The response of grassland productivity, soil carbon content and soil respiration rates to different grazing regimes in a desert steppe in northern China
Xiangyang Hou A C , Zhen Wang A , Schellenberg P. Michael B , Lei Ji A and Xiangjun Yun AA Institute of Grassland Research, Chinese Academy of Agricultural Sciences, Hohhot, 010010, China.
B Semiarid Prairie Agricultural Research Centre (SPARC), AAFC-AAC, Box 1030, Swift Current, Saskatchewan, Canada S9H 3X2.
C Corresponding author. Email: Houxy16@126.com
The Rangeland Journal 36(6) 573-582 https://doi.org/10.1071/RJ13038
Submitted: 26 April 2013 Accepted: 8 September 2014 Published: 30 September 2014
Journal Compilation © Australian Rangeland Society 2014
Abstract
Soil respiration is a major process for organic carbon losses from arid ecosystems. A field experiment was conducted in 2010 and 2012 on the responses to continuous grazing, rotational grazing and no grazing on desert steppe vegetation in northern China. The growing season in 2010 was relatively dry and in 2012 was relatively wet. The results showed that mean soil respiration was the highest with no grazing in both growing seasons. Compared with no grazing, the soil respiration was decreased by 23.0% under continuous grazing and 14.1% under seasonal rotational grazing. Soil respiration increased linearly with increasing soil water gravimetric content, aboveground net primary productivity (ANPP), belowground net primary productivity (BNPP) and soil carbon and nitrogen contents across the 2 years, whereas a negative correlation was detected between soil respiration and soil temperature. A significant decrease in soil respiration was observed under both continuous grazing and in seasonal rotational grazing in the dry growing season, but no significant difference was detected in the wet growing season. In the wet year, only a non-significant difference in soil respiration was observed between different grazing types. Patterns of seasonal precipitation strongly affected the temporal changes of soil respiration as well as its response to different grazing types. The findings highlight the importance of differences in abiotic (soil temperature, soil water gravimetric content and soil carbon and nitrogen contents) and biotic (ANPP, BNPP and litter mass) factors in mediating the responses of soil respiration to the different grazing regimes.
Additional keywords: ANPP, BNPP, grazing management, litter mass, soil carbon content, soil nitrogen content, soil temperature, soil water content.
Introduction
Soil respiration is the primary pathway for carbon dioxide (CO2) flux to the atmosphere because it accounts for up to 25% of the global emissions (Schimel 1995). As the dominant component of ecosystem respiration (Hibbard et al. 2005), the current knowledge of which drivers are important for soil respiration is still poor in comparison with plant leaf photosynthesis and respiration. Soil temperature (Rustad et al. 2001), soil water content (Liu et al. 2002), plant growth (Raich and Tufekcioglu 2000), and soil carbon (C) and nitrogen (N) contents (Xu and Wan 2008) all affect soil respiration. The CO2 emissions from the soil surface reflect the metabolic activity of roots as well as free-living organisms in the soil (Högberg et al. 2001; Wan and Luo 2003). Temperature has been widely recognised as an important factor for regulating soil respiration in most models. Net primary productivity, litter mass and its decomposition also affect the supply of C substrate for plant roots and soil microorganisms, which result in changes in the soil respiration in terrestrial ecosystems (Carreiro et al. 2000). Many models of soil respiration have been developed to attempt to understand the factors influencing soil respiration (Knapp et al. 1998; Fang and Moncrieff 2001), such as soil temperature, soil water content, plant growth (both above- and belowground), litter mass (Davidson et al. 2000) and soil C and N contents (Liu et al. 2007), but a strict theoretical basis is still lacking.
As a major use of grassland, grazing may alter the composition of plant species (Cao et al. 2004) and the composition of C and nutrient pools (Wilsey et al. 2002), as well as the physical and chemical properties of soils (Lal 2001). Long-term livestock grazing is one of the major causes for soil and vegetation degradation on grasslands (Keya 1998), especially in the desert steppe region in China (Li et al. 2008). Grazing potentially affects soil respiration by indirectly altering soil physico-chemical properties (Carreiro et al. 2000; Hook and Burke 2000). Given the spatial variation of soil respiration and its controlling biotic and abiotic factors, the effects of environmental changes (temperature and water availability) on soil respiration could well be affected by different levels of utilisation of the grassland by grazing livestock.
The desert steppe is a vulnerable ecosystem lying between grasslands to the east and desert to the west. All ecosystem processes are influenced by the erratic precipitation, which mostly occurs from May to September. The inter- and intra-annual variation in precipitation has important consequences for soil respiration (Rey et al. 2005; Jarvis et al. 2007). Compared with other ecosystems, future climate changes are predicted to result in large changes in arid ecosystems (Subke et al. 2006; Bond-Lamberty and Thomson 2010). Therefore, it is vitally important to understand the drivers regulating soil respiration in desert steppe ecosystems. To address this knowledge gap, a field experiment was conducted in 2010 and 2012 to examine the potential effects of different grazing management (continuous grazing, seasonal rotational grazing and no grazing) regimes on soil respiration in an arid desert steppe in northern China. In this water-limited ecosystem, we hypothesise that soil respiration will vary with different types of grazing management and that this variation is regulated by grazing-induced changes in abiotic (soil temperature and soil water content) and biotic (plant aboveground and belowground production) factors. Furthermore, the effects of grazing on soil respiration interact with the precipitation, which has a large inter-annual variation in this region.
