Grazing primarily drives the relative abundance change of C4 plants in the typical steppe grasslands across households at a regional scale
Qing Zhang A C D , Yong Ding B D , Wenjing Ma A , Sarula Kang A , Xin Li C , Jianming Niu A C E , Xiangyang Hou B E , Xiliang Li B andA School of Life Sciences, Inner Mongolia University, Hohhot, 010021, China.
B Institute of Grassland Research, Chinese Academy of Agricultural Sciences, Hohhot, 010010, China.
C Sino-US Centre for Conservation, Energy and Sustainability Science in Inner Mongolia University, Hohhot, 010021, China.
D The authors have contributed equally to the paper.
E Corresponding authors. Emails: jmniu2005@163.com; houxy16@126.com
The Rangeland Journal 36(6) 565-572 https://doi.org/10.1071/RJ13050
Submitted: 15 May 2013 Accepted: 19 July 2014 Published: 14 August 2014
Journal Compilation © Australian Rangeland Society 2014
Abstract
Increases in temperature and grazing intensity are believed to promote the relative abundance of C4 plants in grassland communities in Inner Mongolia. However, there is a lack of understanding as to which factor is the primary driver at the household scale. The relative abundance of C4 plants in grassland communities within 32 households was monitored over a 5-year period (2008–12) in the typical steppe region of Inner Mongolia. The relationships between the mean annual temperature, grazing intensity and their combinations on the patterns of the relative abundance of C4 plants across the land managed by these households were analysed. The results showed that (1) the herbage mass of the typical steppe grassland was mainly composed of C3 plants (87%); (2) the C4 plants were more sensitive to, and can be used as indicators of, environmental changes. These C4 species included Cleistogenes squarrosa (Trin.) Keng, Chenopodium glaucum Linn. and Salsola collina Pall.; (3) both increasing temperature and grazing intensity promoted the relative abundance of C4 plants. Grazing intensity was the primary driver of the change in relative abundance of C4 plants in this region. Not only did grazing change the micro-environment of the grasslands, but also the C3 species were preferentially grazed by the livestock. Comparison of the results with previous studies on the temporal variation in the abundance of C4 plants suggests that the relative importance of grazing and climatic factors depends on the spatial scales of the studies, with climate being of greater importance at the regional rather than the household scale.
Additional keywords: grazing intensity, grazing preferences, Inner Mongolian grassland, temperature.
Introduction
Terrestrial vegetation is composed of 95% C3 plants and 5% C4 plants (Su et al. 2011), but C4 plants contribute about 20% of global gross primary productivity (Wand et al. 1999). The C4 pathway of photosynthesis in the dicots originated in arid regions at low latitudes and C4 grasses and sedges dominate nearly all grasslands in the tropics, subtropics and warm temperate zones and are major components of arid landscapes from the temperate regions to the tropics (Archibold 1995; Sage et al. 1999). The relative abundance of C4 plants is not only an aspect of ecosystem structure, but more importantly, it reflects ecosystem functions (Snyder and Tartowski 2006; Sierra et al. 2010). Thus, studies on the relative abundance of C4 plants have become more important in the context of global climate change (Kohn 2010; Wittmer et al. 2010; Scheiter et al. 2012).
The concentration of CO2 in the atmosphere, air temperature, and land-use patterns are generally considered to be the main drivers that influence the relative abundance of C4 and C3 species (Zech et al. 2009; Pushkina et al. 2010; Wittmer et al. 2010). Compared with C3 plants, C4 plants have higher water-use efficiency and greater photosynthetic rates at low atmospheric CO2 concentrations resulting in greater growth under these conditions (Farquhar et al. 1989; Leegood 2013). A series of studies on paleoclimate climate modelling, and vegetation changes have confirmed the fact that the relative abundance of C4 plants will decrease with increasing atmospheric CO2 concentration (Cerling et al. 1993; Scheiter et al. 2012; Ripley et al. 2013). However, some studies did not fully support this view (Owensby et al. 1999; Tooth and Leishman 2013). Then researchers gradually realised the importance of temperature. On one hand, C4 plants appear only if the temperature is above a certain threshold (Teeri and Stowe 1976; Sage 2004). The optimum temperatures for many C4 species are about 35°C and at temperatures lower than about 15°C, the growth of most C4 species virtually stops. The optimum for many C3 species is about 20−25°C and growth will continue down to close to 0°C (McWilliam 1978). On the other hand, a temperature rise would directly affect the relative C3 and C4 plant growth and dry matter accumulation and would ultimately determine the relative abundance of the two groups in the vegetation (Sage and Kubien 2007; Wittmer et al. 2010).
Disturbance, including grazing, fire, and roads related to land-use change, also have different effects on the growth and reproduction of C3 and C4 plants and can lead to changes in their relative abundance (Pushkina et al. 2010; Collins and Calabrese 2012; Scheiter et al. 2012; Way 2012). Animal grazing is the most dominant land use of grasslands (Mavromihalis et al. 2013). Grazing influence on the relative abundance of C4 plants has received much attention in the study of grasslands in North and South America (Reeder et al. 2004; Altesor et al. 2006; Derner et al. 2006), Central Asia (Auerswald et al. 2012; Ren et al. 2012), South Africa (Franz-Odendaal et al. 2002), Australia (Bell et al. 2012) and New Zealand (Crush and Rowarth 2007). Most of these studies suggest that grazing increases the relative abundance of C4 species in some plant communities (Reeder et al. 2004; Waters et al. 2005; Fanselow et al. 2011). Two reasons may explain these results. Firstly, grazing may have changed the micro-environment. Secondly, the foraging preferences of herbivores caused by the different palatability of C3 and C4 plants (McPherson and Rasmussen 1989), as well as differences in their digestibility could mean that the animals select either C3 or C4 plants (Whalley 1994; Norman et al. 2009). However, other studies suggested that grazing had no effects on the relative abundance of C4 plants (Derner et al. 2006; Auerswald et al. 2012).
