Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation
Christopher Malcuit A , Marc Maserati B , Yoshiyuki Takahashi C , Raymond Page B and Rafael A. Fissore A DA Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003, USA.
B Cyagra Inc., Research and Development, 200 Westboro Rd, North Grafton, MA 01536, USA.
C Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Hokkaido University, Sapporo 060-0818, Japan.
D Corresponding author. Email: rfissore@vasci.umass.edu
Reproduction, Fertility and Development 18(2) 39-51 https://doi.org/10.1071/RD05131
Submitted: 3 October 2005 Accepted: 3 October 2005 Published: 14 December 2005
Abstract
Fertilisation by intracytoplasmic sperm injection (ICSI), a technique that bypasses the membrane fusion of the gametes, has been widely used to produce offspring in humans and mice. Success with this technique has lent support to the hypothesis that in mammalian fertilisation, a factor from the sperm, the so-called sperm factor, is responsible for oocyte activation and that the fusion process is not involved in the generation of the hallmark [Ca2+]i signalling seen following fertilisation. However, the success of ICSI has largely eluded large domestic species, such as the bovine, porcine and equine, casting doubt on the current model of oocyte activation at fertilisation in these species. Using Ca2+ imagery and a series of treatments to manipulate the chemical structure of the sperm, we have investigated the early events of oocyte activation in response to ICSI in the bovine. Our results demonstrate, for the first time, that following ICSI, the majority of bovine oocytes are unable to mount [Ca2+]i oscillations, although, in few cases, the initiation of [Ca2+]i oscillations can occur in a manner indistinguishable from in vitro fertilisation. We also show that bull sperm possess a full complement of sperm factor. However, either the release and/or activation of the sperm factor are compromised after ICSI, leading to the delivery of a defective Ca2+ stimulus, which results in premature termination of embryo development.
Introduction
In oocytes of all species studied to date, the fertilising sperm evokes a rise in the concentration of intracellular free calcium ions ([Ca2+]i). In mammals, this increase in [Ca2+]i takes the form of long-lasting oscillations, initiating shortly after sperm–oocyte fusion and terminating around the time of the first mitotic cell cycle. This series of [Ca2+]i oscillations is necessary and sufficient for completing the events of oocyte activation that, in general terms, comprises the exocytosis of cortical granule material (block to polyspermy), the resumption of meiosis and the initiation of embryonic development (for a review, see Schultz and Kopf 1995). Although the mechanism by which the sperm initiates these [Ca2+]i transients has not been fully elucidated, evidence supports the idea that shortly after fusion of the gametes, the sperm delivers a factor that then results in the activation of the phosphoinositide pathway. This factor, that nature of which is not yet known, has collectively been referred to as ‘sperm factor’ and is thought to act either as an activator of an egg phospholipase C (PLC) or is itself a PLC. Phospholipase C enzymes catalyse the hydrolysis of phosphatidyl 4,5-bisphosphate (PIP2) into the two signalling molecules, inositol 1,4,5-trisphosphate (IP3) and 1,2-diacyl glycerol (DAG). Whereas DAG plays a role in several signalling pathways, including protein kinase C activation, IP3 is a direct mediator of intracellular Ca2+ release by binding to its receptor, the IP3R, a tetrameric ligand-gated Ca2+ channel located on the smooth endoplasmic reticulum, the Ca2+ store of the cell.
Although there is consensus in the field for the need of persistent [Ca2+]i oscillations to exit meiosis in mammalian fertilisation, controversy remains regarding how the sperm initiates and maintains the signalling cascade responsible for the delivery of such a persistent stimulus. The advent of intracytoplasmic sperm injection (ICSI; Palermo et al. 1992), which bypasses fusion of the gametes and is able to induce a normal [Ca2+]i responses in the mouse (Nakano et al. 1997; Kurokawa and Fissore 2003), seemed to have consolidated the view that a soluble sperm factor(s) diffuses from the sperm head into the ooplasm and is responsible for triggering the oscillations (Swann 1990). Intracytoplasmic sperm injection has been used widely for the production of offspring in species ranging from the mouse (Kimura and Yanagimachi 1995) to the human (Devroey and Van Steirteghem 2004). However, success with this technique has been very limited in large domestic livestock species, namely the bovine, porcine and equine (Suttner et al. 2000; Bedford et al. 2003; Choi et al. 2004). With the exception of the birth of several calves (Goto et al. 1990; Horiuchi et al. 2002; Wei and Fukui 2002; Galli et al. 2003), conventional ICSI techniques have failed to produce physiological rates of embryonic and fetal development in the absence of an exogenous activation stimulus, such as calcium ionophore and an inhibitor of M-phase cyclins (or their respective cyclin-dependent kinases; Rho et al. 1998; Chung et al. 2000; Suttner et al. 2000). Therefore, the shortcomings of this technique in species such as the bovine pose serious questions as to the mechanism of oocyte activation in these species and raise valid concerns regarding the identity of the sperm factor(s) and its mechanism of activation and/or release in large domestic species. Notably, a recent report has demonstrated that bull sperm subjected to ICSI do not undergo comparable dissolution of the sperm perinuclear theca, a dense cytoskeletal matrix contiguous with the nucleus, as is seen during in vitro fertilisation (IVF; Sutovsky et al. 2003). Because it has been hypothesised that the active sperm factor molecule resides in the perinuclear theca (Kimura et al. 1998; Perry et al. 2000), the inability of the egg to disassemble critical regions of this structure may prevent the release of the active molecule and preclude oocyte activation. With regard to the identity of the sperm factor, a sperm-specific PLC, namely PLCζ, has been identified recently in mouse sperm and it is believed to represent the sperm factor (Saunders et al. 2002). Phospholipase Cζ is expressed in bull sperm (Malcuit et al. 2005) and it is possible that the release or activation of PLCζ may be compromised during ICSI in large domestic species.
If, indeed, the absence of release of the active sperm factor/PLCζ is the limiting factor in bovine ICSI, the very early events of egg activation, namely the presence of [Ca2+]i oscillations and the underlying production of IP3, should not take place. Therefore, in an effort to address the mechanistic failures inherent to bovine ICSI, we made use of Ca2+ imaging to determine whether sperm injected into the ooplasm of bovine oocytes are capable of initiating and maintaining [Ca2+]i oscillations, as seen during fertilisation (Fissore et al. 1992; Nakada et al. 1995). We also sought to determine whether chemical manipulation of the physical properties of the sperm structure would facilitate the initiation of [Ca2+]i release after this procedure. Finally, we investigated whether the rates of preimplantation embryo development after ICSI corresponded with the [Ca2+]i responses triggered by this method in the bovine.