Materials and methods
Study site
The experiment was conducted in Xisu Banner (42°16′45″N, 112°47′44″E, 1184 m a.s.l.), in the desert steppe region in Inner Mongolia, China. The climate of this site is classified as continental. Long-term (1952–2011) mean annual precipitation is ~213 mm, with 90% of the precipitation occurring from May to October; and the long-term mean annual temperature is 4.9°C. The frost-free period is ~200 days. The sandy loam soil at the study site is classified as Kastanozem according to the Food and Agriculture Organisation soil classification system. The dominant species in this desert steppe were Stipa breviflora Griseb., Cleistogenes songorica (Roshev.) Ohwi, Allium polyrrizum L. and Artemisia frigida Willd.
Experiment design
Nine paddocks (100 m × 1200 m) were fenced off for this grazing experiment in 1999. A randomised complete block design was used comprising three different types of grazing and there were three blocks. The grazing regimes were continuous grazing (CG), seasonal rotational grazing (RG) and no grazing (E), with stocking rates of 1.25, 1.25 and 0 sheep ha–1 month–1, respectively. The grasslands were grazed for 6 months (June–November) in every year.
The measurements were conducted in 2010 and 2012, respectively a dry and a wet year compared with the long-term average rainfall. The CG treatment was grazed for 6 months during the period from June to November each year including 2011 in which no data were collected. The seasonal rotational grazing schedule was from 15 to 29 June, 11 to 25 August and 7 to 21 November in every year.
Measurements
Aboveground net primary production (ANPP) was determined, in August of 2010 and 2012, by harvesting 10 1-m2 quadrats inside each no grazing plot (sampling locations were spaced ~100 m apart), whereas 10 1-m2 quadrats were harvested in 10 portable cages (1.5 m × 1.5 m) that were established in both continuous grazing and the seasonal rotational grazing plots before grazing began in the spring. A total of 90 quadrats were in the nine plots. All aboveground plant material was cut at ground level (including living aboveground biomass, standing litter, and ground litter) in each quadrat. We separated plant aboveground tissues (living and dead aboveground biomass) from dead biomass of the previous year; and separated current-year biomass species by species. Harvested biomass was oven-dried at 65°C for 48 h and then weighed. The ANPP was calculated as the sum of aboveground biomass for all plant species.
Belowground net primary production (BNPP) was measured in each plot by using a root growth method (Steingrobe et al. 2000). Ten 30-cm depth soil samples were collected using a soil auger (7 cm in diameter), at the same sites where ANPP was estimated in each experimental plot in early August in 2010 and 2012, making 90 samples in all. All root material greater than 2 mm was separated from each soil sample using sieves. A root bag (meshes 0.2 mm, 7 cm in diameter, and length 30 cm) was then put into each hole and the soil returned to its original hole inside the root bags. In late October, we collected the root growth samples by taking out the root bag from each hole, separated the new root material from the soil by sieving and weighed it after being oven-dried at 65°C for 48 h.
Soil was also sampled in August 2010 and 2012 from the locations along six transect lines within each plot. Six soil cores (3.5 cm diameter) were randomly collected manually, 0–10 cm with a soil core sampler at each sampling site within each plot. Plant material and other debris on the soil surface were removed before the sampling. Soil samples were air-dried and sieved to pass through a 2-mm grid. The 2-mm air-dried soil samples were further ground to pass through a 0.15-mm sieve. Soil organic C was determined using the method of Amundson et al. (1988) and N contents was measured by using Kjeldahl digestion (Liu et al. 2007).
At the beginning of the experiment (May 2010), a PVC collar (11 cm in diameter and 5 cm in height) was inserted 2–3 cm into the soil to measure soil respiration in each plot, according to the method used by Xu and Wan (2008) and Xia et al. (2009). The soil respiration was measured using a LI-Cor 8100 IRGA (LI-Cor, Lincoln, NE, USA), and the measurements were done twice a month, from late May to early October in both 2010 and 2012. The PVC collar was inserted into the soil 48 h before each measurement to allow any disturbance caused by the installation to subside. Living plants inside the soil collar were removed at the soil surface to avoid inclusion of plant leaf respiration. All soil respiration measurements were carried out between 09:00 hours and 11:00 hours (local time) and were performed 2 or 3 days after a rainfall event to avoid any pulse effect of precipitation. At the same time that soil respiration was measured, soil temperature and soil water content were also determined. Soil temperature was measured at 10 cm from each PVC collar by a thermocouple probe connected to the LI-Cor 8100, and soil gravimetric water content was determined by sampling soil at 0–10 cm using a soil core 3.5 cm in diameter. The weight loss from drying the soil samples at 105°C for 24 h was used to calculate soil moisture content.
Data analysis
The monthly mean values were calculated from all measurements in the same month, whereas seasonal mean values of soil temperature, soil water content and soil respiration were calculated from the monthly means. Repeated-measures ANOVA were used to examine the effects of year and different types of grazing management on soil temperature, soil gravimetric water, soil-C content, soil-N content, ANPP, BNPP, litter mass and soil respiration. If the inter-annual variability was significant (year impact P < 0.05), one-way ANOVA was used to examine the effects of the different types of grazing management on soil-C content, soil-N content, ANPP, BNPP and litter mass. Regression, with correction and stepwise multiple linear analyses, was used to examine the relationships of soil respiration with soil temperature, soil water content, ANPP, BNPP, litter biomass and soil-C and soil-N contents. All statistical analyses were done using SAS 8.1 software (SAS Institute 2000).