Since the household is the basic management unit of the grasslands in Inner Mongolia (Hou et al. 2012), the objective of the present work was to understand the relative importance of grazing and temperature in determining the relative abundance of C4 plants in these grasslands at this scale. Bearing in mind that temperature may affect the relative abundance of C4 plants at a large temporal-spatial scale, grazing may play a more important role at a smaller temporal-spatial scale (Franz-Odendaal et al. 2002; Reeder et al. 2004; Bell et al. 2012).
We hypothesise that, at the household scale, grazing is the primary driver for the changes in the relative abundance of C4 plants in grassland communities in Inner Mongolia. To verify this hypothesis, we selected 32 households in the Baiyinxile ranch, located in a typical steppe grassland region in Inner Mongolia, and monitored the relative abundance of C4 plants during 2008–12. We also determined the mean annual temperature and grazing intensity at each household, and analysed the effects of these two factors and their combinations on the relative abundance of C4 plants. This study aimed to enrich the understanding of the changes in the relative abundance of C3 and C4 plants in response to environmental factors, and provide a better basis for assessing the effects of climate and land-use change on grassland ecosystem structure and functioning, and provide support for the development of improved management strategies.
Materials and methods
Study area
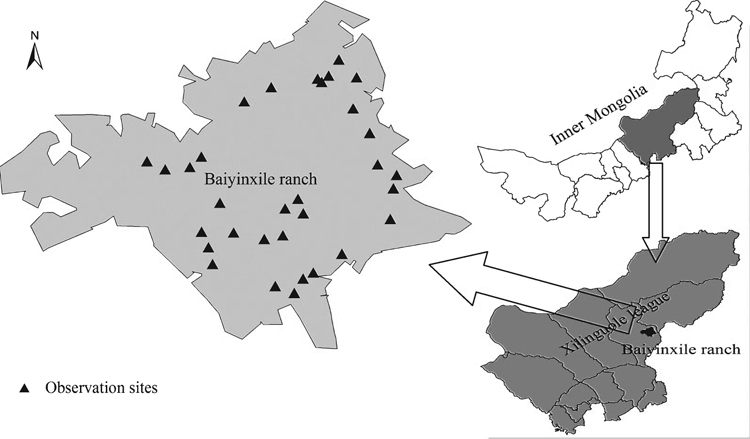
This study was conducted on the Baiyinxile ranch, Xilingol league of Inner Mongolia (Fig. 1), ranging from 116.03°E to 116.79°E and 43.69°N to 44.27°N. Gentle hills and high plains constitute the major terrain with altitude ranging from 1126 m to 1384 m above mean sea level. It is a temperate continental climate, characterised by a mean annual temperature of –0.1°C, and mean annual precipitation of 350 mm. The main soil type is a chestnut soil. The region is covered by typical steppe vegetation with Stipa grandis P.Smirn. and Leymus chinensis (Trin.) Tzvel. as the major dominant species. The ‘double rights one system’ land policy began in 1999 and was fully implemented in 2003. It is the semi-private property rights, which mean that both the livestock and grassland are contracted to herder households, but the grasslands belong to the state (Li et al. 2007). Thus, individual households became the basic production and management units at that time. The most common grazing animals were sheep, goats and cattle in this area.
|
Vegetation survey
Vegetation data were collected from 2008 to 2012 between the end of July and mid-August when the aboveground biomass is at its peak. The grasslands within 32 households (all with long-term grazing from 2003) were investigated. In each household, an area of 10 × 10 m was selected and monitored during the whole period. The grasslands at all these sites were dominated by Stipa species (S. grandis or S. krylovii Roshev). The grazing intensity at each site was estimated based on the distance of the site from the housing and sheepfolds, that is, a greater intensity was estimated for sites close to the housing and sheepfolds. The average distance of a site from housing was 500 m (An and Han 2011). Since topography, grassland community type and plant patches all affect the foraging behaviour of sheep (Senft et al. 1987), we selected the monitoring site within each household to ensure that the selected site was representative of the average grazing intensity within the household. Each site was selected (Wang et al. 1999) on flat land and in an area where the vegetation reflected, as much as possible, the overall vegetation condition of the household and having the lowest heterogeneity. All sites were located with a global positioning system device. At peak plant biomass, three 1 × 1-m plots were randomly placed at each site to determine aboveground plant biomass by harvesting species by species and drying at 65°C (for ~24 h) in the laboratory. Each year, care was taken to ensure that the new sampling sites were not at the same locations that had been previously sampled. All species were grouped into C3 or C4 species (Tang and Liu 2001), and the relative abundance of C4 species was calculated as their proportion of the total aboveground biomass of the grassland at each site. The monitoring of vegetation on all the 32 sites lasted for 5 years, and during this period, six C4 species were recorded: Artemisia sieversiana Ehrhart ex Willd., Cleistogenes squarrosa (Trin.) Keng, Chenopodium aristatum L., Ch. glaucum L., Kochia prostrata (L.) Schrad. and Salsola collina Pall. and 53 C3 species were recorded.
