Using digital photography to monitor changes in biocrusts and ground cover in a savanna rangeland
 A * , Susanne Schmidt A , Andries Potgieter B , Robyn Cowley C , Vincent Mellor
A * , Susanne Schmidt A , Andries Potgieter B , Robyn Cowley C , Vincent Mellor  D , Colin Driscoll A and Yan Zhao B
D , Colin Driscoll A and Yan Zhao B
A NUE Lab, School of Agriculture and Food Sciences, The University of Queensland, St Lucia, Qld 4072, Australia.
B Queensland Alliance of Agriculture and Food Innovation, The University of Queensland, Gatton, Qld 4343, Australia.
C Department of Industry, Tourism and Trade, Darwin, NT 0801, Australia.
D School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
The Rangeland Journal - https://doi.org/10.1071/RJ22019
Submitted: 5 August 2022 Accepted: 1 February 2023 Published online: 9 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Rangeland Society. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Biocrusts form a living soil cover in Australia’s northern savannas, delivering essential ecosystem services. More accessible tools are needed to quantify and monitor ground cover, including biocrusts, as current methodologies are time-consuming, expensive, or specialised. At Victoria River Research Station (Northern Territory, Australia), long-term fire research plots were used to monitor the response of low vegetative ground and soil covers for different burning intervals and seasons. Mobile phone photographs were analysed using machine-learning software and a derived decision tree-based segmentation model (DTSM). The resulting data were compared to visual in-field assessment by trained researchers. Visual assessments and photographs were taken at two time points during the post-fire recovery period, mid-wet and dry seasons, at three burning intervals (2, 4, and 6 years) and for two different burning times, early or late dry season. DTSM-derived grass and litter cover were statistically similar to field observations in the burnt and unburnt plots. Biocrust cover derived from DTSM also matched field observations in fire treatments and unburnt control plots in the dry season, except when obscured by grass or litter. In the wet season, DTSM underestimated biocrust cover in some treatments, and DTSM did not detect biocrust obscured under dense grass cover. Nevertheless, biocrust pigment analysis confirmed a significant presence of biocrusts both on seemingly bare soil and under the grass canopy. We concluded that mobile phone photographs are suitable for monitoring dry-season ground cover. When similar colours of grass and litter cover were combined, the modelled accuracy reached 95–97%. With some refinements, DTSM analysis of photographs could accurately quantify the impact of fire disturbance on biocrusts and grass cover. However, it would be advantageous to improve the model by additional field records to determine how much biocrust occurs under the grass. This study provides land managers with an efficient method of recording ground cover over time to aid land-condition assessments.
Keywords: biocrusts, fire, ground cover, mobile phone photos, monitoring, rangelands, savanna, soil health.
Introduction
Monitoring vast areas of savanna exposed to climate variability spanning decadal timescales has to be carefully considered to implement effective management strategies. Ground-based monitoring of extensive native grasslands provides fine scale data on grass condition, weed invasion, nutrient cycling, and soil surface condition (Eyre et al. 2011). Such data can then be linked to remote sensing platforms using satellite or aircraft and applied to large-scale analysis of landscape function. By connecting scales, remotely sensed ecolological indices of disturbance combined with high resolution ground cover monitoring can provide greater power to models to identify ecological function at a landscape scale (Ward and Kutt 2009; Tongway and Ludwig 2012). Furthermore, there is a need for spatially hierarchical and complimentary measures that use targeted surveillance and landscape-scale monitoring to provide information for rangeland management (Eyre et al. 2011).
The structure of woodlands and grasslands in the northern Australian dry savannas (600–800 mm average annual rainfall) is primarily driven by variations in climate, being conditioned to monsoon-driven summer rains and dry winters. In the lead-in to the wet season, storms with lightning strikes ignite dry vegetation and can cause large-scale hot fires (Kilinc and Beringer 2007; Bradstock 2010). Historically, fire has been used throughout the year by Indigenous Traditional Landowners to manage ecosystems and avoid vast wildfires (Preece 2002), and fire is used by livestock producers to enhance pasture production by managing woody vegetation cover (Cowley et al. 2014). Fire can negatively affect vegetation, fauna survival (Preece 2002) and the integrity of the soil surface (Barger et al. 2016). In addition to fire, extensive grazing by livestock and other herbivores can damage surface stability and disrupt soil nutrient cycling, which is most pronounced during drought periods when affected landscapes lack vegetation cover and soils are prone to degradation and erosion (Williams et al. 2021).
In this study, we addressed the need for easy-to-execute and efficient methods of monitoring dry savanna ground cover at fine spatial scales, with the view of providing indicators that can be scaled to satellite-level imaging (Barnetson et al. 2017). A key factor influencing soil degradation across landscapes is low or non-existent ground cover that ultimately results in the degradation of the soil surface, concomitant with a loss of nutrients and loss of ecosystem function (e.g. water cycling, carbon storage, vegetation growth). Tongway (2010) identified key components of landscapes with low vegetation cover and damaged soil surfaces that directly influence ecological function. These components include grass and canopy cover that reduce the impact of rain splash erosion, and litter fall as indicative of a functional nutrient cycle. An important element of soil and ecosystem integrity is biological soil crusts, i.e. biocrusts, which bind soil particles, regenerate carbon and nitrogen, and are integral to soil stability and nutrient cycling (Evans and Lange 2001; Bowker et al. 2014; Williams et al. 2018; Büdel et al. 2018).
In the rangelands biocrusts form a living soil cover, delivering essential ecosystem services. Accessible tools are needed for quantifying and monitoring ground cover, including biocrusts, as current methodologies are time-consuming, expensive, or specialised. Loss of grass, canopy cover and biocrusts in dry savanna, in combination with an overall low vegetative cover, can rapidly result in a degraded landscape. When such a landscape is affected by fire, the ground cover is burnt, with ash as a transient soil cover and biocrusts integrated into the soil surface, either burnt or inactive, until the next rain (Brianne et al. 2020). At this point, the landscape is at its most vulnerable state with much of the soil bare and prone to wind and water erosion (Flores et al. 2020).
To address the challenges of current methods for analysing biocrusts and their recovery post-disturbance at the field level (e.g. paddocks or grazing regions), we aimed to develop an easy-to-use alternative utilising the power of mobile phones and high-performance computing. Over the past decade, high-resolution photography (red, green, blue, RGB) and digital technologies with machine learning (ML) have enabled new methodologies for measuring plant canopy (Guo et al. 2017), plant nutrition estimations (Shi et al. 2021) and assessment of biomass for grasslands (Possoch et al. 2016).
Following on from successful close-range unmanned aerial vehicle (UAV) imagery analysis of biocrusts (Havrilla et al. 2020), we hypothesised that ground cover including biocrusts could be effectively quantified using ML based on RGB reflectance. To test our hypothesis, we compared field data with mobile phone images of ground cover plots. We expected no significant differences between the two methods. With a focus on rapid assessment tools for monitoring ground cover, our overarching aims were to (i) evaluate whether the proposed methodology would work as a tool for use by land managers, and (ii) examine how it performs under different conditions, such as burning regimes and across seasons.
