Effects of ovarian hyperstimulation on mitochondria in oocytes and early embryos
Jing Shu A B , Li-Li Xing B C , Guo-Lian Ding C D , Xin-Mei Liu C D , Qing-Feng Yan E and He-Feng Huang C D FA Wenzhou Medical University, Wenzhou 325035, PR China.
B Department of Reproductive Endocrinology, Zhejiang Provincial People’s Hospital, No. 158, Shangtang Road, Hangzhou 310014, PR China.
C The Key Laboratory of Reproductive Genetics, Ministry of Education, Hangzhou 310058, PR China.
D International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, 910# Hengshan Road, Xuhui Area, Shanghai, PR China.
E Institute of Genetics, College of Life Science, Zhejiang University, Hangzhou 310058, PR China.
F Corresponding author. Email: huanghefg@hotmail.com
Reproduction, Fertility and Development 28(8) 1214-1222 https://doi.org/10.1071/RD14300
Submitted: 15 August 2014 Accepted: 8 December 2014 Published: 9 February 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
A mouse model was used to compare the number and function of mitochondria in oocytes and embryos obtained by superovulation and in a natural cycle (control group). The superovulation group had a higher number of total oocytes, MII oocytes, embryos with two pronuclei, 2-cell embryos and blastocysts than the control group (P < 0.05 for all). The superovulation group had high proportion of MII oocytes with low number of mitochondrial (mt) DNA copies. The average number of mtDNA copies, ATP level and mitochondrial membrane potential ( Ψm) in MII oocytes in the superovulation were lower than in the control group (P < 0.05 for all). However, at the blastocyst stage, mean mtDNA copies, ATP level and
Ψm) in MII oocytes in the superovulation were lower than in the control group (P < 0.05 for all). However, at the blastocyst stage, mean mtDNA copies, ATP level and  Ψm did not differ significantly between the two groups. These results suggest that ovarian hyperstimulation does not cause damage to the mitochondria in eggs but, rather, more eggs with poor mitochondrial quality are recruited, resulting in a decline in average mitochondrial quality.
Ψm did not differ significantly between the two groups. These results suggest that ovarian hyperstimulation does not cause damage to the mitochondria in eggs but, rather, more eggs with poor mitochondrial quality are recruited, resulting in a decline in average mitochondrial quality.
Additional keywords: mitochondrial DNA, mitochondrial membrane potential.
Introduction
For IVF, eggs are often harvested through superovulation via a high gonadotropin (Gn) dose. However, numerous studies have suggested that eggs obtained via superovulation have low developmental potential with abnormal spindle structures (Van Blerkom and Davis 2001) and an abnormal distribution of actin and cortical granules (Lee et al. 2005). The blastocyst production rate after superovulation is low, which is accompanied by a small inner cell mass (McKiernan and Bavister 1998; Edwards et al. 2005) and increased chance of polyploid embryos (Ma et al. 1997). Conversely, some believe that ovarian hyperstimulation itself is harmless to eggs and that only more low-quality eggs are recruited (Gosden and Lee 2010).
Ovarian hyperstimulation acts on antral follicle development, especially on the late stage, which is a critical period for cytoplasm maturation. Mitochondria are important cytoplasmic organelles involved in the regulation of intracellular metabolism and apoptosis. Mitochondrial function is essential for the normal activity of the oocyte cytoskeleton (Katayama et al. 2006) and normal chromosome segregation (Van Blerkom 2011). Both play an important role in fertilisation and embryonic development (Van Blerkom 2008). The zygotic genome is not activated, and no mitochondrial replication occurs, before embryo implantation (Dumollard et al. 2007). Therefore, the energy for early embryo development is totally dependent on the oocyte mitochondrial reserve.
During antral follicle development, oocyte mitochondria replicate considerably (Dumollard et al. 2007). It is unknown whether ovarian hyperstimulation during this sensitive period interferes with the natural changes occurring in the oocyte mitochondria, and whether ovarian hyperstimulation may result in a low development potential of oocytes and embryos due to mitochondrial replication injury.
In addition, the mitochondrion is the only organelle that contains extranuclear genetic material. Compared with nuclear DNA, mitochondrial (mt) DNA does not have any histone protection, repair or recombination mechanisms. Because of sperm mitochondrial ubiquitinoylation in ooplasma, zygotic mtDNA is only inherited from the mother (Sutovsky et al. 1999). Therefore, oocyte mitochondrial mutations are an important issue for both embryo developmental potential and offspring health. During superovulation, changes in the ovarian metabolic environment are much more severe than during normal physiological follicular development. Jancar et al. (2008) reported that ovarian stimulation increases the incidence of apoptotic granulosa cells, but has no effect on the level of production of reactive oxygen species (ROS). Chao et al. (2005) reported that after repeated superovulation in mice, oxidative damage products and mtDNA mutations in ovarian tissue are increased. Gibson et al. (2005) reported that in rhesus monkeys mtDNA deletion is increased in eggs obtained through ovarian hyperstimulation. Because oxidative damage is a leading cause of mtDNA mutations, the question arises whether ovarian hyperstimulation may pose a potential risk to reproduction and the health of offspring due to ovarian oxidative damage and subsequent mutation of oocyte mtDNA.