Materials and methods
Animal care and welfare
Animals used for gamete collections for the present studies were handled according to the National Research Council Animal Care and Welfare guidelines and these procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts.
Collection of gametes
Germinal vesicle-stage bovine oocytes were isolated from cow ovaries collected from an abattoir located in the mid-western region of the USA. Oocytes were isolated from follicles 2–8 mm in diameter and only those with several layers of compact cumulus cells were selected. Following isolation, oocytes were placed into vials in 2 mL TCM-199 medium (Gibco, Grand Island, NY, USA), supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA), 0.1 U mL−1 luteinising hormone (LH; Sioux Biochemical, Sioux Center, IA, USA) and 1 μg mL−1, oestradiol for 20 h. Oocytes were shipped overnight at 39°C in a portable incubator (Mini Tube of America, Verona, WI, USA). At approximately 20 h after the onset of maturation, oocytes for ICSI experiments were denuded of their cumulus cells by brief vortexing in Tyrode's lactate medium (Parrish et al. 1988) supplemented with 0.3% Fraction V bovine serum albumin (BSA) and 0.001% hyaluronidase (Sigma, St Louis, MO, USA; all chemicals were obtained from Sigma unless indicated otherwise). Cumulus-free oocytes were held in KSOM (Speciality Media, Phillipsburg, NJ, USA) supplemented with ½× amino acids (Life Technologies, Invitrogen, Carlsbad, CA, USA) and 0.1% Fraction V BSA. Cumulus-enclosed oocytes to be used for IVF were washed for 5 min in Tyrode's Albumin Lactate Puruvate (TALP)–HEPES (Eid et al. 1994) and were then transferred into fertilisation medium (Eid et al. 1994). Bull sperm was graciously donated by Dr Marvin Pace (American Breeder Services, DeForest, WI, USA). Live, motile sperm were separated from cryoprotectant using the Percoll method (Parrish et al. 1985).
Mouse oocytes were collected from B6D2F1 female mice between 8 and 12 weeks of age. Mice were superovulated by sequential injections of 5 IU equine chorionic gonadotrophin (eCG) followed by 5 IU human chorionic gonadotrophin (hCG), as described previously (Wu et al. 1998). Metaphase II (MII) oocytes were collected from the oviducts of stimulated females 14 h following the administration of hCG into Tyrode's Lactate (TL)–HEPES supplemented with 10% FCS. Cumulus cells were removed by exposure to 0.025% hyaluronidase for 3–5 min followed by washing in TL–HEPES. Mouse sperm was obtained from the cauda epididymis of 12- to 16-month-old CD-1 males and collected into injection buffer (IB; 75 mm KCl and 20 mm HEPES, pH 7.0).
Boar sperm was obtained from a fresh ejaculate at the University of Massachusetts’ barn and was washed and stored in IB at −80°C for no longer than 1 week before use.
Sperm treatments
Several treatments were applied to sperm before ICSI. All working solutions were prepared fresh daily and sperm were washed several times in IB after treatment, before injection. Sperm decapitation was accomplished by sonication using an XL2020 sonicator (Heat Systems, Farmingdale, NY, USA; Wu et al. 1998) on a setting of 3 for 30 s on ice. Triton X-100 (TX-100), a mild detergent, was used to permeabilise sperm heads. The TX-100 was diluted in IB at a concentration of 1% and sperm were incubated in this solution for 15 min at room temperature. Dithiothreitol (DTT), a reducing agent, was prepared at a concentration of 15 mm in TALP–HEPES and sperm were incubated in this solution for 1 h at room temperature. Capacitation was performed in fertilisation medium containing 6 mg mL−1 fatty acid-free BSA, 25 mm Na2HCO3, 10 μg mL−1 heparin, 1 mm dibutyryl cAMP and 100 μm 3-isobutyl-1-methylxanthine (IBMX; Galantino-Homer et al. 1997) for 2 h at 39°C in 5% CO2. The acrosome reaction was induced by the addition of 5 μm ionomycin (Calbiochem, San Diego, CA, USA) for 10 min immediately after the 2 h capacitation incubation (Larson and Miller 1999).
Intracytoplasmic sperm injection
Intracytoplasmic sperm injection was performed as described previously (Kimura and Yanagimachi 1995; Knott et al. 2003) with some modifications. Briefly, either intact or treated sperm (see above) were resuspended in an arbitrary volume of IB, to which an equal volume of 12% polyvinyl pyrrilidone (PVP) was added. The sperm/PVP suspension was placed in a 30-μL drop onto a 100-mm Petri dish along with 50-μL drops of either TL–HEPES (for bovine) or Chatot-Ziomek-Bavister (CZB)–HEPES (for mouse), each supplemented with 0.05% polyvinyl alcohol (PVA). Bovine oocytes were centrifuged in 200 μL TL–HEPES for 8 min at 5000g to polarise lipid droplets to allow visualisation of sperm injection. Intracytoplasmic sperm injection was performed on a Nikon (Tokyo, Japan) TE200 inverted microscope under 200× phase-contrast magnification. Sperm were aspirated into a 12-μm (outside diameter) blunt, glass pipette preloaded with 1 cm Hg. Oocytes were positioned with a fire-polished holding pipette (75 μm outside diameter) with the first meiotic polar body to either the 12 or 6 o’clock position to avoid disruption of the actin-rich network at the spindle pole. The injection pipette containing sperm was placed against the zona pellucida and a single pulse of a PiezoDrill (Burleigh Instruments, Rochester, NY, USA) was delivered with gentle aspiration to facilitate zona pellucida (ZP) penetration. Once through the ZP, the pipette was placed approximately three-quarters across the oocyte and the oolema was aspirated gently into the injection pipette while applying a second pulse of the PiezoDrill to break the oolema. The sperm was then deposited in the ooplasm and the pipette withdrawn.
In vitro fertilisation
In vitro fertilisation was performed as described previously (Damiani et al. 1996; Sutovsky et al. 1997; Knott et al. 2003). Briefly, cumulus-enclosed oocytes were washed for 5 min in TALP–HEPES, picked up in a 2-μL volume and then placed into 44-μL drops equilibrated fertilisation medium under oil. Oocytes were allowed to incubate for 1 h before insemination. Sperm was prepared by the Percoll method, as described above. Briefly, sperm were centrifuged for 20 min at 400g through a discontinuous gradient of 45% and 90% Percoll. Live, motile sperm were collected from the pellet and sperm was diluted appropriately in TALP–HEPES to yield a final concentration of 1 × 106 in the insemination drop. Immediately following insemination, heparin, diluted in TALP–HEPES, was added to the insemination drops to a final concentration of 10 μg mL−1. For [Ca2+]i-monitoring experiments, inseminated oocytes were removed from the fertilisation medium 8 h after insemination and were denuded of cumulus cells by brief vortexing in warm TALP–HEPES.