Materials and methods
Study site
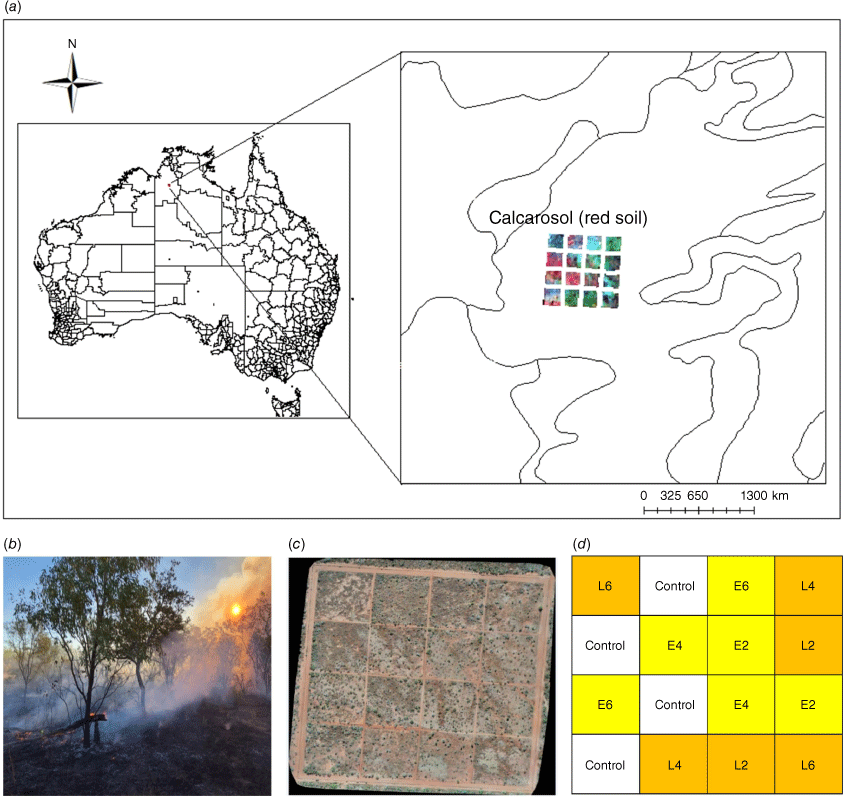
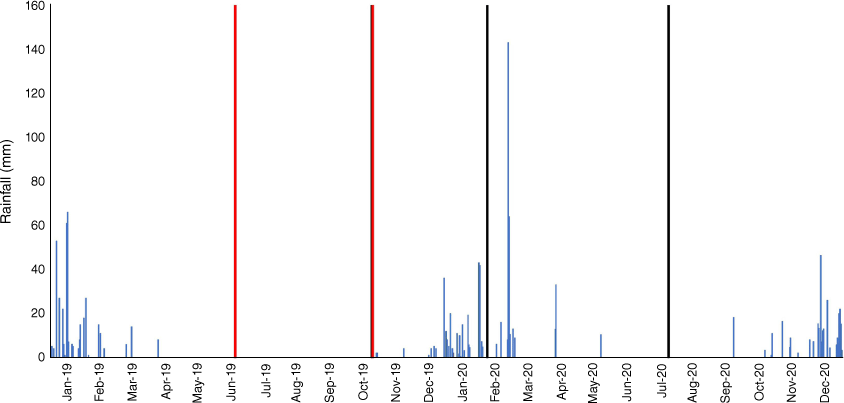
Field data were collected at the Victoria River Research Station (VRRS, Kidman Springs, Northern Territory; 16.12°S, 130.96°E; Fig. 1a). The climate is dominated by a summer wet season from November to April, and a drier period with little to no rain from May to October. The annual average temperature range is 20.1–34.9°C and average annual rainfall is 753.9 mm (Bureau of Meteorology 2021). Rainfall for the 12 months covering the pre-fire and wet season sample times is shown in Fig. 2.
|
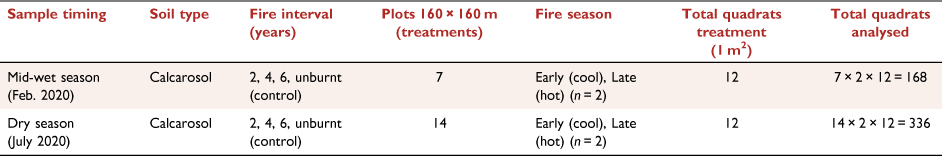
In 1993, a long-term fire research program was established at VRRS to investigate the best timing for fire in terms of burning interval (years between fire) and season (Cowley et al. 2014). Within a fenced-off area, 16 experimental plots (160 m × 160 m) set on a 4 × 4 grid pattern were established (Fig. 1b), with two replicates for each fire treatment: early (E) or late (L) dry-season burning, with fire intervals of 2, 4 and 6 years and four unburnt control plots (Fig. 1c, d); firebreaks separated each plot.
Research data were collected only from the open eucalypt woodland (Conkerberry Paddock) trial site, with pastures dominated by grasses Heteropogon contortus, Enneapogon spp. and Dichanthium fecundum (Cowley et al. 2014). Grasses are underlain by red calcarosol soils, with soil texture from 0 down to 10 cm comprising clay (32%), silt (16%), sand (53%) and pH 7.9 (Allen et al. 2011). VRRS is a cattle research station, and the fire trials are open to cattle at the end of the first wet season following fire, grazing density is at industry recommended continuous stocking rates (Cowley et al. 2014). As we sampled during and at the end of the wet season, there was no grazing during this period.
Field measurements
Biocrusts and ground cover
The assessment of ground cover included both low vegetative cover (annual and perennial grasses) and litter accumulation. Bare soil was divided into visible biocrust cover and bare soil with no apparent biocrust cover. These four parameters (herein called microsites) were selected for ground cover descriptions that could be repeated in future studies to determine key functional indices representative of post-fire recovery such as soil stability and nutrient cycling (as described by Tongway 2010).
Fire plots were burnt in the dry season in mid-June or late October 2019. The first wet-season rains were recorded in early December 2019. Total rainfall for the 2019–2020 wet season was 602.5 mm with the last rain fall in May. The rainfall distribution suggests that the grasses and biocrusts had their maximum chance of recovery post-wet season (Büdel et al. 2018). To measure early recovery, initial data collections for ground cover occurred mid-wet season (February 2020) after approximately 260 mm rainfall in the preceding 2 months. Dry season measurements were made in July 2020. The sampling times were intended to document the recovery of grass and biocrusts post-fire during recovery and following a full wet season.
To assess ground cover, two 30-m transects were established centrally in each fire-treatment plot in the eucalypt woodland site. This study had seven treatments, with two plots each for the early and late fire seasons at 2-, 4-, and 6-year intervals and utilised two of the four unburnt plots as control treatments (Fig. 1c, d). Due to limited site access in the mid-wet season, measurements were carried out on one plot per treatment (n = 7), while two plots per treatment were sampled in the dry season (n = 14; Fig. 1d). At each treatment plot, 1-m2 quadrats were placed at 5-m intervals, commencing at 0 m alongside the 30-m transect (n = 6 per transect; Table 1). Overall covers of grass and small shrubs were visually estimated for each quadrat (Fig. 3c); ground cover, including that under the grass canopy and small shrubs, was also estimated. Biocrust, bare soil, litter and basal area of the grass cover were visually estimated and recorded as a percentage relative to total cover. The four values made up 100% of the ground cover. The overall grass canopy cover was estimated separately. Field observers were trained over several quadrats to assess cover attributes, and data calibrations were performed by experienced biocrust researchers.
|
Taking RGB photographs in the field
RGB photographs of each quadrat were collected for use in EasyPCCr to determine ground-cover attributes. In each plot, photographs of the 1-m2 quadrat were taken at about 1.5-m height with a mobile phone (iPhone 11 pro; Fig. 2c, d, Table 1). There were n = 336 quadrats measured and photographed in the dry season and n = 168 in the wet season.
