts ~0.3 mm long; staminate flowers subsessile; pedicel ~0.7 mm long, densely pubescent; sepals 3, ~1 mm long; stamens 5–6, ~1 mm long, shorter than the sepals. Pistillate inflorescences 5.5–15.0 cm long, the axis 0.5–1.0 mm in diameter, often flattened distally, 1–3(–4)-flowered, pubescent like the stem; pedicel 2–7 mm long; bracts 1.0–4.0 × 0.3 mm; calyx at first apparently suburceolate (only one young flower seen), with 5(?) acute lobes, soon splitting into 5 narrow sepal-like segments ~2 mm long, acute, pubescent; ovary densely long-pubescent and granulose-glandular; stylodia 6–13 mm long. Fruit bilobed, 7–9 mm wide, 5–6 mm high, ~3 mm thick, pubescent with both long and short hairs, densely granulose-glandular, bearing many soft spines ~1 mm long; column 3 mm high, 2.5–3.0 mm wide distally; seeds spherical, 4.0–4.5 mm in diameter, smooth, black.
Recognition
With its narrowly obtuse to narrowly cordate leaf bases, domatia on the abaxial leaf surface, (sub)spiciform staminate inflorescences, and long pistillate inflorescences (5.5–15.0 cm) producing softly spiny fruits, Macaranga latebrosa stands apart from its congeners in New Caledonia. Macaranga vedeliana (Baill.) Müll.Arg. also occurs on Île Art, in coastal forests and also, atypically, on the ultramafic plateau. The two species are very easily distinguished from each other even with sterile material and were not observed growing in sympatry.
Distribution
Macaranga latebrosa is known only from Île Art on both plateaus (southern and northern) within or at the edges of the remnants of the higher-elevation (200–230 m) rainforests. It is not common on the southern plateau.
Etymology
The species is named after its very distinctive acarodomatia (H. Jourdan, pers. comm.), ‘latebrosa’ meaning full of hiding places.
Conservation status
This new micro-endemic species occurs in the narrow ecological ecosystem of higher-elevation rainforests of the island. Like most of this island’s endemic species, M. latebrosa is highly threatened by anthropogenic bushfires that can simultaneously affect the remnant rainforests on both plateaus and that progressively reduce the surface of the remaining patches of forests, resulting in an observed and projected decline in EOO, AOO, habitat quality and the number of mature individuals. Both plateaus represent a single location (sensu IUCN) with respect to the fire threat. The calculated EOO being smaller than the AOO, the AOO value of 12 km2 also applies to the EOO. According to IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, Macaranga latebrosa is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau au nord du terrain d’aviation, D.Bourret 1885 (NOU 025124); Île Art, plateau Nord (rebord Est), 220 m, H.S.MacKee 30459 (MO 3603856, NOU 025109, P 00172334); Île Art, plateau Nord, 200 m, H.S.MacKee 30525 (P 00172335); Île Art, plateau Sud, antenne, 19°44′59″S, 163°40′42″E, J.Munzinger, U.Swenson & L.Barrabé 5737 (NOU 051015); Île Art, plateau au dessus de Wala, ~230 m, J.-M.Veillon 2708 (MO 6053173, NOU 025112).
Lauraceae
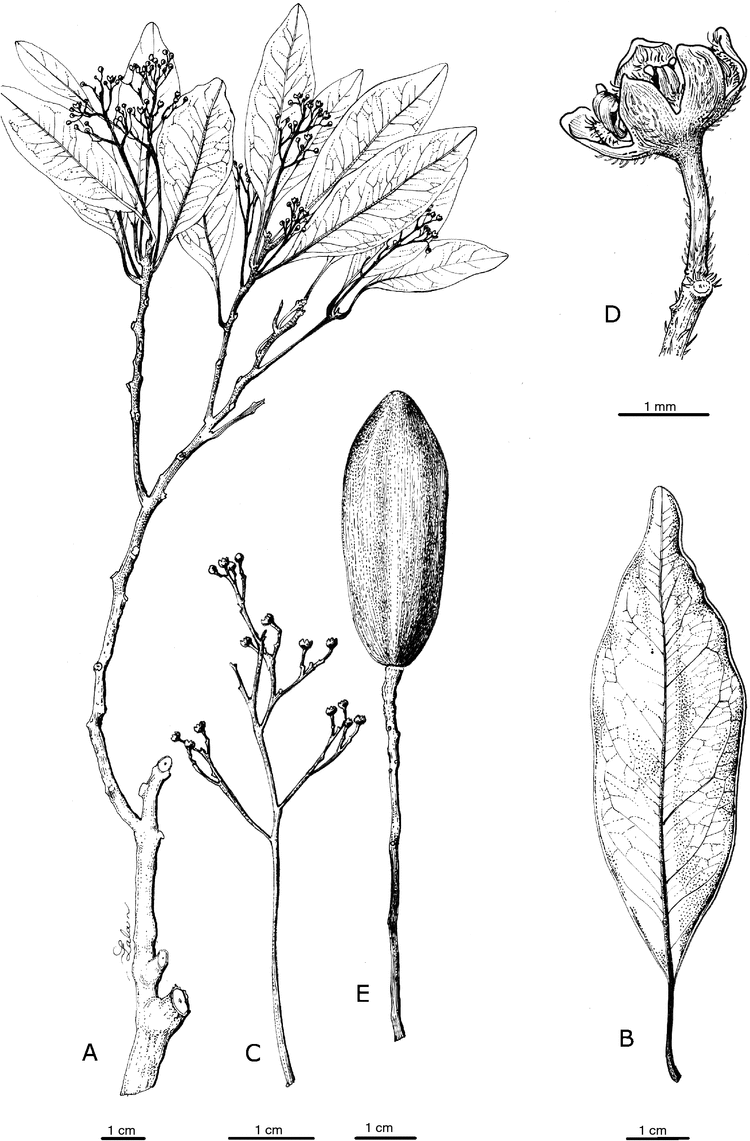
Endiandra artensis Munzinger & McPherson, sp. nov.
Diagnosis: among New Caledonian species of Endiandra, the new species most closely resembles E. lecardii Guillaumin and E. neocaledonica Kosterm. in its mid-sized, glabrescent leaves (blade up to 8 cm long, petiole more than 10 mm long) and glabrescent stems; however, in E. artensis the tepals are shorter (1.0–1.5 mm long v. 1.5–2.0 mm in E. lecardii and E. neocaledonica), the staminodes are at least half as long as the fertile stamens (v. up to one-third as long), the leaf blades are thinner and smooth (v. coriaceous and muricate), and the species is restricted to Île Art (v. widespread on Grande Terre).
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Nord, rebord Est, 220 m, forêt dense humide, terrain rocheux serpentineux, 9.xii.1975, H.S.MacKee 30476 (holo: P 01753198!; iso: MO 6850477!, NOU 016556!, MPU 310777!).