In the present study, a mouse model was used to compare the degree of maturation, IVF rate and embryo development of oocytes obtained by superovulation and in a natural cycle. The number and function of mitochondria in oocytes and embryos at different development stages were compared between the two groups. The morphology, distribution and genetic heterogeneity of mitochondria in oocytes, and oxidative products of the ovarian tissue, were also compared between the two groups.
Materials and methods
This study was approved by the Institutional Review Board and the Institutional Animal Care and Use Committee (IACUC) of the School of Medicine, Zhejiang University, Hangzhou, China. Portions of the methods have been reported previously (Shu et al. 2013).
Study animals, observation of the oestrous cycle and ovarian stimulation
Female (8–10 weeks of age; weight 20–25 g) and male(12–13 weeks of age; weight 30–35 g) C57BL/6J mice purchased from the Experimental Animal Center of Zhejiang University were used in the present study. Mice were raised under a 14-h light–10-h dark cycle at 24°C and had free access to food and drinking water. Vaginal smears of female mice were obtained daily at noon. After haematoxylin and Alcian blue staining, slides were observed and mice were categorised as being in dioestrus, pro-oestrus, oestrus or metoestrus.
In the control group, with typical smear manifestations of the oestrous cycle at noon, mice were injected with human chorionic gonadotropin (hCG), 10 IU, i.p., per mouse on the same day at 1800 hours. In the superovulation group, mice in dioestrus were administered 10 IU, i.p., pregnant mare’s serum gonadotropin (PMSG), followed by 10 IU hCG 48 h later. In both groups, eggs were obtained 14 h after hCG injection and the cumulus–oocyte complex (COC) was collected for IVF and embryo culture.
Oocyte retrieval, fertilisation and embryo culture
After an intraperitoneal injection of an overdose of 1% pentobarbital at 0.01 mL g–1 bodyweight, mice were killed by cervical dislocation. The fallopian tube was cut and placed in modified human tubal fluid (mHTF; Irvine Scientific, Santa Ana, CA, USA). The COCs were released by needle puncture to the oviduct magnum, then were transferred to HTF.
To collect oocytes, COCs were treated with 80 IU mL–1 hyaluronidase (Sigma-Aldrich, St Louis, MO, USA) for 2–3 min before COCs were transferred to mHTF using a fine glass needle. COCs were washed with mHTF to remove granulosa cells. Oocyte morphology was observed under a microscope. If polar body discharge was observed, the egg was classified as an MII oocyte.
For IVF, the COC was cultured in a droplet and covered by mineral oil. Spermatozoa harvested from the epididymis of a male mouse were collected in HTF medium containing 10% serum substitute supplement (SSS) and then cultured in an incubator with 5% CO2 at 37°C for 1 h to allow capacitation. The sperm concentration was adjusted to 1 × 106 mL–1 and 6 µL sperm solution was added to each COC droplet.
Fertilisation status was observed 8 h after insemination. The oocyte was considered to be fertilised normally when two pronuclei (PN; a male PN and female PN) appeared. The zygote was then transferred to HTF medium containing 10% SSS and cultured continuously. Embryo development was observed once a day. The fertilisation rate (number of 2PN embryos/total number of oocytes obtained), cleavage rate (number of 2-cell embryos/number of 2PN embryos) and blastocyst formation rate (number of blastocysts/number of 2PN embryos) were calculated. All 2PN and 2-cell embryos and blastocysts were collected for examination of embryo mtDNA copy number, ATP detection and JC-1 staining.
Analysis of mtDNA copy number in eggs and embryos
In a mature oocyte, each mitochondrion contains one to two mtDNA copies. Thus, the number of mtDNA copies can be used as an index of the quantity of mitochondria (Shoubridge and Wai 2007). The methods used for the analysis of mtDNA copy number in eggs and embryos were as described previously (Shu et al. 2013). Briefly, purified and absolute quantified polymerase chain reaction (PCR) products of the mouse mtDNA 2567–2723 fragment (157 bp) were prepared using the following primers: CGAAAGGACAAGAGAAATAGAG (forward) and GAACAAGGTTTTAAGTCTTACGCA (reverse). The primers were designed according to the GenBank mtDNA standard sequence (GI: 33115104) for C57 mice and produced by Shanghai Sangon Biological Engineering Technology Services (Shanghai, China). Serial external standard templates with a known copy number were produced by serial dilutions of the purified product.
