Reversible infertility in a liver receptor homologue-1 (LRH-1)-knockdown mouse model
Han Gerrits A C , Marc C. B. C. Paradé A , Annemie M. C. B. Koonen-Reemst A , Nicole E. C. Bakker A , Lenita Timmer-Hellings A , Maarten D. Sollewijn Gelpke B and Jan A. Gossen AA Merck Sharp and Dohme Research Laboratories, Women’s Health Department, Molenstraat 110, 5342 CC, Oss, The Netherlands.
B Merck Sharp and Dohme Research Laboratories, Molecular Design and Informatics, Molenstraat 110, 5342 CC, Oss, The Netherlands.
C Corresponding author. Email: han.gerrits@merck.com
Reproduction, Fertility and Development 26(2) 293-306 https://doi.org/10.1071/RD12131
Submitted: 23 April 2012 Accepted: 22 December 2012 Published: 21 February 2013
Abstract
Liver receptor homologue-1 (LRH-1) is an orphan nuclear receptor that has been implicated in steroid hormone biosynthesis and fertility. Herein we describe a transgenic inducible short hairpin (sh) RNA mouse model that was used to study the effect of transient LRH-1 knockdown in vivo. Induction of expression of the shRNA directed against LRH-1 for 2–6 weeks resulted in 80% knockdown of LRH-1 protein in the ovary and complete infertility. Gonadotropin hyperstimulation could not rescue the observed defects in ovulation and corpus luteum formation in LRH-1-knockdown mice. The infertility phenotype was fully reversible because LRH-1-knockdown females became pregnant and delivered normal size litters and healthy pups after cessation of LRH-1 shRNA expression. Timed ovarian microarray analysis showed that, in line with the observed decrease in plasma progesterone levels, key steroid biosynthesis genes, namely Star, Cyp11a1, Hsd3b and Scarb1, were downregulated in LRH-1-knockdown ovaries. In contrast with what has been described previously, no clear effect was observed on oestrogenic activity in LRH-1-knockdown mice. Only Sult1e1 and, surprisingly, Hsd17b7 expression was modulated with potentially opposite effects on oestradiol bioavailability. In conclusion, the fully reversible infertility phenotype of LRH-1-knockdown mice shows the feasibility of an LRH-1 antagonist as new contraceptive therapy with a mechanism of action that most prominently affects cholesterol availability and progesterone production.
Additional keywords: estrogen, follicle culture, folliculogenesis and corpus luteum formation, microarray, NR5A2, ovary, progesterone, steroidogenesis.
Introduction
The orphan nuclear receptor LRH-1 belongs to the nuclear receptor subfamily 5, group A (NR5A), or fushi tarazu factor-1, subfamily of nuclear receptors that bind to DNA as a monomer (Ellinger-Ziegelbauer et al. 1994; Galarneau et al. 1998; Kudo and Sutou 1997; Lin et al. 2000). Compounds modulating the activity of LRH-1 could represent an attractive new therapy for multiple indications because LRH-1 has been implicated in the inflammatory response, tumour formation, bile acid homeostasis and fertility (Botrugno et al. 2004; Schoonjans et al. 2005; Mueller et al. 2006; Venteclef et al. 2006, 2011; Coste et al. 2007; Mataki et al. 2007; Duggavathi et al. 2008; Lee et al. 2008; Out et al. 2011). The crystal structure of LRH-1 in complex with cofactor peptides reveals the presence of phospholipids in the ligand-binding domain, indicating that the activity of LRH-1 can potentially be modulated (Ortlund et al. 2005; Burendahl et al. 2008). This was further delineated by the description of LRH-1 agonists by Whitby et al. (2006, 2011).
LRH-1 is expressed during embryonic development, mostly in tissues of endodermal origin. Inactivation of LRH-1 leads to early embryonic lethality at the epiblast stage due to the loss of pluripotency of embryonic stem cells (Pare et al. 2004; Gu et al. 2005). In adult tissues, LRH-1 mRNA can be detected in the liver, small intestine, adrenal gland, granulosa cells in the ovary, pre-adipocytes and testis (Fayard et al. 2004).
In the liver, LRH-1 has been reported to affect genes involved in lipid metabolism and transport, including Scrab1, CETP and APOA1 (Luo et al. 2001; Schoonjans et al. 2002; Delerive et al. 2004). Analysis of several genetically engineered mouse models revealed an important role of LRH-1 in bile acid homeostasis and subsequent lipid absorption (Mataki et al. 2007; Lee et al. 2008; Out et al. 2011). In these models, LRH-1 was shown to affect Shp and Cyp8b1 expression in the liver, thereby reducing the amount cholic acid in the bile acid pool. In a more recent study, LRH-1 was shown to be critical for adequate regulation of Cyp7a1 under conditions of bile acid sequestration (Out et al. 2011).
A role for LRH-1 in fertility originated from in vitro transactivation studies in which LRH-1 was suggested to control Cyp19a1, Star, Cyp11a1, Cyp17 and Hsd3b2 expression (Britt et al. 2001; Clyne et al. 2002; Sirianni et al. 2002; Hinshelwood et al. 2003). Analysis of heterozygous LRH-1-knockout mice provided the first evidence that LRH-1 is important for steroid hormone biosynthesis and normal fertility in vivo. Progesterone, but not oestradiol, production was reduced in LRH-1+/− mice, and this coincided with reduced fertility due to pregnancy loss (Labelle-Dumais et al. 2007). Analysis of granulosa cell-specific LRH-1-knockout mice confirmed the role of LRH-1 in progesterone production and in the regulation of Scarb1, Star and Cyp11a1 expression (Duggavathi et al. 2008). The Lrh1 gc–/– mice were completely infertile, lacked corpora lutea (CL) in their ovaries and exhibited disturbed cumulus expansion due to dysfunction of the prostaglandin and hyaluronic acid cascades (Duggavathi et al. 2008). In addition, intrafollicular oestradiol levels were increased in these mice, presumably through increased Cyp19a1 expression and reduced Nos3 and Sult1e1 expression (Duggavathi et al. 2008). Together, these findings indicate that LRH-1 is involved in follicular maturation, cumulus–oocyte expansion, CL transition and ovulation in vivo.
Herein we describe an inducible LRH-1-knockdown (KD) mouse model that was used to assess the value of the in vivo KD technology for fertility drug target validation and to further investigate the mechanism of action of LRH-1 in the ovary.
Materials and methods
Animals and husbandry
Mice were housed in macrolon cages (five animals per cage) at 19–21°C at a relative humidity was 50%–60% under an artificial 12-h light–dark cycle. Standard pelleted food (SDS, Essex, UK) and drinking water with either 2 mg mL−1 doxycyclin (Dox; Sigma, St Louis, MO, USA) and 10% sucrose or 10% sucrose alone was provided ad libitum during the in-life period or for the time period described for the individual experiments. During mating experiments, female mice were checked daily for the presence of vaginal plugs. Mice were observed daily for morbidity and mortality during the in-life period. All animal experiments were approved by the local Animal Ethics Committee of NV Organon, Oss, The Netherlands.
Generation of LRH-1-KD mice
The LRH-1-KD mice used in the present study were obtained from Artemis (Cologne, Germany). Recombinase-mediated cassette exchange (RMCE) in embryonic stem (ES) cells was used to introduce an inducible LRH-1 short hairpin (sh) RNA in the ROSA26 locus (Seibler et al. 2007). The following shRNA sequence was used for LRH-1-specific vector construction: 5′-acacagaagtcgcgttcaattcaagagattgaacgcgacttctgtgt-3′. This shRNA sequence was inserted behind the human polymerase III human H1 promoter and an inducible Tet-system. The vectors obtained were used to transfect RMCE ES cells. Three homologous recombinant clones for each shRNA vector were isolated and treated with 1 μg mL−1 Dox (D-9891; Sigma) for 48 h. The LRH-1 KD efficiencies were determined by quantitative polymerase chain reaction (q-PCR). The ES cell clone with the highest level of KD (90% reduction of LRH-1 expression) was used to generate F1 mice by injection into tetraploid blastocysts. Genotyping PCR was performed on isolated genomic DNA from tail biopsies using the following generic primers for the shRNA construct: forward, 5′-ccatggaattcgaacgctgacgtc-3′; reverse, 5′-tatgggctatgaactaatgaccc-3′. As a control for primer functionality, genomic DNA of luciferase-KD mice was used (Artemis). The PCR products were analysed on a 1% Tris/Borate/EDTA (TBE) agarose gel. Adult heterozygous LRH-1-transgenic mice and wild-type littermates were used for all experiments and ranged in age from 12 to 17 weeks, unless stated otherwise.
Ovarian hyperstimulation
Dams with 2-week-old litters were treated with 2 mg mL−1 Dox in 10% sucrose in their drinking water. After 1 week the mice were weaned, a tail biopsy was taken to determine the genotype and treatment with 2 mg mL−1 Dox in 10% sucrose continued. When the mice were 31 days old they were injected with 10 IU, s.c., urinary FSH (Humegon; NV Organon, Oss, The Netherlands) in saline at 1430 hours. Forty-six hours after Humegon injection, the mice were injected with 5 IU, s.c., human chorionic gonadotrophin (hCG; Pregnyl; NV Organon, Oss, The Netherlands) or saline control. Mice were killed by cervical dislocation 24 or 3 h after hCG administration. After 24 h, the mice were killed, the oviduct was isolated and the number of oocytes present in the oviduct were counted.