Temperature and grazing intensity data collection
The vegetation monitoring started in 2008, but portable weather stations (Kestrel 4500, USA) were not established on the 32 sites until 2012. The mean annual temperature data for 2012 were obtained from these portable weather stations, whereas the information for 2008–11 was derived from the nearby Xilingol meteorological station. All the sites were within 40 km of this station but at different altitudes. The established adiabatic lapse rate is –0.6°C 100 m–1, so the mean annual temperature at each site was estimated using following formula:
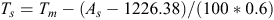
where Ts and AS are the annual mean temperature and altitude at a site, and Tm (oC) and 1226.4 m are the annual mean temperature and altitude, respectively, at Xilinhot meteorological station.
The accuracy of these calculations was verified by comparing the estimated values for 2012 against that recorded by the portable weather stations. The correlation between the estimated and recorded data across the 32 sites was significant (P < 0.01) and indicated the high reliability of the calculation method (Fig. 2).

|
We calculated the grazing intensity at each of the 32 households for each year, using the pasture area divided by standardised sheep units (SU). The average distance of a site from housing, sheepfolds or a water-point was 500 m. Therefore, we can minimise the error caused by the distance to grazing intensity. The number of different livestock at each household were surveyed along with the vegetation survey each year, and were converted into the number of SU based on their feed requirements (Li and Ji 2004), i.e. for cattle, horses, or mules, 1 animal = 5 SU, and 1 lamb = 0.5 SU.
Data analyses
The sensitivity of C3 plants, C4 plants and each of the six C4 species to environmental factors was tested by calculating the coefficient of variation (CV) of their aboveground biomass across the 32 households from 2008 to 2012. We also calculated Pearson correlation coefficients between grazing intensity, mean annual temperature, and the relative abundance of all and each of the six C4 species. To examine the effect of temperature and grazing on the relative abundance of C4 plants, four different detrended canonical correspondence analyses (DCCA) were run based on Borcard points (Borcard et al. 1992) using three matrices of data of the relative abundance of C4 plants, mean annual temperature and grazing intensity (each matrix consisted of 32 households and 5 years). The separate and coupled effect of temperature and grazing, as well as the effects of all other factors on the relative abundance of C4 plants, were assessed using the ratio of the total variation of the relative abundance of C4 plants to the variation of temperature, grazing intensity, temperature × grazing intensity, or all other factors. That is, we first calculated the effect of temperature (Et) and grazing intensity (Eg) on the relative abundance of C4 plants using the constrained ordination analysis with temperature or grazing intensity, respectively, as the constrained factor. Then we calculated the effect of only temperature (Et0) and only grazing intensity (Eg0) by the same approach after exclusion of the effect of grazing (with grazing intensity as a co-variable) or the effect of temperature (with temperature as a co-variable), respectively. Based on these calculations, the coupling effect between temperature and grazing was calculated as (Et-Et0) or (Eg-Eg0), and the effect of all other factors was 1 – (Et + Eg0) or 1 – (Et0 + Eg).
All statistical analyses were performed using SPSS 17.0 and Canaco 4.5 (Braak and Smilauer 2002).
Results
The status of C3 and C4 plants
Averaged over all 32 sites, the percentage of C3 plants in the plant biomass was 87.7% (Fig. 3). The contribution of C4 plants to community biomass decreased from 28.2% in 2008 to 6.9% in 2012, and was 12.3% on average.

|
Variation of C3 and C4 plants across the 32 households
We calculated the CV of aboveground biomass of C3 and C4 plants at the 32 sites from 2008 to 2012. The CV of C4 plants (69%) was greater than that of C3 plants (53%) (Fig. 4). There were also differences in CV among the six C4 species: C. squarrosa, Ch. aristatum, Ch. glaucum and S. collina had a higher CV, while A. sieversiana and K. prostrata had a lower CV. The lower CV of these latter two species was mainly related to their lower frequency in the studied grasslands. Artemesial sieversiana only appeared at two sites in 2009 and 2011, and K. prostrata appeared at three sites in 2008, 2009 and 2012.

|
Relationships between grazing intensity, mean annual temperature and the relative abundance of C4 plants
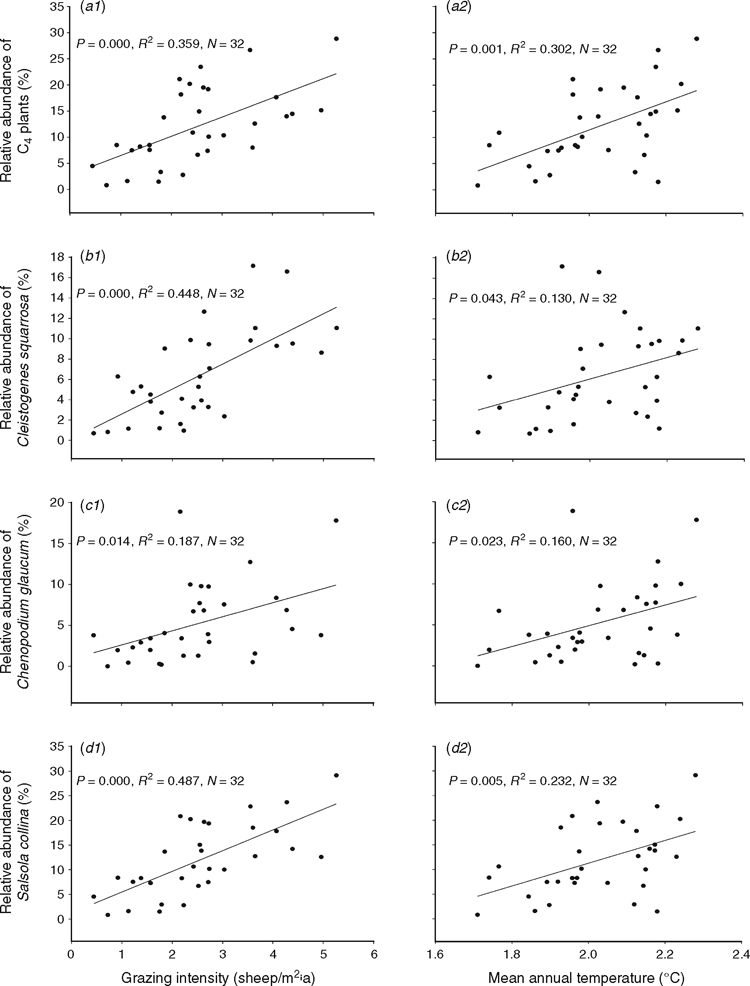
The relative abundance of all the C4 plants showed a positive correlation with grazing intensity and with mean annual temperature across the 32 households (Fig. 5a1, a2). The relative abundance of C. squarrosa, Ch. glaucum, and S. collina was also significantly correlated with grazing intensity or mean annual temperature, respectively (Fig. 5b1, c1, d1, b2, c2 and d2). These results showed that the relative abundance of these three C4 species was higher with a high grazing intensity and a high mean annual temperature.