Results
Soil microclimate
Air temperature showed a one-peak pattern in May–October in both 2010 and 2012 (Fig. 1). Compared with the long-term (1952–2011) mean growing season temperature (16.2°C), 2010 (17.7°C) was higher and 2012 (16.5°C) was very similar. The precipitation in the growing season in 2010 (166.3 mm) was somewhat lower than the long-term (1952–2011) mean (191.5 mm), whereas that in 2012 (290.2 mm) was 52% higher (Fig. 1).
Soil temperature at a depth of 10 cm was higher in 2010 than in 2012 (P < 0.001, repeated ANOVA, Table 1). Soil temperature at a depth of 10 cm was highest under continuous grazing, and lowest under no grazing (P < 0.001). The higher precipitation in 2012 than 2010 led to a significantly higher soil water content in 2012 than 2010 (P < 0.001). The soil water content was the highest in the no grazing treatment for both years (P < 0.001, Table 1).

|
The effect of grazing treatments on soil-C content and soil-N content
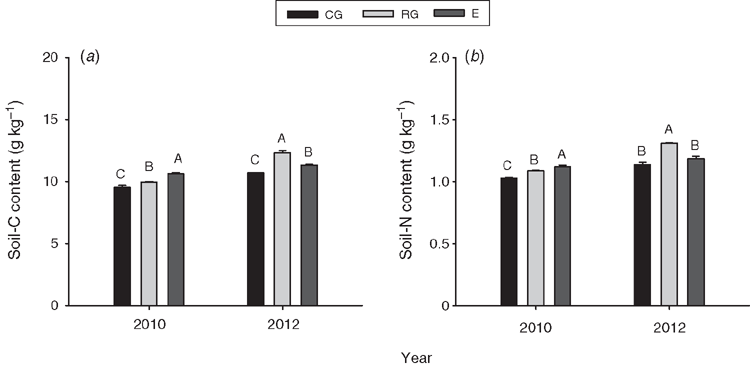
Grazing treatment had significant effects on soil-C content averaged over both years (P < 0.001, Table 2). Compared with the no grazing treatment, continuous grazing significantly decreased soil C by 7.9% (P < 0.001, Table 2), whereas rotational grazing significantly increased soil C by 1.3% averaged over both years (P < 0.001, Table 2). Soil-C content was significantly higher by 13.9% in 2012 (wet) than 2010 (dry) (P < 0.05, Table 2), and was the highest under the no grazing treatment in 2010 (P < 0.05, Fig. 2). Soil-C content was also significantly affected (P < 0.001) by the grazing treatment in 2012 being the highest under rotational grazing and lowest under continuous grazing (Fig. 2).
Similarly, grazing treatment (P < 0.001), year (P < 0.001) and their interaction (P < 0.01) all had significant effects on soil-N content (Table 2). Soil-N content (0–10 cm) was highest under rotational grazing, and lowest under continuous grazing (P < 0.001, Table 2). The soil-N content was 12.0% higher in the wet year (2012) than the dry year (2010) (P < 0.001, Table 2). When analysed separately by year, the soil-N content at the time of peak plant biomass in 2010 had the following trend: no grazing (1.12 g kg–1) > rotational grazing (1.09 g kg–1) > continuous grazing (1.03 g kg–1) (P < 0.05, Fig. 2). The soil-N content was the highest under rotational grazing (1.31 g kg–1) in 2012.
The effect of grazing treatments on plant production
The grazing treatments had significant effects on ANPP averaged over both years (P < 0.001, Table 2). The ANPP with no grazing was significantly higher (19.0%) than under continuous grazing (P < 0.001, Table 2), whereas there was no significant difference between the rotational grazing and no grazing averaged over both years (Table 2). When analysed separately by year, the ANPP was the highest with no grazing in 2010 (P < 0.05, Fig. 3), but highest under rotational grazing in 2012 (P < 0.05, Fig. 3).
The BNPP was also significantly affected by the grazing treatments averaged over both years (P < 0.001, Table 2) with the lowest value under continuous grazing, and no significant difference between rotational grazing and continuous grazing (Table 2). When analysed separately by year, the BNPP was the highest under no grazing in 2010, whereas it was the highest under rotational grazing in 2012 (P < 0.05, Fig. 3). Different grazing treatments, the year and their interaction significantly affected litter mass (P < 0.001, Table 2) with the lowest litter mass found under continuous grazing averaged over both years (P < 0.001, Table 2). Compared with the dry year (2010), the litter mass was significantly higher in the wet year (P < 0.001, Table 2). When analysed separately by year, litter accumulation at the time of peak plant biomass of dry matter was not significantly different (P < 0.05) between the no grazing and rotationally grazed plots in 2010 (Fig. 3). In 2012, the litter accumulation was the highest in the rotationally grazed plots.
