Weak negative responses of spider diversity to short-term ‘kraaling’
Sicelo Sebata A B * , Charles R. Haddad B , Moira J. FitzPatrick C and Stefan H. Foord
A B * , Charles R. Haddad B , Moira J. FitzPatrick C and Stefan H. Foord  D
D
A Department of Crop and Soil Sciences, Faculty of Agricultural Sciences, Lupane State University, Bulawayo, Zimbabwe.
B Department of Zoology and Entomology, Faculty of Natural and Agricultural Sciences, University of Free State, Bloemfontein 9300, Republic of South Africa.
C Natural History Museums of Zimbabwe, Centenary Park, Suburbs, Bulawayo, Zimbabwe.
D NRF-SARChI Chair in Biodiversity Value and Change, University of Venda, Thohoyandou 0950, South Africa.
The Rangeland Journal 44(2) 61-75 https://doi.org/10.1071/RJ22004
Submitted: 28 January 2022 Accepted: 26 May 2022 Published: 16 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Rangeland Society. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The influence of short-duration, concentrated kraaling (enclosure) has been documented for plants, wildlife, and macro-invertebrates. However, limited information is available on its impact on ground-dwelling spiders. The purpose of this study was to assess the effect of short-duration kraaling, time since cattle removal, and microhabitat variables on spider assemblages in Matabeleland North Province, Zimbabwe. We used a matched-pair and space for time design (inside vs outside previously kraaled inclusions) across 11 sites, using four cattle herds (H1, H6, H7 and HNguni). Spiders were sampled in the early and late rainy season with pitfall traps left open for 14-day sampling periods and emptied twice in each period. We captured 634 spiders, comprising 63 species in 44 genera and 18 families. The most abundant family was Lycosidae (37%; 16 spp.), followed by Gnaphosidae (15%; 10 spp.) and Salticidae (14.5%; 7 spp.). Generalised linear mixed models showed that generic richness was greater in sites with more bare ground. However, this effect was reversed in previously kraaled sites, and was particularly evident for spider abundance that responded negatively relative to unkraaled sites. Furthermore, with a U-shaped recovery, generic richness increased with time since kraaling. Model-based multivariate models showed that short-duration kraaling had a significant impact on spider assemblage structure, but this impact was relatively small compared with the effect of seasonality. Most of the species that made significant contributions to this multivariate response were less abundant in kraaled sites. Spider diversity, therefore, had a weak negative response to short-term kraaling. However, these impacts should also be assessed at broader scales, including areas where cattle go to graze during the day.
Keywords: Araneae, cattle, ground dwelling spiders, holistic planned grazing, rangeland, savanna, vegetation, Zimbabwe.
Introduction
Cattle enclosures, referred to as bomas (Stelfox 1986), livestock corrals (Augustine et al. 2009) or kraals (Huruba et al. 2018), have been part of daily management within livestock practices in most African rangelands for decades (Augustine 2003), and are used as overnight protection enclosures from theft and livestock predators. In addition, kraals provide confinements that enable milk extraction and the concentrated manure production for crop cultivation (Abagale and Ayuegabe 2015). Kraals are usually constructed of material ranging from thorn scrub branches (Augustine et al. 2009) and fences (Stelfox 1986). They are commonly circular or rectangular, the former being preferred to avoid bunching of livestock in corners (Borg 1996).
During the day, cattle normally graze within a few kilometres from the kraal. When inside the kraals at night, cattle dung and urine redistribute nutrients consumed during the day, forming heterogeneous nutrient-rich patches (Augustine 2003). Enclosures can be short-term, usually lasting for 7 days or less (Huruba et al. 2018), or long-term, where kraal owners utilise the same location for decades within the vicinity of water resources such as boreholes or shallow pans (Kizza and Areola 2010). The former is a recently developed practice that has since been incorporated as part of the innovative management approach called holistic planned grazing (Savory and Parsons 1980; Savory 1983). The latter comprises the traditional kraaling culture in most semi-arid regions in southern Africa.
The influence of abandoned kraal sites has been well documented (Kizza and Areola 2010; Chikorowondo et al. 2017, 2018). Abandoned kraal sites enhance the soil nutrient status, including nitrogen, phosphorus and potassium (Huruba et al. 2017, 2018; Muvengwi et al. 2018), and are important as nutrient sources utilised in crop production (Kangalawe et al. 2008). The nutrient-rich patches enhanced by kraaling have implications for vegetation diversity (Sibanda et al. 2016; Huruba et al. 2018), as well as their utilisation by wildlife (Huruba et al. 2018), as a result of grass resprouting and an increase in palatable grass species (Sibanda et al. 2016; Huruba et al. 2018).
Apart from work on diversity and abundance (Muvengwi et al. 2018) and functional diversity of macro-invertebrates (Chikorowondo et al. 2018), limited knowledge exists on the influence of previously kraaled enclosures on invertebrates, in particular spiders. Spiders are a megadiverse arthropod order, with almost 50 000 described species (World Spider Catalog 2022). They also occupy almost all possible terrestrial microhabitats (Turnbull 1973; Foelix 2011). Ecologically, spiders are important predators (Nyffeler and Birkhofer 2017) that commonly feed on insects, rendering spiders important natural control agents (Nentwig and Kobelt 2010). Spiders also feed on small mammals such as bats (Nyffeler and Knörnschild 2013), aquatic organisms such as fish (Nyffeler and Pusey 2014), and even other spiders (Wise 2006).
Spider diversity is dependent on several factors (Foelix 2011), including vegetation structure (Baldissera et al. 2004; Roberson et al. 2016), prey availability and competitive exclusion (Dennis et al. 2015; Rodriguez-Artigas et al. 2016). Spiders possess several characteristics that make them good bio-indicators (Churchill 1997; Marc et al. 1999) of environmental disturbances such as fire (Pryke and Samways 2012; Haddad et al. 2015), habitat modification (Haddad et al. 2010), habitat quality (Halaj et al. 1998) and grazing (Fuller et al. 2014; Schwerdt et al. 2018). They have also been used to determine the influence of other environmental changes, including leaf-litter structure (Castro and Wise 2009; Butler and Haddad 2011; Podgaiski et al. 2013), seasonality (Niemela et al. 1994; Weeks and Holtzer 2000; Mineo et al. 2010) and rainfall gradients (Churchill 1998). According to Podgaiski et al. (2013), spiders have the potential to re-occupy affected habitats within less than a month, and are therefore an appropriate taxon for testing the effect of short-duration kraaling (7 days) on spider assemblages inside previously kraaled inclusions and their surroundings.
This study examined the response of spider assemblages to short-duration kraaling. It was predicted that spider assemblages would be less diverse in kraaled sites than in adjacent non-kraaled (control) sites, because of grazing and trampling by cattle. Second, we assessed whether spider assemblages varied with time since kraal occupation. We predicted that spider assemblages between the kraaled and non-kraaled sites would be more diverse with time since disturbance because of vegetation regrowth and recolonisation by mobile spiders. Last, we sought to identify the micro-habitat variables that influence spider assemblages around previously kraaled inclusions and their control sites. We predicted that spider diversity would be more diverse in structurally more complex vegetation, mainly through varied substrates, opportunities for web attachments, and shelter from predators (Fuller et al. 2014).
