Response of the annual biomass production of a typical steppe plant community to precipitation fluctuations
Zhen Wang A D , Qing Zhang B D , Xiaoping Xin C , Yong Ding A E , Xiangyang Hou A E ,A Institute of Grassland Research, Chinese Academy of Agricultural Sciences, Hohhot, 010010, China.
B Department of Ecology and Environment Science, Inner Mongolia University, Hohhot, 010021, China.
C National Hulunber Grassland Ecosystem Observation and Research Station, Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing, 100081, China.
D These authors contributed equally to the paper.
E Corresponding authors. Email: houxy16@126.com; dingyong228@126.com
The Rangeland Journal 36(6) 527-534 https://doi.org/10.1071/RJ14065
Submitted: 13 May 2014 Accepted: 11 October 2014 Published: 11 November 2014
Journal Compilation © Australian Rangeland Society 2014
Abstract
Understanding the relationship between the aboveground net primary production (ANPP) and annual precipitation in arid and semiarid grasslands is crucial for assessing the effects of climate change on grassland ecosystems. The temporal pattern of ANPP, based on long-term data on a semiarid ecosystem in Inner Mongolia, was investigated. The biomass of perennial grasses, perennial forbs and Stipa grandis P. Smirn., showed a positive relationship with annual precipitation. The amount of annual precipitation also changed the annual biomass of 13 other dominant species and consequently the ANPP. The coefficient of variation of the ANPP of the plant community was lower than the coefficient of variation of annual precipitation. Irrespective of the strong inter-annual variation in annual precipitation, the positive relationship found between ANPP and annual precipitation suggests the dependence of ANPP upon hydrological variations in typical steppe. Our findings highlight the importance of dominant perennial species and functional groups in mediating the responses of ANPP to annual precipitation in the typical steppe in northern China.
Additional keywords: ANPP, annuals and biennials, perennial forbs, perennial grass, shrubs and semi-shrubs.
Introduction
Global climate change resulting from anthropogenic activities is occurring (Solomon et al. 2007), including shifts in precipitation regimes and increasing extreme rainfall events. Precipitation is a critical factor affecting plant growth and could well alter inter-specific relationships among plant species (Niu et al. 2007). The fluctuations and cyclical variations in precipitation are closely related to net primary productivity and biomass allocation (Huxman et al. 2004). These changes in community structure and composition are likely to have consequent effects on ecosystem functioning (Tilman et al. 1997) and potentially feed back to climate change (Chapin et al. 2005).
Overgrazing is one of the most important human-induced causes for arid and semiarid grassland degradation, which reduces vegetation cover, increases the abundance of undesirable species, reduces species diversity and destroys soil structure (Li et al. 2006; Zhou et al. 2011; Louhaichi et al. 2012). However, fencing and the exclusion of domestic livestock is the most common management tool used for restoring vegetation productivity in degraded grassland throughout the world (Spooner et al. 2002; Liu et al. 2007). Fencing, as a method of restoring rangelands, has been implemented in many areas in China, including desert steppe (Li et al. 2008) and semiarid steppe (Bai et al. 2004; Deng et al. 2014). The effect of livestock exclusion on grasslands has been studied with a focus on plant diversity, community structure and productivity (Gibson et al. 2000; Wu et al. 2009; Deng et al. 2014). Livestock exclusion has major effects on ecosystem processes, which vary in different grasslands (Pettit et al. 1995; Reeves 2000), and consequently the results are difficult to predict (Spooner et al. 2002).
The existence of a long-term (29 years) fenced exclosure in a typical steppe region has provided an opportunity to better understand the effect of grazing exclusion on several aspects of grassland production. The general objective was to better understand several aspects of the dynamics of grassland production in response to precipitation fluctuations during 1981–2011 in the absence of livestock grazing. We addressed the following questions: (1) how does the aboveground net primary production (ANPP) of the grassland change with the annual variation in precipitation, and (2) how do the plant functional group composition and the contributions of the dominant species change with the annual variation in the precipitation?