Image-classification method
An image-classification software (EasyPCCr) was used to determine the different cover percentages within each photo. EasyPCCr is based on a decision tree-based segmentation model (DTSM) and is an effective method to extract areas of plant foliage from RGB photographs that are taken in natural light conditions. This method defines individual classes for each object relating to specific plant and non-plant characteristics within an area. By this means, ML models are trained to recognise the classes (Guo et al. 2013). We used the EasyPCCr tool to determine the percentage of cover types from RGB photographs of sample quadrats across different fire treatments and time since fire.
Biocrust field collections and pigment analysis
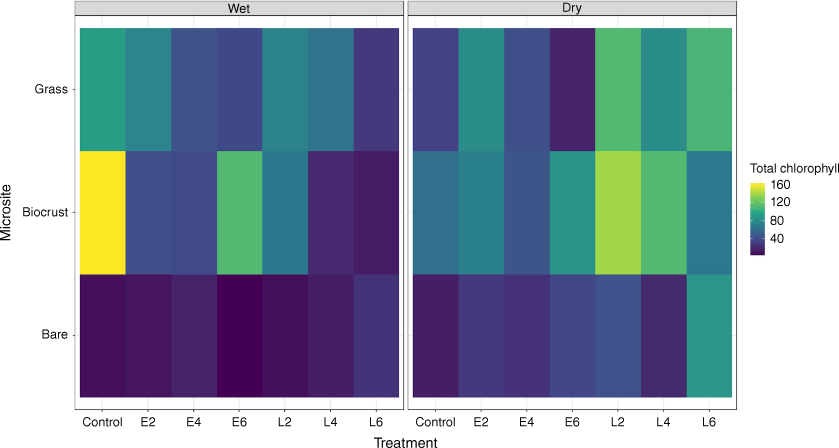
To confirm the presence of biocrusts, replicate micro-core samples of soil surfaces (up to 1 cm depth) were taken from each microsite. We had previously determined that the biocrusts at this site were dominated by photosynthetic cyanobacteria, some of which are surface dwelling and others subsurface (within first few millimetres). These samples provided confirmation of biocrust presence, especially under the grass canopy and litter where they are not always clearly visible. Three micro-cores (3.56 cm2 × 1 cm depth, average weight 5 g) of visible biocrust or apparently bare soil were collected from all treatment microsites (grass, litter, biocrust and bare ground). However, biocrust under litter was collected only during the dry season (July 2020). There was virtually no litter cover mid-wet season to provide sample points, because this had been recently burnt. The samples were dried and individually stored in sealed Falcon tubes and transported back to the laboratory. To preserve the chlorophyll (pigment), analysis was undertaken within 2 weeks of sample collection. Prior to extraction, all samples were moistened with equal amounts of water to facilitate cell rehydration. Biocrust pigment content analysis (chlorophyll a, b, c concentrations) was undertaken using a 2-h dark extraction with a 1:5 ratio of di-methyl-sulfoxide (DMSO; Barnes et al. 1992), and the concentrations were calculated using Wellburn’s (1994) equations. Results are shown as biocrust pigment and data were converted to micrograms of chlorophyll per square metre for all treatments and microsites. Heat maps were used to demonstrate the chlorophyll concentrations across all treatments.
Quantifying ground cover by using decision tree-based segmentation model (DTSM)
The photographs were processed to obtain the cover of grass, biocrusts, litter and bare soil, using DTSM. The model uses a classification and regression tree algorithm (Barnetson et al. 2019) to create a series of nodes to stepwise discriminate the photo elements under different light conditions. Variables of colour in this model include red (R), green (G), blue (B), hue (H), saturation (S), value (V), lightness (L*), red/green intensity (a*) and yellow/blue intensity (b*). This covers any differences in range and texture, works well in situations with clearly ldefined background colour classes. The study used the EasyPCCr tool, which provides a graphical user interface (GUI) for implementing the model using sample data, model training and generating classification maps (Guo et al. 2017).
Preparation of photographs and defining region of interest (ROI)
For each RGB image, the entire frame was used for locating the training pixels and then creating a training dataset for each cover type (Fig. 4). The image was then cropped to the quadrat frame and loaded into the model for comparisons across photographs. This was undertaken using the ROI tool in EasyPCCr. The training dataset was generated for individual photographs because of different light conditions and the DTSM segmentation was also applied to each image.
The vegetation cover included green leaves and stems of annual and perennial plants. The identification of litter captured debris, including stones, dry branches, and dead grasses. Biocrusts were identifiable by their dark colour (pigment) compared to the natural red colour of the soil. Due to the nature of an overhead photograph, ML-based cover estimates were determined from the visible areas of biocrust in RGB image analysis. This contrasts with the field observations where biocrusts that occurred under the grass canopy were included in the ground-cover measurements. Bare soil were areas that had no distinguishable biocrust on the soil surface.
DTSM application and accuracy evaluation
The DTSM algorithm was adopted to create the decision tree, and the noise reduction filter (size 100) was applied to reduce the misclassification of the training photographs. This step was needed to enhance photo quality and thus increase accuracy of the classification and segmentation of individual classes from the model. The analysis was carried out in the EasyPCCr tool that showed the output of percentage cover (%Cov) of individual classes in each photo (Fig. 5). DTSM generates individual class data as cover portions that total 100% and are provided as surface cover for each training photo.

|
The verification process is an important step that determines the accuracy of an algorithm model or ML tool. In this study, verification of the model was implemented by comparing statistical analysis data between DTSM-developed ground cover and field-collected ground cover. The DTSM step in EasyPCCr produced the four ground-cover classes, i.e. grass cover, litter, visible biocrust and bare soil, which were identified in training photographs. This information provided the means to analyse the effects of fire treatments and how the landscape has recovered.
Statistical analysis
For each grass, litter, visible biocrust, and bare soil measurement, a single value was compiled for each plot by averaging values from all quadrats for wet and dry seasons derived from individual experimental plots. This provided two values (mid-wet season and dry season) per treatment. The research data were analysed using R-studio statistic software (Su et al. 2021).
We used DTSM to analyse the grass and litter combinations within 1 m2 and the field-observed biocrust percentage cover (%Cov ± s.e.) for both the mid-wet season and dry season. In the case of the field observations, grass and litter cover were above 100% because litter could occur under the grass canopy. To obtain a more accurate percentage for comparisons with modelled results, we calculated the combination of both and divided it by the total percentage of all ground cover (microsites). Grass and litter combined cover was then analysed to determine the difference between DTSM and field measurements. Biocrust %Cov was analysed in R statistics software. The combination of grass and litter data were analysed using three-way analysis of variance (ANOVA) and Tukey’s honestly significant difference post hoc test (HSD) to identify significant differences. A simple linear regression analysis was applied to explore relationships among various ground covers in both seasons. Biocrust pigment variation (Chl) for microsites was assessed using one-way ANOVA and Tukey’s HSD and the heat maps.