Pooled oocytes or embryos were lysed by proteinase K-containing buffer in a PCR tube, and the average number of mtDNA copies was determined by real-time PCR simultaneously with the serial external standard calibration products. The reaction was performed with 5 μL SYBR, 0.1 μL forward primer (10 μM), 0.2 μL reverse primer (10 μM), 3 μL mtDNA template in DNA lysis buffer and 1.6 μL distilled water. The reaction conditions were 94°C denaturation for 5 min, followed by 40 cycles of 94°C denaturation for 30 s, 61°C annealing for 30 s and 72°C extension for 30 s.
ATP detection
The ATP detection methods used in the present study were as described previously (Shu et al. 2013). ATP assays were performed with an ATP Determination Kit (A22066; Molecular Probes, Life Technologies, Carlsbad, CA, USA). Changes in chemiluminescence were measured with a Thermo Scientific Luminoskan Ascent chemiluminescence plate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Observation of mitochondrial membrane potential
The methods used for the determination of mitochondrial membrane potential ΔΨm were as described previously (Shu et al. 2013). The fluorescent dye JC-1 (catalogue number 1130–5; Biovision) fluoresces red for a high ΔΨm, whereas if ΔΨm is low the dye fluoresces green. Red and green fluorescence distribution and intensity were evaluated using a two-photon confocal microscope (BX61W1-FV1000; Olympus). Each egg was measured at an equatorial axis section. The light excitation and emission wavelengths for red and green fluorescence were 559 and 572 nm, respectively, and 488 and 520 nm, respectively. ImageJ software was used to calculate the ratio of red to green fluorescence intensity (ΔΨm).
Observation of mitochondria distribution in oocytes
MitoTracker Red FM (catalogue no. M2242; Invitrogen) was used for observation of mitochondria distribution. MitoTracker Red is a fluorescent dye that passes through the cell membrane and accumulates in mitochondria. It is oxidised by active mitochondria and exhibits red fluorescence. Mitochondrial staining with MitoTracker does not depend on ΔΨm and, unlike JC-1 staining, can be used to quantify the number of mitochondria.
Oocytes or embryos were transferred into HTF containing 300 nM MitoTracker Red and incubated in the dark at 37°C for 60 min. They were then rinsed three times with mHTF before being placed in a Petri dish for immediate observation. A laser confocal microscope was used to observe the excitation and emission of MitoTracker Red FM probes at 543 and 633 nm, respectively. Each oocyte was scanned at an equatorial plane with a slice thickness of 2 μm. The distribution of mitochondria in oocytes was categorised as follows: (1) uniform distribution, whereby the mitochondria were distributed throughout the cytoplasm as if they were fine particles; (2) cluster-like distribution, whereby large particles were present and the mitochondria were distributed unevenly in the ooplasm or under the plasma membrane; and (3) polar aggregation, whereby large mitochondrial particles gathered in one hemisphere and small mitochondrial particles were found in the other hemisphere.
Observation of the mitochondrial ultrastructure of oocytes
A total of 12 MII oocytes was randomly selected from each of the control and superovulation groups for observation of mitochondrial ultrastructure by electron microscopy (Shu et al. 2013). After fixation, staining and dehydration, the specimens were soaked in 1 : 1 acetone : Epon812 for 1.5 h, embedded and placed into a polymerisation reactor (37°C, 24 h; 45°C, 24 h; 60°C, 48 h). Ultrathin sections (120 nm) were cut and stained with 4% uranyl acetate for 20 min and lead citrate for 5 min. The samples were then observed and photographed under a TECNAI–10 Phillips electron microscope (80–100 kV).
Detection of mtDNA D-loop region heterogeneity
Mouse mtDNA has a total of 16 299 bp and the D-loop region fragments were selected for heterogeneity testing. The D-loop region is located at nucleotides 15 423–16 299. It is a mutation hot spot and is the regulatory region for mitochondrial replication and transcription. Single oocytes were transferred into a sterile PCR tube and oocyte lysis and inactivation of proteinase K procedures were performed similar to those described above for analysis of mtDNA copy number. Samples were stored at –80°C until use.
