Seven days ex vivo perfusion of whole ewe ovaries with follicular maturation and oocyte retrieval: towards the development of an alternative fertility preservation method
Panagiotis Tsiartas A B , Claudia Mateoiu C , Meghshree Deshmukh D , Debashish Banerjee D , Arvind M. Padma A D , Milan Milenkovic B E , Fulvio Gandolfi F , Mats Hellström A D , Pasquale Patrizio G H and Randa Akouri A D *
A D *
A Department of Obstetrics and Gynecology, Sahlgrenska University Hospital, Blå stråket 6, 41345 Gothenburg, Sweden.
B Department of Oncology-Pathology, Karolinska Institute, Stockholm, Sweden.
C Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden.
D Laboratory for Transplantation and Regenerative Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
E BeoGyn Clinic, Belgrade, Serbia.
F Department of Agricultural and Environmental Sciences, University of Milano, Milano, Italy.
G Yale Fertility Center, Yale School of Medicine, New Haven, CT, USA.
H Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology and Reproductive Sciences, University of Miami, Miller School of Medicine, Miami, FL, USA.
Reproduction, Fertility and Development 34(3) 331-342 https://doi.org/10.1071/RD21197
Published online: 28 January 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Fertility preservation methods for prepubertal women about to undergo gonadotoxic chemo and/or radiation therapy are limited. Therefore, the aim of this study was to investigate the feasibility to develop an alternative fertility preservation method based on an ex vivo perfusion platform for whole ewe ovaries. Thirteen ewe ovaries were divided into two groups (group 1 and 2) that were perfused in a bioreactor for up to 7 days. Group 1 (n = 3) were stimulated with human menopausal gonadotropin (hMG) administered in single daily dose, while group 2 (n = 10) were stimulated continuously for 24 h. The perfused ovaries in group 1 showed no significant differences in follicular density, sub-follicular morphology and oocyte quality after ischaemia and after ex vivo perfusion compared with non-perfused control ovaries. The perfused ovaries in group 2 showed a significant decrease in the follicular reserve and oocyte quality compared with the control group. In total, 16 GV–MI oocytes were retrieved from both groups. This study describes for the first time the ex vivo maintenance of viable follicles of ewe ovaries with oocyte integrity and the retrieval of oocytes after ex vivo hormonal perfusion with two different protocols for up to 7 days.
Keywords: bioreactor, endocrinology, ewes, ex vivo, fertility preservation, follicular development, ovary, perfusion.
Introduction
Recent advances in chemo/radiotherapy protocols are providing a cure for many types of cancers. Cancer survivors need to be offered fertility preservation options (Letourneau et al. 2012; Benedict et al. 2018) since several cancer treatments can induce ovarian failure with consequent infertility (Wallace et al. 2005; Donnez et al. 2006, 2010; Bromer and Patrizio 2008). There are many strategies available today for fertility preservation in women requiring gonadotoxic treatments, including ovarian transposition, oocyte and/or embryo vitrification, and ovarian cortex transplantation (OCT) (Donnez 2013; Diaz-Garcia et al. 2018; Moravek et al. 2018). OCT is the only fertility preservation option available for prepubertal girls and for women who need prompt cancer treatment (Bromer and Patrizio 2008; Donnez and Dolmans 2013). Although live births after OCT have been reported, including one in a leukaemia survivor (Shapira et al. 2018), there are serious concerns that systemic cancers may metastasise to the ovaries posing the risk of re-introducing neoplastic cells at the time of OCT (Wallace et al. 2005; Dolmans et al. 2010, 2013; Donnez and Dolmans 2017). Folliculogenesis and retrieval of mature oocytes performed in vitro and ex vivo could be alternative methods for fertility preservation in these patients (Donnez and Dolmans 2017). Some progress has been made in terms of complete follicular growth and isolation of mature oocytes in vitro (Picton et al. 2008; Telfer and Zelinski 2013; Maffei et al. 2016; Chiti et al. 2017; McLaughlin et al. 2018). However, full development, from early follicular stages to a viable offspring, has only been described in rodent models (Eppig and Schroeder 1989; O’Brien et al. 2003; Vanacker et al. 2014; Laronda et al. 2017). The complex molecular events controlling follicular expansion and the long time required for folliculogenesis and oocyte maturity in large mammalian species are still poorly understood, creating challenges and limitations for in vitro studies (Edson et al. 2009; Figueiredo et al. 2011; Songsasen et al. 2012; Shea et al. 2014). A novel idea to overcome these hurdles, might be to explant an ovary from the patient prior to the cancer treatment, perfuse it ex vivo in a bioreactor, and perform ovarian stimulation in this artificial environment, which would have the advantage of exploiting the intact ovarian architecture to support folliculogenesis and oocyte maturation. The aim of this study was to evaluate follicular growth and oocyte survival and integrity after ex vivo whole ovarian perfusion using sheep ovaries. Potential ovarian tissue damage caused by any cold ischaemia events between the times from ovarian explantation to reperfusion, where the ovary is stored in a cold preservation solution, was also considered. We also investigated the possibility of retrieving mature oocytes using two different gonadotropin stimulation protocols during the normothermic perfusion in a bioreactor. Such perfusion bioreactor system could become a useful research platform for continued studies on how to maintain and stimulate fresh and cryopreserved ovaries ex vivo to develop alternative fertility preservation methods for young prepubertal female cancer patients or for patients in high risk of ovarian metastasis.