Parthenogenetic activation of bovine oocytes
At 26 h after the onset of in vitro maturation, bovine oocytes were selected for the presence of a first meiotic polar body. These oocytes were then placed into a solution of TL–HEPES containing 5 μm ionomycin (Calbiochem) for 4 min. Following several washes in TL–HEPES, ionomycin-treated oocytes were placed into a solution of KSOM (described below) containing 5 μg mL−1 dihydro cytochalasin B (Calbiochem) and 10 μg mL−1 cyclohexamide (Calbiochem) for 6 h at 38.5°C in 5% CO2 in air. After four consecutive washes through TL–HEPES, activated oocytes were placed into culture dishes containing KSOM (see culture methods below).
Embryo culture
Immediately after fertilisation, ICSI and parthenogenetic activation, all zygotes were cultured in KSOM containing 1 mg mL−1 BSA and supplemented with ½× essential and non-essential amino acids (Life Technologies) until Day 3 of culture. On Day 4, embryos were transferred to the above medium supplemented with 10% heat-treated FCS (Hyclone, Logan, UT, USA) and cultured for an additional 4 days. All culture was performed at 38.5°C in 5% CO2, 5% O2 and a balance of 90% N2.
Measurement of [Ca2+]i
Bovine and mouse oocytes were injected with the fluorescent dye Fura-2 dextran or loaded with 1 μm Fura-2 acetoxymethylester (Fura-2 AM; Molecular Probes, Eugene, OR, USA) supplemented with 0.02% Pluronic F-127 (Molecular Probes) at room temperature for 20 min. The Ca2+ imaging was performed as described elsewhere (Fissore et al. 1992). In brief, excitation wavelengths were at 340 and 380 nm and the light emitted was quantified, after passing through a 500-nm barrier filter. Neutral density filters attenuated the intensity of the excitation light and the fluorescent signal was averaged for the whole cell. Oocytes were monitored in groups of six to nine in 40-μL drops of TL–HEPES on a glass coverslip on the bottom of a Petri dish covered with paraffin oil. Fluorescence ratios were taken every 20–30 s for various time-points depending on the experiment and are reported as the ratios of 340/380 nm fluorescence.
Assessment of oocyte activation
Approximately 16 h after insemination or 12 h after ICSI, the ZP of the zygotes was removed by brief incubation in acid Tyrode's solution, pH 1.8, at room temperature. After removal of the ZP, zygotes were placed into 3% paraformaldehyde containing 0.25% TX-100. Fixation was performed for 30–60 min at room temperature. Embryos were washed extensively in phosphate-buffered saline (PBS) containing 0.3% PVP for 12–14 h. Groups of up to 10 embryos were then mounted on glass slides in 90% glycerol containing 0.5 mg mL−1 Hoechst 33358. Embryos were visualised for the presence of a pronucleus on a Nikon TE300 inverted microscope with ultraviolet (UV) fluorescence at a magnification of ×200.
Western blot technique
To assess the levels of intact IP3R-1 protein, five oocytes were added to 15 μL double-strength reducing Laemmli buffer (Laemmli 1970), as described previously (He et al. 1999; Jellerette et al. 2000). Samples were boiled for 3 min to denature proteins, loaded onto a 5% sodium dodecyl sulfate (SDS) polyacrylamide gel and proteins were separated by electrophoresis followed by transfer onto nitrocellulose membranes (Micron Separations, Westboro, MA, USA). Membranes were blocked in PBS containing 0.1% Tween (PBST) supplemented with 5% non-fat dry milk for 1.5 h and incubated overnight with one of two rabbit polyclonal antibodies raised against the same C-terminal region of IP3R-1 diluted in PBST (Parys et al. 1995). Membranes were subsequently washed in PBST, followed by incubation for 1 h with a goat anti-rabbit secondary antibody coupled to horseradish peroxidase. Membranes were incubated for 1 min in cheminoluminescence reagent (NEN Life Science Products, Boston, MA, USA) and developed according to the manufacturer’s instructions. Prelabelled molecular weight markers were run in parallel to estimate the molecular weight of the IP3R-1 bands. The intensities of IP3R-1 bands were assessed using a Kodak 440 Image Station (Eastman Kodak, Rochester, NY, USA) and plotted using Sigma Plot (Jandel Scientific Software, San Rafael, CA, USA). The IP3R-1 band from MII oocytes was arbitrarily given the value of 100% and the values from the bands in other lanes were expressed relative to 100%.
Statistical analysis
Statistical analysis was performed for all experiments using a two-tailed t-test assuming unequal variances. Only those values within the 5% confidence interval (P < 0.05) were considered to be statistically significant. All values presented herein are displayed as the mean + the standard error above the mean.
Results
Bull sperm initiate an incomplete [Ca2+]i response after intracytoplasmic sperm injection
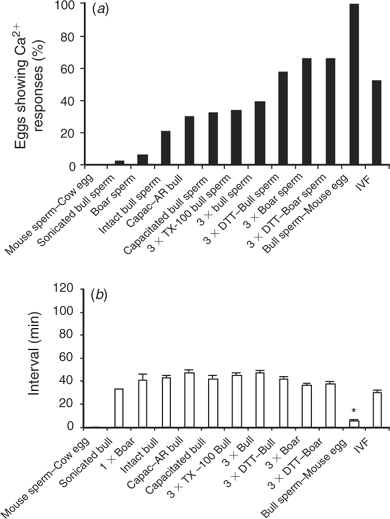
The [Ca2+]i oscillations are required to induce oocyte activation in mammals (for reviews, see Schultz and Kopf 1995; Ducibella et al. 2002). Coincidentally, a dominant feature of oocytes of large domestic species subjected to ICSI is the lack of activation and cleavage (Suttner et al. 2000; Wei and Fukui 2002). Hence, to determine whether the lack of efficiency of ICSI in the bovine could be ascribed, at least in part, to failure to initiate appropriate [Ca2+]i responses, we compared the [Ca2+]i oscillations observed during IVF with those induced by ICSI. As expected, the majority of bovine eggs that underwent IVF showed [Ca2+]i oscillations, which were characteristically separated by prolonged intervals (Fig. 1a; Fissore et al. 1992). Importantly, injection of an intact bull sperm was unable to trigger Ca2+ release/oscillations in the majority of oocytes monitored (data not shown), although, in a few cases, a single [Ca2+]i transient was observed within 1 h of sperm injection (Fig. 1b).