Denaturing HPLC (DHPLC) was used for heterogeneity detection. DNA double strands dissociate when heated to 95°C. During a slow cooling process, samples containing heterogeneous DNA produce hybridisation products. The column hold-up time of the heteroduplex with a mutant sequence is shorter than that of the corresponding homoduplex. Therefore, samples with the mutant sequence exhibit two peaks (heteroduplex and homoduplex) and samples without the mutant sequence only produce the homoduplex peak. Under ideal separation conditions, fragments containing a 1% difference in size can be separated. The temperatures for denaturation in the present study were: A, fragment 15 308–15 703, denaturation at 53.8°C; B, fragment 15 632–15 977, denaturation at 57.8°C; and C, fragment 15 961–16 269, denaturation at 54.2°C. Five mice from each group were used in the experiment and all oocytes harvested from mice in the control and superovulation groups were tallied; there were 41 and 103 eggs, respectively.
Detection of oxidative products and antioxidant capacity of ovarian tissue
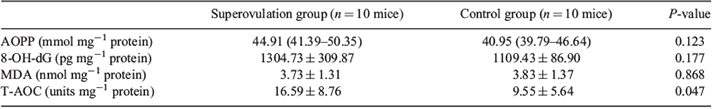
The antioxidant capacity of ovarian tissue has been evaluated previously (Kankofer et al. 2013). Briefly, 8-hydroxy-deoxyguanosine (8-OH-dG) is the product of oxidative damage to DNA caused by free radicals. Advanced oxidation protein products (AOPP) are produced by oxidative damage to proteins caused by free radicals. Maleic dialdehyde (MDA) is a lipid peroxidation product. Total antioxidant capacity (T-AOC) is a measure of the antioxidant capacity of two systems: enzymatic and non-enzymatic.
Both ovaries of mice in the two groups (10 mice from each group) were removed before the mice were injected with hCG and were rapidly frozen in liquid nitrogen for storage. The ovaries were taken out of the liquid nitrogen and placed on ice before the experiment. After addition of 400 µL phosphate-buffered saline (PBS), the ovaries were ground in a grinder and centrifuged at 667g for 10 min at 4°C. The supernatant was collected for measurements. 8-OH-dG was detected using ELISA (8-OH-dG detection kit; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. AOPP was also detected using ELISA (AOPP detection kit; R&D Systems). MDA was detected using a thiobarbituric acid (TBA) assay (MDA detection kit; Nanjing Jiancheng Bioengineering Institute). MDA can condense with TBA to form red products. The product has a maximum absorption at 532 nm. The test was performed and optical density (OD) was measured according to the manufacturer’s instructions. T-AOC was detected using a colorimetric method. Antioxidants were used to reduce Fe3+ to Fe2+; the latter can form a stable complex with a phenanthroline. The whole T-AOC detection procedure was performed according to the T-AOC measurement kit instructions (Nanjing Jiancheng Bioengineering Institute) and OD was measured at 520 nm to determine the concentration of T-AOC.
Statistical analysis
Categorical data are presented as numbers with percentages in parentheses, whereas continuous data are presented as the mean ± s.d. Differences between groups were analysed by Fisher’s exact test for categorical data, and by the two-sample t-test for continuous data. Continuous data with a non-normal distribution are presented as median values with the interquartile range in parentheses and were analysed by the Mann–Whitney U-test. All statistical assessments were two-tailed and P < 0.05 was considered significant. Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
In the control group, we attempted to harvest oocytes from 118 mice in which the oestrus cycle was observed; oocytes were obtained from 90 mice, giving a success rate of egg retrieval of 76.3%. In the superovulation group, we attempted to harvest oocytes from 95 mice; oocytes were obtained from 90 mice, giving a success rate of egg retrieval of 94.7%, which was significantly higher than that of the control group (P < 0.01). The oocytes from all mice were randomly used in subsequent experiments. In all experiments, all oocytes collected were used; no oocytes were discarded.
A comparison of oocyte recovery and embryo development between the superovulation and control groups (22 mice in each group) is given in Table 1. The superovulation group had a higher number of oocytes retrieved, MII oocytes, 2PN embryos, 2-cell embryos and blastocysts per mouse than the control group (P < 0.05 for all). However, the egg maturation rate, fertilisation rate and blastocyst rate were all lower in the superovulation group (P < 0.05 for all; Table 1). Table 2 shows that the median number of mtDNA copies of MII oocytes and 2PN embryos in the superovulation group was lower than in the control group (P < 0.05 for both). The ATP levels of MII oocytes in the superovulation group were lower than in the control group (33.65 ± 20.65 vs 45.41 ± 20.16 fmol, respectively; P = 0.001). The ΔΨm of MII oocytes and 2PN embryos of the superovulation group was lower than in the control group (1.13 ± 0.32 vs 1.39 ± 0.24 for MII oocytes, respectively (P < 0.001); 1.33 (1.17–1.55) vs 1.44 (1.35–1.53) for 2PN embryos, respectively (P = 0.015)). At the blastocyst stage, mtDNA copies, ATP level and ΔΨm were not significantly different between the two groups (Table 2). The JC-1 staining pattern of eggs and embryos in the two groups is shown in Fig. 1. JC-1 red fluorescence showed that hyperpolarised mitochondria were mainly distributed under the cell membrane or under the trophoblast layer.