For ovulation induction in adult mice, non-synchronised adult female transgenic mice were treated with 2 mg mL−1 Dox in 10% sucrose in their drinking water for 2 weeks. After 2 weeks the mice were injected with 5 U, s.c., hCG (Pregnyl) and, 24 h later, they were killed and the number of oocytes in the oviduct was counted.
Histology
Histopathological analysis was performed on the liver, ovary, uterus and vagina of Dox-treated female LRH-1-KD and wild-type mice. At autopsy, all internal organs were examined for visual signs of abnormalities and their weights were recorded. Samples of organs and tissues from the mice were preserved in 4% buffered formaldehyde, dehydrated and embedded in paraffin wax. Sections (3–4 µm) from the different tissues were stained with haematoxylin and eosin (HE) and for subsequent microscopic evaluation.
Blood analyses
Blood was collected via the vena cava from mice under isoflurane anaesthesia. Blood was allowed to clot and the serum was separated by centrifugation at 20 800g for 15 min at room temperature. To obtain plasma samples, blood was collected in centrifuge tubes containing lithium–heparin (9 µL mL−1 blood) and separated by centrifugation at 20 800g for 15 min at room temperature.
Commercial assays were used to measure plasma 17β-oestradiol (EIA-2693; DRG Instruments, Marburg, Germany) and plasma progesterone (catalogue no. 582601; Cayman Chemical, Ann Arbor, MI, USA). Serum concentrations of LH and FSH were determined using an immunofluorescence assay (IFMA), as described previously (van Casteren et al. 2000). Briefly, a maxisorb microtitre plate was coated with a mouse anti-recombinant hCGβ monoclonal antibody (mAb) or mouse anti-recombinant hFHSβ mAb and serum samples were added. Plates were washed and a biotin-labelled mouse anti-recombinant rat LHα polyclonal antibody was added. After the addition of europium-labelled streptavidin and enhancement solution, fluorescence was measured using a Wallac Victor2 1420 multilabel counter (Perkin Elmer, Waltham, MA, USA).
A standard curve was used to calculate a four parameter fit and to determine LH and FSH concentrations.
Real-time PCR analysis
Total RNA was isolated using Trizol (Gibco BRL, Grand Island, NY, USA). The quality of the samples was monitored using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Only RNA with an RNA integrity number (RIN) ≥8.5 was used. First-strand synthesis was performed using Oligo(dT)15 (Promega, Madison, WI, USA) and pd(N)6 (Amersham Pharmacia Biotech, Uppsala, Sweden) primers and Superscript II RNase H-Reverse Transcriptase (Invitrogen) according to the manufacturers’ instructions. Briefly, 1.0 µg Oligo(dT)15 primer and 1.0 µg pd(N)6 were added to 2 µg RNA and the volume was adjusted to 12.0 µL with diethylpyrocarbonate (DEPC) water. The mixture was incubated at 70°C for 10 min and quickly chilled on ice for 2 min. Second-strand synthesis was performed in a volume of 20 µL containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT), 0.5 mM dNTPs and 200 U M-MLV RNase H-reverse transcriptase. The mixture was incubated for 10 min at room temperature and then for 50 min at 40°C. The cDNA from the synthesis reaction was subjected to quantitative real-time PCR with SYBR Green mastermix (Applied Biosystems, Benelux, Bleiswijk, The Netherlands) in an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The program used was 10 min at 95°C 100% ramp, followed by 40 cycles of 15 s at 95°C 100% ramp and 1 min at 60°C 100% ramp, followed by a dissociation curve step of 15 s at 95°C 100% ramp, 15 s at 60°C 100% ramp and 15 s at 95°C 2% ramp. Dissociation curves were checked for non-specific amplification. For quantification, standard curves were prepared for both the target and endogenous reference (HP1) genes. Levels of the target gene in each sample were normalised against those of the endogenous reference gene. The primer sequences used in the present study were as follows: for LRH-1 exon 6–7, 5′-cagaccctgttctccattgttg-3′ (forward) and 5′-tcactccagcagttttgaagca-3′ (reverse); for SHP, 5′-gagctgggtcccaagga-3′ (forward) and 5′-ggcacatctgggttgaaga-3′ (reverse); for Scarb1, 5′-gaaccctaacccaaaggagcat-3′ (forward) and 5′-tgcatcttcacagaacagttcatg-3′ (reverse); for Cyp7a1, 5′-ccacttcatcacaaactccctg-3′ (forward) and 5′-tgcttctgtgtccaaatgcc-3′ (reverse); for Cyp19, 5′-agaacaattcgccctttctttatg-3′ (forward) and 5′-atggactccacacaaacttcca-3′ (reverse); for Star, 5′-tgccgaagacaatcatcaac-3′ (forward) and 5′-tgtcatgggattagtagggaag-3′ (reverse); for HP1, 5′-gcccaagatggacgcaatc-3′ (forward) and 5′-ccgaggcgccagtcttc-3′ (reverse); for LHCGR, 5′-ttgaacccggtgcttttacaa-3′ (forward) and 5′-ccggatgcctgtgttacaga-3′ (reverse); for FSHR, 5′-gaattgcctgatgatgttttcca-3′ (forward) and 5′-gggaatagacctttgtccttgaga-3′ (reverse); for PRLR, 5′-aagtgctcaatccctggtatgg-3′ (forward) and 5′-cgatgctcacctccacagaga-3′ (reverse); and for Cyp11a1, 5′-atcagtgatgacctattccgcttt-3′ (forward) and 5′-ccatgcgctccccaaatata-3′ (reverse).
Western blot analysis
For western blot analysis, tissues were homogenised at approximately –80°C using a Micro Dismembrator (B. Braun Biotech International, Melsungen, Germany). Frozen powder (10–100 mg) was then mixed with 200 μL ice-cold lysis buffer (150 mM NaCl, 2 mM MgCl2, 0.5 mM DTT, 0.25 mM EDTA, 1 mg mL−1 Pefabloc (1585916; Boehringer, Ingelheim am Rhein, Germany), 10% glycerol, 1% Triton-X-100 and 50 mM Tris-HCl (pH 8.0), including protease inhibitor cocktail (1873580; Boehringer)). After 30 min incubation at 4°C, the samples were centrifuged at 14 000g for 15 min and the supernatant stored at –20°C. Protein concentrations were determined using the BCA Protein Assay kit (23227; Pierce, Rockford, IL, USA). From each sample, 30 μg total protein was separated on a 4%–12% Precast NUPAGE Bis-Tris gel (Invitrogen) using NUPAGE 3-[N-morpholino]propanesulphonic acid (MOPS)–sodium dodecyl sulfate (SDS) running buffer (Invitrogen). Separated protein was transferred to a nitrocellulose membrane (Hybond ECL, Amersham Pharmacia Biotech, Uppsala, Sweden) and the blots were blocked with 89.5% non-fat milk, 10% of 10× phosphate-buffered saline (PBS) and 0.5% Tween-20. Antibody incubations were performed in PBS containing 0.5% bovine serum albumin (BSA), 0.05% gelatin, 0.5% Tween-20 and 300 mM NaCl. The primary antibodies used were: anti-LRH-1 rabbit polyclonal antibody (ab18293; Abcam, Cambridge, UK), diluted 1 : 500, for the detection of LRH-1 in the ovary; anti-LRH-1 rabbit polyclonal generated against the C-terminal 20 amino acids of LRH-1, diluted 1 : 1000 for the detection of LRH-1 in the liver; and anti-β-actin rabbit polyclonal antibody (NB600-505; Novus Biologicals, Littleton, CO, USA), diluted 1 : 1500. The secondary antibody used was horseradish peroxidase (HRP)-conjugated goat anti-rabbit Ig (ALI4404; Invitrogen). Antibody binding was visualised using an ECL Western Blotting Detection kit (Amersham) and Hyperfilm ECL (Amersham).
Gene arrays
Immature transgenic LRH-1 shRNA and wild-type mice (n = 10) were treated with Dox as described above. Forty-six hours after Humegon injection, the ovaries of five LRH-1-KD and wild-type mice were isolated and, 2 h after Pregnyl injection, the ovaries of the remaining LRH-1-KD and wild-type mice were isolated for RNA extraction. The RNA was isolated using an RNeasy mini extraction kit (74106; Qiagen, Valencia, CA, USA) and RNA integrity was verified by capillary electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies). Double-stranded cDNA was synthesised from 1.5 µg total RNA using the One-Cycle Target Labelling Kit (Affymetrix, Santa Clara, CA, USA) and used for the preparation of biotin-labelled cRNA using a GeneChip IVT Labelling Kit (Affymetrix). Hybridisation of cRNA and washing of Affymetrix GeneChip Mouse Genome 430 2.0 arrays was performed according to standard Affymetrix protocols. The arrays were laser scanned with a GeneChip Scanner 300 (Affymetrix) according to the manufacturer’s instructions. Data were quantified using Microarray Suite 5.0/GCOS 1.1 (Affymetrix) and were normalised using the GeneChip Robust Multi-array average (GCRMA) method; Limma (R package) was used to calculate ratios and P-values (Linear Models for Microarray Data, open source software). Genes were selected that were present in more than two arrays, had an expression intensity of at least 20 in one of the arrays and showed a differential expression of KD compared with wild-type mice with an adjusted P-value <0.05. The gene expression dataset was subsequently analysed for gene ontology and pathways to relate changes in gene expression to functional alterations using the Ingenuity Pathway Analysis tool (Ingenuite Systems, Redwood City, CA, USA) and the standard settings at the David webserver (da Huang et al. 2009; http://david.abcc.ncifcrf.gov; January 2013).