Materials and methods
Organ collection
Twenty-six ovaries from 13 sexually mature ewes (10–14 months old) of mixed breeds were retrieved at the local slaughterhouse between January 2020 and April 2020. All ovaries (n = 26) with intact vascular pedicles were directly dipped once in a 0.001% chlorhexidine solution dissolved in normal saline (Fresenius Kabi, Uppsala, Sweden) and then dipped three times in a sterile saline solution (0.9 mg/mL, Braun, Melsungen AB, Germany) to minimise contamination risk and tissue damage from the chlorhexidine solution. The collected ovaries were divided into an experimental group (n = 13) and a control group (n = 13) (Table 1). For the ovaries included in the experimental group, the ovarian artery was separated from the ovarian vein using a dissection microscope (Nikon, Tokyo, Japan). The ovarian artery was then cannulated with a 24 G cannula (Becton Dickinson infusion Therapy AB, Helsingborg, Sweden) and the venous outflow was left open. Sterile silk sutures 4–0 were used to fix the cannula to the ovarian artery as previously described (Wallin et al. 2009). The ovaries were then gently perfused with 2 mL ice-cold sterile phosphate-buffered saline (PBS, Gibco, London, UK) supplemented with heparin (50 IU/mL; Apoteket AB, Stockholm, Sweden), xylocaine (0.04 mg/mL; Astra Zeneca, Gothenburg, Sweden), piperacillin/tazobactam (10 μg/mL and 1.25 μg/mL; Stragen Nordic, Hillerød, Denmark) and Gibco’s antibiotic–antimycotic (anti–anti; 1%; penicillin 10 000 U/mL, streptomycin 10 000 μg/mL and fungizone 25 μg/mL; Thermo Fisher Scientific, Stockholm, Sweden) until the organ blanched and clear liquid was seen flowing through the ovarian vein. All experimental ovaries were then submerged in cold (4°C) sterile PBS solution and placed on ice during transport to the laboratory. Each control ovary was dissected along the long axis and divided in two equal halves. One half was placed directly in 4% formaldehyde and served as the fresh control tissue for follicular assessment before ex vivo ovarian perfusion, and the other half was kept in the same cold preservation solution used for the transportation of the intact ovaries used for perfusion to the laboratory thus serving as control tissue to assess potential follicular damage from the cold ischaemia during transport. The average time between ovarian collection, cannulation and vascular perfusion was between 30 and 60 min, and the average cold ischaemia time was between 180 and 240 min (from ovarian collection to perfusion start).
|
Perfusion system
The ovaries (n = 13) were perfused in a modified closed circulation perfusion system assembled using equipment parts from a bioreactor set up previously assessed for ex vivo sheep uterus perfusion (Padma et al. 2019) (Fig. 1a). The ovaries were connected through the cannulated ovarian artery to the perfusion system. The bioreactor system recirculated the perfusate (closed system) which allowed the maintenance of a sterile environment and monitoring of temperature, oxygenation, perfusion pressure and flow throughout the duration of the experiment. The bioreactor consisted of three separate circuit loops: (1) the organ chamber that was combined with the media reservoir and a single ovary was completely submerged in the perfusion medium, (2) carbogen gas (95% O2 and 5% CO2) that oxygenated and buffered the perfusion media and (3) warm water (38°C) transported in a jacketed manner (physically separated from the two other circuits) ensuring a constant physiological temperature for the perfusate and the organ throughout the experiment. A peristaltic pump circulated the perfusate through the oxygenator (including a bubble trap) to the ovary in the organ chamber (Fig. 1a). The perfusion pressure was kept below 80 mmHg by regulating flow rate during the entire perfusion period in order to avoid ovarian vascular damage.
Experimental design
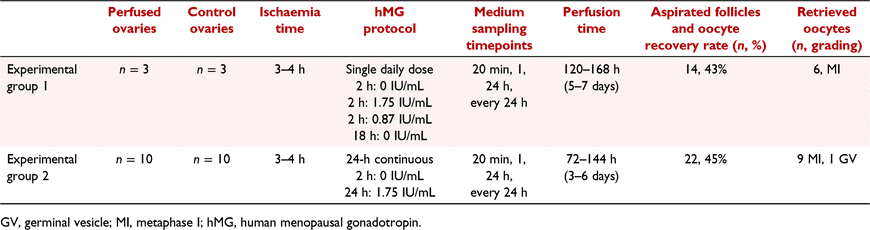
The perfused ovaries were allocated to two groups to assess follicular development and maturation using two different gonadotropin stimulation protocols (Table 1). Experimental group 1 (n = 3) consisted of ovaries that were perfused in the bioreactor (Fig. 1a) between 120 and 168 h with medium consisting of M199 (Thermo Fisher Scientific) supplemented with 2% bovine serum albumin (Roche Diagnostic GmbH, Mannheim, Germany), piperacillin/tazobactam (10 μg/mL and 1.25 μg/mL; Stragen Nordic), anti–anti (Thermo Fisher Scientific), 12 mL/L sodium bicarbonate 7.5% (Thermo Fisher Scientific), fetal bovine serum (FBS) 10% (Thermo Fisher Scientific), IGF-1 (50 ng/mL, Peprotech, Stockholm, Sweden) and HEPES (Sigma Aldrich, Stockholm, Sweden), with the addition of hMG (Menopur, Ferring Pharmaceuticals, Malmö, Sweden) administered in a single daily dose of 1.75 IU/mL for 2 h followed by 0.87 IU/mL for further 2 h. The hMG is a mixture (1:1) of follicle stimulating hormone (FSH) and luteinising hormone (LH) purified from the urine of postmenopausal women, both hormones are essential for follicle development and luteinisation (Campbell et al. 2007). Experimental group 2 (n = 10) consisted of ovaries that were perfused in the bioreactor between 72 and 144 h with the same medium used for group 1, but with hMG administered continuously at the same concentration of 1.75 IU/mL. Since gonadotropin secretion follows a pattern of daily peaks in cycling ewes (Baby and Bartlewski 2011), a single daily dose hMG media supplementation pattern was implemented in group 1 while a continuous hMG administration pattern was implemented in experimental group 2, as detailed in Table 1. The same cycle was repeated every 24 h. Human chorionic gonadotropin (hCG) (Pregnyl, MSD Sverige AB, Stockholm, Sweden) 50 IU/mL was added in the medium of 2 of the 3 ovaries in group 1 and of 6 of the 10 ovaries in group 2 during the last 24 h of perfusion before ovum pick-up (OPU) to trigger final oocyte maturation. OPU was performed at the end of the perfusion from visible follicles with a diameter between 3 and 5 mm (Fig. 1b, c), through gentle manual aspiration with an 18 G needle connected to a tube with an inner diameter of 2 mm (Rodriguez et al. 2006). The perfusion time was not constant among the experiments because the ovaries were retrieved from ewes that were not synchronised in their reproductive cycle. For this reason visible follicles amenable for OPU were achieved in different timepoints during the ovarian perfusion. Additionally, 10 mL of the perfusion medium was sampled from the organ chamber at 20 min, 1 h, 24 h and at every 24 h during the perfusion (before any medium change). These perfusate samples were immediately frozen at −20°C and later biochemically analysed, as described below. At the end of the perfusion time, each ovary was fixed in 4% phosphate buffered formaldehyde for 24–48 h for later analysis.