|
The proportion of MII oocytes with a different mtDNA content level was significantly different between the superovulation and control groups (P < 0.001; Table 3). Approximately 41.5% of MII oocytes in the superovulation group had a small number of mtDNA copies (<1 × 105), compared with only 13.2% of MII oocytes in the control group.

|
The distribution pattern of mitochondria in MII oocytes was also different between the two groups. In the control group, 76.2% of MII oocytes had a uniform distribution of mitochondrial particles, whereas in the superovulation group 51.9% of MII oocytes exhibited a uniform mitochondrial distribution and 48.1% exhibited distribution polarity (Fig. 2; P = 0.017).

|
Observation of mitochondrial ultrastructure showed that all selected MII oocytes in the control group (12/12) had abundant mitochondria in a spherical shape, and the mitochondria had a high matrix density and a small number of ridges (Fig. 3). The endoplasmic reticulum vesicles were surrounded by many crescent-like mitochondria. In the superovulation group, the mitochondrial ultrastructure in 83.3% of selected oocytes (10/12) was similar to that in the control group; however, in 16.7% of oocytes (2/12) the mitochondria were sparse and small in size, and they lacked a condensed mitochondrial structure around the endoplasmic reticulum vesicles.

|
Heterogeneity analysis revealed no heterogeneity in the three D-loop region fragments (15 308–15 703, 15 632–15 977 and 15 961–16 269) in oocytes in either group.
Results of the analysis of oxidative damage and the antioxidant capacity of ovarian tissue in the two groups are given in Table 4. T-AOC was significantly higher in the superovulation group than in the control group (16.59 ± 8.76 vs 9.55 ± 5.64 units mg–1 protein, respectively; P = 0.047), whereas there were no significant differences in AOPP, 8-OH-dG and MDA between the two groups.
Discussion
The results of the present study using a murine model showed that oocytes produced by superovulation are more likely to be immature and have a low number of mitochondria with decreased function, which are likely to be selected out during the fertilisation and cleavage process. However, there was no significant difference between the groups in the number and function of blastocyst mitochondria, indicating that high-quality oocytes can be obtained through ovarian hyperstimulation. The results support the hypothesis that the low embryo development rate of oocytes obtained after high-dose Gn is due to recruitment of a large number of low-potential eggs rather than egg damage induced by high-dose Gn.
Ge et al. (2013) found that superovulation results in a lower mean number of mitochondria and inferred that ovarian hyperstimulation can destroy mitochondria replication. However, in the present study these results are primarily attributed to the higher percentage of eggs with lower mtDNA content in the superovulation group. Mitochondria do not replicate before embryo implantation; thus, the number of mitochondria in a single early embryo can reflect the number of mitochondria in the oocyte, its developmental origin (Thundathil et al. 2005; Wai et al. 2010). In the present study, the mtDNA content increased gradually in the superovulation group from the MII oocyte to the 2PN, 2-cell and the blastocyst (Table 1), which verifies that a low number of mitochondrial copies has a negative impact on the fertilisation of oocytes and early embryo development. Santos et al. (2006) observed that the average number of mtDNA copies was 250 454 and 163 698 in human zygotes and non-fertilised eggs, respectively, compared with 44 629 in degraded egg. Zeng et al. (2007) compared the mtDNA copy numbers in oocytes with and without meiotic spindle formation and found that the number was lower in the latter and that the latter had a low fertilisation rate after intracytoplasmic sperm injection (ICSI).
Fertilisation and cleavage are energy-consuming processes that require energy support from mitochondrial ATP (Dumollard et al. 2004). Eggs with low mitochondrial function cannot mature and the tendency for an increase in ATP and ΔΨm from the MII oocyte to blastocyst stage confirm this. ATP levels and ΔΨm were significantly different at the MII oocyte stage between the two groups, but no differences were observed at the blastocyst stage. Among the large number of oocytes recruited after Gn administration, the percentage of oocytes with a low mitochondrial copy number was high, and we speculate that the oocytes were eliminated because the ATP generated by the mitochondria did not meet the requirements for fertilisation and cleavage. In addition to an insufficient number of mitochondria, the low ΔΨm of the oocytes in the superovulation group may be a possible reason for the low ATP synthesis of oocytes. ΔΨm is low when mitochondrial metabolism is low and is high when the metabolic rate is high. The decline in the average ΔΨm of oocytes in the superovulation group is possibly due to low mitochondrial function in some oocytes obtained through superovulation, or mitochondrial membrane injury in low-potential oocytes and subsequent proton leakage.