Trees hermaphroditic, 4–10 m tall. Diameter ~35 cm. Bark nearly smooth to rather rough, light brown. Terminal buds densely appressed-pubescent with brownish or greyish hairs, the young stems quickly glabrescent, shallowly lined and wrinkled when dry, lenticellate only well below leafy portion, the lenticels somewhat raised. Leaf simple, subopposite to alternate, blade elliptic, 4.0–8.0 × 1.8–4.0 cm, base attenuate to acute, apex obtuse, chartaceous, adaxial and abaxial surfaces sparsely appressed-puberulent at first, quickly glabrescent, muricate only along the midvein, secondary veins 8–9 on each side, slightly raised on both surfaces as is the higher-order venation. Petiole 10.0–16.0 × ~1.5 mm, flat adaxially, appressed-puberulent at first, quickly glabrescent. Inflorescence axillary, paniculate, 1.2–7.5 cm long, the axes sparsely puberulent; peduncle 8–38 mm long, ~0.6 mm in diameter; bracts minute; pedicels 1.0–2.5 mm long. Flowers yellow, 1.5 mm long, up to 3 mm in diameter at full anthesis; tepals ovate, 1.5 mm long, spreading at anthesis, subequal, abaxially glabrous or sparsely pubescent, adaxially densely pubescent except near margins. Fertile stamens 3, ~0.8 mm long, filaments ~0.3 mm long, pubescent, anthers lateral, ~0.5 mm long, glabrous, basal glands sessile, subtriangular, 0.5 mm long, glabrous; staminodes 3, ~0.4–0.6 mm high (i.e. at least half as long as the fertile stamens), pubescent basally. Ovary ovoid, ~1 mm high, glabrous. Fruit ellipsoid, smooth, purple-black, 3.2–4.0 × 1.6–1.9 cm.
Recognition
Endiandra artensis is easily recognised in the field, because it is the only member of its genus known to occur on Île Art. The two species that it most closely resembles, namely, E. lecardii and E. neocaledonica, are restricted to Grande Terre and have more coriaceous leaves with thicker petioles, as well as longer tepals (1.5–2.0 mm v. 1.0–1.5 mm) and shorter staminodes (up to 1/3 the length of the fertile stamens v. at least 1/2 the length of the fertile stamens).
Distribution
Endiandra artensis is endemic to low rainforests of the ultramafic plateaus of Île Art.
Etymology
This species is named after Île Art, where it is a newly recognised micro-endemic.
Conservation status
Endiandra artensis was seen only in the remnants of rainforest of the northern and southern plateaus of Île Art. The plant is threatened by anthropogenic bush fires on both plateaus and as a single fire can affect both plateaus, it is considered as a single location (sensu IUCN). The successive fires are source of an observed and projected decline in EOO, AOO, habitat quality and the number of mature individuals.The calculated EOO being smaller than the AOO, the AOO value of 12 km2 also applies to the EOO. Endiandra artensis is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau Nord, 200 m, H.S.MacKee 30524 (MO 6850478, NOU 016553, P 01753201, P 02116875); Île Art, Ph.Morat 6176 (MO 6850479, NOU 016555, P 02003038); Île Art, partie Sud, P.Cabalion 663 (NOU 016552); Île Art, partie Sud, C.Tirel 1298 (P 02194641); Île Art, plateau Sud, J.-M.Veillon 3703 (MO 6850480, NOU 016554, P 02003056, P 02033072); Île Art, plateau Nord, 220 m, J.-P.Butin 251 (NOU 084485).
Myrtaceae
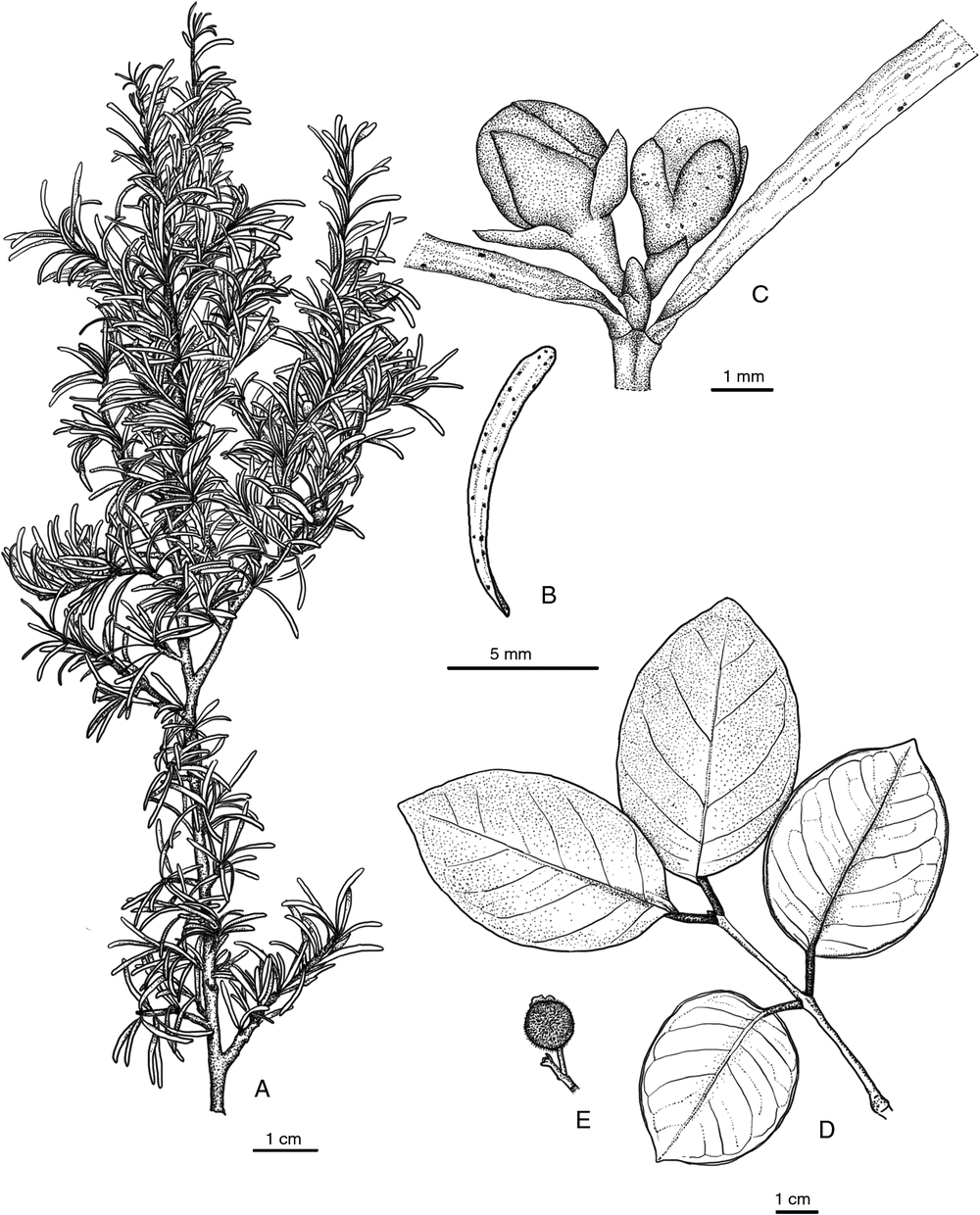
Eugenia belepiana J.W.Dawson ex N.Snow, sp. nov.
Diagnosis: among other New Caledonian members of the genus, Eugenia belepianiana resembles E. ericoides Guillaumin but differs from that species by its prostrate to spreading growth habit, narrower and shorter leaves (6.0–14.0 × 0.4–0.8 mm), and sessile flowers. The reddish flaking bark and spreading growth habit of the new species resemble those of E. horizontalis Pancher ex Brongn. & Gris, but that species differs by its broader leaves (2–15 mm), its long-pedicellate flowers, and its bark, which peels to a greater extent than that of E. belepiana.
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Nord (rebord Est), 250 m, 8.xii.1975, H.S.MacKee 30377 (holo: P 05121251!; iso: NOU 082631!).