Histomorphological examination and immunohistochemistry of ovarian tissue
Formaldehyde-fixed ovarian samples from both the perfused and non-perfused (control) ovaries were dehydrated and embedded in paraffin, sectioned at 4-μm thickness and stained with H&E according to standard protocols. Each specimen was examined in duplicate by two pathologists blinded to the sample groups. A minimum of two sections of each ovary were examined, taking at least ten sections (50 μm) apart from each other to avoid counting the same follicles twice. All ovarian-tissue slides were digitally scanned with a NanoZoomer S210 (Hamamatsu Photonics, Japan) and the surface area of the ovarian cortex on each section was measured using the NanoZoomer Digital Pathology.view 2 analysis software (Hamamatsu Photonics, Hamamatsu, Japan). The degree of injury was scored using digital pathology, according to previously established parameters (Paynter et al. 1999; Gandolfi et al. 2006). Non-damaged primordial follicles were identified as follicles with a spherical oocyte and a non-pyknotic nucleus, surrounded by one layer of flattened granulosa cells. Non-damaged primary follicles were identified as small spherical follicles that contained a GV stage oocyte surrounded by one to two layers of cuboidal granulosa cells. Secondary non-damaged follicles were identified as larger follicles surrounded by several layers of granulosa cells with or without small spaces between cells and containing a GV stage oocyte. Non-damaged antral follicles were identified by their characteristic fluid-filled antral cavity, where the oocyte lies at the edge in a mound made of granulosa epithelial cells (cumulus oophorus). Damaged follicles were classified as grade 1 follicles (G1) when theca and granulosa cells were pulled away from the edge, with disruptions and apparent loss of granulosa cells but with an oocyte maintaining its spherical shape. Grade 2 follicles (G2) were identified as follicles showing a greater disruption, the loss of granulosa cells and with theca cells pulled away from the follicle edge, with severe pyknotic nuclei in the granulosa cells and a misshapen oocyte with or without vacuolation or pyknotic nucleus (Fig. 2a–f). The overall ovarian histomorphological architecture was described with regards to abnormalities in the arteries and veins as endothelial detachment, internal elastic membrane rupture, or smooth muscle cell bloating (Koos et al. 1984; Milenkovic et al. 2011b). Follicular cell proliferation was studied by 3,3′-diaminobenzidine (DAB) immunohistochemistry for Ki-67, a nuclear protein associated with proliferation. Antigen retrieval was conducted in a pressure cooker with Diva Decloaker (Biocare Medical, HistoLab, Gothenburg, Sweden). Sections were exposed to hydrogen peroxide for 5 min, blocked with 5% bovine serum for 10 min and incubated with the Ki-67 antibody (antibody #15580; 1:500, Abcam, Cambridge, UK) overnight. Detection of the primary antibody was achieved by MACH 2 universal HPR (Biocare Medical) for 30 min, then Betazoid DAB for 5 min. Sections were then counterstained with hematoxylin. Apoptosis was confirmed by cleaved caspase 3 immunostaining (antibody Cat No. #9661; 1:300, Cell Signaling Technology, BioNordika, Stockholm, Sweden) followed by the highly sensitive MACH 3 polymer detection kit and by the Warp Red Chromogen kit (both from Biocare Medical) according to the manufacturers’ instructions. This method clearly showed the different steps in the localisation of caspase 3 activation from cytoplasmic to nuclear translocation of the protein. Proliferation and apoptotic index were determined by calculating the percentage of Ki-67 and caspase 3 positive cells in hotspot areas of the ovarian cortex, examining all follicular subclasses separately (Fig. 2g, h).
Oocyte examination and maturation grading
Retrieved oocytes were examined using stereomicroscopy directly after OPU and were graded by an experienced embryologist who was blinded to the experimental groups. The number of retrieved oocytes, recovery rates and maturation grade were noted (Table 1).
Hormonal analysis
Oestradiol and progesterone concentrations in the undiluted sampled media were estimated to assess the follicular steroidogenic activity. The oestradiol concentration was estimated using sheep estrogen ELISA Kit (Reagent Genie, Dublin, Ireland). The coefficient of variation (CV) for oestradiol varied between 10 and 16%, the analytical sensitivity was 9.375 pg/mL and the detection range was 15.625–1000 pg/mL. The progesterone concentration was estimated using sheep progesterone ELISA Kit (Reagent Genie, Dublin, Ireland). The CV for progesterone varied between 6 and 11%, the analytical sensitivity was <0.188 ng/mL and the detection range was 0.0313–20 ng/mL.
Biochemical analysis
The concentrations of glucose, lactate, potassium, sodium, calcium and chloride in the perfusion medium were measured by a standard blood-gas analysis according to the manufacturer’s protocols (RapidPoint 500 Blood Gas System, Siemens Healthcare, Erlangen, Germany) to assess the ovarian metabolic activity.
Statistics
The distribution of the data was assessed using Kolmogorov–Smirnov and Shapiro–Wilk normality tests. The Wilcoxon Signed Rank test for related samples was used to compare the number of non-damaged and damaged follicles before perfusion, after cold ischaemia and after perfusion. Wilcoxon matched-pairs signed-rank test was used to compare the percentage of Ki-67 and cleaved caspase 3 positive follicle cells before and after perfusion. To assess any changes over time for hormonal-, metabolic- and electrolytic biomarker concentrations in the perfusion medium, the coefficient from a univariable linear regression with perfusion time as covariate was calculated for each perfused ovary and the set of coefficients were tested with Wilcoxon signed-rank test. A post hoc analysis was also performed for these biomarkers with Wilcoxon signed-rank test for the changes from the start of perfusion to each measure after perfusion. Data herein is presented as mean (±s.d.) and a two-sided P-value of less than 0.05 was considered significant. All analyses were performed with SAS version 9.4 and Prism version 9.0.0.
Ethics approval and consent to participate
No ethical approval for the study was necessary since the ovaries were collected from animals that were bred for meat production with the possibility to use discarded organs for research (in line with the Swedish and European Union ethical regulations). Consent to participate was not applicable.