Contribution of temperature and grazing to change in the relative abundance of C4 plants
Based on four different DCCA analyses, we determined the separate effects of temperature alone, grazing intensity alone and the coupling effects of temperature and grazing intensity, as well as the effects of all other factors on the variation in the relative abundance of C4 species (Table 1). Grazing intensity explained 53.6% of the variation, among which 41.1% was explained by grazing intensity alone. Temperature explained 42.6% of the variation, and 30.1% of which was explained by temperature alone. Only 12.5% of the variation was explained by the coupled effects of temperature and grazing; and all other factors explained the rest (16.3%). Thus grazing and temperature together led to the differences in relative abundance of C4 species, and the effects of grazing was greater than those of temperature.

|
Discussion
Both temperature and grazing can promote the abundance of C4 plants
It is widely considered that higher temperatures are generally associated with greater abundance of C4 plants (Zheng et al. 2011; Auerswald et al. 2012). Our data supported this statement in that there was a decrease in the abundance of C4 species associated with a decrease in temperature over the years 2008–12 (Figs 3 and 5a2). This is because C4 species have a competitive advantage in environments with higher temperatures, lower CO2 concentrations, higher solar radiation, and lower soil moisture contents (Sage and Kubien 2007). The rising temperatures and associated climatic aridity predicted by climate-change scenarios (Kang et al. 2011) are therefore likely to lead to an increase in the abundance of C4 species in the Inner Mongolia grasslands.
Although there is some dispute about whether grazing can affect the relative abundance of C4 plants (Derner et al. 2006; Fanselow et al. 2011), a series of studies carried out in the Inner Mongolian steppe have found that grazing intensity can indeed increase the relative abundance of C4 plants (Wang 2002; Fanselow et al. 2011; Zheng et al. 2011). Our results at the household scale also support these earlier findings (Fig. 5). Three reasons may explain these results. Firstly, grazing suppresses C3 plants more than C4 plants and C4 plants turn generally green later than C3 plants (by 6 weeks, see Liang et al. 2002), and thus avoid grazing damage in early spring. In addition, C4 species (mainly C. squarrosa) also have a lower canopy (<10 cm) than C3 plants (20–50 cm) and thus avoid being grazed by cattle (Wang et al. 2003). Secondly, removal of the biomass of C3 plants through grazing enhances the intensity of light reaching the ground and the action of wind, creating a drier and warmer micro-environment (Teeri and Stowe 1976; Li et al. 2000), which facilitates the development of C4 plants (Sage 2004). The shade from tall C3 plants not only reduces the light availability for short grasses, but also changes the proportion of infrared/far-infrared (Casal et al. 1987). Controlled experiments have demonstrated that the proportional reduction of infrared/far-infrared light can reduce the tillering of C. squarrosa (Deregibus et al. 1985; Casal et al. 1987), the major C4 species in the studied grassland. Infrared light intensity diminishes to a greater extent than far-infrared when light is filtered through the plant canopy, thus removal of the upper plant canopy by grazing will increase the infrared/far-infrared ratio of the light reaching the short grasses, and will enhance the tillering of C. squarrosa. The last and perhaps the most important reason is the differences in the preference (McPherson and Rasmussen 1989) and digestibility (Norman et al. 2009) of the C3 and C4 species. Yokohama et al. (2011) reported that the species most preferred by grazing livestock on the Mongolian plateau [S. glareosa, P. smirnov, S. krylovii, Achnatherum splendens (Trin.) Nevski, Agropyron cristatum (L.) Gaertn., Elytrigia repens (L.) Nevski, L. chinensis, Poa pratensis L., Artemisia frigid Willd., and Caragana pygmaea (L.) DC. etc.] are all C3 species. These species maintain the forage production of Inner Mongolian grasslands. The results of Norman et al. (2009) indicated that the C4 species in the region have lower digestibility values than C3 species and so the grazing livestock tend to prefer the C3 species.