Materials and methods
Study sites and field sampling
The fenced grassland used in this study, covered an area of ~26.6 ha and was a permanent field site located in typical steppe in the Baiyinxile ranch, Xilinguole of Inner Mongolia, China (115°32ʹE–116°12ʹE, 43°26ʹN–43°39ʹN). Mean annual temperature is 0.7°C and mean annual precipitation is ~350 mm, which falls mainly in the summer from June to August. The growing season runs from early April to late September for perennial plant species. The dominant species in the plant community were Leymus chinensis (Trin.) Tzvel. and Stipa grandis P. Smirn., accompanied by, principally, Achnatherum sibiricum (Linn.) Keng, Agropyron critsatum (L.) P. Beauv., Caragana microphylla Lam., Artemisia commutata Besser, Carex korshinskyi Kom., Kochia prostrata (L.) Schrad., Serratula centauroides L., Cleistogenes squarrosa (Trin.) Keng, Koeleria cristata (L.) Pers., Artemisia frigida Willd. Sp. Pl. and Potentilla bifurca L. This community is representative of one of the most widely distributed grasslands in the Eurasian steppes. The site had been fenced off, preventing grazing by domestic animals since 1979. At the time of enclosure, the site was considered to be in excellent condition, and representative of an undisturbed community. Soils were classified as Calcic Chernozems (IUSS Working Group WRB 2006). A different 50 × 50 m-sample area was selected within the fenced exclosure each year and 20 × 1-m2 quadrats were randomly placed within this area for sampling. Care was taken to ensure that the 50 × 50-m sample area chosen in any one year had not been sampled in previous years.
ANPP was measured in late August each year by clipping all living vascular plant material to ground level within 1 m × 1 m quadrats that were randomly located within plots in 1981–1994 and 1997–2011. No data were collected in 1995–1996. The clipped material represented the total growth since the beginning of the growing season each year. The harvested material was sorted into species, oven-dried at 65°C for 48 h and then weighed. The dry mass of all plant species m–2 averaged over the 20 sampling plots was used to estimate the aboveground community biomass, which approximated the ANPP of the grassland ecosystem. Plant species in the sample plots were grouped into four plant functional groups: perennial grasses, perennial forbs, shrubs and semi-shrubs, and annuals-biennials (short-lived <2 years) (Bai et al. 2008). All meteorological data were obtained from the Xilinhot weather station.
Data analyses
The coefficient of variation (CV) was calculated as: CV = (standard deviation/mean) × 100% for each of the attributes listed above. Rain-use efficiency was calculated directly as the ratio of ANPP to precipitation (RUE = ANPP/Precipitation). The effects of annual precipitation on total ANPP, the biomass of the functional groups and dominant species were analysed using one-way ANOVA with P = 0.05 as the level of significance. Linear regression analysis was used to evaluate the relationships of annual precipitation with the ANPP of the plant community, and the biomass of the different functional groups and the dominant species. Linear regression analysis was also used to examine the relationship between RUE and the biomass of dominant species, RUE and annual precipitation, species richness and ANPP, and species richness and annual precipitation. Stepwise multiple linear analyses were used to examine the relationships of ANPP with the biomass of forbs, grasses, shrubs and semi-shrubs, and annual-biennials. All statistical analyses were conducted using SAS software version 9.1 (SAS Institute 2003).
Results
Plant species recorded in the community
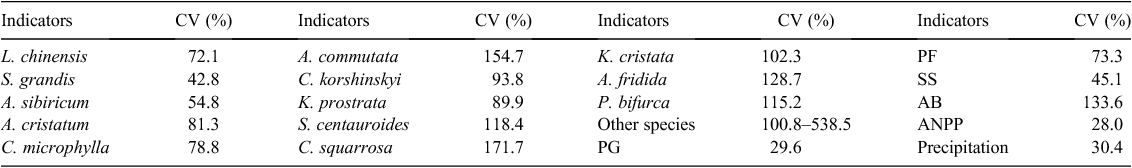
Seventy-seven plant species were recorded in the study and of these, 13 each contributed >1% of the biomass and together accounted for 90.3% of the total biomass of the community (Fig. 1a). Among these 13 species, the matrix species, L. chinensis, and the dominant species, S. grandis, each contributed more than 20.0% and the first five most abundant species accounted for 70.3% of the total community biomass. The percentages of the biomass contributed by the different functional groups were perennial grasses (73.0%) > perennial forbs (14.1%) > shrubs (10.1%) > annual and biennials (2.8%) (Fig. 1b).

|
Compared with the other plant species, the CV for the biomass of S. grandis was the smallest and that of the other species the largest (Table 1). The CV of the ANPP of the plant community was the smallest with the CV of the annual precipitation slightly larger (Table 1). The CV of the biomass of the functional groups was in the order of perennial grasses (29.6%) > shrubs-semi-shrubs (45.1%) > perennial forbs (73.3%) > annual and biennials (133.6%) (Table 1).