Results
Effect of perfusion on ovarian histomorphology, cell proliferation and apoptosis
Group 1
The comparison of the ovarian cortex follicular population between the non-perfused (control) ovaries, ovaries exposed to 3–4 h of cold ischaemia, and ovaries exposed to 120–168 h of normothermic perfusion, showed that the perfused ovaries had a good follicular preservation without a significant reduction in both the total and non-damaged number of primordial follicles per mm2. Intact histomorphology was seen in 67% of the medullary arteries, in 0% of the ovarian arteries, in 100% of the medullary veins and in 50% of the ovarian veins of the perfused ovaries. Moreover, 33% and 100% of the ovaries after perfusion had intact endothelial layer and vascular wall morphology, respectively. However, in the case that one artery presented, for example, endothelial injury or vascular wall injury, it was counted as an injured vessel. All follicular subclasses were negative for Ki-67 (proliferation marker), which indicated no ongoing cell proliferation. Furthermore, cleaved caspase 3 (apoptosis marker) staining indicated that there was no ongoing apoptosis in any follicle type.
Group 2
The comparison between the ovarian cortex follicular population of the non-perfused control ovaries, after 3–4 h of cold ischaemia, and after 72–144 h of perfusion, showed that there was a significant reduction in the number of non-damaged primordial follicles per mm2 after cold ischaemia (median values; 4.05 vs 2.32, P = 0.039), and after perfusion (median values; 4.05 vs 0, P = 0.031). This was also noticed for the total number of secondary follicles per mm2 after ischaemia (median values; 0 vs 0.028, P = 0.047). Moreover, a significant increase was seen in the number of damaged, grade 2 (G2) primordial follicles per mm2 after perfusion (median values; 0.021 vs 3.83, P = 0.031), indicating a low follicular and oocyte stability during the perfusion time. However, the perfused ovaries showed intact histomorphology in 33% of the medullary arteries, in 40% of the ovarian arteries, in 29% of the medullary veins and in 20% of the ovarian veins. Moreover, 14% and 43% of the ovaries after perfusion had intact endothelial layer and vascular wall morphology, respectively. After perfusion, follicular cells positive for cleaved caspase 3 were seen in non-damaged primordial follicles. However, there was no significant difference in follicular cell apoptosis comparing non-perfused (control) ovaries with perfused ovaries. Immunohistochemistry staining showed that primary, secondary and antral follicles were negative for Ki-67 and cleaved caspase 3, both indicating no ongoing apoptosis.
Effect of perfusion on steroid production
After assessment of the perfusion medium with ELISA, no significant changes were seen in oestradiol and progesterone concentrations in group 1 during the perfusion time (Fig. 3a, b). Oestradiol concentrations in the perfusion medium in group 2 significantly decreased between 20 min and all the later time points (Fig. 3c), indicating an insufficient gonadotropin follicle stimulation. In contrast, the significantly increased progesterone concentration in this group after 20 min (Fig. 3d) indicated an ongoing luteinising process of the growing follicles.
|
|
Effect of perfusion on metabolic and electrolytic biomarkers
After assessment of the perfusion medium with blood-gas analysis, no significant changes were seen in the concentration of metabolic and electrolytic biomarkers in group 1 (Figs 4a, b and 5a–d). Lactate, potassium, calcium and chloride concentrations in the perfusion medium in group 2 significantly increased between the perfusion start and the later time points (Figs 4d and 5f–h). In contrast, the glucose concentration significantly decreased in this group after the start of perfusion (Fig. 4c). Both metabolic and electrolytic changes in group 2 indicate active metabolic processes.
|
|
|
|
Effect of perfusion on oocyte recovery rate and quality
From fourteen aspirated follicles in group 1, six metaphase I stage (MI) oocytes were retrieved (oocyte recovery rate 43%) and from 22 follicles of group 2, nine MI and one germinal vesicle stage (GV) oocyte were retrieved (oocyte recovery rate 45%) (Table 1). No metaphase II stage (MII) oocytes were retrieved from both groups. All retrieved oocytes had homogeneous cytoplasm and were partially covered by cumulus cells.
Discussion
In this study, we described an ex vivo perfusion device able to maintain whole sheep ovaries viable for up to 168 h (7 days). Two different gonadotropin stimulation protocols were assessed for ex vivo ovarian stimulation and the subsequent follicular development and histomorphology were evaluated. This study demonstrated successful harvesting of oocytes and to our knowledge, this is the longest ex vivo perfusion time (7 days) described to date for ewe ovaries. A number of studies have been performed on ovarian tissue perfusion from rodents (Koos et al. 1984; Sogn et al. 1984; Brännström et al. 1987; Brännström and Flaherty 1995), rabbit (Lambertsen et al. 1976; Hamada et al. 1977, 1979; Kobayashi et al. 1981a), sheep (Wallin et al. 2009; Milenkovic et al. 2011b; Maffei et al. 2016) and also from human tissue (Stähler et al. 1974; Abrahamsson et al. 1990; Milenkovic et al. 2011a; McLaughlin et al. 2018). However, a complete follicular development in vitro with subsequent oocyte maturation and live offspring has only been successful in rodent models (Eppig and Schroeder 1989; O’Brien et al. 2003). Progress in large animals, e.g. the sheep, has been limited due to the long time required for the in vitro follicular growth and oocyte maturation typical of large mammals (Shea et al. 2014). However, significant advances in organ reperfusion research using bioreactors and normothermic (37°C) conditions provide novel platform ideas on how to overcome these hurdles (Hosgood et al. 2018; Nasralla et al. 2018). In the study described herein, we used similar perfusion principles and found that a hMG-enriched medium used in a closed bioreactor system can maintain the general health of the ovary after explantation, and that the follicular sub-populations could be protected from apoptosis. Moreover, no significant damage was observed in the sub-follicular morphology (the theca and granulosa cells), or in oocyte integrity compared with control ovaries. Interestingly, the primordial follicles were particularly stable in group 1 where ovaries were stimulated with hMG administered in a single daily dose, while a significant depletion of the non-damaged primordial and total secondary follicular density, with concomitant increase in G2 damaged primordial follicular density was seen in group 2. Perhaps, this difference was due to the modifications made in the pulsatile hormone dosage, since a more gradual hormone administration (as used in group 1) may ensure a better protection of the immature follicles pool. Furthermore, no differences in follicular apoptosis were seen after perfusion in any of the follicular subclasses in group 1. However, the relatively intact histomorphology of both the arterial and venous ovarian pedicles in both groups after the extended ovarian perfusion, together with the good cellular survival and minimal tissue damage is a significant achievement considering the long perfusion times explored. Our results are consistent with, and expand on, the previous