Grazing affects the relative abundance of C4 plants more than temperature at the household scale
Scale is widely considered to be the main cause of differences among ecological models because of the operation of different mechanisms at different scales (Wu and Loucks 1995). Temperature is the most important factor in the evolution of paleoclimates (Zhang et al. 2003; Pushkina et al. 2010) and the change in relative abundance of C4 plants on a large scale (Sage 2004; Wittmer et al. 2010). Auerswald et al. (2012) found that C4 abundance in an Inner Mongolia grassland system is driven by a temperature-moisture interaction, not grazing pressure. Our study shows that grazing had a greater effect than temperature in altering the abundance of C4 plants at the small scale across households, which is in agreement with similar conclusions in other studies (Fanselow et al. 2011). Scale must be taken into account when defining the relative effects of different factors on ecological processes and functions, including the effects on the abundance of C4 plants.
However, our results are different from those of Auerswald et al. (2012). In their study, the temporal variation in the abundance of C4 plants was measured at one site that experiences large inter-annual variation in climate (temperature as well as precipitation) but under a small range of grazing intensities (02.25 SU ha–1), while our study considered the spatial variation in the abundance of C4 plants on grasslands across 32 households, where the variation in mean annual temperature was small, and the range of grazing intensities was large (0–5.25 SU ha–1). The contrasting results of Auerswald et al. (2012) and ours indicate that the relative importance was dependent on the relative scales of the different factors. For the same group of factors, those with more variation would have greater effects than those with less variation.
C4 plants, especially C. squarrosa, Ch. glaucum, and S. collina, can be used as important indicators for monitoring Inner Mongolian grasslands
Changes in a range of environmental factors, including grazing, temperature, road construction and precipitation, at both large scale and small scales, can affect changes in the biomass of C4 plants (Jolly and Haxeltine 1997; Pushkina et al. 2010; Sinninghe Damsté et al. 2011; Collins and Calabrese 2012; Scheiter et al. 2012; Way 2012). In our study, the CV of C4 species was higher than that of C3 species (Fig. 4) implying that C4 plants exhibited a greater sensitivity to environmental change than the C3 plants. In particular, three C4 species: C. squarrosa, C. glaucum, and S. collina, not only showed more sensitivity to environmental change (Fig. 4), but they also all showed an increasing abundance with increased grazing pressure and temperature (Fig. 5). Thus, the changes in the abundance of these species could be used as indicators for detecting climate change and human disturbance in Inner Mongolian grasslands.
Acknowledgements
The authors are grateful to Drs John Milne, Frank Yonghong Li and Wal Whalley for their insightful comments and valuable suggestions. We also thank Dr Wal Whalley for improving the English readability of this manuscript. This study was supported by the State Key Basic Research Development Program of China (2014CB138805, 2012CB722201), the National Basic Research Program of China (31200414), Specialised Research Fund for the Doctoral Program of Higher Education of China (20121501120006), Start Research Funding Project of Inner Mongolia University (125106) and Central Nonprofit Research Institutes Fundamental Research Funds (1610332014001).
References
Altesor, A., Piñeiro, G., Lezama, F., Jackson, R., Sarasola, M., and Paruelo, J. (2006). Ecosystem changes associated with grazing in sub-humid South American grasslands. Journal of Vegetation Science 17, 323–332.| Ecosystem changes associated with grazing in sub-humid South American grasslands.Crossref | GoogleScholarGoogle Scholar |
An, B., and Han, G. D. (2011). Effect of grazing intensity on underground biomass and carbon density in meadow steppe. Inner Mongolia Prataculture 23, 42–45.
Archibold, O. W. (1995). ‘Ecology of World Vegetation.’ (Chapman & Hall: London, UK.)
Auerswald, K., Wittmer, M., Bai, Y. F., Yang, H., Taube, F., Susenbeth, A., and Schnyder, H. (2012). C4 abundance in an Inner Mongolia grassland system is driven by temperature-moisture interaction, not grazing pressure. Basic and Applied Ecology 13, 67–75.
| C4 abundance in an Inner Mongolia grassland system is driven by temperature-moisture interaction, not grazing pressure.Crossref | GoogleScholarGoogle Scholar |
Bell, M. J., Eckard, R. J., and Cullen, B. R. (2012). The effect of future climate scenarios on the balance between productivity and greenhouse gas emissions from sheep-grazing systems. Livestock Science 147, 126–138.
| The effect of future climate scenarios on the balance between productivity and greenhouse gas emissions from sheep-grazing systems.Crossref | GoogleScholarGoogle Scholar |
Borcard, D., Legendre, P., and Drapeau, P. (1992). Partialling out the spatial component of ecological variation. Ecology 73, 1045–1055.
| Partialling out the spatial component of ecological variation.Crossref | GoogleScholarGoogle Scholar |
Braak, C., and Smilauer, P. (2002). CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4.5).’ (Microcomputer Power: Ithaca, New York.)
Casal, J. J., Sachz, R. A., and Deregibus, V. A. (1987). Tillering responses of Lolium multiflorum plants to changes of red/far-red ratio typical of sparse canopies. Journal of Experimental Botany 38, 1432–1439.
| Tillering responses of Lolium multiflorum plants to changes of red/far-red ratio typical of sparse canopies.Crossref | GoogleScholarGoogle Scholar |
Cerling, T. E., Wang, Y., and Quade, J. (1993). Expansion of C4 ecosystems as an indicator of global ecological change in the late Miocene. Nature 361, 344–345.
| Expansion of C4 ecosystems as an indicator of global ecological change in the late Miocene.Crossref | GoogleScholarGoogle Scholar |
Collins, S. L., and Calabrese, L. B. (2012). Effects of fire, grazing and topographic variation on vegetation structure in tallgrass prairie. Journal of Vegetation Science 23, 563–575.
| Effects of fire, grazing and topographic variation on vegetation structure in tallgrass prairie.Crossref | GoogleScholarGoogle Scholar |
Crush, J., and Rowarth, J. (2007). The role of C4 grasses in New Zealand pastoral systems. New Zealand Journal of Agricultural Research 50, 125–137.