The effect of grazing treatments on soil respiration
Soil respiration was found to be higher in 2012 than in 2010 (P < 0.001, Table 1) averaged over all grazing treatments. Compared with no grazing, rotational grazing significantly decreased soil respiration and continuous grazing further decreased it, averaged over the 2 years (P < 0.001). The seasonal pattern of soil respiration followed the seasonal pattern of precipitation, with a marked decrease under all grazing treatments in the dry July of 2010 compared with the wet July of 2012 (Figs 1 and 4e, f). When analysed separately by year, soil respiration was the greatest in the no grazing treatment in 2010 (Fig. 4e), whereas the highest value was found under rotational grazing in 2012 and a marginally significant difference was detected between grazing types (P < 0.1, Fig. 4f).
The effect of biotic and abiotic factors on soil respiration
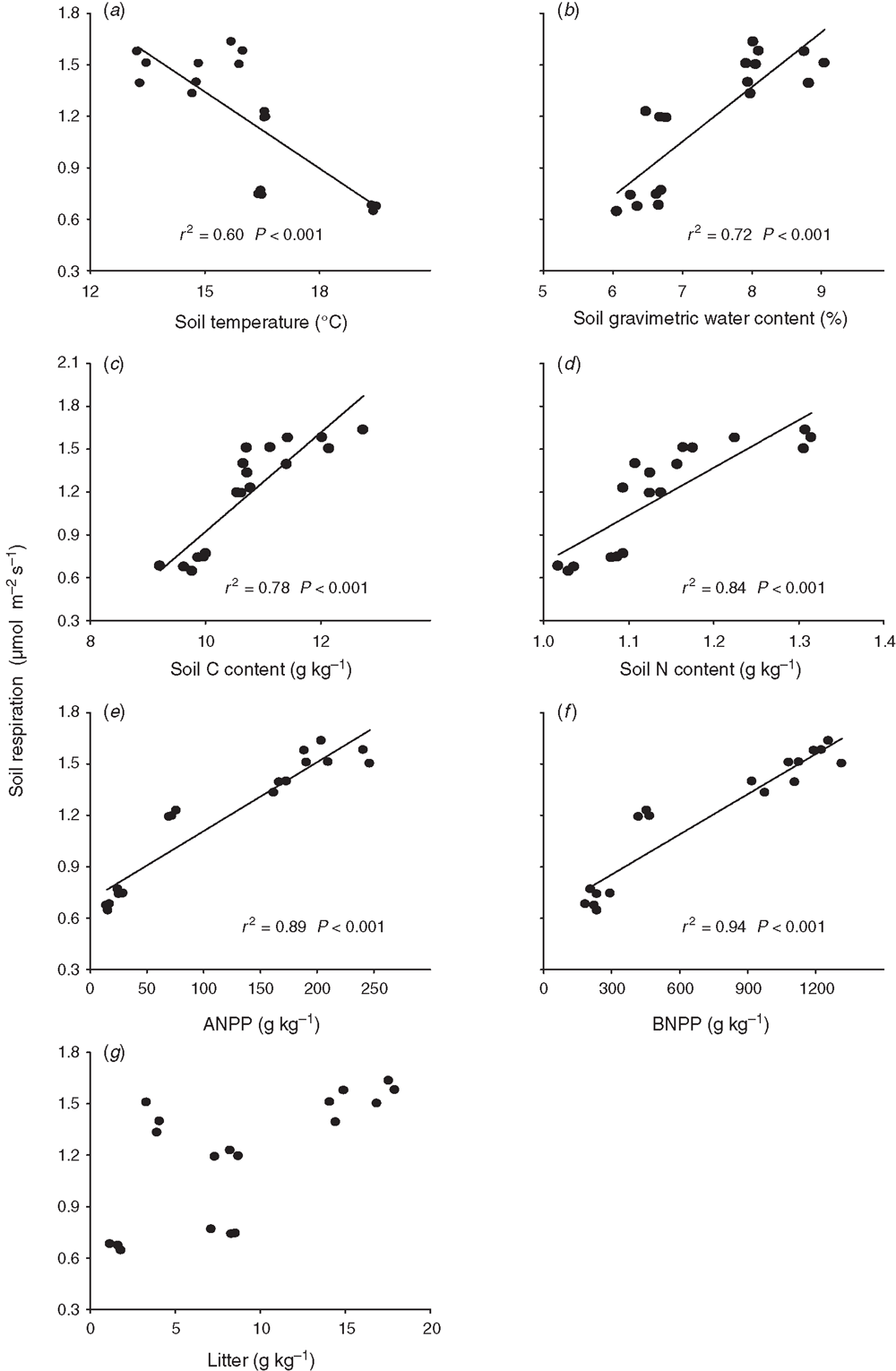
Soil respiration decreased linearly with soil temperature across the 2 years (Fig. 5a). Soil respiration increased linearly with increasing soil water content, ANPP, BNPP and soil-C and soil-N contents across the 2 years, with a steeper slope of regression in BNPP than in the other biotic and abiotic factors (Fig. 5b–f). Stepwise multiple regression analyses showed that ANPP (partial R2 = 0.89, P < 0.001), soil-C content (partial R2 = 0.02, P = 0.05) and litter mass (partial R2 = 0.02, P = 0.04) together accounted for 93.0% of the variation in soil respiration over the 2 years. The BNPP alone explained 69.5% of the seasonal variation in soil respiration by stepwise multiple regression analyses.
