51 DEVELOPMENT OF BOVINE CLONES CONSTRUCTED WITH CYTOPLASM ORIGINATING FROM OOCYTES CULTURED IN RETINOL
J.L. Lawrence A , F.N. Schrick A , J.D. Godkin A and J.L. Edwards ADepartment of Animal Science, The University of Tennessee, Knoxville, TN, USA. email: jedwards@utk.edu
Reproduction, Fertility and Development 16(2) 147-148 https://doi.org/10.1071/RDv16n1Ab51
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
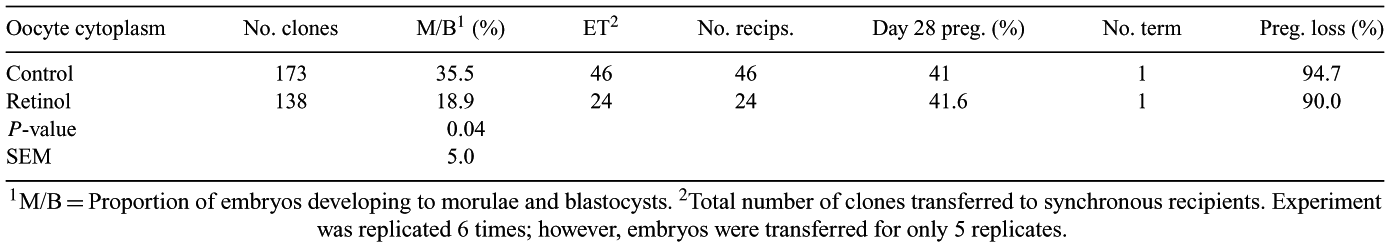
Incomplete reprogramming of a somatic nucleus by the oocyte cytoplasm may contribute in large part to inefficiencies of cloning procedures using somatic cell nuclear transfer. Predominant use of cytoplasts derived from in vitro-matured oocytes may further exacerbate problems. Addition of all-trans-retinol (Livingston TL et al. 2002 Biol. Reprod. 66, 104–105 abst) or 9-cis-retinoic acid (Duque P et al. 2002 Human Reprod. 17, 2706–2714) to culture medium improved developmental competence of oocytes to blastocyst stage. The objective of this study was to evaluate effects of retinol for improving developmental competence of cloned embryos constructed with retinol-treated cytoplasts. After removal from 3–8 mm follicles, COC were matured in the presence of 0 (n = 1005; diluent only) or 5 μM all trans-retinol (n = 1017). Beginning approximately 18 h after placement into culture, oocytes with extruded polar bodies were enucleated of maternal chromatin. Ovarian/granulosa cells were aspirated from adult Jersey cows (n = 2) using an ultrasound-guided transvaginal probe. Primary cell lines were established and, before nuclear transfer, cultured in the presence of 0.5% serum (5–8 days; passage = 3). Ovarian/granulosa cells were fused with cytoplasts originating from control or retinol-treated oocytes using two electrical pulses of 2.2 kV cm−1 for 20 μs between 24–27 h post-maturation (hpm). Cloned embryos were activated between 26.5 and 30 hpm and then cultured in an atmosphere of 7% O2 and 5.5% CO2 in KSOMaa + BSA. Development of cloned embryos to morula and blastocyst stages was assessed on Days 6–7 post-activation. In 5 replicates, compact morulae and blastocysts were transferred to synchronous recipients. Establishment of pregnancy was confirmed 28 days post-estrus by presence of an embryonic heartbeat using ultrasound. With the exception of pregnancy, data were analyzed as a randomized block design using mixed models of SAS (2000) after testing for normality. Proportion of oocytes recovered after denuding (94.5 and 88.9%; SEM = 2.2), those that had visibly lysed (6.4 and 7.3%; SEM = 1.5), and extruded a polar body by 20 hpm (61.7 and 62.5%; SEM = 7.0) was similar for control and retinol-treated oocytes, respectively. In addition, ability of ovarian/granulosa cells to fuse with control or retinol-treated cytoplasts was similar (80.6 and 73.1%; SEM = 4.5). Lysis of cytoplasts after electrical pulses (8.2 v. 13.6%; SEM = 3.3) and activation (11.6 v. 5.0%; SEM = 2.9) did not differ for control and retinol-treated cytoplasts. Development to morula and blastocyst stages was lower in cloned embryos constructed with retinol-treated cytoplasts (Table). However, ability of morulae and blastocysts to establish a pregnancy was comparable. One clone from each treatment developed to term and was born alive. Culture of oocytes in medium containing retinol during maturation did not improve developmental competence of clones.
|