Shrubs hermaphroditic, prostrate, of low stature (height unconfirmed), comprising a few to several thicker spreading branches, each moderately to densely crowded with thinner short shoots bearing moderate to dense foliage. Plants glabrous and eglandular except as noted. Branchlets terete or slightly laterally compressed, the epidermal layers reddish-maroon to dark brown or nearly blackish and flaking irregularly in thin layers continuously (including older stems). Leaf simple, opposite (rarely 3 per node), blade (4.0–)6.0–14.0 × 0.4–0.8(–3.0) mm, linear or somewhat falcate, base tapering slightly into petiole, apex obtuse and sometimes somewhat curving downwards, often diverging widely, margin flat, coriaceous, slightly discolorous, surfaces matte, sparsely sericeous on emergence (trichomes dibrachiate) but otherwise glabrous, oil glands few but pronounced (with magnification) and concentrated near margin; secondary veins (and midvein usually) obscure above and below. Petiole ~0.5 mm. Inflorescence of small sessile flowers, axillary or terminal; bracteoles 0.5–0.9 × < 0.5 mm, narrowly triangular to narrowly elliptic. Hypanthium 0.6–1.3 mm, obconic, sparsely glandular; ovary apex glabrous. Calyx lobes 4, 0.6–1.3 mm, broadly triangular, slightly dimorphic, apex broadly acute, minutely and sparsely ciliate apically. Petals 4, ~1.7–2.5 mm, widely obovate to oblate, minutely sparsely ciliate, white or pinkish. Staminal ring sparsely ciliate or glabrous. Stamens <30; filaments ~2–3 mm, white; anthers 0.3–0.4 mm, globular to subglobular, with terminal gland, tawny. Style 2.5–5 mm; stigma narrow. Fruit unknown.
Recognition
The short, linear to falcate leaf blades coupled with the prostrate or nearly prostrate growth and sessile flowers of E. belepiana are unmistakable in the genus. The species sometimes has three leaves per node, and individuals may have a few narrowly elliptic leaves interspersed with the overwhelmingly linear-falcate ones. Its scraggly, spreading growth habit also helps differentiate it from other species of the genus in New Caledonia, such as an undescribed species Eugenia sp. ‘adenosticta’ from Mont Taom, Unio and Tontouta, which consistently has elliptic leaves.
Distribution
Known only from the northern plateau of Île Art on ultramafic substrate up to ~250 m. The label of the type gathering indicates that the plant was quite common in shaded areas, but that was over 40 years ago. From its recent collection and observation (J.-P. Butin, pers. comm.), there are not that many plants left in a secondary type of vegetation mainly composed of Acacia spirorbis Labill.
Etymology
The species is named after the Belep archipelago, Île Art being part of this archipelago, where it is a newly recognised micro-endemic.
Conservation status
Eugenia belepiana has been confirmed so far only in the remnants of rainforest of the northern plateau of Île Art, in one location. However, one of the co-authors (GG) recalls seeing what he believed was the same species in the rainforest of the southern plateau in 2017, but was unable to collect or photograph the specimen. The main threats to it are the anthropogenic fires that occur frequently in the area that are sources of of an observed and projected decline in EOO, AOO, habitat quality and the number of mature individuals. The calculated EOO being smaller than the AOO, the AOO value of 4 km2 also applies to the EOO. From IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, E. belepiana is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v)+2ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau Nord, T.Jaffré 1538 (NOU 082629, P 05094280); Île Art, plateau vers 220 m, J.-M.Veillon 2899 (NOU 082630); Île Art, plateau Nord, J.-P.Butin 227 (NOU 084469).
Eugenia insulartensis J.W.Dawson ex N.Snow, sp. nov.
Diagnosis: among other New Caledonian members of the genus, Eugenia insulartensis differs by the combination of branchlets that often arch slightly between successive nodes, the glabrous, elliptic to broadly elliptic leaves that sometimes are slightly conduplicate above the petiole, and especially by the densely tomentose fruits bearing a coppery indumentum.
Type: New Caledonia, Province Nord, Belep, Île Art, plateau Nord (rebord Est), 220 m, 9.xii.1975, H.S.MacKee 30468 (holo: P 05094571!; iso: NOU 082595!).
Shrubs hermaphroditic, 3–4 m, slender. Bark of main stem greyish-brown, becoming irregularly roughened. Branchlets terete or compressed (immature), light brown–grey, glabrous, smooth, eglandular, sometimes somewhat arching between successive nodes. Leaf simple, opposite, blade (3.0–)4.5–12.0 × 2.0–6.8 cm, elliptic to broadly elliptic, base rounded to somewhat cuneate and sometimes slightly conduplicate at junction with petiole, apex broadly acute to mostly obtuse, margin flat or slightly revolute, coriaceous, eglandular, surfaces matte or slightly glossy above; venation brochidodromous, discolorous, glabrous, adaxial midvein broadly sulcate proximally, typically flush (or nearly so) distally; abaxial secondary veins faint, more or less straight between midvein and marginal vein, marginal vein indistinct, 1.5–3.0 mm from margin at midpoint of blade. Petiole 8–14 mm, sulcate above, coarsely rugose, minutely grey-tomentose. Inflorescence consisting of monads, triads or short brachyblasts, solitary, cauliflorous, paired, or fascicled ~1–2 cm long; peduncle 5–9 mm, rigid, densely short ferrugineous-velutinous; bracteoles not seen. Hypanthium 3–5 mm, cupulate, pinkish in bud, densely short ferrugineous-velutinous. Calyx lobes 4, 2–4 mm long, broadly rounded to elliptic, apex obtuse, indumentum of both surfaces as on hypanthium, persistent on and more or less crowning mature fruit. Petals 6–7 × 4–5 mm, elliptic to narrowly obovate, minutely ciliate on margins, whitish. Stamens >150, multiseriate; filaments 4–6 mm; anthers 0.5–0.8 mm, globose to subellipsoid, basifixed, eglandular; staminal disk densely short velutinous. Style ~4–5 mm, glabrous; stigma narrowly if at all capitate. Fruit globose or subglobose, rounded at base, ~7–20 mm, sepal lobes persistent and crowning the fruit, densely brownish-velutinous.
Recognition
The glabrous leaves of this new species contrast with the densely pubescent flowers and fruits (of the latter, see especially MacKee 30385 in fragment packet). Among congeners on Île Art, the slightly arching branchlets between successive nodes, elliptic leaves with sulcate petioles, narrow peduncles of the flowers and fruits, and densely velutinous fruits are reliable diagnostic field traits. Leaves that appear to be pruinose, in fact, are densely ingrown by fungal hyphae; the whitish colour, therefore, is not a waxy covering. Eugenia insulartensis might be confused with the widespread E. gacognei Montrouz., but its longer petioles (8–14 mm), pedicillate flowers, and sometimes conduplicate leaf bases differ from the latter. Eugenia insulartensis also somewhat resembles E. mendute Guillaumin in leaf size and shape, but the former has densely velutinous flowers.
Distribution
In dense and (often shaded) rainforests on rocky serpentines on the eastern edge of the northern plateau near the east-central coast on Île Art at 220–250 m. Eugenia insulartensis is presently known from only one locality, the gatherings of which were made on consecutive days.
Etymology
A Latin derivation of Île Art, its only known location.
Conservation status
Eugenia insulartensis has been collected only in the rainforest remnants of the eastern border of the northern plateau of Île Art. The main threats in this area are the anthropogenic fires that progressively reduce the surface of the remaining patches of forests, resulting in an observed and projected decline in EOO, AOO, habitat quality and the number of mature individuals. The calculated EOO being smaller than the AOO, the AOO value of 4 km2 also applies to the EOO. From IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, E. insulartensis is assigned the preliminary status of Critically Endangered CR B1ab(i,ii,iii,v)+2ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau Nord, T.Jaffré 1567 (NOU 054945, P 05094287); Île Art, plateau Nord (rebord Est), 250 m, H.S.MacKee 30385 (NOU 082593, P 05094570).
Rubiaceae
Psychotria neodouarrei Barrabé & A.Martini, sp. nov.