Materials and methods
Study sites
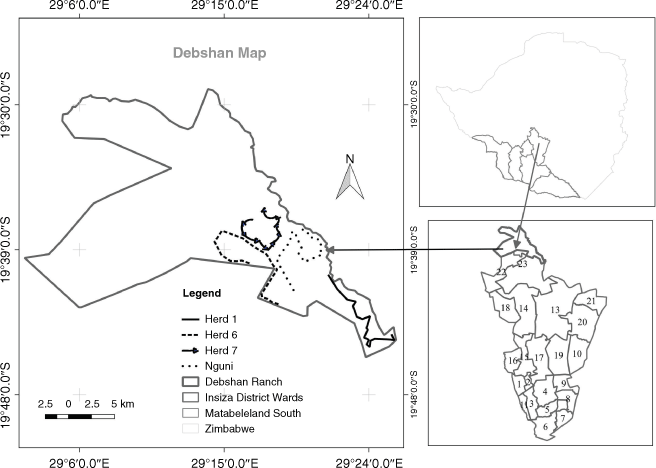
The study was conducted on the Debshan Ranch, a privately owned commercial cattle ranch 100 km northeast of Bulawayo within Insiza district, Matabeleland North Province, Zimbabwe (Fig. 1). The Debshan Ranch (19°35′S, 29°15′E) occupies 800 km2 (Huruba et al. 2018), and utilises a holistic management approach (Mberi 2013). Mean annual rainfall is 639 mm, the rainy season starts in November, peaks in December, averaging 144 mm, and ends in March–April. Annual average temperature is 18°C, October is the hottest month (average temperature 21°C), and July the coldest (average temperature 12.4°C). Average daily humidity is 55% (Climate-data.org 2019).
The ranch falls under agro-ecological natural region IV (Cousins 1992), a livestock production area that also cultivates drought-tolerant crops such as sorghum, millet and rapoko. It is also a semi-extensive savanna biome suitable for forestry, wildlife and tourism (Mugandani et al. 2012). Elevation is between 1230 and 1414 m above sea level (Dunham et al. 2003), and soils are coarse-grained yellowish-brown loamy sands, resulting from granite, forming soils that are usually infertile and poorly drained, ultramafic or mafic rocks that give rise to productive red soils, and dark brown clayey soils (Robertson 2013). The soils support floral types that are normally dispersed in a catenae pattern (Dunham et al. 2003) that comprises bushlands, grasslands, wetlands and woodlands, such as (1) Julbernadia–Stereochlaena woodland, (2) Combretum hereroense–Hyparrhenia mixed bushlands, (3) Colophospermum bushlands, (4) Terminalia–Schizachyrium bushlands, (5) Riverine woodland, and (6) Hyperthelia vlei grassland (Robertson 2013).
Spider sampling
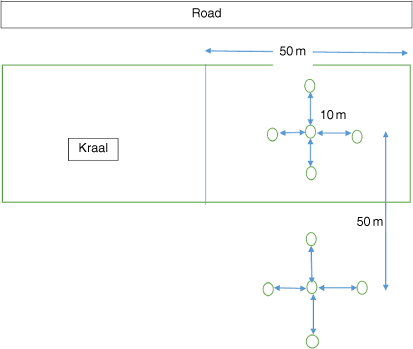
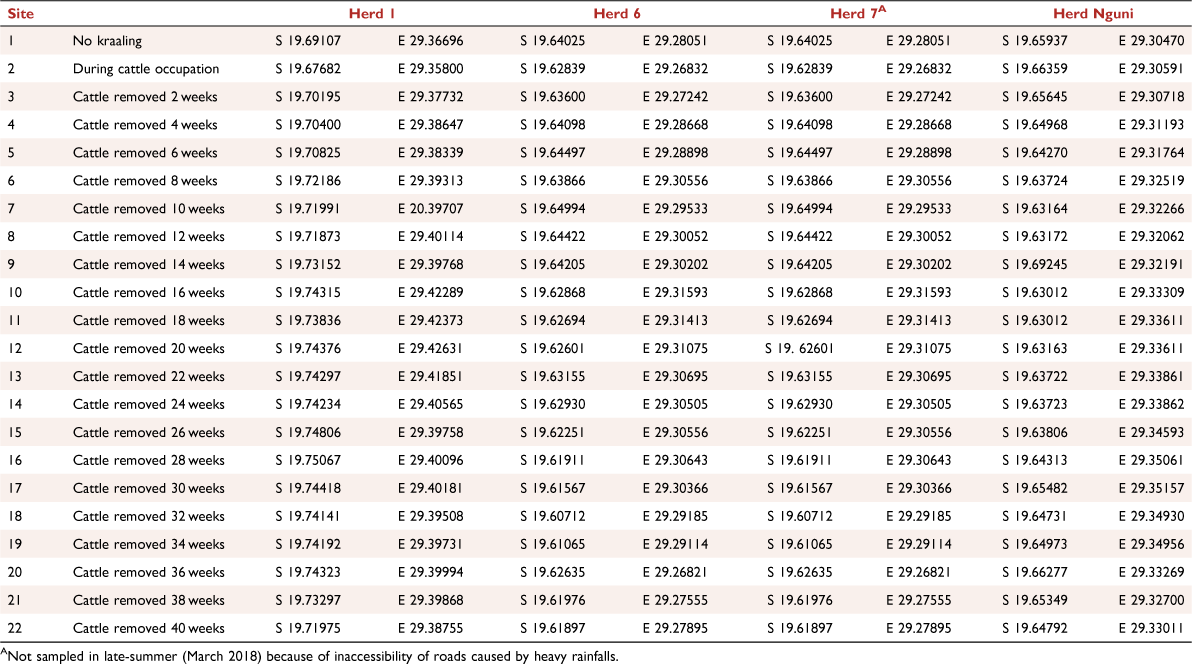
Ground-dwelling spiders were collected using pitfall traps at 22 sites × 4 herds = 88 sites during the early rainy season sampling period (November 2017) and at 22 sites × 3 herds = 66 sites during the late-summer sampling period (March 2018). Herd 7 was not sampled in the late-summer sampling period because of the inaccessibility of roads caused by heavy rainfall (Appendix Table A1). The 88 sites comprised kraaled and adjacent non-kraaled (control) plots (Fig. 1). Each herd was considered a replicate, and contained approximately 350–396 cattle (Huruba et al. 2018). At each site, a matched-pair design was employed, consisting of two plots, each containing five pitfall traps, with one plot inside the kraal and one outside the kraal. The pitfall traps in a plot were arranged in a cross (Fig. 2), for a total of 880 pitfall traps (22 sites × 4 herds × 10 traps) across all sites. Pitfall traps were 10 m apart, and the plots (kraaled and non-kraaled) were 50 m apart (Fig. 2). Pitfall traps consisted of glass bottles 14 cm deep and 9 cm wide at the mouth, filled with 100 mL of 70% propylene glycol, placed inside a plastic PVC pipe and buried to their rims. Pitfall traps were left open for 14day sampling periods, and emptied twice in each sampling period. To reduce seasonal influences on spiders (Whitmore et al. 2002; Muelelwa et al. 2010), sampling was conducted in early summer (November 2017) and late summer (March 2018), because these are the periods when spider activity is considered to be relatively high within the savanna region (Muelelwa et al. 2010). At the end of each sampling period, the contents of the sampling bottles were collected, and sorted. Adult specimens were sorted into morphospecies and identified to species level where possible, with all the juveniles being identified at least to genus level. Spider identification to family level was undertaken using the keys of Dippenaar-Schoeman and Jocqué (1997), and further to morphospecies by the first and third authors. All adult spider specimens collected were deposited in the Department of Arachnology, Natural History Museum in Bulawayo.
|
Micro-habitat variables
Micro-habitat variables were assessed by placing a 1 m × 1 m quadrat over each pitfall trap and taking a photograph. Each sampled pitfall trap was photographed in both November and March. Images were visually inspected and the percentage cover of the following micro-habitat variables was estimated: grass, bare ground, leaf litter, coarse woody debris (wood with a diameter >5 cm), cow dung, and rock, by utilising the methodology of Dethier et al. (1993). In each of the 88 kraaled and adjacent non-kraaled (control) sites, a 40 m transect was established inside and outside the kraal within the plots. Along each transect, 10 grasses approximately 1 m apart were selected and marked with plastic ear tags and the tallest vertical point of each grass with the leaf blade extended was measured, and mean grass height (cm) determined at each site.
Data analysis
All statistical analyses were performed using R statistical software version 4.1.2 (R Core Team 2021). Data for spiders were pooled within a plot, i.e. catches from five pitfall traps in a plot within each 14day sampling period were pooled. Sample coverage was calculated using the iNEXT package (Hsieh et al. 2016), to enable estimation of the percentage of total species obtained in a sample. Its inverse counterpart is the likelihood that the following sampled individual may be a previously unsampled species (Chao and Jost 2012).