The effects of annual precipitation on the dominant species, functional groups and ANPP
Dominant species
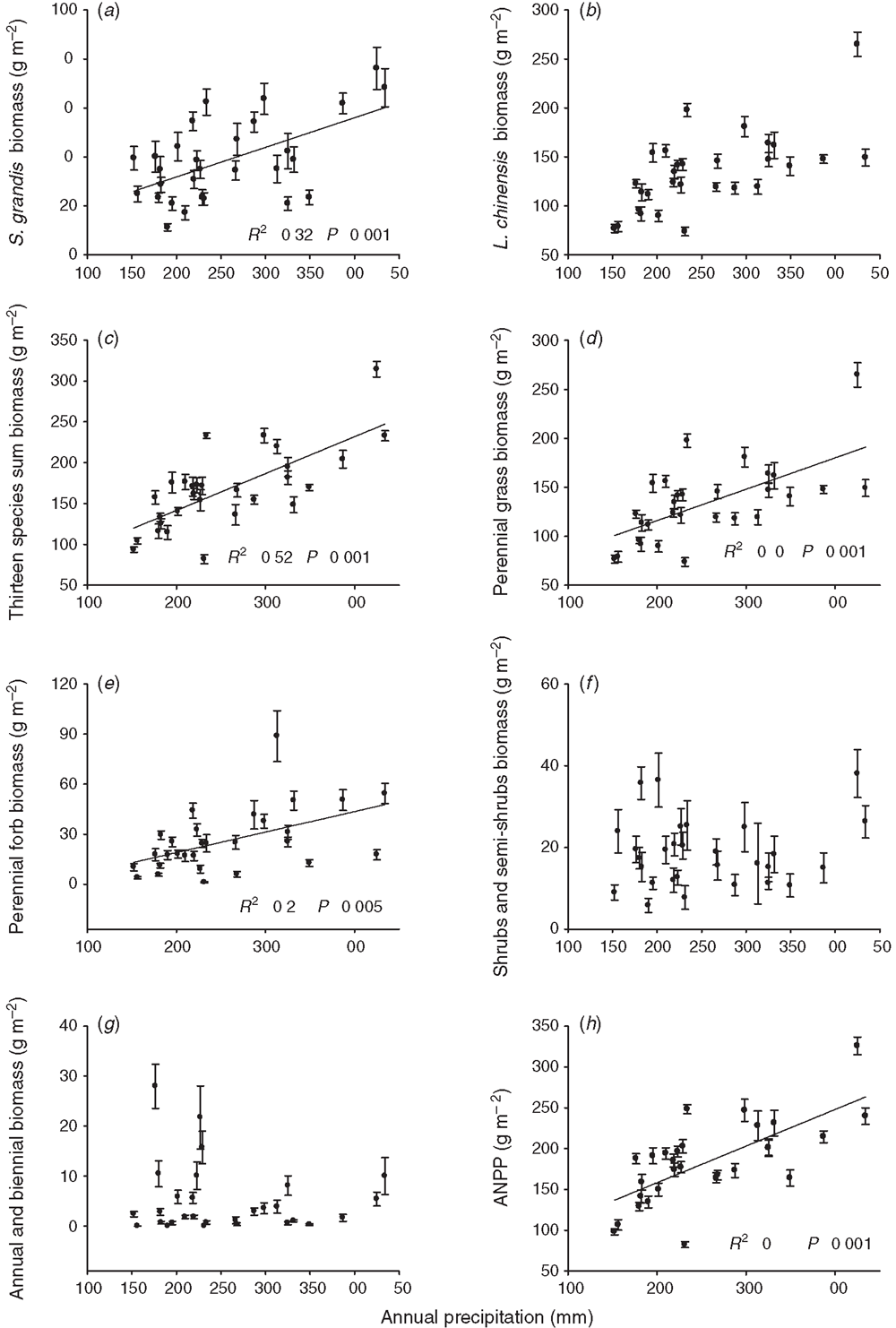
The annual biomass of S. grandis increased with increasing precipitation (R2 = 0.32, P = 0.001; Fig. 2a), whereas its RUE decreased with increasing precipitation over the 29 years (R2 = 0.22, P = 0.009; Fig. 3a). Our results also showed that the annual biomass of S. grandis was positively correlated with precipitation in the periods of January–August, April–August, April–July and January–July (Table 2). No relationship was detected for annual biomass of L. chinensis and annual precipitation (Fig. 2b) but the total biomass of the 13 most abundant species did increase significantly with increasing rainfall (Fig. 2c). Annual biomass of L. chinensis was positively correlated with precipitation in the periods of January–August, April–August, April–July and January–July (Table 2). A marginally positively relationship was detected between annual biomass of S. centauroides and January–August precipitation as well as marginally positively correlations for annual biomass of K. cristata with precipitation in the periods of January–August and April–August (Table 2).

