76 SURVIVAL AND IN VITRO FUNCTIONALITY OF DOMESTIC CAT EPIDIDYMAL SPERMATOZOA FOLLOWING CRYOPRESERVATION IN EXTENDERS WITH OR WITHOUT EGG YOLK
J. R. Saenz A B , C. Dumas B , B. L. Dresser B C , M. C. Gómez B , R. A. Godke A and C. E. Pope BA Louisiana State University Agricultural Center, Baton Rouge, LA;
B Audubon Center for Research of Endangered Species, New Orleans, LA;
C University of New Orleans, New Orleans, LA
Reproduction, Fertility and Development 21(1) 138-139 https://doi.org/10.1071/RDv21n1Ab76
Published: 9 December 2008
Abstract
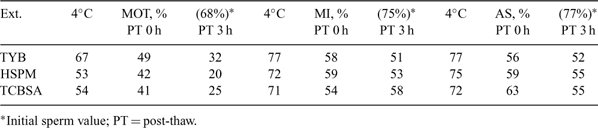
Our purpose was to compare in vitro survivability and functionality of cat epididymal spermatozoa cryopreserved in TEST egg-yolk buffered extender (TYB) with that obtained by use of clear Tris-citrate and HEPES-buffered extenders containing BSA. Testes were transported to the lab in HEPES saline; epididymides were dissected in HEPES-199 medium (HE-199) and repeatedly sliced. The sperm suspension was filtered (40 μm), layered onto a density gradient column (Isolater, Irving Scientific, Santa Ana, CA), and centrifuged at 600g for 20 min. Aliquots of the sperm pellet were extended in TYB, Human Sperm Preservation Medium (HSPM), or Tris-citrate + 10% BSA (TCBSA). After cooling to 4°C, samples were diluted 1:1 with extender + 12% glycerol in 4 steps as modified from Gao DY et al. 1995 Hum. Reprod. 10, 1109–1122. Then, samples were loaded into 0.25-mL straws, sealed, and frozen on a dry ice block (–80°C) for 20 min before storage in LN2. Straws were thawed by exposure to air (22°C) for 5 s and immersion in a 60°C water bath for 5 s. Samples were diluted by addition of HE-199 in 7 steps as modified from Gao DY et al. 1995 Hum. Reprod. 10, 1109–1122, centrifuged at 200g for 10 min and pellets resuspended in HE-199. Motility (MOT, phase contrast, 37°C), membrane integrity (MI, SYBR 14–PI), and acrosomal status (AS, FITC–PNA) were evaluated at 0 h, after gradual cooling to 4°C, and after freezing at 0 h and 3 h post-thaw (37°C). Cumulus oocyte complexes (COC) were placed in modified TCM-199 and cultured for 24 h in 5% O2, 5% CO2, and 90% N2 at 38°C (IVM). For IVF, COC were co-incubated with spermatozoa frozen in either TYB or HSPM in droplets (1 million sperm mL–1) of IVF medium under 5% CO2 in air at 38°C. After 18 h, oocytes were rinsed and cultured using a 3-step system (Pope CE et al. 2006 Theriogenology 66, 59–71) until blastocyst development was evaluated (Day 8). There were no treatment differences at any time/temperature point for the 3 sperm parameters evaluated (one-way ANOVA; P > 0.05). As shown in Table 1, sperm motility in TCBSA and HSPM decreased by 20% after cooling to 4°C and another 20% after freezing, whereas motility in TYB was maintained after cooling and decreased <30% after freezing. Membrane integrity and acrosomal status values were 12 to 15% greater at collection, at 4°C and at 0 h post-thaw, and 25% greater at 3 h post-thaw than were the motility values. Cleavage frequency and blastocyst development rate of 203 IVM oocytes after IVF using sperm frozen in TYB and HSPM was 36 v. 33% and 50 v. 44%, respectively. In summary, we have shown that cat epididymal spermatozoa can be frozen successfully in cryoprotectant solutions that do not contain egg yolk.
|