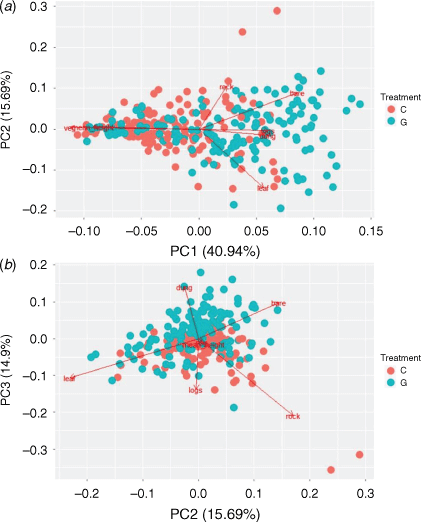
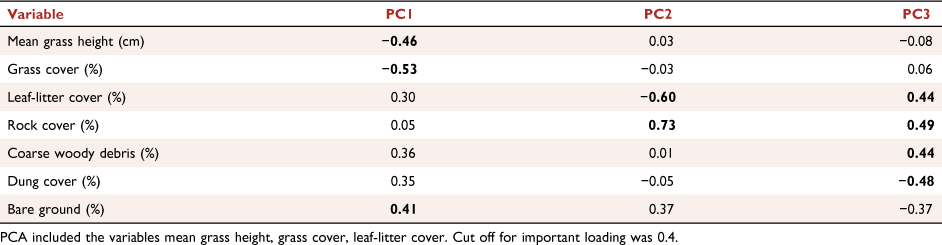
The relationships between micro-habitat structure variables were summarised using principal component analysis (PCA). By reducing data, collinearity that hinders interpretation and analysis of ecological data (Graham 2003) is avoided while retaining information from such variables. These derived variables are then included as predictors in regression models (Ellison 2004). The first component (PC1, explained variance = 41%) was moderately negatively correlated with microhabitat variables such as percentage grass cover (−0.52) and mean grass height (cm; −0.46), and positively with percentage bare ground cover (0.41; Appendix Fig. A1a). The second component (PC2, explained = 16%) was correlated positively with percentage rock cover (0.73) and negatively with percentage leaf litter cover (−0.60; Fig. A1a). The third component (PC3, explained variance = 15%) and was positively correlated with percentage coarse woody debris cover (0.44), percentage leaf litter cover (0.44) and percentage rock cover (0.49), and negatively with percentage dung cover (−0.48; Fig. A1b). Original variables that were strongly correlated with individual PCA axes with a cut-off point of 0.4 (Legendre and Legendre 1998) were utilised in the discussion of the results (Table A2).
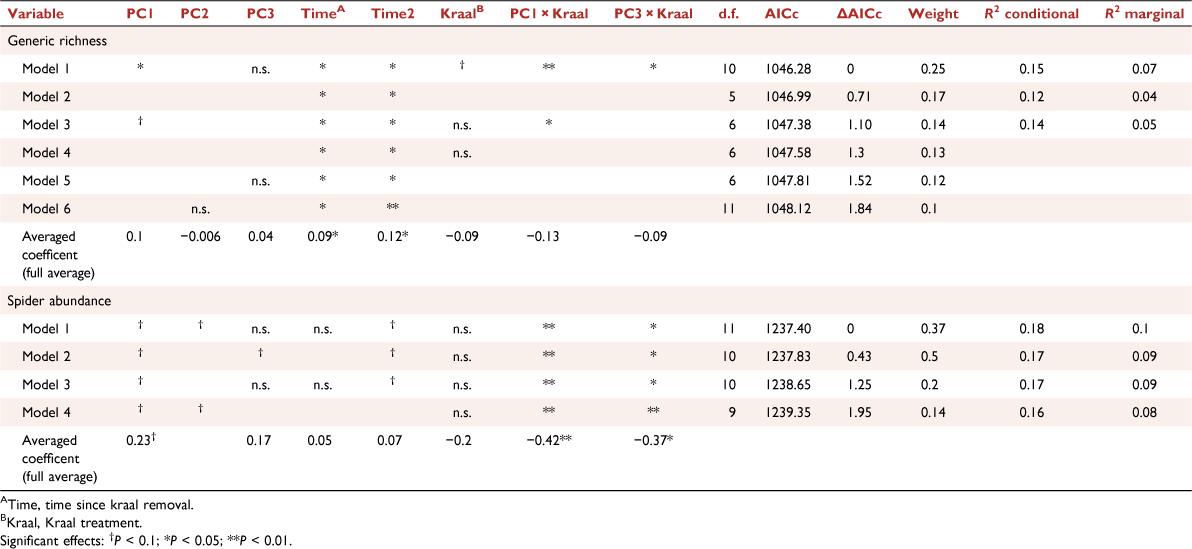
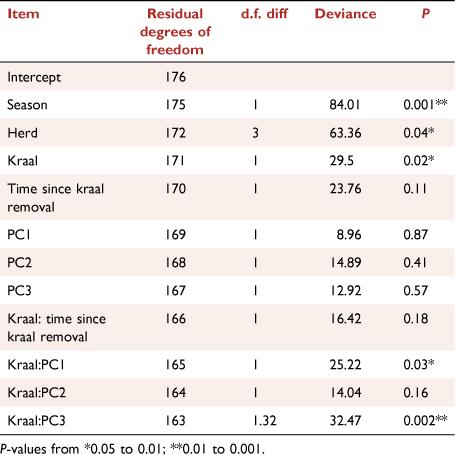
Factors affecting spider abundance and generic richness were modelled using generalised linear mixed models with a log-link function, with either Poisson or negative binomial distributions, using the ‘glmer’ or ‘glmer.nb’ functions in the lme4 package (Bates et al. 2015), depending on the degree of over-dispersion of dependent variables. Candidate models were subsets of the global model that contained all predictors, namely time since kraal removal, PC 1, 2 and 3, and the paired treatment (kraaled vs non-kraaled), as well as the interactions between treatment and these variables. A quadratic term for time since kraaling was also included to account for any non-linear responses of abundance and generic richness over time. Herds nested within survey were included as random factors. Model residuals were inspected for overdispersion, heteroscedacity and normality, as well as influential observations (Zuur and Ieno 2016). Model selection was conducted using an information-theoretic approach based on Akaike Information Criterion (AIC; Burnham and Anderson 2002). The best model was that with the lowest AIC, but we also report on those models that differed by less than 2 AIC values from the best model. To determine variation explained by fixed factors and that explained by the whole model, marginal Rm2 and conditional Rc2 respectively were also calculated (Nakagawa and Schielzeth 2013).
Model-based multivariate analysis using generalised linear models with negative binomial distribution and log-link functions was used to test for differences in composition between kraaling and no kraaling, time since kraaling, PC1, PC2 and PC3. This was undertaken using the R package mvabund (Wang et al. 2018). This approach accounts for confounding mean–variance relationships, which are common in abundance data containing many zeros (Warton et al. 2015). The likelihood-ratio statistics for each species were summed, resulting in a community-level measure for each predictor. The PIT–residual bootstrap method was utilised by resampling 999 rows of the dataset to derive P-values for the variables (Warton et al. 2017). Model assumptions were evaluated by visually examining plots of residuals for any non-random patterns.