|
Functional groups
Across the 29 years of observation, the annual biomass of perennial grasses (R2 = 0.40, P = 0.0002; Fig. 2d) and the annual biomass of perennial forbs (R2 = 0.26, P = 0.005; Fig. 2e) showed a linear increase with annual precipitation, whereas no relationship was found between annual precipitation and the annual biomass of shrubs (Fig. 2f), or annual precipitation and the annual biomass of annuals and biennials (Fig. 2g). The annual biomass of perennial grasses and perennial forbs also showed a linear increase with precipitation in the periods of January–August, April–August, April–July and January–July (Table 2).
Plant communities
Our analysis of the 29 years showed that the ANPP increased linearly with annual precipitation (R2 = 0.48, P < 0.001; Fig. 2h). The RUE decreased with increasing annual precipitation across the 29 years of observation (Fig. 3b). The ANPP was positively correlated with precipitation in the periods of January–August, April–August, April–July and January–July (Table 2). Stepwise multiple regression analyses demonstrated that 94.8% of the variation in ANPP could be explained by a combination of the annual biomass of perennial grasses (partial R2 = 0.84, P < 0.001) and perennial forbs (partial R2 = 0.11, P < 0.001) over 29 years. Plant species richness increased with increasing annual precipitation (R2 = 0.15, P = 0.037; Fig. 4a), and a positive linear relationship was detected between species richness and ANPP (R2 = 0.34, P < 0.001; Fig. 4b).

|
Discussion
Mechanisms of stability in biomass production of the grassland community
It has been proposed that the complementary effects that species or functional groups have on diversity and the mutual compensation effect of resource utilisation among species or functional groups maintains the stability of community productivity (Bai et al. 2004; Firn et al. 2007). However, in our study, the stability of community productivity mainly depended on one or several dominant species and our results supported the idea that communities with higher species richness were more productive probably because such communities are more likely to contain high-yielding dominant species (Bell et al. 2005). Our results also supported the notion that high species richness maintained the stability of plant communities in terms of their functioning and productivity (Tilman 1993). Moreover, the perennial grasses may have contributed to the high ANPP and the low CV of ANPP, whereas the variable growth of annuals and biennials would have contributed little in our study (Table 2, Fig. 1b). Our results suggested community matrix species, dominant species and perennial grasses maintain community stability.
Plant species and functional group-level responses to annual precipitation
Our results demonstrated that the responses of individual species to annual precipitation were highly variable and changed with time, for example as demonstrated by the positive linear correlation found between the biomass of S. grandis and annual precipitation (Fig. 2a), whereas no such relationship was detected for L. chinensis. Bai et al. (2004) concluded that compensatory effects at the species level are important in grassland communities and reductions of biomass of one species are invariably compensated for by increases from other species (Tilman 1999). However, there have been inconsistencies in the ways that different species contribute to community biomass in previous studies. Our results revealed that the responses of dominant species to annual precipitation had important effects on plant community biomass in that S. grandis and L. chinensis, together, accounted for 46.9% of the total aboveground biomass, and their precipitation utilisation within the community was dominant in contributing to the biomass of the community.
Based on our original hypothesis, different plant functional groups should have shown different responses to annual precipitation. A positive linear relationship was found between precipitation and perennial grasses, and perennial forbs, whereas no relationship was shown between annual precipitation and shrubs or annuals and biennials (Fig. 2d, e, f and g). The responses of different functional groups appeared to be driven by the dominant and subdominant species as well as by species interactions. For example, with increasing precipitation (Table 2), enhanced biomass of S. grandis and L. chinensis resulted in an increase in the biomass of perennial grasses irrespective of the small changes in other species. This might be attributable to competitive interactions with the other species and their intrinsically low competitive abilities because the two main perennial grass species are tall and have a high productivity (Fanselow et al. 2011). Our results further showed that the effect of annual precipitation on the biomass of functional groups was mainly due to the responses of the following 13 key species of grasses (S. grandis, L. chinensis, A. sibiricum, A. cristatum, C. korshinskyi, C. squarrosa, K. cristata), forbs (S. centauroides and P. bifurca), shrub (C. microphylla, K. prostrata and A. frigida) and annuals and biennials (A. commutata); together, they accounted for 90.3% of the total aboveground biomass. The above observations suggest that key species, as well as different species interactions, play important roles in regulating the responses of plant functional groups to precipitation.