short-time ovarian perfusion studies: 2 h of perfusion in ewes (Wallin et al. 2009), 5 h in human (Abrahamsson et al. 1990; Milenkovic et al. 2011a), 12 h in rabbit (Lambertsen et al. 1976; Hamada et al. 1977, 1979; Kobayashi et al. 1981a) and 20 h in rat and mouse (Koos et al. 1984; Sogn et al. 1984; Brännström and Flaherty 1995). Our results support the findings of another study that used a complex perfusion medium supplemented with insulin, IGF-I and FSH that resulted in a successful 96-h long perfusion time (Maffei et al. 2016). Perhaps it could be beneficial to supplement ovarian perfusion protocols with growth factors and hMG. An unexpected finding of our study was the observation that, despite the long perfusion time, there was no cell damage and apoptosis of follicular subclasses as shown by the absence of cell proliferation and apoptosis markers (Ki-67 and cleaved caspase 3, respectively). These results may be explained by the low density of higher follicular classes found in the perfused ovaries of both groups, and/or that the gonadotropin insensitivity of the primordial follicles prevented the development of early-stage follicles. This normal hormone insensitivity in small follicles is caused by a low expression of hormone receptors and, in future studies, alternative growth stimulants should be included in the perfusate to better promote primordial follicle development. Correspondingly, oestradiol concentrations in group 1 did not significantly increase during the perfusion, suggesting the need for protocol optimisation (higher gonadotropin levels and addition of growth stimulants) in the perfusate to initiate the primordial follicle growth and to support granulosa and theca cells. Interestingly, the oestradiol concentrations significantly decreased during the perfusion in group 2, suggesting that a continuous (24 h daily) gonadotropin administration is suboptimal. However, another plausible explanation for the low oestradiol concentrations could be related to the repeated perfusate changes, every 24 h, that could have diluted the oestradiol concentrations measured in both groups. These perfusate changes were necessary to prevent accumulation of toxic levels of lactate and other metabolic byproducts associated with a high ovarian metabolic rate and the absence of a systemic clearance of these byproducts in vitro/ex vivo. The progesterone levels significantly increased during perfusion in group 2, but to a lesser extent in group 1. The elevation of progesterone levels in group 2 were likely associated with the high and non-pulsatile hCG supplementation in the perfusate that probably supported early luteinisation of the higher-class follicles. Previous ovarian perfusion studies performed on different models have also shown steroid hormonal secretion in the perfusion medium. For example, the addition of forskolin stimulated an increase of oestradiol and progesterone levels in the perfusate after 2 h in ewe ovary perfusion (Wallin et al. 2009). Therefore, the addition of forskolin to the perfusate in future experiments could be beneficial using our ex vivo ovarian perfusion platform. Steroid hormones were also produced from perfused human postmenopausal ovaries in response to hCG during a 5-h perfusion period (Abrahamsson et al. 1990; Milenkovic et al. 2011a). Previous ovarian perfusion studies with rabbit, rodents and human models used shorter perfusion times than what is biologically required to retrieve fully mature oocytes (Stähler et al. 1974; Lambertsen et al. 1976; Hamada et al. 1977, 1979; Wallach et al. 1978; Kobayashi et al. 1981b; Koos et al. 1984; Sogn et al. 1984; Brännström et al. 1987; Sogn et al. 1987; Brännström and Flaherty 1995). In a recent study, an extended ex vivo whole ewe ovary perfusion was achieved, although no ovum pick-up was performed (Maffei et al. 2016). To our knowledge, our study is the first to report oocyte retrieval after extended ewe ovarian perfusion. A total of 16 oocytes (1 GV and 15 MI) were retrieved from groups 1 and 2 after hMG ovarian stimulation. The oocyte recovery rates (43–45%) were comparable with a previous study where oocyte retrieval was performed post-mortem, using similar ovum pick-up parameters as presented herein (Rodriguez et al. 2006). Despite the absence of MII oocytes, the retrieval of 15 MI oocytes that could be maturated in vitro (IVM) is an encouraging result. It is known that oocyte immaturity (GV/MI), in sheep is expected because the process of in vitro embryo production is still inefficient (approximately 70–90% of immature oocytes undergo maturation). Thus, the use of IVM of retrieved immature oocytes is considered necessary before any in vitro fertilisation (IVF) should be performed (Zhu et al. 2018).
Conclusion
Our study sought to find a novel method for enhancing the options of fertility preservation for prepubertal girls prior to chemo/radiotherapy or for women with cancer at high risk of ovarian metastasis. The strengths of this study are the extended perfusion times achieved herein (up to 7 days) and that a total of 16 GV–MI oocytes were retrieved from both groups. Long perfusion times will be necessary to give sufficient time for ex vivo follicle development and oocyte harvesting. The addition of piperacillin/tazobactam in the perfusate presented herein resulted in extended perfusion times without contamination. Additionally, we assessed all follicular sub-classes separately using cell proliferation and apoptosis indexes to evaluate potential negative influences caused by the perfusion. A limitation to our study includes the collection of ovaries from ewes of unknown cycle and reproductive history. However, organs collected from the slaughterhouse provided a large number of ovaries without the need for costly large animal experiments and this approach does not require any ethical permission within the European Union since the animals are bred and culled for food production. Another limitation is that IVM was not performed to assess the viability of the retrieved oocytes. However, the experimental platform presented herein will serve as an excellent whole ovary perfusion model to conduct future experiments that could elucidate what additional factors may be beneficial to stimulate ex vivo follicular maturation. In clinical settings, such a platform might be used for fertility preservation by promoting in vitro follicular growth and harvesting oocytes. Once perfected, this option could be very beneficial for prepubertal girls of for patients with systemic cancer that have high risk of ovarian metastasis thus precluding ovarian tissue freezing for future regrafting. Future studies should assess the effect on follicular growth, viability and oocyte maturation of higher doses of gonadotropins, and other growth stimulants, administered in the perfusate, in combination with more refined ovum pick up parameters.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was supported by grants from the Hjalmar Svensson’s Research Foundation, Sahlgrenska Academy (Grant number SU-791201) and from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (Grant number ALFGBG-872611).
Author contributions
Conceptualisation: RA, MH, PP; methodology: RA, PT, MD, DB, MH, FG, CM, MM; formal analysis and investigation: PT, AMP; writing – original draft preparation: PT; writing – review and editing: all authors contributed; funding acquisition: RA; resources: RA, MH.