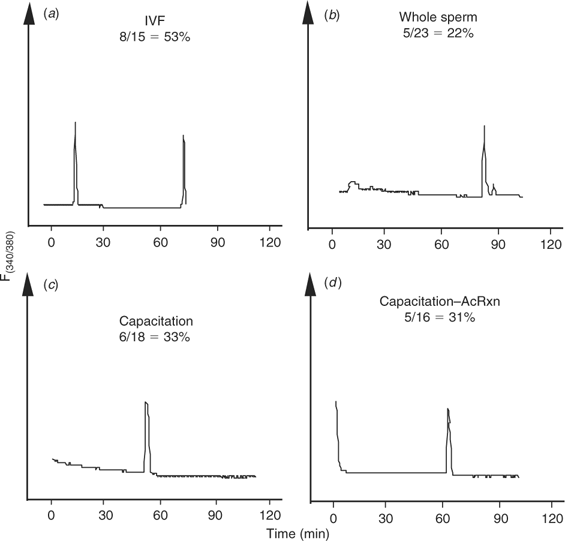
|
Capacitation and the acrosome reaction of bull sperm do not increase the incidence of [Ca2+]i increases after intracytoplasmic sperm injection
The demonstrated inability of bull sperm to initiate oscillations upon injection into bovine oocytes could be attributed to the fact that, during ICSI, certain physiological events that occur before sperm entry are bypassed. For instance, it is well known that mammalian sperm must undergo capacitation and the acrosome reaction to penetrate the oocyte vestments and fuse with the oolema. Accordingly, capacitation and the acrosome reaction were induced in bull sperm before ICSI and [Ca2+]i monitoring. To induce capacitation, sperm were incubated in TALP–HEPES containing dbcAMP, IBMX, 6 mg mL−1 BSA, 10 μg mL−1 heparin and 25 mm NaHCO3 for 2 h at 38°C (Wu et al. 1998). Western blot analysis for tyrosine-phosphorylated proteins, a reliable indicator of sperm capacitation (Visconti et al. 1995), confirmed that capacitation was taking place under our conditions and the maximal intensity of this signal was seen by 2 h (data not shown). After exposure to capacitating conditions, sperm were washed several times in TALP–HEPES and were resuspended in IB–PVP for ICSI. To induce the acrosome reaction, capacitated sperm were treated with 5 μm ionomycin for 10 min in TALP–HEPES, washed and prepared as above. Capacitated and acrosome-reacted sperm (Fig. 1c,d, respectively) injected into MII oocytes failed to consistently initiate repetitive [Ca2+]i oscillations as those observed during IVF. Collectively, these results demonstrate that conventional ICSI techniques are not capable of initiating the [Ca2+]i oscillations typical of those seen after fertilisation in the bovine and that neither capacitation nor the acrosome reaction of sperm induced by in vitro protocols increase the Ca2+-releasing ability of bull sperm.
Bull sperm contain a full complement of [Ca2+]i oscillation-inducing activity
Given the inability of bull sperm to initiate oscillations upon injection into bovine oocytes, it could be thought that, unlike the sperm of other species, bull sperm do not contain the so-called sperm factor. Even though it has been shown that bull sperm has the ability to initiate oscillations upon injection into mouse oocytes (Knott et al. 2003), we further extended those results here by showing that injection of decapitated bull sperm did, indeed, initiate high-frequency oscillations in mouse oocytes (Fig. 2a). Conversely, injection of mouse sperm into bovine oocytes failed to initiate [Ca2+]i responses in this species (Fig. 2b), indicating that the inability to induce oscillations after ICSI in large domestic species may be due to a combination of both sperm and oocyte factors.
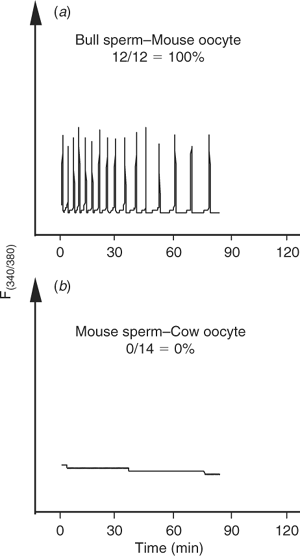
|
Polyspermy increases the number of eggs responding to intracytoplasmic sperm injection, but not the frequency of [Ca2+]i oscillations
Having demonstrated that bull sperm contain a full complement of sperm factor, a possible interpretation of the failure to initiate oscillations in bovine oocytes is that the factor is either not released and/or activated when fertilisation is accomplished via sperm injection. Hence, to test the first possibility, we injected three bull sperm heads into cow oocytes with the notion that partial release from more than one sperm may restore the normal pattern of oscillations. Sperm heads were injected rather than whole sperm because injection of three whole sperm would have required the injection of large volumes likely to result in cell lysis. The injection of three sperm heads increased the proportion of oocytes showing [Ca2+]i responses (12/30 (40%); Fig. 3b) compared with oocytes injected with one sperm head (1/33 (3%); Fig. 3a). Nonetheless, the frequency of oscillations was not greatly affected by this treatment and, only in a few occasions, two [Ca2+]i transients were observed separated by prolonged intervals (Fig. 3b). This result suggests that some of the active sperm factor molecules are released from the sperm, albeit not in the concentrations required to initiate [Ca2+]i oscillations; therefore, injection of three sperm was able to compensate somewhat for this inadequacy.
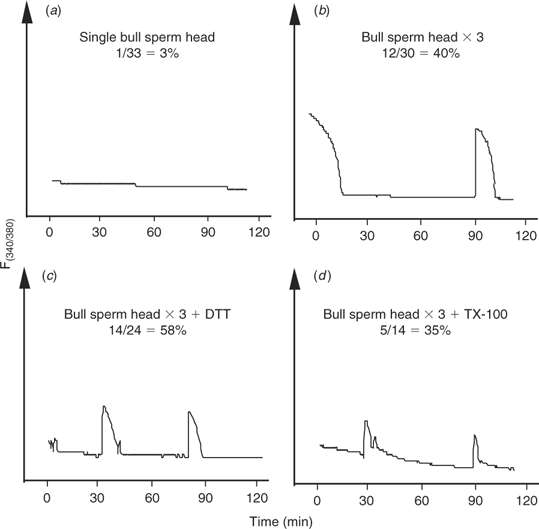
|
Partial sperm head decondensation and disruption of the sperm plasma membrane increases the number of eggs responding to polyspermic intracytoplasmic sperm injection
Although sperm decapitation may facilitate the contact of the sperm membranes with the ooplasm, and consequently enhance the release of the sperm factor, we sought to further stimulate this interaction by artificially manipulating the physical and chemical structure of the sperm by various chemical treatments before ICSI. We first investigated the effect of reducing the disulfide bonds of sperm protamines with DTT, because this treatment has been shown to enhance sperm head decondensation (Perreault et al. 1988) and development after ICSI (Galli et al. 2003). Injection of DTT-treated sperm, which displayed a swelling of the sperm head, as described previously (Kimura et al. 1998), increased the overall number of oocytes displaying [Ca2+]i responses (14/24 (58%); Fig. 3c) but did not consistently initiate oscillations in the injected oocytes. We next chose to permeabilise the sperm’s membranes with the hope of increasing the access of the ooplasm to the perinuclear matrix, which is the structure thought to harbor the sperm’s active Ca2+-releasing molecule (Kimura et al. 1998; Perry et al. 2000). To accomplish this, we exposed sperm to TX-100, followed by polyspermic injection as above. As shown in Fig. 3d, TX-100 treatment of sperm did not appear to increase either the number of responding oocytes or the frequency of [Ca2+]i oscillations. Collectively, these results suggest that factors downstream of the release of sperm factor, such as activation and/or substrate availability, may limit the initiation of [Ca2+]i responses in bovine ICSI.