Response of ANPP to annual precipitation
The study of relationship between precipitation and ANPP in arid and semiarid environments has focussed on the influence of average precipitation at different time scales including a year, a quarter and a month (Briggs et al. 1989; Fang et al. 2001; Knapp and Smith 2001). Bai et al. (2004) considered that precipitation in the period of January–July is the primary climatic factor causing fluctuations in ANPP. However, in our study, the positive association between precipitation and ANPP in the period of January–August was higher than in the periods of April–August, January–July and April–July (Table 2). Thus, our study suggested that the precipitation during January–August (precipitation before and during the whole growing season) played a vital role in determining the response of ANPP to the annual precipitation.
Variations in the seasonality, frequency and amounts of precipitation will have important impacts on ANPP (Knapp et al. 2002; Nippert et al. 2009). Aboveground net primary production is a key integrative measure of ecosystem functioning that increases with increasing annual precipitation (Huxman et al. 2004). Our data support the notion that precipitation controls the inter-annual variations in ANPP (strong linear relationship between annual precipitation and ANPP) in this typical steppe. The fluctuation of annual RUE (0.46–1.10 g m–2 mm–1) for typical steppe in Inner Mongolia is within the broad range of RUE (0.05–1.81 g m–2 mm–1) reported generally for arid and semiarid ecosystems throughout the world (Le Houérou et al. 1988). These results were consistent with a previous study that found that RUE decreased with increasing annual precipitation from an analysis of a 24-year dataset in the typical steppe (Bai et al. 2008). Our results supported previous work that RUE decreased over time with increasing annual precipitation (Paruelo et al. 1999; Lauenroth et al. 2000).
Traits of dominant plant species and functional groups may affect ANPP (Eviner and Chapin 2003). In our study, positive linear relationships between annual precipitation and annual biomass of S. grandis (Fig. 2a) suggest that annual precipitation increases the ANPP. An array of perennial species (particularly perennial grasses) affects ANPP and annual precipitation produced a similar trend with the perennial grasses, in agreement with a previous study (Pérez-Camacho et al. 2012). A dry year can be the major limiting factor for perennial plant growth, as has been shown in previous studies (Tozer et al. 2009; Pérez-Camacho et al. 2012). Perennials and annuals are at the two ends of an adaptation gradient to drought, based on ‘drought tolerance’ and ‘drought avoidance’, respectively. Perennial species maintain relatively low above- and belowground living tissues throughout a dry growing season to ensure survival, whereas annuals survive the drought as seeds (Slatyer 1967). Perennial grasses also may grow less from prolonged closure of stomata to prevent water loss and eventually die from desiccation during the drought (Jackson and Roy 1986). However, the perennial plant species occupy space by their vegetative structure and litter accumulation, and the thick litter or living structures of perennials affects the invasion of annuals and biennials (Noy-Meir et al. 1989). Such grasslands are thus barely colonised by annuals and biennials (Madon and Médail 1997). Therefore, our results highlighted the importance of perennial grasses in modulating the responses of ANPP to precipitation.
Conclusions
In typical steppe, variation in ANPP was significantly correlated with annual precipitation. The dominant species and plant functional groups played a positive role in maintaining the relative stability of the production of the plant community. The variation in the biomass of the dominant species, S. grandis, was most sensitive to annual precipitation. Our study has improved knowledge about the responses of plant communities, functional groups and species to precipitation, and provides useful information to develop grassland management methods under the variable of precipitation within the typical steppe communities.
Acknowledgements
The authors thank Fenghui Guo and Lei Ji for their help with the field measurements. The authors also thank the referees for their comments on the original version of this manuscript. This study was financially supported by the National Key Basic Research Development Program (2014CB138805), the International Science and Technology Cooperation Project of China (2012DFA31290), the National Natural Science Foundation of China (70933004, 71103185 and 71311120089), Central Non-profit Research Institutes Fundamental Research Funds (1610332014001) and Key Technologies Research and Development Program of China (2012BAC19B04).