Results
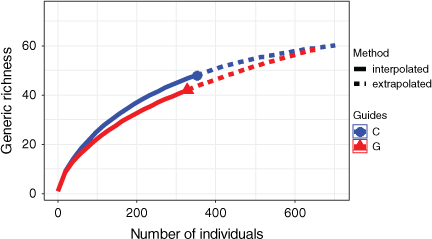
In total, 634 individual spiders in 63 identified species representing 44 genera and 18 families were collected (Table A3). Of these, 451 were adults identified to species level, and 183 were juveniles identified to genus level. In total, 430 individuals were collected in December 2017, and 204 in March 2018. The most common families were Lycosidae and Gnaphosidae (Table A3). Nearly one quarter of the mature spiders were either Allocosa umtalica (Purcell, 1903) (101 specimens) or Asemesthes paynteri Tucker, 1923 (61 specimens). The four most common species represented 40% of the total spiders sampled. One species is possibly new, on the basis of the identifications of specialists; 45 species were encountered only once. Sample-based rarefaction curves showed higher genera richness of spider assemblages in the unkraaled sites than in the kraaled sites, although confidence intervals did overlap (Fig. 3). Furthermore, sample coverage for both the kraaled and the adjacent non-kraaled (control) sites was relatively high (>94%), suggesting that sampling captured a significant portion of the spider assemblages at Debshan Ranch.
|
Effects on spider abundance and generic richness
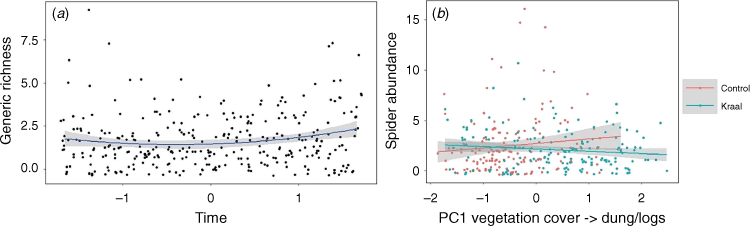
Spider generic richness was related to microhabitat variables, increasing with PC1RICH (Rm2 = 0.15, P = 0.1; Table 1), suggesting more genera and individuals in sites that have lower vegetation cover, i.e. that are more open PC1 (bare ground, logs), and have more dung. However, this effect was reversed in plots that were previously kraaled, and was particularly evident for spider abundance where the interactions of kraaling with PC1ABUN (Rm2 = 0.18, P = 0.05; Table 1, Fig. 4) and PC3ABUN (Rm2 = 0.18, P = 0.1; Table 1, Fig. 4) affected spider abundance negatively relative to plots that were not kraaled. Furthermore, there was also an effect of time since kraal removal, with generic richness increasing with time since kraaling (Rm2 = 0.15, P = 0.1; Table 1, Fig. 4), with a non-linear component where there is an overall decrease in richness and then a recovery.
Effects on spider assemblages
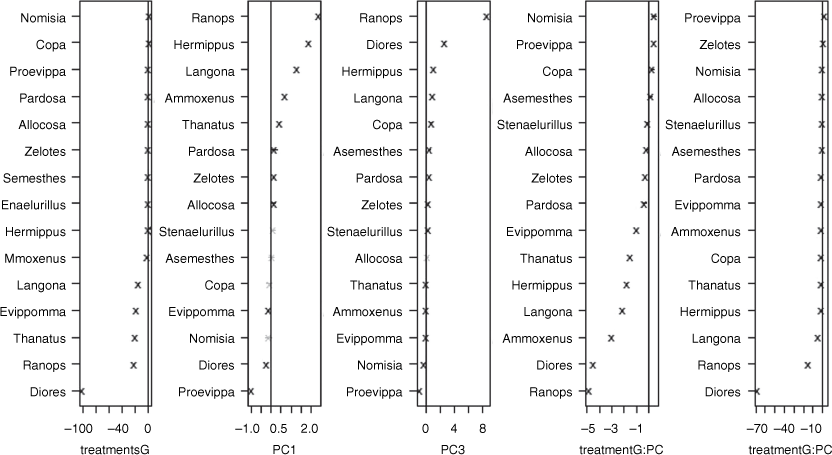
The multivariate generalised linear models showed significant effects of short-duration kraaling, but those were relatively small when compared with that of sampling season and time since kraaling on spider genera composition (Table 2). In other words, the differences among the spider assemblages were more evident between sampling season and time since kraal removal than were those observed between previously kraaled sites and their surroundings. Furthermore, there was a weak but significant effect of two interactions, namely, short-duration kraaling interacted with both PC1 (bare ground, logs) and also PC3 (dung). Most of the genera that responded significantly to short-duration kraaling had a negative response, including Diores, Ranops, Thanatus, Evippomma and Langona. Only Proevippa responded negatively to more open habitat, whereas Ranops and Hermippus, in particular, responded positively. The negative response of spider diversity to short-duration kraaling is further exemplified by the presence of several genera that generally were less diverse within the short-duration kraaled sites with comparable microhabitat characteristics than were those found in the control (non-kraaled) plots (Fig. 5).
|
|
Discussion
Effects of short-duration kraaling
In line with our first prediction, non-kraaled (control) sites were more diverse than kraaled sites. De Keer and Maelfait (1988) observed that spiders mostly oviposit or overwinter in ungrazed areas to avoid unfavourable climatic conditions. Despite the limited variation (18%) explained by the models, even with the random factors (herd nested within survey), there were weak negative responses of spider diversity to short-duration kraaling. We found lower generic richness and abundance in the kraaled sites than in the adjacent non-kraaled (control) sites, with the greatest variation being explained by seasons. These patterns were consistent with previous studies (Gibson et al. 1992a; Bromham et al. 1999; Sebata 2020), which found less diverse spider assemblages in grazed areas. Grazing leads to the modification of vegetation by cattle through trampling (Fuller et al. 2014), reducing the suitability of the biotope, because structure of vegetation is a key element that affects habitat choice by most spiders (Štokmane and Spuņģis 2016; Lafage et al. 2019) and other invertebrates (Crist et al. 2006).
Furthermore, most spiders have excellent dispersal powers, with many Lycosidae and Gnaphosidae ballooning, especially as juveniles (Mrzljak and Wiegleb 2000). Ballooning is a ‘tiptoe’ behaviour, where an individual straightens its legs, balancing on the tips of its tarsi while raising its abdomen, thereby releasing a silken dragline in the air, while a drag-induced lift of the spider’s body by wind is achieved (Weyman 1993; Zhao et al. 2017). Ballooning allows dispersal, but mainly over short distances (Pedley and Dolman 2014). For example, Pardosa monticola (Clerck, 1757) may disperse no more than 280 m over its lifetime (Bonte et al. 2003), with females able to balloon between 30 and 40 m per day during natal dispersal (Bonte et al. 2007). Several negative and positive factors that elicit ballooning behaviour have been reported (Weyman 1993), ranging from temperature, humidity, wind, vibration, light and stress. Grazing and trampling by cattle usually causes vibration that can be detected by the spider legs, and may induce ballooning of spiders away from the kraaled sites. This suggests that the small differences between the inside and outside of kraals may have been the result of the high dispersal power by either walking or ballooning (Weyman et al. 2002), which enabled spatial exchange among spider assemblages, because the sites among the treatments were separated by only 50 m.
Beta diversity has been generally defined as the variation in species composition of assemblages among sites (Whittaker 1960), and is determined by two different phenomena acting on spider assemblages, namely spatial species turnover (the changeover of species between seasons) and nestedness (the loss and gain of species across time; Carvalho et al. 2012, 2013). Whereas richness differences are determined by the net loss (or gain) of species from site to site or from one date to another, replacement refers to the substitution of species between sites or points in time. Although the contribution of both sources of dissimilarity is usually not disentangled (Baselga 2010), the dissimilarity of spider assemblages between the short-duration kraaled sites and their surroundings is most likely due to the change in generic richness and abundance. In all likelihood, short-duration kraaled sites do not complement the unkraaled (control) sites, but are a smaller subset of the species found around the kraals.
Despite the sampling being conducted in the rainy season, seasonality was an important predictor of spider assemblages, with a lower spider abundance caught in the late rainy season (March) than in the early rainy season (December). These findings support those of Muelelwa et al. (2010), who recorded higher spider abundance and species richness in early summer (November) than in autumn (March). They attributed their results to maturing of overwintering juveniles and subadults, captured as adults ready for mating during the rainy season of the early summer. By the late-summer rainy season, most adults have died and assemblages are dominated by juveniles (Foord et al. 2008; Muelelwa et al. 2010). However, in this study, the differences in spider abundance may have been affected by the heavy rains that fell during the late summer season, which may have led to a reduction in spider activity, as reported elsewhere (Haddad et al. 2015; Queiroz and Gasnier 2017).