| The role of C4 grasses in New Zealand pastoral systems.Crossref | GoogleScholarGoogle Scholar |
Deregibus, V. A., Sanchez, R. A., Casal, J. J., and Trlica, M. J. (1985). Tillering responses to enrichment of red light beneath the canopy in a humid natural grassland. Journal of Applied Ecology 22, 199–206.
| Tillering responses to enrichment of red light beneath the canopy in a humid natural grassland.Crossref | GoogleScholarGoogle Scholar |
Derner, J. D., Boutton, T. W., and Briske, D. D. (2006). Grazing and ecosystem carbon storage in the North American Great Plains. Plant and Soil 280, 77–90.
| Grazing and ecosystem carbon storage in the North American Great Plains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhslOrs7w%3D&md5=8c379ba6bcab961ddc7cd238ac87c922CAS |
Fanselow, N., Schoenbach, P., Gong, X. Y., Lin, S., Taube, F., Loges, R., Pan, Q., and Dittert, K. (2011). Short-term regrowth responses of four steppe grassland species to grazing intensity, water and nitrogen in Inner Mongolia. Plant and Soil 340, 279–289.
| Short-term regrowth responses of four steppe grassland species to grazing intensity, water and nitrogen in Inner Mongolia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitFGkt7w%3D&md5=4c50f27791d5c3c10ccd8e6b29b4621dCAS |
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T. (1989). Carbon isotope discrimination and photosynthesis. Annual Review of Plant Biology 40, 503–537.
| Carbon isotope discrimination and photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlKmu70%3D&md5=12b6e7eb49ee78f994a152e286b19830CAS |
Franz-Odendaal, T. A., Lee-Thorp, J. A., and Chinsamy, A. (2002). New evidence for the lack of C4 grassland expansions during the early Pliocene at Langebaanweg, South Africa. Paleobiology 28, 378–388.
| New evidence for the lack of C4 grassland expansions during the early Pliocene at Langebaanweg, South Africa.Crossref | GoogleScholarGoogle Scholar |
Hou, X.-Y., Han, Y., and Li, Y. (2012). The perception and adaptation of herdsmen to climate change and climate variability in the desert steppe region of northern China. The Rangeland Journal 34, 349–357.
| The perception and adaptation of herdsmen to climate change and climate variability in the desert steppe region of northern China.Crossref | GoogleScholarGoogle Scholar |
Jolly, D., and Haxeltine, A. (1997). Effect of low glacial atmospheric CO2 on tropical African montane vegetation. Science 276, 786–788.
| Effect of low glacial atmospheric CO2 on tropical African montane vegetation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXivFOrsro%3D&md5=fea91663cf82dc96cbfdcce3b6056bc6CAS | 9115201PubMed |
Kang, X., Hao, Y., Li, C., Cui, X., Wang, J., Rui, Y., Niu, H., and Wang, Y. (2011). Modeling impacts of climate change on carbon dynamics in a steppe ecosystem in Inner Mongolia, China. Journal of Soils and Sediments 11, 562–576.
| Modeling impacts of climate change on carbon dynamics in a steppe ecosystem in Inner Mongolia, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtVCqsLo%3D&md5=9795b530fd1e4ba3c0dfcd6f90fb70fdCAS |
Kohn, M. J. (2010). Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proceedings of the National Academy of Sciences of the United States of America 107, 19 691–19 695.
| Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVymu7bI&md5=495d2860e7b7b5a34e65b28523e9c1b8CAS |
Leegood, R. C. (2013). Strategies for engineering C4 photosynthesis. Journal of Plant Physiology 170, 378–388.
| Strategies for engineering C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVekt7bL&md5=fb5ec2ef8e7ce4a715c4df01e78f1505CAS | 23245935PubMed |
Li, Y. P., and Ji, J. J. (2004). Assessment of the productivity and livestock carrying capacity of Inner Mongolia grassland by regional scale modeling. Journal of Natural Resources 19, 610–616.
Li, S. G., Harazono, Y., Oikawa, T., Zhao, H. L., He, Z. Y., and Chang, X. L. (2000). Grassland desertification by grazing and the resulting micrometeorological changes in Inner Mongolia. Agricultural and Forest Meteorology 102, 125–137.
| Grassland desertification by grazing and the resulting micrometeorological changes in Inner Mongolia.Crossref | GoogleScholarGoogle Scholar |
Li, W. J., Ali, S. H., and Zhang, Q. (2007). Property rights and grassland degradation: a study of the Xilingol Pasture, Inner Mongolia, China. Journal of Environmental Management 85, 461–470.
| Property rights and grassland degradation: a study of the Xilingol Pasture, Inner Mongolia, China.Crossref | GoogleScholarGoogle Scholar |
Liang, C., Michalk, D., and Millar, G. (2002). The ecology and growth patterns of Cleistogenes species in degraded grasslands of eastern Inner Mongolia, China. Journal of Applied Ecology 39, 584–594.
| The ecology and growth patterns of Cleistogenes species in degraded grasslands of eastern Inner Mongolia, China.Crossref | GoogleScholarGoogle Scholar |
Mavromihalis, J. A., Dorrough, J., Clark, S. G., Turner, V., and Moxham, C. (2013). Manipulating livestock grazing to enhance native plant diversity and cover in native grasslands. The Rangeland Journal 35, 95–108.
| Manipulating livestock grazing to enhance native plant diversity and cover in native grasslands.Crossref | GoogleScholarGoogle Scholar |
McPherson, G. R., and Rasmussen, G. A. (1989). Seasonal herbivory effects on herbaceous plant communities of the Edwards plateau Texas, USA. The Texas Journal of Science 41, 59–70.