References
Bai, Y. F., Han, X. G., Wu, J. G., Chen, Z. Z., and Li, L. H. (2004). Ecosystem stability and compensatory effects in the Inner Mongolia grasslands. Nature 431, 181–184.| Ecosystem stability and compensatory effects in the Inner Mongolia grasslands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntlGks7w%3D&md5=4521d03d8ef508af376edf636aff5f41CAS |
Bai, Y. F., Wu, J. G., Xing, Q., Pan, Q. M., Huang, J. H., Yang, D. L., and Han, X. G. (2008). Primary production and rain-use efficiency across a precipitation gradient on the Mongolian Plateau. Ecology 89, 2140–2153.
| Primary production and rain-use efficiency across a precipitation gradient on the Mongolian Plateau.Crossref | GoogleScholarGoogle Scholar |
Bell, T., Newman, J. A., Silverman, B. W., Turner, S. L., and Liley, A. K. (2005). The contribution of species richness and composition to bacterial services. Nature 436, 1157–1160.
| The contribution of species richness and composition to bacterial services.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXovVOgtro%3D&md5=47d360c2209d248c7ab2b8db2b8a75d9CAS | 16121181PubMed |
Briggs, J. M., Seastedt, T. R., and Gibson, D. J. (1989). Comparative analysis of temporal and spatial variability in above-ground production in a deciduous forest and prairie. Holarctic Ecology 12, 130–136.
Chapin, F. S., Sturm, M., Serreze, M. C., McFadden, J. P., Key, J. R., Lloyd, A. H., Rupp, T. S., Lynch, A. H., Schimel, J. P., Beringer, J., Chapman, W. L., Epstein, H. E., Euskirchen, E. S., Hinzman, L. D., Jia, G., Ping, C. L., Tape, K. D., Thompson, C. D. C., Walker, D. A., and Welker, J. M. (2005). Role of land-surface changes in Arctic summer warming. Science 310, 657–660.
| Role of land-surface changes in Arctic summer warming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFChsLvL&md5=a13d9e33cc5733e8ff9b25f1ccd918f2CAS | 16179434PubMed |
Deng, L., Sweeney, S., and Shangguan, Z. P. (2014). Grassland responses to grazing disturbance: plant diversity changes with grazing intensity in a desert steppe. Grass and Forage Science 69, 524–533.
| Grassland responses to grazing disturbance: plant diversity changes with grazing intensity in a desert steppe.Crossref | GoogleScholarGoogle Scholar |
Eviner, V. T., and Chapin, F. S. (2003). Functional matrix: a conceptual framework for predicting multiple plant effects on ecosystem processes. Annual Review of Ecology, Evolution and Systematics 34, 455–485.
| Functional matrix: a conceptual framework for predicting multiple plant effects on ecosystem processes.Crossref | GoogleScholarGoogle Scholar |
Fang, J. Y., Piao, S. L., and Tang, Z. Y. (2001). Inter-annual variability in net primary production and precipitation. Science 293, 1723–1723a.
| Inter-annual variability in net primary production and precipitation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mrgs1Cjug%3D%3D&md5=ec0cd0ef6f62bddbfeefd7141a5827ebCAS |
Fanselow, N., Schönbach, P., Gong, X. Y., Lin, S., Taube, F., Loges, R., Pan, Q. M., and Dittert, K. (2011). Short-term regrowth responses of four steppe grassland species to grazing intensity, water and nitrogen in Inner Mongolia. Plant and Soil 340, 279–289.
| Short-term regrowth responses of four steppe grassland species to grazing intensity, water and nitrogen in Inner Mongolia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitFGkt7w%3D&md5=4c50f27791d5c3c10ccd8e6b29b4621dCAS |
Firn, J., Erskine, P. D., and Lamb, D. (2007). Woody species diversity influences productivity and soil nutrient availability in tropical plantations. Oecologia 154, 521–533.
| Woody species diversity influences productivity and soil nutrient availability in tropical plantations.Crossref | GoogleScholarGoogle Scholar | 17899202PubMed |
Gibson, R. S., Hewitt, A., Sparling, G., and Bosch, O. (2000). Vegetation change and soil quality in central Otago Tussock grasslands, New Zealand. The Rangeland Journal 22, 190–204.