Effects of time since kraal removal
In line with our second prediction, with a U-shaped recovery, spider generic richness increased with time since kraaling. The lowest generic richness was recorded when cattle occupied the short-duration kraals, most probably a consequence of livestock trampling, which resulted in the development of bare soil (Gibson et al. 1992a, 1992b). Bare soil implies reduced raw materials for minerals and nutrients for most organisms (Rampai 2017), and has been negatively associated with spider species richness under trees (Barton et al. 2017). At the time of abandonment, short-duration kraals are usually bare and mostly covered by dung above the soil-surface layer (Sibanda et al. 2016), which indirectly leads to the subsequent increase in grass cover with time since kraal removal (Huruba et al. 2018). Similar to other studies, grass re-establishment at Debshan was rapid following kraal removal (Reid and Ellis 1995), most probably a consequence of nutrient-reserve patches (a consequence of dung and urine deposition) that develop in previously kraaled inclusions (Augustine 2003). Grass cover can increase faster during shorter periods of cattle occupation (4 and 7 day treatments) than in corals where cattle occupation was longer (14 and 28 day treatments), owing to the more hospitable conditions of moderate versus excessive cattle waste deposits (Veblen and Porensky 2019). In addition, there is usually less grass trampling in short-duration kraals, allowing for more rapid regrowth after rains than with excessive trampling in long-duration kraals. Vegetation structure such as plant height can reflect the diversity of potential invertebrate prey and habitat for spiders (Gallé et al. 2011), thereby potentially supporting the different species composition at each time interval. Spiders can benefit from higher grass cover, which provides essential services to most invertebrates (Gibson et al. 1992a, 1992b); hence, the observed increase in spider diversity recorded with time since kraal removal.
Effects of microhabitat variables
Contrary to our third hypothesis, spider genera abundance and richness did not benefit from greater vegetation structure and cover, but instead, several genera and individuals were obtained in sites that had less vegetation cover, i.e. areas of bare ground and coarse woody debris. Lycosidae have been reported to increase in abundance with increased disturbance (Pedley and Dolman 2014). Similarly, in this study wolf spiders were sampled at higher densities inside than outside kraals, indicating a preference for increased levels of disturbance, making this group good indicators of these changes (Piacentini and Ramírez 2019). There are several possible reasons for this, namely that wolf spiders chase their prey at ground level, thereby making open habitat the optimal foraging ground. Also, because this groups hunts actively by running, warmer and drier ground is usually more suitable than the unkraaled plots with usually lower numbers of suitable prey (Suominen 1999). Furthermore, the most abundant species collected belonged to Lycosidae, which are usually found in high numbers in open and disturbed habitats (Nyffeler 1999; Mallis and Hurd 2005), because they are normally the first species to inhabit disturbed lands (Pedley and Dolman 2014). For example, Pardosa species have been reported to achieve dense populations in open barren lands (Buddle and Rypstra 2003; Mallis and Hurd 2005). Here, two species, P. manubriata Simon, 1898 (15%) and P. crassipalpis Purcell, 1903 (5%), were the second- and third-most abundant lycosid species sampled, after Allocosa umtalica (32%). However, since sampling took place only within the miombo woodlands, further research in other woodland types is recommended, to determine their degree of habitat specificity and biomonitoring potential.
In conclusion, diversity and abundance of ground-dwelling spiders were enhanced in sites with more bare ground within the miombo woodlands of the Debshan Ranch. However, this effect disappeared in sites previously kraaled. The differential response of spider assemblages to short-duration kraaling in the present study showed the importance of structurally complex habitats, essential for a wide variety of invertebrates with contrasting life traits. Despite the initial decrease in spider fauna because of short-duration kraaling, spider richness did show a U-shaped recovery, suggesting that the 10 month resting period is essential to ensure recovery of the spider diversity. Further research on the impacts of spider assemblages within a broader landscape context would contribute to the knowledge on short-duration kraaling effects on spider assemblages, and help develop management recommendations within south-western Zimbabwe.
Conservation significance and management recommendations
In Zimbabwe, conservation approaches currently consider vertebrates and plants, believing that protection given to these groups will benefit arthropods such as spiders. This approach usually does not provide for the protection of threatened and rare spider species (Lovell et al. 2009). Spiders contrast in their responses to disturbance, and managers may decide to protect spider communities by targeting species of concern and implementing species-specific management plans. However, such information requires long-term monitoring of spider assemblages within a region, so as to provide a complete database of all possible spiders in each biotope, which will later contribute to recognising the rare, endemic, and threatened species within that region. Species need to be ranked according to the effects of grazing and trampling of the holistic management approach.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
The authors thank N. J. Oppenheimer and the University of the Free State for funding the fieldwork, and the Debshan Ranch staff for their logistical support during this research. Particular thanks go to the Natural History Museum staff for assistance with fieldwork, sorting invertebrate samples and taxonomic inputs. The authors are also grateful to the anonymous referees for their valuable comments that improved our paper considerably. This paper forms part of the PhD thesis of Sebata (2020) entitled ‘Spider Ecology in South-Western Zimbabwe, with emphasis on the impact of Holistic Planned Grazing Practices’.
References
Abagale, FK, and Ayuegabe, E (2015). Enhancing soil nutrient status using dynamic kraaling strategy in northern Ghana. Elixir International Journal 88, 36279–36283.Augustine, DJ (2003). Long-term, livestock-mediated redistribution of nitrogen and phosphorus in an East African savanna. Journal of Applied Ecology 40, 137–149.
| Long-term, livestock-mediated redistribution of nitrogen and phosphorus in an East African savanna.Crossref | GoogleScholarGoogle Scholar |
Augustine, DJ, Veblen, KE, Goheen, JR, Riginos, C, and Young, TP (2009). Pathways for positive cattle–wildlife interactions in semiarid rangelands. Smithsonian Contributions to Zoology 632, 55–71.
Baldissera, R, Ganade, G, and Fontoura, SB (2004). Web spider response along an edge between pasture and Araucaria forest. Biological Conservation 118, 403–409.
| Web spider response along an edge between pasture and Araucaria forest.Crossref | GoogleScholarGoogle Scholar |
Barton, PS, Evans, MJ, Foster, CN, Cunningham, SA, and Manning, AD (2017). Environmental and spatial drivers of spider diversity at contrasting microhabitats. Austral Ecology 42, 700–710.
| Environmental and spatial drivers of spider diversity at contrasting microhabitats.Crossref | GoogleScholarGoogle Scholar |
Baselga, A (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19, 134–143.
| Partitioning the turnover and nestedness components of beta diversity.Crossref | GoogleScholarGoogle Scholar |
Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bonte, D, Lens, L, Maelfait, J-P, Hoffmann, M, and Kuijken, E (2003). Patch quality and connectivity influence spatial dynamics in a dune wolfspider. Oecologia 135, 227–233.
| Patch quality and connectivity influence spatial dynamics in a dune wolfspider.Crossref | GoogleScholarGoogle Scholar | 12698344PubMed |
Bonte, D, Van Belle, S, and Maelfait, JP (2007). Maternal care and reproductive state-dependent mobility determine natal dispersal in a wolf spider. Animal Behaviour 74, 63–69.
| Maternal care and reproductive state-dependent mobility determine natal dispersal in a wolf spider.Crossref | GoogleScholarGoogle Scholar |
Borg R (1996) ‘Corrals for handling beef cattle.’ (Alberta, Food and Rural Development: Edmonton, Alberta, Canada)
Bromham, L, Cardillo, M, Bennett, AF, and Elgar, MA (1999). Effects of stock grazing on the ground invertebrate fauna of woodland remnants. Australian Journal of Ecology 24, 199–207.
| Effects of stock grazing on the ground invertebrate fauna of woodland remnants.Crossref | GoogleScholarGoogle Scholar |
Buddle, CM, and Rypstra, AL (2003). Factors initiating emigration of two wolf spider species (Araneae: Lycosidae) in an agroecosystem. Environmental Entomology 32, 88–95.