Follicle culture and induction of ovulation in vitro
Ovarian follicles were isolated and according to Rose et al. (1999). Briefly, female immature mice (F1: B6BCA; 21–23 days of age) treated with Dox for 7 days were anaesthetised with isoflurane. Ovaries were removed and preantral follicles were isolated and collected. Follicles 160–210 μm in diameter with normal morphological appearance were cultured individually in Millicell-CM culture plate inserts (PICM 01250; Millipore, Bedford, MA, USA) in 250 μL α-minimum essential medium (αMEM; 22571–020; Gibco BRL) supplemented with 2 mM glutamine, 10 μg mL−1 transferrin, 5 μg mL−1 insulin, 50 μg mL−1 ascorbic acid, 2 ng mL−1 selenium (αMEM culture medium), 5% immature mouse serum, 1 µg mL−1 Dox and 100 U L−1 recombinant FSH (Puregon; NV Organon). Follicles were cultured in a humidified incubator gassed with 5% CO2 in air at 37°C. The culture medium was exchanged every other day. The diameter of the follicles was measured each day at ×100 magnification using a calibrated micrometer. In addition, the survival rate of the follicles was checked by evaluating degeneration (blackening of the follicle) and bursting (loss of the oocyte). After 4–5 days, follicles were induced to ovulate with 5 U mL−1 hCG (Pregnyl; NV Organon) in αMEM supplemented with 5% immature mouse serum and 1 µg mL−1 Dox. After 24 h incubation, follicles were checked for ovulation (follicular rupture, oocyte extrusion, and oocyte maturation).
Statistical analysis
All data are expressed as the mean ± s.e.m. Statistical analyses were performed using non-paired Student’s t-test or analysis of variation (ANOVA). P < 0.05 was considered significant.
Results
LRH-1-KD mouse model
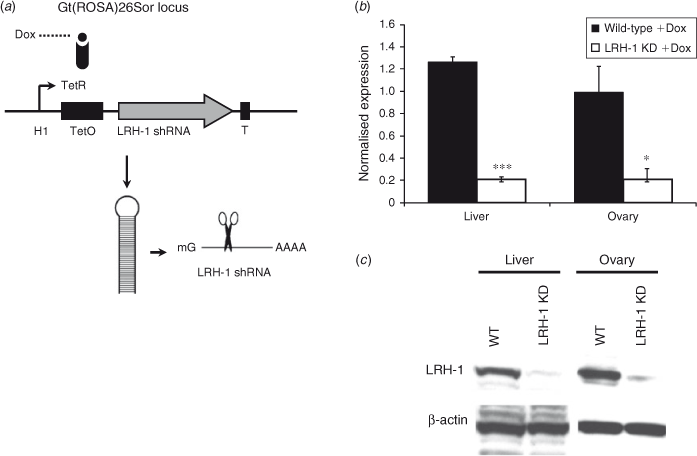
To study the mechanism of action and the reversibility of the LRH-1 infertility phenotype, an in vivo inducible KD mouse model was developed. In this model the ROSA26 locus was targeted with a construct containing the human polymerase III human H1 promoter upstream of an inducible Tet-system and an shRNA directed against a highly specific sequence in exon 8 of LRH-1 (Fig. 1a). In the absence of Dox, the constitutively active Tet repressor (TetR) binds to the Tet operator (TetO) sequence located between the H1 promoter and the shRNA sequence and prevents transcription of the shRNA. Upon administration of Dox, TetR can no longer bind to TetO, and this results in the expression of the shRNA and subsequent KD of LRH-1.
The validity of the model was established by mating female transgenic LRH-1 shRNA mice, treated with 2 mg mL−1 Dox and 10% sucrose in their drinking water, with wild-type male mice. The KD of LRH-1 during embryogenesis should reproduce the embryonic lethality as reported for the LRH-1-knockout mouse. Six plug-positive females delivered a total of 24 wild-type pups but no transgenic pups. It was therefore concluded that Dox-induced expression of the LRH-1 shRNA was sufficient to induce LRH-1 KD in vivo.
Subsequently, adult transgenic LRH-1 shRNA mice were treated with Dox for 2, 4 or 6 weeks. After 6 weeks treatment, the LRH-1-KD mice exhibited normal bodyweight compared with Dox-treated wild-type mice (31.5 ± 4.7 vs 32.3 ± 0.9 g, respectively). The transgenic LRH-1 shRNA mice exposed to 10% sucrose without Dox exhibited significantly reduced bodyweight (27.5 ± 2.9 g). Pathological examination revealed that this was caused mainly by the absence of solid food in their digestive tract. Most likely these mice preferred the intake of 10% sucrose water above solid food. Further examination of the different organs did not reveal any abnormalities in the LRH-1 KD-mice and microscopy of the liver did not show any morphological changes.
To test the efficiency of Dox-induced LRH-1 KD, q-PCR was performed on tissues samples from LRH-1-KD and wild-type mice treated either with 2 mg mL−1 Dox and 10% sucrose or 10% sucrose alone. LRH-1 KD for 4 weeks resulted in an 83.5% reduction in LRH-1 mRNA expression in the liver (Fig. 1b). LRH-1 KD for 2 and 6 weeks also resulted in an 80% reduction in LRH-1 mRNA (data not shown). The transgenic LRH-1 shRNA control mice, receiving 10% sucrose without Dox, also exhibited decreased LRH-1 expression of 17% in the liver and 16% in the ovary (data not shown). This could be caused by leakage of the TetR/O regulatory system or it could be the result of physiological changes in these mice caused by the 10% sucrose treatment. In the ovary, 68% KD of LRH-1 mRNA levels was observed after 4 weeks Dox treatment compared with levels in the Dox-treated wild-type controls (Fig. 1b). We also tested several other tissues in which LRH-1 expression and a functional role for LRH-1 has been reported. In the colon of LRH-1-KD mice, the KD efficiency was approximately 60%. In adipose tissue, LRH-1 expression levels differed markedly between individual mice and no Dox-dependent KD was observed. The expression levels of LRH-1 in the testis, mammary gland and adrenal gland were very low and no Dox-dependent KD of LRH-1 could be observed. Subsequently, we performed western blot analysis of liver and ovary protein extracts using LRH-1-specific antibodies. After 4 weeks Dox treatment, the liver of LRH-1-KD mice contained approximately 10%–20% of the LRH-1 protein in the liver of wild-type control mice treated with Dox (Fig. 1c). The reduction in LRH-1 protein in the ovary was also approximately 80%–90% compared with that in wild-type mice.
LRH-1-KD mice are infertile
To evaluate the fertility phenotype of LRH-1 KD, female transgenic LRH-1 shRNA mice were treated with Dox for 6 weeks and were then mated for 10 days with wild-type male mice; Dox treatment continued for another 2 weeks while the mice were breeding. Of the 17 LRH-1-KD females treated with Dox, none became pregnant (Fig. 2d). Female mice in the LRH-1 shRNA control and wild-type control groups did get pregnant and delivered normal sized litters (Fig. 2d). Even after prolonged mating with male mice for several weeks, the LRH-1-KD mice did not get pregnant.
Histological examination of the ovaries from LRH-1-KD mice that were treated with Dox for 4 weeks revealed the complete absence of CL (Fig. 2b), whereas the ovaries from Dox-treated wild-type mice contained 5.25 ± 0.85 CL (Fig. 2a). Follicular development in the LRH-1 KD ovary proceeded up to the antral stage and the ovaries contained normal numbers of secondary, preantral and antral follicles that were not significantly different from those in wild-type ovaries. Serial sections of the complete ovary revealed that, after 4 weeks Dox treatment, the LRH-1 KD ovary contained 31.0 ± 2.9 secondary follicles (vs 41.0 ± 3.1 in Dox-treated wild-type control mice), 15.5 ± 0.9 preantral follicles (vs 14.3 ± 2.0 in Dox-treated wild-type controls) and 17.3 ± 0.6 antral follicles (vs 19.7 ± 1.5 in Dox-treated wild-type controls). In addition, no changes in the number of follicles were found after 6 weeks Dox treatment. Oocytes of normal appearance were present in the follicles of the LRH-1-KD female mice. The area of the cumulus–oocyte complex was comparable between wild-type and LRH-1-KD antral stage follicles (Fig. 2g, h). Longer treatments with Dox for up to 12 weeks resulted in an identical ovarian phenotype.
Serum hormone analysis was performed in adult non-synchronised LRH-1-KD mice. To determine the stage of the cycle, the vaginal epithelium was examined microscopically after autopsy. This indicated that 14 of 17 Dox-treated LRH-1-KD mice were in oestrus and three of 17 were in metoestrus. Of the 21 wild-type mice examined, two were in pro-oestrus, 10 were in oestrus, two were in metoestrus and seven were in dioestrus. Comparison of serum hormone levels in mice that were in oestrus revealed significant reductions in serum progesterone levels in LRH-1-KD mice compared with Dox-treated wild-type mice (5.9 ± 0.9 vs 20.2 ± 7.7 nmol L−1, respectively; P < 0.05). Serum FSH levels were also reduced in LRH-1-KD mice compared with Dox-treated wild-type mice (0.74 ± 0.10 vs 1.45 ± 0.30 ng mL−1, respectively; P < 0.05). There were no significant differences between LRH-1-KD and Dox-treated wild-type mice in terms of LH (0.169 ± 0.035 vs 0.130 ± 0.048 ng mL−1, respectively) and oestradiol (34.9 ± 1.8 vs 43.4 ± 5.1 pmol L−1, respectively) concentrations.