Diagnosis: Psychotria neodouarrei resembles P. gabriellae (Baill.) Guillaumin in having bifid, caducous and free stipules, glabrous leaves, compound cymes, and funnelformed, not pure white corolla, but differs most notably from the latter species in having obovate (v. elliptic) leaf blades, angular (v. round) buds, bluish-white (v. pink) corolla, and bluish-black (v. white to blue grey) fruits with a thin (v. white and spongy) mesocarp.
Type: New Caledonia, Province Nord, Belep, Île Art, plateau au nord d’Oono, 29.vii.2009, J.Fambart-Tinel (leg. J.-P.Butin) 213 (holo: P!; iso: NOU052690!).
Shrubs hermaphroditic, up to 1 m tall, branched; bark light grey when dry, glabrous, slightly striate longitudinally, without lenticels; young shoots, petioles, and terminal vegetative buds glabrous. Leaf simple, spread along stem, glabrous, blade generally obovate, sometimes elliptic, 2.6–9.0 × 0.9–4.4 cm, acute and slightly decurrent at base, briefly acuminate at apex, margin entire and slightly revolute, chartaceous and leathery, concolorous when dry, midvein brown–red when dry, raised and slightly channeled on adaxial surface, raised on abaxial surface, secondary veins 5–11 on each side, spaced at 1.5–11.0 mm, at 38–55° angle with the midvein, slightly raised to impressed on adaxial surface, slightly raised on abaxial surface, tertiary venation obscure on both surfaces. Petiole slightly wrinkled when dry, 0.4–1.7 cm long, 1.0–1.5 mm thick, plano-convex, slightly channeled on adaxial face, dark brown to black when dry. Stipules free, ovate, 3.0–5.0 × 1.0–2.5 mm, margins entire, bifid, chartaceous, colour unknown, glabrous, deciduous, lobes narrowly triangular with a base of 1.5–3.0 mm; colleters present on the inner surface, narrowly triangular, brown. Inflorescence erect, a compound cyme, 3 or 4 times branched, glabrous, pedunculate; peduncule 3–16 mm long, 1.25–1.5 mm width, slightly striate longitudinally; bracts axillary to each secondary peduncule, lanceolate, tapered, navicular, acute, 1.5–2.0 × 0.5 mm, margin entire, brown when dry, glabrous on adaxial side; pedicel up to 0.5 mm long, 0.75 mm wide; bracteoles 1 or 2, axillary to each flower, narrowly triangular, up to 1.5 mm long. Flowers 5-merous, erect, slightly pedicellate, style heterogeneity unknown; buds obovoid and angular. Hypanthium turbinate, 1.0–1.5 × 1.0 mm, colour unknown, glabrous; nectary disk entire, dome-shaped, 1 mm in diameter, glabrous. Calyx coriaceous, light green, glabrous outside, hirsute inside; tube up to 2 mm long, 1 mm wide, colleters lacking; lobes triangular, up to 0.25 mm long, obtuse at apex with a rounded apicule, erect, margin entire. Corolla actinomorphic, funnelform, chartaceous, bluish-white, glabrous outside, sparsely hirsute inside on lobes and on a cylinder of 5.5 mm high, then glabrous 2.8 mm from the base; tube straight, 7.0–9.2 mm long, throat slightly flared, 3.0–4.5 mm wide at mouth, base 1–2 mm wide; lobes narrowly triangular, 2.0–5.5 × 1.0–2.0 mm, rounded at apex, erect (at anthesis). Stamens partially included, glabrous; filaments linear, ~0.75 mm long, terete, adnate to corolla ~1 mm below the mouth; anthers oblongoid, ~2.9–1.0 mm, medifixed. Style filiform, 9.0–10.5 × 0.3 mm, terete, glabrous, stigma bilobed, papillate, lobes up to 0.3 mm long. Fruit (unripe) globose, 9.7 × 7.5 mm; exocarp smooth, colour unknown, glabrous; mesocarp thin, not spongy; pyrenes (unripe) plano-convex, ovoid, 2.25–2.5 × 1.1 × 4.1 mm, round at base, obtuse at apex; dorsal side convex, strongly wrinkled, 4-channelled; ventral side flat, wrinkled, with a thin raised median crest; pregermination slits lacking, basal aperture present; exotesta unknown.
Recognition
Psychotria neodouarrei shares a similar morphology of its inflorescences, flowers and fruits with a group of 11 New Caledonian species that includes the well known hyperaccumulator P. gabriellae, P. belepensis Barrabé & Mouly, P. ferdinandi-muelleri Guillaumin, P. guillauminiana Barrabé & Mouly, P. oua-tilouensis Guillaumin, P. pininsularis Guillaumin, P. semperflorens (Pancher ex Beauvis.) Guillaumin and four other new species not yet described. The bluish-white corolla and bluish-black fruits of P. neodouarrei recall those of P. oua-tilouensis. However, the latter has narrowly ovate abaxially velutinous leaf blades greater than 9 cm in length, and large, elongated inflorescences with numerous flowers (>50) v. obovate glabrous leaf blades less than 9 cm, and small, compact inflorescences with few flowers (<30). The bifid stipules ressemble those of P. gabriellae, and P. belepensis; however, P. gabriellae and P. belepensis differ from P. neodouarrei in having a pink corolla, elliptic and narrowly ovate leaf blades with an acute apex and generally acute base. The angular buds of P. neodouarrei are similar to those of P. ferdinandi-muelleri and P. guillauminiana but both P. ferdinandi-muelleri and P. guillauminiana have pink corollas and linear leaf blades with a white prominent adaxially midvein. Psychotria ferdinandi-muelleri has also large persistent light green and involute stipules (>1 cm long) v. small caducous and flat stipules (<5 mm long) in P. neodouarrei. Last, P. pininsularis and P. semperflorens differ from P. neodouarrei by having pink corollas, connate stipules and round buds.
Distribution
Psychotria neodouarrei is known from three islands of the Belep archipelago, namely, Île Art, Île Pott, Île Daos du Nord, where it occurs in low maquis at elevations near the sea level. This new species is locally rare (only two individuals recorded at O’ono; J.-P. Butin, pers. comm.).
Etymology
Barrabé et al. (2013) raised the question of an uncertain taxon from the Belep archipelago described in 1860 by Montrouzier as Douarrea alba Montrouz., which was later recognised as Mapouria douarrei Beauvis. by Beauvisage in 1894. The type specimen was considered to have been destroyed, and the diagnosis was insufficient to attribute this name precisely to one of the four species occurring in the Belep. One of these four species clearly belongs to the genus Eumachia DC. (i.e. E. collina (Labill.) Barrabé, C.M.Taylor & Razafim., see Taylor et al. 2017). Another has pink flowers (i.e. Psychotria belepensis), and the last two have white to bluish-white corollas (i.e. P. montrouzieri Barrabé & J.Florence with large sepals and a previously nameless species with small sepals). The original diagnosis of D. alba mentioned that it possesses white flowers and a short calyx that correspond better to the un-named species. However, considering that the Belep archipelago could shelter a fifth species that is not rediscovered and that the type specimen of D. alba is lost, it seems best to attribute this name to the species with bluish-white flowers and small calyx. We have, consequently, decided to consider D. alba as a doubtful species and to describe here the species with bluish-white flower as P. neodouarrrei to recall the names of Montrouzier and Beauvisage.
