The ovarian follicle of ruminants: the path from conceptus to adult
Jennifer L. Juengel A J , Robert A. Cushman B , Joëlle Dupont C , Stéphane Fabre
A J , Robert A. Cushman B , Joëlle Dupont C , Stéphane Fabre  D , Richard G. Lea
D , Richard G. Lea  E , Graeme B. Martin F , Francesca Mossa G , Janet L. Pitman H , Christopher A. Price I and Peter Smith A
E , Graeme B. Martin F , Francesca Mossa G , Janet L. Pitman H , Christopher A. Price I and Peter Smith A
A AgResearch Ltd, Invermay Agricultural Centre, Mosgiel, New Zealand.
B Livestock Biosystems Research Unit, US Department of Agriculture, Agricultural Research Service, US Meat Animal Research Center, Clay Center, NE, USA.*
C INRAE Institute UMR85 Physiologie de la Reproduction et des Comportements, Tours University, France.
D GenPhySE, Université de Toulouse, Institut national de recherche pour l’agriculture, l’alimentation et l’environnement, Institut national polytechnique de Toulouse, Ecole nationale vétérinaire de Toulouse, Castanet Tolosan, France.
E School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, UK.
F UWA Institute of Agriculture, University of Western Australia, Perth, WA, Australia.
G Dipartimento di Medicina Veterinaria, Università degli Studi di Sassari, Italy.
H School of Biological Sciences, Victoria University of Wellington, New Zealand.
I Faculty of Veterinary Medicine, Université de Montréal, Montréal, QC, Canada.
J Corresponding author.# Email: jenny.juengel@agresearch.co.nz
Reproduction, Fertility and Development 33(10) 621-642 https://doi.org/10.1071/RD21086
Submitted: 22 March 2021 Accepted: 6 June 2021 Published: 2 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
This review resulted from an international workshop and presents a consensus view of critical advances over the past decade in our understanding of follicle function in ruminants. The major concepts covered include: (1) the value of major genes; (2) the dynamics of fetal ovarian development and its sensitivity to nutritional and environmental influences; (3) the concept of an ovarian follicle reserve, aligned with the rise of anti-Müllerian hormone as a controller of ovarian processes; (4) renewed recognition of the diverse and important roles of theca cells; (5) the importance of follicular fluid as a microenvironment that determines oocyte quality; (6) the ‘adipokinome’ as a key concept linking metabolic inputs with follicle development; and (7) the contribution of follicle development to the success of conception. These concepts are important because, in sheep and cattle, ovulation rate is tightly regulated and, as the primary determinant of litter size, it is a major component of reproductive efficiency and therefore productivity. Nowadays, reproductive efficiency is also a target for improving the ‘methane efficiency’ of livestock enterprises, increasing the need to understand the processes of ovarian development and folliculogenesis, while avoiding detrimental trade-offs as greater performance is sought.
Keywords: fetal programming, genetics, granulosa cell, nutrition, oocyte, theca cell.
Dedication: David T. Armstrong
A theme of this series of workshops, and thus the reviews, is an appreciation of the intricate interactions among cell types within the ovary, as well as between the ovary and other tissues. The fine-tuning of follicle development by adipose tissue has become accepted, and there is a growing recognition that, within the follicle wall, the well-established interactions between theca and granulosa cells are accompanied by functional relationships with other follicular cells, including endothelial and immune system cells. The ‘two cell theory’ of follicle development has thus expanded into a ‘multicell hypothesis’ since the original ‘two gonadotrophin, two cell theory’ was pioneered by Dr David T. Armstrong in the 1960s. In his publications in Nature (Armstrong 1967) and in Endocrinology (Armstrong and Papkoff 1976), he identified granulosa and theca cells as targets of FSH and LH, respectively. He followed this up in the 1970s with numerous studies demonstrating that both gonadotrophins and both cell types were absolutely required for follicular oestradiol secretion (International Embryo Technology Society 2018). This body of work essentially defined our understanding of follicle development. Armstrong was so excited about the two cell theory that he was known to pull over to the side of the road and stop the car to explain it to students! Remarkably, this was not his only ground-breaking contribution: in the 1950s, while at Cornell University with William Hansel, he was the first to describe the luteolytic action of oxytocin (Armstrong and Hansel 1959) and, later, in the 1970s, his laboratory discovered the role that prostaglandins play in ovulation (Armstrong and Grinwich 1972). These discoveries, and his purification of FSH, paved the way to many practical applications in assisted reproduction, including oestrus synchronisation and superovulation in farm animals.
Although a scientific giant, Armstrong was quite unpretentious and insisted that everyone call him ‘Dave’. Dave’s home base was in his native Canada at the University of Western Ontario (now named Western University) and, in the late 1970s, he took up a post as visiting professor at the University of Adelaide, Australia. His main hobby was sailing, either on Lake Huron, Ontario, or around Hindmarsh Island, South Australia. He was well known to spend northern summers in Canada and northern winters in Australia, the ultimate snowbird practising a ‘two country, two summer’ theory.
Dave’s illustrious career led him to the Presidency of the Society for the Study of Reproduction (SSR) and of the International Embryo Transfer Society (now the International Embryo Technology Society), and to numerous awards and recognitions, including the SSR’s Carl Hartman Award and Fellowship of the Royal Society of Canada. Although Dave retired in 2012 and passed away in 2016, the mark that he left on our field lives on.
Introduction
In ruminants, the number of oocytes released at ovulation (ovulation rate) is tightly regulated, seldom exceeds three and is the outcome of complex processes of follicle development and differentiation. These processes are of major interest in farm animals because they determine litter size, a major component of reproductive efficiency and thus productivity. Litter size has always been important but, these days, it has also become a focus in our plans to reduce the environmental impact of ruminant industries because fecundity and fertility are determinants of the greenhouse gas emissions efficiency of a livestock enterprise.
For investigating folliculogenesis, the ewe has proven to be an excellent experimental model because its ovulation rate is affected by the classical gene × environment interaction. Environmental factors, especially photoperiod and nutrition, have been researched for over 100 years but, in recent decades, our understanding of the genetic component of the phenotype has been reinvigorated by the discoveries of critical major genes. The roles of these genes have transformed our understanding of the molecular and cellular processes that control follicle development and function. The cow has also been useful because the processes leading to ovulation in this species are even more tightly controlled, so multiple ovulations are rare. In other words, the differences between the ovine and bovine models have been informative, if perhaps a little frustrating for those interested in reproductive efficiency in cattle. Moreover, as well as being a determinant of litter size, the processes controlling follicle development and differentiation are critical for an animal’s ability to conceive. Considering these issues, it is no surprise that improving our understanding of the process of folliculogenesis is still seen as essential for all ruminant-based industries worldwide.
Progress in this field over the past 30 years has been marked by international workshops that produced a series of consensus views of the state of knowledge. The first was held in 1991 in Australia and focused mostly on the mechanisms in sheep that caused variation in ovulation rate with breed and with environmental or experimental conditions (Scaramuzzi et al. 1993). The second workshop, held in 2008 in France, updated the scientific consensus and extended the scope to include bovine folliculogenesis. Among the major leaps since the 1993 publication was a revolution in our understanding of the role of the oocyte: it had been transformed from passive passenger into an active controller of its own destiny. We also started to understand the development of ovarian follicles in the fetus, as well as how metabolic factors directly affect the processes leading to ovulation (Scaramuzzi et al. 2011).
The third workshop, the foundation of the present paper, was held in 2020 in New Zealand and was timely, in terms of both scientific advances and global industry context. With respect to the science, another wave of technical advances has led to profound improvements in our understanding of the cellular and molecular processes that underpin follicle development, in particular: (1) the dynamics of development in the fetal ovary (represented as a black box in the 1993 paper!), with inevitable links to environmental influences and the concept of ‘programming’; (2) new perspectives of the value of the major genes; (3) the concept of the ‘ovarian follicle reserve’, aligned with the rise of anti-Müllerian hormone (AMH) as a player in ovarian function and in diagnosis of dysfunction; (4) the resurrection of the theca and its various cell types as important participants in follicular processes; (5) recognition of normal antral fluid as a regulatory environment for the oocyte; and (6) the arrival of the ‘adipokinome’ as a major metabolic input. The 2020 workshop covered these issues and the consensus of those discussions forms the body of this review.
The period 2010–20 has also seen a marked upheaval in societal attitudes to ruminant production systems. Specifically, issues such as climate change, pollution, animal welfare, food safety and the role of animal products in the human diet came to the fore. The view that ruminant industries cause rather than solve problems was predominant in 2010, but it subsequently became clear that we needed to consider all options to ensure human food security. To this conversation, ruminants bring their ‘superpower’: they can digest grass to produce human food (Eisler et al. 2014). Nevertheless, considering this contextual change, we need to develop new visions and goals for our research programs in reproduction. For example, a deeper understanding of metabolic inputs into folliculogenesis is fundamental for ‘clean, green and ethical’ management systems, and ovulation rate now needs to be considered as an avenue for improving the ‘methane efficiency’ of cattle, sheep and goat enterprises (Martin et al. 2009).
Over the three decades between the first and present reviews, there has been a changing of the guard – all except one of the original workshop participants has left the field, although Rex Scaramuzzi instigated the 2020 workshop and contributed to planning the program. The authors of the present review take pleasure in acknowledging the contributions of our predecessors and were pleased to retain a similar scope for the workshop. In particular, the mechanisms at various levels of analysis, from molecular and cellular to whole organism, were considered as we readdressed the functional model of folliculogenesis and the control of ovulation rate, and suggested primary foci for future research.
This paper therefore highlights key advances in our understanding of ovarian function over the past decade. Discoveries of new genes controlling ovulation rate continue to provide new insights into factors controlling follicle development and remain a driver of our changing perspectives in the field. Integrating findings from multiple perspectives has highlighted a potential central role of AMH in ovarian follicle development, and thereby fertility. This review embraces the entire process, from the very beginning (i.e. the formation of primordial follicles during fetal ovary development) through to the mechanisms that underpin the growth and selection of a healthy ovulatory follicle(s) during adult life.
Genetic determination of ovarian function
Studies on the genetic determination of ovulation rate and litter size in sheep have led to very important discoveries about the control of folliculogenesis. For reference, the broad overview of the process from Scaramuzzi et al. (2011) is presented in Fig. 1. In addition, understanding of the concept of ovarian follicular waves and hierarchical follicle development has also added to our understanding of the dynamics of follicle development and its role in the control of reproductive cycles. However, these topics are outside of the scope of this review and so, for further information on them, readers are directed to McNatty et al. (2010), Bartlewski et al. (2011), Forde et al. (2011) and García-Guerra et al. (2018c). Although prolificacy is a lowly heritable trait (h2 = 0.1–0.2; Van Vleck et al. 1991; Árnason and Jónmundsson 2008; Borg et al. 2009), and determined mainly by polygenic processes, numerous major mutations play critical roles. In cattle, genetic mapping studies of animals selected for multiple ovulation or twinning have revealed quantitative trait loci on chromosome 5 (Allan et al. 2009) and identified the insulin-like growth factor (IGF) pathway as a potential regulator of multiple ovulations (Echternkamp et al. 1990, 2004; Aad et al. 2013). Further information on the role of the IGF pathway in follicle development can be found in reviews by Webb and Campbell (2007) and Shimizu (2016). In sheep, there has been particular interest in three major fecundity (Fec) genes, namely bone morphogenetic protein receptor type 1B (BMPR1B), bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9), that control the bone morphogenetic protein (BMP) signalling pathway and have major effects on ovulation rate (for a review, see Fabre et al. 2006; Juengel et al. 2013).
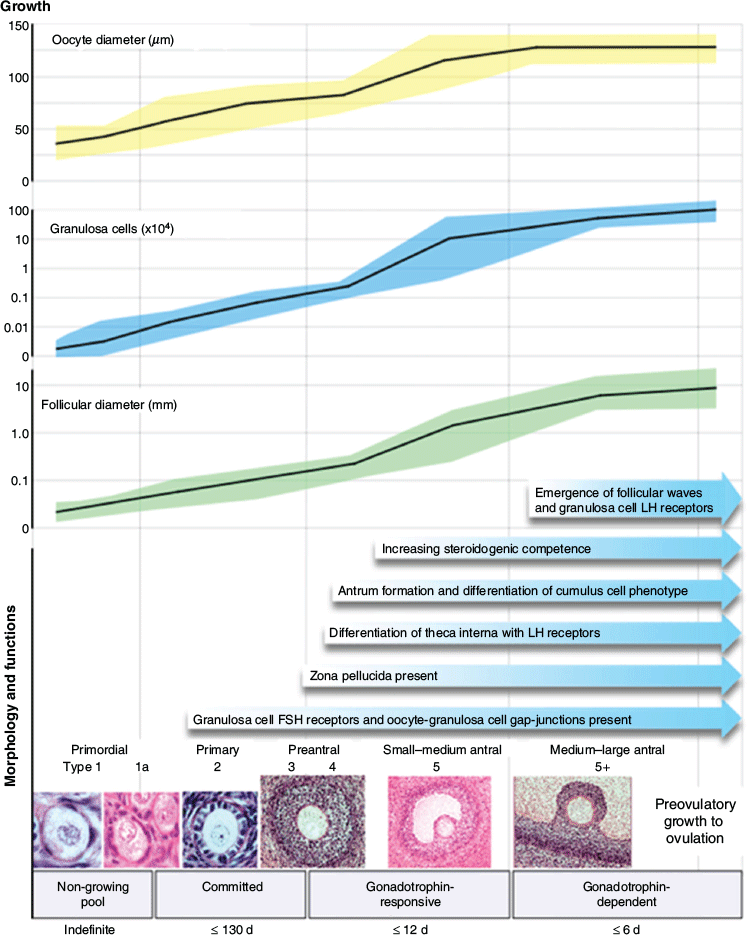
|
Recently, a new major mutation, FecL, has been added to the list of those associated with high ovulation rate and hyperprolificacy. Most importantly, as a mutation of beta-1,4-N-acetyl-galactosaminyltransferase 2 (B4GALNT2), it has provoked new questions about the control of ovarian function because it brings into play a pathway that is not related a priori to the BMP signalling system (Drouilhet et al. 2013). B4GALNT2 encodes a β-1,4-N-acetyl-galactosaminyltransferase, which catalyses a terminal step of glycosylation by adding an N-acetylgalactosamine residue to a subterminal galactose residue of glycosylated target proteins (Montiel et al. 2003). In Lacaune sheep, increased ovulation rate is associated with the ectopic overexpression of B4GALNT2 in granulosa cells (where it is not normally expressed), but only in follicles from the secondary to preovulatory stages. The search for intraovarian targets of B4GALNT2 activity has led to elements of the extracellular matrix and, more interestingly, to the two subunits of inhibin A, INHA and INHBA (Drouilhet et al. 2013). Indeed, differential subunit association, leading to either inhibin A (INHA/INHBA) or activin A (INHBA/INHBA), depends on glycosylation events (Antenos et al. 2007) and it has long been known that immunisation against inhibin can increase ovulation rate and prolificacy in ruminants (O’Shea et al. 1994).
In FecLL carrier ewes, the high ovulation rate is associated with an increase in the number of gonadotrophin-dependent follicles, as well as a reduction in the size of the preovulatory follicles (Drouilhet et al. 2010). These relationships are consistently observed with major genes that control ovulation rate in sheep and cattle (Fabre et al. 2006; García-Guerra et al. 2018a). However, compared with non-carrier ewes, FecLL carriers have greater circulating concentrations of oestradiol during the follicular phase, leading to an increase in LH pulse frequency followed by an earlier preovulatory LH surge. In the luteal phase, circulating progesterone concentrations are also increased, probably due to the increased number of corpora lutea. More importantly, inhibin A concentrations are consistently decreased, possibly due to atypical glycosylation by B4GALNT2. Only the circulating concentrations of FSH and activin A are unaffected by FecLL (Drouilhet et al. 2010; C. Mansanet and S. Fabre, unpubl. data). Apart from FSH, this endocrine scenario contrasts strongly with those for mutations in BMP15, GDF9 or BMPR1B, in which the concentrations of LH, inhibin, oestradiol or progesterone did not differ from those in wild types, as also observed in the Trio model in cattle (Juengel et al. 2013; García-Guerra et al. 2018b).
Conversely, one notable endocrine observation could reconcile, at least in part, the prolific sheep models: circulating AMH concentrations are low in B4GALNT2/FecLL ewes, as well as in BMPR1B/FecBB, GDF9/FecGE and BMP15/FecXR ewes (Lahoz et al. 2014; Estienne et al. 2015; Chantepie et al. 2018; Pinto et al. 2018). Circulating AMH may not be affected by other BMP15 mutations (FecXL and FecXGr), but ovarian AMH signalling is altered by a decrease in the expression of the AMH receptor type 2 (AMHR2) gene, probably through direct action of BMP molecules on AMH and AMHR2 gene expression in granulosa cells (Estienne et al. 2015; Pierre et al. 2016). These findings raise the intriguing possibility that AMH plays a role in the control of ovulation rate and prolificacy, at least in sheep. Notably, this relationship is not obvious in prolific cattle (García-Guerra et al. 2017).
Use of major genes to improve reproductive efficiency
Over the past 40 years, sheep and cattle models of major genes for prolificacy have been crucial in deciphering mechanisms that control ovarian function. However, with respect to reproductive efficiency, major genes for prolificacy can have a much broader impact than simply increasing litter size because, compared with non-carriers, female carriers of prolific mutations (when not homozygous sterile) show several advantages in assisted reproductive technologies. For instance, FecLL carrier Lacaune ewes exhibit precocious puberty, a higher fertility rate to AI and increased responsiveness to equine chorionic gonadotrophin (eCG; Martin et al. 2014; L. Chantepie and S. Fabre, unpubl. data, 2021). In FecBB carrier ewes from the short-tailed Han breed, lambing rate after AI was also improved (Qi et al. 2020). In FecXR carrier Rasa Aragonesa sheep, responsiveness to eCG was increased, as was the recovery of oocytes competent for in vitro development and the rate of embryo survival after transfer (Lahoz et al. 2011, 2013). For double-heterozygous mutant FecGV/FecBB ewes during the breeding season, there is also an increase in responsiveness to eCG but, more interestingly, eCG is not needed during seasonal anoestrus when these ewes continue to produce twin ovulations naturally (Moraes and Souza 2017). Together, these observations strongly suggest that the major genes for prolificacy could also deliver a reduction in the use of hormones in livestock herd management.
