240 EFFECTS OF TIMING AND DIPLOIDIZATION ON ACTIVATION AND DEVELOPMENT OF RAT OOCYTES
J. Zhu A , D. Amarnath A and K. Campbell ASchool of Biosciences, University of Nottingham, Loughborough, Leics, LE 12 5RD, UK
Reproduction, Fertility and Development 21(1) 218-218 https://doi.org/10.1071/RDv21n1Ab240
Published: 9 December 2008
Abstract
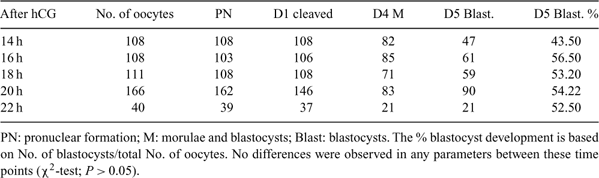
The objectives of these studies were (1) to compare the effects of Cytochalasin B (CB) or 6-dimethyl amino purine (DMAP) in combination with SrCl2 on activation and development of rat oocytes and (2) to determine the optimal timing for collection and activation of rat oocytes following superovulation. In Experiment 1, rat oocytes collected at 16–18 h after hCG injection were immediately cultured in 10 mm SrCl2 alone, 10 mm SrCl2 + 2 mm DMAP for 4 h and 10 mm SrCl2 + 5 μg mL–1 CB for 5–6 h, respectively, they were then cultured in KSOM for 20 h and finally cultured in R1ECM for an additional 4 days, all at 37°C, 5% CO2 in air. There were no significant differences in pronuclear formation (SrCl2 alone: 51/53; DMAP: 139/140; CB: 139/140) and cleavage rate (SrCl2 alone: 52/53; DMAP: 129/140; CB: 130/140) observed among three groups (χ2-test, P > 0.05). On Day 5 the frequency of development to blastocyst was not statistically different (P > 0.05) between DMAP (55.71%, 78/140) and CB (54.23%, 76/140) treated groups; however, no blastocysts were produced in 10 mm SrCl2 alone. In Experiment 2, oocytes were collected at 14, 16, 18, 20 and 22 hours after hCG injections, respectively, and they were immediately cultured in 10 mm SrCl2 + 2 mm DMAP for 4 h and then cultured in the same conditions described for Experiment 1. Although development to blastocyst was slightly lower in the 14-h group (43.50%, 47/108), no significant differences in the frequencies of pronuclear formation, cleavage on Day 1 or development to blastocyst on Day 5 were observed between groups (χ2-test, P > 0.05) (Table 1). All experiments were repeated at least three times. In conclusion, our results showed that SrCl2 combined with either CB or DMAP could equally, effectively activate rat oocytes. In contrast rat haploid oocytes activated in the absence of CB or DMAP were not able to develop to the blastocyst stage. Furthermore, rat oocytes collected between 14 and 22 h after hCG injection had an equal potential to develop to the blastocyst stage after activation by 10 mm SrCl2 + 2 mm DMAP.
|