Porcine sperm are capable of eliciting persistent [Ca2+]i oscillations in cow oocytes after intracytoplasmic sperm injection
Because boar sperm seemingly contain greater Ca2+-releasing ability than both mouse and bull sperm when injected into mouse oocytes (Fig. 4a), we investigated whether some of the treatments tested previously with bull sperm, if applied to boar sperm, could induce fertilisation-like oscillations upon injection into bovine oocytes. In support of this approach, we have shown previously that injection of boar sperm extracts into bovine oocytes initiated fertilisation-like oscillations (Knott et al. 2002). Whereas ICSI with a single boar sperm was unable to increase the frequency of oscillations or the number of responding oocytes (Fig. 4b) compared with injection of a single monospermic bull sperm (from Fig. 1b), the injection of three boar sperm, whether treated or not with DTT, resulted in an increase in both the number of responding oocytes and the presence of oscillatory oocytes (Fig. 4c,d). These results suggest that bovine oocytes can mount oscillatory responses upon sperm injection, although it appears that either the release and/or activation of the bull sperm factor is compromised after injection into these oocytes.
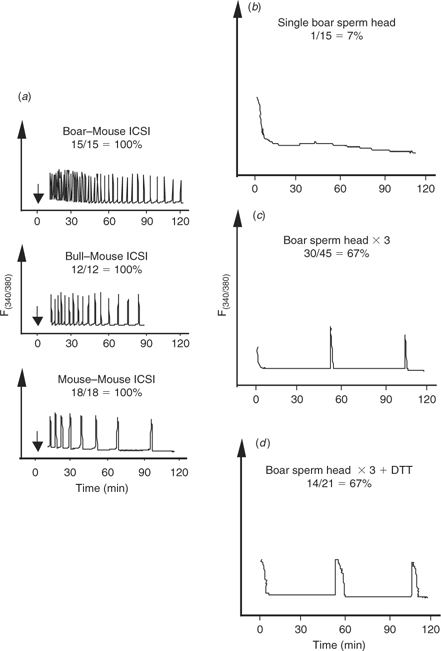
|
Failure of bovine intracytoplasmic sperm injection is due to the inability to induce sustained [Ca2+]i oscillations
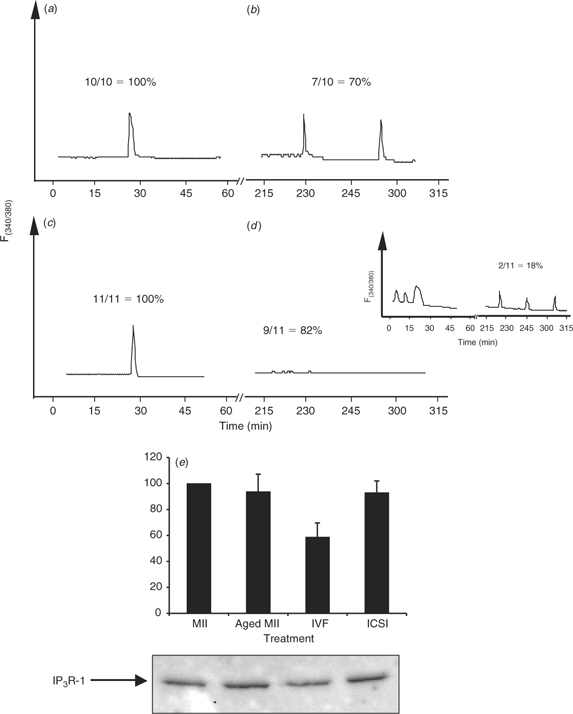
Recent studies have shown that the initiation and completion of the different oocyte activation events have different [Ca2+]i requirements, with late activation events, such as pronuclear formation and recruitment of maternal mRNAs, requiring many more [Ca2+]i increases than early activation events (Ducibella et al. 2002). Therefore, we investigated whether ICSI-derived bovine zygotes that initially displayed [Ca2+]i responses were able to sustain these oscillations, as would be expected to occur after IVF. To accomplish this, we used DTT-treated sperm because this is the only treatment that was able to increase the number of responding oocytes after injection of both bovine and porcine ICSI (Fig. 5a). Single bull sperm were injected to provide, as close as possible, a control to IVF and, as mentioned, only ICSI zygotes with an observed [Ca2+]i response were used for these comparisons. To control for synchronicity, insemintated eggs were assessed for sperm penetration by staining with Hoechst 33342 at 1 h intervals after IVF. Approximately 80% of the total fertilisation observed occurred between 6 and 8 h after insemination (data not shown). At 8 h after insemination, IVF embryos were denuded of their cumulus cells by brief vortexing in warm TL–HEPES, injected with Fura-2 dextran and transferred immediately to a [Ca2+]i imaging dish. After sufficient time had elapsed to determine an accurate number of zygotes oscillated for both ICSI and IVF, all oscillating oocytes for either ICSI or IVF were selected and transferred back into the incubator for a period of 2.5 h. After such time, these zygotes were returned to the microscope for further Ca2+ imaging. Whereas seven of 10 IVF zygotes continued to oscillate for an additional 2 h (time monitored; Fig. 6a,b), only two of 11 ICSI-derived zygotes showed persistent oscillations (Fig. 6c,d and inset). This result implies that one of the major limiting factors in bovine ICSI lies in the inability of the bull sperm to initiate persistent [Ca2+]i oscillations. Because IP3 production underlies the initiation of oscillations in mammalian fertilisation (Jellerette et al. 2000), we ascertained whether the concentration of IP3R-1 in bovine oocytes after ICSI has been reduced. Downregulation of IP3R-1, which is mediated by the proteasome (Oberdorf et al. 1999), is observed after fertilisation (Jellerette et al. 2000) and it is known to be induced exclusively by IP3 binding to the receptor (Zhu and Wojcikiewicz 2000). As shown in Fig. 6e, only IVF-derived zygotes displayed an observable decrease in the mass of intact IP3R-1, whereas ICSI-derived embryos contained similar amounts of IP3R-1 to both MII eggs and aged-matched control eggs (n = 2). This result confirms the hypothesis that bovine ICSI fails to induce robust IP3 production and that this is responsible for the partial [Ca2+]i responses observed after sperm injection in this species.