Mitochondria distribution analysis with MitoTracker Red and observation of mitochondrial ultrastructure with electron microscopy revealed that the superovulation group had more variation in oocyte mitochondrial quality, a greater proportion of oocytes with cluster or polar aggregation and therefore a greater chance of collecting eggs with poor ooplasm quality with few, small mitochondria or a lack of mitochondrion–endoplasmic reticulum complexes.
The D-loop region regulates mtDNA replication and transcription (Fish et al. 2004) and mutations in this region are related to infertility and abortion (Parsons et al. 1997; Seyedhassani et al. 2010). D-Loop heterogeneity has been positively associated with age (Sondheimer et al. 2011), suggesting that long-term repeated oxidative damage may produce mtDNA mutations. No heterogeneity in the D-loop region of the mtDNA of oocytes was noted in the two groups, suggesting that the likelihood of superovulation causing mtDNA damage to the oocytes discharged in the current cycle is not high. Furthermore, we did not find increases in 8-OH-dG, AOPP or MDA in ovarian tissue after a single ovarian hyperstimulation. In contrast, the antioxidant capacity of hyperstimulated ovarian tissue was higher than that in the control group. Miyamoto et al. (2010) thought ovulation itself was an oxidative stress response and that superoxides contributed to the process of ovulation, and reported that after multiple ovulation 8-OH-dG content increased in oocytes, the mtDNA content of the eggs was decreased and mitochondria; distribution was abnormal.
The present study does have some limitations. An animal model was used, and so the results may not be applicable to human reproduction. Detection of heterogeneity may be affected by DHPLC sensitivity because the minimum heterogeneity that can be detected is only 1%. In addition, the fragments that can be used for detection are limited, and we did not examine the entire mtDNA genome. Previous studies (Ge et al. 2013) have performed comparisons using the same number of oocytes (i.e. comparing oocytes with excellent qualities obtained during a natural cycle with oocytes with different qualities harvested during ovarian hyperstimulation) and reported poorer results in the ovarian hyperstimulation group. We tried to compare the actual outcome of development between all the oocytes produced by a single individual during ovarian hyperstimulation and those produced by a single individual during a natural cycle. For this reason we choose the same number of mice for each group to test the efficiency of superovulation.
Conclusions
Superovulation increases the number of oocytes, but they are more likely to be immature and have a low number of mitochondria with decreased function and are likely to be selected out during the fertilisation and cleavage process. The number and function of blastocyst mitochondria did not differ between those obtained through ovarian hyperstimulation and a normal cycle, and a single ovarian hyperstimulation does not increase ovarian oxidative products and the mutation frequency of the mtDNA D-loop region. These results suggest that single ovarian hyperstimulation does not cause damage to the mitochondria in eggs, but rather more eggs of poor mitochondrial quality are recruited, resulting in a decline in average mitochondrial quality.
Acknowledgements
This study was supported by grants from the National Basic Research Program of China (2012CB944900), National Science and Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAI32B00), The Key Program of Zhejiang Provincial Natural Science Foundation of China(Z2110063) and Medicine and Health Care Projects of Zhejiang Province (2011KYA085).
References
Chao, H. T., Lee, S. Y., Lee, H. M., Liao, T. L., Wei, Y. H., and Kao, S. H. (2005). Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann. N. Y. Acad. Sci. 1042, 148–156.| Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXot1Cqsbs%3D&md5=1f05c0080bab1c1c043400b3aaa726e4CAS | 15965057PubMed |
Dumollard, R., Marangos, P., Fitzharris, G., Swann, K., Duchen, M., and Carroll, J. (2004). Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131, 3057–3067.
| Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtFKlsb0%3D&md5=36d1ff554931340655c1201a3b67abb8CAS | 15163630PubMed |
Dumollard, R., Duchen, M., and Carroll, J. (2007). The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 77, 21–49.
| The role of mitochondrial function in the oocyte and embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1Gltb4%3D&md5=ef902ce04a34ede18ce030f99570900eCAS | 17222699PubMed |
Edwards, L. J., Kind, K. L., Armstrong, D. T., and Thompson, J. G. (2005). Effects of recombinant human follicle-stimulating hormone on embryo development in mice. Am. J. Physiol. Endocrinol. Metab. 288, E845–E851.
| Effects of recombinant human follicle-stimulating hormone on embryo development in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktlSgurY%3D&md5=92878c32520fb4ca6e078de2ee2e4858CAS | 15598671PubMed |
Fish, J., Raule, N., and Attardi, G. (2004). Discovery of a major D-loop replication origin reveals two modes of human mtDNA synthesis. Science 306, 2098–2101.