References
Abrahamsson, G, Janson, PO, and Kullander, S (1990). An in vitro perfusion method for metabolic studies on human postmenopausal ovaries. Acta Obstetricia et Gynecologica Scandinavica 69, 527–532.| An in vitro perfusion method for metabolic studies on human postmenopausal ovaries.Crossref | GoogleScholarGoogle Scholar | 2178300PubMed |
Baby, TE, and Bartlewski, PM (2011). Circulating concentrations of ovarian steroids and follicle-stimulating hormone (FSH) in ewes with 3 or 4 waves of antral follicle emergence per estrous cycle. Reproductive Biology 11, 19–36.
| Circulating concentrations of ovarian steroids and follicle-stimulating hormone (FSH) in ewes with 3 or 4 waves of antral follicle emergence per estrous cycle.Crossref | GoogleScholarGoogle Scholar | 21455278PubMed |
Benedict, C, Thom, B, Friedman, DN, Pottenger, E, Raghunathan, N, and Kelvin, JF (2018). Fertility information needs and concerns post-treatment contribute to lowered quality of life among young adult female cancer survivors. Supportive Care in Cancer 26, 2209–2215.
| Fertility information needs and concerns post-treatment contribute to lowered quality of life among young adult female cancer survivors.Crossref | GoogleScholarGoogle Scholar | 29387996PubMed |
Brännström, M, and Flaherty, S (1995). Methodology and characterization of an in vitro perfusion model for the mouse ovary. Journal of Reproduction and Fertility 105, 177–183.
| Methodology and characterization of an in vitro perfusion model for the mouse ovary.Crossref | GoogleScholarGoogle Scholar | 8568758PubMed |
Brännström, M, Johansson, BM, Sogn, J, and Janson, PO (1987). Characterization of an in vitro perfused rat ovary model: ovulation rate, oocyte maturation, steroidogenesis and influence of PMSG priming. Acta Physiologica Scandinavica 130, 107–114.
| Characterization of an in vitro perfused rat ovary model: ovulation rate, oocyte maturation, steroidogenesis and influence of PMSG priming.Crossref | GoogleScholarGoogle Scholar | 3591383PubMed |
Bromer, JG, and Patrizio, P (2008). Preservation and postponement of female fertility. Placenta 29, 200–205.
| Preservation and postponement of female fertility.Crossref | GoogleScholarGoogle Scholar | 18708254PubMed |
Campbell, BK, Kendall, NR, and Baird, DT (2007). The effect of the presence and pattern of luteinizing hormone stimulation on ovulatory follicle development in sheep1. Biology of Reproduction 76, 719–727.
| The effect of the presence and pattern of luteinizing hormone stimulation on ovulatory follicle development in sheep1.Crossref | GoogleScholarGoogle Scholar | 17167168PubMed |
Chiti, MC, Dolmans, M-M, Hobeika, M, Cernogoraz, A, Donnez, J, and Amorim, CA (2017). A modified and tailored human follicle isolation procedure improves follicle recovery and survival. Journal of Ovarian Research 10, 71.
| A modified and tailored human follicle isolation procedure improves follicle recovery and survival.Crossref | GoogleScholarGoogle Scholar | 29061149PubMed |
Diaz-Garcia, C, Domingo, J, Garcia-Velasco, JA, Herraiz, S, Mirabet, V, Iniesta, I, Cobo, A, Remohi, J, and Pellicer, A (2018). Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertility and Sterility 109, 478–485.e2.
| Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 29428307PubMed |
Dolmans, M-M, Marinescu, C, Saussoy, P, Van Langendonckt, A, Amorim, C, and Donnez, J (2010). Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 116, 2908–2914.
| Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe.Crossref | GoogleScholarGoogle Scholar | 20595517PubMed |
Dolmans, M-M, Luyckx, V, Donnez, J, Andersen, CY, and Greve, T (2013). Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertility and Sterility 99, 1514–1522.
| Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue.Crossref | GoogleScholarGoogle Scholar | 23541406PubMed |
Donnez, J (2013). Introduction: fertility preservation, from cancer to benign disease to social reasons: the challenge of the present decade. Fertility and Sterility 99, 1467–1468.
| Introduction: fertility preservation, from cancer to benign disease to social reasons: the challenge of the present decade.Crossref | GoogleScholarGoogle Scholar | 23635347PubMed |
Donnez, J, and Dolmans, M-M (2013). Fertility preservation in women. Nature Reviews Endocrinology 9, 735–749.
| Fertility preservation in women.Crossref | GoogleScholarGoogle Scholar | 24166000PubMed |
Donnez, J, and Dolmans, MM (2017). Fertility preservation in women. New England Journal of Medicine 377, 1657–1665.
| Fertility preservation in women.Crossref | GoogleScholarGoogle Scholar |
Donnez, J, Martinez-Madrid, B, Jadoul, P, Van Langendonckt, A, Demylle, D, and Dolmans, M-M (2006). Ovarian tissue cryopreservation and transplantation: a review. Human Reproduction Update 12, 519–535.
| Ovarian tissue cryopreservation and transplantation: a review.Crossref | GoogleScholarGoogle Scholar | 16849817PubMed |
Donnez, J, Jadoul, P, Squifflet, J, Van Langendonckt, A, Donnez, O, Van Eyck, A-S, Marinescu, C, and Dolmans, M-M (2010). Ovarian tissue cryopreservation and transplantation in cancer patients. Best Practice & Research Clinical Obstetrics & Gynaecology 24, 87–100.
| Ovarian tissue cryopreservation and transplantation in cancer patients.Crossref | GoogleScholarGoogle Scholar |
Edson, MA, Nagaraja, AK, and Matzuk, MM (2009). The mammalian ovary from genesis to revelation. Endocrine Reviews 30, 624–712.
| The mammalian ovary from genesis to revelation.Crossref | GoogleScholarGoogle Scholar | 19776209PubMed |
Eppig, JJ, and Schroeder, AC (1989). Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biology of Reproduction 41, 268–276.
| Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro.Crossref | GoogleScholarGoogle Scholar | 2508774PubMed |
Figueiredo, JR, Rodrigues, APR, Silva, JRV, and Santos, RR (2011). Cryopreservation and in vitro culture of caprine preantral follicles. Reproduction, Fertility and Development 23, 40–47.