Results
Modelled grass cover compared to field measurements
In the wet season, there were no significant differences between the modelled cover and the field measurements (P > 0.05). There was, on average, a difference of 8.4%Cov between modelled (48%Cov ± 2.54) and field measurements (56%Cov ± 3.53; Fig. 6). Across the early season burning treatments, the average difference in modelled grass cover (49.4%Cov ± 8.81) was 8.2% lower than field observations (57.6%Cov ± 8.81). In the wet season, modelled grass cover in the late-season burning treatments was lower than the field observations. In the unburnt control plots, the modelled grass cover was lower than that in the field data.
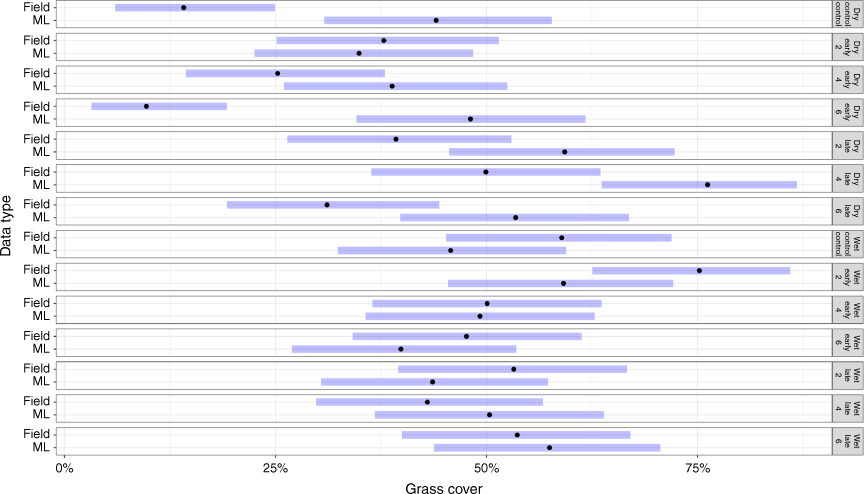
|
In the dry season, there were significant differences between modelled and field methods in estimated grass cover. Modelled grass cover was higher than field observation across fire treatments and control plots (P < 0.01). For example, in the 4-year early burn plots, grass cover (modelled) was 38.8%Cov, with an average difference of 13.5%, compared to field data. In contrast, modelled grass cover for the 2-year early burn plots (34.9%Cov ± 2.52) was a near match to field observations (37.8%Cov ± 6.48). However, there were no significant differences (P = 0.07) between modelled and field data across the early burning treatments, except for the early 6-year burnt plots (P < 0.001). In the late-burn plots, the modelled grass cover for the late 2-year and late 4-year plots was 59.3%Cov (±1.8) and 76.2%Cov (±7.61) respectively, and significantly (P < 0.001) different from the field observations of late burn every 2-year plots, 39.3%Cov ± 6.6, and late burn every 4-year plots 49.9%Cov ± 7.15, (Fig. 6). Field observations of grass cover in the wet season (56% ± 3.53) were higher than those in the dry season (30% ± 5.41), whereas modelled grass cover in the wet season (48% ± 2.54) was lower than that in the dry season (51% ± 5.29).
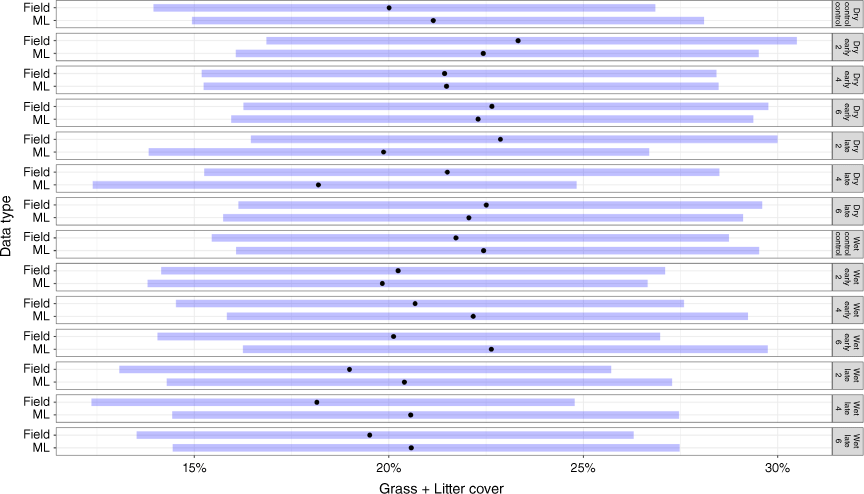
Modelled grass and litter cover compared to field measurements
When grass and litter cover were estimated together, there was greater similarity between methodology estimates. For the wet season, grass and litter %Cov derived from photographs averaged 8.8% (±2.6) across all treatments and was comparable to field measurements, with an average of 1% difference between them (Fig. 7). We also measured grass and litter cover across fire-plot treatments in July 2020 during the dry season (rain ceased in May). Modelled grass and litter %Cov across all treatments was 20.9% (±0.6), compared to field observations of 22.21% (±0.32). Furthermore, no significant differences were observed between fire treatments and the unburnt control plots in either wet or dry seasons (P = 0.99; Fig. 7) where there was an average cover difference of 1%. Both field observations and modelled grass cover in the wet season were slightly lower than those in the dry season.
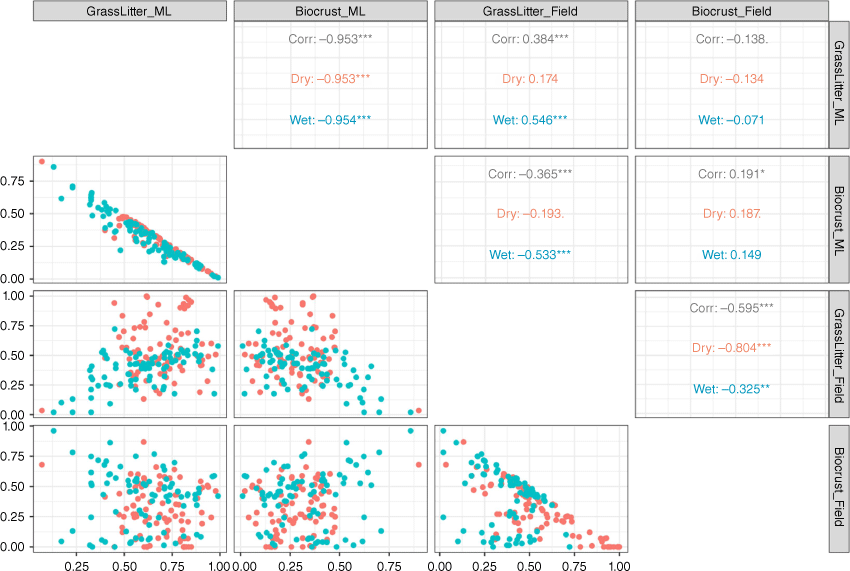
Relationships between microsites
In both seasons, when grass and litter cover were analysed in combination, as their cover increased, the modelled biocrust cover decreased (Fig. 8). There was a strong inverse relationship between grass and litter cover compared with visible biocrust cover in both the wet (R2 = 0.95) and the dry seasons (R2 = 0.95; Fig. 8). Additionally, biocrust from the field observation decreased when the combined grass and litter coverage in the plot increased, especially in the dry season (R2 = 0.80). DTSM can also distinguish between biocrust and bare soil in both seasons (wet, R2 = 0.15; dry, R2 = 0.09). Yet, the relationship between bare and biocrust from field observations in the wet season showed that as bare cover increased, the biocrust cover decreased (R2 = 0.80).