McWilliam, J. R. (1978). Response of pasture plants to temperature. In: ‘Plant Relations in Pastures’. (Ed. J. R. Wilson.) pp. 17–34. (CSIRO Publishing: Melbourne.)
Norman, H. C., Wilmot, M. G., Thomas, D. T., Masters, D. G., and Revell, D. K. (2009). Stable carbon isotopes accurately predict diet selection by sheep fed mixtures of C3 annual pastures and saltbush or C4 perennial grasses. Livestock Science 121, 162–172.
| Stable carbon isotopes accurately predict diet selection by sheep fed mixtures of C3 annual pastures and saltbush or C4 perennial grasses.Crossref | GoogleScholarGoogle Scholar |
Owensby, C. E., Ham, J., Knapp, A., and Auen, L. (1999). Biomass production and species composition change in a tallgrass prairie ecosystem after long-term exposure to elevated atmospheric CO2. Global Change Biology 5, 497–506.
| Biomass production and species composition change in a tallgrass prairie ecosystem after long-term exposure to elevated atmospheric CO2.Crossref | GoogleScholarGoogle Scholar |
Pushkina, D., Bocherens, H., Chaimanee, Y., and Jaeger, J. J. (2010). Stable carbon isotope reconstructions of diet and paleoenvironment from the late Middle Pleistocene Snake Cave in North-eastern Thailand. Naturwissenschaften 97, 299–309.
| Stable carbon isotope reconstructions of diet and paleoenvironment from the late Middle Pleistocene Snake Cave in North-eastern Thailand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslehtrk%3D&md5=e7e3f820e8efa1de143d93f945bcd0baCAS | 20127068PubMed |
Reeder, J. D., Schuman, G. E., Morgan, J. A., and Lecain, D. R. (2004). Response of organic and inorganic carbon and nitrogen to long-term grazing of the shortgrass steppe. Environmental Management 33, 485–495.
| Response of organic and inorganic carbon and nitrogen to long-term grazing of the shortgrass steppe.Crossref | GoogleScholarGoogle Scholar | 15453402PubMed |
Ren, H., Schönbach, P., Wan, H., Gierus, M., and Taube, F. (2012). Effects of grazing intensity and environmental factors on species composition and diversity in typical steppe of Inner Mongolia, China. PLoS ONE 7, e52180.
| Effects of grazing intensity and environmental factors on species composition and diversity in typical steppe of Inner Mongolia, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsVOjtA%3D%3D&md5=20bfa2b3454892421d3bc6595873b440CAS | 23284925PubMed |
Ripley, B. S., Cunniff, J., and Osborne, C. P. (2013). Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres. Global Change Biology 19, 900–910.
| Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres.Crossref | GoogleScholarGoogle Scholar | 23504846PubMed |
Sage, R. F. (2004). The evolution of C4 photosynthesis. New Phytologist 161, 341–370.
| The evolution of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsVymuro%3D&md5=b1c61a8f612aa6f633fac0b3cfa15287CAS |
Sage, R. F., and Kubien, D. S. (2007). The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30, 1086–1106.
| The temperature response of C3 and C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeiurrP&md5=75b7c196aa464b647364fb5ce4224605CAS |
Sage, R. F., Wedin, D. A., and Li, M. (1999). The biogeography of C4 photosynthesis: patterns and controlling factors. In: ‘Plant Biology’. (Eds R. F. Sage and R. K. Monson.) pp. 313–373. (Academic Press: San Diego, CA.)
Scheiter, S., Higgins, S. I., Osborne, C. P., Bradshaw, C., Lunt, D., Ripley, B. S., Taylor, L. L., and Beerling, D. J. (2012). Fire and fire-adapted vegetation promoted C4 expansion in the late Miocene. New Phytologist 195, 653–666.
| Fire and fire-adapted vegetation promoted C4 expansion in the late Miocene.Crossref | GoogleScholarGoogle Scholar | 22712748PubMed |
Senft, R., Coughenour, M., Bailey, D., Rittenhouse, L., Sala, O., and Swift, D. (1987). Large herbivore foraging and ecological hierarchies. Bioscience 37, 789–799.
| Large herbivore foraging and ecological hierarchies.Crossref | GoogleScholarGoogle Scholar |
Sierra, J., Brisson, N., Ripoche, D., and Deque, M. (2010). Modelling the impact of thermal adaptation of soil microorganisms and crop system on the dynamics of organic matter in a tropical soil under a climate change scenario. Ecological Modelling 221, 2850–2858.
| Modelling the impact of thermal adaptation of soil microorganisms and crop system on the dynamics of organic matter in a tropical soil under a climate change scenario.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1ems7zL&md5=f6aaaa7b647bc0dabcc63634a5910d17CAS |
Sinninghe Damsté, J. S., Verschuren, D., Ossebaar, J., Blokker, J., van Houten, R., van der Meer, M. T., Plessen, B., and Schouten, S. (2011). A 25,000-year record of climate-induced changes in lowland vegetation of eastern equatorial Africa revealed by the stable carbon-isotopic composition of fossil plant leaf waxes. Earth and Planetary Science Letters 302, 236–246.
| A 25,000-year record of climate-induced changes in lowland vegetation of eastern equatorial Africa revealed by the stable carbon-isotopic composition of fossil plant leaf waxes.Crossref | GoogleScholarGoogle Scholar |
Snyder, K. A., and Tartowski, S. L. (2006). Multi-scale temporal variation in water availability: implications for vegetation dynamics in arid and semi-arid ecosystems. Journal of Arid Environments 65, 219–234.
| Multi-scale temporal variation in water availability: implications for vegetation dynamics in arid and semi-arid ecosystems.Crossref | GoogleScholarGoogle Scholar |
Su, P. X., Xie, T. T., and Zhou, Z. J. (2011). C4 plant species and geographical distribution in relation to climate in the desert vegetation of China. Sciences in Cold and Arid Regions 3, 381–391.