Conservation status
Psychotria neodouarrei has been found in three localities, namely, Île Art, Île Daos du Nord and Île Pott, which represent three locations with respect to the main threat, the recurrent anthropogenic fires that progressively reduce the surface of the native vegetation and result in an observed and projected decline in EOO, AOO, habitat quality and the number of mature individuals.The calculated EOO and AOO are 31 km2 and 16 km2 respectively. On the basis of IUCN Standards and Petitions Subcommittee (2017) Categories and Criteria, P. neodouarrei is assigned the preliminary status of Endangered EN B1ab(i,ii,iii,v)+2ab(i,ii,iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Belep. Île Art, plateau au nord d’Oono, J.Fambart-Tinel (leg. J.-P.Butin) 256 (NOU 083380); Île Daos du Nord (Dau âc), Butin 30 (NOU 079682); Île Pott, Mouane, 0–60 m, H.S.MacKee 19375 (NOU 032616, P 04531115).
Sapotaceae
Planchonella serpentinicola Swenson & Munzinger, sp. nov.
Diagnosis: Planchonella serpentinicola differs from the other members of the genus in New Caledonia, especially P. contermina and P. povilana, by the combination of its oblanceolate–obovate leaves, with usually four pairs of secondary veins, tubular flowers with fimbriate corolla margin, and ovoid to pear-shaped fruits.
Type: New Caledonia, Province Nord, Belep, Île Art, north plateau, along the most eastern prospecting track, 19°42′06″S, 163°39′49″E, 256 m alt., 26.viii.2009, U.Swenson, J.Munzinger & L.Barrabé 921 (holo: P 01156238!; iso: NOU 051206!, S 09-36604!).
Shrub or small tree hermaphroditic, up to 5 m tall, usually much branched. Leaf simple, blade oblanceolate to obovate, 2.8–5.5 × 1.0–3.0 cm, base cuneate, apex round to slightly retuse, flat with a pronounced margin, first ferruginous tomentulose on both surfaces, soon glabrous above, turning greyish tomentulose below, partly glabrescent; venation brochidodromous, weak on both surfaces; secondary veins straight, meeting the midvein at 40–55°, usually of 4 pairs (sometimes 3 or 5), intersecondaries rarely present, tertiary veins laxly reticulate, very weak. Petiole 3–8 mm long, tomentulose, ferruginous, turning greyish. Flowers usually solitary, axillary, 5-merous, borne on a pedicel 6–12 mm long with the same indument as the petiole. Sepals 5–10 mm long, glabrous inside, with the same indument as the pedicel outside, the inner sepals usually with a glabrous margin. Corolla tubular, cream or pale greenish, 8–10 mm long, glabrous, the lobes with a ciliate margin. Stamens inserted just above the middle of the corolla tube, not exserted; anthers 1.5–1.8 mm long; staminodes flat, linear or lanceolate, entire. Gynoecium flask-shaped, with a proportionally long style (~8 mm), hispid at base, with 5 round stigmatic areas. Fruit ovoid to pear-shaped, not ridged, 15–25 × 6–15 mm, 3–5-seeded, pubescent, partly glabrescent, with a remnant style 4–8 mm long; seeds shaped like segments of an orange, laterally compressed, 10–14 mm long, 3–4 mm wide; seed scar 90–100% of the seed length; testa light brown, shiny, thin, ~0.4 mm thick; cotyledons foliaceous, white, with an exserted radicle below the commissure; endosperm present.
Recognition
Planchonella serpentinicola could be confused with two congeners, P. contermina Pierre ex Dubard and P. povilana Swenson & Munzinger, all belonging to the same clade of 14 endemic species from New Caledonia (Swenson et al. 2007; U. Swenson, unpubl. data). In this clade, P. serpentinicola is sister to the remaining species in the clade. Planchonella serpentinicola is distinguished from these two species by the shape of the leaves, the number of secondary veins, corolla morphology and fruit shape. Leaves of all three are somewhat similar, usually oblanceolate to obovate in P. serpentinicola, spathulate to orbicular in P. povilana, and obovate–oblanceolate–linear in P. contermina, but the former usually has four pairs of secondary veins v. six or more in the latter two species. Moreover, P. serpentinicola has the longest corolla (8–10 mm) with fimbriate corolla-lobe margins v. 6–8 mm long in P. contermina and 7 mm long in P. povilana, both of which have glabrous lobe margins. Around Voh and the Plateau de Tiéa grow populations of P. contermina with small, oblanceolate leaves (Fig. 11H) similar to those of P. serpentinicola, but the fruit of P. contermina is more globose and not ovoid or pear-shaped as in P. serpentinicola (Fig. 11F).
Distribution
Planchonella serpentinicola is known from Île Art in the Belep archipelago, and also occurs in Baaba and Yandé islands (Poum municipality). The species is a naturally common (at least before the severe fire in August 2016) shrub or small tree on Île Art, being a dominant member of the low and dense forest on ultramafic soil, primarily serpentine (Fig. 1B). One population is known from Grande Terre, on the western slope of Mount Tiébaghi, where it occurs in maquis vegetation.
Etymology
This species is named serpentinicola because it occurs (-cola, -dweller) on serpentine.
Conservation status
The known distribution of Planchonella serpentinicola form an EOO of 700 km2 and an AOO of 40 km2. None of the locations is protected, but instead half of the known locations are located in mining concessions, and, thus, are under risk from future mining activities. Anthropogenic fires are also an important threat in that area. With an observed and projected decline in EOO, AOO and the number of mature individuals, Planchonella serpentinicola is assigned an IUCN preliminary status of Endangered, EN B1ab(iii,v)+2ab(iii,v).
Specimens examined
NEW CALEDONIA, Province Nord. Koumac. Western base of Mount Tiébaghi, 50 m, 20°30′22″S, 164°12′50″E, U.Swenson & J.Munzinger 1117 (BRI, G, MO, NOU, P, S 13-19098). Poum. Île Baaba, secteur sud-ouest (Tiomatch), 30–130 m, H.S.MacKee 23165 (NOU 009869, P 00538591); Île Yandé, 50–100 m, H.S.MacKee 22626 (G, MO, MPU, NOU 009870, NY, P 00352923, S 14-30127); Yandé, on the eastern slope, 120 m, 20°02′49″S, 163°49′19″E, U.Swenson & J.Munzinger 715 (NOU 009932, P, S 05-10376). Belep. Île Art, après le creek Weaa, J.-P.Butin 32 (NOU); Île Art Sud, P.Cabalion 667 (NOU 009867, P 00538592); Île Art, Centre-Ouest, J.-M.Veillon 3671 (NOU 009872); Île Art, northern plateau, along the most eastern prospecting track, 256 m, 19°42′10″S, 163°40′01″E, U.Swenson, J.Munzinger & L.Barrabé 910 (MO, NOU 051200, P 06707415, S 09-36482); Île Art, northern plateau, along the most eastern prospecting track, 256 m, 19°42′21″S, 163°39′58″E, U.Swenson, J.Munzinger & L.Barrabé 914 (BRI, MO, NOU 051211, P 06707408, S 09-36486); Île Art, northern plateau, along the most eastern prospecting track, 256 m, 19°42′06″S, 163°39′49″E, U.Swenson, J.Munzinger & L.Barrabé 919 (NOU 051208, S 09-36602); Île Art, partie Nord, 200 m, H.S.MacKee 30450 (G, MO, NOU 009866, P 00428189, S 14-33648); Île Art, partie Sud, 50 m, C.Tirel 1295 (P 00292110); Île Art, plateau, T.Jaffré 1528 (NOU 009871, P, S 07-15266); Île Art, plateau Sud, J.-M.Veillon 3699 (NOU 009865, P 02089456); Île Art, s.loc., J.M.Veillon 2701 (P 02089463, S 14-33697); Île Art, s.loc., J.-M.Veillon 3725 (NOU 009868, P 02089455); Île Art, sentier du plateau au dessus de chez Donatienne, 19°41′58.91″S, 163°38′41.26″E, 200 m, G.Gâteblé, E.Bourguet & G.Templier 859 (NOU, P); Île Art, maquis de Keyani au nord, 19°38′53.08″S, 163°38′35.19″E, 80 m, G.Gâteblé 926 (NOU, P).