| Vegetation change and soil quality in central Otago Tussock grasslands, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Huxman, T. E., Smith, M. D., Fay, P. A., Knapp, A. K., Shaw, M. R., Loik, M. E., Smith, S. D., Tissue, D. T., Zak, J. C., Weltizn, J. F., Pockman, W. T., Sala, O. E., Haddad, B. M., Harte, J., Koch, G. W., Schwinning, S., Small, E. E., and Williams, D. G. (2004). Convergence across biomes to a common rain-use efficiency. Nature 429, 651–654.
| Convergence across biomes to a common rain-use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXks1Kls7k%3D&md5=882e51dd5d8b19a6e184e17dd20c8938CAS | 15190350PubMed |
IUSS Working Group WRB (2006). ‘World Reference Base for Soil Resources 2006.’ World Soil Resources Reports No.103. (FAO: Rome, Italy.)
Jackson, L. E., and Roy, J. (1986). Growth patterns of Mediterranean annual and perennial grasses under simulated rainfall regimes of southern France and California. Acta Oecologica 7, 197–212.
Knapp, A. K., and Smith, M. D. (2001). Variation among biomes in temporal dynamics of above-ground primary production. Science 291, 481–484.
| Variation among biomes in temporal dynamics of above-ground primary production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlslensg%3D%3D&md5=8354bee5ae58cb07741a3bfe02b8423dCAS | 11161201PubMed |
Knapp, A. K., Fay, P. A., Blair, J. M., Collins, S. L., Smith, M. D., Carlisle, J. D., Harper, C. W., Danner, B. T., Lett, M. S., and McCarron, J. K. (2002). Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298, 2202–2205.
| Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsVSktLg%3D&md5=2d8b35ddbe81d75a2962418c5bd9d129CAS | 12481139PubMed |
Lauenroth, W. K., Burke, I. C., and Paruelo, J. M. (2000). Patterns of production and precipitation-use efficiency of winter wheat and native grasslands in the Central Great Plains of the United States. Ecosystems 3, 344–351.
| Patterns of production and precipitation-use efficiency of winter wheat and native grasslands in the Central Great Plains of the United States.Crossref | GoogleScholarGoogle Scholar |
Le Houérou, H. N., Bingham, R. L., and Skerbek, W. (1988). Relationship between the variability of primary production and the variability of annual precipitation in world arid lands. Journal of Arid Environments 15, 1–18.
Li, X. R., Jia, X. H., and Dong, G. R. (2006). Influence of desertification on vegetation pattern variations in the cold semi-arid grasslands of the Qinghai-Tibet plateau, Northwest China. Journal of Arid Environments 64, 505–522.
| Influence of desertification on vegetation pattern variations in the cold semi-arid grasslands of the Qinghai-Tibet plateau, Northwest China.Crossref | GoogleScholarGoogle Scholar |
Li, C. L., Hao, X. Y., Zhao, M. L., Han, G. D., and Willms, W. D. (2008). Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agriculture, Ecosystems & Environment 128, 109–116.
| Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia.Crossref | GoogleScholarGoogle Scholar |
Liu, B., Wu, N., Luo, P., and Tao, Y. P. (2007). Characteristics of soil nutrient distribution in high-altitude meadow ecosystems with different management and degradation scenarios. Chinese Journal of Eco-Agriculture 15, 45–48.
| 1:CAS:528:DC%2BD2sXhtVGjtrrE&md5=b510c9a502b4ef23e52a1ad9c1b663cdCAS |
Louhaichi, M., Ghassali, F., Salkini, A. K., and Petersen, S. L. (2012). Effect of sheep grazing on rangeland plant communities: case study of landscape depressions within Syrian arid steppes. Journal of Arid Environments 79, 101–106.
| Effect of sheep grazing on rangeland plant communities: case study of landscape depressions within Syrian arid steppes.Crossref | GoogleScholarGoogle Scholar |
Madon, O., and Médail, F. (1997). The ecological significance of annuals on a Mediterranean grassland (Mt Ventoux, France). Plant Ecology 129, 189–199.
| The ecological significance of annuals on a Mediterranean grassland (Mt Ventoux, France).Crossref | GoogleScholarGoogle Scholar |
Nippert, J. B., Fay, P. A., Carlisle, J. D., Knapp, A. K., and Smith, M. D. (2009). Ecophysiological responses of two dominant grasses to altered temperature and precipitation regimes. Acta Oecologica 35, 400–408.
| Ecophysiological responses of two dominant grasses to altered temperature and precipitation regimes.Crossref | GoogleScholarGoogle Scholar |
Niu, S. L., Wu, M. Y., Han, Y., Li, L. H., and Wan, S. Q. (2007). Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytologist 177, 209–219.