Subsequently, we investigated whether the infertility phenotype of the LRH-1-KD mice could be rescued by ovarian hyperstimulation treatment of both adult and synchronised immature female LRH-1-KD mice. Adult mice were treated for 4 weeks with Dox or vehicle, after which ovulation was induced via s.c. injection of hCG. Although both wild-type mice treated with Dox and untreated transgenic LRH-1 shRNA mice contained oocytes in the oviduct 24 h after hCG injection, there was no ovulation observed in Dox-treated LRH-1-KD mice, with oocyte numbers in Dox-treated wild-type, untreated LRH-1-KD and Dox-treated LRH-1-KD mice of 6.2 ± 1.0, 6.3 ± 0.8 and 0 ± 0, respectively.
Two-week-old immature female mice were treated with Dox for 17 days. Folliculogenesis was subsequently induced by s.c. injection of human gonadotrophins, followed by the induction of ovulation by hCG injection 2 days later. In Dox-treated LRH-1-KD female mice, there was a marked reduction in the number of oocytes in the oviduct 24 h after hCG injection compared with that in Dox-treated wild-type control (2.3 ± 2.3 vs 23.6 ± 4.0, respectively). Histology of ovaries from immature mice showed that, 24 h after hCG, no preovulatory follicles were present in the wild-type ovaries but that in ovaries from LRH-1-KD mice the follicles had not progressed beyond the antral stage (Fig. 2e, f). In addition, the area of the cumulus–oocyte complexes in the gonadotropin-stimulated immature LRH-1 follicles (Fig. 2i) was comparable to that seen in the adult wild-type and LRH-1-KD follicles (Fig. 2g, h).
Reversible infertility in LRH-1 knockdown mice
To determine whether the infertility of LRH-1-KD mice was reversible, adult female transgenic LRH-1 shRNA and wild-type mice were treated with Dox for 2 weeks and then mated with wild-type male mice. Dox treatment was stopped after 6 weeks when all the wild-type control females had become pregnant. None of the LRH-1-KD female mice had become pregnant at that time. Subsequently, Dox treatment was stopped and, 3 weeks later, the same LRH-1-KD and wild-type female mice were mated again with wild-type male mice. All transgenic LRH-1 shRNA female mice became pregnant and delivered a total of 22 viable pups (Fig. 2d). Analysis of genomic DNA from tail biopsies revealed the presence of both wild-type and LRH-1 shRNA transgenic pups in the litters of the LRH-1 shRNA transgenic mice. Microscopic examination showed that, 10 weeks after Dox treatment stopped, the ovaries of the LRH-1-KD mice were indistinguishable from wild-type ovaries and CL were normally present (Fig. 2c). In addition, serum progesterone levels had returned to those seen in the wild-type mice (data not shown).
Culture of LRH-1-KD follicles
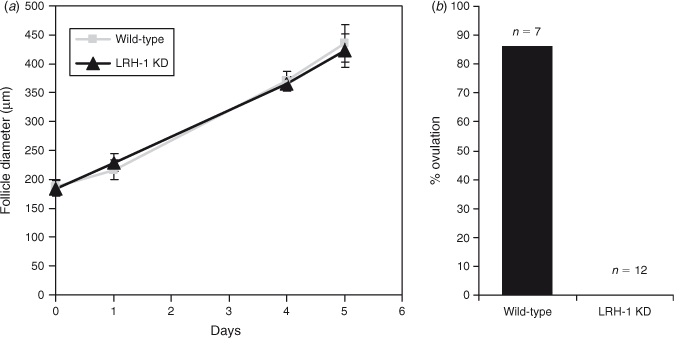
To study the infertility phenotype in vitro, follicles were collected from the ovaries of 28-day-old immature transgenic LRH-1 shRNA mice and their wild-type littermates that had been treated with Dox for 7 days. Follicles ranging in size from 160 to 210 µm in diameter were cultured in the presence of recombinant FSH and 0.1 µg mL−1 Dox for 5 days, and their growth was recorded. The efficiency of LRH-1 KD was confirmed by q-PCR analysis. As shown in Fig. 3a, LRH-1 KD follicles developed at the same rate as wild-type follicles. After 5 days in culture, ovulation was induced by the addition of hCG. Ovulation was completely blocked in the LRH-1-KD follicles, whereas 86% of the wild-type follicles ovulated (Fig. 3b).
Expression of LRH-1 target genes in LRH-1-KD mice
To investigate which pathways and LRH-1 target genes are affected by LRH-1 KD, we determined the expression of several LRH-1 target genes in the liver and ovary and performed microarray analysis on the ovaries of LRH-1-KD and wild-type mice. Quantitative PCR analysis revealed that Shp expression was downregulated by 85% in the liver of LRH-1-KD mice (Fig. 4a), Scrarb1 expression was downregulated by 43% and there was no obvious regulation of Cyp7a1 expression in liver samples from LRH-1-KD mice (Fig. 4a).

|
Furthermore, q-PCR analysis of unsynchronised adult LRH-1-KD ovaries revealed that Star, Cyp11a1, Scarb1, Lhgcr and Prlr expression was significantly reduced compared with that in Dox-treated wild-type mice (Fig. 4b). There were no changes in Cyp19 and Fshr expression. To further study the changes in expression in the LRH-1-KD ovary, we performed a microarray experiment on immature wild-type and LRH-1-KD ovaries. Two-week-old immature mice were treated with Dox for 17 days. Subsequently, the mice were treated with FSH to induce follicle maturation and, 46 h later, with hCG to induce ovulation. RNA was isolated 40 h after FSH treatment and 2 h after hCG treatment and both time points were analysed on microarray. The results showed that 40 h after FSH treatment 637 genes were differentially expressed between the LRH-1-KD and Dox-treated wild-type ovaries (P < 0.05). Two hours after hCG, 794 genes were differentially expressed (P < 0.05; see Table S1 available as Supplementary Material to this paper) and 285 genes were significantly regulated at both time points. Pathway analysis showed that regulated canonical pathways in the LRH-1-KD ovaries 40 h after FSH include C21-Steroid Hormone Metabolism and Androgen and Oestrogen Metabolism. These pathways were not regulated 2 h after hCG. At both time points, the Interferon Signalling pathway and other immunological pathways were among the most significantly regulated pathways (data not shown).
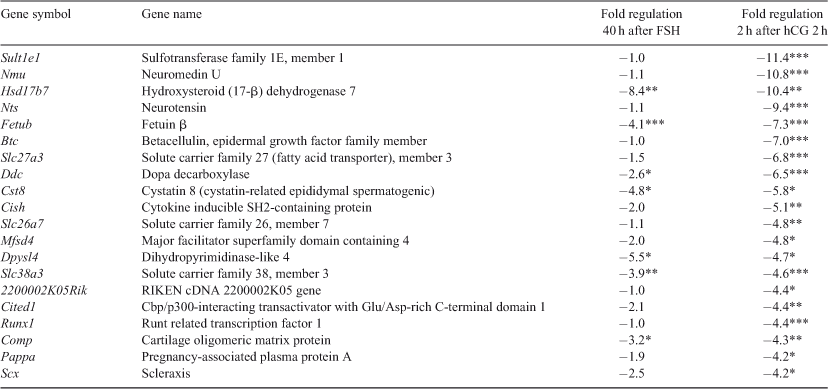
In line with the q-PCR analysis of the adult ovary, LRH-1 expression in the immature LRH-1-KD ovary was significantly reduced by 2.4- and 2.7-fold at 40 h after FSH and 2 h after hCG, respectively, compared with Dox-treated wild-type (see Fig. S1). Microarray analysis further indicated that 40 h after FSH the genes that showed the highest regulation in the LRH-1-KD ovaries were steroidogenic genes Star and Hsd17b7 (Table 1). In addition, Scarb1, Cyp11a1 (Table 1) and Hsd3b (see Fig. S1) were significantly downregulated during folliculogenesis. Expression of Fshr, Cyp19a1 and Nos3 was not found to be significantly modulated; however, Ccnd2 expression was significantly reduced at both time points (see Fig. S1).
Two hours after hCG, Sult1e1 and Hsd17b7 were among the highest regulated genes, but Star, Scarb1 and Cyp11a1 were not significantly regulated at this time point (Table 2). Seven of the highly regulated genes were significantly modulated at both time points (Hsd17b7, Fetub, Ddc, Cst8, Dpysl4, Slc38a3 and Comp).