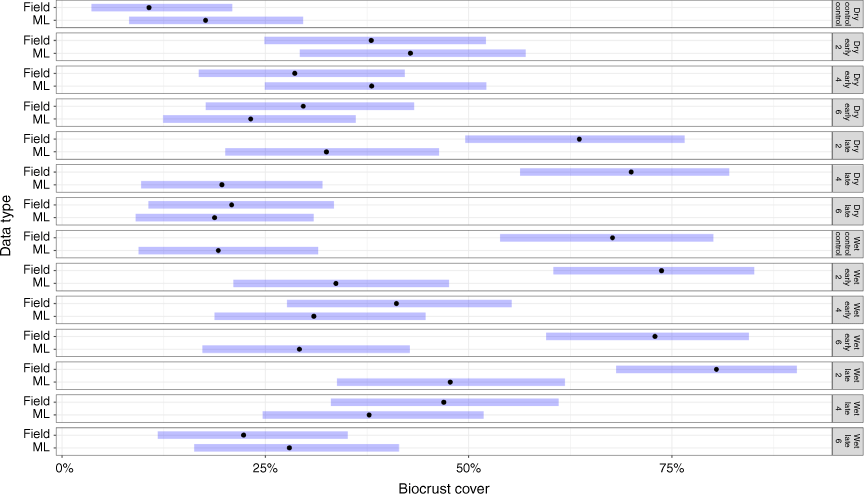
Modelled biocrust cover compared to field measurements
In the wet season, modelled biocrust cover (34%Cov ± 2.0) differed significantly from field measurements when averaged across all treatments (39%Cov ± 3.2; P = 0.04; Fig. 9). These differences were more obvious in early burning treatments, such as the 6-yearly early burning treatment (P < 0.01) where the modelled cover was, on average, 20% lower than the observed field result (Fig. 9). Furthermore, in the unburnt control plots, detected biocrust cover (20%Cov ± 2.1) was also significantly lower than field observations (57%Cov ± 5.8) (P < 0.01). In contrast, modelled biocrust cover (33%Cov ± 3.3) was comparable with field observations (42%Cov ± 6.2) in late-burning treatments (P = 0.21; Fig. 9).
In the dry season, modelled biocrust cover across all burnt and unburnt treatments was comparable to field observations (Fig. 9). Biocrust cover in early burning plots averaged 30%, with no significant differences between DTSM-modelled cover and field observations. Similar data were generated for modelled biocrust cover on control plots (18%Cov ± 2.8) and field observations (15%Cov ± 5.6), although DTSM-modelled biocrust cover in late-burn plots (25%Cov ± 2.7) differed significantly from field observations (53%Cov ± 4.4). However, there were no overall differences between modelled biocrust cover and field observations in either fire treatments and control plots in the dry season (P = 0.06), with the exception of the late 4-year burning treatment (Fig. 9).
Biocrust pigment content across microsites
Biocrust pigment content based on chlorophyll concentration (Chl) was analysed across three microsites, i.e. biocrust, grass and bare soil in both wet and dry seasons (Fig. 10). Overall, bare soil microsites had the lowest and biocrust microsites the highest pigment concentrations. Biocrust pigment was recorded under litter but only in the dry season (Supplementary Fig. S1).
In the wet season, the highest average pigment concentration was detected in biocrust microsites in the control plots (162 ± 35 mg Chl/m2; P < 0.01). In the burnt treatments, the early season 6-yearly (E6) burn plot was not significantly different from the unburnt controls (112 ± 12 mg Chl/m2, P = 0.35) whereas L6 (14 ± 1 mg Chl/m2) and L4 (20 ± 3 mg Chl/m2) had significantly lower pigment concentration compared to the other treatments (P < 0.01), in contrast to E6 which had high concentrations in the biocrust microsites.
Furthermore, in the wet season, microsites under grass canopy also had the highest pigment concentrations in the unburnt control plots (92 ± 19 mg Chl/m2) and E2 (75 ± 5 mg Chl/m2), whereas L6 (29 ± 1 mg Chl/m2) had significantly (P < 0.01) lower pigment concentrations in grass microsites. Microsites under grass in E6 (38 ± 4 mg Chl/m2) and E4 (45 ± 1 mg Chl/m2) had also significantly lower pigment concentrations than did the unburnt control (P < 0.01). In the wet season, although low, L6 bare microsites had significantly (P < 0.05) higher pigment concentrations (26 ± 5 mg Chl/m2) than did other burnt bare microsites, including E2 (10 ± 2 mg Chl/m2), L2 (8 ± 2 mg Chl/m2), unburnt control (8 ± 2 mg Chl/m2) and E6 (2 ± 2 mg Chl/m2; P < 0.01). Pigment concentration in bare microsites E4 (17 ± 4 mg Chl/m2) and L4 (14 ± 1 mg Chl/m2) was significantly higher than E6 (P < 0.04).
In the dry season, L6 had the highest pigment concentrations for all burnt treatments and in the unburnt control plots; however, they were not significantly (P = 0.16) different. The highest pigment in biocrust microsites ranged between (L6) and 128 ± 35 mg Chl/m2 (L2). The lowest pigment recorded was in E4 (45 ± 11 mg Chl/m2) and the unburnt control (61 ± 31 mg Chl/m2). Litter microsites in the control had the highest pigment (236 ± 48 mg Chl/m2) together with E4 (190 ± 116 mg Chl/m2), but both showing the large variability between sample points.
L6 also had the highest pigment concentration under the grass canopy (108 ± 13 mg Chl/m2), whereas E6 had the lowest pigment concentration (17 ± 2 mg Chl/m2; P < 0.01). Similarly, the control had low pigment (34 ± 7 mg Chl/m2), significantly different from L2 (86 ± 5 mg Chl/m2; P < 0.01), L4 (104 ± 7 mg Chl/m2) and E2 (81 ± 17 mg Chl/m2; P < 0.03). E4 (42 ± 7 mg Chl/m2) was significantly (P < 0.01) lower than L6 and L2.
Similarly, L6 had the highest pigment concentration in dry-season bare microsites (86 ± 38 mg Chl/m2) and the lowest in the unburnt control plots (14 ± 3 mg Chl/m2), although differences were not significant (P = 0.06). For example, E6 (37 ± 3 mg Chl/m2), L2 (35 ± 3 mg Chl/m2), E2 (29 ± 9 mg Chl/m2) and E4 and L4 were both 25 ± 3 mg Chl /m2.