Rise of AMH
The major genes have revealed a variety of critical processes in follicle development and function, including the role of AMH. In fact, for sheep and cattle, the importance of AMH produced by granulosa cells was pioneered in the 1980s (Vigier et al. 1984), but was then largely ignored for 20 years until it gained recognition as an endocrine marker of growing follicles in the human in the context of assisted reproductive technologies. Completing the cycle, we now have a greatly renewed interest for ruminant reproduction, where AMH has now been recognised for its role in activating primordial follicles and limiting the growth of activated follicles (Campbell et al. 2012; Yang et al. 2017). This perspective was accompanied by the development of assays allowing accurate measurement of AMH in blood and follicular fluid, and thus the precise delineation of the spatiotemporal expression, regulation and production of AMH within the ovary.
Regulation of AMH production by granulosa cells
In the ruminant ovary, expression of the AMH gene is restricted to granulosa cells, where the rate of expression increases with the transition of the follicle from primary through to early antral stages, before declining through to the preovulatory stage (for a review, see Monniaux et al. 2012). In healthy antral follicles in cattle and sheep, the AMH gene is mostly expressed in the cumulus cells and the outer layers of granulosa cells, close to the theca. Moreover, AMH gene expression in the granulosa cells is accompanied by accumulation of AMH protein in follicular fluid to the point where it becomes easily measurable by using immunoassays. In cattle, sheep and goats, follicular fluid AMH concentrations are greatest in small antral follicles (~300–500 ng mL–1) and then decrease markedly as follicles grow to the preovulatory stage (Monniaux et al. 2012). In atretic follicles, AMH expression is greatly diminished, except for the cumulus cells surrounding the oocyte. These observations suggest that, generally, the oocyte and the theca both produce paracrine factors that support AMH expression in growing follicles.
The pattern of AMH expression (i.e. switching on in primary follicles, maximum production in granulosa cells of preantral and small antral follicles, switching off during atresia or in preovulatory follicles (except cumulus cells)), raises important questions about AMH regulation during the process of folliculogenesis. As indicated above, AMH activity is probably regulated by signals from both the oocyte and the theca cells, and BMPs are likely candidates (Fig. 2). In particular, oocyte-derived BMP15 upregulates AMH gene expression in ruminants (Pierre et al. 2016; Poole et al. 2016; Zhao et al. 2018) and the temporal sequence of BMP15 expression in oocytes parallels that of AMH expression, beginning during primary follicle development and increasing until the antral stages (Bonnet et al. 2011). Moreover, BMP2, BMP4 and BMP6, produced by granulosa or theca cells, stimulate AMH expression and could even sustain AMH production during follicle development in ruminants (Rico et al. 2011; Estienne et al. 2015; Poole et al. 2016). By contrast, activin A and transforming growth factor-β1 (TGFB1), members of the TGFB family, play no role (Monniaux et al. 2012).
In the developing antral follicle, steroid hormones and FSH are also thought to affect AMH expression in granulosa cells, although there are differences among species: (1) androgens inhibit AMH expression in cattle and humans, but stimulate it in mouse granulosa cells (Crisosto et al. 2009); (2) oestradiol inhibits AMH expression in granulosa cells of humans, but not cattle (Crisosto et al. 2009; Grynberg et al. 2012); and (3) in cattle, FSH regulates AMH gene expression in a bimodal, dose-dependent manner, being stimulatory at low concentrations and inhibitory at high concentrations (Scheetz et al. 2012; Umer et al. 2019), yet has no effect in ovine granulosa cells (Monniaux et al. 2012). This complexity could be due to inconsistent methodologies and incomplete control of experimental variables, so it will be difficult to develop a universal theory until such issues are resolved.
AMH in the peripheral circulation
Circulating AMH concentrations have been measured in cattle, sheep and goats, and vary greatly among individuals within species (Ireland et al. 2008; Rico et al. 2009; Monniaux et al. 2011; Lahoz et al. 2014; Torres-Rovira et al. 2014; Gobikrushanth et al. 2018). Conversely, within individuals, circulating AMH concentrations are stable over months or even years (Monniaux et al. 2011; Evans et al. 2012) because the population of growing small antral follicles, where the greatest amounts of AMH are produced, is itself quite stable (Scaramuzzi et al. 2011). A degree of uncertainty has been introduced by the recent discovery that, in cattle, AMH is synthesised and secreted by epithelial cells in the oviduct and endometrium (Ferdousy et al. 2020), suggesting that we can expect changes in plasma AMH concentrations as animals progress through major stages of reproductive life, such as puberty.
In heifers, circulating AMH is almost undetectable at birth, increases markedly by 2–3 months of age, declines but remains quite high until 5–6 months of age, then declines markedly to a stable baseline at puberty (8–9 months of age for dairy cattle; 12 months for beef cattle; Monniaux et al. 2012; Mossa et al. 2017). Similar dynamics have been observed in ewe lambs, with peak concentrations also at age 2–3 months (Lahoz et al. 2014; Torres-Rovira et al. 2014). Interestingly, in both species, the prepubertal peak is preceded by a rise in plasma FSH concentration (Torres-Rovira et al. 2014; El-Sheikh Ali et al. 2017) that probably drives the postnatal increase in the number of antral follicles (Rawlings et al. 2003) and thus the rise in AMH concentrations. Thereafter, FSH concentrations fall to their lowest levels as AMH concentrations reach peak values. As puberty approaches, these changes are reversed, with AMH concentrations decreasing to reach adult values and FSH concentrations rising (Monniaux et al. 2012; El-Sheikh Ali et al. 2017).
Plasma AMH concentration varies little in cattle during the oestrous cycle or between successive cycles. In heifers, it remains quite static around ovulation (Ireland et al. 2008). In adult cows, particularly those with high average values, it does vary slightly within the oestrous cycle, with maximum values at the time of oestrus and minimum values around Days 7–8 after ovulation (Monniaux et al. 2012; Gobikrushanth et al. 2018). The same patterns are seen in natural and synchronised oestrous cycles in dairy cows (Pfeiffer et al. 2014) unless superovulation is induced by exogenous FSH, a treatment that increases plasma AMH concentrations at oestrus but, curiously, decreases it in goats (Rico et al. 2009; Monniaux et al. 2011). There are also significant changes in circulating AMH concentrations during gestation; in cows, for example, levels increase during the two first trimesters and then decline until calving, when basal postpartum concentrations are reached (Monniaux et al. 2012).
Development of the ovary and establishment of the ovarian reserve
Arguably, the establishment of the ovarian reserve is the key outcome of ovarian development in the fetus. Here, we briefly describe the current dogma on the development of the ovary, emphasising processes that are susceptible to disruption by environmental factors with potential long-term consequences on postnatal fertility (Fig. 3). There have been numerous studies, and the results have varied markedly, probably because they differed in the levels and windows of exposure, the species studied and the methodologies used. Moreover, both Lea et al. (2016) and Smith et al. (2019b) have highlighted that, during the period of fetal development, changes in exposure to environmental contaminants or nutritional treatments may perturb development more potently than long periods of consistent exposure. It appears that, given time to adapt, compensatory mechanisms are activated to allow development to cope with at least some level of environmental challenge.
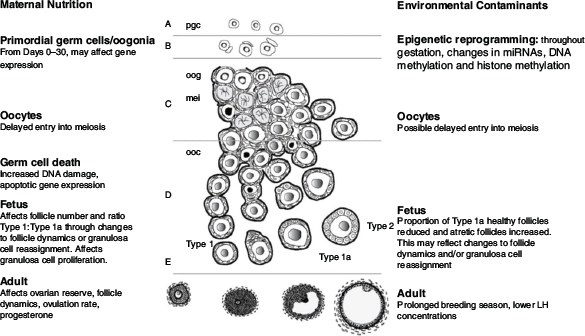
|
Maternal nutrition
Effects of maternal nutrition during the periconception period on fetal ovarian development are associated with changes in oocyte health and the survival and growth of early embryos, potentially mediated by epigenetic alterations to gene expression (Sinclair et al. 2016). The effects of maternal nutrition during early gestation in sheep had been clearly demonstrated by the study of Rae et al. (2001), who underfed ewes during the first 30 days of gestation and observed changes in follicle populations in the fetal ovary on Day 100 of gestation. These outcomes were known to be associated with a delayed germ cell meiosis (Borwick et al. 1997). Importantly, the period of germ cell meiosis overlaps the periods of germ cell mitosis and germ cell death. Germ cell death is particularly significant because, in most mammalian species, 80–90% of germ cells are lost during a brief window (Days 75–90 of gestation in sheep; Days 130–170 of gestation in cattle). Therefore, delayed entry into meiosis alters the dynamics of germ cell death and potentially reduces reproductive performance in adult animals (Borwick et al. 1997).
The loss of germ cells is often attributed to apoptosis, but non-apoptotic pathways have long been recognised (Wartenberg et al. 2001; McClellan et al. 2003). Autophagy plays a role (Zhang et al. 2012; Smith et al. 2019a) but, before follicle formation, autophagy proteins are thought to be more important for germ cell survival, with their role in germ cell death only becoming dominant after follicle formation. During this particularly active period, the classical gradient of germ cell development has been observed in most species, with the least developed cells (oogonia) at the ovarian periphery and the more developed (oocytes and subsequently follicles) located in the inner regions of the ovarian cortex. At a simplistic level, the three developmental processes (mitosis, meiosis, cell death) program the ovarian reserve, and several studies implicate them in environment-induced changes in ovary development (Lea et al. 2006; Smith et al. 2014).
Germ cell meiosis is known to be susceptible to environmental disruptors such as nutrition and infection. Murdoch et al. (2003) proposed that germ cells entering meiosis are susceptible to oxidative DNA damage, with undernutrition leading up to and during meiosis being critically important. Recent gene expression studies by Smith et al. (2019a) have expanded on this concept and suggest that the damage may be caused by an increase in the production of nitric oxide by the germ cells.
The effects of restricted nutrition on the size of the ovarian reserve are not limited to germ cell death. As germ cells die, the associated pregranulosa cells appear to remain healthy and are subsequently redistributed among the remaining germ cells, leading to the formation of follicles with a lopsided appearance, often containing multiple granulosa cell layers on one side (Sawyer et al. 2002). This ‘granulosa cell reassignment hypothesis’ suggests that environmental challenges affecting germ cell proliferation, cell death and meiosis affect the number of germ cells that are available to form follicles, in turn dictating the number of granulosa cells associated with each newly formed primordial follicle. Further, this outcome raises the possibility that, in late-fetal or neonatal animals, an altered ratio of Type 1 to Type 1a follicles, often interpreted as a change in early follicle dynamics, actually reflects variation in granulosa cell reassignment resulting from differences in the numbers of germ cells present in the fetal ovary when follicles are formed. Thus, in addition to influencing the size of the ovarian reserve, environmental challenges have the potential to affect the structure and therefore the function of the follicles in the reserve, including follicle dynamics and granulosa cell expression of proliferation and apoptosis genes (Lea et al. 2006).
Environmental contaminants
In addition to the effects of nutrition on fetal ovarian development, our understanding of the effects of chemical contaminants on fetal ovaries, particularly on primordial germ cells, has increased greatly over the past decade. We have seen the emergence of an ovine model for exploring exposure to a mixture of chemicals during pregnancy, with relevance to human real-life exposure. The model is based on the common global agricultural practice of grazing pregnant ewes on pastures that have been treated with processed human sewage sludge (biosolids) as a fertiliser (Sharma et al. 2017). The biosolids and the treated soil contain a variety of types of chemicals from a variety of sources, including plasticizers (phthalates, bisphenol A (BPA)), industrial surfactants and coolants (polychlorinated biphenyls (PCBs)), flame retardants (polybrominated diphenyl ethers), combustion products (polyaromatic hydrocarbons), pesticides and various pharmaceuticals (Rhind et al. 2002; Rhind et al. 2013). Notably, our understanding of such exposures is complicated by additive, synergistic or antagonistic interactions among the chemicals, reflecting the role of the fundamentally synergistic interaction between oestrogen and progestogen in negative feedback in the ewe (Martin et al. 1983). In addition, both the contaminants and their breakdown products may exhibit androgenic, anti-androgenic and/or oestrogenic activities.
Worryingly, many of the chemicals listed above have been detected in both maternal and fetal tissues collected from mid- and late-gestation pregnant ewes (Rhind et al. 2005, 2009, 2010), and many of the pollutants exhibit endocrine-disrupting activity, leading to concerns about their effects on reproductive development in the fetal and prepubertal lamb.
Ovary development in fetuses, in both mid- and late gestation, that have been exposed in utero to biosolids (via the ewe) is perturbed compared with control non-exposed fetuses (ewes grazed on pastures treated with inorganic fertiliser), and there is a striking reduction in the number of healthy early stage Type 1a transitional follicles (Fowler et al. 2008; Bellingham et al. 2013; Lea et al. 2016). This observation is consistent with that of Fowler et al. (2008) who reported that exposure to biosolids from mating to Day 110 reduced follicle density. Bellingham et al. (2013) compared two periods of exposure, the first from preconception to Day 110 of gestation and the second from mating through to Day 110 of gestation, and included additional ewe cohorts that were switched between treated and control pastures at mating. Intriguingly, ewes that were transferred from treated to control pastures had fewer Type 1a follicles than ewes exposed from before mating through to the end of gestation. Moreover, ovarian proteomics revealed that more protein changes were induced in ewes switched from control to treated pastures at mating than in the other exposure groups (Bellingham et al. 2013). Again, consistent, long-term exposure appears to activate compensatory mechanisms, whereas a change in exposure seems to be more potent in perturbing development.
When examining the effects of exposure of the fetus via the mother consuming the chemical contaminants contained in biosolids, Lea et al. (2016) compared the effects of a period of continuous exposure from mating to exposure limited to 80-day periods during either early, mid- or late gestation. In all exposure groups, regardless of the timing or duration of exposure, there was a reduced percentage of healthy Type 1a follicles in Day 140 fetal ovaries. It is not clear whether this outcome reflects changes in follicle formation mediated through pregranulosa reassignment or changes in the initiation of follicle growth. Again, compared with continuous exposure, exposure during mid- or late gestation had a greater effect on the number of fetal genes and proteins that were altered (Lea et al. 2016). Most of the genes were downregulated, suggesting an underlying epigenetic mechanism, a hypothesis supported by bioinformatic analysis showing that 14 of the differentially expressed genes were regulators of histone methylation (Lea et al. 2016).
The differences in outcome between continuous and short-term exposure raise fundamental questions about both the mechanisms and the biological advantages of the different responses. One possibility centres on a group of differentially expressed genes that code for drug-metabolising enzymes, raising the possibility that changes in these enzymes during early development are an adaptation to counter the effects of the abnormal environment later in gestation. This adaptation would allow logical and biologically advantageous responses to changes in the follicular environment and may also explain the more deleterious effects of subsequent environmental challenges, such as transitory exposure to biosolids. Indeed, this same concept seems to be applicable to pregnant ewes grazing high-salt fodder (Tay et al. 2012). Thus, being able to adapt to changes in the maternal environment would enable the developing fetus to prepare for postnatal life, on the expectation that the maternal and postnatal environments are similar.
Additional insights regarding the long-term effects of environmental contaminants on the developing ovary have been provided by examination of key compounds in isolation or as mixtures of a few defined compounds. Given that the intrauterine endocrine milieu is critical for fetal development, and that many environmental contaminants act as endocrine disruptors, it became essential to characterise the effects of supplementary androgen and oestrogen in pregnant ruminants. In sheep, exposure of the developing lamb to androgens during mid-pregnancy induces an adult ovarian, metabolic and hormonal phenotype that is similar to that seen in women with polycystic ovarian syndrome (Padmanabhan and Veiga-Lopez 2013; Monniaux et al. 2020; Siemienowicz et al. 2020). Smith et al. (2009) focused on the androgen effect and, in 10-month-old neonates, demonstrated an increase in late-gestation early growing follicles but a decrease in primordial follicles. Intriguingly, the decrease in primordial follicles was attributed to oestrogen derived from aromatisation of testosterone propionate. Together, these studies suggest that anthropogenic chemicals with androgenic or oestrogenic activity would cause similar effects. In support of this hypothesis, mid-gestation exposure of the developing female lamb to BPA prolonged the first breeding season and delayed and dampened the preovulatory LH surge (Savabieasfahani et al. 2006). Moreover, in lambs exposed to BPA or diethylstilbestrol from Day 1 to Day 14 after birth, the reserve of primordial follicles was reduced and the transition to primary follicles was increased by Day 30 (Rivera et al. 2011). This outcome was accompanied by a greater rate of proliferation of granulosa and theca cells, and an increased incidence of multi-ovular follicles, suggesting that one or more key processes in early follicle development had been perturbed.
It is now clear that primordial germ cell biology often affects ovary development through changes in DNA methylation. Migrating germ cells begin with a high level of DNA methylation, then undergo demethylation until around birth, followed by a period of remethylation, although the temporal sequence varies with sex and species (Zeng and Chen 2019). In the mouse, for example, male germ cells undergo remethylation in utero, whereas remethylation in female germ cells begins postnatally and continues as the oocytes develop and follicles are recruited. Therefore, environmental changes have more opportunity to alter imprinting patterns in females than in males.