| Factors initiating emigration of two wolf spider species (Araneae: Lycosidae) in an agroecosystem.Crossref | GoogleScholarGoogle Scholar |
Burnham KP, Anderson D (2002) ‘Model selection and inference: a practical information-theoretic approach’, 2nd edn. (Springer-Verlag: New York, NY, USA)
Butler, VP, and Haddad, CR (2011). Spider assemblages associated with leaf litter of three tree species in central South Africa (Arachnida: Araneae). African Journal of Ecology 49, 301–310.
| Spider assemblages associated with leaf litter of three tree species in central South Africa (Arachnida: Araneae).Crossref | GoogleScholarGoogle Scholar |
Carvalho, JC, Cardoso, P, and Gomes, P (2012). Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Global Ecology and Biogeography 21, 760–771.
| Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns.Crossref | GoogleScholarGoogle Scholar |
Carvalho, JC, Cardoso, P, Borges, PAV, Schmera, D, and Podani, J (2013). Measuring fractions of beta diversity and their relationships to nestedness: a theoretical and empirical comparison of novel approaches. Oikos 122, 825–834.
| Measuring fractions of beta diversity and their relationships to nestedness: a theoretical and empirical comparison of novel approaches.Crossref | GoogleScholarGoogle Scholar |
Castro, A, and Wise, DH (2009). Influence of fine woody debris on spider diversity and community structure in forest leaf litter. Biodiversity and Conservation 18, 3705–3731.
| Influence of fine woody debris on spider diversity and community structure in forest leaf litter.Crossref | GoogleScholarGoogle Scholar |
Chao, A, and Jost, L (2012). Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93, 2533–2547.
| Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size.Crossref | GoogleScholarGoogle Scholar | 23431585PubMed |
Chikorowondo, G, Muvengwi, J, Mbiba, M, and Gandiwa, E (2017). Influence of abandoned cattle enclosures on plant assemblages and herbivory in a semi-arid savanna. Ecological Research 32, 1023–1033.
| Influence of abandoned cattle enclosures on plant assemblages and herbivory in a semi-arid savanna.Crossref | GoogleScholarGoogle Scholar |
Chikorowondo, G, Muvengwi, J, Mbiba, M, and Gandiwa, E (2018). Functional diversity of macroinvertebrates on abandoned cattle enclosures in a semi-arid African savannah. African Journal of Ecology 56, 629–640.
| Functional diversity of macroinvertebrates on abandoned cattle enclosures in a semi-arid African savannah.Crossref | GoogleScholarGoogle Scholar |
Churchill, TB (1997). Spiders as ecological indicators: an overview for Australia. Memoirs of the Museum of Victoria 56, 331–337.
| Spiders as ecological indicators: an overview for Australia.Crossref | GoogleScholarGoogle Scholar |
Churchill TB (1998) Spiders as ecological indicators in the Australian tropics: family distribution patterns along rainfall and grazing gradients. In ‘Proceedings of the 17th European Colloquium of Arachnology’. Edinburgh 1997. (Ed. PA Selden) (British Arachnological Society: Burnham Beeches, Buckinghamshire, UK)
Climate-data.org (2019) Climate: Shangani. Available at https://en.climate-data.org/africa/zimbabwe/matabeleland-south/shangani-214843/ [Accessed 16 October 2019]
Cousins, B (1992). Managing communal rangeland in Zimbabwe: experience and lessons. Journal of Experimental Psychology: General 136, 23–42.
Crist, TO, Pradhan-Devare, SV, and Summerville, KS (2006). Spatial variation in insect community and species responses to habitat loss and plant community composition. Oecologia 147, 510–521.
| Spatial variation in insect community and species responses to habitat loss and plant community composition.Crossref | GoogleScholarGoogle Scholar | 16222547PubMed |
De Keer, R, and Maelfait, JP (1988). Observations on the life cycle of Erigone atra (Araneae, Erigoninae) in a heavily grazed pasture. Pedobiologia 32, 201–212.
Dennis, P, Skartveit, J, Kunaver, A, and McCracken, DI (2015). The response of spider (Araneae) assemblages to structural heterogeneity and prey abundance in sub-montane vegetation modified by conservation grazing. Global Ecology and Conservation 3, 715–728.
| The response of spider (Araneae) assemblages to structural heterogeneity and prey abundance in sub-montane vegetation modified by conservation grazing.Crossref | GoogleScholarGoogle Scholar |
Dethier, MN, Graham, ES, Cohen, S, and Tear, LM (1993). Visual versus random-point percent cover estimations: objective is not always better. Marine Ecology Progress Series 96, 93–100.
| Visual versus random-point percent cover estimations: objective is not always better.Crossref | GoogleScholarGoogle Scholar |
Dippenaar-Schoeman AS, Jocqué R (1997) ‘African spiders: an identification manual.’ Plant Protection Research Institute Handbook No. 9. (Agricultural Research Council: Pretoria, South Africa)
Dunham, KM, Robertson, EF, and Swanepoel, CM (2003). Population decline of tsessebe antelope (Damaliscus lunatus) on a mixed cattle and wildlife ranch in Zimbabwe. Biological Conservation 113, 111–124.
| Population decline of tsessebe antelope (Damaliscus lunatus) on a mixed cattle and wildlife ranch in Zimbabwe.Crossref | GoogleScholarGoogle Scholar |
Ellison, AM (2004). Bayesian inference in ecology. Ecology Letters 7, 509–520.
| Bayesian inference in ecology.Crossref | GoogleScholarGoogle Scholar |
Foelix RF (2011) ‘Biology of Spiders.’ (Oxford University Press: New York, NY, USA)
Foord, SH, Mafadza, MM, Dippenaar-Schoeman, AS, and Van Rensburg, BJ (2008). Micro-scale heterogeneity of spiders (Arachnida: Araneae) in the Soutpansberg, South Africa: a comparative survey and inventory in representative habitats. African Zoology 43, 156–174.
| Micro-scale heterogeneity of spiders (Arachnida: Araneae) in the Soutpansberg, South Africa: a comparative survey and inventory in representative habitats.Crossref | GoogleScholarGoogle Scholar |
Fuller, L, Newman, M, Irwin, S, Kelly, T, and O’Halloran, J (2014). Ground-dwelling spider diversity in rare European oak and yew woodlands and the impact of grazing. Biodiversity and Conservation 23, 1911–1929.
| Ground-dwelling spider diversity in rare European oak and yew woodlands and the impact of grazing.Crossref | GoogleScholarGoogle Scholar |
Gallé, R, Vesztergom, N, and Somogyi, T (2011). Environmental conditions affecting spiders in grasslands at the lower reach of the River Tisza in Hungary. Entomologica Fennica 22, 29–38.
| Environmental conditions affecting spiders in grasslands at the lower reach of the River Tisza in Hungary.Crossref | GoogleScholarGoogle Scholar |
Gibson, CWD, Brown, VK, Losito, L, and McGavin, GC (1992a). The response of invertebrate assemblies to grazing. Ecography 15, 166–176.
| The response of invertebrate assemblies to grazing.Crossref | GoogleScholarGoogle Scholar |
Gibson, CW, Hambler, C, and Brown, V (1992b). Changes in spider (Araneae) assemblages in relation to succession and grazing management. Journal of Applied Ecology 29, 132–142.
| Changes in spider (Araneae) assemblages in relation to succession and grazing management.Crossref | GoogleScholarGoogle Scholar |
Graham, MH (2003). Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815.
| Confronting multicollinearity in ecological multiple regression.Crossref | GoogleScholarGoogle Scholar |
Haddad, CR, Honiball, AS, Dippenaar-Schoeman, AS, Slotow, R, and Van Rensburg, BJ (2010). Spiders as potential indicators of elephant-induced habitat changes in endemic sand forest, Maputaland, South Africa. African Journal of Ecology 48, 446–460.
| Spiders as potential indicators of elephant-induced habitat changes in endemic sand forest, Maputaland, South Africa.Crossref | GoogleScholarGoogle Scholar |
Haddad, CR, Foord, SH, Fourie, R, and Dippenaar-Schoeman, AS (2015). Effects of a fast-burning spring fire on the ground-dwelling spider assemblages (Arachnida: Araneae) in a central South African grassland habitat. African Zoology 50, 281–292.
| Effects of a fast-burning spring fire on the ground-dwelling spider assemblages (Arachnida: Araneae) in a central South African grassland habitat.Crossref | GoogleScholarGoogle Scholar |
Halaj, J, Ross, DW, and Moldenke, R (1998). Habitat structure and prey availability as predictors of the abundance and community organization of spiders in western Oregon forest canopies. Journal of Arachnology 26, 203–220.