Concluding remarks
The Belep archipelago, as part of the Zone du Grand Lagon Nord, is a part of a site on the UNESCO World Heritage List in recognition of its reef diversity and associated ecosystems. However, its highly endemic and threatened terrestrial biota also should be considered a major part of this natural heritage. We hope this new taxonomic account will raise public awareness of the unique Belep flora and the need for urgent and concrete actions to ensure its conservation. This could include (1) delineating one or several North Province reserves on both Art and Pott islands, (2) increasing awareness among the local population (custom authorities, local Belep UNESCO management committee) of the importance of preventing deliberately set fires, (3) formalising the memorandum of understanding to ensure that no future nickel mining activities take place on Pott and Art islands and (4) setting up urgent in situ and ex situ conservation programs for the most Critically Endangered and Endangered taxa on the brink of extinction.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Institut Agronomique néo-Calédonien (IAC) supports this paper by way of Open Access publication.
Acknowledgements
The authors thank the curators of all the herbaria listed for permitting access to their collections, as well as Sandrine Isnard and Jacqueline Fambart-Tinel (NOU) for providing scans, and Pete Lowry (MO and P) for arranging to have duplicate Euphorbiaceae specimens sent to MO. The conservation authorities of North Province (Jean-Jérôme Cassan, DDEE) as well as custom authorities of Belep islands kindly granted access to Île Art for fieldwork. Hervé Jourdan, Edouard Bourguet and Gwenaïs Templier (IRD) are thanked for logistics and help in the field. Dominique Fleurot (DDEE) provided useful GIS data on the Belep archipelago. Many thanks go to Roger Lala Andriamiarisoa, Monika Osterkamp and Laurence Ramon for their fine drawings, to Jean-Pierre Butin who provided permission to use his images and who shared his knowledge and to Armelle Tardivel for mounting Fig. 5 and 8. Fabien Albouy (OEIL) provided the most precise and unpublished shapefile for fire occurrence in Île Art for July–December 2016. Gendrilla Warimavute (Endemia Red List Authority) kindly calculated and provided EOO and AOO data used for conservation assessments. N. Snow thanks Kanchi Gandhi (GH) for assistance with nomenclature. L. Barrabé thanks Aurore Martini who participated actively to the description of Psychotria neodouarrei. G. Gâteblé is most thankful to Vincent Tanguy who coordinated the Endemia local IUCN Red List Authority (2014–February 2018) and who provided the in press IUCN evaluations awaiting formal publication. The three reviewers (Pete Lowry, Martin Callmander and Brendan Lepschi) have provided numerous useful comments on the earlier version of the manuscript and are also gratefully acknowledged. Thanks also go to Darren Crayn for inviting us to submit this paper.
References
Bachman S, Moat J, Hill AW, de la Torre J, Scott B (2011) Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. ZooKeys 150, 117–126.Barrabé L (2014) Four new species of Psychotria (Rubiaceae) from New Caledonia, including one presumed to be extinct. Phytotaxa 173, 101–116.
| Four new species of Psychotria (Rubiaceae) from New Caledonia, including one presumed to be extinct.Crossref | GoogleScholarGoogle Scholar |
Barrabé L, Mouly A, Florence J (2013) Psychotriae (Rubiaceae) neocaledonicarum specierum nomenclator. Adansonia 35, 281–357.
| Psychotriae (Rubiaceae) neocaledonicarum specierum nomenclator.Crossref | GoogleScholarGoogle Scholar |
Barrabé L, Maggia L, Pillon Y, Rigault F, Mouly A, Davis AP, Buerki S (2014) New Caledonian lineages of Psychotria (Rubiaceae) reveal different evolutionary histories and the largest documented plant radiation for the archipelago. Molecular Phylogenetics and Evolution 71, 15–35.
| New Caledonian lineages of Psychotria (Rubiaceae) reveal different evolutionary histories and the largest documented plant radiation for the archipelago.Crossref | GoogleScholarGoogle Scholar |
Beauvisage G (1894) Révision de quelques genres de plantes néo-calédoniennes du R. P. Montrouzier. Annales de la société Botanique de Lyon 19, 15–28.
| Révision de quelques genres de plantes néo-calédoniennes du R. P. Montrouzier.Crossref | GoogleScholarGoogle Scholar |
Beauvisage G (1901) Genera Montrouzierana. Annales de la société Botanique de Lyon 26, 1–48.
| Genera Montrouzierana.Crossref | GoogleScholarGoogle Scholar |
Caesar M, Grandcolas P, Pellens R (2017) Outstanding micro-endemism in New Caledonia: more than one out of ten animal species have a very restricted distribution range. PLoS One 12, e0181437
| Outstanding micro-endemism in New Caledonia: more than one out of ten animal species have a very restricted distribution range.Crossref | GoogleScholarGoogle Scholar |
Callmander MW, Munzinger J, Stone BC (2011) Pandanus belepensis (Pandanaceae), a new species from the Belep Archipelago (New Caledonia). Phytotaxa 38, 36–40.
| Pandanus belepensis (Pandanaceae), a new species from the Belep Archipelago (New Caledonia).Crossref | GoogleScholarGoogle Scholar |
Cañadas EM, Fenu G, Peñas J, Lorite J, Mattana E, Bacchetta G (2014) Hotspots within hotspots: endemic plant richness, environmental drivers, and implications for conservation. Biological Conservation 170, 282–291.
| Hotspots within hotspots: endemic plant richness, environmental drivers, and implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Curt T, Borgniet L, Ibanez T, Moron V, Hély C (2015) Understanding fire patterns and fire drivers for setting a sustainable management policy of the New-Caledonian biodiversity hotspot. Forest Ecology and Management 337, 48–60.
| Understanding fire patterns and fire drivers for setting a sustainable management policy of the New-Caledonian biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Dawson JW (1992) ‘Flore de la Nouvelle Calédonie et Dépendances: Myrtacées–Leptospermoïdées. Vol. 18.’ (Muséum National d’Histoire Naturelle: Paris, France)
Eken G, Bennun L, Brooks TM, Darwall W, Fishpool LDC, Foster M, Knox D, Langhammer P, Matiku P, Radford E, Salaman P, Sechrest W, Smith ML, Spector S, Tordoff A (2004) Key biodiversity areas as site conservation targets. Bioscience 54, 1110–1118.
| Key biodiversity areas as site conservation targets.Crossref | GoogleScholarGoogle Scholar |
Endemia Red List Authority (2017) La liste rouge de la flore menacée de Nouvelle-Calédonie, synthèse octobre 2017. Available at http://endemia.nc/files/20171107_Resultats_RLA_Oct2017_FR_web2p.pdf [Verified February 2018]
Fenu G, Mattana E, Congiu A, Bacchetta G (2010) The endemic vascular flora of Supramontes (Sardinia), a priority plant conservation area. Candollea 65, 347–358.
| The endemic vascular flora of Supramontes (Sardinia), a priority plant conservation area.Crossref | GoogleScholarGoogle Scholar |
Gomez C, Mangeas M, Curt T, Ibanez T, Munzinger J, Dumas P, Jérémy A, Despinoy M, Hély C (2015) Wildfire risk for main vegetation units in a biodiversity hotspot: modeling approach in New Caledonia, South Pacific. Ecology and Evolution 5, 377–390.