| Discovery of a major D-loop replication origin reveals two modes of human mtDNA synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOjsLrO&md5=8a565034e18b6c6ac553ba24c6f2e429CAS | 15604407PubMed |
Ge, H. S., Zhang, F., Li, X. H., Chen, H., Xi, H. T., Huang, J. Y., Zhu, C. F., and Lu, J. Q. (2013). Effects of controlled ovarian hyperstimulation on mitochondria copy number and functions in murine oocytes Zhonghua Fu Chan Ke Za Zhi 48, 858–861.
| 24444565PubMed |
Gibson, T. C., Kubisch, H. M., and Brenner, C. A. (2005). Mitochondrial DNA deletions in rhesus macaque oocytes and embryos. Mol. Hum. Reprod. 11, 785–789.
| Mitochondrial DNA deletions in rhesus macaque oocytes and embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFahtrg%3D&md5=b37f72072ea1725fb56964ff9bb94a89CAS | 16373367PubMed |
Gosden, R., and Lee, B. (2010). Portrait of an oocyte: our obscure origin. J. Clin. Invest. 120, 973–983.
| Portrait of an oocyte: our obscure origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVChsrY%3D&md5=df5d04b89c5169b739dfdfe6e3419770CAS | 20364095PubMed |
Jancar, N., Virant-Klun, I., Osredkar, J., and Vrtacnik Bokal, E. (2008). Apoptosis, reactive oxygen species and follicular anti-Mullerian hormone in natural versus stimulated cycles. Reprod. Biomed. Online 16, 640–648.
| Apoptosis, reactive oxygen species and follicular anti-Mullerian hormone in natural versus stimulated cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVOks7g%3D&md5=8b46e8c95152bb9df9cc9b12890eb44fCAS | 18492367PubMed |
Kankofer, M., Wawrzykowski, J., and Giergiel, M. (2013). Sex- and age-dependent activity of glutathione peroxidase in reproductive organs in pre- and post-pubertal cattle in relation to total antioxidant capacity. Aging Clin. Exp. Res. 25, 365–370.
| Sex- and age-dependent activity of glutathione peroxidase in reproductive organs in pre- and post-pubertal cattle in relation to total antioxidant capacity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjgsVCjsA%3D%3D&md5=c793edbce60ca6bdfa6fecc5b94694f9CAS | 23740597PubMed |
Katayama, M., Zhong, Z., Lai, L., Sutovsky, P., Prather, R. S., and Schatten, H. (2006). Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential. Dev. Biol. 299, 206–220.
| Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVygsb7K&md5=5ac42363801a53278f6c4a52c31f8732CAS | 16945363PubMed |
Lee, S. T., Kim, T. M., Cho, M. Y., Moon, S. Y., Han, J. Y., and Lim, J. M. (2005). Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development. Fertil. Steril. 83, 1264–1274.
| Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktFaru7w%3D&md5=721acb28dde8b958baeb6459d91bc5c5CAS | 15831301PubMed |
Ma, S., Kalousek, D. K., Yuen, B. H., and Moon, Y. S. (1997). Investigation of effects of pregnant mare serum gonadotropin (PMSG) on the chromosomal complement of CD-1 mouse embryos. J. Assist. Reprod. Genet. 14, 162–169.
| Investigation of effects of pregnant mare serum gonadotropin (PMSG) on the chromosomal complement of CD-1 mouse embryos.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3ksF2hsg%3D%3D&md5=5b5fc0760451467b26b77300e700a98cCAS | 9090560PubMed |
McKiernan, S. H., and Bavister, B. D. (1998). Gonadotrophin stimulation of donor females decreases post-implantation viability of cultured one-cell hamster embryos. Hum. Reprod. 13, 724–729.
| Gonadotrophin stimulation of donor females decreases post-implantation viability of cultured one-cell hamster embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivF2itLo%3D&md5=17efd07b5cfcfd75398100614675fe5dCAS | 9572442PubMed |
Miyamoto, K., Sato, E. F., Kasahara, E., Jikumaru, M., Hiramoto, K., Tabata, H., Katsuragi, M., Odo, S., Utsumi, K., and Inoue, M. (2010). Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 49, 674–681.
| Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXoslWrsbw%3D&md5=9b24c297dade0d797b5723abe54b966fCAS | 20621580PubMed |
Parsons, T. J., Muniec, D. S., Sullivan, K., Woodyatt, N., Alliston-Greiner, R., Wilson, M. R., Berry, D. L., Holland, K. A., Weedn, V. W., Gill, P., and Holland, M. M. (1997). A high observed substitution rate in the human mitochondrial DNA control region. Nat. Genet. 15, 363–368.