Across the nine plots, seasonal mean soil respiration was positively and linearly correlated with soil-C content (P < 0.001, Fig. 6c), soil-N content (P = 0.009, Fig. 6d), ANPP (P < 0.001, Fig. 6e) and BNPP (P < 0.001, Fig. 6f) in 2010). The BNPP alone explained 99.2% of the seasonal variation in soil respiration by stepwise multiple regression analyses in 2010. In 2012, seasonal mean soil respiration had a positive linear dependence upon soil-C content (P = 0.03, Fig. 6c), soil-N content (P = 0.01, Fig. 6d) and BNPP (P = 0.02, Fig. 6e). Soil-N content alone explained 64.1% (P = 0.009) of the spatial variation in soil respiration in 2012.
Discussion
Soil respiration in the desert steppe
Soil respiration is generally lower in the desert steppe than in other ecosystems (Maestre and Cortina 2003; Carbone et al. 2008). The timing of precipitation was crucial to determining the seasonal soil respiration in the studied desert steppe (Fig. 4), and this result is consistent with those widely observed in other arid ecosystems (Shen et al. 2008; Rey et al. 2011). Thomas et al. (2011) found adding water to the soil crust (2 mm) and to the crust and sub-soil (50 mm) led to increases in soil respiration as a result of increased microbial activity in the Kalahari Desert of southern Africa. Our results found that a large precipitation event (166.6 mm in July 2012) confirmed that such events stimulate large discrete pulses of soil respiration, as shown in a previous study (Scholes et al. 2009).
Effect of grazing regime on soil respiration
Compared with no grazing, soil respiration was lower under continuous grazing (by 23.0%) and rotational grazing (by 14.1%) in the studied desert steppe, which is consistent with the results of other grassland studies (Davidson et al. 1998; Rey et al. 2002). A relatively long-term experiment found that soil respiration was low in a high grazing-intensity grassland ecosystem in East Asia (Cao et al. 2001), but this result was opposite to that of Frank (2002) who found that grazing induced a higher CO2 efflux in a tallgrass prairie. However, in the arid and semiarid grasslands of northern China, soil water content is the primary limiting factor for plant growth (Chen and Wang 2000) and microbial activity (Liu et al. 2007). In our study, a higher soil water content with no grazing or under rotational grazing may lead to greater plant production, belowground C allocation and soil-C content (Tables 1 and 2; Figs 1 and 2, 3), providing more C substrate for the activities and respiration of plant roots and soil microorganisms (Saiz et al. 2006; Liu et al. 2007). The depletion of productivity, plant litter input, water and wind erosion are major mechanisms for soil-C loss under continuous grazing in the long-term (Su et al. 2005; Steffens et al. 2008), whereas the high contents of soil C and N (Fig. 2a and b) under rotational grazing as a result of the enhancing plant litter input and productivity (Fig. 3a, b and c) may increase soil respiration in the desert steppe ecosystem.
Our results are also consistent with those of Lin et al. (2010) that grazing-induced vegetation fragmentation. In arid environments, vegetation patches (especially the large ones) provide favourable habitats for maintaining species richness, improving seedling establishment and increasing community productivity (Maestre and Cortina 2003). However, the break-up of this vegetation patches into smaller ones under continuous grazing results in vegetation fragmentation. This process negatively affects plant reproduction (Aguilar et al. 2006) and increases the risk of plant species loss (Joshi et al. 2006). The vegetation fragmentation and the decrease of BNPP under continuous grazing and the positive relationship between these factors and soil respiration (Fig. 5), suggest a decrease in soil respiration under continuous grazing.
Roles of temporal variations of precipitation
The high rainfall during the plant growing season in 2012 (290.2 mm, May–October) was responsible for the high soil respiration, whereas the lower rainfall in 2010 (166.3 mm) was associated with low soil respiration. Several possible reasons could explain the response pattern of soil respiration to precipitation in this study. Soil water content is the primary limiting factor for plant productivity (Chen and Wang 2000) and microbial activity (Liu et al. 2007) in the arid grasslands of northern China. High soil water content in 2012 led to a greater above- and belowground production, thus provided more C substrate for the respiration of plant root and soil microbes as previously reported (Liu et al. 2007). In addition, greater water availability in 2012 compared with 2010 may also directly stimulate auto- and heterotrophic-microbial activities, increasing soil respiration (Xu and Wan 2008). Therefore, our study highlights the importance of temporal pattern of precipitation events and the associated dynamics of soil water content, on soil respiration in the desert steppe (Sponseller 2007; Cable et al. 2008).
Increasing precipitation may lead to an increase in soil respiration, thus at least partly off-setting the grazing-induced reduction in soil respiration. High soil respiration was detected under no grazing in the dry year (2010), but only a marginally significant difference was detected among the different grazing regimes in a wet year (2012). The increased soil respiration under the different grazing regimes in a wet year are most likely associated with the increased soil-C pools pulsed by increased precipitation (Emmerich 2003; Inglima et al. 2009). In addition, increasing precipitation also enhances C availability for soil microorganisms, thus enhancing soil respiration (Gallo et al. 2009). Given the strong regulating effect of soil moisture on soil respiration, future study is warranted to examine the effects of water availability and other global-change factors on arid grasslands along with the different types of grazing management.
Conclusions
Both biotic and abiotic factors are important in regulating soil respiration under different grazing regimes in the desert steppe of northern China. Continuous grazing significantly decreases ANPP, BNPP, litter mass and soil-C and soil-N contents, and leads to low soil respiration in the desert steppe. Precipitation plays a vital role in regulating the effect of grazing on soil respiration. These findings have improved our understanding of soil respiration under the interactive effects of different types of grazing and precipitation in arid grassland ecosystems.