Noy-Meir, I., Gutman, M., and Kaplan, Y. (1989). Responses of Mediterranean grassland plants to grazing and protection. Journal of Ecology 77, 290–310.
| Responses of Mediterranean grassland plants to grazing and protection.Crossref | GoogleScholarGoogle Scholar |
Paruelo, J. M., Lauenroth, W. K., Burke, I. C., and Sala, O. E. (1999). Grassland precipitation-use efficiency varies across a resource gradient. Ecosystem 2, 64–68.
| Grassland precipitation-use efficiency varies across a resource gradient.Crossref | GoogleScholarGoogle Scholar |
Pérez-Camacho, L., Rebollo, S., Hernández-Santana, V., García-Salgado, G., Pavón-Garcia, J., and Gómez-Sal, A. (2012). Plant functional trait responses to inter-annual rainfall variability, summer drought and seasonal grazing in Mediterranean herbaceous communities. Functional Ecology 26, 740–749.
| Plant functional trait responses to inter-annual rainfall variability, summer drought and seasonal grazing in Mediterranean herbaceous communities.Crossref | GoogleScholarGoogle Scholar |
Pettit, N. E., Froend, R. H., and Ladd, P. G. (1995). Grazing in remnant woodland vegetation: changes in species composition and life form groups. Journal of Vegetation Science 6, 121–130.
| Grazing in remnant woodland vegetation: changes in species composition and life form groups.Crossref | GoogleScholarGoogle Scholar |
Reeves, G. W. (2000). ‘Bushcare Program: Mid-term Review.’ (CSIRO: Canberra, ACT.) Available at: www.nht.gov.au/mtrfinrpt/bushcarefinalreport.pdf (accessed 20 October 2000).
SAS Institute (2003). ‘SAS/STAT User’s Guide Release 9.1 edn.’ (SAS Institute Ltd: Cary, NC.)
Slatyer, R. O. (1967). ‘Plant Water Relationships.’ (Academic Press: London, UK.)
Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Avery, K. B., Tignor, M., and Miller, H. L. (2007). ‘Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK.)
Spooner, P., Lunt, I., and Robinson, W. (2002). Is fencing enough? The short-term effects of stock exclusion in remnant grassy woodlands in southern NSW. Ecological Management & Restoration 3, 117–126.
| Is fencing enough? The short-term effects of stock exclusion in remnant grassy woodlands in southern NSW.Crossref | GoogleScholarGoogle Scholar |
Tilman, D. (1993). Species richness of experimental productivity gradients: how important is colonization limitation? Ecology 74, 2179–2191.
| Species richness of experimental productivity gradients: how important is colonization limitation?Crossref | GoogleScholarGoogle Scholar |
Tilman, D. (1999). The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474.
Tilman, D., Naeem, S., Knops, J., Reich, P., Siemann, E., Wedin, D., Ritchie, M., and Lawton, J. (1997). Biodiversity and ecosystem properties. Science 278, 1865–1869.
| Biodiversity and ecosystem properties.Crossref | GoogleScholarGoogle Scholar |
Tozer, K. N., Chapman, D. F., Cousens, R. D., Quigley, P. E., Dowling, P. M., Kearney, G. A., and Cameron, C. A. (2009). Effects of perennial species on the demography of annual grass weeds in pastures subject to seasonal drought and grazing. Crop & Pasture Science 60, 1088–1096.
| Effects of perennial species on the demography of annual grass weeds in pastures subject to seasonal drought and grazing.Crossref | GoogleScholarGoogle Scholar |
Wu, G. L., Du, G. Z., Liu, Z. H., and Thirgood, S. (2009). Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant and Soil 319, 115–126.
| Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlvFOrs7s%3D&md5=9c2ae16de1df3494ee7bac4651b8f10cCAS |
Zhou, Z. Y., Li, F. R., Chen, S. K., Zhang, H. R., and Li, G. D. (2011). Dynamics of vegetation and soil carbon and nitrogen accumulation over 26 years under controlled grazing in a desert shrubland. Plant and Soil 341, 257–268.
| Dynamics of vegetation and soil carbon and nitrogen accumulation over 26 years under controlled grazing in a desert shrubland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjt1Wjsrg%3D&md5=212fcc50cd5031d5b4839ec936f7f171CAS |