211 QUALITY CHANGES IN BLUE WILDEBEEST (CONNOCHAETES TAURINUS) EPIDIDYMAL SPERMATOZOA MAINTAINED AT 4°C
F. Olivier A , T. Spies A , F. Martinez-Pastor B , D.M. Barry A and P. Bartels CA Department of Animal Sciences, University of Stellenbosch, Stellenbosch, South Africa;;
B Reproduction and Obstetrics, University of Leon, Leon, Spain;;
C Wildlife Biological Resource Centre of The Endangered Wildlife Trust Pretoria, South Africa. email: 13270435@sun.ac.za
Reproduction, Fertility and Development 16(2) 227-227 https://doi.org/10.1071/RDv16n1Ab211
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
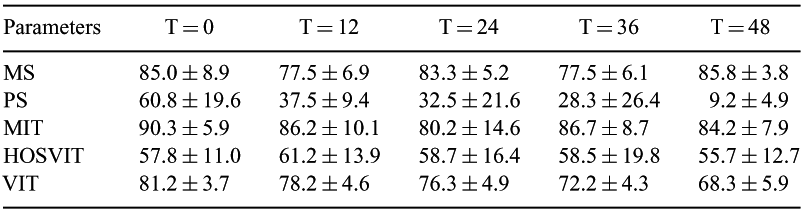
Wildlife management in southern Africa often involves the harvesting of animals on ranches and reserves, providing unique opportunities to collect and assess the quality of epididymal spermatozoa for possible future conservation actions. The black wildebeest (Connochaetes gnu) is facing renewed threats to its survival, including the production of fertile hybrids from crossing with the more common blue wildebeest (Connochaetes taurinus). The close relationship between the two wildebeest species allows for the blue wildebeest to be used as a model to assess epididymal sperm quality over time while maintained at 4°C. Field conditions often preclude the immediate availability of liquid nitrogen, necessitating the development of alternative short-term storage methods. All chemicals were provided by Sigma (South Africa) unless otherwise stated. Testes were harvested from 6 blue wildebeest bulls at a local game farm, Savannah, and kept at 5°C during transportation to the lab. Epididymides were dissected out and spermatozoa were flushed out of the cauda epididymis using 1 mL of Tris-citrate egg yolk extender (Fraction A, Biladyl, Minitub, Germany), followed by storage at 4°C and assessment at 12 h intervals. At each interval, an aliquot was removed, washed with a modified buffered HEPES solution (20 mM HEPES, 355 mM sucrose, 10 mM glucose, 2.5 mM KOH;; 400 mOsm/kg, pH 7; Sigma) and visually assessed with a phase contrast microscope (×200, at 37°C) to determine the percentage of motile (MS) and progressive motile (PS) spermatozoa. In addition, plasma membrane integrity (PMI) was assessed with eosin-nigrosin staining and active mitochondrial status (MIT) assessed with an epifluorescent microscope (×400) using the fluorescent probe JC-1 (Molecular Probes, The Netherlands;; 7.5 μM; 30 min at 37°C). Resilience to hypo-osmotic shock was also evaluated by incubating the sample in a modified hypo-osmotic medium (100 mOsm kg−1; 15 min RT), and staining with PI to assess plasma membrane integrity (HOSPMI). A summary of results is presented in the table 1. The MS, MIT and HOSPMI did not decrease significantly during the 48 h storage period. The only parameters that showed a significant decrease were PS and PMI (P < 0.01, Kruskall-Wallis test). However, PMI showed a slow but steady decrease (13%), whereas PS underwent a significant drop (52%). In conclusion, epididymal spermatozoa from the blue wildebeest, kept at 4°C for 48 h, may still be useful for some assisted-reproduction techniques. The use of spermatozoa from a common but closely related wildebeest species allows for the development of assisted-reproduction techniques that may one day aid the conservation of threatened wildebeest species. Additional research is needed to confirm these findings and to test the effect of longer storage times on spermatozoa of this species as well as closely related endangered species.
|