Acknowledgements
We thank Zhujie Huang and Xiliang Li for their help in setting up the experiment and field soil respiration measurements. This study was financially supported by the National Key Basic Research Program of China (2014CB138806), the Natural Science Foundation of China (70933004), the National Important Research Program of Inner Mongolia (2010ZD08), China Postdoctoral Science Foundation (2013M541096) and Fundamental Research Funds of Central Nonprofit Research Institutes (1610332014010).
References
Aguilar, R., Ashworth, L., Galetto, L., and Marcelo Adrián, A. (2006). Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters 9, 968–980.| Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 16913941PubMed |
Amundson, R. G., Trask, J., and Pendall, E. (1988). A rapid method of soil carbonate analysis using gas chromatography. Soil Science Society of America Journal 52, 880–883.
| A rapid method of soil carbonate analysis using gas chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkslanu7c%3D&md5=59e033222989b37b7cc7b6fd4fbda495CAS |
Bond-Lamberty, B., and Thomson, A. (2010). A global database of soil respiration data. Biogeosciences 7, 1915–1926.
| A global database of soil respiration data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2ksrvP&md5=efa5e772533af0556304b4587339bb6eCAS |
Cable, J. M., Ogle, K., Williams, D. G., Weltzin, J. F., and Huxman, T. E. (2008). Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: implications for climate change. Ecosystems 11, 961–979.
| Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: implications for climate change.Crossref | GoogleScholarGoogle Scholar |
Cao, G. M., Li, Y. N., and Zhang, X. J. X. (2001). Values of carbon dioxide emission from different land-use patterns of alpine meadow. Environmental Sciences 6, 14–19.
Cao, G. M., Tang, Y. H., Mo, W. H., Wang, Y. A., Li, Y. N., and Zhao, X. Q. (2004). Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biology & Biochemistry 36, 237–243.
| Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFGrsg%3D%3D&md5=9a2ea010d68bd2c2a6812f1318feb9b8CAS |
Carbone, M. S., Winston, G. C., and Trumbore, S. E. (2008). Soil respiration in perennial grass and shrub ecosystems: linking environmental controls with plant and microbial sources on seasonal and diel timescales. Journal of Geophysical Research – Biogeosciences 113, G02022.
| Soil respiration in perennial grass and shrub ecosystems: linking environmental controls with plant and microbial sources on seasonal and diel timescales.Crossref | GoogleScholarGoogle Scholar |
Carreiro, M. M., Sinsabaugh, R. L., Repert, D. A., and Parkhurst, D. F. (2000). Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81, 2359–2365.
| Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition.Crossref | GoogleScholarGoogle Scholar |
Chen, Z., and Wang, S. (2000). ‘Typical Steppe Ecosystems of China.’ (Science Press: Beijing, China.)
Davidson, E. A., Belk, E., and Boone, R. D. (1998). Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Global Change Biology 4, 217–227.
| Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest.Crossref | GoogleScholarGoogle Scholar |
Davidson, E. A., Verchot, L. V., Cattanio, J. H., Ackerman, I. L., and Carvalho, J. E. M. (2000). Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48, 53–69.
| Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitVOjsr0%3D&md5=2ca8f67dca30c57a62c978354a003c68CAS |
Emmerich, W. E. (2003). Carbon dioxide fluxes in a semi-arid environment with high carbonate soils. Agricultural and Forest Meteorology 116, 91–102.
| Carbon dioxide fluxes in a semi-arid environment with high carbonate soils.Crossref | GoogleScholarGoogle Scholar |
Fang, C., and Moncrieff, J. B. (2001). The dependence of soil CO2 efflux on temperature. Soil Biology & Biochemistry 33, 155–165.
| The dependence of soil CO2 efflux on temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXht1Kmsbk%3D&md5=b5fa9bf575541ac32bd17674bb077d5cCAS |
Frank, A. B. (2002). Carbon dioxide fluxes over a grazed prairie and seeded pasture in the Northern Great Plains. Environmental Pollution 116, 397–403.
| Carbon dioxide fluxes over a grazed prairie and seeded pasture in the Northern Great Plains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovVersbg%3D&md5=e017fae90254e41b1920532e2dd8ae39CAS | 11822718PubMed |
Gallo, M. E., Porras-Alfaro, A., Odenbach, K. J., and Sinsabaugh, R. L. (2009). Photo-acceleration of plant litter decomposition in an arid environment. Soil Biology & Biochemistry 41, 1433–1441.
| Photo-acceleration of plant litter decomposition in an arid environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnt1Oltbk%3D&md5=ad253e5691112126bfa97e0e92ac63f6CAS |
Hibbard, K. A., Law, B. E., Reichstein, M., and Sulzman, J. (2005). An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 73, 29–70.
| An analysis of soil respiration across northern hemisphere temperate ecosystems.Crossref | GoogleScholarGoogle Scholar |
Högberg, P., Nordgren, A., Buchmann, N., Taylor, A. F. S., Ekblad, A., Högberg, M. N., Nyberg, G., Ottosson-Löfvenius, M., and Read, D. J. (2001). Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411, 789–792.
| Large-scale forest girdling shows that current photosynthesis drives soil respiration.Crossref | GoogleScholarGoogle Scholar | 11459055PubMed |
Hook, P. B., and Burke, I. C. (2000). Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate. Ecology 81, 2686–2703.
| Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate.Crossref | GoogleScholarGoogle Scholar |
Inglima, I., Alberti, G., Bertolini, T., Vaccari, F. P., Gioli, B., Miglietta, F., Cotrufo, M. F., and Peressotti, A. (2009). Precipitation pulses enhance respiration of Mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux. Global Change Biology 15, 1289–1301.
| Precipitation pulses enhance respiration of Mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux.Crossref | GoogleScholarGoogle Scholar |
Jarvis, P., Rey, A., Petsikos, C., Wingate, L., Rayment, M., Pereira, J., Banza, J., David, J., Miglietta, F., Borghetti, M., Manca, G., and Valentini, R. (2007). Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: the “Birch effect”. Tree Physiology 27, 929–940.
| Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: the “Birch effect”.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXoslGgtr4%3D&md5=679f6597f85e20e6d99ecd95003fab9eCAS | 17403645PubMed |
Joshi, J., Stoll, P., Rusterholz, H. P., Schmid, B., Dolt, C., and Baur, B. (2006). Small-scale experimental habitat fragmentation reduces colonization rates in species-rich grasslands. Oecologia 148, 144–152.
| Small-scale experimental habitat fragmentation reduces colonization rates in species-rich grasslands.Crossref | GoogleScholarGoogle Scholar | 16429312PubMed |
Keya, G. A. (1998). Herbaceous layer production and utilization by herbivores under different ecological conditions in an arid savanna of Kenya. Agriculture, Ecosystems & Environment 69, 55–67.
| Herbaceous layer production and utilization by herbivores under different ecological conditions in an arid savanna of Kenya.Crossref | GoogleScholarGoogle Scholar |
Knapp, A. K., Conard, S. L., and Blair, J. M. (1998). Determinants of soil CO2 flux from a sub-humid grassland: effect of fire and fire history. Ecological Applications 8, 760–770.
Lal, R. (2001). The physical quality of soil on grazing lands and its effect on sequestering carbon. In: ‘The Potential of U.S. Grazing Lands to Sequester Carbon and Mitigate the Greenhouse Effect’. (Eds R. F. Follet, J. M. Kimble and R. Lal.) pp. 249–266. (Lewis Publishers: New York.)
Li, C. L., Hao, X. Y., Zhao, M. L., Han, G. D., and Willms, W. D. (2008). Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agriculture, Ecosystems & Environment 128, 109–116.
| Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia.Crossref | GoogleScholarGoogle Scholar |
Lin, Y., Hong, M., Han, G. D., Zhao, M. L., Bai, Y. F., and Chang, S. X. (2010). Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe. Agriculture, Ecosystems & Environment 138, 282–292.
| Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe.Crossref | GoogleScholarGoogle Scholar |
Liu, X., Wan, S., Su, B., Hui, D., and Luo, Y. (2002). Response of soil CO2 efflux to water manipulation in a tallgrass prairie ecosystem. Plant and Soil 240, 213–223.
| Response of soil CO2 efflux to water manipulation in a tallgrass prairie ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltlOnu7c%3D&md5=f1b4491070d6cefa29d08204c4fb54deCAS |
Liu, W. X., Xu, W. H., Han, Y., Wang, C. H., and Wan, S. Q. (2007). Responses of microbial biomass and respiration of soil to topography, burning, and nitrogen fertilization in a temperate steppe. Biology and Fertility of Soils 44, 259–268.
| Responses of microbial biomass and respiration of soil to topography, burning, and nitrogen fertilization in a temperate steppe.Crossref | GoogleScholarGoogle Scholar |
Maestre, F. T., and Cortina, J. (2003). Small-scale spatial variation in soil CO2 efflux in a Mediterranean semi-arid steppe. Applied Soil Ecology 23, 199–209.
| Small-scale spatial variation in soil CO2 efflux in a Mediterranean semi-arid steppe.Crossref | GoogleScholarGoogle Scholar |
Raich, J. W., and Tufekcioglu, A. (2000). Vegetation and soil respiration: correlations and controls. Biogeochemistry 48, 71–90.
| Vegetation and soil respiration: correlations and controls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitVOjsro%3D&md5=653c360c075529e860dd8efdce1e60feCAS |
Rey, A., Pegoraro, E., Tedeschi, V., De Parri, I., Jarvis, P. G., and Valentini, R. (2002). Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Global Change Biology 8, 851–866.
| Annual variation in soil respiration and its components in a coppice oak forest in Central Italy.Crossref | GoogleScholarGoogle Scholar |
Rey, A., Pepsikos, C., Jarvis, P. G., and Grace, J. (2005). The effect of soil temperature and soil moisture on carbon mineralization rates in a Mediterranean forest soil. European Journal of Soil Science 56, 589–599.
| The effect of soil temperature and soil moisture on carbon mineralization rates in a Mediterranean forest soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGlsbrP&md5=7e6cc42c9ec17fecfe889db916646b1eCAS |
Rey, A., Pegoraro, E., Oyonarte, C., Were, A., Escribano, P., and Raimundo, J. (2011). Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biology & Biochemistry 43, 393–403.
| Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGmtQ%3D%3D&md5=8e30b62279e8624effc883991a641881CAS |
Rustad, L. E., Campbell, J. L., Marion, G. M., Norby, R. J., Mitchell, M. J., Hartley, A. E., Cornelissen, J. H. C., and Gurevitch, J. (2001). A meta-analysis of the response of soil respiration, net nitrogen mineralization, and above-ground plant growth to experimental ecosystem warming. Oecologia 126, 543–562.