Other genes that showed high downregulation were previously described to be involved in: (1) the ovulation process and in CL formation and function, namely Nts (Hernandez-Gonzalez et al. 2006), Btc (Ashkenazi et al. 2005; Hernandez-Gonzalez et al. 2006), Cst8 (Hsia et al. 2005), Cish (Anderson et al. 2009), Runx1 (Hernandez-Gonzalez et al. 2006; Jo and Curry 2006), Pappa (Nyegaard et al. 2010), Cited1 (Sriraman et al. 2010) and Sfrp4 (Hernandez-Gonzalez et al. 2006; Maman et al. 2011), in addition to Sult1e1, Star, Hsd17b7, Cyp11a, Lhcgr, Scarb1 (Table 1) and Oxtr (Shirasuna et al. 2007) and Prlr (Le et al. 2012; see Fig. S1 and Table S1); (2) proliferation, cell cycle and apoptosis, namely Btc (Oh et al. 2011), Rasl10a (Elam et al. 2005), Dpysl4 (Kimura et al. 2011), Sfrp4 (Jacob et al. 2012), Dusp1 (Chen et al. 2011) and Rcc2 (Grigera et al. 2012); and (3) neuronal processes, namely Nmu (Chu et al. 2012), Nts (Hernandez-Gonzalez et al. 2006; Hanson et al. 2012) and Ddc (Børglum et al. 2001). Significant regulation was also observed for Ptgs2, Cd44 and Ptx3 genes involved in the cumulus expansion process, but not for C1qbp, Tnfaip6, Mmp2, Mmp9, Mmp19, Adamts4, Adamts1 and Has2 (see Fig. S1; Duggavathi et al. 2008).
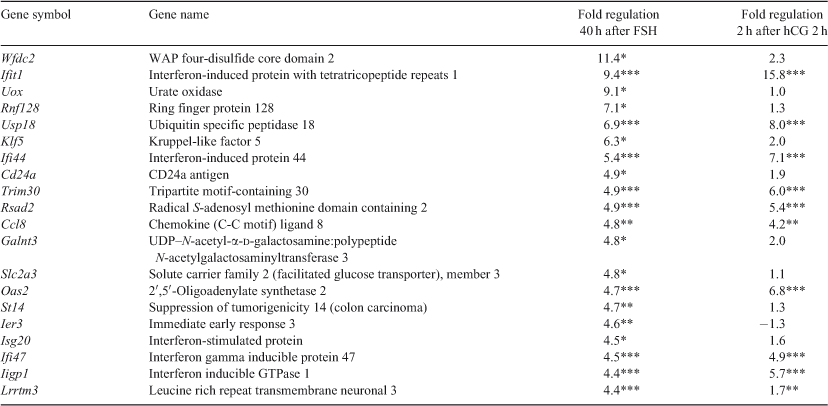
Most of the most upregulated genes at both 40 h after FSH and 2 h after hCG are linked to interferon and immunology-related processes (Wfdc2, Ifit1, Uox, Rnf128, Usp18, Ifi44, Cd24a, Trim30, Rsad2, Ccl8, Oas2, Ier3, Isg20, Ifi47, Iigp1, Ifit2, Cxcl10, Irf7, Igtp, Ifi27, Psmb8 and Sp100; Tables 3, 4; de Veer et al. 2005) and nine these highly modulated genes were significantly regulated at both time points.
Discussion
In the present study, temporal in vivo KD of LRH-1 generally recapitulated phenotypic aspects of previous studies. However, some interesting differences and additional findings were observed. With respect to the liver, Shp and Scarb1 expression was reduced, but Cyp7a1 expression was unaffected (Mataki et al. 2007; Lee et al. 2008; Out et al. 2011). Liver histology was normal and plasma liver parameters were not significantly changed (data not shown); these findings are in line with other studies of LRH-1-deficient mouse models.
In the ovaries of transgenic LRH-1 shRNA mice, Dox treatment resulted in 80–90% KD of LRH-1 protein in the ovary. Remarkably, the LRH-1 mRNA level was only reduced by 68% in the LRH-1-KD ovary. A possible explanation could be the presence of a partial LRH-1 transcript after Dox treatment.
The reduced LRH-1 expression resulted in complete infertility, the absence of CL and an abnormal oestrous cycle. The altered expression profile at 40 h after FSH stimulation, exemplified by the reduced expression of Ccnd2, an FSH/oestradiol-responsive gene involved in granulosa cell proliferation (Sicinski et al. 1996; Robker and Richards 1998), suggests that LRH-1 deficiency also affects folliculogenesis. However, morphologically, follicular maturation (including granulosa cells proliferation) appeared to progress normally up to the antral stage in LRH-1-KD mice both in vitro and ex vivo. Ovulation was blocked in the LRH-1-KD mice and examination of serial sections of the ovaries of LRH-1-KD mice revealed that oocytes were present in all antral stage follicles. Stimulation with gonadotrophins in adult and immature mice and in follicle culture could not force oocyte rupture. This indicates that the observed reduction in serum FSH itself is most likely not causing the infertility phenotype, and this is supported by the normal fertility observed in haploinsufficient FSHβ-knockout mice (Kumar et al. 1997; Sun et al. 2006). Instead of progressing to the luteal stage, late stage follicles in LRH-1-KD mice most likely become atretic, because there was no indication of accumulation of antral follicles or luteinised unruptured follicles in the ovaries.
Plasma progesterone levels were reduced and the expression of progesterone steroid biosynthesis genes Scarb1, Cyp11a, Star and Hsd3b was downregulated in the ovaries of LRH-1-KD mice, most prominently 40 h after FSH treatment. Duggavathi et al. (2008) reported an increase in intrafollicular oestradiol production in granulosa cell-specific LRH-1-knockout mice. This was accompanied by increased Cyp19a1 expression and reduced Nos3 and Sult1e1 expression. We did not observe this in the present study. Plasma oestradiol concentrations were not significantly increased in LRH-1-KD mice, Cyp19a1 and Nos3 expression was not significantly modulated (see Fig. S1) and no effect on the thickness of the epithelial cells of the endometrium was observed (data not shown). We did observe a markedly reduced expression of Sult1e1 2 h after hCG and, surprisingly, Hsd17b7 expression was highly regulated both before and after hCG stimulation. Hsd17b7 has been described to convert oestrone to oestradiol in luteal cells and also has a role in the final steps of cholesterol biosynthesis (Shehu et al. 2008). This finding would support a decrease, rather than an increase, in oestradiol synthesis during folliculogenesis. The regulation of both Sult1e1 and HSD17b7 2 h after hCG would lead to opposite effects on oestrogen bioavailability. Together, these observations suggest that, in the LRH-1 KD model, oestradiol is not the main component that causes the infertility phenotype, and this is further supported by the fact that mouse models of increased oestrogenic activity do not show complete female infertility (Jablonka-Shariff et al. 1998; Gershon et al. 2007). The different effect on oestradiol in LRH-1-KD mice compared with granulose cell-specific LRH-1-knockout mice (Duggavathi et al. 2008) could be explained by the incomplete and temporal nature of KD of LRH-1 in the LRH-1-KD mice. Downstream of oestradiol we found some changes in the expression of genes involved in cumulus expansion (see Fig. S1). Ptgs2 and Ptx3 expression was significantly downregulated in LRH-1-KD mice 2 h after hCG, and Cd44 expression was significantly increased 40 h after FSH. The expression of Tnafaip6, C1qbp, Adamts4, Adamts1 and other proteases was not significantly changed or was undetectable in our experiments. Nevertheless, cumulus expansion appeared to be affected because, in the gonadotropin-stimulated immature LRH-1-KD mice, the cumulus–oocyte complex was still not fully expanded 24 h after hCG (Duggavathi et al. 2008; Richards and Pangas 2010).
The reduced progesterone level in the LRH-1-KD mice is likely to represent a factor with greater impact on fertility. In rodents, ovulation is preceded by a small increase in progesterone as well as an increase in oestradiol, prolactin and LH. The preovulatory rise in progesterone has been shown to be mandatory for ovulation (Hibbert et al. 1996; Robker et al. 2000) and its absence in LRH-1-KD mice could largely explain the observed infertility phenotype. The progesterone receptor-knockout (PRKO) mice, in which both progesterone receptors have been inactivated, are completely infertile (Robker et al. 2000; Conneely et al. 2003), like LRH-1-KD mice. In PRKO mice, folliculogenesis progresses normally, but ovulation is blocked and cannot be induced by exposure to superovulatory levels of gonadotrophins. The main difference between the LRH-1 KD and PRKO phenotypes resides in the observation that PRKO granulosa cells can still differentiate into luteal cells (Robker et al. 2000), indicating that additional defects are present in LRH-1-KD mice.
We were unable to show that progesterone supplementation rescued the LRH-1-KD phenotype. Subcutaneous injection of 100 µL of 10 mg mL−1 progesterone 3 h prior and 2 h after hCG injection in adult LRH-1-KD mice failed to rescue ovulation (data not shown).
The absence of luteinisation may be caused by reduced Lhcgr and Prlr expression in LRH-1-KD mice. This could indicate that LRH-1 KD antral follicles are not fully prepared for ovulation and progression of granulosa cells to the luteal phase and have reduced sensitivity to the LH surge.
Microarray analysis revealed significant upregulation of interferon and immunology pathways in the LRH-1-KD model. This could be caused by LRH-1 shRNA expression provoking cellular antiviral RNA interference machinery (de Veer et al. 2005). However, if the regulation of these genes indeed represents cellular antiviral defence or off-target effects, they do not seem to cause a deviant liver or ovarian phenotype in the LRH-1-KD mice. Alternatively, regulation of these pathways could be a consequence of the role of LRH-1 in immunological responses (Lin et al. 2000; Mueller et al. 2006; Venteclef et al. 2006, 2011; Coste et al. 2007), or an immunological response to the increase cellular debris from atretic antral follicles (Hakuno et al. 1996).
