332 EFFECT OF DIFFERENT PIG OOCYTE ACTIVATION PROTOCOLS ON EMBRYO DEVELOPMENT IN SOF AND NCSU-23
I. Lagutina A , G. Lazzari A and C. Galli A BA Laboratorio di Tecnologie della Riproduzione1, CIZ srl, Istituto Sperimentale Italiano Lazzaro Spallanzani, Cremona, Italy
B Dipartimento Clinico Veterinario, Università di Bologna, Bologna, Italy
Reproduction, Fertility and Development 19(1) 281-282 https://doi.org/10.1071/RDv19n1Ab332
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
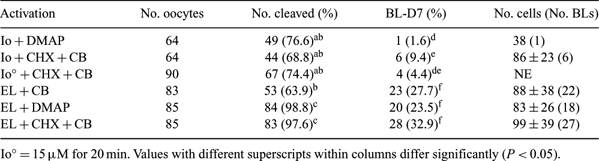
Several factors affect nuclear transfer success. These include efficient parthenogenetic activation and embryo culture medium that should efficiently support pre-implantation development of good quality blastocysts. We investigated pig oocyte activation and embryo development in SOFaa in response to ionomycin (Io = 5 µM Io for 4 min; Io° = 15 µM Io for 20 min) and electric impulse (EL; one 30-µs pulse of DC 1.5 kV cm−1 in the presence of 50 µM Ca) in combination with 2 mM 6-DMAP or 10 µg mL−1 cycloheximide (CHX) +5 µg mL−1 cytochalasin B (CB) for 4 h. In addition, we studied the effect of elevated (1 mM) (Cheong et al. 2002 Mol. Reprod. Dev. 61, 488) in comparison with 50 µM Ca during EL activation on embryo development in SOFaa and NCSUaa-23. Porcine oocytes were recovered from slaughtered donors and matured in vitro for 44 h in DMEM-F12 supplemented with 10% FCS, 0.05 IU LH and FSH (Menogon®, Ferring, Milan, Italy), 0.3 mM cystine, 0.5 mM cysteamine, 50 ng mL−1 long-EGF, 100 ng mL−1 long-IGF1, 5 ng mL−1 bFGF (Sigma-Aldrich, Milan, Italy) in 5% CO2 at 38.5°C. The rates of cleavage, blastocyst formation (BL) and BL cell number on Day 7 (BL-D7) were recorded. All experiments were done with 3 replicates. The data were compared by chi-square test. There was no difference in the ability of Io (all groups) and EL + CB activated oocytes to cleave, whereas the additional treatment of EL-activated oocytes with DMAP and CHX + CB significantly increased cleavage. Io activation resulted in poor blastocyst development in comparison with all EL-activated groups (see Table 1). When calcium levels were elevated during EL activation, significantly more embryos developed in SOFaa (35.6%, n = 191 vs. 26%, n = 192; P < 0.05), but no differences were observed with culture in NCSUaa-23 (about 56%). The BL rate was significantly higher in NCSUaa-23 vs. SOFaa (55.9%, n = 68 vs. 34.8%, n = 69, respectively); however, the BL total cell number was significantly higher in SOFaa (58 ± 18, n = 40 vs. 86 ± 35, n = 56, respectively; P < 0.05). In conclusion, we have found that SOFaa and NCSUaa-23 differ in ability to support pig parthenogenetic embryo development. EL activation combined with elevated Ca significantly increased the embryo developmental capacity in SOFaa but not in NCSUaa-23. NCSUaa-23 was more efficient for embryo culture, whereas SOF produced BLs of higher quality.
|
This work was supported by grants ISS-CS11 and Fondazione Cariplo.