Hsieh, TC, Ma, KH, and Chao, A (2016). iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods in Ecology and Evolution 7, 1451–1456.
| iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers).Crossref | GoogleScholarGoogle Scholar |
Huruba, R, Mundy, PJ, Sebata, A, Purchase, GK, and MacFadyen, DN (2017). Impala, Aepyceros melampus: does browse quality influence their use of sites originally utilised as short-duration kraals in a Southern African savanna? Rangeland Journal 39, 113–121.
| Impala, Aepyceros melampus: does browse quality influence their use of sites originally utilised as short-duration kraals in a Southern African savanna?Crossref | GoogleScholarGoogle Scholar |
Huruba, R, Mlambo, T, Mundy, PJ, Sebata, A, and MacFadyen, DN (2018). Short duration overnight cattle kraaling in natural rangelands: Implications for grass composition, quality, above-ground biomass, species diversity and basal cover. Agriculture, Ecosystems and Environment 257, 144–151.
| Short duration overnight cattle kraaling in natural rangelands: Implications for grass composition, quality, above-ground biomass, species diversity and basal cover.Crossref | GoogleScholarGoogle Scholar |
Kangalawe, RYM, Christiansson, C, and Östberg, W (2008). Changing land-use patterns and farming strategies in the degraded environment of the Irangi Hills, central Tanzania. Agriculture, Ecosystems & Environment 125, 33–47.
Kizza, S, and Areola, O (2010). Analysis of the effects of kraal manure accumulation on soil nutrient status through time. Journal of Soil Science and Environmental Management 1, 217–226.
Lafage, D, Djoudi, EA, Perrin, G, Gallet, S, and Pétillon, J (2019). Responses of ground-dwelling spider assemblages to changes in vegetation from wet oligotrophic habitats of western France. Arthropod-Plant Interactions 13, 653–662.
| Responses of ground-dwelling spider assemblages to changes in vegetation from wet oligotrophic habitats of western France.Crossref | GoogleScholarGoogle Scholar |
Legendre P, Legendre L (1998) ‘Numerical ecology’, 2nd edn. (Elsevier: Amsterdam, Netherlands)
Lovell, S, Hamer, M, Slotow, R, and Herbert, D (2009). An assessment of the use of volunteers for terrestrial invertebrate biodiversity surveys. Biodiversity and Conservation 18, 3295–3307.
| An assessment of the use of volunteers for terrestrial invertebrate biodiversity surveys.Crossref | GoogleScholarGoogle Scholar |
Mallis, RE, and Hurd, LE (2005). Diversity among ground-dwelling spider assemblages: habitat generalist and specialists. Journal of Arachnology 33, 101–109.
| Diversity among ground-dwelling spider assemblages: habitat generalist and specialists.Crossref | GoogleScholarGoogle Scholar |
Marc, P, Canard, A, and Ysnel, F (1999). Spiders (Araneae) useful for pest limitation and bioindication. Agriculture, Ecosystems & Environment 74, 229–273.
Mberi M (2013) Debshan Ranch and the holistic alternative: an overview. In ‘The 4th Annual Diamond Route Research Conference’, 29 October 2013, De Beers Campus, Johannesburg, South Africa.
Mineo, MF, Del-Claro, K, and Brescovit, AD (2010). Seasonal variation of ground spiders in a Brazilian savanna. Zoologia (Curitiba) 27, 353–362.
| Seasonal variation of ground spiders in a Brazilian savanna.Crossref | GoogleScholarGoogle Scholar |
Mrzljak, J, and Wiegleb, G (2000). Spider colonization of former brown coal mining areas - time or structure dependent? Landscape & Urban Planning 51, 131–146.
Muelelwa, MI, Foord, SH, Dippenaar-Schoeman, AS, and Stam, EM (2010). Towards a standardized and optimized protocol for rapid biodiversity assessments: spider species richness and assemblage composition in two savanna vegetation types. African Zoology 45, 273–290.
| Towards a standardized and optimized protocol for rapid biodiversity assessments: spider species richness and assemblage composition in two savanna vegetation types.Crossref | GoogleScholarGoogle Scholar |
Mugandani, R, Wuta, M, Makarau, A, and Chipindu, B (2012). Re-classification of agroecological regions of Zimbabwe in conformity with climate variability and change. African Crop Science Journal 20, 361–369.
Muvengwi, J, Chikorowondo, G, Mbiba, M, and Gandiwa, E (2018). Diversity and abundance of macro-invertebrates on abandoned cattle kraals in a semi-arid savanna. Ecology and Evolution 8, 8477–8489.
| Diversity and abundance of macro-invertebrates on abandoned cattle kraals in a semi-arid savanna.Crossref | GoogleScholarGoogle Scholar | 30250717PubMed |
Nakagawa, S, and Schielzeth, H (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4, 133–142.
| A general and simple method for obtaining R2 from generalized linear mixed-effects models.Crossref | GoogleScholarGoogle Scholar |
Nentwig, W, and Kobelt, M (2010). Spiders (Araneae). In ‘Alien terrestrial arthropods of Europe’. Ch. 7.3. (Eds A. Roques, M. Kenis, D. Lees, C. Lopez-Vaamonde, W. Rabitsch, J.-Y. Rasplus & D. Roy.) BioRisk 4, 131–147.
Niemela, J, Pajunen, T, Haila, Y, Punttila, P, and Halme, E (1994). Seasonal activity of boreal forest-floor spiders (Araneae). Journal of Arachnology 22, 23–31.
Nyffeler, M (1999). Prey selection of spiders in the field. Journal of Arachnology 27, 317–324.
Nyffeler, M, and Birkhofer, K (2017). An estimated 400–800 million tons of prey are annually killed by the global spider community. The Science of Nature 104, 30.
| An estimated 400–800 million tons of prey are annually killed by the global spider community.Crossref | GoogleScholarGoogle Scholar | 28289774PubMed |
Nyffeler, M, and Knörnschild, M (2013). Bat predation by spiders. PLoS One 8, e58120.
| Bat predation by spiders.Crossref | GoogleScholarGoogle Scholar | 23516436PubMed |
Nyffeler, M, and Pusey, BJ (2014). Fish predation by semi-aquatic spiders: a global pattern. PLoS One 9, e99459.
| Fish predation by semi-aquatic spiders: a global pattern.Crossref | GoogleScholarGoogle Scholar | 24940885PubMed |
Pedley, SM, and Dolman, PM (2014). Multi-taxa trait and functional responses to physical disturbance. Journal of Animal Ecology 83, 1542–1552.
| Multi-taxa trait and functional responses to physical disturbance.Crossref | GoogleScholarGoogle Scholar | 24942040PubMed |
Piacentini, LN, and Ramírez, MJ (2019). Hunting the wolf: a molecular phylogeny of the wolf spiders (Araneae, Lycosidae). Molecular Phylogenetics and Evolution 136, 227–240.
| Hunting the wolf: a molecular phylogeny of the wolf spiders (Araneae, Lycosidae).Crossref | GoogleScholarGoogle Scholar | 30953780PubMed |
Podgaiski, LR, Joner, F, Lavorel, S, Moretti, M, Ibanez, S, Mendonça Jr, MDS, and Pillar, VD (2013). Spider trait assembly patterns and resilience under fire-induced vegetation change in South Brazilian grasslands. PLoS One 8, e60207.
| Spider trait assembly patterns and resilience under fire-induced vegetation change in South Brazilian grasslands.Crossref | GoogleScholarGoogle Scholar | 23555927PubMed |
Pryke, JS, and Samways, MJ (2012). Importance of using many taxa and having adequate controls for monitoring impacts of fire for arthropod conservation. Journal of Insect Conservation 16, 177–185.
| Importance of using many taxa and having adequate controls for monitoring impacts of fire for arthropod conservation.Crossref | GoogleScholarGoogle Scholar |
Queiroz, M, and Gasnier, T (2017). Strong negative effect of diurnal rainfall on nocturnal activity of a wandering spider in Central Amazonia. International Journal of Tropical Biology 65, 1152–1160.
| Strong negative effect of diurnal rainfall on nocturnal activity of a wandering spider in Central Amazonia.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing. Version 4.1.2.’ (R Foundation for Statistical Computing: Vienna, Austria)
Rampai RI (2017) Effects of different grazing systems on ecosystem functions in Lesotho and Iceland. Final project report. United Nations University Land Restoration Training Programme, Reykjavik, Iceland.