It should be noted that DNA methylation is only one of the three main epigenetic mechanisms underpinning changes in gene action without needing a change in DNA sequence; the other two are modification of histones, including acetylation and methylation, and the production of non-coding RNAs that inhibit gene expression. All three mechanisms regulate the development of the follicle in the fetal, postnatal and adult ovary (for reviews, see Pan et al. 2012; Cruz et al. 2014). For example, in pregnant ewes, the expression of fetal ovarian microRNAs is modified by exposure to BPA from Days 30 to 90 of gestation, leading to changes in developmental and functional insulin-related genes (Veiga-Lopez et al. 2013). This observation builds on extensive literature suggesting that the prenatal fetal ovary is particularly sensitive to BPA, in both human and animal models, and that low BPA concentrations affect the epigenome of female germ cells in rodents (Eichenlaub-Ritter and Pacchierotti 2015; Chianese et al. 2018; Mathew and Mahalingaiah 2019; Huang and Zeng 2021). The effects of environmental contaminants can also be transgenerational, although to date the most emphatic demonstration has been with rodent models. Exposure of the pregnant rat clearly results in exposure of the F1 fetus, with the F2 generation generated from the exposed F1 germline. By definition, the F3 generation has not been exposed, so any effects observed in the third or fourth generations are truly transgenerational. In 2005, a landmark paper showed that exposure of pregnant rats to the endocrine disruptor vinclozolin results in transgenerational effects on male fertility that are accompanied by alterations in DNA methylation patterns in the germ line (Anway et al. 2005). More recently, in a study of gestating rats exposed to the pesticide methoxychlor (a contaminant known to affect fetal ovarian development and postnatal fertility), there was an increased incidence of disease in both male and female F3 offspring (Manikkam et al. 2014). Remarkably, the increase in disease in the reverse outcross F4 female (but not male) offspring provides strong evidence of transgenerational inheritance through the female germline.
Transgenerational effects in sheep or cattle following maternal exposure to environmental contaminants, or even to natural phytoestrogens, have yet to be reported. Interestingly, differences in ovarian genomic DNA methylation have been reported in high- and low-prolificacy sheep, with lower levels of DNA methyltransferase genes in the high-prolificacy group (Zhang et al. 2017b). The authors of that study also reported differential methylation of genes associated with prolificacy, including BMP7, BMPR1B, FSH receptor (FSHR), LH/choriogonadotrophin receptor (LHCGR), TGFB2 and TGFB3, providing a potential mechanism by which maternal exposure to environmental contaminants or nutritional restriction could alter follicle growth and the ovulation rate in future generations.
Oocyte exposure to environmental contaminants has been associated with perturbations in meiosis. For example, in vitro exposure of cattle oocytes to BPA and to mixtures of PCBs is reported to delay meiotic progression (Pocar et al. 2001; Ferris et al. 2016; Campen et al. 2018); similar observations have been reported in mice (Susiarjo et al. 2007). Therefore, it would appear that meiotic progression in the oocyte is sensitive to both chemical exposure and altered nutrition, as discussed above.
Relationships among ovarian reserve, fertility and AMH
The size of the ovarian reserve varies greatly among young females (Erickson 1966), and there have been several attempts to dissect the relationship between variation in ovarian reserve and fertility. In cattle, phenotypic studies have mostly been positive, showing that a greater number of follicles is associated with: (1) earlier conception in the breeding season (Mossa et al. 2012; Cushman et al. 2014; McNeel and Cushman 2015); (2) greater uterine luminal protein concentrations (McNeel et al. 2017); (3) greater circulating progesterone concentrations (Jimenez-Krassel et al. 2009; Santa Cruz et al. 2018); and (4) a shorter interval from calving to oestrus (Martinez et al. 2016).
Genetic selection for ovarian reserve traits to improve fertility
In cattle, the heritability of most female reproductive traits is usually less than 0.05. In contrast, the genomic heritability of traits linked to ovarian reserve are moderate, thus more responsive to selection. The heritability of circulating AMH concentration is 0.36–0.46 (Gobikrushanth et al. 2018; Nawaz et al. 2018), similar to a heritability of 0.14–0.21 for the number of embryos collected after superovulation (Jaton et al. 2016) and a heritability of 0.31–0.44 for antral follicle count by ultrasonography (Snelling et al. 2012; Walsh et al. 2014). Therefore, fertility may be enhanced by genetic selection to increase the ovarian reserve, with phenotype measured directly by antral follicle count or indirectly by AMH concentration. In Holsteins, it seems likely that we can select based on strongly associated molecular markers on chromosomes 7 and 11, as well as positional candidates for the AMH gene itself on chromosome 11 (Gobikrushanth et al. 2018, 2019; Nawaz et al. 2018). Conversely, the adoption of ultrasonography for assessing reproductive efficiency in cattle has been hindered by the reports of antagonistic genomic relationships between antral follicle number and heifer pregnancy rate, as well as between antral follicle number and age at puberty (Snelling et al. 2012; Oliveira Júnior et al. 2017). The disagreement between phenotypic data and genomic correlations can be explained by the presumption that the relationship is linear in the genomic models, whereas, in fact, it may be quadratic. For example, reproductive longevity increases as the circulating concentrations of AMH increase, but the response is bimodal, with reproductive longevity being shorter in heifers with very high circulating concentrations of AMH (Mossa and Ireland 2019). In sheep, similar studies have not been done and we know only that greater prepubertal plasma AMH concentrations are associated with earlier puberty and greater fertility at first lambing (Lahoz et al. 2012; Torres-Rovira et al. 2014).
Altering the size of the ovarian reserve by management and nutrition
In beef cows, underfeeding during the first 110 days of the first pregnancy resulted in daughters with fewer antral follicles during the first 1.5 years of life (Mossa et al. 2013). It is not known whether fewer antral follicles has consequences for reproductive efficiency. In contrast, in ewes, underfeeding during the first 55 days of gestation followed by ad libitum feeding throughout the remainder of pregnancy resulted in daughters with more antral follicles and a higher ovulation rate at 20 months of age (Smith et al. 2019b). Moreover, at 75 days of gestation, germ cell number and ovarian volume were also increased in the daughters of the feed-restricted ewes (Smith et al. 2019a). For cattle, a comparable histological analysis has not yet been done.
The age of the dam also affects follicle number in daughters, with heifers born to heifers having fewer antral follicles than herd mates born to mature cows (Walsh et al. 2014; McNeel et al. 2017; Tenley et al. 2019). This observation may reflect the effects of nutritional restriction described above (Mossa et al. 2013), because heifers may be less efficient at providing nutrients to their daughters in utero.
With peripubertal heifers, a decrease in energy intake causes an increase in the number of primordial follicles in the ovaries at the time of breeding, a counterintuitive response that is probably explained by a slowing of the rate of activation of primordial follicles (Freetly et al. 2014; Amundson et al. 2015). Whether this increase in primordial follicle number truly improves reproductive function remains unclear, but a large production-setting study recently demonstrated that herd survival, largely influenced by reproductive longevity, is greater in heifers developed at a slower rate of gain (Freetly et al. 2021).
Applications of developmental programming of the ovarian reserve
If nutrition during fetal development or the peripubertal period really does alter primordial follicle numbers (for better or worse), then it may be possible to use nutritional interventions to alter the size of the ovarian reserve and the fertility of replacement females. It will be important to determine whether nutrition-induced changes in primordial follicle number also alter fertility endpoints in the same way that natural increases in ovarian reserve improve uterine luminal protein concentrations, progesterone concentrations and reproductive performance. Until these important questions are answered, we cannot know whether there is any value in manipulating developmental programming to alter the size of the ovarian reserve.
Answering such questions is difficult while various methods are used to estimate the ovarian reserve. Histological counts of primordial follicle number undertaken with stereological principals are the gold standard but are not practical for a production system where less direct indicators would need to be used, such as ultrasound counts of antral follicles or measurements of circulating AMH concentrations. Strong correlations between AMH and the number of growing follicles support the widely held view that AMH is a reliable marker of the ovarian reserve of growing follicles (Ireland et al. 2008; Rico et al. 2011; Monniaux et al. 2012; Baruselli et al. 2015; Mossa et al. 2017). Strong relationships between the numbers of primordial follicles and antral follicle counts (Ireland et al. 2008; Monniaux et al. 2014) have been observed, but the assumption that relationships among these three variables are universally valid may be false. For instance, changes in circulating AMH in relation to the major genes affecting prolificacy could challenge AMH as a marker of growing follicles in the context of assisted reproductive technologies in ovine genotypes where these prolificacy mutations are segregating. Moreover, research questions and practical application often have different endpoints. If the concept of the follicle reserve is to be transferred to production systems, the basic research must always return to histological results to ensure that conclusions based on ultrasound or AMH concentrations are challenged.
In addition to nutrition and environmental contaminants, other environmental factors may affect ovary development and thus postnatal fertility. Among the current gaps in our knowledge are the effects of maternal disease status and heat stress, both of which warrant more attention given the effects of global climate change on disease vectors and thermoregulation. Recently, it has been shown that dairy heifers conceived in the summer, with their mothers having been exposed to a high temperature–humidity index, had lower antral follicle counts and AMH concentrations than those conceived in the winter (Succu et al. 2020), although there appeared to be no association between season and conception or fertility at first conception.
Over the past decade, our understanding of the mechanisms linking environment and fetal development has increased greatly. Over the next decade, our challenge is to put this knowledge to use by determining whether we can reprogram ovary development to produce a desired fertility outcome for livestock, as well as pest animals and companion animals. We also need to test whether we can mitigate negative impacts and maintain fertility in the face of environmental challenges.
Resurrection of the theca
Interactions between granulosa/cumulus cells and the oocyte have received a great deal of attention in the past decade, and the layer of theca cells seems to have been neglected. Indeed, when Young and McNeilly (2010) reviewed our scant knowledge of the development and endocrine activity of the theca cell, they referred to it as ‘the forgotten cell’. According to the dogma at the time, the theca provided physical support for the follicle and had only one essential role, namely the secretion of androgens as a precursor for granulosa cells to synthesise and secrete oestradiol, the basis of the ‘two -cell theory’ originally presented by Armstrong et al. (1979). The only other functional aspect being explored was the effect of members of the TGFB family on thecal androgen secretion (for a review, see Juengel et al. 2018). It has now become clear that this tissue layer plays a more important role in the development and health of the follicle.
The primary endocrine function of theca cells is bidirectional signalling with granulosa cells (Fig. 2), although other cell types are also involved, as discussed below. Theca cells secrete several growth factors, including members of the BMP family, specifically BMP4 and BMP7, that act on granulosa cells to enhance oestradiol secretion and cell proliferation, and are considered to be inhibitors of luteinisation and apoptosis (Glister et al. 2004; Chang et al. 2016a). The signals from granulosa cells to theca cells include BMP6, which, in cultured bovine cells, inhibits androgen production (Glister et al. 2005). Following BMP6 treatment, the strongest downregulation was seen with insulin-like factor 3 (INSL3) protein and mRNA abundance (Glister et al. 2013); INSL3 is a theca-derived protein involved in the paracrine control of theca function that enhances theca androgen secretion, thereby increasing the availability of precursors for oestradiol production by granulosa cells (Glister et al. 2013). Androstenedione and oestradiol stimulate INSL3 secretion from theca cells (Dai et al. 2017), apparently establishing a positive feedback loop that enhances the ability of a growing follicle to produce oestradiol. The inhibition of androgen secretion by BMP6 may involve the inhibition of INSL3 secretion.
The action of BMPs is regulated by four secreted proteins, namely noggin (NOG), gremlin (GREM1), follistatin (FST) and chordin (CHRD). These proteins are produced mainly by the granulosa cell layer, at least in cattle (Glister et al. 2011). In bovine theca cells in vitro, GREM1 and NOG attenuate the inhibition of androgen secretion by granulosa- and theca-derived BMPs (Glister et al. 2019), suggesting that granulosa cells may modulate paracrine and/or autocrine signalling within the theca layer, as well as modulating the bioactivity of their own BMP output.
Several members of the fibroblast growth factor (FGF) family, including FGF2, FGF7 and FGF18, are produced mainly, if not exclusively, by the theca layer, at least in cattle (for a review, see Price 2016), and exert a variety of effects on granulosa and theca cells. It has long been known that FGF7 from the bovine theca increases granulosa cell proliferation without affecting theca cells themselves (Parrott and Skinner 1998). Most interesting is theca-derived FGF18, because, unlike the other FGFs, it stimulates apoptosis in granulosa cells without activating the typical FGF signalling pathways (Jiang et al. 2013; Portela et al. 2015). In contrast, FGF18 acts as a typical growth factor in theca cells by reducing apoptosis and activating typical signalling pathways (Han et al. 2018). Adverse effects on follicle health may not be limited to those caused by FGF18; in cattle, FGF10 mRNA in the theca layer is more abundant in subordinate than dominant follicles (Castilho et al. 2017) and, as seen with FGF18, FGF10 does not elicit the typical cellular response to growth factors in granulosa cells (Jiang and Price 2012).
As a follicle undergoes atresia, apoptosis begins with the granulosa cells and oocyte, and then the theca cells, although the theca layer does seem to be disrupted early in smaller follicles (<5 mm in cattle; Irving-Rodgers et al. 2001). In atretic follicles, theca cells do not show increased expression of genes related to apoptosis, but rather show a decrease in genes involved in the cell cycle and proliferation, such as cyclin B1 (CCNB1) and centromere protein F (CENPF; Hatzirodos et al. 2014). However, theca cells can be affected by environmental factors. For example, prenatal exposure to testosterone impairs androgen production in the theca of small follicles during adult life (Monniaux et al. 2020). In addition, theca cells appear surprisingly susceptible to the mycotoxin metabolite deepoxy-deoxynivalenol, showing increased apoptosis and levels of mRNAs that encode apoptosis-related genes, including BH3 interacting domain death agonist (BID) and Fas ligand (FASLG; Guerrero-Netro et al. 2017). Clearly, the theca cell should not be overlooked when assessing the effects of environmental toxins and endocrine disruptors on ovarian function.
It is important to remember that the theca cell layer is complex, containing vascular cells, immune cells and fibroblasts, all of which are gaining recognition for their roles in follicle function. The thecal vasculature is well developed in healthy follicles and breaks down in atretic follicles. The major proangiogenic factors, FGF2 and vascular endothelial growth factor (VEGF), are expressed in follicles and are under endocrine or paracrine control, as demonstrated by the ability of BMP4 to stimulate VEGFA mRNA levels in vitro in the bovine theca (Nichols et al. 2019). In a bovine theca–endothelial cell coculture system, endothelial tubes form during culture, and angiogenesis is inhibited by TGFB1 or BMP6 and stimulated by a combination of VEGF and FGF2 (Mattar et al. 2020). In these studies, some observations do not agree, perhaps due to differences among the various BMPs in intracellular signalling, the potential for confounding effects with coculture in contrast to monoculture and variations in the additives to the culture media. There is still a lot to be learned about the roles of thecal vascular cells.
Immune cells within the thecal layer may also be involved in follicle development. For example, in the mouse, depletion of macrophages from the ovary causes haemorrhage and loss of vascular integrity in the theca layer (Turner et al. 2011). In cattle, mRNA encoding the macrophage-specific markers CD68 and CD14 is upregulated in the theca layer during atresia (Hatzirodos et al. 2014), suggesting an influx of macrophages, and coculture of theca cells with macrophages suppresses thecal androgen secretion (Samir et al. 2017). Macrophages secrete FGF2 and VEGF, so there are likely to be complex and stage-specific interactions among steroidogenic cells, endothelial cells and immune cells. The challenge now is to determine the local concentrations of these paracrine factors, and to dissect out the degree of cross-talk between the various cell types in a physiological model.
Follicular microenvironment and oocyte maturation
The follicular microenvironment has long been recognised as important for the oocyte to attain developmental competency. However, many of the recent advances in our understanding of key events in the later stages of follicle development have arisen from in vitro experiments, and we are now realising that it is additionally helpful when such experiments simulate, as best they can, physiologically relevant conditions. We appreciate the importance of a whole-follicle setting for supporting key physiological pathways that promote oocyte development, but then often ignore the issue during the commercial production of embryos in vitro.
Follicular fluid contains a myriad of factors obtained from the circulation, the cells of the theca, mural granulosa and cumulus layers, and the oocyte (Fig. 2). Assessment of follicular fluid constituents at each stage of follicle development has revealed changes in the microenvironment, including substrates, energy and metabolites, as the oocyte matures (Murray 2019; Clark et al. 2020). These changes may be essential for enabling each follicle cell type to perform the many critical processes for successful oocyte maturation.
One such process is the active maintenance of meiotic arrest; in all mammals studied to date, inhibiting the resumption of meiosis involves preventing the activation of maturation-promoting factor (MPF), a cyclin-dependent kinase (CDK) 1–cyclin B complex. Activation of MPF is inhibited by high intraoocyte concentrations of cyclic adenosine monophosphate (cAMP) that sustain activation of protein kinase A, which, in turn, phosphorylates nuclear kinase WEE2 oocyte meiosis inhibiting kinase (WEE2) and cell division cycle 25B (CDC25B) phosphatase. The phosphorylated forms of WEE2 and CDC25B phosphatase inhibit MPF activity (Eppig 1993; Tsafriri and Dekel 1994; Han and Conti 2006). The intraoocyte cAMP concentration is the sum of its production in the oocyte by adenylate cyclase and its degradation by phosphodiesterases (PDEs). Hydrolysis of cAMP by species-specific isoforms of PDEs, as well as the conserved PDE3a isoform, is regulated by the presence of cyclic guanosine monophosphate (cGMP), which enters the oocyte via gap junctions from cumulus cells and then competitively binds the catalytic site of PDEs (Franciosi et al. 2014). The production of cGMP in granulosa and cumulus cells is stimulated by the action of granulosa cell-derived C-type natriuretic peptide, via natriuretic peptide receptor (NPR2) on cumulus cells (Zhang et al. 2010; Hiradate et al. 2014). As the follicle matures, this pathway is increasingly supported by gonadotrophin-induced cGMP production, as well as oestradiol, which upregulates NPR2 (Zhang et al. 2011; Wigglesworth et al. 2013).