Previously described LRH-1-deficient mouse models did not establish whether the defects caused by LRH-1 deficiency are reversible. We successfully applied in vivo inducible RNA interference (RNAi) technology in the LRH-1-KD model to address whether fertility is restored after transient LRH-1 KD. Three weeks after withdrawal of Dox treatment, the LRH-1 shRNA transgenic mice were again fully fertile and delivered healthy pups with no signs of irreversible changes due to the transient reduction in LRH-1 expression. Therefore, analysis of the LRH-1-KD mouse model revealed a reversible infertility phenotype that was predominantly caused by reduced progesterone synthesis and, in contrast with earlier studies, with no obvious effects on oestradiol activity. In addition, the data obtained indicated a unique mechanism of action in which the processes of ovulation and luteinisation are disturbed due to defects caused by LRH-1 deficiency during folliculogenesis before the LH surge. Because this model mimics the effects of an LRH-1 antagonist, the results further support the feasibility of the use of LRH-1 antagonising compounds as contraception therapy. It should be mentioned though that the unopposed oestrogenic effects, the possible need for continuous treatment and the potentially teratogenic effects of LRH-1 antagonists during embryogenesis make the development of LRH-1 antagonist for contraception challenging.
Acknowledgements
The authors thank P. W. J. de Leeuw and J. H. M. I. Brands (Merck Sharp and Dohme Research Laboratories, Department of Toxicology and Drug Disposition, Schaijk, The Netherlands) for performing blood biochemistry analyses, Magda Krajnc and Dorette van Ingen Schenau (Merck Sharp and Dohme Research Laboratories, Department of Toxicology and Drug Disposition, Schaijk, The Netherlands) for histopathological analysis, Jos Verhagen (Merck Sharp and Dohme Research Laboratories, Women’s Health Department, Oss, The Netherlands) for performing LH and FSH serum analysis and Eddy van der Struik (Merck Sharp and Dohme Research Laboratories, Target Discovery Department, Oss, The Netherlands) for performing the microarray experiment.
References
Anderson, S. T., Isa, N. N., Barclay, J. L., Waters, M. J., and Curlewis, J. D. (2009). Maximal expression of suppressors of cytokine signaling in the rat ovary occurs in late pregnancy. Reproduction 138, 537–544.| Maximal expression of suppressors of cytokine signaling in the rat ovary occurs in late pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOgtrjM&md5=418f5425119e8d3d515771f785c6a6f0CAS | 19502454PubMed |
Ashkenazi, H., Cao, X., Motola, S., Popliker, M., Conti, M., and Tsafriri, A. (2005). Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146, 77–84.
| Epidermal growth factor family members: endogenous mediators of the ovulatory response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFGmt7jP&md5=9ec0eb753300edfe536e4473dd4f0a75CAS | 15459120PubMed |
Børglum, A. D., Hampson, M., Kjeldsen, T. E., Muir, W., Murray, V., Ewald, H., Mors, O., Blackwood, D., and Kruse, T. A. (2001). Dopa decarboxylase genotypes may influence age at onset of schizophrenia. Mol. Psychiatry 6, 712–717.
| Dopa decarboxylase genotypes may influence age at onset of schizophrenia.Crossref | GoogleScholarGoogle Scholar | 11673800PubMed |
Botrugno, O. A., Fayard, E., Annicotte, J. S., Haby, C., Brennan, O., Wendling, T., Tanaka, T., Kodama, T., Thomas, W., Aurwerx, J., and Schoonjans, K. (2004). Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell 15, 499–509.
| Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXns1ynurs%3D&md5=8fbd4b1c65bf79006e640c7d5022be1bCAS | 15327767PubMed |
Britt, K. L., Drummond, A. E., Dyson, M., Wreford, N. G., Jones, M. E., Simpson, E. R., and Findlay, J. K. (2001). The ovarian phenotype of the aromatase knockout (ArKO) mouse. J. Steroid Biochem. Mol. Biol. 79, 181–185.
| The ovarian phenotype of the aromatase knockout (ArKO) mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFyltbs%3D&md5=d5d9e312dda3083a18c1c04b143d3de1CAS | 11850223PubMed |
Burendahl, S., Treuter, E., and Nilsson, L. (2008). Molecular dynamics simulations of human LRH-1: the impact of ligand binding in a constitutively active nuclear receptor. Biochemistry 47, 5205–5215.
| Molecular dynamics simulations of human LRH-1: the impact of ligand binding in a constitutively active nuclear receptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXks1Wntrc%3D&md5=3785d630467484465280e5b122a8acdaCAS | 18410128PubMed |
Chen, C. C., Hardy, D. B., and Mendelson, C. R. (2011). Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J. Biol. Chem. 286, 43 091–43 102.
| Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1ShurjP&md5=2eac3ad183231054676646ebbe6ff559CAS |
Chu, C. P., Xu, C. J., Kannan, H., and Qiu,, D. L. (2012). Corticotrophin-releasing factor inhibits neuromedin U mRNA expressing neuron in the rat hypothalamic paraventricular nucleus in vitro. Neurosci. Lett. 511, 79–83.
| Corticotrophin-releasing factor inhibits neuromedin U mRNA expressing neuron in the rat hypothalamic paraventricular nucleus in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVOnu7g%3D&md5=9c79db095ddc8c84b0671f0df6a4ef68CAS | 22306094PubMed |
Clyne, C. D., Speed, C. J., Zhou, J., and Simpson, E. R. (2002). Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J. Biol. Chem. 277, 20 591–20 597.
| Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkslWgur4%3D&md5=dc27ecc702dc158fb77f832b433d5c7aCAS |
Conneely, O. M., Mulac-Jericevic, B., and Lydon, J. P. (2003). Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68, 771–778.
| Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXps1Oltb8%3D&md5=33e662c18a09b45e4cb06b116c55d920CAS | 14667967PubMed |
Coste, A., Dubuquoy, L., Barnouin, R., Annicotte, J. S., Magnier, B., Notti, M., Corazza, N., Antal, M. C., Metzger, D., Desreumaux, P., Brunner, T., Auwerx, J., and Schoonjans, K. (2007). LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc. Natl Acad. Sci. USA. 104, 13 098–13 103.
| LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1GgsL4%3D&md5=c0aa17a9b9e0c5579cebf16fe9dfc5aaCAS |
Huang da, W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57.
| Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources.Crossref | GoogleScholarGoogle Scholar | 19131956PubMed |
de Veer, M. J., Sledz, C. A., and Williams, B. R. (2005). Detection of foreign RNA: implications for RNAi. Immunol. Cell Biol. 83, 224–228.
| Detection of foreign RNA: implications for RNAi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtVSitrY%3D&md5=20cc845433971f0bdd7fa0c39a4007ceCAS | 15877599PubMed |
Delerive, P., Galardi, C. M., Bisi, J. E., Nicodeme, E., and Goodwin, B. (2004). Identification of liver receptor homolog-1 as a novel regulator of apolipoprotein AI gene transcription. Mol. Endocrinol. 18, 2378–2387.
| Identification of liver receptor homolog-1 as a novel regulator of apolipoprotein AI gene transcription.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotFamsLg%3D&md5=3fbd1a19ff2268300174980e8e3413dfCAS | 15218078PubMed |
Duggavathi, R., Volle, D. H., Mataki, C., Antal, M. C., Messaddeq, N., Auwerx, J., Murphy, B. D., and Schoonjans, K. (2008). Liver receptor homolog 1 is essential for ovulation. Genes Dev. 22, 1871–1876.
| Liver receptor homolog 1 is essential for ovulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFWlsLo%3D&md5=2cc605570f2b6e211751d8d0a245f77bCAS | 18628394PubMed |
Elam, C., Hesson, L., Vos, M. D., Eckfeld, K., Ellis, C. A., Bell, A., Krex, D., Birrer, M. J., Latif, F., and Clark, G. J. (2005). RRP22 is a farnesylated, nucleolar, Ras-related protein with tumor suppressor potential. Cancer Res. 65, 3117–3125.
| 1:CAS:528:DC%2BD2MXjt1yktr0%3D&md5=98a5f195a6d9ac90d9c477f67cfd327bCAS | 15833841PubMed |
Ellinger-Ziegelbauer, H., Hihi, A. K., Laudet, V., Keller, H., Wahli, W., and Dreyer, C. (1994). FTZ-F1-related orphan receptors in Xenopus laevis: transcriptional regulators differentially expressed during early embryogenesis. Mol. Cell. Biol. 14, 2786–2797.
| FTZ-F1-related orphan receptors in Xenopus laevis: transcriptional regulators differentially expressed during early embryogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksFWhtrg%3D&md5=7d91164e62199f15b948eaded50434cdCAS | 8139576PubMed |
Fayard, E., Auwerx, J., and Schoonjans, K. (2004). LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 14, 250–260.
| LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVemu74%3D&md5=8c5aecfa05bb70255571df6d47de5d5dCAS | 15130581PubMed |
Galarneau, L., Drouin, R., and Belanger, L. (1998). Assignment of the fetoprotein transcription factor gene (FTF) to human chromosome band 1q32.11 by in situ hybridization. Cytogenet. Cell Genet. 82, 269–270.