Discussion
Seasonal impacts on ML accuracy
Building good ‘training’ data was a crucial part of the photographic analysis. If the object was correctly defined, DTSM provided accurate data. In the dry season, even though grass and litter cover were recorded under sunny conditions in the training photographs, it was difficult to obtain accurate training data when these ground-cover classes were presenting the same or similar colours. For example, the grass colour resembled litter, dry leaves and dead grass, and ML over-estimated grass cover. In effect, the grass cover measured by ML was a combination of dry litter and dry grass, whereas observers could visually separate the two. Also, dry-season grass and litter cover in the 2-yearly burning treatments and unburnt control were significantly higher than those in other treatments.
Thus, one of the challenges of the DTSM model was to separate objects of the same colour among different trained classes. We approached this problem in two ways. First, we refined the training with attention to detail and colour differentiation. Second, we confirmed the poorer differentiation by ML, by combining litter and grass. When we performed these steps, there were no significant differences between ML and observers. Future applications could couple these measurements as a positive indicator of ground cover and soil-surface stability (Williams et al. 2021).
For wet-season field measurements, high soil water content and active biological processes indicated by pigment (Belnap et al. 2016) improved biocrust identification of biocrust presence (Blanco-Sacristán et al. 2019, 2021). In this case, as the biocrust was sometimes difficult to see (owing to the early stage of recovery), in situ pigment detection verified its presence. Furthermore, the DTSM model could not detect biocrust cover located under high density of grass, shrubs, or litter cover. This was demonstrated in the regression models (Fig. 7).
Measuring biocrusts using ML
Methods such as UAV (Havrilla et al. 2020; Blanco‐Sacristán et al. 2021), airborne sensing (Weber et al. 2008; Rodríguez-Caballero et al. 2014) and satellite imagery (Panigada et al. 2019) can map landscapes, but have limitations because of environmental conditions obscuring biocrust quantification with dry- versus wet-season variability, sun position without clouds, and using conventional indices (e.g. normalised difference vegetation index (NDVI); Karnieli et al. 2001; Belnap et al. 2016). Such methods are also unsuitable for direct use by most land managers, although there is a need for land manager-based on-ground monitoring.
In the dry season, biocrusts were quite clearly visible because of their darkened colour contrasting against the red calcarosol soils. However, in many cases, there was biocrust under grass and shrubs (recorded by observers) that was not discernible by the ML methods we used, as a consequence of the grass canopy covering the biocrust. During the wet season, biocrust pigment concentrations derived from microsites demonstrated that biocrusts were as prevalent under the grass canopy as they were in the interspaces between the grass plants and, to a lesser extent, in apparently bare soil (Fig. 10). Biocrust pigment was not measured under litter in the wet season because of the scarcity of litter so soon after fire. Nevertheless, dry-season pigment measurements taken in 2020 reconfirmed the presence of biocrusts across all microsites including under litter (data not shown). In some instances, dry-season pigment was less concentrated than the wet-season one, whih is likely to be indicative of the inactive state of the biocrusts.
There was a high correlation between the increase in grass and litter cover and a decreased biocrust cover (Fig. 7). This demonstrated the limitation of this method in detecting biocrust cover that was not visible in the photo as it was obscured by the grass canopy. Another factor that contributed to decreased accuracy in biocrust cover detection was likely to be the colour effect. As there were two visible biocrust colours (light and dark), and light patches of biocrust mixed with bare soil and small grasses, detection was often difficult, leading potentially to underestimation of biocrust presence. In previous studies, the classification of biocrusts was identified as dark and light biocrust by using UAV images, but the identification of light-coloured cyanobacteria has presented challenges in UAV images when separating from bare soil and surface roughness, with shadowing from biocrust microtopography. These surface cover classes are difficult to separate due to the reflectance in the RGB zone and when real-life colours are similar (Havrilla et al. 2020). To resolve these problems, further field measurements that estimate biocrusts under the grass canopy as a separate measure to visible areas could be used to establish a suitable crust cover index for different landscapes.
ML comparisons with alternative methods
We analysed ground cover, including biocrust, grass, litter, and bare soil to determine whether digital photographs were suitable. We applied ML techniques to aid in the quantification of these microsites following different burning treatments and in wet and dry seasons. Comparing data obtained by trained biocrust field-observers with ML analysis of photographs, we demonstrated that it was feasible to monitor microsite recovery post-fire disturbance by using a mobile phone camera.
Information derived from photographs, generated by ML by using the DTSM algorithm, generally showed high accuracies in detecting cover of each class when compared with field observation. There were exceptions in some cases, such as biocrust cover in the wet season when grass tussocks obscured biocrusts. Yet, the proposed method provided a high level of accuracy (88%) for biocrust cover in contrasting conditions, including the dry season, and following late burning in the wet season.
However, it was apparent that there was a lower level of accuracy for early dry-season burning, most likely a consequence of the increase in litter, although it could also be related to observer errors. In addition, observers did not consider any differences in plant species that may influence accuracies. Further ground-truthing and adjustments to the model that are specific to seasonal conditions and biocrusts are therefore required.
Post-fire recovery monitoring tools
The Australian savanna landscapes are extensive and often subject to managed and natural fires (Cowley et al. 2014). Furthermore, rainfall is spatially variable, resulting in better recovery in some paddocks than others, or even within paddocks that can be as large 2000 ha or more (O’Reagain et al. 2009). Even though it appears feasible that UAVs and satellite imagery encompass the large areas, resolution can range between 10 and 30 m (multi-spectral), with small-scale attributes of the ground cover mixed.
The benefit of digital photographs is the capacity to collect relatively large numbers of high-resolution images at a small scale. Photos can be acquired seasonally with minimal effort (e.g. at fixed sentinel points) while performing tasks such as checking cattle, water points and fences. Once the ML tools have been applied for specific regions with similar characteristics, their applications are time- and cost-effective. The EasyPCCr method has been proven efficient in cropping systems (Guo 2018; Tresch et al. 2019) and, as demonstrated, can be readily applied to the savanna grasslands such as the landscapes in this study. Decision-making regarding ground cover and local conditions, especially for post-fire or drought recovery, can then be based on biocrust presence as an indicator of soil health and land condition (Williams et al. 2021). We acknowledge that not all land managers would have the capacity to analyse their photographs, although field extension officers could be trained to develop the DTSM model for their local region.
Conclusions
Advantages of ML methods
The method developed here required minimal time to collect field data and used simple equipment and relatively easy data processing to quantify biocrust and other ground-cover types at a fine scale. Similarly, quantifying grass and litter cover following early and late-season burning provided results between methods with 95–97% similarity. The exception was an underestimation of grass and litter cover produced by the DTSM algorithm for the 2-year early season burning treatment in the wet season, which was likely to be a result of small grasses being shaded by the photographer’s shadow and quadrat frame. However, statistically there was no difference between grass cover in this treatment plot when combined with litter cover when DTSM was compared with field observations. Thus, the DTSM model provided accurate results under various light conditions, and, like other studies, produced the best results in sunny conditions (Guo et al. 2017).
The methodology effectively detected ground-cover classes from photographs taken with a mobile phone in combination with ‘off-the-shelf’ image-analysis software package and DTSM learning algorithm. Our derived sensing metric showed moderate to high accuracies in classifying different ground cover when compared with observations by specialists in the field. We concluded that the methodology developed in this study effectively quantified ground cover, including grass and biocrust, as potential indicators of primary productivity and soil health.