| Wildfire risk for main vegetation units in a biodiversity hotspot: modeling approach in New Caledonia, South Pacific.Crossref | GoogleScholarGoogle Scholar |
Gouvernement de la Nouvelle-Calédonie (2009) Le schéma de mise en valeur des richesses minières de la Nouvelle-Calédonie. Available at https://dimenc.gouv.nc/sites/default/files/download/2009-05_schema_mise_en_valeur_des_richesses_minieres_nc.pdf [Verified February 2018]
Gouvernement de la Nouvelle-Calédonie (2018) Explorateur cartographique Géorep.nc. Available at https://dtsi-sgt.maps.arcgis.com/apps/webappviewer/index.html?id=da224a6ff1c24c029de4024d7ae8af26 [Verified February 2018]
Green PS (1998) ‘Flore de la Nouvelle Calédonie et Dépendances: Oleaceae. Vol. 22.’ (Muséum National d’Histoire Naturelle: Paris, France)
Guillaumin A (1948) ‘Flore analytique et synoptique de la NouvelleCalédonie, phanerogames.’ (Office de la Recherche Scientifique Coloniale: Paris, France)
Hopkins HCF, Pillon Y, Hoogland R (2014) ‘Flore de la Nouvelle Calédonie: Cunoniaceae. Vol. 26.’ (Muséum National d’Histoire Naturelle: Paris et IRD Editions: Marseille, France)
Hopkins HCF, Pillon Y, Stacy EA, Kellermann J (2015) Jaffrea, a new genus of Rhamnaceae endemic to New Caledonia, with notes on Alphitonia and Emmenosperma. Kew Bulletin 70, 42
| Jaffrea, a new genus of Rhamnaceae endemic to New Caledonia, with notes on Alphitonia and Emmenosperma.Crossref | GoogleScholarGoogle Scholar |
Isnard S, L’huillier L, Rigault F, Jaffré T (2016) How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant and Soil 403, 53–76.
| How did the ultramafic soils shape the flora of the New Caledonian hotspot?Crossref | GoogleScholarGoogle Scholar |
IUCN Standards and Petitions Subcommittee (2017) Guidelines for using the IUCN Red List categories and criteria: version 13. Prepared by the Standards and Petitions Subcommittee. Available at http://www.iucn.org/RedListGuidelines.pdf [Verified February 2018]
Joppa LN, Roberts DL, Myers N, Pimm SL (2011) Biodiversity hotspots house most undiscovered plant species. Proceedings of the National Academy of Sciences of the United States of America 108, 13171–13176.
| Biodiversity hotspots house most undiscovered plant species.Crossref | GoogleScholarGoogle Scholar |
Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W (2009) A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences of the United States of America 106, 9322–9327.
| A global assessment of endemism and species richness across island and mainland regions.Crossref | GoogleScholarGoogle Scholar |
Kostermans AJGH (1974) ‘Flore de la Nouvelle Calédonie et Dépendances: Lauracées. Vol. 5.’ (Muséum National d’Histoire Naturelle: Paris, France)
McPherson G, Lowry PP (2004) Hooglandia, a newly discovered genus of Cunoniaceae from New Caledonia. Annals of the Missouri Botanical Garden 91, 260–265.
McPherson G, Tirel C (1987) ‘Flore de la Nouvelle Calédonie et Dépendances: Euphorbiacées I. Vol. 14.’ (Muséum National d’Histoire Naturelle: Paris, France)
Meve U, Gâteblé G, Liede-Schumann S (2018) Two new species from Ile des Pins (New Caledonia), and a not so new species from Grande Terre (New Caledonia). Phytotaxa 349, 201–213.
| Two new species from Ile des Pins (New Caledonia), and a not so new species from Grande Terre (New Caledonia).Crossref | GoogleScholarGoogle Scholar |
Montrouzier X (1860) Flore de l’Île Art (près de la Nouvelle-Calédonie). Mémoires de l’Académie impériale des Sciences, Belles-Lettres et Arts de Lyon. Classe des Sciences 10, 173–254.
Morat P (1993) Our knowledge of the flora of New Caledonia: endemism and diversity in relation to vegetation types and substrates. Biodiversity Letters 1, 72–81.
| Our knowledge of the flora of New Caledonia: endemism and diversity in relation to vegetation types and substrates.Crossref | GoogleScholarGoogle Scholar |
Morat P, Jaffré T, Tronchet F, Munzinger J, Pillon Y, Veillon J-M, Chalopin M, Birnbaum P, Rigault F, Dagostini G, Tinel J, Lowry PP (2012) The taxonomic reference base Florical and characteristics of the native vascular flora of New Caledonia. Adansonia 34, 179–221.
| The taxonomic reference base Florical and characteristics of the native vascular flora of New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Mouly A, Jeanson M (2015) Specialization to ultramafic substrates and narrow endemism of Cyclophyllum (Rubiaceae) in New Caledonia: contribution of novel species to the understanding of these singular patterns. Acta Botanica Gallica: Botany Letters 162, 173–189.
| Specialization to ultramafic substrates and narrow endemism of Cyclophyllum (Rubiaceae) in New Caledonia: contribution of novel species to the understanding of these singular patterns.Crossref | GoogleScholarGoogle Scholar |
Munzinger J (2015) Novitates neocaledonicae I: an additional new species of Planchonella (Sapotaceae) endemic to the Roches de la Ouaième. Phytotaxa 201, 71–78.
| Novitates neocaledonicae I: an additional new species of Planchonella (Sapotaceae) endemic to the Roches de la Ouaième.Crossref | GoogleScholarGoogle Scholar |
Munzinger J, McPherson G (2016) Novitates neocaledonicae IV: three new species of Cryptocarya R.Br. (Lauraceae). Adansonia 38, 165–174.
| Novitates neocaledonicae IV: three new species of Cryptocarya R.Br. (Lauraceae).Crossref | GoogleScholarGoogle Scholar |
Munzinger J, Swenson U (2009) Three new species of Planchonella Pierre (Sapotaceae) with a dichotomous and an online key to the genus in New Caledonia. Adansonia 31, 175–189.
| Three new species of Planchonella Pierre (Sapotaceae) with a dichotomous and an online key to the genus in New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Munzinger J, Swenson U (2015) Revision of Pycnandra subgenus Leptostylis and description of subgenus Wagapensia (Sapotaceae), a genus endemic to New Caledonia. Australian Systematic Botany 28, 91–110.
| Revision of Pycnandra subgenus Leptostylis and description of subgenus Wagapensia (Sapotaceae), a genus endemic to New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Munzinger J, Morat Ph, Jaffré T, Gâteblé G, Pillon Y, Tronchet F, Veillon J-M, Chalopin M (2016) FLORICAL: checklist of the vascular indigenous flora of New Caledonia. ver. 22.IV.2016. Available at http://www.botanique.nc/herbier/florical [Verified February 2018]
Myers N (1988) Threatened biotas: ‘hot spots’ in tropical forests. The Environmentalist 8, 187–208.
| Threatened biotas: ‘hot spots’ in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar |
OEIL (2018) Alerte incendies, géoportail vulcain. (Observatoire de l’environnement Nouvelle-Calédonie) Available at http://geoportail.oeil.nc/AlerteIncendies [Verified February 2018]
Schmid M (1991) ‘Flore de la Nouvelle Calédonie et Dépendances: Euphorbiacées II, Phyllanthoïdées, Phyllanthus. Vol. 17.’ (Muséum National d’Histoire Naturelle: Paris, France)
Schmid M (2009) Contribution à la connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie. II. Le genre Rapanea Aubl. Adansonia 31, 341–395.