| Assignment of the fetoprotein transcription factor gene (FTF) to human chromosome band 1q32.11 by in situ hybridization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksl2isQ%3D%3D&md5=d426dd3c1bf5c54398bf0d4821717e5fCAS | 9858833PubMed |
Gershon, E., Hourvitz, A., Reikhav, S., Maman, E., and Dekel, N. (2007). Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice. FASEB J. 21, 1893–1901.
| Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsVWks7o%3D&md5=6def116c8eede438886c2e30ddd79a7aCAS | 17341680PubMed |
Grigera, P. R., Ma, L., Borgman, C. A., Pinto, A. F., Sherman, N. E., Parsons, J. T., and Fox, J. W. (2012). Mass spectrometric analysis identifies a cortactin-RCC2/TD60 interaction in mitotic cells. J. Proteomics 75, 2153–2159.
| Mass spectrometric analysis identifies a cortactin-RCC2/TD60 interaction in mitotic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xit12ht70%3D&md5=4dc14077d4887cfe8bbe4bb50645499fCAS | 22282019PubMed |
Gu, P., Goodwin, B., Chung, A. C.-K., Xu, X., Wheeler, D., Price, R. R., Galardi, C., Peng, L., Latour, A., Koller, B. H., Gossen, J., Kliever, S. A., and Cooney, A. J. (2005). Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 25, 3492–3505.
| Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjvFSmsbc%3D&md5=67973731639cc23975b355096ef1dfafCAS | 15831456PubMed |
Hakuno, N., Koji, T., Yano, T., Kobayashi, N., Tsutsumi, O., Taketani, Y., and Nakane, P. K. (1996). Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137, 1938–1948.
| Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XisFCqsL4%3D&md5=5b8b9c003eff9fe0e1a34752df01ef68CAS | 8612534PubMed |
Hanson, G. R., Hoonakker, A. J., Alburges, M. E., McFadden, L. M., Robson, C. M., and Frankel, P. S. (2012). Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience 203, 99–107.
| Response of limbic neurotensin systems to methamphetamine self-administration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVOks7w%3D&md5=0f27848a9b5452604310c9e94306b1d8CAS | 22245499PubMed |
Hernandez-Gonzalez, I., Gonzalez-Robayna, I., Shimada, M., Wayne, C. M., Ochsner, S. A., White, L., and Richards, J. S. (2006). Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol. Endocrinol. 20, 1300–1321.
| Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltlSrsLY%3D&md5=861410c17a3ec756c8138b4da9137f05CAS | 16455817PubMed |
Hibbert, M. L., Stouffer, R. L., Wolf, D. P., and Zelinski-Wooten, M. B. (1996). Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc. Natl Acad. Sci. USA 93, 1897–1901.
| Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhsFart78%3D&md5=e873f4c6524b5f669a908294682074e5CAS | 8700855PubMed |
Hinshelwood, M. M., Repa, J. J., Shelton, J. M., Richardson, J. A., Mangelsdorf, D. J., and Mendelson, C. R. (2003). Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol. Cell. Endocrinol. 207, 39–45.
| Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntVyns7c%3D&md5=1d977ecefc1f8ce04988455cb8d0b5b8CAS | 12972182PubMed |
Hsia, N., Brousal, J. P., Hann, S. R., and Cornwall, G. A. (2005). Recapitulation of germ cell- and pituitary-specific expression with 1.6 kb of the cystatin-related epididymal spermatogenic (Cres) gene promoter in transgenic mice. J. Androl. 26, 249–257.
| 1:CAS:528:DC%2BD2MXis1Slu7w%3D&md5=81a87b6ce7993a5eb5fc9ec4d1f2e5deCAS | 15713831PubMed |
Jablonka-Shariff, A., and Olson, L. M. (1998). The role of nitric oxide in oocyte meiotic maturation and ovulation: meiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes. Endocrinology 139, 2944–2954.
| The role of nitric oxide in oocyte meiotic maturation and ovulation: meiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjsFSksrg%3D&md5=817350158a534842942267057e141364CAS | 9607805PubMed |
Jacob, F., Ukegjini, K., Nixdorf, S., Ford, C. E., Olivier, J., Caduff, R., Scurry, J. P., Guertler, R., Hornung, D., Mueller, R., Fink, D. A., Hacker, N. F., and Heinzelmann-Schwarz, V. A. (2012). Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients. PLoS One 7, e31885.
| Loss of secreted frizzled-related protein 4 correlates with an aggressive phenotype and predicts poor outcome in ovarian cancer patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVaqsbc%3D&md5=94c7d8746c381610964f7288e1feb678CAS | 22363760PubMed |
Jo, M., and Curry, T. E., (2006). Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles. Mol. Endocrinol. 20, 2156–2172.
| Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Cqu78%3D&md5=69cd86880dc63ebbf0850782d3095404CAS | 16675540PubMed |
Kimura, J., Kudoh, T., Miki, Y., and Yoshida, K. (2011). Identification of dihydropyrimidinase-related protein 4 as a novel target of the p53 tumor suppressor in the apoptotic response to DNA damage. Int. J. Cancer 128, 1524–1531.
| Identification of dihydropyrimidinase-related protein 4 as a novel target of the p53 tumor suppressor in the apoptotic response to DNA damage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1els7Y%3D&md5=c1bdc780c9ecc07b5afda33957322ebfCAS | 20499313PubMed |
Kudo, T., and Sutou, S. (1997). Molecular cloning of chicken FTZ-F1-related orphan receptors. Gene 197, 261–268.
| Molecular cloning of chicken FTZ-F1-related orphan receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlsFGmsL8%3D&md5=dd15c6ee997adeae0930e13e56259aa9CAS | 9332374PubMed |
Kumar, T. R., Wang, Y., Lu, N., and Matzuk, M. M. (1997). Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 15, 201–204.
| Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtVOks7c%3D&md5=2825d99f3f9b1aa021c4a74c96931c4bCAS | 9020850PubMed |
Labelle-Dumais, C., Paré, J. F., Bélanger, L., Farookhi, R., and Dufort, D. (2007). Impaired progesterone production in Nr5a2+/– mice leads to a reduction in female reproductive function. Biol. Reprod. 77, 217–225.
| Impaired progesterone production in Nr5a2+/– mice leads to a reduction in female reproductive function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1OnsLg%3D&md5=fb2f38a5b58e4bb612a1ef0b1e447f53CAS | 17409375PubMed |
Le, J. A., Wilson, H. M., Shehu, A., Mao, J., Devi, Y. S., Halperin, J., Aguilar, T., Seibold, A., Maizels, E., and Gibori, G. (2012). Generation of mice expressing only the long form of the prolactin receptor reveals that both isoforms of the receptor are required for normal ovarian function. Biol. Reprod. 86, 86.
| Generation of mice expressing only the long form of the prolactin receptor reveals that both isoforms of the receptor are required for normal ovarian function.Crossref | GoogleScholarGoogle Scholar | 22190699PubMed |
Lee, Y. K., Schmidt, D. R., Cummins, C. L., Choi, M., Peng, L., Zhang, Y., Goodwin, B., Hammer, R. E., Mangelsdorf, D. J., and Kliewer, S. A. (2008). Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol. Endocrinol. 22, 1345–1356.
| Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFajtrg%3D&md5=e0868577b89f34547f1d4e275693cc7cCAS | 18323469PubMed |
Lin, W.-W., Wang, H.-W., Sum, C., Liu, D., Hew, C. L., and Chung, B.-C. (2000). Zebrafish ftz-f1 gene has two promoters, is alternatively spliced, and is expressed in digestive organs. Biochem. J. 348, 439–446.
| Zebrafish ftz-f1 gene has two promoters, is alternatively spliced, and is expressed in digestive organs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksF2mtrc%3D&md5=f85dc768b7f8c3ef945d5c9de424e893CAS |
Luo, Y., Liang, C. P., and Tall, A. R. (2001). The orphan nuclear receptor LRH-1 potentiates the sterol-mediated induction of the human CETP gene by liver X receptor. J. Biol. Chem. 276, 24 767–24 773.
| The orphan nuclear receptor LRH-1 potentiates the sterol-mediated induction of the human CETP gene by liver X receptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlt1agtrg%3D&md5=d711fe91e3a419ade4ab8a1b6d49d5a0CAS |
Maman, E., Yung, Y., Cohen, B., Konopnicki, S., Dal Canto, M., Fadini, R., Kanety, H., Kedem, A., Dor, J., and Hourvitz, A. (2011). Expression and regulation of sFRP family members in human granulosa cells. Mol. Hum. Reprod. 17, 399–404.
| Expression and regulation of sFRP family members in human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvVKms7k%3D&md5=d8a60495e9dd796926156a3bd2dd6ce6CAS | 21307090PubMed |
Mataki, C., Magnier, B. C., Houten, S. M., Annicotte, J. S., Argmann, C., Thomas, C., Overmars, H., Kulik, W., Metzger, D., Auwerx, J., and Schoonjans, K. (2007). Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol. Cell. Biol. 27, 8330–8339.
| Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVeisL7L&md5=e74b8ff813a16a9e64a81cd1bf95d624CAS | 17908794PubMed |
Mueller, M., Cima, I., Noti, M., Fuhrer, A., Jakob, S., Dubuquoy, L., Schoonjans, K., and Brunner, T. (2006). The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 203, 2057–2062.