Other critical factors present within the follicular fluid include the oocyte-secreted factors GDF9 and BMP15. These two growth factors are key mediators of follicle growth, affecting both granulosa and theca cell function, and are critical for the attainment of developmental competency in the ruminant oocyte (Gilchrist et al. 2008; Spicer et al. 2008; Juengel 2018; Juengel et al. 2018; D’Occhio et al. 2020). In other species, GDF9 and BMP15 have been reported to suppress expression of Lhcgr (Eppig et al. 1997) and activate numerous key metabolic genes, such as those controlling the enzymes for glycolysis and cholesterol biosynthesis (Su et al. 2008), which aid cumulus cell-specific functions. Recently, GDF9 and BMP15 have also been implicated in the signalling events required for ovulation. Ovulation is triggered when the preovulatory LH surge initiates a cascade of events, resulting in gap junction closure and an immediate decline in intraoocyte cGMP and cAMP levels, allowing meiosis to resume. In contrast with mural granulosa cells, where the expression of LH receptors increases during selection of the dominant follicle (Webb and Campbell 2007; Scaramuzzi et al. 2011), cumulus cells lose LH receptors as the follicle grows (Baltar et al. 2000). Thus, the effect of the LH surge signal is facilitated through the production of epidermal growth-factor-like peptides (EGF-p) from granulosa cells. The EGF-p bind onto epidermal growth factor receptors (EGFR) on cumulus cells to promote the final events in maturation, such as cumulus cell expansion (Sela-Abramovich et al. 2006; Norris et al. 2008; Vaccari et al. 2009). The functionality of EGFRs on cumulus cells is promoted through the cooperation of cAMP, GDF9 and BMP15 (Sugimura et al. 2015).
Clearly, this new understanding of the regulation of oocyte maturation must be used to revise our methodologies if we are to further improve the production of embryos in vitro. For example, we know that the addition of BMP15 and GDF9 to IVM media improves the blastocyst rate following IVF (Hussein et al. 2006). Similarly, we would expect the addition of C-type natriuretic peptide in place of pharmacological agents, such as PDE inhibitors or exogenous cAMP (Gilchrist et al. 2016), to prevent precocious resumption of meiosis and improve oocyte quality (Santiquet et al. 2017; Zhang et al. 2017a). This approach has led to the development of a physiologically relevant IVM system (PR-IVM) that mimics the intrafollicular microenvironment of bovine follicles with respect to the concentrations of amino acids, energy substrates, steroids, hormones, growth factors and metabolites (Murray 2019). With bovine cumulus cell–oocyte complexes, PR-IVM did not improve the blastocyst rate of abattoir-sourced cumulus cell–oocyte complexes after IVF; however, it did decrease the number of pyknotic cells in both the inner cell mass and trophoblast compartments of the resultant embryos, suggesting an improvement in embryo quality (Murray 2019). It is becoming increasingly clear that careful scrutiny of changes in the follicular microenvironment will provide new insights into the regulation of oocyte maturation, better IVM media and greater success in artificial reproductive technologies in any species.
Adipokines as mediators of nutritional and metabolic input to the follicle: the adipokinome
The effects of metabolic state on reproduction and fertility are well documented. In cattle, for example, poor nutritional status, as reflected by a low body condition score, together with offspring presence or suckling, is associated with long postpartum anoestrus, early embryo death and reduced pregnancy and weaning rates (Diskin and Morris 2008). Body condition is related to fertility, especially in the peripartum period, when underfed cows mobilise their lipid reserves, increasing the concentrations of non-esterified fatty acids, reflecting their negative energy balance (Cardoso et al. 2020). The key roles of metabolic hormones, produced by various peripheral tissues, including growth hormone (GH), insulin-like growth factor 1 (IGF1), insulin, ghrelin, tri-iodothyronine, thyroxine and leptin, as well as metabolic factors (glucose, fatty acids, amino acids), that affect each component of the hypothalamic–pituitary–ovarian axis in female cattle have been reviewed many times (Butler 2000; Wettemann et al. 2003; Chagas et al. 2007; Hernandez-Medrano et al. 2012; Dupont et al. 2014; D’Occhio et al. 2019). Body condition score is largely related to the amount of white adipose tissue, which, in addition to playing a key role in energy storage, is an active endocrine tissue that senses metabolic status and secretes numerous biologically active adipokines. For many years, the leptin concentration was viewed as an index of metabolic status, as well as a metabolic signal to the reproductive system. However, studies over the past 10 years have demonstrated the importance of other adipokines produced by adipose tissue, such as adiponectin, resistin, chemerin, visfatin and apelin, in the regulation of bovine ovarian function (Fig. 4; Kurowska et al. 2021).
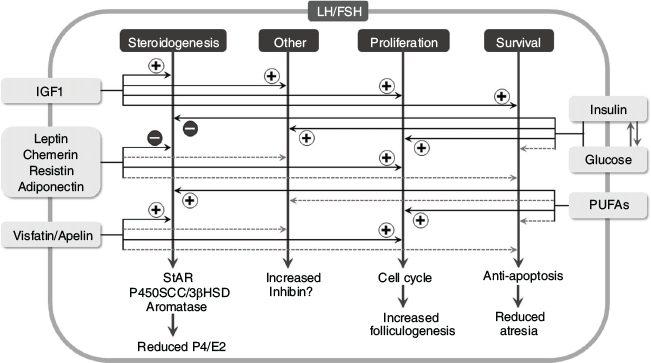
|
Adiponectin plays important roles in the control of lipid metabolism, glucose homeostasis and insulin sensitivity, and evidence is slowly accumulating for its position as a systemic endocrine communicator between metabolism and reproduction. For example, during the normal oestrous cycle of the cow, circulating adiponectin concentrations gradually decrease after ovulation and then increase before the next ovulation (Kafi et al. 2015). Moreover, normal luteal activity starts earlier in high-producing dairy cows that have high postpartum adiponectin concentrations (Kafi et al. 2015).
In addition to their endocrine roles, adipokines can act locally within reproductive tissues; follicles, corpora lutea, oocytes, granulosa cells, theca cells and cumulus cells all have adiponectin and its two receptors, AdipoR1 and AdipoR2 (Lagaly et al. 2008; Maillard et al. 2010; Tabandeh et al. 2010). Similarly, all types of ovarian cells have chemerin and its three G-protein-coupled receptors, chemokine receptor-like 1 (CMKLR1), G-protein-coupled receptor 1 (GPR1) and C-C chemokine receptor-like 2 (CCRL2), apelin and its receptor GPR1, and resistin and visfatin, whose receptors are yet to be identified (Maillard et al. 2011; Spicer et al. 2011; Reverchon et al. 2014, 2016; Roche et al. 2017). In vitro studies with bovine granulosa cells have shown that, like leptin, adiponectin, chemerin and resistin decrease FSH-induced steroidogenesis (Lagaly et al. 2008; Maillard et al. 2010, 2011; Spicer et al. 2011; Reverchon et al. 2014), as illustrated in Fig. 4. Moreover, in vitro studies with bovine theca cells have shown that adiponectin decreases insulin-induced production of androgen and progesterone (Lagaly et al. 2008). Conversely, visfatin and apelin increase steroid secretion by granulosa cells (Reverchon et al. 2016; Roche et al. 2017), as illustrated in Fig. 4. Omentin and asprosin have also been identified as adipokines that may regulate ovarian follicle development in polycystic ovarian disease (Bongrani et al. 2019; Deniz et al. 2021). It is important to recognise that monogastrics typically achieve average body fat percentages that are greater than those achieved by ruminants, so the roles of the adipokines could differ significantly between these two types of animals. However, recent research with heifer tissues has shown strong expression of the precursor of asprosin (fibrillin 1 (FBN1)) in theca cells, with regulation by TGFB1, epidermal growth factor and FGFs; furthermore, asprosin inhibits IGF1-stimulated proliferation and enhances LH-induced androstenedione production (Maylem et al. 2021).
Thus, we have strong indications that adipokines play significant paracrine or autocrine roles within the ovary, but these roles are complex. First, most adipokines are produced in more than one variant (e.g. adiponectin, chemerin) and, to date, it has not proven possible to detect all forms in cattle. Second, most of our knowledge comes from in vitro experiments. In vivo studies are limited; for chemerin, for example, some recombinant forms have been synthesised and specific ELISAs have been developed, but only for humans and mice, not cattle (Chang et al. 2016b). Third, many studies report the effects of one or two adipokines, but it is probably important to evaluate the roles of all adipokines simultaneously because additive, antagonistic or synergistic effects are possible. Indeed, during early lactation in cattle, the various adipokines have different circulatory profiles (Mellouk et al. 2017), clearly indicating the need to consider the full ‘adipokinome’ rather than individual adipokines if we are to understand endocrine effects on reproductive function.
Big data and massive complexity: a return to mathematical modelling?
In the first workshop 30 years ago, a critical part of the vision was a functional model of follicle development, follicle selection and the determination of ovulation rate, based on quantitative knowledge of physiological processes rather than anatomical changes (Scaramuzzi et al. 1993). That change in perspective, from descriptive to functional and quantitative, has since been a foundation for hypothesis development in this field. The intention had been to translate the conceptual models into mathematical models that could be used to define knowledge gaps, and to rank research areas based on sensitivity, and therefore importance, for the outcomes of folliculogenesis. That translation did not eventuate, but it is now time to reinvigorate the idea. The past decade has seen the rise of ‘big data’ and bioinformatics, and the development of vast arrays of transcriptomic, proteomic, metabolomic and genomic techniques, all generating massive volumes of information. We need to meet the challenge of translating that information into a useable form that can drive our understanding of follicle development and create new opportunities for managing ovarian development and function for livestock industries, as well as human health.
Conclusion
Over the past decade we continued our journey towards an understanding of the regulation of ovarian follicle development and ovulation rate in domestic ruminants. Finally, we are coming to grips with the origins of the process: the events during fetal life that can determine, for better or worse, the structure of the ovary and therefore act as the foundation of the future fertility of an animal. Having developed, the ovary contains follicles with two major layers of cells, one of which, the theca, was largely forgotten but is now being recognised as critically important. We have also started to recognise the antrum of the mature follicle, a fluid-filled cavity bathing the oocyte and its cumulus cells, as providing a microenvironment that nurtures the oocyte as it prepares for fertilisation and transformation into a healthy embryo. Achieving this first goal in reproduction involves complex interactions among many genes, including major genes that exert profound effects on, for example, the number of follicles that ovulate. These genes continue to provide novel insights into the processes that affect fecundity and fertility. Among the most important changes in our perspective has been a deeper understanding of the role of AMH as a biomarker for the ovarian reserve and as a regulator of fertility. AMH has already provided a breakthrough to the next level of efficiency in assisted reproductive technologies. Gaining a better understanding of the role AMH plays in determining fertility in natural cycles is a challenge that we are just beginning to confront.
The series of follicle workshops leading to this review were originally driven by the need to understand how nutrition affects ovarian follicle development and ovulation rate in sheep. The past four decades have seen an explosion in the variety of metabolic factors, and the intricacy of nutrition–reproduction relationships, that regulate the development of the fetal ovary and folliculogenesis. This revolution is exemplified by the realisation that there is an ‘adipokinome’ and that it is far more important than any single adipokine as a determinant of ovarian activity. Our need to understand the complexity of these systems is further accentuated by the arrival of human-made environmental pollutants that interfere with the very mechanisms that we need to control.
The major new foci of his review, namely AMH, follicle reserve, theca function, the adipokinome and environmental influences, have arisen primarily as a consequence of an ever-expanding variety of new tools and methodologies, bringing with them an explosion of data. Inevitably, one of the big challenges for the next decade will be the synthesis of the resulting massive datasets into a better understanding of the factors that regulate female fertility. It is difficult to see how we can achieve this goal without continued mathematical modelling.
In addition to the changes flowing through our investigative technologies, changes are flowing through society. The world’s population is growing, putting pressure on food security, the planetary environment and the way food is produced. We now have a focus on sustainability, leading inevitably to questions about food-producing animals: questions about food safety, the environmental footprint of food animals and animal welfare. Given this situation, improvements in reproductive efficiency become even more significant. Clearly, sheep or cows that fail to become pregnant effectively only produce methane, and females that wean single rather than twin offspring increase the environmental impact per unit of product. We must continue investigating the processes that link ovarian development in the fetus to folliculogenesis in the adult to the development of a healthy oocyte to the delivery and growth of healthy offspring.
Conflicts of interest
Graeme Martin is the Editor-in-Chief of Reproduction, Fertility and Development, but was blinded from the peer-review process for this paper. Rex Scaramuzzi is an Associate Editor of Reproduction, Fertility and Development and contributed to the organisation of the workshop from which this review was derived.
Declaration of funding
The authors acknowledge the financial support of AgResearch (New Zealand), CSIRO Publishing (Australia) and the Society for Reproduction and Fertility (UK).
References
Aad, P. Y., Echternkamp, S. E., and Spicer, L. J. (2013). Possible role of IGF2 receptors in regulating selection of 2 dominant follicles in cattle selected for twin ovulations and births. Domest. Anim. Endocrinol. 45, 187–195.| Possible role of IGF2 receptors in regulating selection of 2 dominant follicles in cattle selected for twin ovulations and births.Crossref | GoogleScholarGoogle Scholar | 24209503PubMed |
Allan, M. F., Kuehn, L. A., Cushman, R. A., Snelling, W. M., Echternkamp, S. E., and Thallman, R. M. (2009). Confirmation of quantitative trait loci using a low-density single nucleotide polymorphism map for twinning and ovulation rate on bovine chromosome 5. J. Anim. Sci. 87, 46–56.
| Confirmation of quantitative trait loci using a low-density single nucleotide polymorphism map for twinning and ovulation rate on bovine chromosome 5.Crossref | GoogleScholarGoogle Scholar | 18791147PubMed |
Amundson, O. L., Fountain, T. H., Larimore, E. L., Richardson, B. N., McNeel, A. K., Wright, E. C., Keisler, D. H., Cushman, R. A., Perry, G. A., and Freetly, H. C. (2015). Postweaning nutritional programming of ovarian development in beef heifers. J. Anim. Sci. 93, 5232–5239.
| Postweaning nutritional programming of ovarian development in beef heifers.Crossref | GoogleScholarGoogle Scholar | 26641043PubMed |
Antenos, M., Stemler, M., Boime, I., and Woodruff, T. K. (2007). N-linked oligosaccharides direct the differential assembly and secretion of inhibin alpha- and betaA-subunit dimers. Mol. Endocrinol. 21, 1670–1684.
| N-linked oligosaccharides direct the differential assembly and secretion of inhibin alpha- and betaA-subunit dimers.Crossref | GoogleScholarGoogle Scholar | 17456790PubMed |
Anway, M. D., Cupp, A. S., Uzumcu, M., and Skinner, M. K. (2005). Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469.
| Epigenetic transgenerational actions of endocrine disruptors and male fertility.Crossref | GoogleScholarGoogle Scholar | 15933200PubMed |
Armstrong, D. T. (1967). On the site of action of luteinizing hormone. Nature 213, 633–634.
| On the site of action of luteinizing hormone.Crossref | GoogleScholarGoogle Scholar | 6032265PubMed |
Armstrong, D. T., and Hansel, W. (1959). Alteration of the bovine estrous cycle with oxytocin. J. Dairy Sci. 42, 533–542.
| Alteration of the bovine estrous cycle with oxytocin.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. T., and Grinwich, D. L. (1972). Blockade of spontaneous and LH-induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins 1, 21–28.
| Blockade of spontaneous and LH-induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 4633871PubMed |
Armstrong, D. T., and Papkoff, H. (1976). Stimulation of aromatization of exogenous and endogenous androgens in ovaries of hypophysectomized rats in vivo by follicle-stimulating hormone. Endocrinology 99, 1144–1151.
| Stimulation of aromatization of exogenous and endogenous androgens in ovaries of hypophysectomized rats in vivo by follicle-stimulating hormone.Crossref | GoogleScholarGoogle Scholar | 976193PubMed |
Armstrong, D. T., Goff, A. K., and Dorrington, J. H. (1979). Regulation of follicular estrogen biosynthesis. In ‘Ovarian Follicular Development and Function’. (Eds A. R. Midgley and W. A. Sadler) pp. 169–182. (Raven Press: New York)
Árnason, T., and Jónmundsson, J. V. (2008). Multiple trait genetic evaluation of ewe traits in Icelandic sheep. J. Anim. Breed. Genet. 125, 390–396.
| Multiple trait genetic evaluation of ewe traits in Icelandic sheep.Crossref | GoogleScholarGoogle Scholar | 19134074PubMed |
Baltar, A. E., Oliveira, M. A., and Catanho, M. T. (2000). Bovine cumulus/oocyte complex: quantification of LH/hCG receptors. Mol. Reprod. Dev. 55, 433–437.
| Bovine cumulus/oocyte complex: quantification of LH/hCG receptors.Crossref | GoogleScholarGoogle Scholar | 10694751PubMed |
Bartlewski, P. M., Baby, T. E., and Giffin, J. L. (2011). Reproductive cycles in sheep. Anim. Reprod. Sci. 124, 259–268.
| Reproductive cycles in sheep.Crossref | GoogleScholarGoogle Scholar | 21411253PubMed |
Baruselli, P. S., Batista, E. O. S., Vieira, L. M., and Souza, A. H. (2015). Relationship between follicle population, AMH concentration and fertility in cattle. Anim. Reprod. 12, 487–497.