A constraint for quantifying biocrusts was high grass cover in the wet season such as the unburnt control plots or early 6-yearly burn plots where biocrusts were often obscured by grass tussocks. Nevertheless, we confirmed that there was a considerable biocrust presence under grass tussocks, which was confirmed by the pigment analysis. This created one of the key differences between methods with observer estimations of microsite cover, incorporating cover underneath grass canopy and in the interspaces between grass tussocks. Thus, to accurately analyse biocrust and litter cover, the estimates for biocrusts and litter under the grass canopy need to be built into the model. In terms of landscape function, it appeared that dry-season photographs were more informative. With promising findings, next steps in this research should be expanded to analyse other soil types so that a land manager-based management tool can be developed.
Supplementary material
Supplementary material is available online.
Data availability
The data are available on request from the corresponding author.
Conflicts of interest
The authors declare there are no conflicts of interest.
Declaration of funding
We thank Meat & Livestock Australia’s for funding the research program ‘Boosting natural regeneration of the nitrogen capital in grazing lands’ (B.PAS.0502) in partnership with The University of Queensland, the Northern Territory Department of Industry, Tourism and Trade, and the Department of Agriculture and Fisheries Queensland and Victoria River Research Station. TMS acknowledges the Australian Government for funding his studies in Australia under the Australia Awards program.
Acknowledgements
We thank Stephen Williams, Laiza Sherar, Madeline Dooley, Harry Cosgrove and the Kidman Springs team for the field photographs and data collection.
References
Allen, EB, Steers, RJ, and Dickens, SJ (2011). Impacts of fire and invasive species on desert soil ecology. Rangeland Ecology & Management 64, 450–462.| Impacts of fire and invasive species on desert soil ecology.Crossref | GoogleScholarGoogle Scholar |
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J (2016) Patterns and controls on nitrogen cycling of biological soil crusts. In ‘Biological soil crusts: an organizing principle in drylands’. (Eds B Weber, B Büdel, J Belnap) pp. 257-285. (Springer: Cham, Switzerland)
Barnes, JD, Balaguer, L, Manrique, E, Elvira, S, and Davison, AW (1992). A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environmental and Experimental Botany 32, 85–100.
| A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants.Crossref | GoogleScholarGoogle Scholar |
Barnetson, J, Phinn, S, Scarth, P, and Denham, R (2017). Assessing Landsat fractional ground-cover time series across Australia’s arid rangelands: separating grazing impacts from climate variability. International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences XLII-3/W2, 15–26.
| Assessing Landsat fractional ground-cover time series across Australia’s arid rangelands: separating grazing impacts from climate variability.Crossref | GoogleScholarGoogle Scholar |
Barnetson, J, Phinn, S, and Scarth, P (2019). Mapping woody vegetation cover across Australia’s arid rangelands: utilising a machine-learning classification and low-cost Remotely Piloted Aircraft System. International Journal of Applied Earth Observation and Geoinformation 83, 101909.
| Mapping woody vegetation cover across Australia’s arid rangelands: utilising a machine-learning classification and low-cost Remotely Piloted Aircraft System.Crossref | GoogleScholarGoogle Scholar |
Belnap J, Weber B, Büdel B (2016) Biological soil crusts as an organizing principle in drylands. In ‘Biological soil crusts: an organizing principle in drylands’. (Eds B Weber, B Büdel, J Belnap) pp. 3–13. (Springer)
Blanco-Sacristán, J, Panigada, C, Tagliabue, G, Gentili, R, Colombo, R, Ladrón de Guevara, M, Maestre, FT, and Rossini, M (2019). Spectral diversity successfully estimates the α-diversity of biocrust-forming lichens. Remote Sensing 11, 2942.
| Spectral diversity successfully estimates the α-diversity of biocrust-forming lichens.Crossref | GoogleScholarGoogle Scholar |
Blanco‐Sacristán, J, Panigada, C, Gentili, R, Tagliabue, G, Garzonio, R, Martín, MP, Ladrón de Guevara, M, Colombo, R, Dowling, TPF, and Rossini, M (2021). UAV RGB, thermal infrared and multispectral imagery used to investigate the control of terrain on the spatial distribution of dryland biocrust. Earth Surface Processes and Landforms 46, 2466–2484.
| UAV RGB, thermal infrared and multispectral imagery used to investigate the control of terrain on the spatial distribution of dryland biocrust.Crossref | GoogleScholarGoogle Scholar |
Bowker, MA, Maestre, FT, Eldridge, D, Belnap, J, Castillo-Monroy, A, Escolar, C, and Soliveres, S (2014). Biological soil crusts (biocrusts) as a model system in community, landscape and ecosystem ecology. Biodiversity and Conservation 23, 1619–1637.
| Biological soil crusts (biocrusts) as a model system in community, landscape and ecosystem ecology.Crossref | GoogleScholarGoogle Scholar |
Bradstock, RA (2010). A biogeographic model of fire regimes in Australia: current and future implications. Global Ecology and Biogeography 19, 145–158.
| A biogeographic model of fire regimes in Australia: current and future implications.Crossref | GoogleScholarGoogle Scholar |
Brianne, P, Rebecca, H, and David, L (2020). The fate of biological soil crusts after fire: a meta-analysis. Global Ecology and Conservation 24, e01380.
| The fate of biological soil crusts after fire: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Büdel, B, Williams, WJ, and Reichenberger, H (2018). Annual net primary productivity of a cyanobacteria-dominated biological soil crust in the Gulf Savannah, Queensland, Australia. Biogeosciences 15, 491–505.
| Annual net primary productivity of a cyanobacteria-dominated biological soil crust in the Gulf Savannah, Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2021) Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/averages/tables/cw_014847.shtml [accessed 20 June 2021]
Cowley, RA, Hearnden, MN, Joyce, KE, Tovar-Valencia, M, Cowley, TM, Pettit, CL, and Dyer, RM (2014). How hot? How often? Getting the fire frequency and timing right for optimal management of woody cover and pasture composition in northern Australian grazed tropical savannas. Kidman Springs Fire Experiment 1993–2013. The Rangeland Journal 36, 323–345.
| How hot? How often? Getting the fire frequency and timing right for optimal management of woody cover and pasture composition in northern Australian grazed tropical savannas. Kidman Springs Fire Experiment 1993–2013.Crossref | GoogleScholarGoogle Scholar |
Evans RD, Lange OL (2001) Biological soil crusts and ecosystem nitrogen and carbon dynamics. In ‘Biological soil crusts: structure, function, and management’. (Eds J Belnap, OL Lange) pp. 263–279. (Springer)
Eyre, TJ, Fisher, A, Hunt, LP, and Kutt, AS (2011). Measure it to better manage it: a biodiversity monitoring framework for the Australian rangelands. The Rangeland Journal 33, 239–253.
| Measure it to better manage it: a biodiversity monitoring framework for the Australian rangelands.Crossref | GoogleScholarGoogle Scholar |
Flores, BM, Staal, A, Jakovac, CC, Hirota, M, Holmgren, M, and Oliveira, RS (2020). Soil erosion as a resilience drain in disturbed tropical forests. Plant and Soil 450, 11–25.