| Contribution à la connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie. II. Le genre Rapanea Aubl.Crossref | GoogleScholarGoogle Scholar |
Schmid M (2012) Contribution à la connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie. III. Les genres Tapeinosperma Hook.f. et Mangenotiella gen. nov. Adansonia 34, 279–341.
| Contribution à la connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie. III. Les genres Tapeinosperma Hook.f. et Mangenotiella gen. nov.Crossref | GoogleScholarGoogle Scholar |
Snow N (2009) Kanakomyrtus (Myrtaceae): a new endemic genus from New Caledonia with linear stigma lobes and baccate fruits. Systematic Botany 34, 330–344.
| Kanakomyrtus (Myrtaceae): a new endemic genus from New Caledonia with linear stigma lobes and baccate fruits.Crossref | GoogleScholarGoogle Scholar |
Snow N, Dawson JW, Callmander MW, Gandhi K, Munzinger J (2016a) New species, new combinations, and lectotypifications in New Caledonian Eugenia L. (Myrtaceae). Candollea 71, 67–81.
| New species, new combinations, and lectotypifications in New Caledonian Eugenia L. (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Snow N, Munzinger J, Callmander MW (2016b) Novitates neocaledonicae V: Eugenia plurinervia N.Snow, Munzinger & Callm. (Myrtaceae), a new threatened species with distinct leaves. Candollea 71, 211–215.
| Novitates neocaledonicae V: Eugenia plurinervia N.Snow, Munzinger & Callm. (Myrtaceae), a new threatened species with distinct leaves.Crossref | GoogleScholarGoogle Scholar |
Stone BC (1985) New and noteworthy paleotropical species of Rutaceae. Proceedings. Academy of Natural Sciences of Philadelphia 137, 213–228.
Swenson U, Munzinger J (2010) Revision of Pycnandra subgenus Achradotypus (Sapotaceae), with five new species from New Caledonia. Australian Systematic Botany 23, 185–216.
| Revision of Pycnandra subgenus Achradotypus (Sapotaceae), with five new species from New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Swenson U, Munzinger J, Bartish IV (2007) Molecular phylogeny of Planchonella (Sapotaceae) and eight new species from New Caledonia. Taxon 56, 329–354.
Swenson U, Nylander JAA, Munzinger J (2018) Phylogeny, species delimitation and revision of Pleioluma (Sapotaceae) in New Caledonia, a frequently gynodioecious genus. Australian Systematic Botany 31, 120–165.
| Phylogeny, species delimitation and revision of Pleioluma (Sapotaceae) in New Caledonia, a frequently gynodioecious genus.Crossref | GoogleScholarGoogle Scholar |
Taylor CM, Razafimandimbison SG, Barrabé L, Jardim JG, Barbosa MRV (2017) Eumachia expanded, a pantropical genus distinct from Psychotria (Rubiaceae, Palicoureeae). Candollea 72, 289–318.
| Eumachia expanded, a pantropical genus distinct from Psychotria (Rubiaceae, Palicoureeae).Crossref | GoogleScholarGoogle Scholar |
Tirel C, Veillon JM (2002) ‘Flore de la Nouvelle Calédonie et Dépendances: Pittosporaceae. Vol. 24.’ (Muséum National d’Histoire Naturelle: Paris, France)
Wulff AS, Hollingsworth PM, Ahrends A, Jaffré T, Veillon J-M, L’Huillier L, Fogliani B (2013) Conservation priorities in a biodiversity hotspot: analysis of narrow endemic plant species in New Caledonia. PLoS One 8, e73371
| Conservation priorities in a biodiversity hotspot: analysis of narrow endemic plant species in New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. List of species endemic to the Belep archipelago (New Caledonia) with reference to the vouchers and coordinates used
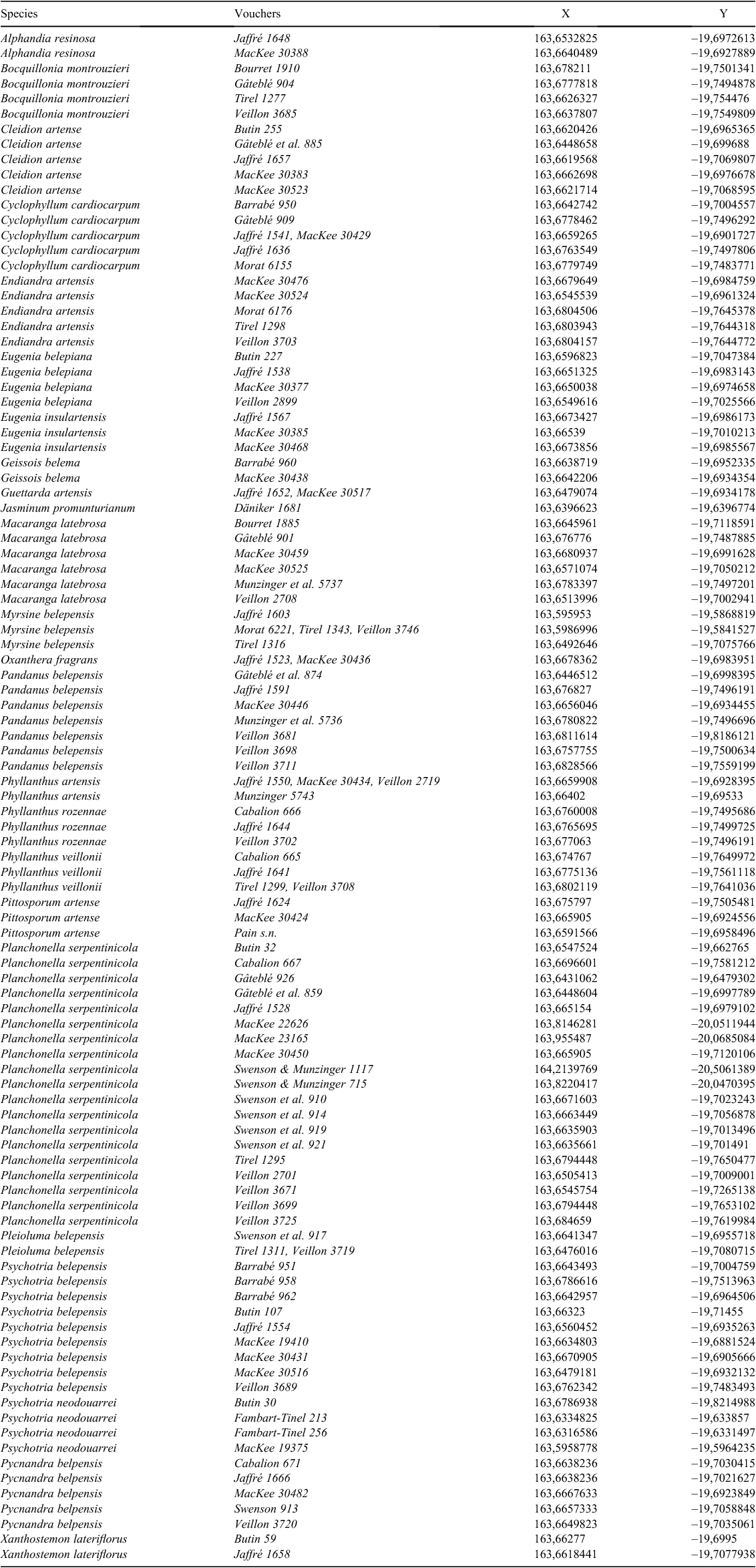
|
Appendix 2. List of published and unpublished (ined.) new taxa for New Caledonia between January 2000 and December 2017
All endemic except for Hymenophyllum braithwaitei and Sphaeromorphaea subintegra. Species statuses are: D, doubtful taxon; H, hybrid taxon; V, valid species; ined., unpublished species

|