| The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptlSgs74%3D&md5=6c7660ccc503595dceef47f34f1fd682CAS | 16923850PubMed |
Nyegaard, M., Overgaard, M. T., Su, Y. Q., Hamilton, A. E., Kwintkiewicz, J., Hsieh, M., Nayak, N. R., Conti, M., Conover, C. A., and Giudice, L. C. (2010). Lack of functional pregnancy-associated plasma protein-A (PAPPA) compromises mouse ovarian steroidogenesis and female fertility. Biol. Reprod. 82, 1129–1138.
| Lack of functional pregnancy-associated plasma protein-A (PAPPA) compromises mouse ovarian steroidogenesis and female fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVCgt7g%3D&md5=46a810378cfd3e9e6c1c66e763b55a2eCAS | 20130263PubMed |
Oh, Y. S., Shin, S., Lee, Y. J., Kim, E. H., and Jun, H. S. (2011). Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors. PLoS One 6, e23894.
| Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1SqtL7L&md5=004a33d619ffb781f1b72365b47f0275CAS | 21897861PubMed |
Ortlund, E. A., Lee, Y., Solomon, I. H., Hager, J. M., Safi, R., Choi, Y., Guan, Z., Tripathy, A., Raetz, C. R., McDonnell, D. P., Moore, D. D., and Redinbo, M. R. (2005). Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat. Struct. Mol. Biol. 12, 357–363.
| Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivFWjtLg%3D&md5=fb421e05812b9660970ba6e7b32a150dCAS | 15723037PubMed |
Out, C., Hageman, J., Bloks, V. W., Gerrits, H., Sollewijn Gelpke, M. D., Bos, T., Havinga, R., Smit, M. J., Kuipers, F., and Groen, A. K. (2011). Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration. Hepatology 53, 2075–2085.
| Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsFCqtLs%3D&md5=aa21c62357f6b87f9ac7e7f8b836333dCAS | 21391220PubMed |
Pare, J. F., Malenfant, D., Courtemanche, C., Jacob-Wagner, M., Roy, S., Allard, D., and Belanger, L. (2004). The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J. Biol. Chem. 279, 21 206–21 216.
| The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvV2ntLg%3D&md5=60a0ee9981d1c70c358d1b82294d8125CAS |
Richards, J. S., and Pangas, S. A. (2010). The ovary: basic biology and clinical implications. J. Clin. Invest. 120, 963–972.
| The ovary: basic biology and clinical implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVChsrk%3D&md5=86ac5ed499d36554efa9be7e9aafc438CAS | 20364094PubMed |
Robker, R. L., and Richards, J. S. (1998). Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol. Endocrinol. 12, 924–940.
| Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXktlOisLo%3D&md5=4a9c0a8beabe37f0f5f85e1212c92bcbCAS | 9658398PubMed |
Robker, R. L., Russell, D. L., Espey, L. L., Lydon, J. P., O’Malley, B. W., and Richards, J. S. (2000). Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc. Natl Acad. Sci. USA 97, 4689–4694.
| Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXivFKjtLw%3D&md5=452860e47b66e6a835c2c625a5b9bb8dCAS | 10781075PubMed |
Rose, U. M., Hanssen, R. G., and Kloosterboer, H. J. (1999). Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol. Reprod. 61, 503–511.
| Development and characterization of an in vitro ovulation model using mouse ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkslKqt7g%3D&md5=4760dad7ddb55cd09fa2c9728e9f6d9dCAS | 10411533PubMed |
Schoonjans, K., Annicotte, J.-S., Huby, T., Botrugno, O. A., Fayard, E., Ueda, Y., Chapman, J., and Auwerx, J. (2002). Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 3, 1181–1187.
| Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVGmtLs%3D&md5=0c6d1fe44ff4802615d57528d16420acCAS | 12446566PubMed |
Schoonjans, K., Dubuquoy, L., Mebis, J., Fayard, E., Wendling, O., Haby, C., Geboes, K., and Auwerx, J. (2005). Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc. Natl Acad. Sci. USA 102, 2058–2062.
| Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhvFGru7Y%3D&md5=560c4a036c7b08d859b6247c4532aa6eCAS | 15684064PubMed |
Seibler, J., Kleinridders, A., Küter-Luks, B., Niehaves, S., Brüning, J. C., and Schwenk, F. (2007). Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 35, e54.
| Reversible gene knockdown in mice using a tight, inducible shRNA expression system.Crossref | GoogleScholarGoogle Scholar | 17376804PubMed |
Shehu, A., Mao, J., Gibori, G. B., Halperin, J., Le, J., Devi, Y. S., Merrill, B., Kiyokawa, H., and Gibori, G. (2008). Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol. Endocrinol. 22, 2268–2277.
| Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Sgt7bL&md5=f4c85beca03446dda1575518f724e406CAS | 18669642PubMed |
Shirasuna, K., Shimizu, T., Hayashi, K. G., Nagai, K., Matsui, M., and Miyamoto, A. (2007). Positive association, in local release, of luteal oxytocin with endothelin 1 and prostaglandin F2alpha during spontaneous luteolysis in the cow: a possible intermediatory role for luteolytic cascade within the corpus luteum. Biol. Reprod. 76, 965–970.
| Positive association, in local release, of luteal oxytocin with endothelin 1 and prostaglandin F2alpha during spontaneous luteolysis in the cow: a possible intermediatory role for luteolytic cascade within the corpus luteum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvFGmsrY%3D&md5=64bd0363552b95ca87ee9d69f11dbe6bCAS | 17287495PubMed |
Sicinski, P., Donaher, J. L., Geng, Y., Parker, S. B., Gardner, H., Park, M. Y., Robker, R. L., Richards, J. S., McGinnis, L. K., Biggers, J. D., Eppig, J. J., Bronson, R. T., Elledge, S. J., and Weinberg, R. A. (1996). Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384, 470–474.
| Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XnsFWqurc%3D&md5=d8306e276cefd9adfec1fb86d529e872CAS | 8945475PubMed |
Sirianni, R., Seely, J. B., Attia, G., Stocco, D. M., Carr, B. R., Pezzi, V., and And Rainey, W. E. (2002). Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J. Endocrinol. 174, R13–R17.
| Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnslGju74%3D&md5=c1a5e156b230e87347886d7af7682cf4CAS | 12208674PubMed |
Sriraman, V., Sinha, M., and Richards, J. S. (2010). Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol. Reprod. 82, 402–412.
| Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVSnsbs%3D&md5=c96e90597c2671d1c9e3b5f8a118c704CAS | 19726735PubMed |
Sun, L., Peng, Y., Sharrow, A. C., Iqbal, J., Zhang, Z., Papachristou, D. J., Zaidi, S., Zhu, L. L., Yaroslavskiy, B. B., Zhou, H., Zallone, A., Sairam, M. R., Kumar, T. R., Bo, W., Braun, J., Cardoso-Landa, L., Schaffler, M. B., Moonga, B. S., Blair, H. C., and Zaidi, M. (2006). FSH directly regulates bone mass. Cell. 125, 247–260.
| FSH directly regulates bone mass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xkt1Oqtbs%3D&md5=9eebca89cc8ab0762c0ccdd1f71cd1d9CAS | 16630814PubMed |
van Casteren, J. I., Schoonen, W. G., and Kloosterboer, H. J. (2000). Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol. Reprod. 62, 886–894.
| Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitFajt7o%3D&md5=e240bb8f2a6c4fc6298e76d8f6c78ba6CAS | 10727257PubMed |
Venteclef, N., Smith, J. C., Goodwin, B., and Delerive, P. (2006). Liver receptor homolog 1 is a negative regulator of the hepatic acute-phase response. Mol. Cell. Biol. 26, 6799–6807.
| Liver receptor homolog 1 is a negative regulator of the hepatic acute-phase response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Clur0%3D&md5=67cc8eb3e49ab4c5f31fd5f5ca4597a6CAS | 16943422PubMed |
Venteclef, N., Jakobsson, T., Steffensen, K. R., and Treuter, E. (2011). Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metab. 22, 333–343.
| Metabolic nuclear receptor signaling and the inflammatory acute phase response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1ymur0%3D&md5=99c2521d0146d7c56552896630686f9bCAS | 21646028PubMed |
Whitby, R. J., Dixon, S., Maloney, P. R, Delerive, P., Goodwin, B. J, Parks, D. J, and Willson, T. M. (2006). Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J. Med. Chem. 49, 6652–6655.
| Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtV2gtLnI&md5=21f05c844aade33d90440a7691f0c2b0CAS | 17154495PubMed |
Whitby, R. J., Stec, J., Blind, R. D., Dixon, S., Leesnitzer, L. M., Orband-Miller, L. A., Williams, S. P., Willson, T. M., Xu, R., Zuercher, W. J., Cai, F., and Ingraham, H. A. (2011). Small molecule agonists of the orphan nuclear receptors steroidogenic factor-1 (SF-1, NR5A1) and liver receptor homologue-1 (LRH-1, NR5A2). J. Med. Chem. 54, 2266–2281.
| Small molecule agonists of the orphan nuclear receptors steroidogenic factor-1 (SF-1, NR5A1) and liver receptor homologue-1 (LRH-1, NR5A2).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFyisrg%3D&md5=63d3a525b6524a6a0c4ae556bd2684e0CAS | 21391689PubMed |