|
|
Intracytoplasmic sperm injection-derived bovine zygotes are capable of reaching the blastocyst stage
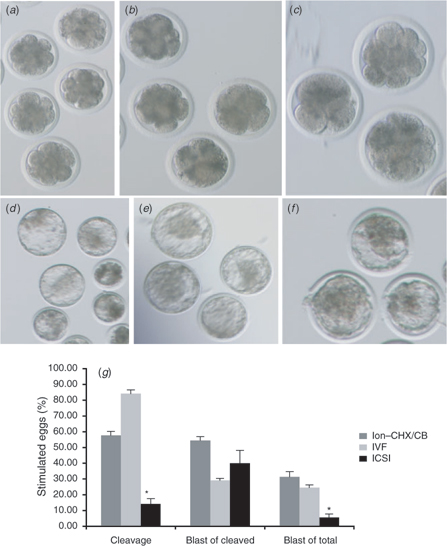
Notwithstanding the findings that ICSI-derived zygotes are not nearly as efficient as IVF-derived zygotes at producing normal [Ca2+]i oscillations, there is a small percentage of oocytes treated by ICSI that does manage to initiate persistent [Ca2+]i responses. In accordance with this observation, it is not surprising, then, that reports in the literature have shown that a small percentage of ICSI-derived zygotes is capable of reaching the blastocyst stage of development (Goto et al. 1990; Wei and Fukui 2002; Galli et al. 2003). Moreover, to confirm this, bovine ICSI (with DTT-treated sperm) and IVF-derived zygotes (produced by the same methodology described in the preceding section) were cultured for 8 days in KSOM, with 10% FBS supplemented at Day 4. As expected, IVF-derived embryos were capable of reaching the blastocyst stage (Fig. 7b,e,g) at levels comparable to parthenogenetic control embryos (Fig. 7a,d,g), whereas ICSI-derived embryos reached this stage at very low levels (Fig. 7c,f,g). However, the most interesting observation here is that the percentage of blastocysts from cleaved embryos was not significantly dissimilar between IVF and ICSI. It becomes apparent, then, that the most significant limiting factor for successful embryonic development in bovine ICSI is the inability to reach the early stages of development, most likely due to a defective activation stimulus caused by the abnormal [Ca2+]i responses that follow sperm injection.
|
Discussion
Intracytoplasmic sperm injection is a very powerful technique in the field of assisted reproduction and has been responsible for the production of thousands of human infants across the globe. Nevertheless, its application in agriculture and conservation biology has been greatly reduced by the low success rate reported for this method in species of economical importance. Specifically, oocyte activation and zygote cleavage are greatly reduced in ICSI-generated zygotes. Hence, it is of importance from an applied stand-point, as well as for a basic understanding of the mechanisms that underlie fertilisation in mammals, that we identify the signalling pathways and cellular events that occur during normal fertilisation but that fail to be activated during ICSI in large domestic species.
Towards this end, the results of our study indicate that ‘the bovine ICSI problem’ can be ascribed, at least in part, to one of the very earlier steps of fertilisation, namely the initiation of [Ca2+]i oscillations. We observed that less than 10% of all oocytes subjected to monospermic ICSI showed any [Ca2+]i responses. Also revealing were the findings that although injection of multiple sperm in conjunction with chemical treatments increased the overall number of eggs responding to ICSI, such treatments failed to increase the frequency of [Ca2+]i oscillations, which is expected in cases of polyspermic fertilisation (Figs 3–5). Finally, whereas the majority of oscillations initiated in response to IVF were sustained, as determined by extended monitoring, the [Ca2+]i responses initiated by ICSI failed to be so. Taken together, the results demonstrate that some of the signalling mechanisms that lead to the activation of the phosphoinositide pathway and generation of oscillations during natural fertilisation are not been replicated by ICSI. The latter is consistent with findings in mouse ICSI, which showed that, in spite of the fact that sperm injection induced normal rates of oocyte activation and embryo development, the pattern of [Ca2+]i oscillations and IP3 production, which was evaluated by assessing IP3R-1 degradation, appeared compromised (Kurokawa and Fissore 2003).
The inability to initiate oscillations and induce oocyte activation appears to be the main reason compromising the success of ICSI in the bovine. To assess the possible association of the presence of [Ca2+]i oscillations and preimplantation in in vitro embryo development, we performed IVF, as described, and ICSI using single sperm heads treated with DTT, which was the only treatment tested herein that increased [Ca2+]i responsiveness following injection of both bull and boar sperm. Interestingly, although the percentage of ICSI-derived zygotes reaching the blastocyst stage after this treatment was low compared with IVF-generated zygotes, this result was greatly influenced by the low incidence of early embryonic cleavage. However, analysis of the rate of ICSI-derived blastocysts out of cleaved embryos reveals that the rates of development between IVF and ICSI are similar (Fig. 7). Importantly, in both cases, the rates of development to the blastocyst stage closely corresponded to the percentage of oocytes that mounted persistent [Ca2+]i responses (Figs 1a,b, 6a–d). Moreover, the notion that appropriate activation may be the most important limiting factor in bovine ICSI also emanates from recent findings showing that parthenogenetic activation after ICSI greatly improves preimplantation embryo development and the production of calves (Oikawa et al. 2005). The culmination of all these results demonstrates that, if the proper stimulus is delivered following fertilisation, be it IVF or ICSI, the resulting zygote is capable of reaching late stages of preimplantation development.