Bellingham, M., Amezaga, M. R., Mandon-Pepin, B., Speers, C. J., Kyle, C. E., Evans, N. P., Sharpe, R. M., Cotinot, C., Rhind, S. M., and Fowler, P. A. (2013). Exposure to chemical cocktails before or after conception—the effect of timing on ovarian development. Mol. Cell. Endocrinol. 376, 156–172.
| Exposure to chemical cocktails before or after conception—the effect of timing on ovarian development.Crossref | GoogleScholarGoogle Scholar | 23791816PubMed |
Bongrani, A., Mellouk, N., Rame, C., Cornuau, M., Guerif, F., Froment, P., and Dupont, J. (2019). Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 20, 3778.
| Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction?Crossref | GoogleScholarGoogle Scholar |
Bonnet, A., Bevilacqua, C., Benne, F., Bodin, L., Cotinot, C., Liaubet, L., Sancristobal, M., Sarry, J., Terenina, E., Martin, P., Tosser-Klopp, G., and Mandon-Pepin, B. (2011). Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection. BMC Genomics 12, 417.
| Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection.Crossref | GoogleScholarGoogle Scholar | 21851638PubMed |
Borg, R. C., Notter, D. R., and Kott, R. W. (2009). Phenotypic and genetic associations between lamb growth traits and adult ewe body weights in western range sheep. J. Anim. Sci. 87, 3506–3514.
| Phenotypic and genetic associations between lamb growth traits and adult ewe body weights in western range sheep.Crossref | GoogleScholarGoogle Scholar | 19648484PubMed |
Borwick, S. C., Rhind, S. M., McMillen, S. R., and Racey, P. A. (1997). Effect of undernutrition of ewes from the time of mating on fetal ovarian development in mid gestation. Reprod. Fertil. Dev. 9, 711–715.
| Effect of undernutrition of ewes from the time of mating on fetal ovarian development in mid gestation.Crossref | GoogleScholarGoogle Scholar | 9623491PubMed |
Butler, W. R. (2000). Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 60–61, 449–457.
| Nutritional interactions with reproductive performance in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 10844215PubMed |
Campbell, B. K., Clinton, M., and Webb, R. (2012). The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 153, 4533–4543.
| The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep).Crossref | GoogleScholarGoogle Scholar | 22778215PubMed |
Campen, K. A., Kucharczyk, K. M., Bogin, B., Ehrlich, J. M., and Combelles, C. M. H. (2018). Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S. Hum. Reprod. 33, 895–904.
| Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S.Crossref | GoogleScholarGoogle Scholar | 29538760PubMed |
Cardoso, F. C., Kalscheur, K. F., and Drackley, J. K. (2020). Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period. J. Dairy Sci. 103, 5684–5693.
| Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period.Crossref | GoogleScholarGoogle Scholar | 32008772PubMed |
Castilho, A. C. S., Price, C. A., Dalanezi, F., Ereno, R. L., Machado, M. F., Barros, C. M., Gasperin, B. G., Goncalves, P. B. D., and Buratini, J. (2017). Evidence that fibroblast growth factor 10 plays a role in follicle selection in cattle. Reprod. Fertil. Dev. 29, 234–243.
| Evidence that fibroblast growth factor 10 plays a role in follicle selection in cattle.Crossref | GoogleScholarGoogle Scholar |
Chagas, L. M., Bass, J. J., Blache, D., Burke, C. R., Kay, J. K., Lindsay, D. R., Lucy, M. C., Martin, G. B., Meier, S., Rhodes, F. M., Roche, J. R., Thatcher, W. W., and Webb, R. (2007). Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. J. Dairy Sci. 90, 4022–4032.
| Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 17699018PubMed |
Chang, H. M., Qiao, J., and Leung, P. C. (2016a). Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum. Reprod. Update 23, 1–18.
| Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors.Crossref | GoogleScholarGoogle Scholar | 27797914PubMed |
Chang, S. S., Eisenberg, D., Zhao, L., Adams, C., Leib, R., Morser, J., and Leung, L. (2016b). Chemerin activation in human obesity. Obesity 24, 1522–1529.
| Chemerin activation in human obesity.Crossref | GoogleScholarGoogle Scholar | 27222113PubMed |
Chantepie, L., Bordes, A., Aletru, M., Burg, C., Debat, F., Rialland, F., Rivemale, F., Tadi, N., Alabart, J. L., Lahoz, B., and Fabre, S. (2018). Search for a pleiotropic effect of the FecLL prolific mutation in Lacaune meat sheep. Reprod. Domest. Anim. 53, 86–87.
| Search for a pleiotropic effect of the FecLL prolific mutation in Lacaune meat sheep.Crossref | GoogleScholarGoogle Scholar |
Chianese, R., Troisi, J., Richards, S., Scafuro, M., Fasano, S., Guida, M., Pierantoni, R., and Meccariello, R. (2018). Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 25, 748–770.
| Bisphenol A in reproduction: Epigenetic effects.Crossref | GoogleScholarGoogle Scholar | 28990514PubMed |
Clark, Z. L., Heath, D. A., O’Connell, A. R., Juengel, J. L., McNatty, K. P., and Pitman, J. L. (2020). The follicular microenvironment in low (++) and high (I+B+) ovulation rate ewes. Reproduction 159, 585–599.
| The follicular microenvironment in low (++) and high (I+B+) ovulation rate ewes.Crossref | GoogleScholarGoogle Scholar | 32069217PubMed |
Crisosto, N., Sir-Petermann, T., Greiner, M., Maliqueo, M., Moreno, M., Aedo, P., and Lara, H. E. (2009). Testosterone-induced downregulation of anti-Mullerian hormone expression in granulosa cells from small bovine follicles. Endocrine 36, 339–345.
| Testosterone-induced downregulation of anti-Mullerian hormone expression in granulosa cells from small bovine follicles.Crossref | GoogleScholarGoogle Scholar | 19714502PubMed |
Cruz, G., Foster, W., Paredes, A., Yi, K. D., and Uzumcu, M. (2014). Long-term effects of early-life exposure to environmental oestrogens on ovarian function: role of epigenetics. J. Neuroendocrinol. 26, 613–624.
| Long-term effects of early-life exposure to environmental oestrogens on ovarian function: role of epigenetics.Crossref | GoogleScholarGoogle Scholar | 25040227PubMed |
Cushman, R. A., McNeel, A. K., and Freetly, H. C. (2014). The impact of cow nutrient status during the second and third trimesters on age at puberty, antral follicle count, and fertility of daughters. Livest. Sci. 162, 252–258.
| The impact of cow nutrient status during the second and third trimesters on age at puberty, antral follicle count, and fertility of daughters.Crossref | GoogleScholarGoogle Scholar |
D’Occhio, M. J., Baruselli, P. S., and Campanile, G. (2019). Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 125, 277–284.
| Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review.Crossref | GoogleScholarGoogle Scholar | 30497026PubMed |
D’Occhio, M. J., Campanile, G., and Baruselli, P. S. (2020). Transforming growth factor-beta superfamily and interferon-tau in ovarian function and embryo development in female cattle: review of biology and application. Reprod. Fertil. Dev. 32, 539–552.
| Transforming growth factor-beta superfamily and interferon-tau in ovarian function and embryo development in female cattle: review of biology and application.Crossref | GoogleScholarGoogle Scholar |
Dai, Y., Ivell, R., and Anand-Ivell, R. (2017). Theca cell INSL3 and steroids together orchestrate the growing bovine antral follicle. Front. Physiol. 8, 1033.
| Theca cell INSL3 and steroids together orchestrate the growing bovine antral follicle.Crossref | GoogleScholarGoogle Scholar | 29311967PubMed |
Deniz, R., Yavuzkir, S., Ugur, K., Ustebay, D. U., Baykus, Y., Ustebay, S., and Aydin, S. (2021). Subfatin and asprosin, two new metabolic players of polycystic ovary syndrome. J. Obstet. Gynaecol. 41, 279–284.
| Subfatin and asprosin, two new metabolic players of polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar | 32608281PubMed |
Diskin, M. G., and Morris, D. G. (2008). Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 43, 260–267.
| Embryonic and early foetal losses in cattle and other ruminants.Crossref | GoogleScholarGoogle Scholar | 18638133PubMed |
Drouilhet, L., Taragnat, C., Fontaine, J., Duittoz, A., Mulsant, P., Bodin, L., and Fabre, S. (2010). Endocrine characterization of the reproductive axis in highly prolific lacaune sheep homozygous for the FecLL mutation. Biol. Reprod. 82, 815–824.
| Endocrine characterization of the reproductive axis in highly prolific lacaune sheep homozygous for the FecLL mutation.Crossref | GoogleScholarGoogle Scholar | 20075395PubMed |
Drouilhet, L., Mansanet, C., Sarry, J., Tabet, K., Bardou, P., Woloszyn, F., Lluch, J., Harichaux, G., Viguie, C., Monniaux, D., Bodin, L., Mulsant, P., and Fabre, S. (2013). The highly prolific phenotype of Lacaune sheep is associated with an ectopic expression of the B4GALNT2 gene within the ovary. PLoS Genet. 9, e1003809.
| The highly prolific phenotype of Lacaune sheep is associated with an ectopic expression of the B4GALNT2 gene within the ovary.Crossref | GoogleScholarGoogle Scholar | 24086150PubMed |
Dupont, J., Scaramuzzi, R. J., and Reverchon, M. (2014). The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal 8, 1031–1044.
| The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants.Crossref | GoogleScholarGoogle Scholar | 24774511PubMed |
Echternkamp, S. E., Spicer, L. J., Gregory, K. E., Canning, S. F., and Hammond, J. M. (1990). Concentrations of insulin-like growth factor-I in blood and ovarian follicular fluid of cattle selected for twins. Biol. Reprod. 43, 8–14.
| Concentrations of insulin-like growth factor-I in blood and ovarian follicular fluid of cattle selected for twins.Crossref | GoogleScholarGoogle Scholar | 2393694PubMed |
Echternkamp, S. E., Roberts, A. J., Lunstra, D. D., Wise, T., and Spicer, L. J. (2004). Ovarian follicular development in cattle selected for twin ovulations and births. J. Anim. Sci. 82, 459–471.
| Ovarian follicular development in cattle selected for twin ovulations and births.Crossref | GoogleScholarGoogle Scholar | 14974544PubMed |
Eichenlaub-Ritter, U., and Pacchierotti, F. (2015). Bisphenol A effects on mammalian oogenesis and epigenetic integrity of oocytes: A case study exploring risks of endocrine disrupting chemicals. BioMed Res. Int. 2015, 698795.
| Bisphenol A effects on mammalian oogenesis and epigenetic integrity of oocytes: A case study exploring risks of endocrine disrupting chemicals.Crossref | GoogleScholarGoogle Scholar | 26339634PubMed |
Eisler, M. C., Lee, M. R., Tarlton, J. F., Martin, G. B., Beddington, J., Dungait, J. A., Greathead, H., Liu, J., Mathew, S., Miller, H., Misselbrook, T., Murray, P., Vinod, V. K., Van Saun, R., and Winter, M. (2014). Agriculture: Steps to sustainable livestock. Nature 507, 32–34.
| Agriculture: Steps to sustainable livestock.Crossref | GoogleScholarGoogle Scholar | 24605375PubMed |
El-Sheikh Ali, H., Kitahara, G., Takahashi, T., Mido, S., Sadawy, M., Kobayashi, I., Hemmi, K., and Osawa, T. (2017). Plasma anti-Mullerian hormone profile in heifers from birth through puberty and relationship with puberty onset. Biol. Reprod. 97, 153–161.
| Plasma anti-Mullerian hormone profile in heifers from birth through puberty and relationship with puberty onset.Crossref | GoogleScholarGoogle Scholar | 28859283PubMed |
Eppig, J. J. (1993) Regulation of mammalian oocyte maturation. In ‘The Ovary’. (Eds E. Y. Adashi and P. C. K. Leung) pp. 185–208. (Raven Press Ltd.: New York)
Eppig, J. J., Wigglesworth, K., Pendola, F., and Hirao, Y. (1997). Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol. Reprod. 56, 976–984.
| Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells.Crossref | GoogleScholarGoogle Scholar | 9096881PubMed |
Erickson, B. H. (1966). Development and senescence of the postnatal bovine ovary. J. Anim. Sci. 25, 800–805.
| Development and senescence of the postnatal bovine ovary.Crossref | GoogleScholarGoogle Scholar | 6007918PubMed |
Estienne, A., Pierre, A., di Clemente, N., Picard, J. Y., Jarrier, P., Mansanet, C., Monniaux, D., and Fabre, S. (2015). Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway. Endocrinology 156, 301–313.
| Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway.Crossref | GoogleScholarGoogle Scholar | 25322464PubMed |
Evans, A. C., Mossa, F., Walsh, S. W., Scheetz, D., Jimenez-Krassel, F., Ireland, J. L., Smith, G. W., and Ireland, J. J. (2012). Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod. Domest. Anim. 47, 31–37.
| Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring.Crossref | GoogleScholarGoogle Scholar | 22827347PubMed |
Fabre, S., Pierre, A., Mulsant, P., Bodin, L., Di Pasquale, E., Persani, L., Monget, P., and Monniaux, D. (2006). Regulation of ovulation rate in mammals: contribution of sheep genetic models. Reprod. Biol. Endocrinol. 4, 20.
| Regulation of ovulation rate in mammals: contribution of sheep genetic models.Crossref | GoogleScholarGoogle Scholar | 16611365PubMed |
Ferdousy, R. N., Kereilwe, O., and Kadokawa, H. (2020). Anti-Müllerian hormone is expressed and secreted by bovine oviductal and endometrial epithelial cells. Anim. Sci. J. 91, e13456.
| Anti-Müllerian hormone is expressed and secreted by bovine oviductal and endometrial epithelial cells.Crossref | GoogleScholarGoogle Scholar |
Ferris, J., Mahboubi, K., MacLusky, N., King, W. A., and Favetta, L. A. (2016). BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reprod. Toxicol. 59, 128–138.
| BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression.Crossref | GoogleScholarGoogle Scholar | 26686065PubMed |
Forde, N., Beltman, M. E., Lonergan, P., Diskin, M., Roche, J. F., and Crowe, M. A. (2011). Oestrous cycles in Bos taurus cattle. Anim. Reprod. Sci. 124, 163–169.
| Oestrous cycles in Bos taurus cattle.Crossref | GoogleScholarGoogle Scholar | 20875708PubMed |
Fowler, P. A., Dora, N. J., McFerran, H., Amezaga, M. R., Miller, D. W., Lea, R. G., Cash, P., McNeilly, A. S., Evans, N. P., Cotinot, C., Sharpe, R. M., and Rhind, S. M. (2008). In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol. Hum. Reprod. 14, 269–280.
| In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep.Crossref | GoogleScholarGoogle Scholar | 18436539PubMed |
Franciosi, F., Coticchio, G., Lodde, V., Tessaro, I., Modina, S. C., Fadini, R., Dal Canto, M., Renzini, M. M., Albertini, D. F., and Luciano, A. M. (2014). Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol. Reprod. 91, 61.
| Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes.Crossref | GoogleScholarGoogle Scholar | 25078681PubMed |
Freetly, H. C., Vonnahme, K. A., McNeel, A. K., Camacho, L. E., Amundson, O. L., Forbes, E. D., Lents, C. A., and Cushman, R. A. (2014). The consequence of level of nutrition on heifer ovarian and mammary development. J. Anim. Sci. 92, 5437–5443.
| The consequence of level of nutrition on heifer ovarian and mammary development.Crossref | GoogleScholarGoogle Scholar | 25403194PubMed |
Freetly, H. C., Cushman, R. A., and Bennett, G. L. (2021). Production performance of cows raised with different post weaning growth patterns. Transl. Anim. Sci , .
| Production performance of cows raised with different post weaning growth patterns.Crossref | GoogleScholarGoogle Scholar | 33659864PubMed |
García-Guerra, A., Motta, J. C. L., Melo, L. F., Kirkpatrick, B. W., and Wiltbank, M. C. (2017). Ovulation rate, antral follicle count, and circulating anti-Mullerian hormone in Trio allele carriers, a novel high fecundity bovine genotype. Theriogenology 101, 81–90.
| Ovulation rate, antral follicle count, and circulating anti-Mullerian hormone in Trio allele carriers, a novel high fecundity bovine genotype.Crossref | GoogleScholarGoogle Scholar | 28708520PubMed |
García-Guerra, A., Canavessi, A. M. O., Monteiro, P. L. J., Mezera, M. A., Sartori, R., Kirkpatrick, B. W., and Wiltbank, M. C. (2018a). Trio, a novel bovine high fecundity allele: III. Acquisition of dominance and ovulatory capacity at a smaller follicle size. Biol. Reprod. 98, 350–365.
| Trio, a novel bovine high fecundity allele: III. Acquisition of dominance and ovulatory capacity at a smaller follicle size.Crossref | GoogleScholarGoogle Scholar | 29425314PubMed |
García-Guerra, A., Kamalludin, M. H., Kirkpatrick, B. W., and Wiltbank, M. C. (2018b). Trio a novel bovine high-fecundity allele: II. Hormonal profile and follicular dynamics underlying the high ovulation rate. Biol. Reprod. 98, 335–349.
| Trio a novel bovine high-fecundity allele: II. Hormonal profile and follicular dynamics underlying the high ovulation rate.Crossref | GoogleScholarGoogle Scholar | 29425274PubMed |
García-Guerra, A., Wiltbank, M. C., Battista, S. E., Kirkpatrick, B. W., and Sartori, R. (2018c). Mechanisms regulating follicle selection in ruminants: lessons learned from multiple ovulation models. Anim. Reprod. 15, 660–679.
| Mechanisms regulating follicle selection in ruminants: lessons learned from multiple ovulation models.Crossref | GoogleScholarGoogle Scholar |
Gilchrist, R. B., Lane, M., and Thompson, J. G. (2008). Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 14, 159–177.
| Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality.Crossref | GoogleScholarGoogle Scholar | 18175787PubMed |
Gilchrist, R. B., Luciano, A. M., Richani, D., Zeng, H. T., Wang, X., Vos, M. D., Sugimura, S., Smitz, J., Richard, F. J., and Thompson, J. G. (2016). Oocyte maturation and quality: role of cyclic nucleotides. Reproduction 152, R143–R157.
