120 EFFECT OF INDIVIDUAL CULTURE SYSTEM USING WOW OR CELL-TAK ON DEVELOPMENT OF IN VITRO-PRODUCED BOVINE EMBRYOS
S. Matoba A B and P. Lonergan AA National Livestock Breeding Center, Nishigo, Fukushima, Japan;
B School of Agriculture, Food Science and Veterinary Medicine, University College Dublin, Belfield, Dublin, Ireland
Reproduction, Fertility and Development 21(1) 160-160 https://doi.org/10.1071/RDv21n1Ab120
Published: 9 December 2008
Abstract
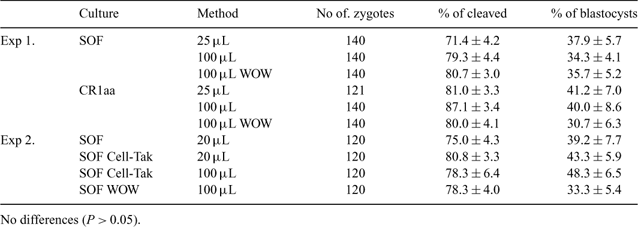
The culture of embryos individually in vitro is generally associated with poorer developmental rates. However, the ability to do this successfully would greatly facilitate studies where identification of individual embryos, or the embryos from a particular donor, is necessary. The objective of this study was to examine the effect of culture system on the development of individual IVP bovine embryos. Presumptive zygotes (n = 1301, 6 replicates), produced by IVM/IVF, were used. The aim of Experiment 1 was to compare development of bovine embryos in SOF or CR1aa supplemented with 5% FCS. Zygotes were cultured in droplets under oil as follows: (i) 20/25 μL, (ii) 20/100 μL or (iii) 20/100 μL individually in the Well of the Well (WOW) system (Vajta et al. 2000 Mol. Reprod. Dev. 55, 254–264). Twenty WOW were prepared in a 100 μL droplet of medium under oil using a sterile rod. The aim of Experiment 2 was to compare development of embryos cultured in groups but individually identifiable on the cell adhesive Cell-Tak (Stokes et al. 2005 Dev. Biol. 284, 62–71) or in the WOW system. Zygotes were cultured as follows: (i) 20/20 μL, (ii) 20/20 μL with Cell-Tak, (iii) 20/100 μL with Cell-Tak or (iv) 20/100 μL in WOW. A drop of Cell-Tak (1 μL/20 μL medium) was placed on the base of the dish, dried for 20 min, washed with sterile water and dried completely. Once dried, the area was covered with 10 μL of FCS-free medium and groups of 20 zygotes were placed on the Cell-Tak in a 5 × 4 grid formation a maximum of 160 μm apart. Then, an additional 10 μL or 90 μL medium supplemented with FCS was added to give a final volume of 20 or 100 μL. Cleavage and blastocyst rates were assessed on Day 2 and Days 7–9, respectively. Data (means ± SE) were analyzed by one way ANOVA. In Experiment 1, there were no differences between SOF and CR1aa with respect to culture of embryos individually in WOW (P > 0.05); therefore, SOF was used as the basal medium for Experiment 2. There were no differences (P > 0.05) between the cleavage and blastocyst rate among drop sizes and individual culture systems; individual culture, irrespective of the system used (Cell-Tak or WOW), resulted in the similar developmental rates to the control. In conclusion, individual embryo culture offers the opportunity to study embryo development in a more powerful manner. Furthermore, the use of the cell adhesive Cell-Tak may be more practical because it removes the potential variability associated with well dimensions in the WOW system and may improve any potential paracrine effects during embryo culture. Further studies are required to establish the viability of such embryos after transfer.
|
Supported by Science Foundation Ireland.