The question that arises then, is why ICSI fertilisation in the bovine does not induce persistent [Ca2+]i oscillations? There are several factors that individually, or collectively, could account for oocyte activation failure in this species. The first possibility is that the release of the sperm factor is compromised after ICSI. Because the sperm undergoes significant changes before fertilisation, such as capacitation and the acrosome reaction, which are bypassed during ICSI, it could be envisioned that some of the molecular underpinning of these events also facilitates the release of the factor upon sperm entry. Our findings that the injection of several sperm partly overcame the faulty [Ca2+]i responses induced by ICSI support this contention. Nonetheless, polyspermic ICSI did not fully re-establish the oscillatory pattern, nor did any of the chemical treatments intended to facilitate the release of the factor. Moreover, our previous results in horse oocytes, which failed to initiate [Ca2+]i oscillations after ICSI but did so immediately after fusing to a mouse MII oocyte, suggest the successful delivery of the sperm factor into the ooplasm (Bedford et al. 2004). Hence, these results raise the possibility that the function and/or activation of the sperm factor after release may also be compromised after ICSI, thereby leading to incomplete [Ca2+]i responses. The mechanism(s) that regulates the function of the sperm factor remains to be established, because the identity of the putative molecule responsible for the sperm’s [Ca2+]i oscillatory activity remains to be fully established. The recent identification of a sperm-specific PLCζ in the mouse and the demonstration that its depletion from hamster sperm extracts abrogates the Ca2+ activity of the extract suggest that PLCζ may be the long sought-after active component of the sperm factor (Saunders et al. 2002). In agreement with this concept, our studies in the bovine showed that PLCζ is expressed in sperm and that injection of mouse PLCζ mRNA triggers fertilisation-like oscillations in this species (Malcuit et al. 2005). How the function of PLCζ is regulated in the sperm, as well as in the oocyte after its release, and whether the function of PLCζ is compromised after ICSI is not presently known. However, it is possible that the activity of PLCζ may be determined, at least in part, by its cellular distribution and/or by interaction with binding partners. With regard to the former, PLCζ possesses a nuclear localisation signal and nuclear sequestration during zygote formation has been shown to abrogate the [Ca2+]i oscillations (Larman et al. 2004); in the sperm, PLCζ seems to be localised to the post-acrosomal region (Fujimoto et al. 2004). With regard to interacting partners, it has been shown recently that the C2 domain of PLCζ has great binding affinity for certain phosphoinositide lipids, the binding to which reportedly inhibits in vitro PLCζ activity (Kouchi et al. 2004). Therefore, it is possible that the release of these inhibitory interactions may be required for the molecule to attain maximal function and that this ‘processing’ of PLCζ may occur more efficiently in sperm that undergo normal fertilisation. Finally, the possibility cannot be discounted that the fusion of gametes, which is circumvented during ICSI, may facilitate the generation of persistent oscillations. Although the success of ICSI in the human and mouse has all but ruled out a role for gamete fusion in the initiation of the signalling cascade leading to activation of the phosphoinositide pathway, a role for this event in the reorganisation of the oocyte cortex such that the PIP2 substrate becomes more accessible to PLCζ warrants further investigation.
In conclusion, our results demonstrate that a significant shortcoming of ICSI in the bovine lies directly with the inability of most injected sperm to mount the persistent and long-lasting [Ca2+]i oscillations that are required for proper embryonic development. Elucidation of the mechanism(s) that fails to be appropriately activated during ICSI not only promises to increase the efficiency of this technique in large domestic species, but will also help in the design of better parthenogenetic methods to be applied in cloning procedures and may aid in the unraveling of the signalling mechanism(s) that underlies mammalian fertilisation.
Acknowledgments
This project was supported, in part, by grants from the National Research Initiative Competitive Grants from the US Department of Agriculture (USDA; 2002-2078), the USDA/Hatch programme and from the National Institutes of Health RO3 to RAF.
Bedford, S. J. , Kurokawa, M. , Hinrichs, K. , and Fissore, R. A. (2003). Intracellular calcium oscillations and activation in horse oocytes injected with stallion sperm extracts or spermatozoa. Reproduction 126, 489–499.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bedford, S. J. , Kurokawa, M. , Hinrichs, K. , and Fissore, R. A. (2004). Patterns of intracellular calcium oscillations in horse oocytes fertilized by intracytoplasmic sperm injection: possible explanations for the low success of this assisted reproduction technique in the horse. Biol. Reprod. 70, 936–944.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Choi, J. Y. , Lee, E. Y. , Cheong, H. T. , Yoon, B. K. , Bae, D. S. , and Choi, D. S. (2004). Effects of activation timing on the fertilization rate and early embryo development in porcine ROSI procedure. J. Assist. Reprod. Genet. 21, 329–334.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chung, J. T. , Keefer, C. L. , and Downey, R. B. (2000). Activation of bovine oocytes following intracytoplasmic sperm injection (ICSI). Theriogenology 53, 1273–1284.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Damiani, P. , Fissore, R. A. , Cibelli, J. B. , Long, C. R. , Balise, J. J. , Robl, J. M. , and Duby, R. T. (1996). Evaluation of developmental competence, nuclear and ooplasmic maturation of calf oocytes. Mol. Reprod. Dev. 45, 521–534.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Devroey, P. , and Van Steirteghem, A. (2004). A review of ten years experience of ICSI. Hum. Reprod. Update 10, 19–28.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ducibella, T. , Huneau, D. , Angelichio, E. , Xu, Z. , Schultz, R. M. , Kopf, G. S. , Fissore, R. , Madoux, S. , and Ozil, J. P. (2002). Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev. Biol. 250, 280–291.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Eid, L. N. , Lorton, S. P. , and Parrish, J. J. (1994). Paternal influence on S-phase in the first cell cycle of the bovine embryo. Biol. Reprod. 51, 1232–1237.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fissore, R. A. , Dobrinsky, J. R. , Balise, J. J. , Duby, R. T. , and Robl, J. M. (1992). Patterns of intracellular Ca2+ concentrations in fertilized bovine eggs. Biol. Reprod. 47, 960–969.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fujimoto, S. , Yoshida, N. , Fukui, T. , Amanai, M. , Isobe, T. , Itagaki, C. , Izumi, T. , and Perry, A. C. (2004). Mammalian phospholipase C zeta induces oocyte activation from the sperm perinuclear matrix. Dev. Biol. 274, 370–383.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Galantino-Homer, H. L. , Visconti, P. E. , and Kopf, G. S. (1997). Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol. Reprod. 56, 707–719.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Galli, C. , Vassiliev, I. , Lagutina, I. , Galli, A. , and Lazzari, G. (2003). Bovine embryo development following ICSI: effect of activation, sperm capacitation and pre-treatment with dithiothreitol. Theriogenology 60, 1467–1480.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Goto, K. , Kinoshita, A. , Takuma, Y. , and Ogawa, K. (1990). Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet. Rec. 127, 517–520.