| Cryopreservation and in vitro culture of caprine preantral follicles.Crossref | GoogleScholarGoogle Scholar |
Gandolfi, F, Paffoni, A, Papasso Brambilla, E, Bonetti, S, Brevini, TA, and Ragni, G (2006). Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertility and Sterility 85, 1150–1156.
| Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models.Crossref | GoogleScholarGoogle Scholar | 16616087PubMed |
Hamada, Y, Bronson, RA, Wright, KH, and Wallach, EF (1977). Ovulation in the perfused rabbit ovary: the influence of prostaglandins and prostaglandin inhibitors. Biology of Reproduction 17, 58–63.
| Ovulation in the perfused rabbit ovary: the influence of prostaglandins and prostaglandin inhibitors.Crossref | GoogleScholarGoogle Scholar | 884187PubMed |
Hamada, Y, Wright, KH, and Wallach, EE (1979). The effects of progesterone and human chorionic gonadotropin on ovulation in the in vitro perfused rabbit ovary. Fertility and Sterility 32, 335–339.
| The effects of progesterone and human chorionic gonadotropin on ovulation in the in vitro perfused rabbit ovary.Crossref | GoogleScholarGoogle Scholar | 573708PubMed |
Hosgood, SA, Thompson, E, Moore, T, Wilson, CH, and Nicholson, ML (2018). Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. British Journal of Surgery 105, 388–394.
| Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors.Crossref | GoogleScholarGoogle Scholar |
Kobayashi, Y, Santulli, R, Wright, KH, and Wallach, EE (1981a). Fertilizability of ova ovulated and recovered from rabbit ovaries perfused in vitro. Science 213, 1127–1128.
| Fertilizability of ova ovulated and recovered from rabbit ovaries perfused in vitro.Crossref | GoogleScholarGoogle Scholar | 7268420PubMed |
Kobayashi, Y, Wright, KH, Santulli, R, and Wallach, EE (1981b). Ovulation and ovum maturation in the rabbit ovary perfused in vitro. Biology of Reproduction 24, 483–490.
| Ovulation and ovum maturation in the rabbit ovary perfused in vitro.Crossref | GoogleScholarGoogle Scholar | 7236814PubMed |
Koos, RD, Jaccarino, FJ, Magaril, RA, and Le Maire, WJ (1984). Perfusion of the rat ovary in vitro: methodology, induction of ovulation, and pattern of steroidogenesis. Biology of Reproduction 30, 1135–1141.
| Perfusion of the rat ovary in vitro: methodology, induction of ovulation, and pattern of steroidogenesis.Crossref | GoogleScholarGoogle Scholar | 6733206PubMed |
Lambertsen, CJ, Greenbaum, DF, Wright, KH, and Wallach, EE (1976). In vitro studies of ovulation in the perfused rabbit ovary. Fertility and Sterility 27, 178–187.
| In vitro studies of ovulation in the perfused rabbit ovary.Crossref | GoogleScholarGoogle Scholar | 1248664PubMed |
Laronda, MM, Rutz, AL, Xiao, S, Whelan, KA, Duncan, FE, Roth, EW, Woodruff, TK, and Shah, RN (2017). A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nature Communications 8, 15261.
| A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice.Crossref | GoogleScholarGoogle Scholar | 28509899PubMed |
Letourneau, JM, Ebbel, EE, Katz, PP, Katz, A, Ai, WZ, Chien, AJ, Melisko, ME, Cedars, MI, and Rosen, MP (2012). Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 118, 1710–1717.
| Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer.Crossref | GoogleScholarGoogle Scholar | 21887678PubMed |
Maffei, S, Galeati, G, Pennarossa, G, Brevini, TAL, and Gandolfi, F (2016). Extended ex vivo culture of fresh and cryopreserved whole sheep ovaries. Reproduction, Fertility and Development 28, 1893–1903.
| Extended ex vivo culture of fresh and cryopreserved whole sheep ovaries.Crossref | GoogleScholarGoogle Scholar |
McLaughlin, M, Albertini, DF, Wallace, WHB, Anderson, RA, and Telfer, EE (2018). Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Molecular Human Reproduction 24, 135–142.
| Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system.Crossref | GoogleScholarGoogle Scholar | 29390119PubMed |
Milenkovic, M, Gharemani, M, Bergh, A, Wallin, A, Mölne, J, Fazlagic, E, Eliassen, E, Kahn, J, and Brännström, M (2011a). The human postmenopausal ovary as a tool for evaluation of cryopreservation protocols towards whole ovary cryopreservation. Journal of Assisted Reproduction and Genetics 28, 453–460.
| The human postmenopausal ovary as a tool for evaluation of cryopreservation protocols towards whole ovary cryopreservation.Crossref | GoogleScholarGoogle Scholar | 21350836PubMed |
Milenkovic, M, Wallin, A, Ghahremani, M, and Brännström, M (2011b). Whole sheep ovary cryopreservation: evaluation of a slow freezing protocol with dimethylsulphoxide. Journal of Assisted Reproduction and Genetics 28, 7–14.
| Whole sheep ovary cryopreservation: evaluation of a slow freezing protocol with dimethylsulphoxide.Crossref | GoogleScholarGoogle Scholar | 20842419PubMed |
Moravek, MB, Confino, R, Smith, KN, Kazer, RR, Klock, SC, Lawson, AK, Gradishar, WJ, and Pavone, ME (2018). Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertility and Sterility 109, 349–355.
| Long-term outcomes in cancer patients who did or did not pursue fertility preservation.Crossref | GoogleScholarGoogle Scholar | 29338854PubMed |
Nasralla, D, Coussios, CC, Mergental, H, Akhtar, MZ, Butler, AJ, Ceresa, CDL, Chiocchia, V, Dutton, SJ, Garcia-Valdecasas, JC, Heaton, N, Imber, C, Jassem, W, Jochmans, I, Karani, J, Knight, SR, Kocabayoglu, P, Malago, M, Mirza, D, Morris, PJ, Pallan, A, Paul, A, Pavel, M, Perera, M, Pirenne, J, Ravikumar, R, Russell, L, Upponi, S, Watson, CJE, Weissenbacher, A, Ploeg, RJ, and Friend, PJ Nasralla, D, Coussios, CC, Mergental, H, Akhtar, MZ, Butler, AJ, Ceresa, CDL, Chiocchia, V, Dutton, SJ, Garcia-Valdecasas, JC, Heaton, N, Imber, C, Jassem, W, Jochmans, I, Karani, J, Knight, SR, Kocabayoglu, P, Malago, M, Mirza, D, Morris, PJ, Pallan, A, Paul, A, Pavel, M, Perera, M, Pirenne, J, Ravikumar, R, Russell, L, Upponi, S, Watson, CJE, Weissenbacher, A, Ploeg, RJ, and Friend, PJ (2018). A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56.