Reid, RS, and Ellis, JE (1995). Impacts of pastoralists on woodlands in South Turkana, Kenya: livestock-mediated tree recruitment. Ecological Applications 5, 978–992.
| Impacts of pastoralists on woodlands in South Turkana, Kenya: livestock-mediated tree recruitment.Crossref | GoogleScholarGoogle Scholar |
Roberson, EJ, Chips, MJ, Carson, WP, and Rooney, TP (2016). Deer herbivory reduces web-building spider abundance by simplifying forest vegetation structure. PeerJ 4, e2538.
| Deer herbivory reduces web-building spider abundance by simplifying forest vegetation structure.Crossref | GoogleScholarGoogle Scholar | 27703868PubMed |
Robertson EF (2013) Vegetation monitoring sites on Shangani Ranch: methods and descriptions of grass species composition and ecological condition for sites 1–27, March–April 2013. Report prepared for E. Oppenheimer and Son (Pty) Limited and Debshan Pvt Ltd, Shangani, Zimbabwe.
Rodriguez-Artigas, SM, Ballester, R, and Corronca, JA (2016). Factors that influence the beta-diversity of spider communities in northwestern Argentinean grasslands. PeerJ 4, e1946.
| Factors that influence the beta-diversity of spider communities in northwestern Argentinean grasslands.Crossref | GoogleScholarGoogle Scholar | 27123380PubMed |
Savory, A (1983). The savory grazing method or holistic resource management. Rangelands 5, 155–159.
Savory, A, and Parsons, SD (1980). The savory grazing method. Rangelands 2, 234–237.
Schwerdt, L, de Villalobos, AE, and Miles, FP (2018). Spiders as potential bioindicators of mountain grasslands health: the Argentine tarantula Grammostola vachoni (Araneae, Theraphosidae). Wildlife Research 45, 64–71.
| Spiders as potential bioindicators of mountain grasslands health: the Argentine tarantula Grammostola vachoni (Araneae, Theraphosidae).Crossref | GoogleScholarGoogle Scholar |
Sebata S (2020) Spider ecology in South-Western Zimbabwe, with an emphasis on the impact of Holistic Planned Grazing Practices. PhD Thesis, University of the Free State, Bloemfontein, South Africa.
Sibanda, P, Sebata, A, Mufandaedza, E, and Mawanza, M (2016). Effect of short-duration overnight cattle kraaling on grass production in a southern African savanna. African Journal of Range and Forage Science 33, 217–223.
| Effect of short-duration overnight cattle kraaling on grass production in a southern African savanna.Crossref | GoogleScholarGoogle Scholar |
Stelfox, JB (1986). Effects of livestock enclosures (bomas) on the vegetation of the Athi Plains, Kenya. African Journal of Ecology 24, 41–45.
| Effects of livestock enclosures (bomas) on the vegetation of the Athi Plains, Kenya.Crossref | GoogleScholarGoogle Scholar |
Štokmane, M, and Spuņģis, V (2016). The influence of vegetation structure on spider species richness, diversity and community organization in the Apšuciems calcareous fen, Latvia. Animal Biodiversity and Conservation 39, 221–236.
| The influence of vegetation structure on spider species richness, diversity and community organization in the Apšuciems calcareous fen, Latvia.Crossref | GoogleScholarGoogle Scholar |
Suominen, O (1999). Mammalian herbivores, vegetation and invertebrate assemblages in boreal forests: feeding selectivity, ecosystem engineering and trophic effects. Annales Universitatis Turkuensis 122, 1–26.
Turnbull, AL (1973). Ecology of the true spiders (Araneomorphae). Annual Reviews of Entomology 18, 305–348.
| Ecology of the true spiders (Araneomorphae).Crossref | GoogleScholarGoogle Scholar |
Veblen, KE, and Porensky, LM (2019). Thresholds are in the eye of the beholder: plants and wildlife respond differently to short-term cattle corrals. Ecological Applications 29, e01982.
| Thresholds are in the eye of the beholder: plants and wildlife respond differently to short-term cattle corrals.Crossref | GoogleScholarGoogle Scholar | 31348560PubMed |
Wang Y, Naumann U, Wright S, Eddelbuettel D, Warton D (2018) Mvabund: statistical methods for analysing multivariate abundance data. R package version 3.13.1. Available at https://rdrr.io/cran/mvabund [Accessed 11 January 2018]
Warton, DI, Foster, SD, De’ath, G, Stoklosa, J, and Dunstan, PK (2015). Model-based thinking for community ecology. Plant Ecology 216, 669–682.
| Model-based thinking for community ecology.Crossref | GoogleScholarGoogle Scholar |
Warton, DI, Thibaut, L, and Wang, YA (2017). The PIT-trap: a ‘model-free’ bootstrap procedure for inference about regression models with discrete, multivariate responses. PLoS One 12, e0181790.
| The PIT-trap: a ‘model-free’ bootstrap procedure for inference about regression models with discrete, multivariate responses.Crossref | GoogleScholarGoogle Scholar | 28738071PubMed |
Weeks, RD, and Holtzer, TO (2000). Habitat and season in structuring ground-dwelling spider (Araneae) communities in a shortgrass steppe ecosystem. Environmental Entomology 29, 1164–1172.
| Habitat and season in structuring ground-dwelling spider (Araneae) communities in a shortgrass steppe ecosystem.Crossref | GoogleScholarGoogle Scholar |
Weyman, GS (1993). A review of the possible causative factors and significance of ballooning in spiders. Ethology Ecology & Evolution 5, 279–291.
Weyman, GS, Sunderland, KD, and Jepson, PC (2002). A review of the evolution and mechanisms of ballooning by spiders inhabiting arable farmland. Ethology Ecology & Evolution 14, 307–326.
Whitmore, C, Slotow, R, Crouch, TE, and Dippenaar-Schoeman, AS (2002). Diversity of spiders (Araneae) in a savanna reserve, Northern Province, South Africa. Journal of Arachnology 30, 344–356.
| Diversity of spiders (Araneae) in a savanna reserve, Northern Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Whittaker, RH (1960). Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs 30, 280–338.
| Vegetation of the Siskiyou Mountains, Oregon and California.Crossref | GoogleScholarGoogle Scholar |
Wise, DH (2006). Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annual Review of Entomology 51, 441–465.
| Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations.Crossref | GoogleScholarGoogle Scholar | 16332219PubMed |
World Spider Catalog (2022) World Spider Catalog, Version 22.5. Natural History Museum Bern. Available at https://wsc.nmbe.ch [Accessed 12 January 2022]
Zhao L, Panayotova IN, Chuang A, Sheldon KS, Bourouiba L, Miller LA (2017) Flying spiders: simulating and modeling the dynamics of ballooning. In ‘Women in mathematical biology’. (Eds AT Layton, LA Miller) pp. 179–210. (Springer: Cham, Switzerland)
Zuur, AF, and Ieno, EN (2016). A protocol for conducting and presenting results of regression-type analyses. Methods in Ecology and Evolution 7, 636–645.
| A protocol for conducting and presenting results of regression-type analyses.Crossref | GoogleScholarGoogle Scholar |
Appendix
|
|
|

|