Tang, H. P., and Liu, S. R. (2001). The list of C4 plants in the Inner Mongolia area. Journal of Inner Mongolia University 4, 431–438.
Teeri, J., and Stowe, L. (1976). Climatic patterns and the distribution of C4 grasses in North America. Oecologia 23, 1–12.
Tooth, I. M., and Leishman, M. R. (2013). Post-fire resprouting responses of native and exotic grasses from Cumberland Plain Woodland (Sydney, Australia) under elevated carbon dioxide. Austral Ecology 38, 1–10.
| Post-fire resprouting responses of native and exotic grasses from Cumberland Plain Woodland (Sydney, Australia) under elevated carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Wand, S. J., Midgley, G., Jones, M. H., and Curtis, P. S. (1999). Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Global Change Biology 5, 723–741.
| Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions.Crossref | GoogleScholarGoogle Scholar |
Wang, R. (2002). Photosynthetic pathway types of forage species along a grazing gradient from the Songnen grassland, Northeastern China. Photosynthetica 40, 57–61.
| Photosynthetic pathway types of forage species along a grazing gradient from the Songnen grassland, Northeastern China.Crossref | GoogleScholarGoogle Scholar |
Wang, S. P., Li, Y. H., and Wang, Y. F. (1999). Relationship between foraging areas of sheep and spatial heterogeneity of grassland landscape. Acta Ecologica Sinica 19, 431–436.
Wang, S. P., Wang, Y. F., and Chen, Z. Z. (2003). Effect of climate change and grazing on populations of Cleistogenes squarrosa in Inner Mongolia steppe. Acta Phytoecologica Sinica 27, 337–343.
Waters, C. M., Garden, D. L., Smith, A. B., Friend, D. A., Sanford, P., and Auricht, G. C. (2005). Performance of native and introduced grasses for low-input pastures. 1. Survival and recruitment. The Rangeland Journal 27, 23–39.
| Performance of native and introduced grasses for low-input pastures. 1. Survival and recruitment.Crossref | GoogleScholarGoogle Scholar |
Way, D. A. (2012). What lies between: the evolution of stomatal traits on the road to C4 photosynthesis. New Phytologist 193, 291–293.
| What lies between: the evolution of stomatal traits on the road to C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitVejs7w%3D&md5=f03b612d09f3b8796026dd641a9caa5fCAS | 22221147PubMed |
Whalley, R. D. B. (1994). State and transition models for rangelands 1. Successional theory and vegetation change. Tropical Grasslands 28, 195–205.
Wittmer, M., Auerswald, K., Bai, Y. F., Schaufele, R., and Schnyder, H. (2010). Changes in the abundance of C3/C4 species of Inner Mongolia grassland: evidence from isotopic composition of soil and vegetation. Global Change Biology 16, 605–616.
| Changes in the abundance of C3/C4 species of Inner Mongolia grassland: evidence from isotopic composition of soil and vegetation.Crossref | GoogleScholarGoogle Scholar |
Wu, J., and Loucks, O. (1995). From balance of nature to hierarchical patch dynamics: a paradigm shift in ecology. The Quarterly Review of Biology 70, 439–466.
| From balance of nature to hierarchical patch dynamics: a paradigm shift in ecology.Crossref | GoogleScholarGoogle Scholar |
Yokohama, M., Shimada, S., Sekiyama, A., Gombojav, A., and Ariunsren, P. (2011). Grassland vegetation and palatability in Mongolian grasslands. Journal of Agricultural Science - Tokyo Nogyo Daigaku 56, 203–211.
Zech, M., Zech, R., Morras, H., Moretti, L., Glaser, B., and Zech, W. (2009). Late Quaternary environmental changes in Misiones, subtropical N-E Argentina, deduced from multi-proxy geochemical analyses in a palaeosol-sediment sequence. Quaternary International 196, 121–136.
| Late Quaternary environmental changes in Misiones, subtropical N-E Argentina, deduced from multi-proxy geochemical analyses in a palaeosol-sediment sequence.Crossref | GoogleScholarGoogle Scholar |
Zhang, Z., Zhao, M., Lu, H., and Faiia, A. M. (2003). Lower temperature as the main cause of C4 plant declines during the glacial periods on the Chinese Loess Plateau. Earth and Planetary Science Letters 214, 467–481.
| Lower temperature as the main cause of C4 plant declines during the glacial periods on the Chinese Loess Plateau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnt1CgtLg%3D&md5=c73824217852bf575caf32da33e7bb69CAS |
Zheng, S., Lan, Z., Li, W., Shao, R., Shan, Y., Wan, H., Taube, F., and Bai, Y. (2011). Differential responses of plant functional trait to grazing between two contrasting dominant C3 and C4 species in a typical steppe of Inner Mongolia, China. Plant and Soil 340, 141–155.
| Differential responses of plant functional trait to grazing between two contrasting dominant C3 and C4 species in a typical steppe of Inner Mongolia, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitFGks7k%3D&md5=a7e393afb0fff8ef7011a67e1d67cef9CAS |