| A high observed substitution rate in the human mitochondrial DNA control region.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXitF2hsbY%3D&md5=ee572ef6517965e8aab4a6edf4075ef2CAS | 9090380PubMed |
Santos, T. A., El Shourbagy, S., and St John, J. C. (2006). Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 85, 584–591.
| Mitochondrial content reflects oocyte variability and fertilization outcome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsVChsr0%3D&md5=44608f7ae2e3ffae80f03b8cd2a33e56CAS | 16500323PubMed |
Seyedhassani, S. M., Houshmand, M., Kalantar, S. M., Modabber, G., and Aflatoonian, A. (2010). No mitochondrial DNA deletions but more D-loop point mutations in repeated pregnancy loss. J. Assist. Reprod. Genet. 27, 641–648.
| No mitochondrial DNA deletions but more D-loop point mutations in repeated pregnancy loss.Crossref | GoogleScholarGoogle Scholar | 20499271PubMed |
Shoubridge, E. A., and Wai, T. (2007). Mitochondrial DNA and the mammalian oocyte. Curr. Top. Dev. Biol. 77, 87–111.
| Mitochondrial DNA and the mammalian oocyte.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1Gltbo%3D&md5=121c7deb6e125bf1e015b31d783cab46CAS | 17222701PubMed |
Shu, J., Xing, L., Ding, G., Luo, Q., Liu, X., Yan, Q., Sheng, J., and Huang, H. (2013). The effect of peritoneal fluid from patients with endometriosis on mitochondrial function and development of early mouse embryos. PLoS ONE 8, e82334.
| The effect of peritoneal fluid from patients with endometriosis on mitochondrial function and development of early mouse embryos.Crossref | GoogleScholarGoogle Scholar | 24386092PubMed |
Sondheimer, N., Glatz, C. E., Tirone, J. E., Deardorff, M. A., Krieger, A. M., and Hakonarson, H. (2011). Neutral mitochondrial heteroplasmy and the influence of aging. Hum. Mol. Genet. 20, 1653–1659.
| Neutral mitochondrial heteroplasmy and the influence of aging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVKrtbc%3D&md5=4e336dab15c88229fcbf95cadce00b95CAS | 21296868PubMed |
Sutovsky, P., Moreno, R. D., Ramalho-Santos, J., Dominko, T., Simerly, C., and Schatten, G. (1999). Ubiquitin tag for sperm mitochondria. Nature 402, 371–372.
| Ubiquitin tag for sperm mitochondria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvVyktrs%3D&md5=388b8a034bf2dc7881b87edaea5ac773CAS | 10586873PubMed |
Thundathil, J., Filion, F., and Smith, L. C. (2005). Molecular control of mitochondrial function in preimplantation mouse embryos. Mol. Reprod. Dev. 71, 405–413.
| Molecular control of mitochondrial function in preimplantation mouse embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvFSltLk%3D&md5=9a7b046ebc00c7c939ee51773ec88e58CAS | 15895466PubMed |
Van Blerkom, J. (2008). Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod. Biomed. Online 16, 553–569.
| Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells.Crossref | GoogleScholarGoogle Scholar | 18413065PubMed |
Van Blerkom, J. (2011). Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 11, 797–813.
| Mitochondrial function in the human oocyte and embryo and their role in developmental competence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWrsrzM&md5=d8fce630c456055ad27de96490482f43CAS | 20933103PubMed |
Van Blerkom, J., and Davis, P. (2001). Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum. Reprod. 16, 757–764.
| Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7ptVWlsQ%3D%3D&md5=9718c54472827e6fe81f1f53fa825ac9CAS | 11278229PubMed |
Wai, T., Ao, A., Zhang, X., Cyr, D., Dufort, D., and Shoubridge, E. A. (2010). The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 83, 52–62.
| The role of mitochondrial DNA copy number in mammalian fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotlWqtLo%3D&md5=46ce3598089e81edd1f349205c92f95eCAS | 20130269PubMed |
Zeng, H. T., Ren, Z., Yeung, W. S., Shu, Y. M., Xu, Y. W., Zhuang, G. L., and Liang, X. Y. (2007). Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 22, 1681–1686.
| Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1ejtL0%3D&md5=121d3047e27534e0e7088da721068d16CAS | 17449512PubMed |




 ) and polar aggregations (
) and polar aggregations ( ). Data are presented as the mean ± s.e.m. Dispersion differed significantly between the two groups (P < 0.05).
). Data are presented as the mean ± s.e.m. Dispersion differed significantly between the two groups (P < 0.05).