324 PRODUCTION OF GENETICALLY MODIFIED PIGS BY ARTIFICIAL REPRODUCTIVE TECHNOLOGIES USING FROZEN EPIDIDYMAL SPERM
K. Honda A , Y. Takeuchi A , T. Matsuda A , T. Kanai A , M. Kuramoto A , M. Maehara A , H. Matsunari B C , K. Nakano A , K. Umeyama A B , M. Watanabe A B , H. Nakauchi C D and H. Nagashima A BA Laboratory of Developmental Engineering, Department of Life Sciences, School of Agriculture, Meiji University, Kawasaki, Kanagawa, Japan;
B Meiji University International Cluster for Bio-Resource Research, Kawasaki, Kanagawa, Japan;
C JST, ERATO, Nakauchi Stem Cell and Organ Regeneration Project, Minato-ku, Tokyo, Japan;
D Center for Stem Cell and Regenerative Medicine, Institute of Medical Science, The University of Tokyo, Minato-ku, Tokyo, Japan
Reproduction, Fertility and Development 25(1) 309-310 https://doi.org/10.1071/RDv25n1Ab324
Published: 4 December 2012
Abstract
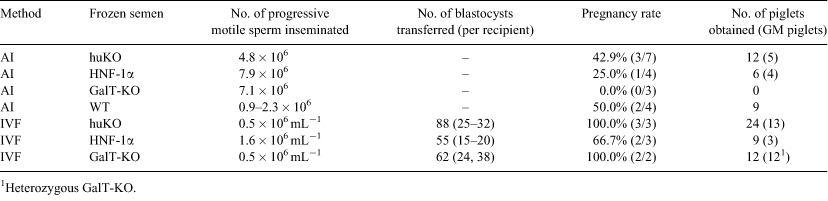
Genetically modified (GM) pigs are useful tools for many types of biomedical research. The objective of this study was to develop a reliable protocol for the reproduction of GM pigs using frozen epididymal sperm. Epididymal sperm were collected from 3 GM boars: (1) humanized Kusabira-Orange (huKO) transgenic, (2) mutant human hepatocyte nuclear factor 1α (HNF-1α) transgenic, and (3) α1,3-galactosyltransferase gene knockout (GalT-KO). Additionally, a wild type (WT) boar was also used for sperm collection. After collection, the sperm were frozen in Niwa and Sasaki freezing medium (Niwa et al. 1989 Manual for Cryopreservation of Pig Spermatozoa 19–23; 1.0 × 109 sperm mL–1) using 0.5-mL straws. The sperm were cooled utilising the 2-step method, from 25 to 15°C at a rate of 0.17°C min–1, and then from 15 to 5°C at 0.08°C min–1. After cooling to 5°C, the straws were frozen in liquid nitrogen with 3% glycerol for storage. After storage for 0.5 to 21 months, the straws were thawed by immersion into a 37°C water bath. The recovered sperm were washed by centrifugation in PBS + 1 mg mL–1 BSA and were resuspended in either Beltsville thawing solution (Pursel and Johnson. 1975 J. Anim. Sci. 40, 99–102) for intra-fallopian AI or in porcine fertilization medium (PFM, Research Institute for the Functional Peptides, Yamagata, Japan) for IVF. Prepubertal gilts (6.5 months), in which oestrus was induced by 1000 IU eCG and 1500 IU hCG given 66 or 72 h apart, were surgically inseminated with 0.9–7.9 × 106 progressive motile sperm 41 to 51 h after receiving an hCG injection. Oocytes, which matured in vitro either in modified porcine oocyte medium (IFP) or modified NCSU23 medium, were inseminated in PFM for 8 h with either 0.5 (huKO, GalT-KO) or 1.6 (HNF-1α) × 106 progressive motile sperm mL–1. The in vitro fertilized oocytes were cultured in porcine zygote medium-5 (Research Institute for the Functional Peptides) for 5 to 6 days until they were transferred. Some of these oocytes were examined for their fertilization rates. Blastocysts were transferred into the uterus of the recipient 150 h after hCG treatment. The fertilization rates for huKO, HNF-1α and GalT-KO sperm were 63.5% (61/96), 62.5% (70/112) and 92.4% (61/66), respectively; 50.8% (31/61), 82.9% (58/70) and 60.7% (37/61) of these were monospermic. The rate at which the IVF embryos developed into blastocysts was 47.8–52.4%. This study demonstrates that IVF using frozen epididymal sperm is more efficient method for reproducing genetically modified pigs than AI (87.5 v. 33.3%).
|
This study was supported by JST, ERATO, NAKAUCHI Stem Cell and Organ Regeneration Project, Tokyo.