| Oocyte maturation and quality: role of cyclic nucleotides.Crossref | GoogleScholarGoogle Scholar | 27422885PubMed |
Glister, C., Kemp, C. F., and Knight, P. G. (2004). Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 127, 239–254.
| Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin.Crossref | GoogleScholarGoogle Scholar | 15056790PubMed |
Glister, C., Richards, S. L., and Knight, P. G. (2005). Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 146, 1883–1892.
| Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling?Crossref | GoogleScholarGoogle Scholar | 15625241PubMed |
Glister, C., Satchell, L., and Knight, P. G. (2011). Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro. Reproduction 142, 581–591.
| Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro.Crossref | GoogleScholarGoogle Scholar | 21821720PubMed |
Glister, C., Satchell, L., Bathgate, R. A., Wade, J. D., Dai, Y., Ivell, R., Anand-Ivell, R., Rodgers, R. J., and Knight, P. G. (2013). Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc. Natl. Acad. Sci. USA 110, E1426–E1435.
| Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production.Crossref | GoogleScholarGoogle Scholar | 23530236PubMed |
Glister, C., Regan, S. L., Samir, M., and Knight, P. (2019). Gremlin, Noggin, Chordin and follistatin differentially modulate BMP induced suppression of androgen secretion by bovine ovarian theca cells. J. Mol. Endocrinol. 62, 15–25.
| Gremlin, Noggin, Chordin and follistatin differentially modulate BMP induced suppression of androgen secretion by bovine ovarian theca cells.Crossref | GoogleScholarGoogle Scholar |
Gobikrushanth, M., Purfield, D. C., Colazo, M. G., Butler, S. T., Wang, Z., and Ambrose, D. J. (2018). The relationship between serum anti-Mullerian hormone concentrations and fertility, and genome-wide associations for anti-Mullerian hormone in Holstein cows. J. Dairy Sci. 101, 7563–7574.
| The relationship between serum anti-Mullerian hormone concentrations and fertility, and genome-wide associations for anti-Mullerian hormone in Holstein cows.Crossref | GoogleScholarGoogle Scholar | 29729909PubMed |
Gobikrushanth, M., Purfield, D. C., Canadas, E. R., Herlihy, M. M., Kenneally, J., Murray, M., Kearney, F. J., Colazo, M. G., Ambrose, D. J., and Butler, S. T. (2019). Anti-Mullerian hormone in grazing dairy cows: Identification of factors affecting plasma concentration, relationship with phenotypic fertility, and genome-wide associations. J. Dairy Sci. 102, 11622–11635.
| Anti-Mullerian hormone in grazing dairy cows: Identification of factors affecting plasma concentration, relationship with phenotypic fertility, and genome-wide associations.Crossref | GoogleScholarGoogle Scholar | 31521342PubMed |
Grynberg, M., Pierre, A., Rey, R., Leclerc, A., Arouche, N., Hesters, L., Catteau-Jonard, S., Frydman, R., Picard, J. Y., Fanchin, R., Veitia, R., di Clemente, N., and Taieb, J. (2012). Differential regulation of ovarian anti-mullerian hormone (AMH) by estradiol through alpha- and beta-estrogen receptors. J. Clin. Endocrinol. Metab. 97, E1649–E1657.
| Differential regulation of ovarian anti-mullerian hormone (AMH) by estradiol through alpha- and beta-estrogen receptors.Crossref | GoogleScholarGoogle Scholar | 22689696PubMed |
Guerrero-Netro, H. M., Estienne, A., Chorfi, Y., and Price, C. A. (2017). The mycotoxin metabolite deepoxy- deoxynivalenol increases apoptosis and decreases steroidogenesis in bovine ovarian theca cells. Biol. Reprod. 97, 746–757.
| The mycotoxin metabolite deepoxy- deoxynivalenol increases apoptosis and decreases steroidogenesis in bovine ovarian theca cells.Crossref | GoogleScholarGoogle Scholar | 29045588PubMed |
Han, S. J., and Conti, M. (2006). New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle 5, 227–231.
| New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate.Crossref | GoogleScholarGoogle Scholar | 16418576PubMed |
Han, P., Guerrero-Netro, H., Estienne, A., and Price, C. A. (2018). Effects of fibroblast growth factors and the transcription factor, early growth response 1, on bovine theca cells. Mol. Cell. Endocrinol. 476, 96–102.
| Effects of fibroblast growth factors and the transcription factor, early growth response 1, on bovine theca cells.Crossref | GoogleScholarGoogle Scholar | 29723542PubMed |
Hatzirodos, N., Irving-Rodgers, H. F., Hummitzsch, K., and Rodgers, R. J. (2014). Transcriptome profiling of the theca interna from bovine ovarian follicles during atresia. PLoS One 9, e99706.
| Transcriptome profiling of the theca interna from bovine ovarian follicles during atresia.Crossref | GoogleScholarGoogle Scholar | 24956388PubMed |
Hernandez-Medrano, J. H., Campbell, B. K., and Webb, R. (2012). Nutritional influences on folliculogenesis. Reprod. Domest. Anim. 47, 274–282.
| Nutritional influences on folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 22827381PubMed |
Hiradate, Y., Hoshino, Y., Tanemura, K., and Sato, E. (2014). C-type natriuretic peptide inhibits porcine oocyte meiotic resumption. Zygote 22, 372–377.
| C-type natriuretic peptide inhibits porcine oocyte meiotic resumption.Crossref | GoogleScholarGoogle Scholar | 23331536PubMed |
Huang, J., and Zeng, H. (2021). The influence of environmental factors on ovarian function, follicular genesis, and oocyte quality. Adv. Exp. Med. Biol. 1300, 41–62.
| The influence of environmental factors on ovarian function, follicular genesis, and oocyte quality.Crossref | GoogleScholarGoogle Scholar | 33523429PubMed |
Hussein, T. S., Thompson, J. G., and Gilchrist, R. B. (2006). Oocyte-secreted factors enhance oocyte developmental competence. Dev. Biol. 296, 514–521.
| Oocyte-secreted factors enhance oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 16854407PubMed |
(2018). Recipient of the 2018 IETS Pioneer Award: David Thomas Armstrong, BSA, MSc, PhD. Reprod. Fertil. Dev. 30, xxii–xxiv.
| Recipient of the 2018 IETS Pioneer Award: David Thomas Armstrong, BSA, MSc, PhD.Crossref | GoogleScholarGoogle Scholar |
Ireland, J. L., Scheetz, D., Jimenez-Krassel, F., Themmen, A. P., Ward, F., Lonergan, P., Smith, G. W., Perez, G. I., Evans, A. C., and Ireland, J. J. (2008). Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 79, 1219–1225.
| Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle.Crossref | GoogleScholarGoogle Scholar | 18768912PubMed |
Irving-Rodgers, H. F., van Wezel, I. L., Mussard, M. L., Kinder, J. E., and Rodgers, R. J. (2001). Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction 122, 761–775.
| Atresia revisited: two basic patterns of atresia of bovine antral follicles.Crossref | GoogleScholarGoogle Scholar | 11690537PubMed |
Jaton, C., Koeck, A., Sargolzaei, M., Malchiodi, F., Price, C. A., Schenkel, F. S., and Miglior, F. (2016). Genetic analysis of superovulatory response of Holstein cows in Canada. J. Dairy Sci. 99, 3612–3623.
| Genetic analysis of superovulatory response of Holstein cows in Canada.Crossref | GoogleScholarGoogle Scholar | 26923051PubMed |
Jiang, Z., and Price, C. A. (2012). Differential actions of fibroblast growth factors on intracellular pathways and target gene expression in bovine ovarian granulosa cells. Reproduction 144, 625–632.
| Differential actions of fibroblast growth factors on intracellular pathways and target gene expression in bovine ovarian granulosa cells.Crossref | GoogleScholarGoogle Scholar | 22956519PubMed |
Jiang, Z., Guerrero-Netro, H. M., Juengel, J. L., and Price, C. A. (2013). Divergence of intracellular signaling pathways and early response genes of two closely related fibroblast growth factors, FGF8 and FGF18, in bovine ovarian granulosa cells. Mol. Cell. Endocrinol. 375, 97–105.
| Divergence of intracellular signaling pathways and early response genes of two closely related fibroblast growth factors, FGF8 and FGF18, in bovine ovarian granulosa cells.Crossref | GoogleScholarGoogle Scholar | 23707615PubMed |
Jimenez-Krassel, F., Folger, J. K., Ireland, J. L., Smith, G. W., Hou, X., Davis, J. S., Lonergan, P., Evans, A. C., and Ireland, J. J. (2009). Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biol. Reprod. 80, 1272–1281.
| Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle.Crossref | GoogleScholarGoogle Scholar | 19211804PubMed |
Juengel, J. L. (2018). How the quest to improve sheep reproduction provided insight into oocyte control of folliculr development. J. R. Soc. N. Z. 48, 143–163.
| How the quest to improve sheep reproduction provided insight into oocyte control of folliculr development.Crossref | GoogleScholarGoogle Scholar |
Juengel, J. L., Davis, G. H., and McNatty, K. P. (2013). Using sheep lines with mutations in single genes to better understand ovarian function. Reproduction 146, R111–R123.
| Using sheep lines with mutations in single genes to better understand ovarian function.Crossref | GoogleScholarGoogle Scholar | 23801782PubMed |
Juengel, J. L., Smith, P. R., Quirke, L. D., French, M. C., and Edwards, S. J. (2018). The local regulation of folliculogenesis by members of the transforming growth factor superfamily and its relevance for advanced breeding programmes. Anim. Reprod. 15, 180–190.
| The local regulation of folliculogenesis by members of the transforming growth factor superfamily and its relevance for advanced breeding programmes.Crossref | GoogleScholarGoogle Scholar | 34178140PubMed |
Kafi, M., Tamadon, A., and Saeb, M. (2015). The relationship between serum adiponectin and postpartum luteal activity in high-producing dairy cows. Theriogenology 83, 1264–1271.
| The relationship between serum adiponectin and postpartum luteal activity in high-producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 25680575PubMed |
Kurowska, P., Mlyczyńska, E., Dawid, M., Sierpowski, M., Estienne, A., Dupont, J., and Rak, A. (2021). Adipokines change the balance of proliferation/apoptosis in the ovarian cells of human and domestic animals: a comparative review. Anim. Reprod. Sci. 228, 106737.
| Adipokines change the balance of proliferation/apoptosis in the ovarian cells of human and domestic animals: a comparative review.Crossref | GoogleScholarGoogle Scholar | 33756403PubMed |
Lagaly, D. V., Aad, P. Y., Grado-Ahuir, J. A., Hulsey, L. B., and Spicer, L. J. (2008). Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol. Cell. Endocrinol. 284, 38–45.
| Role of adiponectin in regulating ovarian theca and granulosa cell function.Crossref | GoogleScholarGoogle Scholar | 18289773PubMed |
Lahoz, B., Alabart, J. L., Jurado, J. J., Calvo, J. H., Martinez-Royo, A., Fantova, E., and Folch, J. (2011). Effect of the FecX(R) polymorphism in the bone morphogenetic protein 15 gene on natural or equine chorionic gonadotropin-induced ovulation rate and litter size in Rasa Aragonesa ewes and implications for on-farm application. J. Anim. Sci. 89, 3522–3530.
| Effect of the FecX(R) polymorphism in the bone morphogenetic protein 15 gene on natural or equine chorionic gonadotropin-induced ovulation rate and litter size in Rasa Aragonesa ewes and implications for on-farm application.Crossref | GoogleScholarGoogle Scholar | 21622876PubMed |
Lahoz, B., Alabart, J. L., Monniaux, D., Mermillod, P., and Folch, J. (2012). Anti-Mullerian hormone plasma concentration in prepubertal ewe lambs as a predictor of their fertility at a young age. BMC Vet. Res. 8, 118.
| Anti-Mullerian hormone plasma concentration in prepubertal ewe lambs as a predictor of their fertility at a young age.Crossref | GoogleScholarGoogle Scholar | 22824005PubMed |
Lahoz, B., Alabart, J. L., Folch, J., Sanchez, P., Echegoyen, E., and Cocero, M. J. (2013). Influence of the FecX(R) allele in heterozygous ewes on follicular population and outcomes of IVP and ET using LOPU-derived oocytes. Reprod. Domest. Anim. 48, 717–723.
| Influence of the FecX(R) allele in heterozygous ewes on follicular population and outcomes of IVP and ET using LOPU-derived oocytes.Crossref | GoogleScholarGoogle Scholar | 23438026PubMed |
Lahoz, B., Alabart, J. L., Cocero, M. J., Monniaux, D., Echegoyen, E., Sanchez, P., and Folch, J. (2014). Anti-Mullerian hormone concentration in sheep and its dependence of age and independence of BMP15 genotype: an endocrine predictor to select the best donors for embryo biotechnologies. Theriogenology 81, 347–357.
| Anti-Mullerian hormone concentration in sheep and its dependence of age and independence of BMP15 genotype: an endocrine predictor to select the best donors for embryo biotechnologies.Crossref | GoogleScholarGoogle Scholar | 24268018PubMed |
Lea, R. G., Andrade, L. P., Rae, M. T., Hannah, L. T., Kyle, C. E., Murray, J. F., Rhind, S. M., and Miller, D. W. (2006). Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction 131, 113–124.
| Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary.Crossref | GoogleScholarGoogle Scholar | 16388015PubMed |
Lea, R. G., Amezaga, M. R., Loup, B., Mandon-Pepin, B., Stefansdottir, A., Filis, P., Kyle, C., Zhang, Z., Allen, C., Purdie, L., Jouneau, L., Cotinot, C., Rhind, S. M., Sinclair, K. D., and Fowler, P. A. (2016). The fetal ovary exhibits temporal sensitivity to a ‘real-life’ mixture of environmental chemicals. Sci. Rep. 6, 22279.
| The fetal ovary exhibits temporal sensitivity to a ‘real-life’ mixture of environmental chemicals.Crossref | GoogleScholarGoogle Scholar | 26931299PubMed |
Maillard, V., Uzbekova, S., Guignot, F., Perreau, C., Rame, C., Coyral-Castel, S., and Dupont, J. (2010). Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrinol. 8, 23.
| Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development.Crossref | GoogleScholarGoogle Scholar | 20219117PubMed |
Maillard, V., Froment, P., Rame, C., Uzbekova, S., Elis, S., and Dupont, J. (2011). Expression and effect of resistin on bovine and rat granulosa cell steroidogenesis and proliferation. Reproduction 141, 467–479.
| Expression and effect of resistin on bovine and rat granulosa cell steroidogenesis and proliferation.Crossref | GoogleScholarGoogle Scholar | 21239528PubMed |
Manikkam, M., Haque, M. M., Guerrero-Bosagna, C., Nilsson, E. E., and Skinner, M. K. (2014). Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One 9, e102091.
| Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline.Crossref | GoogleScholarGoogle Scholar | 25057798PubMed |
Martin, G. B., Scaramuzzi, R. J., and Henstridge, J. D. (1983). Effects of oestradiol, progesterone and androstenedione on the pulsatile secretion of luteinizing hormone in ovariectomized ewes during spring and autumn. J. Endocrinol. 96, 181–193.
| Effects of oestradiol, progesterone and androstenedione on the pulsatile secretion of luteinizing hormone in ovariectomized ewes during spring and autumn.Crossref | GoogleScholarGoogle Scholar | 6827203PubMed |
Martin, G. B., Durmic, Z., Kenyon, P. R., and Vercoe, P. E. (2009). Landcorp Farming Limited Lecture: “Clean, green and ethical” animal reproduction: extension to sheep and dairy systems in New Zealand. Proc. N.Z. Soc. Anim. Prod. 69, 140–147.