| A randomized trial of normothermic preservation in liver transplantation.Crossref | GoogleScholarGoogle Scholar | 29670285PubMed |
O’Brien, MJ, Pendola, JK, and Eppig, JJ (2003). A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biology of Reproduction 68, 1682–1686.
| A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence.Crossref | GoogleScholarGoogle Scholar | 12606400PubMed |
Padma, AM, Truong, M, Jar-Allah, T, Song, MJ, Oltean, M, Brännström, M, and Hellström, M (2019). The development of an extended normothermic ex vivo reperfusion model of the sheep uterus to evaluate organ quality after cold ischemia in relation to uterus transplantation. Acta Obstetricia et Gynecologica Scandinavica 98, 1127–1138.
| The development of an extended normothermic ex vivo reperfusion model of the sheep uterus to evaluate organ quality after cold ischemia in relation to uterus transplantation.Crossref | GoogleScholarGoogle Scholar | 30932168PubMed |
Paynter, SJ, Cooper, A, Fuller, BJ, and Shaw, RW (1999). Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology 38, 301–309.
| Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro.Crossref | GoogleScholarGoogle Scholar | 10413573PubMed |
Picton, HM, Harris, SE, Muruvi, W, and Chambers, EL (2008). The in vitro growth and maturation of follicles. Reproduction 136, 703–715.
| The in vitro growth and maturation of follicles.Crossref | GoogleScholarGoogle Scholar | 19074213PubMed |
Rodriguez, C, Anel, L, Alvarez, M, Anel, E, Boixo, JC, Chamorro, CA, and de Paz, P (2006). Ovum pick-up in sheep: a comparison between different aspiration devices for optimal oocyte retrieval. Reproduction in Domestic Animals 41, 106–113.
| Ovum pick-up in sheep: a comparison between different aspiration devices for optimal oocyte retrieval.Crossref | GoogleScholarGoogle Scholar | 16519714PubMed |
Shapira, M, Raanani, H, Barshack, I, Amariglio, N, Derech-Haim, S, Marciano, MN, Schiff, E, Orvieto, R, and Meirow, D (2018). First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertility and Sterility 109, 48–53.
| First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination.Crossref | GoogleScholarGoogle Scholar | 29198847PubMed |
Shea, LD, Woodruff, TK, and Shikanov, A (2014). Bioengineering the ovarian follicle microenvironment. Annual Review of Biomedical Engineering 16, 29–52.
| Bioengineering the ovarian follicle microenvironment.Crossref | GoogleScholarGoogle Scholar | 24849592PubMed |
Sogn, J, Abrahamsson, G, and Janson, PO (1984). Release of cyclic AMP and progesterone from the isolated perfused luteal ovary of the PMSG treated rat. Acta Endocrinologica 106, 265–270.
| Release of cyclic AMP and progesterone from the isolated perfused luteal ovary of the PMSG treated rat.Crossref | GoogleScholarGoogle Scholar | 6328825PubMed |
Sogn, JH, Curry, TE, Brännström, M, Lemaire, WJ, Koos, RD, Papkoff, H, and Janson, PO (1987). Inhibition of follicle-stimulating hormone-induced ovulation by indomethacin in the perfused rat ovary. Biology of Reproduction 36, 536–542.
| Inhibition of follicle-stimulating hormone-induced ovulation by indomethacin in the perfused rat ovary.Crossref | GoogleScholarGoogle Scholar | 3109504PubMed |
Songsasen, N, Comizzoli, P, Nagashima, J, Fujihara, M, and Wildt, DE (2012). The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reproduction in Domestic Animals 47, 13–18.
| The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro.Crossref | GoogleScholarGoogle Scholar | 23279457PubMed |
Stähler, E, Spätling, L, Bethge, HD, Daume, E, and Buchholz, R (1974). Induction of ovulation in human ovaries perfused in vitro. Archiv für Gynäkologie 217, 1–15.
| Induction of ovulation in human ovaries perfused in vitro.Crossref | GoogleScholarGoogle Scholar | 4479142PubMed |
Telfer, EE, and Zelinski, MB (2013). Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertility and Sterility 99, 1523–1533.
| Ovarian follicle culture: advances and challenges for human and nonhuman primates.Crossref | GoogleScholarGoogle Scholar | 23635350PubMed |
Vanacker, J, Dolmans, M-M, Luyckx, V, Donnez, J, and Amorim, CA (2014). First transplantation of isolated murine follicles in alginate. Regenerative Medicine 9, 609–619.
| First transplantation of isolated murine follicles in alginate.Crossref | GoogleScholarGoogle Scholar | 25372078PubMed |
Wallace, WHB, Anderson, RA, and Irvine, DS (2005). Fertility preservation for young patients with cancer: who is at risk and what can be offered? The Lancet Oncology 6, 209–218.
| Fertility preservation for young patients with cancer: who is at risk and what can be offered?Crossref | GoogleScholarGoogle Scholar |
Wallach, EE, Wright, KH, and Hamada, Y (1978). Investigation of mammalian ovulation with an in vitro perfused rabbit ovary preparation. American Journal of Obstetrics and Gynecology 132, 728–738.
| Investigation of mammalian ovulation with an in vitro perfused rabbit ovary preparation.Crossref | GoogleScholarGoogle Scholar | 102198PubMed |
Wallin, A, Ghahremani, M, Dahm-Kahler, P, and Brannstrom, M (2009). Viability and function of the cryopreserved whole ovary: in vitro studies in the sheep. Human Reproduction (Oxford, England) 24, 1684–1694.
| Viability and function of the cryopreserved whole ovary: in vitro studies in the sheep.Crossref | GoogleScholarGoogle Scholar |
Zhu, J, Moawad, AR, Wang, C-Y, Li, H-F, Ren, J-Y, and Dai, Y-F (2018). Advances in in vitro production of sheep embryos. International Journal of Veterinary Science and Medicine 6, S15–S26.
| Advances in in vitro production of sheep embryos.Crossref | GoogleScholarGoogle Scholar | 30761316PubMed |