| Soil erosion as a resilience drain in disturbed tropical forests.Crossref | GoogleScholarGoogle Scholar |
Guo W (2018) Automated characterization of plant growth and flowering dynamics using RGB images. In ‘Smart plant factory’. (Ed. T Kozai) pp. 385–393. (Springer)
Guo, W, Rage, UK, and Ninomiya, S (2013). Illumination invariant segmentation of vegetation for time series wheat images based on decision tree model. Computers and Electronics in Agriculture 96, 58–66.
| Illumination invariant segmentation of vegetation for time series wheat images based on decision tree model.Crossref | GoogleScholarGoogle Scholar |
Guo, W, Zheng, B, Duan, T, Fukatsu, T, Chapman, S, and Ninomiya, S (2017). EasyPCC: benchmark datasets and tools for high-throughput measurement of the plant canopy coverage ratio under field conditions. Sensors 17, 798.
| EasyPCC: benchmark datasets and tools for high-throughput measurement of the plant canopy coverage ratio under field conditions.Crossref | GoogleScholarGoogle Scholar |
Havrilla, CA, Villarreal, ML, DiBiase, JL, Duniway, MC, and Barger, NN (2020). Ultra‐high‐resolution mapping of biocrusts with unmanned aerial systems. Remote Sensing in Ecology and Conservation 6, 441–456.
| Ultra‐high‐resolution mapping of biocrusts with unmanned aerial systems.Crossref | GoogleScholarGoogle Scholar |
Karnieli A, Kokaly RF, West NE, Clark RN (2001) Remote sensing of biological soil crusts. In ‘Biological soil crusts: structure, function, and management’. (Eds J Belnap, OL Lange) pp. 431–455. (Springer)
Kilinc, M, and Beringer, J (2007). The spatial and temporal distribution of lightning strikes and their relationship with vegetation type, elevation, and fire scars in the Northern Territory. Journal of Climate 20, 1161–1173.
| The spatial and temporal distribution of lightning strikes and their relationship with vegetation type, elevation, and fire scars in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
O’Reagain, P, Bushell, J, Holloway, C, and Reid, A (2009). Managing for rainfall variability: effect of grazing strategy on cattle production in a dry tropical savanna. Animal Production Science 49, 85–99.
| Managing for rainfall variability: effect of grazing strategy on cattle production in a dry tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Panigada, C, Tagliabue, G, Zaady, E, Rozenstein, O, Garzonio, R, Di Mauro, B, De Amicis, M, Colombo, R, Cogliati, S, Miglietta, F, and Rossini, M (2019). A new approach for biocrust and vegetation monitoring in drylands using multi-temporal Sentinel-2 images. Progress in Physical Geography: Earth and Environment 43, 496–520.
| A new approach for biocrust and vegetation monitoring in drylands using multi-temporal Sentinel-2 images.Crossref | GoogleScholarGoogle Scholar |
Possoch, M, Bieker, S, Hoffmeister, D, Bolten, A, Schellberg, J, and Bareth, G (2016). Multi-temporal crop surface models combined with the RGB vegetation index from UAV-based images for forage monitoring in grassland. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences XLI-B1, 991–998.
| Multi-temporal crop surface models combined with the RGB vegetation index from UAV-based images for forage monitoring in grassland.Crossref | GoogleScholarGoogle Scholar |
Preece, N (2002). Aboriginal fires in monsoonal Australia from historical accounts. Journal of Biogeography 29, 321–336.
| Aboriginal fires in monsoonal Australia from historical accounts.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Caballero, E, Escribano, P, and Cantón, Y (2014). Advanced image processing methods as a tool to map and quantify different types of biological soil crust. ISPRS Journal of Photogrammetry and Remote Sensing 90, 59–67.
| Advanced image processing methods as a tool to map and quantify different types of biological soil crust.Crossref | GoogleScholarGoogle Scholar |
Shi, P, Wang, Y, Xu, J, Zhao, Y, Yang, B, Yuan, Z, and Sun, Q (2021). Rice nitrogen nutrition estimation with RGB images and machine learning methods. Computers and Electronics in Agriculture 180, 105860.
| Rice nitrogen nutrition estimation with RGB images and machine learning methods.Crossref | GoogleScholarGoogle Scholar |
Su, Y-g, Liu, J, Zhang, Y-m, and Huang, G (2021). More drought leads to a greater significance of biocrusts to soil multifunctionality. Functional Ecology 35, 989–1000.
| More drought leads to a greater significance of biocrusts to soil multifunctionality.Crossref | GoogleScholarGoogle Scholar |
Tongway, DJ (2010). Teaching the assessment of landscape function in the field: enabling the design and selection of appropriate restoration techniques. Ecological Restoration 28, 182–187.
| Teaching the assessment of landscape function in the field: enabling the design and selection of appropriate restoration techniques.Crossref | GoogleScholarGoogle Scholar |
Tongway DJ, Ludwig JA (2012) Planning and implementing successful landscape-scale restoration. In ‘Restoration ecology’. (Eds J van Andel, J Aronson) pp. 30–42. (John Wiley & Sons, Ltd)
Tresch, L, Mu, Y, Itoh, A, Kaga, A, Taguchi, K, Hirafuji, M, Ninomiya, S, and Guo, W (2019). Easy MPE: extraction of quality microplot images for UAV-based high-throughput field phenotyping. Plant Phenomics 2019, 2591849.
| Easy MPE: extraction of quality microplot images for UAV-based high-throughput field phenotyping.Crossref | GoogleScholarGoogle Scholar |
Ward, DP, and Kutt, AS (2009). Rangeland biodiversity assessment using fine scale on-ground survey, time series of remotely sensed ground cover and climate data: an Australian savanna case study. Landscape Ecology 24, 495–507.
| Rangeland biodiversity assessment using fine scale on-ground survey, time series of remotely sensed ground cover and climate data: an Australian savanna case study.Crossref | GoogleScholarGoogle Scholar |
Weber, B, Olehowski, C, Knerr, T, Hill, J, Deutschewitz, K, Wessels, DCJ, Eitel, B, and Büdel, B (2008). A new approach for mapping of biological soil crusts in semidesert areas with hyperspectral imagery. Remote Sensing of Environment 112, 2187–2201.
| A new approach for mapping of biological soil crusts in semidesert areas with hyperspectral imagery.Crossref | GoogleScholarGoogle Scholar |
Wellburn, AR (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144, 307–313.
| The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution.Crossref | GoogleScholarGoogle Scholar |
Williams, W, Büdel, B, and Williams, S (2018). Cyanobacterial species richness and Nostoc highly correlated to seasonal N enrichment in the northern Australian savannah. Biogeosciences 15, 2149–2159.
| Cyanobacterial species richness and Nostoc highly correlated to seasonal N enrichment in the northern Australian savannah.Crossref | GoogleScholarGoogle Scholar |
Williams, WJ, Schmidt, S, Zaady, E, Alchin, B, Myint Swe, T, Williams, S, Dooley, M, Penfold, G, O’Reagain, P, Bushell, J, Cowley, R, Driscoll, C, and Robinson, N (2021). Resting subtropical grasslands from grazing in the wet season boosts biocrust hotspots to improve soil health. Agronomy 12, 62.
| Resting subtropical grasslands from grazing in the wet season boosts biocrust hotspots to improve soil health.Crossref | GoogleScholarGoogle Scholar |