Martin, P., Raoul, J., and Bodin, L. (2014). Effects of the FecL major gene in the Lacaune meat sheep population. Genet. Sel. Evol. 46, 48.
| Effects of the FecL major gene in the Lacaune meat sheep population.Crossref | GoogleScholarGoogle Scholar | 25158754PubMed |
Martinez, M. F., Sanderson, N., Quirke, L. D., Lawrence, S. B., and Juengel, J. L. (2016). Association between antral follicle count and reproductive measures in New Zealand lactating dairy cows maintained in a pasture-based production system. Theriogenology 85, 466–475.
| Association between antral follicle count and reproductive measures in New Zealand lactating dairy cows maintained in a pasture-based production system.Crossref | GoogleScholarGoogle Scholar | 26489910PubMed |
Mathew, H., and Mahalingaiah, S. (2019). Do prenatal exposures pose a real threat to ovarian function? Bisphenol A as a case study. Reproduction 157, R143–R157.
| Do prenatal exposures pose a real threat to ovarian function? Bisphenol A as a case study.Crossref | GoogleScholarGoogle Scholar | 30689546PubMed |
Mattar, D., Samir, M., Laird, M., and Knight, P. G. (2020). Modulatory effects of TGF-beta1 and BMP6 on thecal angiogenesis and steroidogenesis in the bovine ovary. Reproduction 159, 397–408.
| Modulatory effects of TGF-beta1 and BMP6 on thecal angiogenesis and steroidogenesis in the bovine ovary.Crossref | GoogleScholarGoogle Scholar | 31967968PubMed |
Maylem, E. R. S., Spicer, L. J., Batalha, I., and Schutz, L. F. (2021). Discovery of a possible role of asprosin in ovarian follicular function. J. Mol. Endocrinol. 66, 35–44.
| Discovery of a possible role of asprosin in ovarian follicular function.Crossref | GoogleScholarGoogle Scholar |
McClellan, K. A., Gosden, R., and Taketo, T. (2003). Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev. Biol. 258, 334–348.
| Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary.Crossref | GoogleScholarGoogle Scholar | 12798292PubMed |
McNatty, K. P., Heath, D. A., Hudson, N. L., Reader, K. L., Quirke, L., Lun, S., and Juengel, J. L. (2010). The conflict between hierarchical ovarian follicular development and superovulation treatment. Reproduction 140, 287–294.
| The conflict between hierarchical ovarian follicular development and superovulation treatment.Crossref | GoogleScholarGoogle Scholar | 20501789PubMed |
McNeel, A. K., and Cushman, R. A. (2015). Influence of puberty and antral follicle count on calving day in crossbred beef heifers. Theriogenology 84, 1061–1066.
| Influence of puberty and antral follicle count on calving day in crossbred beef heifers.Crossref | GoogleScholarGoogle Scholar | 26197954PubMed |
McNeel, A. K., Soares, E. M., Patterson, A. L., Vallet, J. L., Wright, E. C., Larimore, E. L., Amundson, O. L., Miles, J. R., Chase, C. C., Lents, C. A., Wood, J. R., Cupp, A. S., Perry, G. A., and Cushman, R. A. (2017). Beef heifers with diminished numbers of antral follicles have decreased uterine protein concentrations. Anim. Reprod. Sci. 179, 1–9.
| Beef heifers with diminished numbers of antral follicles have decreased uterine protein concentrations.Crossref | GoogleScholarGoogle Scholar | 28215453PubMed |
Mellouk, N., Rame, C., Touze, J. L., Briant, E., Ma, L., Guillaume, D., Lomet, D., Caraty, A., Ntallaris, T., Humblot, P., and Dupont, J. (2017). Involvement of plasma adipokines in metabolic and reproductive parameters in Holstein dairy cows fed with diets with differing energy levels. J. Dairy Sci. 100, 8518–8533.
| Involvement of plasma adipokines in metabolic and reproductive parameters in Holstein dairy cows fed with diets with differing energy levels.Crossref | GoogleScholarGoogle Scholar | 28803009PubMed |
Monniaux, D., Baril, G., Laine, A. L., Jarrier, P., Poulin, N., Cognie, J., and Fabre, S. (2011). Anti-Mullerian hormone as a predictive endocrine marker for embryo production in the goat. Reproduction 142, 845–854.
| Anti-Mullerian hormone as a predictive endocrine marker for embryo production in the goat.Crossref | GoogleScholarGoogle Scholar | 21930684PubMed |
Monniaux, D., Drouilhet, L., Rico, C., Estienne, A., Jarrier, P., Touze, J. L., Sapa, J., Phocas, F., Dupont, J., Dalbies-Tran, R., and Fabre, S. (2012). Regulation of anti-Mullerian hormone production in domestic animals. Reprod. Fertil. Dev. 25, 1–16.
| Regulation of anti-Mullerian hormone production in domestic animals.Crossref | GoogleScholarGoogle Scholar | 23244824PubMed |
Monniaux, D., Clement, F., Dalbies-Tran, R., Estienne, A., Fabre, S., Mansanet, C., and Monget, P. (2014). The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol. Reprod. 90, 85.
| The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link?Crossref | GoogleScholarGoogle Scholar | 24599291PubMed |
Monniaux, D., Genet, C., Maillard, V., Jarrier, P., Adriaensen, H., Hennequet-Antier, C., Laine, A. L., Laclie, C., Papillier, P., Plisson-Petit, F., Estienne, A., Cognie, J., di Clemente, N., Dalbies-Tran, R., and Fabre, S. (2020). Prenatal programming by testosterone of follicular theca cell functions in ovary. Cell. Mol. Life Sci.: CMLS 77, 1177–1196.
| Prenatal programming by testosterone of follicular theca cell functions in ovary.Crossref | GoogleScholarGoogle Scholar | 31327046PubMed |
Montiel, M. D., Krzewinski-Recchi, M. A., Delannoy, P., and Harduin-Lepers, A. (2003). Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acalpha2–3Galbeta-R beta1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem. J. 373, 369–379.
| Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acalpha2–3Galbeta-R beta1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain.Crossref | GoogleScholarGoogle Scholar | 12678917PubMed |
Moraes, J. C. F., and Souza, C. J. H. (2017). Ewes carrying the Booroola and Vacaria prolificacy alleles respond differently to ovulation induction with equine chorionic gonadotrophin. Genet. Mol. Res. 16, gmr16039787.
| Ewes carrying the Booroola and Vacaria prolificacy alleles respond differently to ovulation induction with equine chorionic gonadotrophin.Crossref | GoogleScholarGoogle Scholar |
Mossa, F., and Ireland, J. J. (2019). Physiology and endocrinology symposium: Anti-Mullerian hormone: a biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J. Anim. Sci. 97, 1446–1455.
| Physiology and endocrinology symposium: Anti-Mullerian hormone: a biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows.Crossref | GoogleScholarGoogle Scholar | 30668706PubMed |
Mossa, F., Walsh, S. W., Butler, S. T., Berry, D. P., Carter, F., Lonergan, P., Smith, G. W., Ireland, J. J., and Evans, A. C. (2012). Low numbers of ovarian follicles >/=3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 95, 2355–2361.
| Low numbers of ovarian follicles >/=3 mm in diameter are associated with low fertility in dairy cows.Crossref | GoogleScholarGoogle Scholar | 22541464PubMed |
Mossa, F., Carter, F., Walsh, S. W., Kenny, D. A., Smith, G. W., Ireland, J. L., Hildebrandt, T. B., Lonergan, P., Ireland, J. J., and Evans, A. C. (2013). Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88, 92.
| Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring.Crossref | GoogleScholarGoogle Scholar | 23426432PubMed |
Mossa, F., Jimenez-Krassel, F., Scheetz, D., Weber-Nielsen, M., Evans, A. C. O., and Ireland, J. J. (2017). Anti-Mullerian Hormone (AMH) and fertility management in agricultural species. Reproduction 154, R1–R11.
| Anti-Mullerian Hormone (AMH) and fertility management in agricultural species.Crossref | GoogleScholarGoogle Scholar | 28356501PubMed |
Murdoch, W. J., Van Kirk, E. A., Vonnahme, K. A., and Ford, S. P. (2003). Ovarian responses to undernutrition in pregnant ewes, USA. Reprod. Biol. Endocrinol. 1, 6.
| Ovarian responses to undernutrition in pregnant ewes, USA.Crossref | GoogleScholarGoogle Scholar | 12646075PubMed |
Murray, L. A. (2019). Refinement of a physiologically-relevant IVM/IVF system on oocyte developmental competency in New Zealand dairy cows. PhD thesis, Victoria University of Wellington, New Zealand.
Nawaz, M. Y., Jimenez-Krassel, F., Steibel, J. P., Lu, Y., Baktula, A., Vukasinovic, N., Neuder, L., Ireland, J. L. H., Ireland, J. J., and Tempelman, R. J. (2018). Genomic heritability and genome-wide association analysis of anti-Mullerian hormone in Holstein dairy heifers. J. Dairy Sci. 101, 8063–8075.
| Genomic heritability and genome-wide association analysis of anti-Mullerian hormone in Holstein dairy heifers.Crossref | GoogleScholarGoogle Scholar | 30007805PubMed |
Nichols, J. A., Perego, M. C., Schutz, L. F., Hemple, A. M., and Spicer, L. J. (2019). Hormonal regulation of vascular endothelial growth factor A (VEGFA) gene expression in granulosa and theca cells of cattle1. J. Anim. Sci. 97, 3034–3045.
| Hormonal regulation of vascular endothelial growth factor A (VEGFA) gene expression in granulosa and theca cells of cattle1.Crossref | GoogleScholarGoogle Scholar | 31077271PubMed |
Norris, R. P., Freudzon, M., Mehlmann, L. M., Cowan, A. E., Simon, A. M., Paul, D. L., Lampe, P. D., and Jaffe, L. A. (2008). Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135, 3229–3238.
| Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption.Crossref | GoogleScholarGoogle Scholar | 18776144PubMed |
O’Shea, T., Hillard, M. A., Anderson, S. T., Bindon, B. M., Findlay, J. K., Tsonis, C. G., and Wilkins, J. F. (1994). Inhibin immunization for increasing ovulation rate and superovulation. Theriogenology 41, 3–17.
| Inhibin immunization for increasing ovulation rate and superovulation.Crossref | GoogleScholarGoogle Scholar |
Oliveira Júnior, G. A., Perez, B. C., Cole, J. B., Santana, M. H. A., Silveira, J., Mazzoni, G., Ventura, R. V., Santana, M. L. J., Kadarmideen, H. N., Garrick, D. J., and Ferraz, J. B. S. (2017). Genomic study and Medical Subject Headings enrichment analysis of early pregnancy rate and antral follicle numbers in Nelore heifers. J. Anim. Sci. 95, 4796–4812.
| Genomic study and Medical Subject Headings enrichment analysis of early pregnancy rate and antral follicle numbers in Nelore heifers.Crossref | GoogleScholarGoogle Scholar |
Padmanabhan, V., and Veiga-Lopez, A. (2013). Sheep models of polycystic ovary syndrome phenotype. Mol. Cell. Endocrinol. 373, 8–20.
| Sheep models of polycystic ovary syndrome phenotype.Crossref | GoogleScholarGoogle Scholar | 23084976PubMed |
Pan, Z., Zhang, J., Li, Q., Li, Y., Shi, F., Xie, Z., and Liu, H. (2012). Current advances in epigenetic modification and alteration during mammalian ovarian folliculogenesis. J. Genet. Genomics 39, 111–123.
| Current advances in epigenetic modification and alteration during mammalian ovarian folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 22464470PubMed |
Parrott, J. A., and Skinner, M. K. (1998). Developmental and hormonal regulation of keratinocyte growth factor expression and action in the ovarian follicle. Endocrinology 139, 228–235.
| Developmental and hormonal regulation of keratinocyte growth factor expression and action in the ovarian follicle.Crossref | GoogleScholarGoogle Scholar | 9421419PubMed |
Pfeiffer, K. E., Jury, L. J., and Larson, J. E. (2014). Determination of anti-Mullerian hormone at estrus during a synchronized and a natural bovine estrous cycle. Domest. Anim. Endocrinol. 46, 58–64.
| Determination of anti-Mullerian hormone at estrus during a synchronized and a natural bovine estrous cycle.Crossref | GoogleScholarGoogle Scholar | 24211073PubMed |
Pierre, A., Estienne, A., Racine, C., Picard, J. Y., Fanchin, R., Lahoz, B., Alabart, J. L., Folch, J., Jarrier, P., Fabre, S., Monniaux, D., and di Clemente, N. (2016). The bone morphogenetic protein 15 up-regulates the anti-Mullerian hormone receptor expression in granulosa cells. J. Clin. Endocrinol. Metab. 101, 2602–2611.
| The bone morphogenetic protein 15 up-regulates the anti-Mullerian hormone receptor expression in granulosa cells.Crossref | GoogleScholarGoogle Scholar | 27070094PubMed |
Pinto, P. H. N., Balaro, M. F. A., Souza-Fabjan, J. M. G., Ribeiro, L. D. S., Braganca, G. M., Leite, C. R., Arashiro, E. K. N., de Moraes Silva, K., Da Fonseca, J. F., and Brandao, F. Z. (2018). Anti-Mullerian hormone and antral follicle count are more effective for selecting ewes with good potential for in vivo embryo production than the presence of FecG(E) mutation or eCG pre-selection tests. Theriogenology 113, 146–152.
| Anti-Mullerian hormone and antral follicle count are more effective for selecting ewes with good potential for in vivo embryo production than the presence of FecG(E) mutation or eCG pre-selection tests.Crossref | GoogleScholarGoogle Scholar |
Pocar, P., Brevini, T. A., Perazzoli, F., Cillo, F., Modina, S., and Gandolfi, F. (2001). Cellular and molecular mechanisms mediating the effects of polychlorinated biphenyls on oocyte developmental competence in cattle. Mol. Reprod. Dev. 60, 535–541.
| Cellular and molecular mechanisms mediating the effects of polychlorinated biphenyls on oocyte developmental competence in cattle.Crossref | GoogleScholarGoogle Scholar | 11746964PubMed |
Poole, D. H., Ocon-Grove, O. M., and Johnson, A. L. (2016). Anti-Mullerian hormone (AMH) receptor type II expression and AMH activity in bovine granulosa cells. Theriogenology 86, 1353–1360.
| Anti-Mullerian hormone (AMH) receptor type II expression and AMH activity in bovine granulosa cells.Crossref | GoogleScholarGoogle Scholar | 27268296PubMed |
Portela, V. M., Dirandeh, E., Guerrero-Netro, H. M., Zamberlam, G., Barreta, M. H., Goetten, A. F., and Price, C. A. (2015). The role of fibroblast growth factor-18 in follicular atresia in cattle. Biol. Reprod. 92, 14.
| The role of fibroblast growth factor-18 in follicular atresia in cattle.Crossref | GoogleScholarGoogle Scholar | 25411391PubMed |
Price, C. A. (2016). Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J. Endocrinol. 228, R31–R43.
| Mechanisms of fibroblast growth factor signaling in the ovarian follicle.Crossref | GoogleScholarGoogle Scholar | 26542145PubMed |
Qi, M. Y., Xu, L. Q., Zhang, J. N., Li, M. O., Lu, M. H., and Yao, Y. C. (2020). Effect of the Booroola fecundity (FecB) gene on the reproductive performance of ewes under assisted reproduction. Theriogenology 142, 246–250.
| Effect of the Booroola fecundity (FecB) gene on the reproductive performance of ewes under assisted reproduction.Crossref | GoogleScholarGoogle Scholar | 31711699PubMed |
Rae, M. T., Palassio, S., Kyle, C. E., Brooks, A. N., Lea, R. G., Miller, D. W., and Rhind, S. M. (2001). Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction 122, 915–922.
| Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses.Crossref | GoogleScholarGoogle Scholar | 11732987PubMed |
Rawlings, N. C., Evans, A. C., Honaramooz, A., and Bartlewski, P. M. (2003). Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim. Reprod. Sci. 78, 259–270.
| Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats.Crossref | GoogleScholarGoogle Scholar | 12818648PubMed |
Reverchon, M., Rame, C., Cognie, J., Briant, E., Elis, S., Guillaume, D., and Dupont, J. (2014). Resistin in dairy cows: plasma concentrations during early lactation, expression and potential role in adipose tissue. PLoS One 9, e93198.
| Resistin in dairy cows: plasma concentrations during early lactation, expression and potential role in adipose tissue.Crossref | GoogleScholarGoogle Scholar | 24675707PubMed |
Reverchon, M., Rame, C., Bunel, A., Chen, W., Froment, P., and Dupont, J. (2016). VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in bovine granulosa cells. Biol. Reprod. 94, 54.
| VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in bovine granulosa cells.Crossref | GoogleScholarGoogle Scholar | 26792944PubMed |
Rhind, S. M., Smith, A., Kyle, C. E., Telfer, G., Martin, G., Duff, E., and Mayes, R. W. (2002). Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants. J. Environ. Monit. 4, 142–148.
| Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants.Crossref | GoogleScholarGoogle Scholar | 11871695PubMed |
Rhind, S. M., Kyle, C. E., Telfer, G., Duff, E. I., and Smith, A. (2005). Alkyl phenols and diethylhexyl phthalate in tissues of sheep grazing pastures fertilized with sewage sludge or inorganic fertilizer. Environ. Health Perspect. 113, 447–453.
| Alkyl phenols and diethylhexyl phthalate in tissues of sheep grazing pastures fertilized with sewage sludge or inorganic fertilizer.Crossref | GoogleScholarGoogle Scholar | 15811823PubMed |
Rhind, S. M., Kyle, C. E., Mackie, C., and McDonald, L. (2009). Accumulation of endocrine disrupting compounds in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge. J. Environ. Monit. 11, 1469–1476.
| Accumulation of endocrine disrupting compounds in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge.Crossref | GoogleScholarGoogle Scholar | 19657530PubMed |
Rhind, S. M., Kyle, C. E., Mackie, C., McDonald, L., Zhang, Z., Duff, E. I., Bellingham, M., Amezaga, M. R., Mandon-Pepin, B., Loup, B., Cotinot, C., Evans, N. P., Sharpe, R. M., and Fowler, P. A. (2010). Maternal and fetal tissue accumulation of selected endocrine disrupting compounds (EDCs) following exposure to sewage sludge-treated pastures before or after conception. J. Environ. Monit. 12, 1582–1593.
| Maternal and fetal tissue accumulation of selected endocrine disrupting compounds (EDCs) following exposure to sewage sludge-treated pastures before or after conception.Crossref | GoogleScholarGoogle Scholar | 20676422PubMed |
Rhind, S. M., Kyle, C. E., Ruffie, H., Calmettes, E., Osprey, M., Zhang, Z. L., Hamilton, D., and McKenzie, C. (2013). Short- and long-term temporal changes in soil concentrations of selected endocrine disrupting compounds (EDCs) following single or multiple applications of sewage sludge to pastures. Environ. Pollut. 181, 262–270.
| Short- and long-term temporal changes in soil concentrations of selected endocrine disrupting compounds (EDCs) following single or multiple applications of sewage sludge to pastures.Crossref | GoogleScholarGoogle Scholar | 23896644PubMed |
Rico, C., Fabre, S., Medigue, C., di Clemente, N., Clement, F., Bontoux, M., Touze, J. L., Dupont, M., Briant, E., Remy, B., Beckers, J. F., and Monniaux, D. (2009). Anti-mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 80, 50–59.
| Anti-mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow.Crossref | GoogleScholarGoogle Scholar | 18784351PubMed |
Rico, C., Medigue, C., Fabre, S., Jarrier, P., Bontoux, M., Clement, F., and Monniaux, D. (2011). Regulation of anti-Mullerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol. Reprod. 84, 560–571.
| Regulation of anti-Mullerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels.Crossref | GoogleScholarGoogle Scholar | 21076084PubMed |
Rivera, O. E., Varayoud, J., Rodriguez, H. A., Munoz-de-Toro, M., and Luque, E. H. (2011). Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 32, 304–312.
| Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb.Crossref | GoogleScholarGoogle Scholar | 21722727PubMed |
Roche, J., Rame, C., Reverchon, M., Mellouk, N., Rak, A., Froment, P., and Dupont, J. (2017). Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reproduction 153, 589–603.
| Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells.Crossref | GoogleScholarGoogle Scholar | 28250234PubMed |
Samir, M., Glister, C., Mattar, D., Laird, M., and Knight, P. G. (2017). Follicular expression of pro-inflammatory cytokines tumour necrosis factor-alpha (TNFalpha), interleukin 6 (IL6) and their receptors in cattle: TNFalpha, IL6 and macrophages suppress thecal androgen production in vitro. Reproduction 154, 35–49.
| Follicular expression of pro-inflammatory cytokines tumour necrosis factor-alpha (TNFalpha), interleukin 6 (IL6) and their receptors in cattle: TNFalpha, IL6 and macrophages suppress thecal androgen production in vitro.Crossref | GoogleScholarGoogle Scholar | 28432091PubMed |
Santa Cruz, R., Cushman, R. A., and Vinoles, C. (2018). Antral follicular count is a tool that may allow the selection of more precocious Bradford heifers at weaning. Theriogenology 119, 35–42.
| Antral follicular count is a tool that may allow the selection of more precocious Bradford heifers at weaning.Crossref | GoogleScholarGoogle Scholar | 29982134PubMed |
Santiquet, N. W., Greene, A. F., Becker, J., Barfield, J. P., Schoolcraft, W. B., and Krisher, R. L. (2017). A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol. Hum. Reprod. 23, 594–606.
| A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 28586460PubMed |
Savabieasfahani, M., Kannan, K., Astapova, O., Evans, N. P., and Padmanabhan, V. (2006). Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 147, 5956–5966.
| Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function.Crossref | GoogleScholarGoogle Scholar | 16946013PubMed |
Sawyer, H. R., Smith, P., Heath, D. A., Juengel, J. L., Wakefield, S. J., and McNatty, K. P. (2002). Formation of ovarian follicles during fetal development in sheep. Biol. Reprod. 66, 1134–1150.
| Formation of ovarian follicles during fetal development in sheep.Crossref | GoogleScholarGoogle Scholar | 11906935PubMed |
Scaramuzzi, R. J., Adams, N. R., Baird, D. T., Campbell, B. K., Downing, J. A., Findlay, J. K., Henderson, K. M., Martin, G. B., McNatty, K. P., McNeilly, A. S., and Tsonis, C. G. (1993). A model for follicle selection and the determination of ovulation rate in the ewe. Reprod. Fertil. Dev. 5, 459–478.
| A model for follicle selection and the determination of ovulation rate in the ewe.Crossref | GoogleScholarGoogle Scholar | 8190903PubMed |
Scaramuzzi, R. J., Baird, D. T., Campbell, B. K., Driancourt, M. A., Dupont, J., Fortune, J. E., Gilchrist, R. B., Martin, G. B., McNatty, K. P., McNeilly, A. S., Monget, P., Monniaux, D., Vinoles, C., and Webb, R. (2011). Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 23, 444–467.
| Regulation of folliculogenesis and the determination of ovulation rate in ruminants.Crossref | GoogleScholarGoogle Scholar | 21426863PubMed |
Scheetz, D., Folger, J. K., Smith, G. W., and Ireland, J. J. (2012). Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod. Fertil. Dev. 24, 327–336.
| Granulosa cells are refractory to FSH action in individuals with a low antral follicle count.Crossref | GoogleScholarGoogle Scholar | 22281079PubMed |
Sela-Abramovich, S., Edry, I., Galiani, D., Nevo, N., and Dekel, N. (2006). Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147, 2280–2286.
| Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation.Crossref | GoogleScholarGoogle Scholar | 16439460PubMed |
Sharma, B., Sarkar, A., Singh, P., and Singh, R. P. (2017). Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 64, 117–132.
| Agricultural utilization of biosolids: A review on potential effects on soil and plant grown.Crossref | GoogleScholarGoogle Scholar | 28336334PubMed |
Shimizu, T. (2016). Molecular and cellular mechanisms for the regulation of ovarian follicular function in cows. J. Reprod. Dev. 62, 323–329.
| Molecular and cellular mechanisms for the regulation of ovarian follicular function in cows.Crossref | GoogleScholarGoogle Scholar | 27097851PubMed |
Siemienowicz, K., Rae, M. T., Howells, F., Anderson, C., Nicol, L. M., Franks, S., and Duncan, W. C. (2020). Insights into manipulating postprandial energy expenditure to manage weight gain in polycystic ovary syndrome. iScience 23, 101164.
| Insights into manipulating postprandial energy expenditure to manage weight gain in polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar | 32464593PubMed |
Sinclair, K. D., Rutherford, K. M., Wallace, J. M., Brameld, J. M., Stoger, R., Alberio, R., Sweetman, D., Gardner, D. S., Perry, V. E., Adam, C. L., Ashworth, C. J., Robinson, J. E., and Dwyer, C. M. (2016). Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod. Fertil. Dev. 28, 1443–1478.
| Epigenetics and developmental programming of welfare and production traits in farm animals.Crossref | GoogleScholarGoogle Scholar |
Smith, P., Steckler, T. L., Veiga-Lopez, A., and Padmanabhan, V. (2009). Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol. Reprod. 80, 726–736.
| Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep.Crossref | GoogleScholarGoogle Scholar | 19092114PubMed |
Smith, P., Wilhelm, D., and Rodgers, R. J. (2014). Development of mammalian ovary. J. Endocrinol. 221, R145–R161.
| Development of mammalian ovary.Crossref | GoogleScholarGoogle Scholar | 24741072PubMed |
Smith, P., Juengel, J., Maclean, P., Rand, C., and Stanton, J. L. (2019a). Gestational nutrition 2: gene expression in sheep fetal ovaries exposed to gestational under nutrition. Reproduction 157, 13–25.
| Gestational nutrition 2: gene expression in sheep fetal ovaries exposed to gestational under nutrition.Crossref | GoogleScholarGoogle Scholar | 30394704PubMed |
Smith, P., Stanton, J. L., Quirke, L., and Juengel, J. L. (2019b). Gestational nutrition 1: alterations to gestational nutrition can increase indicators of fertility in sheep. Reproduction 157, 199–213.
| Gestational nutrition 1: alterations to gestational nutrition can increase indicators of fertility in sheep.Crossref | GoogleScholarGoogle Scholar | 30817311PubMed |
Snelling, W. M., Cushman, R. A., Fortes, M. R., Reverter, A., Bennett, G. L., Keele, J. W., Kuehn, L. A., McDaneld, T. G., Thallman, R. M., and Thomas, M. G. (2012). Physiology and Endocrinology Symposium: How single nucleotide polymorphism chips will advance our knowledge of factors controlling puberty and aid in selecting replacement beef females. J. Anim. Sci. 90, 1152–1165.
| Physiology and Endocrinology Symposium: How single nucleotide polymorphism chips will advance our knowledge of factors controlling puberty and aid in selecting replacement beef females.Crossref | GoogleScholarGoogle Scholar | 22038989PubMed |
Spicer, L. J., Aad, P. Y., Allen, D. T., Mazerbourg, S., Payne, A. H., and Hsueh, A. J. (2008). Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol. Reprod. 78, 243–253.
| Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9.Crossref | GoogleScholarGoogle Scholar | 17959852PubMed |
Spicer, L. J., Schreiber, N. B., Lagaly, D. V., Aad, P. Y., Douthit, L. B., and Grado-Ahuir, J. A. (2011). Effect of resistin on granulosa and theca cell function in cattle. Anim. Reprod. Sci. 124, 19–27.
| Effect of resistin on granulosa and theca cell function in cattle.Crossref | GoogleScholarGoogle Scholar | 21315524PubMed |
Su, Y. Q., Sugiura, K., Wigglesworth, K., O’Brien, M. J., Affourtit, J. P., Pangas, S. A., Matzuk, M. M., and Eppig, J. J. (2008). Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135, 111–121.
| Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells.Crossref | GoogleScholarGoogle Scholar | 18045843PubMed |
Succu, S., Sale, S., Ghirello, G., Ireland, J. J., Evans, A. C. O., Atzori, A. S., and Mossa, F. (2020). Exposure of dairy cows to high environmental temperatures and their lactation status impairs establishment of the ovarian reserve in their offspring. J. Dairy Sci. 103, 11957–11969.
| Exposure of dairy cows to high environmental temperatures and their lactation status impairs establishment of the ovarian reserve in their offspring.Crossref | GoogleScholarGoogle Scholar | 33041040PubMed |
Sugimura, S., Ritter, L. J., Rose, R. D., Thompson, J. G., Smitz, J., Mottershead, D. G., and Gilchrist, R. B. (2015). Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Dev. Biol. 403, 139–149.
| Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes.Crossref | GoogleScholarGoogle Scholar | 25981108PubMed |
Susiarjo, M., Hassold, T. J., Freeman, E., and Hunt, P. A. (2007). Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 3, e5.
| Bisphenol A exposure in utero disrupts early oogenesis in the mouse.Crossref | GoogleScholarGoogle Scholar | 17222059PubMed |
Tabandeh, M. R., Hosseini, A., Saeb, M., Kafi, M., and Saeb, S. (2010). Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology 73, 659–669.
| Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development.Crossref | GoogleScholarGoogle Scholar | 20047754PubMed |
Tay, S. H., Blache, D., Gregg, K., and Revell, D. K. (2012). Consumption of a high-salt diet by ewes during pregnancy alters nephrogenesis in 5-month-old offspring. Animal 6, 1803–1810.
| Consumption of a high-salt diet by ewes during pregnancy alters nephrogenesis in 5-month-old offspring.Crossref | GoogleScholarGoogle Scholar |
Tenley, S. C., Gomes, R. S., Rosasco, S. L., Northrop, E. J., Rich, J. J. J., McNeel, A. K., Summers, A. F., Miles, J. R., Chase, C. C., Lents, C. A., Perry, G. A., Wood, J. R., Cupp, A. S., and Cushman, R. A. (2019). Maternal age influences the number of primordial follicles in the ovaries of yearling Angus heifers. Anim. Reprod. Sci. 200, 105–112.
| Maternal age influences the number of primordial follicles in the ovaries of yearling Angus heifers.Crossref | GoogleScholarGoogle Scholar | 30563721PubMed |
Torres-Rovira, L., Gonzalez-Bulnes, A., Succu, S., Spezzigu, A., Manca, M. E., Leoni, G. G., Sanna, M., Pirino, S., Gallus, M., Naitana, S., and Berlinguer, F. (2014). Predictive value of antral follicle count and anti-Mullerian hormone for follicle and oocyte developmental competence during the early prepubertal period in a sheep model. Reprod. Fertil. Dev. 26, 1094–1106.
| Predictive value of antral follicle count and anti-Mullerian hormone for follicle and oocyte developmental competence during the early prepubertal period in a sheep model.Crossref | GoogleScholarGoogle Scholar | 24008140PubMed |
Tsafriri, A., and Dekel, N. (1994) Molecular mechanisms in ovulation. In ‘Molecular Biology of the Female Reproductive System’. (Ed. J. K. Findlay) pp. 207–258. (Academic Press: San Diego)
Turner, E. C., Hughes, J., Wilson, H., Clay, M., Mylonas, K. J., Kipari, T., Duncan, W. C., and Fraser, H. M. (2011). Conditional ablation of macrophages disrupts ovarian vasculature. Reproduction 141, 821–831.
| Conditional ablation of macrophages disrupts ovarian vasculature.Crossref | GoogleScholarGoogle Scholar | 21393340PubMed |
Umer, S., Sammad, A., Zou, H., Khan, A., Weldegebriall Sahlu, B., Hao, H., Zhao, X., Wang, Y., Zhao, S., and Zhu, H. (2019). Regulation of AMH, AMHR-II, and BMPs (2,6) genes of bovine granulosa cells treated with exogenous fSH and their association with protein hormones. Genes (Basel) 10, 1038.
| Regulation of AMH, AMHR-II, and BMPs (2,6) genes of bovine granulosa cells treated with exogenous fSH and their association with protein hormones.Crossref | GoogleScholarGoogle Scholar |
Vaccari, S., Weeks, J. L., Hsieh, M., Menniti, F. S., and Conti, M. (2009). Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol. Reprod. 81, 595–604.
| Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 19474061PubMed |
Van Vleck, L. D., Gregory, K. E., and Echternkamp, S. E. (1991). Ovulation rate and twinning rate in cattle: heritabilities and genetic correlation. J. Anim. Sci. 69, 3213–3219.
| Ovulation rate and twinning rate in cattle: heritabilities and genetic correlation.Crossref | GoogleScholarGoogle Scholar | 1894556PubMed |
Veiga-Lopez, A., Luense, L. J., Christenson, L. K., and Padmanabhan, V. (2013). Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884.
| Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression.Crossref | GoogleScholarGoogle Scholar | 23525218PubMed |
Vigier, B., Picard, J. Y., Tran, D., Legeai, L., and Josso, N. (1984). Production of anti-Mullerian hormone: another homology between Sertoli and granulosa cells. Endocrinology 114, 1315–1320.
| Production of anti-Mullerian hormone: another homology between Sertoli and granulosa cells.Crossref | GoogleScholarGoogle Scholar | 6546716PubMed |
Walsh, S. W., Mossa, F., Butler, S. T., Berry, D. P., Scheetz, D., Jimenez-Krassel, F., Tempelman, R. J., Carter, F., Lonergan, P., Evans, A. C., and Ireland, J. J. (2014). Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle. J. Dairy Sci. 97, 4503–4511.
| Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle.Crossref | GoogleScholarGoogle Scholar | 24835969PubMed |
Wartenberg, H., Ihmer, A., Schwarz, S., Miething, A., and Viebahn, C. (2001). Mitotic arrest of female germ cells during prenatal oogenesis. A colcemid-like, non-apoptotic cell death. Anat. Embryol. (Berl.) 204, 421–435.
| Mitotic arrest of female germ cells during prenatal oogenesis. A colcemid-like, non-apoptotic cell death.Crossref | GoogleScholarGoogle Scholar | 11789990PubMed |
Webb, R., and Campbell, B. K. (2007). Development of the dominant follicle: Mechanisms of selection and maintenance of oocyte quality. Soc. Reprod. Fertil. Suppl. 64, 141–163.
| Development of the dominant follicle: Mechanisms of selection and maintenance of oocyte quality.Crossref | GoogleScholarGoogle Scholar | 17491145PubMed |
Wettemann, R. P., Lents, C. A., Ciccioli, N. H., White, F. J., and Rubio, I. (2003). Nutritional- and suckling-mediated anovulation in beef cows. J. Anim. Sci. 81, E48–E59.
| Nutritional- and suckling-mediated anovulation in beef cows.Crossref | GoogleScholarGoogle Scholar |
Wigglesworth, K., Lee, K. B., O’Brien, M. J., Peng, J., Matzuk, M. M., and Eppig, J. J. (2013). Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. USA 110, E3723–E3729.
| Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes.Crossref | GoogleScholarGoogle Scholar | 23980176PubMed |
Yang, M. Y., Cushman, R. A., and Fortune, J. E. (2017). Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol. Hum. Reprod. 23, 282–291.
| Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles.Crossref | GoogleScholarGoogle Scholar | 28333275PubMed |
Young, J. M., and McNeilly, A. S. (2010). Theca: the forgotten cell of the ovarian follicle. Reproduction 140, 489–504.
| Theca: the forgotten cell of the ovarian follicle.Crossref | GoogleScholarGoogle Scholar | 20628033PubMed |
Zeng, Y., and Chen, T. (2019). DNA methylation reprogramming during mammalian development. Genes (Basel) 10, 257.
| DNA methylation reprogramming during mammalian development.Crossref | GoogleScholarGoogle Scholar |
Zhang, M., Su, Y. Q., Sugiura, K., Xia, G., and Eppig, J. J. (2010). Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366–369.
| Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 20947764PubMed |
Zhang, M., Su, Y. Q., Sugiura, K., Wigglesworth, K., Xia, G., and Eppig, J. J. (2011). Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology 152, 4377–4385.
| Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro.Crossref | GoogleScholarGoogle Scholar | 21914782PubMed |
Zhang, M., Jiang, M., Bi, Y., Zhu, H., Zhou, Z., and Sha, J. (2012). Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS One 7, e41412.
| Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice.Crossref | GoogleScholarGoogle Scholar | 23285222PubMed |
Zhang, T., Zhang, C., Fan, X., Li, R., and Zhang, J. (2017a). Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In vitro Cell. Dev. Biol. Anim. 53, 199–206.
| Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation.Crossref | GoogleScholarGoogle Scholar | 27761784PubMed |
Zhang, Y., Li, F., Feng, X., Yang, H., Zhu, A., Pang, J., Han, L., Zhang, T., Yao, X., and Wang, F. (2017b). Genome-wide analysis of DNA methylation profiles on sheep ovaries associated with prolificacy using whole-genome bisulfite sequencing. BMC Genomics 18, 759.
| Genome-wide analysis of DNA methylation profiles on sheep ovaries associated with prolificacy using whole-genome bisulfite sequencing.Crossref | GoogleScholarGoogle Scholar | 28969601PubMed |
Zhao, Z., Guo, F., Sun, X., He, Q., Dai, Z., Chen, X., Zhao, Y., and Wang, J. (2018). BMP15 regulates AMH expression via the p38 MAPK pathway in granulosa cells from goat. Theriogenology 118, 72–79.
| BMP15 regulates AMH expression via the p38 MAPK pathway in granulosa cells from goat.Crossref | GoogleScholarGoogle Scholar | 29885643PubMed |
*The US Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, colour, national origin, age, disability and, where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (not all prohibited bases apply to all programs). Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape etc.) should contact USDA’s TARGET Center at +1 (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250–9410, or call +1 (800) 795–3272 (voice) or +1 (202) 720–6382 (TDD). USDA is an equal opportunity provider and employer.
#Other than the corresponding author, the order of authors was determined alphabetically.