| PubMed |

He, C. L. , Damiani, P. , Ducibella, T. , Takahashi, M. , Tanzawa, K. , Parys, J. B. , and Fissore, R. A. (1999). Isoforms of the inositol 1,4,5-trisphosphate receptor are expressed in bovine oocytes and ovaries: the type-1 isoform is down-regulated by fertilization and by injection of adenophostin A. Biol. Reprod. 61, 935–943.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Horiuchi, T. , Emuta, C. , Yamauchi, Y. , Oikawa, T. , Numabe, T. , and Yanagimachi, R. (2002). Birth of normal calves after intracytoplasmic sperm injection of bovine oocytes: a methodological approach. Theriogenology 57, 1013–1024.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jellerette, T. , He, C. L. , Wu, H. , Parys, J. B. , and Fissore, R. A. (2000). Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev. Biol. 223, 238–250.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kimura, Y. , and Yanagimachi, R. (1995). Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 52, 709–720.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kimura, Y. , Yanagimachi, R. , Kuretake, S. , Bortkiewicz, H. , Perry, A. C. , and Yanagimachi, H. (1998). Analysis of mouse oocyte activation suggests the involvement of sperm perinuclear material. Biol. Reprod. 58, 1407–1415.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Knott, J. G. , Poothapillai, K. , Wu, H. , He, C. L. , Fissore, R. A. , and Robl, J. M. (2002). Porcine sperm factor supports activation and development of bovine nuclear transfer embryos. Biol. Reprod. 66, 1095–1103.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Knott, J. G. , Kurokawa, M. , and Fissore, R. A. (2003). Release of the Ca2+ oscillation-inducing sperm factor during mouse fertilization. Dev. Biol. 260, 536–547.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kouchi, Z. , Fukami, K. , Shikano, T. , Oda, S. , Nakamura, Y. , Takenawa, T. , and Miyazaki, S. (2004). Recombinant phospholipase C zeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 279, 10 408–10 412.
| Crossref | GoogleScholarGoogle Scholar |

Kurokawa, M. , and Fissore, R. A. (2003). ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol. Hum. Reprod. 9, 523–533.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Larman, M. G. , Saunders, C. M. , Carroll, J. , Lai, F. A. , and Swann, K. (2004). Cell cycle-dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLC zeta. J. Cell Sci. 117, 2513–2521.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Larson, J. L. , and Miller, D. J. (1999). Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 52, 445–449.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Malcuit, C. , Knott, J. G. , He, C. , Wainwright, T. , Parys, J. B. , Robl, J. M. , and Fissore, R. A. (2005). Fertilization and inositol 1,4,5-trisphosphate (ip3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol. Reprod. 73, 2–13.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nakada, K. , Mizuno, J. , Shiraishi, K. , Endo, K. , and Miyazaki, S. (1995). Initiation, persistence, and cessation of the series of intracellular Ca2+ responses during fertilization of bovine eggs. J. Reprod. Dev. 41, 77–84.
| Crossref | GoogleScholarGoogle Scholar |

Nakano, Y. , Shirakawa, H. , Mitsuhashi, N. , Kuwabara, Y. , and Miyazaki, S. (1997). Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol. Hum. Reprod. 3, 1087–1093.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oberdorf, J. , Webster, J. M. , Zhu, C. C. , Luo, S. G. , and Wojcikiewicz, R. J. (1999). Down-regulation of types I, II and III inositol 1,4,5-trisphosphate receptors is mediated by the ubiquitin/proteasome pathway. Biochem. J. 339, 453–461.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oikawa, T. , Takada, N. , Kikuchi, T. , Numabe, T. , Takenaka, M. , and Horiuchi, T. (2005). Evaluation of activation treatments for blastocyst production and birth of viable calves following bovine intracytoplasmic sperm injection. Anim. Reprod. Sci. 86, 187–194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Palermo, G. , Joris, H. , Devroey, P. , and Van Steirteghem, A. C. (1992). Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17–18.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parrish, J. J. , Susko-Parrish, J. L. , and First, N. L. (1985). Effect of heparin and chondroitin sulfate on the acrosome reaction and fertility of bovine sperm in vitro. Theriogenology 24, 537–549.
| Crossref | GoogleScholarGoogle Scholar |

Parrish, J. J. , Susko-Parrish, J. , Winer, M. A. , and First, N. L. (1988). Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parys, J. B. , DeSmedt, H. , Missiaen, L. , Bootman, M. D. , Sienaert, I. , and Casteels, R. (1995). Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium 17, 239–249.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Perreault, S. D. , Barbee, R. R. , Elatein, K. H. , Zucker, R. M. , and Keefer, C. L. (1988). Interspecies difference in the stability of mammalian sperm nuclei assessed in vivo by sperm microinjection and in vitro by flow cytometry. Biol. Reprod. 39, 157–167.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Perry, A. C. , Wakayama, T. , Cooke, I. M. , and Yanagimachi, R. (2000). Mammalian oocyte activation by the synergistic action of discrete sperm head components: induction of calcium transients and involvement of proteolysis. Dev. Biol. 217, 386–393.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rho, G. J. , Kawarsky, S. , Johnson, W. H. , Kochhar, K. , and Betteridge, K. (1998). Sperm and oocyte treatments to improve the formation of male and female pronuclei and subsequent development following intracytoplasmic sperm injection into bovine oocytes. Biol. Reprod. 59, 918–924.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Saunders, C. M. , Larman, M. G. , Parrington, J. , Cox, L. J. , Royse, J. , Blayney, L. M. , Swann, K. , and Lai, F. A. (2002). PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129, 3533–3544.
| PubMed |

Schultz, R. M. , and Kopf, G. S. (1995). Molecular basis of mammalian egg activation. Curr. Top. Dev. Biol. 30, 21–62.
| PubMed |

Sutovsky, P. , Oko, R. , Hewitson, L. , and Schatten, G. (1997). The removal of the sperm perinuclear theca and its association with the bovine oocyte surface during fertilization. Dev. Biol. 188, 75–84.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sutovsky, P. , Manandhar, G. , Wu, A. , and Oko, R. (2003). Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc. Res. Tech. 61, 362–378.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Suttner, R. , Zakhartchenko, V. , Stojkovitc, P. , Müller, S. , and Alberio, R. , et al. (2000). Intracytoplasmic sperm injection in bovine: effects of oocyte activation, sperm pre-treatment and injection technique. Theriogenology 54, 935–948.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Swann, K. (1990). A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110, 1295–1302.
| PubMed |

Visconti, P. E. , Bailey, J. L. , Moore, G. D. , Pan, D. , Olds-Clarke, P. , and Kopf, G. S. (1995). Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121, 1129–1137.
| PubMed |

Wei, H. , and Fukui, Y. (2002). Birth of calves derived from intercytoplasmic sperm injection without exogenous oocyte activation. Zygote 10, 149–153.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wu, H. , He, C. L. , and Fissore, R. A. (1998). Injection of a porcine sperm factor induces activation of mouse eggs. Mol. Reprod. Dev. 49, 37–47.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhu, C. C. , and Wojcikiewicz, R. J. (2000). Ligand binding directly stimulates ubiquitination of the inositol 1,4,5-trisphosphate receptor. Biochem. J. 348, 551–556.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