| A meta-analysis of the response of soil respiration, net nitrogen mineralization, and above-ground plant growth to experimental ecosystem warming.Crossref | GoogleScholarGoogle Scholar |
Saiz, G., Byrne, K. A., Butterbach-Bahl, K, Kiese, R., Blujdea, V., and Farrell, E. P. (2006). Stand age-related effects on soil respiration in a first-rotation Sitka spruce chronosequence in central Ireland. Global Change Biology 12, 1007–1020.
| Stand age-related effects on soil respiration in a first-rotation Sitka spruce chronosequence in central Ireland.Crossref | GoogleScholarGoogle Scholar |
SAS Institute (2000). ‘SAS/STAT User’s Guide Release.’ 8.1 edn. (SAS Institute Ltd: Cary, NC.)
Schimel, D. S. (1995). Terrestrial ecosystems and the carbon-cycle. Global Change Biology 1, 77–91.
| Terrestrial ecosystems and the carbon-cycle.Crossref | GoogleScholarGoogle Scholar |
Scholes, R. J., Monteiro, P. M. S., Sabine, C. L., and Canadell, J. G. (2009). Systematic long-term observations of global carbon cycle. Trends in Ecology & Evolution 24, 427–430.
| Systematic long-term observations of global carbon cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MvpvVWjtQ%3D%3D&md5=2a46c7a7404ff56e52a01d1020a4a9a9CAS |
Shen, W. J., Jenerette, G. D., Hui, D. F., Phillips, R. P., and Ren, H. (2008). Effects of changing precipitation regimes on dryland soil respiration and C-pool dynamics at rainfall event, seasonal and interannual scales. Journal of Geophysical Research – Biogeosciences 113, G03024.
| Effects of changing precipitation regimes on dryland soil respiration and C-pool dynamics at rainfall event, seasonal and interannual scales.Crossref | GoogleScholarGoogle Scholar |
Sponseller, R. A. (2007). Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biology 13, 426–436.
| Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem.Crossref | GoogleScholarGoogle Scholar |
Steffens, M., Kolbl, A., Totsche, K. U., and Kogel-Knabner, I. (2008). Grazing effects on soil chemical and physical properties in a semi-arid steppe of Inner Mongolia (PR China). Geoderma 143, 63–72.
| Grazing effects on soil chemical and physical properties in a semi-arid steppe of Inner Mongolia (PR China).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVent77M&md5=ea99225baa655dbf689636d5c6b63c8aCAS |
Steingrobe, B., Schmid, H., and Claassen, N. (2000). The use of the in-growth core method for measuring root production of arable crops – influence of soil conditions inside the ingrowth core on root growth. Journal of Plant Nutrition and Soil Science 163, 617–622.
| The use of the in-growth core method for measuring root production of arable crops – influence of soil conditions inside the ingrowth core on root growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtFGisA%3D%3D&md5=6d6224796a0c6943ae05929d7dbbfae2CAS |
Su, Y. Z., Li, Y. L., Cui, J. Y., and Zhao, W. Z. (2005). Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena 59, 267–278.
| Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China.Crossref | GoogleScholarGoogle Scholar |
Subke, J. A., Inglima, I., and Cotrufo, M. F. (2006). Trends and methodological impacts in soil CO2 efflux partitioning: a meta-analytical review. Global Change Biology 12, 921–943.
| Trends and methodological impacts in soil CO2 efflux partitioning: a meta-analytical review.Crossref | GoogleScholarGoogle Scholar |
Thomas, A. D., Hoon, S. R., and Dougill, A. J. (2011). Soil respiration at five sites along the Kalahari Transect: effects of temperature, precipitation pulses and biological soil crust cover. Geoderma 167–168, 284–294.
| Soil respiration at five sites along the Kalahari Transect: effects of temperature, precipitation pulses and biological soil crust cover.Crossref | GoogleScholarGoogle Scholar |
Wan, S., and Luo, Y. (2003). Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochemical Cycles 17, 1054.
| Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment.Crossref | GoogleScholarGoogle Scholar |
Wilsey, B. J., Parent, G., Roulet, N. T., Moore, T. R., and Potvin, C. (2002). Tropical pasture carbon cycling: relationships between C source/sink strength, above-ground biomass and grazing. Ecology Letters 5, 367–376.
| Tropical pasture carbon cycling: relationships between C source/sink strength, above-ground biomass and grazing.Crossref | GoogleScholarGoogle Scholar |
Xia, J. Y., Han, Y., Zhang, Z., Zhang, Z., and Wan, S. (2009). Effects of diurnal warming on soil respiration are not equal to the summed effects of day and night warming in a temperate steppe. Biogeosciences 6, 1361–1370.
| Effects of diurnal warming on soil respiration are not equal to the summed effects of day and night warming in a temperate steppe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlKjtLnJ&md5=99a82ef9469be72cf0a803766a02fd90CAS |
Xu, W. H., and Wan, S. Q. (2008). Water- and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semi-arid grassland in northern China. Soil Biology & Biochemistry 40, 679–687.
| Water- and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semi-arid grassland in northern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFKksQ%3D%3D&md5=565336244b23536b016bab9555646258CAS |